Abstract
Adult T‐cell leukemia‐lymphoma (ATL) is a T‐cell malignancy that is endemic to Japan. In this latest nationwide study of ATL, we collected the data from 4 nationwide registries of patients diagnosed in 2012‐2013; the Hematology Blood Disease, the Skin Cancer Society, the Hospital‐Based Cancer Registries, and information from the hospitals that participated in the Japanese nationwide survey of ATL in 2010‐2011. In the present study, 2614 patients with ATL were diagnosed based on the registries, and 117 departments registered 1042 patients. Among these patients, 984 were eligible for analysis. The median age at diagnosis was 69 y. A larger proportion of patients with ATL older than 70 y was diagnosed with the lymphoma subtype, and more than half of the patients with ATL in the metropolitan areas were born in the human T‐cell leukemia virus type I (HTLV‐1)‐endemic areas of Kyushu/Okinawa, which are almost identical to the findings in our 2010‐2011 study. Additionally, we identified that patients with ATL migrated from the endemic areas for HTLV‐1 to the non‐endemic metropolitan areas. The present study was able to reduce the burden of searching each hospital and to update the clinico‐epidemiological characteristics of a large number of patients with ATL in Japan, suggesting the usefulness and feasibility of the novel data collection method. The establishment of a more sophisticated database management system for ATL is necessary for future continuous surveys.
Keywords: adult T‐cell leukemia‐lymphoma, epidemiology, HTLV‐1, nationwide survey, registry data
We identified that ATL patients shifted from areas endemic for HTLV‐1 to non‐endemic metropolitan areas. The median age at diagnosis was 69 y, which was significantly older than that in the 1980s and 1990s. We conducted a nationwide survey of ATL during 2012‐2013 by modifying data acquisition from 4 nationwide registries.

Abbreviations
- APP
ATL Prevention Program
- ATL
adult T‐cell leukemia‐lymphoma
- HTLV‐1
human T‐cell leukemia virus type I
- JNSA
Japanese Nationwide Survey of ATL
- JSCSR
Japanese Skin Cancer Society Registry
- JSHBDR
Japanese Society of Hematology Blood Disease Registry
- NHBCR
Nationwide Hospital‐Based Cancer Registry
- RA
rheumatoid arthritis
1. INTRODUCTION
ATL is a mature T‐cell malignancy with diverse clinical features and prognoses,1, 2 and it is etiologically associated with human T‐cell leukemia virus type I (HTLV‐1) infections.3, 4 HTLV‐1 causes non‐neoplastic inflammatory diseases such as HTLV‐1‐associated myelopathy/tropical spastic paraparesis, HTLV‐1 uveitis, and other diseases with suspected but unestablished associations including arthropathy, pneumopathy, dermatitis, exocrinopathy, and myositis.5 The virus is transmitted through 3 routes: mother to child, sexual intercourse, and blood transfusions.6, 7, 8, 9 It has been reported that breastfeeding is the major route in mother‐to‐child transmissions, and that the overall infection rate of HTLV‐1 by seropositive mothers is between 10% and 30%.10 Conversely, only 3%‐5% of HTLV‐1 carriers who were infected through breastfeeding develop ATL, and they often experience a long period of latency.11 The rarity and latency are associated with the multi‐step leukemogenesis of the disease.12 It has been well known that HTLV‐1‐infections are prevalent in southwestern Japan, central South America, sub‐Saharan Africa, a part of the Middle and Far East, a part of Europe, and a part of Oceania and central Australia.13, 14, 15, 16 However, sporadic HTLV‐1 infections and patients with ATL have been observed in other small areas, which are possibly due to population migration from the HTLV‐1‐endemic areas. Globally, approximately 2000‐3000 patients with ATL are newly diagnosed annually.17, 18
Since the first discovery of ATL in Japan in 1977,1, 2 9 biennial nationwide clinico‐epidemiological surveys of ATL have been conducted from 1981 to 1998 by the T‐ and B‐cell Malignancy Study Group.19, 20, 21, 22, 23, 24, 25, 26, 27 These nationwide studies have revealed an unbalanced disease distribution in Japan, and it has established the epidemiological features of ATL and clinical subtypes (smoldering, chronic, lymphoma, and acute) based on the diversity of the clinical features of the patients and its natural history.28 However, after the establishment of the Shimoyama criteria, no nationwide study has been conducted in Japan except for an abridged ATL survey in 2008.29
In 2010, the number of HTLV‐1 carriers in Japan was estimated to be 1 million, based on the seropositivity of HTLV‐1 among blood donations.30 The number of HTLV‐1 carriers in Japan had not significantly decreased from that in the 1990s. From 2013 to 2018, our research team also conducted a nationwide clinico‐epidemiological study of patients with ATL who were diagnosed from 2010 to 2011 in Japan31 using methods similar to those described in previous nationwide studies performed from 1981 to 1998.19, 20, 21, 22, 23, 24, 25, 26, 27, 28 Our research revealed the following characteristics of patients with ATL included in the study: (1) the median age at first diagnosis was 68 y; (2) half of the patients with the lymphoma subtype were older than 70 y, whereas one‐third of the patients with the chronic subtype were younger than 60 y. Therefore, we showed that the clinical and epidemiological characteristics of patients with ATL diagnosed from 2010 to 2011 were significantly different from those of patients diagnosed from 1981 to 1998.19, 20, 21, 22, 23, 24, 25, 26, 27, 28
However, our previous nationwide study of patients with ATL in 2010‐201131 had several drawbacks. The source of data of the patients was limited to hospitals with more than 100 beds that had dedicated hematology and/or dermatology departments and were based on the list of medical institutions in the Regional Bureau of Health and Welfare, which might have caused underestimation and limited generalizability. Furthermore, information on the incidence of ATL has not been reported in the nationwide Japanese cancer registry primarily because ATL, having the word “leukemia,” was previously included under the category of “other leukemia” in the registry; ATL was included in a few limited prefectural cancer registries with high‐quality data.32 Therefore, the additional nationwide surveys of ATL are expected to confirm the characteristics of patients diagnosed with ATL in more recent years.
