Abstract
Objective:
Migraine is a prevalent and disabling neurological disease. Its genesis is poorly understood and there remains unmet clinical need. We aimed to identify mechanisms and thus novel therapeutic targets for migraine using human models of migraine and translational models in animals, with emphasis on amylin, a close relative of calcitonin gene-related peptide (CGRP).
Methods:
Thirty-six migraine without aura patients were enrolled in a randomized, double-blinded, two-way, cross-over, positive-controlled clinical trial study to receive infusion of an amylin analogue pramlintide or human αCGRP on two different experimental days. Furthermore, translational studies in cells and mouse models, and rat and human tissue samples were conducted.
Results:
Thirty patients (88%) developed headache after pramlintide infusion, compared to thirty-three (97%) after CGRP (p = 0.375). Fourteen patients (41%) developed migraine-like attacks after pramlintide infusion, compared to nineteen patients (56%) after CGRP (p = 0.180). The pramlintide induced migraine-like attacks had similar clinical characteristics to those induced by CGRP. There were differences between treatments in vascular parameters. Human receptor pharmacology studies showed that an amylin receptor likely mediates these pramlintide-provoked effects, rather than the canonical CGRP receptor. Supporting this, preclinical experiments investigating symptoms associated with migraine showed that amylin treatment, like CGRP, caused cutaneous hypersensitivity and light aversion in mice.
Interpretation:
Our findings propose amylin receptor agonism as a novel contributor to migraine pathogenesis. Greater therapeutic gains could therefore be made for migraine patients through dual amylin and CGRP receptor antagonism, rather than selectively targeting the canonical CGRP receptor.
Keywords: Amylin, headache, pramlintide, CGRP, CLR, RAMP, migraine pathophysiology, pain, CTR
Introduction
Migraine is a chronic neurological disease, 1 characterised by recurrent attacks of moderate or severe headache with concomitant symptoms including photophobia, phonophobia, and nausea. 2 Migraine afflicts more than 10% of the global adult population and is a leading cause of years lived with disability in women between ages 15 and 49. 2–4 Migraine is highly complex, and its underlying mechanistic basis is elusive. Despite major therapeutic advances, many patients remain refractory to effective treatment. Even recently approved drugs targeting the calcitonin gene-related peptide (CGRP) pathway reduce monthly migraine days only in 40-60% of patients 5,6, with substantial variability in response rate. 6 This suggests that these drugs do not effectively target all components of the CGRP pathway and/or that other targets exist.
Amylin is a member of the CGRP peptide family, sharing substantial amino acid sequence identity with CGRP. Amylin is predominantly expressed in the pancreas, acting as an endocrine hormone together with insulin. 7–9 The beneficial metabolic effects of amylin have resulted in the approved amylin analog drug, pramlintide. 9 However, amylin receptor expression in sensory neurons, rodent studies reporting effects of amylin in pain models and a report of pramlintide causing headache as a side-effect, suggest that amylin and/or its receptors could play an unrecognised role in migraine. 10–14 A further connection between amylin and CGRP is their shared receptor, AMY1.
Human migraine provocation studies are powerful for identifying novel mechanisms and therapeutic targets. 3 Data for CGRP from such a model made a major contribution to solidifying the CGRP system as a target for migraine, translating into six Food and Drug Administration (FDA)-approved drugs. 15 As migraine is thought to be a uniquely human experience, we directly asked the question as to whether amylin could provoke migraine in a clinical trial in migraine patients. For this trial, we selected pramlintide as the amylin agonist because human amylin is not suitable for use as a drug in humans due to its fibrillogenic properties. 9 We conducted a randomized, double-blinded, two-way cross-over, positive-controlled clinical trial study using well-validated human models of migraine (Fig.1). We complemented this with behavioral studies in mice and translational experiments that probed the expression, localization, distribution and receptor pharmacology of amylin in mice, rats and humans.
Figure 1. Experimental design.
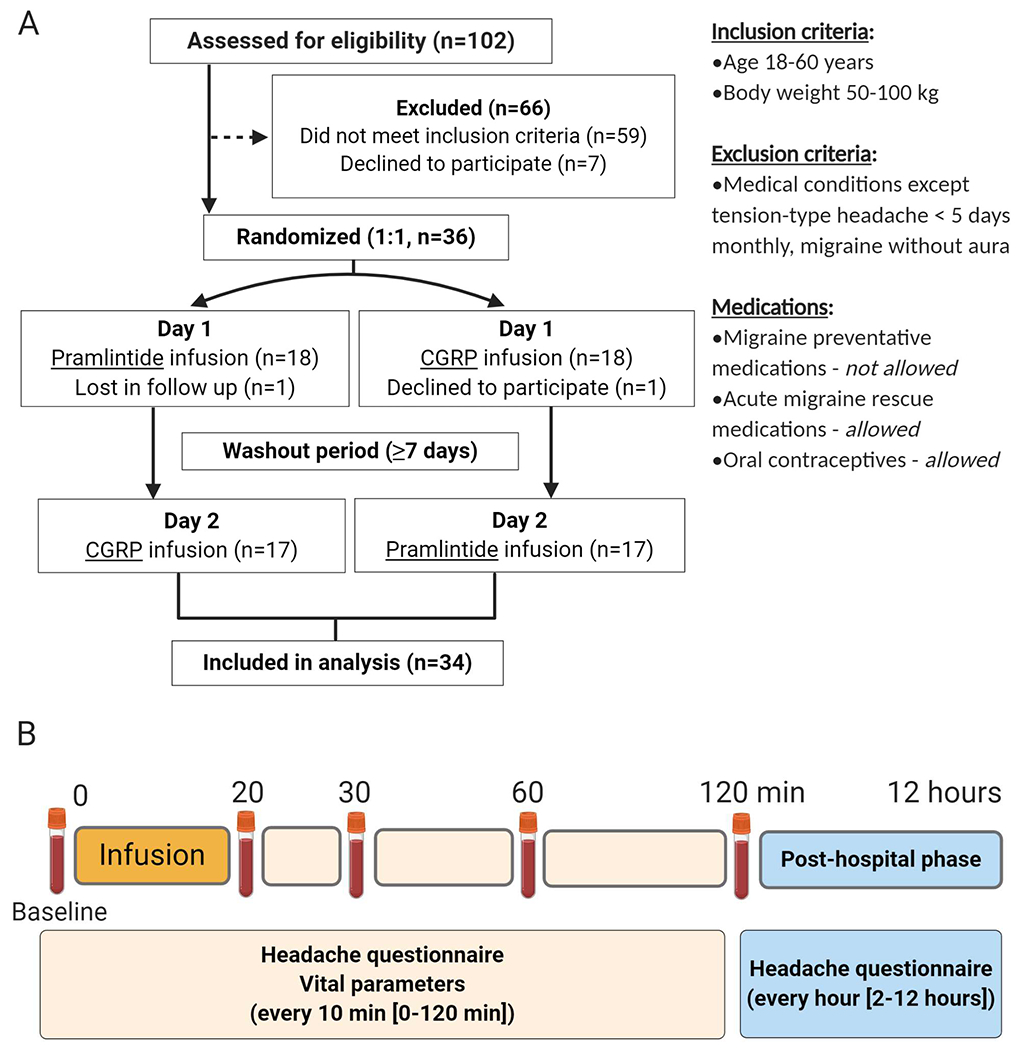
A) Workflow chart, explaining inclusion and exclusion criteria and B) Main study design. For B), hospital phase (0-120 min), post-hospital phase (2-12 hours). In B), infusion was of pramlintide (6 μg/min) or CGRP (1.5 μg/min), Headache and migraine associated symptoms including premonitory symptoms were recorded in the following order using a standard questionnaire: nausea, vomiting, photophobia, phonophobia, unusual fatigue, craving, yawning, thirst, mood swings, facial flushing and difficulty concentrating. We used a numerical rating scale from 0 to 10 to obtain headache and migraine pain intensity.
