Abstract
Purpose of Review
The goal of this article is to highlight how and why urinalyses and urine cultures are misused, review quality improvement interventions to optimize urine culture utilization, and highlight how to implement successful, sustainable interventions to improve urine culture practices in the acute care setting.
Recent Findings
Quality improvement initiatives aimed at reducing inappropriate treatment of asymptomatic bacteriuria often focus on optimizing urine test utilization (i.e., urine culture stewardship). Urine culture stewardship interventions in acute care hospitals span the spectrum of quality improvement initiatives, ranging from strong systems-based interventions like suppression of urine culture results to weaker interventions that focus on clinician education alone. While most urine culture stewardship interventions have met with some success, overall results are mixed, and implementation strategies to improve sustainability are not well understood.
Summary
Successful diagnostic stewardship interventions are based on an assessment of underlying key drivers and focus on multifaceted and complementary approaches. Individual intervention components have varying impacts on effectiveness, provider autonomy, and sustainability. The best urine culture stewardship strategies ultimately include both technical and socio-adaptive components with long-term, iterative feedback required for sustainability.
Keywords: Urine culture, Urinalysis, Diagnostic stewardship, Quality improvement, Implementation science
Background
A positive urine culture is considered the current gold standard for diagnosing urinary tract infection (UTI); however, there are many limitations to this test [1]. A positive urine culture in a person without genitourinary symptoms, or asymptomatic bacteriuria (ASB), is common in many populations, particularly with advancing age, hospitalization, or certain underlying conditions [2–4]. The prevalence of ASB ranges from 1 to 8% in nonpregnant women, 3 to 20% in elderly persons living in the community, and 15 to 50% in older residents of long-term care facilities [5]. ASB occurs in part because the urinary bladder hosts a complex community of asymptomatic colonizing microorganisms [6]. Catheter-associated bacteriuria, or positive urine cultures in catheterized patients, is also a common phenomenon due to bacterial colonization of the indwelling urinary catheter. Most indwelling catheters are colonized at the rate of 3 to 7% per day, reaching 100% colonization in long-term catheters [7, 8]. Asymptomatic pyuria or presence of white blood cells in urine is also quite common and occurs in 32% of young women, 90% of older residents in long-term care facilities, and 90% of patients on hemodialysis with ASB [9–11]. Because asymptomatic pyuria and bacteriuria are more common in those with comorbidities, ASB is considered a marker of aging and frailty [12]. Despite this, there are very few circumstances in adults (e.g., pregnancy, prior to urologic procedures) where antibiotic treatment of ASB improves outcomes. Instead, antibiotics should generally be avoided in most patients with ASB in order to prevent harm from unnecessary antibiotic use [5]. Antibiotic treatment of ASB contributes to adverse outcomes such as antimicrobial resistance, antibiotic-related adverse events, and Clostridioides (C) difficile infections [13, 14]. Nevertheless, many clinicians order urine cultures and prescribe antibiotics inappropriately in asymptomatic patients with bacteriuria or pyuria [3, 4, 15]. Below, we describe the diagnostic challenges associated with UTIs, review current urine testing practices, discuss different urine culture stewardship interventions, and highlight how to address organizational barriers to implementing diagnostic stewardship in the acute care setting.
Diagnostic Challenges with UTIs
Diagnosis of UTI is often a clinical challenge due to the high prevalence of asymptomatic bacteriuria, presence of non-specific symptoms, and lack of knowledge about appropriate indications for urine testing [15]. Surveillance criteria and clinical definitions have been used to diagnose UTI [16, 17], but even these definitions overcall many cases of ASB as UTI [18]. Overdiagnosis occurs because UTI is not a dichotomous outcome [9] but rather exists on a continuum spanning no detectable bacteriuria or pyuria, asymptomatic bacteriuria with or without pyuria, probable UTI, and clinical UTI (see Table 1). High incidence of ASB coupled with non-specific findings such as confusion, fever, or hypotension leads many clinicians to lean on laboratory findings to diagnose UTI [15, 19]. Increasing reliance on urine testing leads to overdiagnosis of UTI and antibiotic overuse, because a positive urine culture has a poor positive predictive value for diagnosing a UTI, in contrast to blood where pathogen detection equates to infection for most pathogens. Overdiagnosis of UTI also may cause alternative or true diagnoses to be missed. Advani et al. found that catheterized patients treated for a positive urine culture experienced a delay in other diagnoses likely due to anchoring to positive urine cultures [15].
Table 1.
Continuum of UTI diagnosis
| Not UTI or ASB | ASB | Probable UTI | UTI |
|---|---|---|---|
| Asymptomatic | No genitourinary symptoms + / − Non-specific symptoms attributable to different source | Cannot endorse symptoms or has non-specific symptoms (fever) not attributable to other source | + Genitourinary symptoms |
| No bacteriuria | + Bacteriuria | + Bacteriuria | + Bacteriuria |
| No pyuria | + / − Pyuria | + / − Pyuria | + Pyuria |
UTI urinary tract infection, ASB asymptomatic bacteriuria
Urine Testing Practices
Urine tests are misused and overused for various reasons. When faced with a patient who presents with fever, leukocytosis, hemodynamic instability, or non-specific symptoms, physicians often use a pan-culturing approach instead of symptom-directed evaluation [20]. Pan-culturing is a deep-rooted practice in medicine [20, 21], driven by fear of missing an infection combined with increasing reliance on laboratory tests over clinical findings. “Pan-culturing” usually provides instant gratification as it often yields positive results, albeit these results may be due to detecting colonization and contamination more often than true infection [15]. Testing may also be influenced by the convenience of sampling site. For example, clinicians order urine cultures as a part of initial workup of confusion more often than ordering a lumbar puncture [20]. Misuse of urine cultures also occurs in response to subjective findings such as change in color or odor of urine, change in mental status, positive urinalysis parameters, and initial workup of fever in patients without genitourinary symptoms [15]. In addition, urine cultures and urinalyses are often included as pre-selected tests in many order sets (e.g., pre-operative, admission). Overtesting is especially relevant to urine cultures, where the positive predictive value is heavily influenced by symptoms and collection techniques [22].
