Abstract
Tumor growth and progression are complex processes mediated by mutual interactions between cancer cells and their surrounding stroma that include diverse cell types and acellular components, which form the tumor microenvironment. In this environment, direct intercellular communications play important roles in the regulation of the biological behaviors of tumors. However, the underlying molecular mechanisms are insufficiently defined. We used an in vitro coculture system to identify genes that were specifically expressed at higher levels in cancer cells associated with stromal cells. Major examples included epithelial membrane protein 1 (EMP1) and stomatin, which positively and negatively regulate tumor progression, respectively. EMP1 promotes tumor cell migration and metastasis via activation of the small GTPase Rac1, while stomatin strongly suppresses cell proliferation and induces apoptosis of cancer cells via inhibition of Akt signaling. Here we highlight important aspects of EMP1, stomatin, and their family members in cancer biology. Furthermore, we consider the molecules that participate in intercellular communications and signaling transduction between cancer cells and stromal cells, which may affect the phenotypes of cancer cells in the tumor microenvironment.
Keywords: intercellular communication, signal transduction, stroma, tumor invasion, tumor microenvironment
Types of cell‐to‐cell contacts between cancer cells and stromal cells. The contacts regulate cancer cell behavior in the tumor microenvironment.
Abbreviations
- EMP1
epithelial membrane protein 1
- HSP90
heat shock protein 90
- PD‐L1
programmed cell death‐ligand 1
- PDPK1
phosphoinositide‐dependent protein kinase 1
- PrS
primary human prostate stroma
- SPFH
stomatin, prohibitin, flotillin, and HflK/C
- TM
tumor microtube
- TNT
tunneling nanotube
1. INTRODUCTION
Tumor growth and progression are complex processes that involve interactions between cancer cells and their surrounding stroma. These interactions are required for the formation of the so‐called tumor microenvironment, which includes numerous types of cells, such as fibroblasts and immune cells, as well as acellular components such as the extracellular matrix and associated soluble factors.1 These cells and components of the tumor microenvironment differ according to type, developmental stage and location of tumors, and function in a context‐dependent manner.2
The outcomes of the association between cancer cells and stroma lead to positive or negative regulation of tumor progression.3 When cancer cells appear during the early stage of tumor development, the surrounding stroma is mainly composed of normal cells that preferentially suppress tumor growth. However, during tumor progression, a subpopulation of cancer cells resist stroma‐mediated suppression, and begin to reprogram and remodel the surrounding stroma to support further tumor progression.4 This transition induces tumor progressive activities of stromal cells through epigenetic modifications and locally acting signals, which serves as a critical driver of malignancy.5 Moreover, the tumor microenvironment exhibits spatiotemporally promotive or suppressive functions that contribute to tumor progression.6
Numerous studies have shown that classical paracrine and endocrine signaling via the secretion of soluble factors, including cytokines and growth factors, into the extracellular space contribute to the regulation of the tumor microenvironment to suppress or promote tumor progression.3, 4, 7 Furthermore, direct contact between cancer cells and stromal cells may crucially affect the biological behavior of cancer cells,8 although few studies focus on this process. Therefore, this review article highlights recent findings that illuminate how direct cell‐to‐cell contact and intercellular communication between cancer cells and stromal cells regulate the characteristics and behaviors of cancer cells during tumor progression.
2. DIRECT CELL‐TO‐CELL CONTACT‐INDUCED GENE EXPRESSION IN CANCER CELLS THROUGH THEIR ASSOCIATIONS WITH STROMAL CELLS
To determine how stromal cells regulate tumor behavior through direct cell‐to‐cell contact in the microenvironment, we have recently screened for genes that are upregulated in prostate cancer LNCaP cells when they directly associate with primary human prostate stroma (PrS) cells. For this purpose, we used an in vitro coculture system that specifically detects the effect of direct cell‐to‐cell contact by limiting the effects of soluble factors secreted from cancer cells and stromal cells.9 We identified 30 genes that were markedly upregulated in cocultured LNCaP cells, including epithelial membrane protein 1 (EMP1) and stomatin, which are associated with the plasma membrane. We have determined the functions of these proteins in cancer cells, particularly in prostate cancer cells.9, 10 In this section, we introduce their detailed roles in cancer biology.
2.1. EMP1
EMP1 is a member of the growth arrest‐specific 3/peripheral myelin protein 22 kDa family, which belongs to the tetraspanin superfamily, and has been clarified to regulate membrane blebbing and inhibit spinal chondrocyte differentiation in cultured human cells.11, 12 Among the members of this family, the amino acid sequences of EMP1, EMP2, and EMP3 are highly conserved and include four predicted transmembrane domains, two extracellular domains, and small intracellular domains.13 Each protein mediates tumor progression and suppression in a cancer type‐dependent manner.14
As mentioned above, expression of EMP1 is upregulated in prostate cancer LNCaP cells cocultured with PrS cells, and increased EMP1 levels promote the progression of prostate cancer in vitro and in vivo.9 Gain of function of EMP1 in several types of cancer cells, including prostate, breast, and colorectal cancer cells, enhances their migration and invasiveness. LNCaP cells stably expressing EMP1 inoculated into the prostate grands of immunodeficient mice exhibit enhanced tumor metastasis into lymph nodes and lungs, compared with the control LNCaP cells. Primary tumors engrafted in the prostate gland similarly proliferate in control and EMP1‐expressing LNCaP cells, suggesting that EMP1 does not affect tumor growth.
