Abstract
As a POU homeodomain transcription factor, POU4F2 has been implicated in regulating tumorigenic processes in various cancers. However, the role of POU4F2 in colorectal cancer (CRC) remains unclear. Here, we revealed that POU4F2 functions as a tumor promotor in CRC. Bioinformatics analysis in specimens from CRC patients and expression analysis in CRC cell lines showed that POU4F2 was upregulated at the mRNA and protein levels in CRC. Depletion of POU4F2 suppressed the metastatic phenotypes of CRC cells, including cell migration, invasion, and the expression of epithelial‐mesenchymal transition (EMT) markers. Moreover, depletion of POU4F2 decreased the number of lung metastatic nodes in nude mice. Mechanistically, POU4F2 positively regulated the Hedgehog signaling pathway, as inferred from the downregulation of the expression of sonic Hedgehog homolog, patched 1, Smoothened, and GLI family zinc finger 1 in vitro and vivo following silencing of POU4F2. Furthermore, the SMO agonist SAG reversed the effects of POU4F2 knockdown in CRC. Functionally, POU4F2 contributed to the Hedgehog signaling‐regulated activation of the EMT process and promotion of CRC cell migration and invasion. Collectively, these findings elucidated the role of POU4F2 as a tumor promotor in CRC through the regulation of Hedgehog signaling‐mediated EMT and suggested that POU4F2 suppression might be a promising therapeutic target in inhibiting CRC metastasis.
Keywords: colorectal cancer, EMT, Hedgehog pathway, migration and invasion, POU4F2
Our work provides novel evidence that POU4F2 facilitates metastatic properties by regulating the Hedgehog axis‐mediated epithelial‐mesenchymal transition of colon cancer cells. This study introduces a potential therapeutic target for colorectal cancer from understanding the roles and mechanism of POU4F2 in the Hedgehog pathway
Abbreviations
- CDK
cyclin‐dependent kinase
- COADREAD
colonic adenocarcinoma and rectal adenocarcinoma
- CRC
colorectal cancer
- EMT
epithelial‐mesenchymal transition
- EMT‐TF
EMT transcription factor
- GLI‐1
GLI family zinc finger 1
- GSEA
Gene Set Enrichment Analysis
- POU4F2
POU domain, class 4, transcription factor 2
- PTCH1
patched 1
- RT‐qPCR
reverse transcription quantitative real‐time PCR
- SAG
Smoothened agonist
- SHH
sonic Hedgehog homolog
- SMO
Smoothened
- TCGA
The Cancer Genome Atlas
1. INTRODUCTION
Colorectal cancer is the second leading cause of cancer‐related mortality worldwide,1 and the fifth leading cause of cancer mortality in China.2 Moreover, the incidence of CRC has continued to increase in countries with medium to high Human Development Index.3 Surgery and endoscopic resection are currently the only two available curative treatments for patients with nonadvanced CRC; however, even after chemotherapy or radiotherapy, the cancer‐specific recurrence rate remains at 21.6% and the 5‐year overall mortality rate at 13.0%.4 Unfortunately, the 5‐year survival rate of CRC patients with distant metastasis is as low as 10%.5 Therefore, there is an urgent need to identify novel therapeutic targets that could improve prognosis for CRC patients.
The POU4F2 transcription factor, which was first identified to be expressed in retinal ganglion cells,6 has since been found in various other tissues, including tumors.7, 8, 9 In particular, POU4F2 is characterized by a highly conserved POU DNA binding domain and regulates the transcription of multiple target genes, such as eomesodermin that is required for optic nerve development,10 as well as CDK4, cyclin D1, and plakoglobin in breast cancer cells.11 Moreover, POU4F2 has been found to regulate various tumorigenic processes.9, 11, 12, 13, 14 However, the expression of POU4F2 and its specific role in the development of CRC is not well understood.
Epithelial‐mesenchymal transition, defined by the gain of the mesenchymal marker vimentin and the loss of the expression of the epithelial marker E‐cadherin, has been closely associated with tumor metastasis.15 The Hedgehog signaling pathway is known to function as a regulator of the growth and differentiation of normal cells, with aberrant Hedgehog signaling resulting in tumorigenesis, tumor progression, and metastasis.16 Furthermore, GLI‐1, downstream of the Hedgehog signaling, has been reported to facilitate EMT in multiple cancers.17, 18 However, it remains unclear whether POU4F2 leads to the progression and metastasis of CRC through a molecular mechanism involving the Hedgehog signaling pathway and EMT.
In this study, we evaluated changes in cell migration, invasion, and EMT markers using shRNA to silence the expression of POU4F2 in two CRC cell lines, HCT116 and RKO. In addition, we also used the SAG agonist to assess the role of the Hedgehog pathway in the POU4F2‐mediated migration and invasion of CRC cells. Our findings revealed that POU4F2 might represent a novel target in the treatment of CRC (Figure 1).
FIGURE 1.
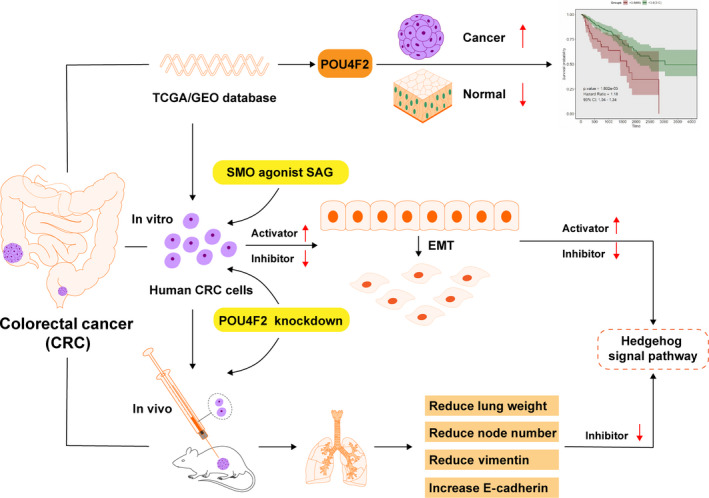
General experimental process
2. MATERIALS AND METHODS
2.1. Bioinformatics analysis
We compared the expression of genes between colorectal and normal tissues using TCGA‐COADREAD,19 GSE24514, and GSE24551 datasets.20 We also analyzed the relationship between the expression of POU4F2 and CRC prognosis using the GSE24514 and GSE24551 datasets (https://www.ncbi.nlm.nih.gov/gds/). Moreover, we used GSEA to analyze the relationships between the expression of POU4F2 and signal pathways.21
2.2. Cell culture
The HCT116, SW620, NCI‐H508, and RKO human CRC and 293T HEK cell lines were obtained from the ATCC; the HCoEpiC human normal colonic epithelial cell line was obtained from Sciencell Research Laboratories. HCT116, HCoEpiC, and HEK 293T cell lines were cultivated in DMEM (Procell Life Science & Technology) supplemented with 10% FBS (WISENT) and 1% penicillin/streptomycin (New Cell & Molecular Biotech). SW620 and NCI‐H508 were cultivated in RPMI‐1640 (Procell Life Science & Technology), whereas RKO was cultivated in minimum essential medium (Procell Life Science & Technology). All cells were incubated at 37℃ in air enriched with 5% CO2. In addition, 500 nM SMO agonist SAG (MCE, HY‐12848B) was added to complete medium for coculturing with HCT116 for 24 hours in the incubator.