Urine Culture Stewardship Interventions
Because overtesting leads to overdiagnosis of UTI, initiatives aimed at reducing ASB-related antibiotic treatment often focus on optimizing urine test utilization—typically by decreasing urine cultures or urinalyses (i.e., urine culture stewardship). Broad categories of urine culture stewardship interventions are shown in Fig. 1. Because overtesting is ubiquitous, urine culture stewardship interventions span from systems-based to persons-focused approaches. Systems-based changes are generally viewed as stronger interventions and include forced functions, automation within the electronic medical record or laboratory, and standardization of processes and order sets [23]. As interventions become move towards more persons-focused, they provide more autonomy to the users but lose effectiveness if used in isolation (e.g., policies, rules, and education). We discuss each of these categories of interventions in detail below:
Fig. 1.
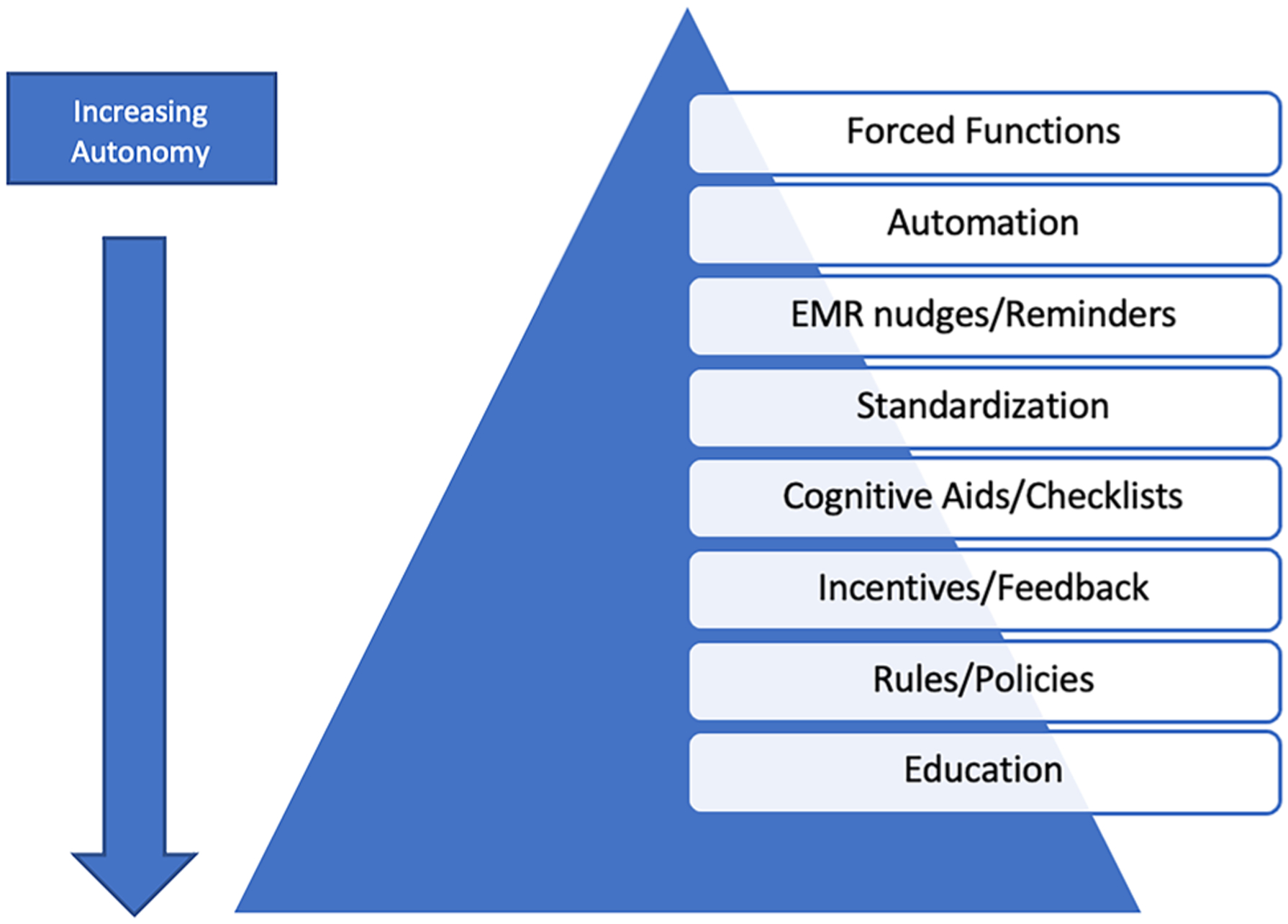
Broad categories of quality improvement interventions focusing on urine culture stewardship
Forced Functions and Automation
Forced functions require users to perform actions in a certain order, by preventing the next action until the first is complete [24]. For example, an academic medical center in New York City developed a new protocol for urine collection that required indwelling catheter replacement prior to urine collection (if the catheter was in place for more than 24 h). This additional step prior to urine collection led to fewer urine culture orders and catheter-associated UTIs (CAUTI) [25]. In a similar approach, an orthopedic hospital changed their laboratory process to require physician communication through a phone call to process any urine culture ordered for pre-operative screening, which led to a substantial reduction in urine cultures ordered and antibiotics prescribed, without an increase in prosthetic joint infections [26].
To reduce ASB treatment, some hospitals use laboratory automation strategies such as “urinalysis with reflex to culture” as a diagnostic stewardship intervention [27]. In this laboratory intervention, a urinalysis order proceeds to culture when specific urinalysis parameters (e.g., leukocyte esterase, white blood cells (WBC), red blood cells (RBC), nitrite, yeast, or bacteria) or combination of parameters are found. Recent surveys of academic and community hospitals revealed more than half of surveyed hospital laboratories offer reflex urine cultures [28, 29]. Use of reflex urine cultures has resulted in a decline in urine cultures and CAUTI rates in many acute care hospitals, emergency departments, and long-term care facilities [30–35]. Similarly, use of stricter UA criteria for reflexing to culture resulted in a 45% reduction in the urine cultures ordered at the University of North Carolina Health System [36]. In another study, a simple change of raising the threshold for reporting urine cultures from 104 to 105 colony-forming units led to a decrease in treatment of almost one-third of cases of asymptomatic bacteriuria and candiduria among hospitalized patients [37].
