Abstract
Cellular senescence is an essential tumor suppressive mechanism that prevents the propagation of oncogenically activated, genetically unstable, and/or damaged cells. Induction of tumor cell senescence is also one of the underlying mechanisms by which cancer therapies exert antitumor activity. However, an increasing body of evidence from preclinical studies demonstrates that radiation and chemotherapy cause accumulation of senescent cells (SnCs) both in tumor and normal tissue. SnCs in tumors can, paradoxically, promote tumor relapse, metastasis, and resistance to therapy, in part, through expression of the senescence-associated secretory phenotype. In addition, SnCs in normal tissue can contribute to certain radiation- and chemotherapy-induced side effects. Because of its multiple roles, cellular senescence could serve as an important target in the fight against cancer. This commentary provides a summary of the discussion at the National Cancer Institute Workshop on Radiation, Senescence, and Cancer (August 10-11, 2020, National Cancer Institute, Bethesda, MD) regarding the current status of senescence research, heterogeneity of therapy-induced senescence, current status of senotherapeutics and molecular biomarkers, a concept of “one-two punch” cancer therapy (consisting of therapeutics to induce tumor cell senescence followed by selective clearance of SnCs), and its integration with personalized adaptive tumor therapy. It also identifies key knowledge gaps and outlines future directions in this emerging field to improve treatment outcomes for cancer patients.
Cells become senescent after extensive replication that causes telomere shortening (1) or from exposure to genotoxic, oncogenic, and/or oxidative stress (2). Senescent cells (SnCs) induced by different stimuli share some common characteristics including an essentially stable growth arrest, relative resistance to apoptosis, persistent DNA damage signaling, changes in heterochromatin, decreased lamin-B1 levels, and increased expression of the cyclin-dependent kinase (CDK) inhibitors, p16INK4a (p16, encoded by the INK4a/ARF locus, also known as Cdkn2a), p21Cip1/Waf1 (p21, encoded by Cdkn1a) (3) and senescence-associated β-galactosidase (SA-β-gal) (4). SnCs secrete a plethora of factors, including proinflammatory cytokines, chemokines, matrix metalloproteinases, bioactive lipids, noncoding nucleotides (miRNAs, mitochondrial DNA), vesicles, and growth factors, collectively termed the senescence-associated secretory phenotype (SASP) (5-10). SnCs can exist in a continuum of states and contribute to a variety of physiological and pathophysiological processes, including organogenesis and wound healing (11). Cellular senescence is also a critical barrier for tumorigenesis, preventing division of cells with oncogene activation and genetic instability and promoting immune clearance of these cells, in part, through the SASP (12).
Senescence occurs after treatment with radiation and/or certain chemotherapies, known as therapy-induced senescence (TIS) (13,14), through induction of DNA double-strand breaks (DSBs) (15). On one hand, senescence can contribute to antitumor effects and treatment outcomes (16); on the other, chronic accumulation of SnCs can stimulate relapse and metastasis (17). Both these effects have been linked to the SASP and clearly suggest the importance of cellular and tissue context. For example, some tumor cells may escape TIS with the acquisition of genomic changes that confer treatment resistance (18), especially under a p53-deficient environment and p21-driven genomic instability (19). Also, malignant cells reprogrammed by TIS to acquire a change in lineage and/or stemness can become self-renewing tumor-initiating cells that cause tumor relapse and promote aggressive growth (20–22). Studies demonstrated that transplanting relatively small numbers of senescent cells into young mice was sufficient to cause persistent physical dysfunction and spread cellular senescence to host tissues (23). Through the SASP, SnCs can contribute to treatment-induced side effects such as myelosuppression, fatigue, and cardiovascular dysfunction (24). Thus, for cancer treatment, inhibiting the induction of senescence is detrimental, whereas promoting posttreatment SnC clearance is beneficial, timing being critical. Therefore, targeting SnCs, with senotherapeutics, including senomorphics (small molecules that partly suppress senescence phenotypes such as the SASP without cell killing) and senolytics (small molecules that induce SnCs death), is an emerging strategy for cancer treatment (25,26).
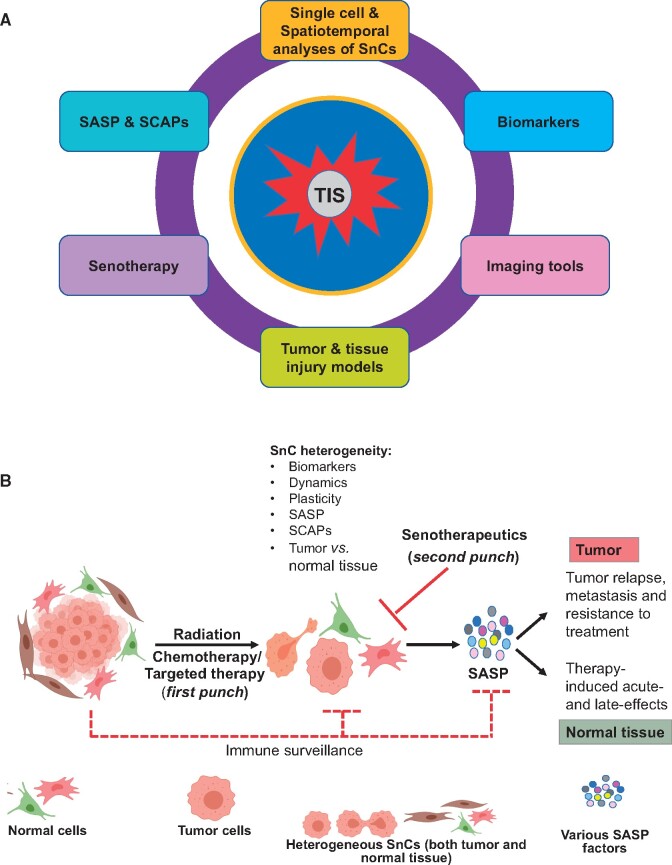
Understanding the molecular pathways that regulate senescence in cancer can generate novel insights to guide the discovery of unique anticancer agents and molecular biomarkers. This approach may spur the development of novel “one-two punch” cancer treatments consisting of agents that induce tumor cell senescence followed by senolytics to selectively clear SnCs (27,28) in tumor and normal tissue, a strategy that has the potential to improve therapy and concurrently mitigate many treatment-related side effects (24). This commentary summarizes the research discussions on important knowledge gaps to exploit TIS (Figure 1, A) and one-two punch cancer therapy on patient outcomes (Figures 1, B and 2) at the National Cancer Institute (NCI) Workshop on Radiation, Senescence, and Cancer (August 10-11, 2020, Bethesda, MD, USA).
Figure 1.
Senescent cell as a target in one-two punch cancer therapy. A) Key knowledge gaps and future directions to advance one-two punch cancer therapy. B) Therapy-induced senescence (TIS) and one-two punch cancer therapy. Cancer therapies (first punch) induce senescence both in tumor and normal tissue. SnCs are normally cleared by immune surveillance but can accumulate after cancer therapy. Therapy-induced SnCs are heterogeneous and dynamic, which is also reflected in biomarkers, cellular plasticity, expression of SASPs and SCAPs, tissue of origin, and cell lineage. Selective clearance of SnCs with a serotherapeutic (second punch) in tumors will prevent tumor relapse, metastasis, and development of resistance to treatment. Similarly, selective clearance of SnCs in normal tissue in a spatiotemporal dynamic environment will prevent, mitigate, and treat therapy-induced side effects and restore tissue homeostasis. However, time of administration of the second punch therapy will be important to improve efficacy. The figure was created with BioRender.com. SnCs = senescent cells; SASP = senescence-associated secretory phenotype; SCAPs = senescent cell anti-apoptotic pathways.
Figure 2.
Schematic diagram of an approach to integrate one-two punch cancer therapy with personalized adaptive tumor therapy. To therapeutically exploit and benefit from the differences in response to treatment between tumor and normal tissue for the best patient outcome, factors that should be considered for pretreatment planning include tumor molecular profiling, tumor heterogeneity, imaging, identification of target(s), metabolic status, and planned integrated biomarkers for tumor diagnosis and treatment matching (136). Similar profiling of normal tissue response to treatment may include determination of genetic susceptibility, immune status, stromal tissue subsets, the impact of the anatomical location of the tumor on normal tissue, metabolic status, and biomarkers that predict response and adverse effects. In a one-two punch therapy, punch 1 may include spatially targeted radiotherapy (eg, dose-boost to hypoxic regions), molecularly targeted drugs, and/or immune therapy to the tumor, which will induce TIS in the tumor, stroma, and bystander tissue. Thus, tumor, stroma, and bystander tissue all need to be evaluated for TIS for the second punch to be successful. Biomarker-driven TIS evaluation will be essential to optimize immune modulation, dose, and schedule of the second punch with a suitable senolytic. Along with dynamic adaptive tumor targeting (with drugs, immune modulators, and radiation), the use of different types of senolytics may be necessary to address spatial, temporal, and tissue heterogeneity among tumors and senescent cells. Repeat treatment courses (punch #n) with senotherapeutics (senolytics or senomorphics) may be necessary to prevent tumor recurrence, drug resistance, plasticity, and normal tissue injury and mitigate and/or treat adverse effects months to years after completing the one-two punch therapy for optimal tissue remodeling and tissue function restoration. Dotted boxes represent current biomarkers and future opportunities to develop diagnostics or therapeutics for precision medicine in TIS. Tissues are indicated by the colors red (tumor), green (normal tissue), blue (stroma and immune related to tumor), and brown (bystander tissues). The figure was created with BioRender.com. Rx = prescription; TIS = therapy-induced senescence.