Therefore, the aim of the present study was to verify our previous clinico‐epidemiological findings of patients with ATL diagnosed from 2010 to 2011 in Japan31 by performing a novel nationwide survey of patients diagnosed with ATL from 2012 to 2013 using an improved method to efficiently collect data from 3 existing nationwide registration systems (Hematology Blood Disease, Skin Cancer Society, and the Hospital‐Based Cancer Registries) in Japan.
2. MATERIALS AND METHODS
2.1. Study design and data sources
We performed a retrospective, multicenter, hospital‐based survey of patients with ATL from April 2016 to March 2020. Subjects included in this study were patients newly diagnosed with ATL between January 2012 and December 2013. The study protocol was approved by the ethics committee and institutional review board (IRB) of the National Cancer Center (approval no. 2016‐065) and Saitama Medical University (approval no. 17‐037) in Japan. The ethical committee waived the need for written informed consent because of the retrospective design and anonymous data collection. The present study was conducted in accordance with the Declaration of Helsinki. All the patients' clinical information was provided anonymously from the participating hospitals.
The study procedure was in accordance with our previous nationwide hospital‐based survey31 with some modifications regarding data acquisition. We used 4 academic registry systems: (1) the Japanese Society of Hematology (JSH) Blood Disease Registry (JSHBDR) 33; (2) the Japanese Skin Cancer Society (JSCS) Registry (JSCSR) 34; (3) the Nationwide Hospital‐Based (NHB) Cancer Registry (NHBCR) 35; and (4) information from the hospitals that participated in our previous nationwide survey for ATL (the Japanese nationwide survey of ATL, JNSA) in 2010‐2011.31
The overall study procedures were as follows. After receiving permission to collect data from the 3 academic databases (JSH registry, JSCS registry, and NHB cancer registry), we obtained the following information from hospitals that registered patients with ATL: 2130 patients were registered from 378 hospitals in the NHBCR; at least 54 patients were registered from 37 hospitals in the JSCSR; 992 patients were registered from 219 hospitals in the JSHBDR; and 844 patients at most were registered from 77 hospitals in the 11th JNSA (Figures S1 and S2).31 After combining the information from the 4 databases, we checked for duplication in the number of cases of ATL. We identified, in total, 2614 unique patients with ATL from the 4 registries. We then asked each of the hospitals to provide anonymized information on each patient with ATL, and we received the records from the hospitals that agreed to participate in this study. Finally, we checked for possible multiple entries and confirmed the diagnosis of ATL based on the study sheets. All data collection and management were performed by a professional clinical research support office (Ata‐Life Inc.). Data collection began in September 2016 and ended in April 2019.
The detailed study procedures for each step were as follows. We obtained the anonymized list of patients from each academic database as shown above and then identified, in total, 2614 patients with ATL. After consolidating hospital names from the 4 registries, we made a list of 465 unique hospitals with dedicated departments for hematology and/or dermatology. We then asked if the hematologists or dermatologists would participate in our study (Figure 1). We also asked participating hospitals to only register once even if a patient was registered across the 4 databases.
FIGURE 1.
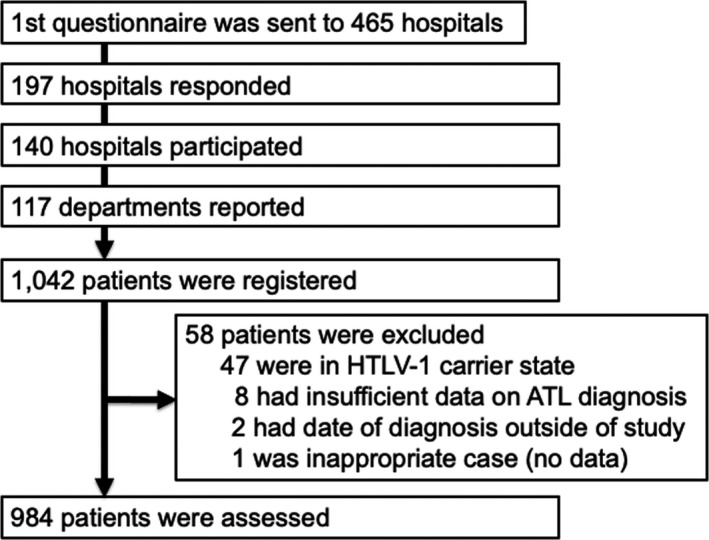
Study flowchart of ATL in Japan, 2012‐2013. Abbreviations: ATL, adult T‐cell leukemia‐lymphoma; HTLV‐1, human T‐cell leukemia virus type I
The initial questionnaires from 197 hospitals (42% of those listed in the first questionnaire) were returned. Of those returned, 140 hospitals (71%) agreed to participate in the study after approval by the IRBs at each respective hospital (Figure 1). We then sent a set of the study sheets to the 140 hospitals. The study sheets contained epidemiological items including age, sex, place of diagnosis, and clinical items including diagnostic criteria and subtype classification; the sheets were almost identical to those used in our previous nationwide survey.31
2.2. Evaluation of patients' eligibility for enrollment
A central review was conducted on all the returned study sheets by 5 hematologists skilled in the field of ATL (AU, SI, KN, YI, and KT). Criteria for ATL included cytologically or histologically confirmed T‐cell malignancy with HTLV‐1 seropositivity,36, 37 and ATL subtype classification was performed.28 To eliminate repeated patient data, we checked the initials, sex, birth date, and hospital location of each patient, as necessary.