Successful interventions often combine multiple approaches, like forced functions combined with laboratory automation (e.g., reflex urine cultures, rejection of contaminated urine samples). In a quasi-experimental study involving two general hospitals and 10 community clinics, urine cultures were processed and reported only if one of the following criteria were met: presence of white blood cells or bacteria on microscopy; labeled as “pregnancy,” “urological procedure,” “renal transplant,” or “neutropenic”; or urine that was from a ureteric, nephrostomy, or suprapubic source [38]. For urine samples that did not fulfill these criteria, the microscopy results and a rejection comment were reported. This intervention led to a significant reduction in antimicrobial use and had no effect on patient mortality. There were no unintended patient consequences (e.g., bacteremia from untreated UTI) in this study [38].
Electronic Medical Record (EMR) Nudges and Reminders
An emerging body of research describes the impact of thoughtful choice architecture design and gentle nudges through the EMR to modify practitioner behavior and practices [39]. Choice architecture is the way in which choices are presented to users (e.g., the number of choices, order of choices, presence of default, and ease of choosing one choice over the other). Nudges include “any aspect of the choice architecture that alters people’s behavior in a predictable way without forbidding any options or significantly changing their economic incentives” [39]. Even small annoyances—such as having to spend a few minutes to enter indications—can cause users to forgo ordering tests [40]. Nudges through the EMR work by making the “right thing” easier and the”wrong thing” harder (but not impossible).
Examples of EMR nudges include removal of routine urinalysis and urine culture from admission, emergency department, or presurgical order sets or adding additional clicks or steps to order a urine culture [26, 36]. In several studies, requiring selection of indications for ordering urine cultures in catheterized patients led to an overall reduction in urine cultures ordered [41, 42]. Similar EMR enhancements increased adherence to guideline-based urine culture ordering for catheterized intensive care unit patients [43]. Similarly, cascading of microbiologic susceptibilities has been used to nudge clinicians to use narrowest antibiotic choice [44].
Nudges can also be outside the EMR. For example, in one study, the hospital laboratory suppressed urine culture results from non-catheterized inpatients and required clinicians to call the laboratory if result was desired [45]. Following this proof-of-concept study, the authors performed a randomized control trial to assess the impact of this modified laboratory reporting of urine cultures. Suppression of low-risk urine culture results resulted in a significant reduction in inappropriate antibiotic treatment without an increase in adverse events [46].
Standardization and Habits
Standardizing processes and workflows is one way to remove variation and confusion and promote predictability and consistency [47]. Dougherty and colleagues saw a decline in urine cultures with implementation of a urine culture standardization program which required order indications, standardized collection techniques, and reflex urine cultures [48]. Similarly, standardizing urine culture processing in the laboratory resulted in a decrease in monthly urine cultures and total number of patients starting a new antimicrobial in adult intensive care units [49]. Likewise, Davies et al. implemented standardized urine collection practices to reduce the risk of false-positive cultures. This resulted in a reduction of inappropriate urine culture orders and CAUTIs over the course of 5 months [50].
Checklists
Checklists were first identified as a way to standardize, avoid reliance on memory, and improve quality outside of medicine (e.g., in aviation) [51]. More recently, checklists have been used to reduce inappropriate urine cultures. For example, one study tested a checklist of approved testing indications for urine cultures in catheterized patients and found the checklist led to > 30% reduction in National Healthcare Safety Network-defined CAUTI rates by decreasing inappropriate urine cultures in intensive care units without a significant change in catheter utilization [52]. Sampathkumar and colleagues found similar reductions in CAUTI rates after implementing a quality improvement project focused on a checklist of bundle elements [53, 54].
Cognitive Aids, Rules, and Policies
As it is difficult to remember the massive amount of information needed to treat all diseases, approaches like cognitive aids, rules, and policies have been used to assist clinicians in the best course of action. Such approaches can have a modest impact on appropriate test utilization [20, 55]. A cluster-randomized trial of a diagnostic and therapeutic algorithm for suspected UTI failed to have any impact on the number of urine cultures ordered [56]. Together, these studies suggest that once “positive” urine culture results are reported, they are difficult to ignore.
Rules and policies are critical infrastructure for any improvement project but are unlikely to change practice without dissemination, integration, and bundling with other interventions. One of the more successful interventions using rules and policies combined a change in urine culture policy with cognitive aids and education. The chief medical officer, patient safety officer, and director of infection prevention issued a campus-wide memo about indications for urine cultures and harms of ordering urine culture as a part of fever workup. This memo was combined with education on the appropriate criteria for inpatient urine cultures to medical and nursing staff in the departments of surgery and medicine. In addition, a hospital-wide screensaver encouraged providers to stop sending urine cultures as part of routine nosocomial fever workups. These combined interventions reduced monthly urine cultures to half across most intensive care units and wards, with a corresponding decrease in CAUTI [57].
Education and Training
Education is a core element of any antibiotic stewardship program [58]. However, providing broad, general education alone is frequently insufficient to improve care. Providing real-time, directed educational feedback to clinicians, in the form of treatment algorithms, or just-in-time coaching, is modestly effective but resource intensive [56, 59, 60]. Education coupled with EMR changes is shown to be more impactful [61]. In a pilot study, nursing education and a clinical tool to enhance discussions on the necessity of urine cultures among nurses and hospitalists were associated with a reduction in urine cultures [62].
Training nurses regarding appropriate culturing practices and urine collection techniques is crucial [63–66].