2.3. Short hairpin RNA transfection
Scramble shRNA sequences were designed using the online RNAi Designer software (http://rnaidesigner.thermofisher.com/rnaiexpress/) and synthesized using the primers listed in Table S1. Subsequently, shRNAs were ligated to the pLVX‐shRNA2‐puro plasmid using a T4 DNA ligase, synthesizing the pLVX‐shPOU4F2‐1‐puro, pLVX‐shPOU4F2‐2‐puro, and pLVX‐shNC‐puro interference plasmids. These plasmids were transformed into DH5α competent cells, and consecutively their correct sequence was verified by restriction enzyme digestion and sequencing. The three lentiviral plasmids were cotransfected into HEK 293T cells to obtain lentiviral particles containing the target fragments. After concentration, the expression of the green fluorescent protein was detected under a fluorescence microscope (Olympus Corporation, Tokyo, Japan) to determine the viral titer (LV‐shPOU4F2‐1, LV‐shPOU4F2‐2, and LV‐shNC) for the infection of HCT116 cells. For cell infection, HCT116 cells were transduced with the lentiviral particles followed by puromycin selection (2 μg/mL).22
2.4. Wound healing assay
We used a marker pen and a ruler to draw even horizontal lines on the back of a 6‐well plate; each line was drawn at every 0.5‐1 cm across the well, with at least five lines per well. Cells were inoculated in 6‐well plates at a density of 5 × 105/well and cultured to 90% confluency. A wound scratch was made on each plate using a 200‐μL tip and plates were washed three times with PBS to remove loose cell fragments. Cells were then cultivated in serum‐free medium at 37℃ in air enriched with 5% CO2. At the time points of 0, 24, and 48 h, we measured the migration distance using an Olympus microscope and calculated the healing rate.23
2.5. Transwell assay
Transwell chambers (Corning) were used to evaluate cell migration in the Transwell assays.24 Cells at a density of 4 × 104/chamber were inoculated with serum‐free medium in the upper chamber, and relevant medium containing 10% FBS was added in the lower chamber as chemoattractant. After incubation at 37℃ for 30 hours, cells in the upper chamber were removed using cotton swabs; cells in the lower chamber were fixed with formaldehyde for 30 seconds and stained with 0.1% crystal violet for 20 minutes. Three fields were randomly selected and positively stained cells were counted using an Olympus microscope.
2.6. Cell invasion assay
Matrigel invasion chambers (BD BioCoat) were used to evaluate cell invasion in the Transwell assays. Cells at a density of 4 × 104/chamber were inoculated in the upper chamber, and relevant medium containing 10% FBS was added in the lower chamber as chemoattractant. After incubation at 37℃ for 48 hours, cells in the upper chamber were removed using cotton swabs; cells in the lower chamber were fixed with formaldehyde for 30 seconds and stained with 0.1% crystal violet for 20 minutes. Three fields were randomly selected and positively stained cells were counted using an Olympus microscope.
2.7. Lung metastasis model in nude mice
Three different HCT116 cell lines were generated following the transduction of HCT116 cells with the LV‐shPOU4F2‐1, LV‐shPOU4F2‐2, and LV‐shNC vectors. To construct a lung metastasis model in vivo, we injected tail veins with 1 × 107 cells of each of the three generated HCT116 cell lines in 5‐ to 6‐week‐old BALB/c athymic nude mice (Hunan SJA Laboratory Animal Co., Ltd). All animal experiments were approved by the Zhejiang Chinese Medical University Laboratory Animal Research Center with the approval number SYXK (Zhejiang) 2018‐0012. Consecutively, 18 mice were randomly divided into three groups, namely the shPOU4F2‐1, shPOU4F2‐2, and shNC groups. On the 50th day after the injection of cells, mice were killed and their lungs were dissected. The obtained lungs were weighed and the number of metastatic nodes was counted.
2.8. Immunofluorescence
Metastatic lung tissues were embedded in paraffin and sectioned, and then immunofluorescence staining was carried out according to a published protocol.25 Primary Abs against E‐cadherin (1:200; 20874‐1‐AP) and vimentin (1:300; 10366‐1‐AP) were obtained from Proteintech. The DAPI DNA dye was obtained from Beyotime Biotechnology. The secondary Ab was a cy3‐conjugated goat anti‐rabbit IgG (Beyotime Biotechnology). All stained tissues were examined and photographed under a NEXcope (NE900) confocal fluorescence microscope (Ningbo Yongxin Optics Co., Ltd).
