Abstract
PURPOSE:
The purpose of the study was to study the relationship between pseudoexfoliation (PES) and other predictors in the development of complications in cataract surgery by phacoemulsification in patients with PES.
METHODS:
A retrospective cohort study of patients undergoing cataract surgery by phacoemulsification in the health area of Cee in northwestern Spain during the 2-year period from 2009 to 2010. Capsule rupture, choroidal hemorrhage, and vitreous loss were included as complications and intraoperative nucleus or lens dislocation as the independent variable. PES, age, hardness, type of cataract, myopia, preoperative visual acuity, antiplatelet use, anticoagulant uses, alpha agonist use, mydriasis prior to surgery, anterior chamber depth, and axial length were included as predictor variables. All predictive hierarchical models were tested using as a selection criterion the one minimizing the Akaike index.
RESULTS:
A total of 551 patients were initially identified from hospital register, of which 48 were excluded due to the presence of an exclusion factor. After the initial selection, the final sample was 681 eyes of 503 patients. Of the 8192 possible models, a model with the following seven variables was selected: PES, steroid use, alpha agonist use, nuclear hardness, mydriasis, anterior chamber depth, and axial length. The selected model had an Akaike index of 435.4 and an area under the curve of 0.7895 corresponding to a sensitivity of 6.2% and a specificity of 98.5%.
CONCLUSION:
PES, nuclear hardness, and alpha agonist use are risk factors strongly predictive of complications.
Keywords: Cataract/complications, cataract/surgery, phacoemulsification, pseudoexfoliation syndrome
INTRODUCTION
Pseudoexfoliation (PES) is an entity characterized by the deposition of fibrillar material in various anatomic locations.[1] PES increases the risk of cataract development and accelerates their progression, in addition to the development of intraoperative complications.[2,3] These complications lead to an increase in postoperative complications with a reduction in final visual acuity,[4] increasing both direct and indirect costs in the development of the disease.
Prophylaxis and prevention of associated complications still remains today a challenge since the multitude of factors involved generates risk stratification systems with a considerable margin of error,[5] and they do not always take into account the interactions of PES with other factors.
Our objective is to try to determine the existence of other predictors that would enable us to stratify the risk of complications in surgery.
METHODS
This retrospective cohort study was conducted based on the data obtained from patients undergoing phacoemulsification cataract surgery in a health area in northwestern Spain during the 2-year period from 2009 to 2010. In the area, 551 patients (730 eyes) underwent cataract surgery. From this initial number of patients, 59 eyes of 48 patients were excluded due to the presence of traumatic cataracts, pharmacologically uncontrolled glaucoma, or previous eye surgery procedures. The remaining 503 patients with 681 eyes were initially followed for a period of 1 year, corresponding to the last checkup obtained in the patient [Figure 1]. Ethical permission for the study was obtained from the Ethics Committee (2015/502) and it complied with the Declaration of Helsinki guidelines.
Figure 1.
