Abstract
Background
The primary goal of human papillomavirus (HPV) vaccination is to reduce morbidity and mortality from HPV-associated disease, especially cervical cancer. We determined the real-world effectiveness of HPV vaccination against cervical cancer.
Methods
The study included women aged 17-30 years living in Denmark October 2006-December 2019. From nationwide registries, information on HPV vaccination and cervical cancer diagnoses were retrieved. Incidence rate ratios (IRRs) with 95% confidence intervals (CIs) for cervical cancer according to vaccination status were estimated using Poisson regression with HPV vaccination treated as a time-varying variable and stratified by age at vaccination. We adjusted for attained age, education, and ethnicity. To address the effect of prevalent disease, different buffer periods were used, with 1-year buffer period as primary analysis.
Results
The cohort comprised 867 689 women. At baseline, 36.3% were vaccinated at age 16 years and younger, and during follow-up, 19.3% and 2.3% were vaccinated at ages 17-19 years and 20-30 years, respectively. For women vaccinated at ages 16 years and younger or 17-19 years, the IRRs of cervical cancer were 0.14 (95% CI = 0.04 to 0.53) and 0.32 (95% CI = 0.08 to 1.28), respectively, compared with unvaccinated women. In women aged 20-30 years at vaccination, the incidence rate was higher than among unvaccinated women (IRR = 1.19, 95% CI = 0.80 to 1.79) but slightly decreased with increasing buffer period (IRR = 0.85, 95% CI = 0.55 to 1.32, with 4-year buffer period).
Conclusion
HPV vaccine effectiveness against cervical cancer at the population level is high among girls vaccinated younger than age 20 years. The lack of immediate effect in women vaccinated at age 20-30 years points to the importance of early age at vaccination.
The first vaccine for prevention of morbidity and mortality attributable to human papillomavirus (HPV)–associated disease was licensed in 2006. HPV is one of the most common sexually transmitted infections and a well-established cause of cervical cancer, other anogenital cancers (anus, vulva, vagina, and penis), and oropharyngeal cancer (1). The total burden of cancers attributable to HPV is substantial and constitutes annually around 630 000 new cases worldwide, of which the majority (n = 530 000) is by far cervical cancers (1). Among HPV-related cancers, cervical cancer has the strongest association with HPV, as the development of virtually all cases is dependent on persistent infection with oncogenic HPV types (1-3). This implies that, in theory, HPV vaccination (in combination with screening and relevant treatment of cervical precancerous lesions) has the potential to eliminate cervical cancer defined by the World Health Organization as less than 4 new cases per 100 000 women-years (4).
All currently available HPV vaccines target HPV16 and HPV18, which cause approximately 70% of all cervical cancers (2,5). With the inclusion of 5 additional oncogenic HPV types (HPV31, HPV33, HPV45, HPV52, and HPV58) in the nonavalent HPV vaccine, the preventable proportion of cervical cancers has been estimated to increase to approximately 90% (6).
Because of the long time from HPV infection to cancer development and the clinical diagnosis of cervical cancer, the clinical vaccine trials (6-8) and postlicensure observational studies (9,10) have used precancerous cervical lesions as proxy endpoints for cervical cancer. It is now 14 years since licensure of the HPV vaccine, and the effectiveness of HPV vaccination against cervical cancer has just been reported in a Swedish population-based study showing that the cervical cancer risk among girls vaccinated before the age of 17 years was 88% lower than among unvaccinated girls (11).
HPV vaccination was implemented in the Danish childhood vaccination program for 12-year-old girls in January 2009. From October 2008, girls 13-15 years of age were offered HPV vaccination in the first catch-up program, and a second catch-up program including women up to the age of 27 years was initiated in August 2012. All these vaccination programs are free of charge. Today, HPV vaccination is offered to girls 12-17 years of age with 2 doses to those vaccinated before age 15 years. The vaccination program included the quadrivalent HPV vaccine from the beginning and up until January 2016. Hereafter, the bivalent Cervarix vaccine was offered for a shorter period of time and followed by the nonavalent vaccine from November 2017.
In Denmark, we have complete and individual-level registration of vaccination status and all histological diagnoses performed at pathology departments all over the country. By use of these data sources, we aimed to investigate the real-world effectiveness of HPV vaccination against cervical cancer among young Danish women.
