Abstract
Background
Cardiac troponin concentrations differ in women and men, but how this influences risk prediction and whether a sex-specific approach is required is unclear. We evaluated whether sex influences the predictive ability of cardiac troponin I and T for cardiovascular events in the general population.
Methods
High-sensitivity cardiac troponin (hs-cTn) I and T were measured in the Generation Scotland Scottish Family Health Study of randomly selected volunteers drawn from the general population between 2006 and 2011. Cox-regression models evaluated associations between hs-cTnI and hs-cTnT and the primary outcome of cardiovascular death, myocardial infarction, or stroke.
Results
In 19 501 (58% women, mean age 47 years) participants, the primary outcome occurred in 2.7% (306/11 375) of women and 5.1% (411/8126) of men during the median follow-up period of 7.9 (IQR, 7.1–9.2) years. Cardiac troponin I and T concentrations were lower in women than men (P < 0.001 for both), and both were more strongly associated with cardiovascular events in women than men. For example, at a hs-cTnI concentration of 10 ng/L, the hazard ratio relative to the limit of blank was 9.7 (95% CI 7.6–12.4) and 5.6 (95% CI 4.7–6.6) for women and men, respectively. The hazard ratio for hs-cTnT at a concentration of 10 ng/L relative to the limit of blank was 3.7 (95% CI 3.1–4.3) and 2.2 (95% CI 2.0–2.5) for women and men, respectively.
Conclusions
Cardiac troponin concentrations differ in women and men and are stronger predictors of cardiovascular events in women. Sex-specific approaches are required to provide equivalent risk prediction.
Keywords: sex, cardiac troponin, risk factors, cardiovascular events
Introduction
Cardiovascular disease remains the main cause of death worldwide with 17.6 million people dying each year (1, 2). The development of approaches to improve the prediction and targeting of effective preventative therapies for those at highest risk may help minimize the impact of cardiovascular disease on the population. It is important that these approaches are equitable for women and men (2). In both primary and secondary care, guidelines have been established in populations where men are over represented and women seem to be disadvantaged and receive fewer preventative treatments (3–5).
Cardiac biomarkers may provide an unbiased aproach toward the prediction of cardiovascular events in women and men. Increasingly, high-sensitivity cardiac troponin is considered a useful marker of risk outwith the setting of acute coronary syndromes to evaluate asymptomatic individuals and guide therapeutic approaches to prevent the onset of cardiovascular disease (6–11). Recent major improvements in analytical performance have greatly enhanced assay sensitivity, such that with high-sensitivity assays we are now able to accurately measure cardiac troponin concentrations in the majority of healthy individuals (12). In a recent meta-analysis of apparently healthy individuals, 43% of participants with cardiac troponin concentrations in the top third developed cardiovascular disease over the next 8 years (13).
It remains unclear in practice how best to harness this prognostic information to guide the use of primary and secondary prevention, and whether sex-specific troponin thresholds should be considered. The use of high-sensitivity assays has identified important differences in troponin concentrations between men and women, with the 99th centile upper reference limits used for diagnosis of myocardial infarction up to 2-fold higher in men (14). In our recent systematic review, we documented that this observation is consistent for all troponin assays across multiple cohorts from different ethnic backgrounds (15). Furthermore, we recently demonstrated in the Generation Scotland Scottish Family Health Study, where both cardiac troponin I and T were measured in the same cohort, that differences in the 99th centile between men and women exist for both biomarkers across all age groups (16). Although several studies have investigated cardiac troponins in relation to cardiovascular outcomes in the general population (7, 11, 13, 17, 18), few have evaluated how sex influences risk prediction, and it remains unclear whether a different approach is required in women and men. Our aim was to determine whether sex influences the predictive ability of cardiac troponin I and T for cardiovascular events in the general population.
Material and Methods
Study Population
The Generation Scotland Scottish Family Health Study is a well-phenotyped family-based cohort that enrolled 24 090 participants aged between 18 and 98 years and has been described previously (7, 16, 19). Briefly, individuals between 35 and 65 years of age were identified at random from participating general medical practices in Scotland between 2006 and 2011. Participants were asked to identify at least 1 first-degree relative who was at least 18 years of age that would also enrol. For this study, we excluded participants with missing cardiac troponin measurements. Study participants provided written informed consent, including linkage to their medical records. The study was conducted according to principles of the Declaration of Helsinki and was approved by the National Health Service Tayside Committee on Medical Research Ethics (REC Reference Number: 05/S1401/89). The study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Clinical Characteristics
Participants completed a health questionnaire, and had physical characteristics and clinical characteristics measured according to a standardized protocol (19). Past medical history, including a diagnosis of diabetes mellitus, previous myocardial infarction or stroke, and use of medications was self-reported. Family history of cardiovascular disease was defined as a self-report of parents or siblings having heart disease or stroke. Blood samples were taken, according to a standard operating procedure, and serum was prepared. Total cholesterol, high-density lipoprotein cholesterol, and serum creatinine, were measured at the time of collection, and additional aliquots were stored at –80 °C for future analyses. The Scottish Index of Multiple Deprivation (2009) scores were derived from participants’ postcodes: they denote nationally compiled composite measures of small-area deprivation (20).
Cardiac Troponin Measurements
Serum cardiac troponin I was measured on ARCHITECT i1000SR high-sensitivity cardiac troponin I assay (Abbott Diagnostics) and cardiac troponin T was measured on Cobas e411 high-sensitivity cardiac troponin T (Roche Diagnostics) assay. During the conduct of this study, we participated in the National External Quality Assurance Scheme (UKNEQAS) for these biomarkers. Both assays were calibrated and quality controlled using the manufacturer’s reagents. Coefficient of variations for cardiac troponin I were 6.2%, 6.0%, and 4.6% for the low, intermediate, and high control, respectively. Coefficients of variation for cardiac troponin T were 5.0% and 3.4% for the low and high control, respectively. Cardiac troponin T has a limit of blank (LoB) of 3 ng/L and limit of detection (LoD) of 5 ng/L. Cardiac troponin I has a LoB of 1.2 ng/L and LoD of 1.9 ng/L (21).