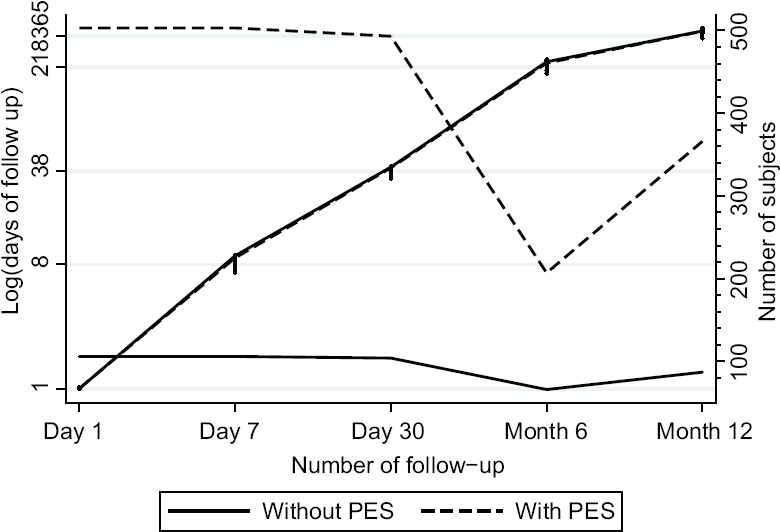
Course of the sample
All data were collected from the patients' medical charts using structured forms containing the demographic characteristics and the variables obtained in the clinical examination pre- and post-pupil dilation. The examination included determination of best-corrected visual acuity, presence of PES using as a criterion, the presence on the surface of the crystalline lens of fibrillar material forming a more or less complete ring,[6] and description of the cataracts in terms of type and grade using a photographic scale according to the LOCS III classification system.[7] The examination also included determination of intraocular pressure, anterior chamber depth, and pupil diameter. In addition, keratometry and contact biometry applying the Sanders-Retzlaff-Kraff II formula were determined.[8]
The surgical procedure included dilation with topical mydriatic therapy: tropicamide 1%, phenylephrine 10% and cyclopentolate 1%; and topical anesthesia with lidocaine 3% in nearly all cases. The surgical technique included performance of bimanual phacoemulsification through a 2.75 mm temporal limbal incision using the “divide and conquer” technique with a phacofragmenter (WHITESTAR Signature® Phacoemulsification System). The hydrophobic acrylic, foldable, one-piece IOL of first intention was implanted in the capsular bag. In those cases, in which zonular support was insufficient, a rigid polymethyl methacrylate lens was implanted. If there was no support, an anterior chamber intraocular lens was implanted in first or second intention.
Intraoperative complications considered in the study were capsule rupture, choroidal hemorrhage, vitreous loss, and lens and nucleus dislocation and the presence of any one of them was considered as a complication. PES, age, hardness, type of cataract, myopia, preoperative visual acuity, antiplatelet use, anticoagulant uses, alpha agonist use, mydriasis before surgery, anterior chamber depth, and axial length were used as predictor variables. The existence of collinearity between the different variables was previously discarded.
To construct the model, logistic regression was applied to all predictor variables involved, for which all possible hierarchical models including all the variables were tested, seeking to minimize the Akaike index.
All statistical analyses were performed using STATA 15 (StataCorp. 2017. Stata Statistical Software: Release 15. College Station, TX, USA: StataCorp LLC).
RESULTS
A total of 551 patients were initially identified from hospital register, of which 42 were excluded due to the presence of an exclusion factor, leaving 681 eyes of 503 patients after the initial selection. The course of the patients can be seen in Figure 1. Of the selected patients, 106 patients had PES [Table 1], with a distribution by gender of 50.94% in men with PES (52 patients) and 39.6% in men without PES (201 patients). Among these patients, there were a total of 34 capsule ruptures (6 with PES and 28 without PES), 13 vitreous loss (2 with PES and 11 without PES), no nuclear dislocation, and 12 zonular dialysis, corresponding to 7 in patients with PES and 5 in patients without PES. The prevalence of intraoperative complications was 8.66% overall.
Table 1.
