ABSTRACT
Potassium is an essential mineral nutrient required by all living cells for normal physiological function. Therefore, maintaining intracellular potassium homeostasis during bacterial infection is a requirement for the survival of both host and pathogen. However, pathogenic bacteria require potassium transport to fulfill nutritional and chemiosmotic requirements, and potassium has been shown to directly modulate virulence gene expression, antimicrobial resistance, and biofilm formation. Host cells also require potassium to maintain fundamental biological processes, such as renal function, muscle contraction, and neuronal transmission; however, potassium flux also contributes to critical immunological and antimicrobial processes, such as cytokine production and inflammasome activation. Here, we review the role and regulation of potassium transport and signaling during infection in both mammalian and bacterial cells and highlight the importance of potassium to the success and survival of each organism.
KEYWORDS: potassium, host-pathogen interactions
INTRODUCTION
Potassium is the most abundant intracellular cation in all living organisms, where it is required for numerous basic cellular functions, including regulating intracellular pH, governing the magnitude of the transmembrane electrical potential, and balancing turgor/osmotic pressure (1). However, a large asymmetric distribution between intracellular and extracellular potassium concentrations nearly always exists. Therefore, eukaryotic and prokaryotic cells alike must acquire potassium across steep membrane concentration gradients, utilizing both active and passive mechanisms, including dedicated channels and specialized transport systems. These mechanisms of potassium homeostasis have been well characterized, and they vary greatly in their regulation, energization, and utilization. Until recently, however, considerably less was known about the role and regulation of potassium acquisition during infection and its function at the host-pathogen interface.
In mammalian systems, potassium has fundamental roles in processes as diverse as kidney function, muscle contraction, and neuronal transmission. Potassium transport in nonexcitable immune and epithelial cells has also been shown to contribute to effective antimicrobial functions (2). For example, potassium flux in monocytes and macrophages during bacterial infection is required for the activation of the NLRP3 and NLRC4 inflammasomes (3, 4). Furthermore, potassium availability acts as a key signal in host-microbiome dysbiosis, directly affecting cytokine and antimicrobial peptide production (5).
While potassium availability in animal systems is tightly controlled through diet and renal release, bacterial pathogens must survive or grow under conditions containing a wide range of potassium concentrations, from low (≤4 mM)-potassium environments, such as water, soil, blood, and extracellular spaces, to those containing high (≥100 mM) potassium concentrations, such as within the host cytoplasm. Environmental potassium concentrations have also been shown to affect virulence gene expression, host cell invasion, antimicrobial resistance, biofilm formation, and host colonization in a variety of bacterial pathogens. Together, these findings have established potassium transport as a conserved mediator of bacterial infection outcome.
Here, we review significant reports that have identified and characterized the crucial role of potassium at the host-pathogen interface. We discuss the effects of potassium transport on antibacterial immune function and describe the contribution of potassium transport to pathogenesis in various bacterial species. Finally, we review recent findings on the role of potassium in bacterial intercellular and interspecies electrical communication. To date, this subject has not been reviewed and, therefore, will shed much-needed light on this important element of host-pathogen interaction.
POTASSIUM AND IMMUNE FUNCTION
In mammalian systems, potassium homeostasis involves the regulation of both intracellular potassium concentrations and extracellular potassium availability (6). Serum potassium levels are maintained at nearly constant levels in healthy individuals (3.5 to 5.0 mM), as potassium excretion from the kidney is balanced by the flux of intracellular potassium in the liver and muscles. Potassium retention from dietary intake replaces any deficits, and postprandial insulin stimulates cellular potassium uptake. As discussed further below, catecholamines also regulate potassium distribution, as β-adrenergic receptors promote and α-adrenergic receptors impair cellular potassium uptake (7). Changes in plasma osmolality and acid-base balance also influence potassium balance (8). Finally, nutritional increases in plasma potassium stimulate adrenal aldosterone secretion, which subsequently stimulates renal potassium excretion until plasma potassium levels return to homeostasis (9).
In most cells, potassium accumulation is largely mediated by the sodium-potassium ATPase, which extrudes intracellular sodium while importing extracellular potassium, leading to a potassium gradient across the membrane. However, multiple voltage-gated (e.g., KV1.3), “leak” (e.g., K2P), and calcium-linked (e.g., KCa3.1) potassium channels play important roles in immune cell function (2, 10, 11). In general, immune cells require mechanisms of potassium flux to maintain a hyperpolarized membrane potential critical for sustaining a calcium gradient. Immune cell activation (e.g., antigen recognition) induces calcium influx, which promotes nuclear factor (NF)-κB activity and inflammatory gene transcription (12). Furthermore, differentiation and activation of mononuclear cells modulates inward-rectifying potassium channel expression and activity (13). Dendritic cells have also been shown to upregulate and utilize KV channel activity for cytokine production, major histocompatibility complex (MHC) class II expression, chemotaxis, and phagocytosis in response to lipopolysaccharide (LPS) (14, 15). Potassium channel activity is required for antimicrobial nitric oxide (NO) production in macrophages (13, 16). Neutrophil phagosomes have also been shown to rapidly accumulate potassium during maturation, which is required for activation of neutrophil elastase and cathepsin G, leading to bacterial killing (17); however, this is not due to large-conductance calcium-activated potassium (BK) channel activity (18). Finally, KV1.3 and KCa3.1 localize to the immunological synapse between a T cell and antigen-presenting cell following T cell receptor (TCR) activation; however, the function of this localization is still unclear (19, 20).
HYPERKALEMIA AND HYPOKALEMIA
Substantial deviations from a normal serum potassium range, termed hyperkalemia and hypokalemia, are often associated with life-threatening health complications, including cardiovascular and neuromuscular dysfunction, which can lead to increased mortality, especially in critically ill patients (21, 22). Potassium intake is considered inadequate in many countries, which may increase the risk for hypertension and cardiovascular disease (23). Changes in potassium distribution can also be affected by disturbances in the acid-base balance due to transcellular exchange of hydrogen and potassium ions, resulting in metabolic acidosis or alkalosis (24). Further, the stimulation of β2-adrenergic receptors modulates the movement of potassium into the intracellular space, while nonselective β2 blockage can induce hyperkalemia (25). Recent studies have demonstrated that hyperkalemia is commonly associated with ventricular arrhythmia, bradycardia, and cardiac arrest (26) as well as a higher incidence of hepatic encephalopathy, bacterial infections, gastrointestinal bleeding, and acute kidney injury (27, 28). Of note, infection was found to be a leading cause of mortality in patients hospitalized with hyperkalemia (28). Transient hyperkalemia can result from either a significant dietary intake or infusion of potassium, while sustained hyperkalemia can result from a combination of decreased renal potassium excretion and excessive potassium intake (29). Hyperkalemia also induces metabolic acidosis by impairing ammonia excretion (30). As a result, potassium is released into the extracellular space to maintain electroneutrality and compensate for hydrogen uptake.
On the other hand, hypokalemia enhances ammonia excretion and affects proteins involved in ammonia metabolism in the kidney (30), thereby resulting in metabolic alkalosis, where extracellular potassium is exchanged for intracellular hydrogen. In animal models, low potassium, even when within the conventional range of serum potassium, may impair myocardial contractile and relaxation responses to epinephrine (31). Hypokalemia is also significantly associated with higher risk of cardiovascular disease, infection, and all-cause mortality by contributing to the hemodynamic hyporesponsiveness that occurs in some patients with septic shock (32, 33). Similarly, clinical reports have implicated hypokalemia with an enhanced generalized susceptibility to bacterial infection (34), as a substantial serum potassium deficiency may be detrimental to the innate immune system by impairing the cellular mechanisms necessary for inflammasome activation and activity (35).
CATECHOLAMINES
In addition to their roles in vascular resistance and neuromodulation, catecholamines act as neurotransmitters and hormones vital to the homeostatic regulation of potassium distribution by the sympathetic nervous system (36). Under normal physiologic conditions, catecholamines target alpha- and beta-adrenergic receptors, which utilize either cyclic AMP (cAMP) or phosphoinositol second messenger systems to impair or promote the cellular entry of potassium, respectively (37–39). Catecholamine signaling is also heavily involved in the regulation of inflammatory responses, including cell activation, proliferation, and cytokine (e.g., tumor necrosis factor [TNF], interleukin-10 [IL-10], and IL-6) production (40, 41). The stimulation of catecholamine signaling through α-adrenergic receptors promotes proinflammatory responses, whereas stimulation of catecholamine signaling through β-adrenergic receptors exerts significant immunoregulatory mechanisms involved in anti-inflammatory responses, specifically through its effect on macrophages (42). In general, myeloid cells typically produce α- and β-adrenergic receptors, whereas lymphocytes mainly express β-adrenergic receptors. The endogenous release of catecholamines, specifically dopamine and norephinephrine, has also been found to increase bacterial proliferation and pathogenesis, including motility, toxin production, iron acquisition, and host cell adherence (43, 44).
POTASSIUM AND INFLAMMASOME ACTIVATION
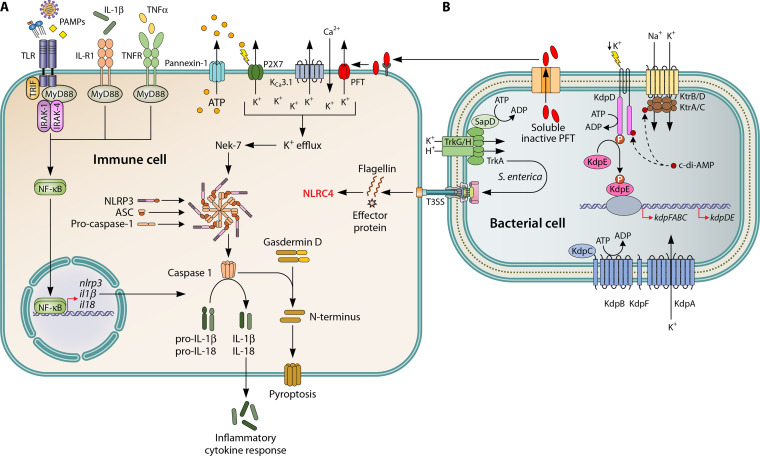
Potassium flux plays a significant role in NLRP3 (3, 45) and NLRC4 (4) inflammasome activation in response to various microbial products, including from a wide variety of Gram-positive and Gram-negative commensals and pathogens (46, 47) (Fig. 1). Canonical NLRP3 inflammasome activation is regulated by two distinct signals. First, toll-like receptor and NF-κB activation by various pathogen-associated molecular patterns (PAMPs) upregulates NLRP3 expression, and then damage-associated molecular patterns (DAMPs) initiate assembly of the NLRP3 inflammasome, including the adaptor protein ASC and procaspase-1 complex (48). Potassium efflux is a well-established DAMP trigger required in canonical NLRP3 activation (45, 49) as well as caspase-11-mediated (noncanonical) activation (50), and high extracellular potassium blocks NLRP3 inflammasome activation (3, 45). This occurs following extracellular ATP binding to the cation-selective P2X7 channel, which opens a pore that triggers potassium efflux down its electrochemical gradient (51). Interestingly, Mycobacterium tuberculosis infection susceptibility has been linked to genetic polymorphisms in the P2RX7 gene (52, 53). Numerous ionophores and bacterial pore-forming toxins, including Listeria monocytogenes listeriolysin O (54) and Staphylococcus aureus α-hemolysin (55, 56), directly facilitate potassium release and promote NLRP3 activation (57). Thus, the NLRP3 inflammasome can be activated in response to signaling pathways triggered by bacterial infections involving PAMPs and pore-forming toxins that deplete intracellular potassium.
