Summary
Anti‐drug antibody (ADA) formation is a major complication in treatment of the X‐linked bleeding disorder haemophilia B (deficiency in coagulation factor IX, FIX). Current clinical immune tolerance protocols are often not effective due to complications such as anaphylactic reactions against FIX. Plant‐based oral tolerance induction may address this problem, as illustrated by the recent first regulatory approval of orally delivered plant cells to treat peanut allergy. Our previous studies showed that oral delivery of plant cells expressing FIX fused to the transmucosal carrier CTB (cholera toxin subunit B) in chloroplasts suppressed ADA in animals with haemophilia B. We report here creation of the first lettuce transplastomic lines expressing a coagulation factor, in the absence of antibiotic resistance gene. Stable integration of the CTB‐FIX gene and homoplasmy (transformation of ˜10 000 copies in each cell) were maintained in both T1 and T2 generation marker‐free plants. CTB‐FIX expression in lyophilized leaves of T1 and T2 marker‐free plants was 1.0–1.5 mg/g dry weight, confirming that the marker excision did not affect antigen levels. Oral administration of CTB‐FIX to Sprague Dawley rats at 0.25, 1 or 2.5 mg/kg did not produce overt adverse effects or toxicity. The no‐observed‐adverse‐effect level (NOAEL) is at least 2.5 mg/kg for a single oral administration in rats. Oral administration of CTB‐FIX at 0.3 or 1.47 mg/kg either mixed in food or as an oral suspension to Beagle dogs did not produce any observable toxicity. These toxicology studies should facilitate filing of regulatory approval documents and evaluation in haemophilia B patients.
Keywords: antibiotic resistance free plants, anaphylaxis, oral protein drug delivery, tolerance induction, toxicology, regulatory approval
Introduction
Anti‐drug antibody (ADA) formation is a major complication in treatment of the X‐linked bleeding disorder haemophilia, which is caused by mutations in coagulation factors VIII (FVIII, haemophilia A) or IX (FIX, haemophilia B; Franchini and Mannucci, 2012). The prevalence of the disease is approximately 1 in 4000 male births for haemophilia A and 1 in 20 000 for haemophilia B (Iorio at al., 2019). Severe disease (<1% coagulation activity) is characterized by frequent bleeding episodes, which can be potentially life‐threatening. Neutralizing antibodies or ‘inhibitors’ form in 20–30% of patients with severe haemophilia A and in ˜3% of severe haemophilia B patients (Barg et al., 2017; Ragni, 2017; Santoro et al., 2018), albeit reported prevalence of FIX inhibitors varies between studies from 2% to 9% (Male et al., 2021; Puetz et al., 2014). Clinical immune tolerance induction (ITI) protocols have been developed to eradicate inhibitors by frequent (typically daily) intravenous infusions of the clotting factor. ITI can take months to years, costing upward of $1 000 000, during which time the patients are at increased risks of morbidity and mortality (Ljung et al., 2019). Moreover, ITI protocols are often not effective or have to be discontinued for FIX inhibitors because of anaphylactic reactions and/or nephrotic syndrome. In the case of haemophilia A, a bispecific antibody, emicizumab, a coagulation factor VIIIa‐mimetic, is in the market and effectively restores haemostasis in inhibitor positive (i.e. with neutralizing antibody against FVIII) as well as in inhibitor negative patients (Butterfield et al., 2020; Kitazawa and Shima, 2020). However, no such drug is available for haemophilia B. Therefore, FIX inhibitors, although comparatively rare, pose a much more challenging problem for treatment.
Inhibitors typically form during the first 50 exposure days to factor product, necessitating tolerance induction in young paediatric patients. Diverse alternative immune tolerance approaches are currently in preclinical development (Daniell et al., 2019a). However, immune suppression or complex cell‐ or gene therapy‐based methods may not be attractive for use in this patient population. Oral antigen delivery however may be acceptable. This approach takes advantage of the small intestine’s natural ability to promote tolerance to food antigen. Antigen processing by the immune system of the small intestine induces regulatory T cells (Treg) that can systemically suppress immune responses (Bertolini et al., 2021; Kuhn and Weiner, 2016; Kumar et al., 2020; Wang et al., 2015).
We addressed previous limitations of the oral tolerance concept including cost of production, delivery to the gut while avoiding degradation in the stomach, release in the small intestine and efficient delivery of sufficient antigen doses across the epithelium to the gut immune system by developing a plant cell‐based platform for immune tolerance induction in haemophilia A and B (Herzog et al., 2017; Kwon et al., 2018; Sherman et al., 2014; Su et al., 2015; Verma et al., 2010). Plant cell biofactories have distinct advantages over microbial and mammalian production systems in that they do not host human pathogens and can be engineered to produce complex mammalian proteins. In addition, transplastomic synthesis ensures enhanced target protein expression due to high copy number of chloroplasts (Daniell et al., 2021).
However, the chloroplast prokaryotic expression system is not optimal for expression of large human proteins. Therefore, new algorithms were recently developed using >130 sequenced chloroplast genomes to optimize expression of human genes in chloroplasts (Kwon et al., 2016, Kwon et al., 2018; Park et al., 2020; Daniell et al., 2020). Bioencapsulation in plant cells protected by cell wall also prevents degradation from harsh degrading enzymes after oral administration until the drugs are acted upon by enzymes produced by specific commensals in the mammalian gut (Kumar et al 2020). When the target antigen is tagged with carrier proteins such as CTB (cholera toxin subunit B), efficient transmucosal uptake and delivery to the immune or circulatory system is accomplished (Daniell et al., 2019; Kwon and Daniell, 2016; Xiao et al., 2016).
In addition, bioencapsulation in plant cells followed by lyophilization ensures long shelf‐life at ambient temperatures, thereby avoiding expensive downstream protein purification and cold chain (Herzog et al., 2017; Kwon and Daniell, 2016; Su et al., 2015). Our prior studies demonstrated prevention of inhibitor formation and IgE formation/anaphylactic reactions against intravenous FIX replacement therapy in haemophilia B mice and dogs by repeated oral delivery of transplastomic lyophilized plant cells expressing CTB‐FIX (Herzog et al., 2017; Su et al., 2015; Verma et al., 2010).
In the current study, we further advance translation of plant‐based oral tolerance for haemophilia B by completing the first toxicity studies in rats and dogs using lyophilized lettuce biomass expressing CTB‐FIX in chloroplasts. We also for the first time generated transplastomic lettuce plants expressing CTB‐FIX without an antibiotic selection marker (‘marker‐free’ CTB‐FIX) and evaluated stability of protein expression in subsequent generations in the absence of the selectable marker gene. Oral administration of marker‐free (MF) CTB‐FIX expressed in chloroplasts in an edible crop together with the availability of established scale‐up protocols (cGMP hydroponics, Daniell et al., 2020) and recent FDA approval of orally delivered Ara h protein expressed in peanut cells to treat peanut allergy (Bird et al., 2018; Patrawala et al., 2020; Tilles and Petroni, 2018; Vickery et al., 2018) should facilitate regulatory approval for clinical studies in humans for sustainable management of haemophilia B.
Results
Generation of marker‐free, MF‐CTB‐FIX homoplasmic lettuce lines
CTB‐FIX cDNA was subcloned into the marker‐free (MF) lettuce chloroplast transformation vector, pLS‐MF under the control of lettuce psbA promoter and terminator (Figure 1a). The pLS‐MF‐CTB‐FIX construct was then used to successfully to bombard lettuce explants, which regenerated shoots after 4 weeks on spectinomycin‐containing media. PCR analysis of the regenerated shoots with 16S‐F/atpB‐R and TpsbA‐F/23S‐R resulted in 2.4‐kb product, which confirmed the site‐specific integration of the cassette between the endogenous 16S and 23S rRNA regions in the host chloroplast genome of positive shoots (Fig S2). In addition, the 16S‐F/aadA‐R primer pair amplified a 3.3‐kb fragment confirming the presence of the marker gene, aadA conferring resistance to the aminoglycoside antibiotic, spectinomycin. Interestingly, this 3.3‐kb band was absent in line 7 which suggested the excision of the antibiotic selection marker by homologous recombination between the two directly repeated atpB fragments at some stage during the regeneration and selection on spectinomycin‐containing media, thereby resulting in a MF‐transplastomic line (Fig S2). This PCR‐positive MF transplastomic shoot was carried forward for further nodal propagation, and the resulting plants (T0) were grown in mini‐hydroponic system and finally transferred to the greenhouse for harvesting biomass and seeds (T1 and repeated up to generation T2).
Figure 1.

Evaluation of site‐specific integration, homoplasmy, expression and pentameric assembly of marker‐free CTB‐FIX lettuce transplastomic lines. Schematic depiction of CTB‐FIX gene in the expression cassette of lettuce chloroplast marker‐free vector and the process of marker excision (a). Confirmation of selectable marker gene‐free lettuce lines by seed germination assay. Seeds of MFCTBFIX (T2) and wild‐type (WT) lettuce were surface‐sterilized and germinated on plates containing half‐MS medium and that supplemented with spectinomycin 50 mg/L. Bleached phenotype of germinated seedlings of LSMFCTBFIX were observed after 10 days on antibiotic marker containing medium (b). PCR analysis to confirm site‐specific integration of LSMFCTBFIX: T0 (Lane 4), T1 (Lane 5) and T2 (Lanes 6‐8) generation plants. Lane 2 represents positive control from LSCTBFIX plant carrying the antibiotic resistance marker and Lane 1 contains the 1‐kb DNA Ladder (c). Southern blot analysis of LSMFCTBFIX transplastomic lines. A single band of 6.6 kb after digestion of gDNA (2 µg) with BglII confirms CTB‐FIX gene integration, marker excision and homoplasmy in MF transplastomic plants. Lane 1,9: 1 kb DNA Ladder, Lane 2: LSCTBFIX with marker DNA as positive control (7.1 kb); Lane 3: LSMFCTBFIX T0 generation, Lane 4: LSMFCTBFIX T1 generation, Lanes 5‐7: LSMFCTBFIX T2 generation; Lane 8: untransformed wild type (WT: 3.8 kb). Expected sizes are indicated in red arrowhead (d). Western blot analysis and quantification of Ls‐MF‐CTB‐FIX in lettuce total leaf protein (TLP) from T0, T1 and T2 transplastomic lines loaded at 5, 10.0 µg TLP per lane. CTB is used as a standard at 2, 4 and 6 ng, respectively, while WT TLP served as the negative control. The expected protein size of CTB‐FIX and CTB is marked (e). GM1‐binding assay of LSMFCTBFIX. Total soluble protein (TSP) from T0, T1 and T2 generation plants at 350 µg was used for the assay. CTB (5, 10 ng) standard was used as the positive control while 3% milk, GM1 and 350 µg TSP from lyophilized untransformed wild‐type (WT) lettuce were used as negative controls. Data shown are mean ± SD of triplicates (f).
Marker‐free homoplasmic status was stably maintained in subsequent generations
To assess homoplasmy and stable maintenance of MF phenotype in subsequent generations, MF‐CTB‐FIX seeds (T1 and T2) were germinated on spectinomycin‐free half‐strength Murashige–Skoog (MS) medium. In addition, for a preliminary evaluation of their MF nature, when T1 and T2 seeds of MF‐CTB‐FIX transplastomic lines were germinated on antibiotic‐containing (50 mg/L spectinomycin) half‐strength MS medium, the seedlings exhibited a bleached phenotype with inhibited growth, 10 days post‐germination indicating the loss of the aadA gene (Figure 1b). PCR analysis of T1 and T2 lines were carried out in a similar manner as performed for the parent T0 transplastomic line. As shown in Figure 1c, the transplastomic plants maintained their marker‐free nature with the absence of the antibiotic resistance gene (3.3‐kb fragment with 16S‐F/aadA‐R primers) in both T1 and T2 generations. In addition, the transplastomic plants were also homoplasmic (absence of WT genome) as confirmed by Southern hybridization using BglII digested total plant gDNA. As can be seen from Figure 1d, the DIG‐labelled probe bound to the genomic DNA resulted in detection of the expected 6.6‐kb digested fragment. The absence of an 8.6‐kb band corresponding to the marker not excised phenotype (Figure 1a) and a 3.8‐kb fragment specific to the wild type confirm the excision of the antibiotic selection marker, and thereby achieving homoplasmy. These observations correlate well with recently published results that the marker‐free status and homoplasmy is stably maintained through subsequent generations in the transplastomic lettuce plants (Daniell et al., 2020; Park et al., 2020).