Main characteristics of the sample
| PES Group, n (%) | No PES Group, n (%) | P | |
|---|---|---|---|
| Number of eyes/patients (n) | 106 (120) | 487 (561) | |
| Males | 52 (50.94) | 201 (39.96) | 0.0373* |
| Age (mean±SD) | 77.87±5.57 | 75.75±6.89 | 0.0031** |
| Maximum mydriasis (mean±SD) | 7.24±0.88 | 7.80±0.88 | 0.0000** |
| Nuclear hardness ≥3 | 34 (30.36) | 150 (32.08) | 0.7288 |
| Anterior chamber depth <3 (van Herick grading) | 16 (15.09) | 72 (14.31) | 0.8355 |
| Glaucoma | 39 (36.79) | 94 (18.69) | 0.0000** |
| Alpha agonists | 22 (20.75) | 104 (20.68) | 0.9855 |
| Antiplatelet use | 36 (33.96) | 128 (25.45) | 0.0725 |
| Anticoagulant use | 27 (25.47) | 97 (19.28) | 0.1510 |
| High myopia | 16 (15.09) | 74 (14.71) | 0.9196 |
| Steroid use | 23 (21.70) | 89 (17.69) | 0.3335 |
*Significant at 0.05, **Significant at 0.01. PES: Pseudoexfoliation, SD: Standard deviation
A total of 8192 models were tested, prioritizing the models that minimized the Akaike criterion. Three models were obtained with equally low values, which included different combinations of PES, steroid use, antiplatelet use, alpha agonist use, nuclear hardness, maximum mydriasis in millimeters, anterior chamber depth, and total axial length. The finally selected model [Table 2] has an Akaike value of 435.4, with an area under the curve of 0.7895 [Figure 2], a sensitivity of 6.2%, and a specificity of 98.5% and includes seven variables: PES, steroid use, alpha agonist use, nuclear hardness, mydriasis, anterior chamber depth, and axial length. Overall, it has a positive predictive value of 46.67% and a negative predictive value of 87.85% and correctly classifies 86.90% of patients, with a R2 of 0.1334.
Table 2.
Characteristics of the finalist models
| Variables | AIC | BIC | AUC | Se | Sp | pfitHL |
|---|---|---|---|---|---|---|
| PES STER ALPHA HARDNESS mydriasis AC AL | 435.4 | 470.9 | 0.783 | 6.2 | 98.5 | 0.096 |
| PES STER ALPHA HARDNESS mydriasis AC AL | 435.4 | 466.4 | 0.777 | 4.9 | 98.3 | 0.048 |
| PES antipla ALPHA HARDNESS mydriasis AC AL | 435.4 | 470.9 | 0.783 | 7.4 | 98.7 | 0.141 |
AIC: Akaike information criterion, BIC: Schwarz Bayesian criterion, AUC: Area under the curve, Se: Sensitivity (%), Sp: Specificity (%), pfitHL: Hosmer-Lemeshow Goodness-of-Fit, PES: Pseudoexfoliation; STER: Steroid use; ALPHA: Alpha agonist use; HARDNESS: Hardness of the nucleus; AC: Anterior chamber length; AL: Axial length
Figure 2.
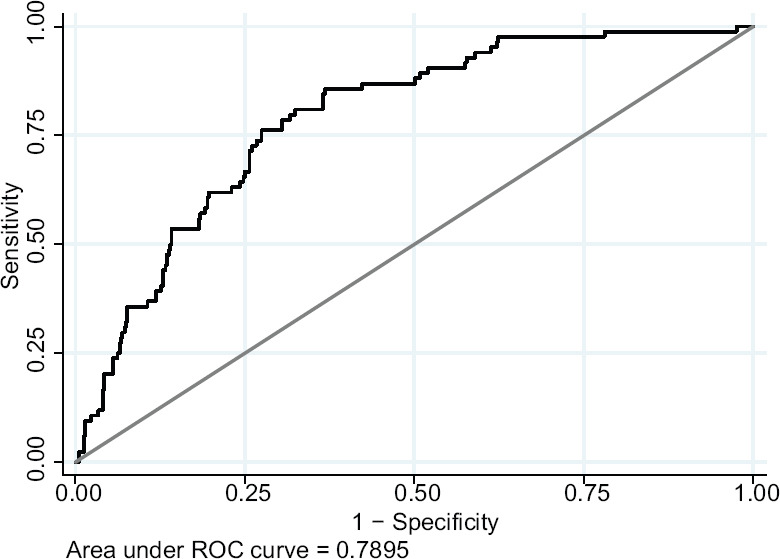
ROC curve of the best model obtained
The selected model is shown in Table 3. There were two risk factors that were found to be significant in the model: Alpha agonist use, odds ratio (OR) = 2.74 (95% confidence interval [CI]: 1.26–5.97), and nuclear hardness, OR = 2.00 (95% CI: 1.44–2.78). PES, OR = 1.38 (95% CI: 0.76–2.50), was not found to be significant in the overall model as a risk factor, largely due to the sample size. On the other hand, mydriasis in mm, OR = 0.58 (95% CI: 0.45–0.75), anterior chamber depth, OR = 0.55 (95% CI: 0.32–0.95), steroid use, OR = 0.49 (95% CI: 0.20–1.20), and axial length, OR = 0.66 (95% CI: 0.39–1.12) were found to be protective, mydriasis, and anterior chamber depth being significant and with a strongly protective effect.