2.9. Western blot analysis
Cell lysates and metastatic tissues were collected, and 30 mg protein from each sample was separated using 8%, 10%, or 15% SDS‐PAGE. Proteins were then electrotransferred to PVDF membranes (Millipore). Membranes were blocked in 10% skimmed milk and then incubated with primary Abs, including anti‐POU4F2 (1:1000; ab235268, Abcam), anti‐rabbit GAPDH (1:6000; 10494‐1‐AP, Proteintech), anti‐mouse GAPDH (1:6000; 60004‐1‐Ig, Proteintech,), anti‐E‐cadherin (1:1000; 20874‐1‐AP, Proteintech), anti‐vimentin (1:4000; 10366‐1‐AP, Proteintech), anti‐Slug (1:1000; 12129‐1‐AP, Proteintech), anti‐Twist (1:500;, 25465‐1‐AP, Proteintech), anti‐Snail1 (1:500; 13099‐1‐AP, Proteintech ), anti‐ZEB1 (1:2000; 21544‐1‐AP, Proteintech), anti‐ZEB2 (1:1000; 14026‐1‐AP, Proteintech), anti‐β‐actin (1:5000; 66009‐1‐Ig, Proteintech), anti‐SHH (1:800; 20697‐1‐AP, Proteintech), anti‐PTCH1 (1:500; ab53715, Abcam), anti‐SMO (1:2000; 66851‐1‐Ig, Proteintech), and anti‐GLI‐1 (1:3000; 66905‐1‐Ig, Proteintech) at 4℃ overnight. Membranes were then incubated with HRP‐conjugated secondary Ab (anti‐mouse IgG [H + L] or anti‐rabbit IgG [H + L]; 1:10 000, 14 709, or 14 708; Cell Signaling Technology) at 25℃ for 2 hours. Enhanced chemiluminescent (New Cell & Molecular Biotech) was used to visualize the protein bands.
2.10. Reverse transcription quantitative real‐time PCR
Total RNA was extracted from CRC cells, and then reverse‐transcribed into cDNA using an RT‐PCR kit (GeneCopoeia).26 The RT‐PCR primers were designed and synthesized as follows: GAPDH forward, 5′‐ACAGCCTCAAGATCATCAGC‐3′; GAPDH reverse, 5′‐GGTCATGAGTCCTTCCACGAT‐3′; POU4F2 forward, 5′‐CAAGCAGCGACGCATCAAG‐3′; and POU4F2 reverse, 5′‐GGGTTTGAGCGCGATCATATT‐3′. The RT‐PCR assays were undertaken using BeyoFast SYBR Green qPCR Mix on a qTower 3.2G system. Human GAPDH was used as the internal reference gene. Relative mRNA expression was calculated according to the 2−ΔΔCq method.26
2.11. Statistical analysis
Comparisons between two groups were undertaken using Student’s t test; comparisons between three or more groups were carried out using one‐way ANOVA. Multiple comparisons among these groups were evaluated using Dunnett’s least significant difference test. A P value less than .05 was considered statistically significant. All data were analyzed using SPSS 22.0 software (IBM).
3. RESULTS
3.1. POU4F2 upregulated in CRC patients and correlated with poor prognosis
To assess the expression of POU4F2 in colon cancer clinical samples, we undertook bioinformatics analyses of the POU4F2 mRNA expression data. The data from TCGA‐COADREAD, GSE24514, and GSE24551 public databases revealed that the levels of POU4F2 mRNA were significantly upregulated in CRC samples compared with normal samples (Figure 2A‐C). In addition, we detected the mRNA and protein expression of POU4F2 in the HCT116, SW620, NCI‐H508, and RKO human CRC and HCoEpiC human normal colonic epithelial cell lines. Compared with HCoEpiC, we found that CRC cell lines highly expressed POU4F2; the highest levels of expression were observed in the HCT116 and RKO cells (Figure 2D,E). Thus, we selected these two cell lines to verify the effects of POU4F2 on the migration and invasion of colon cancer cells. To determine the clinical relevance of POU4F2 in colon cancer, we undertook Kaplan‐Meier survival analyses of the mRNA expression data (n = 376; low expression, 310; high expression, 66) and found that the high expression of POU4F2 was significantly associated with poor prognosis in CRC patients (Figure 2F).
FIGURE 2.
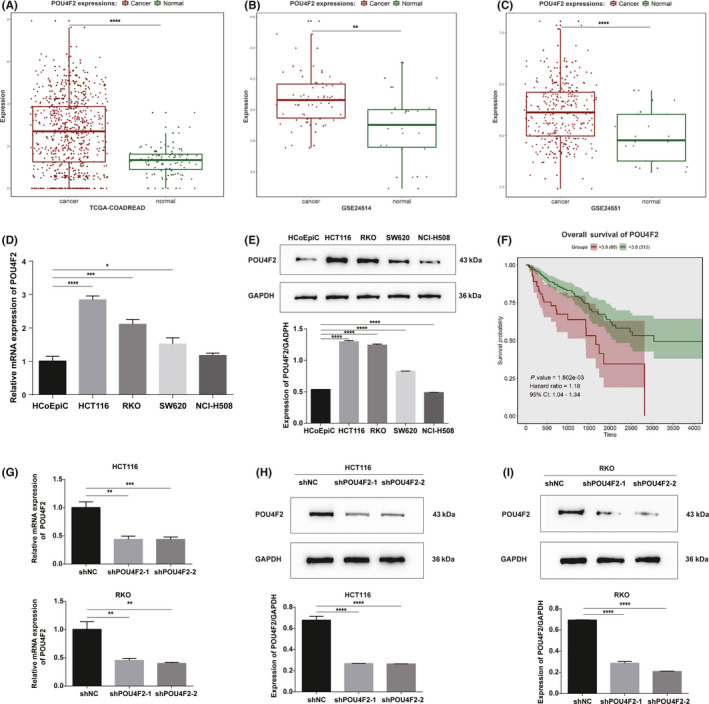
The expression of POU4F2 is increased in colorectal cancer (CRC) and associated with adverse prognosis. A, B, C, Analysis of the expression of POU4F2 in public datasets TCGA‐COADREAD (A), GSE24514 (B), and GSE24551 (C) and in CRC specimens. D, Expression of POU4F2 mRNA in the HCoEpiC human normal colonic epithelial cell line and CRC cell lines. E, Expression of the POU4F2 protein in the HCoEpiC human normal colonic epithelial cell line and CRC cell lines. F, Kaplan‐Meier curve of overall survival (OS) stratified by the median level of POU4F2 using the public database. G, mRNA level of POU4F2 in POU4F2‐knockdown HCT116 cells and POU4F2‐knockdown RKO cells; H, I, Levels of the POU4F2 protein in POU4F2‐knockdown HCT116 (H) and POU4F2‐knockdown RKO (I) cells. All experiments were performed in triplicate. Data are presented as the mean ± SEM (*P < .05, **P < .01, ***P < .001, ****P < .0001)
