Abstract
Cancer‐related microRNAs (miRNAs) are emerging as promising and noninvasive biomarkers for colorectal cancer (CRC). This study aimed to investigate the usefulness of postoperative changes in plasma miR21‐5p levels for recurrence and progressive disease (PD) after surgical resection. This study was a prospective study of 103 CRC patients who underwent surgical resection. Self‐paired plasma samples collected pre‐operation (Pre), 7 days post‐operation (POD7), 1 month post‐operation (POM1), and 6 months post‐operation (POM6) were analyzed. The miRNA levels were evaluated by quantitative reverse transcription PCR. Among the enrolled patients, ten cases (9.7%) of postoperative recurrence and six cases (5.8%) of postoperative PD occurred at POM6. In the recurrence and PD group, plasma miR21‐5p levels significantly increased (POM1: P < .01, POM6: P < .01, respectively). The area under the curve (AUC) value for postoperative changes in plasma miR21‐5p levels at POM1 and POM6 to discriminate recurrence and PD were 0.675 and 0.715, respectively. Combined analysis with postoperative carcinoembryonic antigen (CEA) level in discriminating recurrence and PD increased AUC values (POM1: 0.715 and POM6: 0.789). Furthermore, multivariate analysis for recurrence and PD after surgical resection showed that postoperative changes in the plasma miR21‐5p level at POM1 and POM6 were independent prognostic factors (POM1: P = .03, POM6: P < .01). The postoperative changes in plasma miR21‐5p level could be a useful noninvasive biomarker for monitoring and predicting recurrence and PD after surgical resection of CRC patients. Furthermore, plasma miR21‐5p can predict recurrence and PD after surgical resection.
Keywords: biomarker, colorectal cancer, microRNA, miR21‐5p, progressive disease, recurrence
Postoperative changes in plasma miR21‐5p level could be a useful noninvasive biomarker for monitoring and predicting recurrence and progressive disease after surgical resection of CRC patients.

Abbreviations
- Akt
protein kinase B
- Alb
albumin
- ALT
alanine aminotransferase
- APTT
activated partial thromboplastin time
- AST
aspartate aminotransferase
- AUC
area under the curve
- BMI
body mass index
- BUN
blood urea nitrogen
- CA19‐9
carbohydrate antigen 19‐9
- CEA
carcinoembryonic antigen
- CI
confidence interval
- CRC
colorectal cancer
- Cre
creatinine
- CRP
c‐reactive protein
- CT
computed tomography
- Ct
threshold cycle
- EDTA
ethylenediamine tetra‐acetic acid
- JSCCR
Japanese society of cancer of the colon and rectum
- Ly
lymphatic invasion
- miRNA
microRNA
- mRNA
messenger RNA
- MTAP
methylthioadenosine phosphorylase
- OR
odds ratio
- PD
progressive disease
- PDCD4
programmed cell death 4
- PI3K
phosphoinoside‐3 kinase
- Plt
platelet
- POD7
7 days post‐operation
- POM1
1 month post‐operation
- POM6
6 months post‐operation
- Pre
pre‐operation
- PT
prothrombin time
- PTEN
phosphatase and tensine homolog
- RBC
red blood cell
- ROC
receive operating characteristic
- RT‐qPCR
reverse transcription‐ quantitative polymerase chain reaction
- TGF‐β
Transforming Growth Factor‐β
- TMEM49
transmembrane protein 49
- V
venous invasion
- WBC
white blood cell
1. INTRODUCTION
Colorectal cancer (CRC) is the third most common cancer and the second leading cause of cancer‐related death in both males and females globally.1 However, the prognosis of CRC has improved with the development of multidrug chemotherapy, including molecular targeting drugs. With the advent of immune checkpoint inhibitors, further improvement in prognosis is expected in the future. Therefore, to reduce the mortality associated with CRC, it is important to modify the treatment regimen effectively according to the state of the disease. Thus, there is an urgent need to develop promising noninvasive biomarkers that can diagnose recurrence and progressive disease (PD) as easily as possible.
Recently, potential candidate biomarkers such as cancer‐related microRNAs (miRNAs) are being explored via liquid biopsy. MiRNAs are short RNAs (20‐24 nucleotides) involved in the post‐transcriptional regulation of gene expression in multicellular organisms by affecting both the stability and translation of mRNAs.2, 3, 4 MiRNAs target protein‐coding mRNAs at the post‐transcriptional level via direct cleavage of mRNAs or inhibition of protein synthesis. MiRNA biogenesis is tightly controlled, and their dysregulation is known to be associated with carcinogenesis. In addition, miRNAs function as signaling molecules, influencing the behavior of recipient cells. Recent studies have identified miRNAs in tissue and plasma, and their importance as minimally invasive, liquid biopsy‐based biomarkers for the early detection of cancer has been reported.5, 6, 7 These results are important as they show the potential of the miRNA level to reflect tumor characteristics and status in the body. However, almost all past studies have been retrospective, and the dynamic changes in circulating miRNAs at different time points during follow‐up using self‐paired plasma samples have never been explored. Thus, it is unclear how miRNAs can be used in actual clinical situations, excluding early cancer detection. In our previous prospective study,8 we showed that cancer‐related miRNA expression levels in tissue and plasma reflected clinicopathological features of CRC, and that the plasma expression level significantly changed after surgical resection. These results suggest that cancer‐related miRNAs, especially miR21‐5p, may have the potential to be a predictive biomarker for treatment efficacy.
In this prospective study, we aimed to analyze the time course of self‐paired plasma samples from enrolled CRC patients who underwent surgical resection to clarify (a) the difference in plasma miR21‐5p levels between recurrence and PD group and non‐recurrence group, (b) the potential of postoperative changes in plasma miR21‐5p level as a diagnostic biomarker and a prognostic factor for recurrence and PD, and (c) the diagnostic value of miR21‐5p compared to current tumor markers.
2. MATERIALS AND METHODS
2.1. Study population
Hundred and three patients with CRC who underwent surgical resection of primary colorectal tumors were prospectively enrolled as study subjects at the Gifu University Hospital between July 2019 and March 2020.