Methods
Study Population
Since 1968, all Danish residents have been assigned a unique personal identification number, which is used in all public systems including demographic and health-care registers and enables accurate linkage between registries. The study cohort included all women aged 17-30 years and living in Denmark between October 1, 2006, and December 31, 2019. The women were identified in the Danish Civil Registration System (12), which holds continuously updated information about vital status, emigrations, and immigrations. Use of registry data for this study was approved by the Danish National Board of Health Data (FSEID-0045 and FSEID-4711).
Information on HPV Vaccination
Information about HPV vaccination was obtained from the National Health Service register (13) for women vaccinated in the free-of-charge vaccination program using service codes 808328-808330 and 808334-808336. For women who were not covered by the free-of-charge vaccination program and thus bought the vaccine themselves, vaccination information was retrieved from the National Prescription registry (14) using Anatomical Therapeutical Chemical codes J07BM01 (Gardasil), J07BM02 (Cervarix), and J07BM03 (Gardasil9).
Information on Cervical Cancer
The Danish national screening program for cervical cancer starts inviting women at age 23 years, and screening is recommended every 3 years up until 49 years of age. In addition, opportunistic screening before 23 years of age is not uncommon in Denmark (15). First diagnoses of cervical cancer were identified from the Danish Pathology Registry (16), which contains complete information on all cytological and histological diagnoses performed at all Danish pathology departments since 1997. In addition, many pathology departments also included information dating back to before 1980. The diagnostic information in the Pathology Registry is based on the Systematized Nomenclature of Medicine, and cervical cancers were identified by topography codes T83xxx and morphology codes starting with M8 and M9 and behavior code 3.
Information on Covariates
A priori, we considered attained age, educational level, and ethnicity as potentially confounding factors (17). We obtained information about highest achieved education (short, medium, or long) for the women and their parents from the educational register at Statistics Denmark (18) and used the highest level of the women or their parents. Ethnicity was also derived from Statistics Denmark (19) and defined as Danish, Western immigrant, and non-Western immigrant.
Statistical Analysis
The women were followed from October 1, 2006, or their 17th birthday (cohort entry), whichever came last, until diagnosis of cervical cancer, death, emigration, or December 31, 2019, whichever came first. Thus, women with cervical cancer prior to cohort entry were not included in the cohort. Vaccination status was treated as a time-varying variable, and women contributed person-time as unvaccinated until 1 year after the date of first vaccination. Thus, women could enter the cohort as either unvaccinated or vaccinated depending on the date of first vaccination. Vaccinated women were categorized according to age at vaccination in a priori determined age groups (16 years or younger, 17-19 years, or 20-30 years) based on knowledge from our previous studies on HPV vaccine effectiveness (9,20,21) and reflecting that median age of sexual debut among Danish girls is 16 years (22). Person-time was further divided according to attained age in 1-year intervals to account for the underlying age-specific rate of cervical cancer.
The cumulative incidence was estimated according to age at vaccination. We calculated incidence rates (IRs) of cervical cancer as the ratio of the number of new incident cases and the person-time at risk according to age at vaccination. Poisson regression with a log-link and person-time as offset was used to estimate incidence rate ratios (IRRs) and corresponding 95% confidence intervals (CIs) for cervical cancer comparing vaccinated women with unvaccinated women. We estimated IRRs for each of the 3 age-at-vaccination groups with unvaccinated women as reference, while adjusting for attained age through a restricted cubic spline. Additionally, we adjusted for education, calendar year, and ethnicity. Moreover, to further address the impact of prevalent cases, we considered 5 different buffer periods in additional analyses, where vaccinated women were counted as unvaccinated until date of vaccination (no buffer) or 2, 3, 4, and 5 years after date of vaccination, respectively.
To address missing values in ethnicity and education, we applied 3 strategies: complete case analysis, coding missing as unknown, and nonparametric multiple imputation based on random forest. The results were almost identical, and we therefore only present the results from the complete case analysis.
A statistical significance level of 5% was used in all analyses. The statistical software R version 3.6.3 (23) was used for all analyses.
Results
A total of 867 689 women were included for analysis, of which 314 852 (36.3%) received the first dose before the age of 17 years. During follow-up, 20 063 (2.3%) and 167 607 (19.3%) initiated vaccination at the age of 17-19 and 20-30 years, respectively (Table 1). Among the 502 522 vaccinated women, 78.5% completed 3-dose vaccination, 15.7% received 2 doses, and 5.8% received 1 dose.