Implementing Successful Stewardship Interventions
Though each of these interventions may be somewhat effective in isolation, a combination of interventions targeting both systems and persons is most effective. In an ideal world, the process of designing a successful intervention bundle should involve six crucial steps: (1) assessment of need and defining the underlying problem; (2) identifying which key barriers are modifiable, have the greatest potential for change, and would lead to the most benefit; (3) implementing one change at a time; (4) using complementary approaches; (5) testing the intervention in a pilot population; and (6) assessing outcomes at regular intervals [67]. We describe several complementary interventions in Table 2, which can be used to design a successful urine culture stewardship program.
Table 2.
Multifaceted interventions for urine test stewardship
| Type of intervention | Systems- or persons-focused | Intervention descriptions |
|---|---|---|
| Automation | Systems-based | Automatic rejection of some urine cultures (e.g., contaminated cultures, absence of pyuria) |
| Automation | Systems-based | Institute two-step processes in ED to reduce automatic culturing |
| EMR nudge | Systems-based | Remove urine cultures from admission, ED, and presurgical order sets (except urology) |
| EMR nudge | Systems-based | Suppressing urine culture results in certain scenarios (e.g., inappropriate indications, lower colony counts) |
| EMR nudge | Systems-based | Make ordering inappropriate urine cultures more difficult in EMR by requiring selection of appropriate indications |
| EMR nudge | Systems-based | Frame urine test results (i.e., positive urine cultures often represent ASB) |
| Standardization | Systems-based | Standardize institutional guidelines for urine culture ordering across inpatient, ED, and outpatient practices |
| Standardization | Systems-based | Standardize collection and transport of urine cultures (collect in containers with preservatives that allow specimens to be kept at room temperature; avoid transport delays) |
| Incentives and feedback | System-based | Incorporate outcomes into pay-for performance metrics |
| Incentives and feedback | Persons-focused | Provide just-in-time coaching of urine culture testing and treatment |
| Cognitive aids | User-focused | Use dashboards or monthly report cards that highlight ordering practices for ordering physicians |
| Policies | Systems-based | Update institutional guidelines to recommend against ordering urine cultures in patients without genitourinary treatment or treating patients with ASB |
| Persons-focused | Engage and support champions from each specialty to promote appropriate testing | |
| Education | Persons-focused | Educate nurses, physicians, and other relevant clinicians on indications for testing and treatment |
| Education | Persons-focused | Incorporate education (e.g., decision support or framing) into urine test ordering |
EMR electronic medical record, ED emergency department, ASB asymptomatic bacteriuria
Addressing Organizational Barriers to Urine Culture Stewardship
In addition to cognitive and diagnostic hurdles, organizational barriers like competing initiatives, organizational culture, and sustainability also need to be addressed for successful implementation of urine culture stewardship.
Competing Initiatives
In most health systems, sepsis initiatives and antimicrobial stewardship programs may appear to have opposing messages around antimicrobial prescribing (i.e., early empiric therapy vs. judicious, narrow antibiotic use). In the era of increasing antimicrobial resistance, there is a need for alignment between sepsis and antimicrobial stewardship initiatives. Quality management of sepsis includes time-dependent recognition, resuscitation pathways, and early initiation of antimicrobials [68]. To avoid potential unintended consequences from inappropriate antimicrobial prescribing, antimicrobial stewardship strategies including de-escalation protocols and stopping antimicrobials in non-infectious patients should be a fundamental component of sepsis quality improvement initiatives [69]. Preferably, antibiotic stewardship and sepsis initiatives to be designed with all stakeholders involved so that initiatives harmonize their message (e.g., watchful waiting vs. early empiric therapy based on patient risk and stability).
Organizational Culture
Organizational culture consists of the shared beliefs and values established by leaders, communicated to employees, reinforced through various methods, and ultimately shaping staff perceptions and behaviors [70]. Pertinent to antibiotic stewardship, an organization’s culture can either promote or dissuade antibiotic overuse and overtreatment of ASB. One example of how culture can affect antibiotic use is a multi-center veteran affairs study of urologic procedures which found that facilities with higher rates of excessive antibiotic use for one procedure had higher rates for other procedures [71]. Similarly, in another study of 46 hospitals in the state of Michigan, inappropriate antibiotic treatment of ASB was linked to overdiagnosis of pneumonia (or inappropriate antibiotic treatment for patients without clinical pneumonia) [72]. These studies show the importance of addressing and changing the organizational culture to successfully implement new interventions.
Sustainability
Sustainability of urine test stewardship depends on the adaptive component of interventions and how initiatives align with the organizational culture [73]. There are a number of obstacles to sustainability of interventions including staff engagement, insufficient resources, and lack of stakeholder buy-in. While systems-based interventions may appear to be more effective, they may sometimes be viewed unfavorably due to decreasing prescriber autonomy and may lead to workarounds and diminishing effectiveness. Combining systems-based interventions like forced functions and automation with persons-based approaches like coaching and education are more likely to result in long-term success. To ensure sustainability, it is important to incorporate user feedback, flexibility, and adaptability in interventions to counter workarounds and continually update guidelines and other EMR changes so as not to become out of date.
Unintended Consequences of Urine Culture Stewardship
While diagnostic stewardship interventions generally result in a reduction in the number of urine cultures ordered, their impact on appropriate antimicrobial use or clinician’s response to an abnormal urinalysis is not clear [74]. Abnormal urinalysis parameters in patients without urinary symptoms are a powerful stimulus to start antibiotic treatment, thwarting diagnostic and antibiotic stewardship interventions [75, 76]. The level of pyuria on urinalysis correlates with increasing use of urine cultures and inappropriate antimicrobial prescribing [74]. Routine urinalysis screening is a surprisingly common practice, used in about 25% of emergency department visits, but does not directly impact decisions of care and delays the final disposition in most patients [75, 77]. Similar misuse of urinalysis is associated with reflex urine cultures. In one study of reflex urine cultures, more patients with positive urinalysis but negative culture received antibiotics than those with a negative urinalysis (30.5 vs 7.1%) [78]. The urine culture standardization intervention by Dougherty and colleagues led to a reduction in urine cultures, but it resulted in a non-significant increase in urinalyses orders and surveillance CAUTIs [48]. Dietz et al. reported a reduction in urine culture orders and antibiotic use after stopping their reflex urine culture process [79]. These studies highlight that reflexing all urine samples with positive urinalysis parameters to culture regardless of symptoms can paradoxically lead to an increase in urine culture orders and overtreatment of ASB [28]. Universal use of reflex urine cultures in a hospital leads to the incorrect assumption that “positive” reflexed urine culture is more indicative of a UTI. Hence, future diagnostic stewardship interventions should address precursor tests like urinalysis by uncoupling it from urine cultures, interpreting urinalysis results in the context of their pre-test probability, and ideally, considering selective reporting of urinalysis criteria [80].
