Abstract
Pyroptosis refers to the process of gasdermin (GSDM)‐mediated programmed cell death (PCD). Our understanding of pyroptosis has expanded beyond cells and is known to involve extracellular responses. Recently, there has been an increasing interest in pyroptosis due to its emerging role in activating the immune system. In the meantime, pyroptosis‐mediated therapies, which use the immune response to kill cancer cells, have also achieved notable success in a clinical setting. In this review, we discuss that the immune response induced by pyroptosis activation is a double‐edged sword that affects all stages of tumorigenesis. On the one hand, the activation of inflammasome‐mediated pyroptosis and the release of pyroptosis‐produced cytokines alter the immune microenvironment and promote the development of tumors by evading immune surveillance. On the other hand, pyroptosis‐produced cytokines can also collect immune cells and ignite the immune system to improve the efficiency of tumor immunotherapies. Pyroptosis is also related to some immune checkpoints, especially programmed death‐1 (PD‐1) or programmed death‐ ligand 1 (PD‐L1). In this review, we mainly focus on our current understanding of the interplay between the immune system and tumors that process through pyroptosis, and debate their use as potential therapeutic targets.
Keywords: cancer, immune, immune microenvironment, inflammasome, pyroptosis
The immune response induced by pyroptosis activation is a double‐edged sword that affects all stages of tumorigenesis. On the one hand, the activation of inflammasome‐mediated pyroptosis and the release of pyroptosis‐produced cytokines alter the immune microenvironment and promote the development of tumors by evading immune surveillance. On the other hand, pyroptosis‐produced cytokines can also collect immune cells and ignite the immune system to improve the efficiency of tumor immunotherapies.
1. INTRODUCTION
Environmental factors and genetic mutation have caused cancer incidence and mortality to rapidly increase. Cancer has become one of the crucial causes of death worldwide, and is becoming a major public health problem.1 The traditional core types of cancer treatment are surgery, chemo‐therapy, and radiation therapy, which can reduce tumor cell proliferation by inducing cancer cell death.2 However, accumulating evidence has shown that tumors often relapse and it has been suggested that successful oncotherapy requires prolonged antineoplastic immunity.3 The field of cancer immunotherapy (CIT) has emerged in recent years and aims to stimulate the body's immune system to create a robust immune response that can kill cancer cells.4 This type of treatment can induce immunogenic cell death in different ways and achieve long‐term anticancer immunity.5 Although the application of CIT has been considered for a broad range of tumors, only a minority of patients achieve a satisfactory treatment effect due to immune escape. These results indicate that the immune system is intricate and still not well managed.6 Thus, it is necessary to explore the mechanisms involved in CIT to develop more efficient methods of treatment for better cancer prevention and treatment.
Programmed cell death (PCD) provides inflammatory and immunogenic signals that act as secondary signals that induce the maintenance of homeostasis of the cell environment and drive adaptive immunity.7 Pyroptosis is a gasdermins (GSDM)‐mediated PCD induced by various stimuli and has been widely studied in many disease models.8 The GSDM family includes gasdermin A (GSDMA), gasdermin B (GSDMB), gasdermin C (GSDMC), gasdermin D (GSDMD), gasdermin E (GSDME), and DFNB59.9 With the exception of DFNB59, members of the GSDM family comprise both a C‐terminal domain and an N‐terminal domain. GSDM can be cleaved to release the GSDM‐N domain, which can punch holes in the cell membrane, resulting in characteristic morphological changes involving cytoplasmic swelling, membrane rupture, and the release of inflammatory factors into the extracellular environment, which direct amplify the systemic immune response.10 In the last few years, pyroptosis has become popular for its double‐edged sword effect in cancer and has attracted much attention in studies conducted on the immune system.11
In this review, we provide an overview of the latest findings that have propelled our understanding of the double‐edged role of pyroptosis in the tumor immunity. The crucial function provided by pyroptosis in regulating immunotherapy in cancers, which will undoubtedly become a focused area in coming years, is subsequently discussed to provide a new direction for therapeutic strategies focusing on the clinical prevention and treatment of cancers.
2. IMMUNE SYSTEM AND TUMOR IMMUNITY
The immune system contains many types of immune cells and immune mediators, which together make up the adaptive and innate immune systems. It is well known that innate immune cells are the first line of innate immunity. Dendritic cells (DCs), macrophages, and mast cells perform immunological surveillance and release immune mediators when the body is attacked.12 Then, leukocytes infiltrate, while macrophages and mast cells trigger a vascular reaction. Therefore, the innate immune system can respond to foreign pathogens. However, the adaptive immune system needs DCs and nature kill (NK) cells for foreign antigen uptake and for memory of previous attacks.13 The main function of the immune system is to recognize and protect the body against infectious pathogens and maintain tissue homeostasis.
During the past few years, scholars have amplified our knowledge of the complicated relationship between immune cells and tumors. The immune system is highly plastic and plays paradoxical roles during cancer progression, which is constantly fine‐tuned as the tumor microenvironment (TME) is modified14 through incidences including immune elimination, immune equilibrium, and immune escape. On the one hand, chronic activation of immune cells in tissues form a chronic inflammatory immune microenvironment that can disable tumor‐killing cells and promote immune escape. On the other hand, immune cells can examine the tumor immune microenvironment and recognize tumor‐specific antigens, which are major histocompatibility complex (MHC) molecules found on cancer cells. Thereafter, immune cells are activated and proliferate to attack tumor cells. Therefore, there is an urgent need to explore therapies that can take advantage of the immune system to treat tumors. At present, immune checkpoint inhibitors, adoptive cell therapies, chimeric antigen receptors, oncolytic viruses, and vaccinations have been developed in the field of immunotherapy. However, CIT can stimulate the immune system to reactivate the anticancer immune response to kill cancer cells.15 Nevertheless, the regulation of immune responses in tumors is a highly complicated process that includes promotional and inhibitory cell immunity signaling pathways. Therefore, the infiltration of immune cells into the tumor immune microenvironment is a significant obstacle that needs to be solved for a satisfactory antitumor immune response to occur.14 At present, the enormous potential of pyroptosis in tumor immunity has been recognized. In this study, we focus on the mechanism of pyroptosis and the immune system, and methods by which the immune system can be controlled for the use of its potential abilities that may be beneficial to humans.
3. PYROPTOTIC SIGNALING
Pyroptosis is considered to be an inflammatory form of PCD and is qualitatively distinct from other types of PCD. Pyroptosis was first identified in 1992 in infected macrophages16 and was named by Cookson et al in 2001.17 Researchers have found that pyroptosis may play a double‐edged role in the pathogenesis and treatment of cancer. On the one hand, many inflammatory factors are released during pyroptosis as normal cells are stimulated, which leads to the formation of an inflammatory microenvironment in which normal cells are transformed into cancer cells. On the other hand, pyroptosis of cancer cells can be used as novel therapeutic targets to inhibit the occurrence and development of cancer.11
The pathways of pyroptosis have been investigated during the past few years. The traditional view of pyroptosis is that it is inflammatory caspase‐induced cell death.18 There are two initial pathways of pyroptosis engagement, canonical or noncanonical, that are associated with GSDMD. Caspase‐1‐dependent pyroptosis is known as canonical pyroptosis, while the activation of human caspase‐4/‐5 and mouse caspase‐11 by intracellular lipopolysaccharide (LPS) is known as the noncanonical pathway. Caspases activated through either pathway result in the cleavage of GSDMD, which can liberate the N‐terminal effector domain of GSDMD and trigger pyroptosis.19 Subsequently, the involvement of GSDME, another member of the GSDM family, has also emerged. Wang et al20 found in 2017 that chemotherapy drugs could induce pyroptosis through caspase‐3 cleavage of GSDME. Thereafter, it was discovered that targeted molecular therapies and other stimuli could also activate caspase‐3 and cleave GSDME to induce pyroptosis. Some studies have found that caspase‐8 activation followed by certain stimuli could result in the cleavage of both GSDMD and GSDME, leading to pyroptosis.21 Recently, besides caspases, researchers have also identified other proteases, apart from caspases, which may be key activators of pyroptosis‐direct granzyme (Gzm)‐mediated induction of pyroptosis. Gzms can not only cleave GSDM but also activate caspase to cleave GSDM, which is much more complicated than traditional inflammasome activation. Their detailed relationship is discussed below.22
In summary, pyroptosis can be identified as GSDM‐mediated PCD, which can be cleaved by members of the caspase family to trigger pyroptosis. As shown in Figure 1, caspase‐1 and caspase‐4/‐5/‐11 can cleave GSDMD, while caspase‐3 can cleave GSDME. Interestingly, it has been found that Gzms may interact with GSDM to trigger pyroptosis. However, the potential relationship between Gzms and pyroptosis, as well as pyroptotic signaling remains to be investigated.
FIGURE 1.
