Abstract
ALK, ROS1, and RET kinase fusions are important predictive biomarkers of tyrosine kinase inhibitors (TKIs) in non‐small‐cell lung cancer (NSCLC). Analysis of cell‐free DNA (cfDNA) provides a noninvasive method to identify gene changes in tumor cells. The present study sought to use cfRNA and cfDNA for identifying fusion genes. A reliable protocol was established to detect fusion genes using cfRNA and assessed the analytical validity and clinical usefulness in 30 samples from 20 cases of fusion‐positive NSCLC. The results of cfRNA‐based assays were compared with tissue biopsy and cfDNA‐based liquid biopsy (Guardant360 plasma next‐generation sequencing [NGS] assay). The overall sensitivity of the cfRNA‐based assay was 26.7% (8/30) and that of cfDNA‐based assay was 16.7% (3/18). When analysis was limited to the samples collected at chemo‐naïve or progressive disease status and available for both assays, the sensitivity of the cfRNA‐based assay was 77.8% (7/9) and that of cfDNA‐based assay was 33.3% (3/9). Fusion gene identification in cfRNA was correlated with treatment response. These results suggest that the proposed cfRNA assay is a useful diagnostic test for patients with insufficient tissues to facilitate effective administration of first‐line treatment and is a useful tool to monitor the progression of NSCLC for consideration of second‐line treatments.
Keywords: cell‐free DNA, cell‐free RNA, fusion gene, liquid biopsy, non‐small‐cell lung cancer
cfRNA‐ and cfDNA‐based assays are evaluated in 20 cases of fusion‐positive NSCLC. cfRNA assay was superior to cfDNA assay for the detection of gene fusions. The results of the cfRNA assay were consistent with the therapeutic effect.
1. INTRODUCTION
The discovery of gene rearrangements involving the receptor tyrosine kinase genes ALK, ROS1, and RET has revolutionized management of the subsets of non‐small‐cell lung cancer (NSCLC) with these changes.1, 2, 3 Tyrosine kinase inhibitors (TKIs) targeting these oncogenic signaling pathways have achieved dramatic and durable responses in patients.4, 5 As TKIs are currently available as first‐line treatments for fusion‐positive advanced NSCLC, reliable detection of these fusions is essential in clinical practice. Fluorescence in situ hybridization (FISH), immunohistochemical (IHC) analysis, and next‐generation sequencing (NGS) are often used to detect fusions as companion diagnoses with the use of TKIs.6, 7
Liquid biopsy involves the analysis of tumor‐derived materials obtained by minimally invasive or noninvasive methods, such as sampling of blood or other body fluids.8, 9, 10 Recently, several analytes have been established for liquid biopsy such as circulating tumor cells (CTCs), cell‐free DNA (cfDNA), and circulating microRNA.10, 11, 12 Liquid biopsy has several potential advantages over tissue biopsy, including better resolution of spatial and temporal intratumoral heterogeneity,13 detection of acquired resistance to targeted therapies, and monitoring of disease progression.14
Many studies have reported the use of liquid biopsy for analysis of cfDNA in lung cancers.15, 16, 17 Detection of the EGFR exon 19 mutations p.L858R and p.T790M with cfDNA has been applied as a companion strategy to monitor the efficacy of EGFR‐related therapies.18, 19, 20, 21 In contrast, although studies have suggested that plasma genotyping is sufficient for detection of ALK or ROS1 fusions and kinase domain mutations with a high degree of concordance with tissue genotyping,22, 23, 24 the feasibility of plasma genotyping of fusion‐positive NSCLC has not been fully evaluated due to the limited number of cases.
Gene fusions, which usually arise from the inter‐chromosomal or intra‐chromosomal ligation of distinct introns, are difficult to detect by DNA sequence capture and NGS, especially when the corresponding introns are large and/or contain repetitive sequences.25, 26 In addition, various breakpoints and fusion partners may pose unique barriers to DNA‐based genotyping. In contrast, RNA sequencing (RNA‐Seq) is not influenced by the complication of large introns and is reportedly superior to DNA sequencing for the identification of ROS1 fusion genes in tissue biopsy samples.27, 28, 29
However, the feasibility and performance of cfRNA for fusion detection remain unclear. Therefore, we developed a cfRNA‐based liquid biopsy protocol that may be superior to conventional liquid biopsy with cfDNA for the evaluation of gene fusions.
2. MATERIALS AND METHODS
2.1. Patients and samples
The study cohort consisted of 20 patients with NSCLC who were treated at the National Cancer Center Hospital and Juntendo University Hospital. Tumor tissue specimens were collected by either surgery or diagnostic biopsy and histologically confirmed as NSCLC. All tumors were positive for the ALK, ROS1, or RET fusion, as determined by IHC analysis, FISH, reverse‐transcription polymerase chain reaction (RT‐PCR), or NGS. Blood samples (10‐15 mL) were collected into blood collection tubes (EDTA‐2K) and assayed within 3 h. The study protocol was approved by the Ethics Committees of the National Cancer Center (approval no. 2015‐202) and Juntendo University Hospital (approval no. 2020017). All patients underwent tumor imaging at baseline and subsequent tumor evaluation every 3‐6 mo until disease progression. Tumor response to treatment was assessed according to the Response Evaluation Criteria in Solid Tumors (version 1.1). Written informed consent was obtained from all patients. In addition, whole blood was collected from healthy participants. All procedures involving human participants were performed in accordance with the ethical standards of the Institutional Research Committee and the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
2.2. cfRNA extraction, cDNA synthesis, multiplex PCR, and sequencing
Total cfRNA was extracted from 4‐5 mL of plasma using the Quick‐cfRNA Serum and Plasma Kit (Zymo Research) and reverse‐transcribed into cDNA using the GenNext RamDA‐seq Single Cell Kit according to the manufacturers’ protocols. The resulting template cDNA in a final volume of 15 µL was subjected to multiplex PCR using Go Taq G2 Green Master Mix (Promega Corporation). The fusion genes were amplified with the primers shown in Table S1. The following primer sets were used to detect TKI‐resistance mutations of ALK and ROS1: ALK set 1: 5′‐GGATTTCCTCATGGAAGCCCTG‐3′/5′‐GTCTCTCGGAGGAAGGACTTG‐3′; ROS1 set 1: 5′‐GTGTATGAAGGAACAGCAGTGGAC‐3′/5′‐GAAGGAGGCACATCTGATGAGC‐3′; and set 2: 5′‐GGAATTCAATCTTCTCCTGGTCTG‐3′/5′‐CATCCGGGCTTTACGCAAATAAG‐3′. The amplified products were subjected to Sanger sequencing with the use of a 3130xl Genetic Analyzer (Thermo Fisher Scientific, Waltham, MA, USA) or NGS with a MiSeq benchtop sequencer (Illumina) using the Reagent Nano Kit V2 (300 cycles) with the 150‐bp paired‐end option. Sanger sequencing was performed on 5 samples (cases 4, 5, 7, 8, and 9). A NGS library was generated using the NEBNext Ultra II DNA Library Prep Kit (New England Biolabs) according to the manufacturer’s instructions. The library quality was assessed using a Qubit 2.0 Fluorometer (Thermo Fisher Scientific) and the Agilent 2200 TapeStation System (Agilent).