Table 1.
Baseline characteristics of the study population stratified by vaccination status and age at vaccination
| Baseline characteristic | Unvaccinateda | Age at vaccination |
||
|---|---|---|---|---|
| ≤16 y | 17-19 ya | 20-30 ya | ||
| (n = 365 167) | (n = 314 852) | (n = 20 063) | (n = 167 607) | |
| Educational level, No. (%)b | ||||
| Higher | 143 994 (39.4) | 141 217 (44.9) | 10 897 (54.3) | 60 759 (36.3) |
| Vocational | 160 359 (43.9) | 142 238 (45.2) | 7857 (39.2) | 82 828 (49.4) |
| Basic | 37 789 (10.3) | 28 671 (9.1) | 1198 (6.0) | 22 670 (13.5) |
| Missing | 23 025 (6.3) | 2726 (0.9) | 111 (0.6) | 1350 (0.8) |
| Ethnicity, No. (%) | ||||
| Danish | 302 657 (82.9) | 288 013 (91.5) | 19 275 (96.1) | 154 801 (92.4) |
| Western immigrant | 16 902 (4.6) | 2313 (0.7) | 123 (0.6) | 1707 (1.0) |
| Non-Western immigrant | 41 010 (11.2) | 24 526 (7.8) | 665 (3.3) | 11 099 (6.6) |
| Missing | 4598 (1.3) | 0 | 0 | 0 |
Women can contribute person-time to both unvaccinated and vaccinated after the age of 17 years.
Maximum of own, mother, or father.
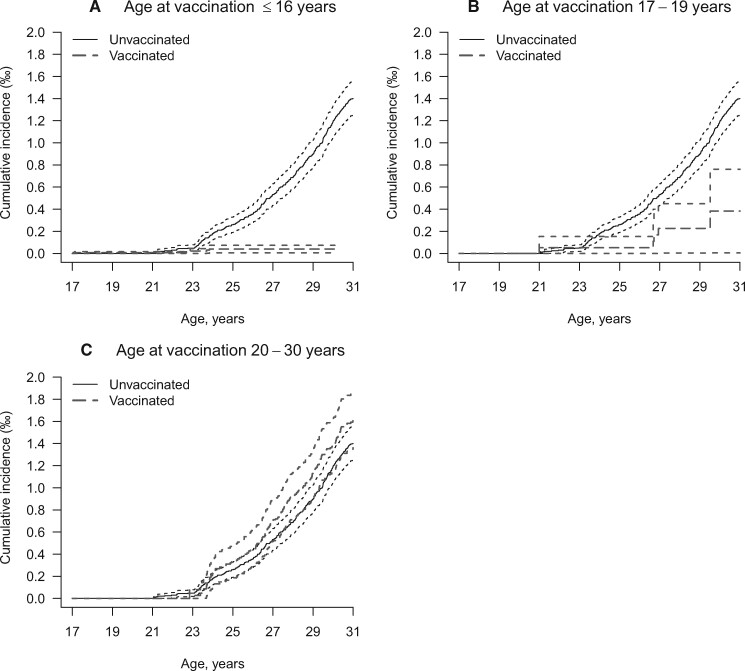
During 5 544 655 person-years, the women developed 504 cervical cancers of which 325 were among unvaccinated women and 179 were among vaccinated women, mostly among women vaccinated at age 20-30 years. The crude incidence of cervical cancer was 11.3 per 100 000 among unvaccinated women and 6.7 per 100 000 among vaccinated women. The cumulative incidence of cervical cancer by age at vaccination and attained age is displayed in Figure 1. In the unvaccinated cohort, the incidence started to rise sharply at age 23 years (entry into the screening program) and reached a maximum of 0.13% at age 30 years, and a similar pattern was seen in women vaccinated at 20-30 years. Among women vaccinated at age 16 years and younger, the incidence of cervical cancer remained low at 0.01% with increasing age.
Figure 1.
Cumulative incidence of cervical cancer by attained age and stratified by age at vaccination. Analyses are shown for women vaccinated (A) age 16 years or younger, (B) 17-19 years, and (C) 20-30 years.