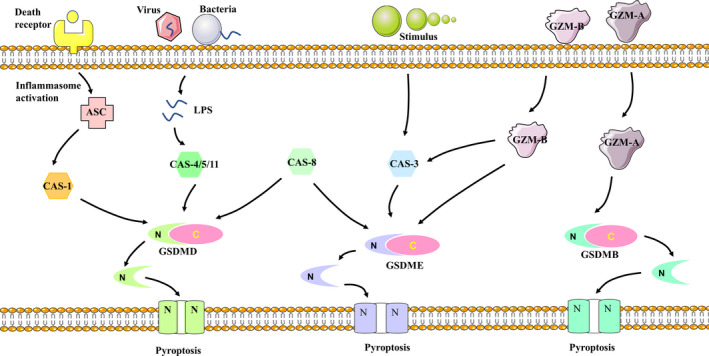
Pyroptotic signaling. When stimulated, caspase‐1 and caspase‐4/‐5/‐11 could cleave GSDMD and caspase‐3 could cleave GSDME to induce pyroptosis. Caspase‐8 can cleave GSDMD and GSDME. Gzm‐A cleaves GSDMB to induce pyroptosis. Gzm‐B could activate or directly cleave GSDME to induce pyroptosis
4. THE ROLE OF PYROPTOSIS IN THE ASSOCIATION BETWEEN CANCER AND THE IMMUNE SYSTEM
It is widely appreciated that there are close interactions between tumors and the immune system that are complex, and the immune system plays both protumorigenic and antitumorigenic roles in tumors.23 Various cancer immunotherapies have been used to identify and destroy tumor cells, but tumorigenesis is augmented while the immune system remains abnormal. Similarly, the relationship between pyroptosis and the immune system relies on complicated cellular communication that involves pyroptosis and immune cells.4 On the one hand, pyroptosis in normal cells may alter the microenvironment and accelerate immune evasion, providing protection from antitumorigenesis.24 On the other hand, there are a number of therapeutic strategies that could stimulate the immune system while pyroptosis is induced in cancer cells.25 In this section, we argue that research on pyroptosis should focus on its role in cancer development and its potential function in tumor immunotherapy.
4.1. Involvement of pyroptosis in the immune microenvironment in tumor progression
Apart from the contribution of the genomic alterations, the immune system is increasingly appreciated for its significant role in tumor progression.23 Furthermore, it has been increasingly realized that cancer cells can adapt to the immune microenvironment and employ immune evasion tactics to help tumor cells to escape from destruction by the immune system and regulate the advancement of primary tumors and metastases. Except in chronic inflammation in protumorigenesis, pyroptosis plays a critical role in exerting potential tumor‐promoting effects along with inflammasome effects, as well as the release of pyroptosis‐produced cytokines in the TME.26 After the original balance between cancer cells and immune microenvironment is tipped, early‐stage cancer needs to escape immune‐mediated surveillance if it is to progress.
4.1.1. Inflammasome activation
Inflammasomes were first described by Tschopp and colleagues in 2002.27 They are multiprotein complexes that enable host defenses, leading to inflammation and pyroptosis, which respond to pathogens and endogenous danger through innate immunity. The human body contains at least three types of inflammasomes: leucine‐rich repeat‐containing proteins (NLRs), AIM2‐like receptors (ALRs) platforms, and pyrin.28 NLRs, in particular, contain nod‐like receptor proteins (NLRPs) and nuclear oligomerization domain proteins subfamily C (NLRC) and are categorized, such as NLRP1b, NLRP3, NLR4 and NLRC4, based on whether the NLRs contain a PYD or a CARD. They can act as core signaling members of the innate immune response and their pattern recognition receptors (PRRs) can identify pathogen‐associated molecular patterns (PAMPs), danger‐associated molecular patterns (DAMPs), and homeostat sis‐altering molecular processes (HAMPs). After the PRR is activated, it can attract the adaptor apoptosis speck protein (ASC), which contains both a pyrin domain (PYD) and a caspase activation and recruitment domain (CARD). The ASC of inflammasomes can interact with the CARD of caspase‐1, subsequently promoting the activation of caspase‐1. In other words, inflammasome complexes can regulate and activate caspase‐1‐mediated pyroptosis to release cytokines, which may be able to provide an inflammatory microenvironment for the development and metastasis of tumors.29
Inflammasomes are notable for their double‐edged sword‐like function in tumors. In this study, we will mainly introduce the molecular mechanisms of inflammasomes and discuss the mechanisms by which pyroptosis‐produced cytokines can trigger cancer development.
4.1.2. Inflammasome‐mediated pyroptosis promotes tumor development
The potential relationship between cancer and inflammation was initially proposed by Rudolf Virchow in 1863. He found that chronic inflammation could promote inflammatory cell proliferation and infiltration in tumors.30 Additionally, Chen et al31 showed that NLRP3 could active IL‐1β, which is associated with antineoplastic immune responses and tumor development in head and neck squamous cell carcinoma. Furthermore, Wang et al32 showed that the accumulation of alcohol in the esophagus could trigger caspase‐1‐mediated pyroptosis and the release of IL‐1β and IL‐18, which may be involved in esophageal cancer. Barrett's esophagus (BE) can also activate caspase‐1‐dependent pyroptosis and cause the release of IL‐1β and IL‐18 to enhance the progression of esophageal cancer.33 Additionally, Gao et al34 described a novel mechanism by which GSDMD‐mediated pyroptosis may aid non‐small‐cell lung cancer (NSCLC) to evade the innate immune response through the dysregulation of immunomodulatory factors, such as IL‐1β. The report published by Pachathundikandi et al reiterated that Helicobacter pylori, which always colonizes in the stomach, could promote NLRP3 inflammasome formation. NLRP3 can stimulate pyroptosis and the release mature IL‐1β or IL‐18 to decelerate host immunity in gastric tumorigenesis.35 Additionally, some studies have confirmed that activated caspase‐1 could induce pyroptosis and release pro‐inflammatory cytokines to exert protumorigenesis effects in human hepatocellular carcinoma (HCC).36 Kofahi et al37 found that the hepatitis C virus (HCV) could activate NLRP3 inflammasomes and induce caspase‐1‐mediated pyroptosis, which could promote a carcinogenesis effect. NLRP3 could also promote tumorigenesis through immune tolerance in pancreatic ductal adenocarcinoma (PDA).38 Previous other studies have shown that NLRP3 can increase IL‐1β secretion and suppress T cell responses in the TME, which are involved in regulating the proliferation of melanoma cells.39 Another study demonstrated that inflammasome‐mediated pyroptosis and the release of IL‐1β could trigger the transport of collections of immune cells, such as myeloid cells into the TME, which promote tumor growth and metastasis of human breast cancer.40 Interestingly, Kaplanov et al41 also found that IL‐1β could recruit monocytes in the TME and act as a master cytokine in promoting breast cancer development. Another study conceptualized the crucial role played by caspase‐1 and IL‐1 in forming the chronic inflammatory microenvironment and promoting the development of human cutaneous squamous cell carcinoma.42 Furthermore, results published by Niebler et al43 strongly suggest that high‐risk human papillomaviruses (HPVs) could stimulate the release of IL‐1β to escape immune surveillance and may be associated with the development of anogenital cancer.
In summary, as shown in Table 1, inflammasomes can trigger caspase‐1‐mediated pyroptosis and the release of pyroptosis‐dependent cytokines, which can create a chronic inflammatory microenvironment that especially includes IL‐1β and IL‐18. This may help tumor cells evade host cell immune response, encouraging local immunosuppression and the subversion of an antigen‐specific immune response, which provides protection against tumor progression. Due to the latent immunosuppressive mechanism by which pyroptosis occurs, researchers have found that overcoming its evasive immune phenotype may be a novel strategy for cancer therapy.44
TABLE 1.
Inflammasome‐mediated pyroptosis promotes tumor development
| Stimulation factor | Pyroptotic signaling | Pro‐inflammatory cytokines | Normal cells | Cancer cells | Reference |
|---|---|---|---|---|---|
| ‐ | NLRP3‐mediated | IL‐1β | Head and neck squamous cell | Head and neck squamous cell carcinoma | (31) |
| Alcohol | Caspase‐1‐mediated | IL‐1β and IL‐18 | Esophagus | Esophagus cancer | (32) |
| ‐ | Caspase‐1‐dependent | IL‐1β and IL‐18 | Barrett's esophagus | Esophagus cancer | (33) |
| ‐ | GSDMD‐mediate | IL‐1β | Lung | Non‑small‐cell lung cancer | (34) |
| Helicobacter pylori | NLRP3‐mediate | IL‐1β and IL‐18 | Stomach | Gastric cancer | (35) |
| ‐ | Caspase‐1‐mediate | ‐ | Hepatocyte | Human hepatocellular carcinoma | (36) |
| Hepatitis C virus | NLRP3‐mediate | ‐ | Hepatocyte | Human hepatocellular carcinoma | (37) |
| ‐ | Caspase‐1‐mediate | ‐ | Pancreas | Pancreatic ductal adenocarcinoma | (38) |
| ‐ | Caspase‐1‐mediate | IL‐1β | Melanoma | Melanoma | (39) |
| ‐ | NLRP3‐mediate | IL‐1β | Breast cell | Breast cancer | (40) |
| ‐ | ‐ | IL‐1β | Breast cell | Breast cancer | (41) |
| ‐ | Caspase‐1‐mediate | IL‐1 | Human cutaneous squamous cell | Human cutaneous squamous cell carcinoma | (42) |
| High‐risk human papillomaviruses | ‐ | IL‐1β | Anogenital | Anogenital cancer | (43) |
The activation of inflammasome could trigger caspase‐1‐mediated pyroptosis and promote tumor development.