3.2. POU4F2 knockdown suppressed migration and invasion of CRC cells
To investigate the potential function of POU4F2 in CRC, we used two different shRNA lentiviral constructs to silence the expression of POU4F2 in HCT116 and RKO cell lines, which expressed high levels of the POU4F2 protein. The effects of knocking down POU4F2 in these cells were confirmed by RT‐PCR and western blot analyses (Figure 2G‐I). We then carried out several cell‐based experiments to explore the biological functions of POU4F2 in CRC cells. We noticed that silencing POU4F2 in HCT116 and RKO cells led to a weakened capacity of cells to migrate (wound healing and Transwell migration assays) and invade (Transwell invasion assay), as well as to an EMT phenotype (Figure 3A‐F). Furthermore, we found that some EMT‐TFs, such as Slug, Twist, Snail1, and ZEB1, were downregulated in the shPOU4F2 groups; interestingly, the expression of the ZEB2 EMT‐TF was not affected (Figure 3G,H). To study the POU4F2‐mediated effects in the pathogenesis of CRC in vivo, we constructed a lung metastatic model in BALB/c athymic nude mice using tail vein injection with POU4F2‐depleted HCT116, and their corresponding control cells, respectively. Consistent with our in vitro results, we observed that both shPOU4F2‐1‐derived and shPOU4F2‐2‐derived tumors displayed decreased metastatic nodes in lungs compared with negative control cell‐derived tumors (Figure 4A). Concomitantly, we detected the expression of POU4F2 in lung metastatic nodes and confirmed the knocking down of POU4F2 in vivo (Figure 4B). We next detected the expression of E‐cadherin and vimentin using western blot analysis and found that, compared with the control group, the shPOU4F2 groups showed higher levels of E‐cadherin and lower levels of vimentin (Figure 4C). Immunofluorescence staining for both E‐cadherin and vimentin verified the above results (Figure 4D).
FIGURE 3.
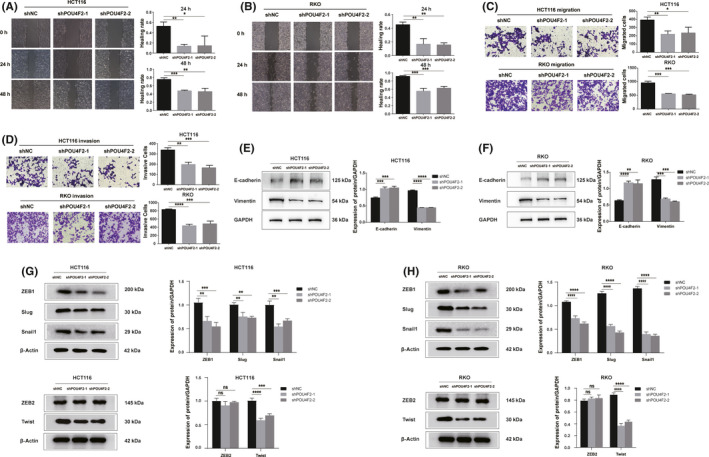
POU4F2 is a tumor promotor in CRC cell lines. A, B , Wound healing ability of POU4F2‐knockdown HCT116 (A) and POU4F2‐knockdown RKO (B) cells. C, D, Migration and invasion abilities of POU4F2‐knockdown HCT116 (C) and POU4F2‐knockdown RKO (D) cells. E, F , Expression of E‐cadherin and vimentin proteins in POU4F2‐knockdown HCT116 (E) and POU4F2‐knockdown RKO (F) cells. G, H, Expression of EMT‐TFs in POU4F2‐knockdown HCT116 (G) and POU4F2‐knockdown RKO (H) cells. All experiments were performed in triplicate. Data were presented as the mean ± SEM (*P < .05, **P < .01, ***P < .001, ****P < .0001)
FIGURE 4.
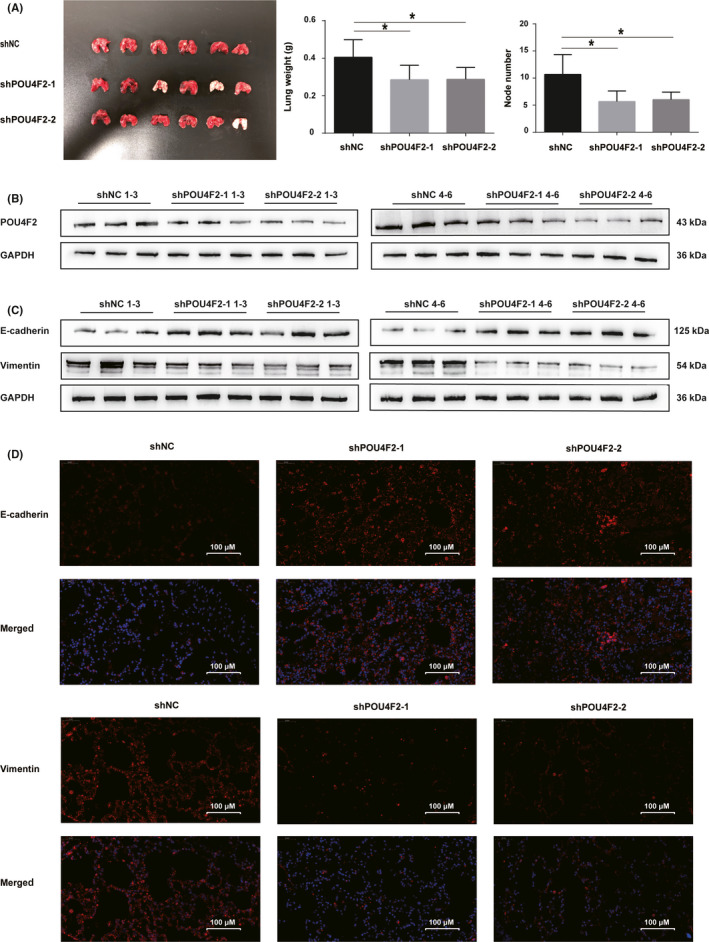
POU4F2 is a tumor promotor in lung metastatic nude mice. A, Lung weight and number of lung metastatic nodes in BALB/c athymic nude mice injected with HCT116 cells transfected with shNC, shPOU4F2‐1, and shPOU4F2‐2. B, Expression of POU4F2 in lung metastatic nodes for each nude mouse. C, Expression of E‐cadherin and vimentin in lung metastatic nodes for each nude mouse. D, Immunofluorescence staining to evaluate the levels of E‐cadherin and vimentin. Fluorescent DAPI staining of cell nucleus (blue) and antibodies against E‐cadherin or vimentin (red). All experiments were performed in triplicate. Data are presented as the mean ± SEM (*P < .05)
3.3. POU4F2 functions as a regulator of Hedgehog signaling pathway
We used GSEA to analyze the correlation between the expression of POU4F2 in CRC and the Hedgehog signaling pathway. Accordingly, we detected a significant positive association between the expression of POU4F2 and the activation of the Hedgehog signaling pathway (Figure 5A). Subsequently, we examined the levels of expression of the SHH, PTCH1, SMO, and GLI‐1 proteins in the HCT116 and RKO cell lines before and after transfection with POU4F2 shRNAs (Figure 5B,C). Western blot analysis showed that the expression of SHH, PTCH1, SMO, and GLI‐1 was significantly decreased in both HCT116 and RKO cells transfected with two different POU4F2 shRNAs. Moreover, we examined the lung metastatic nodes of each mouse using western blotting and found that the expression of SMO and GLI‐1 was significantly downregulated in mice injected with POU4F2 shRNA‐transfected cells (Figure 5D).