2.3. Statistical analyses
Continuous variables were described using the median and range, interquartile range, or mean with standard deviation, and comparisons were evaluated using Wilcoxon's rank‐sum test or the Kruskal‐Wallis test. The continuous variables were also categorized into several groups, as necessary. The differences in the frequency of the categorical variables were compared using the chi‐square test or Fisher exact test. The agreement in the diagnosis of ATL subtypes among the participating hospitals and our central review was assessed by Kappa statistics using QuickCalcs (GraphPad Software online calculator [GraphPad Software.]). For all statistical analyses, a two‐sided P‐value of < .05 was considered significant.
3. RESULTS
3.1. Clinical characteristics of patients
From the 4 databases in Japan, we identified in total 2614 patients with newly diagnosed ATL during a 2‐y period (Figure S1). Among the 140 hospitals to which we sent a set of study sheets, 117 departments reported information on patients with ATL, which included 1042 patients (Figure 1). Among those patients, 58 were excluded from the central review process because of the following reasons: (1) HTLV‐1 carrier state (n = 47); (2) insufficient information to make a diagnosis of ATL (n = 8); (3) date of diagnosis outside the study period (n = 2); and (4) and lack of information (n = 1). Finally, in total, 984 patients were included in the analysis (Figure 1). Approximately 87% of the subtype classification at the participating hospitals was consistent with the central reviewer's classification, indicating substantial agreement (Kappa coefficient, 0.865, 95% CI, 0.837‐0.892) (Table S1).
The characteristics of the 984 patients are summarized in Table 1 and Table S2. The median age of all patients diagnosed from 2012 to 2013 was 69 y (Table 1), and the mean age at diagnosis was 67.9 y (Figure 2A). These findings were almost identical to the median and mean ages (68 and 67.5 y, respectively) of the patients in our previous study from 2010 to 2011.31 However, the ages were approximately 10 y older than those in the 1980s and 1990s (Table S3). Of the 984 patients, 525 (53.3%) were male, and 459 (46.7%) were female. The male‐to‐female ratio was 1.14 (Table 1), which was almost identical to the 1.12 ratio found in our previous 2010‐2011 study (Table S3).31 There was also no significant difference in the age distribution by sex (P = .37) (Figure 2B).
TABLE 1.
Characteristics of adult T‐cell leukemia‐lymphoma patients diagnosed in 2012‐2013 in Japan
| Items | Items (unit) | Summary |
|---|---|---|
| Evaluable patients | n | 984 |
| Gender, Male/Female (M/F ratio) | n | 525/459 (1.14) |
| Age at diagnosis, | median (range), y | 69 (25‐98) |
| Age at diagnosis category, y | ||
| <40 | n (% of all) | 12 (1.2) |
| 40‐49 | 47 (4.8) | |
| 50‐59 | 147 (14.9) | |
| 60‐69 | 322 (32.7) | |
| 70‐79 | 307 (31.1) | |
| 80< | 151 (15.3) | |
| Subtype | ||
| Acute | n (% of all), median age, y | 511 (51.9), 68 |
| Lymphoma | 245 (24.9), 72 | |
| Chronic | 123 (12.5), 64 | |
| Smoldering | 105 (10.7), 67 | |
| Complications and past history | ||
| Malignancy other than | n (% of all) | 107 (10.9%) |
| Hematological malignancies | ||
| Autoimmune disease | 37 (3.7%) | |
FIGURE 2.
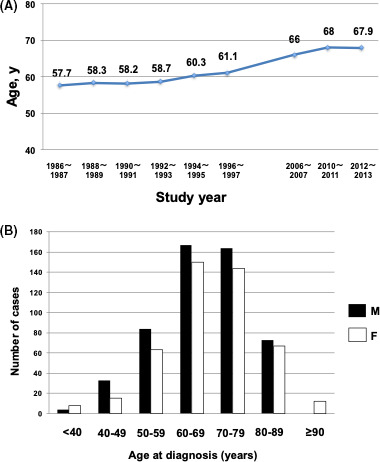
Age distribution of patients with adult T‐cell leukemia‐lymphoma. A, Mean age at diagnosis of patients by year of diagnosis from the 1980s to the present study. B, Age distribution of patients in the present study by sex
The most common subtype of ATL was acute (51.9%) (Figure 3A), followed by lymphoma (24.9%), chronic (12.5%), and smoldering (10.7%); these results were similar to the proportions in our previous 2010‐2011 study (Figure 3A and Table S3).31 However, the proportion of the acute subtype (51.9%) was slightly higher than in the previous 2010‐2011 study (49.5%).31 The proportion of smoldering and chronic subtypes together was 23.2% in the present study, which was almost similar to that in our 2010‐2011 study (24.8%) but was greater than those in the 1990s and 2006‐2007 studies (Figure 3A). In evaluating the age at diagnosis (Figure 3B), we found that the proportion of the lymphoma subtype increased with age, particularly after 70 y old, whereas the chronic subtype decreased with age (Figure 3B). In contrast with the previous 2010‐2011 study, female predominance in the chronic subtype was not observed in this study (Figure S3).
