Abstract
Due to the prevalence of cardiovascular diseases, there is a large need for small diameter vascular grafts that cannot be fulfilled using autologous vessels. Although medium to large diameter synthetic vessels are in use, no suitable small diameter vascular graft has been developed due to the unique dynamic environment that exists in small vessels. To achieve long term patency, a successful tissue engineered vascular graft would need to closely match the mechanical properties of native tissue, be non-thrombotic and non-immunogenic, and elicit the proper healing response and undergo remodeling to incorporate into the native vasculature. Electrospinning presents a promising approach to the development of a suitable tissue engineered vascular graft. This review provides a comprehensive overview of the different polymers, techniques, and functionalization approaches that have been used to develop an electrospun tissue engineered vascular graft.
Keywords: Electrospinning, Scaffold, Vascular tissue engineering
1. Introduction
Cardiovascular diseases (CVDs) remain the leading cause of mortality, accounting for 17.8 million deaths worldwide in 2017 [1]. While the number of deaths attributable to CVDs increased from 2007 to 2017, the death rate from CVDs dropped by 10% over the same time frame [1]. Despite the decrease in death rate, the years of life lost increased for coronary heart disease (CHD) and stroke over that time ranking first and third, respectively [1]. In total nearly 200 million people have CHD and 113 million people suffer from peripheral artery disease (PAD) [2]. In the United States, an estimated 20 million people suffer from CHD and 6.5 million people have PAD, with 365,000 people dying from CHD and 70,000 from PAD in 2018 [3]. Graft bypass surgery is one of the most common treatments for CHD with an estimated 371,000 surgeries performed in 2014 in the United States [3]. Open revascularization surgery for the treatment of PAD is needed in cases of critical limb ischemia or where endovascular intervention is unsuitable [4,5]. The rise of these diseases has created an ever-present demand for suitable vascular grafts.
Autologous vessel grafts are considered the gold standard for bypass and revascularization surgeries, with the greater saphenous vein (GSV) considered the vessel of choice by many [6]. However, between 20 and 40% of patients lack a suitable GSV due to it being too small, previous removal, or disease [6–8]. Alternative vascular conduits include internal mammary, radial, and internal thoracic arteries as well as the lesser saphenous and arm veins [6,9–12]. Many of the same availability issues seen with the GSV arise with these vessels as well and the use of some of these vessels, such as the arm vein, results in reduced patency of the graft [6]. Therefore, there is a need for an alternative to autologous vessel grafts. Allograft and xenograft vessels prepared by cryopreservation or decellularization to reduce immunogenicity have been used as an alternative to autologous vessels but generally suffer from lower patency. This has attributed to increased thrombogenicity, host immune response, and increased calcification [13–16]. Synthetic grafts composed of expanded polytetrafluoroethylene (ePTFE) and poly-ethyleneterephthalate (Dacron) have been used clinically since the 1950s for the replacement of medium to large diameter vessels. For small diameter vessels (<6 mm in diameter), however, these synthetic grafts are prone to failure due to rapid occlusion and acute thrombogenicity. This has been attributed to a mismatch of mechanical properties and poor endothelialization [17,18]. Tissue engineering represents a promising approach to overcome the shortcomings of currently available vessel grafts.
An ideal small diameter tissue engineered vascular graft (TEVG) would replicate the biological and mechanical properties of autologous vessels and be able to function in the low flow, higher resistance hemodynamic environment that exists in small diameter vessels [18,19]. Several design criteria would have to be met to develop a viable TEVG. The TEVG would have to be biocompatible such that it is non-immunogenic and nonthrombogenic, properties that are associated with a fully developed and functional endothelium. It would have to have similar mechanical properties as the native vessel, able to withstand hemodynamic forces immediately upon implantation and over the long term while also not being susceptible to permanent deformation that could lead to an aneurysm. In addition, a successful TEVG would need to elicit a good healing response to minimize inflammation and avoid intimal hyperplasia and fibrosis. A biologically active TEVG would also be beneficial to induce remodeling, allowing for the growth of an endothelium and the infiltration of vascular smooth muscle cells (VSMCs) to successfully integrate into the vasculature. Finally, from a surgical perspective, the TEVG needs to compliant enough for handling and strong enough to hold sutures for implantation [17,20,21].
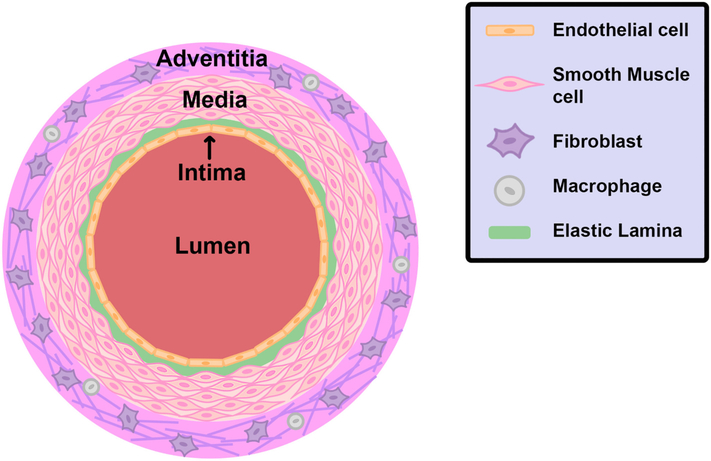
The design of a TEVG should take into account the structure of a native artery to best replicate the function of the artery. Arteries are composed of three distinct layers: the tunica intima, tunica media, and adventitia (Fig. 1). The innermost layer is the tunica intima and is composed of a single layer of endothelial cells that lay a top the elastic lamina, providing for smooth laminar flow of blood. Endothelial cells are responsible for the release of numerous bioactive substances, modulation of vascular tone, and the selective diffusion of various substances [22,23]. The middle layer is the tunica media and is composed of concentric layers of circumferentially aligned VSMCs, with the number of layers depending on the size of the artery. VSMCs are contractile cells and the primary regulator of blood pressure. Interspersed among the VSMCs is an extensive extracellular matrix (ECM) of collagen fibers and elastin. The collagen fibers have high tensile strength and elastic modulus and bear most of the force on the artery. Elastin is a distensible protein that acts as an elastic reservoir allowing the artery to expand and contract to maintain blood pressure [24]. Finally, the outmost layer of an artery is the adventitia. The adventitia is primary composed of connective tissue, with nerve fibers and lymphatic vessels dispersed throughout. Fibroblasts are the main cell type found in the adventitia and regulate inflammatory responses. Immune cells such as macrophages are also present in the adventitia [25].
Fig. 1.
Diagram of an artery. An artery is composed of three layers: the intima, media, and adventitia. The intima is composed of a monolayer of endothelial cells surrounded by the elastic lamina. The media contains several layers of VSMCs interspersed with an extensive ECM of collagen fibers and elastin (not shown). The adventitia is composed of connective tissue and contains fibroblast and macrophages.
Various techniques have been employed to create TEVGs such as casting [26], self-assembly [27], 3D bioprinting [28], and decellularized scaffolds [29]. However, with the exception of decellularized scaffolds, many of these methods fail to mimic the structure of natural extracellular matrix (ECM) which a key component to the regeneration and remodeling of tissue [30]. One promising method for the production of TEVGs that addresses this concern is electrospinning. Electrospinning a polymer solution results in the production nanofibers that recreates the natural structure of ECM. Fiber structure, diameter, topology, and mechanical properties are readily tunable by adjusting various operating parameters. Furthermore, electrospinning can utilize a wide variety of polymers and blends to further tune the scaffold’s properties and various bioactive compounds can be incorporated into the fibers to improve remodeling of the scaffold into the native vasculature.
In this review we examine the current state of electrospinning as viable method for generating small diameter vascular grafts. We present an overview of electrospinning and the most widely used polymers for vascular electrospinning including their benefits and drawbacks. Next, we examine techniques that have been used to improve the structure of the scaffolds to improve cellular responses. Finally, we look at the incorporation of bioactive agents into the grafts to improve the formation of functional tissue.
2. Electrospinning
Electrospinning was first discovered in 1887 and mathematically described in the 1960s in a series of papers by Geoffrey Taylor [31–34]. In the simplest set-up, electrospinning involves a high voltage DC power supply, syringe pump, spinneret (typically a blunt hypodermic needle), and a collector. For vascular tissue engineering a rotating mandrel is generally used as the collector to produce a tubular shaped graft. During electrospinning a droplet of liquid is emitted from the spinneret. This droplet becomes electrically charged and deforms due to electrostatic repulsion forming a Taylor cone. A jet is emitted from the apex of the Taylor cone, forming a long, thin strand of liquid. This strand initially travels straight as it is stretched and elongated within the electric field. At various points the electrostatic repulsion within the strand is great enough for bending instability to occur causing the strand to further stretch into a series of coils and accounts for most of reduction in diameter of the fiber [35]. At smaller diameters, the solvent evaporates quickly allowing the fiber to solidify and accumulate on the collector.
All of the set-up parameters (voltage, distance to collector, flow rate, and needle diameter) are readily adjustable and allow for fine tuning to achieve the desired fiber diameter. In general, higher voltage results in smaller fiber diameters although in some cases a higher voltage may cause more liquid to be emitted and increase fiber diameter [36,37]. Higher flow rates usually result in increased fiber diameter. Increasing the distance between the spinneret and collector generally results in thinner fibers up to a certain distance beyond which the fiber will have solidified and no further extension will occur [37]. However, there is a complex interplay between each of these parameters that often requires numerous experiments to optimize the operating parameters and achieve the desired fiber diameter [36]. Furthermore, additional factors such as the polymer, solvent, and ambient conditions including temperature and humidity influence the operating parameters and require further optimization.
3. Polymers used for the fabrication of vessel grafts by electrospinning
3.1. Synthetic polymer
3.1.1. Polylactic acid
Polylactic acid (PLA) is a renewable, bioresorbable, low-cost manufacturable synthetic material. It has been wildly popular in different fields such as implantable biomedical devices, food packaging, drug delivery system and many more [38]. PLA is an aliphatic hydrophobic polyester. There are three commonly methods to synthesize PLA from the L,D lactic acid monomers, including direct condensation, dehydration condensation and ring-opening polymerization (ROP) [39]. Lactide ROP pathway is the most practiced method in the industrial setting to produce high molecular weight PLA, and polymers can be accustomed to achieving specific properties through the controlled ROP method [39]. PLA degrades naturally via hydrolysis, and its by-product lactic acid is nontoxic to the human body. The degradation rate of PLA is slow due to its hydrophobic methyl group [40]. The molecular weight and stereo-chemistry determine the crystallinity of PLA, which then determines its mechanical performance. Generally, higher crystallinity leads to higher PLA stiffness and toughness, and vice versa [40,41]. A few major disadvantages hinder the function and performance of PLA in biomedical applications. Homopolymer PLA is very brittle, which limits its ability in withstanding high stress settings because it cannot undergo enough plastic deformation. Slow degradation rate makes it unfit for shorter term implants. Hydrophobicity leads to low cell attachment and will further result in inflammation reactions [42]. To optimize PLA’s applicability in the biomedical field, it can be blended with other polymers, such as polyglycolic acid, polyethylene glycol, polycaprolactone, etc. to increase the hydrophilicity, flexibility and elasticity, respectively [39].
In one study, Shalumon et al. [43] created a multilayered PLA tubular scaffold. Initially, aligned PLA fibers were collected on a rotating mandrel after which the aligned mat was wrapped around a tubular form and fused with a concentrated PLA in chloroform solution. Multilayered scaffolds were generated by wrapping additional PLA mats around the form and fusing them. An outer layer composed of a 50:50 PLA/polycaprolactone (PCL) was then electrospun onto the tubular scaffold. The inner aligned PLA was selected to support ECs while the more porous PLA/PCL layer was selected to support the growth and infiltration of VSMCs. The bilayer graft composed of one inner PLA and one outer PLA/PCL layer had good mechanical stability and the multilayered grafts with additional PLA layers showed enhanced mechanical properties. Human umbilical vein endothelial cells (HUVECs) and human umbilical artery smooth muscle cells (HUASMCs) were seeded onto the inner and outer layers, respectively. After 48 h, HUVECs had aligned and elongated in the direction of the aligned PLA fibers while the HUASMCs spread well and showed good actin organization. The scaffold supported the proliferation of both cell types similarly over the course of 96 h. The functionality of the HUVECs was assessed by measuring acetylated-low density lipoprotein uptake and nitric oxide (NO) production and the HUVECs present were found to be functional, mimicking the behavior of ECs in native vessels.
3.1.2. Polyglycolic acid
Among many biodegradable biomaterials, polyglycolic acid (PGA) is considered the first synthetic biodegradable polymer used for biomedical applications [44]. It was used in the first biodegradable synthetic sutures under the tradename “DEXON®” [45]. Although PGA is much like PLA in terms of structure and degradation pathway, it lacks a methyl group thus making it hydrophilic and degrade in the presence of water. PGA possesses great mechanical strength due to its high crystallinity, and excellent controlled degradation while maintaining its shape in organic solvents due to its low solubility. With a glass transitional temperature in the range between 35 °C and 40 °C, PGA degrades under the physiological conditions. However, rapid degradation and a toxic by-product are two major limitations of PGA. The ability to degrade within weeks (depending on specific molecular weight) is beneficial in some instances such as for short-term implants but is also a significant drawback because the mechanical properties are compromised [44]. Although PGA is considered to be non-toxic, its degradation by-product glycolic acid can acidify the local environment and result in inflammation [46]. To resolve these challenges, it is more commonly copolymerized PGA with PLA to form poly(lactic-co-glycolic) acid (PLGA) copolymer to optimize their mechanical properties and degradation rate.
Hodge and Quint took advantage of the rapid degradation of PGA, developing a TEVG by electrospinning PGA while simultaneously electrospraying polyethylene oxide (PEO) [47]. After rinsing with deionized water, the PEO was removed resulting in a more porous scaffold. The PGA grafts were seeded with human dermal fibroblasts to promote collagen deposition and cultured under pulsative flow at 160 cycles per minute for 10 weeks. The PGA rapidly degraded, allowing for rapid migration of the fibroblasts into the scaffold and formation of a collagen-rich construct, with no residual PGA fibers being detected after 10 weeks. The cyclic mechanical stretch provided by pulsative conditions resulted in significantly greater collagen content that was evenly distributed throughout the wall of the graft compared to those cultured under static conditions. Likewise, the pulsative cultured grafts had much better mechanical properties, with significantly higher ultimate tensile strength, elastic modulus, and burst pressure.
3.1.3. Poly(lactic-co-glycolic) acid
Poly(lactic-co-glycolic) acid (PLGA), the co-polymer of PLA and PGA, has been extensively investigated since the 1970s to better tailor the properties of PLA and PGA [48]. PLGA is an attractive polymer for use in tissue engineering as it is FDA approved and is already used in absorbable sutures and meshes under the tradename “Vicryl®”. Furthermore, it has good processibility, controllable degradation times, commercial availability, and excellent biocompatibility [44]. In addition, PLGA is in an amorphous state up to 70% glycolide making it less stiff than either of its co-polymers [48]. PLGA degrades through bulk erosion and hydrolysis of the ester bonds. The degradation rate of PLGA is faster than either of its co-polymers due to morphological changes that disrupt the more crystalline structure of its pure co-polymers and can be adjusted by varying the lactide:glycolide ratio. The 50:50 co-polymer has been shown to be relatively unstable, degrading in 1–2 months. Greater resistance to degradation can be found at higher co-polymer ratios at either end of the spectrum, with the 75:25 co-polymer degrading in 4–5 months and increasing to 5–6 months for the 85:15 blend [44,49].
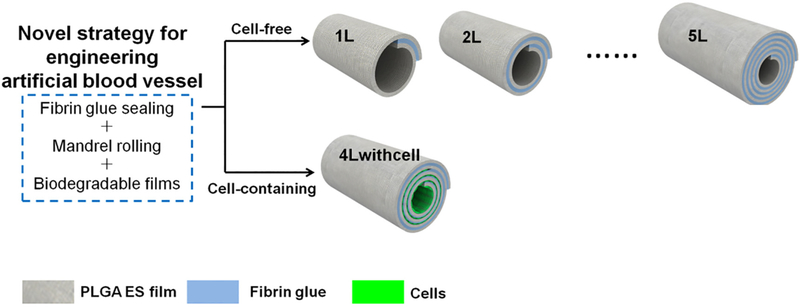
Wang et al. [50] developed a multilayered tubular PLGA graft by rolling an electrospun PLGA sheet coated with fibrin glue around a 2 mm diameter mandrel (Fig. 2). This method allowed for the number of PLGA layers and thus wall thickness to be easily adjusted and allowed for easy manipulation of mechanical properties, with a 5-layer graft having a burst pressure similar to the GSV. Cells could be easily encapsulated within the scaffold by seeding cells on the PLGA sheet prior to rolling the graft. C2C12 mouse myoblast cells seeded in the scaffolds demonstrated good viability and proliferation. Importantly, the cell-seeded scaffolds also showed reduced leakage.
Fig. 2.
Wang et al. [50] rolled PLGA mats with fibrin glue to create a multilayered scaffold. Cells seeded in the scaffolds demonstrated good viability and proliferation. In addition, the cell-seeded scaffolds also showed reduced leakage. Reproduced with permission of MDPI.
The degradation properties of PLGA pose a potential issue in its use for a TEVG. In one study, porcine smooth muscle cells grew on PLGA nanofibers for 30 days after which growth slowed [51]. PLGA nanofibers have been shown to shrink in fluids including Dulbecco’s Modified Eagle Medium (DMEM), simulated body fluid (SFB), and saliva [52]. This is due to fluid-induced relaxation of the polymer chain and can increase hydrolytic and enzymatic degradation. Any implantable medical device also has to undergo sterilization, which has also been shown to speed the degradation of PLGA. Chor et al. [52] found that gamma irradiated electrospun PLGA scaffolds had low polymer molecular weight and enhanced hydrolytic degradation in SBF and saliva compared to nonirradiated scaffolds. Since PLGA undergoes bulk erosion, the mechanical properties of PLGA decrease as it degrades and may pose a problem for the long-term patency of a PLGA TEVG, especially, if it degrades before substantial remodeling and integration can occur.