After adjusting for attained age, the IRRs were lower among women vaccinated before the age of 20 years compared with unvaccinated women (Table 2). This persisted after adjusting for calendar year, education, and ethnicity (16 years and younger: IRR = 0.14, 95% CI = 0.04 to 0.53; 17-19 years: IRR = 0.32, 95% CI = 0.08 to 1.28) although only statistically significant for women vaccinated before the age of 17 years. The IRR of cervical cancer among all women vaccinated before 20 years of age (16 years and younger and 17-19 years combined) was 0.19 (95% CI = 0.06 to 0.59) compared with unvaccinated women (data not shown). For women vaccinated at the age of 20 years or older, the IRR tended to be higher in vaccinated than in unvaccinated women (IRR = 1.19, 95% CI = 0.80 to 1.79).
Table 2.
Incidence rate ratios (IRRs) of cervical cancer comparing vaccinated with unvaccinated women according to age at vaccination and with 1-year buffer period
| Vaccination status | Person-years | Events | Age-adjusted | Adjusteda | Adjustedb |
|---|---|---|---|---|---|
| IRR (95% CI) | IRR (95% CI) | IRR (95% CI) | |||
| Unvaccinated | 2 884 778 | 325 | 1 | 1 | 1 |
| Vaccinated, age ≤16 y | 1 643 967 | 6 | 0.13 (0.04 to 0.40) | 0.13 (0.04 to 0.41) | 0.14 (0.04 to 0.53) |
| Vaccinated, age 17-19 y | 174 679 | 5 | 0.29 (0.08 to 1.01) | 0.31 (0.09 to 1.07) | 0.32 (0.08 to 1.28) |
| Vaccinated, age 20-30 y | 841 231 | 168 | 1.15 (0.88 to 1.50) | 1.14 (0.87 to 1.49) | 1.19 (0.80 to 1.79) |
Adjusted for attained age and maximum educational level of own, mother, or father. CI = confidence interval.
Adjusted for attained age; maximum educational level of own, mother, or father; calendar year; and ethnicity.
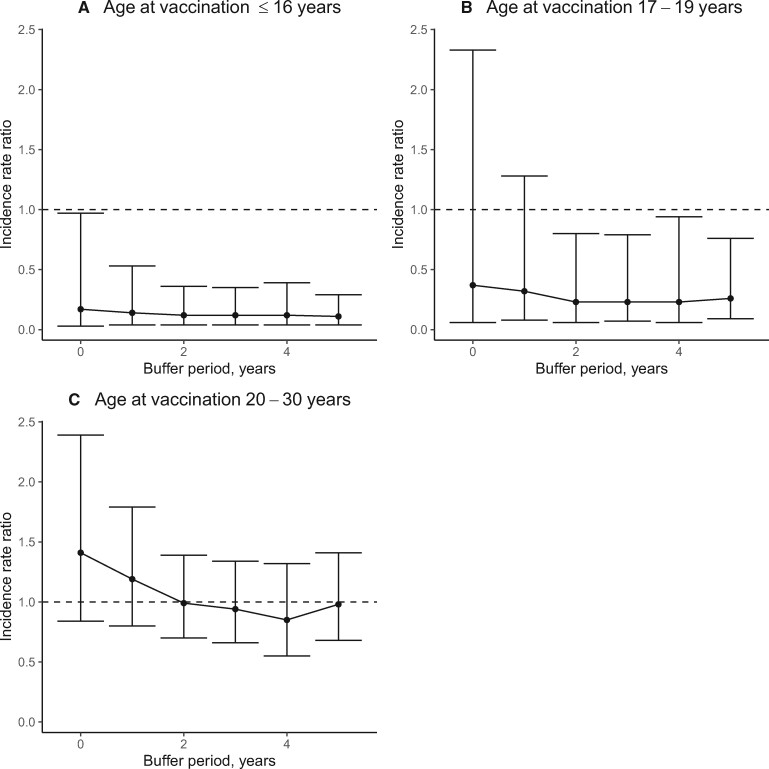
Figure 2 shows the adjusted IRR in relation to different buffer periods after vaccination. The largest change was seen for women vaccinated at older age (20-30 years) where the IRR was 1.41 (95% CI = 0.84 to 2.39) at the time at first dose (no buffer period) and 0.85 (95% CI = 0.55 to 1.32) with 4-year buffer period. In contrast, longer buffer period had little effect for women vaccinated before the age of 20 years.