Conclusion
Urine culture stewardship interventions in acute care hospitals span across a spectrum of quality improvement initiatives, ranging from strong systems-based interventions to weaker interventions that focus on clinician education alone. While many interventions have been successful, overall results are mixed, and sustainability is poorly understood. To be most successful, urine culture stewardship interventions should be implemented after a detailed assessment of underlying key drivers and barriers and should utilize multifaceted and complementary approaches [81, 82]. For long-term results, interventions must rely on both the technical and socio-adaptive components to sustain improved patient care [83–85].
Acknowledgements
We would like to thank Dr. David Weber, Professor of Medicine, Infectious diseases, University of North Carolina, for review of the manuscript.
Funding
SA reports grants from the CDC, NIH NIDDK K12 (K12DK100024), SHEA and consulting fees from IPEC Experts, LLC (co-owner) and IDSA. VV reports grants from AHRQ, Gordon and Betty Moore Foundation, NHBLI, NIA, and SHEA.
Footnotes
Conflict of Interest The authors declare no competing interests.
References
- 1.Wilson ML, Gaido L. Laboratory diagnosis of urinary tract infections in adult patients. Clin Infect Dis. 2004;38(8):1150–8. [DOI] [PubMed] [Google Scholar]
- 2.Nicolle LE, Gupta K, Bradley SF, Colgan R, DeMuri GP, Drekonja D, et al. Clinical practice guideline for the management of asymptomatic bacteriuria: 2019 update by the Infectious Diseases Society of America. Clin Infect Dis. 2019;68(10):1611–5. [DOI] [PubMed] [Google Scholar]
- 3.Trautner BW, Petersen NJ, Hysong SJ, Horwitz D, Kelly PA, Naik AD. Overtreatment of asymptomatic bacteriuria: identifying provider barriers to evidence-based care. Am J Infect Control. 2014;42(6):653–8. [DOI] [PubMed] [Google Scholar]
- 4.Trautner BW. Asymptomatic bacteriuria: when the treatment is worse than the disease. Nat Rev Urol. 2011;9(2):85–93. [DOI] [PubMed] [Google Scholar]
- 5.Nicolle LE, Gupta K, Bradley SF, Colgan R, DeMuri GP, Drekonja D, et al. Clinical practice guideline for the management of asymptomatic bacteriuria: 2019 update by the Infectious Diseases Society of America. Clin Infect Dis. 2019. [DOI] [PubMed] [Google Scholar]
- 6.Advani SD, Fakih MG. The evolution of catheter-associated urinary tract infection (CAUTI): is it time for more inclusive metrics? Infect Control Hosp Epidemiol. 2019:1–5. [DOI] [PubMed] [Google Scholar]
- 7.Garibaldi RA, Mooney BR, Epstein BJ, Britt MR. An evaluation of daily bacteriologic monitoring to identify preventable episodes of catheter-associated urinary tract infection. Infect Control. 1982;3(6):466–70. [DOI] [PubMed] [Google Scholar]
- 8.Eddeland A, Hedelin H. Bacterial colonization of the lower urinary tract in women with long-term indwelling urethral catheter. Scand J Infect Dis. 1983;15(4):361–5. [DOI] [PubMed] [Google Scholar]
- 9.Trautner BW. Urinary tract infection as a continuum-implications for diagnostic and antibiotic stewardship. Clin Infect Dis. 2020. [DOI] [PubMed] [Google Scholar]
- 10.Tambyah PA, Maki DG. The relationship between pyuria and infection in patients with indwelling urinary catheters: a prospective study of 761 patients. Arch Intern Med. 2000;160(5):673–7. [DOI] [PubMed] [Google Scholar]
- 11.Nicolle LE. Asymptomatic bacteriuria: review and discussion of the IDSA guidelines. Int J Antimicrob Agents. 2006;28(Suppl 1):S42–8. [DOI] [PubMed] [Google Scholar]
- 12.Biggel M, Heytens S, Latour K, Bruyndonckx R, Goossens H, Moons P. Asymptomatic bacteriuria in older adults: the most fragile women are prone to long-term colonization. BMC Geriatr. 2019;19(1):170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petty LA, Vaughn VM, Flanders SA, Patel T, Malani AN, Ratz D, et al. Assessment of testing and treatment of asymptomatic bacteriuria initiated in the emergency department. Open Forum Infect Dis. 2020;7(12):ofaa537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petty LA, Vaughn VM, Flanders SA, Malani AN, Conlon A, Kaye KS, et al. Risk factors and outcomes associated with treatment of asymptomatic bacteriuria in hospitalized patients. JAMA Intern Med. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Advani SD, Gao CA, Datta R, Sann L, Smith C, Leapman MS, et al. Knowledge and practices of physicians and nurses related to urine cultures in catheterized patients: an assessment of adherence to IDSA guidelines. Open Forum Infect Dis. 2019;6(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.CDC. National Healthcare Safety Network (NHSN) Patient safety component manual. January2017. Available from: https://www.cdc.gov/nhsn/pdfs/pscmanual/pcsmanual_current.pdf.
