Abstract
Aim:
Diverticular disease is widespread worldwide. Mainstay approach is non-operative treatment with bowel rest and broad-spectrum intravenous antibiotics. However, extra-colic abscess larger than 4 cm may require percutaneous trans-abdominal drainage. We report a single centre case series of patients underwent to trans-luminal endoscopic ultrasound (EUS)-guided drainage of pelvic abscess in diverticular disease with temporary placement of lumen apposing metal stent (LAMS).
Methods:
All patients referred to our tertiary centre from January 2019 to July 2020 were enrolled in a prospective data base that was retrospectively analysed. Procedural steps were as follows: pre-operative computed tomography scan, broad-spectrum antibiotic therapy, EUS-guided deployment of LAMS for 15 days, LAMS removal and deployment of pigtail stent in case of pseudo-cavity persistence.
Results:
Ten patients (6F) with an average of 59.6 years were enrolled with deployment of 10 LAMS. One patient was excluded after EUS evaluation and 1 patient had 2 LAMS for 2 separate abscesses. Technical and clinical success was achieved in 88.8% (8/9).
Conclusions:
Management of diverticulitis has shifted from primary surgical intervention towards a non-operative approach of bowel rest and broad-spectrum intravenous antibiotics in conjunction with interventional procedures to drain abscesses whenever necessary. EUS-guided drainage with LAMS for the management of diverticular abscesses seems an efficient treatment modality for encapsulated abscesses more than 4 cm in size and close to colonic wall. In expert centres, it may avoid radiologic intervention and/or surgery in a relevant percentage of cases.
Keywords: Abscess, colon, diverticula disease, double pigtail, drainage, endoscopic ultrasound, LAMS, rectum
INTRODUCTION
Diverticular disease is widespread worldwide due to increase of obesity, low-fibre diet and reduced physical activity.[1] Although diverticular disease is more common in the elderly population, the incidence of left-sided acute diverticulitis (ALCD) is recently increasing even in younger patients.[2] ALCD may cause perforation, abscess and fistula formation that may require surgery.[3] International guidelines have established well-defined treatment principles.[4] Modified Hinchey classification stage 0 and I are mainly managed with antibiotic therapy only and additionally drainage for larger pericolic abscesses.[1] Whereas, in case of extra-colonic abscesses (Hinchey Stage II) of more than 4 cm in size, an intra-abdominal drainage is usually required. Percutaneous abscess drainage (PAD) fails in more than 35% of cases.[5] Moreover, it may represent the ‘primum movens’ for entero-cutaneous fistula in up to 12.5% of cases.[5] Finally, recent studies showed that percutaneous drainage combined with antibiotics did not seem to be superior to antibiotics only and percutaneous drainage had no independent positive effect on short- and long-term outcomes.[6]
Surgical drainage is more effective, especially for larger abscesses; however, surgery is not recommended as the first-line treatment due to high morbidity and mortality.[5] Nowadays, a conservative approach is preferred over surgery ‘tout-court’ whenever feasible. Endoscopic ultrasound (EUS)-guided drainage of intra-abdominal encapsulated collection is a well-established technique both for upper and lower gastrointestinal tract collections.[7,8] Here we report our experience, evaluating technical/clinical success rates and long-term follow-up of patients underwent trans-luminal EUS-guided drainage of diverticular pelvic abscess with temporary placement of covered lumen apposing metal stent (LAMS).
METHODS
All patients referred to our tertiary centre from January 2019 to July 2020 for EUS-guided drainage of any intra-abdominal collection were enrolled in a prospective data base that was retrospectively analysed. Among all patients underwent to EUS-guided drainage, only those with a collection related to ALCD were considered for this study. The study received institutional review board approval from Ramsay Santé Ethical Committee.
