Abstract
To explore the effect of insulin‐like growth factor 2 mRNA‐binding protein 2 (IGF2BP2) on colorectal cancer (CRC) by recognizing the m6A modification of YAP mRNA thus activating ErbB2 expression. High expressions of IGF2BP2, YAP, and ErbB2 promoted the proliferation, migration and invasion of CRC cells and reduced their apoptosis. IGF2BP2 recognized the m6A on YAP mRNA and promoted the translation of mRNA. YAP regulated ErbB2 expression by promoting TEAD4 enrichment in ErbB2 promoter region. Therefore, IGF2BP2 promoted the expression of ErbB2 to enhance the proliferation, invasion and migration of CRC cells, to repress cell apoptosis, and to promote solid tumor formation in nude mice. IGF2BP2 activates the expression of ErbB2 by recognizing the m6A of YAP, thus affecting the cell cycle of CRC, inhibiting cell apoptosis, and promoting proliferation.
Keywords: apoptosis, colorectal cancer, ErbB2, IGF2BP2, invasion, m6A, migration, proliferation, YAP
IGF2BP2 recognizes m6A modification of YAP mRNA. YAP acts as a tumor promoter in colorectal cancer by promoting ErbB2. IGF2BP2 recognizes YAP m6A and upregulates ErbB2 to induce colorectal cancer.
Abbreviations
- ChIP
chromatin immunoprecipitation
- CRC
colorectal cancer
- DEGs
differentially expressed genes
- HER2
human epidermal growth factor receptor‐2
- HIECs
human intestinal epithelial cells
- IGF2BP2
insulin‐like growth factor 2 mRNA‐binding protein 2
- IHC
immunohistochemistry
- m6A
methylation of the 6th adenosine
- mRNA
messenger RNA
- TP
total protein
- YAP
Yes‐associated protein
1. INTRODUCTION
Colorectal cancer (CRC) is the one of the most frequently diagnosed malignancies in the world, accounting for more than 700 000 deaths annually.1 Surgery is regarded as the gold‐standard treatment for patients with CRC at early stages, while conventional cytotoxic chemotherapy and targeted therapeutic regimens are commonly adopted to treat patients with high risks of developing recurrent or metastatic disease.2 Due to the exorbitant costs of chemotherapy, it is prudent to diagnose CRC at earlier stages and prevent deterioration for better prognosis.3 Meanwhile, the hard‐done work of our peers has highlighted the vast implications of epigenetic modification, including DNA methylation, histone modifications, and noncoding RNAs with CRC.4 Genome‐wide hypomethylation is a characteristic of CRC, which constitutes an early event in colorectal carcinogenesis by causing chromosomal instability.5 Thus, an elaborate understanding of the epigenetic regulatory mechanisms in CRC might enable their future clinical applications as prognosis biomarkers or their potential as therapeutic targets.
Methylation of the 6th adenosine (m6A) is the most frequent messenger RNA (mRNA) modification and plays an important role in the regulation of gene expression at a post‐transcriptional level.6, 7 m6A is dynamically deposited, removed, and recognized by m6A methyltransferases (“writers”), demethylases (“erasers”), and m6A‐specific binding proteins (“readers”), respectively. What is notable is that m6A and its regulators are often dysregulated in a variety of malignancies, including breast cancer and CRC.8, 9, 10, 11 Additionally, single‐nucleotide polymorphisms in the m6A modification core genes are also known to contribute to the etiology of cancer; for instance, the rs7766006, YTHDF2 rs3738067, and FTO rs9939609 variants confer an amplified risk of glioma.12 Meanwhile, rs62328061 and rs298982 exhibit significant association with decreased susceptibility to neuroblastoma, whereas rs4834698 and rs9884978 can augment the risk of neuroblastoma.13 Additionally, the insulin‐like growth factor 2 mRNA‐binding protein 2 (IGF2BP2) is recognized as a crucial reader for m6A modification and further participates in the pathogenesis of various malignancies.12 Moreover, a prior study suggested that IGFBP2 plays a key tumor‐promotive role in the progression of CRC.14 Furthermore, another study further identified that insulin‐like growth factor‐1 receptor serves as a vital mediator in the development of intratumor hypoxia by regulating the Yes‐associated protein (YAP).15 Recent evidence suggests that YAP serves as an important driver of cancer development, tumor growth, and metastasis.16 A large proportion of the YAP protein is localized in the nucleus in breast cancer with high YAP expression levels.17 Similarly, overexpression of YAP can significantly augment the proliferation of ovarian granulosa cells.18 On the other hand, knockdown of YAP can reduce the growth and ability to trigger tumor formation in human CRC lines.19 However, there is lack of research on the epigenetic regulation of YAP expression in CRC, particularly in regard to YAP mRNA methylation. Nevertheless, YAP possesses the ability to regulate liver cell proliferation by directly targeting the ErbB2 promoter.20 In recent years, the genetic mutation of ErbB2, also known as human epidermal growth factor receptor‐2 (HER2), has been documented in multiple cancer types including breast cancer, gastric cancer, and CRC.21, 22 In addition, preclinical studies have further confirmed a causative role of ErbB2 in tumorigenesis.21 Therefore, a deep understanding of the mechanism underlying ErbB2 in regulating tumor cell development will provide more strategy for the treatment of CRC.
Consequently, we collected CRC samples from patients and analyzed the relationship between IGF2BP2 and clinical features. Furthermore, we elucidated the mechanism of IGF2BP2 in regulating YAP m6A modification and ErbB2 expression, which can provide a new strategy in treating CRC.
2. MATERIALS AND METHODS
2.1. In silico analysis
First, Colon adenocarcinoma (COAD)‐related expression dataset was retrieved from The Cancer Genome Atlas (TCGA) database (https://portal.gdc.cancer.gov/). A total of 512 samples were obtained, comprising 41 control samples and 471 COAD samples. In addition, human genome data were obtained from GENCODE (https://www.gencodegenes.org/human/). The perl language (https://www.perl.org/) was then adopted to convert gene names in the COAD dataset to GeneSymbol, and then 22 genes related to m6A modification were obtained from m6A2target (http://m6a2target.canceromics.org/#/home). The R language (https://www.r‐project.org/) was employed to extract the m6A modification–related genes from the COAD dataset, and the number of zero values and null values was retrieved in the data. As IGF2BP1 and IGF2BP3 presented with more zero values, 20 m6A modification–related genes were selected for subsequent analyses. In order to accurately analyze the relationship among genes in CRC, the COAD dataset after deleting the control sample was designated as COAD#.
The “limma” package of R language (https://bioconductor.org/packages/limma/) was adopted again to analyze the difference between the control and COAD sample in COAD to obtain the expression of m6A modified genes, and the differentially expressed genes (DEGs) were screened with a threshold of |log fold change (FC)| > 0.2 and P < .01. The “pheatmap” package (version 1.0.12, https://CRAN.R‐project.org/package =pheatmap) was employed to plot a heat map of m6A modification–related genes, and the “vioplot” package (https://CRAN.R‐project.org/package=vioplot) was adopted to draw a violin map of m6A modification–related genes. Pearson correlation among the expressions of genes was analyzed using R language built‐in function “cor.test(),” and the top 300 memories were regarded as the most relevant genes.