The enrolled patients in this study met all the following criteria: (a) no previous history of cancer‐related disease, (b) no diagnosis as synchronous double cancer, (c) diagnosed with CRC pathologically following surgery, (d) no clinical diagnosis of familial adenomatous polyposis or hereditary nonpolyposis, and (e) no clinical diagnosis of inflammatory bowel disease.
The present study was conducted in accordance with the World Medical Association Declaration of Helsinki and was approved by the Ethics Committee of Gifu University (approval number: 2019‐074). As this study was a prospective study and included potentially identifiable patient data, informed consent was obtained from the enrolled patients.
2.2. Treatment and evaluation after surgical resection
In this study, as a general rule, stage III and high‐risk stage II cases were indicated for postoperative adjuvant chemotherapy for 6 months based on the Japanese Society for Cancer of the Colon and Rectum guidelines for the treatment of CRC.9 High‐risk stage II cases were defined as (a) pathological T4 stage, (b) the initial symptom was intestinal perforation or obstruction, (c) the number of harvested lymph nodes was less than 12, (d) histologically highly venous or lymphatic invasion positive, and (e) poorly differentiated adenocarcinoma.
Recurrence after surgery and response to systemic chemotherapy were evaluated by contrast‐enhanced computed tomography (CT) on postoperative 6months according to Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST ver 1.1).10 The therapeutic response criteria were classified into four groups: Complete response (CR), partial response (PR), stable disease (SD), and PD. In this study, cases without recurrence, PR, and SD cases were classified into non‐recurrence group, and cases with recurrence and PD cases were classified into recurrence and PD group (Figure 1).
FIGURE 1.
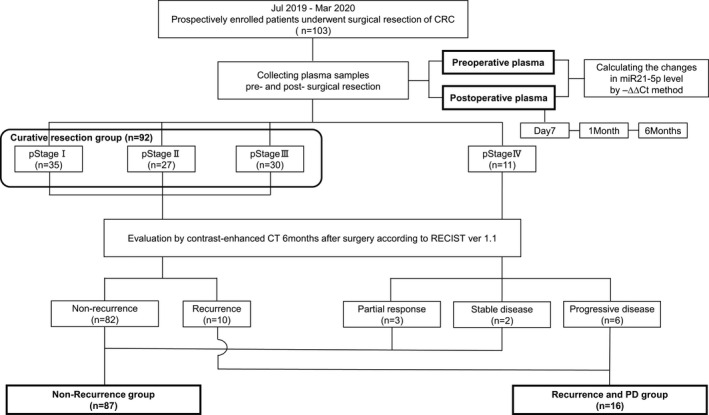
Patients and study flow chart
2.3. Sample processing
Peripheral blood samples for miRNA measurement were collected from each patient pre‐operation (Pre), 7 days post‐operation (POD7), 1 month post‐operation (POM1), and 6 months post‐operation (POM6). Peripheral blood (4 mL) was added to an EDTA‐treated anticoagulant tube, and plasma was isolated by centrifugation at 1500 g for 15 minutes at 4°C immediately after the blood collection. The plasma was carefully moved to a new 2 mL microfuge tube and stored at −80°C until measurement of miRNA levels could be performed. To minimize RNA degradation, we only used samples that were freeze‐thawed once.
2.4. Extraction of miRNA from plasma samples
MiRNAs from the frozen plasma were extracted using the NucleoSpin miRNA Plasma kit (MACHEREY‐NAGEL GmbH, Düren, Germany) according to the standard protocol. Frozen plasma samples (300 µL) were thawed and centrifuged for 3 minutes at 11 000×g to remove residual cell debris. Proteins in the supernatant were precipitated using a reagent in the kit and removed by centrifugation. After adjustment of the binding conditions with isopropanol, miRNAs were bound to a miRNA collection column. The miRNA was then eluted into 30 µL of RNase‐free water. The yield of circulating miRNA from plasma is usually very small, and a micro‐volume spectrophotometer can hardly detect them, unlike total miRNA from tissues. The miRNA quality was assessed by measuring circulating miR16‐5p levels. MiR16‐5p is abundantly and stably found in plasma, similar to ribosomal RNA in cellular RNAs; thus, its levels reflect miRNA degradation and the quality of the plasma samples. The purified miRNA was immediately used for reverse transcription to prevent degradation of RNA before the PCR step.
2.5. Quantitative reverse transcription PCR
A miRNA quantitative reverse transcription PCR (qRT‐PCR) was performed to measure the miRNA levels. Total RNA was reverse‐transcribed using the TaqMan Advanced miRNA cDNA Synthesis Kit (Applied Biosystems, Waltham, MA, USA).QRT‐PCR was performed using Thermal Cycler Dice Real Time System II (TaKaRa, Shiga, Japan) according to the manufacturer's protocol. Considering the effect of qPCR efficiency on measured values, we always conducted qPCR for self‐paired plasma samples from the same patient all at once using the same plate.
We designed primers for RT‐PCR using TaqMan Advanced miR Assays (Applied Biosystems) for hsa‐miR21‐5p (Assay ID: 478587_mir) and hsa‐miR16‐5p (Assay ID: 477860_mir). PCR amplification was performed in triplicate using THUNDERBIRD Probe qPCR Mix (Toyobo, Osaka, Japan) following the manufacturer's procedures.
The threshold cycle (Ct) was automatically calculated using the second derivative maximum method to minimize the errors due to the variation of manual threshold determination and differences in background fluorescence in the samples and runs. The Ct values were identified at the cycle of maximum fluorescence acceleration, the beginning point of the log‐linear phase in the amplification curve.