Materials and Methods
Pharmacology of CGRP and pramlintide at calcitonin-family receptors
Human receptors: CGRP (calcitonin-like receptor [CLR]/receptor activity-modifying protein [RAMP] RAMP1), adrenomedullin (AM) AM1, (CLR/RAMP2), AM2 (CLR/RAMP3), calcitonin (calcitonin receptor [CTR, CT(a) splice variant]), AMY1 (CTR/RAMP1), AMY2 (CTR/RAMP2), AMY3 (CTR/RAMP3), were transiently transfected into Cos7 cells as previously described. 16,17 CGRP and pramlintide-stimulated cAMP production was measured as previously described. 18 Data are the mean ± SEM, fit to a three-parameter logistic equation with significance accepted at p <0.05.
Clinical study
Participants
Forty-two participtants (six healthy volunteers [pilot study], 36 migraine without aura patients [main study)] were recruited via a website for recruitment of volunteers to clinical research (forsoegsperson.dk) or out-patient clinics. Participants completed a physical and neurological examination with a trained physician (HG). The study protocol was approved by the Capital Region Ethics Committee of Denmark (H-17035931) and registered at Clinicaltrials.gov (ID: NCT03598075). In accordance with the Declaration of Helsinki (2013 version), informed consent was obtained after oral and written information had been provided. The study was performed between July 2018 and September 2019. Pramlintide and CGRP peptides were synthesized in-house using methods, purification, and analysis described in Supplementary Table 1. The intravenous solution was prepared by the Capital Region Central Pharmacy, Denmark.
Pilot dose finding study
All migraine inducing substances identified to date are vasoactive 1,3 therefore, we conducted a randomized, single-blind multiple-ascending-dose finding (1.5, 3, 6 μg/min over 20 min) pilot study, to investigate the safety, tolerability and potential vasoactive properties of pramlintide in six healthy participants. Inclusion criteria are as Fig. 1A and specific exclusion criteria were: daily intake of medication except contraceptives, serious somatic disease, history of migraine including family predisposition, any other type of headache except episodic tension-type headache less than once a month. Participants received intravenous pramlintide on three separate days with a washout period of ≥5 days. The highest dose (6 μg/min) was well tolerated and appeared to have a slight vasodilatory effect, increasing the diameter of the superficial temporal artery (STA) and radial artery (RA) and inducing facial flushing, compared to baseline. This dose was therefore used for the main study. Four out of six subjects reported mild headache of diffuse intensity, three out of six reported transient mild nausea for a period of 10-20 minutes post infusion which resolved without intervention. Given this, a glucose clamp system was available for the main study but was not required for any subject. The small sample size of this pilot precludes detailed analysis and these observations should be considered with reservation.
Main study
Participants with episodic migraine without aura and with a minimum of two migraine attacks per month as defined according to the International Classification of Headache Disorder 3 beta version (ICHD-3 beta) 2 were recruited for the main study. Inclusion and exclusion criteria (for both sexes) are described in Fig. 1. Participants were randomly assigned receive infusion of pramlintide or CGRP over 20 min in a double-blinded, two-way cross-over, positive-controlled fashion on two different experimental days (Fig. 1) following a screening visit and separated by ≥1 week. A balanced in-house randomization and drug allocation were carried out by personnel unrelated to the study to ensure study staff, participants and investigator remained blinded.
Experimental procedures
The study is outlined in Figure 1B. Participants arrived for the experiment ≥ 48 hours migraine and headache-free without taking analgesics, caffeine, alcohol or smoking within twelve hours of the experiment. Female participants were booked for experiments at least three days before or after menstruation and pregnancy tests were conducted on both study days. Patients were placed in a supine position and venous catheters inserted into each antecubital vein; one for peptide infusion and the other for blood sampling. After 20 min of rest in a temperature and light controlled laboratory, baseline values were recorded. A well-validated headache questionnaire 19 was used to record headache/migraine intensity and characteristics, mean arterial pressure (MAP) and heart rate (HR), were monitored. The criteria for experimentally-induced migraine attacks were used. 3 High resolution ultrasonography (Dermascan C; Cortex Technology: 20MHz, bandwidth 5MHz) was used to measure the diameter of left STA and left RA, as previously described. 20 Facial skin blood flow was measured using the laser speckle contrast technique (moorFLPI, Moor instruments, Devon UK) as previously described. 21 We found no carry-over or period effect for baseline values of headache, HR, MAP, STA and RA, facial flushing (p > 0.05). Infusion was initiated (time 0) after completion of baseline measurements, with measurements repeated every 10 min. After 120 min participants were discharged with a headache questionnaire to complete hourly for 12 hours post infusion.
Blood collection and processing
Blood was collected at five different time points (Fig. 1B). After discarding the first 2-3 mL sample, blood was transferred into pre-cooled (4°C) blood collection tubes (BD P100 8.5 mL, BD, USA) for pramlintide and (VACUETTE TUBE 10 mL K3E K3EDTA Greiner Bio-One International GmbH, Germany) for CGRP measurement, respectively. Tubes were inverted several times and stored in a cool box for 15 min until centrifugation (4°C, 2000 g, 10 min). Plasma was transferred to pre-cooled 1.5 mL protein LoBind microcentrifuge tubes (Eppendorf, Germany) and immediately stored at −50°C, then transferred to −80°C until analysis. Blood glucose, pH, hemoglobin, lactate were analyzed at the lab (ABL, Radiometer, Copenhagen, Denmark).
Statistics and data analysis
The sample size was calculated by determining the risk of type 1 error at 5% and defined a power at 80% and type 2 error was fixed at 20%. The migraine induction rate after infusion of CGRP in this human experimental model is approximately 60% and the migraine induction rate after pramlintide infusion hypothesized to be at the level of nocebo, selected as 20%.3 Using a cross-over design to detect a difference in migraine induction between the treatment arms with a 40% difference, we estimated that 15 patients would be sufficient to observe a difference. However, to ensure that the study had adequate power to answer the primary endpoint of the study, we included 36 patients in each arm. We set the difference between incidence of migraine attack between pramlintide and CGRP as the primary endpoint of the study.
The incidence of migraine attacks and headache were analyzed using McNemar test of equal probability for paired data. We used logistic regression with log-link, taking account of the correlation between the two binary observations for each patient.
For the all quantitative outcomes the Mixed model for repeated measurements, allowing for correlation between the two series of measurements for each patient (and possibly different variances for the two treatments) and additional autocorrelation within each longitudinal series, was used. The mean values were set to be equal at time 0 for the two treatments but allowed to vary freely for the rest of the measurement times. Overall equality of treatments were tested and estimates of difference for each time was calculated. Mann-Whitney test was used to test for period and carry-over effects for all baseline variables.
Clinical data are presented as mean ± SEM, except the headache intensity scores which are individual data sets and median score. Statistical analyses were carried out using SPSS version 23.0 (Chicago, IL, USA) with no adjustments made for multiple analyses, p < 0.05 was accepted as the level of significance.