3.1.4. Polycaprolactone
PCL has many versatile properties including excellent biocompatibility, biodegradability, and nontoxicity. It is a competitive biomaterial candidate for medical and clinical uses such as tissue engineering scaffolds, drug delivery, surgical sutures, wound dressings, etc., attributed to these merits [44]. There are two methods to synthesize PCL, including 6-hydroxyhexanoic acid polycondensation, and epsilon-caprolactone ring-opening polymerization (ROP). ROP pathway, in particular, is more commonly practiced considering it yields a more superior end product [53]. PCL has a semi-crystalline structure. It has a low melting point at about 60 °C and a glass transition temperature at about −60 °C. Therefore, under the physiological conditions, it maintains great mechanical properties such as high toughness, strength, and elasticity. A general degradation period for PCL implants is about 2 to 3 years and the degradation mechanism is usually undergone by the indwelling micro-organism’s digestion and through the ester linkage hydrolysis [54]. Caproic acid, one of the degradation products, is non-toxic to the local tissues and can be reabsorbed by metabolic processes or excreted through the renal system [55]. Although PCL possesses many talents as a biomaterial, its limitations in tissue engineering need to be addressed and overcome with new solutions. PCL alone as a tissue engineering scaffold exhibits slow degradation rate, low cell attachment, and poorly matched mechanical properties. Blending PCL with other materials is believed to be a remediation to overcome these challenges [56].
In a long-term study in a rat model, de Valence et al. implanted PCL vascular grafts into infrarenal abdominal aorta for up to 18 months [57]. Over the time course of the study, the complia nce of the PCL grafts was hypothesized to improve due to degradation and remodeling. While the grafts did experience significant degradation, especially after 6 months, little change in compliance was found. The lumen of the grafts was rapidly covered in ECs and a small amount of intimal hyperplasia developed after 1.5 months and remained stable through 18 months. However, by 12 months VSMCs began to regress and the intimal hyperplasia was primarily composed of collagen layers. Furthermore, calcification was noted to begin at 1.5 months and by 12 to 18 months extensive calcification was noted with the presence of compact bone and osteocytes. Within the graft wall, macrophages infiltrated for the first 6 months then retreated. A similar process was noted with vascularization of the grafts with capillaries forming over the course of the first 6 months and then regressing at 12 to 18 months. At 18 months, the interior of the graft wall was observed to be devoid of cells and only contained collagen, which could explain the lack of change in compliance. It was noted that this could be due to the lack of stimulating cues to direct the remodeling of the graft wall. Despite these issues, all grafts remained patent over the course of the study.
3.1.5. Poly(L-lactide-co-ε-caprolactone)
Poly(L-lactide-co-ε-caprolactone) (PLCL), a co-polymer of PLA and PCL, has been investigated for its potential use in constructing TEVGs. PLCL, an amorphous polymer with the PCL disrupting the more crystalline nature of PLA, exhibits better mechanical properties than PLA. Similar to PLGA, the properties of PLCL can be tailored by adjust the ratio of the co-polymers. Increasing the caprolactone content increases the elastomeric properties of the polymer with a 70:30 PLCL co-polymer having a Young’s modulus of 12 MPa, significantly softer than PLA and comparable to the stiffness of native vessels [58,59]. However, PLCL co-polymers do have relatively low glass transition temperatures, making several blends metastable at temperatures ranging from room temperature to body temperature and may undergo drastic change in mechanical properties. The 70:30 PLCL co-polymer has been shown to undergo significant changes during storage at room temperature in as little as 3 weeks with its elastic modulus increasing from 12 to 128 MPa [59]. Despite this, PLCL nanofibers have been shown to support VSMC growth up to 3 months with the nanofibers having a half-life of 110 days [51].
In one study, Mun et al. [60] developed an electrospun vascular graft using 50:50 PLCL. 2D mats of electrospun PLCL were seeded on both sides with New Zealand white rabbit VSMCs and then rolled on 4 mm diameter mandrels using fibrin to glue the layers together. The grafts were then cultured under static or dynamic conditions. For the dynamic conditions, a peristaltic pump was used with gradually increasing pressure over the first week after which the pump head was switched to a pulsative one for the second week to better mimic in vivo conditions. Pressures were gradually increased such that over the last 4 days of culture, the graft was subject to systolic/diastolic pressures of 120/80 mmHg. After 2 weeks, dynamically cultured grafts had significantly higher burst pressure and collagen content compared to the statically cultured grafts indicating significant remodeling of the graft was occurring. A confluent layer of VSMCs was observed on the outer surface and were present on all layers of the dynamically cultured graft compared to just a few on the statically cultured one.
Horakova et al. [61] created a tubular graft from 70:30 PLCL. In vitro studies showed that PLCL scaffolds better supported viability and proliferation of 3 T3 mouse fibroblasts and endothelial cells after 14 days in static culture compared to PCL scaffolds. This was attributed to the more hydrophilic PLCL allowing for better cell adhesion. Long-term patency studies were done in New Zealand white rabbits using an end-to-side coronary bypass procedure. Full cell penetration was observed at 10 weeks and after 6 months a confluent endothelium and layers of VSMCs were found. After 10 weeks, however, 4 of 5 grafts remained patent, while after 6 months only 1 of 5 grafts maintained full patency. These were attributed to thrombotic events (both at 10 weeks and 6 months) and the loss of mechanical properties from degradation of the grafts (at 6 months). It was noted that the long-term mechanical properties could be improved with a thicker graft as the graft wall was only 100 μm thick compared to 200–300 μm for the coronary artery wall.
3.1.6. Polyurethanes
Polyurethanes (PUs) are a diverse group of polymers with a wide variety of mechanical properties, good biocompatibility, and anti-thrombogenic properties. PUs are thermoplastics composed of hard and soft segments. Under strain, the soft segments will deform and elongate while the hard segments stabilize the structure and ultimately allow it to return to its original shape. Electrospun PU has been shown to closely match the circumferential tensile strength of human bloods vessels and a nonlinear stress-strain curve or J-shaped response mimicked those seen in natural vessels that was maintained after cyclic loading. Specifically, the toe region (a region of low slope prior to the linear region) that corresponds to the unfolding of elastin fibers was reproduced by collecting PUs on a mandrel with a wavy cross section [62]. Adjusting the length and type of the hard and soft segments allows PUs to achieve unique and desirable properties. By utilizing polyesters such as PLA, PGA, and PCL or polyethers as the soft segments, PUs can be made to be biodegradable. Typically, the hard segments of PUs are composed of diisocyanates, the most common being 2,4-toluene diisocyanate and 4,4′-methylenediphenyl diisocyanate and are toxic degradation products. To overcome this, non-toxic diisocyanates such as lysine-diisocyanate, hexmethylene diisocyanate, and 1,4-diisocyanatobutane have been used [44,63]. A potential issue with the use of PUs is biostability for those containing polyesters and polyethers as soft segments, which undergo hydrolysis and oxidation, thus reducing mechanical properties and can lead to graft failure [64]. The PUs Tecoflex and Pellethane have been shown to maintain their mechanical properties for 6 months after implantation into the abdominal aorta of Wistar rats [65]. Using next generation sequencing gene expression profiling, HUVECs cultured on electrospun Tecoflex scaffolds showed similar gene expression to HUVECs cultured on decellularized human umbilical vein [66].
Several groups have created TEVGs from PUs. Bergmeister et al. [67] implanted Pellethane electrospun grafts into the infrarenal aorta of male Sprague-Dawley rats. Across all time points 95% of the graft remained patent. One-week post-implantation, CD34+ hematopoietic stem cells were observed on the luminal surface and after 1 month, the luminal surface was covered with endothelial cells. After 3 months, actin+ myofibroblasts and vimentin+ fibroblasts were observed throughout the graft wall while desmin+ SMCs were only found in the outer third of the graft wall. However, at 6 months, the population of actin+, vimentin+, and desmin+ cell decreased compared to 3 months and the portion of cell-free voids also increased. This was attributed to a mix of cell death and lack of cell penetration and was hypothesized that larger voids could improve this. Another study compared HUVEC attachment and proliferation on electrospun PU grafts to PTFE grafts and found HUVECs had better attachment and proliferation on the electrospun PU grafts. Furthermore, the grafts had good tensile strength (5.85 ± 0.62 MPa), flexibility (elongation 294.5 ± 19.4%), and porosity (50–60%) [68].
One newer PU that has shown promise for vascular tissue engineering is degradable polar hydrophobic ionic PU (D-PHI). The hydrophobic, ionic, and polar regions of D-PHI can be selected to optimized physical properties and while maintaining good cytocompatibility and promoting cell adhesion [69]. D-PHI has been shown to have only slight effect on blood clotting over negative control albumin-coated substrates, with slightly higher whole blood clotting and platelet activation. No activation of the intrinsic blood clotting pathway was observed while there was mild activation of the extrinsic pathway, which may play a beneficial role in the wound healing response rather than excessive blood clotting [70]. Chan et al. [71] developed an electrospun scaffold containing D-PHI. To achieve the proper viscosity for electrospinning, D-PHI was mixed with a polycarbonate PU (PCNU) and UV crosslinked in midair before being collected on a rotating mandrel. The D-PHI/PCNU scaffolds were more hydrophilic and significantly softer than PCNU films. Scaffolds implanted into Wistar rats showed slow degradation over 90 days, maintaining their thickness while also having good integration with the surrounding tissue. VSCMs showed good viability and proliferation on the scaffolds and continued to express α-smooth muscle actin (α-SMA) after 7 days of culture.
3.2. Natural polymers
3.2.1. Collagen
Collagen is the most abundant mammalian protein and is an excellent biomaterial for tissue engineering applications. Collagen proteins have four structural hierarchies and at least 28 different types of collagen exist in vertebrate tissues varying in the length of the molecule and the number and characteristics of the carbohydrates attached on the helix, but they all have triple helix structure in common [72]. There are six collagens found in the vascular wall with collagen types I and III being the most prevalent, constituting a major portion of ECM, and types IV, VI, XV, and XVIII found in basement membranes [73]. Having similar chemical compositions as the native tissue, collagen scaffolds aid in cell attachment and migration, therefore they can interact and be integrated with the local tissues over time [74]. They also have an excellent biodegradability and can be degraded by the enzymes within human body, particularly by matrix metalloproteases (MMPs) [72]. Regardless of these merits of collagen, the major limitations prevent it from being wildly used for the biomedical applications are such as its poor mechanical properties and rapid degradation rate [74]. A promising solution is to blend collagen with other materials such as proteoglycans and glycosaminoglycans (GAGs) to reduce the degradation rate, and to improve the mechanical behavior such as elasticity and fracture energy. Gelatin, a derivative of collagen, is commonly used in polymer blends for electrospinning to improve the biocompatibility of synthetic polymers, though it must be crosslinked to prevent rapid degradation [75].
3.2.2. Silk fibroin
Silk is renowned for its strength and elasticity, often exceeding those of synthetic polymers, making it of particular interest in the field of tissue engineering [76]. The most extensively used silk in tissue engineering comes from the domesticated silkworm Bombyx mori. Raw silk cocoons are composed of two proteins: the adhesive sericin and silk fibroin (SF). SF is extracted through a multistep purification process involving degumming to remove sericin, dissolution, and dialysis to remove impurities [77]. SF is composed of a light chain and heavy chain polypeptide linked together by a disulfide bridge. The heavy chain polypeptide consists of hydrophilic and hydrophobic domains. The hydrophobic domain form crystalline β-sheets while the hydrophilic regions form interspersed semi-amorphous regions. The crystalline β-sheets give SF its strength and stiffness while the semi-amorphous region lend SF its extensibility and toughness. However, regenerated silk fibroin does lose a great deal of its mechanical properties due to the degumming and dissolution processes [78]. Similar to synthetic co-polymers and PUs, the mechanical properties and degradation of SF is tunable, particularly by modulating the formation of β-sheets via heating or solvent exposure. β-sheet formation essentially renders SF immune to degradation in water or biological salt solutions and SF mainly degrades by surface erosion through the action of proteases and immune cells. Increased β-sheet content slows degradation as most proteases act outside of β-sheets [79]. Like all implantable polymers, SF does elicit an immune response. The formation of fibrosis tissue has been noted early after implantation and decreases with time and unlike synthetic polymers does not result in fibrotic encapsulation [79]. The thrombogenic properties of SF are disputed. Studies of silk sutures and vascular grafts from the 1980s demonstrated the thrombogenic potential of SF [80,81]. However, like mechanical and degradation properties, the processing parameters of SF have been shown to influence its thrombogenicity, with no single parameter successfully predicting blood compatibility [82].
Electrospun TEVG were produced by Marelli et al. [83] using SF dissolved in formic acid. After electrospinning, the grafts were treated with methanol which enhanced β-sheet formation and the crystallinity of SF. The compliance at physiological pressures was 3.51 ± 0.42% radial deformation per mmHg × 10−2, slightly lower than the compliance of the saphenous and umbilical veins. The SF graft was able to withstand pressures up to four times physiological levels (575 ± 17 mmHg), but much lower than the coronary artery (5000 mmHg) and saphenous vein (2250 mmHg). Despite the somewhat lackluster mechanical properties, the SF tubes showed good cytocompatibility when cultured with 3 T3 fibroblasts. Importantly, 3 T3 fibroblasts were observed to pass through the graft wall after 5 days, indicating sufficient porosity to allow cell migration.
In a novel manufacturing approach, Alessandrino et al. [84] creating a three-layer SF scaffold called SilkGraft composed of an inner and outer layer of electrospun SF with a braided degummed SF yarn layer in between, which lent strength to the scaffold. A burst pressure of 2308 mmHg was close to that of the internal mammary artery (3196 ± 1264 mmHg) and the saphenous vein (1599 ± 877 mm Hg). The SilkGrafts were seeded with human endothelial cells on the luminal surface and with human VSMCs and adventitial fibroblasts on the outer surface. Cell adhesion on SilkGraft was two-fold better compared to polystyrene tissue culture plates and after 20 days normalized cell number was significantly higher for VSMCs and fibroblasts on SilkGraft. The SilkGraft did not induce biologically relevant compliment activation, nor did it exert hemolytic activity or alter red and white blood cell counts. Long-term in vivo pilot studies were performed in the carotid artery of minipigs and sheep. No thrombosis was observed after 4 weeks in either minipigs or sheep. SilkGrafts in minipigs did have slight neointimal hyperplasia and endothelium lined the lumen. No hyperplasia was observed in sheep and endothelialization was restricted to the ends of grafts which was attributed to the slower proliferation of endothelial cells in sheep. SilkGrafts in both animals elicited a foreign body response similar to other SF grafts.
3.2.3. Polysaccharides
Polysaccharide-based biomaterials have been investigated as a candidate for tissue engineering applications because their biomimicry, non-toxicity, biodegradability and ease of manufacture [85]. Common polysaccharides used in biomedical applications can be found in naturally occurring plants and animals, such as cellulose, amylose from plants; chitin from insects and crustaceans; and glycosaminoglycans from the mammalian connective tissues [85]. They are generally high molecular weight carbohydrates composed of repeating or alternating unites of long monosaccharide, linked by glycosidic linkages. Because polysaccharides have similar chemical composition to human tissue, therefore it is easier for cell attachment and integration with the replacing tissue. The biggest challenge of this type of biomaterial is weak mechanical strength, and an effective solution is to blend it with other natural polymers to increase the mechanical performance. Polysaccharide biomaterials are usually degraded by specific enzymes and the rate of degradation depends on the type and molecular weight of the material [86]. Recent research has found that cellulose-based scaffolds provide excellent mechanical strength for bone tissue engineering [87]. Sulfated-polysaccharide-based scaffolds shows great potential in orthopedic tissue engineering scaffolds credited with its high porosity, biomimicry, competent mechanical performance [88]. Li et al. demonstrated that oxidized pectin electrospun mats supported the differentiation of mesenchymal stem cells into ECs and VSMCs, with the softer 25% oxidized mats better supporting EC differentiation and the stiffer 50% oxidized mats supporting VSMC differentiation [89].
Chitosan is a natural amino polysaccharide derived from chitin with a structure analogous to GAGs found in the ECM [90]. Chitosan is generally used in blends for vascular tissue engineering due to its lack of suitable mechanical properties. A particularly common blend is one with chitosan and collagen due to their ability to form a complex with one another and mimic the natural ECM [90–92]. Chen et al. demonstrated that ECs and VSMCs proliferated well on chitosan/collagen electrospun mats, particularly at chitosan concentrations of 20% and 50%. However, the addition of chitosan greatly reduced the ultimate tensile strength and elastic modulus of the mats [90]. PLA has been added, as a blend and co-electrospun, to improve the mechanical properties of the chitosan/collagen blend which has the added benefit of reducing the hydrophobic nature of PLA [91,92]. PCL has also been utilized to improve the mechanical properties of chitosan for vascular grafts while also using chitosan to deliver the anti-thrombotic drug heparin and vascular endothelial growth factor (VEGF) [93,94].
3.3. Polymer summary
A wide variety of synthetic and natural polymers have seen use in the development of an electrospun TEVG. A brief summary of the advantages and disadvantages of the discussed synthetic polymers is listed in Table 1. These polymers share similar disadvantage namely degradation/biostability (PGA, PLGA, PLCL, and PUs), hydrophobicity (PLA and PCL), and mismatched mechanical properties (PLA and PCL). Furthermore, all of these benefit from the addition of bioactive molecules to improve cell attachment and proliferation. On the other hand, the main issue with natural polymers is lack of mechanical properties making the use of unmodified natural polymers unlikely. PCL is by far the most commonly used polymer in vascular grafts and its main drawback of hydrophobicity can be overcome by blending with natural polymers or through the addition of bioactive molecules. However, PUs represent a more intriguing option for future research due to their unique mechanical properties that better mimic those of native vessels. In addition, the biostability issue of PUs can be overcome by using newer PUs such as PCNU.
Table 1.