MiR16‐5p was selected as an internal control of plasma samples in this study because it was the best internal control available for normalizing plasma miRNAs according to our previous study.11 Thus, the expression levels of miR21‐5p were normalized to that of miR16‐5p, and the postoperative changes in plasma miR21‐5p level were calculated using the ‒ΔΔCt method with the following equation:
2.6. Collection of clinical and pathological characteristics
We collected information on patient characteristics, including sex, age, body mass index (BMI), primary colorectal tumor location, pre‐ and postoperative tumor markers including carcinoembryonic antigen (CEA; normal upper limit at 5 ng/mL) and carbohydrate antigen 19‐9 (CA19‐9; normal upper limit at 37 ng/mL), pathological Union for International Cancer Control‐tumor, node, and metastasis classification (8th edition),12 histological lymphatic invasion (Ly), and histological venous invasion (V). All histological characteristics were based on the Japanese Classification of Colorectal, Appendiceal, and Anal Carcinoma: the 3d English Edition.13
2.7. Statistical analysis
The Mann‐Whitney U test was used in independent cases for comparisons of variables between groups and the Wilcoxon signed‐rank test was used in paired cases for continuous variables. Multivariate correlation analysis was performed using Spearman's rank correlation coefficient to examine the correlation between plasma miR21‐5p level and other clinical parameters. Receiver operating characteristic (ROC) curve analysis was used to calculate the area under the curve (AUC) value for assessing the validity of discriminating recurrence and PD from enrolled patients. The Youden's index was used to determine the optimal cut‐off point to calculate the specificities and sensitivities in ROC curve analysis.
A logistic regression model was used to calculate the odds ratio (OR) and 95% confidence interval (CI) on multivariate analysis for recurrence and PD after surgical resection. Univariate analysis was conducted for each factor, and multivariate analysis was performed for factors with P value <.10 in univariate analysis. The limit of statistical significance for all analyses was defined as a 2‐sided P =.05. All statistical analyses were performed using JMP software (SAS Institute, Cary, NC, USA).
3. RESULTS
3.1. Patient characteristics
Patient characteristics and primary colorectal tumors are listed in Table 1. The cohort consisted of 56 male (54.4%) and 47 female (45.6%). The age ranged from 42 to 86 years, with a median of 70 years. The primary tumor location was the colon in 52 cases (50.5%) and the rectum in 51 cases (49.5%). The pathological stage was stage I in 35 cases (34.0%), stage II in 27 cases (26.2%), stage III in 30 cases (29.1%), and stage IV in 11 cases (10.7%). Compared with the cancer statistics in Japan in 2019,14 the enrolled patient population distribution of sex, age, tumor location, and pathological stage (surgical cases only) was generally in agreement. Recurrence and PD on 6months after surgery occurred 10 (9.7%; Table S1) and 6 (5.8%) cases, respectively.
TABLE 1.
Characteristics of patients and primary colorectal tumor
| Characteristics | Patient total n = 103 |
|---|---|
| Sex, n (%) | Male 56 (54.4) Female 47 (45.6) |
| Age, median [range] | 70 [42‐86] |
| Primary colorectal tumor location, n (%) | Cecum 8 (7.8) Ascending 17 (16.5) Transverse 11 (10.7) Descending 3 (2.9) Sigmoid 13 (12.6) Rectum 51 (49.5) |
| Colon 52 (50.5) Rectum 51 (49.5) | |
| Right side 67 (65.0) Left side 36 (35.0) | |
| Pathological T Stagea, n (%) | T1 25 (24.2) |
| T2 15 (14.6) | |
| T3 43 (41.7) | |
| T4 20 (19.4) | |
| Pathological N Stagea, n (%) | N0 67 (65.0) |
| N1 22 (21.4) | |
| N2 14 (13.6) | |
| Distant metastasis, n (%) | Positive 11 (10.7) Negative 92 (89.3) |
| Liver 7 (6.8) Lung 3 (2.9) Peritoneal dissemination 2 (1.9) Ovary 1 (1.0) | |
| Pathological Stagea, n (%) | I 35 (34.0) |
| II 27 (26.2) | |
| III 30 (29.1) | |
| IV 11 (10.7) | |
| Preoperative CEA level, n (%) | Normal 72 (69.9) Elevated 31 (30.1) |
| Preoperative CA19‐9 level, n (%) | Normal 84 (81.6) Elevated 19 (18.4) |
| Postoperative chemotherapy, n (%) | Present 44 (42.7) Absent 59 (57.3) |
| Adjuvant chemotherapy 36 (35.0): Stage II:12 (33.3) Stage III 24 (66.7) | |
| Capecitabine 13 (12.6) CapeOX 20 (19.4) UFT 3 (2.9) | |
| Systemic chemotherapy 8 (7.8): | |
| CapeOX 2 (1.9) CapeOX + Bmab 4 (3.9) FOLFOX + Bmab 1 (1.0) FOLFOX + Pmab 1 (1.0) | |
| Recurrence and progressive diseaseb, n (%) | Present 16 (15.5) Absent 87 (84.5) |
| Recurrent 10 (9.7) | |
| Liver 2 (1.9) Lung 5 (4.9) Peritoneal dissemination 1 (1.0) Distant lymph node 4 (3.9) | |
| Progressive disease 6 (5.8) |
Abbreviations: CA19‐9, Carbohydrate antigen 19‐9 level, normal upper limit at 37 ng/mL; CapeOX, Capecitabine + Oxaliplatin; Bmab, Bevacizumab; Pmab: Panitumumab; CEA, Carcinoembryonic antigen level, normal upper limit at 5 ng/mL.
UICC TNM classification(the 8th edition).
Revised Response Evaluation Criteria in Solid Tumors (RECIST) guideline (version 1.1).
3.2. Relationship between changes in plasma miR21‐5p levels and recurrence and PD
We previously estimated the plasma levels of several miRNAs in these patients.8 Among the miRNAs tested, we focused on plasma miR21‐5p levels by performing qRT‐PCR between pre‐ and post‐surgical resection. In the recurrence and PD group, the plasma miR21‐5p level showed a significant increase after surgical resection at POM1 and POM6 (POD7: P = .19, POM1: P < .01, and POM6: P < .01). On the other hand, in the non‐recurrence group, there was no significant difference (POD7: P = .45, POM1: P = .38, and POM6: P = .82; Figure 2A‐C).
FIGURE 2.