4.1.3. Targeting inflammasome‐mediated pyroptosis to inhibit tumor development
The analysis provided above shows that many studies have been conducted to explore methods of pyroptosis‐mediated inflammation control to inhibit tumor development. There are also many other studies that have investigated drugs that can target NLRP3. Curcumin is the primary bioactive polyphenolic element in turmeric and it can inhibit inflammation and oxidation in tissues. Hasanzadeh and his colleagues found that curcumin could upregulate NLRP3 to restrain the activity of caspase‐1 and pyroptosis, leading to an antitumor effect.45 Furthermore, an unpublished study conducted by Chen et al31 found that MCC950 is a small molecule inhibitor of NLRP3 that can inhibit pyroptosis and that MCC950 may halt tumorigenesis in head and neck squamous cells. Arctigenin is the primary active component of Fructus arctii. Qiao et al investigated whether arctigenin could inhibit the growth of various tumors through the inhibition of NLRP3‐mediated pyroptosis.46
Apart from the inhibitor of NLRP3, studies have also explored the advantages of IL‐1β, IL‐1, IL‐1β, IL‐1α, and IL‐18 blocking therapies to inhibit cancer development. Canakinumab, a monoclonal antibody that binds to IL‐1β and inhibits its biological function, could significantly decrease the incidence and mortality rate of lung cancer.47 Additionally, Anakinra, an IL‐1 inhibitor, could remarkably inhibit the development of bone tumors.48 Rilonacept, an inhibitor of IL‐1β and IL‐1α, could control tumorigenesis.49 Last but not least, the GSDMD inhibitor may be able to inhibit pyroptosis from suppressing the development of cancer. Necrosulfonamide (NSA) is an inhibitor of mixed‐lineage kinase domain‐like protein (MLKL), which can modify Cys191, a crucial residue of the GSDMD protein. In other words, NSA may function as a GSDME inhibitor. Therefore, NSA may be able to inhibit NLRP3‐mediated pyroptosis and significantly delay cancer development.50
Overall, targeting inflammasome‐mediated pyroptosis may be a potential method of tumorigenesis inhibition. However, the functions of these pyroptosis‐mediated cytokines under these circumstances and the manner in which they are controlled have not been fully elucidated. Therefore, future studies that investigate the mechanism of action of inflammasome‐mediated pyroptosis and their potential therapeutic role in human anticancer immunotherapies need to be carried out.
4.2. Pyroptosis induces an immunogenic response in cancer
Along with increased knowledge of pyroptosis‐mediated immune responses, therapies that induce pyroptosis to kill tumor cells have achieved extraordinary success in a clinical setting. Pyroptosis‐induced inflammation in a tumor environment can stimulate the cancer immune system through the proliferation and activation of immune cells and immune factors that enhance the efficiency of cancer immunotherapies.51 Therefore, this section will review the methods used to activate pyroptosis in cells and induce an immunogenic response in tumors.
4.2.1. The pyroptosis of cancer cells improves the efficacy of cancer immunotherapies
Various factors can induce inflammatory pyroptosis in cancer cells. Pyroptosis can highly efficiently inhibit primary tumor growth and distant tumor metastasis. It is thought that the level of tumor inhibition is correlated with increased activation of antineoplastic immunity, which can result in strong immune cell maturation, as well as the accumulation and upregulation of several immune cytokines.52 In other words, pyroptosis can stimulate the immune system by increasing the number of immune cells and immune factors to improve the efficacy of cancer immunotherapies.
Wang et al established a bioorthogonal chemical system for GSDMA3, which could trigger pyroptosis to remove a whole tumor graft in breast cancer. The population of immune cells increased significantly. Chemotactic cytokines or antineoplastic effector genes were upregulated in the tumors, while genes that encoded for protumoral or immunosuppressive genes were downregulated. The results suggested that the pyroptosis of cancer cells triggered the activation of robust antineoplastic immunity.53 Veronica et al detailed a tumor ablation technique that used high‐frequency irreversible electroporation (H‐FIRE) that stimulated the pyroptosis of cancer cells and a pro‐inflammatory shift. It activated cellular immunity along with the excitation of immune cells and significantly inhibited tumor progression.54 Furthermore, Zhao et al created a biomimetic nanoparticle (BNP) to induce a change in the cytoplasmic Ca2+ concentration to encourage caspase‐3 activation, which triggered the cleavage of GSDME and the pyroptosis of cancer cells. This resulted in an enhanced level of cytokine secretions and DC accumulation. This was followed by a robust immune response for tumor immunotherapy, indicating the inhibition of primary tumor growth and distant tumor metastasis.52 Oncolytic virotherapy could also induce pyroptosis and the release of TNF‐α and IL‐1β, which resulted in the accumulation of granulocytes and macrophages to inhibit the expression of the negative immune checkpoint for the treatment of melanoma.55 Moreover, Fan et al56 proposed a new hypothesis that combined decitabine (DAC) with chemotherapy nanodrugs that could reverse GSDME silencing to stimulate pyroptosis, leading to the maturation of immune cells which further enhanced the immunological effect of the antitumor drug. In addition, Li et al57 designed a therapeutic nanoreactor that could trigger pyroptosis and boost the level of immune stimulants, especially the pro‐inflammatory cytokine, IL‐1β, HMGB1, and calreticulin, which potentiate the antineoplastic immune system. Furthermore, sea hare hydrolysates (SHH) were used to inhibit STAT3 and induce pyroptosis in non‐small‐cell lung cancer cells. SHH could also reverse the polarization of macrophages for the treatment of cancer, which indicated the conserved function of SHH in the activation of the immune environment to kill tumor cells.58 Additionally, cisplatin (CDDP) could activate caspase‐3 and cleave GSDME to induce pyroptosis in NSCLC. Thereafter, it was able to release chemokines and activate T cells to stimulate the immune system.59 Additionally, Gao et al60 discovered plasma‐membrane microvesicles that contained methotrexate, which could trigger GSDME‐induced pyroptosis and as a result activate macrophages, subsequently stimulating the immune system for the treatment of cholangiocarcinoma.
Therefore, as shown in Figure 2, we analyzed immunologically relevant parameters, as well as GSDMD and GSDME levels in 20 solid tumors based on data obtained from the Cancer Immunome Atlas (https://tcia.at/) and described the results in two heat maps. The results of multiple detection methods found that GSDMD and GSDME expression were associated with the activation of immune cells in a large proportion of these solid tumors, such as CD8+ T cells, CD4+ T cells, dendritic cells, NK cells, macrophages, monocyte, and neutrophil. In Figure 3, we also analyze immunologically relevant parameters, as well as GSDMD and GSDME levels in stomach adenocarcinoma (STAD) and head and neck squamous cell carcinoma (HNSC) based on data obtained from the GEPIA (http://gepia.cancer‐pku.cn/). We found that GSDMD and GSDME expression were associated with immune factors including IFN‐g, IL‐18, IL‐1α, TNF, IL‐1β, and IL‐6. That indicated that pyroptosis could stimulate the immune system through the activation of immune cells and immune factors.
FIGURE 2.