Figure 2.
Incidence rate ratios (IRRs) of cervical cancer comparing vaccinated with unvaccinated women by length of buffer period and stratified by age at vaccination. Analyses are shown by age at vaccination for (A) age ≤16 years or younger, (B) age 17-19 years, and (C) age 20-30 years. Error bars indicate 95% confidence intervals.
Discussion
In this nationwide cohort study, we find that HPV vaccination reduces the incidence rate of cervical cancer by 86% and 68% among girls and women vaccinated at age 16 years and younger or 17-19 years, respectively. The picture was less clear for women vaccinated at age 20-30 years, where the incidence was similar to that of unvaccinated women when limiting the potential effect of prevalent cases by including a buffer period. These findings provide evidence, with cervical cancer as the endpoint, for the greater utility of vaccinating women at a young age.
To our knowledge, HPV vaccine effectiveness against cervical cancer has been investigated in only 1 previous study from Sweden, and our results for the women vaccinated before age 20 years agree with the Swedish data showing that HPV vaccine effectiveness against cervical cancer was 88% among girls vaccinated younger than age 17 years (11). Our results are also in line with what could be expected from recent findings on HPV-vaccine effectiveness against severe cervical lesions (9,10,24), and we previously reported that among Danish women vaccinated younger than 17 years of age, HPV vaccine effectiveness against cervical intraepithelial neoplasia grade 3 or worse (CIN3+) was 63% (IRR = 0.37, 95% CI = 0.30% to 0.45%) (9). The greater protection against cervical cancer compared with our previous findings for CIN3+ is most likely explained by differences in the prevalence of specific HPV types in cervical cancer vs CIN3. HPV16 and HPV18 account for approximately 70% of cervical cancers and some 50% of CIN3 lesions, and other oncogenic HPV types (not included in the quadrivalent vaccine) such as HPV31 and HPV33 seem to be more common in CIN3 (5,25).
Our finding of no or limited effect among women vaccinated at an older age (20-30 years) is in line with previous findings from Denmark and Sweden on vaccine effectiveness against CIN3+ (26), and also other studies have reported a similar trend in HPV vaccine effectiveness by age at first dose (10,21). This may partly be explained by selection bias, as a higher proportion of women vaccinated at older age have been vaccinated outside the free-of-charge vaccination program, and it is possible that they have been vaccinated for reasons related to a higher risk of cervical cancer including sexual behavior and lifestyle and health factors. Another potential explanation is that these women have a much higher likelihood of already being exposed to HPV before vaccination, and the increased IRR for women aged 20 years and older at vaccination and zero buffer period suggest indication for vaccination is risk related in some of the women vaccinated at older age. To account for potential prevalent cervical cancers at time of vaccination, we used a buffer period of 1-5 years with 1-year intervals. The buffer period had virtually no effect on the IRR of cervical cancer among women vaccinated younger than 17 years of age, of which the majority likely were HPV naive at time of vaccination. In women vaccinated at 20-30 years, however, the IRR for cervical cancer decreased with increasing length of buffer period. Optimal length of buffer period depends on the lead time of the outcome, and buffer periods even longer than 5 years might be required for evaluation of HPV-vaccine effectiveness against cervical cancer (27).
The substantial vaccine effectiveness against cervical cancer among women vaccinated at age younger than 20 years is very encouraging. Thus, when combining vaccination with screening, it should potentially be possible to eliminate cervical cancer (4). In Denmark, organized cervical cancer screening was initiated in the 1960s, and in 1996, a nationwide program was implemented (28). Although the Danish screening program has reduced cervical cancer incidence markedly (29,30), there are still around 350 new cases each year corresponding to an age-standardized incidence of approximately 9 per 100 000 women (31). The corresponding global incidence is 13 per 100 000 women (32).
Over time, the overall incidence of cervical cancer will also benefit from the increasing number of birth cohorts being vaccinated and because of a more pronounced herd protection. Moreover, because the nonavalent vaccine was first introduced in the Danish vaccination program in November 2017, our results reflect primarily protection against HPV16 and HPV18. Adding the additional 5 oncogenic HPV types in the nonavalent HPV vaccine has been estimated to increase the potential preventable proportion of cervical cancers from approximately 70% to nearly 90% (6). Cervical cancers associated with HPV16 and HPV18 seem to be diagnosed at an earlier age than cancers associated with other oncogenic HPV types (5,25), and this age-specific type distribution most likely explains the great vaccine effectiveness of 86%-88% among the youngest women vaccinated in our study and the Swedish (11), where maximum age at end of follow-up was 30 years.