2.3. NGS analytical pipeline for fusion detection and RNA mutation call
Reference sequences were constructed from the fusion junction data of 689 previously reported fusions retrieved from the annotation database of the University of California at Santa Cruz (refGene.txt.gz) and the human reference genome (hg38). RNA‐Seq reads for fusion detection were aligned to the created reference sequences using the Burrows‐Wheeler Alignment Tool (BWA‐MEM; http://bio‐bwa.sourceforge.net/). The number of split reads to support the junction points spanning ≥60 mer was counted. RNA‐Seq reads for mutation detection were mapped to hg38 using the Spliced Transcripts Alignment to a Reference (STAR) RNA‐Seq read mapper (https://github.com/alexdobin/STAR/) and Gencode release 31 GTF (https://www.gencodegenes.org/human/release_31.html). Mutations were detected using the samtools “mpileup” function (http://www.htslib.org/) and discarded if (a) the read depth was <50 or the variant allele frequency (VAF) was <0.01, (b) supported by only one strand of the genome, or (c) present in the 1000 Genomes Project dataset (http://www.internationalgenome.org/). Gene mutations were annotated with the SnpEff genomic variant annotations and functional effect prediction toolbox (https://pcingola.github.io/SnpEff/).
2.4. Tissue NGS analysis
Formalin‐fixed paraffin‐embedded samples were subjected to the Oncomine Comprehensive Assay v3 or Oncomine Dx Target Test (Thermo Fisher Scientific). The Oncomine Dx Target Test is a companion diagnostic test approved in Japan to evaluate patient tumor samples for multiple biomarkers associated with targeted therapies for NSCLC.
2.5. RT‐PCR
RT‐PCR to assess the expression of GAPDH was performed using the Power SYBR Green Master Mix (Thermo Fisher Scientific) and a 7500HT Fast Real‐Time PCR System (Thermo Fisher Scientific). Each 20‐μL reaction volume contained 5 μL of cDNA, 0.2 μmol/L primers, and 10 μL of SYBR Green Master Mix. GAPDH was amplified with the primers (sense) 5'‐CTGCCAACGTGTCAGTGGTG‐3' and (antisense) 5'‐GTCGCTGTTGAAGTCAGAGGAG‐3'. Thermal cycling was conducted according to the kit protocol.
2.6. cfDNA analysis
cfDNA samples were examined with the Guardant360 plasma NGS assay (Guardant Health) as previously described.30, 31, 32 In brief, cfDNA was extracted from 2 mL of plasma using the QIAamp Circulating Nucleic Acid Kit (QIAGEN), and 5‐30 ng of the extracted cfDNA were labeled with nonrandom oligonucleotide barcodes for preparation of a sequencing library. The target regions of 74 genes and 90 microsatellite loci were then enriched using the hybrid capture method. The libraries were sequenced by paired synthesis using the NextSeq 500 or HiSeq 2500 Sequencing System (Illumina). Sequencing reads were mapped to the hg19/GRCh37 human reference sequence, and genomic changes were identified using a proprietary analysis pipeline (Guardant Health). The data reported single nucleotide variations, insertions/deletions, and fusions with each VAF, copy number amplification, and microsatellite instability status.
3. RESULTS
3.1. Establishment of cfRNA‐based fusion gene detection assay
The sensitivity of cfRNA‐based fusion gene analysis is dependent on the yield of cfRNA and efficiency of cDNA synthesis.33 To maximize sensitivity, the whole process from cfRNA extraction to NGS analysis was optimized. For library preparation, an amplicon‐based method was used to enrich target fusion points by multiplex PCR with specific primers, which requires less input cDNA. An overview of the proposed cfRNA‐based fusion gene detection assay is presented in Figure 1.
FIGURE 1.
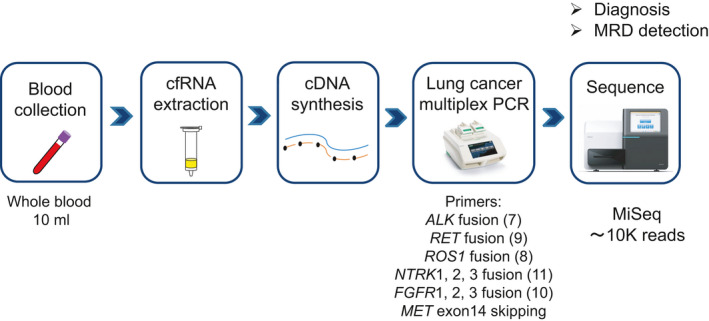
Protocol for cfRNA extraction, cDNA synthesis, multiplex PCR, and sequencing. An overview of the cfRNA‐based assay in this study. Whole blood samples were obtained from NSCLC patients. cfRNA extracted from 4‐5 mL of plasma was converted to cDNA by the RT‐RamDA method and subsequently subjected to PCR with primers for the lung cancer‐related fusion genes (7 ALK fusions, 9 RET fusions, 8 ROS1 fusions, 11 NTRK1/2/3 fusions, and 10 FGFR1/2/3 fusions) and MET exon 14 skipping. An NGS library was generated that contained c. 10 K reads on the MiSeq platform. (Image courtesy of Bio‐Rad Laboratories and Illumina)
Reverse transcription with random displacement amplification (RT‐RamDA) is reported to provide global cDNA amplification directly from RNA during RT.34 First, the amplification efficiency of the RT‐RamDA method was verified with the use of Seraseq Fusion RNA Mix (SeraCare Life Sciences Inc, Milford, MA, USA), a reference standard of 16 clinically relevant fusion transcripts. Serially diluted RNA samples, equivalent to 1‐600 fusion copies, were prepared for cDNA synthesis and subsequently subjected to PCR using primers for the fusion genes EML4‐ALK and KIF5B‐RET, which were successfully detected from a sample containing 3 copies of fusion RNA (Figure 2A).