The intracellular domain of EMP1 directly binds to copine‐III, which triggers an intracellular signaling cascade mediated by the protein tyrosine kinase Src and the Rac guanine nucleotide exchange factor Vav2 to activate the small GTPase Rac1, resulting in enhanced cell migration and invasiveness.9 EMP1 is uniformly distributed on the plasma membrane of cancer cells,9 while signaling molecules including Src and Rac1 are highly accumulated at the leading edge of migrating cells. One possible explanation for this different molecular accumulation is that growth factor signals, such as ErbB2 amplification,15 might support the recruitment of copine‐III to the leading edge for inducing its interaction with EMP1 and subsequent activation of signaling molecules downstream of EMP1 and copine‐III. In human prostate tumors, higher levels of EMP1 correlate with the degree of malignancy of prostate cancer. Together, these data support the conclusion that EMP1 promotes tumor metastasis by enhancing the movement of cancer cells (Figure 1). However, insufficient data are available to show how EMP1 expression is increased in cancer cells through direct association with stromal cells.
FIGURE 1.
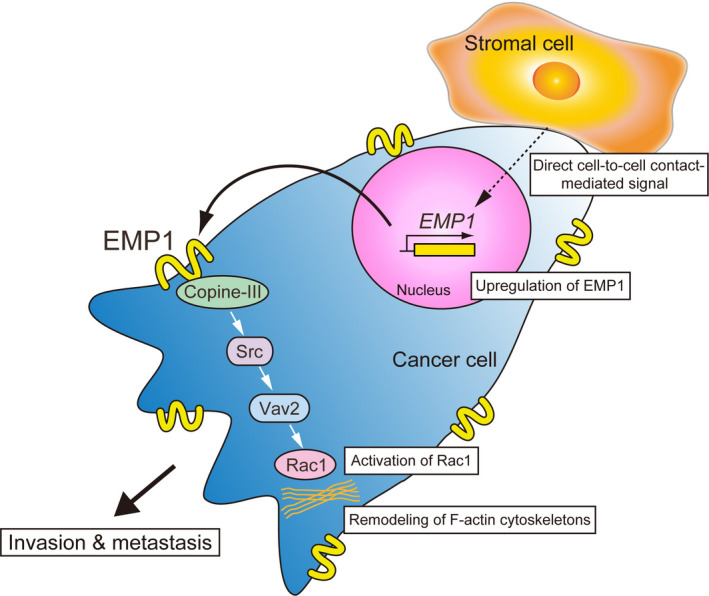
EMP1‐induced signal transduction promotes cancer metastasis. Interactions between cancer and stromal cells enhance EMP1 expression through an unidentified molecular mechanism, leading to activation of Rac1 to promote the migration of cancer cells and subsequent tumor invasion and metastasis
These findings have important clinical implications because they indicate that the development of a blocking antibody against EMP1 may inhibit metastasis. For example, inhibition of EMP2, which has pro‐metastatic functions in certain cancers, by a recombinant anti‐EMP2 bivalent antibody fragment reduces the aggressiveness of endometrial cancer.16 In our preliminary examination, the EMP1 antibody that we generated by using the epitope at the second extracellular loop of this protein showed an approximately 60% reduction of prostate cancer cell migration, which may contribute to inhibition of tumor metastasis. It is speculated that the binding of this antibody to EMP1 might change its conformation, followed by impairment of the EMP1‐copine‐III interaction. Further molecular structural study in the future would provide clear evidence for this speculation. In addition, small molecules and other agents that block the interaction between EMP1 and copine‐III may serve as anti‐metastatic cancer therapeutics.
In contrast with our findings, it has been reported that the levels of EMP1 are higher in normal tissues rather than prostate tumors, and that overexpression of EMP1 in PC3 prostate cancer cells decreases their migration and invasiveness.17 However, EMP1 is selected as one of the five novel predictive markers for poor outcomes of prostate cancer in African American men.18 Such a discrepancy in EMP1 function is also observed in breast cancer. For example, lobular carcinomas, highly malignant breast tumors, express significantly higher levels of EMP1, compared with ductal carcinomas.19, 20 In contrast, reduced expression of EMP1 is associated with shorter survival of patients with breast cancer.21 Furthermore, evidence indicates that EMP1 positively and negatively regulates tumor progression, including metastasis, depending on tumor type.17, 22 Future studies are required to identify the factor(s) that switches between the tumor progressive and suppressive functions of EMP1.
2.2. Stomatin
Stomatin is a member of the stomatin, prohibitin, flotillin, and HflK/C (SPFH) superfamily, which comprises stomatin, stomatin‐like proteins, prohibitins, flotillin/reggie proteins, and HflK/C proteins. Members of the SPFH protein family, which are highly conserved among species, are expressed in red blood cells, fibroblasts, and cancer cells.23 These proteins localize different intracellular compartments including membrane lipid rafts, and promote or inhibit diverse cellular functions, such as induction of endocytosis and reduction of protein synthesis.24, 25 Stomatin was originally called “erythrocyte band 7.2b” and later given its name “stomatin,” because this protein is not expressed in patients with hereditary stomatocytosis, a form of hemolytic anemia.26 However, different from humans, homozygous deletion of the gene encoding murine stomatin causes no obvious physiologically significant phenotype in mice.27
Stomatin is a 31 kDa integral membrane protein possessing an intramembrane domain, two palmitoylated cysteines and a coiled‐coil domain in addition to the conserved SPFH domain (Figure 2A), and mainly localizes on lipid rafts, to regulate the activities of several channels and transporters.28, 29, 30 Furthermore, stomatin is involved in the determination of cell morphology through binding to cortical actin in epithelial cells,31, 32 and increased expression of stomatin promotes cell fusion.33 Together, these findings provide compelling evidence that stomatin mediates events that occur on the plasma membrane. However, little information is known about the function of stomatin in cancer cells.
FIGURE 2.
