Abstract
Tendons are specialized matrix-rich connective tissues that transmit forces from muscle to bone and are essential for movement. As tissues that frequently transfer large mechanical loads, tendons are commonly injured in patients of all ages. Following injury, mammalian tendons heal poorly through a slow process that forms disorganized fibrotic scar tissue with inferior biomechanical function. Current treatments are limited and patients can be left with a weaker tendon that is likely to re-rupture and an increased chance of developing degenerative conditions. More effective, alternative treatments are needed. However, our current understanding of tendon biology remains limited. Here, we emphasize why expanding our knowledge of tendon development, healing, and regeneration is imperative for advancing tendon regenerative medicine. We provide a comprehensive review of the current mechanisms governing tendon development and healing and further highlight recent work in regenerative tendon models including the neonatal mouse and zebrafish. Importantly, we discuss how present and future discoveries can be applied to both augment current treatments and design novel strategies to treat tendon injuries.
Keywords: connective tissues, stem cells, injury, repair
1. Introduction
The musculoskeletal system is an interconnected network of cartilage, muscle, and bone that coordinates movement. Central to the proper function of this network are tendons, specialized uniaxial connective tissues that bridge muscle and bone. Tendons are viscoelastic, highly ordered, extracellular matrix (ECM)-rich tissues that can transmit mechanical forces as large as 12.5 times the human body weight.1,2 Their dynamic role in daily movement and athletic activities also makes them prone to injury. Although tendons are proliferative during embryonic development and postnatal life, adult tendons exhibit little to no cellular turnover and extremely poor healing capacities.3 Tendon injuries can therefore be especially devastating for an athlete’s career as well as a strain on the quality of life for patients of all ages in the general population.
Injuries to tendons and other joint tissues including ligaments, which connect bones, comprise an estimated 45% of the approximate 32 million musculoskeletal injuries reported annually in the United States.4 Although ligaments are considered molecularly indistinguishable from tendons during development, they are functionally distinct. While injuries to ligaments such as the anterior cruciate ligament (ACL) are also common, in this review we will focus on tendons. Tendon injuries are often caused by overuse, athletic activity, and/or aging in predominantly heavy load-bearing limb tendons and can range from chronic pain-inducing disorders called tendinopathies to severe acute pain induced by partial or full ruptures.5–7 Despite their prevalence, treatment options for tendon injuries remain limited and largely rely on surgical re-attachment or replacement followed by a slow recovery period lasting several months. Trofa et al. (2017)8 reported that 30% of professional athletes across a variety of sports were unable to return following Achilles tendon injury, and those that did return following treatment performed significantly worse than prior to injury within the first year. Hence, even with treatment, injured tendons form scar tissue with inferior biomechanical properties that can only sustain a maximum of 60% of the force of their healthy counterparts, leading to a high chance of re-rupture and a gradual progression of other musculoskeletal diseases including osteoarthritis.9–11 Likewise, chronic pain-inducing tendon disorders called tendinopathies can also lead to ruptures,12 altogether contributing substantially to a growing financial healthcare burden and underscoring the pressing need for the development of better alternative therapeutics for tendon injuries and disorders.
Although adult mammalian tendons exhibit limited reparative capacity, recent studies have demonstrated that tendons in neonatal mice and zebrafish can fully regenerate. Understanding the mechanisms driving successful regeneration in these models will yield valuable insights into the restoration of ordered matrix-rich tissues that can be applied towards designing new therapeutic approaches to stimulate regenerative outcomes in patients. In this review, we provide an overview of our current understanding of tendon development and repair and how tendon regeneration models can be exploited as powerful systems that can open up new frontiers in tendon regenerative medicine.
2. The molecular and cellular architecture of tendons
As a force transmitting and mechanosensing tissue, the tendon harbors a unique structural morphology critical to its function.13,14 Tendons are hypocellular, ECM-rich tissues. The ECM is tightly packed with collagen I fibrils that run parallel to the tendon axis from muscle to bone (Figure 1A). Collagen I fibrils are comprised of a triple helical arrangement of collagen I polypeptides, which are ultimately assembled into successively higher order structures, i.e. fibers and then fascicles. Each fascicle is separated by a connective tissue layer called the endotenon. Although collagen I is the predominant ECM component and comprises approximately 80% of the tendon dry mass, other ECM proteins are present and required for the structural integrity of higher order collagen I arrangement.15 For instance, other collagens (COL5A1, COL12A1, COL14A1, COL22A1) and ECM proteins including Decorin (DCN), Fibromodulin (FMOD), Laminin 2 (LAMA2), and Tenascin C (TNC), crosslink and act as structural scaffolds between collagen I fibrils.16 Altogether, these ECM components construct a hierarchically ordered structure with properly aligned collagen I fibrils, which is key to providing strength, durability, and stability to the tissue during force transmission.
Figure 1. Tendon structure.
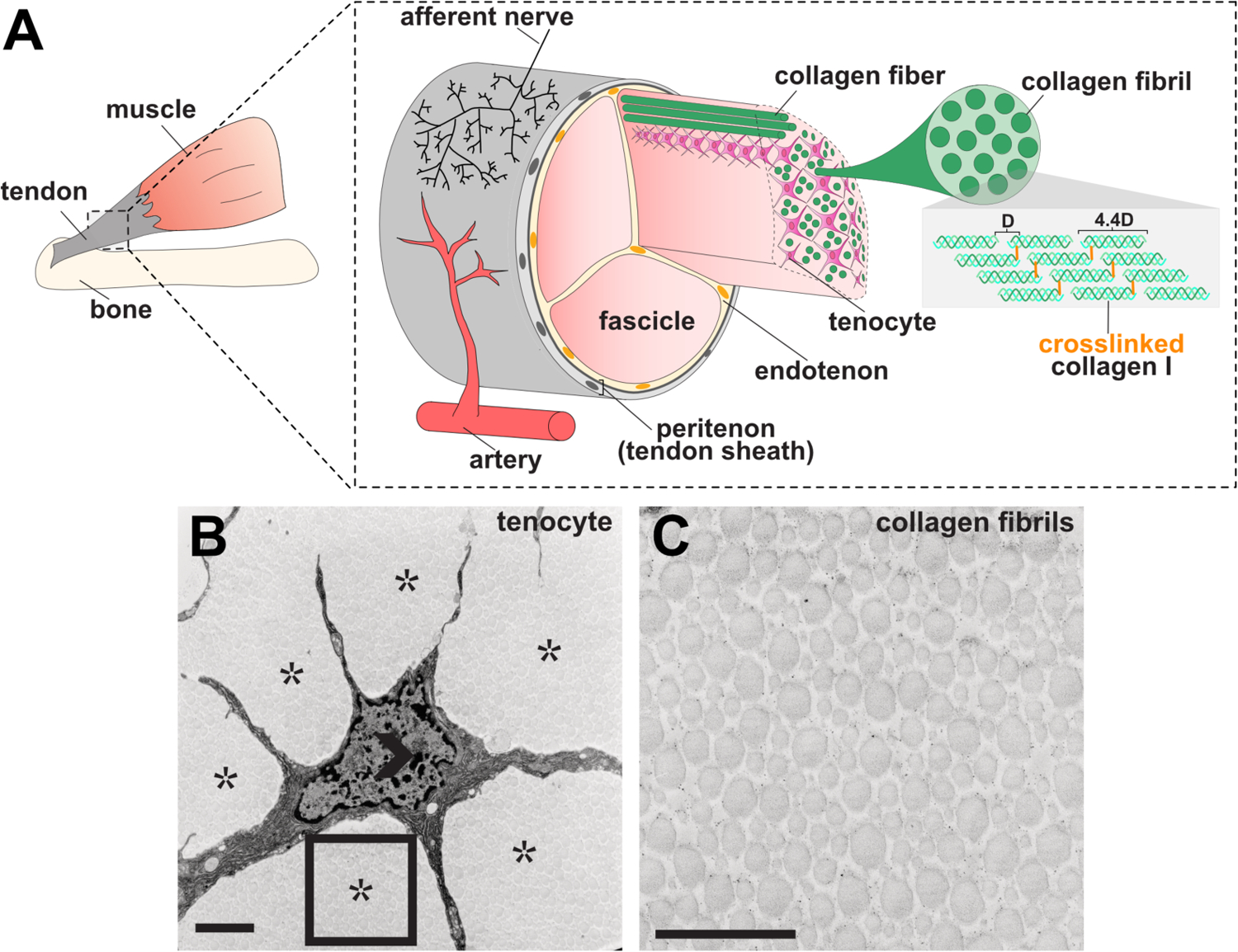
(A) Graphical representation of tendon morphology. The tendon midsubstance is comprised of collagen molecules which are spaced apart at a distance of 67 nm (letter D in diagram) and cross-linked to form stable fibrils199. Tenocytes are interspersed between collagen fibrils, which together generally form higher order bundles called fascicles. Fascicles are held together by connective tissue called the endotenon. The tendon midsubstance is encased in the peritenon, or tendon sheath, which is comprised of a basement membrane and epithelial cell layer.200 (B–C) Transmission electron micrograph (TEM) of 6-week old mouse tenocyte (B) and collagen fibrils (C). Open arrowhead marks cell nuclei and asterisks mark collagen fibrils. Rectangle in B is shown at higher magnification in C. Scale bar, 1 um. Images in B–C were adapted from Kalson et al. (2015).37
Interspersed amongst collagen I fibrils are tendon cells, called tenocytes, which are arrayed in parallel rows along the tendon axis (Figure 1B–C). Tenocytes have stellate morphologies with thin, elongated cellular protrusions extending in all directions. These protrusions form cell-cell contacts with neighboring tenocytes from adjacent rows and within the same row, building an intricate tenocyte network. Gap junction proteins including Connexin-43 (CX43) are expressed at these cell-cell contacts and are thought to regulate the transfer of molecules between tenocytes.17,18 However, the significance of these gap junction proteins in regulating intercellular communication as well as what molecules pass between the cells is not well understood. Tenocytes and collagen fibrils are encased in an outer cellular sheath called the peritenon that is composed of two layers, the epitenon and the paratenon.5 In addition, while the interior of the tendon is largely devoid of vasculature or innervation, blood vessels and nerve endings penetrate and run through the epitenon and paratenon.19,20
Tendons are not a homogenous tissue and contain regions that are specialized for their function at their muscle and bone attachments. Tendons can broadly be broken into three general sections along their axes: the muscle-tendon attachment, termed the myotendinous junction (MTJ), the tendon midbody, and the tendon-bone attachment, called osteo-tendinous junction or the enthesis. While the structure of the tendon midbody is as described above, the MTJ and enthesis have distinct structural, cellular, and molecular characteristics required for muscle and bone attachment.
