Abstract
Background
There is a critical need to identify patient characteristics associated with long-term ovarian cancer survival.
Methods
Quality of life (QOL), measured by the Functional Assessment of Cancer Therapy-Ovarian-Trial Outcome Index (FACT-O-TOI), including physical, functional, and ovarian-specific subscales, was compared between long-term survivors (LTS) (8+ years) and short-term survivors (STS) (<5 years) of GOG 218 at baseline; before cycles 4, 7, 13, 21; and 6 months post-treatment using linear and longitudinal mixed models adjusted for covariates. Adverse events (AEs) were compared between survivor groups at each assessment using generalized linear models. All P values are 2-sided.
Results
QOL differed statistically significantly between STS (N = 1115) and LTS (N = 260) (P < .001). Baseline FACT-O-TOI and FACT-O-TOI change were independently associated with long-term survival (odds ratio = 1.05, 95% confidence interval = 1.03 to 1.06 and odds ratio = 1.06, 95% confidence interval = 1.05 to 1.07, respectively). A 7-point increase in baseline QOL was associated with a 38.0% increase in probability of LTS, and a 9-point increase in QOL change was associated with a 67.0% increase in odds for LTS. QOL decreased statistically significantly with increasing AE quartiles (cycle 4 quartiles: 0-5 vs 6-8 vs 9-11 vs ≥12 AEs, P = .01; cycle 21 quartiles: 0-2 vs 3 vs 4-5 vs ≥6 AEs, P = .001). Further, LTS reported statistically significantly better QOL compared with STS (P = .03 and P = .01, cycles 4 and 21, respectively), with similar findings across higher AE grades.
Conclusions
Baseline and longitudinal QOL change scores distinguished LTS vs STS and are robust prognosticators for long-term survival. Results have trial design and supportive care implications, providing meaningful prognostic value in this understudied population.
Advances in ovarian cancer treatments have improved 5-year survival rates for advanced-stage disease from 16% to 24% in the past 25 years (1). During the same period, only negligible improvements in long-term survival were observed (1). Therefore, there is a critical need to identify and understand differences between long and short-term ovarian cancer survivor characteristics to better stratify patients to conventional or experimental therapies, with the hope of statistically significantly improving survival rates overall.
Tumor biology (eg, cancer stage, grade, histology, cytology) and age at diagnosis have clear associations with overall survival (2). Considerable evidence has associated quality of life (QOL) at study initiation (3-11), as well as QOL changes over time (3), with statistically significant overall survival improvement. Although these relationships are not well understood, it is known that a cancer patient’s responsiveness to cancer therapy may positively affect QOL by decreasing disease burden, thereby influencing survival. By extension, it is reasonable to expect that QOL is related to adverse events (AEs), because adherence to cancer treatment (12) and maintenance regimens (13–15) are clearly affected by experiencing AEs (16,17).
Examining both QOL and AEs across a clinical trial trajectory could assist in identifying the points in cancer treatment at which the probability for becoming a short-term survivor (STS) vs long-term survivor (LTS) can be detected and potentially addressed. Moreover, the extent to which the relationship between QOL and AEs might have prognostic value for long-term survival in this understudied population is unknown. Therefore, this study examines the relationship between QOL, measured by the Functional Assessment of Cancer Therapy-Ovarian-Trial Outcome Index (FACT-O-TOI) subscales examining physical, functional, and ovarian cancer-specific concerns, together with AEs, to identify characteristics associated with becoming a LTS of advanced ovarian cancer.
Methods
Patients
We used data from Gynecologic Oncology Group 218 (ClinicalTrials.gov numbers NCT02321735, NCT00262847), a phase III randomized clinical trial testing the efficacy of bevacizumab incorporated into standard frontline treatment of patients with stage III or stage IV ovarian epithelial, primary peritoneal, or fallopian tube cancer (18–20). All enrolled patients signed written informed consent for study participation to include receipt of study drug regimens, surveillance for treatment response and toxicity, and completion of QOL assessments at protocol-specified intervals in accordance with institutional and federal guidelines.
Selected patients were classified into 3 groups based on the time interval between enrollment and death or last follow-up. LTS included those who survived 8 and more years from enrollment (n = 260); 73.1% were still alive at latest follow-up. STS included those who died within less than 5 years after enrollment (n = 1115). Patients who were alive at their last follow-up less than 5 years (n = 70, 5.9%) were excluded. Based on the natural history of the disease where median survival is known to be between 4 and 5 years, this group served as the reference population. Intermediate-term survivors (ITS) included those who died between 5 and 8 years from enrollment (n = 215). Patients still alive at last follow-up 5-8 years post enrollment (n = 42, 16.3%) who could belong to the LTS group were excluded from analyses for ITS.
Measures
QOL was measured using the FACT-O-TOI (21). The FACT-O-TOI is a 26-item summary score with a possible total of 112 points that captures the FACT-General QOL dimensions of Physical Well-Being (7 items), Functional Well-Being (7 items), and an Ovarian Cancer Subscale (12 items). The Functional Assessment of Cancer Therapy–Abdominal Discomfort (22) includes items on abdominal pain, swelling, and cramps. QOL and abdominal discomfort were measured at baseline; before cycles 4, 7, 13, 21; and 6 months after completing protocol-directed therapy. Patients completed questionnaires at scheduled assessment time points regardless of disease progression or if protocol-directed therapy was stopped secondary to toxicity.
AEs were graded using the National Cancer Institute Common Terminology Criteria for Adverse Events (version 3) (23) and reported until 30 days after the last study treatment had been administered. AEs were summarized for patients who received at least 1 cycle of bevacizumab or placebo (18). AE data collected during the QOL assessment time points are reported herein.
Statistical Analysis
Primary interest was in the association between QOL measurements and the probability of long-term survival in women newly diagnosed with advanced-stage ovarian cancer. These were quantified as the odds ratio (OR) of LTS (reference was STS) associated with a 1-unit increase in QOL or a 1-unit increase in the arithmetic change in QOL from baseline. QOL change across the treatment period was calculated as continuous change from baseline to the longest QOL follow-up data available from data collected at cycles 13 and 21 and post-treatment. Odds ratio estimates greater than 1.0 generally indicate better survival prognosis. All multivariable models included main-effect adjustment for age at diagnosis (continuous), stage (3 vs 4), grade (1 vs 2, 3), performance status (0, 1 vs 2), residual disease (≤1 cm vs >1 cm), treatment (3 levels), and baseline QOL (continuous).