FIG 1.
Role of potassium during bacterial infection. (A) Activation of the NLRP3 inflammasome is dependent on two distinct signals. The priming signal is provided by recognition of pathogen-associated molecular patterns (PAMPs) via pattern recognition receptors (e.g., toll-like receptors; TLRs) and inflammatory cytokines (e.g., TNF-α and IL-1β). These signals induce MyD88-dependent activation of NF-κB, which promotes the transcription of NLRP3, pro-IL-1β, and pro-IL-18. The activation signal is commonly provided by potassium (K+) efflux via P2X7, an ATP-gated cation channel, and the calcium (Ca2+)-linked potassium channel KCa3.1. Similarly, the insertion and oligomerization of bacterial pore-forming toxins (PFT; e.g., alpha-toxin and listeriolysin O) promotes the efflux of potassium and other small molecules (e.g., ATP) that trigger inflammatory responses. The activation signal promotes the assembly of NLRP3, ASC, and procaspase-1 to form an active NLRP3 inflammasome, leading to the maturation and release of caspase-1, which, in turn, cleaves pro-IL-1β and pro-IL-18 into their mature forms prior to release. Gasdermin D is also cleaved and inserts into the cellular membrane to form a transmembrane pore and induce pyroptosis. Additionally, bacterial flagellin and components of the Gram-negative type three secretion system (T3SS) induce host NLRC4 inflammasome activation (not fully depicted). (B) The Trk, Ktr, and Kdp systems are three of the most common, multicomponent potassium transport systems in bacteria. The Trk system is composed of a transmembrane component (e.g., TrkG/H), cytoplasmic regulatory proteins (e.g., TrkA) that function to cotransport potassium and protons (H+), and, in some cases, an ATPase (e.g., SapD). The Ktr system is also multimeric and consists of dimeric transmembrane components (e.g., KtrB/D) and respective octameric cytoplasmic regulatory components (e.g., KtrA/C). The Ktr system is thought to energize potassium uptake via sodium (Na+) cotransport. Activity of the Ktr system is often promoted by ATP and inhibited by c-di-AMP binding to the cytoplasmic regulatory component. The inducible Kdp system is transcriptionally regulated by the two-component KdpDE system, where the histidine kinase sensor, KdpD, senses environmental potassium concentration and phosphorylates the transcription factor, KdpE, to activate the expression of kdpFABC. This high-affinity transporter component is composed of a potassium channel (KdpA), ATPase (KdpB), a secondary membrane component thought to mediate affinity (KdpC), and a hydrophobic protein implicated in stabilizing the Kdp complex (KdpF).
Both NLRP3 and NLRC4 combine with active caspase-1 to cleave several proinflammatory IL-1 family cytokines to their mature form, including IL-1β and IL-18, and induce pyroptosis, a distinctive and highly inflammatory form of programmed cell death (58). The activation of caspase-1 and the subsequent secretion of mature IL-1β and IL-18 play fundamental roles in innate immunity and the inflammatory response. Unlike NLRP3, the NLRC4 inflammasome is activated in response to a restricted set of stimuli, the intracellular bacterial flagellin and type III secretion system (T3SS) machinery (59, 60) (Fig. 1). While not described in detail here, it has also been shown that NLRC4 inflammasome activation is dependent on potassium efflux, albeit to a lesser degree than NLRP3 inflammasome activation (4).
POTASSIUM AND BACTERIAL PATHOGENESIS
Not only do bacterial pathogens need to produce virulence factors and/or immune modulators to enable invasion, survival, and proliferation within their host but they must also carry out processes necessary for all living organisms, including nutrient acquisition and metabolism. Additionally, maintenance of chemiosmotic homeostasis during infection dictates overall cellular health by regulating membrane turgor and components of the proton motive force, including ΔpH and transmembrane electrical potential. Ion flux is central to establishing electrochemical energy, and, as mentioned above, the most abundant bacterial intracellular cation is potassium. Bacteria use a variety of potassium transport systems that differ in their structure, affinity, kinetics, transcriptional and posttranslational regulation, and energy coupling. These systems have been compared elsewhere (61, 62) and, thus, will not be discussed in great detail. Rather, this section will focus on general mechanisms of potassium transport used by various bacterial pathogens and how potassium sensing and uptake contributes to their pathogenesis.
The conserved structure and function of bacterial potassium channels have been appreciated for decades (63, 64), and high-resolution crystal structures of numerous channel proteins have been resolved, thereby permitting the detailed assessment of ion conductance mechanisms (65, 66). Essentially, a central selectivity pore permits the passage of potassium across the membrane while simultaneously excluding other ions. The gating of ion conductance through the pore is an essential component of potassium transport, as evidenced by the numerous transport systems that are comprised of a channel protein and cognate cytoplasmic regulatory protein(s). For example, the multimeric Trk and Ktr transport systems, which satisfy the general potassium requirements of various bacteria, consist of an ion-conducting transmembrane subunit and a separate regulatory subunit (67) (Fig. 1B). Furthermore, as opposed to stand-alone potassium channels, most bacterial transport systems are “active,” utilizing either ATP hydrolysis (e.g., Kdp), the proton motive force, or cotransport of another ion to energize potassium uptake (61). High‐affinity Kdp systems often rely on the KdpDE two-component system to sense low extracellular potassium and induce the expression of the KdpABC ATPase and channel proteins (68); however, some Bacillus species lack KdpDE and instead regulate the expression of kdpFABC using a riboswitch (69). The presence of and variation among potassium transport systems likely reflects evolutionary adaptations made to enhance survival in specific environmental niches. Interestingly, many intracellular pathogens lack major potassium uptake systems (64, 67).
The role of potassium in bacterial pathogenesis ranges from its function as a compatible solute under times of hyperosmotic shock, adaptation to pH stress, modulating resistance to antimicrobials, to mediating virulence factor expression. As described below, mechanisms of potassium transport have also been shown to play crucial roles during infection and colonization in a variety of animal models (Table 1). Bacterial potassium transporters may also serve as potential drug targets, as they have no close homologues in animals (67). This review highlights the evolving appreciation for the role of potassium beyond mediating chemiosmotic homeostasis and demonstrates how potassium is a well-conserved mediator of bacterial pathogenesis.
TABLE 1.
Bacterial potassium transporters and their roles in pathogenesis
| Bacterial pathogen | Characterized transport system(s) | Role in pathogenesis | Reference(s) |
|---|---|---|---|
| Staphylococcus aureus | Ktr, Kdp | Hyperosmotic tolerance, virulence factor production, antimicrobial resistance, murine abscess formation | 73–77, 79 |
| Listeria monocytogenes | Ktr, Kdp, Kim | NDa | 82, 83 |
| Streptococcus pneumoniae | Trk | Possibly linked to c-di-AMP levels | 87, 88 |
| Streptococcus mutans | Trk | Acidic stress, hyperosmotic tolerance, biofilm formation | 101, 102 |
| Mycobacterium tuberculosis | Trk, Kdp | Acidic stress, macrophage intracellular survival, murine intranasal infection | 105 – 109 |
| Salmonella enterica | Trk, Kdp, Kup | Acidic stress, virulence factor production, epithelial cell invasion, replication within macrophages, protamine resistance, murine intragastric and intraperitoneal infection | 115 – 122 |
| Acinetobacter baumannii | Trk, Kdp | Virulence factor production, murine pneumonia infection | 124 |
| Helicobacter pylori | Kch (channel) | Murine orogastric infection | 127 |
| Francisella tularensis | Trk, Kdp | Virulence factor production, hyperosmotic tolerance, survival in blood, murine intraperitoneal infection | 131 – 134 |
| Pseudomonas aeruginosa | Trk | Antimicrobial resistance, biofilm formation | 137 – 139 |
| Vibrio vulnificus | Trk | Protamine and polymyxin B resistance, growth in serum, murine subcutaneous and intraperitoneal infection | 141, 142 |
| Haemophilus influenzae | Trk | Antimicrobial resistance, murine nasopharynx and middle ear colonization | 145 |
ND, not determined.
GRAM-POSITIVE PATHOGENS
Staphylococcus aureus.
S. aureus is a leading cause of both health care- and community-associated infections, often manifesting as skin and soft-tissue infections, sepsis, endocarditis, and osteomyelitis. Also characterized as a halotolerant organism, S. aureus is well known for its ability to grow in salt concentrations ranging from trace amounts to greater than 2 mol/liter. In fact, mannitol salt agar, which contains 7.5% (1.3 M) NaCl, is recommended as a selective and differential medium for the clinical isolation of staphylococci based on their well-established high salt tolerance (70). S. aureus is also capable of withstanding considerable changes in environmental osmolarity, salt concentration, pH, and mechanical stress, which mediates colonization and survival on human skin (71). In addition, high osmotic tolerance is thought to enable colonization of host epithelial and mucosal surfaces and contamination of cured foods (72). Elevated salinity also induces broad alteration of S. aureus gene expression, including genes involved in capsule polysaccharide synthesis, virulence, central metabolism, and amino acid utilization (73). Consistent with many other bacteria, the growth and survival of S. aureus in high-salinity environments requires mechanisms of potassium transport that have only come to light within the last decade (73–76). These studies identified and characterized two distinct potassium transporters: a multicomponent Ktr system and a Kdp transporter.