The diagnosis of ALCD was established combining clinical evaluation (left lower pain, left-sided tenderness and fever), blood chemistry tests (C-reactive protein [CRP] and white blood cell [WBC]) and computed tomography (CT) findings. All patients underwent EUS-guided drainage with temporary placement of LAMS after referral to our centre from peripheral hospital. EUS approach was chosen due to technical difficulties to perform CT-guided drainage. The inclusion criteria were as follows: age ≥18 years, intra-abdominal encapsulated abscess related to ALCD and absence of generalised peritonitis. Pre-procedural abdominal-pelvic CT scan) was performed in all patients to confirm diverticular aetiology, rule out generalised peritonitis and to help planning the most suitable segment of the colon to be used for accessing the abscess. Prior total colonoscopy was never performed. EUS-guided drainage did not require full bowel preparation but only multiple enemas. This approach was in line with current guidelines that stand against full bowel preparation.
Our protocol was as follows: multiple trans-anal enemas before endoscopic procedure, EUS-guided drainage of intra-abdominal or pelvic abscess collection by the placement of LAMS and sample fluid collection for bacteria culture test. All patients received broad-spectrum antibiotic therapy since hospital admission (4.5 g Piperacillin/Tazobactam). Antibiotic therapy was eventually modified according to bacteria culture test results and was continued for at least 7 days after endoscopic drainage. All procedures were performed in an endoscopic suite equipped with fluoroscopy with patients in the supine position under general anaesthesia. EUS drainage was performed by an experienced interventional surgical endoscopist with a case load of more than 200 therapeutic EUS procedures per year. Therapeutic EUS (Olympus®, Tokyo, Japan) was used to locate and to define morphological features of the peri-rectal or peri-colic encapsulated collections. Under EUS guidance, the most suitable puncture site was located choosing the shortest route between colonic wall and collection and avoiding intervening vessels or interposing structures. Electrocautery-enhanced LAMS (Axios®, Boston Scientifics, Massachusetts, USA) was delivered with distal flange within the collection and intra-channel release of the proximal one. Pure Cut effect 4 mode was used. LAMS was left in place for 15 days. Patients re-started oral liquid diet 6 h after the procedure and were discharged from hospital at post-procedural day 3 with ‘tailored’ antibiotic therapy according to bacteria-sensitivity results. Per protocol, a CT scan was performed to all patients at post-operative day 7. Whereas, the first follow-up endoscopy was scheduled after 15 days from LAMS deployment on an outpatient setting. LAMS was withdrawn in all cases. In case of persistent abscess cavity, a double pigtail stent (its length varying according to the size of pseudo-cavity) was inserted with one end within the collection and the other one within the colonic lumen. Second follow-up endoscopy was scheduled after 3 months. If the stent was not yet evacuated, it was removed irrespective to the presence of residual pseudo-cavity.
Technical success was defined as proper drainage of the collection and correct deployment of LAMS with no evidence of complications such as diffuse peritonitis, sepsis, bleeding and refractory pain. Clinical success was defined as complete radiological obliteration (assumption of complete healing) of the abscess and CRP normalisation with no need for prolonged antibiotic or analgesic therapy for at least 6 months after the end of endoscopic treatment.
SSPS software version 20.0 (IBM Corp., Armonk, New York, USA) was employed to run the statistical analyses. Descriptive statistics were calculated for all demographic and clinical variables data and are expressed as mean and median or as proportions for the categorical variables.
RESULTS
Ten patients (6F), with an average age of 59.6 years (23–91) were referred to our hospital for EUS drainage of intra-abdominal or pelvic collection following ALCD.
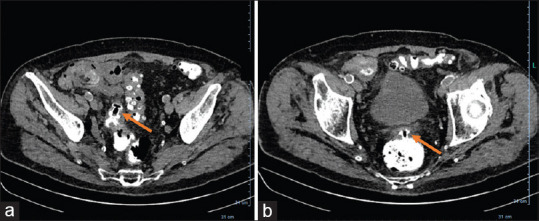
Pre-procedure CRP and WBCs were both markedly elevated with an average value of 126.5 U/ml (range 40–312; standard deviation [SD] 84.9) and 12,550 (range 5500–18,000; SD 3858), respectively. The median abscess size was 7.45 cm (range 3–4 cm; SD 2.8). Abscess location was as follows: 6 in the pelvis and 5 intra-abdominal. One patient had two separate collections that required deployment of 2 LAMS (one trans-colonic and the other one trans-rectal). A trans-rectal approach was used in six cases, whereas in the remaining 5 a trans-colonic one (sigmoid) was adopted [Figures 1 and 2].