FIGURE 2.
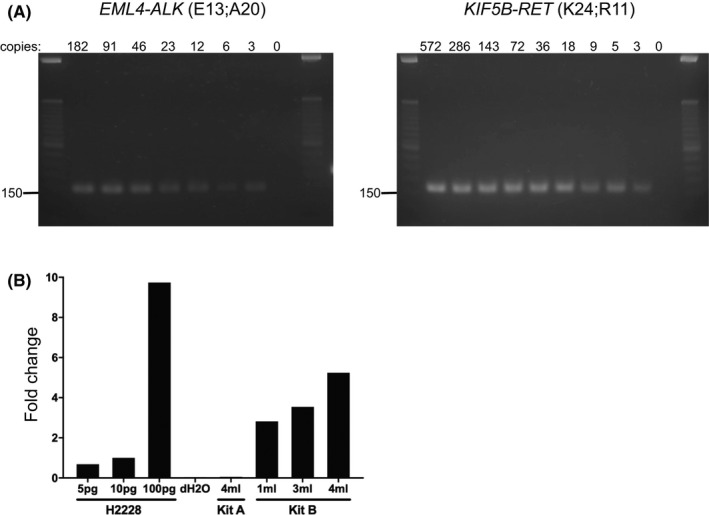
Validation of cDNA synthesis and cfRNA extraction. A, Agarose gel electrophoresis of PCR amplicons of EML4‐ALK and KIF5B‐RET gene‐specific cDNA fragments from a standard RNA Mix. Estimated size bands were detected in all samples with indicated copy numbers of fusion genes. B, Real‐time PCR of GAPDH mRNA expression. Total cfRNA was extracted from plasma using the MagMAX Cell‐Free Total Nucleic Acid Isolation Kit (Kit A) or the Quick‐cfRNA Serum and Plasma Kit (Kit B). As a control, total RNA of H2228 cells was extracted using the RNeasy Mini Kit. cfRNA and RNA of H2288 cells were converted to cDNA using the GenNext RamDA‐seq Single Cell Kit. Real‐time PCR was performed using the Power SYBR Green Master Mix on the 7500HT Fast Real‐Time PCR System. Fold change of GAPDH mRNA expression was calculated by comparison with the value from 10 pg H2228 mRNA
Two commercially available kits for cfRNA extraction were compared: the MagMAX Cell‐Free Total Nucleic Acid Isolation Kit (referred to as Kit A) (Thermo Fisher Scientific) and the Quick‐cfRNA Serum and Plasma Kit (referred to as Kit B) (Zymo Research). Kit A uses magnetic beads for the purification of nucleic acids, while Kit B uses a spin column‐based method. A pilot study was conducted using 4 mL of plasma collected from a healthy volunteer. The cfRNA was extracted with each kit and converted to cDNA using the RT‐RamDA method. GAPDH mRNA expression was quantified by RT‐PCR. Total RNA extracted from human lung carcinoma H2228 cells was prepared as a control. The amount of GAPDH mRNA extracted with Kit B was higher compared with that with Kit A (Figure 2B). The cfRNA extracted from 1‐ml plasma with Kit B was equivalent to 10 pg of H2228 total RNA.
To optimize the protocol for cfRNA extraction and cDNA synthesis, the ability of the assay to detect spiked RNA of fusion genes in cfRNA extracted from plasma was investigated. Approximately 3‐500 copies of EML4‐ALK, CD74‐ROS1, and KIF5B‐RET fusion RNA were spiked into cfRNA extracted from 500 μL of plasma from a healthy volunteer. The samples were converted to cDNA and then amplified by PCR with multiplex primers for detection of the ALK, ROS1, and RET fusions. The PCR products were subjected to NGS to confirm the fusion reads. The number of obtained fusion reads was well correlated with the number of spiked fusion copies (Figure S1). Even less than 10 copies of each fusion spiked as the initial input were detected by NGS analysis, suggesting high sensitivity of the assay for multiplex detection of fusion genes. Similarly, analytical validation was conducted for the TPM3‐NTRK1, ETV6‐NTRK3, FGFR3‐TACC3, and LMNA‐NTRK1 fusions and MET exon 14 skipping (Figure S2).
3.2. Clinical validation of the cfRNA‐based assay for fusion gene detection
Samples were collected from fusion‐positive NSCLC patients to validate the ability of the cfRNA‐based assay to detect fusion genes in the clinical setting. The demographic and clinicopathologic characteristics of the patients enrolled in this study are shown in Table 1. The numbers of males and females were equal and the ALK fusion was the most common (70%). Most patients had clinically advanced stages of disease (stage III, 20%; stage IV, 65%) and had received molecular‐targeted therapies (75%) or cytotoxic chemotherapy (10%) as an initial treatment.
TABLE 1.