The role of KdpDE in responding to extracellular potassium and regulating the transcription of kdpFABC in S. aureus remains unclear, as shown by several conflicting reports (73, 76, 77). Initial findings suggested that the KdpE response regulator directly suppresses kdpFABC expression, even under potassium-limiting conditions (76, 77); however, subsequent findings from another group indicate that KdpE indeed activates kdpFABC expression (73), consistent with that of other bacteria. Moreover, the overall contribution of the S. aureus Kdp system to potassium uptake is contradictory, with two reports describing little to no role for KdpFABC in low-potassium growth (75, 76) and another identifying Kdp as the primary potassium transporter (73). The S. aureus KdpDE two-component system has also been shown to regulate the expression ofseveral virulence-associated genes, including protein A, capsular polysaccharide, alpha-toxin, aureolysin, lipase, and gamma-hemolysin (73, 76; reviewed in reference 78). Together, these studies have begun to illuminate the role of KdpDE in S. aureus pathogenesis, but several key aspects of the system, including the contribution of the KdpFABC transporter itself, require additional examination.
The S. aureus Ktr system is unique compared to other bacterial Ktr systems; it harbors two ion‐conducting membrane proteins, KtrB and KtrD, but only one cytoplasmic gating protein, KtrC (also termed KtrA) (73, 75). Interestingly, only one channel protein is necessary and sufficient for full S. aureus Ktr function (75). This type of Ktr system was the first to be described, and its existence appears to be conserved among most staphylococcal species (75). The Ktr system is required for normal growth in medium containing less than 10 mM potassium (73–75), while the role for the Kdp system, as described above, ranges from primary to functioning as a potassium scavenger in the absence of a functional Ktr system. The Ktr system has also been shown to play a significant role in S. aureus antimicrobial resistance; mutation of KtrC substantially increased susceptibility to aminoglycoside antibiotics, host cationic antimicrobial peptides, polymyxin B, and gramicidin (75). The Ktr system is also required for pathogenesis in a mouse sepsis model of kidney abscess infection (75), likely owing to the combination of its role in osmotolerance, antimicrobial resistance, and central metabolism (79).
Finally, the essential bacterial nucleotide second messenger cyclic di-AMP (c-di-AMP) binds and inhibits KtrC activity in S. aureus (74, 80). C-di-AMP also binds the sensor kinase KdpD and inhibits the upregulation of kdpFABC under salt stress (74, 81). Recent studies have shown that c-di-AMP also binds a putative S. aureus KimA transporter (82), similar to that in B. subtilis (83), which permits growth in low potassium when expressed in Listeria monocytogenes. As described for other bacterial species below, the conserved interaction of c-di-AMP with potassium transporters has identified novel mechanisms of regulating channel activity, and the implications are only just being revealed.
Listeria monocytogenes.
L. monocytogenes is an opportunistic foodborne pathogen commonly found in diverse environments such as soil, water, food products, and the gastrointestinal tract of animals (84). Ingestion of L. monocytogenes is often associated with self-limited acute febrile gastroenteritis in healthy individuals but can present as bacteremia, meningitis, and meningoencephalitis in immunocompromised, pregnant, and elderly individuals. As a facultative intracellular pathogen, transition of L. monocytogenes from the environment to the cytosol requires efficient adjustment to ionic conditions. Early studies showed that L. monocytogenes superoxide dismutase, catalase, and listeriolysin O activity are increased in high-potassium environments akin to intracellular conditions (85). L. monocytogenes encodes both Kdp and Ktr systems and a newly identified KimA potassium transporter (82, 83). Interestingly, the L. monocytogenes Kdp system was not able to support growth of E. coli strain LB650, which lacks all potassium uptake systems, suggesting that it is not functional (82). Similar to S. aureus, c-di-AMP binds and inhibits L. monocytogenes KtrC to prevent overaccumulation and osmotic swelling. Furthermore, c-di-AMP binds L. monocytogenes KdpD and KimA from both L. monocytogenes and S. aureus (82). While c-di-AMP contributes to L. monocytogenes virulence, to date, no studies have investigated the role of the Ktr, Kdp, or KimA potassium transporter in L. monocytogenes pathogenesis.
Streptococcus pneumoniae.
S. pneumoniae is an opportunistic pathogen that commonly colonizes the mucosal surfaces of the human upper respiratory tract. When aspirated or locally spread, S. pneumoniae can cause invasive infections, including pneumonia, septicemia, meningitis, and otitis media (86). S. pneumoniae encodes two potassium uptake systems, a TrkH-CabP (c-di-AMP binding protein) system and another putative Trk-like system; however, only TrkH-CabP appears to play the central role in potassium uptake (87, 88). Bai et al. examined the role of c-di-AMP in TrkH-CabP function and found that c-di-AMP inhibits potassium uptake by preventing interaction between TrkH and CabP (87). This mechanism of inhibition may also be present in the putative Trk and Ktr systems of group B Streptococcus (89), although functional analyses of these transporters have yet to be performed. Interestingly, deletion of S. pneumoniae CabP, but not TrkH, markedly reduced intracellular c-di-AMP levels (88), which influences pneumococcal growth and virulence (90, 91), indicating that CabP has additional roles besides potassium uptake. This may be attributed to CabP interacting with another Trk/Ktr channel protein (SPD_0429) that was not assessed in these studies (92). Finally, it was also found that pneumococcal diadenylate cyclase expression was affected by environmental potassium concentration (88), as shown in other organisms (83).
Streptococcus mutans.
S. mutans is an opportunistic human oral pathogen notable for its association with dental caries and the formation of dental plaque. Following adherence to the tooth surface, S. mutans proliferates and forms biofilms, which metabolize dietary sugars to produce acidic end products leading to tooth demineralization and decay (93, 94). Numerous studies have demonstrated that S. mutans requires potassium to survive and metabolize under acidic (pH ≤5) conditions (95–97). Furthermore, several putative Trk genes were found to be significantly upregulated when S. mutans was placed under acid stress, further suggesting their involvement in acid tolerance (98). Glutamate transport, which plays a major role in bacterial acid tolerance, also utilizes potassium ions for function in S. mutans (99, 100).
S. mutans requires relatively high concentrations of available potassium (25 mM) for optimal growth, and growth is limited in ≤5 mM potassium (101). S. mutans encodes four putative potassium transporters, annotated Trk1 (trkB, trk, and pacL), Trk2 (trkA and trkH), Kch, and the glutamate transporter GlnQHMP. Of the four transport systems, Trk2 was found to be critical for growth under low potassium conditions, membrane potential, survival during acidic and osmotic stress, and biofilm formation, while Trk1 played a minor role, particularly in the absence of Trk2 (101). The defect in biofilm formation of the trk2 mutant was attributed to decreased glucan production as a result of impaired glucosyltransferase activity. Because potassium acquisition is necessary for bacterial growth under adverse environmental conditions, efficient potassium transport systems, including Trk2, would be crucial for bacterial cell survival and persistence of S. mutans and phylogenetically related bacteria. Finally, a report published at the same time as Binepal et al. found two S. mutants Trk proteins that bind c-di-AMP, therein termed CabPA (trk) and CabPB (trkA), and that they also play a role in biofilm formation (102).
Mycobacterium tuberculosis.
M. tuberculosis, the causative agent of tuberculosis, is the leading cause of bacterial infectious disease deaths worldwide. Inhaled M. tuberculosis disseminates to regional lung lymph nodes followed by hematogenous spread to other parts of the lung and throughout the body (103). Following phagocytosis by alveolar macrophages, M. tuberculosis is retained in the early endosome by preventing phagosome-lysosome fusion. However, survival within the phagosome, which has a pH of 6.2 to 6.4, is essential to M. tuberculosis pathogenesis (104). M. tuberculosis encodes both Trk and Kdp potassium transport systems (105, 106), and Trk is required for adaptation to acidic pH stress and survival within macrophages (107). M. tuberculosis lacking the Trk system was also attenuated in a mouse intranasal infection model (107). However, several reports indicate that the M. tuberculosis Trk system has a unique function related to potassium acquisition and cell physiology, including no detectable role in low-potassium growth, intracellular pH homeostasis, or regulation of membrane potential (105, 107, 108). It is possible that increased kdp gene expression compensates for the lack of a functional Trk system (108).
M. tuberculosis responds to low-potassium environments by differentially regulating a variety of genes, the most prominent of which are those of the Kdp system (106, 107). As with most Kdp systems, kdpFABC expression in low potassium is dependent on KdpDE. Furthermore, membrane lipoproteins LprF and LprJ have been shown to function as accessory proteins that directly interact with KdpD and affect its kinase activity to modulate KdpE-dependent kdpFABC expression (106). A kdpF::GFP reporter showed no induction during growth under acidic pH stress or high osmolarity but was enhanced in a transcriptional repressor mutant, rv0500A, when grown in potassium-free media, although the mechanisms responsible remain unclear (107). An earlier study of six M. tuberculosis two-component regulatory systems found that deletion of kdpDE increased virulence, as determined by a significantly shorter survival time in SCID infected mice (109). Interestingly, numerous studies have shown that susceptibility to M. tuberculosis infection is linked to mutations in the potassium efflux gene p2rx7, which contributes to the antibacterial response of P2RX7-mediated inflammasome activation in macrophages (reviewed in reference 110).
GRAM-NEGATIVE PATHOGENS
Salmonella enterica.
S. enterica is major cause of community-acquired bloodstream infections (typhoidal Salmonella) and gastrointestinal disease (nontyphoidal Salmonella), transmitted predominantly through contaminated food and water (111, 112). Consumed S. enterica cells must initially survive passage through the stomach, where gastric acid presents an initial barrier to colonization (113). Under these conditions, the well-described Salmonella inducible acid tolerance response permits survival under low pH conditions (114) mediated, in part, through potassium/proton transport and upregulation of the Kdp system (115). S. enterica encodes Trk, Kdp, and Kup transport systems (116), and the latter is considered essential under acidic conditions. Consistent with other bacteria, transcription and translation of kdpA in S. enterica is highly induced after high salt challenge and potassium limitation (117, 118). Upon entering the small intestine, S. enterica must traverse the mucus layer, attach, and invade small intestinal epithelial or microfold (M) cells. Here, the KdpA channel protein is required for S. enterica invasion into colonic epithelial cells and replication within macrophages (115). In a screen for genes involved in facilitating S. enterica serovar Typhimurium invasion into human epithelial cells, mechanisms of potassium transport were by far the most enriched molecular function (119), indicating that the sensing and transportation of potassium is used as a cue to promote Salmonella invasion.
