Abstract
Purpose:
A specialized Helmholtz-style 13C volume transmit “clamshell” coil is currently being utilized for 13C excitation in pre-clinical and clinical hyperpolarized 13C MRI studies aimed at probing metabolic activity of tumors in various target anatomy. Due to widespread use of this 13C clamshell coil design, it is important that the effects of the 13C clamshell coil B1+ profile on HP signal evolution and quantification are well understood. The goal of this study was to characterize the B1+ field of the 13C clamshell coil and assess the impact of inhomogeneities on semi-quantitative and quantitative hyperpolarized MR imaging biomarkers of metabolism.
Methods:
The B1+ field of the 13C clamshell coil was mapped by hand using a network analyzer equipped with a S-parameter test set. Pharmacokinetic models were used to simulate signal evolution as a function of position dependent local excitation angles, for various nominal excitation angles, which were assumed to be accurately calibrated at isocenter. These signals were then quantified according to the normalized lactate ratio (nLac) and the apparent rate constant for the conversion of pyruvate to lactate (kPL). The percent difference between these metabolic imaging biomarker maps and the reference value observed at isocenter of the clamshell coil was calculated to estimate the potential for error due to position within the clamshell coil. Finally, regions were identified within the clamshell coil where deviations in B1+ field inhomogeneity or imaging biomarker errors imparted by the B1+ field were within ±10% of the value at isocenter.
Results:
The B1+ field maps show that a limited volume encompassed by a region measuring approximately 12.9 × 11.5 × 13.4 cm (X-direction, Y-direction, Z-direction) centered in the 13C clamshell coil will produce deviations in the B1+ field within ±10% of that at isocenter. For the metabolic imaging biomarkers that we evaluated, the case when the pyruvate excitation angle (θP) and lactate excitation angle (θL) were equal to 10° produced the largest volumetric region with deviations within ±10% of the value at isocenter. Higher excitation angles yielded higher signal and SNR, but the size of the region in which uniform measurements could be collected near the isocenter of the coil was reduced at higher excitation angles. The tradeoff between the size of the homogenous region at isocenter and signal intensity must be weighed carefully depending on the particular imaging application.
Conclusion:
This work identifies regions and optimal excitation angles (θP and θL) within the 13C clamshell coil where deviations in B1+ field inhomogeneity or imaging biomarker errors imparted by the B1+ field were within ±10% of the respective value at isocenter, and thus where excitation angles are reproducible and well calibrated. Semi-quantitative and quantitative metabolic imaging biomarkers can vary with position in the clamshell coil as a result of B1+ field inhomogeneity, necessitating care in patient positioning and the selection of an excitation angle set that balances reproducibility and SNR performance over the target imaging volume.
Keywords: MRI, Hyperpolarized Pyruvate, Transmit Inhomogeneity, Molecular Biomarkers, Molecular Imaging, Cancer, Metabolism
Introduction
Dissolution dynamic nuclear polarization (dDNP) has attracted interest in recent years as a way to enhance the sensitivity of various molecular imaging agents beyond the capabilities of conventional magnetic resonance imaging (MRI).1,2 dDNP can be utilized to create hyperpolarized (HP) substrates which enable the investigation of metabolic pathways in vivo with sensitivity and spatiotemporal resolution that was previously impossible.3 HP 13C MRI can be used to quantify the conversion of HP pyruvate into lactate as an indicator for glycolytic exchange in vivo.4 HP [1‐13C]-pyruvate and its downstream metabolites have shown tremendous potential for characterization of tumor metabolism and detection of tumor response to therapy.5–9 Robust quantification of HP 13C data will improve the clinical utility of this metabolic imaging method. Therefore, a major goal for the field of HP MRI is the establishment of HP imaging methods that are quantitative, robust, and reproducible.
The standard 1H body coil within clinical MR systems is used to achieve homogeneous signal excitations for traditional MRI. However, because the 1H body coil does not support 13C signal transmit, specialized 13C transmit and receive coils must be integrated into the imaging setup in order to conduct 13C imaging studies. A specialized Helmholtz-style 13C volume transmit “clamshell” coil10,11 was originally designed to support the acquisition of clinical 13C data in the setting of prostate cancer7 and has supported acquisitions in other anatomical regions, such as the heart, brain, kidneys, and elsewhere.12–15 At present there are twelve sites internationally carrying out HP [1-13C]-pyruvate studies in human subjects and almost all of these sites utilize the 13C clamshell coil for signal excitation outside of brain.