Novel Cellular, Molecular, and Epigenetic Mechanisms of Senescence
Whether senescence is a state of permanent growth arrest or reversible is controversial (29,30). Senescence is one avenue whereby tumor cells evade the direct cytotoxic impact of therapy, thereby allowing for prolonged survival in a dormant state, with the potential to recover self-renewal capacity and contribute to disease recurrence (31). Although unrepaired DSBs are a well-recognized trigger of senescence, it can also be acquired in the absence of DNA damage response (DDR) or following DDR pathway activation in the absence of DNA damage (32). Senescence can occur after treatment with inhibitors of CDK4/6 (33,34), Polo and Aurora kinases (34,35), histone deacetylases, and other epigenetic modifiers (36). Furthermore, cells may reenter the cell cycle subsequent to a prolonged senescence arrest to produce progeny with chromosomal instability or a cancer stem cell-like phenotype (20) providing a survival advantage. Therefore, it is important to distinguish “irreversible senescence arrest” from “senescence-like arrest” (37), because the cells reentering the cell cycle after senescence-like arrest may contribute to treatment failure.
Although TIS is commonly associated with unrepaired DSBs, it may also be induced after damage to mitochondria (38–41), which in turn leads to increased production of reactive oxygen species (42) and DNA damage (43). Senescence in quiescent endothelial cells can be induced through 2 independent pathways: 1) activation of DDR and p53 and 2) dysfunction of mitochondria (41,44). In addition, other mitochondrial and cytoplasmic metabolic pathways, including glycolysis and glutaminolysis, are critical mediators of DSB repair and DNA damage checkpoint responses that regulate senescence. The hexosamine biosynthetic pathway and downstream protein O-GlcNAcylation are attractive druggable targets to modulate radiation-induced senescence (45,46). Therefore, studies on regulation of the mitochondrial and other metabolic pathways impacting chromosomal integrity and senescence are essential for the development of novel mitigators of therapy-induced toxicities.
There is now evidence for metabolic (47) and stem cell-like remodeling (20,48) among SnCs and for the presence of an immunogenic switch that renders SnCs susceptible to an adaptive T-cell attack (49). Given this dynamic nature of the senescent state and the occasional cell-cycle reentry of previously SnCs, plasticity-associated functional capabilities may become particularly relevant to selective senescence escape and tumor reprogression.
Gopal and colleagues (50) used single-cell RNA-sequencing (RNAseq) and fluorescence reporters representing distinct transcriptional cell states to model cell-state dynamics and TIS at the single-cell level. They found that phenotypic switching during chemotherapy, influenced by a range of possible senescence scenarios, caused the persistence of quiescent tumor programs. Some of these cells subsequently reverted to more proliferative states with variable time intervals across individual tumors, resulting in the development of treatment resistance and aggressive recurrence. Understanding distinct cell states and how single-cell behaviors establish phenotypic equilibrium in cancer populations and the role that SnCs play in this process is pivotal in elucidating the critical cell fate switches that may underlie treatment resistance (workshop presentations: “Senescent Cells as both Drivers and Suppressors of Radiation-Induced Cancers,” J. Campisi, Buck Institute, Novato, CA, and “Animal Models to Study the Role of Senescence in Diseases and Cancer,” Jan van Deursen, Rochester, MN).
Nevertheless, therapeutic targeting of SnCs is a balancing act that must not affect the beneficial effects of senescence while targeting pathways of pro-tumorigenic and pathological senescence. Future studies could evaluate strategies to integrate senolytic therapies effectively into aggressive anticancer regimens to reduce late toxicities while enhancing cure.
Heterogeneity of SnCs
Heterogeneity among SnCs is contextual, influenced by the cell type and tissue of origin, the nature of the insult causing senescence, and the elapsed time after the insult occurs. Characterization of SnC heterogeneity is fundamental to understanding its role in tumorigenesis and developing cancer treatments, such as one-two punch cancer therapy (27,28) (detailed below). Box 1 provides some important considerations regarding SnC heterogeneity.
Box 1.
Important gaps and considerations in the understanding of the heterogeneity of therapy-induced senescence (TIS)
TIS is heterogeneous and context (e.g., tissue of origin, nature of stress, and time after insults) dependent, and therefore, characterization in a variety of contexts is important for the development of novel approaches to cancer therapeutics such as one-two punch therapy.
Senescent cell (SnC) heterogeneity is also reflected in the use of different SnC anti-apoptotic pathways to resist cell death.
Many TIS-induced SnCs escape from growth arrest, which can result in the acquisition of plasticity, stemness, tumorigenic, and aggressive growth phenotypes.
SnC transcriptomes are also heterogenous, can exert contextually dichotomous effects on responses to therapy, and tumorigenesis.
Specific senescence-associated secretory phenotype factors can modulate the responses of tumor cells and normal tissues to therapy, resulting in inhibition and/or promotion of tumorigenesis and induction of normal tissue injury.
The creation of a comprehensive atlas of SnCs may accelerate the discovery and development of novel biomarkers and senotherapeutics as next-generation anticancer agents to achieve better outcomes for cancer patients.
Time of administration of senotherapeutics may be an essential determinant in the efficacy and toxicity profiles of anticancer agents.
First, heterogeneity of SnCs is reflected in their ability to employ different senescent cell anti-apoptotic pathways (SCAPs) (51,52). For example, senescent endothelial cells rely on the anti-apoptotic protein Bcl-xL for survival and thus are sensitive to Bcl-xL inhibitors, whereas senescent adipocyte progenitors are more sensitive to a pan-tyrosine kinase inhibitor, Dasatinib (52).
Second, many SnCs can escape growth arrest by a variety of mechanisms. For example, in TIS lymphoma cells, inactivation of the H3K9 histone methyltransferase, Suv39h1, or p53 can reverse their growth arrest (11,20,53). Similar observations were made in senescent melanocytes induced by RAS/BRAF oncogenes after ectopic transfection with the lysine-specific demethylase-1 and the Jumonji C domain-containing histone demethylase, JMJD2C (53). Infrequent but spontaneous escape from TIS is seen in breast, non-small cell lung, colon, and ovarian cancer cells after therapy (54). Senescence escape is often associated with high expression of CDC2 and polyploidy (55). TIS cells can reprogram to acquire stemness and become more tumorigenic and drug resistant after senescence escape (54). Thus, strategies to induce and subsequently remove residual SnCs at the right time after treatment may improve therapeutic efficacy and help mitigate treatment morbidity, thus improving patient quality of life (QOL).
Third, the SnC transcriptome is highly heterogeneous and can exert opposite effects on tumorigenesis and response to therapy. The composition and quantity of individual SASP factors secreted by SnCs can vary among cell types and depend on the stimuli (8,17,56). The comprehensive soluble SASP atlases of senescent human fibroblasts induced by radiation, RAS overexpression, or atazanavir (a HIV protease inhibitor), and radiation-induced senescent renal epithelial cells indicated that only 17 soluble SASP factors are shared among many SnCs, whereas several other factors varied depending on tissue type and insults (8). In contrast, mesenchymal stromal cells exposed to different stressors showed common senescent phenotypes characterized by 4 classes of SASP components among several phenotypes: extracellular matrix and cytoskeleton and/or cell junctions, metabolic processes, redox factors, and regulators of gene expression (57). Specific SASP factors can modulate response to therapy. The SASP factors IL-1α, IL-6, TGF-β, CXCL1, and CXCL2 secreted by oncogene-induced senescence in human fibroblasts can reinforce senescence in an autocrine manner and also induce senescence in adjacent cells through a paracrine mechanism (17,58,59). Interestingly, similar paracrine signaling is mediated by small extracellular vesicles (evSASP or extracellular vesicle SASP) released by human fibroblasts undergoing oncogene-induced senescence and in MCF7 cancer cells treated with palbociclib (a CDK4/6 inhibitor) (7).
Through secretion of SASP proinflammatory factors, senescent tumor and stromal cells can promote immune cell infiltration to clear SnCs from the tumor microenvironment (34). Similarly, activation of p53 in hepatic stellate cells due to senescence induced by CCl4 and the carcinogen diethyl-nitrosamine in mice reduced fibrosis and cirrhosis, besides inhibiting liver epithelial tumorigenesis because of immune clearance of SnCs (60). Moreover, combining a CDK4/6 inhibitor or an Aurora kinase A inhibitor with an MDM2 inhibitor resulted in induction of senescence, followed by SASP-induced tumor infiltration of cytotoxic T cells and immune clearance of the senescent tumor cells (61,62). Further, inducing TIS with combination of Trametinib (a MEK inhibitor) and palbociclib activates an immune-modulatory SASP, which in the KrasG12D/+; Trp53−/− lung cancer mouse model results in tumor regression and prolonged survival through activation of natural killer cells. However, TIS in KrasG12D/+; Trp53−/− pancreas cancer renders immunologically “cold” tumors “hot,” and the resulting cytotoxic T-cell infiltration, when combined with immune checkpoint blockade, stimulates tumor regression and prolongs survival (12,63). Further characterization of the impact of SnC clearance with senotherapeutics using immunocompetent mice will help validate the ability of such anticancer agents to improve the efficacy of treatments without compromising antitumor immunity.
Studies on the heterogeneity of SnCs provide unique opportunities to mechanistically dissect the “molecular web” of various pathways defined by contexts, such as specific cancer types, host tissues, stressors, and mechanism-based clinically relevant interventions. Despite many advances in the visualization, quantification, and characterization of SnCs in tissues and organs, several aspects of senescence biology remain unknown. Some noteworthy efforts to characterize SnCs include the creation of a “tumor SnC atlas” with technologies, such as single-cell sequencing (64,65), gene ontology, and ingenuity pathway analyses (56), and establishment of spatial, temporal, and dynamic relationships between SnCs and tumor cells using cytometry by time of flight (66). Single-cell RNAseq data presented at the workshop demonstrated that TIS cells in culture and those isolated from aged mouse kidney exhibit different characteristics, and only a small percentage of p16+ SnCs from the kidney are primarily responsible for the production of profibrotic SASP factors (workshop presentations: “Senescent Cells as Both Drivers and Suppressors of Radiation-Induced Cancers,” Judith Campisi, Buck Institute, Novato, CA, and “Animal Models to Study the Role of Senescence in Diseases and Cancer,” Jan van Deursen, Rochester, MN).