FIGURE 5.
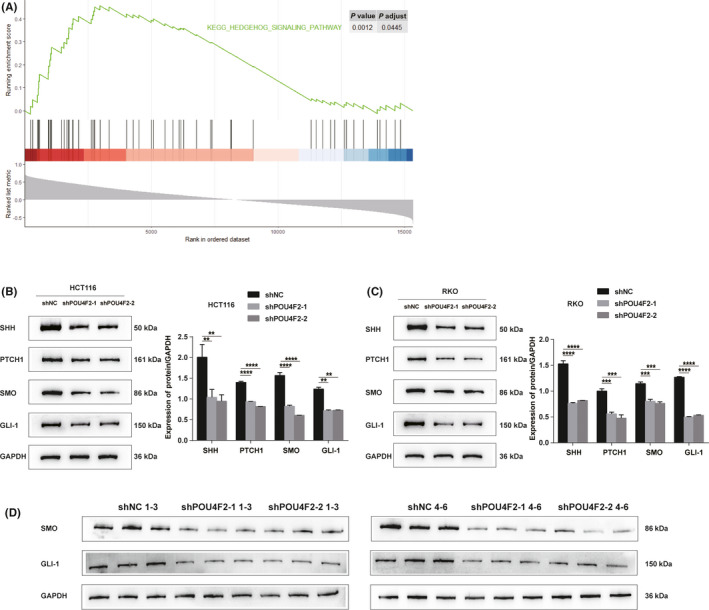
POU4F2 activates the Hedgehog pathway in vitro and in vivo. A, The association between POU4F2 and the Hedgehog pathway in gene set enrichment analysis. B, Protein expression of the Hedgehog pathway in HCT116 cells with or without a POU4F2 knock‐down. C, Protein expression of the Hedgehog pathway in RKO cells with or without a POU4F2 knock‐down. D, Protein expression of the Hedgehog pathway in lung metastatic nodes for each mouse. All experiments were performed in triplicate. Data are presented as the mean ± SEM (**P < .01, ***P < .001, ****P < .0001)
3.4. POU4F2 plays a role in Hedgehog signaling‐mediated inhibition of cell migration and invasion
To further determine whether the mechanism of the inhibition of cell invasion and metastasis by POU4F2 is accomplished through the Hedgehog pathway, we treated shPOU4F2‐1‐ or shNC‐transfected HCT cells with the SMO agonist SAG. As shown in Figure 6A,B, treatment with SAG rescued the migration and invasion phenotype (wound healing and Transwell assays), which was inhibited by the knockdown of POU4F2 in HCT116 cells. Moreover, we noticed that, in contrast to shPOU4F2‐transfected HCT116 cells, the levels of SHH, PTCH1, SMO, and GLI‐1 were not downregulated in SAG‐treated POU4F2‐depleted HCT116 cells (Figure 6C).
FIGURE 6.