The MTJ displays a “zipper”-like morphology, where skeletal muscle fibers and collagen I fibrils in the tendon interdigitate in an alternating manner. At the tendon-muscle interface, the cytoskeletal network and basement membrane of the adjacent bundle of muscle fibers, or sarcolemma, directly attaches to collagen I fibrils in the tendon through binding interactions between different ECM proteins that are highly enriched at the MTJ including Thrombospondin 4 (Tsp4) and various laminins and integrins.16 Notably, the interdigitating pattern at the MTJ maximizes contact surface area, which strengthens the connection point between the two tissues and ensures stable transfer of mechanical forces.21,22
Tendon-bone attachment sites are composed of either fibrous or fibrocartilaginous connections.23 In fibrous entheses, dense tissue directly attaches to the bone or the periosteum via a large surface area, which helps to distribute forces and minimize stress levels. In contrast, the more common fibrocartilaginous enthesis has a comparatively narrow insertion site with a characteristic gradient comprised of four transition zones that contain increased mineralization and proteoglycan levels from the tendon towards the bone. The first zone includes an area that resembles the tendon midbody with linearly aligned type I collagen fibers surrounding fibroblast cells and it connects to the second zone of unmineralized fibrocartilage, which is mostly composed of types I, II, and III collagen and aggrecan. The third zone consists of mineralized fibrocartilage containing type I and II collagen, type X collagen (ColX) and aggrecan and joins to the fourth zone of bone and mineralized type I collagen. This gradual change in structure reduces stress levels exerted on two tissues with different biomechanical properties (tensile tendon and brittle bone), while effectively transferring the muscle force to the bone.24
3. Applying lessons from tendon development to augment existing treatments
Current tendon injury and disorder treatments are limited in the clinic with varying success rates. In this section, we will discuss known mechanisms underlying tendon development as well as how this knowledge can be applied to enhance current mainstream treatments or advance novel stem cell-based treatment strategies. As injuries most often occur in the tendon midbody and tendon-bone attachment, we will focus on these two areas of the tendon. For a comprehensive review of MTJ development, we refer the reader to the following reviews: Subramanian and Schilling, 201516 and Charvet et al. 2012.25
3.1. Tendon and tendon-bone attachment development
Tendons are found in craniofacial, axial, and limb regions of the body, each with distinct developmental origins. Craniofacial tendons are derived from the neural crest,26–28 whereas axial and limb tendons are derived from the paraxial/somitic and lateral plate mesoderm, respectively.29–31 Tendon development begins during embryogenesis and continues through post-natal periods. During early embryonic stages, tendon progenitors are specified and aggregate, forming primordia adjoining developing muscle and skeletal elements.32 These progenitors proliferate33 and differentiate throughout later stages of embryogenesis, secreting collagen I and other ECM proteins including Tenomodulin (Tnmd)34,35 that are required for proper collagen I matrix assembly and structural integrity. The process of tendon maturation continues through postnatal stages, in which tenocytes continue to proliferate and collagen I fibrils grow vastly in diameter and length.36,37 The expansion of the ECM during matrix maturation significantly decreases cell density and is accompanied by a decrease in proliferation as well as the adoption of the elongated, stellate morphology of mature tenocytes.3,37 Proliferation levels continue to decrease through adulthood, in which very limited cellular turnover is observed.
Our understanding of transcriptional regulation of tendon development is limited to the identification of a small subset of transcription factors.38 The basic-loop-helix transcription factor Scleraxis, Scx,32 is highly enriched in tendon progenitors regardless of function and anatomical location. Scx is also expressed in the adult tendon, as well as some other tissues such as the heart valves.39 Developmental studies in mice and zebrafish have determined that Scx is dispensible for the initiation of tendon formation, but required for the maintenance and maturation of tendon cell fate and matrix during later stages of development.40,41 Canonical Transforming Growth Factor β (TGF-β) signaling through intracellular SMAD2/3 can promote expression of Scx and other tendon markers and is required for maintenance of tendon cell fate. However, Tgf-β2−/−;Tgf-β3−/− double null and Prx1-Cre;Tgfβrflox mutant embryos exhibited defects in tendon maturation, not specification,42,43 suggesting other TGF-β ligands and/or additional signaling pathways regulate tendon fate induction. Consistent with these findings, TGF-β superfamily members including Myostatin (Gdf-8) are also involved in tendon maturation.44
The transcription factors Mohawk (Mkx) and Early Growth Response 1 (Egr1) and 2 (Egr2) also play a role in tendon development. Although Mkx and Egr1/Egr2 are highly expressed in many other tissues, loss of function animal models for these genes suggest they regulate specific aspects of tendon matrix development. Mkx−/− null mice display defects in the ECM maturation during late stages of embryogenesis as well as post-natal development and have lower expression of tendon genes (Tnmd, Dcn, and Fmod), resulting in hypoplastic tendons.45,46 Egr1−/− and Egr2−/− null mice both display lower expression of many tendon-enriched collagens, leading to weaker tendons.47,48 Interestingly, tendons from Egr1−/− null mice exhibit lower Scx expression, whereas those from Mkx−/− null mice have unchanged Scx expression, indicating Mkx regulates tendon maturation either downstream or through a parallel mechanism to Scx. While all three transcription factors regulate Col1a1 transcription, none are master regulators of tendon cell fate.
Tendons can be divided into force-transmitting (energy storing or positional)49 or anchoring connective tissues. In general, limb and craniofacial tendons are largely force transferring and directly involved in musculoskeletal movement, whereas axial tendons such as those that connect the intercostal muscles to the ribs anchor and dissipate forces to prevent displacement of the muscle from the bone. Evidence suggests that there may be discrete differences in their developmental programs. For example, Scx−/− null mouse mutants display developmental defects specifically in force transmitting tendons, but not anchoring tendons, due to impaired tendon elongation,40,50 indicating Scx function may vary depending on the functional role and developmental program of the tendon. Interestingly, despite differences in developmental origin, both craniofacial and limb tendons do not require the presence of muscle to initiate tendon specification during embryogenesis, whereas axial tendons do.27,51–53 Furthermore, Chen et al. (2020)54 discovered that skeletal progenitors can be re-specified to a tendon cell fate via the inhibition of the mevalonate pathway during zebrafish cranial and fin, but not axial, tendon development. Together, these data interestingly suggest that similarities in the mode of regulation between craniofacial and limb tendons may outweigh the importance of their developmental cellular origins.
Neighboring muscle and skeletal tissues play significant roles in tendon development. Both the presence and movement of muscle is required for the maintenance of tendon cell fate and maturation.28,30,32,55,56 In particular, the dependence of tendon development on muscle movements has been tied to mechanically-induced changes in TGF-β and Fibroblast Growth Factor (FGF) signaling.55,56 Tendon induction in the developing autopod (hand region) has also been shown to be dependent on developing cartilage in mice.57 Furthermore, evidence suggests that similar extracellular signals can induce different cellular fate outcomes in developing muscle, tendon, and bone. For example, high levels of Bone Morphogenetic Signaling (BMP) signaling promote cartilage formation,58 but inhibit tenogenesis,32 illustrating the existence of discrete genetic regulatory circuits in neighboring tissues originating from a gradient of extracellular signals.
Skeletal and tendon development are intricately linked during formation of the tendon-bone attachment. While muscle, tendon and cartilage first appear during embryonic development, the murine enthesis forms distinctly from these structures and matures during early postnatal stages. Unique multipotent progenitors at the tendon-bone interface specify the position of the bone eminence for the attachment site.59 These progenitors express both Scx and the transcription factor SRY-Box Transcription Factor 9 (Sox9), which is required for skeletal development. Lineage tracing studies have determined that Scx+/Sox9+ cells give rise to the tendon-bone attachment unit and the associated bone eminence.60 Tenocytes and chondrocytes further from the enthesis are derived from Scx+ and Sox9+ single-positive progenitors, respectively. Early formation of the bone eminence, specifically the tendon-humoral tuberosity, requires BMP4 expression from tendon cells as deleting BMP4 in Scx-expressing cells affected the formation of the enthesis and associated bone ridge.
Although muscle loading is not involved in early phases of enthesis development, it is crucial for its maturation. Tatara et al. (2014)61 showed that reducing muscle loading in the maturing enthesis during postnatal stages leads to impaired mineral deposition, reduced fibrocartilage formation, changes in the bone architecture and consequently inferior mechanical properties. Recent studies have begun to reveal molecular signals that translate the mechanical stimuli exerted from the muscle to the tendon-bone insertion site. Schwartz et al. (2015)62 examined hedgehog (Hh) signaling during enthesis formation and found a Hh-responsive Zinc finger protein Gli1-expressing cell population at the developing enthesis, which is distinct from tenocytes and epiphyseal chondrocytes before mineralization. Interestingly, muscle paralysis during postnatal stages increased the number of Gli1+ cells at the enthesis while ablation of these cells disrupts fibrocartilage formation in the mature enthesis. Dyment et al. (2015)63 further showed that Hh signaling drives mineralization via these Gli1+ cells in the unmineralized fibrocartilage. In particular, Indian Hedgehog (IHh) is a likely candidate Hh ligand involved in mineralization. Mineralization proceeds from the bone towards the tendon midbody and is characterized by expression of alkaline phosphatase and ColX in the mineralized fibrocartilage. The mature enthesis retains very few of these Gli1+ cells within the area of unmineralized fibrocartilage which has been associated with its poor healing capabilities (Schwartz et al. 2017)64.