- 17.Hooton TM, Bradley SF, Cardenas DD, Colgan R, Geerlings SE, Rice JC, et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clin Infect Dis. 2010;50(5):625–63. [DOI] [PubMed] [Google Scholar]
- 18.Al-Qas Hanna F, Sambirska O, Iyer S, Szpunar S, Fakih MG. Clinician practice and the National Healthcare Safety Network definition for the diagnosis of catheter-associated urinary tract infection. Am J Infect Control. 2013;41(12):1173–7. [DOI] [PubMed] [Google Scholar]
- 19.Petty LA, Vaughn VM, Flanders SA, Malani AN, Conlon A, Kaye KS, et al. Risk factors and outcomes associated with treatment of asymptomatic bacteriuria in hospitalized patients. JAMA Intern Med. 2019;179(11):1519–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vaughn VM, Chopra V. Revisiting the panculture. BMJ Qual Saf. 2017;26(3):236–9. [DOI] [PubMed] [Google Scholar]
- 21.Strand CL, Wajsbort RR, Sturmann K. Effect of iodophor vs iodine tincture skin preparation on blood culture contamination rate. JAMA. 1993;269(8):1004–6. [PubMed] [Google Scholar]
- 22.Fakih MG, Advani SD, Vaughn VM. Diagnosis of urinary tract infections: need for a reflective rather than reflexive approach. Infect Control Hosp Epidemiol. 2019:1–2. [DOI] [PubMed] [Google Scholar]
- 23.Fakih MG, Khatib R. Improving the culture of culturing: critical asset to antimicrobial stewardship. Infect Control Hosp Epidemiol. 2017;38(3):377–9. [DOI] [PubMed] [Google Scholar]
- 24.Awadalla R, Gnjidic D, Patanwala A, Sakiris M, Penm J. The effectiveness of stewardship interventions to reduce the prescribing of extended-release opioids for acute pain: a systematic review. Pain Med. 2020;21(10):2401–11. [DOI] [PubMed] [Google Scholar]
- 25.Frontera JA, Wang E, Phillips M, Radford M, Sterling S, Delorenzo K, et al. Protocolized urine sampling is associated with reduced catheter-associated urinary tract infections: a pre- and post-intervention study. Clin Infect Dis. 2020. [DOI] [PubMed] [Google Scholar]
- 26.Lamb MJ, Baillie L, Pajak D, Flynn J, Bansal V, Simor A, et al. Elimination of screening urine cultures prior to elective joint arthroplasty. Clin Infect Dis. 2017;64(6):806–9. [DOI] [PubMed] [Google Scholar]
- 27.Claeys KC, Blanco N, Morgan DJ, Leekha S, Sullivan KV. Advances and challenges in the diagnosis and treatment of urinary tract infections: the need for diagnostic stewardship. Curr Infect Dis Rep. 2019;21(4):11. [DOI] [PubMed] [Google Scholar]
- 28.Sullivan KV, Morgan DJ, Leekha S. Use of diagnostic stewardship practices to improve urine culturing among SHEA Research Network hospitals. Infect Control Hosp Epidemiol. 2019;40(2):228–31. [DOI] [PubMed] [Google Scholar]
- 29.Ling D, Seidelman J, Dodds-Ashley E, Lewis S, Moehring RW, Anderson DJ, et al. Navigating reflex urine culture practices in community hospitals: need for a validated approach. Am J Infect Control. 2020. [DOI] [PubMed] [Google Scholar]
- 30.Redwood R, Knobloch MJ, Pellegrini DC, Ziegler MJ, Pulia M, Safdar N. Reducing unnecessary culturing: a systems approach to evaluating urine culture ordering and collection practices among nurses in two acute care settings. Antimicrob Resist Infect Control. 2018;7:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Humphries RM, Dien BJ. Point-counterpoint: reflex cultures reduce laboratory workload and improve antimicrobial stewardship in patients suspected of having urinary tract infections. J Clin Microbiol. 2016;54(2):254–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Epstein L, Edwards JR, Halpin AL, Preas MA, Blythe D, Harris AD, et al. Evaluation of a novel intervention to reduce unnecessary urine cultures in intensive care units at a tertiary care hospital in Maryland, 2011–2014. Infect Control Hosp Epidemiol. 2016;37(5):606–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lynch CS, Appleby-Sigler A, Bork JT, Dave R, Agnes K, Sanikop M, et al. Effect of urine reflex culturing on rates of cultures and infections in acute and long-term care. Antimicrob Resist Infect Control. 2020;9(1):96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Howard-Anderson JR, Ashraf S, Overton EC, Reif L, Murphy DJ, Jacob JT. Sustained decrease in urine culture utilization after implementing a reflex urine culture intervention: a multi-center quasi-experimental study. Infect Control Hosp Epidemiol. 2020;41(3):369–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Claeys KC, Zhan M, Pineles L, Lydecker A, Clore G, Goto M, et al. Conditional reflex to urine culture: evaluation of a diagnostic stewardship intervention within the Veterans’ Affairs and Centers for Disease Control and Prevention Practice-Based Research Network. Infect Control Hosp Epidemiol. 2021;42(2):176–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Munigala S, Rojek R, Wood H, Yarbrough ML, Jackups RR, Burnham CD, et al. Effect of changing urine testing orderables and clinician order sets on inpatient urine culture testing: analysis from a large academic medical center. Infect Control Hosp Epidemiol. 2019;40(3):281–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith MA, Puckrin R, Lam PW, Lamb MJ, Simor AE, Leis JA. Association of increased colony-count threshold for urinary pathogens in hospitalized patients with antimicrobial treatment. JAMA Intern Med. 2019;179(7):990–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee ALH, Leung ECM, Lee MKP, Lai RWM. Diagnostic stewardship programme for urine culture: impact on antimicrobial prescription in a multi-centre cohort. J Hosp Infect. 2021;108:81–9. [DOI] [PubMed] [Google Scholar]
- 39.Vaughn VM, Linder JA. Thoughtless design of the electronic health record drives overuse, but purposeful design can nudge improved patient care. BMJ Qual Saf. 2018;27(8):583–6. [DOI] [PubMed] [Google Scholar]
- 40.Thaler RHSR. Nudge: Penguin Books; 2009.