In addition to the observed protection against cervical cancer in the present study, we have previously documented substantial real-world effectiveness of HPV vaccination against cervical high-grade lesions (9), vulvovaginal high-grade lesions (20), and genital warts (21). Real-world effectiveness data constitute an important supplement to the documentation from the clinical vaccine trials, because it concerns the general population. Denmark experienced a dramatic decline in vaccine uptake during 2015-2016 because of extensive negative media coverage, and similar declines have been observed in other countries (33). Suspected adverse reactions included primarily postural orthostatic tachycardia syndrome and chronic fatigue syndrome (34). A Danish study, however, showed that women who suspected adverse reactions had symptoms and a health-care–seeking pattern different from the matched population even before receiving the first vaccine dose (34). Suspected adverse pregnancy outcomes and infant mortality associated with unintended HPV vaccination during pregnancy have also not been documented (35). Overall, there is a large amount of evidence that supports the safety of the HPV vaccine (36). Fortunately, confidence in the HPV vaccine has been restored in the Danish population, and today, uptake is almost at the level before the crisis (33). Thus, the occurrence of HPV-associated precancers and cancers are expected to decrease substantially in the future with decreased morbidity and mortality and additional benefits such as less need for conization, which is associated with premature delivery (37).
One strength of this study is the nationwide design including the entire Danish female population aged 17-30 years in 2006-2019. HPV vaccine coverage in our study was high because the decline in Danish HPV-vaccine uptake during 2015-2016 did not substantially affect the birth cohorts in the present study (33). Individual-level information on HPV vaccination and cervical cancer diagnoses was derived from nationwide high-quality registries with high completeness. Virtually no women were lost to follow-up, and we were able to exclude women with cervical cancer prior to HPV vaccination. Finally, educational level and ethnicity were included as socioeconomic measures in our analyses as socioeconomic status has been shown to be associated with both participation in the Danish vaccination program (17) and risk of cervical cancer (38) and is associated with lifestyle factors such as smoking.
There are also some limitations that should be mentioned. We had no information on sexual behavior and exposure to HPV prior to vaccination. To address this potential limitation, we stratified the analysis according to age at vaccination assuming that prevalent HPV infections occurred more often with higher age. Although we adjusted for socioeconomic measures, we had no information on potential confounding lifestyle and health factors such as smoking, which is associated with development of cervical cancer (39). Finally, we have previously shown that cervical cancer screening rates do not differ markedly between vaccinated and unvaccinated women (9), indicating that differences in screening is an unlikely explanation for our results.
In conclusion, we find that HPV vaccination is associated with a substantial protection against cervical cancer among girls vaccinated at age 19 years or younger. Thus, the present nationwide study documents a high real-world effectiveness at the population level.
Funding
This work was supported by the Mermaid project (Mermaid 2).
Notes
Role of the funder: The funders had no role in the design of the study, the data collection, the analysis or interpretation of the data, the writing of the article, or the decision to submit the manuscript for publication.
Disclosures: SKK has previously received speakers from Merck and a research grant through her research institution from Merck. All remaining authors have declared no conflicts of interest.
Author contributions: SKK: Conceptualization, Methodology, Investigation, Resources, Writing—Original Draft, Writing—Review & Editing, Supervision. CD: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Writing—Original Draft, Writing—Review & Editing. FB: Methodology, Validation, Formal analysis, Writing—Review & Editing. LB: Conceptualization, Methodology, Investigation, Writing—Original Draft, Writing—Review & Editing.
Data Availability
The data underlying this article cannot be shared publicly due to ethical/privacy reasons.
References
- 1.de Martel C, Plummer M, Vignat J, Franceschi S.. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int J Cancer. 2017;141(4):664–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Serrano B, de Sanjosé S, Tous S, et al. Human papillomavirus genotype distribution for HPVs 6, 11, 16, 18, 31, 33, 45, 52 and 58 in female anogenital lesions. Eur J Cancer. 2015;51(13):1732–1741. [DOI] [PubMed] [Google Scholar]
- 3.Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189(1):12–19. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. A global strategy for elimination of cervical cancer; 2020. . https://www.who.int/publications/i/item/9789240014107. Accessed February 25, 2021.