3.2. Common tendon injuries/disorders and mainstream treatments
Tendon ailments encompass acute injuries (partial or full ruptures) or tendinopathies, chronic disorders that cause prolonged pain in patients. Tendon injuries/disorders most frequently occur in the midbody or enthesis of major force-bearing limb tendons including the Achilles, patellar, supraspinatus, and flexor/extensor tendons (Figure 2). Like acute injuries, tendinopathies also affect the major limb tendons. However, while less studied, they also occur in craniofacial tendons including the temporalis tendon which is involved in jaw movement. Tendinopathies affecting the enthesis are called enthesopathies. One example is lateral epicondylitis, commonly called “tennis elbow”, which specifically causes pain around the extensor tendon insertions into the lateral epicondyle.65
Figure 2. Major force-bearing limb tendons in the human body.
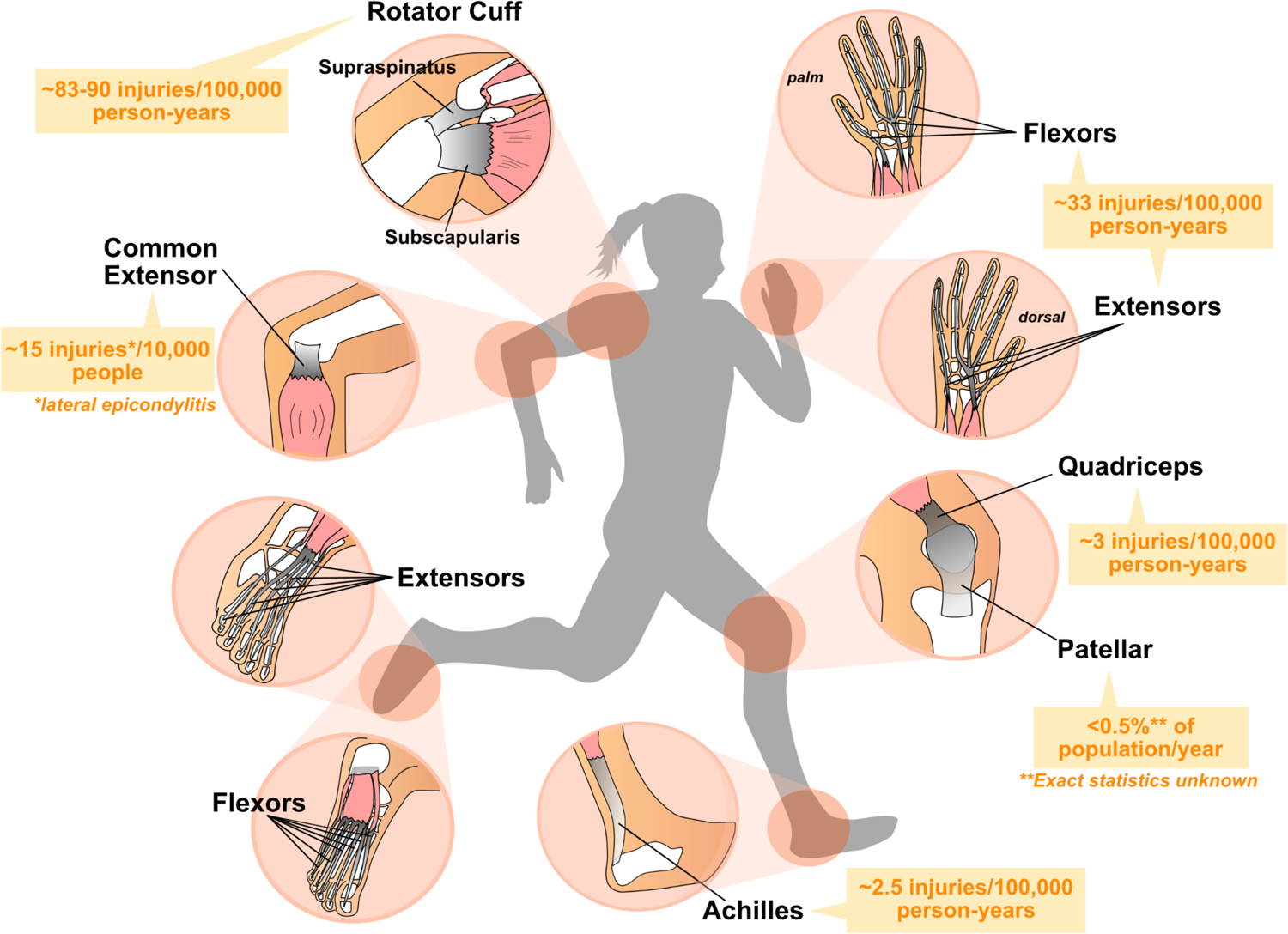
Diagram depicting the structure of major limb tendons that are frequently injured including the digital flexor and extensor tendons, common extensor, Achilles, rotator cuff, and patellar tendons. Muscles are depicted in pink, tendons in grey, and bone in white. Average incidence of injuries amongst the general population (predominantly US statistics) are shown in orange.201–206 Note: Rates of tendon rupture or disease vary with age and population demographics of geographical location or study.
The typical treatments for tendon injuries and tendinopathies are conservative, non-invasive strategies, which predominantly seek to alleviate pain rather than treat the tendon condition itself. These include rest, immobilization of the injured area, anti-inflammatory drugs, as well as physical, cryo-, ultrasound, or laser therapy. Injections of growth factors such as platelet-derived growth factor (PDGF) or via platelet-rich plasma (PRP) are also available courses of treatment.66,67 However, the efficacy of all of these treatments varies substantially depending on the mode and severity of injury or tendinopathy. Oftentimes conservative treatments fail and surgical intervention is required, especially in cases where the tendon has fully ruptured or in advanced tendinopathic patients. For partial and full tears, surgical repair of the ruptured tendon entails suturing the ruptured ends of the tendon together or re-attaching the tendon to the bone for enthesis tears. Overall, surgical intervention in general leads to lower rates of re-rupture and improved biomechanical performance compared to conservative treatments.68 In severe cases of full tears or advanced tendinopathies, the damaged tendon is replaced with a healthy tissue graft. Tissue grafts can be autografts (usually quadriceps or hamstring tendon from the patient depending on which tendon is injured), allografts (from donor cadavers), or xenografts (from large animals). Each of these methods has associated complications. Autografts induce donor site morbidity, while allografts and xenografts run the risk of immune rejection. Surgical intervention is followed by functional rehabilitation with several months of physical therapy. However, even following treatment, repaired or reconstructed tendons in patients may function at as low as only a third of the tensile strength of a healthy tendon.5
3.3. Emerging cell-based treatments and engineering strategies
In the last two decades, tendon regenerative medicine has increasingly moved towards the development of stem cell approaches to treat tendon injuries and tendinopathies.69 In particular, the use of mesenchymal stem cells (MSCs) for tendon treatments has become popularized.70,71 The exact definition and nature of MSCs has been controversial and evolved over time (reviewed in Bianco, 201472). The term MSC was originally coined by Caplan (1991)73 to describe cells isolated from the bone marrow in seminal studies from the 1960–80s that exhibited multipotency in vivo and in vitro, with the ability to differentiate into various skeletal cell types including fat, bone, and cartilage (reviewed in Friedenstein, 199074). At the time, these cells were also separately referred to as osteogenic74 or stromal stem cells75. Caplan (1991) along with subsequent work further implied these cells could also generate and replace non-skeletal tissues including skeletal muscle and tendons/ligaments76; however, this notion became rooted in evidence generated in vitro, rather than in vivo. While the aforementioned bone marrow-derived stem cells have now been re-defined as skeletal stem cells based on their demonstrated function in bone maintenance, the hematopoietic niche, fracture healing, and ability to form fat, cartilage, and bone in vivo (reviewed in Ambrosi et al., 201977), the identification of an MSC as per the original definition that differentiates into tissues derived from different germ layers during normal development and homeostasis has yet to be shown in vivo. For the purposes of this review, we will discuss MSCs in the context of tendon research, which includes cells isolated from various adult tissues including bone marrow, adipose tissue, and umbilical cord blood that exhibit the potential to differentiate into different cell types in vitro.
MSCs have been considered an ideal cellular source clinically because of their relative ease of extraction from adult tissues with minimal manipulation. Treatments using MSCs were not regulated by the Food and Drug Administration (FDA) until recently. MSC injections have been shown to improve tendon repair in animal models in vivo78–82 and some studies have also reported beneficial effects of MSC injections on human tendon healing including pain reduction and improved healing.83 At present, there are several ongoing clinical trials investigating whether autologous and allogeneic MSCs derived from bone marrow or adipose tissue can effectively treat tendon injuries and tendinopathies predominantly in the rotator cuff.71 MSCs have been shown to exhibit tenogenic differentiation potential in vitro through the modulation of various pathways involved in tendon development and healing including FGF, TGF-β, BMP, and Wnt84,85 and the overexpression of Scx, Mkx, and Egr1.48,86,87 While these cells can augment tendon healing in different animal models, less work has been done to demonstrate how well these findings translate to human MSCs in the clinical setting. Several studies have also identified heterogeneity in the molecular signature, differentiation capacity, and proliferative potential within individual MSC populations or between MSCs isolated from different sources.88 How these differences may impact tenogenic differentiation potential and tendon healing outcomes is unknown. Furthermore, ectopic ossification of tendons is commonly observed in patients with tendon injuries or tendinopathies.89 As transplanted MSCs have been shown to form ectopic bone during tendon healing,90 the multipotent differentiation potential of MSCs may call into question of whether transplantation of committed tendon progenitor cells with restricted differentiation capacities would be a better treatment strategy. While MSCs show therapeutic potential, many questions remain unanswered surrounding the mechanisms underlying their beneficial effects. For instance, whether MSCs directly differentiate into tenocytes and contribute to human tendon healing in vivo, indirectly exert beneficial effects through the secretion of anti-inflammatory cytokines and growth factors, or both remains unclear. Long-term engraftment of MSCs in vivo has proven to be challenging and has yet to be demonstrated, indicating MSCs may indirectly affect tendon healing.91,92 Indeed, a role for MSCs as a cellular source of signals that promote healing of the endogenous tissue has been underscored by a recent effort to rename the cells as medicinal signaling cells.93
As an alternative to adult stem cell-based cell therapies, studies in the last decade have shown the promise of directed stem cell differentiation approaches for cell replacement therapies to treat a variety of diseases and conditions.94–96 Devising directed differentiation protocols to generate adult tendon tissue in vitro from human embryonic stem cells (hESCs) or induced pluripotent stem cells (iPSCs) is a tenable goal to improve and replace modern tendon grafts as well as provide an alternative model system for studying mechanisms of human tendon biology. However, these cell sources come with their own associated concerns clinically. The potential tumorigenicity of hESCs and iPSCs, risks of immune rejection with the use of hESCs, and safety issues with virally induced pluripotency in iPSCs remain primary concerns. However, the use of these cell sources for other disease treatments will likely pave the way for their more widespread use97.