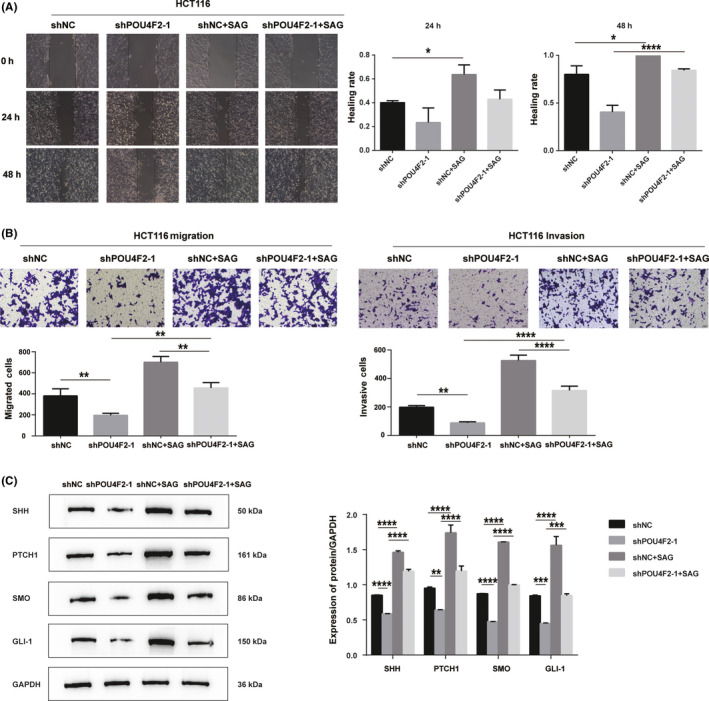
The SMO agonist SAG reverses the effects of knocking down POU4F2 on CRC. A, Wound healing ability of HCT116 cells transfected with shNC or shPOU4F2‐1 and treated with SAG. B, Transwell assays showing the migration and invasion ability of HCT116 cells transfected with shNC or shPOU4F2‐1 and treated with SAG. C, Expression of the SHH, PTCH1, SMO, and GLI‐1 proteins in HCT116 cells transfected with shNC or shPOU4F2‐1 and treated with SAG. All experiments were performed in triplicate. Data are presented as the mean ± SEM (*P < .05, **P < .01, ***P < .001, ****P < .0001)
4. DISCUSSION
Colorectal cancer is one of the most common malignancies worldwide, with a poor survival outcome.27 The local invasion and distant metastasis of tumor cells are major obstacles to the treatment of CRC patients and could strongly influence the outcome as these factors are relevant to poor prognosis and shortened survival. However, the specific molecular mechanisms underlying these associations are not fully understood. As a transcription factor, POU4F2 is known to function in the development of the auditory and visual system.28 To date, few studies have explored the role of POU4F2 in tumor progression. Growing evidence has revealed that POU4F2 is overexpressed and plays a significant role in some aggressive tumors, such as breast,9, 11 cervical,12 small‐cell lung cancer,13 and lung adenocarcinoma,14 as well as neuroblastoma.29 Based on numerous studies reporting that the overexpression of POU4F2 promoted cell growth in vitro and tumor growth in vivo,30, 31 POU4F2 has been considered to be a dominating regulator that controls the expression of numerous downstream genes in a cell‐cycle specific manner. Moreover, POU4F2 has been listed as one of the methylated genes in the genome‐wide profiling for the identification of novel targets in melatonin‐treated breast cancer cells,32 and for the early detection of bladder cancer.33, 34 However, the role of POU4F2 in the progression of CRC has not been clarified. In addition, it should be mentioned that the expression of POU4F2 was high in SKOV3 cells originating from metastatic cells of primary ovarian endometrial adenocarcinoma.35 As the elevated levels of POU4F2 have been associated with growth, invasion, and migratory potential in breast cancer, we speculated that this protein could also play the same role in limiting the anchorage‐independent growth and progressive behavior of CRC cells. In the present study, we investigated the expression level of POU4F2 in colon cancer cells, and evaluated the effects of silencing POU4F2 on HCT116 and RKO cells as well as in a lung metastatic model in nude mice.
We initially found that POU4F2 was highly expressed in colon cancer tissues and cell lines, consistent with studies in ovarian cancer. In addition, the high expression of POU4F2 was correlated with poor prognosis. We then showed that silencing POU4F2 suppressed the migration and invasion of colon cancer cell lines. Wound healing and Transwell assays revealed a significant decline in the capability of POU4F2‐knockdown HCT116 and RKO cells to metastasize. Furthermore, depletion of POU4F2 in HCT116 cells also led to a decrease in the number of lung metastatic nodes in nude mice. Similarly, it was reported that elevated levels of POU4F2 enhanced the invasive ability of neuroblastoma cells.29 In addition, we found that knocking down POU4F2 significantly upregulated the expression of E‐cadherin and downregulated the expression of vimentin in vitro and in vivo, which was found to be relevant with the function of POU4F2 in upregulating the expression of some EMT‐TFs (eg Slug, Twist, Snail1, and ZEB1). The E‐cadherin epithelial marker and vimentin mesenchymal marker are usually used as markers of EMT. Epithelial‐mesenchymal transition is an evolutionarily conserved process whereby epithelial cells lose cell junctions and apical‐basal polarity, and are eventually converted into a migratory mesenchymal phenotype,36 which is considered to be the initiating factor of tumor invasion and metastasis.37 Our results indicated that knocking down POU4F2 inhibited the migration and invasion of colon cancer cells by inhibiting the EMT process.
However, as the exact mechanism of function of POU4F2 has not yet been fully elucidated, its effective targeting remains difficult. An increasing number of recent studies have shown that multiple targets can control the growth and behavior of cancer cells. For instance, POU4F2 was recognized as a critical target for those whose decrement contributed to the activation of cell‐cycle regulators, such as CDK4 and cyclin D1,30, 38 and also for tumor suppressors including BRCA139 and γ‐catenin.40 Decrease in the expression of POU4F2 was also reported to regulate gene expression and reverse growth effects of tumor cells. Therefore, to successfully inhibit the expression of POU4F2, we should delineate the specific mechanism resulting in its elevated expression in CRC cells.
To further dissect the molecular mechanism of POU4F2 in our study, we undertook GSEA and found that the expression of POU4F2 was positively correlated with the Hedgehog signaling pathway. The Hedgehog signaling pathway is known to regulate EMT,41 and previous studies have reported its significant role in the metastasis of CRC.42, 43 Therefore, we further studied the potential role of the Hedgehog pathway in the POU4F2‐mediated EMT of colon cancer cells. We found that knocking down POU4F2 downregulated Hedgehog signaling (SHH, PTCH1, SMO, and GLI‐1), thereby suppressing EMT and inhibiting the migration and invasion of colon cancer cells. The SMO agonist, SAG, could partially reverse the effects of the POU4F2 knockdown on the migration and invasion of colon cancer cells, as well as in the expression of EMT markers. These data further revealed the contribution of POU4F2, the Hedgehog pathway, and EMT to the migration and invasion of colon cancer, which has never been reported before. More specifically, we found that POU4F2 enhanced the expression of SHH, which could bind with PTCH1 to result in the derepression of SMO, activating the Hedgehog signaling, and contributing to the translocation of GLI‐1 to the nucleus, consequently regulating the expression of EMT markers and promoting the migration and invasion of colon cancer cells. These results indicated that knocking down POU4F2 inhibits EMT in colon cancer through the suppression of the Hedgehog pathway. However, there were two main limitations in our study. One limitation is that the promoting metastatic function of POU4F2 was only verified in public databases rather than our own cohort. The other is that the genes that POU4F2 could regulate by transcription factor binding sites to promote metastasis of colon cancer were unclear, which might be the focus in further studies.