Figure 1.
Computed tomography Scan showing: (a) Intra-abdominal abscess close to sigmoid colon; (b) pelvic abscess close to the rectum
Figure 2.
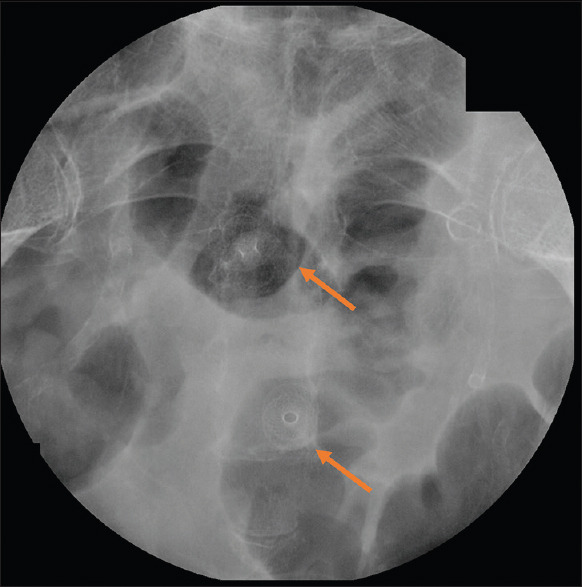
Fluoroscopy showing (red arrows) deployment of 2 LAMS for the management of 2 separate acute diverticulitis related abscesses
One patient did not undergo LAMS deployment due to clinical improvement soon after start of antibiotic therapy and EUS evidence of a small collection of 3 cm in diameter.
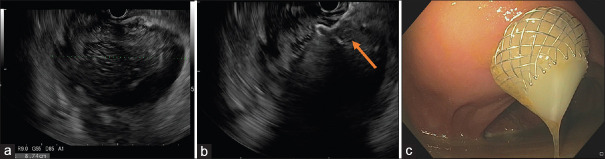
Therefore, deployment of 10 LAMS was performed in nine patients. The procedural times (range 4 minute and 30 seconds/12 minutes) [Figure 3].
Figure 3.
(a) Perirectal abscess; (b) endoscopic ultrasound guided deployment of LAMS; (c) endoscopic view of pus drainage from the collection
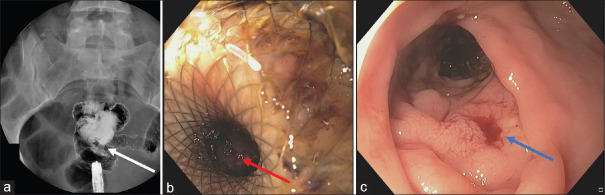
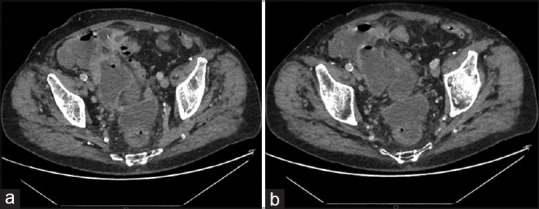
Technical success was achieved in 8 out of 9 patients (88.8%). Technical failure occurred in 1 patient (11.2%) due to diffuse peri-colonic fat inflammation, localised peritonitis and presence of a non-capsulated abscess. After LAMS deployment, endoscopic control with contrast study across the stent highlighted an iatrogenic anastomosis between the rectum and distal sigmoid colon [Figure 4]. LAMS was removed without any further complication. Nonetheless, the patient underwent Hartmann colonic resection 1 week later due to persistent sepsis related to ALCD. Other than the aforementioned mis-deployment, no procedural adverse event occurred. CT scan control (at 7 day) highlighted collection resolution and LAMS correctly in place [Figure 5]. In five patients, a residual pseudo-cavity was detected. At the first endoscopic follow-up, all LAMS were correctly in place. Of note, all patients had CRP and WBC normalisation. In 5 out of 8 patients, the metal stent was replaced with a double pigtail plastic stent due to persistency of a pseudo-cavity. At 3 months control, the plastic stent was found in only 2 out of 5 patients. In the remaining 3 cases, a spontaneous evacuation had occurred. After a median follow-up of 11 months (range 6–17; SD 3.3): 3 pts underwent elective laparoscopic sigmoidectomy, whereas 5 did not undergo surgery and showed no ALCD recurrency. Overall clinical success was achieved in 8 out of 9 patients (88.8%) [Table 1].