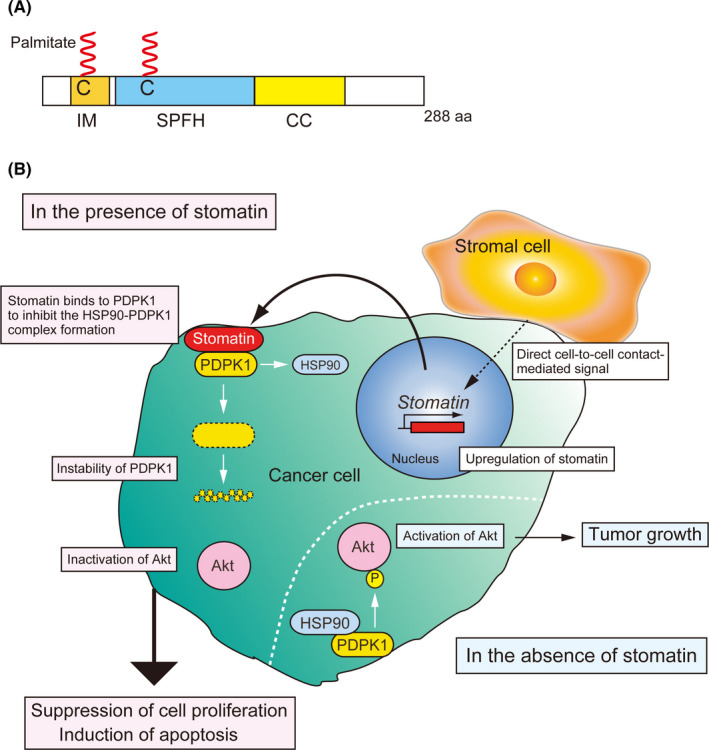
Stomatin‐mediated inhibitory mechanism of tumor growth. A, Schematic models of stomatin. C, palmitoylated cysteine; CC, coiled‐coil domain; IM, intramembrane domain; SPFH, stomatin, prohibitin, flotillin, and HflK/C domain. B, Increased stomatin expression induced by the cancer cell‐stromal cell contact inhibits Akt activation by decreasing PDPK1 expression, followed by suppression of cancer cell proliferation and induction of apoptosis. In contrast, loss of stomatin facilitates tumor growth
Similar to EMP1, LNCaP cells cocultured with PrS cells express significantly higher levels of stomatin.10 Induction of stomatin expression in LNCaP and PC3M cells, both of which do not express endogenous stomatin in the normal condition, strongly suppresses cell proliferation and induces apoptosis, leading to the inhibition of tumor growth.10 Stomatin‐mediated tumor suppression is caused by the inhibition of the Akt signaling pathway, which is crucial for cell proliferation and survival.34 Akt activation is mainly induced by phosphoinositide‐dependent protein kinase 1 (PDPK1), and the stability of PDPK1 is maintained by its binding to heat shock protein 90 (HSP90).35 Stomatin binds to PDPK1 and inhibits the formation of the PDPK1‐HSP90 complex to inhibit PDPK1 expression. Conversely, loss of function of stomatin in prostate cancer 22Rv1 cells, which express a certain degree of stomatin endogenously on the plasma membrane, elevates Akt activation and enhances tumor growth.10 Clinically, stomatin levels are significantly decreased in human prostate cancers with high Gleason scores, and lower levels of stomatin are associated with increased recurrence of prostate cancer after surgery.10 These findings demonstrate the tumor‐suppressive effect of stomatin on cancer cells (Figure 2B).
Consistent with our results, stomatin expression is decreased in malignant breast cancer positive for human epidermal growth factor receptor 2,36 and stomatin inhibits tumor metastasis of non–small‐cell lung cancer.37 For other SPFH family members in cancer biology, stomatin‐like protein 2 enhances the progression of several types of cancers,38, 39 and its overexpression in cancer cells predicts the poor prognosis of patients with colorectal and ovarian cancers.40, 41 Prohibitin possesses tumor‐suppressive and promotive functions.42 These activities depend on the intracellular localization of prohibitin. In the nucleus of a prostate cancer cell, prohibitin recruits Rb to suppress the transcriptional activity of E2F to inhibit the cell cycle.43 In contrast, localization of prohibitin on the plasma membrane activates c‐Raf and promotes cell adhesion and migration.44 Flotillin expression, which is increased in multiple types of cancer, promotes tumorigenesis.45 Together, these findings show that each member of the SPFH family plays a different role in tumor progression in a cell type‐dependent manner.
Although stomatin possesses tumor‐suppressive activity through targeting the HSP90‐PDPK1‐Akt signaling axis (Figure 2B), further studies are required to identify the mechanism of upregulation of stomatin expression in cancer cells subsequent to their direct contact with surrounding stromal cells. It has been reported that hypoxia and dexamethasone treatment induce stomatin expression in lung adenocarcinoma cells, and that dexamethasone effectively induces stomatin expression in concert with IL‐6 stimulation.31, 32, 46 The wide range of pharmacological effects of dexamethasone against numerous diseases, including inflammatory diseases and cancers,47, 48 indicate that it may be useful for anti‐cancer therapy through increasing the expression of stomatin. Therefore, identifying the mechanism that regulates stomatin expression may lead to the development of novel anti‐cancer therapeutics via stomatin‐mediated tumor‐suppressive effects.
3. DIRECT CELL‐TO‐CELL CONTACT‐MEDIATED REGULATION OF TUMOR BEHAVIOR
Several types of cell‐to‐cell contacts and communications occur during the direct interaction between cancer cells and stromal cells. These events are mainly categorized into the types as follows: contact via the molecules of the cell adhesion apparatus, contact between membrane‐tethered ligands and receptors, and contact mediated by tunneling nanotube (TNTs and tumor microtubes (TMs) (Figure 3). These molecules exert tumor‐suppressive or promotive functions, or both, in the tumor microenvironment.8
FIGURE 3.