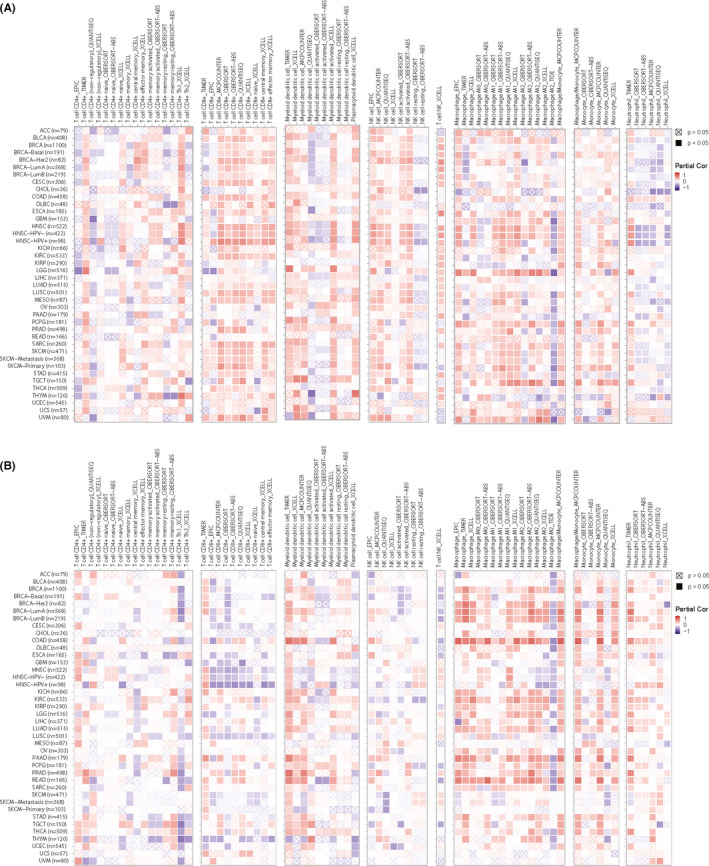
The expression of GSDMD and GSDME are related to immune infiltrates. A, The Cancer Immunome Atlas (https://tcia.at/) showed the expression of GSDMD is related to immune infiltrates through the activation of T cells, NK cells, DC cells, neutrophil, monocyte, macrophage, and T cell NK in 20 solid tumors. B, The Cancer Immunome Atlas (https://tcia.at/) showed the expression of GSDME is related to immune infiltrates through the activation of T cells, NK cells, DC cells, neutrophil, monocyte, macrophage, and T cell NK in 20 solid tumors
FIGURE 3.
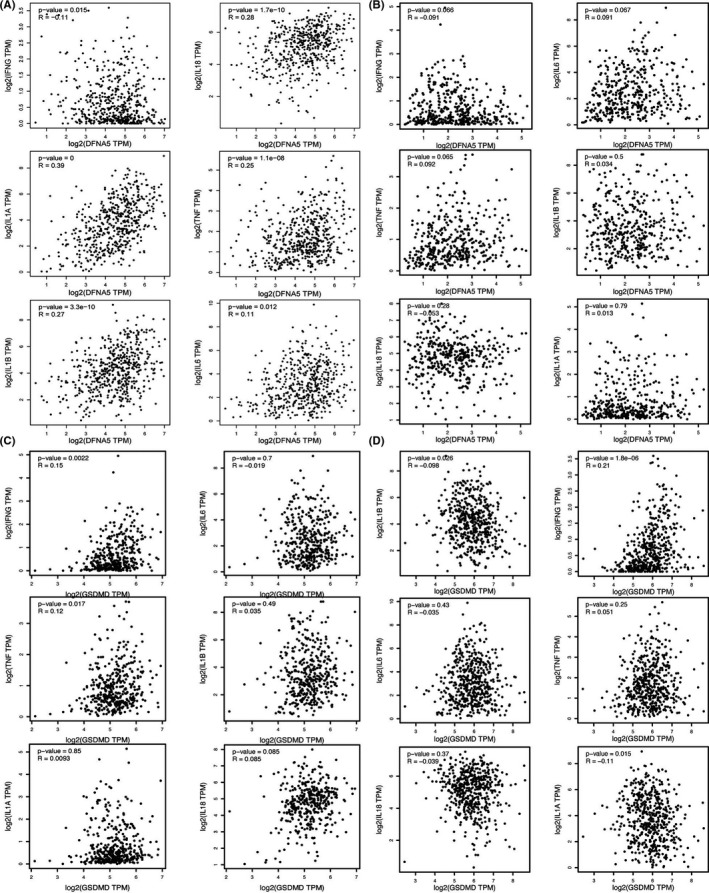
GSDMD and GSDME expression are associated with immune factors. A, GEPIA (http://gepia.cancer‐pku.cn/) showed that the expression of GSDME is related to the expression of IL‐1, IL‐1β, IL‐6, IL‐18, TNF, and IFN in STAD. B, GEPIA (http://gepia.cancer‐pku.cn/) showed that the expression of GSDME is related to the expression of IL‐1, IL‐1β, IL‐6, IL‐18, TNF, and IFN in HNSC. C, GEPIA (http://gepia.cancer‐pku.cn/) showed that the expression of GSDMD is related to the expression of IL‐1, IL‐1β, IL‐6, IL‐18, TNF, and IFN in STAD. D, GEPIA (http://gepia.cancer‐pku.cn/) showed that the expression of GSDMD is related to the expression of IL‐1, IL‐1β, IL‐6, IL‐18, TNF, and IFN in HNSC
Collectively, as summarized in Table 2, these therapeutic approaches could induce pyroptosis in cancer cells and release immune cytokines, which could attract immune cells to magnify the lethal effect induced. The immune system can be stimulated and rendered responsive to immunotherapies.28 However, the results of these research studies have not confirmed the process by which pyroptosis amplifies the immune response for the treatment of tumors. Inflammatory signaling coupled with an increase in immune cytokine expression and immune cells may result in significant changes, but strategies that can be used to control the efficiency of such immunotherapies remain poorly defined.
TABLE 2.
The pyroptosis of cancer cells improves the efficacy of cancer immunotherapies
| Stimulation factor | Cancer type | Pyroptosis signaling | Immune factors | Increased Immune cells | Decreased immune genes | Reference |
|---|---|---|---|---|---|---|
| A bioorthogonal chemical system | Breast cancer | GSDMA3‐mediated | Increase IL‐1β; IL‐18; HMGB1; IFNg; Gzm‐A, Gzm‐B; Gzm‐K; Fasl | Macrophage; CD4+ cells; CD8+ cells; NK cells; cytotoxic T cells | PD‐L1; PD‐L2 | (53) |
| High‐frequency irreversible electroporation | Breast cancer | NLRP3‐mediated | Decrease TAN, MDSC, and TAM populations, | Long‐term memory T cell; leukocyte | ‐ | (54) |
| Biomimetic nanoparticle | Breast cancer | GSDE‐mediated | Increase IFN‐γ | CD4+ cells; CD8+ cells; dendritic cells | ‐ | (52) |
| Oncolytic virotherapy | Melanoma | Caspase‐1‐mediated | Increase TNF‐α; IL‐1β; IL‐10 | Granulocytes; macrophage; NK cell; cytotoxic T‐cells | ‐ | (55) |
| Combining decitabine with chemotherapy nanodrugs | Breast cancer; colon adenocarcino | Caspase‐3‐mediated | IL‐1β and IL‐18 | CD3+ cells; CD8+ cells; cytotoxic T lymphocytes; dendritic cells | ‐ | (56) |
| Therapeutic nanoreactor | Breast cancer; fibrosarcoma cells | Caspase‐3‐mediated | IL‐1β | ‐ | ‐ | (57) |
| Sea hare hydrolysates | Non‐small cell lung cancer cells | Caspase‐1‐mediated | IL‐1β | RAW264.7 cells; peritoneal macrophage; THP‐1 cells | ‐ | (58) |
| Cisplatin | Non‐small‐cell lung cancer | Caspase‐3‐mediated | MIP‐1α; MIP‐1β; MIP‐2; IP‐10 | T cells; CD3+ cells | ‐ | (59) |
| Plasma‐membrane microvesicles | Cholangiocarcinoma | Caspase‐3‐mediated | IL‐1β | Macrophages | ‐ | (60) |
Different therapeutic approaches could induce pyroptosis in cancer cells and release immune cytokines, which could attract immune cells to magnify the lethal effect induced.
4.2.2. The pyroptosis of immune cells triggers robust antineoplastic immunity
Pyroptosis was first identified in macrophages but has subsequently been identified in a variety of immune cells.16 It was recently discovered that pyroptosis of immune cells might promote a robust immune response for the treatment of tumors.
Sorafenib is a spectrum kinase inhibitor that acts as an anticancer agent that targets hepatocellular carcinoma (HCC). Hage et al proved that sorafenib could induce pyroptosis in macrophages and pro‐inflammasome‐cytokine releasing primed NK cell cytotoxicity to kill tumor cells.61 Maltez et al8 also found that IL‐18 was released through pyroptosis, which could stimulate NK cells and trigger robust antineoplastic immunity. DPP8 and DPP9 (DPP8/9) inhibitors are nonselective inhibitors of serine peptidases that are potential anticancer agents. The original and extraordinary immunomodulatory mechanism by which inhibitors are involved in monocyte and macrophage pyroptosis has been reported on and was found to be dependent on caspase‐1 and GSDMD. In other words, these molecules could actuate the innate immune system and induce anticancer immune effects. Furthermore, Yokoyama et al reported on a novel discovery that secretoglobin (SCGB)3A2, which is a protein mainly generated in human airways, could induce pyroptosis in the macrophages of RAW264.7 cells. This was driven by caspase‐11, which proceeded via immune‐modulation to exert its antineoplastic function.62 Moreover, the results of Yang et al demonstrated that pyridoxine (vitamin B6) could selectively induce monocyte macrophages to undergo GSDME‐mediated pyroptosis. This effect could subsequently promote a wider inflammatory response for the treatment of acute myeloid leukemia (AML).63
Collectively, dissimilar pathogenic stimulus could selectively trigger pyroptosis in immune cells, followed by elevated inflammatory cytokine expression. Subsequently, the proliferation and activation of other immune cells were stimulated to encourage the innate immune system to kill cancer cells. However, the mechanism by which immune cells mediate their anticancer amplification effects is still unclear. These studies have provided us with a wide variety of mechanisms that can be used to exploit the immunogenic potential of pyroptosis in immune cells to develop highly effective antineoplastic immunotherapies.