Intriguing mechanisms by which different SnCs use different SCAPs to resist apoptosis have to be elucidated (67). However, at the core of SnC heterogeneity, characterization of whole-transcriptome datasets seems to provide a fingerprint of 55 senescence-associated gene transcriptomes in fibroblasts (56). Such tissue-specific characterization in a variety of cancers, particularly the specific SCAPs used by different SnCs in a TME, can lead to the discovery of novel targets and biomarkers. This will allow specific targeting of the SnCs that are more tumorigenic and immune-suppressive.
Given the heterogeneity of SnCs, there is currently limited knowledge relating to its drivers and consequences and how these processes could be harnessed to improve human health. The National Institutes of Health has recently identified 5 broad areas, in general, to advance senescence research: identification and characterization of SnCs, creation of senescent cell atlases, studies of biomarkers, model systems, and imaging tools (68). Research in these cross-disciplinary areas will also stimulate and synergize research on TIS and its application to improve anticancer therapy. However, biomarkers, model systems, and imaging tools will require validation projects in which pathways of senescence are perturbed to induce, eliminate, and modulate senescence and then assess the impact of those perturbations on health and disease (68). Such demonstration projects could ideally use radiation at different doses and schedules to induce and study cellular perturbations, including generation and characterization of SnCs (69).
Senotherapeutics
The advent of senotherapeutics over the last 5 years has enabled many proof-of-concept studies for age-related diseases (66,67,70,71). Although senomorphics, such as the mTOR inhibitor rapamycin (72), JAK1/2 inhibitor ruxolitinib (73), and BET inhibitor JQ1 (74) may also be useful, the focus has been on senolytics. Table 1 provides a summary of senotherapeutics currently under various stages of development. Over the last decade, many targets for senolytics have been discovered, and several senolytics have been tested in preclinical models. However, the translation of senolytics to the clinic has been challenging for a number of reasons. These include, but are not limited to, SnCs heterogeneity across different tissues, organs, and model systems; the selectivity of drugs to deleterious SnCs; systemic toxicities; and development of drug resistance (75). Box 2 summarizes important pitfalls, challenges, and opportunities underlying the development of senolytics as anticancer agents for clinical use.
Table 1.
Summary of senotherapeutics at various stages of developmenta
| Drug class | Agent (company) | Mechanism of action | Developmental stage | Reference(s) |
|---|---|---|---|---|
| Natural products and derivatives | Alvespimycin, Geldanamycin, and Tanespimycin | HSP inhibitors | Optimization | Fuhrmann-Stroissnigg et al. 2017 (125) |
| Curcumin analog, EF24 | Promotes degradation of anti-apoptotic Bcl-2 proteins | Discovery | Li et al. 2017 (126) | |
| Piperlongumine and analogs | OXR1 and other | Discovery | Liu et al. 2018 (127), Wang et al. 2016 (128), Zhang et al. 2018 (129) | |
| Cardiac glycosides: Digoxin, Ouabain, and Proscillaridin A | Na+/K+ ATPase inhibitor | Discovery/Drug repurposing | Guerrero et al. 2019 (93), Triana-Martinez et al. 2019 (94) | |
| Fisetin | Blocks PI3K/AKT/mTOR pathways | Clinical trials:
Skeletal health (NCT04313634) Frail elderly (NCT03675724) Osteoarthritis (NCT04210986) Chronic kidney disease, Diabetes mellitus, and diabetic nephropathies (NCT03325322) Mild cognitive impairment (NCT02741804) COVID-19 (NCT04476953) |
Zhu et al. 2017 (71) Yousefzadeh et al. 2018 (130) |
|
| Quercetinb | Activates estrogen receptors and inhibits PI3 kinase | Clinical trials:
Alzheimer’s disease (NCT04063124) Chronic kidney disease (NCT02848131) Hematopoietic stem cell transplant (NCT02652052) Skeletal health in older humans (NCT04313634) |
Zhu et al. 2015 (67) | |
| Targeted therapeutics | A1155463 (Abbvie, North Chicago, IL) | Bcl-xL inhibitor | Preclinical tool compound | Zhu et al. 2017 (71) |
| A1331852 (Abbvie, North Chicago, IL) | Bcl-xL inhibitor | Preclinical tool compound | Zhu et al. 2017 (71) | |
| Navitoclax (ABT-263) (Abbvie, North Chicago, IL) | Bcl-2/Bcl-xL inhibitor | Preclinical | Zhu et al. 2015 (67), Chang et al. 2016 (97) |
|
| ABT-737 (Abbvie, North Chicago, IL) | Bcl-2/Bcl-xL inhibitor | Preclinical | Yosef et al. 2016 (131) | |
| Dasatinibb | Pan receptor tyrosine kinase inhibitor | Clinical trials:
Alzheimer’s disease (NCT04063124) Chronic kidney disease (NCT02848131) Hematopoietic stem cell transplant (NCT02652052) Skeletal health in older humans (NCT04313634) |
Zhu et al. 2015 (67) | |
| JQ1 | BET inhibitor | Preclinical | Tasdemir et al. 2016 (74) | |
| P5091 (DFCI, Boston, MA) | USP7 inhibitor | Discovery | He et al. 2020 (132) | |
| Panobinostat | Pan HDAC inhibitor | Unknown | Samaraweera et al. 2017 (133) | |
| Proxofimc | FOXO4/P53 protein interaction inhibitor | Preclinical | Baar et al. 2017 (134) | |
| UBX010 (Unity Biotechnology, South San Francisco, CA) | MDM2/p53 protein interaction inhibitor |
Preclinical Clinical trial terminated |
Vilgelm et al. 2019 (61) Vilgelm et al. 2015 (62) Jeon et al. 2017 (101) |
|
| UBX-1325 (Unity Biotechnology, South San Francisco, CA) | Bcl-2/Bcl-xL inhibitor | Clinical trial:
Diabetic macular edema (NCT04537884) |
Kirkland et al. 2020 (102) | |
| Senescence cell-targeting prodrugs | Duocarmycin galactose conjugate | DNA alkylating agent | Discovery | Guerrero et al. 2020 (79) |
| Gemcitabine galactose conjugate | Nucleoside analog | Discovery | Cai et al. 2020 (80) | |
| PROTACs | ARV825 (Avnias Inc, New Haven, CT) | BET family protein degrader | Discovery | Waikita et al. 2020 (135) |
| PZ15227 (University of FL, Gainesville, FL) | Bcl-xL degrader | Discovery | He et al. 2020 (82) |
aThis is not a comprehensive list of senotherapeutics but provides some examples of classes of drugs developed as senotherapeutics.
bUsed in combination with each other.
cAll drugs in the Table have been reported to have anticancer effects except for Proxofim.
Box 2.
Pitfalls, challenges, and opportunities in the development of senolytics as anticancer agents for clinical use
Discovery. Current efforts involving structure-activity relationships and lead optimization are lagging in senolytic discovery. Medicinal chemistry-based research is needed.
Experimental models. Suitable, reliable, and efficient in vitro and in vivo models for evaluation of the safety and efficacy of senolytics are needed.
Specificity. Further characterization of senescent cells (SnCs) is necessary to allow improved targeting of “harmful SnCs” with sparing of immune-modulating SnCs.
-
Toxicities. Most senolytics developed from anticancer agent pipelines demonstrate on- and off-target toxicities.
For example, Bcl-xL specific inhibitors A1155463 and A1331852 can cause severe thrombocytopenia because platelets depend on Bcl-xL for survival. Similarly, Bcl-xL/Bcl-2 dual inhibitors ABT-263, ABT-737, and UBX-1325 can also cause severe neutropenia because neutrophils depend on Bcl-2 for survival. Improved selective targeting with proteolysis targeting chimeras may reduce toxicities.
Senolytic prodrugs rely on senescence-associated β-galactosidase for SnC selective activation. However, macrophages also express high levels of β-gal. To reduce off-target toxicities by using prodrugs, however, SnCs specific activation enzymes need to be identified.
Time of administration. The effectiveness of senolytics may depend on the time of administration following cancer therapy. Senolytics are most effective when administered in a hit-and-run fashion reducing potential toxicities and off-target effects.
Drug resistance. SnCs in tumors may not be in a state of “permanent growth arrest” but can subsequently acquire “stemness” and/or plasticity, leading to drug resistance and metastasis.
Mechanisms of action. A clearer understanding of the mechanisms of action of senolytics is necessary, particularly for those natural product senolytics or their derivatives including quercetin, fisetin, piperlongumine and analogs, curcumin analog EF-24, and cardiac glycosides digoxin, ouabain, and proscillaridin A.
Intellectual property rights. Most senolytics are obtained from natural products, or derivatives of natural products, repurposed anticancer agents, or off-patent. Therefore, they are not commercially viable for pharmaceutical industries.
Regulatory barriers. Some senolytics are botanical products or derivatives, which need to be registered as investigational drugs with the US Food and Drug Administration. Because of the heterogeneity and possible uncertainty about active constituents, the efficacy of the drugs can vary from batch to batch.
-
Clinical studies.
Safety requirement for senolytics is relatively high as these drugs are intended to treat elderly patients or patients undergoing cancer treatments who have low tolerability to toxicity. Although multiple trials of senolytics have begun, it will take several years to complete these trials.
Data on senescence from clinical studies are relatively sparse.
In mouse models, senolytics such as Navitoclax (ABT-263) selectively eliminate TIS cells and prevent or delay cancer relapse and metastasis (24,76). ABT-263, a Bcl-2/Bcl-xL inhibitor, used as an adjuvant therapy with radiation increases the survival of glioblastoma multiforme tumor-bearing mice by eliminating senescent astrocytes with a tumor-promoting role (workshop presentation: “Prevention of Glioblastoma Recurrence after Radiotherapy by Elimination of Senescent Astrocytes,” Sandeep Burma, University of Texas Health Science Center, San Antonio, TX). Similarly, DNA-replication kinase CDC7 inhibitor–induced senescent liver and lung cancer cells with TP53 mutations can be selectively cleared by mTOR inhibitors, and the combination of inhibitors of CDC7 and mTOR statistically significantly reduced the tumor burden and increased survival in liver cancer xenograft mouse models (77). Recently, Ruscetti et al. (12) demonstrated in a pancreatic ductal adenocarcinoma model, in which combination therapy–induced senescence (MEK and CDK4/6 inhibitors targeting oncogenic signaling) triggers SASP-dependent vascular remodeling, facilitating chemotherapy uptake and SASP-mediated endothelial activation, driving T-cell infiltration into the tumors, and potentiating PD-1 blockade.