QOL was compared between LTS and STS at each assessment interval using multivariable linear models. Trends over time were compared using a longitudinal mixed model adjusted for covariates. Odds ratios for LTS (reference was STS) associated with QOL at each timepoint were estimated using the timepoint-specific multivariable logistic regression model, with a main effect for Trial Outcome Index at the measured time point. Odds ratio estimates for a 7-point increase in QOL are also provided. To further elucidate the association between QOL and survival for the ITS group in addition to LTS and STS, adjusted ORs associated with QOL and QOL change for LTS (reference was STS) and ITS (reference was STS) associated with a 1-unit QOL increase in the furthest assessed timepoint were estimated using a multivariable polychotomous regression model. Additional analyses were conducted to examine differences between LTS and STS in baseline QOL and QOL change scores stratified by treatment response category, classified as responders, nonresponders, and those nonmeasurable. Treatment response and completed cycles of treatment were added to the multivariable model to investigate the prognostic value of QOL and QOL change independent of treatment response.
Mean number of all AEs and grade 2 or higher AEs were compared across the treatment period using generalized linear models. Differences between LTS and STS in QOL across increasing quartiles of all AEs and grade 2 or higher AEs were investigated using multivariable linear models.
Missing data are assumed to be missing at random. P values less than .05 were deemed statistically significant, with no multiple testing adjustment. All P values are 2-sided.
Results
Baseline Characteristics
Analyses comparing LTS and STS included 1375 patients. Of those, 260 were identified as LTS (8+ years), and 1115 were identified as STS (<5 years). STS and LTS did not differ statistically significantly with respect to age, ethnicity, or treatment (Table 1). However, LTS were less likely to have stage IV disease (18.1% vs 30.5%, P < .001), high-grade disease (93.0% vs 96.9% grade 2 or 3, P = .02), poor performance status (4.2% vs 7.8%, P < .001), and residual disease greater than 1 cm (44.6% vs 57.0%, P < .001).
Table 1.
Demographic and patient characteristics for short- and long-term survivors
| Characteristics | Survive ≤5 y (n = 1115) | Survive 8+ y (n = 260) | P a |
|---|---|---|---|
| Mean age (SE), y [No.] | 60.43 (0.33) [1003] | 58.01 (0.68) [244] | .001 |
| Race or ethnicity, No. (%) | |||
| American Indian | 4 (0.4) | 1 (0.4) | .07 |
| Asian | 58 (5.2) | 23 (8.8) | — |
| Black | 50 (4.5) | 8 (3.1) | — |
| White non-Hispanic | 949 (85.1) | 211 (81.2) | — |
| Hispanic | 37 (3.3) | 14 (5.4) | — |
| Unknown | 17 (1.5) | 3 (1.2) | — |
| Treatment, No. (%) | |||
| Chemotherapy | 387 (34.7) | 91 (35.0) | .18 |
| Chemotherapy + bevacizumab | 376 (33.7) | 74 (28.5) | — |
| Chemotherapy + bevacizumab + bevacizumab | 352 (31.6) | 95 (36.5) | — |
| Stage, No. (%) | |||
| III | 775 (69.5) | 213 (81.9) | <.001 |
| IV | 340 (30.5) | 47 (18.1) | — |
| Grade, No. (%) | |||
| 1 | 31 (3.1) | 17 (7.01) | .02 |
| 2 | 125 (12.5) | 30 (12.3) | — |
| 3 | 847 (84.4) | 197 (80.7) | — |
| Performance status, No. (%) | |||
| 0 | 477 (47.0) | 90 (54.2) | <.001 |
| 1 | 458 (45.2) | 69 (41.6) | — |
| 2 | 79 (7.8) | 7 (4.2) | — |
| Residual disease, No. (%) | |||
| ≤1 cm | 479 (43.0) | 144 (55.4) | <.001 |
| >1 cm | 636 (57.0) | 116 (44.6) | — |
| FACT-O-TOIb at baseline, mean (SE) [No.] | 66.26 (0.49) [968] | 68.89 (0.99) [238] | .02 |
| FACT-O-Physical Well-Beingb at baseline, mean (SE) [No.] | 19.83 (0.17) [967] | 21.04 (0.36) [238] | .002 |
| FACT-O-Functional Well-Beingb at baseline, mean (SE) [No.] | 14.30 (0.19) [968] | 14.94 (0.40) [238] | .15 |
| FACT-O-Additional Concernsb at baseline, mean (SE) [No.] | 32.15 (0.20) [968] | 32.93 (0.40) [238] | .08 |
| Abdominal Discomfortc at baseline, mean (SE) [No.] | 11.48 (0.12) [968] | 12.32 (0.24) [238] | .002 |
Differences for categorical variables are tested with a 2-sided Pearson χ2 test; difference in mean age is tested using a 2-sided t test.
Means for FACT-O-TOI and subdomains are adjusted for age, stage, grade, performance status, residual disease, and treatment. Higher scores reflect better quality of life. Differences are tested with a 2-sided F test.
Mean scores are adjusted for age, stage, grade, performance status, residual disease, and treatment. Higher scores reflect less abdominal discomfort. Differences are tested with a 2-sided F test.
Relationship Between QOL and Long-Term Survival
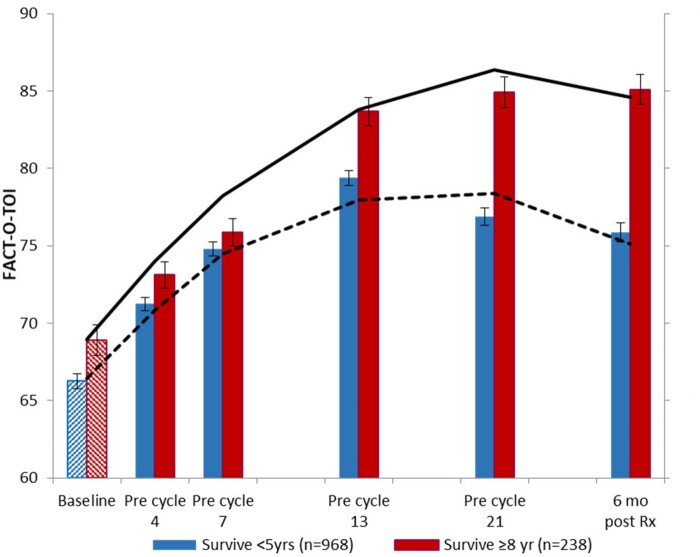
After adjusting for covariates, we identified a statistically significant difference in QOL over time between the STS and LTS (P < .001; Figure 1) and demonstrated a statistically significantly increased probability of being an LTS (Table 2). The difference between the groups increased over time, with the exception of cycle 7 (Figure 1; Table 2). Further, a 7-point change (0.5 SD) in the FACT-O-TOI, which is considered clinically meaningful (24), was established first before cycle 4 as being associated with a 9.5% increase in the likelihood of long-term survival, expanding to a 44.9% increased likelihood of long-term survival at 6 months posttreatment (Table 2).