Analysis of CGRP and pramlintide in plasma samples
Pramlintide plasma concentrations were measured using a competitive sandwich enzyme human amylin immunoassay (EIA; Phoenix Pharmaceuticals, CA, USA EK-017-03). Assay conditions were optimized; 1) A pramlintide standard curve showed equivalence to the kit standard curve and therefore pramlintide was used as the standard for all assays, 2) The specificity of this assay is reportedly 100% for human amylin, amylin-amide or pramlintide but human αCGRP amounts (100-30,000 ng/mL) were tested for cross-reactivity in the EIA, with no signal above buffer only. 3) Control or untreated plasma samples were diluted 1:2, 1:4, 1:8 or 1:16 in assay buffer and the recovery of a 1 ng/mL pramlintide spike determined. The optimal plasma dilution was 1:16, exhibiting the least pronounced background effect, whilst maintaining pramlintide recovery and was therefore used for all patient samples. For analysis, patient plasma samples were thawed to room temperature, diluted and assayed in duplicate. Radioimmunoassay was used to measure CGRP plasma concentrations as described previously. 22
Light aversion and plantar von Frey assays
Male and female C57BL/6J mice (Jackson Laboratories, California and Maine facilities) were housed in groups of five per cage on a 12 h light cycle with food and water ad libitum. Blinded experiments were performed between 7:00 A.M. and 4:00 PM. on mice aged 10-20 weeks, at 20-35 g. Animal procedures were approved by the University of Iowa Animal Care and Use Committee and performed in accordance with the standards set by the National Institutes of Health. Rat α-CGRP (Sigma-Aldrich) or rat/mouse amylin (Bachem) were diluted in the vehicle, which was Dulbecco phosphate buffered solution (Hyclone) and administered at 10 μl/g bodyweight with a 30 g x 0.5-inch needle by intraperitoneal (i.p.) injection in mice held gently but not anesthetized during injection. Different cohorts of mice were used for light aversion and cutaneous hypersensitivity tests.
For light aversion assays light/dark boxes with infrared beam tracking were used (Med Associates), using bright light (25-27,000 lux) and pre-exposure to the chamber twice prior to treatment, as previously described. 23,24 Mice were either immediately placed in the light/dark chamber for testing (amylin) or returned to their home cages for 30 min (CGRP) before testing. Data were analyzed by 2-way ANOVA (line graph) or unpaired t test (scatter plot) for vehicle vs treatment for all conditions as only one experimental condition was performed in a single day. For comparison of amylin vs CGRP and male vs female comparisons, mice were normalized to their respective vehicle control.
For cutaneous hypersensitivity testing, mice were habituated to an acrylic chamber (114 x 80 cm) over a grid support (Bioseb, France), for 1 h on the day of baseline testing. Mice were treated the following day using eight von Frey filaments from A (0.008 g) to H (1 g) (Bioseb, France) using the up and down method. 25,26 The 50% thresholds (g) were log-transformed to obtain normally distributed data suitable for parametric analysis by paired t-test (treatment vs. baseline) or unpaired t-test (treatment vs. vehicle).
Detection of CGRP and amylin-like immunoreactivity in mouse, rat and human trigeminal ganglia
Immunofluorescence was conducted in C57/BL/6 mouse, Sprague-Dawley rat and post-mortem human trigeminal ganglia (TG). All procedures involving the use of animals were conducted in accordance with the New Zealand animal welfare act (1999) and approved by the University of Auckland Animal Ethics Committee. Rodents were housed in pairs in a controlled environment (12-hour light-dark cycle; room temperature, 22±2°C) with ad libitum access to water and standard chow (Teklad TB 2018; Harlan, Madison, WI) and water. Cages also contained an additional enrichment item (house or toy).
Post-mortem human TG were obtained from the University of Auckland Human Anatomy Laboratory, obtained with informed consent by the donor before death and next of kin after death as part of the University of Auckland Human Body Bequest Program for teaching and research. This program and its procedures operate under the Human Tissue Act 2008 and overseen by the New Zealand Police Inspector of Anatomy. Case details are provided in (Table 1). Primary antibody, secondary antibody details and staining procedures for the TG samples are outlined and compared in Supplementary Tables 2, 3.
Table 1.
Details of human cases used for immunofluorescence.
| Case | Sex | Age | Cause of death | Clinical notes |
|---|---|---|---|---|
| 15A | M | 94 | Bronchopneumonia | Slow, steady dementia |
| 3A | F | 92 | Aspiration pneumonia/respiratory failure | Mild, slowly progressive dementia |
| 4A | M | 87 | Myocardial infarction | No cognitive decline reported |
Rodent TG sections were imaged using a Zeiss LSM 710 inverted confocal microscope (20x [0.8 NA] objective, Zeiss, Oberkochen, Germany) or an Operetta high-content imaging system in confocal fluorescence mode (20x high numerical aperture [0.75] objective, PerkinElmer). Human TG sections were imaged using a Zeiss LSM 710 inverted confocal microscope as above. Images were saved as 12-bit files and adjusted for color, contrast and brightness in FIJI.
Results
Pramlintide potently activates AMY receptors but not the CGRP receptor
Amylin activates three unusual G protein-coupled receptors (GPCR) consisting of the CTR and a RAMP; the AMY1, AMY2 and AMY3 receptors. 17 The human AMY1 receptor is a dual receptor for amylin and CGRP. 17 CGRP also activates the canonical CGRP receptor, CLR/RAMP1. CLR also forms complexes with RAMP2 and RAMP3 to form AM receptors. We conducted experiments to validate the receptor pharmacology of pramlintide (a hybrid of human and rat amylin) and human αCGRP in a paired experimental design where both peptides were always tested simultaneously at the seven major human calcitonin family receptors. 17 These experiments used the same batches of peptide as our human clinical study. CGRP was a potent agonist of the CGRP receptor (Fig.2A), with weaker activity at all other receptors (Fig.2D-H), except the AMY1 receptor (Fig.2B), where it was equipotent to the CGRP receptor (Fig.2C, Table 2). Pramlintide had a pharmacological profile that was distinct from CGRP. Pramlintide was a very weak agonist of the CGRP receptor (Fig.2A), being >1000-fold less potent than CGRP at this receptor. Pramlintide activated the AMY1 receptor equally to CGRP (Fig.2B) and was also active at the CTR, AMY2 and AMY3 receptors (Fig.2E, G, H). Pramlintide had comparatively weak activity at the AM2 receptor (Fig.2F), and no measurable activity at the AM1 receptor (Fig.2D, Table 2). Pramlintide can therefore distinguish CLR from CTR-based receptors making it a useful tool to probe activation of amylin-responsive receptors in humans.
Figure 2. Receptor pharmacology of hαCGRP and pramlintide at human calcitonin family receptors.
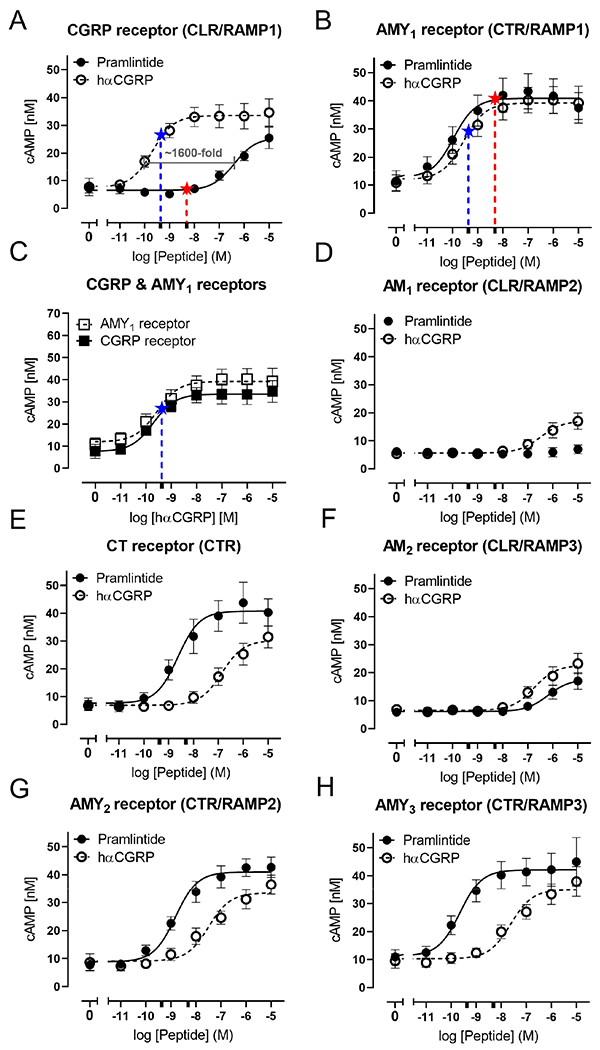
A) CGRP receptor, B) AMY1 receptor, C) comparing hαCGRP at CGRP and AMY1 receptors, D) AM1 receptor, E) CT receptor, F) AM2 receptor, G) AMY2 receptor, H) AMY3 receptor. cAMP production from transiently transfected Cos-7 cells in response to each peptide ligand is shown. Data are mean ± SEM. combined from five independent experiments, each with three technical replicates. Adrenomedullin was used to confirm functionality of the AM receptors (data not shown). Stars on A)-C) indicate the mean peak plasma concentration achieved for each peptide in the assays used in this study; pramlintide (4943 pM, red), CGRP (428 pM, blue) marked on the corresponding concentration-response curve. In A)-H) this is also marked for the relevant peptide with an extra tick on the X axis to show the log molar concentration.