FIGURE 3.
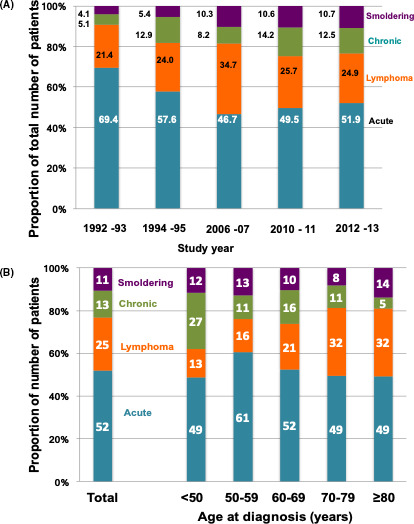
Subtype distribution of patients with ATL. A, The proportion of each subtype of ATL by study‐year from 1992‐1993 to the present study. B, The proportion of each subtype of ATL by age at diagnosis in the present study. Abbreviations: ATL, adult T‐cell leukemia‐lymphoma
3.2. Distribution of location of participating hospitals and patients' birthplaces
When we checked the geographical areas of the hospitals, Kyushu was still the area with the largest number of patients with ATL (69.8% of all patients) (Table S3), and the majority of patients diagnosed in Kyushu were also born in Kyushu where HTLV‐1 is most endemic (Figure 4A,B). In addition, almost 30% of patients with ATL who were diagnosed in the Kanto region, which includes the Tokyo metropolitan area, were born in Kyushu. Furthermore, almost 60% of patients with ATL who were diagnosed in Kinki and Chubu (the second and third largest metropolitan areas, respectively) were born in Kyushu (Figure 4A,B). In Hokkaido, Tohoku, Chugoku, and Shikoku, few patients with ATL were born in Kyushu. All the known patients in the northern non‐endemic and non‐metropolitan areas (Hokkaido and Tohoku) and most patients in the western non‐endemic and non‐metropolitan areas (Chugoku and Shikoku) had non‐immigrant origins (Figure 4A,B). If movement of the parents to the metropolitan area from Kyushu is associated with the increased number of patients with ATL in the metropolitan area, the proportion of young patients diagnosed in the metropolitan area might be higher than those diagnosed in Kyushu. To confirm this hypothesis, we next examined the proportion of young patients diagnosed in the metropolitan areas, Kyushu, and other areas. As shown in Figure S4, the proportions of patients with ATL younger than the median age (69 y) diagnosed in the metropolitan areas were 63.5%, 61.0%, and 63.2% in Kanto, Chubu, and Kinki, respectively. In contrast, the proportion in Kyushu was 38.7%.
FIGURE 4.
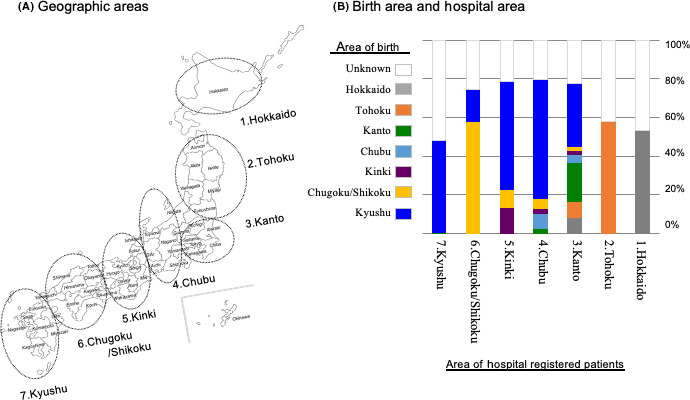
Distribution of patients with adult T‐cell leukemia‐lymphoma by area of birth and by area of hospital where registered. A, Seven geographic areas in Japan. B, The proportion of each birth area among areas of hospitals where patients were registered
3.3. Comorbidities of patients
In evaluating the prevalence of comorbid malignant diseases and history of malignant diseases other than ATL, we found that 107 (10.9%) patients had non‐hematological malignancies (Table 1), which was also similar to that found in the previous 2010‐2011 study (11.6%). However, the proportion of those with autoimmune disease was greater in the present study (3.7%) than in the previous 2010‐2011 study (0.7%).31 All the reported malignancies and autoimmune diseases are listed in Table S4. The most common malignant disease was colorectal cancer, which was similar to the previous 2010‐2011 study. The most common non‐malignant disease was RA (1.0%), which was similar to the estimates of the prevalence of RA (0.6%‐1.0% of the Japanese population) mentioned in a Japan RA cohort study.38
4. DISCUSSION
The present study renewed the epidemiological characteristics of patients with ATL diagnosed from 2012 to 2013 in Japan by using patient information from hospitals in 3 existing patient registry systems and those that registered patients in our previous nationwide ATL study in 2010‐2011.31 By cooperating with these existing academic data sources, the present study was able to reduce the burden of searching each hospital, and it enabled the updating of the clinico‐epidemiological characteristics of a large number of patients with ATL in Japan. The epidemiological features of patients with ATL in the present study were as follows: (1) the median age at diagnosis was 69 y; (2) the subtype distribution revealed that patients with smoldering and chronic subtypes comprised more than 20%; (3) approximately 70% of patients with ATL were diagnosed in the HTLV‐1‐endemic Kyushu/Okinawa areas; (4) even among the patients diagnosed in the HTLV‐1‐non‐endemic metropolitan areas, more than half of the patients were born in Kyushu where HTLV‐1 is highly endemic; (5) among the patients in Hokkaido, Tohoku, and Chugoku/Shikoku (HTLV‐1‐non‐endemic and non‐metropolitan areas), few patients were born in Kyushu/Okinawa. Therefore, the clinico‐epidemiological findings observed in the present study were distinct from those in the 1980s to 1990s and almost identical to those in our previous 2010‐2011 survey despite the difference in the data collection method between the present study and the 2010‐2011 study (Table S3). These results suggest the validity of the novel data collection method in the present study. As shown in the present and previous 2010‐2011 surveys,31 the age at diagnosis increased compared with the earlier nationwide surveys. One possible reason is the discontinuation of breastfeeding in HTLV‐1‐positive mothers. A prefecture‐wide intervention