Figure 1.
Analysis compares Functional Assessment of Cancer Therapy–Ovary Trial Outcome Index (FACT-O-TOI) between short-term survivors (STS) and long-term survivors (LTS) at each assessment interval using mixed hierarchical regression, adjusting for age, stage, grade, performance status, residual disease, treatment, and baseline FACT-O-TOI for cycle 4 and later. Solid line represents FACT-O-TOI in LTS from mixed model; dotted line represents FACT-O-TOI in STS from mixed model. This analysis shows statistically significant differences between survivor groups at baseline that continue to grow over time. Error bars represent ±1 standard error. Rx = treatment.
Table 2.
Odds ratios for prediction of long-term survival (dependent variable) associated with FACT-O-TOI at different treatment time points (adjusted for age, stage, grade, performance status, residual disease, treatment, and baseline FACT-O-TOI)
| Dependent variable | STS, No. | LTS, No. | OR (95% CI) | SE | Pa | Increase in adjusted OR for LTS with 7 pt increase in FACT-O-TOI |
|---|---|---|---|---|---|---|
| TOI at pre-cycle 4 | 863 | 225 | 1.01 (1.00 to 1.03) | 0.01 | .04 | 1.10 |
| TOI at pre-cycle 7 | 832 | 219 | 1.01 (1.00 to 1.02) | 0.01 | .27 | 1.05 |
| TOI at pre-cycle 13 | 729 | 215 | 1.03 (1.02 to 1.04) | 0.01 | <.001 | 1.22 |
| TOI at pre-cycle 21 | 614 | 215 | 1.05 (1.04 to 1.07) | 0.01 | <.001 | 1.40 |
| TOI at 6 mo post treatment | 510 | 209 | 1.06 (1.04 to 1.07) | 0.01 | <.001 | 1.45 |
Data tested using 2-sided z-test. CI = confidence interval; FACT-O-TOI = Functional Assessment of Cancer Therapy–Ovary Trial Outcome Index; LTS = long-term survivor; OR = odds ratio; SE = standard error; STS = short-term survivor; TOI = Trial Outcome Index.
In polychotomous logistic regression adjusting for covariates, both baseline FACT-O-TOI and change in FACT-O-TOI were statistically significantly and independently associated with an increased probability of being an LTS relative to STS (OR = 1.05, 95% confidence interval [CI] =1.03 to 1.06 and OR = 1.06, 95% CI =1.05 to 1.07, respectively; Table 3). Moreover, a clinically meaningful change of 0.5 SD in baseline QOL (7 points) was associated with a 38.0% increase in probability of being an LTS, and a change of 0.5 SD in QOL change (9 points) was associated with a 67.0% increase in odds for long-term survival. When comparing factors associated with survival for the intermediate group surviving 5-8 years relative to STS, baseline FACT-O-TOI and change in FACT-O-TOI were again statistically significantly associated with survival, but at a level intermediate between the STS and LTS (OR = 1.04, 95% CI = 1.02 to 1.05 and OR = 1.04, 95% CI =1.03 to 1.05, respectively).
Table 3.
Odds ratios for survival of 5-8 years and 8 years and more, relative to less than 5 years associated with FACT-O-TOI and change in FACT-O-TOI adjusted for patient covariatesa
| Parameter | Estimate | SE | P b | ORa (95% CI) |
|---|---|---|---|---|
| Survive 5-8 y vs <5 y (ref) | ||||
| FACT-O-TOI baseline | 0.036 | 0.007 | <.001 | 1.04 (1.02 to 1.05) |
| Change in FACT-O-TOIc | 0.039 | 0.006 | <.001 | 1.04 (1.03 to 1.05) |
| Survive ≥8 y vs <5 y (ref) | ||||
| FACT-O-TOI baseline | 0.046 | 0.007 | <.001 | 1.05 (1.03 to 1.06) |
| Change in FACT-O-TOId | 0.057 | 0.006 | <.001 | 1.06 (1.05 to 1.07) |
Reference: survive less than 5 years: 827; survive 5-8 years: 227; survive 8 years and more: 233. Deleted for missing data: 415. CI = confidence interval; FACT-O-TOI = Functional Assessment of Cancer Therapy–Ovary Trial Outcome Index; OR = odds ratio; QOL = quality of life; ref = reference; SE = standard error.
Data tested using 2-sided z-test.
Odds ratios adjusted for age, stage, grade, performance status, residual disease, and treatment.
Change measured from baseline to longest QOL follow-up of cycle 13, cycle 21, or post-treatment.
We further examined the associations of each subscale of the FACT-O-TOI with LTS. When the subscales of Physical Well-Being, Functional Well-Being, and Additional Concerns were included as separate variables in the logistic regression model, change in each subscale contributed statistically significantly and independently to the likelihood of being an LTS. Odds ratios for long-term survival associated with change in Physical Well-Being, Functional Well-Being, and Additional Concerns were, respectively, 1.06 (95% CI =1.03 to 1.11), 1.06 (95% CI = 1.03 to 1.09), and 1.05 (95% CI = 1.02 to 1.08) after adjusting for baseline QOL and patient covariates (data not shown).
We examined the differences in baseline QOL and QOL change stratified by treatment response, classified as nonresponder, responder (partial or complete), and nonmeasurable disease. Baseline QOL scores did not differ statistically significantly by response category adjusted for long-term survival status, with baseline Trial Outcome Index values of 68.2, 68.0, and 68.7 for nonresponders, responders, and nonmeasurables, respectively (P = .87). QOL change scores of the nonmeasurable patients (N = 398) were similar to the responders (N = 550) with a mean QOL change of 11.4 and 11.1, respectively, whereas the nonresponders (N = 185) had a statistically significantly smaller QOL change (mean = 4.6; P = .03). Further, when comparing prognostic factors of LTS compared with STS, QOL change was greater for LTS than STS for each treatment response category (P < .0005), with no statistically significant variability by response status (ie, no interaction, P = .70). In multivariable analysis, after adjustment for completed cycles of treatment and response to treatment, both baseline QOL and QOL change retained statistically significant prognostic value for long-term survival (P < .001 for each) with minimal change (<0.4%) in the respective adjusted ORs for long-term survival.