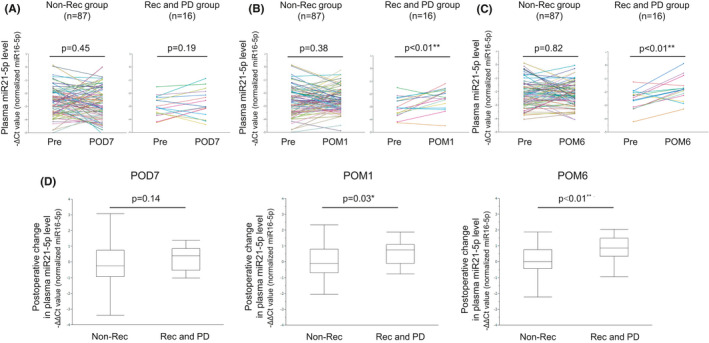
(A) The expression levels of plasma microRNA 21‐5p (miR21‐5p) pre‐operation (Pre) and 7 d post‐operation (POD7) in non‐recurrence (n = 87) and recurrence / PD groups (n = 16). (B) The expression levels of plasma miR21‐5p at Pre and 1 mo post‐operation (POM1) in non‐recurrence (n = 87) and recurrence / PD groups (n = 16). (C) The expression levels of plasma miR21‐5p at Pre and 6 mo post‐operation (POM6) in non‐recurrence (n = 87) and recurrence/PD groups (n = 16). The Wilcoxon signed‐rank test was performed for comparisons between groups. *P < .05; **P < .01. (D) Postoperative changes in plasma miR21‐5p levels at POD7, POM1, and POM6 in the non‐recurrence (n = 87) and recurrence/PD groups (n = 16). The Mann‐Whitney U test was performed for comparisons between groups. *P < .05; **P < .01
Based on these results, we next analyzed the postoperative changes in plasma miR21‐5p level using the –ΔΔCt method at three different time points (POD7, POM1, and POM6) between the recurrence and PD group and non‐recurrence group. We found that the difference between the two groups gradually increases with time after surgery, and at POM6, the postoperative change in plasma miR21‐5p level in the recurrence and PD group became significantly higher than that in the non‐recurrence group (POD7: P = .14, POM1: P = .03, and POM6: P < .01; Figure 2D).
3.3. Correlation analysis between plasma miR21‐5p level and the other clinical parameters
There would be a potential weakness of plasma miRNAs as tumor biomarker if their expression levels are affected by various other clinical parameters such as age, BMI, concurrent disorders such as cytopenia, inflammation, or hepatic or renal damage, and tumor markers. Thus, we assessed the correlation between the plasma miR21‐5p level and other parameters at each point (Pre, POM1, and POM6), including blood parameters such as blood cell count (white and red blood cell), coagulation (platelet and prothrombin time‐international normalized ratio), kidney function (blood urea nitrogen/creatinine ratio), hepatic injury (aspartate aminotransferase, alanine aminotransferase, and albumin), inflammation (C‐reactive protein), and tumor markers (CEA and CA19‐9).
As a result, there were no other clinical parameters except for age and RBC that showed significant correlation with the plasma miR21‐5p levels. Although there was a significant correlation with age at Pre and RBC at Pre and POM1, the correlation coefficient was 0.22, −0.33, and −0.34, respectively, all of which were weak correlations (Table 2).
TABLE 2.
Correlation analysis between the plasma miR21‐5p level and various clinical parameters
| Age | BMI | WBC | RBC | Plt | PT‐INR | BUN/Cre | AST | ALT | Alb | CRP | CEA | CA19‐9 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (a) Preoperative value at 1 mo post‐operation | |||||||||||||
| P‐value | .02* | .95 | .27 | <.001*** | .98 | .78 | .76 | .14 | .84 | .98 | .28 | .44 | .19 |
| Correlation coefficient(ρ)a | 0.22 | 0.01 | 0.11 | −0.33 | −0.003 | −0.03 | −0.03 | 0.14 | 0.02 | 0.002 | 0.11 | 0.08 | 0.13 |
| (b) Postoperative value at 1 mo post‐operation | |||||||||||||
| P‐value | .12 | .93 | .29 | <.001*** | .07 | .21 | .27 | .29 | .58 | .06 | .06 | .80 | .81 |
| Correlation coefficient(ρ)b | 0.15 | −0.01 | 0.11 | −0.34 | 0.18 | 0.18 | −0.11 | 0.11 | 0.05 | −0.19 | 0.18 | −0.02 | 0.02 |
| (c) Postoperative value at 6 mo post‐operation | |||||||||||||
| P‐value | .11 | .10 | .89 | .08 | .69 | .17 | .42 | .34 | .45 | .13 | .31 | .15 | .61 |
| Correlation coefficient(ρ)c | 0.16 | −0.17 | 0.01 | −0.18 | −0.04 | 0.24 | −0.08 | 0.10 | −0.08 | −0.16 | 0.11 | 0.15 | −0.05 |
*P < .05; **P < .01; ***P < .001.
Spearman's rank correlation coefficient with preoperative plasma miR21‐5p level.
Spearman's rank correlation coefficient with postoperative plasma miR21‐5p level at postoperative 1 mo.
Spearman's rank correlation coefficient with postoperative change in plasma miR21 level at postoperative 6 mo.
3.4. Diagnostic value of postoperative change in plasma miR21‐5p level for recurrence and PD after surgical resection
To test the diagnostic value for recurrence and PD after surgical resection, we performed ROC curve analysis on the postoperative change in plasma miR21‐5p level and the postoperative tumor marker levels (CEA and CA19‐9).
At POM6, ROC curve analyses showed that both postoperative changes in plasma miR21‐5p and CEA levels could predict recurrence and PD in enrolled patients with an AUC of 0.715 for miR21‐5p and 0.731 for CEA, respectively (Figure 3A and B). At the cut‐off value of +0.54 for the postoperative change in plasma miR21‐5p level, the sensitivity and specificity were 73.3% and 70.5%, respectively.
FIGURE 3.