Demographic and clinicopathologic characteristics of the 20 patients enrolled in the study
| Feature | No. of patients (N = 20) |
|---|---|
| Median age, y (range) | 68 (29‐86) |
| Sex, N (%) | |
| Male | 10 (50) |
| Female | 10 (50) |
| Smoking status, N (%) | |
| Ever | 11 (55) |
| Never | 9 (45) |
| T‐stage, N (%) | |
| 1 | 4 (20) |
| 2 | 1 (5) |
| 3 | 5 (25) |
| 4 | 10 (50) |
| N‐stage, N (%) | |
| 0 | 1 (5) |
| 1 | 3 (15) |
| 2 | 2 (10) |
| 3 | 14 (70) |
| M‐stage, N (%) | |
| 0 | 5 (25) |
| 1 | 15 (75) |
| Clinical or pathologic stage, N (%) | |
| Ⅰ | 1 (5) |
| Ⅱ | 2 (10) |
| Ⅲ | 4 (20) |
| Ⅳ | 13 (65) |
| Driver genes in tissue, N (%) | |
| ALK | 14 (70) |
| ROS1 | 4 (20) |
| RET | 2 (10) |
| Initial treatment, N (%) | |
| Operation | 3 (15) |
| Chemotherapy | 2 (10) |
| Alectinib | 10 (50) |
| Crizotinib | 3 (15) |
| Selpercatinib | 2 (10) |
Plasma samples were collected from 11 patients (cases 1‐11) who were chemotherapy‐naïve or diagnosed with progressive disease (PD). The sensitivity of the cfRNA‐based assay for detection of fusion genes in this cohort was 72.7% (8/11) (Table 2 and Figure S3). Among the 8 identified fusions, 4 were confirmed as identical to the variants (including the fusion partners) detected by tissue biopsies (cases 1, 4, 5, and 7). The fusion partner genes of the other 4 fusions were not identified by tissue biopsy analyses. The fusion partner genes of 3 patients classified as chemotherapy‐naïve or diagnosed with PD, but negative for fusion genes by cfRNA‐based assay (cases 2, 10, and 11), were also not identified (Table 2). Interestingly, the cfRNA‐based assay failed to detect fusion genes in the samples from patients with disease status of complete response, partial response (PR), and stable disease.
TABLE 2.
Summary of 20 NSCLC cases analyzed for fusion detection by cfRNA‐based assay
| Case # | Sex | Age | Stage | Tissue analysis | Metastasis | Treatment | Treatment at sample collection | Status at sample collection | Liquid fusion detection | Gene alteration cfDNA | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fusion | Method | 1st | 2nd | 3rd | cfRNA | cfDNA | ||||||||
| #1 | F | 30 | IVB | EML4‐ALK | NGS | Bo, Li | Alectinib | Chemo | ‐ | 1st | PD | EML4‐ALK | NA | NA |
| #2 | F | 85 | IVB | ALK | IHC | Bo, Ly | Alectinib | ‐ | ‐ | Pre | Naïve | ND | NA | NA |
| #3 | F | 55 | IVB | ALK | IHC | Bo, Li | Alectinib | Chemo | ‐ | 1st | PD | EML4‐ALK | EML4‐ALK |
TP53 G266E (9.07%) ALK I1171N (0.54%) |
|
TP53 G266E (9.07%) ALK I1171N (0.54%) | ||||||||||||||
| #4 | M | 53 | IVB | EZR‐ROS1 | NGS | Pa, Ly | Crizotinib | Chemo | ‐ | 1st | PD | EZR‐ROS1 | ND | TP53 E204* (0.14%) |
| 2nd | PR | ND | ND | ND | ||||||||||
| #5 | F | 36 | IIIC | CD74‐ROS1 | NGS | Ly | Crizotinib | ‐ | ‐ | Pre | Naïve | CD74‐ROS1 | ND | TP53 L145P (0.3%) |
| 1st | PR | ND | ND | ND | ||||||||||
| #6 | F | 36 | IVB | RET | NGS | Ly | Selpercatinib | ‐ | ‐ | Pre | Naïve | KIF5B‐RET | KIF5B‐RET | ND |
| 1st | PR | ND | ND | ND | ||||||||||
| #7 | M | 57 | IIIA | EML4‐ALK | RT‐PCR | Ly | Operation | Chemo | Alectinib | 3rd | PD | EML4‐ALK | ND | ND |
| #8 | F | 29 | IVB | ALK | NGS | Br | Alectinib | ‐ | ‐ | Pre | Naïve | EML4‐ALK | ND |
BRCA1 S1212P (0.56%) FBXW7 T536R (0.12%) |
| #9 | F | 70 | IVA | RET | NGS | Pl | Selpercatinib | ‐ | ‐ | Pre | Naïve | KIF5B‐RET | KIF5B‐RET | ND |
| #10 | M | 49 | IVA | ALK | NGS | Pl | Alectinib | ‐ | ‐ | Pre | Naïve | ND | ND | ND |
| #11 | M | 44 | IV | ALK | IHC | Br, Bo, Ly | Alectinib | Chemo | Lorlatinib | 3rd | PD | ND | ND | ND |
| #12 | F | 65 | IIB | EML4‐ALK | RT‐PCR | Ly | Operation | Alectinib | ‐ | 2nd | PR | ND | ND | ND |
| #13 | M | 61 | IIA | ALK | IHC | Ly | Operation | Alectinib | ‐ | 2nd | PR | ND | ND | AR G300A (1.89%) |
| #14 | F | 66 | IIIB | ALK | IHC | Ly | Chemo | Alectinib | ‐ | 2nd | CR | ND | NA | NA |
| 2nd | CR | ND | ND | TP53 V274A (0.18%) | ||||||||||
| #15 | M | 64 | IV | ALK | IHC | Br | Alectinib | Chemo | Lorlatinib | 3rd | PR | ND | NA | NA |
| 3rd | PR | ND | NA | NA | ||||||||||
| #16 | M | 58 | IVB | ALK | FISH | Br, Bo | Alectinib | Pembro | Lorlatinib | 3rd | SD | ND | NA | NA |
| 3rd | SD | ND | NA | NA | ||||||||||
| #17 | M | 78 | IV | ALK | FISH | Pl | Alectinib | Lorlatinib | Chemo | 3rd | PR | ND | NA | NA |
| 3rd | PR | ND | NA | NA | ||||||||||
| #18 | M | 63 | IVB | ALK | IHC | Br, Bo | Alectinib | ‐ | ‐ | 1st | PR | ND | NA | NA |
| 1st | PR | ND | ND | ND | ||||||||||
| #19 | F | 86 | IV | EZR‐ROS1 | RT‐PCR | Pl | Chemo | Pembro | Crizotinib | 3rd | PR | ND | NA | NA |
| 3rd | PR | ND | ND | ND | ||||||||||
| #20 | F | 84 | IVA | CD74‐ROS1 | RT‐PCR | Pl | Crizotinib | ‐ | ‐ | 1st | PR | ND | NA | NA |
| 1st | PR | ND | ND |
TP53 R213P (0.14%) RAF1 S26N (0.11%) ERBB2 (CN = 2.19) |
||||||||||
Variant allele frequencies detected with the cfDNA‐based assay are shown in parentheses. *, stop codon.