Marker excision did not affect CTB‐FIX expression and pentameric assembly in Ls‐MF‐CTB‐FIX lettuce plants
Expression of MF‐CTB‐FIX was confirmed and quantified by Western blots using CTB antibody. The anti‐CTB antibody detected a protein corresponding to the expected size of the target protein (59.2 kDa; Figure 1e). Intact monomeric MF‐CTB‐FIX protein indicated lack of any cleaved products, confirming stable expression. The ˜40‐kDa band observed in all the extracts, including the wild type, is possibly an endogenous lettuce protein cross‐reacting with the CTB antibody (Figure S1), as observed previously (Su et al., 2015). Based on the standard curve generated using the CTB protein, the amount of CTB‐FIX expressed in lyophilized lettuce leaves of T1 and T2 MF plants harvested from greenhouse was in the range of 1.0‐1.5 mg/g dry weight, which is comparable with that obtained previously from greenhouse grown Ls‐CTB‐FIX plants containing the antibiotic resistance gene (Figure S1; Herzog et al., 2017; Su et al., 2015) indicating that the marker excision did not affect protein expression.
The formation of pentameric structures of full‐length CTB‐FIX in the marker‐free plants was confirmed using GM1‐ganglioside receptor‐binding ELISA. CTB is a transmucosal carrier transporting proteins across the intestinal epithelial cells. In its stable pentameric form (an assembly of five identical 11.6‐kDa monomers), CTB binds to the GM1 ganglioside receptor of the gut epithelial cells. The GM1 receptor‐bound CTB fusion proteins are transported into gut epithelial cells by retrograde trafficking and transcytosis (Kwon and Daniell, 2016). The cleavage site (RRKR) between CTB and the protein is recognized by Fu, an ubiquitous protease in mammalian cells, that cleaves off the mature protein for delivery into the immune or circulatory system. As shown in Figure 1f, the absorbance values measured at 450 nm of the proteins extracted from T1 and T2 Ls‐MF‐CTB‐FIX biomass showed strong binding affinity to GM1 receptor. The values are comparable to that of the CTB standard (positive control) and no significant absorbance was observed in the negative controls (BSA and WT lettuce protein extract). These results confirmed the expression, pentameric assembly and binding ability (to the GM1 receptor) of CTB‐FIX protein produced by Ls‐MF‐CTB‐FIX lyophilized lettuce biomass and that the marker excision event did not affect the protein expression and functional stability.
Lack of observable toxicity in rats upon oral administration of Ls‐CTB‐FIX lettuce biomass
A single‐dose range‐finding (DRF) toxicity study was carried out to determine potential toxicity of orally administered Ls‐CTB‐FIX expressed in transplastomic lettuce, in 7‐ to 8‐week‐old male Sprague Dawley rats. Ls‐CTB‐FIX lettuce was grown in a cGMP hydroponic facility, the biomass harvested, lyophilized and the expression was quantified as 2.94 mg/gDW of CTB‐FIX. The lyophilized plant powder was suspended in sterile PBS and used for oral administration to animals and the observations recorded (Table S1). All animals were normal and survived until their scheduled sacrifice. Animals maintained body weight in the normal range during the study (Table S2). The haematology and clinical chemistry observations are summarized in Tables 1 and 2. Haematological parameters (white and red blood cell count, haemoglobin/haematocrit levels, platelet counts, granulocyte/lymphocyte/monocyte and reticulocyte counts) remained in the normal range. Except for a slight, but statistically significant, decrease (by 12%) in mean red blood cell distribution width (RDW) in animals of Group IV receiving 2.5 mg CTB‐FIX/kg on Day 3, no adverse effects were seen. In addition, this observed change was also within the normal historical range and hence considered to be of minimal toxicological significance. Similarly, serum chemistry was normal, including kidney and liver/metabolic functions. A slight but statistically significant increase (43%) in alkaline phosphatase (ALP) was observed in Group III animals on Day 3 which could be of minimal toxicological significance as it did not correlate with either dose or changes in other evaluation endpoints. In addition, there was a minimal increase in phosphorous (PHO) levels in animals of Groups II (29%), III (23%) and IV (27%) on Day 3, which, although statistically significant, are still within the range of normal reference values and could be considered toxicologically insignificant. With relation to gross necropsy observations on Day 8, enlarged spleens were observed in one animal each from Groups I (vehicle control), II (low‐dose) and III (mid‐dose) respectively, which were insignificant minor changes commonly observed in rats with minimal toxicological significance. No visible lesions were found in the remaining tissues.
Table 1.
Summary of haematological parameters of male Sprague Dawley rats as observed on Day 3 (relative to the start date) after a single oral gavage of LS‐CTB‐FIX
| Haematology | |||||
|---|---|---|---|---|---|
| Parameter | Group I | Group II | Group III | Group IV | |
| LSCTBFIX | 0 mg/kg | 0.25 mg/kg | 1.0 mg/kg | 2.5 mg/kg | |
| White Blood Cells (×103/µL) | Mean | 11.147 I † | 10.683 | 10.693 | 9.147 |
| SD | 2.399 | 0.860 | 1.055 | 0.436 | |
| N | 3 | 3 | 3 | 3 | |
| Red Blood Cells (×106/µL) | Mean | 5.897 I † | 5.947 | 6.273 | 6.000 |
| SD | 0.319 | 0.445 | 0.350 | 0.286 | |
| N | 3 | 3 | 3 | 3 | |
| Haemoglobin (g/dL) | Mean | 12.37 I † | 12.47 | 13.00 | 13.20 |
| SD | 0.47 | 0.95 | 0.92 | 0.78 | |
| N | 3 | 3 | 3 | 3 | |
| Haematocrit (%) | Mean | 42.13 I † | 42.30 | 44.37 | 44.40 |
| SD | 1.96 | 3.30 | 3.69 | 1.84 | |
| N | 3 | 3 | 3 | 3 | |
| MCV (fL) | Mean | 71.43 I † | 71.13 | 70.70 | 73.97 |
| SD | 2.04 | 1.08 | 2.15 | 1.60 | |
| N | 3 | 3 | 3 | 3 | |
| MCH (pg) | Mean | 20.97 I † | 20.97 | 20.70 | 22.00 |
| SD | 0.78 | 0.15 | 0.52 | 0.70 | |
| N | 3 | 3 | 3 | 3 | |
| MCHC (g/dL) | Mean | 29.33 I † | 29.50 | 29.33 | 29.73 |
| SD | 0.32 | 0.17 | 0.45 | 0.57 | |
| N | 3 | 3 | 3 | 3 | |
| RDW (%) | Mean | 14.90 I † | 13.80 | 14.60 | 13.10 d ‡ |
| SD | 0.20 | 0.62 | 1.14 | 0.62 | |
| N | 3 | 3 | 3 | 3 | |
| Platelet Count (×103/µL) | Mean | 935.0 I † | 930.0 | 1035.0 | 777.7 |
| SD | 117.5 | 94.0 | 225.8 | 127.2 | |
| N | 3 | 3 | 3 | 3 | |
| Mean Platelet Volume (fL) | Mean | 7.60 I † | 7.63 | 7.53 | 7.63 |
| SD | 0.60 | 0.21 | 0.40 | 0.06 | |
| N | 3 | 3 | 3 | 3 | |
| Per cent Neutrophils (%) | Mean | 13.77 I † | 14.13 | 11.43 | 11.70 |
| SD | 0.61 | 3.32 | 3.44 | 0.36 | |
| N | 3 | 3 | 3 | 3 | |
| Per cent Lymphocytes (%) | Mean | 82.40 I † | 81.23 | 84.30 | 84.17 |
| SD | 2.14 | 2.16 | 4.78 | 0.35 | |
| N | 3 | 3 | 3 | 3 | |
| Per cent Monocytes (%) | Mean | 2.37 I † | 3.20 | 2.87 | 2.80 |
| SD | 0.42 | 0.95 | 1.29 | 0.00 | |
| N | 3 | 3 | 3 | 3 | |
| Per cent Eosinophils (%) | Mean | 0.87 I † | 0.90 | 0.73 | 0.73 |
| SD | 0.42 | 0.20 | 0.06 | 0.2 | |
| N | 3 | 3 | 3 | 3 | |
| Per cent Basophils (%) | Mean | 0.67 I † | 0.53 | 0.67 | 0.67 |
| SD | 0.23 | 0.06 | 0.06 | 0.31 | |
| N | 3 | 3 | 3 | 3 | |
| Neutrophils (Absolute) (×103/µL) | Mean | 1.557 R § | 1.490 | 1.233 | 1.070 |
| SD | 0.515 | 0.243 | 0.438 | 0.020 | |
| N | 3 | 3 | 3 | 3 | |
| Lymphocytes (Absolute) (×103/µL) | Mean | 9.150 I † | 8.687 | 9.000 | 7.700 |
| SD | 1.730 | 0.934 | 0.812 | 0.373 | |
| N | 3 | 3 | 3 | 3 | |
| Monocytes (Absolute) (×103/µL) | Mean | 0.263 I † | 0.347 | 0.317 | 0.257 |
| SD | 0.084 | 0.133 | 0.162 | 0.012 | |
| N | 3 | 3 | 3 | 3 | |
| Eosinophils (Absolute) (×103/µL) | Mean | 0.103 I † | 0.097 | 0.077 | 0.067 |
| SD | 0.055 | 0.031 | 0.006 | 0.015 | |
| N | 3 | 3 | 3 | 3 | |
| Basophils (Absolute) (×103/µL) | Mean | 0.080 I † | 0.057 | 0.070 | 0.060 |
| SD | 0.036 | 0.012 | 0.017 | 0.026 | |
| N | 3 | 3 | 3 | 3 | |
| Per cent Reticulocyte (%) | Mean | 10.560 I † | 10.353 | 9.603 | 9.577 |
| SD | 0.810 | 1.115 | 0.594 | 0.657 | |
| N | 3 | 3 | 3 | 3 | |
| Reticulocyte (Absolute) (×109/µL) | Mean | 621.87 I † | 613.33 | 601.73 | 574.37 |
| SD | 38.65 | 43.44 | 38.52 | 39.43 | |
| N | 3 | 3 | 3 | ||
MCV, Mean Corpuscular Volume; MCH, Mean Corpuscular Haemoglobin; MCHC, Mean Corpuscular Haemoglobin Concentration; RDW, Red Blood Cell Distribution Width.
I – Automatic Transformation: Identity (No Transformation).
d – Test: Dunnett two‐sided P < 0.05.
R – Automatic Transformation: Rank.
Table 2.