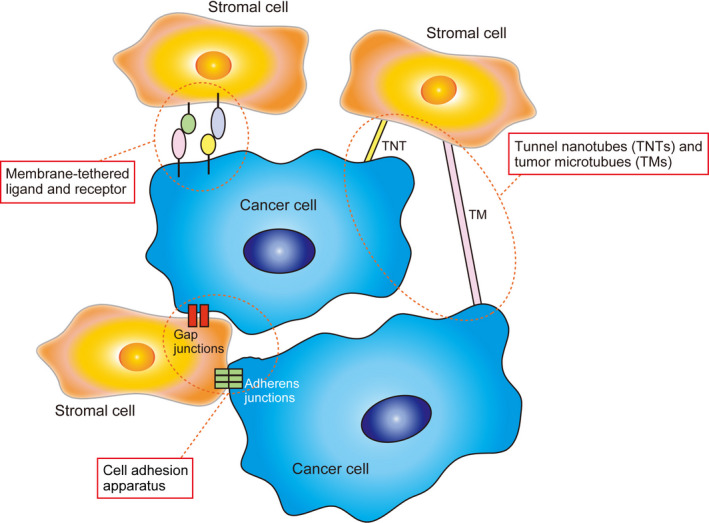
Intercellular communication between cancer cells and stromal cells mediated by the cell adhesion apparatus, membrane‐tethered ligands and receptors, and tunneling nanotubes (TNTs) and tumor microtubes (TMs)
3.1. Intercellular contact via the molecules of the cell adhesion apparatus
Adherens junctions and gap junctions, which participate in forming direct connections between neighboring cells including cancer cells and stromal cells, are essential for diverse cellular functions.8, 49 Adherens junctions mainly consist of transmembrane cell adhesion molecules, such as cadherins and nectins, and scaffold proteins that bind to the intracellular region of cell adhesion molecules to stabilize their cell surface localization.50 Invasive cancer cells usually contact stromal cells through cell adhesion molecules of adherens junctions.51, 52
Heterotypic interactions between N‐cadherin in cancer‐associated fibroblasts and E‐cadherin in cancer cells generate intercellular physical forces to promote collective invasion of cancer cells.52 Expression of E‐cadherin in cancer cells often switches to that of N‐cadherin, particularly during the epithelial‐mesenchymal transition.53 This switch promotes cancer cell invasiveness and metastasis via N‐cadherin‐enhanced fibroblast growth factor signaling. Such signaling increases the expression and secretion of matrix metalloprotease‐9 and the physical contact between cancer cells and the endothelium and stroma.54 Invasive cancer cells and stromal cells express other cadherins such as cadherin‐11 and cadherin‐23, which support the physical contact between cancer and stromal cells, contributing to tumor aggressiveness.55, 56 Cancer cells that express VE‐cadherin interact with endothelial cells to promote neovascularization.57 Furthermore, such vasculogenicity mediated by the cooperation of tumor cells with endothelial cells in the tumor microenvironment occurs in several types of aggressive cancers.58
Gap junctions comprise arrays of intercellular channels formed by connexin proteins. Connexins are integral membrane proteins that constitute a family of 21 members in humans. Connexin‐mediated junctions permit the bidirectional transfer of ions, metabolites, and secondary messengers between adjacent cells.59 Evidence indicates that connexins function as tumor suppressors or promoters, depending on the isoform, tumor stage, and tissue.60 Gap junctions formed between cancer cells and immune cells suppress tumor growth.61, 62 Gap junctions between cancer cells and endothelial cells negatively regulate angiogenesis and positively regulate metastasis.63, 64 The involvement of connexins in establishing intercellular communication between malignant tumors and astrocytes provides advantages for tumor invasiveness and resistance to chemotherapy via transfer of tumor‐protective miRNAs and cyclic GMP‐AMP (a second messenger that stimulates the production of interferon) from astrocytes to cancer cells.65, 66
3.2. Intercellular contact between membrane‐tethered ligands and their receptors
Direct contact of cancer cells with stromal cells in the tumor microenvironment is mediated by the interaction between a membrane‐tethered ligand and its receptor. Eph receptor tyrosine kinases and their ligands ephrins bidirectionally signal to mediate tumor progression.67 Early during the development of the tumor microenvironment, the tumor‐suppressive effect of Eph‐induced signaling is enhanced by ephrins expressed in surrounding normal cells, inhibiting the expansion and invasiveness of tumors that express Eph receptors.68, 69 However, during the late stage, unconventional ephrin‐independent Eph signaling activities promote cancer progression.70 In addition, activation of EphA4 signaling in breast cancer cells is induced by tumor‐associated macrophages expressing ephrins, facilitating cytokine release from cancer cells to sustain the cancer stem cell niche.71 Another signaling between membrane‐tethered ligands and their receptors is Notch signaling between cancer cells and stromal cells that contributes to tumor progression at various stages.72 In colorectal cancer, activation of the Notch1 signal triggered by its ligand delta‐like ligand 4 in endothelial cells stimulates endothelial transmigration, resulting in the promotion of metastasis.73 Endothelial cells provide another Notch ligand, Jagged‐1, to activate Notch1 on glioblastoma cells to nurture self‐renewal of cancer stem‐like cells.74 Activation of the Notch3 signal in breast and ovarian cancer cells by Jagged‐1 in their surrounding cells increases their chemotherapy resistance and proliferation.75, 76
It is well known that the interaction of the membrane‐tethered ligand programmed cell death‐ligand 1 (PD‐L1) in cancer cells with its receptor PD‐1 in effector T cells suppresses the T cell‐mediated anti‐tumor immune response and induces cancer progression.77 Furthermore, this interaction may provide cancer cells with multidrug resistance by upregulation of P‐glycoprotein expression through the phosphatidylinositol 3‐kinase/Akt and mitogen‐activated protein kinase pathways downstream of PD‐L1.78
3.3. Molecules involved in intercellular communication mediated by TNTs and TMs
Recently, newly identified types of intercellular communication, designated TNTs and TMs, function in normal cells as well as in cancer cells (Figure 4). TNTs and TMs are F‐actin‐containing thin membranous channels that differ in size to enable direct communication between cancer cells and stromal cells over long distances. These tubular structures allow the rapid exchange of cellular components and molecules, including organelles, vesicles, molecules, and ions.79 The formation of TNTs requires a complex comprising M‐Sec and the GTPase RALA to regulate the generation of F‐actin.80 In macrophages, loss of function of M‐Sec disrupts TNT‐mediated communications with cancer cells, and suppresses invasive tumor morphology.81 Deficiency of connexin‐43, which stabilizes the connection of TMs to cells, reduces the TM‐mediated network between tumor cells and astrocytes, resulting in the suppression of glioblastoma cell proliferation.82 However, TNTs and TMs are newly identified mediators for direct intercellular communications and, therefore, further investigations are required to establish their importance in the reciprocal interaction between cancer cells and stromal cells.