In summary, our study provided novel evidence that POU4F2 facilitates the invading and metastatic properties of colon cancer cells by regulating their Hedgehog axis‐mediated EMT. This study has introduced a potential therapeutic target for CRC. Furthermore, future studies will focus on the development of POU4F2 mAbs or inhibitors for targeting CRC.
CONFLICT OF INTEREST
The authors have no conflict of interest.
Supporting information
Table S1
ACKNOWLEDGMENTS
This study was financially supported by: General Research Program for Education of Zhejiang Province from Education of Zhejiang Province (Leitao Sun, No. Y202045212); Research Project of the Zhejiang Chinese Medical University from Zhejiang Chinese Medical University (Leitao Sun, No. 2021JKJNTZ016A); Zhejiang Provincial Program for Planted Talent Plan Zhejiang Provincial Department of Science and Technology (Jieru Yu, No. 2020R410043); and National Training Program of Innovation and Entrepreneurship for Undergraduates from Ministry of Education of the People’s Republic of China (Yuxuan Chen, No. 202010344005). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Guo K, Wang P, Zhang L, et al. Transcription factor POU4F2 promotes colorectal cancer cell migration and invasion through hedgehog‐mediated epithelial‐mesenchymal transition. Cancer Sci. 2021;112:4176–4186. 10.1111/cas.15089
Kaibo Guo, Peipei Wang, and Leyin Zhang contributed equally to this work.
Funding information
General Research Program for Education of Zhejiang Province from Education of Zhejiang Province, Grant/Award Number: Y202045212; Research Project of the Zhejiang Chinese Medical University from Zhejiang Chinese Medical University, Grant/Award Number: 2021JKJNTZ016A; Zhejiang Provincial Program for Planted Talent Plan Zhejiang Provincial Department of Science and Technology, Grant/Award Number: 2020R410043; and National Training Program of Innovation and Entrepreneurship for Undergraduates from Ministry of Education of the People’s Republic of China, Grant/Award Number: 202010344005.
Contributor Information
Jieru Yu, Email: jieruyu@zcmu.edu.cn.
Shanming Ruan, Email: shanmingruan@zcmu.edu.cn.
Leitao Sun, Email: sunnylt@zcmu.edu.cn.
REFERENCES
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394‐424. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115‐132. [DOI] [PubMed] [Google Scholar]
- 3.Wong MCS, Huang J, Lok V, et al. Differences in incidence and mortality trends of colorectal cancer worldwide based on sex, age, and anatomic location. Clin Gastroenterol Hepatol. 2020;19:955‐966. [DOI] [PubMed] [Google Scholar]
- 4.Wille‐Jørgensen P, Syk I, Smedh K, et al. Effect of more vs less frequent follow‐up testing on overall and colorectal cancer‐specific mortality in patients with stage II or III colorectal cancer: The COLOFOL randomized clinical trial. JAMA. 2018;319:2095‐2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McQuade RM, Stojanovska V, Bornstein JC, Nurgali K. Colorectal cancer chemotherapy: the evolution of treatment and new approaches. Curr Med Chem. 2017;24:1537‐1557. [DOI] [PubMed] [Google Scholar]
- 6.Xiang M, Zhou L, Peng YW, Eddy RL, Shows TB, Nathans J. Brn‐3b: a POU domain gene expressed in a subset of retinal ganglion cells. Neuron. 1993;11:689‐701. [DOI] [PubMed] [Google Scholar]
- 7.Bitsi S, Ali H, Maskell L, Ounzain S, Mohamed‐Ali V, Budhram‐Mahadeo VS. Profound hyperglycemia in knockout mutant mice identifies novel function for POU4F2/Brn‐3b in regulating metabolic processes. Am J Physiol Endocrinol Metab. 2016;310:E303‐312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mele L, Maskell LJ, Stuckey DJ, Clark JE, Heads RJ, Budhram‐Mahadeo VS. The POU4F2/Brn‐3b transcription factor is required for the hypertrophic response to angiotensin II in the heart. Cell Death Dis. 2019;10:621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Budhram‐Mahadeo VS, Latchman DS. Targeting Brn‐3b in breast cancer therapy. Expert Opin Ther Targets. 2006;10:15‐25. [DOI] [PubMed] [Google Scholar]
- 10.Mao CA, Kiyama T, Pan P, Furuta Y, Hadjantonakis AK, Klein WH. Eomesodermin, a target gene of Pou4f2, is required for retinal ganglion cell and optic nerve development in the mouse. Development. 2008;135:271‐280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ounzain S, Bowen S, Patel C, Fujita R, Heads RJ, Budhram‐Mahadeo VS. Proliferation‐associated POU4F2/Brn‐3b transcription factor expression is regulated by oestrogen through ERα and growth factors via MAPK pathway. Breast Cancer Res. 2011;13:R5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ndisang D, Budhram‐Mahadeo V, Pedley B, Latchman DS. The Brn‐3a transcription factor plays a key role in regulating the growth of cervical cancer cells in vivo. Oncogene. 2001;20:4899‐4903. [DOI] [PubMed] [Google Scholar]
- 13.Ishii J, Sato H, Yazawa T, et al. Class III/IV POU transcription factors expressed in small cell lung cancer cells are involved in proneural/neuroendocrine differentiation. Pathol Int. 2014;64:415‐422. [DOI] [PubMed] [Google Scholar]
- 14.Shi J, Hua X, Zhu B, et al. Somatic genomics and clinical features of lung adenocarcinoma: a retrospective study. PLoS Medicine. 2016;13:e1002162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pastushenko I, Blanpain C. EMT transition states during tumor progression and metastasis. Trends Cell Biol. 2019;29:212‐226. [DOI] [PubMed] [Google Scholar]
- 16.Doheny D, Manore SG, Wong GL, Lo HW. Hedgehog signaling and truncated GLI1 in cancer. Cells. 2020;9:2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liang M, Liu XC, Liu T, et al. GLI‐1 facilitates the EMT induced by TGF‐β1 in gastric cancer. Eur Rev Med Pharmacol Sci. 2018;22:6809‐6815. [DOI] [PubMed] [Google Scholar]