Changes in clinical chemistry of male Sprague Dawley rats as observed on Day 3 (relative to the start date) after a single oral gavage of LS‐CTB‐FIX
| Clinical chemistry | |||||
|---|---|---|---|---|---|
| Parameter | Group I | Group II | Group III | Group IV | |
| LSCTBFIX | 0 mg/kg | 0.25 mg/kg | 1.0 mg/kg | 2.5 mg/kg | |
| Blood Urea Nitrogen (mg/dL) | Mean | 13.7 I † | 13.3 | 15.0 | 15.7 |
| SD | 1.5 | 1.5 | 1.0 | 0.6 | |
| N | 3 | 3 | 3 | 3 | |
| Creatinine (mg/dL) | Mean | – | – | 0.170 | 0.190 |
| SD | – | – | – | – | |
| N | 0 | 0 | 1 | 1 | |
| Glucose (mg/dL) | Mean | 150.3 I † | 151.7 | 167.7 | 160.7 |
| SD | 5.5 | 5.5 | 17.5 | 2.5 | |
| N | 3 | 3 | 3 | 3 | |
| AST (U/L) | Mean | 92.0 I † | 91.3 | 97.0 | 99.3 |
| SD | 15.0 | 5.9 | 7.2 | 8.5 | |
| N | 3 | 3 | 3 | 3 | |
| ALT (U/L) | Mean | 53.0 R ‡ | 58.0 | 61.7 | 56.0 |
| SD | 6.1 | 6.2 | 5.7 | 6.1 | |
| N | 3 | 3 | 3 | 3 | |
| Alkaline phosphatase (U/L) | Mean | 343.0 I † | 325.0 | 490.7 d § | 378.0 |
| SD | 13.5 | 53.0 | 77.8 | 78.0 | |
| N | 3 | 3 | 3 | 3 | |
| Total Bilirubin (mg/dL) | Mean | – | – | – | – |
| SD | – | – | – | – | |
| N | 0 | 0 | 0 | 0 | |
| Sodium (mmol/L) | Mean | 144.7 R ‡ | 143.7 | 144.3 | 145.3 |
| SD | 1.2 | 1.2 | 0.6 | 0.6 | |
| N | 3 | 3 | 3 | 3 | |
| Potassium (mmol/L) | Mean | 6.40 I † | 6.27 | 6.97 | 6.67 |
| SD | 0.53 | 0.32 | 0.51 | 0.49 | |
| N | 3 | 3 | 3 | 3 | |
| Chloride (mmol/L) | Mean | 101.0 I † | 100.3 | 100.3 | 101.7 |
| SD | 1.0 | 1.2 | 0.6 | 1.2 | |
| N | 3 | 3 | 3 | 3 | |
| Calcium (mg/dL) | Mean | 11.40 I † | 11.50 | 12.03 | 12.17 |
| SD | 0.26 | 0.53 | 0.74 | 0.67 | |
| N | 3 | 3 | 3 | 3 | |
| Phosphorous (mg/dL) | Mean | 8.70 I † | 11.27 d § | 10.73 d § | 11.07 d § |
| SD | 0.95 | 0.91 | 0.86 | 0.57 | |
| N | 3 | 3 | 3 | 3 | |
| Total protein (g/dL) | Mean | 5.67 R ‡ | 5.47 | 5.63 | 5.57 |
| SD | 0.15 | 0.23 | 0.29 | 0.40 | |
| N | 3 | 3 | 3 | 3 | |
| Albumin (g/dL) | Mean | 3.83 I † | 3.60 | 3.73 | 3.60 |
| SD | 0.06 | 0.20 | 0.06 | 0.17 | |
| N | 3 | 3 | 3 | 3 | |
| Globulin (g/dL) | Mean | 1.83 I † | 1.87 | 1.90 | 1.97 |
| SD | 0.15 | 0.12 | 0.26 | 0.23 | |
| N | 3 | 3 | 3 | 3 | |
| Alb/Glo Ratio | Mean | 2.0 R ‡ | 2.0 | 2.0 | 2.0 |
| SD | 0.0 | 0.0 | 0.0 | 0.0 | |
| N | 3 | 3 | 3 | 3 | |
| Cholesterol (mg/dL) | Mean | 86.7 I † | 91.7 | 91.3 | 89.0 |
| SD | 20.6 | 9.2 | 8.7 | 6.1 | |
| N | 3 | 3 | 3 | 3 | |
| Triglyceride (mg/dL) | Mean | 124.3 I † | 144.3 | 169.7 | 195.7 |
| SD | 30.2 | 56.0 | 35.9 | 41.2 | |
| N | 3 | 3 | 3 | 3 | |
AST, Aspartate aminotransferase; ALT, Alanine aminotransferase; Alb/Glo, Albumin‐to‐Globulin ratio.
“–“ : Not Applicable.
I – Automatic Transformation: Identity (No Transformation).
R – Automatic Transformation: Rank.
d – Test: Dunnett two‐sided P < 0.05.
In summary, oral administration of Ls‐CTB‐FIX to male Sprague Dawley rats for a single day at 0.25, 1 or 2.5 mg/kg did not produce overt adverse effects and did not have any toxicity. When normalized for body surface area, these reflect a human equivalent dose (HED) of 0.04 mg/kg, 0.16 mg/kg, and 0.4 mg/kg, respectively (using km values of 6 for rat and 37 for adult human). Therefore, the maximum tolerated dose (MTD) of Ls‐CTB‐FIX was not determined but is believed to be greater than 2.5 mg/kg for a single oral dose in rats (a HED of 0.4 mg/kg). The no‐observed‐adverse‐effect level (NOAEL) is at least 2.5 mg/kg for a single oral administration in rats. In prior preclinical efficacy studies, Ls‐CTB‐FIX suppressed inhibitor formation in haemophilia B mice at doses ranging from 0.06–0.6 mg/kg, a HED range of 0.005–0.05 mg/kg (Su et al., 2015). Therefore, the NOAEL observed in rats is at least 8× higher than the highest and 80× higher than the lowest effective dose tested in haemophilia B mice.
Lack of observable toxicity in dogs upon oral administration of Ls‐CTB‐FIX lettuce biomass
A combined single‐dose toxicity and pharmacokinetic study was performed in 9‐ to 10‐month‐old haemostatically normal male Beagle dogs (n = 2 per dose group). The Ls‐CTB‐FIX antigen (2.94 mg/g DW of lettuce biomass) doses received by the animals for oral delivery were 0.3 mg/kg (Groups I and III) and 1.47 mg/kg (Groups II and IV, see Table S3). All animals appeared normal and survived until the end of the study. As seen from Table S4, the animals maintained normal body weight during the study. No altered clinical signs were observed, including gross motor and behavioural activity, or changes in appearance. As evident from data shown in Tables 3, 4 and S5, on Day 3 post‐dose, all dogs showed normal haematology, clinical chemistry and coagulation parameters that were not significantly different from pre‐dose values.
Table 3.
Summary of haematological parameters of male Beagle dogs after a single oral gavage of LS‐CTB‐FIX
| Haematology | ||||||
|---|---|---|---|---|---|---|
| Sex: male | Group I | Group II | Group III | Group IV | ||
| Day(s) relative to start date | 102 mg/kg in food | 500 mg/kg in food | 102 mg/kg PO Gavage | 500 mg/kg PO Gavage | ||
| White blood cells (×103/µL) | −1 | Mean | 6.705 | 7.000 | 5.740 | 5.965 |
| SD | 1.648 | 1.541 | 1.075 | 0.728 | ||
| N | 2 | 2 | 2 | 2 | ||
| 3 | Mean | 6.895 | 6.955 | 5.545 | 5.410 | |
| SD | 1.039 | 0.898 | 1.025 | 1.527 | ||
| N | 2 | 2 | 2 | 2 | ||
| Red blood cells (×106/µL) | −1 | Mean | 6.420 | 6.525 | 6.020 | 6.520 |
| SD | 0.071 | 0.375 | 0.198 | 0.014 | ||
| N | 2 | 2 | 2 | 2 | ||
| 3 | Mean | 6.415 | 6.355 | 6.140 | 6.325 | |
| SD | 0.177 | 0.488 | 0.156 | 0.262 | ||
| N | 2 | 2 | 2 | 2 | ||
| Haemoglobin (g/dL) | −1 | Mean | 14.90 | 15.80 | 14.30 | 16.05 |
| SD | 0.42 | 1.56 | 0.71 | 0.07 | ||
| N | 2 | 2 | 2 | 2 | ||
| 3 | Mean | 15.25 | 14.95 | 14.25 | 15.15 | |
| SD | 0.49 | 1.63 | 0.35 | 0.92 | ||
| N | 2 | 2 | 2 | 2 | ||
| Haematocrit (%) | −1 | Mean | 43.95 | 43.60 | 41.50 | 44.35 |
| SD | 0.21 | 2.97 | 1.56 | 0.07 | ||
| N | 2 | 2 | 2 | 2 | ||
| 3 | Mean | 42.80 | 43.10 | 41.20 | 43.55 | |
| SD | 1.56 | 3.54 | 1.56 | 1.91 | ||
| N | 2 | 2 | 2 | 2 | ||
| MCV (fL) | −1 | Mean | 68.45 | 66.85 | 68.80 | 68.10 |
| SD | 0.35 | 0.78 | 0.28 | 0.14 | ||
| N | 2 | 2 | 2 | 2 | ||
| 3 | Mean | 66.70 | 67.85 | 67.10 | 68.80 | |
| SD | 0.57 | 0.35 | 0.85 | 0.28 | ||
| N | 2 | 2 | 2 | 2 | ||
| Haematology | ||||||
|---|---|---|---|---|---|---|
| Sex: male | Group I | Group II | Group III | Group IV | ||
| Day(s) relative to start date | 102 mg/kg in food | 500 mg/kg in food | 102 mg/kg PO Gavage | 500 mg/kg PO Gavage | ||
| MCH (pg) | −1 | Mean | 23.25 | 24.20 | 23.70 | 24.65 |
| SD | 0.35 | 0.99 | 0.42 | 0.07 | ||
| N | 2 | 2 | 2 | 2 | ||
| 3 | Mean | 23.80 | 23.50 | 23.20 | 23.95 | |
| SD | 0.14 | 0.71 | 0.00 | 0.35 | ||
| N | 2 | 2 | 2 | 2 | ||
| MCHC (g/dL) | −1 | Mean | 33.95 | 36.15 | 34.40 | 36.25 |
| SD | 0.78 | 1.06 | 0.42 | 0.21 | ||
| N | 2 | 2 | 2 | 2 | ||
| 3 | Mean | 35.65 | 34.60 | 34.60 | 34.80 | |
| SD | 0.07 | 0.99 | 0.42 | 0.42 | ||
| N | 2 | 2 | 2 | 2 | ||
| RDW (%) | −1 | Mean | 12.40 | 12.25 | 12.80 | 12.70 |
| SD | 0.14 | 0.64 | 0.00 | 0.00 | ||
| N | 2 | 2 | 2 | 2 | ||
| 3 | Mean | 12.35 | 12.40 | 12.65 | 12.90 | |
| SD | 0.35 | 0.57 | 0.21 | 0.14 | ||
| N | 2 | 2 | 2 | 2 | ||
| Platelet Count (×103/µL) | −1 | Mean | 179.5 | 179.0 | 238.5 | 241.0 |
| SD | 16.3 | 18.4 | 82.7 | 53.7 | ||
| N | 2 | 2 | 2 | 2 | ||
| 3 | Mean | 178.5 | 201.5 | 219.5 | 247.0 | |
| SD | 0.7 | 13.4 | 71.4 | 42.4 | ||
| N | 2 | 2 | 2 | 2 | ||
| Mean Platelet Volume (fL) | −1 | Mean | 10.70 | 10.55 | 8.50 | 8.55 |
| SD | 0.57 | 0.21 | 0.42 | 0.64 | ||
| N | 2 | 2 | 2 | 2 | ||
| 3 | Mean | 10.80 | 11.00 | 8.55 | 8.60 | |
| SD | 0.00 | 0.42 | 0.35 | 0.71 | ||
| N | 2 | 2 | 2 | 2 | ||
| Haematology | ||||||
|---|---|---|---|---|---|---|
| Sex: male | Group I | Group II | Group III | Group IV | ||
| Day(s) relative to start date | 102 mg/kg in food | 500 mg/kg in food | 102 mg/kg PO Gavage | 500 mg/kg PO Gavage | ||
| Per cent neutrophils (%) | −1 | Mean | 57.30 | 52.65 | 56.00 | 53.30 |
| SD | 3.25 | 3.75 | 4.53 | 6.79 | ||
| N | 2 | 2 | 2 | 2 | ||
| 3 | Mean | 54.60 | 60.50 | 53.50 | 54.05 | |
| SD | 0.14 | 3.54 | 4.95 | 4.88 | ||
| N | 2 | 2 | 2 | 2 | ||
| Per cent lymphocytes (%) | −1 | Mean | 33.45 | 35.30 | 32.90 | 35.50 |
| SD | 3.61 | 2.40 | 1.56 | 2.26 | ||
| N | 2 | 2 | 2 | 2 | ||
| 3 | Mean | 34.25 | 28.70 | 32.95 | 35.75 | |
| SD | 0.78 | 5.23 | 2.76 | 0.21 | ||
| N | 2 | 2 | 2 | 2 | ||
| Per cent monocytes (%) | −1 | Mean | 2.50 | 4.20 | 4.30 | 5.60 |