Potassium transport also plays significant roles in S. enterica virulence factor production and pathogenesis. Early studies found that TrkA (SapG) is required for low-potassium growth, protamine resistance, and pathogenesis in mouse models of intragastric and intraperitoneal infection (120, 121). Subsequently, Lu and colleagues found that TrkA is necessary for production of SipA and SipC, effector proteins of the type III secretion system (TTSS) of Salmonella pathogenicity island 1 (SPI-1) (116, 122). These downstream affects may also influence potassium-dependent host inflammasome activation (Fig. 1). In addition, potassium transport is required for S. enteric motility, mammalian cell invasion, and pathogenesis in both mouse and chick models of infection (116, 122). These results demonstrate that potassium uptake plays important roles in pathogenesis beyond maintaining the physiology of Salmonella, including a direct effect on the function of the SP-1 TTSS.
Acinetobacter baumannii.
The spread of multidrug-resistant A. baumannii has raised clinical concern worldwide as a major cause of serious infections, including ventilator-associated pneumonia, urinary tract infections, and bacteremia commonly occurring in hospitalized and/or immunocompromised patients (123). To date, Trk and Kdp systems have been identified in A. baumannii, with the latter being characterized in potassium transport and pathogenesis (124). The A. baumannii KdpDE two-component system was shown to be induced >70-fold and play a significant role during growth under potassium-limited conditions (124). Furthermore, a ΔkdpE mutant was shown to be significantly attenuated in lung colonization during a mouse pneumonia model of infection. Histopathological analyses showed that unlike wild-type A. baumannii, the ΔkdpE-infected lungs demonstrated markedly less congestion, edema, and neutrophil infiltration in the alveoli and interstitial tissue (124). In addition to its role in potassium sensing, this phenotype was attributed to the differential production of 135 proteins in the ΔkdpE mutant, including those involved in the heme pathway, capsule and cell wall synthesis, amino acid biosynthesis, and gene regulation.
Helicobacter pylori.
H. pylori colonization of the human stomach, unless treated, can persist indefinitely and cause chronic gastric diseases, such as gastritis, gastric and duodenal ulcers, and cancer (125). Gastric acid secretion largely determines the ability of H. pylori to colonize, as it must pass through the gastric lumen to reach the neutral pH of the gastric mucosa, its specific niche for proliferation. Here, the potassium concentration is expected to be in the low micromolar range, as it is critical for secretion of hydrochloric acid (126). Despite passage through acidic environments, H. pylori lacks homologues of the Kdp, Trk, Kup, and Ktr systems but encodes a two-transmembrane potassium channel, HpKchA (127, 128). Growth of a ΔhpKchA mutant was decreased in potassium concentrations as high as 17.3 mM, and despite not being required for acid tolerance in vitro, the ΔhpKchA mutant was completely attenuated in a 4-week murine model of orogastric infection (127). The presence of a sole potassium channel in H. pylori might reflect an adaptation to the potassium‐rich, competitor‐free gastric environment (127).
Francisella tularensis.
The causative agent of tularemia, F. tularensis is a facultative intracellular pathogen known for its high infectivity and lethality and as a potential agent in biowarfare and bioterrorism (129). F. tularensis pathogenesis relies heavily on its ability to replicate within host macrophages; however, it must also survive extracellular transmission and dissemination (130). In a screen to identify novel extracellular nutritional requirements of F. tularensis, Alkhuder et al. identified TrkH as being required for growth under hyperosmotic conditions and media containing less than 18 mM potassium (131). While TrkH was not required for macrophage intercellular survival in vitro, growth and survival of a ΔtrkH mutant was attenuated ex vivo in murine blood (131). Mouse survival studies further demonstrated that F. tularensis virulence is severely attenuated in the ΔtrkH mutant following intraperitoneal injection and that the ΔtrkH mutant is unable to replicate within the spleen, liver, and blood. Thus, Trk-mediated potassium uptake appears to play a fundamental role in dissemination and the extracellular phase of F. tularensis pathogenesis.
F. tularensis also encodes a Kdp system, but some strains and subspecies harbor deleterious mutations in the kdpA, kdpB, kdpD, and/or kdpE genes (131, 132). The sensor kinase KdpD was identified in a screen for genes required for F. tularensis survival in the mouse spleen following intraperitoneal injection (133). KdpD is thought to be responsible primarily for phosphorylating PmrA, which regulates the expression of the Francisella pathogenicity island (FPI) virulence genes (134); however, it remains to be determined if F. tularensis KdpD functions to sense environmental potassium concentrations.
Pseudomonas aeruginosa.
P. aeruginosa is an opportunistic and ubiquitous bacterial pathogen present in diverse environmental settings due to its minimal nutritional requirements and tolerance of extreme conditional variances (135). Despite its global prevalence, most infections are associated with health care settings, where P. aeruginosa is the causative agent of pneumonia, urinary tract infections, surgical site infections, and bloodstream infections in immunosuppressed patients. While resistant to multiple classes of antibiotics, the survival and pathogenesis of P. aeruginosa also requires the successful formation of biofilm (136). Mutation of the P. aeruginosa TrkHA system significantly increases sensitivity to carbenicillin and numerous aminoglycoside antibiotics (137, 138). An earlier report also identified a putative novel potassium importer (PA1207) that was also shown to be important for P. aeruginosa biofilm formation and virulence in a barley germination assay (139). Further investigations of Trk and other P. aeruginosa potassium importers are required to establish their contribution to animal pathogenesis.
Vibrio vulnificus.
V. vulnificus is an opportunistic zoonotic pathogen that causes severe wound infections, gastroenteritis, and sepsis after contact with seawater or consumption of raw shellfish (140). Survival and replication under a range of saline conditions is required in the various ecological niches that V. vulnificus inhabits. Moreover, virulence factor expression and survival in serum are essential to infection, including the production of capsular polysaccharide, toxins, and iron acquisition, resulting in a septicemia mortality rate of over 50% (140). Furthermore, resistance to the bactericidal effects of serum plays a fundamental role in the systemic dissemination of pathogenic bacteria. Chen et al. screened a library of V. vulnificus transposon mutants for decreased survival in human serum and identified a trkA mutant that was required for resistance to complement-mediated killing (141). The requirement for TrkA in V. vulnificus serum survival was confirmed in a recent report (142). TrkA is also necessary for resistance to polymyxin B and protamine, and in mouse models of lethal intraperitoneal and subcutaneous infection, the 50% lethal doses (LD50) of the trkA mutant were 80- and 7-fold higher, respectively, than those of wild-type V. vulnificus (141). In a model of elevated iron, the LD50 of the trkA mutant was 150- and 300-fold higher than that of the wild type in the intraperitoneal and subcutaneous models, respectively (141). Together, these studies clearly implicate the Trk system as a major contributor to V. vulnificus pathogenesis.
Haemophilus influenzae.
H. influenzae is a commensal organism of the nasopharynx and is often implicated in systemic and respiratory tract infections, meningitis, and otitis media (143). The species is divided into encapsulated and nonencapsulated (nontypeable; NTHi) strains, depending on the presence or absence of a polysaccharide capsule, respectively. Following widespread use of the H. influenzae type B vaccine, the vast majority of respiratory disease now arises from NTHi strains (143). According to genomic analyses, NTHi encodes a TrkHA system, but Mason et al. showed that a SapD homologue, known to provide ATPase activity to Trk potassium transporters in other bacteria (144), is also required for potassium uptake in NTHi (145). They also demonstrated that the Sap operon was upregulated upon exposure to antimicrobial peptides and that SapD was necessary for resistance to killing by human beta defensin‐3 and cathelicidin LL-37 (146). Furthermore, a ΔsapD mutant was completely attenuated in both nasopharynx and middle ear survival using adult chinchilla models of colonization (145). It is speculated that in addition to its role in providing ATPase activity to the SapA complex, SapD provides ATPase activity to the Trk system to mediate high-rate potassium uptake. In this respect, SapD activity may counter the rapid loss of potassium that occurs after exposure to antimicrobial peptides.
Electrical signaling in bacterial biofilms.
Cell-to-cell signal transmission is essential to the survival of a wide variety of biological systems. Bacterial biofilms depend on cooperative behavior and division of labor but cells also compete for limited resources (147). While quorum sensing is the best-characterized intercellular signaling process in bacteria (148), electrical signaling via ion transport is a major form of communication in many other biological systems. In recent years, the study of bacterial potassium transporters has provided fundamental insights into novel mechanisms that mediate spatial and temporal signaling within biofilms (149–151). Essentially, synchronized oscillations in membrane potential correlate with the active uptake and release of potassium (but not sodium) within and among biofilm cells. Nutrient-starved cells (i.e., glutamate) in the biofilm interior release propagating waves of potassium to temporarily depolarize neighboring cells, resulting in a cell-cell relay wave to the biofilm periphery that allows nutrients to diffuse internally (151). Beyond dictating system behavior in response to variations in nutrient availability, further studies in biofilm electrical signaling have demonstrated that such electrical signals could extend beyond the biofilm periphery to influence distant and unrelated cells (149). In this study, electrical signaling mediated through the Bacillus subtills YugO potassium transporter activity attracted distant P. aeruginosa cells by modulating their membrane potential and motility, demonstrating a mechanism of long-range, cross-species bacterial communication through gradients of potassium (149). Interestingly, P. aeruginosa also directed their motility toward noncellular sources of potassium, indicating that potassium alone serves as a compass rose for pathogenic bacterial motility. Taken together, these results demonstrate the fundamental role of potassium and potassium ion transport in mediating electrical signaling both within and beyond a bacterial biofilm. Importantly, potassium-mediated electrical signaling does not require dedicated receptors or signaling pathways (required for quorum sensing), thereby allowing for cross-species communication using transporters and/or channels already produced for basic cellular survival. However, the role of potassium in biofilm electrical signaling has yet to be demonstrated in any bacterial pathogen and should be considered in the focus of future studies of bacterial pathogenesis.
CONCLUSION
Potassium availability at the host-pathogen interface is required for regulating several host immune functions and antimicrobial activities. Bacterial pathogens also require potassium for virulence factor expression and antimicrobial resistance. As a result, competition for potassium is likely to be a critical factor in determining infection outcomes. Given the importance of potassium in bacterial pathogenesis and immune activity, ongoing studies will continue to identify novel mechanisms of potassium utilization during infection. The study of c-di-AMP, for example, is of active interest, as its role in modulating bacterial potassium transport implicates it as a critical determinant in pathogenesis. C-di-AMP released from bacterial pathogens elicits a type I interferon immune response, mediated by the host receptor protein STING (152, 153). Additionally, continued investigation into the contribution of potassium to bacterial colony signal transduction will undoubtedly shed new light on its role during infection. Finally, further understanding how both host and pathogen utilize potassium to combat each other may identify novel therapeutic strategies to promote a productive immune response and thwart bacterial infection.