4.2.3. Gzms participate in pyroptosis and immunology
Cytotoxic lymphocytes, which contain NK cells and cytotoxic T lymphocytes, are essential executors of cells during tumor immunotherapy. Gzms are cytolytic granules that can be released together with the pore‐forming protein‐perforin through the granule exocytosis pathway in cytotoxic lymphocytes. Gzms is a serine protease family that exerts cleavage specificity. The Gzms family includes granzyme A (Gzm‐A), granzyme B (Gzm‐B), granzyme H (Gzm‐H), and granzyme K (Gzm‐K) in humans, while Gzm‐A and Gzm‐B are the most abundant. On the one hand, Gzms can target cells with the help of perforin; on the other hand, Gzms may be released into microenvironments as pro‐inflammatory mediators that exert extracellular functions.64 Recently, Gzms have drawn increased attention for their novel function in pyroptosis.
Researchers have found that Gzms can activate caspases to cleave members of the GSDM family. Zhou et al demonstrated that Gzm‐A, which is also released by NK cells and cytotoxic lymphocytes, can cleave GSDMB. This action can significantly induce pyroptosis and may augment the immune system.22 Accordingly, researchers have observed that Gzm‐B could also activate caspase‐3 in target cells and induce pyroptosis by cleaving GSDME, increasing the number of macrophages, NK cells, and CD8+ T lymphocytes, thereby activating antineoplastic immunity.22, 51 Moreover, the results of Xi et al and this review demonstrate that GSDMD may be cleaved by caspase‐4/‐11, which is activated by Gzm‐B secreted from cytotoxic T lymphocytes (CTLs). The release of the GSDMD‐N domain resulted in pyroptosis, suggesting that Gzm‐B is involved in pyroptosis‐mediated attack on CTL.65
Zhang et al66 investigated whether Gzm‐B, which is interceded by killer cytotoxic lymphocytes, could cleave GSDME in D270 cells to trigger pyroptosis. Xi et al65 have proposed that the colocalization between GSDMD and Gzm‐B in the vesicles could lead to the cleavage of GSDMD in CTLs, indicating that Gzm‐B is required for the pyroptosis of cancer cells. Additionally, Fong et al67 demonstrated that NK cells can trigger pyroptosis and the release of intracellular contents, such as IL‐1β and Gzm‐B, when attacked by bacteria. Additionally, Olivier et al68 observed that 25‐OH oxysterol could induce both apoptosis and pyroptosis along with the release of Gzm‐B.
In brief, as shown in Figure 4, these results suggest that Gzms, which is released into the microenvironment by immune cells, may be involved in pyroptosis in various ways. However, these research studies have not yet provided a unitive standpoint of the mechanism by which Gzms participates in pyroptotic signaling. Although much remains to be investigated, these studies have broadened our understanding of the mechanism by which GSDM‐mediated pyroptosis occurs in tumor immunity.
FIGURE 4.
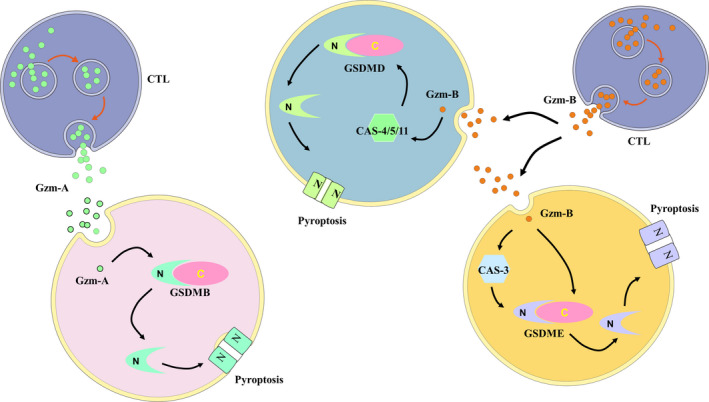
Gzms participate in the process of pyroptosis and immunology. Gzms are released by CTLs. Gzm‐A cleaves GSDMD to induce pyroptosis. Gzm‐B activates caspase‐4/‐5/‐11 to cleave GSDMD. Gzm‐B can activate or directly cleave GSDME to induce pyroptosis
5. SPECIFIC RELATIONSHIP BETWEEN PYROPTOSIS AND IMMUNE CHECKPOINTS
Due to immune editing and immune suppression during tumor ontogenesis, the reactivation and increase in the visibility and accessibility of tumor antigens to the immune profile are of paramount importance for the activation of the tumor immune system. Therefore, immune checkpoint inhibitors that block receptors to inhibit adaptive immunity have been constructed. Immune checkpoint inhibitor‐dependent inhibition may exert a pro‐inflammatory immunity response against cancer neoantigens and trigger a boost for continued anticancer immune responses. These discoveries have provided a competent therapy to improve treatment and survival rates. Pyroptosis may also play a significant role in this therapeutic effect. In this section, we mainly discussed the relationship between immune checkpoint inhibitors and pyroptosis, as well as the potential possibility of using immune checkpoints to modulate pyroptosis activation for antitumor activity.
5.1. The role of pyroptosis in PD‐1
Programmed death‐1 (PD‐1) and programmed death‐ligand 1 (PD‐L1) are well known for their role as immune checkpoint regulators. The primary ligand that targets the interactions of PD‐1 is PD‐L1. Recently, it has been found that pyroptosis is closely associated with PD‐1 and PD‐L1. In this study, we summarized our current understanding of the involvement of PD‐1 and PD‐L1 in pyroptosis to induce an antitumor immune response, with an emphasis on mechanisms by which PD‐1 and PD‐L1 exert their immune checkpoint functions and nonimmune checkpoint functions.
5.1.1. The immune checkpoint function of PD‐1 in pyroptosis
The PD‐1 pathway is a core pathway of immunosuppression in the human TME. Inhibition of the PD‐1 and PD‐L1 pathways is an important for cancer immunosuppression and can generate endogenous antineoplastic immunity to inhibit cancer development.44 However, the response rate may be low. One important reason is that inflammation within the cancer immune microenvironment is ineffective for competent infiltration and activation of immune cells. In other words, the cancer immune microenvironment is inactive towards the resistance against checkpoint blockade. Therefore, it has been found that the efficiency of anti‐PD‐1 or PD‐L1 treatments can be improved under pyroptosis‐induced inflammation in the TME through chemotherapy, radiotherapy, and other therapies.
Published clinical trials have shown that PD‐L1 inhibitors combined with chemotherapy or radiotherapy can kill cancer cells by triggering the pyroptosis of cancer cells. This may improve the survival of patients compared with those who are only administered a single type of therapy to increase the efficiency of PD‐LI inhibitors.69 Moreover, another study aimed to identify a drug that could promote the selective release of GSDMA3 in tumors. It was found that the N‐terminal of GSDMA3 could trigger pyroptosis and release IL‐18 to increase T cell‐mediated inhibition of tumor cells. Importantly, the combined treatment could sensitize breast cancer cells to anti‐PD‐1 therapy due to pyroptosis‐induced inflammation within the cancer immune environment.
In other words, if anti‐PD‐1 or PD‐L1 treatments are the "match that ignites the fire" in tumors, pyroptosis‐induced inflammation may supply the "fuel that feeds the flames". Therefore, these results may provide the theoretical basis and a range of possibilities for the development of combined therapies involving PD‐L1 inhibitors and other therapies to inhibit tumor development.
5.1.2. The nonimmune checkpoint function of PD‐1 in pyroptosis
Apart from their well‐known immune checkpoint function, PD‐1 and PD‐L1 can also exert nonimmune checkpoint functions inside cancer cells.
Hou et al70 found that antibiotic chemotherapy drugs could promote the combined effect of STAT3 and PD‐L1 to upregulate gasdermin C (GSDMC) under hypoxic conditions. It is worth noting that caspase‐8 can activate caspase‐3 to induce GSDMC‐mediated pyroptosis in breast cancer cells,71 while STAT1 may also be associated with PD‐L1. Interestingly, it was also found that GSDMD and GSDME could directly induce pyroptosis, and that this mechanism is regarded as a significant biomarker that can predict the response to anti‐PD‐1 antibodies.