Unlike conventional MRI, HP 13C MRI magnetization is non-recoverable and is limited by 13C signal decay and losses due to signal excitation. Excitations effect signal evolution and quantification by depleting spin labels on metabolic precursors and products in and around target anatomy. Accurate knowledge of excitation angles is critical for HP imaging because excitation losses must be accounted for in order to accurately determine apparent chemical conversion rates in vivo.16 A simple 13C reference standard is often used to calibrate the 13C center frequency and transmit power for imaging prior to injection of HP [1‐13C]-pyruvate.7,17–19 Unfortunately, calibration using a small reference phantom only provides information about excitation fields at the location of the phantom.
Methods to calibrate and map B1 for 13C coils have been proposed to improve the efficient use of HP signal and the accuracy and robustness of HP 13C imaging. Grist, et al. used the endogenous signal derived from 23Na nuclei as a reference for 13C imaging.20 Tang, et al. used regional bolus tracking to trigger Bloch-Siegert B1 mapping and real-time B1 calibration based on regional B1 measurements.21 These methods are useful in reducing B1 transmit errors and accounting for transmit errors due to B1 inhomogeneities that can’t be controlled by careful placement of anatomy within the 13C coil. Comprehensive B1+ maps of the entire volumetric region of the coil could also provide valuable information about the spatial dependencies of semi-quantitative and quantitative imaging biomarkers, along with critical guidance for placement of anatomical structures within the coil to minimize experimental variations. This information can be used to determine the location and extent of a region in the 13C clamshell coil where B1+ inhomogeneity is acceptably low. Accurate positioning of target anatomy in this homogeneous region will ensure accurate excitation and improve quantitative accuracy.4,16
In this research, we measured the B1+ field of a 13C volume transmit clamshell coil by hand and created comprehensive B1+ field maps that provide information about the inhomogeneity across the entire field-of-view (FOV). We then used these B1+ field maps to generate synthetic pyruvate and lactate curves using several excitation angle cases in order to evaluate how semi-quantitative and quantitative metabolic imaging biomarkers can vary with position in the clamshell coil. We focus on the normalized lactate ratio (nLac) and the apparent rate constant for the conversion of pyruvate into lactate (kPL). The resultant biomarker maps permitted identification of acceptable regions within the 13C clamshell coil where deviations in B1+ field inhomogeneity or imaging biomarker errors imparted by the B1+ field were within ±10% of their nominal values at isocenter.
Methods
Bench Measurement of the B1+Field Sensitivity
Local B1+ field sensitivity of the 13C clamshell coil (GE Healthcare, Waukesha, WI, USA) was measured by hand using a network analyzer (4395A, Agilent, Santa Clara, CA, USA) equipped with a S-parameter test set (87511A, Agilent, Santa Clara, CA, USA). S21 at the Larmor frequency for 13C at 3T (32.12 MHz) was measured as a function of position using a custom-built, small untuned 13 mm diameter loop “sniffer” probe. A purpose-built and electromagnetically transparent platform was constructed to ensure consistent and repeatable positioning and measurements (Figure 1). 238 measurements were collected in the XZ plane, over a 40 × 40 cm grid, and these measurements were repeated at eight heights in the Y-direction for a total of 1,904 measurements covering the entire FOV of the clamshell coil.
Figure 1.
The electromagnetically transparent B1+ field sensitivity bench measurement setup used to produce consistent and repeatable positioning and hand measurements. Position in the X- and Z-direction was measured consistently by utilizing the evenly spaced holes present in the pegboard. The sniffer probe was held in place using a plastic clamp and the clamshell coil was placed on a plastic cart to minimize perturbation of the RF field distribution.
The relative magnetic field intensity produced by the 13C clamshell coil at the position of the sniffer probe was calculated from S21 measurements and analyzed using MATLAB R2020b (The MathWorks, Natick, MA, USA). Bilinear interpolation of the data was performed throughout the volumetric region of the clamshell coil and all values were normalized to the value at isocenter. Axial, sagittal, and coronal B1+ field maps at coil isocenter were created, as well as a multi-plane 3D B1+ field map showing an intersecting axial, sagittal, and coronal quadrant at isocenter. Isosurface contour values from −70% to +50% were used to display the percent deviation in B1+ field inhomogeneity from the isocenter of the clamshell coil.