Abbreviations: Bo, bone; Br, brain; Chemo, cytotoxic chemotherapy; CN, copy number; FISH, fluorescence in situ hybridization; IHC, immunohistochemical; Li, liver; Ly, lymph node; NA, not analyzed; ND, not detected; NGS, next‐generation sequencing; Pa, pancreas; PD, progressive disease; Pl, pleura; PR, partial response; RT‐PCR, reverse‐transcription PCR; SD, stable disease.
In total, 18 cfDNA samples collected from 15 cases were subjected to the Guardant360 plasma NGS assay, a cfDNA‐based liquid biopsy approved by the Food and Drug Administration for all advanced solid tumors. The sensitivity of the cfRNA‐ and cfDNA‐based assays was compared with the use of 9 plasma samples collected from 9 patients who were chemotherapy‐naïve or diagnosed with PD (cases 3‐11) (Figure 3). The same plasma samples were used for both assays. Of the 9 plasma samples, fusions were detected in 7 (78%) with the cfRNA‐based assay, but only 3 (33%) with the cfDNA‐based assay. The sensitivity of ROS1 fusion detection was 100% (2/2) and 0% (0/2) with the cfRNA‐ and cfDNA‐based assay, respectively. All fusions identified with the cfDNA‐based assay were also identified with the cfRNA‐based assay.
FIGURE 3.
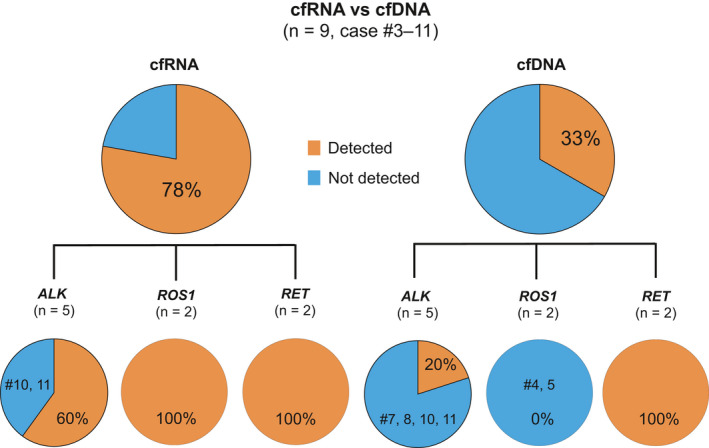
Fusion detection sensitivities of the cfRNA‐based and cfDNA‐based assays for patients who were untreated or with PD. The sensitivities of the cfRNA‐based and cfDNA‐based assays were compared with the use of 9 plasma samples collected from 9 patients who were chemotherapy‐naïve or diagnosed with PD (cases 3‐11). The same plasma was used for both assays
Liquid biopsy was performed at multiple time points in 10 cases (Figures 4 and S4). In case 4, EZR‐ROS1 was detected only by the cfRNA‐based assay, after the patient had advanced to PD following first‐line treatment with crizotinib (Figure 4A). After 3 mo of second‐line chemotherapy when the patient was diagnosed as PR, the cfRNA‐based assay failed to detect the EZR‐ROS1 fusion. Similarly, in cases 5 and 6, the fusions detected before treatment initiation were negative after 3 mo of treatment when the patients achieved PR (Figure 4B, C).
FIGURE 4.
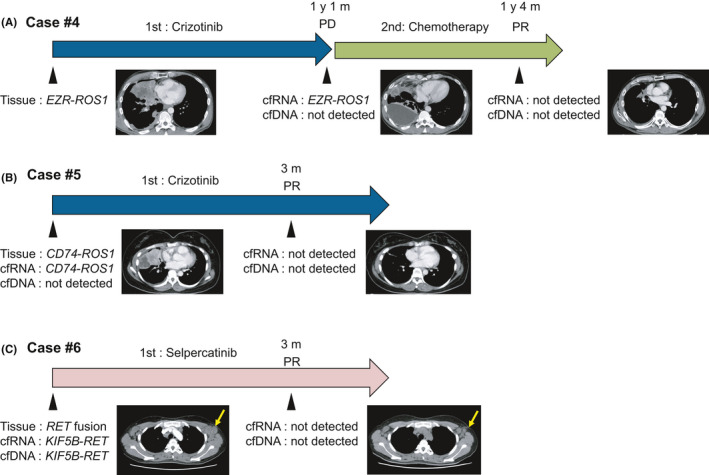
Representative cases of liquid biopsy at multiple time points. The cfRNA‐based assay was performed during treatment to monitor disease status. The arrows indicate the treatment courses and the arrowheads indicate the timings of sample collection and computed tomography scanning. (A), EZR‐ROS1 fusion was detected in cfRNA but not cfDNA when the patient exhibited PD to first‐line treatment with crizotinib. After 3 mo of second‐line chemotherapy, when the patient exhibited PR, the EZR‐ROS1 fusion gene was not detected with the RNA‐based assay. (B), The CD74‐ROS1 fusion was detected with the cfRNA‐based assay before treatment initiation and then was negative after 3 mo of treatment when the patients exhibited PR. (C), The KIF5B‐RET fusion was detected by both cfRNA‐based and cfDNA‐based assays before treatment initiation and switching to negative after 3 mo of treatment when the patients exhibited PR. The yellow arrows indicate tumor shrinkage
3.3. Analytical characterization of cfRNA and cfDNA
The values of RNA integrated number and DV200, cfRNA and cfDNA yield are summarized in Table S2. The relationship between the status of metastatic disease and the cfRNA yield was investigated. No significant difference in cfRNA yield was observed between samples collected chemotherapy‐naïve or PD status (9 samples) and those of CR, PR and stable disease (SD) status (14 samples, Wilcoxon rank‐sum test P = .34; Figure S5).
The correlation between the yield of cfDNA and cfRNA was investigated in 10 cases. There was no positive correlation between the yields of cfDNA and cfRNA (r = −0.58; Figure S6).