| SD | 0.71 | 1.70 | 0.57 | 0.28 | ||
| N | 2 | 2 | 2 | 2 | ||
| 3 | Mean | 4.95 | 4.05 | 5.15 | 4.95 | |
| SD | 1.06 | 1.48 | 0.21 | 1.48 | ||
| N | 2 | 2 | 2 | 2 | ||
| Per cent eosinophils (%) | −1 | Mean | 4.40 | 4.15 | 5.90 | 4.75 |
| SD | 1.98 | 1.63 | 5.66 | 4.31 | ||
| N | 2 | 2 | 2 | 2 | ||
| 3 | Mean | 5.45 | 5.05 | 6.50 | 4.60 | |
| SD | 0.49 | 1.34 | 6.36 | 3.39 | ||
| N | 2 | 2 | 2 | 2 | ||
| Per cent basophils (%) | −1 | Mean | 0.40 | 0.20 | 0.80 | 0.85 |
| SD | 0.57 | 0.28 | 0.14 | 0.07 | ||
| N | 2 | 2 | 2 | 2 | ||
| 3 | Mean | 0.75 | 0.20 | 0.35 | 0.70 | |
| SD | 0.49 | 0.28 | 0.49 | 0.28 | ||
| N | 2 | 2 | 2 | 2 | ||
| Haematology | ||||||
|---|---|---|---|---|---|---|
| Sex: male | Group I | Group II | Group III | Group IV | ||
| Day(s) relative to start date | 102 mg/kg in food | 500 mg/kg in food | 102 mg/kg PO Gavage | 500 mg/kg PO Gavage | ||
| Neutrophils (Absolute) (×103/µL) | −1 | Mean | 3.869 | 3.718 | 3.240 | 3.200 |
| SD | 1.162 | 1.078 | 0.863 | 0.792 | ||
| N | 2 | 2 | 2 | 2 | ||
| 3 | Mean | 3.765 | 4.191 | 2.990 | 2.960 | |
| SD | 0.573 | 0.296 | 0.820 | 1.089 | ||
| N | 2 | 2 | 2 | 2 | ||
| Lymphocytes (Absolute) (×103/µL) | −1 | Mean | 2.212 | 2.458 | 1.895 | 2.110 |
| SD | 0.308 | 0.384 | 0.431 | 0.127 | ||
| N | 2 | 2 | 2 | 2 | ||
| 3 | Mean | 2.365 | 2.020 | 1.842 | 1.925 | |
| SD | 0.417 | 0.622 | 0.492 | 0.530 | ||
| N | 2 | 2 | 2 | 2 | ||
| Monocytes (Absolute) (×103/µL) | −1 | Mean | 0.170 | 0.304 | 0.240 | 0.335 |
| SD | 0.084 | 0.179 | 0.014 | 0.021 | ||
| N | 2 | 2 | 2 | 2 | ||
| 3 | Mean | 0.335 | 0.290 | 0.286 | 0.255 | |
| SD | 0.021 | 0.142 | 0.063 | 0.007 | ||
| N | 2 | 2 | 2 | 2 | ||
| Eosinophils (Absolute) (×103/µL) | −1 | Mean | 0.308 | 0.304 | 0.310 | 0.270 |
| SD | 0.201 | 0.179 | 0.255 | 0.226 | ||
| N | 2 | 2 | 2 | 2 | ||
| 3 | Mean | 0.375 | 0.345 | 0.330 | 0.220 | |
| SD | 0.092 | 0.049 | 0.283 | 0.113 | ||
| N | 2 | 2 | 2 | 2 | ||
| Basophils (Absolute) (×103/uL) | −1 | Mean | 0.030 | 0.015 | 0.045 | 0.050 |
| SD | 0.042 | 0.021 | 0.021 | 0.014 | ||
| N | 2 | 2 | 2 | 2 | ||
| 3 | Mean | 0.050 | 0.015 | 0.025 | 0.040 | |
| SD | 0.028 | 0.021 | 0.035 | 0.028 | ||
| N | 2 | 2 | 2 | 2 | ||
| Haematology | ||||||
|---|---|---|---|---|---|---|
| Sex: male | Group I | Group II | Group III | Group IV | ||
| Day(s) relative to start date | 102 mg/kg in food | 500 mg/kg in food | 102 mg/kg PO Gavage | 500 mg/kg PO Gavage | ||
| Per cent Reticulocyte (%) | −1 | Mean | 0.575 | 0.615 | 0.370 | 0.615 |
| SD | 0.078 | 0.389 | 0.014 | 0.148 | ||
| N | 2 | 2 | 2 | 2 | ||
| 3 | Mean | 0.540 | 0.565 | 0.525 | 0.530 | |
| SD | 0.170 | 0.177 | 0.191 | 0.198 | ||
| N | 2 | 2 | 2 | 2 | ||
| Reticulocyte (Absolute) (109/L) | −1 | Mean | 36.90 | 41.05 | 22.35 | 39.95 |
| SD | 5.23 | 27.79 | 0.35 | 9.69 | ||
| N | 2 | 2 | 2 | 2 | ||
| 3 | Mean | 34.85 | 36.45 | 32.35 | 33.75 | |
| SD | 11.53 | 14.07 | 12.37 | 13.65 | ||
| N | 2 | 2 | 2 | 2 | ||
MCV, Mean Corpuscular Volume.
MCH, Mean Corpuscular Haemoglobin; MCHC, Mean Corpuscular Haemoglobin Concentration; RDW, Red Blood Cell Distribution Width.
Statistical significance indicated on a group with an N < 3 is not valid.
Table 4.
Changes in clinical chemistry of male Beagle dogs after a single oral gavage of LS‐CTB‐FIX
| Clinical Chemistry | ||||||
|---|---|---|---|---|---|---|
| Sex: male | Group I | Group II | Group III | Group IV | ||
| Day(s) relative to start date | 102 mg/kg in food | 500 mg/kg in food | 102 mg/kg PO Gavage | 500 mg/kg PO Gavage | ||
| Blood urea nitrogen (mg/dL) | −1 | Mean | 12.0 | 12.0 | 11.0 | 12.0 |
| SD | 0.0 | 1.4 | 1.4 | 2.8 | ||
| N | 2 | 2 | 2 | 2 | ||
| 3 | Mean | 12.0 | 11.5 | 11.0 | 13.0 | |
| SD | 0.0 | 0.7 | 0.0 | 4.2 | ||
| N | 2 | 2 | 2 | 2 | ||
| Creatinine (mg/dL) | −1 | Mean | 0.770 | 0.720 | 0.670 | 0.680 |
| SD | 0.028 | 0.028 | 0.184 | 0.071 | ||
| N | 2 | 2 | 2 | 2 | ||
| 3 | Mean | 0.700 | 0.745 | 0.650 | 0.670 | |
| SD | 0.014 | 0.049 | 0.099 | 0.085 | ||
| N | 2 | 2 | 2 | 2 | ||
| Glucose (mg/dL) | −1 | Mean | 99.5 | 104.5 | 99.5 | 104.5 |
| SD | 2.1 | 2.1 | 0.7 | 2.1 | ||
| N | 2 | 2 | 2 | 2 | ||
| 3 | Mean | 102.0 | 97.0 | 98.5 | 97.5 | |
| SD | 2.8 | 4.2 | 0.7 | 3.5 | ||
| N | 2 | 2 | 2 | 2 | ||
| AST (U/L) | −1 | Mean | 25.5 | 23.5 | 27.5 | 29.0 |
| SD | 2.1 | 0.7 | 2.1 | 1.4 | ||
| N | 2 | 2 | 2 | 2 | ||
| 3 | Mean | 25.5 | 24.5 | 26.5 | 29.5 | |
| SD | 0.7 | 0.7 | 3.5 | 7.8 | ||
| N | 2 | 2 | 2 | 2 | ||
| ALT (U/L) | −1 | Mean | 24.5 | 24.5 | 25.5 | 27.5 |
| SD | 3.5 | 0.7 | 4.9 | 7.8 | ||
| N | 2 | 2 | 2 | 2 | ||
| 3 | Mean | 24.5 | 24.0 | 28.5 | 27.0 | |
| SD | 0.7 | 2.8 | 7.8 | 4.2 | ||
| N | 2 | 2 | 2 | 2 | ||
| Clinical chemistry | ||||||
|---|---|---|---|---|---|---|
| Sex: male | Group I | Group II | Group III | Group IV | ||
| Day(s) relative to start date | 102 mg/kg in food | 500 mg/kg in food | 102 mg/kg PO Gavage | 500 mg/kg PO Gavage | ||
| Alkaline phosphatase (U/L) | −1 | Mean | 49.0 | 51.0 | 43.0 | 45.5 |
| SD | 2.8 | 2.8 | 7.1 | 12.0 | ||
| N | 2 | 2 | 2 | 2 | ||
| 3 | Mean | 47.5 | 50.0 | 41.5 | 44.0 | |
| SD | 3.5 | 4.2 | 6.4 | 11.3 | ||
| N | 2 | 2 | 2 | 2 | ||
| Total bilirubin (mg/dL) | −1 | Mean | – | – | – | – |
| SD | – | – | – | – | ||
| N | 0 | 0 | 0 | 0 | ||
| 3 | Mean | – | – | – | – | |
| SD | – | – | – | – | ||
| N | 0 | 0 | 0 | 0 | ||
| Sodium (mmol/L) | −1 | Mean | 146.0 | 147.0 | 146.0 | 148.0 |
| SD | 2.8 | 1.4 | 0.0 | 0.0 | ||
| N | 2 | 2 | 2 | 2 | ||
| 3 | Mean | 148.0 | 148.0 | 148.0 | 150.0 | |
| SD | 1.4 | 1.4 | 1.4 | 0.0 | ||
| N | 2 | 2 | 2 | 2 | ||
| Potassium (mmol/L) | −1 | Mean | 4.35 | 4.35 | 4.25 | 4.30 |
| SD | 0.21 | 0.21 | 0.07 | 0.00 | ||
| N | 2 | 2 | 2 | 2 | ||
| 3 | Mean | 4.45 | 4.30 | 4.35 | 4.45 | |
| SD | 0.07 | 0.00 | 0.07 | 0.21 | ||
| N | 2 | 2 | 2 | 2 | ||
| Chloride (mmol/L) | −1 | Mean | 106.0 | 107.0 | 107.0 | 108.0 |
| SD | 4.2 | 0.0 | 0.0 | 0.0 | ||
| N | 2 | 2 | 2 | 2 | ||
| 3 | Mean | 107.0 | 107.5 | 108.0 | 108.5 | |
| SD | 0.0 | 2.1 | 0.0 | 0.7 | ||
| N | 2 | 2 | 2 | 2 | ||
| Clinical chemistry | ||||||
|---|---|---|---|---|---|---|
| Sex: male | Group I | Group II | Group III | Group IV | ||
| Day(s) relative to start date | 102 mg/kg in food | 500 mg/kg in food | 102 mg/kg PO Gavage | 500 mg/kg PO Gavage | ||
| Calcium (mg/dL) | −1 | Mean | 10.70 | 10.55 | 10.55 | 10.50 |
| SD | 0.28 | 0.21 | 0.35 | 0.14 | ||
| N | 2 | 2 | 2 | 2 | ||
| 3 | Mean | 10.35 | 10.40 | 10.40 | 10.40 | |
| SD | 0.07 | 0.14 | 0.00 | 0.00 | ||
| N | 2 | 2 | 2 | 2 | ||
| Phosphorus (mg/dL) | −1 | Mean | 4.85 | 4.45 | 4.35 | 4.30 |
| SD | 0.49 | 0.64 | 0.21 | 0.42 | ||
| N | 2 | 2 | 2 | 2 | ||
| 3 | Mean | 4.60 | 4.45 | 4.15 | 4.10 | |
| SD | 0.57 | 1.20 | 0.21 | 0.28 | ||
| N | 2 | 2 | 2 | 2 | ||
| Total Protein (g/dL) | −1 | Mean | 5.70 | 5.60 | 5.55 | 5.70 |
| SD | 0.14 | 0.42 | 0.07 | 0.00 | ||
| N | 2 | 2 | 2 | 2 | ||
| 3 | Mean | 5.50 | 5.60 | 5.50 | 5.70 | |
| SD | 0.28 | 0.28 | 0.14 | 0.00 | ||
| N | 2 | 2 | 2 | 2 | ||
| Albumin (g/dL) | −1 | Mean | 3.55 | 3.55 | 3.60 | 3.65 |
| SD | 0.07 | 0.21 | 0.14 | 0.07 | ||
| N | 2 | 2 | 2 | 2 | ||
| 3 | Mean | 3.35 | 3.45 | 3.50 | 3.60 | |
| SD | 0.07 | 0.07 | 0.14 | 0.00 | ||
| N | 2 | 2 | 2 | 2 | ||
| Globulin (g/dL) | −1 | Mean | 2.15 | 2.05 | 1.95 | 2.05 |
| SD | 0.07 | 0.21 | 0.07 | 0.07 | ||
| N | 2 | 2 | 2 | 2 | ||
| 3 | Mean | 2.15 | 2.15 | 2.00 | 2.10 | |
| SD | 0.21 | 0.21 | 0.00 | 0.00 | ||
| N | 2 | 2 | 2 | 2 | ||
| Clinical chemistry | ||||||
|---|---|---|---|---|---|---|
| Sex: male | Group I | Group II | Group III | Group IV | ||
| Day(s) relative to start date | 102 mg/kg in food | 500 mg/kg in food | 102 mg/kg PO Gavage | 500 mg/kg PO Gavage | ||
| Alb/Glo Ratio | −1 | Mean | 2.0 | 2.0 | 2.0 | 2.0 |
| SD | 0.0 | 0.0 | 0.0 | 0.0 | ||
| N | 2 | 2 | 2 | 2 | ||
| 3 | Mean | 1.5 | 2.0 | 2.0 | 2.0 | |
| SD | 0.7 | 0.0 | 0.0 | 0.0 | ||
| N | 2 | 2 | 2 | 2 | ||
| Cholesterol (mg/dL) | −1 | Mean | 173.0 | 162.5 | 160.5 | 170.5 |
| SD | 17.0 | 31.8 | 12.0 | 2.1 | ||
| N | 2 | 2 | 2 | 2 | ||
| 3 | Mean | 166.0 | 162.5 | 162.5 | 163.5 | |
| SD | 24.0 | 27.6 | 12.0 | 2.1 | ||
| N | 2 | 2 | 2 | 2 | ||
| Triglyceride (mg/dL) | ‐1 | Mean | 29.0 | 27.5 | 28.5 | 33.5 |
| SD | 0.0 | 6.4 | 2.1 | 6.4 | ||
| N | 2 | 2 | 2 | 2 | ||
| 3 | Mean | 30.5 | 22.5 | 33.0 | 29.0 | |
| SD | 0.7 | 2.1 | 2.8 | 1.4 | ||
| N | 2 | 2 | 2 | 2 | ||
AST, Aspartate Aminotransferase; ALT, Alanine Aminotransferase.