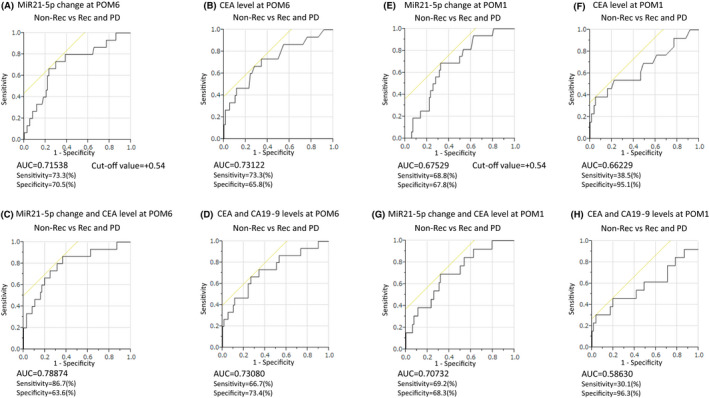
Receiver operating characteristics (ROC) curve analysis of postoperative change in plasma miR21‐5p level and tumor markers (carcinoembryonic antigen [CEA] and carbohydrate antigen 19‐9 [CA19‐9]) levels at POM6 and POM1 for discriminating to recurrence and PD after surgical resection. Postoperative changes in plasma miR21‐5p level at POM6 yielded an area under curve (AUC) of 0.715 with 73.3% sensitivity and 70.5% specificity (A), and CEA level at POM6 yielded an AUC of 0.731 (B). Combined ROC analysis revealed that the combination of miR21‐5p and CEA on POM6 yielded an AUC of 0.788 with 86.7% sensitivity and 63.6% specificity (C), and the combination of CEA and CA19‐9 at POM6 yielded an AUC of 0.730 (D). Postoperative changes in plasma miR21‐5p level at POM1 yielded an AUC of 0.675 with 68.8% sensitivity and 67.8% specificity (E), and CEA level at POM1 yielded an AUC of 0.662 (F). Combined ROC analysis revealed that the combination of miR21‐5p and CEA on POM1 yielded an AUC of 0.707 with 69.2% sensitivity and 68.3% specificity (G), and the combination of CEA and CA19‐9 at POM1 yielded an AUC of 0.586 (H). Youden's index was used to determine the optimal cut‐off point to calculate the specificities and sensitivities in the ROC analysis
Combination ROC analyses also revealed a slightly increased AUC value of 0.788 with 86.7% sensitivity and 63.6% specificity (Figure 3C). Interestingly, the combination of miR21‐5p + CEA showed better AUC and sensitivity than that of CEA + CA19‐9, which is currently widely used (Figure 3D).
Subsequently, we performed the same analysis at POM1. ROC curve analyses showed that both the postoperative change in plasma miR21‐5p level and CEA level could predict recurrence and PD in enrolled patients with an AUC of 0.675 for miR21‐5p and 0.662 for CEA, respectively (Figure 3E and F).
At the cut‐off value of +0.54 for the postoperative change in plasma miR21‐5p level, the sensitivity and specificity were 68.8% and 67.8%, respectively. The calculated cut‐off value was the same as that at POM6, but AUC, sensitivity, and specificity were all inferior to the results at POM6. However, unlike the POM6 results, AUC and sensitivity of the postoperative miR21‐5p level could exceeded that of CEA. Combination ROC analyses also revealed that a little increased AUC value of 0.707 with 69.2% sensitivity and 68.3% specificity (Figure 3G). The combination of miR21p‐5 and CEA showed better AUC and sensitivity than that of CEA + CA19‐9 as well as POM6 (Figure 3H).
3.5. Prognostic factors for recurrence and PD after surgical resection in CRC
The results of univariate and multivariate logistic regression analyses for recurrence and PD after surgical resection are listed in Table 3. The variables included sex, age, tumor location, pathological T and N stages, histological venous and lymphatic invasion, postoperative chemotherapy, postoperative CEA and CA19‐9 levels, and postoperative changes in plasma miR21‐5p levels. The postoperative changes in plasma miR21‐5p levels were classified into two groups (elevated/normal) based on the cut‐off value calculated from the ROC analysis (cut‐off value: +0.54).
TABLE 3.
Prognostic factors for recurrence and PD after surgical resection in clinicopathological characters of 6 and 1 mo post‐operation
| Prognostic factors | POM6 | POM1 | ||||||
|---|---|---|---|---|---|---|---|---|
| n (%) | Univariate | Multivariate | n (%) | Univariate | Multivariate | |||
| P‐value | P‐value | OR (95%CI) | P‐value | P‐value | OR (95%CI) | |||
|
Sex (Male/Female) |
56 (54.4) 47 (45.6) |
.70 |
56 (54.4) 47 (45.6) |
.70 | ||||
|
Age (years) (75≧/<75) |
24 (23.3) 79 (76.7) |
.43 |
24 (23.3) 79 (76.7) |
.43 | ||||
| Primary tumor location (Colon/Rectum) |
52 (50.5) 51 (49.5) |
.97 |
52 (50.5) 51 (49.5) |
.97 | ||||
|
Pathological T stageb (T3+T4/T1+T2) |
63 (61.2) 40 (38.8) |
<.01** | .07 | – |
63 (61.2) 40 (38.8) |
<.01** | .14 | – |
|
Pathological N stageb (N1 + N2/N0) |
36 (35.0) 67 (65.0) |
.01* | .29 | – |
36 (35.0) 67 (65.0) |
.01* | .66 | – |
|
Venous invasion (Positive/Negative) |
69 (67.0) 34 (33.0) |
.048* | .53 | – |
69 (67.0) 34 (33.0) |
.048* | .68 | – |
|
Lymphatic invasion (Positive/Negative) |
70 (68.0) 33 (32.0) |
<.01** | .61 | – |
70 (68.0) 33 (32.0) |
<.01** | .35 | – |
|
Postoperative chemotherapy (Present/Absent) |
44 (42.7) 59 (57.3) |
.96 |
44 (42.7) 59 (57.3) |
.96 | ||||
|
Postoperative CEA level (Elevated/normal) |
19 (18.4) 84 (81.6) |
.01* | .04* | 4.45 (1.10‐ 19.76) |
13 (12.6) 90 (87.4) |
.04* | .04* | 5.04 (1.9‐24.79) |
|
Postoperative CA19‐9 level (Elevated/normal) |
9 (87.3) 94 (91.3) |
.17 |
7 (6.8) 96 (93.2) |
.28 | ||||
|
Postoperative change in plasma miR21‐5p levela Elevated/Normal) |
34 (33.0) 69 (67.0) |
<.01** | <.01** | 7.09 (1.64‐41.82) |
39 (37.9) 64 (62.1) |
<.01** | .03* | 4.33 (1.15‐18.86) |
Abbreviations: OR, Odds ratio; 95% CI, 95% confidence interval; CEA, Carcinoembryonic antigen level, normal upper limit at 5 ng/mL; CA19‐9, Carbohydrate antigen 19‐9 level, normal upper limit at 37 ng/mL.