- 5.Dovey de la Cour C, Guleria S, Nygård M, et al. Human papillomavirus types in cervical high-grade lesions or cancer among Nordic women–potential for prevention. Cancer Med. 2019;8(2):839–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joura EA, Giuliano AR, Iversen OE, et al. ; Broad Spectrum HPV Vaccine Study. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in Women. N Engl J Med. 2015;372(8):711–723. [DOI] [PubMed] [Google Scholar]
- 7.Hildesheim A, Wacholder S, Catteau G, et al. ; CVT Group. Efficacy of the HPV-16/18 vaccine: final according to protocol results from the blinded phase of the randomized Costa Rica HPV-16/18 vaccine trial. Vaccine. 2014;32(39):5087–5097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Munoz N, Kjaer SK, Sigurdsson K, et al. Impact of human papillomavirus (HPV)-6/11/16/18 vaccine on all HPV-associated genital diseases in young women. J Natl Cancer Inst .2010;102(5):325–339. [DOI] [PubMed] [Google Scholar]
- 9.Verdoodt F, Dehlendorff C, Kjaer SK.. Dose-related effectiveness of quadrivalent human papillomavirus vaccine against cervical intraepithelial neoplasia: a Danish nationwide cohort study. Clin Infect Dis. 2020;70(4):608–614. [DOI] [PubMed] [Google Scholar]
- 10.Drolet M, Bénard É, Pérez N, et al. ; HPV Vaccination Impact Study Group. Population-level impact and herd effects following the introduction of human papillomavirus vaccination programmes: updated systematic review and meta-analysis. Lancet. 2019;394(10197):497–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lei J, Ploner A, Elfström KM, et al. HPV vaccination and the risk of invasive cervical cancer. N Engl J Med. 2020;383(14):1340–1348. [DOI] [PubMed] [Google Scholar]
- 12.Schmidt M, Pedersen L, Sørensen HT.. The Danish Civil Registration System as a tool in epidemiology. Eur J Epidemiol. 2014;29(8):541–549. [DOI] [PubMed] [Google Scholar]
- 13.Andersen JS, Olivarius Nde F, Krasnik A.. The Danish National Health Service Register. Scand J Public Health. 2011;39(suppl 7):34–37. [DOI] [PubMed] [Google Scholar]
- 14.Kildemoes HW, Sorensen HT, Hallas J.. The Danish National Prescription Registry. Scand J Public Health. 2011;39(suppl 7):38–41. [DOI] [PubMed] [Google Scholar]
- 15.Baldur-Felskov B, Dehlendorff C, Munk C, Kjaer SK.. Early impact of human papillomavirus vaccination on cervical neoplasia—nationwide follow-up of young Danish women. J Natl Cancer Inst. 2014;106(3). [DOI] [PubMed] [Google Scholar]
- 16.Bjerregaard B, Larsen OB.. The Danish Pathology Register. Scand J Public Health. 2011;39(suppl 7):72–74. [DOI] [PubMed] [Google Scholar]
- 17.Schreiber SMS, Juul KE, Dehlendorff C, Kjær SK.. Socioeconomic predictors of human papillomavirus vaccination among girls in the Danish childhood immunization program. J Adolesc Health. 2015;56(4):402–407. [DOI] [PubMed] [Google Scholar]
- 18.Jensen VM, Rasmussen AW.. Danish Education Registers. Scand J Public Health. 2011;39(suppl 7):91–94. [DOI] [PubMed] [Google Scholar]
- 19.Statistics Denmark, StatBank Denmark [online resource]. https://www.statistikbanken.dk. Accessed January 05, 2021.
- 20.Dehlendorff C, Baandrup L, Kjaer SK.. Real-world effectiveness of human papillomavirus vaccination against vulvovaginal high-grade precancerous lesions and cancers. J Natl Cancer Inst. 2021;113(7):869–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baandrup L, Dehlendorff C, Kjaer SK.. One-dose human papillomavirus vaccination and the risk of genital warts: a Danish Nationwide Population-based Study [published online ahead of print October 13, 2020]. Clin Infect Dis. 2020. doi:10.1093/cid/ciaa1067. [DOI] [PubMed] [Google Scholar]
- 22.Olesen TB, Jensen KE, Munk C, Tolstrup JS, Kjaer SK.. “Liva”-population survey of female sexual habits [in Danish]. Ugeskr Laeger. 2010;172(47):3254–3259. [PubMed] [Google Scholar]
- 23.R Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria; 2020. https://www.R-project.org/. Accessed February 25, 2021.