The “survival” package of R language (version 3.1‐11, https://CRAN.R‐project.org/package =survival) was then employed for Kaplan‐Meier survival analysis, and the top 25% samples were selected as the high‐expression group, while the rest 75% samples were regarded as low expression for IGF2BP2. The difference in overall survival between the high‐expression group and low‐expression group was subsequently analyzed, and the overall survival curve was plotted. The Gene‐list Enrichment module of KO‐Based Annotation System (KOBAS) (http://kobas.cbi.pku.edu.cn/kobas3) was used for Goal‐Oriented (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analyses.
2.2. Clinical sample collection
A total of 66 patients diagnosed with CRC at the General Hospital of Ningxia Medical University from August 2012 to August 2014 were enrolled in the current study. None of the included patients received radiotherapy or preoperative chemotherapy. Obtaiend tumor tissues and adjacent normal tissues were dissected and stored in liquid nitrogen for subsequent experiments.
For further details regarding the materials and methods used, please refer to the Supplementary Information.
2.3. Statistical analysis
Statistical analyses were performed using the SPSS version 19.0 (/20.0/21.0/22.0) (IBM Corp.). Measurement data were shown as mean ± standard deviation. Data between two groups were compared by unpaired t‐test, while one‐way analysis of variance (ANOVA) was adopted for data comparisons among multiple groups. The optical density values at different time points were compared by two‐factor ANOVA. The survival rate of patients was calculated using the Kaplan‐Meier method, and univariate analysis was performed with the log‐rank test. The correlation between the expression of IGF2BP2 and clinical indicators of CRC patients was analyzed by the chi‐square test.
3. RESULTS
3.1. Silencing of IGF2BP2 restrained the proliferation, migration, and invasion of CRC cells and promoted their apoptosis
First, 22 genes related to m6A modification were obtained from the m6A2target. In addition, COAD expression data were obtained through the TCGA database, and the expression patterns of 22 m6A modification–related genes were assessed using the R language. It was found that IGF2BP1 and IGF2BP3 presented with 186 and 30 zero value, respectively, while the remaining 20 genes exhibited no zero value; thus, the expression data of IGF2BP1 and IGF2BP3 were deleted. Subsequent differential analyses of these 20 genes revealed that all genes exhibited significant differences in COAD, among which METL14, ALKBH5, YTHDF3, and YTHDC2 were poorly expressed genes, while WTAP, YTHDC1, YTHDF2, RBM15B, FTO, HNRNPC, HNRNPA2B1, ZC3H13, IGF2BP2, CBLL1, METL16, VIRMA, RBM15, ZCCHC4, YTHDF1, and METL3 were highly expressed genes (Figure 1A, B). The survival time and survival status of each COAD sample were further obtained through TCGA, and the relationship between the expression of these 20 genes and patient survival was analyzed. The patients were then divided into a high‐expression group and a low‐expression group based on the first 25% and last 75% of the gene expression value. It was found that solely the high expression of IGF2BP2 was associated with reduced survival rate of COAD patients (Figure 1C, Table S3). RT‐qPCR was further employed to measure the expression patterns of IGF2BP2 in 66 cases of CRC tissues and the corresponding adjacent normal normal tissues, which revealed that the IGF2BP2 expression in CRC tissues was 1.75 times higher than that in adjacent normal tissues (Figure 1D). In addition, the expression patterns of IGF2BP2 in human CRC cells (SW480, SW620, and HCT‐8) and HIEC‐6 were detected with RT‐qPCR, which illustrated that compared with HIEC‐6 cells, the expression of IGF2BP2 in CRC cell lines was remarkably increased, and the difference was the most pronounced in SW480 cells (Figure 1E). Therefore, the SW480 cells were selected for subsequent experimentation.
FIGURE 1.
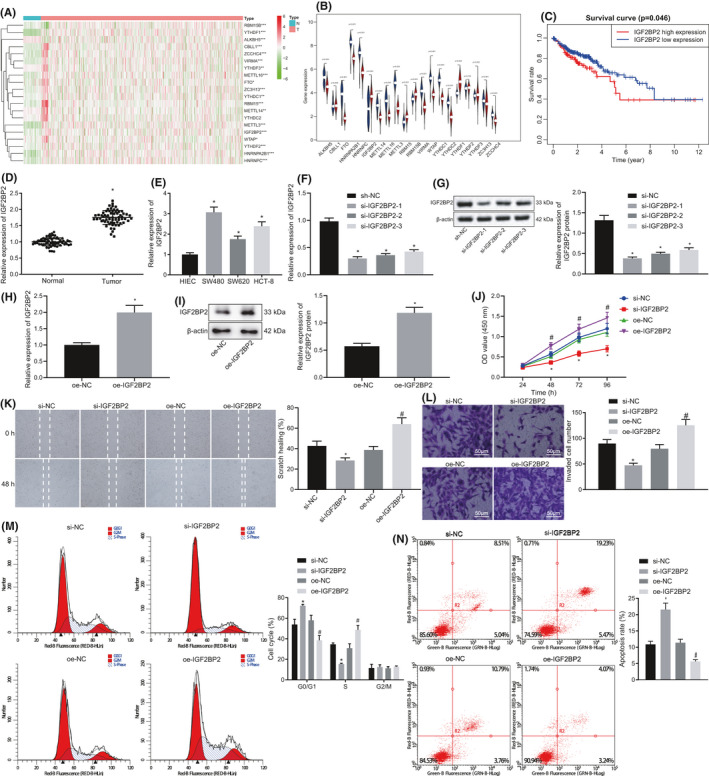
Silencing of insulin‐like growth factor 2 mRNA‐binding protein 2 (IGF2BP2) inhibited the proliferation, migration, and invasion of colorectal cancer (CRC) cells and promoted apoptosis. A, Expression heat map of methylation of the 6th adenosine (m6A)‐modified genes in the COAD dataset obtained from the TCGA database, *P < .05, **P < .01, ***P < .001. B, Violin chart of m6A‐modified gene expression, wherein blue represents the control group and red represents the cancer group. C, Survival curve of IGF2BP2 in COAD. D, Expression of IGF2BP2 in CRC tissues and adjacent normal tissues tested by RT‐qPCR. n = 66. *P < .05, compared with the normal group. E, Expression of IGF2BP2 in four kinds of CRC cells and normal human intestinal epithelial cells tested by RT‐qPCR. *P < .05, compared with human intestinal epithelial cells (HIEC‐6) cells. F, mRNA expression of IGF2BP2 in cells transfected with si‐IGF2BP2‐1, si‐IGF2BP2‐2, and si‐IGF2BP2‐3 tested by RT‐qPCR. G, Protein expression of IGF2BP2 in cells transfected with si‐IGF2BP2‐1, si‐IGF2BP2‐2, and si‐IGF2BP2‐3 measured by Western blot analysis. *P < .05, compared with cells transfected with si‐normal control (NC). H‐I, mRNA expression of IGF2BP2 in the oe‐NC group and the oe‐IGF2BP2 group detected by RT‐qPCR. I, Protein expression of IGF2BP2 in the oe‐NC group and the oe‐IGF2BP2 group measured by Western blot analysis. *P < .05, compared with cells transfected with oe‐NC. J, Proliferation of cells tested by CCK‐8 assay. K, Cell migration measured by the scratch test. L, Cell invasion detected by Transwell assay. M, Cell cycle distribution detected by flow cytometric analysis. N, Cell apoptosis assessed by flow cytometric analysis. *P < .05, compared with cells transfected with si‐NC. #P < .05 compared with cells transfected with oe‐NC. The results are measurement data, shown as mean ± standard deviation. Unpaired t‐test was used to compare the data between two groups, and one‐way ANOVA was used to compare the data among multiple groups. The survival rate of patients was calculated by the Kaplan‐Meier method, and univariate analysis was performed by the log‐rank test. The cell experiments were repeated three times