Alb/Glo Ratio Albumin/Globulin Ratio; ‘–' indicates Not Applicable; Statistical significance indicated on a group with an N < 3 is not valid.
Thus, a single oral dose administration of Ls‐CTB‐FIX at 0.3 or 1.47 mg/kg (equivalent to 102 or 500 mg/kg lyophilized green powder, respectively) either mixed in food or as an oral suspension formulation in PBS to male Beagle dogs did not produce any observable toxicity, even at a dose 5× higher than a dose that was effective in suppressing inhibitor formation in haemophilia B dogs (0.3 mg/kg, a HED of 0.17 mg/kg) (Herzog et al., 2017).
Lack of detectable human FIX antigen in circulation of animals
In order to measure human FIX levels that may have entered the circulation after oral delivery of Ls‐CTB‐FIX to the dogs, blood was collected pre‐dose, 30 min, 1, 2, 3, 4, 6, 8 and 24 h post‐dose (Table S3). For Groups I and II, the time post‐dose started counting once the animals consume all the wet dog food mixed with Ls‐CTB‐FIX lyophilized powder. FIX levels in plasma were determined by ELISA with a greater sensitivity of 0.068 ng/mL (after 1:2 dilution of samples; this bioanalytical method is specific for human FIX and does not detect endogenous canine FIX). However, no evidence for human FIX antigen within this limit of detection was obtained (Table S7).
A similar pharmacokinetics study of Ls‐CTB‐FIX following a single oral dose administration was performed in 8‐week‐old male Sprague Dawley rats. Three animals by study Group I‐III received 0.49, 0.98 or 1.96 mg CTB‐FIX antigen/kg, respectively, by oral gavage in a volume of 0.75 mL, 1.5 mL or 3 mL per animal (Table S6). All animals appeared normal and survived until the day of sacrifice. Daily observations and measurements of weight showed no abnormalities in general health or changes in body weight (Table 5). FIX levels in plasma were determined by ELISA with a greater sensitivity of 0.136 ng/mL (after 1:3 dilution of samples; this bioanalytical method is specific for human FIX and does not detect endogenous rat FIX). FIX antigen was not detected within this limit of sensitivity in any plasma samples, except for a single animal that had 0.14 ng/mL at 30 min (Table S8).
Table 5.
Clinical observations and body weights summary following a single oral dose administration of Ls‐CTB‐FIX in male Sprague Dawley rats for pharmacokinetic study
| Clinical Observations | |||
|---|---|---|---|
| Male | |||
|
Observation type: all types From Day 1 (Start Date) to 2 (Start Date) |
Group I 0.49 mg/kg PO |
Group II 0.98 mg/kg PO |
Group III 1.96 mg/kg PO |
| Normal | |||
| Number of Animals Affected | 3 | 3 | 3 |
| First to Last seen | 1–1 | 1–1 | 1–1 |
| Replacement Animal | |||
| Number of Animals Affected | 0 | 0 | 1 |
| First to Last seen | – | – | 1–1 |
| Main Sacrifice | |||
| Number of Animals Affected | 3 | 3 | 3 |
| First to Last seen | 2–2 | 2–2 | 2–2 |
| Body Weight (kg) | ||||
|---|---|---|---|---|
|
Sex: male Day(s) Relative to Start Date |
Group I 0.49 mg/kg PO |
Group II 0.98 mg/kg PO |
Group III 1.96 mg/kg PO |
|
| 1 | Mean | 258.7 I † | 251.7 | 245.3 |
| SD | 8.1 | 10.8 | 14.0 | |
| N | 3 | 3 | 3 | |
Statistical Test: Anova and Dunnett's Transformation: Automatic; ‘–’ indicates Not Applicable.
In conclusion, Ls‐CTB‐FIX was not detected (within the limit of sensitivity) in circulation of male Sprague Dawley rats (except one) and Beagle dogs following a single oral dose administration. These results may be used to determine dose levels for subsequent toxicity studies and the suitability of the proposed human dose.
Discussion
Development of anti‐drug antibodies (ADA) is a major complication in replacement therapy for inherited protein deficiencies. In contrast, oral delivery of antigens (or) oral immunotherapy (OIT) takes advantage of a naturally tolerogenic route, resulting in processing of the antigen by the gut immune system and ultimately inducing Treg cells that suppress immune responses at other sites. In a successful demonstration of plant‐based OIT, the Food and Drug Administration (FDA) recently approved Palforzia [Peanut (Arachis hypogaea) Allergen Powder] for children aged 4‐17 suffering from peanut allergy to mitigate allergic/anaphylactic reactions occurring upon accidental exposure to peanuts (Bird et al., 2018). Patients are desensitized to the allergen by initial exposure to low doses of the drug, gradually escalating the dose levels over scheduled intervals until they reach their tolerance limit which will then be maintained for a specific period (Patrawala et al., 2020; Tilles and Petroni, 2018; Vickery et al., 2018).
Similarly, plant‐based OIT can also be utilized to prevent ADA formation in protein replacement therapy for genetic diseases such as haemophilia B. Given the limitations of traditional ITI in FIX inhibitor patients, in part owing to the potential for severe allergic reactions, and a lack of a drug equivalent to emicizumab, there continues to be an urgent medical need to suppress inhibitor formation (Barg et al., 2017; Ljung et al., 2019; Mancuso et al., 2021; Santoro et al., 2018). To address this problem, we developed a cost‐effective oral tolerance approach that utilizes transplastomic lettuce cells expressing CTB‐FIX (Herzog et al., 2017; Su et al., 2015). Transgenic antigens are expressed as CTB fusion proteins that are present in pentameric form. CTB functions as a transmucosal carrier, while plant cell wall provides bioencapsulation. The enzymatic activity provided by commensal bacteria causes the CTB fusion protein to be released in small intestine (Kumar et al., 2020; Xiao et al., 2016). Pentameric CTB fusion proteins then bind to the GM1 receptor that is abundantly present on the surface of gut epithelial cells and on dendritic cells. Transmucosal delivery to the immune system leads to induction of FoxP3+ and LAP+ Treg through a complex mechanism, ultimately suppressing antibody formation against the antigen that normally occurs against the intravenously delivered protein (Kuhn and Weiner, 2016; Lacroix‐Desmazes et al., 2020; Pabst and Mowat, 2012; Rezende and Weiner, 2017; Rezende and Weiner, 2018; Wang et al., 2015). Treg induction, including LAP+ Treg, occurs in the lamina propria of the small intestine (Kumar et al., 2020). While the capacity for microbial degradation of plant cell wall is greater in the large intestine, our recent microbiome analysis shows that bacterial species producing enzyme that hydrolyse several cell wall components are present in the small intestine, in particular in sections closer to the stomach such as the duodenum (Kumar et al., 2020).
Berglund et al (2017) recently established key regulatory criteria for FDA approval of orally delivered plant cells expressing therapeutic proteins including detailed product characterization, product safety, integrity, dose, potency, stability, moisture content and bioburden. In the FDA approval process of commercially grown peanut powder, there was no requirement for growth of plants under cGMP hydroponic conditions, but safety of product was evaluated by bioburden analysis. Likewise, the therapeutic proteins (Ara h) were not purified but orally delivered as plant cells, along with all other plant proteins. Product production in cGMP facilities and purification to eliminate host cell proteins are the most expensive aspects of previously approved processes by FDA, making vaccines or therapeutics unaffordable for developing countries. For clinical applications in this study, transplastomic lettuce plants were grown in a cGMP hydroponic system under optimized conditions, enabling robust scale‐up. Upon harvest, the leaf biomass is freeze‐dried and ground into a powder and stored in the dark at ambient temperature free of moisture. Under these conditions CTB‐FIX is stable for at least three years (Herzog et al., 2017), similar to several other human blood proteins expressed in chloroplasts (Daniell et al., 2020; Kwon et al., 2018; Park et al., 2020). Very similar conditions will be used for large‐scale production and characterization of marker‐free FIX plants.
In the past three decades, genetically modified soybean and corn containing antibiotic resistance genes have been consumed regularly in the United States. Although federal regulatory agencies in the United States have not yet mandated removal of antibiotic resistance genes from food products that are consumed daily, European regulatory agencies have raised this concern, although never implemented. This is largely because negative impact on the presence of antibiotic resistance genes in genetically modified food has not yet been demonstrated. Nevertheless, in order to decrease hurdles in regulatory approval around the globe and in further advancing this life‐saving affordable drug to the clinic, we eliminated the antibiotic resistance gene. We used a direct repeat sequence of the promoter region of lettuce ATPase beta subunit (atpB) to flank the antibiotic resistance marker gene, aadA, and utilizing the chloroplast homologous recombination system for marker excision. We were able to observe the excision of the aadA gene in the regenerated transplastomic shoot which was confirmed by PCR and Southern analysis. In addition, stable maintenance of homoplasmy and CTB‐FIX protein levels were observed in subsequent generations (up to T2) confirming that marker excision event did not have any negative effect on the expression of the protein of interest. The observed results of complete marker excision and comparable FIX protein expression (with respect to marker containing system) correlate very well with prior results obtained for the production of other therapeutic proteins and industrial enzymes (Daniell et al., 2020; Daniell et al., 2019; Kumari et al., 2019; Park et al., 2020).