Only a few studies have begun to explore tenogenic induction from hESCs and human iPSCs. One strategy that has been demonstrated is the derivation of multipotent mesenchymal progenitors including MSCs connective tissue progenitors (CTPs), or neural crest cells from hESCs or iPSCs to improve tendon healing.98–101 These approaches primarily rely on priming the multipotent progenitors to form tendon-like structures in vitro via modulating mechanical stimulation, Scx overexpression, or cellular density and inducing terminal tenogenic differentiation and maturation post-transplantation in vivo. Alternatively, Dale et al. (2018)102 demonstrated that modulation of BMP signaling induces the differentiation of tendon-like cells from hESCs; however, Scx expression was notably not induced in these cells. While not in human, Komura et al. (2020)103 have also devised a stepwise differentiation protocol differentiating mouse iPSCs into tenocyte-like cells that improve tendon healing when implanted in vivo, highlighting the potential applicability of a similar strategy for human clinical applications.
Stem cell-based therapeutic approaches can be further enhanced when combined with tissue engineering strategies. Maximizing cell survival, long-term engraftment, and tenocyte differentiation potential of transplanted cells have been formidable hurdles to the practical application of stem-cell based treatments. To this end, bio-engineering approaches aimed at producing cellular scaffolds that re-create endogenous tendon or tendon healing microenvironments have shown promise.104 For example, seeding multipotent progenitors primed for the tenogenic lineage in artificially engineered or decellularized ECM tendon scaffolds from animals including pig and horses has led to improved engraftment of transplanted tissue, tenocyte differentiation, and healing.66,105 Transplantation of MSCs embedded in a collagen matrix together with growth factors such as BMP-12 has also been shown to increase M2 macrophage infiltration and accelerate flexor tendon healing.106 It is therefore likely that the success of directed differentiation approaches will rely on merging tissue engineering principles with developmental and stem cell biology.
Other bioengineering-based strategies are being heavily explored as potential treatment options, which we briefly summarize here. For more in depth discussion on these approaches, we refer the readers to the following reviews: Docheva et al., 2015107 and Freedman and Mooney, 2019.104 Some methods aim to generate artificial constructs that mimic native tendon to replace injured tendon including silk-based scaffolds or FDA-regulated biodegradable synthetic materials including poly(lactic-co-glycolic acid), or PLGA. These materials can mimic collagen fiber alignment and tendon mechanics as well as promote cellular ingrowth and healing post-implantation. However, as tendon matrix is heterogeneous along the tendon axis, accurately re-creating the natural viscoelastic properties with synthetic materials, especially at the muscle and bone attachment points, has proven challenging. Alternatively, surgically replacing only the damaged area of the tendon with synthetic grafts including commercially available devices like GraftJacket™ or Artelon® has shown promise in enhancing endogenous healing instead of replacing the entire tendon.108,109 Another therapeutic avenue that has been long explored is the implantation of engineered scaffolds or surgical sutures embedded with biologics including Connective Tissue Growth Factor (CTGF)110 or PRP111 into the damaged area that may enhance tendon healing. Recent efforts have also focused on an integrative approach in which materials are designed to elicit proper cellular responses to form more complex tissues such as the enthesis.112
3.4. Augmenting stem cell-based strategies by building a complete picture of tendon development
While stem cell differentiation approaches have great potential, major challenges lie ahead in terms of generating functional tendon tissue in vitro. These hurdles are rooted in fundamental gaps in our knowledge of mechanisms underlying tendon development in vivo. First and foremost, tendon development is a complex multidimensional process dependent on coordinated signals from developing muscle and bone as mentioned above. While some important signaling pathways involved in tendon development have been identified including TGF-β and FGF, many others have yet to be deeply explored. Elucidating these signaling mechanisms will greatly aid the design of directed differentiation protocols to generate tendon tissue in vitro going forward. Moreover, our understanding of how each of these pathways is spatiotemporally integrated in the development of the entire musculoskeletal circuit remains incomplete.105,113,114 Not to mention, mechanical force is also important for tendon development and maturation at both embryonic and postnatal stages. While much work has been done in assessing how various mechanical stimulation regimens, substrates, and growth factors can affect tenogenic differentiation potential in vitro,115 understanding how these pathways and mechanical signals can be integrated in 3-D in vivo during healing will require a combination of developmental biology and bioengineering approaches. This interdisciplinary approach will facilitate the combination of innovative engineering solutions and stem-cell based tendon replacement strategies. Lastly, the lack of many tendon-specific molecular markers presents perhaps the biggest challenge in terms of assessing the exact molecular identity, maturation stage, and functionality of tendon tissue generated in vitro. While the structure of the tendon is critical to its function, many questions remain as to the cellular heterogeneity within the tendon as well as the functional relevance of tendon subpopulations. Recent studies have unearthed tendon subpopulations in both adult mouse116,117 and human118 tendon via single cell RNA-sequencing approaches; however the functional importance of these subpopulations particularly during homeostatic activities including running have yet to be explored. These discoveries may provide vital insight into how tendon tissue generated in a lab may accurately recapitulate the cellular composition and functionality of a natural tendon. In all, while applying the knowledge we currently harbor serves as a great starting point, a complete roadmap of tendon development in vivo will be paramount to aid in efforts to generate human tendons in a dish.
4. Major questions surrounding mechanisms underlying tendon healing and disorders
The development of effective, alternative treatments for tendon injuries and tendinopathies requires a mechanistic understanding of tendon healing in vivo. A bulk of tendon research has thus primarily been focused on understanding the cellular and molecular mechanisms underlying tendon healing both in natural and surgical repair contexts in animal models to improve existing treatments for patients. In this section, we review current overarching themes of ongoing tendon healing and repair research.
4.1. General overview of tendon healing
Tendons generally heal in three sequentially semi-overlapping phases following acute injury to either the midbody or enthesis: inflammation, cellular proliferation, and remodeling.11,119 Within the first week post-injury, there is a large influx of leukocytes to the wound site. Similar to other wound healing contexts, researchers have shown in both canine and rat tendon injury and repair models that neutrophils are the first immune responders to arrive on site, peaking at approximately 1 day post-injury, and followed by monocyte-derived macrophages in the subsequent days.120,121 Pro-inflammatory and chemotactic cytokines including Interleukin 1-β (Il1-β) and Tumor Necrosis Factor α (Tnfα) as well as collagen-degrading matrix metalloproteinases (Mmp-1, 3, 13) are highly expressed during this early period as the collagen matrix begins to degrade. VEGF expression also increases, stimulating angiogenesis into the wound area.19 Towards the end of the inflammatory phase, cells begin to proliferate and migrate towards the injury site. Unlike neutrophils, macrophages remain on site during most of the tendon healing process to aid in cell proliferation and ECM deposition.122 During this 2 to 3-week period, new collagen III fibrils are deposited at the wound site as cells accumulate. Finally, cells re-differentiate into mature tenocytes, elongate, and align longitudinally as collagen I fibrils gradually replace collagen III fibrils throughout the remodeling phase. Vascularity in the repaired tendon tissue also decreases during these later stages. Matrix maturation entails the assembly of collagen I fibrils into larger bundles and can take up to a year, ultimately resulting in fibrotic scar tissue with inferior biomechanical properties to a healthy tendon.
4.2. The cellular basis of tendon healing
To date, studies have shown that genes involved in tendon development and matrix maturation including Scx and Tnmd are also required for regulating various aspects of tendon healing (cell proliferation, vascular infiltration, matrix assembly, immune cell accumulation etc.).5,7,123 Yet, while the general progression of mammalian tendon healing and repair has been well-documented, many key questions remain unanswered as to the cellular and molecular mechanisms controlling this process. In particular, the cellular origins of the healed scar tissue post-injury have been debated.
Histological and in vivo transplantation studies from the 1970s and 80s in large animal models of flexor tendon repair including canines and rabbits suggested that both tenocytes and cells from the surrounding sheath possess the capacity to respond to injury.11 With the discovery of resident stem cells that drive tissue homeostasis and repair in a variety of adult tissues including the gut, skin, and muscle,124–127 the idea that such a population may also exist in the tendon became an attractive hypothesis. Although homeostatic proliferation levels and cell turnover rates are extremely low in adult tendons, researchers have indeed identified subpopulations of cells isolated from either interior tendon tissue or peritenon that exhibit stem-cell like characteristics in vitro. Commonly referred to as tendon stem progenitor cells (TSPCs), these cells can expand clonally and differentiate into multiple lineages in vitro, as well as generate tendon tissue upon subsequent transplantation in vivo.117,128–132 Yet to date, only a few studies have taken advantage of Cre-based lineage tracing methods in murine models to examine whether such a stem or progenitor cell exists in vivo and participates in natural tendon homeostasis and healing. Collectively, these findings have pointed to cells both in the surrounding peritenon and within the tendon proper as participating in tendon healing, but the functional significance of each distinct population remains unclear.