Some approaches described below to reduce toxicities and increase efficacy are noteworthy. First, taking advantage of the high expression of SA-β-gal in SnCs, gemcitabine, ABT-263, and duocarmycin have been converted into promising prodrugs with galactose as a pro-moiety (78–80). Such prodrugs are preferentially activated in SnCs by conversion into the active parent drug by SA-β-gal resulting in targeting of SnCs. However, activated macrophages also express SA-β-gal (81), so such agents will not likely be fully selective against SnCs. The second is to improve selectivity by constructing proteolysis targeting chimeras (PROTACs; heterobifunctional molecules that link a ligand for a protein of interest to an E3 ligase ligand) (82). To improve the potency and reduce the severe thrombocytopenia induced by ABT-263, taking advantage of the low expression levels of E3 ligase Cereblon (CRBN) in platelets, PROTAC PZ15227, constructed by linking ABT-263 to a CRBN ligand pomalidomide, has shown improved efficacy and reduced toxicities compared with ABT-263 (82). This approach could have broad applications if SnCs-specific E3 ligases could be identified (70,82). However, although this approach may protect against thrombocytopenia, it may not be effective in protecting against the neutropenia that can be induced by ABT-263 (83). Third, the design of dendrimer-conjugated Bcl-2/xL inhibitor, AZD0466, to optimize drug-release rate has shown promise in reducing cardiovascular toxicities and improved therapeutic index in preclinical models, which allowed its progression to clinical studies (84). However, as some senolytics are repurposed anticancer agents with known on- and off-target toxicities, their dose and scheduling need to be optimized for clinical applications.
Different types of SnCs use different SCAPs to resist apoptosis. Therefore, different TIS cells may require different senolytics (67). The continued discovery of new SCAPs, senolytic targets, and senolytics, and optimization of their dose regimen is essential. However, current efforts involving structure-activity relationships and lead optimization are lagging in senolytic discovery. Several biotechnology companies are currently developing new senolytics. Many of these efforts do not directly focus on developing senolytics as anticancer agents. However, it includes the development of senolytics to target the transition between quiescence and senescence state, modulators of senescence signaling pathways and DNA repair enhancers, approaches to enhance the efficacy and reduce toxicities using PROTACs, and antibodies to promote immune clearance of SnCs as well as novel discovery platforms (85). Similarly, there are also opportunities to repurpose radiation-effect modulators (86–88) as senotherapeutics. Thus, we anticipate the development of a steady pipeline of senotherapeutics in the near future, which may be used in one-two punch cancer therapy.
One-Two Punch Cancer Therapy
To target TIS, a novel one-two punch cancer therapy approach represents an exciting area of research (27,89), which is illustrated in Figure 1, B. Cancer therapies at clinical doses, while accomplishing tumor cell killing (first punch), also induce senescence in both tumor and normal tissues (13,54). SnCs are normally cleared by immune surveillance (90). Therapy-induced SnCs are heterogeneous and dynamic, also reflected in biomarkers, cellular plasticity, expression of SASPs and SCAPs, the tissue of origin, and cell lineage (8,13,52). Selective clearance of SnCs with a senotherapeutic (second punch) in tumors can prevent tumor relapse, metastasis, and development of resistance to treatment (27,89). Similarly, selective clearance of SnCs in normal tissue in a dynamic spatiotemporal environment will prevent, treat, and mitigate therapy-induced side effects and help restore tissue homeostasis. However, the time of administration of the “second punch” therapy will be important to improve efficacy. The report on the one-two punch approach to selectively eliminate chemotherapy-induced senescent lymphoma cells in mice was achieved by using a metabolic senolytic to block glucose utilization or autophagy (47).
Genetic and pharmacological clearance of TIS cells reduces side effects and inhibits tumor relapse and metastasis (24). A combination of chemotherapy and a senolytic therapy (eg, ABT263 or cardiac glycosides) is effective in the treatment of many cancer types in mouse models (77,91–94). The combined treatment with XL413 (a potent CDC7 inhibitor) and AZD8055 (mTOR inhibitor) resulted in pronounced growth inhibition of liver cancer (77). Recently, chimeric antigen receptor T cells targeting the cell surface protein, urokinase-type plasminogen activator receptor (which is broadly present in SnCs), were found effective as a senolytic in a KrasG12D; p53−/− lung adenocarcinoma mouse model demonstrating proof-of-principle for synthetic senolytic cell-based therapy (95).
Clearance of TIS cells can improve posttreatment QOL by mitigating cancer treatment-induced acute and late effects such as radiation-induced tissue fibrosis and chemotherapy-induced neuropathy (76,96,97). Interestingly, in some immunocompetent mouse tumor models, TIS is found to be beneficial, as it can also promote immune clearance of tumor cells through SASP factors along with clearance of SnCs (34,60,63,98). The underlying causes of the differential effects of SnCs on tumorigenesis and response to therapy have not been fully elucidated. For example, SnCs induced immediately after therapy and those that accumulate over time may have different effects regarding antitumor immunity and tumor relapse, metastasis, and drug resistance (17). In addition, the SASP itself may change over time.
The first report on the combination of dasatinib and quercetin (D + Q) as an effective senolytic therapy in a preclinical model was discovered using a hypothesis-driven, mechanism-based, and bioinformatics approach (67). The results of 2 early phase clinical trials have now been published: 1) the first-in-human open-label pilot study (NCT02874989) demonstrated the feasibility and provided initial evidence that senolytic intervention with the above combination in participants with idiopathic pulmonary fibrosis can alleviate physical dysfunction (99), and 2) subsequently, it was demonstrated that treatment with D + Q administered to subjects with diabetic kidney disease statistically significantly reduces the SnCs burden (NCT02848131) (51,100). Thus, several senolytics, including D + Q (51,67,100), fisetin (71), UBX0101 (101), and UBX1325 (102), have now progressed into clinical trials (see Table 1), and some also have progressed to phase 2 studies.
The timing of senotherapeutic administration is a key determinant of the efficacy of the one-two punch strategy, because surveillance of SnCs is executed by cytokines and chemokines; release of which is time dependent. For example, the role for CCR2+ myeloid cells in liver cancer is context specific during disease progression. Although hepatocyte-secreted chemokines suppress liver cancer initiation, they may also promote and accelerate the growth of fully established liver cancers (103). Because the effect of dose and scheduling of senolytics on the clearance of SnCs is not known and the range of potential beneficial or deleterious outcomes elicited by a one-two punch therapy is also not known, additional studies are necessary in a variety of models for their effective translation.
Promises, Pitfalls, and Barriers for Clinical Translation of Senotherapy and One-Two Punch Cancer Therapy
Increased expression of biomarkers associated with but not specific for SnCs is detected in numerous human cancers. For example, increased expression of p16INK4a is associated with an increased risk of tumor relapse and poor prognosis in breast cancer (104,105), and the senescence-associated gene signature in peritumoral tissue correlates with shorter recurrence-free survival in hepatocellular carcinoma (11). In contrast, in malignant pleural mesothelioma patients after chemotherapy, increased expression of p16INK4a is associated with better survival (106). This incongruity may reflect human tumor heterogeneity in response to treatment. Therefore, the juxtaposed roles of SnCs in tumorigenesis and treatment response may be contextual; it may be dependent on tumor and tissue type of origin. In this regard, our understanding of SnC heterogeneity is limited by our inability to spatiotemporally and unambiguously detect, characterize, and monitor SnCs in patients during the course of treatment because of the lack of reliable SnC biomarkers and tools to detect them (68).
Since discovery of the first potential senescence biomarker detectable in normal human skin (4), it has become clear that there are no universal senescence-specific biomarkers, whether in normal (young or old) or malignant tissue. Hence, there has been a concerted effort to develop biomarker panels for identifying senescent cells in vivo (6,68,107,108). Among the recognized senescence, biomarkers include CDK inhibitors 2 A (p16/CDKN2A) and 1 A (p21/CDKN1A), which suppress cell proliferation. However, recent tissue diagnostic array studies of cells expressing p16 and p21 indicated that although different organs express different levels of proteins as a function of age across the human life span, some tissues such as muscle do not appear to have either of these markers (109). Moreover, senescence biomarkers are highly variable, whether in normal or tumor tissue, and they can depend on the cell type, senescence inducer, and time after senescence induction (8). Therefore, reliable SnC biomarkers to spatially identify and longitudinally track SnCs are urgently needed (eg, noninvasive imaging) for successful translation. Of note, Dr Jesus Gil showed preliminary data from his collaboration with Dr Lars Zender at the University of Tübingen, Germany, on using positron emission tomography imaging in the brain of a glioblastoma multiforme patient to monitor TIS cells and their clearance by Digoxin (workshop presentation: “Strategies to Target Senescence,” Jesus Gil, Imperial College, London, UK).
However, a major limitation to the clinical translation of senotherapeutics is the inability of animal models (human xenografts and/or genetically engineered models) to recapitulate fully key mechanisms and predict outcomes for human patients. In this regard, patient-derived xenograft or spontaneous tumor models may serve to better recapitulate tumor biology and heterogeneity of human diseases and predict clinical outcomes (110). Similarly, studies with human tumor organoids and tissue explants may also be useful (111).