FIGURE 4.
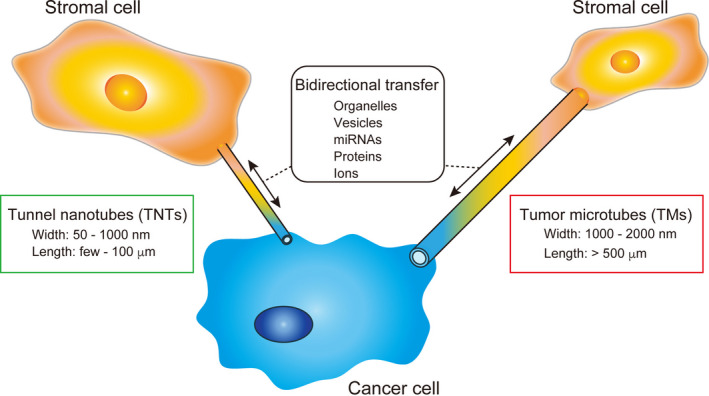
Structure and function of tunneling nanotubes (TNTs) and tumor microtubes (TMs)
4. CONCLUSIONS
Cell‐to‐cell contact‐mediated communications between cancer cells and surrounding stromal cells direct tumor behavior through promotive or suppressive activities, which often depend on the stage of tumor development and the types of tumors and stromal cells. We have recently found that the genes encoding EMP1 and stomatin are upregulated in cancer cells specifically associated with stromal cells. Among the abovementioned three types of direct cell‐to‐cell contacts, cell adhesion might be important for induction of the expression of EMP1 and stomatin by the following studies. A member of epithelial cell adhesion molecule, trophoblast cell surface antigen 2, maintains the expression of EMP1 in cholangiocarcinoma cells.83 In stomatin expression, it is upregulated by IL‐6,46 for which secretion is regulated by cell adhesion molecule cadherin‐11 as well as inflammatory cytokines.84
Furthermore, EMP1 and stomatin positively and negatively regulate cancer progression, respectively. Therefore, in addition to the actions of extracellularly secreted factors, direct cell‐to‐cell contact‐mediated gene expression and direct intercellular communication spatiotemporally contribute to the determination of tumor characteristics. Identification of other molecules that mediate mutual intercellular communication between cancer and stromal cells may provide key insights into the regulation of cancer progression.
DISCLOSURE
The authors declare no conflict of interest.
ACKNOWLEDGMENTS
We thank members at Division of Molecular Medical Biochemistry, Department of Biochemistry and Molecular Biology, Shiga University of Medical Science for their supportive advice to this article.
Sato A, Rahman NIA, Shimizu A, Ogita H. Cell‐to‐cell contact‐mediated regulation of tumor behavior in the tumor microenvironment. Cancer Sci. 2021;112:4005–4012. 10.1111/cas.15114
REFERENCES
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646‐674. [DOI] [PubMed] [Google Scholar]
- 2.Balkwill FR, Capasso M, Hagemann T. The tumor microenvironment at a glance. J Cell Sci. 2012;125:5591‐5596. [DOI] [PubMed] [Google Scholar]
- 3.Alkasalias T, Moyano‐Galceran L, Arsenian‐Henriksson M, Lehti K. Fibroblasts in the tumor microenvironment: shield or spear? Int J Mol Sci. 2018;19:1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Z, Yang Q, Tan Y, et al. Cancer‐associated fibroblasts suppress cancer development: the other side of the coin. Front Cell Dev Biol. 2021;9: 613534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chakraborty S, Sinha S, Sengupta A. Emerging trends in chromatin remodeler plasticity in mesenchymal stromal cell function. FASEB J. 2021;35:e21234. [DOI] [PubMed] [Google Scholar]
- 6.Rinn JL, Bondre C, Gladstone HB, Brown PO, Chang HY. Anatomic demarcation by positional variation in fibroblast gene expression programs. PLoS Genet. 2006;2:e119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adekoya TO, Richardson RM. Cytokines and chemokines as mediators of prostate cancer metastasis. Int J Mol Sci. 2020;21:4449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dominiak A, Chełstowska B, Olejarz W, Nowicka G. Communication in the cancer microenvironment as a target for therapeutic interventions. Cancers (Basel). 2020;12:1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahmat Amin MKB, Shimizu A, Zankov DP, et al. Epithelial membrane protein 1 promotes tumor metastasis by enhancing cell migration via copine‐III and Rac1. Oncogene. 2018;37:5416‐5434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rahman NIA, Sato A, Tsevelnorov K, et al. Stomatin‐mediated inhibition of the Akt Signaling axis suppresses tumor growth. Cancer Res. 2021;81:2318‐2331. [DOI] [PubMed] [Google Scholar]
- 11.Wilson HL, Wilson SA, Surprenant A, North RA. Epithelial membrane proteins induce membrane blebbing and interact with the P2X7 receptor C terminus. J Biol Chem. 2002;277:34017‐34023. [DOI] [PubMed] [Google Scholar]
- 12.Li ZY, Xiong SH, Hu M, Zhang CS. Epithelial membrane protein 1 inhibits human spinal chondrocyte differentiation. Anat Rec (Hoboken). 2011;294:1015‐1024. [DOI] [PubMed] [Google Scholar]
- 13.Ashki N, Gordon L, Wadehra M. Review of the GAS3 family of proteins and their relevance to cancer. Crit Rev Oncog. 2015;20:435‐447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahmat Amin MKB, Shimizu A, Ogita H. The pivotal roles of the epithelial membrane protein family in cancer invasiveness and metastasis. Cancers (Basel). 2019;11:1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heinrich C, Keller C, Boulay A, et al. Copine‐III interacts with ErbB2 and promotes tumor cell migration. Oncogene. 2010;29:1598‐1610. [DOI] [PubMed] [Google Scholar]