Metabolic Imaging Biomarkers
The conversion of HP pyruvate into lactate in vivo is often semi-quantitatively summarized using normalized lactate (nLac), which is the ratio of the area-under-the-curve (AUC) over time for HP lactate to the combined sum of the AUCs for HP pyruvate and lactate. Alternatively, HP lactate production may be quantitatively summarized using kPL, the apparent rate constant for the conversion of pyruvate to lactate obtained through pharmacokinetic (PK) modeling. The effect of B1+ inhomogeneity on nLac and kPL was assessed for six excitation angle cases, including four cases in which pyruvate and lactate were subjected to equal excitation angles (θP = θL = 10°, 20°, 30°, and 60°), and two cases in which lactate was excited at a higher level than pyruvate (θP = 10°, θL = 30° and θP = 20°, θL = 30°). In current practice, constant excitation angle cases are often used with typical values ranging from 10–30°, and spectral-spatial RF pulses are often used to impart differing excitation angles on pyruvate and lactate.18 We assessed these excitation angle cases, as well as a 60° constant excitation angle case in order to explore the effects of B1+ inhomogeneity on nLac and kPL at higher excitation angles. These results were then used to estimate how nLac and kPL could vary spatially within the volume of the 13C clamshell coil due only to variations in B1+.
Pharmacokinetic Modeling
In this work, we consider two PK models.4,19,22 Model A (Equation 1) contains two chemical pools (HP pyruvate and lactate) and one physical compartment. This precursor-product relationship between pyruvate and lactate is an approximation that assumes all observed substrate has the ability to interact with intracellular enzymes that enable exchange:
| (1) |
Here, ML,z(n) refers to the longitudinal lactate magnetization just prior to the nth excitation pulse. ML,z(0) refers to the initial lactate magnetization. T1,L, the longitudinal relaxation rate for HP [1-13C]-lactate, was assumed to be accurately known a priori in these simulations. The transverse magnetization is calculated from longitudinal magnetization according to the excitation angle.
Model B (Equations 2–4) contains two chemical pools and two physical compartments, where the intravascular (iv) space is separated from an extravascular space (ev) that is assumed to be well-mixed and homogenized by rapid diffusion and membrane transport. Chemical conversion of HP pyruvate to lactate occurs only in the extravascular space, and the reverse reaction is assumed to be negligible. Nuisance parameters that must be included but are not of particular interest in the analysis, including T1,P and T1,L, and parameters that account for microvascular function, were assumed to be accurately known throughout. The rate of pyruvate extravasation is given by kve, while vb denotes the vascular blood volume fraction. In this model, extravascular HP signal evolution follows:
| (2) |
Where
| (3) |
The observed transverse magnetization is calculated from the longitudinal magnetization according to excitation angles for pyruvate and lactate, and the volume-weighted combination of intravascular and extravascular compartments follows:
| (4) |
More physiologically accurate PK models for HP [1-13C]-pyruvate have been described4, which account for transport of HP agents across cellular membranes prior to interaction between HP pyruvate and the intracellular enzymes that mediate chemical conversion. However, these models are rarely used because of their complexity and the difficulty in resolving signals in the extravascular/extracellular space from those in the intracellular space.
Synthetic Data Generation
Synthetic dynamic pyruvate and lactate curves were generated for the six previously defined excitation angle cases using a two compartment model (Model B). Hand measured B1+ maps were scaled to achieve the correct excitation angle at isocenter. The default value for T1,P was assumed to be 43 s and T1,L was assumed to be 33 s.23 The vascular input function (VIF) shape was modeled as a gamma-variate function.24 The rate constant of pyruvate extravasation (kve) was set to 0.020 s−1 and the vascular blood volume fraction (vb) was 0.050. The input kPL was defined to be 0.050 s-1. We simulated data using TR = 2 s over a time interval of 128 s.
Analysis of Synthetic Data
nLac was calculated by taking the ratio of the time integrated AUC for HP lactate to the combined AUCs for HP pyruvate and lactate, using synthetic data generated by model B as described above.
The kPL for each set of synthetic signal curves was fit using both model A and model B. All fitting was completed with a non-linear least-squares algorithm in MATLAB using the PK models described above. Data generated by model B was not only fit using model B (denoted hereafter as kPLBB) but also fit using model A (denoted as kPLBA). For analysis using model A, we fit kPLBA and for analysis using model B, we fit kPLBB and a scaling factor for the VIF. All other parameters were assumed to be known and identical to the parameters previously stated in the synthetic data generation section above. Isosurface contour lines were used to display the spatially dependent percent deviation in nLac, as well as kPLBA and kPLBB reference values at isocenter of the coil. It is important to note that the excitation angle value at isocenter of the clamshell coil was assumed to be calibrated accurately.