To further elucidate the effect of IGF2BP2 on the proliferation, migration, invasion, and apoptosis of SW480 cells, we treated SW480 cells with three si‐IGF2BP2 sequences to silence the expression of IGF2BP2, and detected the mRNA and protein expression patterns of IGF2BP2 in SW480 cells using RT‐qPCR and Western blot analysis, respectively. Compared with cells transfected with si‐normal control (NC), the mRNA and protein expressions of IGF2BP2 were found to be significantly reduced following transfection with si‐IGF2BP2‐1, si‐IGF2BP2‐2, or si‐IGF2BP2‐3, among which si‐IGF2BP2‐1 brought about the lowest expression of IGF2BP2 and the highest interference efficiency. Therefore, si‐IGF2BP2 was chosen for subsequent experimentation (Figure 1F, G). In addition, SW480 cells were transfected with oe‐IGF2BP2 plasmids to overexpress IGF2BP2, and the mRNA and protein expression patterns of IGF2BP2 in each group were measured by RT‐qPCR and Western blot analysis, respectively. Compared with cells transfected with oe‐NC, the mRNA and protein expressions of IGF2BP2 were found to be markedly increased after transfection with oe‐IGF2BP2 plasmids (Figure 1H, I). At the same time, the relationship between IGF2BP2 levels and clinical indicators of CRC patients was statistically analyzed, which revealed that IGF2BP2 levels were notably correlated with tumor size, TNM stage, and tumor differentiation (Table S4). Meanwhile, the results of CCK‐8 assay showed that the cell proliferation was significantly decreased following transfection with si‐IGF2BP2, while the cells transfected with oe‐IGF2BP2 presented with increased cell proliferation (Figure 1J). Moreover, cell migration was assessed with the help of a scratch test, which demonstrated that migration of cells transfected with si‐IGF2BP2 was decreased compared with that of cells transfected with si‐NC, whereas the opposing trends were documented in cells transfected with oe‐IGF2BP2 compared with cells transfected with oe‐NC (Figure 1K). Furthermore, Transwell assay results illustrated that the cell invasion was reduced following transfection with si‐IGF2BP2, while being increased after transfection with oe‐IGF2BP2 (Figure 1L). Meanwhile, flow cytometric data suggested that compared with cells transfected with si‐NC, the proportion of G0/G1 phase–arrested cells was remarkably increased and that of S phase–arrested cells was notably reduced, while apoptosis was markedly increased in cells transfected with si‐IGF2BP2. Besides, compared with cells transfected with oe‐NC, the proportion of G0/G1 phase–arrested cells was markedly reduced and that of S phase–arrested cells was considerably increased, whereas apoptosis was significantly reduced in cells transfected with oe‐IGF2BP2 (Figure 1M, N). Altogether, these findings indicated that silencing of IGF2BP2 inhibited the proliferation, migration, and invasion of CRC cells and promoted apoptosis.
3.2. IGF2BP2 increased the stability of YAP by binding to m6A‐modified YAP mRNA in CRC cells
In order to further determine the downstream genes of IGF2BP2, genes presenting with more than 10 zero value in the expression data of COAD were excluded, and differential analysis was subsequently performed using the R language. After screening with the threshold of |logFC| > 0.2 and P < .01, 8823 DEGs were obtained. Moreover, the normal samples in the COAD data (COAD#) were deleted, and the correlation between the expression of IGF2BP2 in COAD# and other genes was analyzed in order to uncover the genes related to IGF2BP2 in CRC. The top 300 related genes were screened, among which the smallest absolute value of correlation was |cor| = 0.353, and its significance was P = 2.83E ‐ 15 (Table S5). It was found that IGF2BP2 was predicted by m6A2target to mediate the m6A modification of 7653 genes. By intersecting the DEGs in COAD, IGF2BP2‐related genes in COAD#, and the predicted IGF2BP2‐mediated genes by m6A2target, a total of 59 shared genes were obtained (Figure 2A). m6A modification of HMGA2 mediated by IGF2BP2 has been widely studied, and the number of documents related to CRC and CCND1 is 331, which is about 3.3 times that of YAP1. Consequently, YAP1 was selected as subject of focus for further investigation. YAP1 expression data were subsequently obtained and analyzed, and the results validated that YAP1 was indeed highly expressed in CRC (Figure 2B). In addition, the results of RT‐qPCR showed that the expression of YAP in CRC tissues was 1.592 times higher than that in adjacent normal tissues (P < .05) (Figure 2C). Meanwhile, YAP was also found to be highly expressed in human CRC cell lines (SW480, SW620, HCT‐8) relative to human normal intestinal epithelial cells HIEC‐6, with the SW480 cells exhibiting the highest YAP expression (Figure 2D). Moreover, the results of RT‐qPCR and Western blot analysis illustrated that compared with cells transfected with si‐NC, YAP expression in cells transfected with si‐IGF2BP2 group was much lower (P < .05). On the other hand, compared with cells transfected with oe‐NC, YAP expression in cells transfected with oe‐IGF2BP2 was increased (Figure 2E, F). Meanwhile, IGF2BP2 expression was found to be positively correlated with YAP1 expression in COAD# (Figure 2G). Furthermore, Pearson correlation analysis suggested that IGF2BP2 expression was positively correlated with YAP expression in clinical samples of CRC (Figure 2H). Furthermore, Me‐RIP results illustrated that compared with cells transfected with si‐NC, YAP m6A modification levels were markedly decreased in cells transfected with si‐IGF2BP2. On the contrary, YAP m6A modification levels were remarkably increased in cells transfected with oe‐IGF2BP2 compared with cells transfected with oe‐NC (Figure 2I). The detection results of the YAP downstream target gene expression further demonstrated that knockdown of IGF2BP2 decreased the expressions of CYR61 and TGF, while overexpression of IGF2BP2 brought about the opposite trends (Figure 2J). Altogether, these findings indicated that IGF2BP2 bound to m6A‐modified YAP mRNA and then increased the stability of YAP in CRC cells.
FIGURE 2.