- 24.Thamsborg LH, Napolitano G, Larsen LG, Lynge E.. High-grade cervical lesions after vaccination against human papillomavirus: a Danish cohort study. Acta Obstet Gynecol Scand. 2020;99(10):1290–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tjalma WA, Fiander A, Reich O, et al. ; HERACLES/SCALE Study Group. Differences in human papillomavirus type distribution in high-grade cervical intraepithelial neoplasia and invasive cervical cancer in Europe. Int J Cancer. 2013;132(4):854–867. [DOI] [PubMed] [Google Scholar]
- 26.Dehlendorff C, Sparén P, Baldur-Felskov B, et al. Effectiveness of varying number of doses and timing between doses of quadrivalent HPV vaccine against severe cervical lesions. Vaccine. 2018;36(43):6373–6378. [DOI] [PubMed] [Google Scholar]
- 27.Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S.. Human papillomavirus and cervical cancer. Lancet. 2007;370(9590):890–907. [DOI] [PubMed] [Google Scholar]
- 28.Pedersen K, Fogelberg S, Thamsborg LH, et al. An overview of cervical cancer epidemiology and prevention in Scandinavia. Acta Obstet Gynecol Scand. 2018;97(7):795–807. [DOI] [PubMed] [Google Scholar]
- 29.Kyndi M, Frederiksen K, Kjaer SK.. Cervical cancer incidence in Denmark over six decades (1943-2002). Acta Obstet Gynecol Scand. 2006;85(1):106–111. [DOI] [PubMed] [Google Scholar]
- 30.Baldur-Felskov B, Munk C, Nielsen TSS, et al. Trends in the incidence of cervical cancer and severe precancerous lesions in Denmark, 1997-2012. Cancer Causes Control. 2015;26(8):1105–1116. [DOI] [PubMed] [Google Scholar]
- 31.Nordcan Database. https://www-dep.iarc.fr/NORDCAN.htm. Accessed December 20, 2020.
- 32.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A.. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. [DOI] [PubMed] [Google Scholar]
- 33.Hansen PR, Schmidtblaicher M, Brewer NT.. Resilience of HPV vaccine uptake in Denmark: decline and recovery. Vaccine. 2020;38(7):1842–1848. [DOI] [PubMed] [Google Scholar]
- 34.Mølbak K, Hansen ND, Valentiner-Branth. Pre-vaccination care-seeking in females reporting severe adverse reactions to HPV vaccine. A registry based case-control study. PLoS One. 2016;11(9):e0162520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Faber MT, Duun-Henriksen AK, Dehlendorff C, Tatla MK, Munk C, Kjaer SK.. Adverse pregnancy outcomes and infant mortality after quadrivalent HPV vaccination during pregnancy. Vaccine. 2019;37(2):265–271. [DOI] [PubMed] [Google Scholar]
- 36.Phillips A, Patel C, Pillsbury A, Brotherton J, Macartney K.. Safety of human papillomavirus vaccines: an updated review. Drug Safety. 2018;41(4):329–346. [DOI] [PubMed] [Google Scholar]
- 37.Nøhr B, Tabor A, Frederiksen K, Kjær SK.. Loop electrosurgical excision of the cervix and the subsequent risk of preterm delivery. Acta Obstet Gynecol Scand. 2007;86(5):596–603. [DOI] [PubMed] [Google Scholar]
- 38.Jensen KE, Hannibal CG, Nielsen A, et al. Social inequality and incidence of and survival from cancer of the female genital organs in a population-based study in Denmark, 1994-2003. Eur J Cancer. 2008;44(14):2003–2017. [DOI] [PubMed] [Google Scholar]
- 39.Jensen KE, Schmiedel S, Frederiksen K, Norrild B, Iftner T, Kjær SK.. Risk for cervical intraepithelial neoplasia grade 3 or worse in relation to smoking among women with persistent human papillomavirus infection. Cancer Epidemiol Biomarkers Prev. 2012;21(11):1949–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article cannot be shared publicly due to ethical/privacy reasons.