Brief summary of the advantages and disadvantages of selected synthetic polymers.
| Polymer | Advantages | Disadvantages |
|---|---|---|
| Polylactic acid | Slow degradation | Hydrophobicity limits cell attachment without modification |
| Currently used in several biomedical applications | Brittle | |
| Polyglycolic acid | High strength and crystallinity Current use in biomedical applications such as sutures | Rapid degradation (within several weeks) severely limits potential use in TEVG without combining with other polymers |
| Poly(lactic-co-glycolic) acid | Tunable mechanical properties and degradation rates by adjusting lactide: glycolide ratio | Relatively fast degradation (up to 6 months) which can be sped up by sterilization and enzymatic degradation |
| Current use in biomedical applications including sutures and meshes | Undergoes bulk erosion which impacts mechanical properties | |
| Polycaprolactone | Slow degradation | Hydrophobic character limits cell attachment and proliferation |
| Great mechanical properties including strength and elasticity | Mismatched mechanical properties poses a potential issue | |
| Poly(l-lactide-co-ε-caprolactone) | Tunable mechanical properties by adjusting co-polymer ratio Slow degradation Stiffness comparable to native vessels More hydrophilic than its co-polymers |
Several blends are metastable and can undergo drastic changes in mechanical properties In vivo studies showed a loss of mechanical properties after 6 months which could be improved with thicker grafts |
| Polyurethanes | Tunable properties based on chosen soft and hard segments Capable of replicating the J-shaped response seen in the stress-strain curves of native vessels |
Potential biostability issues depending on PU used |
3.4. Polymer blends
Very few electrospun grafts are made using a single pure polymer without modification. Synthetic polymers generally have great, tunable mechanical properties including stiffness and elasticity but are typically hydrophobic and lack a suitable interface for cells to adhere to, spread, and proliferate on. On the other hand, though they possess great biocompatibility and cell affinity, natural polymers lack the mechanical strength needed for the dynamic environment found in arteries. Thus, most research today focuses on combinations of polymers, typically a mix of synthetic and natural, either mixed together in solution as a blend, co-electrospinning of polymers simultaneously, or in a multilayer fashion, which will be discussed in the following section. Here we discuss some promising polymer blends and co-electrospun polymers for use in the construction of TEVGs.
One common polymer blend used in the development TEVGs is an elastic PU mixed with a stiffer polymer to mimic the behavior of elastin and collagen, respectively. The goal of these blends is to mimic the nonlinear mechanical behavior of natural vessels; in particular the region of low strain referred to as the toe region or a J-shaped response. In the J-shaped response low strain produces low stress corresponding to the elastic response of elastin fibers. At higher strains, stress is greatly increased due to the stretching of collagen fibers. In a study using synthetic polymers, Montini-Ballarin et al. [95] developed electrospun TEVGs using segmented PU and PLA. PLA and the PU PHD were chosen to mimic the mechanical behavior of elastin and collagen, respectively, with the ultimate goal of creating a J-shaped mechanical response. Two different ratios of ratios of PLA/PHD were chosen: a 50:50 and a 90:10 blend. The PLA/PHD grafts had two layers, created by spinning different concentrations of polymer solution (20% wt/v for the inner layer and 15% wt/v for the outer layer) and resulted in a layer separation of 100–200 μm. This was a desired outcome as the J-shaped response was hypothesized to result from the response of the inner layer to low pressure ranges followed by support from the external layer after a certain level of deformation is achieved. Anisotropy between the axial and circumferential directions was found, similar to the behavior seen in natural vessels but to a lesser extent and a J-shaped mechanical response was noted at low strains (<10%). Young’s modulus, ultimate tensile strength, and ultimate tensile strain were in the range of those seen in the coronary, mammary, and radial arteries and the saphenous vein. A J-shaped response was more clearly seen in the mechanical behavior in response to pressure though the response was more rigid (lower compliance) than that seen in young vessels and more similar to older vessels. Burst pressures were less than native vessels but exceeded physiological pressures. Mi et al. [96] used another synthetic blend of PU and PCL. A 7:3 and 1:1 blend of PU/PCL had modulus and circumferential strength closest to that of native vessel, particularly at lower electrospinning volumes of 0.6 and 1.1 mL. The blends also showed good recovery from cyclic loads after initial loading due to hysteresis. Compliance values of 1.5 to 5.4% per mmHg × 10−2 were similar to that of vein but lower than that of arteries. In addition, the PU/PCL grafts showed sufficient suture retention strength and burst pressure. Live/Dead stain and MTS proliferation assay on HUVECs cultured on the 1.1 mL 1:1 PU/PCL showed high biocompatibility though proliferation on pure PCL was greater. In another study, Yu et al. [97] utilized the PU Tecoflex blended with the natural polymer SF at different ratios with a concentration of 12% wt/v in hexafluoroisopropanol (HFIP). PU/SF ratios of 2:1, 1:1, and 1:2 were used in the study. The 1:1 PU/SF scaffolds had the highest ultimate tensile stress and strain and the lowest modulus of the three ratios tested. The 2:1 and 1:2 blends had similar mechanical properties due to the greater amount of the stiff SF in the 1:2 blend and conglutination of fibers in the 2:1 blend which increased stiffness and limited extensibility despite the presence of more PU. All blends produced cyclic loading results similar to that of the coronary artery with the 1:1 PU/SF blend’s behavior falling in the middle of the upper and lower bounds for the coronary artery. The 1:1 blend was chosen for cell studies due its similar mechanical properties and decent suture retention strength and surface wettability. Live/Dead staining showed good EC viability after 14 days in static culture. When seeded at a higher density, ECs were able to completely cover the surface after 5 days and more cells were clearly present on the PU/SF blend graft compared to a PU graft indicating that SF greatly enhanced the biocompatibility of PU.
Fibrin is a natural polymer that forms the protein scaffolding of blood clots and plays a major role in the wound healing response and displays very favorable qualities for use in vascular tissue engineering including high biocompatibility, non-immunogenic, tunable biodegradability, and high affinity for biological surfaces [98,99]. However, fibrin alone lacks the mechanical strength needed for use in TEVGs [98]. To take advantage of the excellent biological properties of fibrin, Yang et al. [100] constructed a TEVG using a blend of electrospun fibrin and PU to provide mechanical support. PU was dissolved in a mix of tetrahydrofuran (THF) and dimethylformamide (DMF) and fibrin was dissolved in formic acid to prepare PU/fibrin blends of 5:95, 15:85, and 25:75. As the PU content of the graft increased so too did the mechanical properties and hydrophobicity, though the elastic modulus did decrease, and degradation rates also decreased. The suture retention strength and burst pressure of all three were sufficient for implantation and all three showed good hemocompatibility. However, at 25% PU in vitro proliferation of mesenchymal stromal cells was significantly reduced. Thus the 15:85 PU/fibrin graft was selected for animal studies in SD rats. At 3 months post-implantation the PU/fibrin grafts were visually indistinguishable from the native artery. The mechanical strength and function were similar to native artery and were better than a PU graft. Decent remodeling of the PU/fibrin graft took place in vivo with deposition of ECM proteins similar to the native artery and the formation of an endothelium and intima, all of which were greatly reduced on the PU graft. The 15:85 PU/fibrin graft proved to be viable candidate for a TEVG with the addition of fibrin greatly improving the biocompatibility of PU grafts. The same group also found that a 20:80 PCL/fibrin graft possessed good hemocompatibility and cytocompatibility as well good mechanical and degradation properties [101]. Nine months post-implantation in rats, the 20:80 PCL/fibrin grafts had undergone extensive remodeling and were virtually indistinguishable visually from the native tissue. The PCL/fibrin graft showed an improved response to vasoconstrictors compared to a PCL graft and similar response to vasodilators as the native artery. In addition, a nearly continuous endothelium was observed on the luminal surface and VSMC layer of comparable thickness to the native artery with similar expression of contractile phenotype proteins. Similarly, the deposition and arrangement of ECM proteins including collagen, elastin, and GAGs not significantly different from the native tissue.
For one of the few preclinical large animal studies, Ju et al. [102] fabricated a TEVG from a PCL/collagen type 1 blend. The graft was constructed as bilayer with a 5% w/v PCL/collagen blend for the inner layer and 18% PCL/collagen for the outer layer a 4.75 mm diameter mandrel. After electrospinning, the PCL/collagen grafts were crosslinked with 2.5% glutaraldehyde. The grafts were then seeded with ECs and VSMCs and preconditioned in pulsatile bioreactor for 7 days. The pre-seeded grafts remained patent after 6 months in ovine carotid artery while all 3 unseeded control grafts failed. The pre-seeded grafts underwent extensive remodeling and had a well-organized ECM of collagen, elastin, and GAGs as well as a full endothelial and VSMC layer. The seeded grafts had an improved contractility response compared to the unseeded grafts, though much lower than the native vessel. However, the mechanical properties of the pre-seeded grafts were much improved after 6 months and quite similar to the native artery.
4. Modified electrospinning techniques for vascular tissue engineering
4.1. Multilayered electrospun vascular grafts
Multilayered electrospun vascular grafts are a promising choice as can replicate the natural multilayered structure of native vessels. A multilayered design allows for the tailoring of various properties through the choice of polymer, electrospinning parameters, or addition of bioactive molecules to best suit the needs of the cell types found in each layer of the graft. Han et al. [103] made a three-layered vascular graft designed for spatio-temporal delivery of VEGF and platelet derived growth factor (PDGF) (Fig. 3). An inner layer composed of 75:25 PLCL was chosen for the quick release of VEGF to promote rapid endothelialization while a middle layer of 75:25 PLGA was selected for a slow release of PDGF for the formation of a VSMC layer. The outer layer of PCL was improved the mechanical strength of the graft, while also delaying the release of PDGF. The mechanical properties of the three-layer graft were considerably higher than that of the porcine coronary artery used for comparison (tensile strength 5.2 ± 0.7 MPa vs 2.6 MPa, Young’s modulus 35.9 ± 7.7 MPa vs 1 MPa, elongation at break 146.7 ± 0.6% vs 100%, respectively). However, burst pressure and suture retention strength were comparable to native vessels. Though not seen in their 8-week in vivo study in New Zealand white rabbits, the compliance mismatch could lead to intimal hyperplasia and restenosis.
Fig. 3.
Han F. et al. [103] made a three-layered graft composed of an outer PCL/gelatin layer, middle PLGA/gelatin + PDGF layer, and inner PLCL/gelatin + VEGF layer. The gelatin fibers on the inner layer support rapid EC attach and once degraded increased the porosity of the outer and middle layers allowing for VSMC ingrowth. Reproduced with permission of Elsevier.
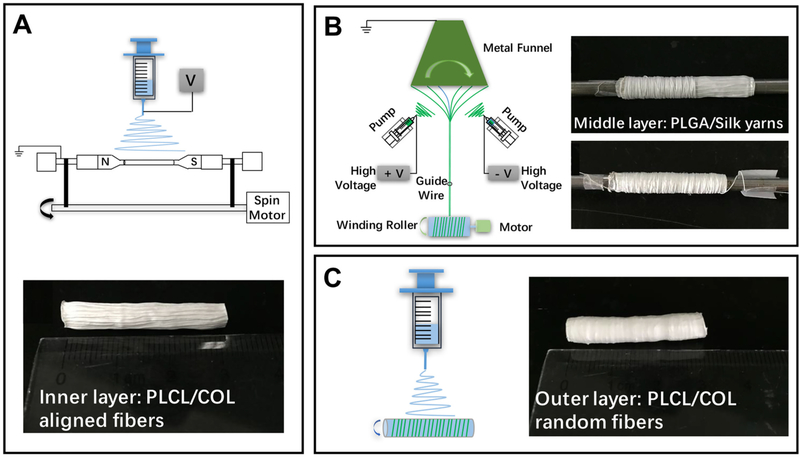
Wu et al. [104] fabricated a three-layer graft with the inner two layers tailored to ECs and VSMCs (Fig. 4). The inner layer was composed of circumferentially aligned PLCL/collagen nanofibers. HUVECs proliferated well on the aligned nanofibers but no difference was observed between the aligned and random fibers. The HUVECs were observed to orientate themselves along the nanofibers which may help promote the growth of an organized endothelium and functioning ECs. The middle layer was composed of a PLGA/SF electrospun yarn. PLGA/SF nanofibers were collected on a rotating funnel used to twist the fiber into yarn and collected on a rod. The yarn was wrapped around the aligned PLCL layer. VSMCs proliferated significantly better on the PLGA/SF yarns compared to PLGA/SF fibers due to its more porous three-dimensional structure and were found to orientate themselves along the yarn. This would help VSMC function in the media wall. Finally, an outer layer of random PLCL/collagen nanofibers was collected to keep the whole structure intact.
Fig. 4.
Schematic used by Wu T. et al. [104] to create a three-layered graft. (A) Aligned PLCL/collagen fibers were collected on a rotating mandrel. (B) A customized electrospinning set up was used to generated PLGA/SF yarns comprised the middle layer by twining on the inner layer. (C) A thin outer layer of random electrospun PCLC/collagen nanofibers was used to bind the construct together. Reproduced with permission of Elsevier.
In order to mimic the structure of a native artery, Wu et al. [105] created a three-layered graft from PLGA and crosslinked gelatin. The inner and outer layers were comprised of coaxial electrospun PLGA/gelatin, with a PLGA core for strength and gelatin shell. HUVECs proliferated well on the PLGA/gelatin fibers, nearly covering the surface after 5 days. The middle had electrospun PLGA and served to enhance the mechanical strength of the graft. The radial tensile strength of the graft was comparable to the human aorta but much higher than the coronary artery (5.13 MPa vs 5.49 vs 1.43 MPa, respectively). The axial tensile strength of the graft was also higher than the aorta or coronary artery (3.14 MPa vs 1.47 MPa vs 1.30 MPa, respectively). However, the three-layer graft did have sufficient suture retention strength.
Fiber orientation can also be used to delineate layers in a multilayered graft [106–108]. Elsayed et al. [106] orientated the syringe at 45° relative to the collector resulting in the collected gelatin nanofibers having a 45° orientation. The syringe was flipped every 4 min resulting in alternating layers with orientations of ±45° for a total of 16 layers after which the gelatin was crosslinked. While the reported mechanical properties were lower than that of the coronary artery, they were similar to those of the tunica media, the layer this method attempts to replicate. Considering the additional 45° layers as well as axially orientated layers for the tunica intima are intended to be added, the final version of this graft could have sufficient mechanical properties. The alternating 45° orientation of the layers provided a highly porous structure for human umbilical vein smooth muscle cell migration (HUVSMC), allow them to migrate up to 240 μm into the scaffold after 9 days. The more porous structure also allowed increased HUVSMC proliferation.
4.2. Fiber morphology and alignment
Electrospun fiber morphology and mechanics play an important role in cell growth and proliferation. Generally, bead-free fibers without conglutination are desired in electrospinning applications. Fiber diameter in particular is known to have a strong influence on cell behavior. 3T3 fibroblasts have been shown to exhibit increased cell area and aspect ratio in response to larger diameter fibers while no effect on proliferation was observed [109]. In one study, Han D. et al. [110] reported the effect of increasing fiber diameter on VSMC proliferation, infiltration, and phenotype. PCL fibers with diameters ranging from 0.5 to 10 μm generated by varying PCL concentration, solvent mix, and voltage. While increasing fiber diameter had no effect on VSMC survival over the course of 10 days, slower proliferation was found with increasing diameter. In contrast, infiltration of VSMCs into the PCL scaffold was noted to improve with increasing fiber diameter, associated with the increased pore size found with the larger diameter fibers. Somewhat quixotically, the ratio of VSMCs with a synthetic phenotype to total VSMCs was observed to increase with larger fiber diameters after 10 days of culture. This same trend was also observed in in vivo studies 7, 14, and 28 days after implantation into mice. These results are intriguing as the synthetic phenotype is associated with VSMC proliferation and may have been due to the greater infiltration of VSMCs in the larger fiber diameter scaffolds. Furthermore, the number of activated macrophages was also observed to increase on scaffolds with larger fiber diameters, which is important for the remodeling of the scaffold. In another study, Shen et al. [111] analyzed the role of fiber diameter on cells ability to resist shear stress. Using electrospun zein, the primary protein found in corn, aligned fibers with diameters of 112 ± 31 nm, 513 ± 80 nm, and 959 ± 147 nm were generated. While no difference in endothelial cell (EA.hy926 cells) retention was observed on randomly orientated fibers of different diameter under 15 dynes cm−2 shear stress after 4 h, aligned medium diameter fibers had significantly higher cell retention at 1, 2, and 4 h under both parallel and perpendicular flow compared to the small and large diameter fibers (the small diameter fibers also had significantly higher retention compared to the large diameter fibers). When allowed to adhere on fibers for 2 days, EA.hy926 cells on medium diameter fibers resisted shear stress under both parallel and perpendicular flow and under parallel flow elongated in the direction of flow. Cells on the large diameter fibers, which were initially aligned along the fibers, changed to a more rounded morphology and eventually washed away under both parallel and perpendicular shear stress.
Fiber morphology can also alter the mechanic of electrospun fibers. Within tissues, collagen fibers have a crimped morphology that contributes to their elasticity. Chao et al. [112] recreated this crimped morphology in PLA nanofibers. It was found that by heating PLA at 85 °C for 15 min, PLA fibers developed a stable crimped morphology hypothesized to be due to changes in its crystallinity, going from an amorphous to semi-crystalline state. A similar result was achieved by soaking PLA nanofibers in 95% ethanol at 37 °C for two days and this method could also be applied to PCL and PLGA nanofibers to achieve crimped fibers. There was a decrease in elastic modulus for heat-treated (362.5 ± 110.8 MPa) and ethanol-soaked (441.6 ± 46.1 MPa) compared to as spun fibers (478.3 ± 77.8 MPa), though not significantly different. In addition, a significant increase in transition state strain was observed. Importantly, the stress-strain curve showed a J-shaped response that is characteristic of native vessels. This behavior was associated with a decrease in “crimpness” at higher strains. Crimpness of the fibers was also maintained after dynamic loading. ACL fibroblasts aligned themselves with the crimped fibers taking on an undulating morphology and had significantly increased collagen type 1 expression which was further increased under dynamic loading conditions.