Results
The hand measured B1+ field maps (Figure 2) show that a volumetric region (X-direction, Y-direction, Z-direction) measuring 12.9 × 11.5 × 13.4 cm centered at coil isocenter will contain deviations in the B1+ field within ±10% of the field at the center of the coil. The axial (Figure 2a) and sagittal (Figure 2b) views of the hand measured B1+ field maps show increased B1+ field intensity in the Y-direction near the coil elements, with a reduction in B1+ field intensity in the X- and Z-direction which produces a distinct hourglass shape in the transmit field profile. The axial, sagittal, and coronal multi-plane 3D view (Figure 2d) better illustrates a portion of the volume that will produce deviations in the B1+ field within ±10% of the value at isocenter.
Figure 2.
(a) Axial, (b) sagittal, (c) coronal, and (d) multi-plane 3D view of hand measured B1+ field maps for the 13C clamshell coil, referenced from coil isocenter. The red contour lines in all subfigures highlight the boundaries of the volumetric region that produces deviations in the B1+ field within ±10% of the value of at isocenter.
nLac values calculated at isocenter for the six excitation angle cases (Figure 3) reveal that the θP = 10°, θL = 30° case (Figure 3e) produced the largest value of nLac (0.64), followed by the θP = θL = 10° case (Figure 3a) which produced a nLac value at isocenter of 0.55. The θP = θL = 60° case (Figure 3d) produced the smallest value of nLac at isocenter (0.12). As excitation angles increased, the observed nominal value of nLac generally decreased and the physical extent of the uniform region decreased in size. A summary of nLac values as a function of excitation angle and the volumetric region that will produce deviations within ±10% nLac of the nLac value found at isocenter for the six excitation angle cases can be found in Table I. The case when θP = θL = 10° (Figure 3a) produced the largest volumetric region that produces deviations within ±10% nLac at isocenter. However, the exact dimensions of this region are outside of the FOV.
Figure 3.
Axial, sagittal, and coronal 3D nLac maps as a function of nominal excitation angles for pyruvate and lactate, when: (a) θP = θL = 10°; (b) θP = θL = 20°; (c) θP = θL = 30°; (d) θP = θL = 60°; (e) θP = 10°, θL = 30°; (f) θP = 20°, θL = 30°. The dashed red contour lines in (a) and (b) highlight the boundaries of the volume that produces deviations in nLac within ±5% of the nLac value found at isocenter of the coil. The solid red contour lines in (b) through (f) highlight the boundaries of the volume that produces deviations in nLac within ±10% of the nLac value found at isocenter.
Table I.
The volumetric region that produces deviations within ±10% nLac of the nLac value found at isocenter of the coil.
| Excitation Angle Case | nLac | X (cm) | Y (cm) | Z (cm) |
|---|---|---|---|---|
| θP = θL = 10°* | 0.55 | - | - | - |
| θP = θL = 20° | 0.46 | 20.5 | 15.6 | 20.8 |
| θP = θL = 30° | 0.35 | 13.5 | 12.1 | 13.9 |
| θP = θL = 60° | 0.12 | 8.0 | 7.7 | 7.9 |
| θP = 10°, θL = 30° | 0.64 | 21.4 | 16.0 | 21.7 |
| θP = 20°, θL = 30° | 0.46 | 15.9 | 13.5 | 16.4 |
10% contour lines were outside the FOV
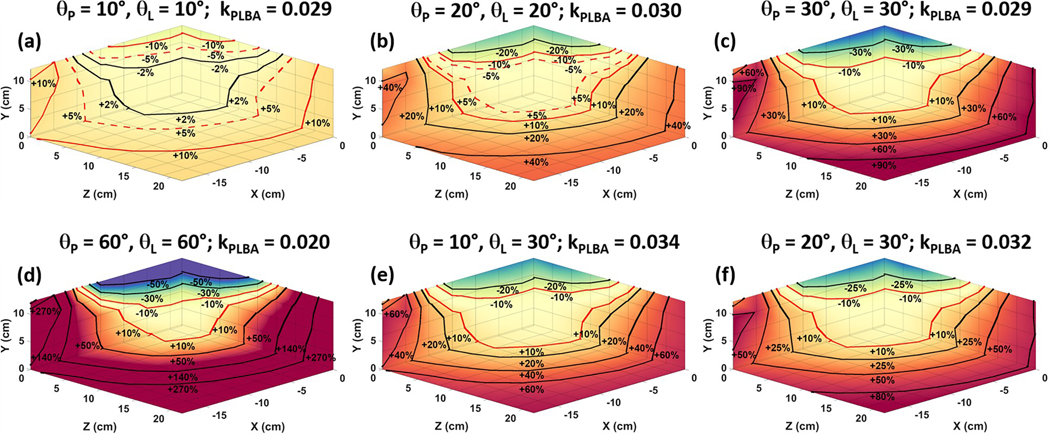