Previously, we have shown that oral delivery of bioencapsulated CTB‐FIX not only protected from formation of antibodies and anaphylaxis against FIX, but studies in haemophilic B dogs also support applicability to non‐rodent species and safety, as extensively assessed by general health, haematology and serum chemistry (Herzog et al., 2017). In this study, we further evaluate safety of oral administration of lyophilized lettuce biomass expressing CTB‐FIX by single oral dose administration studies in accordance with standard laboratory practices. No dose‐limiting toxicities were detected. Toxicology studies in Sprague Dawley rats showed that the maximum tolerated dose (MTD) was not reached at an eightfold higher dose/kg than that used for tolerance induction in the haemophilia B dogs. Similarly, the MTD in Beagle dogs was not reached at a dose/kg fivefold higher than doses given for tolerance induction in haemophilia B dogs. Combined with studies in haemophilia B dogs that received bioencapsulated CTB‐FIX twice per week for up to 6 months (Herzog et al., 2017), an increasing body of results in different species and animal models supports a lack of toxicity, with oral delivery having no negative effects on general health, organ and metabolic functions (including liver and kidney), coagulation or other haematological parameters. Furthermore, no allergic reactions were observed against the orally delivered transplastomic lettuce biomass. CTB is FDA‐approved antigen and has been used for decades and is now available without prescription to prevent traveller’s diarrhoea in several countries (Mottram et al., 2020).
Pharmacokinetics analysis of Ls‐CTB‐FIX revealed that single oral dosing in rats and dogs rarely resulted in detectable FIX antigen in plasma. On contrary, in our previously published data from mice, repeated oral dosing of tobacco or lettuce cells expressing CTB‐FIX (2–3 times over a 24‐h period) resulted in modest delivery of FIX antigen to the circulation in a subset of animals, which was detectable 2–5 h after the last feeding (Su et al., 2015; Verma et al., 2010). Interestingly, some of our initial studies achieved tolerance using a CTB‐FIX variant lacking a Furin cleavage site between CTB transmucosal carrier and FIX, thus resulting in ineffective systemic delivery by design (Verma et al., 2010). Therefore, delivery to the circulation is not required for tolerance induction. Rather, drug absorption from the gastrointestinal tract (GI), resulting in delivery to the gut immune system, has its tolerogenic effects within the GI tract itself. Limited systemic delivery should further facilitate development of the oral tolerance drug. It should also be pointed out that the FIX sequence lacks the pre‐pro‐peptide that is required for gamma‐carboxylation, which in turn is strictly required for enzymatic activity in the coagulation cascade. Therefore, the chloroplast‐produced FIX entirely lacks coagulation activity but is identical in amino acid sequence to mature FIX used to treat haemophilia B patients, thus containing all possible CD4+ T‐cell epitopes. The combination of these safety features further supports clinical testing.
In summary, the results from the current study reiterate the lack of toxicity upon single oral dose administration of transplastomic lettuce expressed CTB‐FIX in rat and canine models. Even though long‐term (>300 days) repeat dose CTB‐FIX studies have been conducted in haemophilia B dogs and support safety, it is possible that FDA could demand GLP compliant repeat dose studies in healthy animals, as well as longer follow‐up evaluation of animals that received single drug doses. Furthermore, we developed antibiotic marker‐free lettuce transplastomic plants which stably expressed CTB‐FIX, although GM food (corn, soybean) consumed daily, for three decades, around the globe contain antibiotic resistance genes. Our study is the first report on stable, antibiotic marker‐free transplastomic plant expressing a human blood clotting factor for oral tolerance induction. We envisage large‐scale production of this Ls‐MF‐CTB‐FIX plants in a hydroponic system allowing for GMP compliant manufacturing, although Food and Drug Administration (FDA) approved orally delivered peanut cells were not grown under cGMP hydroponic conditions. Clinical testing of CTB‐FIX could result in the first prophylactic immune tolerance protocol for haemophilia and compensate for the limited treatment options for patients with FIX inhibitors. Based on these results, we propose a first‐in‐man safety study as an open‐label single‐arm, single‐dose trial followed by multiple dose exposures and dose escalation study of bioencapsulated Ls‐MF‐CTB‐FIX in adult volunteers with haemophilia B.
Proposed Phase I clinical study
Clinical testing of the approach could result in the first prophylactic immune tolerance protocol for haemophilia benefitting those with FIX gene deletions or other mutations that have the highest risk of inhibitor formation and compensate for the limited treatment options for patients with FIX inhibitors. The proposed trial would present an opportunity to establish immune assays and initiate biomarker development of oral tolerance for haemophilia. Based on these results, we propose a first‐in‐man safety study as an open‐label single‐arm, single‐dose trial followed by multiple dose exposures and dose escalation study of bioencapsulated Ls‐MF‐CTB‐FIX in adult volunteers with haemophilia B (Figure 2).
Figure 2.
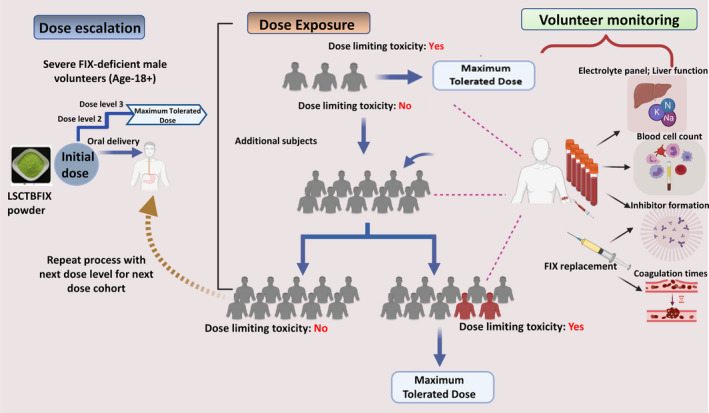
Proposed Phase I clinical study. Dose escalation: Doses will be informed by preclinical data. Subjects will start at an initial dose. Dose would be escalated once enrolled subjects in each dose cohort fail to show dose‐limiting toxicities. Dose Exposure: A combined single and multiple dose trial design is shown. Initial subjects are exposed to a single dose. Only once safety parameters are met would subjects be moved into the final multiple dose cohort. Volunteer monitoring: Blood is collected throughout cohorts of the study for laboratory analysis, including inhibitory antibody titres, cellular immune response, haematological parameters and coagulation activity to establish safety at each dose.
A growing body of evidence from patients with food allergies suggests that oral administration has to be initiated early in life in order to have a sustained effect in humans. This concept applies well to the treatment of haemophilia, where replacement therapy is typically initiated within the first year of life, and inhibitors mostly form within the first 20 exposure days and rarely form after 50 exposure days. Therefore, a subsequent trial would have to be performed in young boys with haemophilia B once this study has established safety in adult human subjects. In this case, oral delivery of plant cells will likely occur via a liquid carrier food. Importantly, a landmark clinical trial in patients with elevated risk of peanut allergy termed LEAP (Learning Early About Peanut allergy) established that long‐lasting tolerance is best achieved when OIT is initiated early in life, that is before 5 years of age (Du Toit et al., 2008). Similar results have now been documented for milk allergy (Boné Calvo et al., 2020). These observations are encouraging for prevention of inhibitor formation in haemophilia by oral antigen delivery, which could be initiated in the first year of infancy. Lack of toxicity in animal studies at doses considerably higher than considered in children further supports the approach.
In conclusion, lack of toxicity in small (rat) and large (canine) animal models and availability of transplastomic edible crop plants devoid of selection marker genes as demonstrated in the current work represent major steps forward towards clinical translation of oral tolerance induction to coagulation factors in patients with severe haemophilia.
Materials and methods
Development of marker‐free CTB‐FIX homoplasmic lettuce plants
The CTB‐FIX fusion gene in the pLS chloroplast vector (Su et al., 2015) was amplified using gene‐specific primers and cloned into the NdeI/PshAI restriction sites of the lettuce marker‐free chloroplast expression vector, pLS‐MF, as reported previously (Daniell et al., 2020; Park et al., 2020). The gene codes for the non‐toxic, cholera toxin B subunit (CTB) fused to mature human FIX (N‐terminal truncated version of FR846240.1, 47‐461 aa/1245 bp long) via a GPGP hinge and furin cleavage site (RRKR). This can induce tolerance as it carries all potential CD4+ T‐cell epitopes but does not produce a functional protein since it lacks the signal peptide and the propeptide (site of gamma‐carboxylation). A commercial lettuce (Lactuca sativa) cultivar, Simpson Elite, was used for generating transplastomic plants.
The pLS‐MF cassette is distinct from the previously used pLs‐LF vector for generating the Ls‐CTB‐FIX transplastomic plants (Herzog et al., 2017; Su et al., 2015) in that it harbours a 2 direct repeat sequences of the 649‐bp long, chloroplast encoded ATP synthase beta subunit (atpB) promoter region, flanking the antibiotic selection marker gene, aadA (Figure 1a). The sequence and expression of pLS‐MF‐CTB‐FIX was initially verified in E. coli and then used for biolistic transformation of 3‐week‐old lettuce explants according to Ref.(Verma et al., 2008). The bombarded explants were incubated on shoot regeneration medium (RMOP) containing 50 mg/L spectinomycin, and positive transformants were screened and selected as described in Park et al., 2020. Briefly, 3‐ to 6‐week‐old regenerated shoots were subject to a first round of PCR screening using the following primers: 16s‐F,5′‐CAGCAGCCGCGGTAATACAGAGGATGCAAGC‐3′; aadA‐R,5′‐CCGCGTTGTTTCATCAAGCCTTACGGTCACC‐3′; atpB‐R,5′GAATTAACCGATCGACGTGCTAGCGGACA TT‐3′; TpsbA‐F,5′‐AGGAGCAATAACGCCCTCTTGATAAAAC‐3′; 23s‐R,5′‐TGCACCCCTACCTCCTTTAT CACTGAGC‐3′. PCR‐positive shoots were checked for marker excision (absence of aadA gene) in a second round of PCR with the same primer set and transferred to rooting medium without spectinomycin. After rooting, the plants (T0) were transferred to a mini‐hydroponics set‐up (AeroGarden, USA) for 2 weeks, before finally transferring to the greenhouse for enhancing biomass and for seed collection. The seeds (T1) were germinated on half‐strength MS medium without 50 mg/L of spectinomycin, respectively, and maintained in magenta boxes in a sterile plant tissue culture facility. Three‐week‐old T1 seedlings were then transferred to a mini‐hydroponic set‐up (AeroGarden, USA) for 14 days, before potting in the greenhouse (soil system) and grown for up to 4 months. The leaves from 2.5‐month‐old lettuce plants were harvested every 3 weeks, washed, frozen at −80°C, lyophilized and ground into a fine powder according to Su et al., 2015. The process was repeated to generate T2 marker‐free plants.
Southern analysis was also carried out to confirm homoplasmy using the leaf genomic DNA of T0, T1 and T2 plants (2 µg) digested with BglII (NEB, USA) and probed using the DIG‐high prime DNA labelling and detection starter kit II according to manufacturer’s protocols (Roche, Germany).
Quantitation of CTB‐FIX
To quantify the expression levels of CTB‐FIX in the marker‐free (Ls‐MF‐CTB‐FIX) transplastomic lettuce plants, the total protein from the lyophilized biomass powder was extracted. 10 mg of the powder was suspended in 500 µL of the extraction buffer (100 mM NaCl, 10 mM EDTA, 200 mM Tris‐Cl pH 8.0, 0.05% v/v Tween20, 0.1% v/v SDS, 400 mM Sucrose, 14 mM β‐Mercaptoethanol, 100 mM DTT, 2 mM phenylmethyl sulphonylfluoride and proteinase inhibitor cocktail). The amount of total protein was then quantified by Bradford assay, denatured, loaded on SDS–polyacrylamide gels and electroblotted onto nylon membranes following protocols described in Daniell et al., 2020. For detection of chemiluminescence, Invitrogen iBright FL1500 imaging system (Thermo Fischer, USA) was used and the expression of CTB‐FIX was quantified from relative band intensities in comparison to a CTB standard (Sigma, USA), using the iBright image analysis software (Thermo Fischer, USA).