Pre-existing tenocytes in the main tendon body have been shown to contribute to tendon healing in certain contexts. Sakabe et al. (2018)133 showed that Scx is necessary for tendon healing in a partial Achilles tendon transection injury. Best and Loiselle (2019)134 performed conditional lineage tracing of Scx+ tenocytes during flexor digitorum longus (FDL) tendon repair and demonstrated their contribution to healed scar tissue. A separate study from the same group also demonstrated that the fibrosis-promoting factor S100a4 is expressed in resident tenocytes and that these cells also contribute to the formation of scar tissue following FDL repair.135 Lineage tracing of both populations following FDL repair intriguingly revealed largely separate contributions to the scar tissue, with Scx+ tenocytes mainly contributing to the aligned bridging tendon tissue and S100 Calcium Binding Protein A4-positive (S100a4+) tenocytes comprising the majority neighboring fibrotic tissue (Figure 3A). Ackermann et al. (2019)135 further found that S100a4-haploinsufficiency and S100a4-expressing cell ablation during FDL repair improved tendon healing outcomes. However, other cell types including macrophages that infiltrated the repair site following injury also expressed S100a4, making it unclear whether impairing S100a4 production from resident tenocytes was the prime cause for this improvement. Finally, Moser et al. (2018)136 showed that a subpopulation of tenocytes expressing Axin2 contributes to healed scar tissue in a full tear enthesis injury and repair model of the supraspinatus tendon. These studies clearly show contribution of Scx-lineage cells to tendon repair, however whether their contribution to healing is mediated via dedifferentiation to a progenitor-like state or proliferation of differentiated tenocytes remains unknown. Notably, these studies performed surgical re-attachment post-injury and the mechanisms governing healing in natural healing contexts may differ. For example, Howell et al. (2017)137 demonstrated that Scx+ tenocytes do not contribute to healed scar tissue following full transection of the mouse Achilles tendon (Figure 3B), illuminating distinct healing mechanisms potentially due to differences in tendon type or load-bearing following surgical repair versus natural healing.
Figure 3. Summary of lineage tracing studies in mouse tendon healing models.
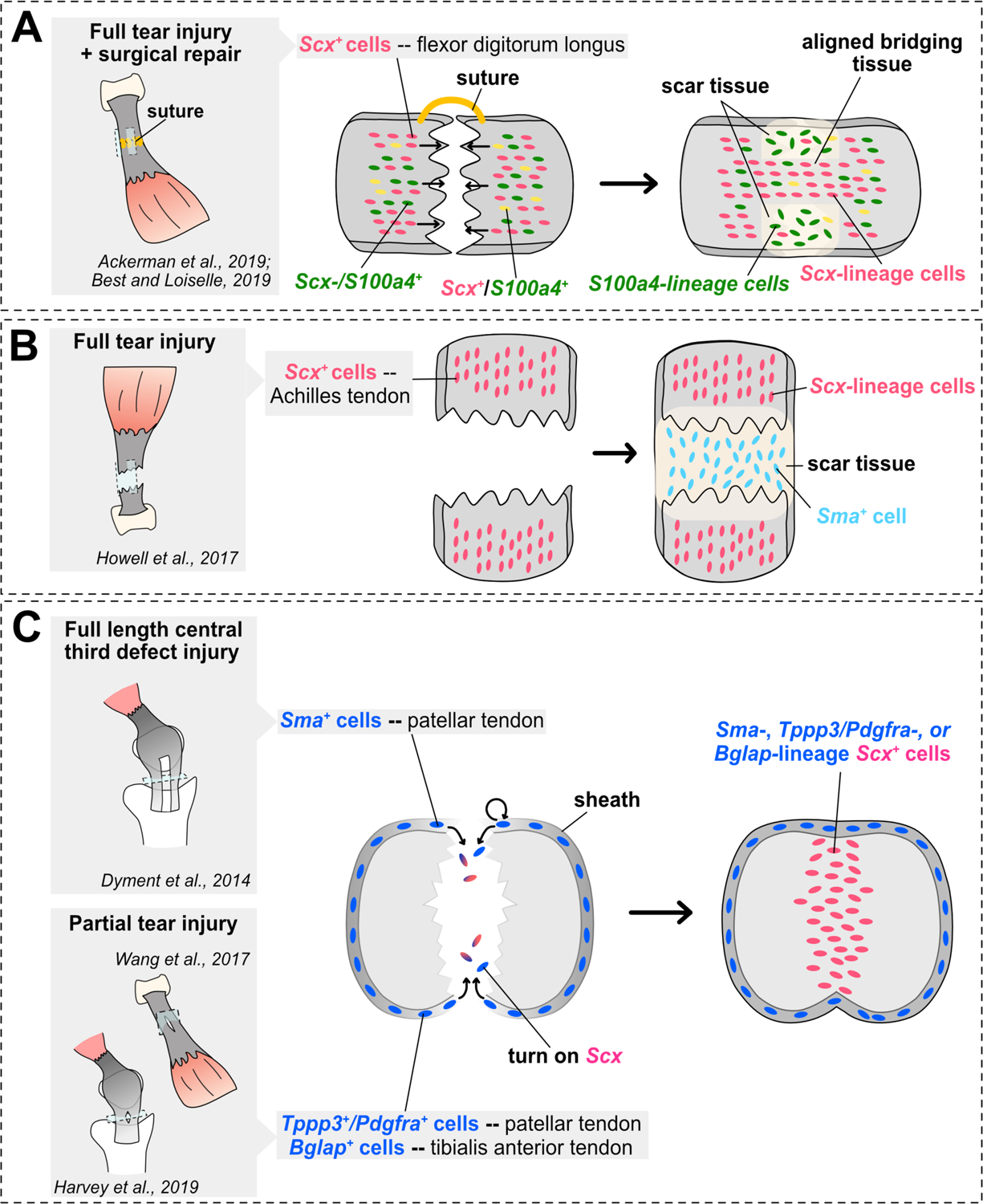
(A–C) Schematics illustrating main conclusions of mouse tendon healing genetic lineage tracing studies in various tendon midsubstance injury models and limb tendons. Studies assessing contribution of cells from the midsubstance are shown in A–B, while studies assessing contribution of cells from the tendon sheath are shown in C. Sagittal views are shown in the cartoons in A–B, while a transverse view is shown in C.
Recent studies in natural tendon healing models have demonstrated that cells derived from the peritenon harbor injury-responsive progenitor populations that comprise a majority of healed tendon tissue (Figure 3C). Dyment et al. (2014)138 identified an α-smooth muscle actin (αSMA)-expressing progenitor population that contributed to both patellar tendon growth and healing in a full length, central third patellar tendon injury model. The authors found that αSMA-expressing progenitors in the paratenon expand upon injury, migrate to bridge the defect, and differentiate into Scx+ tenocytes (Figure 3B). Additionally, Wang et al. (2017)131 identified a progenitor population derived from Osteocalcin+ (Bglap+) tendon sheath cells that proliferates and contributes to both tibialis anterior tendon and Achilles tendon healing following partial injury in mice. They further demonstrated that active Hedgehog (Hh) signaling was both sufficient to induce proliferation in the tendon sheath and necessary for proper sheath-mediated repair. Most recently, an injury-responsive progenitor population co-expressing Tubulin polymerization promoting protein 3 (Tppp3) and Platelet-derived growth factor receptor A (Pdgfra) was identified in the patellar tendon sheath of mice. Harvey et al. (2019)117 demonstrated that Tppp3+Pdgfra+ progenitor cells contribute to healed tendon tissue following a partial biopsy punch-induced injury in the patellar tendon. They further showed that these cells self-renew in the sheath, a stem cell property that was not examined in prior studies. Interestingly, these cells did not contribute to tenocytes in the interior portion of the tendon tissue during homeostasis, suggesting they are only activated upon injury. The authors further demonstrated that PDGF signaling was necessary for stimulating proper pro-reparative responses from Tppp3+Pdgfra+ cells. However, PDGF signaling also appeared to augment fibrotic responses from a separate population of Pdgfra+ only cells, indicating it may be involved in coordinating multiple aspects of tendon healing. Altogether, the identification of these populations collectively represents significant advances in the field, and provides a starting point to assess whether similar populations exist and respond to injury in other tendons.
It is important to note that each of these studies traced tendon sheath-derived subpopulations using different markers (Sma, Tppp3, and Bglap), making it unclear whether the same subpopulation responded in each model or whether the cellular origin of the healed tissue differed depending on the type of injury and/or tendon. In fact, the type of injury appears to be an important factor in determining which cellular populations respond. For example, Axin2+ tenocytes contribute to healed tissue only following a full, but not partial, enthesis injury in the supraspinatus tendon following repair.136 Moreover, midbody versus enthesis injuries involve different cell types and may differ in repair mechanisms. As previously mentioned, calcified fibrocartilage formation depends on Hh signaling mediated by Gli1 expression in the mouse enthesis. Though this Gli1+ cell population persists in mature entheses of adult mice, the number of cells expressing the transcription factor is strongly diminished.62 Schwartz et al. (2017)64 showed that this Gli1+ cell population contributes to enthesis regeneration after injury and maintains high levels of expression throughout the healing process in postnatal stages. In contrast, mature entheses show an even lower number of Gli1+ cells right after injury and no contribution to the healing from this cell type. The authors speculate that this may be one of the reasons why mature entheses are incapable of regenerating and do not restore their original morphology after injury.