Nanofiber alignment has also been identified as a method to direct the behavior of cells on electrospun scaffolds. Within a normal blood vessel different cell types align in different ways, forming a key aspect of their function. ECs are aligned longitudinally with the direction of blood flow while VSMCs are aligned circumferentially and thus able to contract the vessel. When electrospinning tubular scaffolds, circumferentially aligned fibers can be obtained by rotating the mandrel at a sufficient speed. The rotational speed depends on the exact set up as the surface velocity of the mandrel must exceed the velocity of the fibers being deposited. He et al. [68] found that a 4 mm diameter mandrel rotated at 1000 rpm resulted in aligned nanofibers. At higher and lower rotational speeds, random fiber orientation was observed. As this is set up dependent, either mandrel diameter and rotation speed or linear velocity of the mandrel should be reported. Fusaro et al. [113] found that EC proliferation and function was enhanced on circumferentially aligned nanofibers. Longitudinally aligned fibers can be obtained by using two parallel plates as a collector. The deposited fibers will bridge the gap between the plates, orientated perpendicular to the plates and aligned parallel to each other. The fibers can then be collected by rolling a mandrel over then such that they are orientated along the length of the mandrel (Fig. 5) [107,108]. Tan et al. [107] showed that the longitudinally aligned PLC nanofibers promoted HUVEC adhesion and proliferation over randomly aligned PCL fibers.
Fig. 5.
(A) Electrospinning schematic used by Tan et al. [107] (B) Electrospun PCL nanofibers were collected between two parallel rods and (C, D) collected on a mandrel resulting in longitudinally aligned fibers. (E) PCL and PEG were co-electrospun to create the outer layer and (F) finished scaffold. Reproduced with permission of Elsevier.
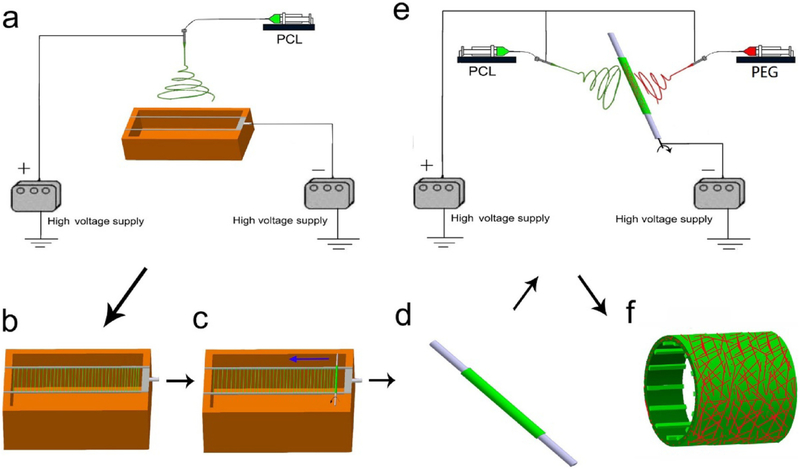
One method for altering the mechanical behavior of an electrospun is to alter the shape of the mandrel. Of particular interest is grafts with a wavy cross section that allow circumferential expansion of the graft to better replicate the nonlinear mechanical behavior of native vessels. In the simplest form a mandrel with a wavy cross section has been utilized with fibers PU fibers being collected directly on the mandrel forming a dense electrospun wall. The wavy shape was maintained after removal from the mandrel through subsequent cyclic loading (100 cycles). The stress-strain curve displayed nonlinear behavior reminiscent of native vessels [62]. In a novel approach, Yu et al. and Mi et al. [96,97] both used a round mandrel surrounded by thin (0.8 mm diameter) rods (Fig. 6). At sufficient rotational speed the thin rods flexed in a manner analogous to a jump rope. As nanofibers were collected on the satellite rods, they would become constrained and shrink back against the mandrel resulting in a wavy structure. As an added benefit, the satellite rods facilitated the removal of the graft from the mandrel without damaging the graft. A flat, dense region was present in areas in contact with outermost area of the rods while a looser, wavy region was found in areas between the rods. The choice of polymer blend and concentration aided in the formation of the wavy structure. Both used elastic PU due to its ability to mimic the behavior of elastin. In order to mimic the stiffer collagen Yu et al. used SF and Mi et al. used PCL. In both cases an intermediate blend of the elastic and stiff polymers best held the wavy shape with pure stiff polymer unable to contract the satellite rods and pure elastic polymer contracting to a round shape after removal of the mandrel.
Fig. 6.
Schematic used by Mi et al. [96]. The set up composed of a hollow metal mandrel surrounded by satellite rods. When rotating the metal rods deform and fibers are collected. As the fibers are deposited the rods contract with the fibers forming a wavy structure. Reproduced with permission of Elsevier.
4.3. Increasing porosity
One major limitation of electrospinning in tissue engineering is the inherently small pore size and porosity as electrospun scaffolds are essentially 2D mats. The lack of porosity hinders cellular infiltration of the grafts, which limits remodeling and integration of the graft into native tissue, as well as the exchange of nutrients and waste products. Porosity can be changed by adjusting the electrospinning parameters including voltage, flow rate, distance to collector, and rotational speed [114]. Thicker fibers are also associated with larger pore size and increased porosity. Wang et al. [115] obtained thicker PCL fibers by increasing PCL concentration while using a higher flow rate and reducing the voltage and collector distance. The thick fibers were 5.59 ± 0.67 μm in diameter compared to 0.69 ± 0.54 μm for the thinner fibers. This resulted in a nearly 8-fold increase in pore size and marked increase in porosity. The increased porosity influenced macrophage polarization and allowed for the remodeling of the grafts and the formation of a tunica media after 100 days in vivo. Using a novel electrospinning technique, Yin et al. [116] used a porous mandrel through which pressurized air was pumped. Termed air-impedance electrospinning, the pressurized air impeded the deposition of PCLC/SF nanofibers resulting in larger pore sizes. Interfiber distance was largest at 50 kPa and decreased with higher pressures. However, cell infiltration depth was still high at 200 and 300 kPa. This was hypothesized to be caused by increased fiber alignment at higher pressures, which resulted in VSMCs assuming a more spindle-like shape and thus could migrate through the smaller gaps between fibers.
Another common approach to increase scaffold porosity is to utilize sacrificial fibers. With this technique, a soluble polymer such as non-crosslinked gelatin [103], polyvinyl alcohol (PVA) [117], polyethylene glycol (PEG) [118], or PEO [119–122] is co-electrospun with a non-soluble polymer. In a three-layer graft, Han et al. [103] co-electrospun non-crosslinked gelatin with an inner PLCL layer, middle PLGA layer, and outer PCL layer. The loss of the gelatin nanofibers in the outer two layers would function to increase pore size and allow VSMC infiltration while the gelatin in the inner layer was primarily used to promote early EC adhesion on the lumen. Within several days the gelatin was completely degraded and in vivo results in New Zealand white rabbits showed VSMCs migrating into the middle layer and forming a thick layer. Tan et al. [117] co-electrospun PVA with a PCL/gelatin blend. After electrospinning, the PVA was removed by rinsing with phosphate buffer saline (PBS) for 24 h at 37 °C. The PVA containing grafts had an 8% increase in porosity without a detrimental decrease in mechanical properties compared to the PCL/gelatin only graft. The grafts were implanted subcutaneously in rats and after 8 weeks cells infiltrated much deeper (about 300 μm) compared to the PCL/gelatin only grafts (about 100 μm). In a different method, Hodge and Quint [122] electrosprayed PEO nanoparticles while simultaneously electrospinning PGA, PCL, or PLGA. Electrospraying is a process similar to electrospinning except the voltage used is high enough to overcome the viscosity of the solution resulting in the formation of particles rather than fibers. After the grafts were constructed, PEO was removed by rinsing with a series of ethanol solutions of decreasing concentration for 1 h each. The PGA/PEO graft had similar mechanical properties but faster degradation compared to the PGA graft while the PLC/PEO and PLGA/PEO graft saw a decrease in mechanical properties, but similar degradation times compared to their respective single polymer grafts. The porous PEO grafts did allow for significantly increase infiltration and proliferation of human dermal fibroblasts after 3 and 7 days of culture.
Composite grafts utilizing electrospinning and another method of graft formation have also been used to generate porous grafts. In these grafts the electrospun layer typically provides increased mechanical support that the porous polymer structure is unable to provide. The simplest method to produce a porous scaffold is freeze drying a hydrogel which leaves behind a highly porous scaffold. In an early study, Jeong et al. [123] created a porous jellyfish collagen layer by casting the hydrogel in a mold with a mandrel in the center followed by lyophilization and crosslinking. A layer of PLGA nanofiber was then collected on the outer surface via electrospinning to reinforce and improve the mechanical properties of the graft. Under pulsative perfusion, the grafts supported the survival and proliferation of VSMCs and ECs, which was enhanced compared to a static culture. In a more recent study, Norouzi and Shamloo [124] co-electrospun a PCL/gelatin inner layer around which a gelatin hydrogel was cast. After freeze drying and crosslinking with glutaraldehyde a porous outer layer was generated (Fig. 7). The graft displayed good mechanical properties due to the PCL nanofiber. VSMC proliferation was supported by the outer porous layer while inner electrospun PCL/gelatin layer successfully allowed adhesion and proliferation of VSMCs. Another method to create a porous layer is thermally induced phase separation (TIPS). During the TIPS process, hot polymer is poured in mold and rapidly cooled to −80 °C. The solvent crystals are then leached out from the scaffold and freeze-dried leaving behind a porous scaffold. Solenti et al. [125] used TIPS to create a porous poly(ester-urethane)urea (PEUU) inner layer enveloped by electrospun PEUU layer. The TIPS/electrospun grafts showed sufficient burst pressure and suture retention strength to withstand the arterial environment while the mechanical properties were similar to native vessel. Under dynamic culture conditions, the grafts supported muscle derived stem cells though decrease in cell number was found between day 0 and day 3, attributed to the high initial seeding density which saturated pore space and limited proliferation.
Fig. 7.
Schematic used by Norouzi and Shamloo [124]. A crosslinked gelatin hydrogel was cast around an inner electrospun PCL/gelatin layer. After freezing drying, the hydrogel formed a porous outer layer Reproduced with permission of Elsevier.
4.4. Reducing thrombogenicity by including an anticoagulant molecule
One of the main causes of vascular graft failure is thrombosis. This is attributed to the inherent thrombogenicity of the graft itself, causing failure shortly after implantation, or the failure to develop a confluent endothelium covering the lumen of the graft, which affects long-term patency. From a processing perspective, fiber diameter has been shown to influence the thrombogenicity of electrospun grafts with larger fibers (2–3 μm) inducing platelet adhesion and activation of the coagulation cascade [126]. A common method to address acute thrombosis and reduce the thrombogenicity of the TEVG is to include an anticoagulant molecule into the graft, either by direct mixing into the polymer or post hoc modification of the lumen surface. By far the most common used and highly effective drugs for this are heparins. Heparins are highly sulfated glycosaminoglycans that inhibit the action of thrombin and clotting factor FXa [127]. Commonly used methods for incorporating heparin into vascular grafts are immobilization [94,128–131], plasma treatment [130,132,133], and coaxial electrospinning [134,135]. Additionally, emulsion electrospinning has been utilized to incorporate heparin directly into the fibers of the graft. In emulsion electrospinning, two or more immiscible solutions (generally a water-soluble drug and a synthetic polymer) are mixed together with the aid of a surfactant resulting in microscopic droplets of the drug being dispersed throughout the polymer [136]. Emulsion electrospinning can take advantage of the drug release kinematics of different polymers to time release of heparin [124].
Immobilization of heparin involves directly linking heparin to the graft surface. Some polymers such as chitosan allow for heparin immobilization without the need for further processing. Yao et al. [94] showed that increasing the mass ratio of chitosan in a PCL/chitosan blend greatly increased the amount of heparin immobilized on the graft surface, improving the anticoagulant properties and reducing platelet adhesion while having no significant effect on the mechanical strength of the graft. In order to immobilize heparin onto the surface of synthetic polymers the surface must first be functionalized through the addition of amine groups. Several groups have successfully utilized modified PEG as a linker, using PEG bis(amine) to functionalize ester and urethane linkages in PLA and PU, respectively, and isocyanate linked PEG on PU, which convert to amine groups in the presence of water [129,131]. Heparin can then be bound to the amine group with the use of activators such as 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC) and hydroxysulfosuccinimide (NHS). In a recent study, Kuang et al. [137] immobilized heparin onto mesoporous silica nanoparticles (MSN) using PEG as the linker. The MSN-PEG-heparin nanoparticles were then dispersed into a PLGA/collagen solution for electrospinning. With the MSN-PEG-heparin nanoparticles dispersed throughout the fibers, the nanoparticles would be exposed gradually as the fibers degraded, increasing the duration of heparin’s effect as opposed to directly grafting to the surface. In vivo studies demonstrated the nanoparticle containing grafts remained patent up to 8 weeks.
Another common method surface modification of a vascular graft is plasma treatment. Plasma treatment uses inert gases such as argon to create radicals on the graft surface allowing heparin to bind to the surface [132]. Other gases can be used to graft functional groups onto the graft surface. Li et al. [133] used NH3 plasma treatment to graft amine groups to polycarbonate polyurethane (PCU) and subsequently graft heparin to the surface. In a comparison of the efficacy of heparin grafting techniques, Qui et al. [130] used two immobilization techniques: aminolysis with a PEG amine linker and a polydopamine (PDA) coating for the absorption of heparin; and compared them to an allylamine plasma treatment on a PCU graft. The plasma treated grafts had a higher amine group density than either the aminolysis or PDA coated grafts and resulted in a higher heparin density. In addition, the heparin-plasma treated grafts also showed greater heparin activity after 7 days in vitro compared to the aminolysis and PDA coated grafts, sustaining the same level of activity seen on day 0.
Several groups have explored alternatives to heparin to improve the antithrombogenicity of electrospun vascular grafts. Liu et al. [138] generated sulfated SF by treating SF with chlorosulphonic acid. The sulfated SF was hypothesized to imitate the highly sulfated structure of heparin. The sulfated SF grafts had greatly improved anticoagulant behavior while also increasing the proliferation of ECs and VSMCs and maintaining higher expression of phenotypic markers. Eldurini et al. [139] constructed a novel electrospun mat using a PCL-wintergreen oil polymer solution. Wintergreen oil significantly improved the blood clotting tendency of PCL and the addition of a PEO coating further improved the anticoagulant effect. An increase in hemolysis seen with the wintergreen oil was reduced with the addition of heparin to the graft. Furthermore, the wintergreen oil had antioxidant activity over the course of 32 h, which could be a favorable behavior. However, the amount of wintergreen oil has to be carefully managed due to its potential toxicity and hemolytic activity of pure wintergreen oil. Conjugated linoleic acid (CLA) has also demonstrated potential as an antithrombosis agent. As a smaller molecule compared to heparin, CLA has the potential to be easier to graft to polymers due to its lower steric hinderance. Tran et al. [140] grafted CLA to a PU/PCL graft via plasma treatment. Despite not changing the hydrophobicity of the PU/PCL, CLA significantly increased clotting time and saw reductions in platelet adhesion and activation. Furthermore, CLA improved proliferation of fibroblasts and especially EC although there appeared to be an optimal density of CLA above which proliferation was consistently, but not significantly slowed. Salicin, a compound extracted from white willow Salix alba, has anticoagulant and anti-inflammatory properties. When incorporated in to an extruded ePTFE-SF graft, salicin significantly reduced platelet adhesion and increased clotting time, demonstrating its anticoagulant effect. In addition, the ePTFE-SF-salicin material had greatly improved HUVEC adhesion and proliferation compared to neat ePTFE [141].
4.5. Improving endothelialization by incorporating endothelial growth compounds
Although various compounds have demonstrated anticoagulant activity in vascular grafts, the key to long-term thrombosis prevention is regeneration of the endothelium. The endothelium lining of blood vessels plays a critical role in the function of the cardiovascular system, providing a smooth, antithrombogenic surface for blood flow and regulating vascular tone through the release of NO among other functions. Inducing rapid and successful endothelialization is an essential step to generating a viable TEVG. A number of proteins, small peptides, and various drugs have incorporated into vascular to promote endothelialization and we will highlight a few of them here.
One popular growth factor used to improve endothelialization is vascular endothelial growth factor (VEGF). VEGF binds to tyrosine kinase cell receptors and has an important role in vasculogenesis and angiogenesis and mainly targets ECs, promoting proliferation and migration among other things [142,143]. VEGF and heparin immobilized via a PDA coating have been shown to greatly improve the hydrophilicity of PU, attributed the abundant hydrophilic groups in the coatings. In combination, heparin and VEGF showed the greatest HUVEC adhesion, spreading, and proliferation, better than either alone [144]. Sevostyanova et al. [145] incorporated VEGF into a PCL tubular scaffold using emulsion electrospinning. The diameter of the PCL fibers decreased with the addition of VEGF, which could help improve cell adhesion. In Wistar rats, the VEGF/PCL improved patency after 1 and 3 months. The improved patency coincided with faster endothelialization and the appearance of a mature, functional endothelium after 6 months which was not apparent on the PCL grafts. Emechebe et al. [146] adhered VEGF to a PDA coated co-spun polydioxanone (PDO)/PCL + dipyridamole (DY) (PDO/PCL + DY) vascular graft. Fibrinogen absorption, which has been shown to increase platelet adhesion and activation, was significantly decreased with the addition of VEGF to the graft and more inactivated platelets were found adhered to the PDO/PCL + DY and PDO/PCL + DY+PDA + VEGF grafts. Increasing VEGF concentration increased HUVEC adhesion and proliferation over the course of 5 days, with 3350 ng mL−1 VEGF showing the highest proliferation and confocal microscopy showed nearly continuous EC coverage after 7 days. In a porcine model, the PDO/PCL + DY+PDA + VEGF grafts had the lowest thrombosis formation and had confluent ECs orientated with blood flow. However, patency and thrombosis formation were only monitored for two weeks so longer-term studies are needed to better evaluate the efficacy of the graft. Oxidative stress induced by cardiovascular diseases are known to induce EC and EPC apoptosis. Dai et al. [147] sought to limit oxidative stress-induced apoptosis and improve the efficacy of VEGF by incorporating PEG-coated cerium oxide nanoparticles (CNPs-PEG) into an electrospun PU scaffold. CNPs have antioxidant properties and the ability to decompose H2O2 into O2 and H2O. The PEG coating on the CNPs reduced their cytotoxicity and did not limit the growth of EPCs over 3 days in culture. The CNP-PEGs reduced H2O2-induced EPC apoptosis and maintained their antioxidant function after electrospinning. In vitro, the CNP-PEGs enhanced the effects of VEGF resulting increased numbers of migrating EPCs and promoting increased differentiation of EPCs with high mRNA and protein expression of several EC markers.