Figure 4.
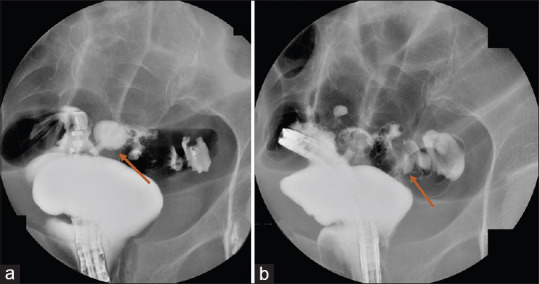
(a and b) Technical failure with LAMS mis-deployment in the distal sigmoid colon with erroneous creation of a colo-rectal anastomosis
Figure 5.
(a and b) Computed tomography scan before LAMS removal showing complete collapse of the abscess without residual pseudo-cavity
Table 1.
Patients characteristics and results
| Age (years) | Sex | CRP (mg/ml) | Abcess size (cm) | Trans-colic/trans rectal | LAMS (N°) | LAMS (size) (mm) | Technical success | Clinical success | Follow-up (months) |
|---|---|---|---|---|---|---|---|---|---|
| 91 | Male | 98 | 10 | C/R | 2 | 8×8 | Y | Y | 6 |
| 8 | 6×8 | ||||||||
| 23 | Female | 235 | 6 | C | 1 | 8×8 | Y | Y | 15 |
| 35 | Female | 312 | 9 | R | 1 | 6×8 | Y | Y | 9 |
| 56 | Female | 67 | 3 | R | 1 | - | N/A | N/A | N/A |
| 39 | Female | 44 | 5 | R | 1 | 6×8 | Y | Y | 17 |
| 45 | Male | 187 | 14 | R | 1 | 10×10 | Y | Y | 10 |
| 61 | Female | 89 | 8 | C | 1 | 8×8 | Y | Y | 8 |
| 77 | Female | 81 | 6 | R | 1 | 8×8 | F | F | F |
| 84 | Male | 112 | 7 | R | 1 | 8×8 | Y | Y | 11 |
| 85 | Male | 40 | 6 | C | 1 | 10×10 | Y | Y | 12 |
*Patients 4 undergo to a diagnostic EUS only with no LAMS deployment due to collection size>3 cm coupled with clinical improvement after antibiotic therapy. CPR: C reactive protein, LAMS: Lumen apposing metal stent, EUS: Endoscopic ultrasound, N/A: Not available
DISCUSSION
Diverticular disease is common in the Western society and is increasing in the young population.[9] Management of diverticulitis has shifted from primary surgical intervention towards a non-operative approach of bowel rest and broad-spectrum intravenous antibiotics in conjunction with interventional procedures to drain abscesses whenever necessary. In clinical practice, it is mandatory to rapidly differentiate between diffuse peritonitis requiring emergency surgery and localised, encapsulated peritonitis that may benefit a mini-invasive approach. The presence of free air, volume and location of the diverticular abscess are the key factors in determining the success of conservative management.[9] PAD seems to reduce recurrence of ALCD compared to both antibiotic treatment only and surgical drainage; the latter, even burdened by an increased mortality rate.[10] US-guided PAD fails in up to 35%,[4] and it requires drainage replacement/repositioning in 15.5% of cases. Nowadays, CT scan-guided PAD is preferred due to higher anatomical definition and possibility of three-dimensional image rendering which helps preventing damage to vital nearby structure.[11] Nonetheless, CT-guided PAD failure has been described in up to 30% of cases,[12] especially in case of multilocular abscess or in the presence of a fistula.[12,13] Catheter malfunctioning and other complications seem higher in case of deep seated collections.[12] There are no studies comparing the outcome of percutaneous versus endoscopic drainage for ALCD-related abscesses. However, several studies comparing the two techniques for gall bladder drainage and pancreatic fluid collections highlighted that EUS drainage with LAMS had a higher success rate with lower complications, shorter hospital stay and fewer reinterventions and hospital readmissions.[14,15,16] Endoscopic transluminal approach has been proposed as a feasible and effective treatment option; however, high quality evidence for endoscopic transluminal drainage in cases of diverticular abscess is lacking in the literature. To our knowledge, this is the biggest consecutive case series on diverticular abscess drainage by means of LAMS.[8,11,17]