program, named the APP, started in 1987 in Nagasaki Prefecture to prevent milk‐borne transmission. Results from the study that analyzed the changes in HTLV‐1 seroprevalence in first time blood donors who donated blood from 2000 to 2006 showed that among the birth cohort of 1981‐1990, the seroprevalence was lower among those born during 1987‐1990, whereas it was stable among those born during 1981‐1986,39 indicating the effect of APP on reducing HTLV‐1 carriers. Conversely, a nationwide HTLV‐1 antibody screening test for pregnant women was started in 2011 in Japan. However, the subjects included in the present study were patients newly diagnosed with ATL between 2012 and 2013. In our cohort, APP and the HTLV‐1 antibody screening test for pregnant women might be able to reduce the number of asymptomatic carriers younger than 30 y old but not that of patients with ATL. The effect of the discontinuation of breastfeeding of HTLV‐1‐positive mothers on reducing the number of patients with ATL may probably be obtained a few decades from now.
In the present study, the most common subtype of ATL was acute, followed by lymphoma, chronic, and smoldering. This indicates that the proportion of indolent ATL is significantly lower than that of aggressive ATL, which is consistent with the results of previous studies. There are 2 possible reasons for a lower incidence of patients with indolent ATL compared to aggressive ATL; in most cases, the indolent‐type ATL is not diagnosed properly due to a lack of symptoms or abnormalities in the complete blood count. In addition, patients with acute‐type ATL may develop directly from HTLV‐1 carriers in most cases. A recent report on the sequential follow‐up of 6 HTLV‐1 carriers who developed aggressive ATL 2‐14 y later showed clonal evolution of HTLV‐1‐infected cells.40
Epidemiological studies for ATL have been conducted after it was initially described in 1977, and the responsible virus, HTLV‐1, was subsequently discovered.13, 14 A recent study in Australia revealed a high prevalence of HTLV‐1 in Aboriginal individuals but found limited development to ATL.41 Outside of Japan, epidemiological studies of ATL have been scarce compared with those of HTLV‐1, possibly reflecting the difficulty in collecting data on patients with ATL because of its rarity. The most unique aspect of the present study was collecting information on patients with ATL from candidate hospitals via existing large academic data resources that included hematological malignancies, skin cancers, and the newly established NHB cancer registry system in Japan. As a result, we were able to include, for the first time, more than 1000 patients with ATL who were diagnosed over a 2‐y period. Estimation of the annual number of newly diagnosed patients with ATL has been debated.14 An NHB survey was conducted recently, in which in total 910 new cases of ATL were diagnosed from 2006 to 2007; the estimate of the annual number of ATL cases in Japan was approximately 1000.29 The present study, using the 4 ATL registries in Japan, revealed that more than 2614 patients with ATL were newly registered during a 2‐y period. The registered number of patients in the present study suggested that the annual incidence of ATL will be approximately 30% more than that previously estimated.29 Furthermore, the number of total patients with ATL in the JSCSR and 11th JNSA is unknown, resulting in the underestimation of ATL incidence in this study. However, after a central review of the registered data, 5% of the patients were not eligible. Further efforts using database registry systems should lead to the improved accuracy of estimating the annual incidence of rare diseases such as ATL. We recognize that studies that use multiple existing databases have several shortcomings because ATL can involve both lympho‐hematological and skin systems. Two independent site‐specific databases for ATL such as the blood disease registry33 and skin cancer registry34 may cause double registration; therefore, we carefully checked the birth date, birthplace, and diagnosis date of each patient and did not find double‐entries in the present study. Previously, Takezaki et al41 suggested that the annual incidence of ATL based on the NHB survey may be underestimated because approximately 65% of ATL cases might have been missed due to a low response of the participating hospitals in endemic areas. However, in the present study, the proportion (69.8%) of registered patients from Kyushu was identical to the finding in our previous 2010‐2011 study (Table S4).31 Therefore, we believe that the low responses of the participating hospitals would be minimal. Nevertheless, further efforts should establish a more sophisticated database management system for ATL. Although the incidence of ATL is expected to decrease in the near future, there are no data supporting the estimate so far. Therefore, it is important to continue such nationwide surveys to evaluate the incidence of ATL.