Stromal cell-derived factor (SDF) 1α is a CXC chemokine that binds to CXC chemokine receptor type-4 (CXCR-4). SDF1α is a chemoattractant that recruits hematopoietic stem cells (HSCs) and EPCs. EPCs circulate in the blood and have the capacity to regenerate the endothelium making them a promising source for in situ endothelialization [148,149]. In one study, CD34+ and CD117+ cells were recruited to vessel grafts with a fibronectin (FN)-SDF-1α coating at a significantly higher level compared to controls and resulted in nearly twice the endothelium coverage 3 months after implantation in sheep [150]. SDF1α experiences a strong electrostatic interaction with heparin and this can be used to immobilize SDF1α. In a recent study, a heparin-SDF1α coating was used in a TIPS vascular graft. In addition to the anticoagulant activity of the heparin, SDF1α enhanced the recruitment of EPCs after 24 h in vitro while also stimulating HUVEC migration proliferation. Heparin upregulated the expression of endothelial functional genes CD144 and endothelial nitric oxide synthase (eNOS), with the addition of SDF1α resulting in further upregulation of the genes [151]. Moreover, the technique used to generate the heparin-SDF1α could easily be applied to electrospun grafts. In a canine study, Guo et al. [152] transplanted VEGF/SDF1α PU electrospun grafts into the femoral arteries. The VEGF/SDF1α grafts had higher patency than the neat PU graft after 6 months (5/8 vs 0/8, respectively). Explanted VEGF/SDF1α grafts showed a continuous neointima composed of ECs while a neointima was absent on the neat PU grafts, making VEGF/SDF1α functionalized grafts a promising candidate for a TEVG.
One major challenge with the use of SDF1α is its short plasma half-life, measured at 25.8 ± 4.6 min, due to its cleavage by dipeptidyl peptidase-4 and MMPs [153,154]. To improve SDF-1α release dynamics, Lee et al. [155] created a charge-based self-assembled SDF1α coacervate with heparin and poly(ethylene argininylaspartate diglyceride) and coated a porous poly(glycerol sebacate) (PGS) scaffold with it. The SDF-1α coacervate limited the initial burst release and showed a sustained release over the course of 28 days under flow conditions. EPCs and mesenchymal progenitor cells (MPCs) were shown to migrate upwards through a fibrin gel toward the SDF-1α coacervate-laden scaffolds. When seeded on top of the SDF-1α coacervate scaffold, EPCs and MPCs both penetrated deeper into the scaffold and had higher proliferation at days 7 and 10 compared to control and free SDF-1α. This system is versatile, designed to load any heparin-binding growth factor and could be used on electrospun grafts. In a more recent study, Muylaert et al. [156] synthesized SDF1α-derived peptides containing the CXCR-4 binding domain with and without the MMP2 cleavage site to limit rapid proteolytic degradation. The SDF-1α-derived peptides were modified with ureidopyrimidinone (UPy) and mixed with chain-extended UPy-PLCL for electrospinning. Both the proteolytic resistant (UPy-SDF1α(R)) and non-resistant (UPy- SDF1α(NR)) peptides attracted and increased adhesion of peripheral blood mononuclear cells (PBMCs) and reduced expression of proinflammatory proteins. The latter effect was greater on the UPy-SDF1α(R), providing some evidence of increased longevity, though longer-term studies are needed. Likewise, in vivo results showed greater cellularity and penetration with both SDF1α-derived proteins and an increase in CD68+ macrophages compared to control.
The use of small peptides represents another method used to improve endothelialization. Generally, the small peptides consist of a few amino acids that correspond to cell attachment domains of ECM proteins and seek to improve EC adhesion. The Arg-Gly-Asp (RGD) sequence is present in many ECM proteins and a number of integrins recognize this domain making it an important recognition site for cell adhesion [157]. A number of studies have demonstrated the ability of RGD to promote endothelialization of vascular grafts [158–161]. RGD also has been shown have equal efficiency at improving primary patency and endothelialization as VEGF [162]. Zheng et al. [160] fabricated tubular RGD containing PCL grafts. To overcome the inherent difficulty in directly immobilizing RGD to PCL, a molecule containing RGD and naphthalene groups (Nap-FFGRGD) was used, which is capable of self-assembly on the PCL surface. The RGD surface greatly reduced platelet adhesion, reducing the risk of thrombosis. In New Zealand white rabbits, the RGD-PCL grafts maintained 100% patency after 4 weeks and showed greater endothelialization compared to PCL grafts (51.1 ± 6.4% vs 11.5 ± 3.2%, respectively). Kuwabara et al. [163] reported on a promising Cys-Ala-Gly (CAG) sequence derived from collagen type IV with a high affinity for ECs and a low affinity for VSMCs. Although the patency of CAG-PCL grafts in rats was not significantly different from PCL grafts, the CAG grafts did have a higher endothelialization ratio at 1, 2, and 6 weeks. Expression of eNOS was significantly higher in CAG grafts, indicative of more functional ECs. The function of CAG peptides can be improved with the addition of the antithrombogenic peptide LTFPRIVFVLG (ACH11). CAG and ACH11 have a synergistic effect, outperforming their single peptide counterparts in thrombogenicity, HUVEC proliferation and migration, and NO production. In New Zealand white rabbits, the CAG-AHC11 grafts remained patent and had much improved endothelialization after 6 weeks compared to neat PLCL scaffolds [164].
4.6. Modulation of the VSMC layer formation on TEVG by VSMC regulating compounds
The formation of a VSMC layer is critical to the overall functioning of a vascular graft. A mature VSMC layer helps to stabilize the endothelium, preventing EC apoptosis and promoting its long-term survival, without which the endothelium could degenerate [165,166]. However, the proliferation of VSMCs must be carefully managed to avoid neointima hyperplasia and stenosis, which reduces the patency of the vessel graft and can lead to graft failure. VSMCs maintain one of two phenotypes: a synthetic phenotype with the capacity for migration, proliferation, and ECM secretion and a quiescent contractile phenotype. Ideally, early migration and proliferation of VSMCs with the synthetic phenotype into the graft would give rise to predominantly contractile VSMCs with few synthetic VSMCs to maintain homeostasis [167]. Heparin has been noted as having inhibitory effects on VSMC proliferation in several papers [94,129,151,168,169]. This effect is similar to that seen in native vessels where the mature endothelium releases heparin-like substances that maintain VSMCs in a contractile state [167]. Furthermore, heparin has been shown to stimulate elastogenesis and elastic fiber formation from VSMCs on SF films while simultaneously inhibiting VSMC proliferation and may help promote vascular remodeling [168]. Elastin, one of the primary constituents of the vessel wall ECM, may help promote VSMC adhesion and function. Recombinant elastin-like polypeptide-4 (ELP4), which mimics the structure of tropoelastin, was crosslinked to the surface of aligned nanofiber PCNU and flat PCNU films. On the electrospun scaffolds, VSMCs exhibited high viability, alignment, and F-actin organization. Interestingly on flat films, ELP4 substantially improved viability and had cell alignment and F-actin organization comparable to the electrospun scaffolds. Moreover, ELP4 increased smooth muscle myosin heavy chain (SM-MHC) expression on electrospun scaffolds and displayed a marked increase on the flat films compared to tissue culture plates. Since proliferation was limited (i.e. cell number was maintained) on the electrospun and flat films compared tissue culture plats and in light of the increased SM-MHC expression, ELP4 may help to maintain VSMCs in a contractile phenotype [170]. Other ECM-like peptides may also help modulate VSMC behavior and formation of a medial layer. The peptide RGD, distributed in many ECM proteins, promoted the formation of VSMC layer in rabbits as identified by α-SMA and SM-MHC staining [160]. On the other hand, CAG, the peptide sequence found in collagen type-4 which forms the elastic lamina that separates the intima from the media, has been demonstrated to limit VSMC adhesion and has the potential to inhibit intimal hyperplasia [163,164]. Thus, careful consideration of the placement of ECM protein-like peptides in the design of an electrospun TEVG may be a viable option to form a functional VSMC layer.
Other peptides have been developed to deliver microRNAs (miRNAs) to VSMCs. Wen et al. [171] developed a Val-Ala-Pro-Gly (VAPG) peptide-modified trimethyl chitosan-g-PEG (TPV) for the targeted delivery of miRNA-145 and incorporated it into electrospun PLCL. miRNA-145 can regulate contractile VSMCs and avoid excessive proliferation and intimal hyperplasia. The TPV was able to release miRNA-145 over the course of 56 days, avoiding the usual quick degradation of miRNAs. TPV/miRNA-145 was able limit the proliferation of VSMCs for 56 days while maintaining contractile phenotype, evidenced by morphology and α-SMA expression.
The combination of the growth factors VEGF and PDGF have been identified to assist neovasculature formation and maturation [166]. As discussed in a previous section, Han et al. [103] devised a multilayered electrospun graft to generate a spatio-temporal delivery of VEGF and PDGF. PDGF alone stimulated the adhesion and proliferation of VSMCs but also restenosis in rabbits. However, with the quick release of VEGF and slow release of PDGF, VSMC proliferation was slowed and generated a VSMC layer 8 weeks after implantation and had the best results in comparison to grafts loaded with a single growth factor.
Rapamycin (RM) is an effective inhibitor of VSMC migration and proliferation and is used to prevent restenosis. As a hydrophobic drug it can be readily electrospun in a variety of synthetic polymers. Han J. et al. [174] electrospun RM in PU at different weight ratios and with different concentrations of RM or RM powder added to 5% PU. After an initial burst release, RM had a sustained release over the course of 77 days. At 10% RM/PU (w/w%) and above, VSMC proliferation was inhibited after 5 days. The 20% RM/PU from powder had a high encapsulation efficiency (>94%) and was still able to inhibit VSMC proliferation after 77 days of release. Yang et al. [172] incorporated RM into an electrospun PCL layer a top an inner decellularized rat aorta layer. After 12 weeks, the RM containing grafts were able to significantly reduce neointima hyperplasia without inhibiting endothelialization or M2 macrophage polarization. One issue with the use of RM is that the dosage must be carefully controlled to not prevent vascular remodeling as high RM concentrations can kill VSMCs. Another drug with VSMC modulation potential is the antithrombogenic DY. At concentrations of 5 and 10% in polyurethane urea, DY has been shown to limit VSMC proliferation and maintain cell number over 7 days in vitro [173].
5. Future work and conclusion
Despite the large amount of progress that has been made in recent years in developing an electrospun TEVG, several areas need to be addressed to achieve a suitable electrospun TEVG. A glaring issue in the development on TEVGs is a lack of consensus on targets for mechanical properties. Research groups have used a variety of native vessels for comparison including the coronary artery, internal mammary artery, and saphenous vein in addition to vessels from other animals such as porcine. Although the saphenous vein is the gold standard for autologous grafts, its usefulness as a comparison for TEVG is debatable owing to the different mechanical properties it possesses compared to arteries. In addition, the forces and hemodynamic environment in a bypass graft can be different from the native vessel, especially near anastomoses, meaning mechanical comparisons with native vessels may be somewhat limited. Computer modeling may be useful in this regard to develop better mechanical targets. Furthermore, there is also variation in the mechanical properties reported in the literature. Burst pressure and suture retention strength are critical to the successful application of a TEVG and thus are commonly reported. However, compliance, the circumferential deformation in response to pressure, is seldom reported explicitly despite compliance mismatch having been previously identified as primary cause failure of small-diameter vessel grafts. To an extent compliance can be inferred from modulus and elongation values but should be a reported value. Expanding upon the compliance mismatch issue, in a number of promising studies, the reported mechanical properties, particularly elastic modulus, greatly exceeds that of any native vessel, which may also pose an issue for translation to clinical use.
Not only is there a need to better match static mechanical properties, but also to better reproduce the mechanical behavior of native blood vessels, in particular the J-shaped response of the stress-strain curve. Some studies, particularly those involving PU blends, have focused on developing an electrospun TEVG with a J-shaped response with success. This is due to the ability of PU to mimic the elastic behavior of elastin while a stiffer polymer can be used to mimic the behavior of collagen. However, beyond research involving PUs, this stress-strain behavior is seldom reported on and may be an issue for grafts using other polymers. It is also not known how well this behavior is maintained post-implantation and how this would impact the patency of the graft. Furthermore, in vivo remodeling of the vessel graft may also allow for a J-shaped response to develop. This also highlights a need to access how the mechanical properties and behavior of TEVGs change during the degradation and remodeling process in vivo. Most studies access patency, endothelialization, VSCM proliferation, and ECM deposition at the end of in vivo studies but few reassess how the mechanical properties changed. Remodeling, if successful, can greatly improve the mechanical properties of the grafts potentially to better match those of native vessels or if it fails result vastly different properties if calcifications form or neointimal hyperplasia occurs. If remodeling occurs quickly and successfully some of the aforementioned issues with differences in mechanical properties may be less impactful on the long-term patency of grafts though this would have to occur before other issues such as calcification and neointimal hyperplasia arise. Thus, assessing the changes to mechanical properties post in vivo should be an area of greater focus for future work.
One of the areas of greatest need is for more large animal studies. To date, only a few studies evaluating electrospun grafts have been conducted in porcine and ovine models with most animal testing having been conducted on rats and rabbits. However, it is known that small mammals usually have faster rates of endothelialization than humans which complicates translating results to humans and highlights the need for more large animal studies [102]. In addition, longer term (>12 months) studies need to be conducted to access the ability of the graft to sustain remodeling and prevent calcification. A major challenge to conducting these studies is the high cost involved. To better identify promising grafts for animal studies, grafts should be tested under dynamic conditions in vitro, such as in a pulsative bioreactor that is able to mimic physiological pressure and environment as a few studies have done. Furthermore, more work should also be done to assess the dynamic mechanical properties of the graft. Some studies have conducted cyclic loading tests to assess changes in elastic modulus. However, these are limited to comparatively few cycles and further testing would be needed to assess other properties such as fatigue failure and creep.
Another area that needs to be better assessed is the use of vascular grafts preseeded with cells. The general consensus for electrospun TEVG and most studies involving them has been to implant the grafts without preseeding of cells due to the additional time to culture cells as well as limited sources of cells. One potential solution for the sourcing of cells is to use stem cells and directing differentiation within the graft and deserves further research. Along with this, further research needs to be conducted into the formation of both the EC layer and the VSMC layer. Both layers are critical to the function of native vessels and work to maintain and regulate each other and some combination of bioactive components such as growth factors, drugs, and others is likely needed to address and guide their formation. Many studies focus on the formation of the endothelium and preventing intimal hyperplasia, both critical concerns for a TEVG. However, careful consideration also needs to be brought to the formation of the VSMC layer as it is needed to sustain the endothelium in the longer term. Thus, bioactive components should be carefully chosen to modulate the formation of the neomedia, avoiding neointima hyperplasia but not completely inhibiting VSMC proliferation as to allow the neomedia layer to form. Ultimately the best approach to developing a suitable electrospun TEVG will likely combine finding a combination of polymers or polymer blends with suitable mechanical behavior in a multilayer graft with a porous layer to allow for the infiltration of VSMCs and some combination of bioactive components to promote endothelialization, VSMC layer formation, and produce an antithrombogenic graft.
In summary, electrospinning shows great potential to develop a successful TEVG that meets all the needs of a synthetic graft, with number of approaches showing great promise. However, there is a clear need for more research to advance the field and develop a TEVG with similar performance to that of the gold standard autologous vessels. As a multidisciplinary field involving material science, mechanics, and biology, strong collaboration between different expertise is needed to fulfill its potential and develop a successful, long lasting electrospun TEVG to meet the needs of an aging population.
Acknowledgment
This work was supported, in part, by South Dakota Board of Regents IMAGEN: Imaging, Materials & Genetic Engineering: Biomaterials Research in South Dakota (D. E.) and NIH R15HL147214 (Z. H.).
Footnotes
Declaration of competing interest
The authors have no conflicts of interest to declare.