Clinical Outcome
We used the Information Services Division National Health Service record linkage for Scotland to collect clinical outcome data until the end of September 2017. Information on cause of death was obtained using the National Health Service Central Register. Clinical outcomes were classified using the 10th revision of the International Classification of Diseases (ICD-10). The primary outcome was a composite endpoint of cardiovascular events including the following component endpoints: (a) cardiovascular death (I00 to I99), (b) myocardial infarction (I21, I22), and (c) stroke (I63, I64, G45). Secondary outcomes were cardiovascular death, noncardiovascular death, and all-cause death.
Statistical Analysis
Continuous variables are presented as mean (SD) or median (25th–75th percentile), as appropriate. Categorical variables are presented as absolute numbers (%). For continous analyses, troponin values below the LoB were set to the LoB value divided by 2. The correlation between cardiac troponin I and T was assessed by Spearman correlation. Sex-specific incidence rates were calculated per 1000 person-years for clinical outcomes.
Statistical Learning Using Cox Proportional Hazard Regression Models
Unadjusted and adjusted multiple fractional polynomial Cox proportional hazard regression analysis were conducted to quantify the relationship between cardiac troponin as a continuous variable with the primary outcome, stratified by sex. The multivariable model is adjusted for age, total cholesterol, high-density lipoprotein cholesterol, systolic blood pressure, cigarettes smoked per day, rheumatoid arthritis, diabetes mellitus, Scottish Index of Multiple Deprivation score, family history of cardiovascular disease, use of blood pressure medications, and use of cholesterol-lowering medications. Each continuous variable was chosen through backward-stepwise selection of the best fractional polynomial transformation. We created hazard ratio (HR) plots for the primary outcome at 5 years of the unadjusted and adjusted cardiac troponin I and T models for women and men, and evaluated the HR relative to the LoB. Due to the low proportion of missing covariates (<6%) and the large number of available samples we did not use imputation techniques, focusing on complete case analysis instead. We constructed receiver operating characteristic (ROC) curves and determined the area under the curve (AUC) to assess discrimination of cardiac troponin for predicting the primary outcome at 5 years in women and men. Individuals with no events by the 5-year mark were censored. Comparisons between unpaired AUCs were tested according to the DeLong method. In secondary analyses, we evaluated the HR relative to the LoD, and we evaluated the additional outcomes of cardiovascular death, noncardiovascular death, and all-cause death. All statistical analysis was performed using R v.3.6.2.
Results
Clinical Characteristics of Study Population
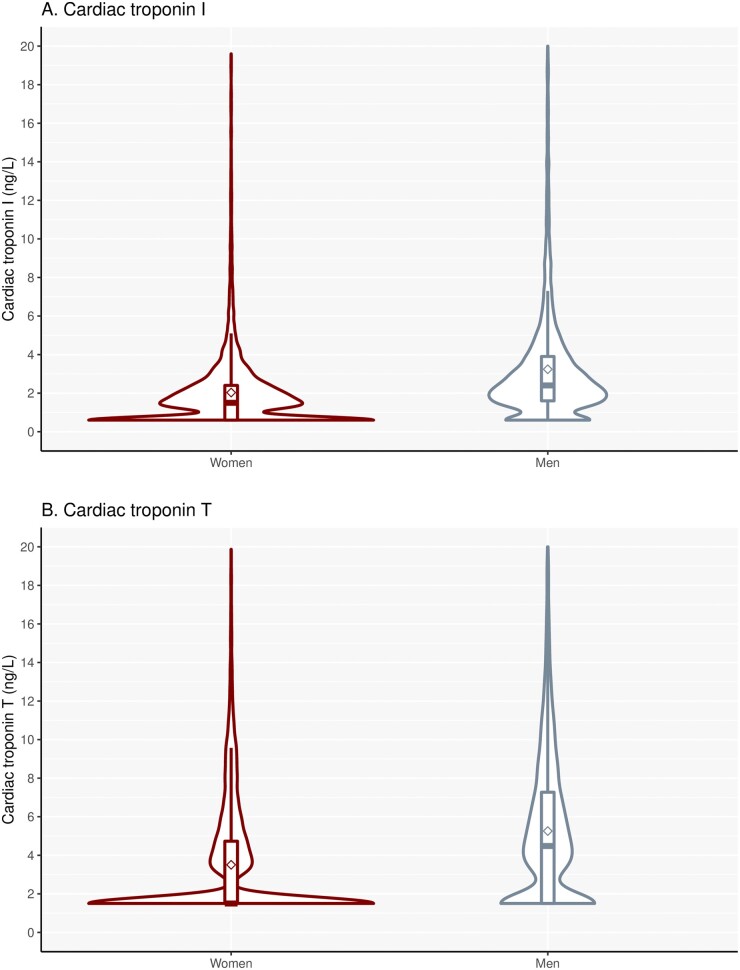
Our study population included 19 501 individuals (58% women; Table 1) with a measured cardiac troponin I and T concentration available. On enrolment women and men were at similar age (47 ± 15 years), but men were more likely to have risk factors, such as hypertension or diabetes mellitus, or to have a history of prior cardiovascular disease. Cardiac troponin concentrations were lower in women than men [cardiac troponin I, women 1.5 (1.2 to 2.5) ng/L versus men 2.5 (1.6 to 4.0) ng/L; cardiac troponin T, women ≤3.0 (3.0 to 4.8) ng/L versus men 4.6 (3.0 to 7.5) ng/L; P < 0.001 for both; Fig. 1]. The proportion of women and men with cardiac troponin I concentrations above the LoD was 66.1% (7523/11 375) and 86.8% (7056/8126), and for cardiac troponin T it was 42.4% (4826/11 375) and 68.5% (5569/8126). The correlation between cardiac troponin I and T concentrations was lower in women than men (r = 0.351, 95% CI 0.334 to 0.370 versus r = 0.446, 95% CI 0.428 to 0.463; P < 0.001).