Table 3.
Variables in the selected model
| Variable | OR | SE | P>Z | 95% CI |
|---|---|---|---|---|
| PES | 1.38 | 0.42 | 0.2879 | 0.76-2.50 |
| STEROIDS | 0.49 | 0.22 | 0.1185 | 0.20-1.20 |
| ALPHA | 2.74 | 1.09 | 0.0112 | 1.26-5.97 |
| HARDNESS | 2.00 | 0.34 | 0 | 1.44-2.78 |
| Mydriasis | 0.58 | 0.08 | 0 | 0.45-0.75 |
| AC | 0.55 | 0.15 | 0.0319 | 0.32-0.95 |
| AL | 0.66 | 0.18 | 0.1279 | 0.39-1.12 |
| CONSTANT | 4.43 | 99.66 | 0.0640 | 0.80-2855.84 |
PES: Pseudoexfoliation, STEROIDS: Steroid use, ALPHA: alpha agonist use, HARDNESS: Hardness of the nucleus, AC: Anterior chamber length, AL: Axial length, CONSTANT: Constant value, OR: Odds ratio, SE: Standard error, CI: Confidence interval
DISCUSSION
Cataract surgery performed under routine conditions with topical anesthesia, with a multitude of circumstances that can modify an uneventful surgery at any moment, poses a challenge for any predictive model, and in fact, there are few models that have been widely tested to stratify risk.[9]
In our model, very high odds ratios for intraoperative complications were obtained in key variables previously described in other studies such as PES,[10,11,12,13] nuclear hardness,[14] alpha agonist use,[15] and other important protective effects such as mydriasis[14] or anterior chamber depth.[16] However, recognized factors such as age,[4,12,17] though it is not universally accepted,[18] gender,[12,13] arterial hypertension,[10] type of cataract,[14] or the presence of diabetes,[12,19] were not significant.
Among published studies, PES is the most frequently mentioned determinant. In the study by Scorolli et al.[11] with 1052 patients, although the anesthetic technique was not topical but peribulbar, PES achieved a OR value of 5.1 for the development of capsule rupture and vitreous loss. Other authors have given equally high values in other source populations, such as Drolsum,[20] who, in a cohort of 1152 patients undergoing cataract surgery by phacoemulsification, obtained an OR of 2.39 for posterior capsule rupture, which was significant. In the study by Salowi et al.,[12] based on 36 public hospitals in Malaysia with a total of 150,213 patients, and though only capsule rupture was considered as a complication, gave it an OR value of 1.36, though it is true that age was included in the adjustment of the logistic regression, which due to the effect of accumulation of pseudoexfoliative material over time, may result in a slight decrease in the OR value versus other studies. Indeed, the study of Thevi et al. in 12,992 patients from Melaka Hospital in Malaysia, also provided an OR value of 2.18, which was the only intraocular risk factor recognized by the authors as significant. This very high value found in studies in the Malaysian area, however, is not universally accepted since after adjusting for age, gender, and ethnicity, Thanigasalam et al.[21] found an OR of 3.083 for posterior capsule rupture with confidence intervals of 0.689–13.801, which was not significant. In the same line, Menkhaus et al.,[22] in a sample of 1210 patients, found no difference in intraoperative complications between patients with and without PES.