Table 2.
Pharmacology of CGRP and pramlintide at human calcitonin-family receptors.
| Receptor | hαCGRP | Pramlintide | ||
|---|---|---|---|---|
| pEC50 (EC50 nM) | Emax (cAMP [nM]) | pEC50 (EC50 nM) | Emax (cAMP [nM]) | |
| CGRP | 9.70 ± 0.12^ (0.20) | 33.7 ± 4.17 | 6.48 ± 0.28* (331) | 26.7 ± 3.96 |
| AM1 | 6.43 ± 0.11* (372) | 17.2 ± 2.95 | <5 (<10,000) | NC |
| AM2 | 6.64 ± 0.14*^ (229) | 22.8 ± 3.52 | 6.23 ± 0.06* (588) | 20.4 ± 1.63 |
| CTR | 6.80 ± 0.09*^ (158) | 30.5 ± 3.81 | 8.59 ± 0.12* (2.6) | 41.1 ± 5.85 |
| AMY1 | 9.48 ± 0.22 (0.33) | 38.2 ± 4.31 | 9.91 ± 0.22 (0.12) | 40.4 ± 6.10 |
| AMY2 | 7.51 ± 0.11*^ (31) | 32.6 ± 3.99 | 8.79 ± 0.08* (1.62) | 40.1 ± 4.39 |
| AMY3 | 7.50 ± 0.23*^ (32) | 35.1 ± 5.02 | 9.67 ± 0.17 (0.21) | 41.6 ± 6.61 |
Data are the mean ± SEM. of five independent experiments, with the following exception; at the AM2 receptor data for pramlintide are from four experiments as no curve (NC) could be fitted to the data in one further experiment. At the AM1 receptor, no curve could be fitted to the pramlintide data in any experiment and the pEC50 is thus estimated as <5. For each peptide, statistical comparisons to the other receptors were made by one-way ANOVA, followed by Tukey’s test; this was for each receptor versus the CGRP receptor for CGRP and for each receptor versus the AMY1 receptor for pramlintide. These statistical differences (p < 0.05) are shown as
Comparisons between peptides at each receptor was by unpaired t test and significant differences (p < 0.05) are shown as
Statistical analysis was conducted on the pEC50 values with the mean EC50 in nM also shown for ease of comparison. Emax values were compared only between peptides at each receptor by unpaired t test, with no significant differences.
Pramlintide induces migraine-like attacks in migraine without aura patients
Thirty-four (31 female, 3 male) migraine without aura patients completed the study (Fig. 1). We used CGRP instead of placebo for two reasons, 1) Due to its structural and biological similarity to amylin with well-documented migraine-inducing properties and adverse events profile in human experimental models 27–29, 2) A meta-analysis of 12 randomized, double-blind, placebo-controlled, two-way crossover design studies using human models of migraine (n=182) shows a low nocebo response rate of 8.1% (95% CI = 2.5-15.5%, I2=50.8%) in human models of migraine. 30 Therefore, the cross-over design of our trial, enabled direct comparison of a known migraine inducer (CGRP) to the test substance (pramlintide).
Thirty patients (88%) developed headache after pramlintide infusion, compared to thirty-three (97%) after CGRP (p = 0.375), (Supplementary Table 4). Fourteen patients (41%) developed migraine-like attacks after pramlintide infusion, compared to nineteen (56%) after CGRP (p = 0.180). Twelveout of 34 (35%) developed migraine on both days. Seven patients responded only to CGRP and two only to pramlintide. The AUC(0–12h) for headache intensity score after CGRP infusion was significantly larger than after pramlintide (p < 0.001). Some migraine associated symptoms were significantly different between the two treatments: photophobia (pramlintide 12/34 vs. CGRP 20/34, p = 0.008) and phonophobia (pramlintide 8/34 vs. CGRP 15/34, p = 0.016). However, we found no difference in incidence of either photophobia or phonophobia in patients that developed migraine on both days (p > 0.05), Supplementary Table 4. No difference was found in nausea incidence between treatments (Figure 3D). The median time for initiation of attacks and median time to intake of rescue medication is shown in Fig.3A,B, Supplementary Table 4). All patients reported their induced migraine attacks mimicked their usual migraine attacks with pain predominantly localized in frontal and temporal regions of the cranium (Fig.3C). Our data suggest that migraine patients could be hypersensitive to amylin as well as CGRP.
Figure 3. Headache intensity, adverse events and plasma measurements.
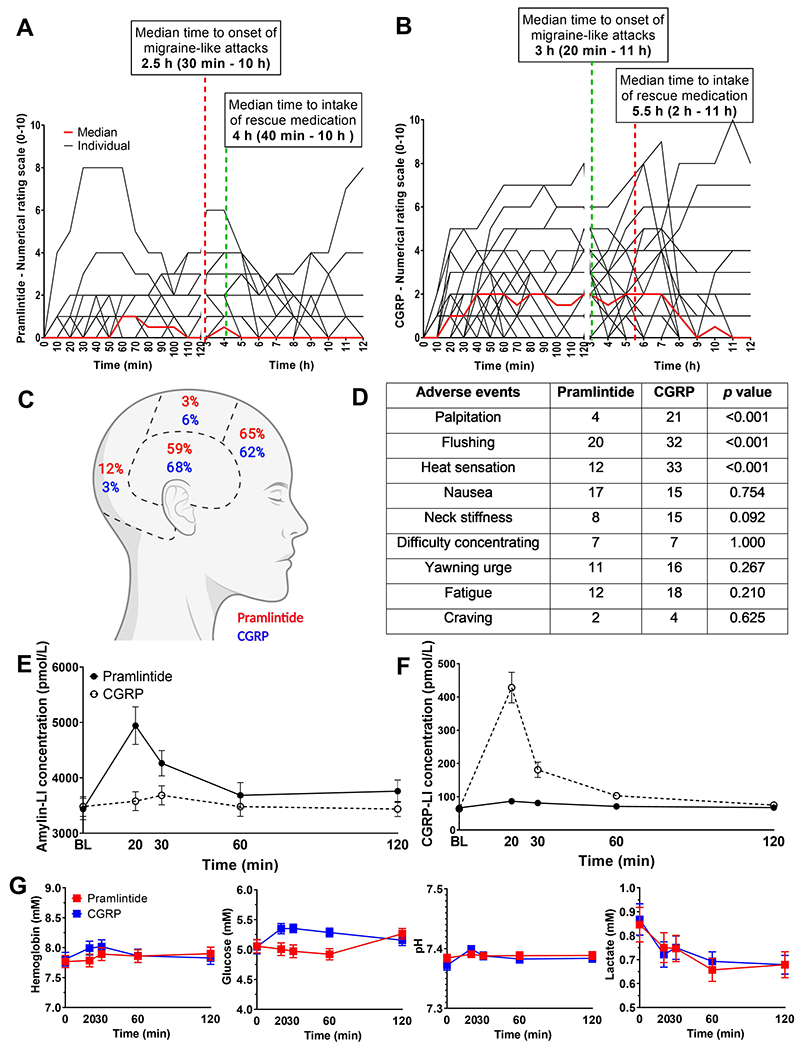
A) and B) Headache intensity in response to pramlintide or CGRP, respectively. Individual (black lines) and median (red line). C) Headache localization after pramlintide and CGRP. D) Incidence of adverse events (0-12 h) after CGRP or pramlintide, with statistical comparison between treatments E) and F) amylin-LI and CGRP-LI, respectively, in plasma samples from patients infused with pramlintide or CGRP, respectively. G) The concentration of hemoglobin, glucose, lactate and measurement of pH. Data are presented as mean ± SEM, except the headache intensity scores which are presented with individual data sets and median score. No statistical differences were found in incidence of headache and migraine-like attacks after pramlintide infusion compared to CGRP p = 0.375, p = 0.180.