GM1‐ganglioside receptor‐binding assay
To test the efficiency of the lettuce chloroplast‐derived MF‐CTB‐FIX fusion protein to form pentamers that facilitate binding to the intestinal epithelial GM1‐ganglioside receptor, a GM1‐binding assay was performed according to previously published protocols (Daniell et al., 2020; Kwon et al., 2018).
Toxicity and pharmacokinetic studies in animals
While not formally performed as GLP studies, toxicity and pharmacokinetic studies in rats and dogs were nonetheless planned, performed, recorded and reported in accordance with standard practices to ensure data quality and integrity. These studies were carried out by SRI Biosciences (Menlo Park, CA). Because haemophilia B is an X‐linked disorder, only male animals were included. The CTB‐FIX fusion protein reported in our previous studies (Herzog et al., 2017; Su et al., 2015; Verma et al., 2010) was used for animal experiments. Oral suspension formulations of lyophilized lettuce cells in sterile PBS were used within 2 h after preparation and maintained at room temperature until administration to the animals. Human FIX levels in plasma of the animals were measured using the ELISA kit (LS‐F10414) from LSBio (Seattle, WA). The lowest detected level of human FIX for this ELISA through standard dilution was 0.1 ng/mL. Detection limit of human FIX according to manufacturer is 0.034 ng/mL.
Single oral dose toxicity in rats
To determine potential toxicity of the lettuce chloroplast‐derived CTB‐FIX in rats, a single oral dose range‐finding toxicity study was performed in male Sprague Dawley rats (7–8 weeks old, ˜186–207 g weight). The animals were quarantined for 2 days. Lyophilized lettuce (grown in a cGMP hydroponic facility) expressing 2.94 mg/kg of Ls‐CTB‐FIX was suspended in sterile PBS. Ls‐CTB‐FIX antigen doses at 0 mg/kg (Group I), 0.25 mg/kg (Group II), 1 mg/kg (Group III) and 2.5 mg/kg (Group IV) were given as oral gavage in a volume of 10 mL/kg to the animals (n = 3 per dose group) on Day 1 (Table ). A control group (n = 3) was given a single oral dose of vehicle, PBS, at an equivalent volume on the same day (Table S1). The dose formulations were prepared fresh, mixed well using a magnetic stirrer for 15 min and used within 2 h after preparation and maintained at room temperature until administration to the animals. The duration of the animals’ in‐life phase was 8 days with the following parameters evaluated: mortality/morbidity, clinical observations, body weights, clinical pathology (haematology and serum chemistry) and gross necropsy observation. For necropsy observations, external examination of all body orifices and an examination of all cranial, thoracic and abdominal organs were performed, and all gross findings were recorded. No tissues were retained. All animals were sacrificed on Day 8. Animals were evaluated 2–4 h after gavage followed by daily observations for any signs of morbidity. The clinical observations were recorded approximately 2–4 h post‐dose on Day 1, and once daily thereafter or more often as clinical signs warrant, and on the day of necropsy (Day 8). Body weights were recorded on Day 1 (pre‐dose), for the purpose of dose calculation and on Day 3 and prior to necropsy on Day 8. Animals were examined for any altered clinical signs, including gross motor and behavioural activity, and observable changes in appearance. Blood samples were collected from the retro‐orbital sinus under anaesthesia (60% CO2/40% O2) on Day 3. Haematology samples were collected using K3EDTA anticoagulant, while no anticoagulant was used for clinical chemistry samples.
Single‐dose pharmacokinetics study in rats
In a single‐dose pharmacokinetics study, 8‐week‐old male Sprague Dawley rats (n = 3 per group), weighing 231–266 g received single oral gavage of plant cell suspension adjusted to doses of Ls‐CTB‐FIX at 0.49 (Group I), 0.98 (Group II) and 1.96 mg/kg (Group III), in a total volume of 0.75 to 3 mL (Table S6). The dose formulations were prepared fresh, mixed well using a magnetic stirrer for 30 min and used within 2 h after preparation and maintained at room temperature until administration to the animals. The duration of the animals’ in‐life phase was 2 days with the following parameters evaluated: mortality/morbidity, clinical observations, body weights and plasma drug levels. All animals were euthanized 2 days after last blood collection. Animals were checked at least once daily and clinical observations were recorded approximately 2–4 h post‐dose on Day 1. Animals were examined for any altered clinical signs, including gross motor and behavioural activity, and observable changes in appearance. Body weights were recorded pre‐dose, for the purpose of dose calculation. Blood (˜120 μL total whole blood or ˜50 μL of plasma per sample) was collected into pre‐chilled tubes containing sodium heparin anticoagulant at pre‐dose, 30 min, 1, 2, 3, 4, 6, 8 and 24‐h post‐dose time points for measurement of human FIX antigen by ELISA (Herzog et al., 2017; Su et al., 2015).
Combined single oral dose toxicity and pharmacokinetics study in dogs
A combined single oral dose toxicity and pharmacokinetics study was performed in 9‐ to 10‐month‐old haemostatically normal male Beagle dogs (n = 2 per group). The animals were quarantined for 15 days prior to study and fasted prior to oral dose administration. Plant cell suspension was given as single dose on Day 1 (Table S3) by oral gavage either as suspension formulations mixed in PBS (Groups III and IV) or fed by mixing into dog food which served as a carrier (Groups I and II). Antigen doses for oral delivery were 0.3 mg/kg of Ls‐CTB‐FIX (Groups I and III) and 1.47 mg/kg (Groups III and IV). Groups I and III were dosed first. The same 4 dogs were used for Groups II and IV, respectively, after washout. The duration of the animals’ in‐life phase was 10 days (including minimal 7‐day washout period between each dose administration of the four dose groups). The following parameters were evaluated: mortality/morbidity, clinical observations, body weights and plasma drug levels. Animals were checked at least once daily for signs of morbidity. Clinical observations were recorded approximately 2–4 h post‐dose on Day 1 and once daily on Days 2–4. Animals were examined for any altered clinical signs, including gross motor and behavioural activity, and observable changes in appearance. In addition, the animals received cage side observations during washout periods. Body weights were recorded on Day 1 (pre‐dose), for the purpose of dose calculation and on Day 3 and repeated for each dose level. Evaluation of clinical pathology parameters included haematological parameters (including haematocrit and complete blood cell counts), clinical chemistry and coagulation (prothrombin time, activated partial thromboplastin time and fibrinogen). Blood samples for these analyses were collected on Day −1 and on Day 3.
In order to estimate human FIX levels that may have entered the circulation after oral delivery of Ls‐CTB‐FIX to the dogs, blood (maximum ˜2 mL whole blood or ˜800 μL of plasma per sample) was collected from the cephalic, saphenous or jugular veins into pre‐chilled tubes containing K3EDTA using the following schedule: pre‐dose, 30 min, 1, 2, 3, 4, 6, 8 and 24‐h post‐dose. To measure human FIX antigen, collected blood was processed to plasma and then stored frozen at temperatures less than −60°C. The plasma FIX antigen levels was measured by ELISA as described previously (Herzog et al., 2017; Su et al., 2015).
Statistical analysis
For clinical pathology and body weight calculation, data are presented as mean±SD at each evaluation interval. One‐way analysis of variance (ANOVA), followed by Dunnett’s test (if the ANOVA is significant), was performed using ® version 9.3.1.1, MS Excel 2007 and 2010. For clinical pathology data, parameter values not within the detection threshold were excluded from statistical evaluation. All other numeric parameters were evaluated by Student’s t‐test, unless specified otherwise.
Conflicts of Interest
Henry Daniell and Roland Herzog are inventors in several patents on expression of blood clotting factors in chloroplasts and oral tolerance induction. Their haemophilia research was funded in the past by several major pharmaceutical companies including Bayer, Novo and Nordisk and is currently funded by Shire/Takeda. All other authors declare no conflict to report.
Author contributions
HD and RWH conceived this project, designed experiments, interpreted data, participated in SMARTT study design/discussion and wrote/edited several versions of this manuscript. A. Srinivasan characterized CTB‐FIX marker‐free transplastomic lines and wrote these sections. IK performed GM1 assay (Figure 1F). A. Sherman developed assay and measured FIX in plasma (Tables S7 and S8). TB compiled and organized toxicity, haematology and pharmacokinetics data and wrote parts of materials and methods. TW developed clinical protocol (Figure 2) and wrote corresponding results and discussion sections.
Supporting information
Figure S1. Western blot of LS‐CTB‐FIX (with antibiotic marker) from lyophilized hydroponic grown lettuce biomass used for animal studies. Lane 1: WT, untransformed, Lane 2, 3: LS‐CTB‐FIX loaded in duplicates, Lanes 4‐6: CTB standard.
Figure S2. PCR analysis to screen the regenerated shoots with the three sets of primers to confirm site‐specific integration. Primer set designations are provided below each gel and annealing sites are described in the main text. Lanes 1‐8: Different regenerated shoot lines, Lane 9: WT, Lane M: 1 kb DNA ladder.
Table S1. Experimental design for single oral dose administration of LS‐CTB‐FIX in male Sprague Dawley Rats.
Table S2. Summary of body weights of male Sprague Dawley rats after a single oral gavage of LS‐CTB‐FIX.
Table S3. Experimental design for a combined single oral dose administration and pharmacokinetic study of LS‐CTB‐FIX in male Beagle dogs.
Table S4. Summary of body weights of male Beagle dogs after a single oral gavage of LS‐CTB‐FIX.
Table S5. Changes in coagulation after a single oral gavage of LS‐CTB‐FIX in male Beagle dogs.
Table S6. Experiment design for pharmacokinetic study of Ls‐CTB‐FIX following a Single Oral Dose Administration in Male Sprague Dawley Rats.
Table S7. Levels of Ls‐CTB‐FIX in plasma samples collected for pharmacokinetic study of Ls‐CTB‐FIX following a Single Oral Dose Administration in Male Beagle dogs.
Table S8. Levels of Ls‐CTB‐FIX in plasma samples collected for pharmacokinetic study of Ls‐CTB‐FIX following a Single Oral Dose Administration in Male Sprague Dawley rats.
Acknowledgements
This research was supported by NIH grant R01 GM 63879, R01 EY 024564, R01 107904 to HD, R01 HL 109442, R01 133191, Novo Nordisk, Shire/Takeda grants to RWH and HD. The National Heart Lung and Blood Institute, National Institutes of Health, Department of Health and Human Services, through the Science Moving TowArds Research Translation and Therapy (SMARTT) program contract # HHSN268201600014C/ RSA‐0000342 was awarded to HD. Authors specifically thank Drs. Maria Arolfo and Dr. Hanna Ng for toxicology and pharmacokinetic studies conducted at SRI International and Drs. Cindy McClintock and Diana Severynse‐Stevens for all regulatory studies conducted at RTI International. The authors thank Dr. Stephen Streatfield at Fraunhofer USA for hydroponic production of lettuce transplastomic plants.