*P < .05; **P < .01.
−∆∆Ct value = ‐{(CtmiR21‐5p‐CtmiR16‐5p)postoperative‐(CtmiR21‐5p‐CtmiR16‐5p)preoperative}, cut off value = +0.54.
UICC TNM classification (the 8th edition).
At both POM6 and POM1, in the univariate analysis, pathological T and N stages, histological venous and lymphatic invasion, postoperative CEA levels, and postoperative changes in plasma miR21‐5p levels were identified as significant variables for recurrence, but only postoperative CEA level and postoperative changes in plasma miR21‐5p level remained significant in multivariate analysis.
Next, we also performed the same analysis limited to recurrence after curative resection in Stage I to Ⅲ patients (n = 92). Only change in plasma miR21‐5p level was remained significance in multivariate analysis {POM1: P = .03, OR(95%CI) = 5.00 (1.16‐26.90), POM6: P < .01, OR(95%CI) = 15.09 (2.20‐323.04), respectively} (Table S2).
4. DISCUSSION
In this study, we analyzed 103 paired plasma samples pre‐ and post‐operation from prospectively enrolled CRC patients, and found that the postoperative changes in plasma miR21‐5p level in each patient could be a useful noninvasive blood‐based biomarker for not only monitoring but also predicting recurrence and PD after surgical resection. To our knowledge, this is the first prospective study to collect four paired plasma samples with time (Pre/POD7/POM1/POM6) from the same patient and analyze the relationship between the postoperative changes in plasma miRNA levels and condition of CRC patients after surgical resection.
MiR21‐5p is located at the tenth intron of the coding region of transmembrane protein 49 at chromosome 17q23, and is one of the most prominent miRNAs implicated in the carcinogenesis and progression of human malignancy.15 The tissue miR21‐5p level is notably upregulated in many cancers, including CRC, lung cancer, glioblastoma, hepatocellular carcinoma, and gastric cancer. In addition, its elevated level has been causally associated with tumor aggressiveness and poor prognosis.16, 17, 18, 19, 20, 21, 22, 23, 24 The target genes of miR21‐5p include PTEN, programmed cell death protein 4, methylthioadenosine phosphorylase, and transforming growth factor‐β, all of which regulate the cell cycle. Numerous reports have demonstrated that miR21‐5p acts as a potential oncogene in CRC by promoting tumorigenesis, invasion, and metastasis through the regulation of these genes. Wu et al25 reported that PTEN protein level in CRC tissues and cells is inversely correlated with miR21‐5p expression. PTEN was reported to be a tumor suppressor gene by inhibiting the phosphoinositide‐3 kinase/protein kinase B pathway. They suggested that miR21‐5p modulates malignant phenotypes such as proliferation, anti‐apoptosis, cell cycle progression, and invasion in CRC cells by downregulating PTEN protein expression.
Circulating plasma miR21‐5p is the most representative miRNA biomarker, as it has been extensively explored in a range of studies on CRC.26, 27, 28, 29, 30 Considerable research has been conducted on the use of plasma miR21‐5p expression level to distinguish between CRC patients and healthy controls, suggesting plasma miR21‐5p expression has great promise as a novel biomarker for CRC screening. According to the diagnostic meta‐analysis of plasma miR21‐5p expression,31 the overall pooled results for sensitivity, specificity, and AUC were 69.0% (95% CI: 54.0‐80.0), 86.0% (95% CI: 75.0‐92.0), and 0.85 (95% CI: 0.83‐0.89), respectively. However, most studies merely have focused on the utility of plasma miR21‐5p expression level as a potential biomarker for the diagnosis or prognosis of CRC. For this reason, the miRNA measured at only one time‐point was used as a parameter in past studies. However, variations in measured values and the effects of differences between patients have limited its clinical application. Identification of cancer‐related miRNAs is hampered by the fact that blood also contains miRNAs derived from other physiological or pathological activities, whose levels correlate with individual differences.32 Therefore, circulating plasma miRNAs generally fluctuate between different patients, and it is difficult to define a normal range of circulating plasma miRNA levels. To solve this problem, we focused on the postoperative changes in plasma miR21‐5p levels by conducting a prospective study focusing on recurrence after curative resection calculated using the –ΔΔCt method. It is thought that a paired comparative study pre‐ and post‐treatment can provide more accurate results to predict and monitor tumor recurrence, as in our study.29, 33 In this study, we demonstrated that the plasma miR21‐5p level were clinically unaffected by other clinical parameters. Furthermore, the postoperative changes in plasma miR21‐5p level could predict recurrence and PD as effectively as currently used tumor markers.
CEA has been recommended as a tumor marker in the follow‐up of CRC according to most guidelines. However, there is still controversy regarding its diagnostic applicability due to its relatively low sensitivity. According to a randomized clinical trial on CEA trend for diagnosing recurrence after curative resection,34 no individual trend threshold achieved a sensitivity above 70%. Furthermore, the AUC for CEA at the end of the first year of follow‐up was only 0.623. Some reports revealed that CEA is insufficiently sensitive to be used alone, even with a low threshold.34, 35, 36 Therefore, they suggested that it is essential to argument CEA monitoring with another diagnostic modality in order to avoid missed recurrence cases. Interestingly, CEA showed improved diagnostic ability in combination with plasma miR21‐5p rather than CA19‐9 in our study. The clinical use of plasma miR21‐5p for improving the diagnostic accuracy of current tumor markers is feasible and may contribute to the efficacy of CRC treatment. Moreover, the postoperative change in plasma miR21‐5p level at POM1 showed potential to be a predictive biomarker and prognostic factor for recurrence and PD after surgical resection, and can be expected to surpass current tumor markers. We performed similar analyses on other well‐known tumor‐related miRNAs, miR29a‐3p and 92a‐3p,37, 38, 39, 40 but only miR21‐5p showed this feature (Figures S1 and S2). This new discovery may decrease the recurrence rate after curative resection as an indication criterion for adjuvant chemotherapy.