Due to the fact that kPLBA (Figure 4) data was generated using a two compartment model (Model B) and fit using a simple precursor-product relationship (Model A), there was a discrepancy between the fitted kPLBA and the kPL (0.050 s−1) used for data generation. Analysis using the precursor-product relationship is known to underestimate kPL because it assumes that all observed pyruvate is in direct biological contact with enzymes that mediate the conversion of pyruvate into lactate. The excitation angle case when θP = θL = 60° (Figure 4d) produced the lowest kPLBA value at isocenter of the coil (0.020 s−1) and the θP = 10°, θL = 30° case (Figure 4e) produced the largest value of kPLBA (0.034 s−1). Therefore, at higher excitation angle cases, the value of kPLBA at isocenter decreases as well as the size of the volumetric region that will produce deviations within ±10% kPLBA. A summary of kPLBA values and the volumetric region that will produce deviations within ±10% kPLBA of the kPLBA value found at isocenter for the six excitation angle cases (Figure 4) can be found in Table II. The case when θP = θL = 10° (Figure 4a) produced the largest volumetric region (29.2 × 19.6 × 32.6 cm) that produces spatially dependent deviations within ±10% kPLBA of the kPLBA value found at isocenter.
Figure 4.
Axial, sagittal, and coronal 3D kPLBA maps as a function of changes in pyruvate and lactate excitation angle, when: (a) θP = θL = 10°; (b) θP = θL = 20°; (c) θP = θL = 30°; (d) θP = θL = 60°; (e) θP = 10°, θL = 30°; (f) θP = 20°, θL = 30°. The dashed red contour lines in (a) and (b) highlight the boundaries that produce deviations in kPLBA within ±5% of the kPLBA value found at isocenter. The solid red contour lines in (a) through (f) highlight the boundaries that produce deviations in kPLBA within ±10% of the kPLBA value found at isocenter.
Table II.
The volumetric region that produces deviations within ±10% kPLBA of the kPLBA value found at isocenter of the coil.
| Excitation Angle Case | kPLBA (s−1) | X (cm) | Y (cm) | Z (cm) |
|---|---|---|---|---|
| θP = θL = 10° | 0.029 | 29.2 | 19.6 | 32.6 |
| θP = θL = 20° | 0.030 | 15.6 | 13.3 | 16.1 |
| θP = θL = 30° | 0.029 | 11.3 | 11.0 | 12.1 |
| θP = θL = 60° | 0.020 | 7.5 | 7.3 | 7.4 |
| θP = 10, θL = 30° | 0.034 | 12.5 | 11.7 | 13.1 |
| θP = 20, θL = 30° | 0.032 | 12.0 | 11.4 | 12.7 |
kPLBB (Figure 5) values were calculated by using the same two compartment PK model (Model B) for synthesis and analysis, and as a result, the value of kPLBB at isocenter remained unchanged (0.050 s−1), regardless of the excitation angle case employed. However, at higher excitation angle cases (Figure 5d), the size of the volumetric region that will produce deviations within ±10% kPLBB decreased considerably when compared to the lowest excitation angle case (Figure 5a). A summary of kPLBB values as a function of excitation angle and the volumetric region that will produce deviations within ±10% kPLBB of the kPLBB value found at isocenter of the coil for the six excitation angle cases can be found in Table III. The case when θP = θL = 10° produced the largest volumetric region that produces spatially dependent deviations within ±10% kPLBB of the kPLBB value found at isocenter of the 13C clamshell coil. However, the exact dimensions of this region are outside of the FOV.
Figure 5.
Axial, sagittal, and coronal 3D kPLBB maps as a function of changes in pyruvate and lactate excitation angle, when: (a) θP = θL = 10°; (b) θP = θL = 20°; (c) θP = θL = 30°; (d) θP = θL = 60°; (e) θP = 10°, θL = 30°; (f) θP = 20°, θL = 30°. The dashed red contour lines in (a) and (b) highlight the boundaries that produce deviations in kPLBB within ±5% of the kPLBB value found at isocenter. The solid red contour lines in (b) through (f) highlight the boundaries that produce deviations in kPLBB within ±10% of the kPLBB value found at isocenter.