References
- [1].C.O.D.C. GBD, Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017, Lancet 392 (2018) 1736–1788, 10.1016/S0140-6736(18)32203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].D.A.I.C. GBD, Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019, Lancet 396 (2020) 1204–1222, 10.1016/S0140-6736(20)30925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Cheng S, Delling FN, Elkind MSV, Evenson KR, Ferguson JF, Gupta DK, Khan SS, Kissela BM, Knutson KL, Lee CD, Lewis TT, Liu J, Loop MS, Lutsey PL, Ma J, Mackey J, Martin SS, Matchar DB, Mussolino ME, Navaneethan SD, Perak AM, Roth GA, Samad Z, Satou GM, Schroeder EB, Shah SH, Shay CM, Stokes A, VanWagner LB, Wang NY, Tsao CW, H.A.C.O.E.A.P.S.C.A.S.S.S. American, Heart disease and stroke statistics-2021 update: A report from the American Heart Association, in: Circulation vol. 143, 2021, pp. e254–e743, 10.1161/CIR.0000000000000950. [DOI] [PubMed] [Google Scholar]
- [4].Morley RL, Sharma A, Horsch AD, Hinchliffe RJ, Peripheral artery disease, BMJ 360 (2018), j5842, 10.1136/bmj.j5842. [DOI] [PubMed] [Google Scholar]
- [5].T.A.S.C. S.C., Jaff MR, White CJ, Hiatt WR, Fowkes GR, Dormandy J, Razavi M, Reekers J, Norgren L, An update on methods for revascularization and expansion of the TASC lesion classification to include below-the-knee arteries: a supplement to the inter-society consensus for the management of Peripheral Arterial Disease (TASC II), Vasc. Med. 20 (2015) 465–478, 10.1177/1358863X15597877. [DOI] [PubMed] [Google Scholar]
- [6].Chew DK, Owens CD, Belkin M, Donaldson MC, Whittemore AD, Mannick JA, Conte MS, Bypass in the absence of ipsilateral greater saphenous vein: safety and superiority of the contralateral greater saphenous vein, J. Vasc. Surg. 35 (2002) 1085–1092, 10.1067/mva.2002.124628. [DOI] [PubMed] [Google Scholar]
- [7].Taylor LM, Edwards JM, Porter JM, Present status of reversed vein bypass grafting: five-year results of a modern series, J. Vasc. Surg. 11 (1990) 193–206, 10.1016/0741-5214(90)90262-9. [DOI] [PubMed] [Google Scholar]
- [8].Taylor LM, Edwards JM, Brant B, Phinney ES, Porter JM, Autogenous reversed vein bypass for lower extremity ischemia in patients with absent or inadequate greater saphenous vein, Am. J. Surg. 153 (1987) 505–510, 10.1016/0002-9610(87)90803-8. [DOI] [PubMed] [Google Scholar]
- [9].Goldman S, Zadina K, Moritz T, Ovitt T, Sethi G, Copeland JG, Thottapurathu L, Krasnicka B, Ellis N, Anderson RJ, Henderson W, V.A. C.S. G., Long-term patency of saphenous vein and left internal mammary artery grafts after coronary artery bypass surgery: results from a Department of Veterans Affairs Cooperative Study, J. Am. Coll. Cardiol. 44 (2004) 2149–2156, 10.1016/j.jacc.2004.08.064. [DOI] [PubMed] [Google Scholar]
- [10].Navaei M, Jannati M, Ronizi L, A comparative review of the outcomes of using arterial versus venous conduits in Coronary Artery Bypass Graft (CABG), Journal of Family Medicine and Primary Care 8 (2019) 2768, 10.4103/jfmpc.jfmpc_367_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Sarwar U, Chetty G, Sarkar P, The short saphenous vein: a viable alternative conduit for coronary artery bypass grafts harvested using a novel technical approach, J Surg Tech Case Rep 4 (2012) 61–63, 10.4103/2006-8808.100359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Tranbaugh RF, Dimitrova KR, Lucido DJ, Hoffman DM, Dincheva GR, Geller CM, Balaram SK, Ko W, Swistel DG, The second best arterial graft: a propensity analysis of the radial artery versus the free right internal thoracic artery to bypass the circumflex coronary artery, J. Thorac. Cardiovasc. Surg. 147 (2014) 133–140, 10.1016/j.jtcvs.2013.08.040. [DOI] [PubMed] [Google Scholar]
- [13].Englberger L, Noti J, Immer FF, Stalder M, Eckstein FS, Carrel TP, The Shelhigh No-React bovine internal mammary artery: a questionable alternative conduit in coronary bypass surgery, Eur. J. Cardiothorac. Surg. 33 (2008) 222–224, 10.1016/j.ejcts.2007.11.006. [DOI] [PubMed] [Google Scholar]
- [14].Leoce BM, Montoya M, Dardik H, Bernik TR, Rapid degradation and subsequent endovascular salvage of upper extremity cryogenic allograft bypass, Ann. Vasc. Surg. 57 (2019) 276.e5–276.e8, 10.1016/j.avsg.2018.10.052. [DOI] [PubMed] [Google Scholar]
- [15].Mitchell IM, Essop AR, Scott PJ, Martin PG, Gupta NK, Saunders NR, Nair RU, Williams GJ, Bovine internal mammary artery as a conduit for coronary revascularization: long-term results, Ann. Thorac. Surg. 55 (1993) 120–122, 10.1016/0003-4975(93)90485-z. [DOI] [PubMed] [Google Scholar]
- [16].Reilly B, Khan S, Dosluoglu H, Harris L, O’Brien-Irr M, Lukan J, Dryjski M, Blochle R, Comparison of autologous vein and bovine carotid artery graft as a bypass conduit in arterial trauma, Ann. Vasc. Surg. 61 (2019) 246–253, 10.1016/j.avsg.2019.05.017. [DOI] [PubMed] [Google Scholar]
- [17].Ercolani E, Del Gaudio C, Bianco A, Vascular tissue engineering of small-diameter blood vessels: reviewing the electrospinning approach, J. Tissue Eng. Regen. Med. 9 (2015) 861–888, 10.1002/term.1697. [DOI] [PubMed] [Google Scholar]
- [18].Tara S, Kurobe H, Maxfield MW, Rocco KA, Yi T, Naito Y, Breuer CK, Shinoka T, Evaluation of remodeling process in small-diameter cell-free tissue-engineered arterial graft, J. Vasc. Surg. 62 (2015) 734–743, 10.1016/j.jvs.2014.03.011. [DOI] [PubMed] [Google Scholar]
- [19].Teebken OE, Haverich A, Tissue engineering of small diameter vascular grafts, Eur. J. Vasc. Endovasc. Surg. 23 (2002) 475–485, 10.1053/ejvs.2002.1654. [DOI] [PubMed] [Google Scholar]
- [20].Pashneh-Tala S, MacNeil S, Claeyssens F, The tissue-engineered vascular graft-past, present, and future, Tissue Eng Part B Rev 22 (2016) 68–100, 10.1089/ten.teb.2015.0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Song HG, Rumma RT, Ozaki CK, Edelman ER, Chen CS, Vascular tissue engineering: progress, challenges, and clinical promise, Cell Stem Cell 22 (2018) 340–354, 10.1016/j.stem.2018.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Waller BF, Orr CM, Slack JD, Pinkerton CA, Van Tassel J, Peters T, Anatomy, histology, and pathology of coronary arteries: a review relevant to new interventional and imaging techniques–part I, Clin. Cardiol. 15 (1992) 451–457, 10.1002/clc.4960150613. [DOI] [PubMed] [Google Scholar]
- [23].Wang D, Wang Z, Zhang L, Wang Y, Roles of cells from the arterial vessel wall in atherosclerosis, Mediat. Inflamm. 2017 (2017), 8135934, 10.1155/2017/8135934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kelleher CM, McLean SE, Mecham RP, Vascular extracellular matrix and aortic development, Curr. Top. Dev. Biol. 62 (2004) 153–188, 10.1016/S0070-2153(04)62006-0. [DOI] [PubMed] [Google Scholar]
- [25].Milutinović A, Šuput D, Zorc-Pleskovič R, Pathogenesis of atherosclerosis in the tunica intima, media, and adventitia of coronary arteries: an updated review, Bosn J Basic Med Sci 20 (2020) 21–30, 10.17305/bjbms.2019.4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Gupta P, Lorentz KL, Haskett DG, Cunnane EM, Ramaswamy AK, Weinbaum JS, Vorp DA, Mandal BB, Bioresorbable silk grafts for small diameter vascular tissue engineering applications: in vitro and in vivo functional analysis, Acta Biomater. 105 (2020) 146–158, 10.1016/j.actbio.2020.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].L’Heureux N, Pâquet S, Labbé R, Germain L, Auger FA, A completely biological tissue-engineered human blood vessel, FASEB J. 12 (1998) 47–56, 10.1096/fasebj.12.1.47. [DOI] [PubMed] [Google Scholar]
- [28].Zhou X, Nowicki M, Sun H, Hann SY, Cui H, Esworthy T, Lee JD, Plesniak M, Zhang LG, 3D bioprinting-tunable small-diameter blood vessels with biomimetic biphasic cell layers, ACS Appl. Mater. Interfaces 12 (2020) 45904–45915, 10.1021/acsami.0c14871. [DOI] [PubMed] [Google Scholar]
- [29].Li N, Rickel AP, Sanyour HJ, Hong Z, Vessel graft fabricated by the on-site differentiation of human mesenchymal stem cells towards vascular cells on vascular extracellular matrix scaffold under mechanical stimulation in a rotary bioreactor, J. Mater. Chem. B 7 (2019) 2703–2713, 10.1039/c8tb03348j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Swinehart IT, Badylak SF, Extracellular matrix bioscaffolds in tissue remodeling and morphogenesis, Dev. Dyn. 245 (2016) 351–360, 10.1002/dvdy.24379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Boys CV, On the production, properties, and some suggested uses of the finest threads, Proc. Phys. Soc. Lond. 9 (1887) 8–19, 10.1088/1478-7814/9/1/303. [DOI] [Google Scholar]
- [32].Taylor GI, Disintegration of water drops in an electric field, Proc R Soc Lond A Math Phys Sci 280 (1964) 383–397, 10.1098/rspa.1964.0151. [DOI] [Google Scholar]
- [33].Taylor GI, The force exerted by an electric field on a long cylindrical conductor, Proc R Soc Lond A Math Phys Sci 291 (1966) 145–158, 10.1098/rspa.1966.0085. [DOI] [Google Scholar]
- [34].Taylor GI, Electrically driven jets, Proc R Soc Lond A Math Phys Sci 313 (1969) 453–475, 10.1098/rspa.1969.0205. [DOI] [Google Scholar]
- [35].Polymeric Nanofibers, American Chemical Society, Washington, DC, 2006. [Google Scholar]
- [36].Chomachayi MD, Solouk A, Mirzadeh H, Electrospun silk-based nanofibrous scaffolds: fiber diameter and oxygen transfer, Progress in Biomaterials 5 (2016) 71–80, 10.1007/s40204-016-0046-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Xue J, Wu T, Dai Y, Xia Y, Electrospinning and electrospun nanofibers: methods, materials, and applications, Chem. Rev. 119 (2019) 5298–5415, 10.1021/acs.chemrev.8b00593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Mehta R, Kumar V, Bhunia H, Upadhyay SN, Synthesis of poly(lactic acid): a review, J. Macromol. Sci. Polym. Rev. 45 (2005) 325–349, 10.1080/15321790500304148. [DOI] [Google Scholar]
- [39].Li G, Zhao M, Xu F, Yang B, Li X, Meng X, Teng L, Sun F, Li Y, Synthesis and biological application of polylactic acid, Molecules 25 (2020), 10.3390/molecules25215023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Casalini T, Rossi F, Castrovinci A, Perale G, A perspective on polylactic acid-based polymers use for nanoparticles synthesis and applications, Front Bioeng Biotechnol 7 (2019) 259, 10.3389/fbioe.2019.00259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Li H, Huneault MA, Effect of nucleation and plasticization on the crystallization of poly(lactic acid), Polymer 48 (2007) 6855–6866, 10.1016/j.polymer.2007.09.020. [DOI] [Google Scholar]
- [42].Farah S, Anderson DG, Langer R, Physical and mechanical properties of PLA, and their functions in widespread applications - a comprehensive review, Adv. Drug Deliv. Rev. 107 (2016) 367–392, 10.1016/j.addr.2016.06.012. [DOI] [PubMed] [Google Scholar]
- [43].Shalumon KT, Sreerekha PR, Sathish D, Tamura H, Nair SV, Chennazhi KP, Jayakumar R, Hierarchically designed electrospun tubular scaffolds for cardiovascular applications, J. Biomed. Nanotechnol. 7 (2011) 609–620, 10.1166/jbn.2011.1337. [DOI] [PubMed] [Google Scholar]
- [44].Nair LS, Laurencin CT, Biodegradable polymers as biomaterials, Prog. Polym. Sci. 32 (2007) 762–798, 10.1016/j.progpolymsci.2007.05.017. [DOI] [Google Scholar]
- [45].Chatterjee S, Comparative trial of Dexon (polyglycolic acid), collagen, and silk sutures in ophthalmic surgery, Br. J. Ophthalmol. 59 (1975) 736–740, 10.1136/bjo.59.12.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Bogan SL, Teoh GZ, Birchall MA, Tissue engineered airways: a prospects article, J. Cell. Biochem. 117 (2016) 1497–1505, 10.1002/jcb.25512. [DOI] [PubMed] [Google Scholar]
- [47].Hodge J, Quint C, Tissue engineered vessel from a biodegradable electrospun scaffold stimulated with mechanical stretch, Biomed. Mater. 15 (2020), 055006, 10.1088/1748-605X/ab8e98. [DOI] [PubMed] [Google Scholar]
- [48].Gilding DK, Reed AM, Biodegradable polymers for use in surgery—polyglycolic/poly(actic acid) homo- and copolymers: 1, Polymer 20 (1979) 1459–1464, 10.1016/0032-3861(79)90009-0. [DOI] [Google Scholar]
- [49].Miller RA, Brady JM, Cutright DE, Degradation rates of oral resorbable implants (polylactates and polyglycolates): rate modification with changes in PLA/PGA copolymer ratios, J. Biomed. Mater. Res. 11 (1977) 711–719, 10.1002/jbm.820110507. [DOI] [PubMed] [Google Scholar]
- [50].Wang N, Zheng W, Cheng S, Zhang W, Liu S, Jiang X, In vitro evaluation of essential mechanical properties and cell behaviors of a novel polylactic-co-glycolic acid (PLGA)-based tubular scaffold for small-diameter vascular tissue engineering, Polymers (Basel) 9 (2017), 10.3390/polym9080318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Dong Y, Yong T, Liao S, Chan CK, Stevens MM, Ramakrishna S, Distinctive degradation behaviors of electrospun polyglycolide, poly(DL-lactide-co-glycolide), and poly(L-lactide-co-epsilon-caprolactone) nanofibers cultured with/without porcine smooth muscle cells, Tissue Eng Part A 16 (2010) 283–298, 10.1089/ten.tea.2008.0537. [DOI] [PubMed] [Google Scholar]
- [52].Chor A, Gonçalves RP, Costa AM, Farina M, Ponche A, Sirelli L, Schrodj G, Gree S, Andrade LR, Anselme K, Dias ML, In vitro degradation of electrospun poly(lactic-co-glycolic acid) (PLGA) for oral mucosa regeneration, Polymers (Basel) 12 (2020), 10.3390/polym12081853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Labet M, Thielemans W, Synthesis of polycaprolactone: a review, Chem. Soc. Rev. 38 (2009) 3484–3504, 10.1039/b820162p. [DOI] [PubMed] [Google Scholar]
- [54].Díaz E, Sandonis I, Valle MB, In vitro degradation of poly(caprolactone)/nHA composites, J. Nanomater. (2014) 1–8, 10.1155/2014/802435,2014. [DOI] [Google Scholar]
- [55].Dwivedi R, Kumar S, Pandey R, Mahajan A, Nandana D, Katti DS, Mehrotra D, Polycaprolactone as biomaterial for bone scaffolds: review of literature, J Oral Biol Craniofac Res 10 (2020) 381–388, 10.1016/j.jobcr.2019.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Hajiali F, Tajbakhsh S, Shojaei A, Fabrication and properties of polycaprolactone composites containing calcium phosphate-based ceramics and bioactive glasses in bone tissue engineering: a review, Polym. Rev. 58 (2018) 164–207, 10.1080/15583724.2017.1332640. [DOI] [Google Scholar]
- [57].de Valence S, Tille JC, Mugnai D, Mrowczynski W, Gurny R, Möller M, Walpoth BH, Long term performance of polycaprolactone vascular grafts in a rat abdominal aorta replacement model, Biomaterials 33 (2012) 38–47, 10.1016/j.biomaterials.2011.09.024. [DOI] [PubMed] [Google Scholar]
- [58].Donovan DL, Schmidt SP, Townshend SP, Njus GO, Sharp WV, Material and structural characterization of human saphenous vein, J. Vasc. Surg. 12 (1990) 531–537. [PubMed] [Google Scholar]
- [59].Fernández J, Etxeberria A, Sarasua JR, Synthesis, structure and properties of poly(L-lactide-co-ε-caprolactone) statistical copolymers, J. Mech. Behav. Biomed. Mater. 9 (2012) 100–112, 10.1016/j.jmbbm.2012.01.003. [DOI] [PubMed] [Google Scholar]
- [60].Mun CH, Jung Y, Kim SH, Kim HC, Kim SH, Effects of pulsatile bioreactor culture on vascular smooth muscle cells seeded on electrospun poly (lactide-co-ε-caprolactone) scaffold, Artif. Organs 37 (2013) E168–E178, 10.1111/aor.12108. [DOI] [PubMed] [Google Scholar]
- [61].Horakova J, Mikes P, Lukas D, Saman A, Jencova V, Klapstova A, Svarcova T, Ackermann M, Novotny V, Kalab M, Lonsky V, Bartos M, Rampichova M, Litvinec A, Kubikova T, Tomasek P, Tonar Z, Electrospun vascular grafts fabricated from poly(L-lactide-co-ε-caprolactone) used as a bypass for the rabbit carotid artery, Biomed. Mater. 13 (2018), 065009, 10.1088/1748-605X/aade9d. [DOI] [PubMed] [Google Scholar]