Biochemical changes in plasma samples
CGRP-like and pramlintide-LI (like immunoreactivity) measurement in plasma samples confirmed that both peptides were successfully infused (Fig.3E,F). These measurements also allow a direct comparison between time of headache onset and plasma levels over time for both peptides for the first time. Pramlintide infusion did not increase plasma CGRP levels (Fig.3F) and CGRP infusion did not increase plasma amylin levels (Fig.3E). The peak pramlintide and CGRP plasma concentrations (20 minutes) are indicated on Fig.2 to illustrate the possible relationship between receptor pharmacology and plasma concentration. The peak concentration of CGRP appears sufficient to activate both the CGRP and AMY1 receptors (Fig.2C), whereas, the concentration of pramlintide may activate the AMY1 receptor (Fig.2B) or other CTR-based receptors (Fig.2E,G,H), but is unlikely to activate the CGRP receptor (Fig.2A). However, it is important to note that measurement of plasma peptides has many confounding factors which can affect absolute concentrations and thus these values are therefore indicative only. 31 We speculate that the ability of CGRP to act at the AMY1 receptor as well as the canonical CGRP receptor could underlie its slightly higher migraine induction rate, compared to pramlintide.
We found a significant difference between infusion of pramlintide and CGRP on glucose (p = 0.0005) but no differences between hemoglobin, pH or lactate concentrations (Fig.3G).
Pramlintide and CGRP differentially affect arterial dilation and vital parameters
Although both peptides were able to provoke migraine, an important difference in clinical observations between patients was the relative vascular activity of both peptides. We found significant differences in HR, MAP, facial skin blood flow and dilation of both the STA and RA after CGRP, compared to pramlintide (Fig.4A-E). The incidence of palpitation and heat sensation was significantly higher after CGRP compared to pramlintide but there were no differences in incidence of other adverse events (Fig.3D).
Figure 4. Effects of pramlintide and CGRP on arterial dilation and vital parameters.

A) Mean arterial pressure (MAP) and B) heart rate (HR) after infusion of pramlintide or CGRP. C) Direct measurement of facial flushing and quantification of facial flushing after infusion of pramlintide or CGRP. Diameter of D) a. temporalis and E) radialis after infusion of pramlintide or CGRP. All graphs show the percentage change. Data are presented as mean ± SEM. *p <0.05, **p <0.01, ***p <0.001, ****p <0.0001 vs. baseline.
Amylin causes cutaneous hypersensitivity and light aversive behavior in mice
We next tested whether amylin could induce migraine-like symptoms in mice. The approach was based on previous studies showing that i.p. CGRP causes cutaneous hypersensitivity and light aversion in mice analogous to touch and light sensitivity (photophobia) in migraine patients 23,32 While allodynia, which includes touch sensitivity, is not a diagnostic criteria of migraine, it is reported by 63-79% of patients and often extends beyond the head 33–35. Thus, paw sensitivity is a commonly used assay in preclinical migraine models 36. Amylin reduced the threshold at which mice withdrew their paws from a von Frey filament (Fig.5A-C), which was more pronounced in female than male mice (Fig. 5.B,C). We then examined light aversive behavior following amylin injection. While the combination of both sexes did not show a significant change (Fig.5D), the female mice showed a significant reduction in time spent in the light compared to vehicle treatment (Fig.5E). In contrast, amylin did not have a significant effect in male mice, although we cannot rule out the possibility of a small effect masked by the slight decrease observed with vehicle (Fig.5F). For comparison and as a control, CGRP caused light aversion in both sexes (Fig.5D-F). Unlike the amylin-treated mice, CGRP was effective in both males and females. While the CGRP response showed a trend to be greater than amylin, it was not significantly different. However, a direct comparison cannot be made since a five-fold greater dose of amylin than CGRP was used, and amylin and CGRP responses were measured at different times post-injection (amylin at 0-30 min and CGRP at 30-60 min). Pilot studies indicated that amylin did not show a consistent response at the later times. Overall, these preclinical results provide independent validation of the human studies and a foundation for further investigation of the underlying mechanisms of action of amylin in migraine.
Figure 5. Plantar von Frey and time in light in mice in response to peptide treatment.
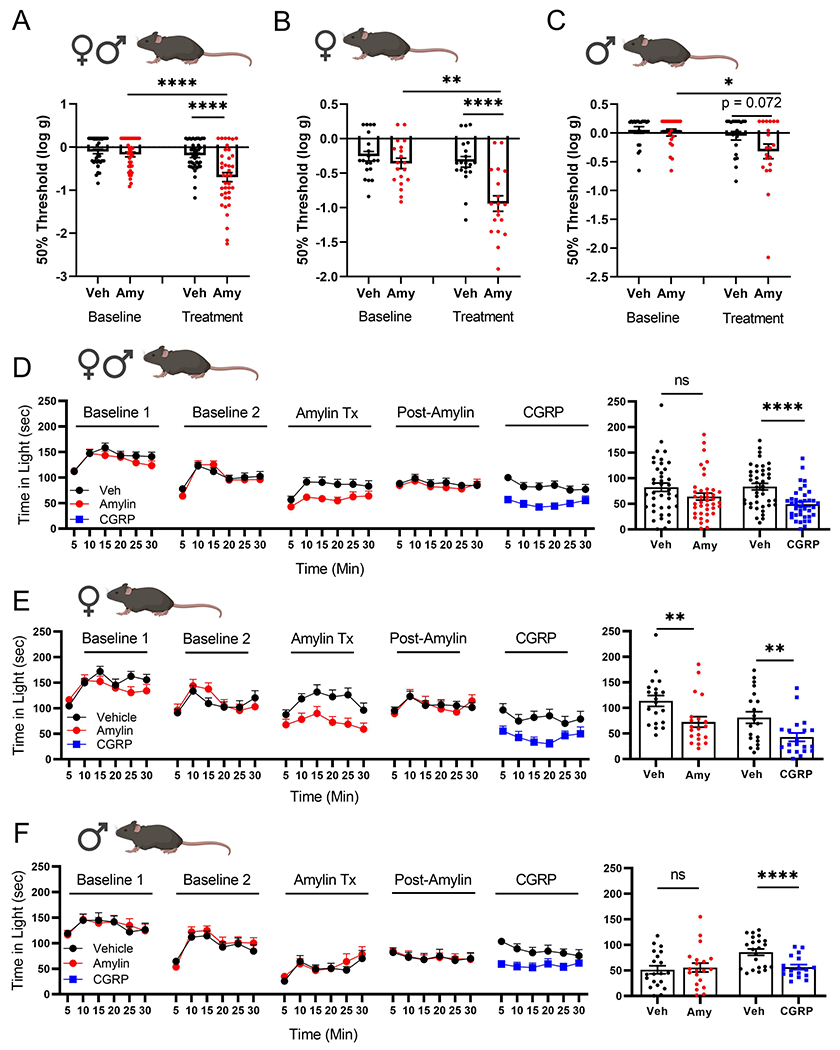
A)-C) Averaged 50% threshold for plantar von Frey. Mice were injected with either vehicle or 0.5 mg/kg amylin and tested within 5-15 min after injection. A) Both male and female mice. B) Female mice. C) Male mice. Male and female cohorts were tested both separately and together in two independent experiments. Females show a greater response to amylin than males (p=0.0306). D)-F) Time in light expressed as a function of time over the 30 min assay with the average for each cohort per 5 min interval (left panels) and as the average time per 5 min for each individual mouse (right panels). Following baseline measurements, mice were injected with vehicle or 0.5 mg/kg amylin, re-tested after 2 days, then treated 2 days later with 0.1 mg/kg CGRP. D) Both male and female mice. E) Female mice. F) Male mice. The CGRP response trends (p=0.0704) toward a greater response than amylin and females show a greater response to amylin than males (p=0.0127). Male and female cohorts were tested separately. ns = not significant, *p <0.05, **p <0.01, ***p <0.001, ****p <0.0001. All data are mean ± SEM. For statistical analyses and n values, see Supplementary Table 5.