To complicate the issue further, transcriptional heterogeneity across different tendons and within tenocytes has been reported.134,139,140 For instance, Bglap was reported to be a reliable marker of adult tendon sheaths in mice,131 but was not detected within the adult patellar tendon sheath.117 Whether and to what extent these differences are important for tendon development, function, and repair remains relatively uncharacterized. Another limitation of these studies is that at present there are no markers that fully differentiate between the midsubstance and the peritenon. For instance, while Scx or αSMA expression predominantly labels tenocytes in the midsubstance or cells in the peritenon, respectively, their expression can also label a small subset of cells in the peritenon or midsubstance. Both the heterogeneous nature of gene expression within different tendons and the lack of robust markers for tendon subpopulations has made generalizing conclusions challenging within the field. While tendons have been relatively understudied compared to other musculoskeletal tissues, the burgeoning set of sequencing technologies and molecular tools to study tendon tissues will aid in our understanding of tendon healing and repair in the future.141,142
4.3. Emerging views on the pathology of tendinopathies
Acute tendon injuries can oftentimes be the end result of tendinopathies, chronic pathological tendon disorders that lead to increased morbidity. Tendinopathies comprise approximately 30% of general practice musculoskeletal consultations and are clinically diagnosed following patient presentation of chronic pain, reduced activity-based movement, and swelling in some cases coupled with either ultrasonography or magnetic resonance imaging (MRI).12,143 In contrast to healthy tendons which are white and exhibit a firm matrix, tendinopathic tendons are discolored, have a loose, disorganized collagen matrix, are hypercellular, and develop increased vasculature and innervation, as well as chondroplasia in some cases.7,144 Notably, many of these characteristics resemble early stages of tendon healing following acute injury including collagen I matrix degradation, deposition of collagen III, as well as neoangiogenesis. These morphological changes are thought to occur in three progressive stages according to the continuum model originally proposed by Cook and Purdam (2008)145: reactive tendinopathy, tendon dysrepair, and degenerative tendinopathy. According to the model, acute tensile or compressive overloading triggers tendon thickening via excess cell proliferation and matrix growth in early reactive tendinopathy. If overloading becomes repetitive, more severe matrix degeneration, cell infiltration and proliferative responses occur in what mirrors a tendon healing response. Finally, in degenerative or advanced tendinopathy, the tendon exhibits acellular areas, neurovascular infiltration, disorganized matrix, dysfunction, and higher levels of cell death. Tendinopathies are therefore believed to be a result of the improper resolution of healing and gradual ECM degradation triggered by the initial formation of microtears in the tendon tissue caused by excessive mechanical loading.
Although the principal causes of tendinopathy are generally accepted as overuse, aging, and genetics,144,146 the molecular mechanisms driving disease initiation and progression remain poorly understood. Most patient biopsy samples taken for research are typically obtained from advanced stages of the disease when surgical intervention is necessary, making study of early stages of human tendinopathy difficult. In fact, many patients are asymptomatic during early stages of tendinopathy.6,147 These limiting circumstances have altogether made it challenging to devise experimental injury models that replicate the general progression of human tendinopathy. Nevertheless, various methods have been widely implemented in animal models to mimic certain aspects of tendinopathy such as ECM degradation in different tendons including treadmill running to simulate overuse/fatigue or chemical induction of tendinopathy via injection of collagenase, TGF-β, or Substance P.148–151 Although artificially induced, experimental tendinopathic animal models have been valuable for uncovering general mechanisms of tendon disease. In some cases, comparison between animal overuse models and patient tissue has even spurred new human models of tendinopathy. For instance, Millar et al. (2010)152 demonstrated that matched intact subcapularis tendon in patients with torn supraspinatus tendons demonstrated signs of early tendinopathy including matrix degeneration, increased apoptosis, and increased expression of inflammatory cytokines. As these characteristics reflected similar disease progression in a rat supraspinatus overuse tendinopathic model, the authors proposed that matched intact subcapularis tendon in patients with full thickness rotator cuff tears were a suitable model for early stages of human tendinopathy.
The role of inflammation in chronic tendon disorders has been particularly controversial and represents perhaps the most striking shift in the tendinopathy field over the last decade. Historically, tendinopathy was primarily considered a chronic degenerative condition that was non-inflammatory. This notion stemmed from several older histological studies reporting a lack of infiltration of immune cells in tendinopathic tissue153–156 as well as lack of clinical diagnostic evidence, as patients typically did not present with signs of inflammation including redness of the skin.12,157 However, more recent studies incorporating modern molecular techniques and cell-type specific markers have shown that immune cells do indeed infiltrate tendinopathic tissue in patients, particularly during earlier stages of disease. Schubert et al. (2005)158 provided one of the first pieces of experimental evidence demonstrating the infiltration of myeloid cells and lymphocytes in Achilles tendinopathy patients. Many studies have since corroborated and extended these findings in human patellar, Achilles, and rotator cuff tendinopathies6,152,159–168 and animal models.121,151,169–172 Comparison of Achilles tendinopathic tissue with spontaneously ruptured Achilles tendons revealed higher levels of granulocytes in ruptured tendons, while macrophages, mast cells, and lymphocytes were more prevalent in diseased tendons, suggesting immune-related events differ between tendinopathy versus spontaneous acute injury.158
Likewise, inflammatory signatures appear to evolve as tendinopathy progresses. In a comparative study between healthy and tendinopathic human supraspinatus tendon, Dakin et al. (2015)162 showed that early and intermediate-stage tendinopathic tendons displayed a distinct inflammatory signature compared to that of advanced stages, indicating separate treatments may be needed to effectively treat different stages of disease. The authors further revealed that two well-known modulators of resolution of inflammation, formyl peptide receptor 2 (FPR2) and chemokine-like receptor 1 (ChemR23), were highly expressed in early and intermediate, but not advanced tendinopathic samples, collectively suggesting that diseased tendons can initiate, but not sustain a response to resolve inflammation. Moreover, a clear link between pain and inflammation in tendinopathy was demonstrated as biopsy samples from patients who still exhibited pain post-surgical treatment displayed continual activation of inflammatory interferon signaling, while tendon biopsies from patients in which pain resolved post-treatment expressed higher levels of two mediators of inflammatory resolution, arachidonate 15-Lipoxygenase (ALOX15) and cluster of differentiation 206 (CD206). Given CD206 is a common surface marker for M2 macrophages, which are generally polarized to an anti-inflammatory, pro-reparative phenotype (Roszer et al., 2015, Watanabe et al., 2019), these data suggest a link between failure to resolve inflammation and chronic pain. Notably, tendon fibroblasts derived from diseased versus healthy tendons in vitro responded to inflammatory insults in a distinct manner compared to their healthy counterparts. Adding to this framework, Stolk et al. (2017) performed macrophage co-cultures with tenocytes derived from ruptured human supraspinatus tendons and demonstrated that tenocytes change their secretory inflammatory profile in response to immune stimulation, which can in turn alter the phenotypic polarization state of macrophages. Taken together, these findings suggest that tenocytes can directly influence inflammatory response outcomes including augmenting or resolving inflammation in vivo. Importantly, similar phenomena were observed in human Achilles tendinopathic tissue as well167, collectively suggesting that chronic inflammation key for the early progression of human tendinopathy.
How inflammation fits into the larger picture of tendinopathy progression remains less clear. As overuse is the most common immediate cause of chronic tendinopathy in patients, genetic studies in mice have examined mechanisms that may link mechanical overloading in the tendon to the onset of inflammation. Mechanical forces in the tendon are critical for homeostasis and physical disruptions in normal tensile forces lead to changes in morphology due to stress/necrosis, tenocyte apoptosis, matrix degeneration, and increased expression of inflammatory markers.173,174 Recent studies have demonstrated that alarmins are important triggers for the onset of tendinopathy. Alarmins are ubiquitous, endogenous molecules that are released from cells upon stress or necrosis and act as ‘danger’ signals to the innate immune system.175,176 Several classes of canonical alarmins including cytokines, heat shock proteins (HSPs), S100 proteins, and high mobility group box 1 (HMGB1) are highly up-regulated in early human tendinopathic patients.164,177–179 Genetic mouse studies have identified important roles for two alarmins in tendinopathy progression, Interleukin-33 (Il-33) and Hmgb1. IL-33 is highly expressed in endothelial cells and tenocytes during early stages of human tendinopathy and acts as both a molecular switch from collagen I to collagen III production in resident tendon fibroblasts and an inflammatory signal to the immune system164. Hmgb1 also plays a key role in the initiation and progression of tendinopathy in mice180. Upon mechanical overloading, Hmgb1 is highly induced and released into the matrix. Ectopic application of Hmgb1 in normal tendons triggered degenerative tendinopathic changes including hypercellularity, the infiltration of immune cells, and neovascularization. Most notably, chemical inhibition of HMGB1 activity with glycyrhizzin (GL) was sufficient to inhibit the onset of tendinopathy in overloaded mice by lowering levels of pro-inflammatory molecules and matrix degrading enzymes in addition to reducing chondroplasia. The release of alarmins from stressed and necrotic cells is therefore a vital event in the onset of tendinopathy. As alarmins serve multi-functional roles in different cell types, understanding the molecular interactions between damaged tenocytes, immune cells, as well as other cells including endothelial cells will be essential for elucidating targetable aspects of tendinopathy for treatment. More broadly, these studies provide a molecular basis for how mechanical disruption and cellular stress in the overloaded tendon can initiate multiple facets of tendinopathy progression including inflammation.
How inflammation and matrix degeneration during mammalian tendinopathy ultimately lead to a chronic ‘failed healing’ response instead of tissue restoration is poorly understood. Studying animal models capable of tendon regeneration such as zebrafish and neonatal mice can therefore provide invaluable insight into answering these questions. Given their extensive appendage and organ regenerative abilities, it stands to reason that damaged cells in regenerative organisms/models may intrinsically respond in a different manner to tissue damage than their adult mammalian counterparts, driving restorative outcomes versus disease and scarring. In fact, some studies have determined that regenerative properties are likely linked to intrinsic cellular responses in the tendon, rather than extrinsic cellular and environmental cues. For example, fetal sheep tendons retain their regenerative properties even when transplanted into an adult microenvironment181 and fetal murine tendon fibroblasts are better at engrafting compared to those from adults when seeded in scaffolds for tendon tissue engineering.182 Recent research has further attributed the loss of neonatal regenerative properties and cellular plasticity in other mammalian organ systems including the heart and brain to the deposition of ECM.183,184 Given adult tendon is an ECM-rich tissue, similar mechanisms may underlie the transition from fetal and neonatal tendon regeneration versus adult disease and scarring in mammals.