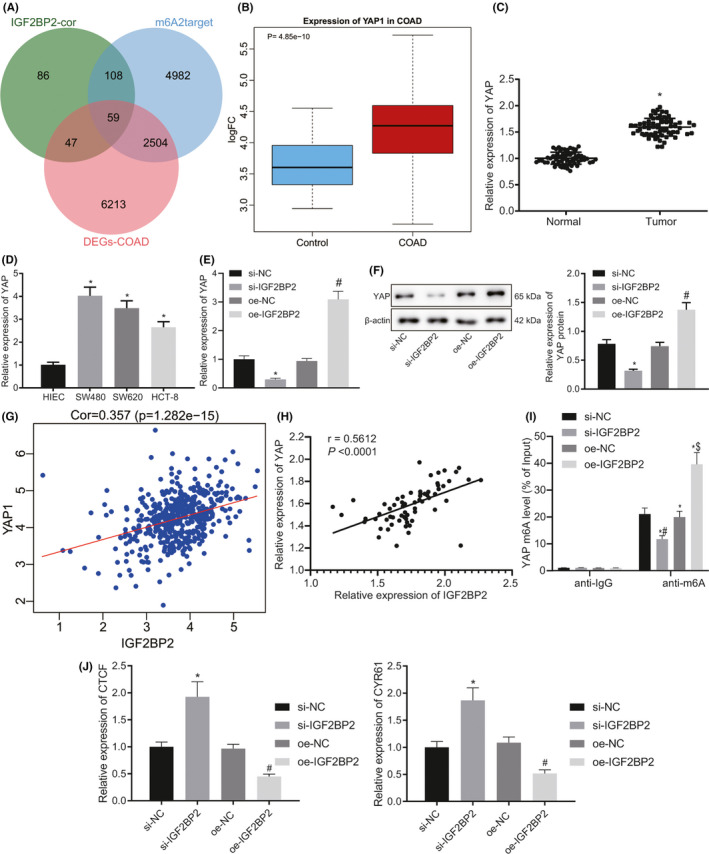
Insulin‐like growth factor 2 mRNA‐binding protein 2 (IGF2BP2) increased the stability of Yes‐associated protein (YAP) by binding to methylation of the 6th adenosine (m6A)‐modified YAP mRNA in colorectal cancer (CRC) cells. A, Venn diagram of COAD differential genes, IGF2BP2‐related genes in COAD#, and the IGF2BP2‐mediated genes predicted by m6A2target showing 59 intersection genes. B, Box plot of YAP1 expression in the COAD dataset, where the left blue box is the control group and the red box on the right is the cancer group. C, Expression of YAP in CRC tissues and adjacent normal tissues detected by RT‐qPCR. n = 66. *P < .05, compared with adjacent normal tissues. D, Expression of YAP in four kinds of CRC cells and normal human intestinal epithelial cells detected by RT‐qPCR. *P < .05, compared with HIEC‐6 cells. E, Expression of YAP in cells when IGF2BP2 was silenced or overexpressed tested by RT‐qPCR. *P < .05, compared with cells transfected with si‐normal control (NC). #P < .05, compared with cells transfected with oe‐NC. F, Expression of YAP in cells when IGF2BP2 was silenced or overexpressed tested by Western blot analysis. *P < .05, compared with cells transfected with si‐NC. #P < .05 compared with cells transfected with oe‐NC. G, Expression correlation diagram of YAP1 and IGF2BP2 in the COAD# dataset. H, Correlation analysis of IGF2BP2 and YAP in 66 cases of human CRC tissues by Pearson correlation analysis. I, m6A level of MYC mRNA detected by Me‐RIP. *P < .05, compared with cells treated by IgG. #P < .05, compared with cells transfected with si‐NC. $P < .05, compared with cells transfected with oe‐NC. J, Expression of the YAP downstream target genes CYR61 and TGF detected by RT‐qPCR in cells following IGF2BP2 silencing or overexpression. The results are measurement data, shown as mean ± standard deviation. Unpaired t‐test was used to compare the data between two groups, and one‐way ANOVA was used to compare the data among multiple groups. The cell experiments were repeated three times
3.3. IGF2BP2 stimulated the proliferation, migration, and invasion of CRC cells while suppressing apoptosis by stabilizing YAP expression
To further explore the effect of IGF2BP2 on CRC cells, CRC cells were transfected with si‐IGF2BP2 or combined with oe‐YAP. Subsequent results of RT‐qPCR and Western blot analysis demonstrated that compared with cells transfected with si‐NC + oe‐NC, the mRNA and protein expression levels of IGF2BP2 and YAP were dramatically reduced in cells transfected with si‐IGF2BP2 + oe‐NC. Meanwhile, there was no significant alteration in the mRNA and protein expressions of IGF2BP2 in cells following transfection of si‐IGF2BP2 + oe‐YAP, while mRNA and YAP protein expressions were found to be increased in comparison with cells transfected with si‐IGF2BP2 + oe‐NC (P < .05) (Figure 3A, B). In addition, cell proliferation was tested by means of a CCK‐8 assay, which illustrated that the cell proliferation was reduced after treatment of si‐IGF2BP2 + oe‐NC compared with that of cells transfected with si‐NC + oe‐NC. Conversely, the proliferation of cells transfected with si‐IGF2BP2 + oe‐YAP was significantly increased compared with that of cells transfected with si‐IGF2BP2 + oe‐NC (P < .05) (Figure 3C). Furthermore, the results of scratch test demonstrated that the migration of cells transfected with si‐IGF2BP2 + oe‐NC was reduced compared with that of cells transfected with si‐NC + oe‐NC, while being remarkably increased following transfection with si‐IGF2BP2 + oe‐YAP compared with cells transfected with si‐IGF2BP2 + oe‐NC (P < .05) (Figure 3D). In addition, Transwell assay data showed that the invasion of cells transfected with si‐IGF2BP2 + oe‐NC was notably reduced compared with that of cells transfected with si‐NC + oe‐NC, while the invasion of cells transfected with si‐IGF2BP2 + oe‐YAP was increased compared with that of cells transfected with si‐IGF2BP2 + oe‐NC (P < .05) (Figure 3E). Flow cytometric analysis further demonstrated that the proportion of G0/G1 phase–arrested cells was significantly increased after treatment with si‐IGF2BP2 + oe‐NC, while that of S phase–arrested cells was reduced, and the apoptosis ability was increased compared with cells transfected with si‐NC + oe‐NC (P < .05). Meanwhile, the proportion of G0/G1 phase–arrested cells was reduced after treatment with si‐IGF2BP2 + oe‐YAP and that of S phase–arrested cells was increased, while cell apoptosis was decreased compared with that of cells transfected with si‐IGF2BP2 + oe‐NC (P < .05) (Figure 3F, G). Together, these findings indicated that IGF2BP2 could boost the proliferation, migration, and invasion of CRC cells but repressed apoptosis by stabilizing the expression of YAP.
FIGURE 3.