3.4. Evaluation of resistance mutations
Several studies have reported the use of cfDNA‐based assays to identify TKI‐resistance mutations within ALK or ROS1 fusions.15, 24 So, the ability of the cfRNA‐based assay to detect such resistance mutations was evaluated. Plasma samples were collected from 3 patients (cases 3, 4, and 7) upon resistance to crizotinib or alectinib for analysis. Multiplex PCR was performed with cfRNA to account for known resistance mutations in the tyrosine kinase domains of ALK and ROS1. Subsequently, the PCR products were subjected to deep NGS. As a result, the cfRNA‐based assay detected no resistance mutations (Figure S7). Although the ALK p.I1171T and ROS1 p.S1986F mutations were present in cases 3 and 4 at VAFs of 0.21 and 0.14%, respectively, both were likely to be classified as sequence errors because each was also found at a VAF of 0.20% in the H2228 cells, which are known to be negative for these mutations. In contrast, the Gurardant360 assay successfully detected the ALK p.I1171N mutation at a VAF of 0.54% in case 3 (Table 2). In addition, the results of the Guardant360 plasma NGS assay of 18 cfDNA samples from 13 cases are summarized in Table 2. Genomic changes were identified in 9 (50%) of 18 samples, including TP53 mutations, which were detected in multiple cases (cases 3, 4, 5, 14, and 20).
4. DISCUSSION
To the best of our knowledge, this is the first report to compare cfDNA‐based and cfRNA‐based assays for the detection of fusion genes in NSCLC. The key advantages of the proposed protocol are highly efficient isolation of cfRNA from plasma and subsequent cDNA conversion.
RT‐RamDA with “not so random” primers (NSRs) improves sensitivity and reproducibility by eliminating the necessity of PCR amplification, which often results in amplification bias of other conventional methods.34 NSRs contribute to high efficiency of capturing poly(A) and non‐poly(A) RNA with multiple primers. Taking advantage of this cutting‐edge technology, the proposed cfRNA‐based assay is able to detect as low as a few copies of fusion transcripts in plasma.
Although cfDNA has emerged as the favored nucleic acid for analysis of liquid biopsy samples, in some situations this marker fails to provide necessary information. In such cases, cfRNA might help fill this void, which is particularly pertinent for NTRK fusions and translocations. DNA‐based NGS fails to capture NTRK fusions with breakpoints that involve long intron sequences, as these extended DNA stretches cannot be covered by hybridization‐capture probes. However, this issue is avoided when analyzing fusion events using RNA.
MSK‐IMPACT DNA sequencing analysis of driver‐negative lung cancer was further refined with a custom RNA‐Seq panel (MSK‐Fusion) specifically focused on select RNA.27 Of 254 samples that lacked driver mutations by DNA testing, 14% were subsequently found by RNA testing to harbor fusion changes, with the great majority matched to targeted therapy.
Compared with the Guardant360 plasma NGS assay, the proposed cfRNA‐based assay can sensitively detect ALK and ROS1 fusion genes. Considering the technical difficulty of the cfDNA‐based assay to capture ROS1 gene rearrangements, which occur in ROS1 gene introns with many repetitive sequences, it was reasonable to suggest that the cfRNA‐based assay is superior to the Guardant360 plasma NGS assay for detection of ROS1 fusions.28 However, it was surprising that even the ALK fusion was more sensitively detected with the cfRNA‐based assay.
Of the 11 cases classified as chemotherapy‐naïve or PD, the cfRNA‐based assay failed to detect fusion genes in 3. Case 10 had carcinomatous pleurisy but no metastasis to distant organs. Therefore, relatively small amounts of ctDNA were shed into the bloodstream. The tumors of the other 2 cases had metastasized to distant organs at the time of blood collection. Case 2 had a chemotherapy‐naïve primary tumor that had metastasized to the bone and lymph node. In case 11, the blood sample was collected following metastasis to the brain during third‐line treatment with lorlatinib. Considering that cases 2 and 11 were also negative by the Guardant360 plasma NGS assay, tissue biopsy is recommended if no driver mutation is identified by liquid biopsy.
Theoretically, the proposed protocol may be applicable for the isolation of any type of fusion gene. The cfRNA genotyping system, therefore, can potentially help with an initial diagnosis in patients with insufficient tissue specimens for tumor genotyping, which can facilitate the administration of effective first‐line therapy.
Moreover, cfRNA‐based assays may be applicable for the identification of fusion genes acquired during TKI treatments. Tyrosine kinase and BRAF fusions are reportedly rare events that mediate resistance to TKIs in patients with advanced EGFR‐mutated NSCLC.35 So far, ALK, ROS1, RET, BRAF, FGFR3, and NTRK1 have been identified as fusions that are potentially responsible for acquired resistance to first‐line or second‐line treatment with osimertinib.36, 37, 38 Notably, in some cases, combination therapies of osimertinib and fusion‐targeting TKIs were effective at overcoming resistance in the presence of fusions.39, 40 Recently, ST7‐MET rearrangements were identified as potential mechanisms of acquired resistance to ALK TKIs. Considering that repeated biopsy of a tumor at the same site may not reflect intratumoral heterogeneity, cfRNA‐based fusion detection should be considered to evaluate fusion genes in patients with PD.
For cases in which blood was collected at 2 points (before and after treatment), the detection of cfRNA was consistent with the therapeutic effect, suggesting that cfRNA might also be a useful predictor of metastases and the treatment response of NSCLC.
This study has some limitations. First, the sample size was small due to the rarity of fusions among patients with NSCLC. However, the main scope of this study is to develop a new application for liquid biopsy and provide novel information about cfRNA focusing analytical and clinical validity of the assay. Therefore, future studies with larger number of cases will be needed to clarify the clinical utility of the assay, including studies to compare the clinical utility of cfRNA‐based vs. cfDNA‐based assays such as Gurdatnt 360 and FoundationOne liquid. Especially, as this study did not include fusion negative cases, the specificities of the assays were not evaluated. Second, there is still room to improve the sensitivity of the proposed cfRNA‐based assays for detection of fusion genes. A possible reason for the less than optimal sensitivity may be due to the quality and quantity of the assayed cfRNA. Cryopreserved plasma was used in this study and the freeze‐thaw event may have affected the cfRNA quality. The cfRNA yield could be further improved by attempting other nucleic extraction methods that minimize cfRNA decay. Third, the sensitivity of cfRNA‐based assays for detection of single nucleotide variations or insertions/deletions is not as high as that of cfDNA‐based assays. The use of unique molecular identifiers in library construction may improve the limit of detection of mutational analysis with cfRNA‐based assays. Fourth, the current assay does not have high quantitation. One applicable way to obtain quantitative data in amplicon‐based NGS analysis is the compensation of fusion read number using the expression of housekeeping genes. Finally, although this method was highly sensitive for detection of known fusions, novel fusions theoretically cannot be detected, which is a major trade‐off with the use of amplicon‐based sequencing.