In summary, PD‐1 and PD‐L1 are closely associated with pyroptosis. On the one hand, certain therapies may trigger pyroptosis to create an inflammation microenvironment that can enhance cancer cell sensitivity to anti‐PD‐L1 therapies. On the other hand, PD‐L1 can regulate GSDMC. Moreover, GSDMD and GSDME may function as biomarkers for anti‐PD‐1 therapies.
5.2. The exploitation of novel immune checkpoints
Apart from PD‐1 and PD‐L1, researchers have also explored other potential immune checkpoints that may be able to eliminate tumor cells.
It was found that the inhibitor of BRAF–MEK could induce pyroptosis in melanoma. Erkes et al showed that BRAF and MEK inhibitors could block the ERK1/2 pathway and activate caspase‐3 to trigger GSDME‐mediated pyroptosis. Furthermore, they identified alterations in the tumor immune microenvironment that resulted from the activation of DCs and the proliferation of T cells.72 The inhibitor of MEK could also cleave GSDME to induce pyroptosis in lung cancer and hepatocellular carcinoma cells.
Recently, Elion et al73 suggested that RIG‐I agonists may be widely applicable for the treatment of breast cancer, and that the RIG‐I agonist could induce pyroptosis through the activation of caspase‐1 and GSDMD‐mediation in breast cancer. The induction of pyroptosis could also increase STAT1 and NF‐κB expression levels and release IFN to activate innate immunity along with an increase in the number of lymphocytes to inhibit cancer growth and metastasis.
Furthermore, Samir et al also found that stress granules and NLRP3 may compete for DDX3X under stress conditions and that the activator of DDX3X could not only assemble stress granules but could also activate NLRP3‐mediated pyroptosis to release IL‐1β and IL‐18. Subsequently, these reactions could trigger the activation of the immune system.74
Additionally, Okondo et al25 revealed that val‐boroPro could lead to caspase‐1‐independent pyroptosis in monocytes and macrophages for the treatment of cancer. This was the first study to indicate that DPP8/9 may be a new immune checkpoint for the activation of the antitumor immune system. In the meantime, Cornelius et al explored the notion that GSDMD is indispensable for caspase‐1‐mediated pyroptosis under the influence of DPP8/9 inhibitors. In other words, a lack of GSDMD and caspase‐1 would activate caspase‐3/‐7 to induce apoptosis.75 Chui et al76 found that DPP8/9 inhibitors could cleave NLRP1b and release the C‐terminus to trigger caspase‐1‐mediated pyroptosis. This reaction could also lead to the degradation of the N‐terminus to activate the innate immune system. Recently, it was also shown that DPP8/9 inhibitors could activate CARD8 and trigger NLRP1b‐mediated pyroptosis in lymphocytes.
Additionally, as shown in Figure 5, we further evaluated CTLA‐4 and anti‐PD‐1 antibodies and showed that GSDMD and GSDME were outstanding predictors of response to checkpoint blockers in STAD and HNSC, which revealed that pyroptosis might be activated by immune checkpoints to induce antitumor activity.
FIGURE 5.
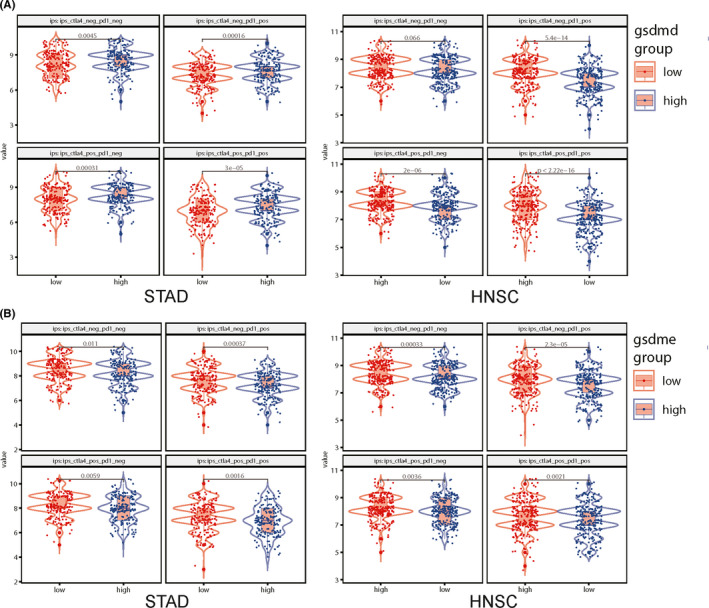
The sensitivity of CTLA‐4 and anti‐PD‐1 are higher in the GSDMD and GSDME overexpression group in STAD and HNSC. A, The sensitivity of CTLA‐4 and anti‐PD‐1 are higher in the GSDMD overexpression group in STAD and HNSC. B, The sensitivity of CTLA‐4 and anti‐PD‐1 is higher in the GSDME overexpression group in STAD and HNSC
In this section, we discussed the close relationship between pyroptosis and immune checkpoints, especially in relation to the immune checkpoint functions and nonimmune checkpoint functions of PD‐1 or PD‐L1, which are shown in Figure 6. We also discussed the potential of immune checkpoints for the induction of pyroptosis in cancer cells. We trust that these results will not only lead to a greater understanding of the mechanism of action of immune checkpoints, but will also promote the development of other potential immune checkpoints that can modulate pyroptosis activation to exert antitumor activity.
FIGURE 6.
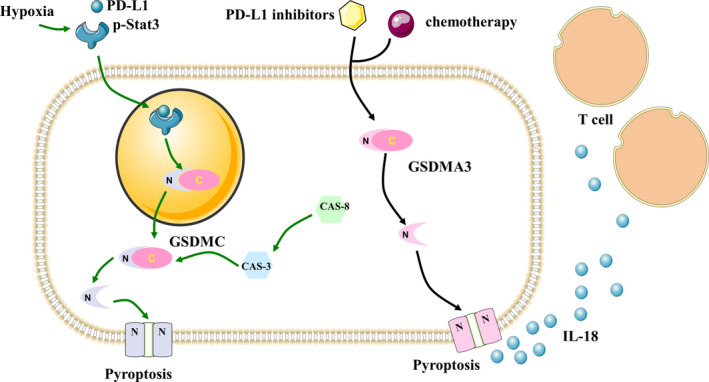
The immune checkpoint function and nonimmune checkpoint function of PD‐1 in pyroptosis. PD‐L1 inhibitors combined with chemotherapy could release the N‐terminal of GSDMA3 and trigger pyroptosis and release IL‐18 to increase T cell‐mediated inhibition of tumor cells. Antibiotic chemotherapy drugs could promote the combined effect of STAT3 and PD‐L1 to upregulate GSDMC under hypoxic conditions. Caspase‐8 can activate caspase‐3 to induce GSDMC‐mediated pyroptosis
6. HOW CAN THE KNOWLEDGE OF PYROPTOSIS SIGNALING BENEFIT TUMOR IMMUNOLOGY?
For the past several years, scholars have mainly focused on the field of cell death itself, and pyroptosis in cancer cells and its differential modulation of the downstream immune system has not been widely investigated.4 Apart from the well‐characterized significant functions of pyroptosis, which is an inflammatory caspase‐induced form of PCD, its role in tumors is complex and has a double‐edged sword‐like effect on the tumor immune system. On the one hand, the activation of pyroptosis in cancer cells and immune cells will lead to the liberation of inflammatory chemokines and the subsequent infiltration of immune cells. This effect can activate the TME and magnify the efficiency of immunotherapies. Furthermore, Gzms have drawn increasing attention to pyroptosis during recent years. On the other hand, the effects of inflammasomes and cytokines produced by pyroptosis could create chronic levels of inflammation that help tumor cells escape from the immune system and promote the development of tumors. Therefore, as shown in Figure 7, pyroptosis acts as a bridge between the immune system and tumors, and its potential as a therapeutic target for antitumor activity is highly debated.
FIGURE 7.
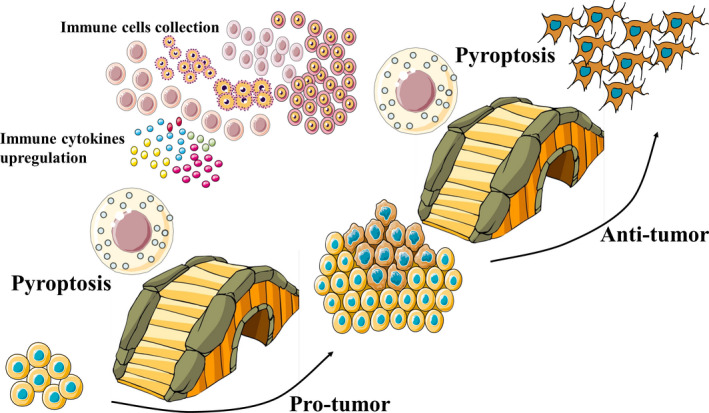
Pyroptosis acts as a bridge between immune system and tumor. Pyroptosis releases immune cytokines and triggers immune cell collection, which influences the tumor immune microenvironment. That plays not only protumor role but also the antitumor role in tumors
Consequently, to further understand the potential function of pyroptosis on the immune system, future studies that address the following are needed. First, potential pathways of pyroptosis must be explored to discover other mechanisms by which pyroptosis exerts antitumor effects. Thereafter, factors that induce pyroptosis activation during protumor processes should be investigated to understand strategies that can be applied to avoid tumorigenesis. Most importantly, we should also identify the function of pyroptosis on the regulation of immune cells and the immune response to enhance the efficiency of cancer treatments and explore more antineoplastic therapies. Last but not least, the possible molecular interactions between pyroptosis and checkpoints should be researched to expand our knowledge of immunotherapy. In brief, the potential of targeting pyroptosis for the development of tumor immunotherapies may be an attractive and relatively unexplored area in the field of cancer research.