Table 1.
Baseline characteristics of entire study population and stratified by composite cardiovascular events.
| Study population |
No incident cardiovascular event |
Incident cardiovacular event |
||||
|---|---|---|---|---|---|---|
| Women (n = 11 375) | Men (n = 8126) | Women (n = 11 069) | Men (n = 7715) | Women (n = 306) | Men (n = 411) | |
| Age (years) | 47 (15) | 47 (15) | 47 (15) | 46 (15) | 65 (14) | 62 (11) |
| Body mass index (kg/m2) | 26.5 (5.6) | 26.9 (4.5) | 26.5 (5.6) | 26.8 (4.4) | 27.8 (5.6) | 28.3 (4.8) |
| Systolic blood pressure (mmHg) | 128 (18) | 136 (16) | 128 (18) | 136 (16) | 141 (22) | 142 (19) |
| Total cholesterol (mmol/L) | 5.2 (1.1) | 5.0 (1.1) | 5.2 (1.1) | 5.0 (1.06) | 5.3 (1.3) | 4.9 (1.2) |
| HDL cholesterol (mmol/L) | 1.6 (0.4) | 1.3 (0.3) | 1.6 (0.4) | 1.3 (0.3) | 1.5 (0.4) | 1.2 (0.4) |
| SIMD (score/10) | 1.2 (0.7–2.4) | 1.1 (0.7–2.1) | 1.2 (0.7–2.4) | 1.1 (0.7–2.1) | 1.5 (0.8–2.9) | 1.4 (0.8–2.6) |
| eGFR (mL/min/1.73 m2) | 94 (17) | 96 (17) | 95 (17) | 97 (17) | 76 (20) | 88 (25) |
| Cigarettes (per day) | 2.3 (6.4) | 2.7 (7.6) | 2.2 (6.3) | 2.6 (7.5) | 4.3 (9.3) | 4.9 (11.9) |
| Family history of CVD (yes) | 4516 (40.4%) | 2888 (36.5%) | 4401 (40.5%) | 2732 (36.4%) | 115 (38.1%) | 156 (38.5%) |
| Rheumatoid arthritis (yes) | 213 (1.9%) | 101 (1.2%) | 195 (1.8%) | 88 (1.1%) | 18 (5.9%) | 13 (3.2%) |
| Baseline CVD (yes) | 369 (3.2%) | 508 (6.3%) | 302 (2.7%) | 401 (5.2%) | 67 (21.9%) | 107 (26.0%) |
| Diabetes mellitus (yes) | 256 (2.3%) | 306 (3.8%) | 228 (2.1%) | 252 (3.3%) | 28 (9.2%) | 54 (13.1%) |
| Lipid-modifying medication (yes) | 604 (5.3%) | 678 (8.3%) | 548 (5.0%) | 587 (7.6%) | 56 (18.3%) | 91 (22.1%) |
| Antihypertensive medication (yes) | 832 (7.3%) | 742 (9.1%) | 761 (6.9%) | 646 (8.4%) | 71 (23.2%) | 96 (23.4%) |
| Cardiac troponin I (ng/L) | 1.5 (1.2–2.5) | 2.5 (1.6–4.0) | 1.5 (1.2–2.4) | 2.4 (1.6–3.9) | 2.9 (1.8–5.9) | 3.9 (2.3–7.3) |
| Cardiac troponin T (ng/L) | ≤3.0 (3.0–4.8) | 4.6 (3.0–7.5) | ≤3.0 (3.0–4.7) | 4.5 (3.0–7.3) | 5.7 (3.0–10.5) | 7.0 (3.8–12.2) |
| Detectable cardiac troponin I (≥1.2 ng/L) | 7523 (66.1%) | 7056 (86.8%) | 7252 (65.5%) | 6663 (86.4%) | 271 (88.6%) | 393 (95.6%) |
| Detectable cardiac troponin T (≥3.0 ng/L) | 4826 (42.4%) | 5569 (68.5%) | 4610 (41.6%) | 5238 (67.9%) | 216 (70.6%) | 331 (80.5%) |
Categorical data are presented as n (%). Continuous variables are presented as mean (SD) or median (ranges, 25th–75th percentile), as appropriate. Abbreviatons: CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate, HDL, high-density lipoprotein; SIMD, Scottish Index of Multiple Deprivation.
Fig. 1.
Distribution of cardiac troponins in women and men. Violin plots of cardiac troponin I (A) and T (B) distribution, stratified by sex (cardiac troponin I, women, 1.5 (1.2–2.5) ng/L versus men 2.5 (1.6–4.0) ng/L; cardiac troponin T, women ≤3.0 (3.0–4.8) ng/L versus men 4.6 (3.0–7.5) ng/L, P < 0.001 for both; n = 11 375 for women, and n = 8126 for men).
Cardiac Troponins and Cardiovascular Events in Women and Men
The median follow-up period was 7.9 (7.1 to 9.2) years, and a total number of 717 (3.7%) individuals experienced a primary outcome event. In those participants with an incident cardiovascular event (Table 1), women were on average 3 years older than men (65 versus 62 years), but otherwise prior cardiovascular disease and risk factors were similar. Women had fewer events than men, with the primary outcome occurring in 306 (2.7%) women and 411 (5.1%) men during the follow-up period (Table 2).
Table 2.