5. Learning from tendon regenerative models to enhance endogenous tendon healing strategies in humans
It is clear that missing or misregulated cues underlie the inability of adult mammalian tendons to regenerate following injury. In contrast, tendons have been shown to perfectly regenerate in larval zebrafish and neonatal mice.137,185 Howell et al. (2017) discovered that Scx+ tenocytes proliferate and regenerate the Achilles tendon following full transection in neonatal, but not adult mice. A population of αSMA-expressing cells infiltrate the injury site during early stages post-injury in both neonatal and adult mice. However, while this population persists and eventually forms scar tissue in adults, these cells largely leave regenerating neonatal tendons as Scx+ cells are recruited into the injury site to restore the tendon. Surprisingly, Scx expression was re-activated in tenocytes in the injured tendon stubs in the adult, though these cells underwent aberrant differentiation into cartilaginous nodules, reminiscent of ectopic calcification seen in patients. In light of the differences between the cellular basis of neonatal tendon regeneration versus adult scar formation uncovered in this study, it is tempting to speculate that the extrinsic αSMA-expressing cells originated from the exterior sheath as in other non-regenerative natural tendon healing models.138 Although this was not addressed, such a finding would hint that the key to initiating proper regeneration may be to limit the contribution of cells from the sheath during later stages of healing or properly induce Scx expression in these cells. A recent follow up study further determined that TGF-β signaling is required for tenocyte recruitment during neonatal regeneration,186 demonstrating functional mechanisms of regeneration may recapitulate tendon development.
Most recently, the zebrafish has emerged as a new model for tendon regeneration. Following genetic ablation of scxa-expressing tendon cells, larval zebrafish completely regenerate their tendons and tendon attachments to muscle and bone. Focusing on the craniofacial tendon which attaches the sternohyoideus muscle to the ceratohyal cartilage and functions in jaw movement during feeding, the authors demonstrated that tendon regeneration occurs via BMP signaling-mediated activation and recruitment of neighboring SRY-box transcription factor 10-positive (sox10+) and nk2 homeobox 5-positive (nkx2.5+) progenitors, which surround the cartilage and muscle, respectively. These findings highlight the plasticity of connective tissues in the context of injury and regeneration, similar to other regenerative models including the axolotl.187–190 Interestingly, complete ablation of tendon cells led to defects in both cartilage and muscle morphology and function, which are restored upon regeneration. As the musculoskeletal system functions as an interconnected unit, these results further imply that aberrations in muscle and skeletal tissues in patients may pathologically stem from defects in tendon. Given the remarkable capacity of adult zebrafish to regenerate other appendages and organs,191 it will be important to examine whether tendon regenerative properties in larvae extend through to adulthood using various modes of injury. While the likelihood of developing an injury or tendinopathy increases with age in mammals, regenerative models including the zebrafish can retain their regenerative abilities throughout their lifespan.192 Furthermore, immune cells including macrophages and T-cells are critical for successful regeneration of other organs in the zebrafish193–195 and differences in immune responses have been linked to the success or failure of regenerative outcomes.196–198 Whether zebrafish are resilient to developing chronic tendon disorders remains an unexplored space, therefore providing the tendon community with a unique model to investigate interesting questions pertaining to the roles of aging and inflammation in the onset and progression of tendinopathy.
Identifying the appropriate cues that are required for successful regeneration may lead to both the design of novel therapeutic strategies or the augmentation of existing biologics treatments to correct or supply missing signaling mechanisms at the appropriate times during tendon healing using pharmacological drugs or extracellular signaling molecules. As tendon regeneration is a dynamic and complex process, it likely relies on the execution of not one, but rather a sequence of required molecular mechanisms. It is thus possible that a temporally managed approach with shifting treatments may be most successful at improving healing outcomes in patients. This body of research may further identify the required signaling mechanisms in specific cell types, adding another layer of complexity to treatment design. Altogether, uncovering the molecular and cellular basis of tendon regeneration in vivo will be an invaluable step to advancing the combined efforts of bioengineers, biologists, and medical professionals in the orthopedic community towards designing effective strategies for treating tendon ailments.
6. Concluding remarks and future perspectives
We are at the precipice of an exciting era for tendon regenerative medicine. Many open questions remain as to the mechanisms underlying tendon development, healing and regeneration. Here, we have detailed our current understanding in each of these areas and further discuss how increasing our knowledge of these processes is a necessary prerequisite to the enhancement of current treatment strategies and design of novel therapeutic avenues. Notably, the study of emerging tendon regenerative models may also provide insight into mechanisms that may be leveraged for stimulating better outcomes in patients. The collective effort to build a complete molecular and cellular blueprint of tendon development, healing, and regeneration will ultimately accelerate advances in tendon regenerative medicine.
Acknowledgements
We’d like to acknowledge members of the Galloway lab for helpful comments and suggestions for the manuscript. We also thank Karl Kadler for permission to adapt images from his published work in Figure 1.
Grants:
S.L.T., M.T.N, and J.L.G. were supported by NIH/NICHD HD069533, NIH/NIDCR DE024771, NIH/NIAMS AR071554, and awards from the Charles H. Hood Foundation and the Harvard Stem Cell Institute.
References
- 1.Komi PV. Relevance of in vivo force measurements to human biomechanics. J Biomech. 1990;23Suppl 1:23–34. 10.1016/0021-9290(90)90038-5. [DOI] [PubMed] [Google Scholar]
- 2.Komi PV, Fukashiro S, Jarvinen M. Biomechanical loading of Achilles tendon during normal locomotion. Clin Sports Med. July1992;11(3):521–31. [PubMed] [Google Scholar]
- 3.Grinstein M, Dingwall HL, O’Connor LD, Zou K, Capellini TD, Galloway JL. A distinct transition from cell growth to physiological homeostasis in the tendon. Elife. September192019;8. 10.7554/eLife.48689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butler DL, Juncosa N, Dressler MR. Functional efficacy of tendon repair processes. Annu Rev Biomed Eng. 2004;6:303–29. 10.1146/annurev.bioeng.6.040803.140240. [DOI] [PubMed] [Google Scholar]
- 5.Nourissat G, Berenbaum F, Duprez D. Tendon injury: from biology to tendon repair. Nat Rev Rheumatol. April2015;11(4):223–33. 10.1038/nrrheum.2015.26. [DOI] [PubMed] [Google Scholar]
- 6.Dean BJF, Dakin SG, Millar NL, Carr AJ. Review: Emerging concepts in the pathogenesis of tendinopathy. Surgeon. December2017;15(6):349–354. 10.1016/j.surge.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steinmann S, Pfeifer CG, Brochhausen C, Docheva D. Spectrum of Tendon Pathologies: Triggers, Trails and End-State. Int J Mol Sci. January282020;21(3). 10.3390/ijms21030844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trofa DP, Miller JC, Jang ES, Woode DR, Greisberg JK, Vosseller JT. Professional Athletes’ Return to Play and Performance After Operative Repair of an Achilles Tendon Rupture. Am J Sports Med. October2017;45(12):2864–2871. 10.1177/0363546517713001. [DOI] [PubMed] [Google Scholar]
- 9.Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. February2004;86(2):219–24. 10.2106/00004623-200402000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Bishop J, Klepps S, Lo IK, Bird J, Gladstone JN, Flatow EL. Cuff integrity after arthroscopic versus open rotator cuff repair: a prospective study. J Shoulder Elbow Surg. May-Jun 2006;15(3):290–9. 10.1016/j.jse.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 11.Hope M, Saxby TS. Tendon healing. Foot Ankle Clin. December2007;12(4):553–67, v. 10.1016/j.fcl.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 12.Millar NL, Murrell GA, McInnes IB. Inflammatory mechanisms in tendinopathy - towards translation. Nat Rev Rheumatol. January252017;13(2):110–122. 10.1038/nrrheum.2016.213. [DOI] [PubMed] [Google Scholar]
- 13.Ezura Y, Chakravarti S, Oldberg A, Chervoneva I, Birk DE. Differential expression of lumican and fibromodulin regulate collagen fibrillogenesis in developing mouse tendons. J Cell Biol. November132000;151(4):779–88. 10.1083/jcb.151.4.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grinstein M, Galloway JL. Developmental Biology in Tendon Tissue Engineering. In: Stoddart JM, Craft AM, Pattappa G, Gardner OFW, eds. Developmental Biology and Musculoskeletal Tissue Engineering. Boston: Academic Press; 2018. Chapter 8:181–206. [Google Scholar]
- 15.Lin TW, Cardenas L, Soslowsky LJ. Biomechanics of tendon injury and repair. J Biomech. June2004;37(6):865–77. 10.1016/j.jbiomech.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 16.Subramanian A, Schilling TF. Tendon development and musculoskeletal assembly: emerging roles for the extracellular matrix. Development. December152015;142(24):4191–204. 10.1242/dev.114777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stanley RL, Fleck RA, Becker DL, Goodship AE, Ralphs JR, Patterson-Kane JC. Gap junction protein expression and cellularity: comparison of immature and adult equine digital tendons. J Anat. September2007;211(3):325–34. 10.1111/j.1469-7580.2007.00781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shen H, Schwartz AG, Civitelli R, Thomopoulos S. Connexin 43 Is Necessary for Murine Tendon Enthesis Formation and Response to Loading. J Bone Miner Res. August2020;35(8):1494–1503. 10.1002/jbmr.4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tempfer H, Traweger A. Tendon Vasculature in Health and Disease. Front Physiol. 2015;6:330. 10.3389/fphys.2015.00330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ackermann PW, Salo P, Hart DA. Tendon Innervation. Adv Exp Med Biol. 2016;920:35–51. 10.1007/978-3-319-33943-6_4. [DOI] [PubMed] [Google Scholar]