Relationship Between QOL, AEs, and Long-Term Survival
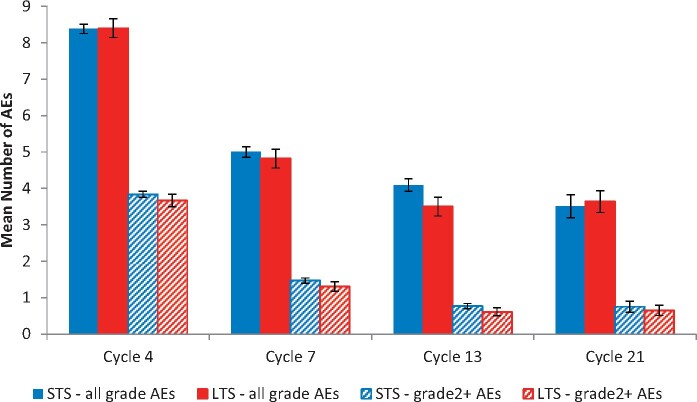
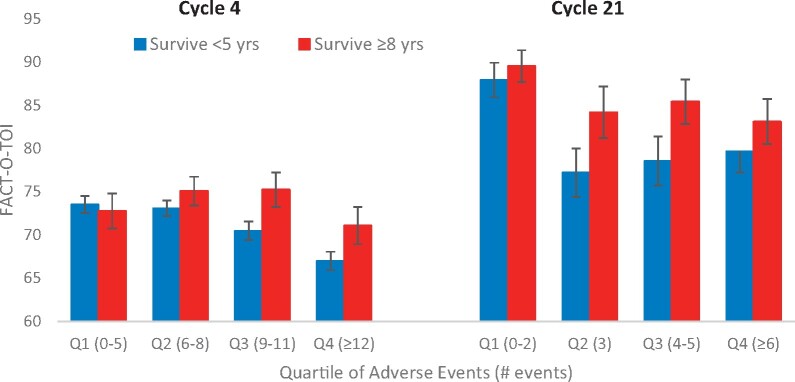
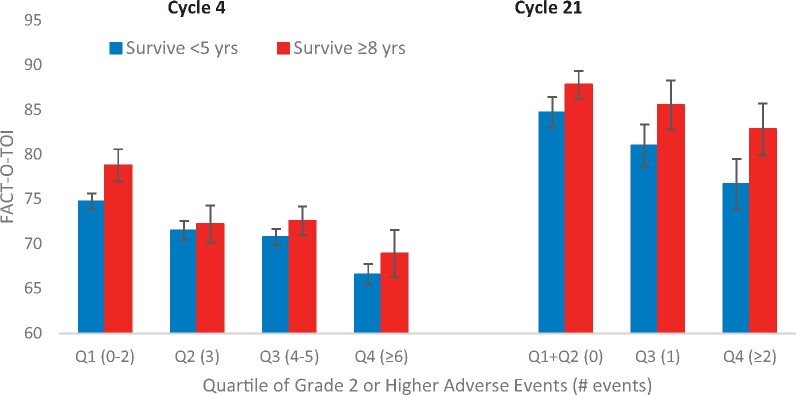
AEs were most frequent at cycle 4, decreasing across the remainder of the treatment period. When AEs were compared between survivor groups, there were no statistically significant differences between the 2 survivor groups with respect to number of all AEs at cycles 4, 7, 13, or 21 after adjusting for baseline characteristics (Figure 2). In early vs later treatment cycle comparisons, QOL decreased statistically significantly with increasing quartiles of AEs (P = .01 at cycle 4 and P = .001 at cycle 21) (Figure 3), and LTS had statistically significantly better QOL compared with STS for each quartile of AEs (P = .03 and P = .01 for cycles 4 and 21, respectively). Similarly, statistically significant QOL decreases were observed across quartiles representing increasing levels of grade 2 or higher AEs (P < .001 at cycle 4, P = .02 at cycle 21) with higher levels of QOL in LTS vs STS at each level of AEs (Figure 4). Associations between QOL and AEs were similar at cycles 7 and 13, with statistically significant decreases in QOL across AE quartiles but nonstatistically significantly higher QOL in LTS.
Figure 2.
Mean number of adverse events (AEs) by survivorship across treatment period. Total number of AEs does not differ statistically significantly between long-term survivors (LTS) and short-term survivors (STS) at cycle 4 (P = .95), cycle 7 (P = .53), cycle 13 (P = .06), or cycle 21 (P = .76). Number of grade 2 or higher AEs does not differ statistically significantly between LTS and STS at cycle 4 (P = .38), cycle 7 (P = .26), cycle 13 (P = .18), or cycle 21 (P = .65). Data are tested using a 2-sided t test. Error bars represent ±1 standard error.
Figure 3.
Functional Assessment of Cancer Therapy–Ovary Trial Outcome Index (FACT-O-TOI) by survivorship and quartiles of all adverse events (AEs). Long-term survivors have higher FACT-O-TOI at cycle 4 (P = .03) and cycle 21 (P = .01). FACT-O-TOI decreases with increasing AEs at cycle 4 (P = .01) and cycle 21 (P = .001). Data are tested using a 2-sided F test. Error bars represent ±1 standard error. Q = quartile.
Figure 4.
Functional Assessment of Cancer Therapy–Ovary Trial Outcome Index (FACT-O-TOI) by survivorship and quartiles of grade 2 or higher adverse events (AEs). Long-term survivors have higher FACT-O-TOI at cycle 4 (P = .05) and cycle 21 (P = .02). FACT-O-TOI decreases with increasing grade 2 or higher AEs at cycle 4 (P < .001) and cycle 21 (P = .02). Data are tested using a 2-sided F test. Error bars represent ±1 standard error. Q = quartile.
We further examined abdominal discomfort, all AEs, and grade 2 or higher AEs at each time point in separate logistic regression models to determine if these measures were statistically significantly related to long-term survival. After adjusting for patient baseline covariates, baseline QOL, and QOL change, abdominal discomfort measured at cycle 4, 7, 13, or 21 was not statistically significantly associated with long-term survival. Further, after adjusting for patient covariates and QOL in multivariable logistic regression models, all AEs and grade 2 or higher AEs measured at any time point were not statistically significantly associated with the probability of long-term survival.
Finally, to potentially provide an additional explanation for short- vs long-term survival, the relationship between AEs, treatment discontinuation, and survival was examined. As reported (18), 66% of patients discontinued treatment prematurely, with progressive disease cited as the most common reason for discontinuation (39% of patients), whereas only 15% discontinued treatment due to AEs. Among those who discontinued due to AEs, no statistically significant differences between LTS and STS in total number of AEs, or grade 2 or higher AEs at cycle 4, cycle 7, or cycle 13 were observed (insufficient data to test at cycle 21). When patients were stratified by quartile of treatment cycles received, mean total AEs, or grade 2+ AEs at cycle 4, 7, 13, or 21, they were not statistically significantly different between LTS and STS. However, after adjusting for cycles of treatment completed, a difference between LTS and STS in QOL and QOL change persisted.