Despite these limitations, progress has been made in monitoring and understanding systemic SnC burden in cancer patients and the role of SnCs in therapy-induced side effects. Increased expression of p16INK4a mRNA in peripheral blood T cells (PBTC, specifically CD3+ cells) can be used as a biomarker for measuring SnC burden and accelerated aging induced by chemotherapy (112,113). Frail patients had higher levels of PBTC p16INK4a mRNA than healthy subjects, suggesting that chemotherapy accelerates aging (113). These biomarkers may be useful for monitoring the posttreatment QOL of patients. In this regard, an open-label intervention collaborative clinical trial (1U01CA246510-01; principal investigators: Dr Gregory Armstrong, St Jude Children’ s Hospital, Memphis, TN, and Dr James Kirkland, Mayo Clinic, Rochester, MN) sponsored by the NCI is noteworthy (https://reporter.nih.gov/search/Lw5x_tbPW0ONWC7cl7etWA/projects). This trial is testing whether D + Q or fisetin alone can modify the biological markers of aging including PBTC p16INK4a mRNA and frailty in adult survivors of childhood cancer by the clearance of SnCs.
Senolytics appear to be most effective when administered in a hit-and-run fashion. Following chemotherapy and/or radiotherapy, it can only take a few days to a couple of weeks for cells to acquire SnC phenotypes and be regarded as logical therapeutic targets. TIS in tumors and normal tissue, following the completion of a cancer treatment regimen, can occur between 10 days and 6 weeks. Senolytics act quickly, within 18 hours, eliminating the SnCs induced by therapy (23) and do so in a hit-and-run fashion (26,114). For example, in mice with age-related osteoporosis, treatment with D + Q, which has an elimination half-life of 4 hours and 11 hours, respectively, for 1 day every month for 4 months, was effective in restoring bone mass (115). This intermittent treatment with senolytics greatly reduced toxicities as well as those off-target effects caused by continuous presence of the drugs, such as those due to sustained occupancy of a receptor or activation or inhibition of an enzyme. Hence, senolytics could be administered starting from 0.5 days to 2 weeks after each round of therapy. Further, every chemotherapy/radiotherapy cycle could be followed by senolytics to prevent tumor recurrence and decrease adverse effects (Figure 2).
Tissue-specific heterogeneity of responses of tumor and normal cells to chemotherapy has been demonstrated with CDK4/6 inhibitors, which induce a “transient cell-cycle block” to exert a protective effect in healthy cells due to Ink4 deficiency, which decreases treatment-related toxicities (workshop presentation, keynote address: “Protecting the Bone Marrow from Ionizing Radiation,” Norman Sharpless, NCI, Bethesda, MD) (116). For example, selective, transient, and reversible inhibition of CDK4/6 kinase activity in the bone marrow with trilaciclib protects against hematopoietic stem cell exhaustion induced by serial treatments with 5-fluorouracil in a murine model (117). CDK4/6 inhibitors were also found to preserve hematopoietic stem cell function in NSCLC patients treated with etoposide and carboplatin without reducing treatment efficacy (118). Further, CDK4/6 inhibitors exhibit excellent in vivo pharmacology and tolerability in patients with metastatic triple-negative breast cancer (119). Trilaciclib is now approved to treat hormone receptor–positive and HER2-negative advanced breast cancer through induction of G1/S cell cycle arrest and senescence in tumor cells (120,121). In combination with MDM2 inhibition, preclinical studies show CDK4/6 inhibition combined with MDM2 inhibition is quite effective in inhibiting NRAS mutant melanoma tumor growth by induction of senescence, followed by immune-mediated tumor cell clearance (61). Studies with CDK4/6 inhibitors are examples of several possibilities of combining agents with differential effects and exploiting differential vulnerabilities in tumor and normal cells to improve treatment efficacy in cancers and decrease toxicities. However, tissue-specific heterogeneity in TIS is a challenge to be overcome and an opportunity to improve patient outcomes.
Discussion
Since the discovery of cellular senescence by Hayflick and Moorhead (122), senescence is now understood to be a fundamental biological process that governs a variety of pathophysiological functions and diseases including cancer. As the number of cancer survivors is growing because of ever-improving treatments, focus on treatment-induced adverse effects has become even more imperative, as is the opportunity to study and mitigate TIS to improve further the treatment and also the QOL of survivors. With SnCs emerging as an anticancer target, the NCI is developing promising strategies to prevent, slow, and reverse age-related consequences of cancer and its treatment (123). To continue with these efforts, this workshop focused on TIS to identify key knowledge gaps in the discovery, development, and translation of senotherapeutics for clinical use.
Although many drugs could be used to study TIS, a simpler approach is to use radiation in experimental models because of its well-defined ability to induce senescence in vivo by a single total body dose without the confounding pharmacokinetic effects of drugs (124). The work described herein demonstrates the feasibility of using established cancer treatments to induce and study cellular senescence, which provides the opportunity to discover novel targets, biomarkers, and senotherapeutics to improve efficacy and reduce toxicities of cancer therapies. Accordingly, studies on the many shared and pivotal molecular pathways between cancer and senescence and the possibility of using therapies such as radiation to induce and study defined cellular perturbations, including senescence, is one of many opportunities. Similarly, the promise of using a one-two punch therapy to improve treatment’s efficacy and reduce SnC burden to improve outcomes is exciting. Figure 2 illustrates one approach to integrate one-two punch cancer therapy with adaptive tumor therapy to improve efficacy by reducing drug resistance, preventing tumor recurrence and injury to normal tissue, and mitigate and treat adverse effects.
Many questions listed in Box 3 are yet to be fully understood, as discussed at the workshop. For example, the data whether senescence occurs in response to chemotherapy (or radiation) in the clinic are relatively sparse. Also, we must understand if the induction and promotion of senescence are treatment specific, and further, if senescence response is uniform or limited to occur in the malignancies of certain organ/sites or drugs or radiation. Similarly, is there a difference in the occurrence of senescence for each drug and malignancy? Addressing such questions is vital to design appropriate clinical trials and get the full benefit from the one-two punch therapy.
Box 3.
Key knowledge gaps in therapy-induced senescence (TIS) research for the discovery and development of senotherapeutics as a novel anticancer strategy and to guide the personalization of cancer treatment
Can we use radiation/chemotherapy as a model to induce and study cellular perturbations that cause senescence and/or cancer and improve outcomes?
Which is the critical type and level of biological damage (DNA mainly) shifting the balance toward senescence or apoptosis?
What are the differences between TIS in normal tissue and the tumor microenvironment?
Is induction and promotion of senescence treatment specific? Is the response uniform or heterogeneous across tumor types? Is the time frame of senescence occurrence after treatment dependent on treatment and tumor types?
Can TIS be used to unravel the intersecting mechanisms of carcinogenesis and senescence?
Will TIS be a viable approach to discover and develop novel senotherapeutics that can also serve as novel cancer therapies?
Can senotherapeutics improve the efficacy of radiation and chemotherapy without compromising antitumor immunity?
Is the timing of senotherapeutics following treatment important for determining the efficacy of one-two punch cancer therapeutics?
Are there any biomarkers that can predict or monitor the effectiveness of senotherapeutics?
How TIS effects the release of heterogeneous senescence-associated secretory phenotypes (SASPs) formed by metabolites, proteins, and vesicles and how the heterogeneous SASP influences the tumor microenvironment?
By understanding TIS, we can anticipate a steady pipeline of novel biomarkers and senotherapeutics and can plan to translate senotherapeutics into next-generation anticancer agents. Furthermore, the identification of novel biomarkers should enable more personalized treatments, which could be more specific to a given patient, type of malignancy and treatment. Successful translation of senotherapeutics will ultimately involve obtaining regulatory approval from the US Food and Drug Administration. Studies of senotherapeutics present unique regulatory challenges, especially when used as combined treatment modalities with chemotherapy and radiation: different toxicity profiles and timelines are likely for the development of adverse acute and late effects between systemic therapies and radiation. Cancer therapeutic-senotherapeutic drug combinations will also present challenges in clinical trial design. Therefore, an early interaction with the US Food and Drug Administration is important if the laboratory data are compelling to consider general aspects related to the development and translation of senotherapeutics and to obtain further guidance.
Funding
This work was supported by the National Cancer Institute (P30CA060553 and R37CA222294 to MEA; RO1 CA197796 and R01 CA246807 to SB; 1R01CA214025-01 to D Gius; RO1CA239706 to D Gewirtz; AG063543, ES029603, AG056278 and P01AG062413 to LJN; R01CA164492 and R01CA217182 to SJK; CA116021 to AR; R37CA233770 to AEV; R37AG013925, P01AG062413 and R33AG061456 to JLK; P30CA008748 to PBR and SWL; K12 CA184746 to PBR; R01CA211963, R01CA219836, R01CA242003, and R01AG063801 to GZ and DZ; and R01CA218596 (Ewing/S Brown) and R21CA205660 (Jae Ho Kim). Further, laboratory studies done in Dr Citrin’s laboratory are supported by the intramural research program of the National Cancer Institute (grant BC10850). Dr Burma is also supported the National Aeronautics and Space Administration (NNX16AD78G). Dr Gius is also supported by the Avon Foundation for Breast Cancer Research and the Lynn Sage Cancer Research Foundation and by the Cancer Prevention and Research Institute of Texas (CPRIT) grant no. RR20012. Studies done in Dr Gil’s laboratory are supported by the Core support from MRC (MC-U120085810) and grants from Worldwide Cancer Research (18- 0215) and CRUK (C15075/A28647). Studies done in Dr Kirkland’s laboratory are also supported by the Alzheimer’s Association Part the Cloud Program, Robert and Arlene Kogod, the Connor Group, Robert J. and Theresa W. Ryan, and the Noaber Foundation. Studies done in Dr O’Loghlen’s lab were funded by the BBSRC (BB/P000223/1) and Barts Charity Grant (MGU0497). Studies done in Dr Richmond’s lab was also funded by a VA MERIT Award (101BX002301) and a VA Senior Research Career Scientist Award. Dr Schmitt’s laboratory is supported by grants to C.A.S. from the Deutsche Krebshilfe (No. 110678), the BMBF e: Med program project SeneSys (No. 031L0189A), the Deutsche Forschungsgemeinschaft DFG (GO 2688/1-1 | SCHM 1633/11-1, SCHM 1633/9-1.
Notes
Role of the funders: The NCI convened the workshop, “Radiation, Senescence, and Cancer” (August 2020) that informed the manuscript’s scope. The funders had no role in the writing of this commentary or the decision to submit it for publication. The manuscript was reviewed and approved for submission through the NCI Division of Cancer Diagnosis and Treatment manuscript clearance process.