We have already reported a case of transrectal drainage of an organised hematoma using a LAMS (without integrated electrocautery) following obesity surgery.[18]
The advent of LAMS has been an important breakthrough for therapeutic EUS allowing a minimally invasive approach for the management of a wide range of conditions such as biliary and gall bladder drainage, biliary access in altered anatomy and relief of gastric outlet obstruction.[19,20] The specific design of LAMS ‘dumbbell-shaped with wide-bore saddle regions’ should guarantee lumen apposition of two adjacent organs thus avoiding fluid leak and reduce the risk of stent migration.
LAMS has different intrinsic advantages over double pigtail stents (DPS) for the management of ALCD-related abscesses. First, LAMS deployment procedure is much faster, safer and relatively easier compared to DPS. Moreover, its design allows a better tissue apposition reducing the risk of spillage of infected fluid within the abdominal or pelvic cavity. DPS drainage requires multiple steps (19 G puncture, guidewire looping within the cavity, tract dilation, catheter exchange and DPS deployment) each one carrying an independent risk of complication. (i.e., perforation, fluid leak after dilation, needle damage if EUS scope is bent too much).[8,21] Second, LAMS larger Calibre induce a fast and complete drainage of the abscess allowing clearance of necrotic debris, as well.
According to our experience, EUS-guided drainage and LAMS deployment may be feasible and favourable for encapsulated ALCD related abscesses larger than 3 cm in size and close (≤1 cm) to the colonic wall. It is of paramount importance to perform EUS drainage only in case of well delimited and encapsulated collection. Our technical failure occurred in case of a not capsulated abscess. In this patient, due to major inflammation, sigmoid colon was misinterpreted as abscess cavity. Most probably, EUS drainage was performed too early and the abscess did not have time to delimit itself.
The diameter of LAMS was chosen according to the size of abscess; however, we suggest to use maximum a 10 mm diameter stent.
Systematic LAMS removal was performed after 15 days in all patients. This timeframe was primarily decided to reduce the risk of the most common LAMS’s related complication, namely bleeding due to erosion of surrounding vascular structures. When the abscess is completely drained and cavity collapsed, if LAMS remains in place for too long time, friction between the stent and vessels in the wall of the pseudo-cavity may induce severe bleeding. Moreover, stent embedding within the colonic wall may occur as well.[22] In our small series neither bleeding nor stent embedding occurred. Of note, after LAMS removal the entry site was hardly visible in almost all the patients [Figure 6]. Subsequent DPS deployment was fundamental to guarantee further fluid drainage and to allow second intention pseudo-cavity obliteration. DPS acted as a foreign body promoting granulation tissue formation.[23] The plastic stent migrated spontaneously in 3 out of 5 cases due to complete closure of the pseudo-cavity.