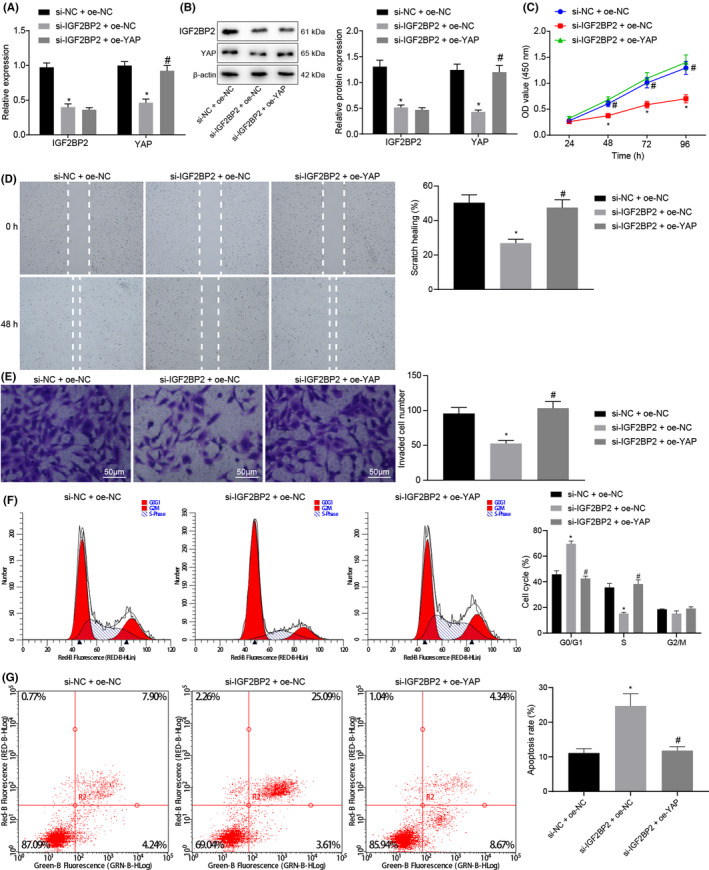
Insulin‐like growth factor 2 mRNA‐binding protein 2 (IGF2BP2) promoted the proliferation, migration, and invasion of colorectal cancer (CRC) cells while attenuating cell apoptosis by stabilizing the expression of Yes‐associated protein (YAP). CRC cells were transfected with si‐IGF2BP2 or combined with oe‐YAP. A, mRNA expression of IGF2BP2 and YAP in CRC cells detected by RT‐qPCR. B, Protein expression of IGF2BP2 and YAP in CRC cells measured by Western blot analysis. C, Cell proliferation tested by CCK‐8 assay. D, Cell migration measured by scratch test. E, Invasion of cells measured by Transwell assay. F, Cell cycle distribution detected by flow cytometry. G, Cell apoptosis assessed by flow cytometry. *P < .05, compared with cells transfected with si‐normal control (NC) + oe‐NC. #P < .05, compared with cells transfected with si‐IGF2BP2 + oe‐NC. The results are measurement data, shown as mean ± standard deviation. Unpaired t‐test was used to compare the data between two groups, and one‐way ANOVA was used to compare the data among multiple groups. Data at different time points were compared by two‐way ANOVA. The cell experiments were repeated three times
3.4. IGF2BP2 increased the expression of ErbB2 by stabilizing YAP
A ChIP assay was conducted to detect the enrichment of transcription factor TEAD4 at the ErbB2 promoter in the presence of YAP overexpression. The results illustrated that TEAD4 was enriched at the ErbB2 promoter after YAP overexpression (Figure 4A). The results of RT‐qPCR further revealed that the expression of ErbB2 in CRC tissues was 1.629 times higher than that in adjacent normal tissues (Figure 4B). Moreover, RT‐qPCR data also revealed increased expressions of ErbB2 in human CRC lines (SW480, SW620, and HCT‐8) compared with human normal intestinal epithelial cells HIEC‐6, and the difference was the most pronounced in SW480 cells (Figure 4C). Subsequent analyses of the correlation between the expression of ErbB2 in COAD# and other genes revealed a total of 300 most relevant genes (Table S6). These genes and the 8823 DEGs in COAD were then intersected, and 72 more important related genes were obtained (Figure 4D). Through the GO and KEGG enrichment analyses of these 72 related genes by KOBAS, the GO annotations primarily enriched in ErbB2 included cellular anatomical entity, chemorepellent activity, regulation of developmental growth, transcription coregulator activity, and apical junction complex (Figure 4E), and the main enriched KEGG pathways contained the Notch signaling pathway, Axon guidance, Tight junction, Fanconi anemia pathway, and Glycerolipid metabolism (Figure 4F).
FIGURE 4.
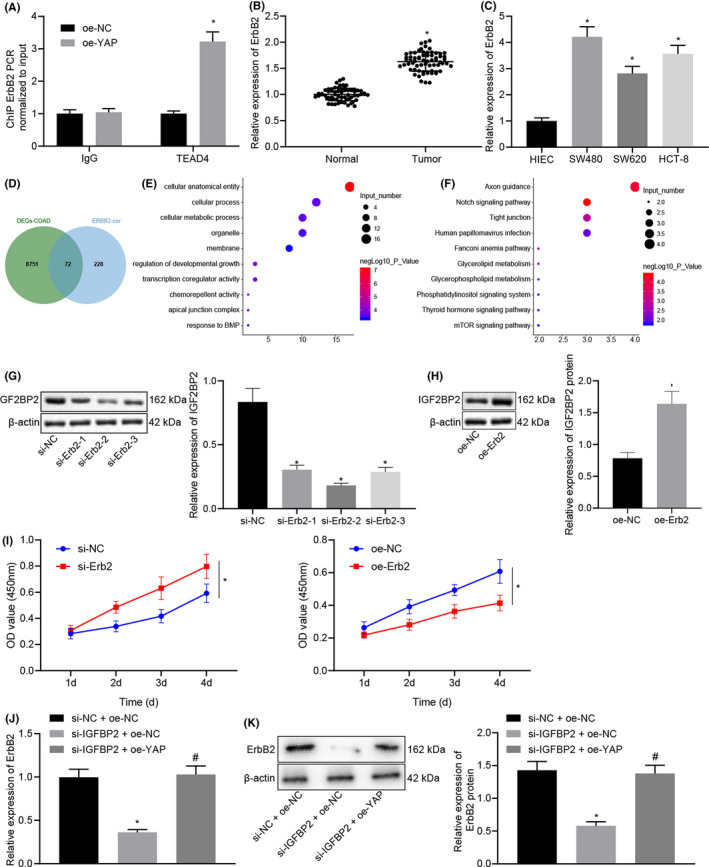
Insulin‐like growth factor 2 mRNA‐binding protein 2 (IGF2BP2) upregulated the expression of ErbB2 by stabilizing the expression of Yes‐associated protein (YAP). A, Enrichment of TEAD in the ErbB2 promoter upon YAP overexpression detected by ChIP assay. *P < .05, compared with cells transfected with oe‐normal control (NC). B, Expression of ErbB2 in colorectal cancer (CRC) tissues and adjacent normal tissues detected by RT‐qPCR. n = 66. *P < .05, compared with adjacent normal tissues. C, Expression of ErbB2 in CRC cells and normal human intestinal epithelial cells detected by RT‐qPCR. *P < .05, compared with human intestinal epithelial cells (HIEC‐6) cells. D, Venn diagram of DEGs in COAD and ErbB2‐related genes in COAD# showing 72 intersection genes. E, Bubble chart of the top 10 GO entries of 72 intersection genes obtained using KOBAS, wherein the ordinate indicates the enriched item, the horizontal indicates the number of genes enriched in the entry, and the color of the bubble indicates the significance of the enrichment‐lgP value. F, Bubble chart of the top 10 genes in the 72 intersection genes according to the KEGG analysis by KOBAS, wherein the ordinate indicates the enriched entry, the abscissa indicates the number of genes enriched in the item, and the color of the bubble indicates the significance of the enrichment‐lgP value. G, Protein expression of ErbB2 measured by Western blot analysis in cells transfected with si‐ErbB2‐1, si‐ErbB2‐2, or si‐ErbB2‐3. H, Protein expression of ErbB2 measured by Western blot analysis in cells transfected with oe‐ErbB2. *P < .05, compared with cells transfected with si‐NC or oe‐NC. I, Cell proliferation tested by CCK‐8 assay. **P < .01, compared with cells transfected with si‐NC or oe‐NC. J, Protein expression of ErbB2 detected by RT‐qPCR. K, Protein expression of ErbB2 measured by Western blot analysis. *P < .05, compared with cells transfected with si‐NC + oe‐NC. #P < .05, compared with cells transfected with si‐IGF2BP2 + oe‐NC. The results are measurement data, shown as mean ± standard deviation. Unpaired t‐test was used to compare the data between two groups and one‐way ANOVA was used to compare the data among multiple groups. The cell experiments were repeated three times
SW480 cells were subsequently transfected with si‐ErbB2 and oe‐ErbB2, and the results of Western blot analysis showed that ErbB2 expressions were reduced in the cells transfected with si‐ErbB2‐1, si‐ErbB2‐2, and si‐ErbB2‐3, with si‐ErbB2‐2 exhibiting superior knockdown efficiency (Figure 4G, H), which was thus selected for further experimentation. In addition, compared with the oe‐NC–transfected cells, the expression of ErbB2 was found to be increased in the oe‐ErbB2–transfected cells. Meanwhile, CCK‐8 results indicated an enhancement in the proliferation of CRC cells transfected with oe‐ErbB2, while a decline in proliferation was noted in the presence of si‐ErbB2 (Figure 4I).