Disclosures: The main organizers of the workshop, Drs. Pataje G. Prasanna, Deborah E. Citrin, and C. Norman Coleman are employees of the US government and have nothing to disclose and performed this work in the interest of general public. Drs. Jeffrey Hildesheim, Mansoor Ahmed, Sundar Venkatachalam, Gabriela Riscuta, Dan Xi, and Mitchell S. Anscher are employees of the US government, and other authors, Drs. David Gius, Goronzy, O’Loghlen, and Mendonca have nothing to disclose. Drs. Guangrong Zheng and Daohong Zhou are inventors of three pending patent applications for use of Bcl-xL PROTACs as senolytic and antitumor agents and are co-founders of and have equity in Dialectic Therapeutics, which develops Bcl-xL PROTACs to treat cancer. Dr Abazeed discloses research grant and travel support from Siemens Healthcare and research grant, travel support, and honorarium from Bayer AG in subject matter unrelated to this work. Dr Jesus Gil has acted as a consultant for Unity Biotechnology, Geras Bio, Myricx Pharma, and Merck KGaA; owns equity in Unity Biotechnology and Geras Bio, and is a named inventor in Imperial College and MRC patents related to senolytic therapies. Drs. Judith Campisi, Jan van Deursen, and Daohong Zhou are co-founders and stockholders of Unity Biotechnology that develops senolytics to treat age-related diseases. Dr James Kirkland has a financial interest related to this research. Patents on senolytic drugs are held by the Mayo Clinic. This research has been reviewed by the Mayo Clinic conflict of interest review board and was conducted in compliance with Mayo Clinic’s Conflict of Interest policies. Dr Laura Niedernhofer is a founder of NRTK Biosciences. Dr Paul Romesser reports prior research funding from EMD Serono, has received travel support from Elekta, and is a consultant for EMD Serono. Dr Scott Lowe is listed as an inventor on a patent application describing use of CAR T-cells as senolytic agents. Dr François Paris is a French State employee and has nothing to disclose.
Author contributions: Pataje Prasanna: Conceptualization, methodology, validation, writing—original draft, review and editing, project administration, and funding acquisition, Deborah Citrin: Conceptualization, validation, writing—original draft, review and editing, Jeffrey Hildesheim, Sundaresan Venkatachalam, Gabriela Riscuta, and Dan Xi: Conceptualization, writing—original draft, review and editing, Mansoor Ahmed: Conceptualization, writing—original draft, review and editing, and visualization, Guangron Zheng: Writing—original draft, review and editing, and visualization, Judith Campisi and James Kirkland: Writing original draft, review and editing, and validation, Jan van Deursen, Jorg Goronzy, Stephen Kron, Mithell Anscher, Norman Sharpless, Stephen Brown, Laura Niedernhofer, Ana O’Loghlen, Alexandros Georgakilas, Francois Paris, David Gius, David Gewirtz, Clemens Schmitt, Mohamed Abazeed, Ann Richmond, Paul Romesser, Scott Lowe, Jesus Gil, Marc Mendonca, and Sandeep Burma: Writing original draft—review and editing, Daohong Zhou: Conceptualization, validation, writing—original draft, review and editing, and visualization, C. Norman Coleman: Conceptualization, validation, writing—original draft, review and editing, visualization, and funding acquisition.
Acknowledgements: The authors would like to acknowledge the assistance of Drs Michael G. Espey, Jeffrey Buchsbaum, Jacek Capala, Bhadrasain Vikram, and Ceferino Obcemia of NCI’s Radiation Research Program in planning the workshop.
Disclaimer: The views and opinions expressed in this article are those of the authors and do not necessarily reflect the views and the opinions of the institutes/organizations they represent.
Data Availability
The data underlying this article are available in the article.
References
- 1. Bernadotte A, Mikhelson VM, Spivak IM.. Markers of cellular senescence. Telomere shortening as a marker of cellular senescence. Aging. 2016;8(1):3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Munoz-Espin D, Serrano M.. Cellular senescence: from physiology to pathology. Nat Rev Mol Cell Biol. 2014;15(7):482–496. [DOI] [PubMed] [Google Scholar]
- 3. Baker DJ, Childs BG, Durik M, et al. Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature. 2016;530(7589):184–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dimri GP, Lee X, Basile G, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci USA. 1995;92(20):9363–9367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Campisi J. Aging, cellular senescence, and cancer. Annu Rev Physiol. 2013;75:685–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sharpless NE, Sherr CJ.. Forging a signature of in vivo senescence. Nat Rev Cancer. 2015;15(7):397–408. [DOI] [PubMed] [Google Scholar]
- 7. Borghesan M, Fafian-Labora J, Eleftheriadou O, et al. Small extracellular vesicles are key regulators of non-cell autonomous intercellular communication in senescence via the interferon protein IFITM3. Cell Rep. 2019;27(13):3956–3971.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Basisty N, Kale A, Jeon OH, et al. A proteomic atlas of senescence-associated secretomes for aging biomarker development. PLoS Biol. 2020;18(1):e3000599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Iske J, Seyda M, Heinbokel T, et al. Senolytics prevent mt-DNA-induced inflammation and promote the survival of aged organs following transplantation. Nat Commun. 2020;11(1):4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Colpani O, Spinetti G.. MicroRNAs orchestrating senescence of endothelial and vascular smooth muscle cells. Vasc Biol. 2019;1(1):H75–H81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee S, Schmitt CA.. The dynamic nature of senescence in cancer. Nat Cell Biol. 2019;21(1):94–101. [DOI] [PubMed] [Google Scholar]
- 12. Ruscetti M, Morris JPM, Mezzadra R, et al. Senescence-induced vascular remodeling creates therapeutic vulnerabilities in pancreas cancer. Cell. 2020;181(2):424–441.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ewald JA, Desotelle JA, Wilding G, et al. Therapy-induced senescence in cancer. J Natl Cancer Inst. 2010;102(20):1536–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shao L, Feng W, Li H, et al. Total body irradiation causes long-term mouse BM injury via induction of HSC premature senescence in an Ink4a- and Arf-independent manner. Blood. 2014;123(20):3105–3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. White RR, Vijg J.. Do DNA double-strand breaks drive aging? Mol Cell. 2016;63(5):729–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schmitt CA, Fridman JS, Yang M, et al. A senescence program controlled by p53 and p16INK4a contributes to the outcome of cancer therapy. Cell. 2002;109(3):335–346. [DOI] [PubMed] [Google Scholar]
- 17. Faget DV, Ren Q, Stewart SA.. Unmasking senescence: context-dependent effects of SASP in cancer. Nat Rev Cancer. 2019;19(8):439–453. [DOI] [PubMed] [Google Scholar]
- 18. Romanov SR, Kozakiewicz BK, Holst CR, et al. Normal human mammary epithelial cells spontaneously escape senescence and acquire genomic changes. Nature. 2001;409(6820):633–637. [DOI] [PubMed] [Google Scholar]
- 19. Georgakilas AG, Martin OA, Bonner WM.. p21: a two-faced genome guardian. Trends Mol Med. 2017;23(4):310–319. [DOI] [PubMed] [Google Scholar]
- 20. Milanovic M, Fan DNY, Belenki D, et al. Senescence-associated reprogramming promotes cancer stemness. Nature. 2018;553(7686):96–100. [DOI] [PubMed] [Google Scholar]
- 21. Cahu J, Bustany S, Sola B.. Senescence-associated secretory phenotype favors the emergence of cancer stem-like cells. Cell Death Dis. 2012;3:e446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Guillon J, Petit C, Moreau M, et al. Regulation of senescence escape by TSP1 and CD47 following chemotherapy treatment. Cell Death Dis. 2019;10(3):199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xu M, Pirtskhalava T, Farr JN, et al. Senolytics improve physical function and increase lifespan in old age. Nat Med. 2018;24(8):1246–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Demaria M, O’Leary MN, Chang J, et al. Cellular senescence promotes adverse effects of chemotherapy and cancer relapse. Cancer Discov. 2017;7(2):165–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Myrianthopoulos V, Evangelou K, Vasileiou PVS, et al. Senescence and senotherapeutics: a new field in cancer therapy. Pharmacol Ther. 2019;193:31–49. [DOI] [PubMed] [Google Scholar]
- 26. Robbins PD, Jurk D, Khosla S, et al. Senolytic drugs: reducing senescent cell viability to extend health span. Annu Rev Pharmacol Toxicol. 2021;61:779–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang L, Leite de Oliveira R, Wang C, et al. High-throughput functional genetic and compound screens identify targets for senescence induction in cancer. Cell Rep. 2017;21(3):773–783. [DOI] [PubMed] [Google Scholar]
- 28. Leite de Oliveira R, Bernards R.. Anti-cancer therapy: senescence is the new black. EMBO J. 2018;37(10):e99386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chakradeo S, Elmore LW, Gewirtz DA.. Is senescence reversible? Curr Drug Targets. 2016;17(4):460–466. [DOI] [PubMed] [Google Scholar]
- 30. Sapieha P, Mallette FA.. Cellular senescence in postmitotic cells: beyond growth arrest. Trends Cell Biol. 2018;28(8):595–607. [DOI] [PubMed] [Google Scholar]
- 31. Saleh T, Tyutyunyk-Massey L, Gewirtz DA.. Tumor cell escape from therapy-induced senescence as a model of disease recurrence after dormancy. Cancer Res. 2019;79(6):1044–1046. [DOI] [PubMed] [Google Scholar]
- 32. Bielak-Zmijewska A, Mosieniak G, Sikora E.. Is DNA damage indispensable for stress-induced senescence? Mech Ageing Dev. 2018;170:13–21. [DOI] [PubMed] [Google Scholar]
- 33. Wagner V, Gil J.. Senescence as a therapeutically relevant response to CDK4/6 inhibitors. Oncogene. 2020;39(29):5165–5176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vilgelm AE, Johnson CA, Prasad N, et al. Connecting the dots: therapy-induced senescence and a tumor-suppressive immune microenvironment. J Natl Cancer Inst. 2016;108(6):djv406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liu Y, Hawkins OE, Su Y, et al. Targeting aurora kinases limits tumour growth through DNA damage-mediated senescence and blockade of NF-kappaB impairs this drug-induced senescence. EMBO Mol Med. 2013;5(1):149–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Petrova NV, Velichko AK, Razin SV, et al. Small molecule compounds that induce cellular senescence. Aging Cell. 2016;15(6):999–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Macedo JC, Vaz S, Bakker B, et al. FoxM1 repression during human aging leads to mitotic decline and aneuploidy-driven full senescence. Nat Commun. 2018;9(1):2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chapman J, Fielder E, Passos JF.. Mitochondrial dysfunction and cell senescence: deciphering a complex relationship. FEBS Lett. 2019;593(13):1566–1579. [DOI] [PubMed] [Google Scholar]