Figure 6.
Endoscopic and fluoroscopic control at 15 days showing (a) no pseudo-cavity opacification (b) LAMS correctly in place (c) closure of the rectal wall soon after stent removal
CONCLUSIONS
This is the first case series applying pure EUS-guided drainage with LAMS deployment for the management of ALCD abscess. This seems an effective approach for encapsulated abscesses more than 4 cm in size and close to the colonic wall. The proposed technique appears fairly safe at expert hands avoiding radiologic intervention and/or surgery in almost all cases (88.8%). However, future studies comparing EUS-guided drainage with the current gold standard treatment of percutaneous CT-guided drainage are needed for further assessment on the efficacy and safety of EUS approach.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Galetin T, Galetin A, Vestweber KH, Rink AD. Systematic review and comparison of national and international guidelines on diverticular disease. Int J Colorectal Dis. 2018;33:261–72. doi: 10.1007/s00384-017-2960-z. [DOI] [PubMed] [Google Scholar]
- 2.Schoetz DJ., Jr Diverticular disease of the colon: A century-old problem. Dis Colon Rectum. 1999;42:703–9. doi: 10.1007/BF02236921. [DOI] [PubMed] [Google Scholar]
- 3.Nagata N, Ishii N, Manabe N, Tomizawa K, Urita Y, Funabiki T, et al. Guidelines for colonic diverticular bleeding and colonic diverticulitis: Japan gastroenterological association. Digestion. 2019;99(Suppl 1):1–26. doi: 10.1159/000495282. [DOI] [PubMed] [Google Scholar]
- 4.Sartelli M, Weber DG, Kluger Y, Ansaloni L, Coccolini F, Abu-Zidan F, et al. 2020 update of the WSES guidelines for the management of acute colonic diverticulitis in the emergency setting. World J Emerg Surg. 2020;15:32. doi: 10.1186/s13017-020-00313-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mali J, Mentula P, Leppaniemi A, Sallinen V. Determinants of treatment and outcomes of diverticular abscesses. World J Emerg Surg. 2019;14:31. doi: 10.1186/s13017-019-0250-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lambrichts DP, Bolkensteint HE, Van des Does DC, Dieleman D, Crolla RM, Dekker JW, et al. Multicentre study of non-surgical management of diverticulitis with abscess formation. Br J Surg. 2019;106:458–66. doi: 10.1002/bjs.11129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donatelli G, Fuks D, Cereatti F, Pourcher G, Perniceni T, Dumont JL, et al. Endoscopic transmural management of abdominal fluid collection following gastrointestinal, bariatric, and hepato-bilio-pancreatic surgery. Surg Endosc. 2018;32:2281–7. doi: 10.1007/s00464-017-5922-1. [DOI] [PubMed] [Google Scholar]
- 8.Poincloux L, Caillol F, Allimant C, Bories E, Pesenti C, Mulliez A, et al. Long-term outcome of endoscopic ultrasound-guided pelvic abscess drainage: A two-center series. Endoscopy. 2017;49:484–90. doi: 10.1055/s-0042-122011. [DOI] [PubMed] [Google Scholar]
- 9.Chua TC, Jeyakumar A, Ip JC, Yuide PJ, Burstow MJ. Conservative management of acute perforated diverticulitis: A systematic review. J Dig Dis. 2020;21:63–8. doi: 10.1111/1751-2980.12838. [DOI] [PubMed] [Google Scholar]
- 10.Gregersen R, Mortensen LQ, Burcharth J, Pommergaard HC, Rosenberg J. Treatment of patients with acute colonic diverticulitis complicated by abscess formation: A systematic review. Int J Surg. 2016;35:201–8. doi: 10.1016/j.ijsu.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 11.Lambrichts DP, Birindelli A, Tonini V, Cirocchi R, Cervellera M, Lange JF, et al. The multidisciplinary management of acute complicated diverticulitis. Inflamm Intest Dis. 2018;3:80–90. doi: 10.1159/000486677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Golfieri R, Cappelli A. Computed tomography-guided percutaneous abscess drainage in coloproctology: Review of the literature. Tech Coloproctol. 2007;11:197–208. doi: 10.1007/s10151-007-0354-x. [DOI] [PubMed] [Google Scholar]