Discussion
In this phase III advanced ovarian cancer clinical trial of more than 1500 patients, we compared differences between STS (<5 years) and LTS (8+ years) on QOL and AE variables. We found that both baseline QOL and longitudinal change in QOL were statistically significantly and independently associated with increased probabilities of being an LTS, reinforcing the prognostic value of both baseline and change scores. Further, the increasing probability of being an LTS, illustrated through the 0.5-SD change in scores, demonstrates a clinically meaningful magnitude of change considered relevant in determining effect sizes for patient-reported outcomes (PROs) (24) and is thought to be large enough to have implications for a patient’s treatment or care (25,26).
With respect to the LTS population, the widening QOL gap is readily apparent as patients are both undergoing (eg, cycle 4) and completing (eg, cycle 21) treatment, with increasing odds of becoming an LTS across the treatment cycles. It is therefore noteworthy that longitudinal change, as measured by the FACT-O-TOI, is a meaningful prognosticator of long-term survival, likely capturing both disease effects and response to treatment. With respect to treatment response, this becomes evident when linking QOL to the treatment response categories as responders, nonmeasurables, or nonresponders. QOL did reflect treatment response and could serve as a marker of clinical benefit. However, independent of response to treatment, there remains a statistically significant difference between LTS and STS in QOL change that is not attributable to the treatment. Therefore, we interpret these data as indications that QOL change over the treatment period is statistically significantly associated with treatment response categories but also stands as an independently statistically significant and robust prognosticator for long- vs short-term survival. These LTS vs STS score gaps are particularly notable because each assessment time point introduces a meaningful opportunity to examine patient-reported deteriorations that might be amenable to supportive care interventions earlier in the treatment trajectory.
With recognition that the number or severity of AEs might influence QOL, we examined that relationship and its association with short- vs long-term survival. This question has important implications for treatment direction, because it is often the case that toxicities could result in treatment delays, dose reductions, or treatment discontinuation, which might in turn affect progression-free survival or overall survival. In this study, where 15% of patients discontinued treatment due to AEs, there were no statistically significant differences between LTS and STS in either mean total AEs or grade 2+ AEs. However, experiencing more or worse AEs was associated with decreased QOL, which is not a surprising finding. It is interesting, however, that the QOL of LTS was consistently better than that of STS for every quartile of both number or increasing grade of AEs, suggesting a certain resilience or tolerance among the LTS, which is being captured specifically through QOL measurement. In short, QOL measurement did differentiate between the STS and LTS groups, whereas the AEs recorded as mean total or 2+ grade did not. Some of this difference, at least related to symptom reports, may be attributed to discrepancies between patient-reported and physician-reported measures of toxicity to the extent they are related to QOL, specifically recognizing that patients are more likely to report more serious toxicities (27) and more AEs across symptoms compared with clinicians (28). Our results provide additional support to recommendations that incorporating PROs into clinical practice can be complementary to clinician-reported data (29), which could improve patients’ health-related QOL, increase treatment adherence (30), and may positively affect overall survival (28).
There is an important interplay between PROs and AEs that deserves further attention, because novel analytic methods accounting for the burden of multiple toxicities, such as those analyzed through a toxicity index (31), may provide a more complete description of the treatment experience. This consideration may be particularly valuable in the recurrent ovarian cancer setting, where the impact of cumulative toxicity associated with multiple lines of therapy on QOL must be weighed against potential impact on progression-free survival (32).
This study supports early work in oncology clinical trials (33,34) as well as recent work (4,9,35) hypothesizing that PRO data are statistically significantly associated with survival because they inform prognostically relevant decreases in well-being earlier than other measures (34). Similarly, patient-reported symptoms and toxicities have been mapped to objective responses and functional status changes during chemotherapy (36), and calculated patient-reported symptoms and physical well-being change scores have been linked to best and worst responses to treatment as well as survival. Taken together, PRO data have been linked to toxicity development, treatment response, and treatment outcomes, including ovarian cancer outcomes (3). This study not only supports this prior literature, but also contributes to this body of work by emphasizing the importance of QOL independently serving as a robust prognostic indicator specifically for long-term ovarian cancer survival, particularly related to QOL change during treatment. This adds value that is not explained by patient covariates, cancer treatment, or treatment adherence. Therefore, real-time QOL score changes could be monitored to identify thresholds for treatment reevaluation and/or supportive care strategies.
By examining QOL across the treatment trajectory in a large, well-controlled clinical trial together with AE development, we identified additional characteristics associated with becoming an LTS of advanced ovarian cancer. We propose that these results underscore the opportunities suggested by many that clinical trial precision can be enhanced by using QOL as a stratification factor (34,35) as well as guiding ovarian cancer care delivery in a meaningful, measurable way to improve QOL, symptom management, and perhaps improve on the numbers of women becoming LTS.
We acknowledge certain limitations of this study. First, the importance of biobehavioral factors, such as social and emotional determinants of well-being, should not be overlooked because they relate to QOL, survival, and long-term survivorship. Psychosocial dimensions assist in characterizing LTS (37), including the importance of high social attachment (38), with strong implications for future directions. Secondly, the low proportion of minorities in this clinical trial does not reflect the distribution of ovarian cancer by race. Because the population in this study was relatively homogeneous, the ability to detect differences that may be present in a more diverse or broader population is limited (39,40). The social determinants of health, including race, ethnicity, and socioeconomic status, are linked to disparities in ovarian cancer health outcomes such as survival (41) and suboptimal practice patterns delivered to underserved women (42). Transportation issues or travel burden experienced by those outside of urban areas (39) are rarely incorporated into disparities of health outcomes in ovarian cancer trials. As clinical trialists develop more robust recruitment efforts to adequately represent the population of ovarian cancer patients, these key issues should be strongly considered.
In summary, our analysis of a large US ovarian cancer clinical trial demonstrates the important contribution of measuring QOL to assist in identifying on-treatment PROs associated with the likelihood of long-term survival. PROs are increasingly important in ovarian cancer clinical trial design, informing regulatory procedures, and adding prognostic value over clinico-pathological factors alone (43,44). Although many have previously identified QOL as prognostic for overall survival, which may be just a matter of months, we believe that recognizing prognostic factors for those who have lived beyond 8 years with this life-threatening illness is very meaningful to the survivor community and may have implications for future treatment considerations. QOL scores, and their change over time, are able to distinguish LTS vs STS, thereby adding clinically meaningful prognostic value in advanced ovarian cancer. Future directions will include development of a long-term survival profile incorporating biologic platforms with patient-reported and clinical factors to improve prognostic accuracy. This direction could directly improve the management possibilities for the majority of advanced ovarian cancer patients.