Our study had some limitations. First, as the sample size was small and further validation in large samples is required to calculate the appropriate cut‐off value. Second, as the interval between pre‐ and post‐operation was short at only six months, it is highly possible that the non‐recurrence group included potential recurrence cases. Therefore, long‐term patient monitoring and correlation analysis is required. In the future, by conducting large‐scale prospective research in multiple institutions, it will be possible to build a biomarker system that enables a more accurate understanding of tumor status in CRC patients.
5. CONCLUSIONS
Our results indicate that the postoperative changes in plasma miR21‐5p level could be a useful noninvasive biomarker for monitoring recurrence and PD after surgical resection of CRC patients. Combination diagnosis with current tumor markers may be practical for clinical application. Furthermore, plasma miR21‐5p can predict recurrence and PD after surgical resection. Therefore, our new findings may suggest a new criterion for adjuvant chemotherapy.
DISCLOSURE
K. Yoshida has received honoraria for research funding from Chugai Pharmaceutical Co., Ltd., Taiho Pharmaceutical Co., Eli Lilly and Company, Sanofi K.K, and Yakult Honsha Co., Ltd. outside the submitted work. T. Takahashi has received honoraria for lectures from Takeda Pharmaceutical Co., Ltd. All remaining authors declare that they have no conflicts of interests.
AUTHORS’ CONTRIBUTIONS
MF, KY, and YA conceived the study concept and planned the design as the principal investigators. MF interpreted the results and wrote the manuscript draft. NS, KH, NM, and KY revised the manuscript draft by adding intellectual insights and provided critical advice. MF, NM, TT, NS, KH, KY, and YA obtained the data and provided their critical comments to improve the manuscript and gave final approval for submission. All authors have read and agreed to the published version of the manuscript.
ETHICAL APPROVAL
The present study was conducted in accordance with the World Medical Association Declaration of Helsinki and was approved by the Ethics Committee of Gifu University (approval number: 2019‐074). As this study was a prospective study and included potentially identifiable patient data, informed consent was obtained from the enrolled patients.
CONSENT FOR PUBLICATION
Written informed consents were obtained from the enrolled patients for publication of this study.
Supporting information
Fig S1‐S2
Table S1‐S2
Fukada M, Matsuhashi N, Takahashi T, et al. Postoperative changes in plasma miR21‐5p as a novel biomarker for colorectal cancer recurrence: A prospective study. Cancer Sci. 2021;112:4270–4280. 10.1111/cas.15065
DATA AVAILABILITY STATEMENT
The datasets used during this study are available from the corresponding author on reasonable request.
REFERENCES
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394‐424. [DOI] [PubMed] [Google Scholar]
- 2.Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin‐4 encodes small RNAs with antisense complementarity to lin‐14. Cell. 1993;75:843‐854. [DOI] [PubMed] [Google Scholar]
- 3.Ambros V. The functions of animal microRNAs. Nature. 2004;431:350‐355. [DOI] [PubMed] [Google Scholar]
- 4.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15‐20. [DOI] [PubMed] [Google Scholar]
- 5.Schwarzenbach H, Nishida N, Calin GA, Pantel K. Clinical relevance of circulating cell‐free microRNAs in cancer. Nat Rev Clin Oncol. 2014;11:145‐156. [DOI] [PubMed] [Google Scholar]
- 6.Mitchell PS, Parkin RK, Kroh EM, et al. Circulating microRNAs as stable blood‐based markers for cancer detection. Proc Natl Acad Sci U S A. 2008;105:10513‐10518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen X, Ba Y, Ma L, et al. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008;18:997‐1006. [DOI] [PubMed] [Google Scholar]
- 8.Fukada M, Matsuhashi N, Takahashi T, et al. Tumor Tissue MIR92a and Plasma MIRs21 and 29a as Predictive Biomarkers Associated with Clinicopathological Features and Surgical Resection in a Prospective Study on Colorectal Cancer Patients. J Clin Med. 2020;9:2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hashiguchi Y, Muro K, Saito Y, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int J Clin Oncol. 2019;2020(25):1‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228‐247. [DOI] [PubMed] [Google Scholar]
- 11.Heishima K, Mori T, Ichikawa Y, et al. MicroRNA‐214 and MicroRNA‐126 Are Potential Biomarkers for Malignant Endothelial Proliferative Diseases. Int J Mol Sci. 2015;16:25377‐25391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brierley JD, Gospodarowicz MK, Wittekind C. International Union Against Cancer. TNM Classification of Malignant Tumors, 8th edn. Hoboken, NJ: Wiley‐Blackwell; 2017. [Google Scholar]
- 13.Japanese Society for Cancer of the Colon and Rectum . Japanese Classification of Colorectal, Appendiceal, and Anal Carcinoma: the 3d English Edition. J Anus Rectum Colon. 2019;3:175‐195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The Editorial BOARD of the Cancer Statistics in Japan . Cancer Statistics in Japan, 2019. Tokyo: Foundation of Promotion of Cancer Research, 2020. [Google Scholar]
- 15.Esquela‐Kerscher A, Slack FJ. Oncomirs‐microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259‐269. [DOI] [PubMed] [Google Scholar]