ACKNOWLEDGMENTS
We thank Zainab Rangwala for her assistance with early drafts of the manuscript.
This work was supported by a pilot grant to C.M.G. from the University of California Riverside School of Medicine.
Contributor Information
Casey M. Gries, Email: Casey.Gries@medsch.ucr.edu.
Anthony R. Richardson, University of Pittsburgh
REFERENCES
- 1.Williams RJ, Wacker EC. 1967. Cation balance in biological systems. JAMA 201:96–100. 10.1001/jama.201.1.96. [DOI] [PubMed] [Google Scholar]
- 2.Feske S, Wulff H, Skolnik EY. 2015. Ion channels in innate and adaptive immunity. Annu Rev Immunol 33:291–353. 10.1146/annurev-immunol-032414-112212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petrilli V, Papin S, Dostert C, Mayor A, Martinon F, Tschopp J. 2007. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ 14:1583–1589. 10.1038/sj.cdd.4402195. [DOI] [PubMed] [Google Scholar]
- 4.Arlehamn CS, Petrilli V, Gross O, Tschopp J, Evans TJ. 2010. The role of potassium in inflammasome activation by bacteria. J Biol Chem 285:10508–10518. 10.1074/jbc.M109.067298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yost S, Duran-Pinedo AE, Krishnan K, Frias-Lopez J. 2017. Potassium is a key signal in host-microbiome dysbiosis in periodontitis. PLoS Pathog 13:e1006457. 10.1371/journal.ppat.1006457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gumz ML, Rabinowitz L, Wingo CS. 2015. An integrated view of potassium homeostasis. N Engl J Med 373:60–72. 10.1056/NEJMra1313341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palmer BF. 2015. Regulation of potassium homeostasis. Clin J Am Soc Nephrol 10:1050–1060. 10.2215/CJN.08580813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aronson PS, Giebisch G. 2011. Effects of pH on potassium: new explanations for old observations. J Am Soc Nephrol 22:1981–1989. 10.1681/ASN.2011040414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Todkar A, Picard N, Loffing-Cueni D, Sorensen MV, Mihailova M, Nesterov V, Makhanova N, Korbmacher C, Wagner CA, Loffing J. 2015. Mechanisms of renal control of potassium homeostasis in complete aldosterone deficiency. J Am Soc Nephrol 26:425–438. 10.1681/ASN.2013111156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di L, Srivastava S, Zhdanova O, Ding Y, Li Z, Wulff H, Lafaille M, Skolnik EY. 2010. Inhibition of the K+ channel KCa3.1 ameliorates T cell-mediated colitis. Proc Natl Acad Sci U S A 107:1541–1546. 10.1073/pnas.0910133107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meuth SG, Bittner S, Meuth P, Simon OJ, Budde T, Wiendl H. 2008. TWIK-related acid-sensitive K+ channel 1 (TASK1) and TASK3 critically influence T lymphocyte effector functions. J Biol Chem 283:14559–14570. 10.1074/jbc.M800637200. [DOI] [PubMed] [Google Scholar]
- 12.Vig M, Kinet JP. 2009. Calcium signaling in immune cells. Nat Immunol 10:21–27. 10.1038/ni.f.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vicente R, Escalada A, Coma M, Fuster G, Sanchez-Tillo E, Lopez-Iglesias C, Soler C, Solsona C, Celada A, Felipe A. 2003. Differential voltage-dependent K+ channel responses during proliferation and activation in macrophages. J Biol Chem 278:46307–46320. 10.1074/jbc.M304388200. [DOI] [PubMed] [Google Scholar]
- 14.Matzner N, Zemtsova IM, Nguyen TX, Duszenko M, Shumilina E, Lang F. 2008. Ion channels modulating mouse dendritic cell functions. J Immunol 181:6803–6809. 10.4049/jimmunol.181.10.6803. [DOI] [PubMed] [Google Scholar]
- 15.Mullen KM, Rozycka M, Rus H, Hu L, Cudrici C, Zafranskaia E, Pennington MW, Johns DC, Judge SI, Calabresi PA. 2006. Potassium channels Kv1.3 and Kv1.5 are expressed on blood-derived dendritic cells in the central nervous system. Ann Neurol 60:118–127. 10.1002/ana.20884. [DOI] [PubMed] [Google Scholar]
- 16.Lowry MA, Goldberg JI, Belosevic M. 1998. Induction of nitric oxide (NO) synthesis in murine macrophages requires potassium channel activity. Clin Exp Immunol 111:597–603. 10.1046/j.1365-2249.1998.00536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reeves EP, Lu H, Jacobs HL, Messina CG, Bolsover S, Gabella G, Potma EO, Warley A, Roes J, Segal AW. 2002. Killing activity of neutrophils is mediated through activation of proteases by K+ flux. Nature 416:291–297. 10.1038/416291a. [DOI] [PubMed] [Google Scholar]
- 18.Essin K, Salanova B, Kettritz R, Sausbier M, Luft FC, Kraus D, Bohn E, Autenrieth IB, Peschel A, Ruth P, Gollasch M. 2007. Large-conductance calcium-activated potassium channel activity is absent in human and mouse neutrophils and is not required for innate immunity. Am J Physiol Cell Physiol 293:C45–C54. 10.1152/ajpcell.00450.2006. [DOI] [PubMed] [Google Scholar]
- 19.Nicolaou SA, Neumeier L, Steckly A, Kucher V, Takimoto K, Conforti L. 2009. Localization of Kv1.3 channels in the immunological synapse modulates the calcium response to antigen stimulation in T lymphocytes. J Immunol 183:6296–6302. 10.4049/jimmunol.0900613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cahalan MD, Chandy KG. 2009. The functional network of ion channels in T lymphocytes. Immunol Rev 231:59–87. 10.1111/j.1600-065X.2009.00816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tongyoo S, Viarasilpa T, Permpikul C. 2018. Serum potassium levels and outcomes in critically ill patients in the medical intensive care unit. J Int Med Res 46:1254–1262. 10.1177/0300060517744427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Viera AJ, Wouk N. 2015. Potassium disorders: hypokalemia and hyperkalemia. Am Fam Physician 92:487–495. [PubMed] [Google Scholar]
- 23.WHO. 2012. Guideline: potassium intake for adults and children. WHO, Geneva, Switzerland. [PubMed] [Google Scholar]
- 24.Bia MJ, DeFronzo RA. 1981. Extrarenal potassium homeostasis. Am J Physiol 240:F257–F268. 10.1152/ajprenal.1981.240.4.F257. [DOI] [PubMed] [Google Scholar]
- 25.Bia MJ, Lu D, Tyler K, De Fronzo RA. 1986. Beta adrenergic control of extrarenal potassium disposal. A beta-2 mediated phenomenon. Nephron 43:117–122. 10.1159/000183809. [DOI] [PubMed] [Google Scholar]
- 26.Truhlář A, Deakin CD, Soar J, Khalifa GEA, Alfonzo A, Bierens JJLM, Brattebø G, Brugger H, Dunning J, Hunyadi-Antičević S, Koster RW, Lockey DJ, Lott C, Paal P, Perkins GD, Sandroni C, Thies K-C, Zideman DA, Nolan JP, Barelli A, Böttiger BW, Georgiou M, Handley AJ, Lindner T, Midwinter MJ, Monsieurs KG, Wetsch WA. 2015. European Resuscitation Council guidelines for resuscitation 2015: section 4. Cardiac arrest in special circumstances. Resuscitation 95:148–201. 10.1016/j.resuscitation.2015.07.017. [DOI] [PubMed] [Google Scholar]
- 27.Cai JJ, Wang K, Jiang HQ, Han T. 2019. Characteristics, risk factors, and adverse outcomes of hyperkalemia in acute-on-chronic liver failure patients. Biomed Res Int 2019:1–9. 10.1155/2019/6025726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.An JN, Lee JP, Jeon HJ, Kim DH, Oh YK, Kim YS, Lim CS. 2012. Severe hyperkalemia requiring hospitalization: predictors of mortality. Crit Care 16:R225. 10.1186/cc11872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Udensi UK, Tchounwou PB. 2017. Potassium homeostasis, oxidative stress, and human disease. Int J Clin Exp Physiol 4:111–122. 10.4103/ijcep.ijcep_43_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harris AN, Grimm PR, Lee HW, Delpire E, Fang L, Verlander JW, Welling PA, Weiner ID. 2018. Mechanism of hyperkalemia-induced metabolic acidosis. J Am Soc Nephrol 29:1411–1425. 10.1681/ASN.2017111163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fitzovich DE, Hamaguchi M, Tull WB, Young DB. 1991. Chronic hypokalemia and the left ventricular responses to epinephrine and preload. J Am Coll Cardiol 18:1105–1111. 10.1016/0735-1097(91)90774-4. [DOI] [PubMed] [Google Scholar]
- 32.Ribeiro SC, Figueiredo AE, Barretti P, Pecoits-Filho R, de Moraes TP. 2015. Low Serum Potassium Levels Increase the Infectious-Caused Mortality in Peritoneal Dialysis Patients: a Propensity-Matched Score Study. PLoS One 10:e0127453. 10.1371/journal.pone.0127453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Torlen K, Kalantar-Zadeh K, Molnar MZ, Vashistha T, Mehrotra R. 2012. Serum potassium and cause-specific mortality in a large peritoneal dialysis cohort. Clin J Am Soc Nephrol 7:1272–1284. 10.2215/CJN.00960112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chuang YW, Shu KH, Yu TM, Cheng CH, Chen CH. 2009. Hypokalaemia: an independent risk factor of Enterobacteriaceae peritonitis in CAPD patients. Nephrol Dial Transplant 24:1603–1608. 10.1093/ndt/gfn709. [DOI] [PubMed] [Google Scholar]
- 35.Carone FA, Kashgarian M, Epstein FH. 1959. Effect of acute potassium deficiency on susceptibility to infection with particular reference to the kidney. Yale J Biol Med 32:100–108. [PMC free article] [PubMed] [Google Scholar]
- 36.Reid JL, Whyte KF, Struthers AD. 1986. Epinephrine-induced hypokalemia: the role of beta adrenoceptors. Am J Cardiol 57:23F–27F. 10.1016/0002-9149(86)90884-2. [DOI] [PubMed] [Google Scholar]
- 37.Williams ME, Gervino EV, Rosa RM, Landsberg L, Young JB, Silva P, Epstein FH. 1985. Catecholamine modulation of rapid potassium shifts during exercise. N Engl J Med 312:823–827. 10.1056/NEJM198503283121304. [DOI] [PubMed] [Google Scholar]
- 38.Moratinos J, Reverte M. 1993. Effects of catecholamines on plasma potassium: the role of alpha- and beta-adrenoceptors. Fundam Clin Pharmacol 7:143–153. 10.1111/j.1472-8206.1993.tb00228.x. [DOI] [PubMed] [Google Scholar]
- 39.Gosmanov AR, Wong JA, Thomason DB. 2002. Duality of G protein-coupled mechanisms for beta-adrenergic activation of NKCC activity in skeletal muscle. Am J Physiol Cell Physiol 283:C1025–32. 10.1152/ajpcell.00096.2002. [DOI] [PubMed] [Google Scholar]