Funding
This work was supported by the United States Department of Defense (W81XWH-16–2-0038); National Cancer Institute grants NRG Oncology Statistics and Data Management Center (U10 CA180822) and NRG Oncology Operations (U10CA 180868 and UG1CA189867).
Notes
Role of the funder: The funders of this study had no role in study design, data collection, data analysis, data interpretation, writing of the report, or the decision to submit this paper for publication.
Disclosures: David Cella received grants from and consults with Clovis, Bristol Myers Squibb, Abbvie, GSK, AstraZeneca, Novartis, and Pfizer. He is also the President of FACIT.org. George Maxwell received honoraria from Merck and Thermo Fischer and served as an advisor to Kiatek during the period noted. Thomas Conrads is a member of the ThermoFisher Scientific Inc. advisory board. He also received funding from AbbVie Inc. Warner Huh is a consultant for Li-Cor, Altum and Pathovax. He also serves on the data safety and monitoring board for Inovio, though none are related to the field of ovarian cancer and quality of life measures. Mario Leitao received honoraria from Intuitive Surgical. He serves as an advisory board member for JnJ/Ethicon and Takeda and consults for Medtronic. Alessandro Santin received grants from Puma, Immunomedics, Gilead, Synthon, Merck, Boehinger-Ingelheim, Genentech, and Tesaro during the conduct of the study. Robert Burger is Principal Medical Director at Genentech as of May, 2020. He received consulting income from AstraZeneca, Tesaro, Clovis Oncology, Inc, Genentech/Roche, Merck, VGL Therapeutics. He also received income related to service on data monitoring committees for Gradalis, Janssen R&D, and Morphotek. Brad Monk received honoraria from the following companies: Abbvie, Advaxis, Agenus, Amgen, Aravive, AstraZeneca, Asymmetric Therapeutics, Boston Biomedical, ChemoCare, ChemoID, Circulogene, Clovis, Conjupro, Easai, Geistlich, Genmab/Seattle Genetics, GOG Foundation, Gradalis, ImmunoGen, Immunomedics, Incyte, Janssen/Johnson&Johnson, Laekna Health Care, Mateon (formally Oxigene), Merck, Mersana, Myriad, Nucana, Oncomed, Oncoquest, Oncosec, Perthera, Pfizer, Precison Oncology, Puma, Regeneron, Roche/Genentech, Samumed, Takeda, TESARO/GSK, VBL, and Vigeo. All other co-authors have no conflicts of interest to declare.
Author contributions: LW: Conceptualization; Methodology; Investigation; Resources; Writing—Original Draft; Writing—Review and Editing; Visualization; Supervision; Project administration; Funding acquisition. KO: Conceptualization; Methodology; Formal Analysis; Investigation; Data Curation; Writing—Original Draft; Writing—Review and Editing; Visualization. CM: Writing—Original Draft; Writing—Review and Editing; Visualization; Project administration. DC: Conceptualization; Methodology; Writing—Review and Editing. GF: Conceptualization; Methodology; Investigation; Writing—Review and Editing. MJS: Conceptualization; Methodology; Writing—Review and Editing. HAL: Conceptualization; Methodology; Investigation; Writing—Review and Editing. VW: Conceptualization; Methodology; Investigation; Data Curation; Writing—Review and Editing. KPN: Conceptualization; Methodology; Investigation; Writing—Review and Editing. GLM: Conceptualization; Methodology; Investigation; Writing—Review and Editing. SCM: Conceptualization; Methodology; Investigation; Writing—Review and Editing. TPC: Conceptualization; Methodology; Investigation; Writing—Review and Editing. AM: Conceptualization; Methodology; Investigation; Data Curation; Writing—Review and Editing. RSM: Conceptualization; Methodology; Writing—Review and Editing. HJG: Conceptualization; Methodology; Writing—Review and Editing. PH: Conceptualization; Methodology; Writing—Review and Editing. WKH: Conceptualization; Methodology; Writing—Review and Editing. NS: Conceptualization; Methodology; Writing—Review and Editing. MMLJ.: Conceptualization; Methodology; Writing—Review and Editing.GG: Conceptualization; Methodology; Writing—Review and Editing. SKS: Conceptualization; Methodology; Writing—Review and Editing. ADS: Conceptualization; Methodology; Writing—Review and Editing. PS: Conceptualization; Methodology; Writing—Review and Editing. SBL: Conceptualization; Methodology; Writing—Review and Editing. RAB: Conceptualization; Methodology; Investigation; Writing—Review and Editing. BJM: Conceptualization; Methodology; Investigation; Writing—Review and Editing. MB: Conceptualization; Methodology; Investigation; Resources; Writing—Review and Editing; Visualization; Funding acquisition.