- 16.Lee KS, Nam SK, Koh J, et al. Stromal Expression of MicroRNA‐21 in Advanced Colorectal Cancer Patients with Distant Metastases. J Pathol Transl Med. 2016;50:270‐277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiong Y, Zhang YY, Wu YY, et al. Correlation of over‐expressions of miR‐21 and Notch‐1 in human colorectal cancer with clinical stages. Life Sci. 2014;106:19‐24. [DOI] [PubMed] [Google Scholar]
- 18.Zhou X, Wang X, Huang Z, et al. Prognostic value of miR‐21 in various cancers: an updating meta‐analysis. PLoS One. 2014;9:e102413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu ZL, Wang H, Liu J, Wang ZX. MicroRNA‐21 (miR‐21) expression promotes growth, metastasis, and chemo‐ or radioresistance in non‐small cell lung cancer cells by targeting PTEN. Mol Cell Biochem. 2012;372:35‐45. [DOI] [PubMed] [Google Scholar]
- 20.Zhan BG, Li JF, Yu BQ, Zhu ZG, Liu BY, Yan M. microRNA‐21 promotes tumor proliferation and invasion in gastric cancer by targeting PTEN. Oncol Rep. 2012;27:1019‐1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qin X, Yan L, Zhao X, Li C, Fu Y. microRNA‐21 overexpression contributes to cell proliferation by targeting PTEN in endometrioid endometrial cancer. Oncol Lett. 2012;4:1290‐1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu C, Yu J, Yu S, et al. MicroRNA‐21 acts as an oncomir through multiple targets in human hepatocellular carcinoma. J Hepatol. 2010;53:98‐107. [DOI] [PubMed] [Google Scholar]
- 23.Lei BX, Liu ZH, Li ZJ, Li C, Deng YF. miR‐21 induces cell proliferation and suppresses the chemosensitivity in glioblastoma cells via downregulation of FOXO1. Int J Clin Exp Med. 2014;7:2060‐2066. [PMC free article] [PubMed] [Google Scholar]
- 24.Yamamichi N, Shimomura R, Inada K, et al. Locked nucleic acid in situ hybridization analysis of miR‐21 expression during colorectal cancer development. Clin Cancer Res. 2009;15:4009‐4016. [DOI] [PubMed] [Google Scholar]
- 25.Wu Y, Song Y, Xiong Y, et al. MicroRNA‐21 (Mir‐21) Promotes Cell Growth and Invasion by Repressing Tumor Suppressor PTEN in Colorectal Cancer. Cell Physiol Biochem. 2017;43:945‐958. [DOI] [PubMed] [Google Scholar]
- 26.Zhang H, Li P, Ju H, et al. Diagnostic and prognostic value of microRNA‐21 in colorectal cancer: an original study and individual participant data meta‐analysis. Cancer Epidemiol Biomark Prev. 2014;23:2783‐2792. [DOI] [PubMed] [Google Scholar]
- 27.Kanaan Z, Rai SN, Eichenberger MR, et al. Plasma miR‐21: a potential diagnostic marker of colorectal cancer. Ann Surg. 2012;256:544‐551. [DOI] [PubMed] [Google Scholar]
- 28.Luo X, Stock C, Burwinkel B, Brenner H. Identification and evaluation of plasma microRNAs for early detection of colorectal cancer. PLoS One. 2013;8:e62880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zanutto S, Pizzamiglio S, Ghilotti M, et al. Circulating miR‐378 in plasma: a reliable, haemolysis‐independent biomarker for colorectal cancer. Br J Cancer. 2014;110:1001‐1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Du M, Liu S, Gu D, et al. Clinical potential role of circulating microRNAs in early diagnosis of colorectal cancer patients. Carcinogenesis. 2014;35:2723‐2730. [DOI] [PubMed] [Google Scholar]
- 31.Peng Q, Zhang X, Min M, Zou L, Shen P, Zhu Y. The clinical role of microRNA‐21 as a promising biomarker in the diagnosis and prognosis of colorectal cancer: a systematic review and meta‐analysis. Oncotarget. 2017;8:44893‐44909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cortez MA, Bueso‐Ramos C, Ferdin J, Lopez‐Berestein G, Sood AK, Calin GA. MicroRNAs in body fluids ‐ the mix of hormones and biomarkers. Nat Rev Clin Oncol. 2011;8:467‐477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Konishi H, Ichikawa D, Komatsu S, et al. Detection of gastric cancer‐associated microRNAs on microRNA microarray comparing pre‐ and post‐operative plasma. Br J Cancer. 2012;106:740‐747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shinkins B, Primrose JN, Pugh SA, et al. Serum carcinoembryonic antigen trends for diagnosing colorectal cancer recurrence in the FACS randomized clinical trial. Br J Surg. 2018;105:658‐662. [DOI] [PubMed] [Google Scholar]
- 35.Nicholson BD, Shinkins B, Pathiraja I, et al. Blood CEA levels for detecting recurrent colorectal cancer. Cochrane Database Syst Rev. 2015;12:CD011134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sørensen CG, Karlsson WK, Pommergaard HC, Burcharth J, Rosenberg J. The diagnostic accuracy of carcinoembryonic antigen to detect colorectal cancer recurrence ‐ A systematic review. Int J Surg. 2016;25:134‐144. [DOI] [PubMed] [Google Scholar]
- 37.Wang LG, Gu J. Serum microRNA‐29a is a promising novel marker for early detection of colorectal liver metastasis. Cancer Epidemiol. 2012;36:61‐67. [DOI] [PubMed] [Google Scholar]
- 38.Zhi ML, Liu ZJ, Yi XY, Zhang LJ, Bao YX. Diagnostic performance of microRNA‐29a for colorectal cancer: A meta‐analysis. Genet Mol Res. 2015;14:18018‐18025. [DOI] [PubMed] [Google Scholar]
- 39.Wu CW, Ng SS, Dong YJ, et al. Detection of miR‐92a and miR‐21 in stool samples as potential screening biomarkers for colorectal cancer and polyps. Gut. 2012;61:739‐745. [DOI] [PubMed] [Google Scholar]
- 40.Huang Z, Huang D, Ni S, Peng Z, Sheng W, Du X. Plasma microRNAs are promising novel biomarkers for early detection of colorectal cancer. Int J Cancer. 2010;127:118‐126. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1‐S2
Table S1‐S2
Data Availability Statement
The datasets used during this study are available from the corresponding author on reasonable request.