- [62].Yan S, Napiwocki B, Xu Y, Zhang J, Zhang X, Wang X, Crone WC, Li Q, Turng LS, Wavy small-diameter vascular graft made of eggshell membrane and thermoplastic polyurethane, Mater. Sci. Eng. C Mater. Biol. Appl. 107 (2020), 110311, 10.1016/j.msec.2019.110311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Kucinska-Lipka J, Gubanska I, Janik H, Sienkiewicz M, Fabrication of polyurethane and polyurethane based composite fibres by the electrospinning technique for soft tissue engineering of cardiovascular system, Mater. Sci. Eng. C Mater. Biol. Appl. 46 (2015) 166–176, 10.1016/j.msec.2014.10.027. [DOI] [PubMed] [Google Scholar]
- [64].Kim S, Liu S, Smart and biostable polyurethanes for long-term implants, ACS Biomater Sci Eng 4 (2018) 1479–1490, 10.1021/acsbiomaterials.8b00301. [DOI] [PubMed] [Google Scholar]
- [65].Gostev AA, Shundrina IK, Pastukhov VI, Shutov AV, Chernonosova VS, Karpenko AA, Laktionov PP, In vivo stability of polyurethane-based electrospun vascular grafts in terms of chemistry and mechanics, Polymers 12 (2020) 845, 10.3390/polym12040845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Chernonosova VS, Laktionov PP, Murashov IS, Karpenko AA, Laktionov PP, Comparative gene expression profiling of human primary endotheliocytes cultivated on polyurethane-based electrospun 3D matrices and natural decellularized vein, Biomed. Mater. 15 (2020), 045012, 10.1088/1748-605X/ab7d84. [DOI] [PubMed] [Google Scholar]
- [67].Bergmeister H, Grasl C, Walter I, Plasenzotti R, Stoiber M, Schreiber C, Losert U, Weigel G, Schima H, Electrospun small-diameter polyurethane vascular grafts: ingrowth and differentiation of vascular-specific host cells, Artif. Organs 36 (2012) 54–61, 10.1111/j.1525-1594.2011.01297.x. [DOI] [PubMed] [Google Scholar]
- [68].He W, Hu Z, Xu A, Liu R, Yin H, Wang J, Wang S, The preparation and performance of a new polyurethane vascular prosthesis, Cell Biochem. Biophys. 66 (2013) 855–866, 10.1007/s12013-013-9528-5. [DOI] [PubMed] [Google Scholar]
- [69].Sharifpoor S, Labow RS, Santerre JP, Synthesis and characterization of degradable polar hydrophobic ionic polyurethane scaffolds for vascular tissue engineering applications, Biomacromolecules 10 (2009) 2729–2739, 10.1021/bm9004194. [DOI] [PubMed] [Google Scholar]
- [70].Brockman KS, Kizhakkedathu JN, Santerre JP, Hemocompatibility studies on a degradable polar hydrophobic ionic polyurethane (D-PHI), Acta Biomater. 48 (2017) 368–377, 10.1016/j.actbio.2016.11.005. [DOI] [PubMed] [Google Scholar]
- [71].Chan JP, Battiston KG, Santerre JP, Synthesis and characterization of electrospun nanofibrous tissue engineering scaffolds generated from in situ polymerization of ionomeric polyurethane composites, Acta Biomater. 96 (2019) 161–174, 10.1016/j.actbio.2019.06.046. [DOI] [PubMed] [Google Scholar]
- [72].Ricard-Blum S, The collagen family, Cold Spring Harb. Perspect. Biol. 3 (2011), a004978, 10.1101/cshperspect.a004978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Manon-Jensen T, Kjeld NG, Karsdal MA, Collagen-mediated hemostasis, J. Thromb. Haemost. 14 (2016) 438–448, 10.1111/jth.13249. [DOI] [PubMed] [Google Scholar]
- [74].Copes F, Pien N, Van Vlierberghe S, Boccafoschi F, Mantovani D, Collagen-based tissue engineering strategies for vascular medicine, Front Bioeng Biotechnol 7 (2019) 166, 10.3389/fbioe.2019.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Aldana AA, Abraham GA, Current advances in electrospun gelatin-based scaffolds for tissue engineering applications, Int. J. Pharm. 523 (2017) 441–453, 10.1016/j.ijpharm.2016.09.044. [DOI] [PubMed] [Google Scholar]
- [76].Omenetto FG, Kaplan DL, New opportunities for an ancient material, Science 329 (2010) 528–531, 10.1126/science.1188936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Rockwood DN, Preda RC, Yücel T, Wang X, Lovett ML, Kaplan DL, Materials fabrication from Bombyx mori silk fibroin, Nat. Protoc. 6 (2011) 1612–1631, 10.1038/nprot.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Koh L-D, Cheng Y, Teng C-P, Khin Y-W, Loh X-J, Tee S-Y, Low M, Ye E, Yu H-D, Zhang Y-W, Han M-Y, Structures, mechanical properties and applications of silk fibroin materials, Prog. Polym. Sci. 46 (2015) 86–110, 10.1016/j.progpolymsci.2015.02.001. [DOI] [Google Scholar]
- [79].Thurber AE, Omenetto FG, Kaplan DL, In vivo bioresponses to silk proteins, Biomaterials 71 (2015) 145–157, 10.1016/j.biomaterials.2015.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Dahlke H, Dociu N, Thurau K, Thrombogenicity of different suture materials as revealed by scanning electron microscopy, J. Biomed. Mater. Res. 14 (1980) 251–268, 10.1002/jbm.820140307. [DOI] [PubMed] [Google Scholar]
- [81].Christenson JT, Eklof B, Al-Huneidi W, Owunwanne A, Elastic and thrombogenic properties for different vascular grafts and its influence on graft patency, Int. Angiol. 6 (1987) 81–87. [PubMed] [Google Scholar]
- [82].Seib FP, Maitz MF, Hu X, Werner C, Kaplan DL, Impact of processing parameters on the haemocompatibility of Bombyx mori silk films, Biomaterials 33 (2012) 1017–1023, 10.1016/j.biomaterials.2011.10.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Marelli B, Alessandrino A, Farè S, Freddi G, Mantovani D, Tanzi MC, Compliant electrospun silk fibroin tubes for small vessel bypass grafting, Acta Biomater. 6 (2010) 4019–4026, 10.1016/j.actbio.2010.05.008. [DOI] [PubMed] [Google Scholar]
- [84].Alessandrino A, Chiarini A, Biagiotti M, Dal Prà I, Bassani GA, Vincoli V, Settembrini P, Pierimarchi P, Freddi G, Armato U, Three-layered silk fibroin tubular scaffold for the repair and regeneration of small caliber blood vessels: from design to in vivo pilot tests, Front Bioeng Biotechnol 7 (2019) 356, 10.3389/fbioe.2019.00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Lee KY, Jeong L, Kang YO, Lee SJ, Park WH, Electrospinning of polysaccharides for regenerative medicine, Adv. Drug Deliv. Rev. 61 (2009) 1020–1032, 10.1016/j.addr.2009.07.006. [DOI] [PubMed] [Google Scholar]
- [86].Witzler M, Büchner D, Shoushrah SH, Babczyk P, Baranova J, Witzleben S, Tobiasch E, Schulze M, Polysaccharide-based systems for targeted stem cell differentiation and bone regeneration, Biomolecules 9 (2019), 10.3390/biom9120840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Kumbar SG, Toti US, Deng M, James R, Laurencin CT, Aravamudhan A, Harmon M, Ramos DM, Novel mechanically competent polysaccharide scaffolds for bone tissue engineering, Biomed. Mater. 6 (2011), 065005, 10.1088/1748-6041/6/6/065005. [DOI] [PubMed] [Google Scholar]
- [88].Dinoro J, Maher M, Talebian S, Jafarkhani M, Mehrali M, Orive G, Foroughi J, Lord MS, Dolatshahi-Pirouz A, Sulfated polysaccharide-based scaffolds for orthopaedic tissue engineering, Biomaterials 214 (2019), 119214, 10.1016/j.biomaterials.2019.05.025. [DOI] [PubMed] [Google Scholar]
- [89].Li N, Xue F, Zhang H, Sanyour HJ, Rickel AP, Uttecht A, Fanta B, Hu J, Hong Z, Fabrication and characterization of pectin hydrogel nanofiber scaffolds for differentiation of mesenchymal stem cells into vascular cells, ACS Biomaterials Science & Engineering 5 (2019) 6511–6519, 10.1021/acsbiomaterials.9b01178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Chen ZG, Wang PW, Wei B, Mo XM, Cui FZ, Electrospun collagen-chitosan nanofiber: a biomimetic extracellular matrix for endothelial cell and smooth muscle cell, Acta Biomater. 6 (2010) 372–382, 10.1016/j.actbio.2009.07.024. [DOI] [PubMed] [Google Scholar]
- [91].Fiqrianti IA, Widiyanti P, Manaf MA, Savira CY, Cahyani NR, Bella FR, Poly-L-lactic acid (PLLA)-chitosan-collagen electrospun tube for vascular graft application, J Funct Biomater 9 (2018), 10.3390/jfb9020032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Zhu C, Ma X, Xian L, Zhou Y, Fan D, Characterization of a co-electrospun scaffold of HLC/CS/PLA for vascular tissue engineering, Biomed. Mater. Eng. 24 (2014) 1999–2005, 10.3233/BME-141009. [DOI] [PubMed] [Google Scholar]
- [93].Du F, Wang H, Zhao W, Li D, Kong D, Yang J, Zhang Y, Gradient nanofibrous chitosan/poly ε-caprolactone scaffolds as extracellular microenvironments for vascular tissue engineering, Biomaterials 33 (2012) 762–770, 10.1016/j.biomaterials.2011.10.037. [DOI] [PubMed] [Google Scholar]
- [94].Yao Y, Wang J, Cui Y, Xu R, Wang Z, Zhang J, Wang K, Li Y, Zhao Q, Kong D, Effect of sustained heparin release from PCL/chitosan hybrid small-diameter vascular grafts on anti-thrombogenic property and endothelialization, Acta Biomater. 10 (2014) 2739–2749, 10.1016/j.actbio.2014.02.042. [DOI] [PubMed] [Google Scholar]
- [95].Montini-Ballarin F, Calvo D, Caracciolo PC, Rojo F, Frontini PM, Abraham GA, Guinea GV, Mechanical behavior of bilayered small-diameter nanofibrous structures as biomimetic vascular grafts, J. Mech. Behav. Biomed. Mater. 60 (2016) 220–233, 10.1016/j.jmbbm.2016.01.025. [DOI] [PubMed] [Google Scholar]
- [96].Mi HY, Jing X, Yu E, Wang X, Li Q, Turng LS, Manipulating the structure and mechanical properties of thermoplastic polyurethane/polycaprolactone hybrid small diameter vascular scaffolds fabricated via electrospinning using an assembled rotating collector, J. Mech. Behav. Biomed. Mater. 78 (2018) 433–441, 10.1016/j.jmbbm.2017.11.046. [DOI] [PubMed] [Google Scholar]
- [97].Yu E, Mi H-Y, Zhang J, Thomson JA, Turng L-S, Development of biomimetic thermoplastic polyurethane/fibroin small-diameter vascular grafts via a novel electrospinning approach, J. Biomed. Mater. Res. A 106 (2018) 985–996, 10.1002/jbm.a.36297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Shaikh FM, Callanan A, Kavanagh EG, Burke PE, Grace PA, McGloughlin TM, Fibrin: a natural biodegradable scaffold in vascular tissue engineering, Cells Tissues Organs 188 (2008) 333–346, 10.1159/000139772. [DOI] [PubMed] [Google Scholar]
- [99].Weisel JW, Litvinov RI, Fibrin formation, structure and properties, Subcell. Biochem. 82 (2017) 405–456, 10.1007/978-3-319-49674-0_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Yang L, Li X, Wu Y, Du P, Sun L, Yu Z, Song S, Yin J, Ma X, Jing C, Zhao J, Chen H, Dong Y, Zhang Q, Zhao L, Preparation of PU/fibrin vascular scaffold with good biomechanical properties and evaluation of its performance in vitro and in vivo, Int. J. Nanomedicine 15 (2020) 8697–8715, 10.2147/IJN.S274459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Yang L, Li X, Wang D, Mu S, Lv W, Hao Y, Lu X, Zhang G, Nan W, Chen H, Xie L, Zhang Y, Dong Y, Zhang Q, Zhao L, Improved mechanical properties by modifying fibrin scaffold with PCL and its biocompatibility evaluation, J. Biomater. Sci. Polym. Ed. 31 (2020) 658–678, 10.1080/09205063.2019.1710370. [DOI] [PubMed] [Google Scholar]
- [102].Ju YM, Ahn H, Arenas-Herrera J, Kim C, Abolbashari M, Atala A, Yoo JJ, Lee SJ, Electrospun vascular scaffold for cellularized small diameter blood vessels: a preclinical large animal study, Acta Biomater. 59 (2017) 58–67, 10.1016/j.actbio.2017.06.027. [DOI] [PubMed] [Google Scholar]
- [103].Han F, Jia X, Dai D, Yang X, Zhao J, Zhao Y, Fan Y, Yuan X, Performance of a multilayered small-diameter vascular scaffold dual-loaded with VEGF and PDGF, Biomaterials 34 (2013) 7302–7313, 10.1016/j.biomaterials.2013.06.006. [DOI] [PubMed] [Google Scholar]
- [104].Wu T, Zhang J, Wang Y, Li D, Sun B, El-Hamshary H, Yin M, Mo X, Fabrication and preliminary study of a biomimetic tri-layer tubular graft based on fibers and fiber yarns for vascular tissue engineering, Mater. Sci. Eng. C Mater. Biol. Appl. 82 (2018) 121–129, 10.1016/j.msec.2017.08.072. [DOI] [PubMed] [Google Scholar]
- [105].Wu C, Zhang H, Hu Q, Ramalingam M, Designing biomimetic triple-layered nanofibrous vascular grafts via combinatorial electrospinning approach, J. Nanosci. Nanotechnol. 20 (2020) 6396–6405, 10.1166/jnn.2020.17878. [DOI] [PubMed] [Google Scholar]
- [106].Elsayed Y, Lekakou C, Labeed F, Tomlins P, Fabrication and characterisation of biomimetic, electrospun gelatin fibre scaffolds for tunica media-equivalent, tissue engineered vascular grafts, Mater. Sci. Eng. C Mater. Biol. Appl. 61 (2016) 473–483, 10.1016/j.msec.2015.12.081. [DOI] [PubMed] [Google Scholar]
- [107].Tan Z, Gao X, Liu T, Yang Y, Zhong J, Tong C, Tan Y, Electrospun vein grafts with high cell infiltration for vascular tissue engineering, Mater. Sci. Eng. C Mater. Biol. Appl. 81 (2017) 407–415, 10.1016/j.msec.2017.08.034. [DOI] [PubMed] [Google Scholar]
- [108].Hu Q, Su C, Zeng Z, Zhang H, Feng R, Feng J, Li S, Fabrication of multilayer tubular scaffolds with aligned nanofibers to guide the growth of endothelial cells, J. Biomater. Appl. 35 (2020) 553–566, 10.1177/0885328220935090. [DOI] [PubMed] [Google Scholar]
- [109].Bashur CA, Dahlgren LA, Goldstein AS, Effect of fiber diameter and orientation on fibroblast morphology and proliferation on electrospun poly(D,L-lactic-co-glycolic acid) meshes, Biomaterials 27 (2006) 5681–5688, 10.1016/j.biomaterials.2006.07.005. [DOI] [PubMed] [Google Scholar]
- [110].Han DG, Ahn CB, Lee J-H, Hwang Y, Kim JH, Park KY, Lee JW, Son KH, Optimization of electrospun poly(caprolactone) fiber diameter for vascular scaffolds to maximize smooth muscle cell infiltration and phenotype modulation, Polymers 11 (2019) 643, 10.3390/polym11040643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Shen N, Zhang Y, Raza A, Chang L, Wang JY, Effects of the micro/nanostructure of electrospun zein fibres on cells in simulated blood flow environment, Mater. Sci. Eng. C Mater. Biol. Appl. 122 (2021), 111900, 10.1016/j.msec.2021.111900. [DOI] [PubMed] [Google Scholar]
- [112].Grace Chao PH, Hsu HY, Tseng HY, Electrospun microcrimped fibers with nonlinear mechanical properties enhance ligament fibroblast phenotype, Biofabrication 6 (2014), 035008, 10.1088/1758-5082/6/3/035008. [DOI] [PubMed] [Google Scholar]
- [113].Fusaro L, Gualandi C, Antonioli D, Soccio M, Liguori A, Laus M, Lotti N, Boccafoschi F, Focarete ML, Elastomeric electrospun scaffolds of a biodegradable aliphatic copolyester containing PEG-like sequences for dynamic culture of human endothelial cells, Biomolecules 10 (2020), 10.3390/biom10121620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Bergmeister H, Schreiber C, Grasl C, Walter I, Plasenzotti R, Stoiber M, Bernhard D, Schima H, Healing characteristics of electrospun polyurethane grafts with various porosities, Acta Biomater. 9 (2013) 6032–6040, 10.1016/j.actbio.2012.12.009. [DOI] [PubMed] [Google Scholar]
- [115].Wang Z, Cui Y, Wang J, Yang X, Wu Y, Wang K, Gao X, Li D, Li Y, Zheng XL, Zhu Y, Kong D, Zhao Q, The effect of thick fibers and large pores of electrospun poly(ε-caprolactone) vascular grafts on macrophage polarization and arterial regeneration, Biomaterials 35 (2014) 5700–5710, 10.1016/j.biomaterials.2014.03.078. [DOI] [PubMed] [Google Scholar]
- [116].Yin A, Li J, Bowlin GL, Li D, Rodriguez IA, Wang J, Wu T, Ei-Hamshary HA, Al-Deyab SS, Mo X, Fabrication of cell penetration enhanced poly (l-lactic acid-co-ε-caprolactone)/silk vascular scaffolds utilizing air-impedance electrospinning, Colloids Surf. B: Biointerfaces 120 (2014) 47–54, 10.1016/j.colsurfb.2014.04.011. [DOI] [PubMed] [Google Scholar]
- [117].Tan Z, Wang H, Gao X, Liu T, Tan Y, Composite vascular grafts with high cell infiltration by co-electrospinning, Mater. Sci. Eng. C Mater. Biol. Appl. 67 (2016) 369–377, 10.1016/j.msec.2016.05.067. [DOI] [PubMed] [Google Scholar]
- [118].Yin A, Zhuang W, Liu G, Lan X, Tang Z, Deng Y, Wang Y, Performance of PEGylated chitosan and poly (L-lactic acid-co-ε-caprolactone) bilayer vascular grafts in a canine femoral artery model, Colloids Surf. B: Biointerfaces 188 (2020), 110806, 10.1016/j.colsurfb.2020.110806. [DOI] [PubMed] [Google Scholar]