DISCLOSURE
The authors have no conflict of interest.
ACKNOWLEDGMENT
We are grateful to the teachers at the Central Cancer Laboratory of Harbin Medical University for their kind help.
Li L, Jiang M, Qi L, et al. Pyroptosis, a new bridge to tumor immunity. Cancer Sci. 2021;112:3979–3994. 10.1111/cas.15059
Funding information
Wu Jieping Medical Foundation (Grant/Award Number: 320.6750.2020‐05‐17), Scientific Research Foundation of Harbin Medical University Cancer Hospital, (Grant/Award Number: BJQN2018‐02, JJZD2021‐08), and the National Natural Science Foundation of China (Grant/Award Number: 81602662).
Contributor Information
Yanjing Li, Email: liyanjing_hmu@126.com.
Yuxian Bai, Email: bai_yuxian@126.com.
REFERENCES
- 1.Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144:1941‐1953. [DOI] [PubMed] [Google Scholar]
- 2.Liu J, Kuang F, Kang R, Tang D. Alkaliptosis: a new weapon for cancer therapy. Cancer Gene Ther. 2020;27:267‐269. [DOI] [PubMed] [Google Scholar]
- 3.Ma Y, Pitt JM, Li Q, Yang H. The renaissance of anti‐neoplastic immunity from tumor cell demise. Immunol Rev. 2017;280:194‐206. [DOI] [PubMed] [Google Scholar]
- 4.Legrand AJ, Konstantinou M, Goode EF, Meier P. The diversification of cell death and immunity: memento mori. Mol Cell. 2019;76:232‐242. [DOI] [PubMed] [Google Scholar]
- 5.Messmer MN, Snyder AG, Oberst A. Comparing the effects of different cell death programs in tumor progression and immunotherapy. Cell Death Differ. 2019;26:115‐129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hegde PS, Chen DS. Top 10 challenges in cancer immunotherapy. Immunity. 2020;52:17‐35. [DOI] [PubMed] [Google Scholar]
- 7.Yatim N, Cullen S, Albert ML. Dying cells actively regulate adaptive immune responses. Nat Rev Immunol. 2017;17:262‐275. [DOI] [PubMed] [Google Scholar]
- 8.Maltez VI, Tubbs AL, Cook KD, et al. Inflammasomes coordinate pyroptosis and natural killer cell cytotoxicity to clear infection by a ubiquitous environmental bacterium. Immunity. 2015;43:987‐997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li L, Li Y, Bai Y. Role of GSDMB in pyroptosis and cancer. Cancer Manag Res. 2020;12:3033‐3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sarhan J, Liu BC, Muendlein HI, et al. Caspase‐8 induces cleavage of gasdermin D to elicit pyroptosis during Yersinia infection. Proc Natl Acad Sci USA. 2018;115:E10888‐E10897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xia X, Wang X, Cheng Z, et al. The role of pyroptosis in cancer: pro‐cancer or pro‐"host"? Cell Death Dis. 2019;10:650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kennedy LB, Salama AKS. A review of cancer immunotherapy toxicity. CA Cancer J Clin. 2020;70:86‐104. [DOI] [PubMed] [Google Scholar]
- 13.Sun JY, Lu XJ. Cancer immunotherapy: current applications and challenges. Cancer Lett. 2020;480:1‐3. [DOI] [PubMed] [Google Scholar]
- 14.de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6:24‐37. [DOI] [PubMed] [Google Scholar]
- 15.Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348:56‐61. [DOI] [PubMed] [Google Scholar]
- 16.Zychlinsky A, Prevost MC, Sansonetti PJ. Shigella flexneri induces apoptosis in infected macrophages. Nature. 1992;358:167‐169. [DOI] [PubMed] [Google Scholar]
- 17.Cookson BT, Brennan MA. Pro‐inflammatory programmed cell death. Trends Microbiol. 2001;9:113‐114. [DOI] [PubMed] [Google Scholar]
- 18.Gaidt MM, Hornung V. Pore formation by GSDMD is the effector mechanism of pyroptosis. EMBO J. 2016;35:2167‐2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruhl S, Shkarina K, Demarco B, Heilig R, Santos JC, Broz P. ESCRT‐dependent membrane repair negatively regulates pyroptosis downstream of GSDMD activation. Science. 2018;362:956‐960. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y, Gao W, Shi X, et al. Chemotherapy drugs induce pyroptosis through caspase‐3 cleavage of a gasdermin. Nature. 2017;547:99‐103. [DOI] [PubMed] [Google Scholar]
- 21.Schwarzer R, Laurien L, Pasparakis M. New insights into the regulation of apoptosis, necroptosis, and pyroptosis by receptor interacting protein kinase 1 and caspase‐8. Curr Opin Cell Biol. 2020;63:186‐193. [DOI] [PubMed] [Google Scholar]
- 22.Zhou Z, He H, Wang K, et al. Granzyme A from cytotoxic lymphocytes cleaves GSDMB to trigger pyroptosis in target cells. Science. 2020;368:eaaz7548. [DOI] [PubMed] [Google Scholar]
- 23.Van Gorp H, Lamkanfi M. The emerging roles of inflammasome‐dependent cytokines in cancer development. EMBO Rep. 2019;20:e47575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chauhan D, Vande Walle L, Lamkanfi M. Therapeutic modulation of inflammasome pathways. Immunol Rev. 2020;297:123‐138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okondo MC, Johnson DC, Sridharan R, et al. DPP8 and DPP9 inhibition induces pro‐caspase‐1‐dependent monocyte and macrophage pyroptosis. Nat Chem Biol. 2017;13:46‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Binnewies M, Roberts EW, Kersten K, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med. 2018;24:541‐550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL‐beta. Mol Cell. 2002;10:417‐426. [DOI] [PubMed] [Google Scholar]
- 28.Karki R, Kanneganti TD. Diverging inflammasome signals in tumorigenesis and potential targeting. Nat Rev Cancer. 2019;19:197‐214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weichand B, Popp R, Dziumbla S, et al. S1PR1 on tumor‐associated macrophages promotes lymphangiogenesis and metastasis via NLRP3/IL‐1β. J Exp Med. 2017;214:2695‐2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539‐545. [DOI] [PubMed] [Google Scholar]
- 31.Chen L, Huang CF, Li YC, et al. Blockage of the NLRP3 inflammasome by MCC950 improves anti‐tumor immune responses in head and neck squamous cell carcinoma. Cell Mol Life Sci. 2018;75:2045‐2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang F, Li G, Ning J, et al. Alcohol accumulation promotes esophagitis via pyroptosis activation. Int J Biol Sci. 2018;14:1245‐1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barber G, Anand A, Katarzyna O, et al. Characterizing caspase‐1 involvement during esophageal disease progression. Cancer Immunol Immunother. 2020;69:2635‐2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gao J, Qiu X, Xi G, et al. Downregulation of GSDMD attenuates tumor proliferation via the intrinsic mitochondrial apoptotic pathway and inhibition of EGFR/Akt signaling and predicts a good prognosis in nonsmall cell lung cancer. Oncol Rep. 2018;40:1971‐1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pachathundikandi SK, Blaser N, Bruns H, Backert SHelicobacter pylori avoids the critical activation of NLRP3 inflammasome‐mediated production of oncogenic mature IL‐1β in human immune cells. Cancers (Basel). 2020;12:803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luan J, Ju D. Inflammasome: a double‐edged sword in liver diseases. Front Immunol. 2018;9:2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kofahi HM, Taylor NG, Hirasawa K, Grant MD, Russell RS. Hepatitis C virus infection of cultured human hepatoma cells causes apoptosis and pyroptosis in both infected and bystander cells. Sci Rep. 2016;6:37433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Daley D, Mani VR, Mohan N, et al. NLRP3 signaling drives macrophage‐induced adaptive immune suppression in pancreatic carcinoma. J Exp Med. 2017;214:1711‐1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ellis LZ, Liu W, Luo Y, et al. Green tea polyphenol epigallocatechin‐3‐gallate suppresses melanoma growth by inhibiting inflammasome and IL‐1beta secretion. Biochem Biophys Res Commun. 2011;414:551‐556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ershaid N, Sharon Y, Doron H, et al. NLRP3 inflammasome in fibroblasts links tissue damage with inflammation in breast cancer progression and metastasis. Nat Commun. 2019;10:4375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaplanov I, Carmi Y, Kornetsky R, et al. Blocking IL‐1beta reverses the immunosuppression in mouse breast cancer and synergizes with anti‐PD‐1 for tumor abrogation. Proc Natl Acad Sci USA. 2019;116:1361‐1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Drexler SK, Bonsignore L, Masin M, et al. Tissue‐specific opposing functions of the inflammasome adaptor ASC in the regulation of epithelial skin carcinogenesis. Proc Natl Acad Sci USA. 2012;109:18384‐18389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Niebler M, Qian X, Hofler D, et al. Post‐translational control of IL‐1beta via the human papillomavirus type 16 E6 oncoprotein: a novel mechanism of innate immune escape mediated by the E3‐ubiquitin ligase E6‐AP and p53. PLoS Pathog. 2013;9:e1003536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garg AD, Agostinis P. Cell death and immunity in cancer: from danger signals to mimicry of pathogen defense responses. Immunol Rev. 2017;280:126‐148. [DOI] [PubMed] [Google Scholar]