- 16.Shimazaki K, Lepin EJ, Wei B, et al. Diabodies targeting epithelial membrane protein 2 reduce tumorigenicity of human endometrial cancer cell lines. Clin Cancer Res. 2008;14:7367‐7377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun GG, Wang YD, Cui DW, Cheng YJ, Hu WN. EMP1 regulates caspase‐9 and VEGFC expression and suppresses prostate cancer cell proliferation and invasion. Tumour Biol. 2014;35:3455‐3462. [DOI] [PubMed] [Google Scholar]
- 18.Echevarria MI, Awasthi S, Cheng CH, et al. African American specific gene panel predictive of poor prostate cancer outcome. J Urol. 2019;202:247‐255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turashvili G, Bouchal J, Baumforth K, et al. Novel markers for differentiation of lobular and ductal invasive breast carcinomas by laser microdissection and microarray analysis. BMC Cancer. 2007;7:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arpino G, Bardou VJ, Clark GM, Elledge RM. Infiltrating lobular carcinoma of the breast: tumor characteristics and clinical outcome. Breast Cancer Res. 2004;6:R149‐R156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun GG, Wang YD, Lu YF, Hu WN. EMP1, a member of a new family of antiproliferative genes in breast carcinoma. Tumour Biol. 2014;35:3347‐3354. [DOI] [PubMed] [Google Scholar]
- 22.Miao L, Jiang Z, Wang J, et al. Epithelial membrane protein 1 promotes glioblastoma progression through the PI3K/AKT/mTOR signaling pathway. Oncol Rep. 2019;42:605‐614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lapatsina L, Brand J, Poole K, Daumke O, Lewin GR. Stomatin‐domain proteins. Eur J Cell Biol. 2012;91:240‐245. [DOI] [PubMed] [Google Scholar]
- 24.Browman DT, Hoegg MB, Robbins SM. The SPFH domain‐containing proteins: more than lipid raft markers. Trends Cell Biol. 2007;17:394‐402. [DOI] [PubMed] [Google Scholar]
- 25.Wang D, Tabti R, Elderwish S, et al. SFPH proteins as therapeutic targets for a myriad of diseases. Bioorg Med Chem Lett. 2020;30:127600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stewart GW. Stomatin. Int J Biochem Cell Biol. 1997;29:271‐274. [DOI] [PubMed] [Google Scholar]
- 27.Zhu Y, Paszty C, Turetsky T, et al. Stomatocytosis is absent in “stomatin”‐deficient murine red blood cells. Blood. 1999;93:2404‐2410. [PubMed] [Google Scholar]
- 28.Genetet S, Desrames A, Chouali Y, Ripoche P, Lopez C, Mouro‐Chanteloup I. Stomatin modulates the activity of the Anion Exchanger 1 (AE1, SLC4A1). Sci Rep. 2017;7:46170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rungaldier S, Oberwagner W, Salzer U, Csaszar E, Prohaska R. Stomatin interacts with GLUT1/SLC2A1, band 3/SLC4A1, and aquaporin‐1 in human erythrocyte membrane domains. Biochim Biophys Acta. 2013;1828:956‐966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brand J, Smith ES, Schwefel D, et al. A stomatin dimer modulates the activity of acid‐sensing ion channels. EMBO J. 2012;31:3635‐3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen JC, Cai HY, Wang Y, et al. Up‐regulation of stomatin expression by hypoxia and glucocorticoid stabilizes membrane‐associated actin in alveolar epithelial cells. J Cell Mol Med. 2013;17:863‐872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Snyers L, Thinès‐Sempoux D, Prohaska R. Colocalization of stomatin (band 7.2b) and actin microfilaments in UAC epithelial cells. Eur J Cell Biol. 1997;73:281‐285. [PubMed] [Google Scholar]
- 33.Lee JH, Hsieh CF, Liu HW, et al. Lipid raft‐associated stomatin enhances cell fusion. FASEB J. 2017;31:47‐59. [DOI] [PubMed] [Google Scholar]
- 34.Porta C, Paglino C, Mosca A. Targeting PI3K/Akt/mTOR signaling in cancer. Front Oncol. 2014;4:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fujita N, Sato S, Ishida A, Tsuruo T. Involvement of Hsp90 in signaling and stability of 3‐phosphoinositide‐dependent kinase‐1. J Biol Chem. 2002;277:10346‐10353. [DOI] [PubMed] [Google Scholar]
- 36.Chen CY, Yang CY, Chen YC, Shih CW, Lo SS, Lin CH. Decreased expression of stomatin predicts poor prognosis in HER2‐positive breast cancer. BMC Cancer. 2016;16:697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.An H, Ma X, Liu M, et al. Stomatin plays a suppressor role in non‐small cell lung cancer metastasis. Chin J Cancer Res. 2019;31:930‐944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang L, Ding F, Cao W, et al. Stomatin‐like protein 2 is overexpressed in cancer and involved in regulating cell growth and cell adhesion in human esophageal squamous cell carcinoma. Clin Cancer Res. 2006;12:1639‐1646. [DOI] [PubMed] [Google Scholar]