- 40.Flierl MA, Rittirsch D, Huber-Lang M, Sarma JV, Ward PA. 2008. Catecholamines-crafty weapons in the inflammatory arsenal of immune/inflammatory cells or opening pandora's box? Mol Med 14:195–204. 10.2119/2007-00105.Flierl. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pongratz G, Straub RH. 2014. The sympathetic nervous response in inflammation. Arthritis Res Ther 16:504. 10.1186/s13075-014-0504-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barnes MA, Carson MJ, Nair MG. 2015. Non-traditional cytokines: how catecholamines and adipokines influence macrophages in immunity, metabolism and the central nervous system. Cytokine 72:210–219. 10.1016/j.cyto.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang Q, Anh ND, Bossier P, Defoirdt T. 2014. Norepinephrine and dopamine increase motility, biofilm formation, and virulence of Vibrio harveyi. Front Microbiol 5:584. 10.3389/fmicb.2014.00584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Freestone P. 2013. Communication between Bacteria and Their Hosts. Scientifica 2013:361073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Munoz-Planillo R, Kuffa P, Martinez-Colon G, Smith BL, Rajendiran TM, Nunez G. 2013. K(+) efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity 38:1142–1153. 10.1016/j.immuni.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hayward JA, Mathur A, Ngo C, Man SM. 2018. Cytosolic Recognition of Microbes and Pathogens: inflammasomes in Action. Microbiol Mol Biol Rev 82:e00015-18. 10.1128/MMBR.00015-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen K, Shanmugam NK, Pazos MA, Hurley BP, Cherayil BJ. 2016. Commensal bacteria-induced inflammasome activation in mouse and human macrophages is dependent on potassium efflux but does not require phagocytosis or bacterial viability. PLoS One 11:e0160937. 10.1371/journal.pone.0160937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jo EK, Kim JK, Shin DM, Sasakawa C. 2016. Molecular mechanisms regulating NLRP3 inflammasome activation. Cell Mol Immunol 13:148–159. 10.1038/cmi.2015.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Di A, Xiong S, Ye Z, Malireddi RKS, Kometani S, Zhong M, Mittal M, Hong Z, Kanneganti TD, Rehman J, Malik AB. 2018. The TWIK2 potassium efflux channel in macrophages mediates NLRP3 inflammasome-induced inflammation. Immunity 49:56–65. 10.1016/j.immuni.2018.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ruhl S, Broz P. 2015. Caspase-11 activates a canonical NLRP3 inflammasome by promoting K(+) efflux. Eur J Immunol 45:2927–2936. 10.1002/eji.201545772. [DOI] [PubMed] [Google Scholar]
- 51.Alves LA, de Melo Reis RA, de Souza CA, de Freitas MS, Teixeira PC, Neto Moreira Ferreira D, Xavier RF. 2014. The P2X7 receptor: shifting from a low- to a high-conductance channel - an enigmatic phenomenon? Biochim Biophys Acta 1838:2578–2587. 10.1016/j.bbamem.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 52.Azad AK, Sadee W, Schlesinger LS. 2012. Innate immune gene polymorphisms in tuberculosis. Infect Immun 80:3343–3359. 10.1128/IAI.00443-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fernando SL, Saunders BM, Sluyter R, Skarratt KK, Goldberg H, Marks GB, Wiley JS, Britton WJ. 2007. A polymorphism in the P2X7 gene increases susceptibility to extrapulmonary tuberculosis. Am J Respir Crit Care Med 175:360–366. 10.1164/rccm.200607-970OC. [DOI] [PubMed] [Google Scholar]
- 54.Hamon MA, Cossart P. 2011. K+ efflux is required for histone H3 dephosphorylation by Listeria monocytogenes listeriolysin O and other pore-forming toxins. Infect Immun 79:2839–2846. 10.1128/IAI.01243-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Walev I, Klein J, Husmann M, Valeva A, Strauch S, Wirtz H, Weichel O, Bhakdi S. 2000. Potassium regulates IL-1 beta processing via calcium-independent phospholipase A2. J Immunol 164:5120–5124. 10.4049/jimmunol.164.10.5120. [DOI] [PubMed] [Google Scholar]
- 56.Craven RR, Gao X, Allen IC, Gris D, Bubeck Wardenburg J, McElvania-Tekippe E, Ting JP, Duncan JA. 2009. Staphylococcus aureus alpha-hemolysin activates the NLRP3-inflammasome in human and mouse monocytic cells. PLoS One 4:e7446. 10.1371/journal.pone.0007446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Greaney AJ, Leppla SH, Moayeri M. 2015. Bacterial exotoxins and the inflammasome. Front Immunol 6:570. 10.3389/fimmu.2015.00570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bergsbaken T, Fink SL, Cookson BT. 2009. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol 7:99–109. 10.1038/nrmicro2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guo H, Callaway JB, Ting JP. 2015. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med 21:677–687. 10.1038/nm.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhao Y, Shao F. 2015. The NAIP-NLRC4 inflammasome in innate immune detection of bacterial flagellin and type III secretion apparatus. Immunol Rev 265:85–102. 10.1111/imr.12293. [DOI] [PubMed] [Google Scholar]
- 61.Epstein W. 2003. The roles and regulation of potassium in bacteria. Prog Nucleic Acids Res Mol Biol 75:293–320. 10.1016/s0079-6603(03)75008-9. [DOI] [PubMed] [Google Scholar]
- 62.Diskowski M, Mikusevic V, Stock C, Hanelt I. 2015. Functional diversity of the superfamily of K(+) transporters to meet various requirements. Biol Chem 396:1003–1014. 10.1515/hsz-2015-0123. [DOI] [PubMed] [Google Scholar]
- 63.Doyle DA, Morais Cabral J, Pfuetzner RA, Kuo A, Gulbis JM, Cohen SL, Chait BT, MacKinnon R. 1998. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science 280:69–77. 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- 64.Kuo MM, Haynes WJ, Loukin SH, Kung C, Saimi Y. 2005. Prokaryotic K(+) channels: from crystal structures to diversity. FEMS Microbiol Rev 29:961–985. 10.1016/j.femsre.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 65.Cao Y, Jin X, Huang H, Derebe MG, Levin EJ, Kabaleeswaran V, Pan Y, Punta M, Love J, Weng J, Quick M, Ye S, Kloss B, Bruni R, Martinez-Hackert E, Hendrickson WA, Rost B, Javitch JA, Rajashankar KR, Jiang Y, Zhou M. 2011. Crystal structure of a potassium ion transporter, TrkH. Nature 471:336–340. 10.1038/nature09731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vieira-Pires RS, Szollosi A, Morais-Cabral JH. 2013. The structure of the KtrAB potassium transporter. Nature 496:323–328. 10.1038/nature12055. [DOI] [PubMed] [Google Scholar]
- 67.Corratge-Faillie C, Jabnoune M, Zimmermann S, Very AA, Fizames C, Sentenac H. 2010. Potassium and sodium transport in non-animal cells: the Trk/Ktr/HKT transporter family. Cell Mol Life Sci 67:2511–2532. 10.1007/s00018-010-0317-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Walderhaug MO, Polarek JW, Voelkner P, Daniel JM, Hesse JE, Altendorf K, Epstein W. 1992. KdpD and KdpE, proteins that control expression of the kdpABC operon, are members of the two-component sensor-effector class of regulators. J Bacteriol 174:2152–2159. 10.1128/JB.174.7.2152-2159.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang X, Cai X, Ma H, Yin W, Zhu L, Li X, Lim HM, Chou SH, He J. 2019. A c-di-AMP riboswitch controlling kdpFABC operon transcription regulates the potassium transporter system in Bacillus thuringiensis. Commun Biol 2:151. 10.1038/s42003-019-0414-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chapman GH. 1945. The significance of sodium chloride in studies of staphylococci. J Bacteriol 50:201–203. 10.1128/JB.50.2.201-203.1945. [DOI] [PubMed] [Google Scholar]
- 71.Otto M. 2010. Staphylococcus colonization of the skin and antimicrobial peptides. Expert Rev Dermatol 5:183–195. 10.1586/edm.10.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sleator RD, Hill C. 2002. Bacterial osmoadaptation: the role of osmolytes in bacterial stress and virulence. FEMS Microbiol Rev 26:49–71. 10.1111/j.1574-6976.2002.tb00598.x. [DOI] [PubMed] [Google Scholar]
- 73.Price-Whelan A, Poon CK, Benson MA, Eidem TT, Roux CM, Boyd JM, Dunman PM, Torres VJ, Krulwich TA. 2013. Transcriptional profiling of Staphylococcus aureus during growth in 2 M NaCl leads to clarification of physiological roles for Kdp and Ktr K+ uptake systems. mBio 4:e00407-13. 10.1128/mBio.00407-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Corrigan RM, Campeotto I, Jeganathan T, Roelofs KG, Lee VT, Grundling A. 2013. Systematic identification of conserved bacterial c-di-AMP receptor proteins. Proc Natl Acad Sci U S A 110:9084–9089. 10.1073/pnas.1300595110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gries CM, Bose JL, Nuxoll AS, Fey PD, Bayles KW. 2013. The Ktr potassium transport system in Staphylococcus aureus and its role in cell physiology, antimicrobial resistance and pathogenesis. Mol Microbiol 89:760–773. 10.1111/mmi.12312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xue T, You Y, Hong D, Sun H, Sun B. 2011. The Staphylococcus aureus KdpDE two-component system couples extracellular K+ sensing and Agr signaling to infection programming. Infect Immun 79:2154–2167. 10.1128/IAI.01180-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhao L, Xue T, Shang F, Sun H, Sun B. 2010. Staphylococcus aureus AI-2 quorum sensing associates with the KdpDE two-component system to regulate capsular polysaccharide synthesis and virulence. Infect Immun 78:3506–3515. 10.1128/IAI.00131-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Freeman ZN, Dorus S, Waterfield NR. 2013. The KdpD/KdpE two-component system: integrating K(+) homeostasis and virulence. PLoS Pathog 9:e1003201. 10.1371/journal.ppat.1003201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gries CM, Sadykov MR, Bulock LL, Chaudhari SS, Thomas VC, Bose JL, Bayles KW. 2016. Potassium uptake modulates Staphylococcus aureus metabolism. mSphere 1:e00125-16. 10.1128/mSphere.00125-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kim H, Youn SJ, Kim SO, Ko J, Lee JO, Choi BS. 2015. Structural studies of potassium transport protein KtrA regulator of conductance of K+ (RCK) C domain in complex with cyclic diadenosine monophosphate (c-di-AMP). J Biol Chem 290:16393–16402. 10.1074/jbc.M115.641340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Moscoso JA, Schramke H, Zhang Y, Tosi T, Dehbi A, Jung K, Grundling A. 2016. Binding of cyclic di-AMP to the Staphylococcus aureus sensor kinase KdpD occurs via the universal stress protein domain and downregulates the expression of the Kdp potassium transporter. J Bacteriol 198:98–110. 10.1128/JB.00480-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gibhardt J, Hoffmann G, Turdiev A, Wang M, Lee VT, Commichau FM. 2019. c-di-AMP assists osmoadaptation by regulating the Listeria monocytogenes potassium transporters KimA and KtrCD. J Biol Chem 294:16020–16033. 10.1074/jbc.RA119.010046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gundlach J, Herzberg C, Kaever V, Gunka K, Hoffmann T, Weiß M, Gibhardt J, Thürmer A, Hertel D, Daniel R, Bremer E, Commichau FM, Stülke J. 2017. Control of potassium homeostasis is an essential function of the second messenger cyclic di-AMP in Bacillus subtilis. Sci Signal 10:eaal3011. 10.1126/scisignal.aal3011. [DOI] [PubMed] [Google Scholar]