In evaluating the geographic distribution of the place of diagnosis and birthplace of patients with ATL, we found that Kyushu was the most common area of ATL diagnosis and the most common birthplace of patients with ATL. Interestingly, almost 30% of patients with ATL in the Kanto region and almost 60% of those in Kinki and Chubu were born in Kyushu (Figure 4B). These distribution patterns are identical to those in HTLV‐1‐infected persons among blood donors.5, 30 In contrast, all known patients in the northern non‐endemic and non‐metropolitan areas (Hokkaido and Tohoku) and most in the western non‐endemic and non‐metropolitan areas (Chugoku and Shikoku) had non‐immigrant origins (Figure 4B). These findings suggest the importance of the clustering of HTLV‐1 carriers in the development of ATL in non‐endemic and non‐metropolitan areas, which is a similar phenomenon observed in endemic areas (Kyushu) and in contrast with the confirmed importance of immigrants in metropolitan regions.30 Additionally, we showed that the proportion of patients with ATL younger than the median age in a metropolitan area was higher than those diagnosed in Kyusyu (Figure S4). These results suggest that the relatively high incidence of patients with ATL in the 3 metropolitan areas (Tokyo, Kinki, and Chubu) (Figure 4B) was due to population migration from HTLV‐1‐endemic areas (Kyushu/Okinawa) after the high economic growth era in Japan around 1970.42, 43 Because of population migration, sporadic cases have also been observed in North America, particularly in New York and Miami, and Europe, particularly in France and the United Kingdom. The incidence of ATL is rising in non‐endemic regions of the world.41, 44, 45
In Japan, the official mortality statistics from the Ministry of Health indicates that approximately 1000 individuals have died from ATL from 1999 to 2017, with no indication of a decline in the trend.46 Therefore, HTLV‐1 infection has consistently contributed approximately 1000 deaths annually in Japan, with a clustering in individuals older than 50 y. Patients with ATL are extremely difficult to treat; a national objective is necessary to develop new agents for the treatment of patients in addition to reducing new HTLV‐1 infections in both endemic and non‐endemic areas in Japan. To tackle this problem, several vaccine candidates for HTLV‐1 are being developed not only in Japan but also internationally.47, 48, 49 In the near future, new HTLV‐1 infections are expected to be eliminated, and new ATL development should be limited in Japan.
In conclusion, the present study showed the clinico‐epidemiological features of patients with ATL who were recently diagnosed in Japan using recent advances in malignancy registration. The novel data collection method using 4 nationwide registries is feasible. To continuously and more accurately identify the clinico‐epidemiological features of patients with ATL, the establishment of a more sophisticated database management system for ATL is necessary.
CONFLICT OF INTEREST
KT received research grants from Chugai Pharmaceutical.
Supporting information
Fig S1
Fig S2
Fig S3
Fig S4
Table S1
Table S2
Table S3
Table S4
Appendix S1
ACKNOWLEDGMENTS
We thank the Blood Disease Registry of the JSH, the JSCS registry, the NHB cancer registry, and the staff at each institute for their cooperation. We thank Dr. Masanori Shimoyama for the expert opinion on this study. This work was partially supported by Grants‐in‐Aid from the Ministry of Health, Labor and Welfare of Japan (grant no. H26‐GanSeisaku‐Ippan‐006) and Grants‐in‐Aid from the AMED (grant no. 18ck0106338s0502) to SI, MI, KN, YI, KI, AU, KU, TW, and KT. We thank Enago (www.enago.jp) for the English language review.
Ito S, Iwanaga M, Nosaka K, et al; Collaborative Investigators . Epidemiology of adult T‐cell leukemia‐lymphoma in Japan: An updated analysis, 2012‐2013. Cancer Sci. 2021;112:4346–4354. 10.1111/cas.15097
Ito, Iwanaga and Tsukasaki contributed equally to this work.
Collaborative Investigators are listed in Appendix S1.
Contributor Information
Shigeki Ito, Email: shigei@iwate-med.ac.jp.
Masako Iwanaga, Email: masakoiwng@gmail.com.
Kunihiro Tsukasaki, Email: tsukasak@saitama-med.ac.jp.