Incidence rates of clinical outcomes in women and men.
| Women (n = 11 375) |
Men (n = 8126) |
|||
|---|---|---|---|---|
| Total events (%) | Incidence rate | Total events (%) | Incidence rate | |
| Composite cardiovascular event | 306 (2.7%) | 3.3/1000 person-years | 411 (5.1%) | 6.3/1000 person-years |
| Myocardial infarction | 81 (0.7%) | 0.9/1000 person-years | 178 (2.2%) | 2.7/1000 person-years |
| Ischemic stroke | 93 (0.8%) | 1.6/1000 person-years | 112 (1.4%) | 2.3/1000 person-years |
| Cardiovascular death | 128 (1.1%) | 1.4/1000 person-years | 138 (1.7%) | 2.1/1000 person-years |
| Noncardiovascular death | 206 (1.8%) | 2.2/1000 person-years | 168 (2.1%) | 2.5/1000 person-years |
| All-cause death | 334 (2.9%) | 3.6/1000 person-years | 306 (3.8%) | 4.6/1000 person-years |
Composite cardiovascular event = myocardial infarction, ischemic stroke, or cardiovascular death.
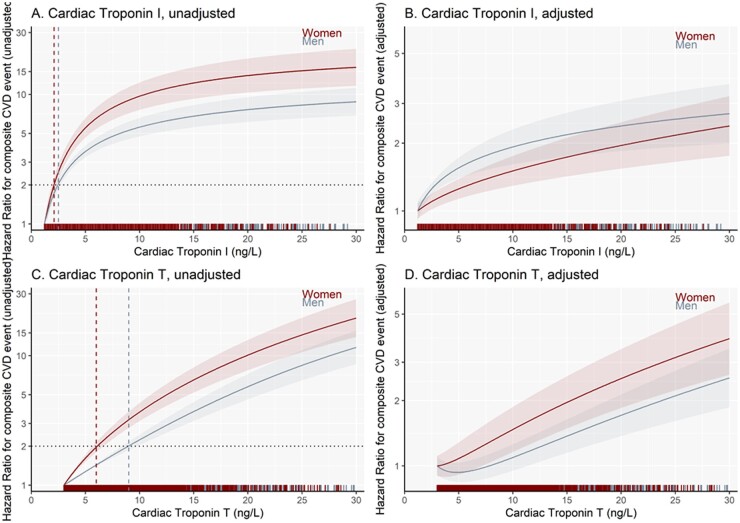
Based on our unadjusted and adjusted regression models we illustrate the HR of a cardiovascular event at 5 years according to cardiac troponin I and troponin T concentrations in men and women (Fig. 2). For estimation of HRs, covariates were standardized for both women and men to illustrate the relationship between cardiac troponin and events in women and men with similar characteristics. Both cardiac troponin I and T concentrations were more strongly associated with the primary outcome in women than men (Fig. 2, A and C). For example, at a cardiac troponin I threshold of 10 ng/L, the unadjusted HR relative to the LoB was 9.7 (95% CI 7.6 to 12.4) for women compared to 5.6 (95% CI 4.7 to 6.6) for men. The unadjusted HR for a cardiac troponin T threshold of 10 ng/L relative to LoB was 3.7 (95% CI 3.1 to 4.3) for women and 2.2 (95% CI 2.0 to 2.5) for men. Cardiac troponin I and T thresholds of 2.1 ng/L and 6.0 ng/L, respectively, were associated with a doubling of cardiovascular risk in women. For men, a doubling of cardiovascular risk required higher thresholds of 2.5 ng/L and 9.0 ng/L for cardiac troponin I and T, respectively. Both cardiac troponin I and T remained strongly associated with cardiovascular events in women and men after adjustment of other risk factors, but the divergence between women and men was attenuated (Fig. 2, B and D).
Fig. 2.
Hazard ratio plots for 5-year risk composite cardiovascular events. Troponin I: (A), unadjusted model; (B), adjusted model and T: (C), unadjusted model; and (D), adjusted model concentrations in relation to composite cardiovascular events, stratified by sex (referent = LoB value). The horizontal dashed line represents the doubling in risk of having a cardiovascular event within 5 years and the vertical dashed lines (red, women; gray, men) respresents the sex-specific thresholds of the 2-fold higher likelihood experiencing a cardiovascular event, accordingly.
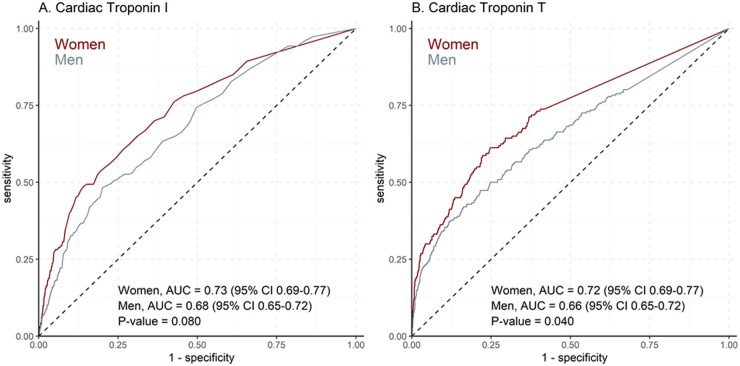
Overall, cardiac troponin I and T concentrations had a good discriminative ability to predict 5-year cardiovascular risk (Fig. 3). For cardiac troponin I (AUC 0.73 in women versus AUC 0.68 in men, P = 0.080), and for cardiac troponin T (AUC 0.72 in women versus AUC 0.66 in men, P = 0.040) there was a trend toward better discrimination in women than in men.
Fig. 3.
Comparison of the discrimination of cardiac troponins for the prediction of the composite cardiovascular event in women and men. Receiver-operating-curve for cardiac troponin I (A) and cardiac troponin T (B) to predict the composite cardiovascular event at 5 years in women and men.