- [119].Wang K, Zhu M, Li T, Zheng W, Li L, Xu M, Zhao Q, Kong D, Wang L, Improvement of cell infiltration in electrospun polycaprolactone scaffolds for the construction of vascular grafts, J. Biomed. Nanotechnol. 10 (2014) 1588–1598, 10.1166/jbn.2014.1849. [DOI] [PubMed] [Google Scholar]
- [120].Wang K, Zheng W, Pan Y, Ma S, Guan Y, Liu R, Zhu M, Zhou X, Zhang J, Zhao Q, Zhu Y, Wang L, Kong D, Three-layered PCL grafts promoted vascular regeneration in a rabbit carotid artery model, Macromol. Biosci. 16 (2016) 608–618, 10.1002/mabi.201500355. [DOI] [PubMed] [Google Scholar]
- [121].Voorneveld J, Oosthuysen A, Franz T, Zilla P, Bezuidenhout D, Dual electrospinning with sacrificial fibers for engineered porosity and enhancement of tissue ingrowth, J Biomed Mater Res B Appl Biomater 105 (2017) 1559–1572, 10.1002/jbm.b.33695. [DOI] [PubMed] [Google Scholar]
- [122].Hodge J, Quint C, The improvement of cell infiltration in an electrospun scaffold with multiple synthetic biodegradable polymers using sacrificial PEO microparticles, J. Biomed. Mater. Res. A 107 (2019) 1954–1964, 10.1002/jbm.a.36706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Jeong SI, Kim SY, Cho SK, Chong MS, Kim KS, Kim H, Lee SB, Lee YM, Tissue-engineered vascular grafts composed of marine collagen and PLGA fibers using pulsatile perfusion bioreactors, Biomaterials 28 (2007) 1115–1122, 10.1016/j.biomaterials.2006.10.025. [DOI] [PubMed] [Google Scholar]
- [124].Norouzi SK, Shamloo A, Bilayered heparinized vascular graft fabricated by combining electrospinning and freeze drying methods, Mater. Sci. Eng. C Mater. Biol. Appl. 94 (2019) 1067–1076, 10.1016/j.msec.2018.10.016. [DOI] [PubMed] [Google Scholar]
- [125].Soletti L, Hong Y, Guan J, Stankus JJ, El-Kurdi MS, Wagner WR, Vorp DA, A bilayered elastomeric scaffold for tissue engineering of small diameter vascular grafts, Acta Biomater. 6 (2010) 110–122, 10.1016/j.actbio.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Milleret V, Hefti T, Hall H, Vogel V, Eberli D, Influence of the fiber diameter and surface roughness of electrospun vascular grafts on blood activation, Acta Biomater. 8 (2012) 4349–4356, 10.1016/j.actbio.2012.07.032. [DOI] [PubMed] [Google Scholar]
- [127].Onishi A, St Ange K, Dordick JS, Linhardt RJ, Heparin and anticoagulation, Front. Biosci. 21 (2016) 1372–1392, 10.2741/4462. Landmark Ed. [DOI] [PubMed] [Google Scholar]
- [128].Seib FP, Herklotz M, Burke KA, Maitz MF, Werner C, Kaplan DL, Multifunctional silk-heparin biomaterials for vascular tissue engineering applications, Biomaterials 35 (2014) 83–91, 10.1016/j.biomaterials.2013.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Choi WS, Joung YK, Lee Y, Bae JW, Park HK, Park YH, Park JC, Park KD, Enhanced patency and endothelialization of small-caliber vascular grafts fabricated by coimmobilization of heparin and cell-adhesive peptides, ACS Appl. Mater. Interfaces 8 (2016) 4336–4346, 10.1021/acsami.5b12052. [DOI] [PubMed] [Google Scholar]
- [130].Qiu X, Lee BL, Ning X, Murthy N, Dong N, Li S, End-point immobilization of heparin on plasma-treated surface of electrospun polycarbonate-urethane vascular graft, Acta Biomater. 51 (2017) 138–147, 10.1016/j.actbio.2017.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Caracciolo PC, Rial-Hermida MI, Montini-Ballarin F, Abraham GA, Concheiro A, Alvarez-Lorenzo C, Surface-modified bioresorbable electrospun scaffolds for improving hemocompatibility of vascular grafts, Mater. Sci. Eng. C Mater. Biol. Appl. 75 (2017) 1115–1127, 10.1016/j.msec.2017.02.151. [DOI] [PubMed] [Google Scholar]
- [132].Wang S, Zhang Y, Wang H, Dong Z, Preparation, characterization and biocompatibility of electrospinning heparin-modified silk fibroin nanofibers, Int. J. Biol. Macromol. 48 (2011) 345–353, 10.1016/j.ijbiomac.2010.12.008. [DOI] [PubMed] [Google Scholar]
- [133].Li Q, Mu L, Zhang F, Mo Z, Jin C, Qi W, Manufacture and property research of heparin grafted electrospinning PCU artificial vascular scaffolds, Mater. Sci. Eng. C Mater. Biol. Appl. 78 (2017) 854–861, 10.1016/j.msec.2017.04.148. [DOI] [PubMed] [Google Scholar]
- [134].Wang S, Mo XM, Jiang BJ, Gao CJ, Wang HS, Zhuang YG, Qiu LJ, Fabrication of small-diameter vascular scaffolds by heparin-bonded P(LLA-CL) composite nanofibers to improve graft patency, Int. J. Nanomedicine 8 (2013) 2131–2139, 10.2147/IJN.S44956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Hu YT, Pan XD, Zheng J, Ma WG, Sun LZ, In vitro and in vivo evaluation of a small-caliber coaxial electrospun vascular graft loaded with heparin and VEGF, Int. J. Surg. 44 (2017) 244–249, 10.1016/j.ijsu.2017.06.077. [DOI] [PubMed] [Google Scholar]
- [136].Agarwal S, Greiner A, On the way to clean and safe electrospinning-green electrospinning: emulsion and suspension electrospinning, Polym. Adv. Technol. 22 (2011) 372–378, 10.1002/pat.1883. [DOI] [Google Scholar]
- [137].Kuang H, Yang S, Wang Y, He Y, Ye K, Hu J, Shen W, Morsi Y, Lu S, Mo X, Electrospun bilayer composite vascular graft with an inner layer modified by polyethylene glycol and haparin to regenerate the blood vessel, J. Biomed. Nanotechnol. 15 (2019) 77–84, 10.1166/jbn.2019.2666. [DOI] [PubMed] [Google Scholar]
- [138].Liu H, Li X, Zhou G, Fan H, Fan Y, Electrospun sulfated silk fibroin nanofibrous scaffolds for vascular tissue engineering, Biomaterials 32 (2011) 3784–3793, 10.1016/j.biomaterials.2011.02.002. [DOI] [PubMed] [Google Scholar]
- [139].Eldurini S, Abd El-Hady BM, Shafaa MW, Gad AAM, Tolba E, A multicompartment vascular implant of electrospun wintergreen oil/polycaprolactone fibers coated with poly(ethylene oxide), Biom. J. (2020), 10.1016/j.bj.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Tran N, Le A, Ho M, Dang N, Thi Thanh HH, Truong L, Huynh DP, Hiep NT, Polyurethane/polycaprolactone membrane grafted with conjugated linoleic acid for artificial vascular graft application, Sci. Technol. Adv. Mater. 21 (2020) 56–66, 10.1080/14686996.2020.1718549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Yan S, Li Y, Jiang Y-C, Xu Y, Wang D, Zhang X, Li Q, Turng L-S, Expanded polytetrafluoroethylene/silk fibroin/salicin vascular graft fabrication for improved endothelialization and anticoagulation, Appl. Surf. Sci. 542 (2021), 148610, 10.1016/j.apsusc.2020.148610. [DOI] [Google Scholar]
- [142].Melincovici CS, Boşca AB, Şuşman S, Mărginean M, Mihu C, Istrate M, Moldovan IM, Roman AL, Mihu CM, Vascular endothelial growth factor (VEGF) - key factor in normal and pathological angiogenesis, Romanian J. Morphol. Embryol. 59 (2018) 455–467. [PubMed] [Google Scholar]
- [143].Apte RS, Chen DS, Ferrara N, VEGF in signaling and disease: beyond discovery and development, Cell 176 (2019) 1248–1264, 10.1016/j.cell.2019.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Davoudi P, Assadpour S, Derakhshan MA, Ai J, Solouk A, Ghanbari H, Biomimetic modification of polyurethane-based nanofibrous vascular grafts: a promising approach towards stable endothelial lining, Mater. Sci. Eng. C Mater. Biol. Appl. 80 (2017) 213–221, 10.1016/j.msec.2017.05.140. [DOI] [PubMed] [Google Scholar]
- [145].Sevostyanova VV, Antonova LV, Velikanova EA, Matveeva VG, Krivkina EO, Glushkova TV, Mironov AV, Burago AY, Barbarash LS, Endothelialization of polycaprolactone vascular graft under the action of locally applied vascular endothelial growth factor, Bull. Exp. Biol. Med. 165 (2018) 264–268, 10.1007/s10517-018-4144-4. [DOI] [PubMed] [Google Scholar]
- [146].Emechebe GA, Obiweluozor FO, Jeong IS, Park JK, Park CH, Kim CS, Merging 3D printing with electrospun biodegradable small-caliber vascular grafts immobilized with VEGF, Nanomedicine 30 (2020), 102306, 10.1016/j.nano.2020.102306. [DOI] [PubMed] [Google Scholar]
- [147].Dai WW, Guo HF, Qian DH, Qin ZX, Lei Y, Hou XY, Wen C, Improving endothelialization by the combined application of polyethylene glycol coated cerium oxide nanoparticles and VEGF in electrospun polyurethane scaffolds, J. Mater. Chem. B 5 (2017) 1053–1061, 10.1039/c6tb02391f. [DOI] [PubMed] [Google Scholar]
- [148].Pearson JD, Endothelial progenitor cells–an evolving story, Microvasc. Res. 79 (2010) 162–168, 10.1016/j.mvr.2009.12.004. [DOI] [PubMed] [Google Scholar]
- [149].Peters EB, Endothelial progenitor cells for the vascularization of engineered tissues, Tissue Eng Part B Rev 24 (2018) 1–24, 10.1089/ten.TEB.2017.0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].De Visscher G, Mesure L, Meuris B, Ivanova A, Flameng W, Improved endothelialization and reduced thrombosis by coating a synthetic vascular graft with fibronectin and stem cell homing factor SDF-1α, Acta Biomater. 8 (2012) 1330–1338, 10.1016/j.actbio.2011.09.016. [DOI] [PubMed] [Google Scholar]
- [151].Wang W, Liu D, Li D, Du H, Zhang J, You Z, Li M, He C, Nanofibrous vascular scaffold prepared from miscible polymer blend with heparin/stromal cell-derived factor-1 alpha for enhancing anticoagulation and endothelialization, Colloids Surf. B: Biointerfaces 181 (2019) 963–972, 10.1016/j.colsurfb.2019.06.065. [DOI] [PubMed] [Google Scholar]
- [152].Guo HF, Dai WW, Qian DH, Qin ZX, Lei Y, Hou XY, Wen C, A simply prepared small-diameter artificial blood vessel that promotes in situ endothelialization, Acta Biomater. 54 (2017) 107–116, 10.1016/j.actbio.2017.02.038. [DOI] [PubMed] [Google Scholar]
- [153].Misra P, Lebeche D, Ly H, Schwarzkopf M, Diaz G, Hajjar RJ, Schecter AD, Frangioni JV, Quantitation of CXCR4 expression in myocardial infarction using 99mTc-labeled SDF-1alpha, J. Nucl. Med. 49 (2008) 963–969, 10.2967/jnumed.107.050054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Bromage DI, Davidson SM, Yellon DM, Stromal derived factor 1α: a chemokine that delivers a two-pronged defence of the myocardium, Pharmacol. Ther. 143 (2014) 305–315, 10.1016/j.pharmthera.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Lee KW, Johnson NR, Gao J, Wang Y, Human progenitor cell recruitment via SDF-1α coacervate-laden PGS vascular grafts, Biomaterials 34 (2013) 9877–9885, 10.1016/j.biomaterials.2013.08.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Muylaert DE, van Almen GC, Talacua H, Fledderus JO, Kluin J, Hendrikse SI, van Dongen JL, Sijbesma E, Bosman AW, Mes T, Thakkar SH, Smits AI, Bouten CV, Dankers PY, Verhaar MC, Early in-situ cellularization of a supramolecular vascular graft is modified by synthetic stromal cell-derived factor-1α derived peptides, Biomaterials 76 (2016) 187–195, 10.1016/j.biomaterials.2015.10.052. [DOI] [PubMed] [Google Scholar]
- [157].Ruoslahti E, RGD and other recognition sequences for integrins, Annu. Rev. Cell Dev. Biol. 12 (1996) 697–715, 10.1146/annurev.cellbio.12.1.697. [DOI] [PubMed] [Google Scholar]
- [158].Hsu SH, Sun SH, Chen DC, Improved retention of endothelial cells seeded on polyurethane small-diameter vascular grafts modified by a recombinant RGD-containing protein, Artif. Organs 27 (2003) 1068–1078, 10.1111/j.1525-1594.2003.07141.x. [DOI] [PubMed] [Google Scholar]
- [159].Li J, Ding M, Fu Q, Tan H, Xie X, Zhong Y, A novel strategy to graft RGD peptide on biomaterials surfaces for endothelization of small-diamater vascular grafts and tissue engineering blood vessel, J. Mater. Sci. Mater. Med. 19 (2008) 2595–2603, 10.1007/s10856-007-3354-5. [DOI] [PubMed] [Google Scholar]
- [160].Zheng W, Wang Z, Song L, Zhao Q, Zhang J, Li D, Wang S, Han J, Zheng XL, Yang Z, Kong D, Endothelialization and patency of RGD-functionalized vascular grafts in a rabbit carotid artery model, Biomaterials 33 (2012) 2880–2891, 10.1016/j.biomaterials.2011.12.047. [DOI] [PubMed] [Google Scholar]
- [161].Hoesli CA, Garnier A, Juneau PM, Chevallier P, Duchesne C, Laroche G, A fluorophore-tagged RGD peptide to control endothelial cell adhesion to micropatterned surfaces, Biomaterials 35 (2014) 879–890, 10.1016/j.biomaterials.2013.09.076. [DOI] [PubMed] [Google Scholar]
- [162].Antonova LV, Seifalian AM, Kutikhin AG, Sevostyanova VV, Matveeva VG, Velikanova EA, Mironov AV, Shabaev AR, Glushkova TV, Senokosova EA, Vasyukov GY, Krivkina EO, Burago AY, Kudryavtseva YA, Barbarash OL, Barbarash LS, Conjugation with RGD peptides and incorporation of vascular endothelial growth factor are equally efficient for biofunctionalization of tissue-engineered vascular grafts, Int. J. Mol. Sci. 17 (2016), 10.3390/ijms17111920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Kuwabara F, Narita Y, Yamawaki-Ogata A, Kanie K, Kato R, Satake M, Kaneko H, Oshima H, Usui A, Ueda Y, Novel small-caliber vascular grafts with trimeric peptide for acceleration of endothelialization, Ann. Thorac. Surg. 93 (2012) 156–163, discussion 163, 10.1016/j.athoracsur.2011.07.055. [DOI] [PubMed] [Google Scholar]
- [164].Zhao J, Bai L, Ren XK, Guo J, Xia S, Zhang W, Feng Y, Co-immobilization of ACH11 antithrombotic peptide and CAG cell-adhesive peptide onto vascular grafts for improved hemocompatibility and endothelialization, Acta Biomater. 97 (2019) 344–359, 10.1016/j.actbio.2019.07.057. [DOI] [PubMed] [Google Scholar]
- [165].Conway EM, Collen D, Carmeliet P, Molecular mechanisms of blood vessel growth, Cardiovasc. Res. 49 (2001) 507–521, 10.1016/s0008-6363(00)00281-9. [DOI] [PubMed] [Google Scholar]
- [166].Chen RR, Silva EA, Yuen WW, Mooney DJ, Spatio-temporal VEGF and PDGF delivery patterns blood vessel formation and maturation, Pharm. Res. 24 (2007) 258–264, 10.1007/s11095-006-9173-4. [DOI] [PubMed] [Google Scholar]
- [167].Chan-Park MB, Shen JY, Cao Y, Xiong Y, Liu Y, Rayatpisheh S, Kang GC, Greisler HP, Biomimetic control of vascular smooth muscle cell morphology and phenotype for functional tissue-engineered small-diameter blood vessels, J. Biomed. Mater. Res. A 88 (2009) 1104–1121, 10.1002/jbm.a.32318. [DOI] [PubMed] [Google Scholar]
- [168].Saitow C, Kaplan DL, Castellot JJ, Heparin stimulates elastogenesis: application to silk-based vascular grafts, Matrix Biol. 30 (2011) 346–355, 10.1016/j.matbio.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Yang Z, Tu Q, Wang J, Huang N, The role of heparin binding surfaces in the direction of endothelial and smooth muscle cell fate and re-endothelialization, Biomaterials 33 (2012) 6615–6625, 10.1016/j.biomaterials.2012.06.055. [DOI] [PubMed] [Google Scholar]
- [170].Blit PH, Battiston KG, Yang M, Paul Santerre J, Woodhouse KA, Electrospun elastin-like polypeptide enriched polyurethanes and their interactions with vascular smooth muscle cells, Acta Biomater. 8 (2012) 2493–2503, 10.1016/j.actbio.2012.03.032. [DOI] [PubMed] [Google Scholar]
- [171].Wen M, Zhou F, Cui C, Zhao Y, Yuan X, Performance of TMC-g-PEG-VAPG/miRNA-145 complexes in electrospun membranes for target-regulating vascular SMCs, Colloids Surf. B: Biointerfaces 182 (2019), 110369, 10.1016/j.colsurfb.2019.110369. [DOI] [PubMed] [Google Scholar]
- [172].Yang Y, Lei D, Zou H, Huang S, Yang Q, Li S, Qing FL, Ye X, You Z, Zhao Q, Hybrid electrospun rapamycin-loaded small-diameter decellularized vascular grafts effectively inhibit intimal hyperplasia, Acta Biomater. 97 (2019) 321–332, 10.1016/j.actbio.2019.06.037. [DOI] [PubMed] [Google Scholar]
- [173].Punnakitikashem P, Truong D, Menon JU, Nguyen KT, Hong Y, Electrospun biodegradable elastic polyurethane scaffolds with dipyridamole release for small diameter vascular grafts, Acta Biomater. 10 (2014) 4618–4628, 10.1016/j.actbio.2014.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Han J, Farah S, Domb A, Lelkes P, Electrospun rapamycin-eluting polyurethane fibers for vascular grafts, Pharm. Res. 30 (2013) 1735–1748, 10.1007/s11095-013-1016-5. [DOI] [PubMed] [Google Scholar]