Table III.
The volumetric region that produces deviations within ±10% kPLBB of the kPLBB value found at isocenter of the coil.
| Excitation Angle Case | kPLBB (s−1) | X (cm) | Y (cm) | Z (cm) |
|---|---|---|---|---|
| θP = θL = 10°* | 0.050 | - | - | - |
| θP = θL = 20° | 0.050 | 19.4 | 14.6 | 19.7 |
| θP = θL = 30° | 0.050 | 12.3 | 11.3 | 13.0 |
| θP = θL = 60° | 0.050 | 7.1 | 7.1 | 7.1 |
| θP = 10°, θL = 30° | 0.050 | 15.6 | 13.0 | 13.4 |
| θP = 20°, θL = 30° | 0.050 | 14.3 | 12.2 | 15.0 |
10% contour lines were outside the FOV
Discussion
There are several observable trends that can be seen when looking at the nLac maps (Figure 3 a–d), kPLBA maps (Figure 4 a–d), and kPLBB maps (Figure 5 a–d) for equal excitation angles. As excitation angle is increased, the volumetric region that produces deviations within ±10% error from the reference value of nLac, kPLBA, and kPLBB observed at clamshell isocenter decreases. As a result, when excitation angles increase, nLac or kPL becomes more sensitive to location in the coil due to B1+ non-uniformity. Furthermore, as excitation angles increase, error in measurements that are taken further from coil isocenter become large, further highlighting the importance of target anatomy placement within the central region of the clamshell coil where B1+ deviations are minimal.
Generally, lactate signal in 13C studies is much lower than pyruvate signal, and it is sometimes beneficial to utilize strategies where the lactate excitation angle is greater than the pyruvate excitation angle. The use of a lower excitation angle for pyruvate allows for the preservation of hyperpolarized pyruvate magnetization, which could be chemically converted into lactate. The results from this work show that using an excitation angle case of θP = θL = 10° produces the largest volumetric region that produces deviations within ±10% nLac, kPLBA, and kPLBB. However, low excitation angles can lead to low signal-to-noise ratio (SNR). Lower excitation angles may produce better absolute excitation uniformity, but this will come at the expense of a reduction in SNR. Therefore, a tradeoff must be made in order to balance SNR and excitation angle homogeneity. Lower excitation angles could also promote a temporal averaging effect that will reduce sensitivity to dynamic content with a higher temporal frequency.25
In this work, synthetic data was used to evaluate how semi-quantitative and quantitative metabolic imaging biomarkers can vary with position in the clamshell coil due solely to variations in the B1+ field. At present, no means exist to generate perfectly uniform magnetization and chemical conversion over the large interior volume of this coil, and therefore it would be impractical to perform this study experimentally.
Another minor limitation of this study is that the B1+ field of the clamshell coil was measured by hand, without loading. It is possible that our results may change slightly once the coil is loaded, however, we don’t expect the B1+ field to change dramatically because the RF wavelength is much larger than body size at the 13C resonance frequency of 32.12 MHz, and thus the B1+ field obtained by hand measurements should be a fair representation of the fields inside the body.26
While it may be possible to utilize the hand measured B1+ maps in this work to correct the effects of B1+ inhomogeneities in acquired image data, this would require very careful registration between the B1+ maps, clamshell coil, and patient/target of interest. While we expect perturbations due to body loading to be small, any mismatch could lead to miscalibration and/or errors when correcting image data for the excitation angle at each point in space. This correction would be particularly error prone in regions where excitation angles and SNR are too low, or where excitation angles are too high and HP magnetization is depleted too quickly. Also, semi-quantitative approaches, such as nLac and Model A, will be challenging to correct because different pyruvate excitation angles will deplete pyruvate signal, and thus subsequent lactate signal, at different rates. These errors can be seen in Table I and Table II where there is a clear variation in nLac and kPLBA values at isocenter, as a function of known excitation angles. However, this error is not observed in the kPLBB maps (Table III) because Model B more accurately accounts for excitation losses in extravascular pyruvate. Our primary focus in this body of work was to characterize potential errors induced by transmit inhomogeneities and to identify the range over which we can make accurate measurements without additional sets of corrections. The framework outlined in this manuscript can be used to evaluate the effects of inhomogeneities in other transmit coils that are used for HP MRI studies.