The development of a predictive model as in this study implies two possible options, either to use previously described predictor variables and formulate a theoretical model that is then tested in a reference population[5] or to develop a regression model discarding the variables that have less weight in calculation of the overall odds of complications applying criteria that allow us to obtain a parsimonious model with a reduced number of involved variables.[23] We used the latter approach since it allows us to assess jointly all the involved variables, so that concurrent events functioning as modifiers can be assessed. However, there are certain limitations to this type of approach, since it requires large sample sizes, especially if the risk factors are uncommon, lest nonsignificant confidence intervals be obtained.
Our study suffered from several handicaps, as, for example, we had a small number of events and a relatively long list of factors to be studied. This was revealed in the level of significance of the risk factors, which showed wide large intervals, despite being risk factors for known complications such as PES. In our favor, the existence of strict inclusion and exclusion criteria reduced the variability of the comparison between groups which, together with a high prevalence of complications, allowed us to obtain several variables with high predictive value within the model. Moreover, in a model of this nature, our objective is not significance but to obtain an adequate classification of patients into risk groups with a view to their stratification. With the model constructed, we obtained an adequate classification in nearly 86.90% of the cases.
CONCLUSION
PES, nuclear hardness, and alpha agonist use are risk factors strongly predictive of complications.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Shingleton BJ, Marvin AC, Heier JS, O'Donoghue MW, Laul A, Wolff B, et al. Pseudoexfoliation: High risk factors for zonule weakness and concurrent vitrectomy during phacoemulsification. J Cataract Refract Surg. 2010;36:1261–9. doi: 10.1016/j.jcrs.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 2.Govetto A, Lorente R, Vázquez de Parga P, Rojas L, Moreno C, Lagoa F, et al. Frequency of pseudoexfoliation among patients scheduled for cataract surgery. J Cataract Refract Surg. 2015;41:1224–31. doi: 10.1016/j.jcrs.2014.09.039. [DOI] [PubMed] [Google Scholar]
- 3.Kanthan GL, Mitchell P, Burlutsky G, Rochtchina E, Wang JJ. Pseudoexfoliation syndrome and the long-term incidence of cataract and cataract surgery: The blue mountains eye study. Am J Ophthalmol. 2013;155:83–8.e1. doi: 10.1016/j.ajo.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 4.González N, Quintana JM, Bilbao A, Vidal S, Fernández de Larrea N, Díaz V, et al. Factors affecting cataract surgery complications and their effect on the postoperative outcome. Can J Ophthalmol. 2014;49:72–9. doi: 10.1016/j.jcjo.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 5.Muhtaseb M, Kalhoro A, Ionides A. A system for preoperative stratification of cataract patients according to risk of intraoperative complications: A prospective analysis of 1441 cases. Br J Ophthalmol. 2004;88:1242–6. doi: 10.1136/bjo.2004.046003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schlötzer-Schrehardt U, Naumann GO. Ocular and systemic pseudoexfoliation syndrome. Am J Ophthalmol. 2006;141:921–37. doi: 10.1016/j.ajo.2006.01.047. [DOI] [PubMed] [Google Scholar]
- 7.Chylack LT., Jr The lens opacities classification system III. the longitudinal study of cataract study group. Arch Ophthalmol. 1993;111:831–6. doi: 10.1001/archopht.1993.01090060119035. [DOI] [PubMed] [Google Scholar]
- 8.Sanders DR, Retzlaff J, Kraff MC. Comparison of the SRK II formula and other second generation formulas. J Cataract Refract Surg. 1988;14:136–41. doi: 10.1016/s0886-3350(88)80087-7. [DOI] [PubMed] [Google Scholar]