- 41.Shirley D, Scholtz H, Osterby K, Musuuza J, Fox B, Safdar N. Optimizing inpatient urine culture ordering practices using the electronic medical record: a pilot study. Infect Control Hosp Epidemiol. 2017;38(4):486–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Watson KJ, Trautner B, Russo H, Phe K, Lasco T, Pipkins T, et al. Using clinical decision support to improve urine culture diagnostic stewardship, antimicrobial stewardship, and financial cost: a multi-center experience. Infect Control Hosp Epidemiol. 2020;41(5):564–70. [DOI] [PubMed] [Google Scholar]
- 43.Lin G, Knowlson S, Nguyen H, Cooper K, Pryor RJ, Doll M, et al. Urine test stewardship for catheterized patients in the critical care setting: provider perceptions and impact of electronic order set interventions. Am J Infect Control. 2019;47(10):1277–9. [DOI] [PubMed] [Google Scholar]
- 44.Liao S, Rhodes J, Jandarov R, DeVore Z, Sopirala MM. Out of sight-out of mind: impact of cascade reporting on antimicrobial usage. Open Forum Infect Dis. 2020;7(2):ofaa002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leis JA, Rebick GW, Daneman N, Gold WL, Poutanen SM, Lo P, et al. Reducing antimicrobial therapy for asymptomatic bacteriuria among noncatheterized inpatients: a proof-of-concept study. Clin Infect Dis. 2014;58(7):980–3. [DOI] [PubMed] [Google Scholar]
- 46.Daley P, Garcia D, Inayatullah R, Penney C, Boyd S. Modified reporting of positive urine cultures to reduce inappropriate treatment of asymptomatic bacteriuria among nonpregnant, noncatheterized inpatients: a randomized controlled trial. Infect Control Hosp Epidemiol. 2018;39(7):814–9. [DOI] [PubMed] [Google Scholar]
- 47.Duhigg C The power of habit: why we do what we do in life and business.: New York Random House; 2012. [Google Scholar]
- 48.Dougherty DF, Rickwa J, Guy D, Keesee K, Martin BJ, Smith J, et al. Reducing inappropriate urine cultures through a culture standardization program. Am J Infect Control. 2020;48(6):656–62. [DOI] [PubMed] [Google Scholar]
- 49.Sarg M, Waldrop GE, Beier MA, Heil EL, Thom KA, Preas MA, et al. Impact of changes in urine culture ordering practice on antimicrobial utilization in intensive care units at an academic medical center. Infect Control Hosp Epidemiol. 2016;37(4):448–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Davies PE, Daley MJ, Hecht J, Hobbs A, Burger C, Watkins L, et al. Effectiveness of a bundled approach to reduce urinary catheters and infection rates in trauma patients. Am J Infect Control. 2018;46(7):758–63. [DOI] [PubMed] [Google Scholar]
- 51.Gawande A The checklist manifesto. London, England: Profile Books; 2011. [Google Scholar]
- 52.Mullin KM, Kovacs CS, Fatica C, Einloth C, Neuner EA, Guzman JA, et al. A multifaceted approach to reduction of catheter-associated urinary tract infections in the intensive care unit with an emphasis on “stewardship of culturing”. Infect Control Hosp Epidemiol. 2017;38(2):186–8. [DOI] [PubMed] [Google Scholar]
- 53.Sampathkumar P, Barth JW, Johnson M, Marosek N, Johnson M, Worden W, et al. Mayo Clinic reduces catheter-associated urinary tract infections through a Bundled 6-C Approach. Jt Comm J Qual Patient Saf. 2016;42(6):254–61. [DOI] [PubMed] [Google Scholar]
- 54.Sampathkumar P Reducing catheter-associated urinary tract infections in the ICU. Curr Opin Crit Care. 2017;23(5):372–7. [DOI] [PubMed] [Google Scholar]
- 55.Huang GC, Kriegel G, Wheaton C, Sternberg S, Sands K, Richards J, et al. Implementation of diagnostic pauses in the ambulatory setting. BMJ Qual Saf. 2018;27(6):492–7. [DOI] [PubMed] [Google Scholar]
- 56.Loeb M, Brazil K, Lohfeld L, McGeer A, Simor A, Stevenson K, et al. Effect of a multifaceted intervention on number of antimicrobial prescriptions for suspected urinary tract infections in residents of nursing homes: cluster randomised controlled trial. BMJ. 2005;331(7518):669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Luu A, Dominguez F, Yeshoua B, Vo C, Nallapa S, Chung D, et al. Reducing catheter associated urinary tract infections via cost-saving diagnostic stewardship. Clin Infect Dis. 2020. [DOI] [PubMed] [Google Scholar]
- 58.CDC. Core elements of antibiotic stewardship. 2021. Available from: https://www.cdc.gov/antibiotic-use/core-elements/index.html.