Given the results of the volumetric regions that produce deviations within ±10% nLac, kPLBA, and kPLBB, as well as SNR limitations, we suggest employing excitation angle cases of θP = 10°, θL = 30° and θP = θL = 20° for nLac, kPLBA, and kPLBB measurements when utilizing the clamshell coil. These excitation angle cases would yield the highest nLac and kPL values as well as the largest volumetric regions that produce deviations within ±10% error from the reference value of nLac, kPLBA, and kPLBB observed at clamshell isocenter. Furthermore, these excitation angle cases would reduce B1+ errors in metabolic analysis of hyperpolarized pyruvate when utilizing the clamshell coil while still maintaining lactate and pyruvate SNR. It is important to note that the tradeoff between the size of the homogenous region at isocenter and signal intensity must be weighed carefully depending on the particular imaging application.
Conclusion
We explored the effect of B1+ field inhomogeneity using a 13C volume transmit body coil and produced various B1+ field maps and molecular biomarker deviation maps in order to aid in the positioning of target anatomy for various HP MRI studies. Kinetic modeling and metabolic biomarkers are highly influenced by RF excitation angles16,27 which require precise B1+ calibration for accurate quantitative measurements. By defining acceptable limits of error to be within ±10% deviation in nLac, kPLBA, and kPLBB we were able to create maps that highlight these boundaries across the range of several excitation angle cases that are currently used in research studies today. These hand measured maps will serve as a valuable visualization tool when placing target anatomy in the clamshell coil to aid in reducing B1+ field inhomogeneity and its effect on nLac and kPL measurements. Establishing HP methods that are quantitative, robust, and reproducible is critical for the field of HP MRI. In order to facilitate the widespread clinical application of HP metabolic imaging methods, collaboration and the comparison of clinical HP data gathered at different institutions will require uniform standards in image acquisition protocols and data analysis that enable multicenter trials. Better understanding of the spatial variations of transmit field heterogeneity is important for guiding the physical setup of imaging measurements, and for establishing imaging protocols that will maximize reproducibility and accuracy. This work identifies regions and optimal excitation angles (θP and θL) within the 13C volume transmit clamshell coil where excitation angles will be reproducible and well calibrated, leading to a reduction of errors in metabolic quantification.
Acknowledgements
This work was supported in part by funding from the National Cancer Institute of the National Institutes of Health (R01CA211150). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflict of Interest
GDR receives salary support from GE Healthcare.
References
- 1.Ardenkjaer-Larsen JH, Fridlund B, Gram A, et al. Increase in signal-to-noise ratio of > 10,000 times in liquid-state NMR. Proc Natl Acad Sci U S A. 2003;100(18):10158–10163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Golman K, Ardenkjaer-Larsen JH, Petersson JS, Mansson S, Leunbach I. Molecular imaging with endogenous substances. Proc Natl Acad Sci U S A. 2003;100(18):10435–10439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Golman K, Zandt RI, Lerche M, Pehrson R, Ardenkjaer-Larsen JH. Metabolic imaging by hyperpolarized 13C magnetic resonance imaging for in vivo tumor diagnosis. Cancer Res. 2006;66(22):10855–10860. [DOI] [PubMed] [Google Scholar]
- 4.Bankson JA, Walker CM, Ramirez MS, et al. Kinetic Modeling and Constrained Reconstruction of Hyperpolarized [1–13C]-Pyruvate Offers Improved Metabolic Imaging of Tumors. Cancer Res. 2015;75(22):4708–4717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kurhanewicz J, Vigneron DB, Ardenkjaer-Larsen JH, et al. Hyperpolarized (13)C MRI: Path to Clinical Translation in Oncology. Neoplasia. 2019;21(1):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kurhanewicz J, Vigneron DB, Brindle K, et al. Analysis of cancer metabolism by imaging hyperpolarized nuclei: prospects for translation to clinical research. Neoplasia. 2011;13(2):81–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nelson SJ, Kurhanewicz J, Vigneron DB, et al. Metabolic imaging of patients with prostate cancer using hyperpolarized [1-(1)(3)C]pyruvate. Sci Transl Med. 2013;5(198):198ra108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aggarwal R, Vigneron DB, Kurhanewicz J. Hyperpolarized 1-[(13)C]-Pyruvate Magnetic Resonance Imaging Detects an Early Metabolic Response to Androgen Ablation Therapy in Prostate Cancer. Eur Urol. 2017;72(6):1028–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Woitek R, McLean MA, Gill AB, et al. Hyperpolarized (13)C MRI of Tumor Metabolism Demonstrates Early Metabolic Response to Neoadjuvant Chemotherapy in Breast Cancer. Radiol Imaging Cancer. 