Furthermore, the results of RT‐qPCR and Western blot analysis indicated that the mRNA and protein expressions of ErbB2 were decreased in the presence of si‐IGF2BP2 + oe‐NC compared with si‐NC + oe‐NC, while opposing trends were observed following transfection with si‐IGF2BP2 + oe‐YAP relative to transfection with si‐IGF2BP2 + oe‐NC (Figure 4J, K). Overall, these findings indicated that IGF2BP2 could upregulate the expression of ErbB2 by stabilizing the expression of YAP.
3.5. IGF2BP2 facilitated tumorigenesis of CRC cells in vivo by stabilizing the expression of YAP and upregulating ErbB2
To investigate whether IGF2BP2 affected tumor growth in vivo by regulating the YAP/ErbB2 axis, SW480 cells transfected with oe‐NC + si‐NC, oe‐IGF2BP2 + si‐NC, and oe‐IGF2BP2 + si‐YAP were injected subcutaneously into nude mice. The average tumor volume and weight of mice were found to be remarkably increased in the presence of IGF2BP2 overexpression, while being markedly reduced following YAP silencing (Figure 5A‐C). Meanwhile, IHC analysis results illustrated that ErbB2 positive expression in the tumor tissues of mice was considerably increased following IGF2BP2 overexpression, while contrasting trends were observed after further YAP silencing (Figure 5D). Collectively, these findings supported that IGF2BP2 promoted the tumorigenesis of CRC cells in vivo via YAP‐mediated ErbB2 upregulation.
FIGURE 5.
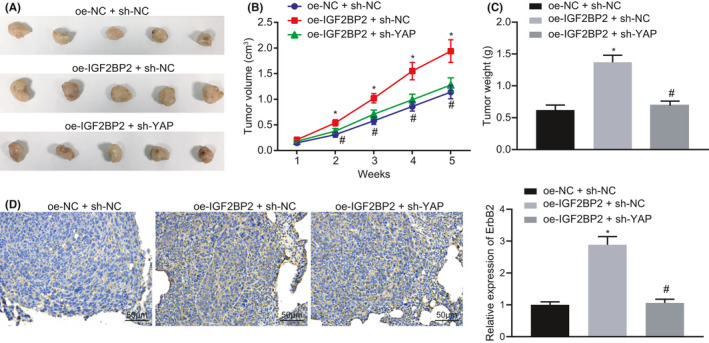
Insulin‐like growth factor 2 mRNA‐binding protein 2 (IGF2BP2) stabilized the expression of Yes‐associated protein (YAP) and upregulated the expression of ErbB2 to promote the tumorigenesis of colorectal cancer (CRC) cells in vivo. Mice were injected with cells stably transfected with oe‐IGF2BP2 + si‐normal control (NC) or oe‐IGF2BP2 + si‐YAP. A, Representative images of xenograft tumors in nude mice. B, Tumor volume in mice. C, Tumor weight in mice. D, Positive expression of ErbB2 in tumor tissues of nude mice tested by immunohistochemistry (IHC). *P < .05, compared with mice injected with cells stably transfected with oe‐NC + si‐NC. #P < .05, compared with mice injected with cells stably transfected with oe‐IGF2BP2 + si‐NC. The results are measurement data, shown as mean ± standard deviation. One‐way ANOVA was used to compare the data among multiple groups. Data of tumor volume at different time points were compared by repeated‐measures ANOVA. n = 6 for mice upon each treatment
4. DISCUSSION
Despite significant advancements in the treatment of CRC over the past few years, the medical burden of CRC is expected to surge upward in the next two decades due to a series of problems attributed to the Western lifestyle.23 Meanwhile, a number of studies have indicated the significance of genetic mutations as specific molecular markers for CRC.24 The current study clarified the molecular mechanism of m6A modification and function of YAP, which can be regulated by IGF2BP2 proteins, in the modulation of CRC cell proliferation, invasion, and apoptosis. Furthermore, we explored the relationship between YAP and ErbB2 and uncovered its significant role in regulating CRC tumor growth and metastasis. Altogether, our findings indicated the IGF2BP2‐YAP‐ErbB2 axis (Figure 6) as a potential novel target for CRC alleviation.
FIGURE 6.