- 84.Hamon M, Bierne H, Cossart P. 2006. Listeria monocytogenes: a multifaceted model. Nat Rev Microbiol 4:423–434. 10.1038/nrmicro1413. [DOI] [PubMed] [Google Scholar]
- 85.Myers ER, Dallmier AW, Martin SE. 1993. Sodium chloride, potassium chloride, and virulence in Listeria monocytogenes. Appl Environ Microbiol 59:2082–2086. 10.1128/AEM.59.7.2082-2086.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bogaert D, De Groot R, Hermans PW. 2004. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect Dis 4:144–154. 10.1016/S1473-3099(04)00938-7. [DOI] [PubMed] [Google Scholar]
- 87.Bai Y, Yang J, Zarrella TM, Zhang Y, Metzger DW, Bai G. 2014. Cyclic di-AMP impairs potassium uptake mediated by a cyclic di-AMP binding protein in Streptococcus pneumoniae. J Bacteriol 196:614–623. 10.1128/JB.01041-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zarrella TM, Metzger DW, Bai G. 2018. Stress suppressor screening leads to detection of regulation of cyclic di-AMP homeostasis by a Trk family effector protein in Streptococcus pneumoniae. J Bacteriol 200:e00045-18. 10.1128/JB.00045-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Devaux L, Sleiman D, Mazzuoli MV, Gominet M, Lanotte P, Trieu-Cuot P, Kaminski PA, Firon A. 2018. Cyclic di-AMP regulation of osmotic homeostasis is essential in group B Streptococcus. PLoS Genet 14:e1007342. 10.1371/journal.pgen.1007342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bai Y, Yang J, Eisele LE, Underwood AJ, Koestler BJ, Waters CM, Metzger DW, Bai G. 2013. Two DHH subfamily 1 proteins in Streptococcus pneumoniae possess cyclic di-AMP phosphodiesterase activity and affect bacterial growth and virulence. J Bacteriol 195:5123–5132. 10.1128/JB.00769-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cron LE, Stol K, Burghout P, van Selm S, Simonetti ER, Bootsma HJ, Hermans PW. 2011. Two DHH subfamily 1 proteins contribute to pneumococcal virulence and confer protection against pneumococcal disease. Infect Immun 79:3697–3710. 10.1128/IAI.01383-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Commichau FM, Stulke J. 2018. Coping with an essential poison: a genetic suppressor analysis corroborates a key function of c-di-AMP in controlling potassium ion homeostasis in gram-positive bacteria. J Bacteriol 200:e00166-18. 10.1128/JB.00166-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Matsui R, Cvitkovitch D. 2010. Acid tolerance mechanisms utilized by Streptococcus mutans. Future Microbiol 5:403–417. 10.2217/fmb.09.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Krzyściak W, Jurczak A, Kościelniak D, Bystrowska B, Skalniak A. 2014. The virulence of Streptococcus mutans and the ability to form biofilms. Eur J Clin Microbiol Infect Dis 33:499–515. 10.1007/s10096-013-1993-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dashper SG, Reynolds EC. 1992. pH regulation by Streptococcus mutans. J Dent Res 71:1159–1165. 10.1177/00220345920710050601. [DOI] [PubMed] [Google Scholar]
- 96.Iwami Y, Guha-Chowdhury N, Yamada T. 1997. Effect of sodium and potassium ions on intracellular pH and proton excretion in glycolyzing cells of Streptococcus mutans NCTC 10449 under strictly anaerobic conditions. Oral Microbiol Immunol 12:77–81. 10.1111/j.1399-302X.1997.tb00621.x. [DOI] [PubMed] [Google Scholar]
- 97.Marsh PD, Williamson MI, Keevil CW, McDermid AS, Ellwood DC. 1982. Influence of sodium and potassium ions on acid production by washed cells of Streptococcus mutans ingbritt and Streptococcus sanguis NCTC 7865 grown in a chemostat. Infect Immun 36:476–483. 10.1128/IAI.36.2.476-483.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gong Y, Tian XL, Sutherland T, Sisson G, Mai J, Ling J, Li YH. 2009. Global transcriptional analysis of acid-inducible genes in Streptococcus mutans: multiple two-component systems involved in acid adaptation. Microbiology 155:3322–3332. 10.1099/mic.0.031591-0. [DOI] [PubMed] [Google Scholar]
- 99.Krastel K, Senadheera DB, Mair R, Downey JS, Goodman SD, Cvitkovitch DG. 2010. Characterization of a glutamate transporter operon, glnQHMP, in Streptococcus mutans and its role in acid tolerance. J Bacteriol 192:984–993. 10.1128/JB.01169-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Noji S, Sato Y, Suzuki R, Taniguchi S. 1988. Effect of intracellular pH and potassium ions on a primary transport system for glutamate/aspartate in Streptococcus mutans. Eur J Biochem 175:491–495. 10.1111/j.1432-1033.1988.tb14221.x. [DOI] [PubMed] [Google Scholar]
- 101.Binepal G, Gill K, Crowley P, Cordova M, Brady LJ, Senadheera DB, Cvitkovitch DG. 2016. Trk2 potassium transport system in Streptococcus mutans and its role in potassium homeostasis, biofilm formation, and stress tolerance. J Bacteriol 198:1087–1100. 10.1128/JB.00813-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Peng X, Zhang Y, Bai G, Zhou X, Wu H. 2016. Cyclic di-AMP mediates biofilm formation. Mol Microbiol 99:945–959. 10.1111/mmi.13277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Smith I. 2003. Mycobacterium tuberculosis pathogenesis and molecular determinants of virulence. Clin Microbiol Rev 16:463–496. 10.1128/CMR.16.3.463-496.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sturgill-Koszycki S, Schlesinger PH, Chakraborty P, Haddix PL, Collins HL, Fok AK, Allen RD, Gluck SL, Heuser J, Russell DG. 1994. Lack of acidification in Mycobacterium phagosomes produced by exclusion of the vesicular proton-ATPase. Science 263:678–681. 10.1126/science.8303277. [DOI] [PubMed] [Google Scholar]
- 105.Cholo MC, Boshoff HI, Steel HC, Cockeran R, Matlola NM, Downing KJ, Mizrahi V, Anderson R. 2006. Effects of clofazimine on potassium uptake by a Trk-deletion mutant of Mycobacterium tuberculosis. J Antimicrob Chemother 57:79–84. 10.1093/jac/dki409. [DOI] [PubMed] [Google Scholar]
- 106.Steyn AJ, Joseph J, Bloom BR. 2003. Interaction of the sensor module of Mycobacterium tuberculosis H37Rv KdpD with members of the Lpr family. Mol Microbiol 47:1075–1089. 10.1046/j.1365-2958.2003.03356.x. [DOI] [PubMed] [Google Scholar]
- 107.MacGilvary NJ, Kevorkian YL, Tan S. 2019. Potassium response and homeostasis in Mycobacterium tuberculosis modulates environmental adaptation and is important for host colonization. PLoS Pathog 15:e1007591. 10.1371/journal.ppat.1007591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cholo MC, van Rensburg EJ, Osman AG, Anderson R. 2015. Expression of the genes encoding the Trk and Kdp potassium transport systems of Mycobacterium tuberculosis during growth in vitro. Biomed Res Int 2015:1–11. 10.1155/2015/608682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Parish T, Smith DA, Kendall S, Casali N, Bancroft GJ, Stoker NG. 2003. Deletion of two-component regulatory systems increases the virulence of Mycobacterium tuberculosis. Infect Immun 71:1134–1140. 10.1128/iai.71.3.1134-1140.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Savio LEB, de Andrade Mello P, da Silva CG, Coutinho-Silva R. 2018. The P2X7 receptor in inflammatory diseases: angel or demon? Front Pharmacol 9:52. 10.3389/fphar.2018.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Crump JA, Sjolund-Karlsson M, Gordon MA, Parry CM. 2015. Epidemiology, clinical presentation, laboratory diagnosis, antimicrobial resistance, and antimicrobial management of invasive salmonella infections. Clin Microbiol Rev 28:901–937. 10.1128/CMR.00002-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fabrega A, Vila J. 2013. Salmonella enterica serovar Typhimurium skills to succeed in the host: virulence and regulation. Clin Microbiol Rev 26:308–341. 10.1128/CMR.00066-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tennant SM, Hartland EL, Phumoonna T, Lyras D, Rood JI, Robins-Browne RM, van Driel IR. 2008. Influence of gastric acid on susceptibility to infection with ingested bacterial pathogens. Infect Immun 76:639–645. 10.1128/IAI.01138-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wilmes-Riesenberg MR, Bearson B, Foster JW, Curtis R, III.. 1996. Role of the acid tolerance response in virulence of Salmonella typhimurium. Infect Immun 64:1085–1092. 10.1128/IAI.64.4.1085-1092.