- 18.Zhuang H, Cao G, Kou C, Liu T. CCL2/CCR2 axis induces hepatocellular carcinoma invasion and epithelial‐mesenchymal transition in vitro through activation of the Hedgehog pathway. Oncol Rep. 2018;39:21‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hutter C, Zenklusen JC. The cancer genome atlas: creating lasting value beyond its data. Cell. 2018;173:283‐285. [DOI] [PubMed] [Google Scholar]
- 20.Guo Y, Bao Y, Ma M, Yang W. Identification of key candidate genes and pathways in colorectal cancer by integrated bioinformatical analysis. Int J Mol Sci. 2017;18:722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Powers RK, Goodspeed A, Pielke‐Lombardo H, Tan AC, Costello JC. GSEA‐InContext: identifying novel and common patterns in expression experiments. Bioinformatics. 2018;34:i555‐i564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hua F, Shang S, Yang YW, et al. TRIB3 interacts with beta‐catenin and TCF4 to increase stem cell features of colorectal cancer stem cells and tumorigenesis. Gastroenterology. 2019;156:708‐721. [DOI] [PubMed] [Google Scholar]
- 23.Liang CC, Park AY, Guan JL. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc. 2007;2:329‐333. [DOI] [PubMed] [Google Scholar]
- 24.Albini A, Iwamoto Y, Kleinman HK, et al. A rapid in vitro assay for quantitating the invasive potential of tumor cells. Cancer Res. 1987;47:3239‐3245. [PubMed] [Google Scholar]
- 25.Yang G, Chang B, Yang F, et al. Aurora kinase A promotes ovarian tumorigenesis through dysregulation of the cell cycle and suppression of BRCA2. Clin Cancer Res. 2010;16:3171‐3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real‐time quantitative PCR and the 2(‐Delta Delta C(T)) Method. Methods. 2001;25:402‐408. [DOI] [PubMed] [Google Scholar]
- 27.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7‐30. [DOI] [PubMed] [Google Scholar]
- 28.Erkman L, McEvilly RJ, Luo L, et al. Role of transcription factors Brn‐3.1 and Brn‐3.2 in auditory and visual system development. Nature. 1996;381:603‐606. [DOI] [PubMed] [Google Scholar]
- 29.Irshad S, Pedley RB, Anderson J, Latchman DS, Budhram‐Mahadeo V. The Brn‐3b transcription factor regulates the growth, behavior, and invasiveness of human neuroblastoma cells in vitro and in vivo. J Biol Chem. 2004;279:21617‐21627. [DOI] [PubMed] [Google Scholar]
- 30.Budhram‐Mahadeo VS, Irshad S, Bowen S, et al. Proliferation‐associated Brn‐3b transcription factor can activate cyclin D1 expression in neuroblastoma and breast cancer cells. Oncogene. 2008;27:145‐154. [DOI] [PubMed] [Google Scholar]
- 31.Dennis JH, Budhram‐Mahadeo V, Latchman DS. The Brn‐3b POU family transcription factor regulates the cellular growth, proliferation, and anchorage dependence of MCF7 human breast cancer cells. Oncogene. 2001;20:4961‐4971. [DOI] [PubMed] [Google Scholar]
- 32.Lee SE, Kim SJ, Yoon HJ, et al. Genome‐wide profiling in melatonin‐exposed human breast cancer cell lines identifies differentially methylated genes involved in the anticancer effect of melatonin. J Pineal Res. 2013;54:80‐88. [DOI] [PubMed] [Google Scholar]
- 33.Reinert T, Modin C, Castano FM, et al. Comprehensive genome methylation analysis in bladder cancer: identification and validation of novel methylated genes and application of these as urinary tumor markers. Clin Cancer Res. 2011;17:5582‐5592. [DOI] [PubMed] [Google Scholar]
- 34.Wu Y, Jiang G, Zhang N, et al. HOXA9, PCDH17, POU4F2, and ONECUT2 as a urinary biomarker combination for the detection of bladder cancer in chinese patients with hematuria. Eur Urol Focus. 2020;6:284‐291. [DOI] [PubMed] [Google Scholar]
- 35.Lee SA, Ndisang D, Patel C, et al. Expression of the Brn‐3b transcription factor correlates with expression of HSP‐27 in breast cancer biopsies and is required for maximal activation of the HSP‐27 promoter. Cancer Res. 2005;65:3072‐3080. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Y, Weinberg RA. Epithelial‐to‐mesenchymal transition in cancer: complexity and opportunities. Front Med. 2018;12:361‐373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brabletz T. To differentiate or not–routes towards metastasis. Nat Rev Cancer. 2012;12:425‐436. [DOI] [PubMed] [Google Scholar]
- 38.Samady L, Dennis J, Budhram‐Mahadeo V, Latchman DS. Activation of CDK4 gene expression in human breast cancer cells by the Brn‐3b POU family transcription factor. Cancer Biol Ther. 2004;3:317‐323. [PubMed] [Google Scholar]
- 39.Budhram‐Mahadeo V, Ndisang D, Ward T, Weber BL, Latchman DS. The Brn‐3b POU family transcription factor represses expression of the BRCA‐1 anti‐oncogene in breast cancer cells. Oncogene. 1999;18:6684‐6691. [DOI] [PubMed] [Google Scholar]
- 40.Samady L, Faulkes DJ, Budhram‐Mahadeo V, et al. The Brn‐3b POU family transcription factor represses plakoglobin gene expression in human breast cancer cells. Int J Cancer. 2006;118:869‐878. [DOI] [PubMed] [Google Scholar]
- 41.Sharma A, De R, Javed S, Srinivasan R, Pal A, Bhattacharyya S. Sonic hedgehog pathway activation regulates cervical cancer stem cell characteristics during epithelial to mesenchymal transition. J Cell Physiol. 2019;234(9):15726‐15741. [DOI] [PubMed] [Google Scholar]
- 42.Kim BR, Na YJ, Kim JL, et al. RUNX3 suppresses metastasis and stemness by inhibiting Hedgehog signaling in colorectal cancer. Cell Death Differ. 2020;27:676‐694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu C, Zhu X, Liu W, Ruan T, Tao K. Hedgehog signaling pathway in colorectal cancer: function, mechanism, and therapy. Onco Targets Ther. 2017;10:3249‐3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