Srinivasan, A. , Herzog, R. W. , Khan, I. , Sherman, A. , Bertolini, T. , Wynn, T. and Daniell, H. (2021) Preclinical development of plant‐based oral immune modulatory therapy for haemophilia B. Plant Biotechnol J, 10.1111/pbi.13608
References
- Barg, A.A. , Livnat, T. and Kenet, G. (2017) Inhibitors in hemophilia: treatment challenges and novel options. Semin. Thromb. Hemost. 44, 544–550. [DOI] [PubMed] [Google Scholar]
- Berglund, J.P. , Szczepanski, N. , Penumarti, A. , Beavers, A. , Kesselring, J. , Orgel, K. , Burnett, B. et al. (2017) Preparation and analysis of peanut flour used in oral immunotherapy clinical trials. J. Allergy Clin. Immunol. Pract. 5, 1098–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolini, T.B. , Biswas, M. , Terhorst, C. , Daniell, H. , Herzog, R.W. and Piñeros, A.R. (2021) Role of orally induced regulatory T cells in immunotherapy and tolerance. Cell. Immunol. 359, 104251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird, J.A. , Spergel, J.M. , Jones, S.M. , Rachid, R. , Assa'ad, A.H. , Wang, J. , Leonard, S.A. et al. (2018) Efficacy and safety of AR101 in oral immunotherapy for peanut allergy: results of ARC001, a randomized, double‐blind, placebo‐controlled phase 2 clinical trial. J. Allergy Clin. Immunol. Pract. 6, 476–485. [DOI] [PubMed] [Google Scholar]
- Boné Calvo, J. , Clavero Adell, M. , Guallar Abadía, I. , Laliena Aznar, S. , Sancho Rodríguez, M.L. , Claver Monzón, A. and Aliaga Mazas, Y. (2020) As soon as possible in IgE‐cow’s milk allergy immunotherapy. Eur. J. Pediatr. 180, 291–294. [DOI] [PubMed] [Google Scholar]
- Butterfield, J.S.S. , Hege, K.M. , Herzog, R.W. and Kaczmarek, R. (2020) A molecular revolution in the treatment of hemophilia. Mol. Ther. 28, 997–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniell, H. , Jin, S.X. , Zhu, X.G. , Gitzendanner, M.A. , Soltis, D.E. and Soltis, P.S. (2021) Green giant‐a tiny chloroplast genome with mighty power to produce high‐value proteins: history and phylogeny. Plant Biotechnol. J. 19, 430–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniell, H. , Kulis, M. and Herzog, R.W. (2019a) Plant cell‐made protein antigens for induction of Oral tolerance. Biotechnol. Adv. 37, 107413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniell, H. , Mangu, V. , Yakubov, B. , Park, J. , Habibi, P. , Shi, Y. , Gonnella, P.A. et al. (2020) Investigational new drug enabling angiotensin oral‐delivery studies to attenuate pulmonary hypertension. Biomaterials, 233, 119750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniell, H. , Ribeiro, T. , Lin, S. , Saha, P. , McMichael, C. , Chowdhary, R. and Agarwal, A. (2019) Validation of leaf and microbial pectinases: commercial launching of a new platform technology. Plant Biotechnol. J. 17, 1154–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Toit, G. , Katz, Y. , Sasieni, P. , Mesher, D. , Maleki, S.J. , Fisher, H.R. , Fox, A.T. et al. (2008) Early consumption of peanuts in infancy is associated with a low prevalence of peanut allergy. J. Allergy Clin. Immunol. 122, 984–991. [DOI] [PubMed] [Google Scholar]
- Franchini, M. and Mannucci, P.M. (2012) Past, present and future of hemophilia: A narrative review. Orphanet. J. Rare Dis. 7, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog, R.W. , Nichols, T.C. , Su, J. , Zhang, B. , Sherman, A. , Merricks, E.P. , Raymer, R. et al. (2017) Oral tolerance induction in hemophilia B dogs fed with transplastomic lettuce. Mol. Ther. 25, 512–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iorio, A. , Stonebraker, J.S. , Chambost, H. , Makris, M. , Coffin, D. , Herr, C. , Germini, F. et al. (2019) Establishing the prevalence and prevalence at birth of hemophilia in males a meta‐analytic approach using national registries. Ann. Intern. Med. 171, 542–546. [DOI] [PubMed] [Google Scholar]
- Kitazawa, T. and Shima, M. (2020) Emicizumab, a humanized bispecific antibody to coagulation factors IXa and X with a factor VIIIa‐cofactor activity. Int. J. Hematol. 111, 20–30. [DOI] [PubMed] [Google Scholar]
- Kuhn, C. and Weiner, H.L. (2016) How does the immune system tolerate food? Science, 351, 810–811. [DOI] [PubMed] [Google Scholar]
- Kumar, S.R.P. , Wang, X. , Avuthu, N. , Bertolini, T.B. , Terhorst, C. , Guda, C. , Daniell, H. et al. (2020) Role of small intestine and gut microbiome in plant‐based oral tolerance for hemophilia. Front. Immunol. 11, 844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari, U. , Singh, R. , Ray, T. , Rana, S. , Saha, P. , Malhotra, K. and Daniell, H. (2019) Validation of leaf enzymes in the detergent and textile industries: launching of a new platform technology. Plant Biotechnol. J. 17, 1167–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon, K.C. and Daniell, H. (2016) Oral delivery of protein drugs bioencapsulated in plant cells. Mol. Ther. 24, 1342–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon, K.C. , Sherman, A. , Chang, W.J. , Kamesh, A. , Biswas, M. , Herzog, R.W. and Daniell, H. (2018) Expression and assembly of largest foreign protein in chloroplasts: oral delivery of human FVIII made in lettuce chloroplasts robustly suppresses inhibitor formation in haemophilia A mice. Plant Biotechnol. J. 16, 1148–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacroix‐Desmazes, S. , Voorberg, J. , Lillicrap, D. , Scott, D.W. and Pratt, K.P. (2020) Tolerating factor VIII: recent progress. Front. Immunol. 10, 2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljung, R. , Auerswald, G. , Benson, G. , Dolan, G. , Duffy, A. , Hermans, C. , Jiménez‐Yuste, V. et al. (2019) Inhibitors in haemophilia A and B: Management of bleeds, inhibitor eradication and strategies for difficult‐to‐treat patients. Eur. J. Haematol. 102, 111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Male, C. , Andersson, N.G. , Rafowicz, A. , Liesner, R. , Kurnik, K. , Fischer, K. , Platokouki, H. et al. (2021) Inhibitor incidence in an unselected cohort of previously untreated patients with severe hemophilia B: A PedNet study. Haematologica, 106, 123–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancuso, M.E. , Mahlangu, J.N. and Pipe, S.W. (2021) The changing treatment landscape in haemophilia: from standard half‐life clotting factor concentrates to gene editing. Lancet, 397, 630–640. [DOI] [PubMed] [Google Scholar]
- Mottram, L. , Lundgren, A. , Svennerholm, A. and Leach, S. (2020) Booster vaccination with a fractional dose of an oral cholera vaccine induces comparable vaccine specific antibody avidity as a full dose: A randomized clinical trial. Vaccine, 38, 655–662. [DOI] [PubMed] [Google Scholar]
- Pabst, O. and Mowat, A.M. (2012) Oral tolerance to food protein. Mucosal Immunol. 5, 232–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, J. , Yan, G. , Kwon, K.C. , Liu, M. , Gonnella, P.A. , Yang, S. and Daniell, H. (2020) Oral delivery of novel human IGF‐1 bioencapsulated in lettuce cells promotes musculoskeletal cell proliferation, differentiation and diabetic fracture healing. Biomaterials, 233, 119591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrawala, M. , Shih, J. , Lee, G. and Vickery, B. (2020) Peanut oral immunotherapy: a current perspective. Curr. Allergy Asthma Rep. 20, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puetz, J. , Soucie, J.M. , Kempton, C.L. and Monahan, P.E. (2014) Prevalent inhibitors in haemophilia B subjects enrolled in the Universal Data Collection database. Haemophilia, 20, 25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragni, M.V. (2017) Novel alternate hemostatic agents for patients with inhibitors: Beyond bypass therapy. Hematology, 2017, 605–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezende, R.M. and Weiner, H.L. (2017) History and mechanisms of oral tolerance. Semin. Immunol. 30, 3–11. [DOI] [PubMed] [Google Scholar]
- Rezende, R.M. and Weiner, H.L. (2018) Cellular components and mechanisms of oral tolerance induction. Crit. Rev. Immunol. 38, 207–231. [DOI] [PubMed] [Google Scholar]
- Santoro, C. , Quintavalle, G. , Castaman, G. , Baldacci, E. , Ferretti, A. , Riccardi, F. and Tagliaferri, A. (2018) Inhibitors in hemophilia B. Semin. Thromb. Hemost. 44, 578–589. [DOI] [PubMed] [Google Scholar]
- Sherman, A. , Su, J. , Lin, S. , Wang, X. , Herzog, R.W. and Daniell, H. (2014) Suppression of inhibitor formation against FVIII in a murine model of hemophilia A by oral delivery of antigens bioencapsulated in plant cells. Blood, 124, 1659–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su, J. , Zhu, L. , Sherman, A. , Wang, X. , Lin, S. , Kamesh, A. , Norikane, J.H. et al. (2015) Low cost industrial production of coagulation factor IX bioencapsulated in lettuce cells for oral tolerance induction in hemophilia B. Biomaterials, 70, 84–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilles, S.A. and Petroni, D. (2018) FDA‐approved peanut allergy treatment: The first wave is about to crest. Ann. Allergy Asthma Immunol. 121, 145–149. [DOI] [PubMed] [Google Scholar]
- Verma, D. , Moghimi, B. , LoDuca, P.A. , Singh, H.D. , Hoffman, B.E. , Herzog, R.W. and Daniell, H. (2010) Oral delivery of bioencapsulated coagulation factor IX prevents inhibitor formation and fatal anaphylaxis in hemophilia B mice. Proc. Natl. Acad. Sci. 107, 7101–7106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma, D. , Samson, N.P. , Koya, V. and Daniell, H. (2008) A protocol for expression of foreign genes in chloroplasts. Nat. Protoc. 3, 739–758. [DOI] [PubMed] [Google Scholar]
- Vickery, B.P. , Vereda, A. , Casale, T.B. , Beyer, K. , Du Toit, G. , Hourihane, J.O. , Jones, S.M. et al. (2018) AR101 oral immunotherapy for peanut allergy. N. Engl. J. Med. 379, 1991–2001. [DOI] [PubMed] [Google Scholar]
- Wang, X. , Su, J. , Sherman, A. , Rogers, G.L. , Liao, G. , Hoffman, B.E. , Leong, K.W. et al. (2015) Plant‐based oral tolerance to hemophilia therapy employs a complex immune regulatory response including LAP+CD4+ T cells. Blood, 125, 2418–2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao, Y. , Kwon, K.C. , Hoffman, B.E. , Kamesh, A. , Jones, N.T. , Herzog, R.W. and Daniell, H. (2016) Low cost delivery of proteins bioencapsulated in plant cells to human non‐immune or immune modulatory cells. Biomaterials, 80, 68–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Western blot of LS‐CTB‐FIX (with antibiotic marker) from lyophilized hydroponic grown lettuce biomass used for animal studies. Lane 1: WT, untransformed, Lane 2, 3: LS‐CTB‐FIX loaded in duplicates, Lanes 4‐6: CTB standard.
Figure S2. PCR analysis to screen the regenerated shoots with the three sets of primers to confirm site‐specific integration. Primer set designations are provided below each gel and annealing sites are described in the main text. Lanes 1‐8: Different regenerated shoot lines, Lane 9: WT, Lane M: 1 kb DNA ladder.
Table S1. Experimental design for single oral dose administration of LS‐CTB‐FIX in male Sprague Dawley Rats.
Table S2. Summary of body weights of male Sprague Dawley rats after a single oral gavage of LS‐CTB‐FIX.
Table S3. Experimental design for a combined single oral dose administration and pharmacokinetic study of LS‐CTB‐FIX in male Beagle dogs.
Table S4. Summary of body weights of male Beagle dogs after a single oral gavage of LS‐CTB‐FIX.
Table S5. Changes in coagulation after a single oral gavage of LS‐CTB‐FIX in male Beagle dogs.
Table S6. Experiment design for pharmacokinetic study of Ls‐CTB‐FIX following a Single Oral Dose Administration in Male Sprague Dawley Rats.
Table S7. Levels of Ls‐CTB‐FIX in plasma samples collected for pharmacokinetic study of Ls‐CTB‐FIX following a Single Oral Dose Administration in Male Beagle dogs.
Table S8. Levels of Ls‐CTB‐FIX in plasma samples collected for pharmacokinetic study of Ls‐CTB‐FIX following a Single Oral Dose Administration in Male Sprague Dawley rats.