Amylin expression in TG
To further translate our clinical findings to a deeper understanding of how endogenous amylin might contribute to migraine pathogenesis it was important to determine whether amylin itself is expressed in the TG and how this may relate to CGRP expression. Extra-pancreatic sources of amylin have occasionally been reported, including within the TG 37 but the validity of these findings is questionable because many antibodies used to detect amylin cross-react with CGRP due to similarities in their structures and sequences. 38 We directly compared amylin and CGRP-LI using two amylin antibodies from a panel of eight that we characterized (see 38 and Supplementary Table 3). Immunofluorescent staining was compared in mouse, rat and human TG with the antibodies separately (data not shown) and together (Fig.6). Whilst limited amylin-LI was evident, co-localization experiments indicated that the brightest cells with amylin-LI were also positive for CGRP-LI. The detection of “amylin”-LI was proportional to how cross-reactive the antibody was with CGRP, being greater for the more cross-reactive T4145 than for the less cross-reactive Ab254259. 38 This strongly implies that most of the amylin-LI detected is not genuinely amylin, but is actually detection of CGRP by the antibodies because CGRP is present at high concentrations in sensory neurons. 39 Small amounts of amylin could be present in the TG but this is unlikely to be a major source of the peptide. However, a limitation of our study is that human samples were only available from older adults and it is possible that amylin expression could change with age. The method of tissue processing could also affect the level of expression that is detectable. Nevertheless, if endogenous amylin does contribute to migraine pathophysiology, it is more likely to originate from the pancreas or other sites of expression, rather than TG neurons. Thus, the endogenous ligand(s) for CTR-based receptors within the TG are likely to be locally acting CGRP (via AMY1 receptors), and/or pancreatic amylin.
Figure 6. Peptide immunoreactivity in mouse, rat and human trigeminal ganglia.

Co-localization of CGRP-like (Ab36001; 1:500) and amylin-like (Ab254259; 1:200 and T4145; 1:500) immunoreactivity, together with the neuronal marker β-tubulin III, in fixed adult mouse, rat or human sagittal trigeminal ganglia sections. In all three species, robust CGRP-LI is found in neurons. Relatively weak amylin-LI was observed in mouse and rat, which was more intense for the T4145 antibody. In human trigeminal ganglia amylin-LI was only observed with the T4145 antibody. Amylin-like immunoreactivity with both antibodies co-localized with CGRP-like immunoreactivity in mouse and rat trigeminal ganglia, whereas in human trigeminal ganglia co-localization was observed only with the T4145 amylin antibody. In mouse and rat, for Ab254259, there was very little immunoreactivity above the no primary control, and thus very little co-localization. Merged images appear white and nuclei are stained blue with DAPI or Hoechst (as indicated). Solid arrowheads indicate examples of positively stained cells, empty arrowheads indicate examples of cells that were not stained and * indicates auto-fluorescence due to lipofuscin. Scale bar = 100 μM. Images are confocal at 20x magnification. Images are representative of experiments using six mice, three rats and three human cases. Images are edited for brightness and contrast for presentation purposes; this was uniformly applied to the no primary controls (not shown).
Discussion
Our main finding is that an amylin analogue, pramlintide, induced migraine-like attacks in migraine without aura patients. The characteristics of these attacks were similar for pramlintide and CGRP. These findings are supported by amylin provoking migraine-like symptoms of cutaneous sensitivity and light aversion in mice, which were similar to those caused by CGRP 23,32 As with the patients, amylin appears to be less potent than CGRP in mice, although the experiments were not designed for direct comparisons. Interestingly, unlike CGRP, amylin had significantly greater effects in female mice. Because nearly all the patients were female, we cannot yet say if pramlintide has a similar sex bias in humans.
Amylin and pramlintide are thought to mediate their beneficial metabolic effects primarily through the circumventricular organs which are brain regions that are freely exposed to circulating hormones. So how might pramlintide provoke migraine? The most likely mechanisms are through activation of amylin-responsive receptors in: the peripheral sensory neurons 40, the circumventricular organs, or within the brain, although the amounts of amylin that cross the blood brain barrier are very small. 41–43 A recent study reported that amylin levels were higher in chronic migraine patients compare to episodic migraine patients 44. At present, it is unknown if plasma amylin is increased during spontaneous migraine attacks. We speculate that a likely site of action is nociceptors innervating blood vessels and TG neurons that are exposed to substances from the blood.
Pramlintide, like amylin, is a very weak agonist of the canonical CGRP receptor (CLR/RAMP1), with even less activity at the AM2 receptor, and no measurable activity at the AM1 receptor (Fig.2). These data strongly suggest that pramlintide does not provoke migraine-like attacks through CLR-based receptors but does so via one or more of the CTR/RAMP complexes, where it has potent activity. Our clinical study showed that a subgroup of patients only responded to CGRP and a few only to pramlintide infusion. This is an important observation which needs further investigation. However, we suggest that differences between patients, such as in receptor expression or sensitivity could contribute to differences in migraine induction between these two peptides.
Amylin receptor components are found in many locations, including migraine relevant structures. 45 CTR either alone or together with RAMP1-LI is widely expressed in the brain, including within the spinal trigeminal complex. 37,40 Importantly, the human and rat TG contains CTR either alone, or CTR together with RAMP1-LI. 37,40 However, a significant challenge in confidently identifying the precise receptor responsible for pramlintides effect is the complexity of the receptor system, paired with a lack of certainty around the anatomical location(s) responsible for provoked migraine attacks. Each RAMP needs to be directly co-localized with CTR in migraine-relevant human cell types in situ. RAMP2 and RAMP3 antibodies are not sufficiently specific or reliable to warrant these investigations at present. Based on the available evidence for functional CTR/RAMP1 co-expression in TG neurons, the AMY1 receptor is therefore implicated as a likely target for both pramlintide and CGRP in inducing migraine. 40 This finding has important implications for understanding what contribution the AMY1 receptor may make to the mechanism of action of the different classes of CGRP blocking drugs.