REFERENCES
- 1.Uchiyama T, Yodoi J, Sagawa K, Takatsuki K, Uchino H. Adult T‐cell leukemia: clinical and hematologic features of 16 cases. Blood. 1977;50:481‐492. [PubMed] [Google Scholar]
- 2.Takatsuki K. Adult T‐cell leukemia. Intern Med. 1995;34:947‐952. [DOI] [PubMed] [Google Scholar]
- 3.Poiesz BJ, Ruscetti FW, Gazdar AF, Bunn PA, Minna JD, Gallo RC. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T‐cell lymphoma. Proc Natl Acad Sci USA. 1980;77:7415‐7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoshida M, Miyoshi I, Hinuma Y. Isolation and characterization of retrovirus from cell lines of human adult T‐cell leukemia and its implication in the disease. Proc Natl Acad Sci USA. 1982;79:2031‐2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Watanabe T. HTLV‐1‐associated diseases. Int J Hematol. 1997;66:257‐278. [DOI] [PubMed] [Google Scholar]
- 6.Yamaguchi K. Human T‐lymphotropic virus type I in Japan. Lancet. 1994;343:213‐216. [DOI] [PubMed] [Google Scholar]
- 7.Hino S, Yamaguchi K, Katamine S, et al. Mother‐to‐child transmission of human T‐lymphotropic virus type I. Jpn J Cancer Res. 1985;76:474‐480. [PubMed] [Google Scholar]
- 8.Stuver SO, Tachibana N, Okayama A, et al. Heterosexual transmission of human T cell leukaemia/lymphoma virus type 1 among married couples in southwestern Japan: an initial report from the Miyazaki cohort study. J Infect Dis. 1993;167:57‐65. [DOI] [PubMed] [Google Scholar]
- 9.Okochi K, Sato H, Hinuma Y. A retrospective study on transmission of adult T cell leukemia virus by blood transfusion: seroconversion in recipients. Vox Sang. 1984;46:245‐253. [DOI] [PubMed] [Google Scholar]
- 10.Hino S. Milk‐borne transmission of HTLV‐1 as a major route in the endemic cycle. Acta Paediatr Jpn. 1989;31:428‐435. 10.1111/j.1442-200X.1989.tb01329.x [DOI] [PubMed] [Google Scholar]
- 11.Tsukasaki K, Tobinai K. Human T‐cell lymphotropic virus type I‐associated adult T‐cell leukemia‐lymphoma: new directions in clinical research. Clin Cancer Res. 2014;20:5217‐5225. [DOI] [PubMed] [Google Scholar]
- 12.Kataoka K, Nagata Y, Kitanaka A, et al. Integrated molecular analysis of adult T cell leukemia/lymphoma. Nat Genet. 2015;47:1304‐1315. [DOI] [PubMed] [Google Scholar]
- 13.Gessain A, Cassar O. Epidemiological aspects and world distribution of HTLV‐1 infection. Front Microbiol. 2012;3:388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iwanaga M, Watanabe T, Yamaguchi K. Adult T‐cell leukemia: a review of epidemiological evidence. Front Microbiol. 2012;3:322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taylor GP. The epidemiology of HTLV‐I in Europe. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;13:S8‐S14. [DOI] [PubMed] [Google Scholar]
- 16.Einsiedel LJ, Pham H, Woodman RJ, Pepperill C, Taylor KA. The prevalence and clinical associations of HTLV‐1 infection in a remote Indigenous community. Med J Aust. 2016;205:305‐309. [DOI] [PubMed] [Google Scholar]
- 17.de Martel C, Ferlay J, Franceschi S, et al. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol. 2012;13:607‐615. [DOI] [PubMed] [Google Scholar]
- 18.Plummer M, de Martel C, Vignat J, Ferlay J, Bray F, Franceschi S. Global burden of cancers attributable to infections in 2012: a synthetic analysis. Lancet Glob Health. 2016;4:e609‐e616. [DOI] [PubMed] [Google Scholar]
- 19.The T‐ and B‐Cell Malignancy Study Group . Statistical analysis of immunologic, clinical and histopathologic data of lymphoid malignancies in Japan. Jpn J Clin Oncol. 1981;11:15‐38. [Google Scholar]
- 20.The T‐ and B‐Cell Malignancy Study Group . Statistical analyses of clinico‐pathological, virological and epidemiological data on lymphoid malignancies with special reference to adult T‐cell leukemia/lymphoma: a report of the second nationwide study of Japan. Jpn J Clin Oncol. 1985;15:517‐535. [PubMed] [Google Scholar]
- 21.The T‐ and B‐Cell Malignancy Study Group . The third nationwide study of adult T‐cell leukemia/lymphoma (ATL) in Japan: Characteristic patterns of HLA antigen and HTLV‐I infection in ATL patients and their relatives. Int J Cancer. 1988;41:505‐512. 10.1002/ijc.2910410406 [DOI] [PubMed] [Google Scholar]
- 22.Tajima K, The T‐ and B‐cell Malignancy Study Group , co‐authors. The 4th nation‐wide study of adult T‐cell leukemia/lymphoma (ATL) in Japan: estimates of risk of ATL and its geographical and clinical features. Int J Cancer. 1990;45:237‐243. 10.1002/ijc.2910450206 [DOI] [PubMed] [Google Scholar]
- 23.The T‐ and B‐cell Malignancy Study Group . The 5th nation‐wide study of adult T‐cell leukemia/lymphoma (ATL) in Japan. Gan No Rinsho. 1992;38:405‐412.[Japanese]. [Google Scholar]
- 24.The T‐ and B‐cell Malignancy Study Group . The 6th nation‐wide study of adult T‐cell leukemia/lymphoma (ATL) in Japan. Gan No Rinsho. 1994;40:229‐246.[Japanese]. [Google Scholar]
- 25.The T‐ and B‐cell Malignancy Study Group . The 7th nation‐wide study of adult T‐cell leukemia/lymphoma (ATL) in Japan. Gan No Rinsho. 1996;42:231‐247.[Japanese]. [Google Scholar]