Acknowledgements: This study was supported by National Cancer Institute grants NRG Oncology SDMC grant U10 CA180822, NRG Oncology Operations grant U10CA 180868 and UG1CA189867 (NCORP). The clinical trial upon which this manuscript is based was sponsored by the Cancer Therapy Evaluation Program (CTEP) of the National Cancer Institute (NCI), under the Collaborative Research and Development Agreement (CRADA) for bevacizumab between NCI and Genentech, Inc. The authors also wish to acknowledge the support of the Chao Family Comprehensive Cancer Center Biobehavioral Shared Resource, supported by the National Cancer Institute of the National Institutes of Health under award number P30CA062203. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The following NRG Oncology/Gynecologic Oncology Group member institutions participated in the primary treatment studies: University of Oklahoma Health Sciences Center, Washington University School of Medicine, Abington Memorial Hospital—Asplundh Cancer Pavilion, University of Alabama at Birmingham, Women's Cancer Center of Nevada, Memorial Sloan-Kettering Cancer Center, University of Kentucky, Sudarshan K Sharma MD Limited—Gynecologic Oncology, Yale University, Metro-Minnesota CCOP, Roswell Park Comprehensive Cancer Center, Women and Infants Hospital, Mount Sinai School of Medicine, Northwestern University, Morristown Medical Center, Abramson Cancer Center of the University of Pennsylvania, Duke University Medical Center, University of Hawaii, Center of Hope at Renown Medical Center, Washington University School of Medicine, Fox Chase Cancer Center, The Hospital of Central Connecticut, Ohio State University Comprehensive Cancer Center, University of California Medical Center At Irvine-Orange Campus, Mayo Clinic, Walter Reed National Military Medical Center, Saitama Medical University International Medical Center, Gynecologic Oncology Network/Brody School of Medicine, Rush University Medical Center, University of Iowa Hospitals and Clinics, Fred Hutchinson Cancer Research Center, University of Chicago, Cleveland Clinic Foundation, University of Mississippi Medical Center, University of North Carolina at Chapel Hill, Cooper Hospital University Medical Center, Indiana University Hospital/Melvin and Bren Simon Cancer Center, Seoul National University Hospital, University of Colorado Cancer Center—Anschutz Cancer Pavilion, University of California at Los Angeles Health System, Wake Forest University Health Sciences, University of New Mexico, Stony Brook University Medical Center, University of Virginia, Case Western Reserve University, Fletcher Allen Health Care, Georgia Center for Oncology Research and Education (CORE), Cancer Research for the Ozarks NCORP, Wayne State University/Karmanos Cancer Institute, University of Minnesota Medical Center-Fairview, Northern Indiana Cancer Research Consortium, Tufts-New England Medical Center, University of Pittsburgh Cancer Institute (UPCI), State University of New York Downstate Medical Center, M D Anderson Cancer Center, Moffitt Cancer Center and Research Institute, University of Wisconsin Hospital and Clinics, University of Texas—Galveston, Gynecologic Oncology of West Michigan PLLC, Carle Cancer Center, Cancer Research Consortium of West Michigan NCORP, Central Illinois CCOP, Virginia Commonwealth University, Saint Vincent Hospital, Penn State Milton S Hershey Medical Center, New York University Medical Center, Michigan Cancer Research Consortium Community Clinical Oncology Program, Northern New Jersey CCOP, University of Cincinnati, Memorial Sloan Kettering Cancer Center, University of Massachusetts Memorial Health Care, Aurora Women's Pavilion of Aurora West Allis Medical Center, Kansas City CCOP, Wisconsin NCI Community Oncology Research Program, Missouri Valley Cancer Consortium CCOP, Delaware/Christiana Care CCOP, William Beaumont Hospital, Saint Louis-Cape Girardeau CCOP, and Wichita CCOP.
Data Availability
The data supporting published research results will be transferred to a public data repository and made available to the public at the time of initial publication.
References
- 1. Timmermans M, Sonke GS, Van de Vijver KK, van der Aa MA, Kruitwagen RFPM.. No improvement in long-term survival for epithelial ovarian cancer patients: a population-based study between 1989 and 2014 in the Netherlands. Eur J Cancer. 2018;88:31–37. [DOI] [PubMed] [Google Scholar]
- 2. Cress RD, Chen YS, Morris CR, Petersen M, Leiserowitz GS.. Characteristics of long-term survivors of epithelial ovarian cancer. Obstet Gynecol. 2015;126(3):491–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Phippen NT, Secord AA, Wolf S, et al. Quality of life is significantly associated with survival in women with advanced epithelial ovarian cancer: an ancillary data analysis of the NRG Oncology/Gynecologic Oncology Group (GOG-0218) study. Gynecol Oncol. 2017;147(1):98–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Quinten C, Martinelli F, Coens C, et al. ; on behalf of Patient Reported Outcomes and Behavioral Evidence (PROBE) and the European Organization for Research and Treatment of Cancer (EORTC) Clinical Groups. A global analysis of multitrial data investigating quality of life and symptoms as prognostic factors for survival in different tumor sites. Cancer. 2014;120(2):302–311. [DOI] [PubMed] [Google Scholar]
- 5. Wenzel L, Huang HQ, Monk BJ, Rose PG, Cella D.. Quality-of-life comparisons in a randomized trial of interval secondary cytoreduction in advanced ovarian carcinoma: a Gynecologic Oncology Group study. J Clin Oncol. 2005;23(24):5606–5612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. von Gruenigen VE, Huang HQ, Gil KM, Frasure HE, Armstrong DK, Wenzel LB.. The association between quality of life domains and overall survival in ovarian cancer patients during adjuvant chemotherapy: a Gynecologic Oncology Group study. Gynecol Oncol. 2012;124(3):379–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Greimel E, Daghofer F, Petru E.. Prospective assessment of quality of life in long-term ovarian cancer survivors. Int J Cancer. 2011;128(12):3005–3011. [DOI] [PubMed] [Google Scholar]
- 8. Carey MS, Bacon M, Tu D, Butler L, Bezjak A, Stuart GC.. The prognostic effects of performance status and quality of life scores on progression-free survival and overall survival in advanced ovarian cancer. Gynecol Oncol. 2008;108(1):100–105. [DOI] [PubMed] [Google Scholar]
- 9. Ediebah DE, Quinten C, Coens C, et al. ; for the Canadian Cancer Trials Group and the European Organization for Research and Treatment of Cancer. Quality of life as a prognostic indicator of survival: a pooled analysis of individual patient data from Canadian Cancer Trials Group clinical trials. Cancer. 2018;124(16):3409–3416. [DOI] [PubMed] [Google Scholar]
- 10. Quinten C, Coens C, Mauer M, et al. Baseline quality of life as a prognostic indicator of survival: a meta-analysis of individual patient data from EORTC clinical trials. Lancet Oncol. 2009;10(9):865–871. [DOI] [PubMed] [Google Scholar]
- 11. Ediebah DE, Coens C, Zikos E, et al. Does change in health-related quality of life score predict survival? Analysis of EORTC 08975 lung cancer trial. Br J Cancer. 2014;110(10):2427–2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Greer JA, Amoyal N, Nisotel L, et al. A systematic review of adherence to oral antineoplastic therapies. Oncologist. 2016;21(3):354–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cheville AL, Alberts SR, Rummans TA, et al. Improving adherence to cancer treatment by addressing quality of life in patients with advanced gastrointestinal cancers. J Pain Symptom Manage. 2015;50(3):321–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mazzotti E, Cappellini GCA, Buconovo S, et al. Treatment-related side effects and quality of life in cancer patients. Support Care Cancer. 2012;20(10):2553–2557. [DOI] [PubMed] [Google Scholar]
- 15. Chau I, Fuchs C, Ohtsu A, et al. Associations of quality of life (QoL) with adverse events and tumor response in patients with advanced gastric cancer: exploratory analyses from RAINBOW and REGARD. Ann Oncol. 2017;28(suppl_3):III142–III143. [Google Scholar]
- 16. Basch E, Jia X, Heller G, et al. Adverse symptom event reporting by patients vs clinicians: relationships with clinical outcomes. J Natl Cancer Inst. 2009;101(23):1624–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Laugsand EA, Sprangers MA, Bjordal K, Skorpen F, Kaasa S, Klepstad P.. Health care providers underestimate symptom intensities of cancer patients: a multicenter European study. Health Qual Life Outcomes. 2010;8(1):104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Burger RA, Brady MF, Bookman MA, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med. 2011;365(26):2473–2483. [DOI] [PubMed] [Google Scholar]
- 19. Monk BJ, Huang HQ, Burger RA, et al. Patient-reported outcomes of a randomized, placebo-controlled trial of bevacizumab in the front-line treatment of ovarian cancer: a Gynecologic Oncology Group study. Gynecol Oncol. 2013;128(3):573–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tewari KS, Burger RA, Enserro D, et al. Final overall survival of a randomized trial of bevacizumab for primary treatment of ovarian cancer. J Clin Oncol. 2019;37(26):2317–2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Basen-Engquist K, Bodurka-Bevers D, Fitzgerald MA, et al. Reliability and validity of the functional assessment of cancer therapy-ovarian. J Clin Oncol. 2001;19(6):1809–1817. [DOI] [PubMed] [Google Scholar]
- 22. Wenzel L, Huang HQ, Cella D, Walker JL, Armstrong DK, Gynecologic Oncology Group. Validation of FACT/GOG-AD subscale for ovarian cancer-related abdominal discomfort: a Gynecologic Oncology Group study. Gynecol Oncol. 2008;110(1):60–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Cancer Institute. Cancer Therapy Evaluation Program. Common Terminology Criteria for Adverse Events v3.0. April 9, 2006. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf2006.