- 13.Akıncı D, Ergun O, Topel Ç, Çiftçi T, Akhan O. Pelvic abscess drainage: Outcome with factors affecting the clinical success. Diagn Interv Radiol. 2018;24:146–52. doi: 10.5152/dir.2018.16500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luk SW, Irani S, Krishnamoorthi R, Lau JY, Ng EK, Teoh AY. Endoscopic ultrasound-guided gallbladder drainage versus percutaneous cholecystostomy for high risk surgical patients with acute cholecystitis: A systematic review and meta-analysis. Endoscopy. 2019;51:722–32. doi: 10.1055/a-0929-6603. [DOI] [PubMed] [Google Scholar]
- 15.Yang J, Chen YI, Friedland S, Holmes I, Paiji C, Law R, et al. Lumen-apposing stents versus plastic stents in the management of pancreatic pseudocysts: A large, comparative, international, multicenter study. Endoscopy. 2019;51:1035–43. doi: 10.1055/a-0759-1353. [DOI] [PubMed] [Google Scholar]
- 16.Chen YI, Yang J, Friedland S, Holmes I, Law R, Hosmer A, et al. Lumen apposing metal stents are superior to plastic stents in pancreatic walled-off necrosis: A large international multicenter study. Endosc Int Open. 2019;7:E347–54. doi: 10.1055/a-0828-7630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramesh J, Bang JY, Trevino J, Varadarajulu S. Comparison of outcomes between endoscopic ultrasound-guided transcolonic and transrectal drainage of abdominopelvic abscesses. J Gastroenterol Hepatol. 2013;28:620–5. doi: 10.1111/jgh.12081. [DOI] [PubMed] [Google Scholar]
- 18.Donatelli G, Dumont JL, Cereatti F, Randone B, Meduri B, Lainas P, et al. EUS-Guided transrectal evacuation of organized pelvic collection following roux-en-y Gastric bypass after failure of radiological and surgical approach. Obes Surg. 2018;28:595–6. doi: 10.1007/s11695-017-3031-9. [DOI] [PubMed] [Google Scholar]
- 19.Donatelli G, Cereatti F, Derhy S. Endoscopic ultrasound-guided duodenojejunostomy for management of refractory benign hepaticojejunal anastomotic stricture. Endoscopy. 2019;51:E261–2. doi: 10.1055/a-0896-2310. [DOI] [PubMed] [Google Scholar]
- 20.Iqbal U, Khara HS, Hu Y, Kumar V, Tufail K, Confer B, et al. EUS-guided gastroenterostomy for the management of gastric outlet obstruction: A systematic review and meta-analysis. Endosc Ultrasound. 2020;9:16–23. doi: 10.4103/eus.eus_70_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Donatelli G, Cereatti F. Fracture of 19G needle during transrectal endoscopic ultrasound-guided drainage of perirectal collection: An unusual complication. Endoscopy. 2019;51:E263–4. doi: 10.1055/a-0896-2336. [DOI] [PubMed] [Google Scholar]
- 22.Bang JY, Navaneethan U, Hasan MK, Sutton B, Hawes R, Varadarajulu S. Non-superiority of lumen-apposing metal stents over plastic stents for drainage of walled-off necrosis in a randomised trial. Gut. 2019;68:1200–9. doi: 10.1136/gutjnl-2017-315335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Donatelli G, Dumont JL, Cereatti F, Ferretti S, Vergeau BM, Tuszynski T, et al. Treatment of leaks following sleeve gastrectomy by endoscopic internal drainage (EID) Obes Surg. 2015;25:1293–301. doi: 10.1007/s11695-015-1675-x. [DOI] [PubMed] [Google Scholar]