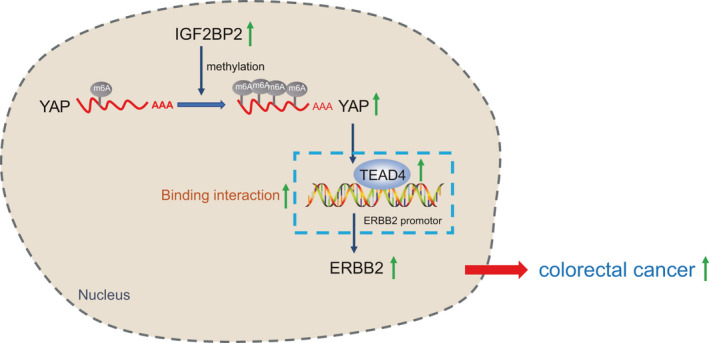
Schematic diagram of the mechanism by which insulin‐like growth factor 2 mRNA‐binding protein 2 (IGF2BP2) affects colorectal cancer (CRC). IGF2BP2 binds to methylation of the 6th adenosine (m6A)‐modified Yes‐associated protein (YAP) mRNA and increases the stability of YAP, thus upregulating ErbB2 expression. By this mechanism, the proliferation of CRC cells is promoted, while apoptosis is inhibited, as well as the accelerated progression of CRC
Firstly, findings obtained in our study illustrated that IGF2BP2 was highly expressed in CRC tissues relative to corresponding adjacent normal tissues. Similarly, a prior study documented upregulation of IGF2BP2 in pancreatic cancer tissues, such that these high expressions were associated with short overall survival in pancreatic cancer patients.25 Meanwhile, aberrantly robust expressions of IGF2BP2 were previously detected in head and neck squamous cell carcinoma tissues and further correlated with poor patient prognoses.26 Furthermore, we established si‐IGF2BP2 and oe‐IGF2BP2 cell lines for in vitro analyses, and found that overexpression of IGF2BP2 augmented tumor cell proliferation, migration, and invasion, whereas its inhibition brought about a significant increase in tumor cell apoptosis. Likewise, deficiency of IGF2BP2 was previously shown to diminish the growth and metastasis of glioma.27 More importantly, a prior study validated the suppressing role of downregulated IGF2BP2 in the progression of CRC, which is in accordance with our findings.28
M6A is the most common RNA modification,29 accounting for more than 60% of all RNA modifications.30 Interestingly, IGF2BP2 was recently identified as a key regulator of m6A modification.31 Furthermore, accumulating evidence has highlighted the importance of m6A as a hallmark of cancer. For instance, Jin et al32 demonstrated the regulatory mechanisms of m6A‐modified integrin subunit alpha 6 in bladder cancer development and progression, and highlighted it as a potential therapeutic target against bladder cancer. Meanwhile, our findings revealed that the expression of YAP was closely associated with IGF2BP2: Both YAP and IGF2BP2 were highly expressed in CRC, whereas inhibition of IGF2BP2 decreased both mRNA and protein YAP expression levels. Similarly, another study identified that YAP expression was decreased by IGF2BP2, such that this downregulation attenuated proliferation and cell cycle entry in diffuse large B‐cell lymphoma cells.33 Moreover, we observed that overexpression of YAP increased CRC cell proliferation, migration, and invasion. This can be possibly explained by the sustained expression of YAP augmenting aberrant cell proliferation by targeting cell cycle–related pathways including DNA synthesis, S‐phase entry, and completion of mitosis.34, 35 Additionally, our findings corroborated the interaction between IGF2BP2 and YAP, thus indicating the role of IGF2BP2 as a m6A modifier of YAP. In addition, we investigated the effects of the IGF2BP2‐YAP axis on the biological characteristics of CRC cells and found that inhibition of IGF2BP2 not only led to low YAP expressions but also precipitated disordered tumor cell biological functions including cell proliferation, migration, and invasion. On the other hand, overexpression of YAP reduced cell apoptosis, which may be attributed to the fact that YAP can bind to transcription factors of the TEAD family to stimulate antiapoptotic genes.36, 37 Overall, it would be plausible to suggest that IGF2BP2‐YAP exerts important functions in regulating tumor cell growth and development and thus may serve as a potential therapeutic target for CRC.
Receptor tyrosine‐protein kinase ErbB2, also known as HER2, is recognized as a member of the epidermal growth factor receptor family.38 ErbB2 is overexpressed in tumor tissues relative to normal tissues, and these increased ErbB2 expressions can trigger various signaling pathways, including the mitogen‐activated protein kinase, phosphatidylinositol 3‐kinase, and protein kinase C pathway, resulting in elevated cellular proliferation and tumorigenesis.39 Interestingly, ErbB2 mutations are present in 7% of cases of CRC, with some studies suggesting that the presence of these alterations can be a potential marker for the prognosis of CRC patients.40 Meanwhile, our findings revealed that inhibition of IGF2BP2 reduced the expression of ErbB2 at both mRNA and protein levels, while overexpressed YAP augmented the expressions of ErbB2 and IGF2BP2, hinting at the presence of a mutual regulation relationship between the three proteins in CRC. However, in ovarian cancer, ErbB4 underwent dramatic changes as a result of YAP activation, whereas ErbB2 expression remained stable,41 which suggests that YAP‐ErbB2 interaction may be cell type dependent. Previous studies have further reported that YAP and TEAD4 directly bind to and activate a regulatory element in the ErbB2 promoter in liver cells.20 It is noteworthy that ChIP analyses in our study also illustrated that TEAD4 was enriched in the ErbB2 promoter as a result of YAP overexpression. In addition, luciferase analyses showed that YAP activated the regulatory element in the ErbB2 promoter, thus validating that YAP could regulate ErbB2 by activating transcription. Besides, the interaction between ErbB2 and YAP was previously implicated with suppressed tumor growth in breast, prostate, and colon cancers.42 Furthermore, YAP and TEAD4 enrichment in the ErbB2 promoter is also conserved in liver cell line,20 which highlights that this regulation may extend to other cancer cells as well.
Collectively, findings obtained in our study indicated that IGF2BP2 can activate ErbB2 expression by recognizing the m6A modification of YAP, thereby affecting the cycle distribution of CRC cells, inhibiting its apoptosis, and promoting its proliferation, and this pathway appears to have important clinical implications. Future studies aiming at modulation of the dynamic balance among IGF2BP2, YAP, and ErbB2 may lead to high‐throughput drug screening strategy to uncover suitable drug regimens for CRC treatment.
DISCLOSURE
The authors have declared that no competing interest exists.
CONSENT FOR PUBLICATION
The authors confirm that they have obtained written consent from each patient to publish the manuscript.
Supporting information
Table S1
Table S2
Table S3
Table S4
Table S5
Table S6
Data S1
ACKNOWLEDGMENTS
We appreciate Professor Dong Li (Shanghai Tongji Hospital) for critical reading of the manuscript.
Cui J, Tian J, Wang W, et al. IGF2BP2 promotes the progression of colorectal cancer through a YAP‐dependent mechanism. Cancer Sci. 2021;112:4087–4099. 10.1111/cas.15083
Jie Cui, Jiale Tian, and Weiwei Wang contributed equally to this work.
Funding information
This work was supported by the National Natural Science Foundation of China (81802084), the Shanghai Post‐doctoral Excellence Program (2020409), the Postdoctoral Science Foundation of China (2020M681399), the Medical Research Project of Jiangsu Provincial Health and Health Commission (2019179), and the 2021 National Natural Science Foundation of Shanghai Tongji Hospital Incubation Project (TJ202010).
[Correction added on 16 Sept 2021, after first online publication: The affiliation details of Jiale Tian and Chenzheng Gu have been updated]
Contributor Information
Lixin Wang, Email: wanglixin16@163.com.
Jian Wu, Email: wj18914645776@zju.edu.cn.
Anquan Shang, Email: shanganquan@tongji.edu.cn.
DATA AVAILABILITY STATEMENT
All data supporting the findings of this study are included in the manuscript and are available from the corresponding author upon request.