- 9.Kim BZ, Patel DV, Sherwin T, McGhee CN. The auckland cataract study: Assessing preoperative risk stratification systems for phacoemulsification surgery in a teaching hospital. Am J Ophthalmol. 2016;171:145–50. doi: 10.1016/j.ajo.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 10.Abbasoğlu OE, Hoşal B, Tekeli O, Gürsel E. Risk factors for vitreous loss in cataract surgery. Eur J Ophthalmol. 2000;10:227–32. doi: 10.1177/112067210001000306. [DOI] [PubMed] [Google Scholar]
- 11.Scorolli L, Scorolli L, Campos EC, Bassein L, Meduri RA. Pseudoexfoliation syndrome: A cohort study on intraoperative complications in cataract surgery. Ophthalmologica. 1998;212:278–80. doi: 10.1159/000027307. [DOI] [PubMed] [Google Scholar]
- 12.Salowi MA, Salowi MA, Chew FL, Adnan TH, King C, Ismail M, et al. The Malaysian Cataract Surgery Registry: Risk indicators for posterior capsular rupture. Br J Ophthalmol. 2017;101:1466–1470. doi: 10.1136/bjophthalmol-2016-309902. doi: 10.1136/bjophthalmol-2016-309902. [DOI] [PubMed] [Google Scholar]
- 13.Thevi T, Maizura Z, Abas AL. The Melaka Hospital cataract complications study analysis of 12,992 eyes. Indian J Ophthalmol. 2017;65:24–9. doi: 10.4103/ijo.IJO_452_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Artzén D, Lundström M, Behndig A, Stenevi U, Lydahl E, Montan P. Capsule complication during cataract surgery: Case-control study of preoperative and intraoperative risk factors: Swedish Capsule Rupture Study Group report 2. J Cataract Refract Surg. 2009;35:1688–93. doi: 10.1016/j.jcrs.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 15.Chatziralli IP, Peponis V, Parikakis E, Maniatea A, Patsea E, Mitropoulos P, et al. Risk factors for intraoperative floppy iris syndrome: A prospective study. Eye (Lond) 2016;30:1039–44. doi: 10.1038/eye.2016.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Küchle M, Viestenz A, Martus P, Händel A, Jünemann A, Naumann GO. Anterior chamber depth and complications during cataract surgery in eyes with pseudoexfoliation syndrome. Am J Ophthalmol. 2000;129:281–5. doi: 10.1016/s0002-9394(99)00365-7. [DOI] [PubMed] [Google Scholar]
- 17.Hashemi H, Rezvan F, Etemad K, Gilasi H, Asgari S, Mahdavi A, et al. Intraoperative complications of cataract surgery in Tehran Province, Iran. Optom Vis Sci. 2016;93:266–71. doi: 10.1097/OPX.0000000000000795. [DOI] [PubMed] [Google Scholar]
- 18.Robbie SJ, Muhtaseb M, Qureshi K, Bunce C, Xing W, Ionides A. Intraoperative complications of cataract surgery in the very old. Br J Ophthalmol. 2006;90:1516–8. doi: 10.1136/bjo.2006.098764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pershing S, Morrison DE, Hernandez-Boussard T. Cataract surgery complications and revisit rates among three states. Am J Ophthalmol. 2016;171:130–8. doi: 10.1016/j.ajo.2016.08.036. [DOI] [PubMed] [Google Scholar]
- 20.Drolsum L, Haaskjold E, Sandvig K. Phacoemulsification in eyes with pseudoexfoliation. J Cataract Refract Surg. 1998;24:787–92. doi: 10.1016/s0886-3350(98)80132-6. [DOI] [PubMed] [Google Scholar]
- 21.Thanigasalam T, Sahoo S, Kyaw Soe HH. Posterior capsule rupture during phacoemulsification among patients with pseudoexfoliation-is there a correlation? Malays J Med Sci. 2014;21:51–3. [PMC free article] [PubMed] [Google Scholar]
- 22.Menkhaus S, Motschmann M, Kuchenbecker J, Behrens-Baumann W. Pseudoexfoliation (PEX) syndrome and intraoperative complications in cataract surgery. Klin Monbl Augenheilkd. 2000;216:388–92. doi: 10.1055/s-2000-10585. [DOI] [PubMed] [Google Scholar]
- 23.Vrieze SI. Model selection and psychological theory: a discussion of the differences between the Akaike information criterion (AIC) and the Bayesian information criterion (BIC) Psychol Methods. 2012;17:228–43. doi: 10.1037/a0027127. [DOI] [PMC free article] [PubMed] [Google Scholar]