- 39.Yang CT, Li JM, Li LF, Ko YS, Chen JT. Stomatin‐like protein 2 regulates survivin expression in non‐small cell lung cancer cells through β‐catenin signaling pathway. Cell Death Dis. 2018;9:425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou C, Li Y, Wang G, et al. Enhanced SLP‐2 promotes invasion and metastasis by regulating Wnt/β‐catenin signal pathway in colorectal cancer and predicts poor prognosis. Pathol Res Pract. 2019;215:57‐67. [DOI] [PubMed] [Google Scholar]
- 41.Sun F, Ding W, He JH, Wang XJ, Ma ZB, Li YF. Stomatin‐like protein 2 is overexpressed in epithelial ovarian cancer and predicts poor patient survival. BMC Cancer. 2015;15:746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang J, Li B, He QY. Significance of prohibitin domain family in tumorigenesis and its implication in cancer diagnosis and treatment. Cell Death Dis. 2018;9:580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang S, Nath N, Adlam M, Chellappan S. Prohibitin, a potential tumor suppressor, interacts with RB and regulates E2F function. Oncogene. 1999;18:3501‐3510. [DOI] [PubMed] [Google Scholar]
- 44.Rajalingam K, Wunder C, Brinkmann V, et al. Prohibitin is required for Ras‐induced Raf‐MEK‐ERK activation and epithelial cell migration. Nat Cell Biol. 2005;7:837‐843. [DOI] [PubMed] [Google Scholar]
- 45.Gauthier‐Rouvière C, Bodin S, Comunale F, Planchon D. Flotillin membrane domains in cancer. Cancer Metastasis Rev. 2020;39:361‐374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Snyers L, Content J. Induction of metallothionein and stomatin by interleukin‐6 and glucocorticoids in a human amniotic cell line. Eur J Biochem. 1994;223:411‐418. [DOI] [PubMed] [Google Scholar]
- 47.Cain DW, Cidlowski JA. Immune regulation by glucocorticoids. Nat Rev Immunol. 2017;17:233‐247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cook AM, McDonnell AM, Lake RA, Nowak AK. Dexamethasone co‐medication in cancer patients undergoing chemotherapy causes substantial immunomodulatory effects with implications for chemo‐immunotherapy strategies. Oncoimmunology. 2016;5:e1066062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Giehl K, Menke A. Microenvironmental regulation of E‐cadherin‐mediated adherens junctions. Front Biosci. 2008;13:3975‐3985. [DOI] [PubMed] [Google Scholar]
- 50.Niessen CM, Gottardi CJ. Molecular components of the adherens junction. Biochim Biophys Acta. 2008;1778:562‐571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takai Y, Miyoshi J, Ikeda W, Ogita H. Nectins and nectin‐like molecules: roles in contact inhibition of cell movement and proliferation. Nat Rev Mol Cell Biol. 2008;9:603‐615. [DOI] [PubMed] [Google Scholar]
- 52.Labernadie A, Kato T, Brugués A, et al. A mechanically active heterotypic E‐cadherin/N‐cadherin adhesion enables fibroblasts to drive cancer cell invasion. Nat Cell Biol. 2017;19:224‐237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Loh CY, Chai JY, Tang TF, et al. The E‐Cadherin and N‐Cadherin switch in epithelial‐to‐mesenchymal transition: signaling, therapeutic implications, and challenges. Cells. 2019;8:1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hazan RB, Qiao R, Keren R, Badano I, Suyama K. Cadherin switch in tumor progression. Ann N Y Acad Sci. 2004;1014:155‐163. [DOI] [PubMed] [Google Scholar]
- 55.McAndrews KM, Yi J, McGrail DJ, Dawson MR. Enhanced adhesion of stromal cells to invasive cancer cells regulated by Cadherin 11. ACS Chem Biol. 2015;10:1932‐1938. [DOI] [PubMed] [Google Scholar]
- 56.Apostolopoulou M, Ligon L. Cadherin‐23 mediates heterotypic cell‐cell adhesion between breast cancer epithelial cells and fibroblasts. PLoS One. 2012;7:e33289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kaessmeyer S, Bhoola K, Baltic S, Thompson P, Plendl J. Lung cancer neovascularisation: cellular and molecular interaction between endothelial and lung cancer cells. Immunobiology. 2014;219:308‐314. [DOI] [PubMed] [Google Scholar]
- 58.Delgado‐Bellido D, Serrano‐Saenz S, Fernández‐Cortés M, Oliver FJ. Vasculogenic mimicry signaling revisited: focus on non‐vascular VE‐cadherin. Mol Cancer. 2017;16:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Villanelo F, Escalona Y, Pareja‐Barrueto C, Garate JA, Skerrett IM, Perez‐Acle T. Accessing gap‐junction channel structure‐function relationships through molecular modeling and simulations. BMC Cell Biol. 2017;18:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Aasen T, Leithe E, Graham SV, et al. Connexins in cancer: bridging the gap to the clinic. Oncogene. 2019;38:4429‐4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Saccheri F, Pozzi C, Avogadri F, et al. Bacteria‐induced gap junctions in tumors favor antigen cross‐presentation and antitumor immunity. Sci Transl Med. 2010;2:44ra57. [DOI] [PubMed] [Google Scholar]