2020;2(4):e200017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tropp J, Lupo JM, Chen A, et al. Multi-channel metabolic imaging, with SENSE reconstruction, of hyperpolarized [1-(13)C] pyruvate in a live rat at 3.0 tesla on a clinical MR scanner. J Magn Reson. 2011;208(1):171–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Autry AW, Gordon JW, Carvajal L, et al. Comparison between 8- and 32-channel phased-array receive coils for in vivo hyperpolarized (13) C imaging of the human brain. Magn Reson Med. 2019;82(2):833–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cunningham CH, Lau JY, Chen AP, et al. Hyperpolarized 13C Metabolic MRI of the Human Heart: Initial Experience. Circ Res. 2016;119(11):1177–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miloushev VZ, Granlund KL, Boltyanskiy R, et al. Metabolic Imaging of the Human Brain with Hyperpolarized (13)C Pyruvate Demonstrates (13)C Lactate Production in Brain Tumor Patients. Cancer Res. 2018;78(14):3755–3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park I, Larson PEZ, Gordon JW, et al. Development of methods and feasibility of using hyperpolarized carbon-13 imaging data for evaluating brain metabolism in patient studies. Magn Reson Med. 2018;80(3):864–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tran M, Latifoltojar A, Neves JB, et al. First-in-human in vivo non-invasive assessment of intra-tumoral metabolic heterogeneity in renal cell carcinoma. BJR Case Rep. 2019;5(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun CY, Walker CM, Michel KA, Venkatesan AM, Lai SY, Bankson JA. Influence of parameter accuracy on pharmacokinetic analysis of hyperpolarized pyruvate. Magn Reson Med. 2018;79(6):3239–3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harlan CJ, Xu Z, Michel KA, et al. Technical Note: A deuterated (13) C-urea reference for clinical multiparametric MRI prostate cancer studies including hyperpolarized pyruvate. Med Phys. 2020;47(7):2931–2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gordon JW, Chen HY, Autry A, et al. Translation of Carbon-13 EPI for hyperpolarized MR molecular imaging of prostate and brain cancer patients. Magn Reson Med. 2019;81(4):2702–2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Larson PEZ, Chen H- Y, Gordon JW, et al. Investigation of analysis methods for hyperpolarized 13C-pyruvate metabolic MRI in prostate cancer patients. NMR in Biomedicine. 2018;31(11):e3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grist JT, Hansen ESS, Sanchez-Heredia JD, et al. Creating a clinical platform for carbon-13 studies using the sodium-23 and proton resonances. Magn Reson Med. 2020;84(4):1817–1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang S, Milshteyn E, Reed G, et al. A regional bolus tracking and real-time B1 calibration method for hyperpolarized (13) C MRI. Magn Reson Med. 2019;81(2):839–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walker CM, Gordon JW, Xu Z, et al. Slice profile effects on quantitative analysis of hyperpolarized pyruvate. NMR Biomed. 2020;33(10):e4373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kazan SM, Reynolds S, Kennerley A, et al. Kinetic modeling of hyperpolarized (13)C pyruvate metabolism in tumors using a measured arterial input function. Magn Reson Med. 2013;70(4):943–953. [DOI] [PubMed] [Google Scholar]
- 24.Walker CM, Chen Y, Lai SY, Bankson JA. A novel perfused Bloch-McConnell simulator for analyzing the accuracy of dynamic hyperpolarized MRS. Med Phys. 2016;43(2):854–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bankson JA, Michel KA, Xu Z, Harlan CJ, Martinez G, Walker CM. The Filtering Effect of Signal Excitations on Hyperpolarized Imaging Agents. Abstract from ISMRM 28th Annual Meeting & Exhibition. 2020. [Google Scholar]
- 26.Vaidya MV, Collins CM, Sodickson DK, Brown R, Wiggins GC, Lattanzi R. Dependence of B1+ and B1- Field Patterns of Surface Coils on the Electrical Properties of the Sample and the MR Operating Frequency. Concepts Magn Reson Part B Magn Reson Eng. 2016;46(1):25–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bahrami N, Swisher CL, Von Morze C, Vigneron DB, Larson PE. Kinetic and perfusion modeling of hyperpolarized (13)C pyruvate and urea in cancer with arbitrary RF flip angles. Quant Imaging Med Surg. 2014;4(1):24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]