Overall, this study highlights new applications for liquid biopsy and novel information about RNA shed from tumor cells. The accuracy of the cfRNA‐based assay for fusion gene detection was markedly improved. The evaluation of cfRNA by liquid biopsy can be useful for diagnostic, treatment prediction, and even prognostic purposes. Future laboratory testing is needed to apply this protocol for molecular profiling and monitoring recurrence and therapeutic effects, which will increase opportunities for treatment and eventually improve patient prognosis.
DISCLOSURE
The authors certify that no actual or potential conflict of interest in relation to this article exists.
Supporting information
Fig S1‐S7
Table S1‐S2
ACKNOWLEDGMENT
The authors would like to thank S. Kaneko and A. Maruyama for technical assistance. This study was financially supported in part through grants from the Practical Research for Innovative Cancer Control (MK received grant no. JP20ck0106536) and the Project for Cancer Research and Therapeutic Evolution (SK received grant no. JP20cm0106502) from the Japan Agency for Medical Research and Development. This work was also supported in part by The National Cancer Center Research and Development Fund (SK received grant no. 31‐A‐1).
Hasegawa N, Kohsaka S, Kurokawa K, et al. Highly sensitive fusion detection using plasma cell‐free RNA in non‐small‐cell lung cancers. Cancer Sci. 2021;112:4393–4403. 10.1111/cas.15084
Funding information
Japan Agency for Medical Research and Development, (Grant/Award Number: JP20ck0106536, JP20cm0106502), The National Cancer Center Research and Development Fund (Grant/Award Number: 31‐A‐1).
Contributor Information
Shinji Kohsaka, Email: skohsaka@ncc.go.jp.
Hiroyuki Mano, Email: hmano@ncc.go.jp.
REFERENCES
- 1.Lin JJ, Shaw AT. Recent advances in targeting ROS1 in lung cancer. J Thorac Oncol. 2017;12:1611‐1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Camidge DR, Bang YJ, Kwak EL, et al. Activity and safety of crizotinib in patients with ALK‐positive non‐small‐cell lung cancer: updated results from a phase 1 study. Lancet Oncol. 2012;13:1011‐1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drilon A, Rekhtman N, Arcila M, et al. Cabozantinib in patients with advanced RET‐rearranged non‐small‐cell lung cancer: an open‐label, single‐centre, phase 2, single‐arm trial. Lancet Oncol. 2016;17:1653‐1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drilon A, Oxnard GR, Tan DSW, et al. Efficacy of selpercatinib in RET fusion‐positive non‐small‐cell lung cancer. N Engl J Med. 2020;383:813‐824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peters S, Camidge DR, Shaw AT, et al. Alectinib versus crizotinib in untreated ALK‐positive non‐small‐cell lung cancer. N Engl J Med. 2017;377:829‐838. [DOI] [PubMed] [Google Scholar]
- 6.Teixidó C, Karachaliou N, Peg V, Gimenez‐Capitan A, Rosell R. Concordance of IHC, FISH and RT‐PCR for EML4‐ALK rearrangements. Transl Lung Cancer Res. 2014;3:70‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Letovanec I, Finn S, Zygoura P, et al. Evaluation of NGS and RT‐PCR Methods for ALK Rearrangement in European NSCLC Patients: Results from the European Thoracic Oncology Platform Lungscape Project. J Thorac Oncol. 2018;13:413‐425. [DOI] [PubMed] [Google Scholar]
- 8.Wan JCM, Massie C, Garcia‐Corbacho J, et al. Liquid biopsies come of age: towards implementation of circulating tumour DNA. Nat Rev Cancer. 2017;17:223‐238. [DOI] [PubMed] [Google Scholar]
- 9.Miller AM, Shah RH, Pentsova EI, et al. Tracking tumour evolution in glioma through liquid biopsies of cerebrospinal fluid. Nature. 2019;565:654‐658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y, Springer S, Mulvey CL, et al. Detection of somatic mutations and HPV in the saliva and plasma of patients with head and neck squamous cell carcinomas. Sci Transl Med. 2015;7:293ra104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haber DA, Velculescu VE. Blood‐based analyses of cancer: circulating tumor cells and circulating tumor DNA. Cancer Discov. 2014;4:650‐661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng G. Circulating miRNAs: roles in cancer diagnosis, prognosis and therapy. Adv Drug Deliv Rev. 2015;81:75‐93. [DOI] [PubMed] [Google Scholar]
- 13.Guibert N, Pradines A, Favre G, Mazieres J. Current and future applications of liquid biopsy in nonsmall cell lung cancer from early to advanced stages. Eur Respir Rev. 2020;29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pasini L, Ulivi P. Liquid Biopsy for the Detection of Resistance Mechanisms in NSCLC: Comparison of Different Blood Biomarkers. J Clin Med. 2019;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dagogo‐Jack I, Rooney M, Lin JJ, et al. Treatment with next‐generation ALK inhibitors fuels plasma ALK mutation diversity. Clin Cancer Res. 2019;25:6662‐6670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zugazagoitia J, Gómez‐Rueda A, Jantus‐Lewintre E, et al. Clinical utility of plasma‐based digital next‐generation sequencing in oncogene‐driven non‐small‐cell lung cancer patients with tyrosine kinase inhibitor resistance. Lung Cancer. 2019;134:72‐78. [DOI] [PubMed] [Google Scholar]
- 17.Leighl NB, Page RD, Raymond VM, et al. Clinical Utility of Comprehensive Cell‐free DNA Analysis to Identify Genomic Biomarkers in Patients with Newly Diagnosed Metastatic Non‐small Cell Lung Cancer. Clin Cancer Res. 2019;25:4691‐4700. [DOI] [PubMed] [Google Scholar]