Secondary Outcomes
When using the LoD as reference value, we observed that the differences in the association with cardiac troponin I and T on the primary outcome between women and men were similar but attenuated (Fig. 1 in the online Data Supplement). Consistent with our observations for the primary composite outcome, the incidence of cardiovascular death was lower in women than men (Table 2). In contrast, no difference was observed in the incidence of noncardiovascular death between sexes. Both cardiac troponin I and T were strongly associated with cardiovascular death (online Supplemental Figs. 2 and 3, P < 0.001 for both) and all-cause death (online Supplemental Figs. 4 and 5, P < 0.001 for both) in women and men in fully adjusted models. Cardiac troponin I was not associated with noncardiovascular death in either women (P = 0.597) or men (P = 0.364), whereas cardiac troponin T was for both sexes (P = <0.001 in women, P = 0.004 in men; online Supplemental Figs. 6 and 7).
Discussion
We have evaluated whether sex influences the prediction of cardiac troponin I and T for cardiovascular events in the general population. Our study has 3 main findings. First, cardiac troponin I and T are independent predictors of cardiovascular events in both women and men in the general population. Second, cardiac troponin concentrations differ between women and men and are stronger predictors of cardiovascular events in women. Use of the same thresholds to guide risk of future cardiovascular events in women and men would not provide equivalent prediction. Third, differences in prediction between women and men are largely explained by the prevalence of cardiovascular risk factors and prior disease, as the divergence between women and men was attenuated after adjustment of other risk factors. These findings highlight the importance of a sex-specific approach when using high-sensitivity cardiac troponin testing in isolation for risk stratification and targeting treatments to prevent cardiovascular disease. Ideally, cardiac troponin would be used as a continuous measure in a cardiovascular risk prediction tool that incorporates sex and other clinical features.
Our study has several strengths. First, the Generation Scotland Scottish Family Health Study enrolled approximately 20 000 individuals and a high proportion were women. Second, we were able to evaluate both cardiac troponin I and T in almost the entire cohort, permitting direct comparisons between markers and ensuring our findings are both representative and generalizable. Third, complete follow-up for almost 8 years ensured we had a sufficient number of cardiovascular events to evaluate prediction in men and women separately.
We found that cardiac troponins are strong independent predictors of cardiovascular events and that in their unadjusted, “raw” status, they are more strongly associated in women than men. This observation is in line with the Atherosclerosis Risk in Communities (ARIC) study, the Nord-Trøndelag Health Study (HUNT) study and the Activity and Function in the Elderly in Ulm (ActiFE) study, showing an interaction between troponin and sex in relation to future cardiovascular events across different ethnicities and age groups (18, 22, 23). Apart from differences in left ventricular mass that could explain the lower troponin concentrations in women than men (24–26), sex hormones may play a role in the divergent cardiovascular risk prediction of cardiac troponin concentrations for women and men (27). Estrogens seems to have a cardioprotective effect in premenopausal women, either directly or indirectly (28–31). Differences in body fat distribution between women and men may lead to a different cardiometabolic risk profile (32), which could influence cardiac troponin concentrations. Also, differences in the prevalence of microvascular disease may play a role (33, 34). However, in line with the ARIC study (22), ActiFE study (23), the Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) study (35), the Age, Gene/Environment Susceptibility-Reykjavik (AGES-Reykjavik) study (36), and the Multi-Ethnic study of Atherosclerosis (MESA) (37), we showed that after adjustment of other risk factors the associations between cardiac troponins and outcome in women and men became similar. This points out that the divergent risk between sexes is at least partly explained by differences in cardiovascular risk profile. We determined previously that age, diabetes, prior cardiovascular disease, and lipid-lowering and antihypertensive medication use are important determinants for elevated cardiac troponin concentrations (16), and adjusting for these factors resulted in similar risk prediction for cardiac troponins in women and men.
What are the implications of these observed sex differences? Women tend to be undertreated for cardiovascular risk (38), and cardiac troponin might be a tool to bridge this imbalance. We believe that differences in prediction between women and men are largely explained by the prevalence of cardiovascular risk factors and prior disease, and therefore ideally cardiac troponin would be used as a continuous measure in a risk prediction tool that incorporates sex and other clinical features. However, if cardiac troponin is used in isolation to stratify patients into low- or high-risk groups for screening purposes, it is essential that a sex-specific approach is adopted. We believe that future research should focus on using cardiac troponin as a continuous variable in a multivariable cardiovascular risk tool that stratifies individuals based on their likelihood of cardiovascular disease. This would be in line with the development of the use of troponins in the acute cardiac setting (8), as the awareness has been raised that consideration of cardiac troponin in a continuous fashion improves risk assessment. Another major advantage of such an approach is that cardiac troponin could be corrected for other relevant risk factors and that would eliminate the problem of under- or overestimation for other important subgroups apart from sex.
In contrast to the acute care setting, the general population contains a high proportion of individuals with cardiac troponin values below the LoD. As our study was not designed to develop a risk prediction tool, but rather to evaluate sex differences between troponins in this setting, we have used cardiac troponins over their entire concentration range. We therefore cannot exclude that the imprecision profile of these assays in individuals with very low cardiac troponin concentrations may have affected the accuracy of our results. When using the LoD rather than the LoB as the reference, differences between women and men were less pronounced. Women have lower troponin concentrations than men and therefore a greater proportion of women have undetectable cardiac troponin concentrations. Our analyses suggest that discrimination in the modeling of future cardiovascular events is partly dependent on being able to identify those individuals who are very low risk with the lowest cardiac troponin values. The clinical implications of this are important. For example, in the USA, cardiac troponin values below the LoD are not reported because of concerns about assay imprecision. While precision is greater in those with higher values and therefore the user can be more confident in actioning the results of those identified as higher risk, it is less clear that based on current analytical precision (and reporting requirements) we are fully harnessing the potential of these tests to identify those who are lower risk. Those developing clinical tools to guide primary prevention approaches that incorporate cardiac troponin should be aware that including troponin values below the LoD may affect the accuracy of prediction and limit the future application of these tools in practice.