- 62.Aucher A, Rudnicka D, Davis DM. MicroRNAs transfer from human macrophages to hepato‐carcinoma cells and inhibit proliferation. J Immunol. 2013;191:6250‐6260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang WK, Chen MC, Leong HF, Kuo YL, Kuo CY, Lee CH. Connexin 43 suppresses tumor angiogenesis by down‐regulation of vascular endothelial growth factor via hypoxic‐induced factor‐1α. Int J Mol Sci. 2014;16:439‐451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Karpinich NO, Caron KM. Gap junction coupling is required for tumor cell migration through lymphatic endothelium. Arterioscler Thromb Vasc Biol. 2015;35:1147‐1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sin WC, Aftab Q, Bechberger JF, Leung JH, Chen H, Naus CC. Astrocytes promote glioma invasion via the gap junction protein connexin43. Oncogene. 2016;35:1504‐1516. [DOI] [PubMed] [Google Scholar]
- 66.Chen Q, Boire A, Jin X, et al. Carcinoma‐astrocyte gap junctions promote brain metastasis by cGAMP transfer. Nature. 2016;533:493‐498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pasquale EB. Eph‐ephrin bidirectional signaling in physiology and disease. Cell. 2008;133:38‐52. [DOI] [PubMed] [Google Scholar]
- 68.Cortina C, Palomo‐Ponce S, Iglesias M, et al. EphB‐ephrin‐B interactions suppress colorectal cancer progression by compartmentalizing tumor cells. Nat Genet. 2007;39:1376‐1383. [DOI] [PubMed] [Google Scholar]
- 69.Guo H, Miao H, Gerber L, et al. Disruption of EphA2 receptor tyrosine kinase leads to increased susceptibility to carcinogenesis in mouse skin. Cancer Res. 2006;66:7050‐7058. [DOI] [PubMed] [Google Scholar]
- 70.Pasquale EB. Eph receptors and ephrins in cancer: bidirectional signalling and beyond. Nat Rev Cancer. 2010;10:165‐180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lu H, Clauser KR, Tam WL, et al. A breast cancer stem cell niche supported by juxtacrine signalling from monocytes and macrophages. Nat Cell Biol. 2014;16:1105‐1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Meurette O, Mehlen P. Notch signaling in the tumor microenvironment. Cancer Cell. 2018;34:536‐548. [DOI] [PubMed] [Google Scholar]
- 73.Sonoshita M, Aoki M, Fuwa H, et al. Suppression of colon cancer metastasis by Aes through inhibition of Notch signaling. Cancer Cell. 2011;19:125‐137. [DOI] [PubMed] [Google Scholar]
- 74.Zhu TS, Costello MA, Talsma CE, et al. Endothelial cells create a stem cell niche in glioblastoma by providing NOTCH ligands that nurture self‐renewal of cancer stem‐like cells. Cancer Res. 2011;71:6061‐6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Boelens MC, Wu TJ, Nabet BY, et al. Exosome transfer from stromal to breast cancer cells regulates therapy resistance pathways. Cell. 2014;159:499‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Choi JH, Park JT, Davidson B, Morin PJ, Shih IM, Wang TL. Jagged‐1 and Notch3 juxtacrine loop regulates ovarian tumor growth and adhesion. Cancer Res. 2008;68:5716‐5723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Han Y, Liu D, Li L. PD‐1/PD‐L1 pathway: current researches in cancer. Am J Cancer Res. 2020;10:727‐742. [PMC free article] [PubMed] [Google Scholar]
- 78.Liu S, Chen S, Yuan W, Wang H, Chen K, Li D. PD‐1/PD‐L1 interaction up‐regulates MDR1/P‐gp expression in breast cancer cells via PI3K/AKT and MAPK/ERK pathways. Oncotarget. 2017;8:99901‐99912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Roehlecke C, Schmidt MHH. Tunneling nanotubes and tumor microtubes in cancer. Cancers (Basel). 2020;12:857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hase K, Kimura S, Takatsu H, et al. M‐Sec promotes membrane nanotube formation by interacting with Ral and the exocyst complex. Nat Cell Biol. 2009;11:1427‐1432. [DOI] [PubMed] [Google Scholar]
- 81.Hanna SJ, McCoy‐Simandle K, Leung E, Genna A, Condeelis J, Cox D. Tunneling nanotubes, a novel mode of tumor cell‐macrophage communication in tumor cell invasion. J Cell Sci. 2019;132:jcs223321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Osswald M, Jung E, Sahm F, et al. Brain tumour cells interconnect to a functional and resistant network. Nature. 2015;528:93‐98. [DOI] [PubMed] [Google Scholar]
- 83.Sawanyawisuth K, Tantapotinan N, Wongkham C, et al. Suppression of trophoblast cell surface antigen 2 enhances proliferation and migration in liver fluke‐associated cholangiocarcinoma. Ann Hepatol. 2016;15:71‐81. [DOI] [PubMed] [Google Scholar]
- 84.Bowler MA, Bersi MR, Ryzhova LM, Jerrell RJ, Parekh A, Merryman WD. Cadherin‐11 as a regulator of valve myofibroblast mechanobiology. Am J Physiol Heart Circ Physiol. 2018;315:H1614‐H1626. [DOI] [PMC free article] [PubMed] [Google Scholar]