REFERENCES
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359‐E386. [DOI] [PubMed] [Google Scholar]
- 2.Fang L, Lu W, Choi HH, et al. ERK2‐dependent phosphorylation of CSN6 is critical in colorectal cancer development. Cancer Cell. 2015;28(2):183‐197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mármol I, Sánchez‐de‐Diego C, Pradilla Dieste A, Cerrada E, Rodriguez Yoldi MJ. Colorectal carcinoma: a general overview and future perspectives in colorectal cancer. Int J Mol Sci. 2017;18:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goel A, Boland CR. Epigenetics of colorectal cancer. Gastroenterology. 2012;143:1442‐1460.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suter CM, Martin DI, Ward RL. Hypomethylation of L1 retrotransposons in colorectal cancer and adjacent normal tissue. Int J Colorectal Dis. 2004;19:95‐101. [DOI] [PubMed] [Google Scholar]
- 6.Dominissini D, Moshitch‐Moshkovitz S, Schwartz S, et al. Topology of the human and mouse m6A RNA methylomes revealed by m6A‐seq. Nature. 2012;485:201‐206. [DOI] [PubMed] [Google Scholar]
- 7.Meyer KD, Saletore Y, Zumbo P, Elemento O, Mason CE, Jaffrey SR. Comprehensive analysis of mRNA methylation reveals enrichment in 3' UTRs and near stop codons. Cell. 2012;149:1635‐1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang H, Weng H, Chen J. m6A Modification in coding and non‐coding RNAs: roles and therapeutic implications in cancer. Cancer Cell. 2020;37:270‐288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cai X, Wang X, Cao C, et al. HBXIP‐elevated methyltransferase METTL3 promotes the progression of breast cancer via inhibiting tumor suppressor let‐7g. Cancer Lett. 2018;415:11‐19. [DOI] [PubMed] [Google Scholar]
- 10.Uddin MB, Roy KR, Hosain SB, et al. An N6‐methyladenosine at the transited codon 273 of p53 pre‐mRNA promotes the expression of R273H mutant protein and drug resistance of cancer cells. Biochem Pharmacol. 2019;160:134‐145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li T, Hu PS, Zuo Z, et al. METTL3 facilitates tumor progression via an m6A‐IGF2BP2‐dependent mechanism in colorectal carcinoma. Mol Cancer. 2019;18:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu X, Peng WX, Zhou H, et al. IGF2BP2 regulates DANCR by serving as an N6‐methyladenosine reader. Cell Death Differ. 2020;27(6):1782‐1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhuo Z, Lu H, Zhu J, et al. METTL14 gene polymorphisms confer neuroblastoma susceptibility: an eight‐center case‐control study. Mol Ther Nucleic Acids. 2020;22:17‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ye S, Song W, Xu X, Zhao X, Yang L. IGF2BP2 promotes colorectal cancer cell proliferation and survival through interfering with RAF‐1 degradation by miR‐195. FEBS Lett. 2016;590:1641‐1650. [DOI] [PubMed] [Google Scholar]
- 15.Zhu H, Wang DD, Yuan T, et al. Multikinase inhibitor CT‐707 targets liver cancer by interrupting the hypoxia‐activated IGF‐1R‐YAP axis. Cancer Res. 2018;78:3995‐4006. [DOI] [PubMed] [Google Scholar]
- 16.Warren JSA, Xiao Y, Lamar JM. YAP/TAZ activation as a target for treating metastatic cancer. Cancers (Basel). 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu L, Yang X. Targeting the hippo pathway for breast cancer therapy. Cancers. 2018;10(11):422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fu D, Lv X, Hua G, et al. YAP regulates cell proliferation, migration, and steroidogenesis in adult granulosa cell tumors. Endocr Relat Cancer. 2014;21:297‐310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yuen HF, McCrudden CM, Huang YH, et al. TAZ expression as a prognostic indicator in colorectal cancer. PLoS One. 2013;8:e54211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang EY, Cheng JC, Thakur A, Yi Y, Tsai SH, Hoodless PA. YAP transcriptionally regulates ErbB2 to promote liver cell proliferation. Biochim Biophys Acta Gene Regul Mech. 2018;1861(9):854‐863. [DOI] [PubMed] [Google Scholar]
- 21.Chmielecki J, Ross JS, Wang K, et al. Oncogenic alterations in ERBB2/HER2 represent potential therapeutic targets across tumors from diverse anatomic sites of origin. Oncologist. 2015;20:7‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ross JS, Fakih M, Ali SM, et al. Targeting HER2 in colorectal cancer: the landscape of amplification and short variant mutations in ERBB2 and ERBB3. Cancer. 2018;124:1358‐1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66:683‐691. [DOI] [PubMed] [Google Scholar]
- 24.Kudryavtseva AV, Lipatova AV, Zaretsky AR, et al. Important molecular genetic markers of colorectal cancer. Oncotarget. 2016;7:53959‐53983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu X, Yu Y, Zong K, Lv P, Gu Y. Up‐regulation of IGF2BP2 by multiple mechanisms in pancreatic cancer promotes cancer proliferation by activating the PI3K/Akt signaling pathway. J Exp Clin Cancer Res. 2019;38:497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deng X, Jiang Q, Liu Z, Chen W. Clinical Significance of an m6A reader gene, IGF2BP2, in head and neck squamous cell carcinoma. Front Mol Biosci. 2020;7:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ding L, Wang L, Guo F. microRNA188 acts as a tumour suppressor in glioma by directly targeting the IGF2BP2 gene. Mol Med Rep. 2017;16:7124‐7130. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y, Lu JH, Wu QN, et al. LncRNA LINRIS stabilizes IGF2BP2 and promotes the aerobic glycolysis in colorectal cancer. Mol Cancer. 2019;18:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fazi F, Fatica A. Interplay between N (6)‐Methyladenosine (m(6)A) and non‐coding RNAs in cell development and cancer. Front Cell Dev Biol. 2019;7:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li J, Yang X, Qi Z, et al. The role of mRNA m(6)A methylation in the nervous system. Cell Biosci. 2019;9:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Z, Cheng H, Xu H, Yu X, Sui D. A five‐gene signature derived from m6A regulators to improve prognosis prediction of neuroblastoma. Cancer Biomark. 2020;28:275‐284. [DOI] [PubMed] [Google Scholar]
- 32.Jin H, Ying X, Que B, et al. N(6)‐methyladenosine modification of ITGA6 mRNA promotes the development and progression of bladder cancer. EBioMedicine. 2019;47:195‐207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou X, Chen N, Xu H, et al. Regulation of Hippo‐YAP signaling by insulin‐like growth factor‐1 receptor in the tumorigenesis of diffuse large B‐cell lymphoma. J Hematol Oncol. 2020;13:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kapoor A, Yao W, Ying H, et al. Yap1 activation enables bypass of oncogenic Kras addiction in pancreatic cancer. Cell. 2014;158:185‐197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zanconato F, Forcato M, Battilana G, et al. Genome‐wide association between YAP/TAZ/TEAD and AP‐1 at enhancers drives oncogenic growth. Nat Cell Biol. 2015;17:1218‐1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao B, Ye X, Yu J, et al. TEAD mediates YAP‐dependent gene induction and growth control. Genes Dev. 2008;22:1962‐1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu S, Liu Y, Zheng Y, Dong J, Pan D. The TEAD/TEF family protein Scalloped mediates transcriptional output of the Hippo growth‐regulatory pathway. Dev Cell. 2008;14:388‐398. [DOI] [PubMed] [Google Scholar]
- 38.Simpson J, Loh Z, Ullah MA, et al. Respiratory syncytial virus infection promotes necroptosis and HMGB1 release by airway epithelial cells. Am J Respir Crit Care Med. 2020;201:1358‐1371. [DOI] [PubMed] [Google Scholar]
- 39.Iqbal N, Iqbal N. Human Epidermal Growth Factor Receptor 2 (HER2) in cancers: overexpression and therapeutic implications. Mol Biol Int. 2014;2014: 852748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pectasides E, Bass AJ. ERBB2 emerges as a new target for colorectal cancer. Cancer Discov. 2015;5:799‐801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.He C, Lv X, Hua G, et al. YAP forms autocrine loops with the ERBB pathway to regulate ovarian cancer initiation and progression. Oncogene. 2015;34:6040‐6054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Serrano I, McDonald PC, Lock F, Muller WJ, Dedhar S. Inactivation of the Hippo tumour suppressor pathway by integrin‐linked kinase. Nat Commun. 2013;4:2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Table S2
Table S3
Table S4
Table S5
Table S6
Data S1
Data Availability Statement
All data supporting the findings of this study are included in the manuscript and are available from the corresponding author upon request.