Another important observation in our study is that cardiac troponin T, but not cardiac troponin I predicts noncardiovascular death in both women and men. This extends our previous finding that cardiac troponin I has a greater specificity for future cardiovascular risk (7, 16). Although the underlying mechanism of this divergence is not well understood and remains speculative, cardiac troponin T elevations appear more strongly related to chronic kidney and neuromuscular diseases (39, 40). Furthermore, the curvilinear relationships between cardiovascular risk and cardiac troponin I and T concentrations differ. For cardiac troponin I, the risk increases in the low troponin range, while for cardiac troponin T the risk accelerates more at higher cardiac troponin values. This divergence may reflect differences in assay precision at very low concentrations and could be an important consideration for the development and implementation of risk prediction tools incorporating troponin, as model performance is likely to be very sensitive to assay choice.
Several limitations merit attention. First, no cardiac imaging data were available and studying the possible structural microvascular cardiac differences between women and men in relation to troponin and outcome was not possible, although this is of secondary value as we have incident cardiovascular outcomes. Second, most of the Generation Scotland Family Health Study participants are Caucasian and generalizing our findings to other ethnic groups should be done with caution. Third, although high-sensitivity testing was used, still a high proportion of individuals had undetectable cardiac troponin concentrations, particularly for cardiac troponin T and particularly in women. Imprecision in those with very low cardiac troponin concentrations might have influenced the accuracy of our model estimates. Finally, cardiac troponin I was only measured using one manufacturer’s assay, which precludes the direct extrapolation of our findings to other cardiac troponin I assays.
In conclusion, cardiac troponin I and T are independent predictors of cardiovascular events in both women and men in the general population. Cardiac troponin concentrations differ in women and men and are stronger predictors of cardiovascular events in women. Sex-specific approaches are required to provide equivalent risk prediction when using high-sensitivity cardiac troponin testing in isolation for risk prediction and the prevention of cardiovascular disease. Ideally, cardiac troponin would be used as a continuous measure in a cardiovascular risk prediction tool that incorporates sex and other clinical features.
Supplemental Material
Supplemental material is available at Clinical Chemistry online
Supplementary Material
Nonstandard Abbreviations:
- Hs-cTn
high-sensitivity cardiac troponin
- STROBE
Strengthening the Reporting of Observational Studies in Epidemiology
- LoB
limit of blank
- LoD
limit of detection
- ICD-10
10th revision of the International Classification of Diseases
- HR
hazard ratio
- ROC
receiver operating characteristic
- AUC
area under the curve
- CI
confidence interval
- ARIC
Atherosclerosis Risk in Communities study
- HUNT
Nord-Trøndelag Health Study
- ActiFE
Activity and Function in the Elderly in Ulm study
- PIVUS
Prospective Investigation of the Vasculature in Uppsala Seniors study
- AGES-Reykjavik
Age, Gene/Environment Susceptibility-Reykjavik study
- MESA
Multi-Ethnic study of Atherosclerosis study
Author Contributions
All authors confirmed they have contributed to the intellectual content of this paper and have met the following 4 requirements: (a) significant contributions to the conception and design, acquisition of data, or analysis and interpretation of data; (b) drafting or revising the article for intellectual content; (c) final approval of the published article; and (d) agreement to be accountable for all aspects of the article thus ensuring that questions related to the accuracy or integrity of any part of the article are appropriately investigated and resolved.
Authors’ Disclosures or Potential Conflicts of Interest
Upon manuscript submission, all authors completed the author disclosure form. Disclosures and/or potential conflicts of interest:
Employment or Leadership
None declared.
Consultant or Advisory Role
N. Sattar, Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Merck Sharp & Dohme, Novartis, Novo Nordisk, Pfizer, and Sanofi; N.L. Mills, Abbott Diagnostics, Roche, Beckman-Coulter, and Singulex.
Stock Ownership
None declared.
Honoraria
A.S.V. Shah, Abbott Diagnostics. S.J.R. Meex, lecture fees from Abbott Diagnostics and Roche Diagnostics; N. Sattar, Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Merck Sharp & Dohme, Novartis, Novo Nordisk, Pfizer, Sanofi.
Research Funding
S.J.R. Meex, Abbott Diagnostics and Roche Diagnostics; N. Sattar, Boehringer Ingelheim; P. Welsh, Roche, Boehringer Ingelheim, Chief Scientist Office, Scotland, Astrazeneca, Novartis. Troponin measurements and analysis were supported by a Stratified Medicine Grant from the Chief Scientist Office of the Scottish Government Health Directorates (ASM/14/1). Generation Scotland received core support from the Chief Scientist Office of the Scottish Government Health Directorates (CZD/16/6) and the Scottish Funding Council (HR03006). The study was supported by Health Data Research UK, which receives its funding from HDR UK Ltd (HDR-5012) funded by the UK Medical Research Council, Engineering and Physical Sciences Research Council, Economic and Social Research Council, Department of Health and Social Care (England), Chief Scientist Office of the Scottish Government Health and Social Care Directorates, Health and Social Care Research and Development Division (Welsh Government), Public Health Agency (Northern Ireland), British Heart Foundation, and the Wellcome Trust. D.M. Kimenai is supported by a Kootstra Talent Fellowship (19.1314). C. Hayward is supported by an MRC University Unit Programme Grant MC_UU_00007/10 (QTL in Health and Disease). N.L. Mills is supported by the British Heart Foundation through a Senior Clinical Research Fellowship (FS/16/14/32023), Programme Grant (RG/20/10/34966) and a Research Excellence Award (RE/18/5/34216).
Expert Testimony
None declared.