Another consideration is the potential contribution of extracerebral arteries to migraine provocation. Several pharmacologically triggered migraine studies demonstrate that extracerebral arterial vasodilation is associated with onset of migraine in the head pain side. 3,46–48 However, in spontaneous migraine attacks, extracerebral vasodilation of arteries did not occur after onset of attack (median time 5 h 45 min (15 min to 21 h)), suggesting that dilatation alone is not sufficient to induce migraine attack in migraine patients. 49 The precise role of arterial hypersensitivity in migraine remains highly debatable. There have been some investigations of amylin vasodilatory activity in isolated vessel preparations and in vivo animal studies. 50 These generally show that amylin is very weak, approximately 100 times less potent compared to CGRP, and implicate the canonical CGRP receptor in these actions. 50,51 This includes a recent study using pramlintide in isolated human coronary and middle meningeal arteries.52 There are a small number of reports of potent amylin responses, though with a reduced maximum response compared to CGRP. 50 We are the first to study the vasodilatory effects of pramlintide in a human clinical study. This showed much less prominent facial flushing and a smaller magnitude of STA dilation with pramlintide infusion compared to CGRP infusion, consistent with the generally weaker prior reports of amylin/pramlintide on vessels. Although the core amylin receptor subunit, CTR has occasionally been found in vessels 40,53, CTR expression is hardly studied in vessels, with researchers defaulting to CLR. Therefore, wider expression of CTR cannot be ruled out and it is possible that amylin receptors have a role in vascular reactivity. In future studies it will be important to determine which CLR and CTR-based receptors may be expressed in different vessel types.
Pramlintide is an approved drug for insulin-requiring diabetes. Our findings suggest that it could cause or exacerbate migraine in patients treated with this drug. Other amylin receptor agonists are in development for diabetes and obesity. 54 Our findings therefore have implications for these drug development efforts.
The AMY1 receptor is a class B peptide GPCR and is therefore a highly tractable novel drug target. 55 Pursuing the development of further probes for this receptor and determining whether there is dysregulated expression or function in migraine is important. Furthermore, our study supports a possible role for amylin in migraine pathophysiology. This expands the repertoire of candidates for further investigation as biomarkers and therapeutic candidates. We propose that therapeutic gains could be made for migraine patients through dual amylin and CGRP receptor antagonism, rather than selectively targeting the canonical CGRP receptor.
Supplementary Material
Acknowledgements
We thank Drs. Thein Phu Do and Lanfranco Pellesi for excellent logistic support, lab technicians Lene Elkjær and Winnie Grønning for assistance with data collection and handling. The authors gratefully thank participating healthy volunteers and migraine patients. Confocal imaging data reported in this paper were obtained at the Biomedical Imaging Research Unit (BIRU), operated and funded by Technical Services (Faculty of Medical and Health Sciences, UoA). We also acknowledge the support services of the Centre for Genomics and Proteomics at the University of Auckland and the microscopy facility at the School of Biological Sciences, University of Auckland. Biorender.com was used for some figures. The study received financial support from the Lundbeck Foundation (R155-2014-171), the NIH (RF1 NS113839 and R01 NS075599), and the University of Auckland. DLH was supported by a James Cook Fellowship from the Royal Society of New Zealand and a Gavin and Ann Kellaway Medical Research Fellowship from the Auckland Medical Research Foundation.
Footnotes
Potential Conflict of interest
Nothing to report.
References
- 1.Ashina M. Migraine. N. Engl. J. Med 2020;383(19):1866–1876. [DOI] [PubMed] [Google Scholar]
- 2.IHS. The International Classification of Headache Disorders, 3rd edition (beta version), Headache Classification Committee of the International Headache Society. Cephalalgia 2018;33(9):629–808. [DOI] [PubMed] [Google Scholar]
- 3.Ashina M, Hansen JM, á Dunga BO, Olesen J. Human models of migraine — short-term pain for long-term gain. Nat. Rev. Neurol 2017;13(12):713–724. [DOI] [PubMed] [Google Scholar]
- 4.GBD 2015 Disease and Injury Incidence and Prevalence Collaborators T, Allen C, Arora M, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet (London, England) 2016;388(10053):1545–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christensen CE, Younis S, Deen M, et al. Migraine induction with calcitonin gene-related peptide in patients from erenumab trials. J. Headache Pain 2018;19(1):105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Vries T, MaassenVanDenBrink A. CGRP-targeted antibodies in difficult-to-treat migraine. Nat. Rev. Neurol 2019;15(12):688–689. [DOI] [PubMed] [Google Scholar]
- 7.Symlin ( pramlintide acetate ) injection. FDA Appl. No 021332 2005;2:1–21. [Google Scholar]
- 8.Westermark P, Wernstedt C, Wilander E, Sletten K. A novel peptide in the calcitonin gene related peptide family as an amyloid fibril protein in the endocrine pancreas. Biochem. Biophys. Res. Commun 1986;140(3):827–831. [DOI] [PubMed] [Google Scholar]
- 9.Hay DL, Chen S, Lutz TA, et al. Amylin: Pharmacology, Physiology and Clinical Potential. 2015;(July):564–600. [DOI] [PubMed] [Google Scholar]
- 10.Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet (London, England) 2012;380(9859):2163–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Potes CS, Pestana AC, Pontes M, et al. Amylin modulates the formalin-induced tonic pain behaviours in rats. Eur. J. Pain (United Kingdom) 2016;20(10):1741–1752. [DOI] [PubMed] [Google Scholar]
- 12.Khoshdel Z, Takhshid MA, Owji AA. Effects of intrathecal amylin on formalin-induced nociception and on cAMP accumulation in the rat embryonic spinal cells. Neuropeptides 2016;57:95–100. [DOI] [PubMed] [Google Scholar]
- 13.Heise T, Heinemann L, Heller S, et al. Effect of pramlintide on symptom, catecholamine, and glucagon responses to hypoglycemia in healthy subjects. Metabolism. 2004;53(9): 1227–1232. [DOI] [PubMed] [Google Scholar]
- 14.Gebre-Medhin S, Mulder H, Zhang Y, et al. Reduced nociceptive behavior in islet amyloid polypeptide (amylin) knockout mice. Mol. Brain Res 1998;63(1):180–183. [DOI] [PubMed] [Google Scholar]
- 15.Charles A, Pozo-Rosich P. Targeting calcitonin gene-related peptide: a new era in migraine therapy. Lancet 2019;394(10210):1765–1774. [DOI] [PubMed] [Google Scholar]
- 16.Bailey RJ, Hay DL. Pharmacology of the human CGRP1 receptor in Cos 7 cells. Peptides 2006;27(6):1367–1375. [DOI] [PubMed] [Google Scholar]
- 17.Hay DL, Garelja ML, Poyner DR, Walker CS. Update on the pharmacology of calcitonin/CGRP family of peptides: IUPHAR Review 25. Br. J. Pharmacol 2018;175(1):3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walker CS, Raddant AC, Woolley MJ, et al. CGRP receptor antagonist activity of olcegepant depends on the signalling pathway measured. Cephalalgia 2018;38(3):437–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Russell MB, Rasmussen BK, Brennum J, et al. Presentation of a New Instrument: The Diagnostic Headache Diary. Cephalalgia 1992;12(6):369–374. [DOI] [PubMed] [Google Scholar]