- 39. Wiley CD, Velarde MC, Lecot P, et al. Mitochondrial dysfunction induces senescence with a distinct secretory phenotype. Cell Metab. 2016;23(2):303–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Correia-Melo C, Marques FD, Anderson R, et al. Mitochondria are required for pro-ageing features of the senescent phenotype. EMBO J. 2016;35(7):724–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lafargue A, Degorre C, Corre I, et al. Ionizing radiation induces long-term senescence in endothelial cells through mitochondrial respiratory complex II dysfunction and superoxide generation. Free Radic Biol Med. 2017;108:750–759. [DOI] [PubMed] [Google Scholar]
- 42. Wolf DA. Is reliance on mitochondrial respiration a “chink in the armor” of therapy-resistant cancer? Cancer Cell. 2014;26(6):788–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Vizioli MG, Liu T, Miller KN, et al. Mitochondria-to-nucleus retrograde signaling drives formation of cytoplasmic chromatin and inflammation in senescence. Genes Dev. 2020;34(5-6):428–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wang Y, Boerma M, Zhou D.. Ionizing radiation-induced endothelial cell senescence and cardiovascular diseases. Radiat Res. 2016;186(2):153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Efimova EV, Takahashi S, Shamsi NA, et al. Linking cancer metabolism to DNA repair and accelerated senescence. Mol Cancer Res. 2016;14(2):173–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Efimova EV, Appelbe OK, Ricco N, et al. O-GlcNAcylation enhances double-strand break repair, promotes cancer cell proliferation, and prevents therapy-induced senescence in irradiated tumors. Mol Cancer Res. 2019;17(6):1338–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dorr JR, Yu Y, Milanovic M, et al. Synthetic lethal metabolic targeting of cellular senescence in cancer therapy. Nature. 2013;501(7467):421–425. [DOI] [PubMed] [Google Scholar]
- 48. Martinez-Zamudio RI, Roux PF, de Freitas J, et al. AP-1 imprints a reversible transcriptional programme of senescent cells. Nat Cell Biol. 2020;22(7):842–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Reimann M, Schrezenmeier JF, Richter-Pechanska P, et al. Adaptive T-cell immunity controls senescence-prone MyD88- or CARD11-mutant B-cell lymphomas. Blood. 2020. doi: 10.1182/blood.2020005244. [DOI] [PubMed] [Google Scholar]
- 50. Gopal P, Rogacki K, Peacock CD , et al. Dynamic transdifferentiation programs in small cell lung carcinoma (Abstract nr 2897). Cancer Res 2019;79(suppl 13). doi: 10.1158/1538-7445.AM2019-2897 [Google Scholar]
- 51. Hickson LJ, Langhi Prata LGP, Bobart SA, et al. Senolytics decrease senescent cells in humans: Preliminary report from a clinical trial of Dasatinib plus Quercetin in individuals with diabetic kidney disease. EBioMedicine. 2019;47:446–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kirkland JL, Tchkonia T.. Cellular senescence: a translational perspective. EBioMedicine. 2017;21:21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yu Y, Schleich K, Yue B, et al. Targeting the senescence-overriding cooperative activity of structurally unrelated H3K9 demethylases in melanoma. Cancer Cell. 2018;33(4):785. [DOI] [PubMed] [Google Scholar]
- 54. Saleh T, Bloukh S, Carpenter VJ, et al. Therapy-Induced senescence: an “old” friend becomes the enemy. Cancers (Basel). 2020;12(4):822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Le Duff M, Gouju J, Jonchère B, et al. Regulation of senescence escape by the CDK4-EZH2-AP2M1 pathway in response to chemotherapy. Cell Death Dis. 2018;9(2):199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hernandez-Segura A, de Jong TV, Melov S, et al. Unmasking transcriptional heterogeneity in senescent cells. Curr Biol. 2017;27(17):2652–2660.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ozcan S, Alessio N, Acar MB, et al. Unbiased analysis of senescence associated secretory phenotype (SASP) to identify common components following different genotoxic stresses. Aging. 2016;8(7):1316–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Acosta JC, O’Loghlen A, Banito A, et al. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell. 2008;133(6):1006–1018. [DOI] [PubMed] [Google Scholar]
- 59. Acosta JC, Banito A, Wuestefeld T, et al. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat Cell Biol. 2013;15(8):978–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lujambio A, Akkari L, Simon J, et al. Non-cell-autonomous tumor suppression by p53. Cell. 2013;153(2):449–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Vilgelm AE, Saleh N, Shattuck-Brandt R, et al. MDM2 antagonists overcome intrinsic resistance to CDK4/6 inhibition by inducing p21. Sci Transl Med. 2019;11(505):eaav7171. doi: 10.1126/scitranslmed.aav7171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Vilgelm AE, Pawlikowski JS, Liu Y, et al. MDM2 and aurora kinase a inhibitors synergize to block melanoma growth by driving apoptosis and immune clearance of tumor cells. Cancer Res. 2015;75(1):181–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ruscetti M, Leibold J, Bott MJ, et al. NK cell-mediated cytotoxicity contributes to tumor control by a cytostatic drug combination. Science. 2018;362(6421):1416–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wiley CD, Flynn JM, Morrissey C, et al. Analysis of individual cells identifies cell-to-cell variability following induction of cellular senescence. Aging Cell. 2017;16(5):1043–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Tang H, Geng A, Zhang T, et al. Single senescent cell sequencing reveals heterogeneity in senescent cells induced by telomere erosion. Protein Cell. 2019;10(5):370–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Robbins PD, Jurk D, Khosla S, et al. Senolytic drugs: reducing senescent cell viability to extend health span. Annu Rev Pharmacol Toxicol. 2021;61:779–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zhu Y, Tchkonia T, Pirtskhalava T, et al. The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell. 2015;14(4):644–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Roy AL, Sierra F, Howcroft K, et al. A blueprint for characterizing senescence. Cell. 2020;183(5):1143–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ahmed MM, Coleman CN, Mendonca M, et al. Workshop report for cancer research: defining the shades of Gy: Utilizing the biological consequences of radiotherapy in the development of new treatment approaches-meeting viewpoint. Cancer Res. 2018;78(9):2166–2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. He Y, Zheng G, Zhou D.. Senolytic drug development. In: Muñoz-Espin D, Demaria M, eds. Senolytics in Diseases, Ageing and Longevity. Healthy Ageing and Longevity. Cham: Springer; 2020. [Google Scholar]
- 71. Zhu Y, Doornebal EJ, Pirtskhalava T, et al. New agents that target senescent cells: the flavone, fisetin, and the BCL-X-L inhibitors, A1331852 and A1155463. Aging. 2017;9(3):955–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Laberge RM, Sun Y, Orjalo AV, et al. MTOR regulates the pro-tumorigenic senescence-associated secretory phenotype by promoting IL1A translation. Nat Cell Biol. 2015;17(8):1049–U416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Xu M, Palmer AK, Ding H, et al. Targeting senescent cells enhances adipogenesis and metabolic function in old age. Elife. 2015;4:e12997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Tasdemir N, Banito A, Roe JS, et al. BRD4 connects enhancer remodeling to senescence immune surveillance. Cancer Discov. 2016;6(6):612–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Carpenter VJ, Saleh T, Gewirtz DA.. Senolytics for cancer therapy: Is all that glitters really gold? Cancers. 2021;13(4):723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Pan J, Li D, Xu Y, et al. Inhibition of Bcl-2/xl with ABT-263 selectively kills senescent type II pneumocytes and reverses persistent pulmonary fibrosis induced by ionizing radiation in mice. Int J Radiat Oncol Biol Phys. 2017;99(2):353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wang C, Vegna S, Jin H, et al. Inducing and exploiting vulnerabilities for the treatment of liver cancer. Nature. 2019;574(7777):268–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Gonzalez-Gualda E, Paez-Ribes M, Lozano-Torres B, et al. Galacto-conjugation of Navitoclax as an efficient strategy to increase senolytic specificity and reduce platelet toxicity. Aging Cell. 2020;19(4):e13142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Guerrero A, Guiho R, Herranz N, et al. Galactose-modified duocarmycin prodrugs as senolytics. Aging Cell. 2020;19(4):e13133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Cai Y, Zhou H, Zhu Y, et al. Elimination of senescent cells by beta-galactosidase-targeted prodrug attenuates inflammation and restores physical function in aged mice. Cell Res. 2020;30(7):574–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Hall BM, Balan V, Gleiberman AS, et al. Aging of mice is associated with p16(Ink4a)- and beta-galactosidase-positive macrophage accumulation that can be induced in young mice by senescent cells. Aging. 2016;8(7):1294–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. He Y, Zhang X, Chang J, et al. Using proteolysis-targeting chimera technology to reduce navitoclax platelet toxicity and improve its senolytic activity. Nat Commun. 2020;11(1):1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. de Vos S, Leonard JP, Friedberg JW, et al. Safety and efficacy of navitoclax, a BCL-2 and BCL-XL inhibitor, in patients with relapsed or refractory lymphoid malignancies: results from a phase 2a study. Leuk Lymphoma. 2021;62(4):810–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Patterson CM, Balachander SB, Grant I, et al. Design and optimisation of dendrimer-conjugated Bcl-2/xL inhibitor, AZD0466, with improved therapeutic index for cancer therapy. Commun Biol. 2021;4(1):112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Dolgin E. Send in the senolytics. Nat Biotechnol. 2020;38(12):1371–1377. [DOI] [PubMed] [Google Scholar]