Patents
None declared.
Other Remuneration
N. Sattar, support for attending meetings and/or travel from Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Novo Nordisk, Pfizer, Sanofi.
Role of Sponsor
The funding organizations played no role in the design of study, choice of enrolled patients, review and interpretation of data, preparation of manuscript, or final approval of manuscript.
Acknowledgments
We thank J. Cooney and P. Stewart (University of Glasgow, UK) for excellent technical support. We are grateful to all the families who took part, the general practitioners, and the Scottish School of Primary Care for their help in recruiting them, and the whole Generation Scotland team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists, healthcare assistants, and nurses.
References
- 1.Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, et al. American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2020 update: a report from the American Heart Association. Circulation 2020;141:e139–e596. [DOI] [PubMed] [Google Scholar]
- 2.Shaw LJ, Pepine CJ, Xie J, Mehta PK, Morris AA, Dickert NW, et al. Quality and equitable health care gaps for women: attributions to sex differences in cardiovascular medicine. J Am Coll Cardiol 2017;70:373–88. [DOI] [PubMed] [Google Scholar]
- 3.Regitz-Zagrosek V, Oertelt-Prigione S, Prescott E, Franconi F, Gerdts E, Foryst-Ludwig A, Group EUCCS, et al. Gender in cardiovascular diseases: impact on clinical manifestations, management, and outcomes. Eur Heart J 2016;37:24–34. [DOI] [PubMed] [Google Scholar]
- 4.Peters SAE, Colantonio LD, Zhao H, Bittner V, Dai Y, Farkouh ME, et al. Sex differences in high-intensity statin use following myocardial infarction in the United States. J Am Coll Cardiol 2018;71:1729–37. [DOI] [PubMed] [Google Scholar]
- 5.Haider A, Bengs S, Luu J, Osto E, Siller-Matula JM, Muka T, et al. Sex and gender in cardiovascular medicine: presentation and outcomes of acute coronary syndrome. Eur Heart J 2020;41:1328–36. [DOI] [PubMed] [Google Scholar]
- 6.Ford I, Shah AS, Zhang R, McAllister DA, Strachan FE, Caslake M, et al. High-sensitivity cardiac troponin, statin therapy, and risk of coronary heart disease. J Am Coll Cardiol 2016;68:2719–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Welsh P, Preiss D, Hayward C, Shah ASV, McAllister D, Briggs A, et al. Cardiac troponin T and troponin I in the general population. Circulation 2019;139:2754–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neumann JT, Twerenbold R, Ojeda F, Sorensen NA, Chapman AR, Shah ASV, et al. Application of high-sensitivity troponin in suspected myocardial infarction. N Engl J Med 2019;380:2529–40. [DOI] [PubMed] [Google Scholar]
- 9.Omland T, Pfeffer MA, Solomon SD, de Lemos JA, Rosjo H, Saltyte Benth J, et al. Prognostic value of cardiac troponin I measured with a highly sensitive assay in patients with stable coronary artery disease. J Am Coll Cardiol 2013;61:1240–9. [DOI] [PubMed] [Google Scholar]
- 10.Omland T, de Lemos JA, Sabatine MS, Christophi CA, Rice MM, Jablonski KA, et al. A sensitive cardiac troponin T assay in stable coronary artery disease. N Engl J Med 2009;361:2538–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blankenberg S, Salomaa V, Makarova N, Ojeda F, Wild P, Lackner KJ, et al. Troponin I and cardiovascular risk prediction in the general population: the BiomarCaRE consortium. Eur Heart J 2016;37:2428–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kimenai DM, Martens RJH, Kooman JP, Stehouwer CDA, Tan FES, Schaper NC, et al. Troponin I and T in relation to cardiac injury detected with electrocardiography in a population-based cohort—The Maastricht Study. Sci Rep 2017;7:6610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Willeit P, Welsh P, Evans JDW, Tschiderer L, Boachie C, Jukema JW, et al. High-sensitivity cardiac troponin concentration and risk of first-ever cardiovascular outcomes in 154,052 participants. J Am Coll Cardiol 2017;70:558–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kimenai DM, Henry RM, van der Kallen CJ, Dagnelie PC, Schram MT, Stehouwer CD, et al. Direct comparison of clinical decision limits for cardiac troponin T and I. Heart 2016;102:610–6. [DOI] [PubMed] [Google Scholar]
- 15.Kimenai DM, Janssen E, Eggers KM, Lindahl B, den Ruijter HM, Bekers O, et al. Sex-specific versus overall clinical decision limits for cardiac troponin I and T for the diagnosis of acute myocardial infarction: a systematic review. Clin Chem 2018;64:1034–43. [DOI] [PubMed] [Google Scholar]
- 16.Welsh P, Preiss D, Shah ASV, McAllister D, Briggs A, Boachie C, et al. Comparison between high-sensitivity cardiac troponin T and cardiac troponin I in a large general population cohort. Clin Chem 2018;64:1607–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lyngbakken MN, Aagaard EN, Kvisvik B, Berge T, Pervez MO, Brynildsen J, et al. Cardiac troponin I and T are associated with left ventricular function and structure: data from the Akershus Cardiac Examination 1950 study. Clin Chem 2020;66:567–78. [DOI] [PubMed] [Google Scholar]
- 18.Omland T, de Lemos JA, Holmen OL, Dalen H, Benth JS, Nygard S, et al. Impact of sex on the prognostic value of high-sensitivity cardiac troponin I in the general population: the HUNT study. Clin Chem 2015;61:646–56. [DOI] [PubMed] [Google Scholar]
- 19.Smith BH, Campbell A, Linksted P, Fitzpatrick B, Jackson C, Kerr SM, et al. Cohort Profile: Generation Scotland: Scottish Family Health Study (GS:SFHS). The study, its participants and their potential for genetic research on health and illness. Int J Epidemiol 2013;42:689–700. [DOI] [PubMed] [Google Scholar]
- 20.Scottish Government. The Scottish Index of Multiple Deprivation. http://www.gov.scot/Topics/Statistics/SIMD (Accessed February 2021).