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ryan D, Pati NB, Ojha UK, Padhi C, Ray S, Jaiswal S, Singh GP, Mannala GK, Schultze T, Chakraborty T, Suar M. 2015. Global transcriptome and mutagenic analyses of the acid tolerance response of Salmonella enterica serovar Typhimurium. Appl Environ Microbiol 81:8054–8065. 10.1128/AEM.02172-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Liu Y, Ho KK, Su J, Gong H, Chang AC, Lu S. 2013. Potassium transport of Salmonella is important for type III secretion and pathogenesis. Microbiology 159:1705–1719. 10.1099/mic.0.068700-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Balaji B, O'Connor K, Lucas JR, Anderson JM, Csonka LN. 2005. Timing of induction of osmotically controlled genes in Salmonella enterica serovar Typhimurium, determined with quantitative real-time reverse transcription-PCR. Appl Environ Microbiol 71:8273–8283. 10.1128/AEM.71.12.8273-8283.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Frymier JS, Reed TD, Fletcher SA, Csonka LN. 1997. Characterization of transcriptional regulation of the kdp operon of Salmonella typhimurium. J Bacteriol 179:3061–3063. 10.1128/jb.179.9.3061-3063.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Thornbrough JM, Gopinath A, Hundley T, Worley MJ. 2016. Human genome-wide RNAi screen for host factors that facilitate salmonella invasion reveals a role for potassium secretion in promoting internalization. PLoS One 11:e0166916. 10.1371/journal.pone.0166916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Parra-Lopez C, Lin R, Aspedon A, Groisman EA. 1994. A Salmonella protein that is required for resistance to antimicrobial peptides and transport of potassium. EMBO J 13:3964–3972. 10.1002/j.1460-2075.1994.tb06712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Groisman EA, Parra-Lopez C, Salcedo M, Lipps CJ, Heffron F. 1992. Resistance to host antimicrobial peptides is necessary for Salmonella virulence. Proc Natl Acad Sci U S A 89:11939–11943. 10.1073/pnas.89.24.11939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Su J, Gong H, Lai J, Main A, Lu S. 2009. The potassium transporter Trk and external potassium modulate Salmonella enterica protein secretion and virulence. Infect Immun 77:667–675. 10.1128/IAI.01027-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Peleg AY, Seifert H, Paterson DL. 2008. Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev 21:538–582. 10.1128/CMR.00058-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Samir R, Hussein SH, Elhosseiny NM, Khattab MS, Shawky AE, Attia AS. 2016. Adaptation to potassium-limitation is essential for Acinetobacter baumannii pneumonia pathogenesis. J Infect Dis 214:2006–2013. 10.1093/infdis/jiw476. [DOI] [PubMed] [Google Scholar]
- 125.Kusters JG, van Vliet AH, Kuipers EJ. 2006. Pathogenesis of Helicobacter pylori infection. Clin Microbiol Rev 19:449–490. 10.1128/CMR.00054-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Geibel JP. 2005. Role of potassium in acid secretion. World J Gastroenterol 11:5259–5265. 10.3748/wjg.v11.i34.5259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Stingl K, Brandt S, Uhlemann EM, Schmid R, Altendorf K, Zeilinger C, Ecobichon C, Labigne A, Bakker EP, de Reuse H. 2007. Channel-mediated potassium uptake in Helicobacter pylori is essential for gastric colonization. EMBO J 26:232–241. 10.1038/sj.emboj.7601471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.van Vliet AHM, Bereswill S, Kusters JG. 2001. Ion metabolism and transport. In Mobley HLT, Mendz GL, Hazell SL (ed), Helicobacter pylori: physiology and genetics. ASM Press, Washington, DC. [PubMed] [Google Scholar]
- 129.McLendon MK, Apicella MA, Allen LA. 2006. Francisella tularensis: taxonomy, genetics, and Immunopathogenesis of a potential agent of biowarfare. Annu Rev Microbiol 60:167–185. 10.1146/annurev.micro.60.080805.142126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Forestal CA, Malik M, Catlett SV, Savitt AG, Benach JL, Sellati TJ, Furie MB. 2007. Francisella tularensis has a significant extracellular phase in infected mice. J Infect Dis 196:134–137. 10.1086/518611. [DOI] [PubMed] [Google Scholar]
- 131.Alkhuder K, Meibom KL, Dubail I, Dupuis M, Charbit A. 2010. Identification of trkH, encoding a potassium uptake protein required for Francisella tularensis systemic dissemination in mice. PLoS One 5:e8966. 10.1371/journal.pone.0008966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.van Hoek ML, Hoang KV, Gunn JS. 2019. Two-component systems in Francisella species. Front Cell Infect Microbiol 9:198. 10.3389/fcimb.2019.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Weiss DS, Brotcke A, Henry T, Margolis JJ, Chan K, Monack DM. 2007. In vivo negative selection screen identifies genes required for Francisella virulence. Proc Natl Acad Sci U S A 104:6037–6042. 10.1073/pnas.0609675104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bell BL, Mohapatra NP, Gunn JS. 2010. Regulation of virulence gene transcripts by the Francisella novicida orphan response regulator PmrA: role of phosphorylation and evidence of MglA/SspA interaction. Infect Immun 78:2189–2198. 10.1128/IAI.00021-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lister PD, Wolter DJ, Hanson ND. 2009. Antibacterial-resistant Pseudomonas aeruginosa: clinical impact and complex regulation of chromosomally encoded resistance mechanisms. Clin Microbiol Rev 22:582–610. 10.1128/CMR.00040-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Prince AS. 2002. Biofilms, antimicrobial resistance, and airway infection. N Engl J Med 347:1110–1111. 10.1056/NEJMcibr021776. [DOI] [PubMed] [Google Scholar]
- 137.Lee S, Hinz A, Bauerle E, Angermeyer A, Juhaszova K, Kaneko Y, Singh PK, Manoil C. 2009. Targeting a bacterial stress response to enhance antibiotic action. Proc Natl Acad Sci U S A 106:14570–14575. 10.1073/pnas.0903619106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gallagher LA, Shendure J, Manoil C. 2011. Genome-scale identification of resistance functions in Pseudomonas aeruginosa using Tn-seq. mBio 2:e00315-10. 10.1128/mBio.00315-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ueda A, Wood TK. 2008. Potassium and sodium transporters of Pseudomonas aeruginosa regulate virulence to barley. Appl Microbiol Biotechnol 79:843–858. 10.1007/s00253-008-1483-5. [DOI] [PubMed] [Google Scholar]
- 140.Jones MK, Oliver JD. 2009. Vibrio vulnificus: disease and pathogenesis. Infect Immun 77:1723–1733. 10.1128/IAI.01046-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chen YC, Chuang YC, Chang CC, Jeang CL, Chang MC. 2004. A K+ yptake protein, TrkA, is required for serum, protamine, and polymyxin B resistance in Vibrio vulnificus. Infect Immun 72:629–636. 10.1128/iai.72.2.629-636.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Carda-Dieguez M, Silva-Hernandez FX, Hubbard TP, Chao MC, Waldor MK, Amaro C. 2018. Comprehensive identification of Vibrio vulnificus genes required for growth in human serum. Virulence 9:981–993. 10.1080/21505594.2018.1455464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.King P. 2012. Haemophilus influenzae and the lung (Haemophilus and the lung). Clin Transl Med 1:10. 10.1186/2001-1326-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Harms C, Domoto Y, Celik C, Rahe E, Stumpe S, Schmid R, Nakamura T, Bakker EP. 2001. Identification of the ABC protein SapD as the subunit that confers ATP dependence to the K+-uptake systems Trk(H) and Trk(G) from Escherichia coli K-12. Microbiology 147:2991–3003. 10.1099/00221287-147-11-2991. [DOI] [PubMed] [Google Scholar]
- 145.Mason KM, Bruggeman ME, Munson RS, Bakaletz LO. 2006. The non-typeable Haemophilus influenzae Sap transporter provides a mechanism of antimicrobial peptide resistance and SapD-dependent potassium acquisition. Mol Microbiol 62:1357–1372. 10.1111/j.1365-2958.2006.05460.x. [DOI] [PubMed] [Google Scholar]
- 146.Vogel AR, Szelestey BR, Raffel FK, Sharpe SW, Gearinger RL, Justice SS, Mason KM. 2012. SapF-mediated heme-iron utilization enhances persistence and coordinates biofilm architecture of Haemophilus. Front Cell Infect Microbiol 2:42. 10.3389/fcimb.2012.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Oliveira NM, Niehus R, Foster KR. 2014. Evolutionary limits to cooperation in microbial communities. Proc Natl Acad Sci U S A 111:17941–17946. 10.1073/pnas.1412673111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Miller MB, Bassler BL. 2001. Quorum sensing in bacteria. Annu Rev Microbiol 55:165–199. 10.1146/annurev.micro.55.1.165. [DOI] [PubMed] [Google Scholar]
- 149.Humphries J, Xiong L, Liu J, Prindle A, Yuan F, Arjes HA, Tsimring L, Suel GM. 2017. Species-independent attraction to biofilms through electrical signaling. Cell 168:200–209. 10.1016/j.cell.2016.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Larkin JW, Zhai X, Kikuchi K, Redford SE, Prindle A, Liu J, Greenfield S, Walczak AM, Garcia-Ojalvo J, Mugler A, Suel GM. 2018. Signal percolation within a bacterial community. Cell Syst 7:137–145. 10.1016/j.cels.2018.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Prindle A, Liu J, Asally M, Ly S, Garcia-Ojalvo J, Suel GM. 2015. Ion channels enable electrical communication in bacterial communities. Nature 527:59–63. 10.1038/nature15709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gries CM, Bruger EL, Moormeier DE, Scherr TD, Waters CM, Kielian T. 2016. Cyclic di-AMP released from Staphylococcus aureus biofilm induces a macrophage type I interferon response. Infect Immun 84:3564–3574. 10.1128/IAI.00447-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Woodward JJ, Iavarone AT, Portnoy DA. 2010. c-di-AMP secreted by intracellular Listeria monocytogenes activates a host type I interferon response. Science 328:1703–1705. 10.1126/science.1189801. [DOI] [PMC free article] [PubMed] [Google Scholar]