- 24. Yost KJ, Eton DT, Garcia SF, Cella D.. Minimally important differences were estimated for six PROMIS-Cancer scales in advanced-stage cancer patients. J Clin Epidemiol. 2011;64(5):507–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wyrwich KW, Bullinger M, Aaronson N, et al. ; Clinical Significance Consensus Meeting Group. Estimating clinically significant differences in quality of life outcomes. Qual Life Res. 2005;14(2):285–295. [DOI] [PubMed] [Google Scholar]
- 26. Norman GR, Sloan JA, Wyrwich KW.. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care. 2003;41(5):582–592. [DOI] [PubMed] [Google Scholar]
- 27. Basch E, Iasonos A, McDonough T, et al. Patient versus clinician symptom reporting using the National Cancer Institute Common Terminology Criteria for Adverse Events: results of a questionnaire-based study. Lancet Oncol. 2006;7(11):903–909. [DOI] [PubMed] [Google Scholar]
- 28. Basch E, Dueck AC, Rogak LJ, et al. Feasibility assessment of patient reporting of symptomatic adverse events in multicenter cancer clinical trials. JAMA Oncol. 2017;3(8):1043–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kluetz PG, Slagle A, Papadopoulos EJ, et al. Focusing on core patient-reported outcomes in cancer clinical trials: symptomatic adverse events, physical function, and disease-related symptoms. Clin Cancer Res. 2016;22(7):1553–1558. [DOI] [PubMed] [Google Scholar]
- 30. Basch E, Deal AM, Kris MG, et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol. 2016;34(6):557–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gresham G, Diniz MA, Razaee ZS, et al. Evaluating treatment tolerability in cancer clinical trials using the toxicity index. J Natl Cancer Inst. 2020;112(12):1266–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Friedlander M, Gebski V, Gibbs E, et al. Health-related quality of life and patient-centred outcomes with olaparib maintenance after chemotherapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT Ov-21): a placebo-controlled, phase 3 randomised trial. Lancet Oncol. 2018;19(8):1126–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dancey J, Zee B, Osoba D, et al. Quality of life scores: an independent prognostic variable in a general population of cancer patients receiving chemotherapy. The National Cancer Institute of Canada Clinical Trials Group. Qual Life Res. 1997;6(2):151–158. [DOI] [PubMed] [Google Scholar]
- 34. Gotay CC, Kawamoto CT, Bottomley A, Efficace F.. The prognostic significance of patient-reported outcomes in cancer clinical trials. J Clin Oncol. 2008;26(8):1355–1363. [DOI] [PubMed] [Google Scholar]
- 35. Gotay C. Fatigue and mortality: from description to action. Lancet Oncol. 2015;16(15):1445–1446. [DOI] [PubMed] [Google Scholar]
- 36. Wagner LI, Beaumont JL, Ding B, et al. Measuring health-related quality of life and neutropenia-specific concerns among older adults undergoing chemotherapy: validation of the Functional Assessment of Cancer Therapy-Neutropenia (FACT-N). Support Care Cancer. 2008;16(1):47–56. [DOI] [PubMed] [Google Scholar]
- 37. Lutgendorf SK, Shinn E, Carter J, et al. Quality of life among long-term survivors of advanced stage ovarian cancer: a cross-sectional approach. Gynecol Oncol. 2017;146(1):101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lutgendorf SK, De Geest K, Bender D, et al. Social influences on clinical outcomes of patients with ovarian cancer. J Clin Oncol. 2012;30(23):2885–2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Moss JL, Murphy J, Filiaci VL, Wenzel LB, Minasian L, Temkin SM.. Disparities in health-related quality of life in women undergoing treatment for advanced ovarian cancer: the role of individual-level and contextual social determinants. Support Care Cancer. 2019;27(2):531–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mishkin G, Minasian LM, Kohn EC, Noone AM, Temkin SM.. The generalizability of NCI-sponsored clinical trials accrual among women with gynecologic malignancies. Gynecol Oncol. 2016;143(3):611–616. [DOI] [PubMed] [Google Scholar]
- 41. Bandera EV, Lee VS, Rodriguez-Rodriguez L, Powell CB, Kushi LH.. Racial/ethnic disparities in ovarian cancer treatment and survival. Clin Cancer Res. 2016;22(23):5909–5914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bristow RE, Chang J, Ziogas A, Anton-Culver H, Vieira VM.. Spatial analysis of adherence to treatment guidelines for advanced-stage ovarian cancer and the impact of race and socioeconomic status. Gynecol Oncol. 2014;134(1):60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Roncolato FT, Gibbs E, Lee CK, et al. Quality of life predicts overall survival in women with platinum-resistant ovarian cancer: an AURELIA substudy. Ann Oncol. 2017;28(8):1849–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hilpert F, Du Bois A.. Patient-reported outcomes in ovarian cancer: are they key factors for decision making? Expert Rev Anticancer Ther. 2018;18(sup1):3–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting published research results will be transferred to a public data repository and made available to the public at the time of initial publication.