- 59.Bonnal C, Baune B, Mion M, Armand-Lefevre L, L’Heriteau F, Wolmark Y, et al. Bacteriuria in a geriatric hospital: impact of an antibiotic improvement program. J Am Med Dir Assoc. 2008;9(8):605–9. [DOI] [PubMed] [Google Scholar]
- 60.Linares LA, Thornton DJ, Strymish J, Baker E, Gupta K. Electronic memorandum decreases unnecessary antimicrobial use for asymptomatic bacteriuria and culture-negative pyuria. Infect Control Hosp Epidemiol. 2011;32(7):644–8. [DOI] [PubMed] [Google Scholar]
- 61.Jackson EC, Thai XC, Bruno-Murtha LA, Barner AE. Impact of tiered interventions to decrease routine urine cultures in asymptomatic patients undergoing arthroplasty. Infect Control Hosp Epidemiol. 2019;40(1):109–10. [DOI] [PubMed] [Google Scholar]
- 62.Fabre V, Pleiss A, Klein E, Demko Z, Salinas A, Jones G, et al. A pilot study to evaluate the impact of a nurse-driven urine culture diagnostic stewardship intervention on urine cultures in the acute care setting. Jt Comm J Qual Patient Saf. 2020;46(11):650–5. [DOI] [PubMed] [Google Scholar]
- 63.Sumner S, Forsyth S, Collette-Merrill K, Taylor C, Vento T, Veillette J, et al. Antibiotic stewardship: the role of clinical nurses and nurse educators. Nurse Educ Today. 2018;60:157–60. [DOI] [PubMed] [Google Scholar]
- 64.Edwards R, Drumright L, Kiernan M, Holmes A. Covering more territory to fight resistance: considering nurses’ role in antimicrobial stewardship. J Infect Prev. 2011;12(1):6–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Olans RN, Olans RD, DeMaria A Jr. The critical role of the staff nurse in antimicrobial stewardship–unrecognized, but already there. Clin Infect Dis. 2016;62(1):84–9. [DOI] [PubMed] [Google Scholar]
- 66.Olans RD, Olans RN, Witt DJ. Good nursing is good antibiotic stewardship. Am J Nurs. 2017;117(8):58–63. [DOI] [PubMed] [Google Scholar]
- 67.Wight D, Wimbush E, Jepson R, Doi L. Six steps in quality intervention development (6SQuID). J Epidemiol Community Health. 2016;70(5):520–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Levy MM, Evans LE, Rhodes A. The surviving sepsis campaign bundle: 2018 update. Crit Care Med. 2018;46(6):997–1000. [DOI] [PubMed] [Google Scholar]
- 69.Ashiru-Oredope D, Sharland M, Charani E, McNulty C, Cooke J, Group AAS. Improving the quality of antibiotic prescribing in the NHS by developing a new antimicrobial stewardship programme: start smart–then focus. J Antimicrob Chemother. 2012;67(Suppl 1):i51–63. [DOI] [PubMed] [Google Scholar]
- 70.Saint S, Kowalski CP, Banaszak-Holl J, Forman J, Damschroder L, Krein SL. The importance of leadership in preventing healthcare-associated infection: results of a multisite qualitative study. Infect Control Hosp Epidemiol. 2010;31(9):901–7. [DOI] [PubMed] [Google Scholar]
- 71.Khaw C, Oberle AD, Lund BC, Egge J, Heintz BH, Erickson BA, et al. Assessment of guideline discordance with antimicrobial prophylaxis best practices for common urologic procedures. JAMA Netw Open. 2018;1(8):e186248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vaughn VM, Gupta A, Petty LA, Gandhi TN, Flanders S, Swaminathan L, Hsaiky L, Ratz D, Horowitz J, McLaughlin E, Chopra V. Misdiagnosis of urinary tract and misdiagnosis of pneumonia linked at the hospital level: a multi-hospital cohort study. Society of Hospital Medicine Annual meeting. 2020;187. [Google Scholar]
- 73.Wiltsey Stirman S, Kimberly J, Cook N, Calloway A, Castro F, Charns M. The sustainability of new programs and innovations: a review of the empirical literature and recommendations for future research. Implement Sci. 2012;7:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gupta K, O’Brien W, Gallegos-Salazar J, Strymish J, Branch-Elliman W. How testing drives treatment in asymptomatic patients: level of pyuria directly predicts probability of antimicrobial prescribing. Clin Infect Dis. 2020;71(3):614–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Anand A, Ballinger B, Ganti L. Impact of urinalysis on medical decision-making and length of stay. Cureus. 2018;10(4):e2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Khawcharoenporn T, Vasoo S, Ward E, Singh K. Abnormal urinalysis finding triggered antibiotic prescription for asymptomatic bacteriuria in the ED. Am J Emerg Med. 2011;29(7):828–30. [DOI] [PubMed] [Google Scholar]
- 77.Neyman G, Dalsey W. A Quantification of the impact of awaiting results of a urinalysis upon emergency department length of stay. J Emerg Med. 2021;60(2):158–64. [DOI] [PubMed] [Google Scholar]
- 78.Klein CN, Elman MR, Townes JM, Lewis JS, McGregor JC. Unintended consequences of a reflex urine culture order set on appropriate antibiotic use. Infect Control Hosp Epidemiol. 2020;41(9):1090–2. [DOI] [PubMed] [Google Scholar]
- 79.Dietz J, Lo TS, Hammer K, Zegarra M. Impact of eliminating reflex urine cultures on performed urine cultures and antibiotic use. Am J Infect Control. 2016;44(12):1750–1. [DOI] [PubMed] [Google Scholar]
- 80.Advani S, Polage C, Fakih M. Deconstructing the urinalysis: A novel approach to diagnostic and antimicrobial stewardship. Antimicrobial Stewardship & Healthcare Epidemiology 2021;1(1), E6. 10.1017/ash.2021.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fakih MG, Krein SL, Edson B, Watson SR, Battles JB, Saint S. Engaging health care workers to prevent catheter-associated urinary tract infection and avert patient harm. Am J Infect Control. 2014;42(10 Suppl):S223–9. [DOI] [PubMed] [Google Scholar]
- 82.Reynolds SS, Sova CD, Lewis SS, Smith BA, Wrenn RH, Turner NA, et al. Sustained reduction in catheter-associated urinary tract infections using multi-faceted strategies led by champions: a quality improvement initiative. Infect Control Hosp Epidemiol. 2021:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Saint S, Greene MT, Krein SL, Rogers MA, Ratz D, Fowler KE, et al. A Program to prevent catheter-associated urinary tract infection in acute care. N Engl J Med. 2016;374(22):2111–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fakih MG, George C, Edson BS, Goeschel CA, Saint S. Implementing a national program to reduce catheter-associated urinary tract infection: a quality improvement collaboration of state hospital associations, academic medical centers, professional societies, and governmental agencies. Infect Control Hosp Epidemiol. 2013;34(10):1048–54. [DOI] [PubMed] [Google Scholar]
- 85.Carayon P, Wetterneck TB, Rivera-Rodriguez AJ, Hundt AS, Hoonakker P, Holden R, et al. Human factors systems approach to healthcare quality and patient safety. Appl Ergon. 2014;45(1):14–25. [DOI] [PMC free article] [PubMed] [Google Scholar]