- 20.Iversen HK, Nielsen TH, Olesen J, Tfelt-Hansen P. Arterial responses during migraine headache. Lancet 1990;336(8719):837–839. [DOI] [PubMed] [Google Scholar]
- 21.Ghanizada H, Al-Karagholi MA-M, Amgrim N, et al. PACAP27 induces migraine-like attacks in migraine patients. Cephalalgia 2020;40(1):57–67. [DOI] [PubMed] [Google Scholar]
- 22.Schifter S Circulating concentrations of calcitonin gene-related peptide (CGRP) in normal man determined with a new, highly sensitive radioimmunoassay. Peptides 1991;12(2):365–369. [DOI] [PubMed] [Google Scholar]
- 23.Mason BN, Kaiser EA, Kuburas A, et al. Induction of Migraine-Like Photophobic Behavior in Mice by Both Peripheral and Central CGRP Mechanisms. J. Neurosci 2017;37(1):204–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaiser EA, Kuburas A, Recober A, Russo AF. Modulation of CGRP-Induced Light Aversion in Wild-Type Mice by a 5-HT1B/D Agonist. J. Neurosci 2012;32(44):15439–15449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dixon WJ. The Up-and-Down Method for Small Samples. J. Am. Stat. Assoc 1965;60(312):967–978. [Google Scholar]
- 26.Chaplan SR, Bach FW, Pogrel JW, et al. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods 1994;53(1):55–63. [DOI] [PubMed] [Google Scholar]
- 27.Lassen LH, Haderslev PA, Jacobsen VB, et al. CGRP may play a causative role in migraine. Cephalalgia 2002;22(1):54–61. [DOI] [PubMed] [Google Scholar]
- 28.Guo S, Vollesen ALH, Olesen J, Ashina M. Premonitory and nonheadache symptoms induced by CGRP and PACAP38 in patients with migraine. Pain 2016;157(12):2773–2781. [DOI] [PubMed] [Google Scholar]
- 29.Asghar MS, Hansen AE, Kapijimpanga T, et al. Dilation by CGRP of middle meningeal artery and reversal by sumatriptan in normal volunteers. Neurology 2010;75(17):1520–1526. [DOI] [PubMed] [Google Scholar]
- 30.Ghanizada H, Iljazi A, Ashina H, et al. Nocebo response in human models of migraine: A systematic review and meta-analysis of randomized, double-blind, placebo-controlled, two-way crossover trials in migraine without aura and healthy volunteers. Cephalalgia 2021;41(1):99–111. [DOI] [PubMed] [Google Scholar]
- 31.Food and Drug Administration. Bioanalytical Method Validation Guidance. Food Drug Adm. 2018;1043(May):25. [Google Scholar]
- 32.Wattiez A-S, Castonguay WC, Gaul OJ, et al. Different forms of traumatic brain injuries cause different tactile hypersensitivity profiles. Pain 2020;PublishAh(0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burstein R, Yarnitsky D, Goor-Aryeh I, et al. An association between migraine and cutaneous allodynia. Ann. Neurol 2000;47(5):614–624. [PubMed] [Google Scholar]
- 34.Bigal ME, Ashina S, Burstein R, et al. Prevalence and characteristics of allodynia in headache sufferers: A population study. Neurology 2008;70(17):1525–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lipton RB, Bigal ME, Ashina S, et al. Cutaneous allodynia in the migraine population. Ann. Neurol 2008;63(2):148–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vuralli D, Wattiez A-S, Russo AF, Bolay H. Behavioral and cognitive animal models in headache research. J. Headache Pain 2019;20(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Edvinsson L, Grell A, Warfvinge K. Expression of the CGRP Family of Neuropeptides and their Receptors in the Trigeminal Ganglion. J. Mol. Neurosci 2020;70(6):930–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rees TA, Hay DL, Walker CS. Amylin antibodies frequently display cross-reactivity with CGRP: characterization of eight amylin antibodies. Am. J. Physiol. Regul. Integr. Comp. Physiol 2021;ajpregu.00338.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Russo AF. Overview of Neuropeptides: Awakening the Senses? Headache J. Head Face Pain 2017;57:37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walker CS, Eftekhari S, Bower RL, et al. A second trigeminal CGRP receptor: function and expression of the AMY1 receptor. Ann. Clin. Transl. Neurol 2015;2(6):595–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zakariassen HL, John LM, Lykkesfeldt J, et al. Salmon calcitonin distributes into the arcuate nucleus to a subset of NPY neurons in mice. Neuropharmacology 2020;167:107987. [DOI] [PubMed] [Google Scholar]
- 42.Banks WA, Kastin AJ. Differential permeability of the blood-brain barrier to two pancreatic peptides: Insulin and amylin. Peptides 1998;19(5):883–889. [DOI] [PubMed] [Google Scholar]
- 43.Banks WA, Kastin AJ, Maness LM, et al. Permeability of the blood-brain barrier to amylin. Life Sci. 1995;57(22):1993–2001. [DOI] [PubMed] [Google Scholar]
- 44.Irimia P, Martínez-Valbuena I, Mínguez-Olaondo A, et al. Interictal amylin levels in chronic migraine patients: A case-control study. Cephalalgia 2020;33310242097710. [DOI] [PubMed] [Google Scholar]
- 45.Hendrikse ER, Bower RL, Hay DL, Walker CS. Molecular studies of CGRP and the CGRP family of peptides in the central nervous system. Cephalalgia 2019;39(3):403–419. [DOI] [PubMed] [Google Scholar]
- 46.Khan S, Mohammad Amin F, Emil Christensen C, et al. Meningeal contribution to migraine pain: a magnetic resonance angiography study. Brain 2019;142(1):93–102. [DOI] [PubMed] [Google Scholar]
- 47.Asghar MS, Hansen AE, Amin FM, et al. Evidence for a vascular factor in migraine. Ann. Neurol 2011;69(4):635–45. [DOI] [PubMed] [Google Scholar]
- 48.Al-Karagholi MA-M, Ghanizada H, Hansen JM, et al. Levcromakalim, an Adenosine Triphosphate-Sensitive Potassium Channel Opener, Dilates Extracerebral but not Arteries, Cerebral. Headache 2019;1468–1480. [DOI] [PubMed] [Google Scholar]
- 49.Amin FM, Asghar MS, Hougaard A, et al. Magnetic resonance angiography of intracranial and extracranial arteries in patients with spontaneous migraine without aura: A cross-sectional study. Lancet Neurol. 2013;12(5):454–461. [DOI] [PubMed] [Google Scholar]
- 50.Edvinsson L, Goadsby PJ, Uddman R. Amylin: Localization, Effects on Cerebral Arteries and on Local Cerebral Blood Flow in the Cat. Sci. World J 2001;1:168–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brain SD, Wimalawansa S, MacIntyre I, Williams TJ. The demonstration of vasodilator activity of pancreatic amylin amide in the rabbit. Am. J. Pathol 1990;136(3):487–490. [PMC free article] [PubMed] [Google Scholar]
- 52.Rivera-Mancilla E, Rubio-Beltrán E, de Vries T, Vincent A, van den Bogaerdt A, Danser AHJ, AM van den B Vasodilatory effects of CGRP receptor agonists in isolated human coronary and middle meningeal arteries. EHF Congress, Berlin, Germany, 2020, e-poster ID 176; 2020 [Google Scholar]
- 53.Wookey PJ, Zulli A, Buxton BF, Hare DL. Calcitonin receptor immunoreactivity associated with specific cell types in diseased radial and internal mammary arteries. Histopathology 2008;52(5):605–612. [DOI] [PubMed] [Google Scholar]
- 54.Srivastava G, Apovian C. Future Pharmacotherapy for Obesity: New Anti-obesity Drugs on the Horizon. Curr. Obes. Rep 2018;7(2):147–161. [DOI] [PubMed] [Google Scholar]
- 55.Davenport AP, Scully CCG, de Graaf C, et al. Advances in therapeutic peptides targeting G protein-coupled receptors. Nat. Rev. Drug Discov 2020; [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