- 18.Gray JE, Okamoto I, Sriuranpong V, et al. Tissue and Plasma EGFR Mutation Analysis in the FLAURA Trial: Osimertinib versus Comparator EGFR Tyrosine Kinase Inhibitor as First‐Line Treatment in Patients with EGFR‐Mutated Advanced Non‐Small Cell Lung Cancer. Clin Cancer Res. 2019;25:6644‐6652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jenkins S, Yang JC, Ramalingam SS, et al. Plasma ctDNA analysis for detection of the EGFR T790M mutation in patients with advanced non‐small cell lung cancer. J Thorac Oncol. 2017;12:1061‐1070. [DOI] [PubMed] [Google Scholar]
- 20.Iwama E, Sakai K, Azuma K, et al. Monitoring of somatic mutations in circulating cell‐free DNA by digital PCR and next‐generation sequencing during afatinib treatment in patients with lung adenocarcinoma positive for EGFR activating mutations. Ann Oncol. 2017;28:136‐141. [DOI] [PubMed] [Google Scholar]
- 21.Oxnard GR, Thress KS, Alden RS, et al. Association between plasma genotyping and outcomes of treatment with osimertinib (AZD9291) in advanced non‐small‐cell lung cancer. J Clin Oncol. 2016;34:3375‐3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dagogo‐Jack I, Brannon AR, Ferris LA, et al. Tracking the Evolution of Resistance to ALK Tyrosine Kinase Inhibitors through Longitudinal Analysis of Circulating Tumor DNA. JCO Prec Oncol. 2018;(2):1‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCoach CE, Blakely CM, Banks KC, et al. Clinical utility of cell‐free DNA for the detection of ALK fusions and genomic mechanisms of ALK inhibitor resistance in non‐small cell lung cancer. Clin Cancer Res. 2018;24:2758‐2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dagogo‐Jack I, Rooney M, Nagy RJ, et al. Molecular analysis of plasma from patients with ROS1‐positive NSCLC. J Thorac Oncol. 2019;14:816‐824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wong D, Yip S, Sorensen PH. Methods for Identifying Patients with Tropomyosin Receptor Kinase (TRK) Fusion Cancer. Pathol Oncol Res. 2020;26:1385‐1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Treangen TJ, Salzberg SL. Repetitive DNA and next‐generation sequencing: computational challenges and solutions. Nat Rev Genet. 2011;13:36‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Benayed R, Offin M, Mullaney K, et al. High yield of RNA sequencing for targetable kinase fusions in lung adenocarcinomas with no mitogenic driver alteration detected by DNA sequencing and low tumor mutation burden. Clin Cancer Res. 2019;25:4712‐4722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davies KD, Le AT, Sheren J, et al. Comparison of molecular testing modalities for detection of ROS1 rearrangements in a cohort of positive patient samples. J Thorac Oncol. 2018;13:1474‐1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kohsaka S, Tatsuno K, Ueno T, et al. Comprehensive assay for the molecular profiling of cancer by target enrichment from formalin‐fixed paraffin‐embedded specimens. Cancer Sci. 2019;110:1464‐1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lanman RB, Mortimer SA, Zill OA, et al. Analytical and clinical validation of a digital sequencing panel for quantitative, highly accurate evaluation of cell‐free circulating tumor DNA. PLoS One. 2015;10:e0140712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Odegaard JI, Vincent JJ, Mortimer S, et al. Validation of a plasma‐based comprehensive cancer genotyping assay utilizing orthogonal tissue‐ and plasma‐based methodologies. Clin Cancer Res. 2018;24:3539‐3549. [DOI] [PubMed] [Google Scholar]
- 32.Willis J, Lefterova MI, Artyomenko A, et al. Validation of microsatellite instability detection using a comprehensive plasma‐based genotyping panel. Clin Cancer Res. 2019;25:7035‐7045. [DOI] [PubMed] [Google Scholar]
- 33.Beck TN, Boumber YA, Aggarwal C, et al. Circulating tumor cell and cell‐free RNA capture and expression analysis identify platelet‐associated genes in metastatic lung cancer. BMC Cancer. 2019;19:603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hayashi T, Ozaki H, Sasagawa Y, Umeda M, Danno H, Nikaido I. Single‐cell full‐length total RNA sequencing uncovers dynamics of recursive splicing and enhancer RNAs. Nat Commun. 2018;9:619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schrock AB, Zhu VW, Hsieh WS, et al. Receptor tyrosine kinase fusions and BRAF kinase fusions are rare but actionable resistance mechanisms to EGFR tyrosine kinase inhibitors. J Thorac Oncol. 2018;13:1312‐1323. [DOI] [PubMed] [Google Scholar]
- 36.Oxnard GR, Hu Y, Mileham KF, et al. Assessment of resistance mechanisms and clinical implications in patients with EGFR T790M‐positive lung cancer and acquired resistance to osimertinib. JAMA Oncol. 2018;4:1527‐1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Le X, Puri S, Negrao MV, et al. Landscape of EGFR‐dependent and ‐independent resistance mechanisms to osimertinib and continuation therapy beyond progression in EGFR‐mutant NSCLC. Clin Cancer Res. 2018;24:6195‐6203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Offin M, Somwar R, Rekhtman N, et al. Acquired ALK and RET gene fusions as mechanisms of resistance to osimertinib in EGFR‐Mutant Lung Cancers. JCO Prec Oncol. 2018;2:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Piotrowska Z, Isozaki H, Lennerz JK, et al. Landscape of acquired resistance to osimertinib in EGFR‐Mutant NSCLC and clinical validation of combined EGFR and RET inhibition with osimertinib and BLU‐667 for acquired RET Fusion. Cancer Discov. 2018;8:1529‐1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zeng L, Yang N, Zhang Y. GOPC‐ROS1 rearrangement as an acquired resistance mechanism to osimertinib and responding to crizotinib combined treatments in lung adenocarcinoma. J Thorac Oncol. 2018;13:e114‐e116. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1‐S7
Table S1‐S2