- 45.Hasanzadeh S, Read MI, Bland AR, Majeed M, Jamialahmadi T, Sahebkar A. Curcumin: an inflammasome silencer. Pharmacol Res. 2020;159:104921. [DOI] [PubMed] [Google Scholar]
- 46.Qiao S, Lv C, Tao Y, et al. Arctigenin disrupts NLRP3 inflammasome assembly in colonic macrophages via downregulating fatty acid oxidation to prevent colitis‐associated cancer. Cancer Lett. 2020;491:162‐179. [DOI] [PubMed] [Google Scholar]
- 47.Ridker PM, MacFadyen JG, Thuren T, Everett BM, Libby P, Glynn RJ. Effect of interleukin‐1β inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double‐blind, placebo‐controlled trial. Lancet. 2017;390:1833‐1842. [DOI] [PubMed] [Google Scholar]
- 48.Shahriari K, Shen F, Worrede‐Mahdi A, et al. Cooperation among heterogeneous prostate cancer cells in the bone metastatic niche. Oncogene. 2017;36:2846‐2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dinarello CA. An expanding role for interleukin‐1 blockade from gout to cancer. Mol Med. 2014;20(Suppl 1):S43‐S58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu X, Zhou M, Mei L, et al. Key roles of necroptotic factors in promoting tumor growth. Oncotarget. 2016;7:22219‐22233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Minton K. Pyroptosis heats tumour immunity. Nat Rev Immunol. 2020;20:274‐275. [DOI] [PubMed] [Google Scholar]
- 52.Zhao P, Wang M, Chen M, et al. Programming cell pyroptosis with biomimetic nanoparticles for solid tumor immunotherapy. Biomaterials. 2020;254:120142. [DOI] [PubMed] [Google Scholar]
- 53.Wang Q, Wang Y, Ding J, et al. A bioorthogonal system reveals antitumour immune function of pyroptosis. Nature. 2020;579:421‐426. [DOI] [PubMed] [Google Scholar]
- 54.Ringel‐Scaia VM, Beitel‐White N, Lorenzo MF, et al. High‐frequency irreversible electroporation is an effective tumor ablation strategy that induces immunologic cell death and promotes systemic anti‐tumor immunity. EBioMedicine. 2019;44:112‐125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bollino D, Colunga A, Li B, Aurelian L. DeltaPK oncolytic activity includes modulation of the tumour cell milieu. J Gen Virol. 2016;97:496‐508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fan JX, Deng RH, Wang H, et al. Epigenetics‐based tumor cells pyroptosis for enhancing the immunological effect of chemotherapeutic nanocarriers. Nano Lett. 2019;19:8049‐8058. [DOI] [PubMed] [Google Scholar]
- 57.Li J, Anraku Y, Kataoka K. Self‐boosting catalytic nanoreactors integrated with triggerable crosslinking membrane networks for initiation of immunogenic cell death by pyroptosis. Angew Chem Int Ed Engl. 2020;59:13526‐13530. [DOI] [PubMed] [Google Scholar]
- 58.Nyiramana MM, Cho SB, Kim EJ, et al. Sea hare hydrolysate‐induced reduction of human non‐small cell lung cancer cell growth through regulation of macrophage polarization and non‐apoptotic regulated cell death pathways. Cancers (Basel). 2020;12:726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Peng Z, Wang P, Song W, et al. GSDME enhances cisplatin sensitivity to regress non‐small cell lung carcinoma by mediating pyroptosis to trigger anti‐tumor immunocyte infiltration. Signal Transduct Target Ther. 2020;5:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gao Y, Zhang H, Zhou N, et al. Methotrexate‐loaded tumour‐cell‐derived microvesicles can relieve biliary obstruction in patients with extrahepatic cholangiocarcinoma. Nat Biomed Eng. 2020;4:743‐753. [DOI] [PubMed] [Google Scholar]
- 61.Hage C, Hoves S, Strauss L, et al. Sorafenib induces pyroptosis in macrophages and triggers natural killer cell‐mediated cytotoxicity against hepatocellular carcinoma. Hepatology. 2019;70:1280‐1297. [DOI] [PubMed] [Google Scholar]
- 62.Yokoyama S, Cai Y, Murata M, et al. A novel pathway of LPS uptake through syndecan‐1 leading to pyroptotic cell death. Elife. 2018;7:e37854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang W, Liu S, Li Y, et al. Pyridoxine induces monocyte‐macrophages death as specific treatment of acute myeloid leukemia. Cancer Lett. 2020;492:96‐105. [DOI] [PubMed] [Google Scholar]
- 64.Garzon‐Tituana M, Arias MA, Sierra‐Monzon JL, et al. The multifaceted function of granzymes in sepsis: some facts and a lot to discover. Front Immunol. 2020;11:1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xi G, Gao J, Wan B, et al. GSDMD is required for effector CD8(+) T cell responses to lung cancer cells. Int Immunopharmacol. 2019;74:105713. [DOI] [PubMed] [Google Scholar]
- 66.Zhang Z, Zhang Y, Xia S, et al. Gasdermin E suppresses tumour growth by activating anti‐tumour immunity. Nature. 2020;579:415‐420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fong JJ, Tsai CM, Saha S, Nizet V, Varki A, Bui JD. Siglec‐7 engagement by GBS beta‐protein suppresses pyroptotic cell death of natural killer cells. Proc Natl Acad Sci USA. 2018;115:10410‐10415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Olivier E, Dutot M, Regazzetti A, Laprevote O, Rat P. 25‐Hydroxycholesterol induces both P2X7‐dependent pyroptosis and caspase‐dependent apoptosis in human skin model: new insights into degenerative pathways. Chem Phys Lipids. 2017;207:171‐178. [DOI] [PubMed] [Google Scholar]
- 69.Reck M, Schenker M, Lee KH, et al. Nivolumab plus ipilimumab versus chemotherapy as first‐line treatment in advanced non‐small‐cell lung cancer with high tumour mutational burden: patient‐reported outcomes results from the randomised, open‐label, phase III CheckMate 227 trial. Eur J Cancer. 2019;116:137‐147. [DOI] [PubMed] [Google Scholar]
- 70.Hou J, Zhao R, Xia W, et al. PD‐L1‐mediated gasdermin C expression switches apoptosis to pyroptosis in cancer cells and facilitates tumour necrosis. Nat Cell Biol. 2020;22:1264‐1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Blasco MT, Gomis RR. PD‐L1 controls cancer pyroptosis. Nat Cell Biol. 2020;22(10):1157‐1159. [DOI] [PubMed] [Google Scholar]
- 72.Erkes DA, Cai W, Sanchez IM, et al. Mutant BRAF and MEK inhibitors regulate the tumor immune microenvironment via pyroptosis. Cancer Discov. 2020;10:254‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Elion DL, Jacobson ME, Hicks DJ, et al. Therapeutically active RIG‐I agonist induces immunogenic tumor cell killing in breast cancers. Cancer Res. 2018;78:6183‐6195. [DOI] [PubMed] [Google Scholar]
- 74.Samir P, Kesavardhana S, Patmore DM, et al. DDX3X acts as a live‐or‐die checkpoint in stressed cells by regulating NLRP3 inflammasome. Nature. 2019;573:590‐594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chui AJ, Griswold AR, Taabazuing CY, et al. Activation of the CARD8 inflammasome requires a disordered region. Cell Rep. 2020;33:108264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chui AJ, Okondo MC, Rao SD, et al. N‐terminal degradation activates the NLRP1B inflammasome. Science. 2019;364:82‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]