- 21.Shah AS, Griffiths M, Lee KK, McAllister DA, Hunter AL, Ferry AV, et al. High sensitivity cardiac troponin and the under-diagnosis of myocardial infarction in women: prospective cohort study. BMJ 2015;350:g7873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jia X, Sun W, Hoogeveen RC, Nambi V, Matsushita K, Folsom AR, et al. High-sensitivity troponin I and incident coronary events, stroke, heart failure hospitalization, and mortality in the ARIC study. Circulation 2019;139:2642–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dallmeier D, Denkinger M, Peter R, Rapp K, Jaffe AS, Koenig W, et al. ; for the ActiFE Study Group. Sex-specific associations of established and emerging cardiac biomarkers with all-cause mortality in older adults: the ActiFE study. Clin Chem 2015;61:389–99. [DOI] [PubMed] [Google Scholar]
- 24.Natori S, Lai S, Finn JP, Gomes AS, Hundley WG, Jerosch-Herold M, et al. Cardiovascular function in multi-ethnic study of atherosclerosis: normal values by age, sex, and ethnicity. AJR Am J Roentgenol 2006;186:S357–65. [DOI] [PubMed] [Google Scholar]
- 25.de Lemos JA, Drazner MH, Omland T, Ayers CR, Khera A, Rohatgi A, et al. Association of troponin T detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. JAMA 2010;304:2503–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neeland IJ, Drazner MH, Berry JD, Ayers CR, deFilippi C, Seliger SL, et al. Biomarkers of chronic cardiac injury and hemodynamic stress identify a malignant phenotype of left ventricular hypertrophy in the general population. J Am Coll Cardiol 2013;61:187–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Subramanya V, Zhao D, Ouyang P, Lima JA, Vaidya D, Ndumele CE, et al. Sex hormone levels and change in left ventricular structure among men and post-menopausal women: the Multi-Ethnic Study of Atherosclerosis (MESA). Maturitas 2018;108:37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eggers KM, Lindahl B.. Impact of sex on cardiac troponin concentrations—a critical appraisal. Clin Chem 2017;63:1457–64. [DOI] [PubMed] [Google Scholar]
- 29.Westerman S, Wenger NK.. Women and heart disease, the underrecognized burden: sex differences, biases, and unmet clinical and research challenges. Clin Sci (Lond) 2016;130:551–63. [DOI] [PubMed] [Google Scholar]
- 30.Donaldson C, Eder S, Baker C, Aronovitz MJ, Weiss AD, Hall-Porter M, et al. Estrogen attenuates left ventricular and cardiomyocyte hypertrophy by an estrogen receptor-dependent pathway that increases calcineurin degradation. Circ Res 2009;104:265–75. 11p following 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Piro M, Della Bona R, Abbate A, Biasucci LM, Crea F.. Sex-related differences in myocardial remodeling. J Am Coll Cardiol 2010;55:1057–65. [DOI] [PubMed] [Google Scholar]
- 32.Lawlor DA, Ebrahim S, Whincup P, Sterne J, Papacosta O, Wannamethee G, et al. Sex differences in body fat distribution and carotid intima media thickness: cross sectional survey using data from the British regional heart study. J Epidemiol Community Health 2004;58:700–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reis SE, Holubkov R, Conrad Smith AJ, Kelsey SF, Sharaf BL, Reichek N, et al. Coronary microvascular dysfunction is highly prevalent in women with chest pain in the absence of coronary artery disease: results from the NHLBI WISE study. Am Heart J 2001;141:735–41. [DOI] [PubMed] [Google Scholar]
- 34.Han SH, Bae JH, Holmes DR Jr., Lennon RJ, Eeckhout E, Barsness GW, et al. Sex differences in atheroma burden and endothelial function in patients with early coronary atherosclerosis. Eur Heart J 2008;29:1359–69. [DOI] [PubMed] [Google Scholar]
- 35.Eggers KM, Johnston N, Lind L, Venge P, Lindahl B.. Cardiac troponin I levels in an elderly population from the community–the implications of sex. Clin Biochem 2015;48:751–6. [DOI] [PubMed] [Google Scholar]
- 36.Thorsteinsdottir I, Aspelund T, Gudmundsson E, Eiriksdottir G, Harris TB, Launer LJ, et al. High-sensitivity cardiac troponin I is a strong predictor of cardiovascular events and mortality in the AGES-Reykjavik community-based cohort of older individuals. Clin Chem 2016;62:623–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sandoval Y, Bielinski SJ, Daniels LB, Blaha MJ, Michos ED, DeFilippis AP, et al. Atherosclerotic cardiovascular disease risk stratification based on measurements of troponin and coronary artery calcium. J Am Coll Cardiol 2020;76:357–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mosca L, Hammond G, Mochari-Greenberger H, Towfighi A, Albert MA, American Heart Association Cardiovascular D. Fifteen-year trends in awareness of heart disease in women: results of a 2012 American Heart Association national survey. Circulation 2013;127:1254–63, e1–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Starnberg K, Friden V, Muslimovic A, Ricksten SE, Nystrom S, Forsgard N, et al. A possible mechanism behind faster clearance and higher peak concentrations of cardiac troponin I compared with troponin T in acute myocardial infarction. Clin Chem 2020;66:333–41. [DOI] [PubMed] [Google Scholar]
- 40.Schmid J, Liesinger L, Birner-Gruenberger R, Stojakovic T, Scharnagl H, Dieplinger B, et al. Elevated cardiac troponin T in patients with skeletal myopathies. J Am Coll Cardiol 2018;71:1540–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.