- 86. Prasanna PG, Narayanan D, Hallett K, et al. Radioprotectors and radiomitigators for improving radiation therapy: the Small Business Innovation Research (SBIR) Gateway for Accelerating Clinical Translation. Radiat Res. 2015;184(3):235–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Zakeri K, Narayanan D, Prasanna PGS, et al. Development of novel radiosensitizers through the National Cancer Institute’s Small Business Innovation Research Program. Radiat Res. 2020;193(5):425–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Zakeri K, Narayanan D, Vikram B, et al. Decreasing the toxicity of radiation therapy: radioprotectors and radiomitigators being developed by the National Cancer Institute through small business innovation research contracts. Int J Radiat Oncol Biol Phys. 2019;104(1):188–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Sieben CJ, Sturmlechner I, van de Sluis B, et al. Two-step senescence-focused cancer therapies. Trends Cell Biol. 2018;28(9):723–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Hoenicke L, Zender L.. Immune surveillance of senescent cells–biological significance in cancer- and non-cancer pathologies. Carcinogenesis. 2012;33(6):1123–1126. [DOI] [PubMed] [Google Scholar]
- 91. Fleury H, Malaquin N, Tu V, et al. Exploiting interconnected synthetic lethal interactions between PARP inhibition and cancer cell reversible senescence. Nat Commun. 2019;10(1):2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Saleh T, Carpenter VJ, Tyutyunyk-Massey L, et al. Clearance of therapy-induced senescent tumor cells by the senolytic ABT-263 via interference with BCL-XL -BAX interaction. Mol Oncol. 2020;14(10):2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Guerrero A, Herranz N, Sun B, et al. Cardiac glycosides are broad-spectrum senolytics. Nat Metab. 2019;1(11):1074–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Triana-Martinez F, Picallos-Rabina P, Da Silva AS, et al. Identification and characterization of cardiac glycosides as senolytic compounds. Nat Commun. 2019;10(1):4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Amor C, Feucht J, Leibold J, et al. Senolytic CAR T cells reverse senescence-associated pathologies. Nature. 2020;583(7814):127–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Acklin S, Zhang M, Du W, et al. Depletion of senescent-like neuronal cells alleviates cisplatin-induced peripheral neuropathy in mice. Sci Rep. 2020;10(1):14170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Chang JH, Wang YY, Shao LJ, et al. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat Med. 2016;22(1):78–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Vilgelm AE, Richmond A.. Chemokines modulate immune surveillance in tumorigenesis, metastasis, and response to immunotherapy. Front Immunol. 2019;10:article333. doi: 10.3389/fimmu.2019.00333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Justice JN, Nambiar AM, Tchkonia T, et al. Senolytics in idiopathic pulmonary fibrosis: results from a first-in-human, open-label, pilot study. EBioMedicine. 2019;40:554–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Hickson LJ, Langhi PL, Bobart SA, et al. Corrigendum to ‘Senolytics decrease senescent cells in humans: Preliminary report from a clinical trial of Dasatinib plus Quercetin in individuals with diabetic kidney disease’ EBioMedicine 47 (2019) 446-456. EBioMedicine. 2019;52:102595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Jeon OH, Kim C, Laberge RM, et al. Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat Med. 2017;23(6):775–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Kirkland JL, Tchkonia T.. Senolytic drugs: from discovery to translation. J Intern Med. 2020;288(5):518–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Eggert T, Wolter K, Ji J, et al. Distinct functions of senescence-associated immune responses in liver tumor surveillance and tumor progression. Cancer Cell. 2016;30(4):533–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Pare R, Shin JS, Lee CS.. Increased expression of senescence markers p14(ARF) and p16(INK4a) in breast cancer is associated with an increased risk of disease recurrence and poor survival outcome. Histopathology. 2016;69(3):479–491. [DOI] [PubMed] [Google Scholar]
- 105. Pare R, Soon PS, Shah A, et al. Differential expression of senescence tumour markers and its implications on survival outcomes of breast cancer patients. PLoS One. 2019;14(4):e0214604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Jennings CJ, Murer B, O’Grady A, et al. Differential p16/INK4A cyclin-dependent kinase inhibitor expression correlates with chemotherapy efficacy in a cohort of 88 malignant pleural mesothelioma patients. Br J Cancer. 2015;113(1):69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Bernardes de Jesus B, Blasco MA.. Assessing cell and organ senescence biomarkers. Circ Res. 2012;111(1):97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Gorgoulis V, Adams PD, Alimonti A, et al. Cellular senescence: defining a path forward. Cell. 2019;179(4):813–827. [DOI] [PubMed] [Google Scholar]
- 109. Idda ML, McClusky WG, Lodde V, et al. Survey of senescent cell markers with age in human tissues. Aging. 2020;12(5):4052–4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Coleman CN, Higgins GS, Brown JM, et al. Improving the predictive value of preclinical studies in support of radiotherapy clinical trials. Clin Cancer Res. 2016;22(13):3138–3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Hu JL, Todhunter ME, LaBarge MA, et al. Opportunities for organoids as new models of aging. J Cell Biol. 2018;217(1):39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Sanoff HK, Deal AM, Krishnamurthy J, et al. Effect of cytotoxic chemotherapy on markers of molecular age in patients with breast cancer. J Natl Cancer Inst. 2014;106(4):dju057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Smitherman AB, Wood WA, Mitin N, et al. Accelerated aging among childhood, adolescent, and young adult cancer survivors is evidenced by increased expression of p16(INK4a) and frailty. Cancer. 2020;126(22):4975–4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Wyld L, Bellantuono I, Tchkonia T, et al. Senescence and cancer: a review of clinical implications of senescence and senotherapies. Cancers. 2020;12(8):2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Farr JN, Xu M, Weivoda MM, et al. Targeting cellular senescence prevents age-related bone loss in mice. Nat Med. 2017;23(9):1072–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Johnson SM, Torrice CD, Bell JF, et al. Mitigation of hematologic radiation toxicity in mice through pharmacological quiescence induced by CDK4/6 inhibition. J Clin Invest. 2010;120(7):2528–2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. He S, Roberts PJ, Sorrentino JA, et al. Transient CDK4/6 inhibition protects hematopoietic stem cells from chemotherapy-induced exhaustion. Sci Transl Med. 2017;9(387):eaal3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Weiss JM, Csoszi T, Maglakelidze M, et al. ; G1T28-02 Study Group. Myelopreservation with the CDK4/6 inhibitor trilaciclib in patients with small-cell lung cancer receiving first-line chemotherapy: a phase Ib/randomized phase II trial. Ann Oncol. 2019;30(10):1613–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Tan AR, Wright GS, Thummala AR, et al. Trilaciclib plus chemotherapy versus chemotherapy alone in patients with metastatic triple-negative breast cancer: a multicentre, randomised, open-label, phase 2 trial. Lancet Oncol. 2019;20(11):1587–1601. [DOI] [PubMed] [Google Scholar]
- 120. Goel S, DeCristo MJ, McAllister SS, et al. CDK4/6 inhibition in cancer: beyond cell cycle arrest. Trends Cell Biol. 2018;28(11):911–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Pernas S, Tolaney SM, Winer EP, et al. CDK4/6 inhibition in breast cancer: current practice and future directions. Ther Adv Med Oncol. 2018;10:1758835918786451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Hayflick L, Moorhead PS.. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961;25:585–621. [DOI] [PubMed] [Google Scholar]
- 123. . Guida JL, Agurs-Collins T, Ahles TA, et al. Strategies to prevent or remediate cancer and treatment-related aging. J Natl Cancer Inst. 2021;113(2):112–122.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Terasima T, Tolmach LJ.. Variations in several responses of HeLa cells to x-irradiation during the division cycle. Biophys J. 1963;3:11–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Fuhrmann-Stroissnigg H, Ling YY, Zhao J, et al. Identification of HSP90 inhibitors as a novel class of senolytics. Nat Commun. 2017;8:article 422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Li W, He Y, Zhang R, et al. The curcumin analog EF24 is a novel senolytic agent. Aging. 2019;11(2):771–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Liu X, Wang Y, Zhang X, et al. Senolytic activity of piperlongumine analogues: synthesis and biological evaluation. Bioorg Med Chem. 2018;26(14):3925–3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Wang Y, Chang J, Liu X, et al. Discovery of piperlongumine as a potential novel lead for the development of senolytic agents. Aging. 2016;8(11):2915–2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Zhang X, Zhang S, Liu X, et al. Oxidation resistance 1 is a novel senolytic target. Aging Cell. 2018;17(4):e12780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Yousefzadeh MJ, Zhu Y, McGowan SJ, et al. Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine. 2018;36:18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Yosef R, Pilpel N, Tokarsky-Amiel R, et al. Directed elimination of senescent cells by inhibition of BCL-W and BCL-XL. Nat Commun. 2016;7:11190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. He Y, Li W, Lv D, et al. Inhibition of USP7 activity selectively eliminates senescent cells in part via restoration of p53 activity. Aging Cell. 2020;19(3):e13117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Samaraweera L, Adomako A, Rodriguez-Gabin A, et al. A novel indication for panobinostat as a senolytic drug in NSCLC and HNSCC. Sci Rep. 2017;7:1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Baar MP, Brandt RMC, Putavet DA, et al. Targeted apoptosis of senescent cells restores tissue homeostasis in response to chemotoxicity and aging. Cell. 2017;169(1):132–147.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Wakita M, Takahashi A, Sano O, et al. A BET family protein degrader provokes senolysis by targeting NHEJ and autophagy in senescent cells. Nat Commun. 2020;11(1):1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Malone ER, Oliva M, Sabatini PJB, et al. Molecular profiling for precision cancer therapies. Genome Med. 2020;12(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article are available in the article.