- 26.The T‐ and B‐cell Malignancy Study Group . The 8th nation‐wide study of adult T‐cell leukemia/lymphoma (ATL) in Japan. Gan No Rinsho. 1998;44:381‐397.[Japanese]. [Google Scholar]
- 27.The T‐ and B‐cell Malignancy Study Group . The 9th nation‐wide study of adult T‐cell leukemia/lymphoma (ATL) in Japan. Gan No Rinsho. 2001;47:341‐357.[Japanese]. [Google Scholar]
- 28.Shimoyama M, The Lymphoma Study Group . Diagnostic criteria and classification of clinical subtypes of adult T‐cell leukaemia‐lymphoma. Br J Haematol. 1991;79(3):428‐437. [DOI] [PubMed] [Google Scholar]
- 29.Yamada Y, Atogami S, Hasegawa H, et al. Nationwide survey of adult T‐cell leukemia/lymphoma (ATL) in Japan. Rinsho Ketsueki. 2011;52:1765‐1771. [Japanese]. [PubMed] [Google Scholar]
- 30.Satake M, Yamaguchi K, Tadokoro K. Current prevalence of HTLV‐1 in Japan as determined by screening of blood donors. J Med Virol. 2012;84:327‐335. [DOI] [PubMed] [Google Scholar]
- 31.Nosaka K, Iwanaga M, Imaizumi Y, et al. Epidemiological and clinical features of adult T‐cell leukemia‐lymphoma in Japan, 2010–2011: a nationwide survey. Cancer Sci. 2017;108:2478‐2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chihara D, Ito H, Matsuda T, et al. Differences in incidence and trends of haematological malignancies in Japan and the United States. Br J Haematol. 2014;164:536‐545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Japanese Society of Hematology (JSH) . http://www.jshem.or.jp/modules/en
- 34.Japanese Skin Cancer Society (JSCS) . http://www.skincancer.jp/index‐e.html
- 35.The Nationwide Hospital‐based (NHB) Cancer Registry in the National Cancer Center in Japan . https://www.ncc.go.jp/en/cis/divisions/stat/index.html
- 36.Tsukasaki K, Hermine O, Bazarbachi A, et al. Definition, prognostic factors, treatment, and response criteria of adult T‐cell leukemia–lymphoma: a proposal from an international consensus meeting. J Clin Oncol. 2009;27:453‐459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ohshima K, Jaffe ES, Yoshino T, et al. Adult T‐cell leukaemia/lymphoma. In: Swerdlow SH, Campo E, Harris NL, eds. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, 4th ed. IARC press; 2017:363‐367. [Google Scholar]
- 38.Yamanaka H, Sugiyama N, Inoue E, Taniguchi A, Momohara S. Estimates of the prevalence of and current treatment practices for rheumatoid arthritis in Japan using reimbursement data from health insurance societies and the IORRA cohort (I). Mod Rheumatol. 2014;24:33‐40. [DOI] [PubMed] [Google Scholar]
- 39.Iwanaga M, Chiyoda S, Kusaba E, Kamihira S. Trends in the seroprevalence of HTLV‐1 in Japanese blood donors in Nagasaki Prefecture, 2000–2006. Int J Hematol. 2009;90:186‐190. [DOI] [PubMed] [Google Scholar]
- 40.Rowan AG, Dillon R, Witkover A, et al. Evolution of retrovirus‐infected premalignant T‐cell clones prior to adult T‐cell leukemia/lymphoma diagnosis. Blood. 2020;135:2023‐2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takezaki T, Hirose K, Hamajima N, Kuroishi T, Tajima K. Estimation of adult T‐cell leukemia incidence in Kyushu District from vital statistics Japan between 1983 and 1982: comparison with a nationwide survey. Jpn J Clin Oncol. 1997;27:140‐145. [DOI] [PubMed] [Google Scholar]
- 42.Chihara D, Ito H, Katanoda K, et al. Increase in incidence of adult T‐cell leukemia/lymphoma in non‐endemic areas of Japan and the United States. Cancer Sci. 2012;103:1857‐1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamaguchi T Population Redistribution of Japan: within the context of the national settlement system. The Proceedings of the Department of Humanities, College of Arts and Sciences. University of Tokyo 78 (Series of Human Geography 8). 1983;78:1‐18.
- 44.Ishikawa Y. The 1970s Migration Turnaround in Japan revised: a shift‐share approach. Pap Reg Sci. 1992;71:153‐173. [Google Scholar]
- 45.Cook LB, Fuji S, Hermine O, et al. Revised adult T–cell leukemia–lymphoma international consensus meeting report. J Clin Oncol. 2019;37:677‐687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Iwanaga M. Epidemiology of HTLV‐1 infection and ATL in Japan: an update. Front Microbiol. 2020;11:1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cook LB, Elemans M, Rowan AG, Asquith B. HTLV‐1: persistence and pathogenesis. Virology. 2013;435:131‐140. [DOI] [PubMed] [Google Scholar]
- 48.Willems L, Hasegawa H, Accolla R, et al. Reducing the global burden of HTLV‐1 infection: an agenda for research and action. Antiviral Res. 2017;137:41‐48. [DOI] [PubMed] [Google Scholar]
- 49.Kannagi M, Hasegawa A, Nagano Y, Kimpara S, Suehiro Y. Impact of host immunity on HTLV‐1 pathogenesis: potential of Tax‐targeted immunotherapy against ATL. Retrovirology. 2019;16:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2
Fig S3
Fig S4
Table S1
Table S2
Table S3
Table S4
Appendix S1