- 21.Curzi D, Ambrogini P, Falcieri E, Burattini S. Morphogenesis of rat myotendinous junction. Muscles Ligaments Tendons J. October2013;3(4):275–80. [PMC free article] [PubMed] [Google Scholar]
- 22.Knudsen AB, Larsen M, Mackey AL, et al. The human myotendinous junction: an ultrastructural and 3D analysis study. Scand J Med Sci Sports. February2015;25(1):e116–23. 10.1111/sms.12221. [DOI] [PubMed] [Google Scholar]
- 23.Benjamin M, McGonagle D. Entheses: tendon and ligament attachment sites. Scand J Med Sci Sports. August2009;19(4):520–7. 10.1111/j.1600-0838.2009.00906.x. [DOI] [PubMed] [Google Scholar]
- 24.Lu HH, Thomopoulos S. Functional attachment of soft tissues to bone: development, healing, and tissue engineering. Annu Rev Biomed Eng. 2013;15:201–26. 10.1146/annurev-bioeng-071910-124656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Charvet B, Ruggiero F, Le Guellec D. The development of the myotendinous junction. A review. Muscles Ligaments Tendons J. April2012;2(2):53–63. [PMC free article] [PubMed] [Google Scholar]
- 26.Noden DM. The role of the neural crest in patterning of avian cranial skeletal, connective, and muscle tissues. Dev Biol. March1983;96(1):144–65. 10.1016/0012-1606(83)90318-4. [DOI] [PubMed] [Google Scholar]
- 27.Grenier J, Teillet MA, Grifone R, Kelly RG, Duprez D. Relationship between neural crest cells and cranial mesoderm during head muscle development. PLoS One. 2009;4(2):e4381. 10.1371/journal.pone.0004381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen JW, Galloway JL. The development of zebrafish tendon and ligament progenitors. Development. May2014;141(10):2035–45. 10.1242/dev.104067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ordahl CP, Le Douarin NM. Two myogenic lineages within the developing somite. Development. February1992;114(2):339–53. [DOI] [PubMed] [Google Scholar]
- 30.Brent AE, Schweitzer R, Tabin CJ. A somitic compartment of tendon progenitors. Cell. April182003;113(2):235–48. 10.1016/s0092-8674(03)00268-x. [DOI] [PubMed] [Google Scholar]
- 31.Gaut L, Duprez D. Tendon development and diseases. Wiley Interdiscip Rev Dev Biol. Jan-Feb 2016;5(1):5–23. 10.1002/wdev.201. [DOI] [PubMed] [Google Scholar]
- 32.Schweitzer R, Chyung JH, Murtaugh LC, et al. Analysis of the tendon cell fate using Scleraxis, a specific marker for tendons and ligaments. Development. October2001;128(19):3855–66. [DOI] [PubMed] [Google Scholar]
- 33.Esteves de Lima J, Bonnin MA, Bourgeois A, Parisi A, Le Grand F, Duprez D. Specific pattern of cell cycle during limb fetal myogenesis. Dev Biol. August152014;392(2):308–23. 10.1016/j.ydbio.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 34.Docheva D, Hunziker EB, Fassler R, Brandau O. Tenomodulin is necessary for tenocyte proliferation and tendon maturation. Mol Cell Biol. January2005;25(2):699–705. 10.1128/MCB.25.2.699-705.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dex S, Alberton P, Willkomm L, et al. Tenomodulin is Required for Tendon Endurance Running and Collagen I Fibril Adaptation to Mechanical Load. EBioMedicine. June2017;20:240–254. 10.1016/j.ebiom.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Birch HL, Thorpe CT, Rumian AP. Specialisation of extracellular matrix for function in tendons and ligaments. Muscles Ligaments Tendons J. January2013;3(1):12–22. 10.11138/mltj/2013.3.1.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kalson NS, Lu Y, Taylor SH, Starborg T, Holmes DF, Kadler KE. A structure-based extracellular matrix expansion mechanism of fibrous tissue growth. Elife. May202015;4. 10.7554/eLife.05958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Delgado Caceres M, Pfeifer CG, Docheva D. Understanding Tendons: Lessons from Transgenic Mouse Models. Stem Cells Dev. September12018;27(17):1161–1174. 10.1089/scd.2018.0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jelinsky SA, Archambault J, Li L, Seeherman H. Tendon-selective genes identified from rat and human musculoskeletal tissues. J Orthop Res. March2010;28(3):289–97. 10.1002/jor.20999. [DOI] [PubMed] [Google Scholar]
- 40.Murchison ND, Price BA, Conner DA, et al. Regulation of tendon differentiation by scleraxis distinguishes force-transmitting tendons from muscle-anchoring tendons. Development. July2007;134(14):2697–708. 10.1242/dev.001933. [DOI] [PubMed] [Google Scholar]
- 41.Kague E, Hughes SM, Lawrence EA, et al. Scleraxis genes are required for normal musculoskeletal development and for rib growth and mineralization in zebrafish. FASEB J. August2019;33(8):9116–9130. 10.1096/fj.201802654RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pryce BA, Watson SS, Murchison ND, Staverosky JA, Dunker N, Schweitzer R. Recruitment and maintenance of tendon progenitors by TGFbeta signaling are essential for tendon formation. Development. April2009;136(8):1351–61. 10.1242/dev.027342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tan GK, Pryce BA, Stabio A, et al. Tgfbeta signaling is critical for maintenance of the tendon cell fate. Elife. January212020;9. 10.7554/eLife.52695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mendias CL, Bakhurin KI, Faulkner JA. Tendons of myostatin-deficient mice are small, brittle, and hypocellular. Proc Natl Acad Sci U S A. January82008;105(1):388–93. 10.1073/pnas.0707069105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ito Y, Toriuchi N, Yoshitaka T, et al. The Mohawk homeobox gene is a critical regulator of tendon differentiation. Proc Natl Acad Sci U S A. June82010;107(23):10538–42. 10.1073/pnas.1000525107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu W, Watson SS, Lan Y, et al. The atypical homeodomain transcription factor Mohawk controls tendon morphogenesis. Mol Cell Biol. October2010;30(20):4797–807. 10.1128/MCB.00207-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lejard V, Blais F, Guerquin MJ, et al. EGR1 and EGR2 involvement in vertebrate tendon differentiation. J Biol Chem. February182011;286(7):5855–67. 10.1074/jbc.M110.153106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guerquin MJ, Charvet B, Nourissat G, et al. Transcription factor EGR1 directs tendon differentiation and promotes tendon repair. J Clin Invest. August2013;123(8):3564–76. 10.1172/JCI67521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Quigley AS, Bancelin S, Deska-Gauthier D, Legare F, Kreplak L, Veres SP. In tendons, differing physiological requirements lead to functionally distinct nanostructures. Sci Rep. March132018;8(1):4409. 10.1038/s41598-018-22741-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang AH, Watson SS, Wang L, et al. Requirement for scleraxis in the recruitment of mesenchymal progenitors during embryonic tendon elongation. Development. October42019;146(20). 10.1242/dev.182782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kardon G. Muscle and tendon morphogenesis in the avian hind limb. Development. October1998;125(20):4019–32. [DOI] [PubMed] [Google Scholar]
- 52.Brent AE, Braun T, Tabin CJ. Genetic analysis of interactions between the somitic muscle, cartilage and tendon cell lineages during mouse development. Development. February2005;132(3):515–28. 10.1242/dev.01605. [DOI] [PubMed] [Google Scholar]
- 53.Bonnin MA, Laclef C, Blaise R, et al. Six1 is not involved in limb tendon development, but is expressed in limb connective tissue under Shh regulation. Mech Dev. April2005;122(4):573–85. 10.1016/j.mod.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 54.Chen JW, Niu X, King MJ, Noedl MT, Tabin CJ, Galloway JL. The mevalonate pathway is a crucial regulator of tendon cell specification. Development. June242020;147(12). 10.1242/dev.185389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Edom-Vovard F, Schuler B, Bonnin MA, Teillet MA, Duprez D. Fgf4 positively regulates scleraxis and tenascin expression in chick limb tendons. Dev Biol. July152002;247(2):351–66. 10.1006/dbio.2002.0707. [DOI] [PubMed] [Google Scholar]
- 56.Subramanian A, Kanzaki LF, Galloway JL, Schilling TF. Mechanical force regulates tendon extracellular matrix organization and tenocyte morphogenesis through TGFbeta signaling. Elife. November262018;7. 10.7554/eLife.38069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huang AH, Riordan TJ, Pryce B, et al. Musculoskeletal integration at the wrist underlies the modular development of limb tendons. Development. July152015;142(14):2431–41. 10.1242/dev.122374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kozhemyakina E, Lassar AB, Zelzer E. A pathway to bone: signaling molecules and transcription factors involved in chondrocyte development and maturation. Development. March12015;142(5):817–31. 10.1242/dev.105536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Blitz E, Sharir A, Akiyama H, Zelzer E. Tendon-bone attachment unit is formed modularly by a distinct pool of Scx- and Sox9-positive progenitors. Development. July2013;140(13):2680–90. 10.1242/dev.093906. [DOI] [PubMed] [Google Scholar]
- 60.Sugimoto Y, Takimoto A, Akiyama H, et al. Scx+/Sox9+ progenitors contribute to the establishment of the junction between cartilage and tendon/ligament. Development. June2013;140(11):2280–8. 10.1242/dev.096354. [DOI] [PubMed] [Google Scholar]
- 61.Tatara AM, Lipner JH, Das R, et al. The role of muscle loading on bone (Re)modeling at the developing enthesis. PLoS One. 2014;9(5):e97375. 10.1371/journal.pone.0097375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schwartz AG, Long F, Thomopoulos S. Enthesis fibrocartilage cells originate from a population of Hedgehog-responsive cells modulated by the loading environment. Development. January12015;142(1):196–206. 10.1242/dev.112714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dyment NA, Breidenbach AP, Schwartz AG, et al. Gdf5 progenitors give rise to fibrocartilage cells that mineralize via hedgehog signaling to form the zonal enthesis. Dev Biol. September12015;405(1):96–107. 10.1016/j.ydbio.2015.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schwartz AG, Galatz LM, Thomopoulos S. Enthesis regeneration: a role for Gli1+ progenitor cells. Development. April12017;144(7):1159–1164. 10.1242/dev.139303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brummel J, Baker CL 3rd, Hopkins R, Baker CL Jr. Epicondylitis: lateral. Sports Med Arthrosc Rev. September2014;22(3):e1–6. 10.1097/JSA.0000000000000024. [DOI] [PubMed] [Google Scholar]
- 66.Lim WL, Liau LL, Ng MH, Chowdhury SR, Law JX. Current Progress in Tendon and Ligament Tissue Engineering. Tissue Eng Regen Med. December2019;16(6):549–571. 10.1007/s13770-019-00196-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Childress MA, Beutler A. Management of chronic tendon injuries. Am Fam Physician. April12013;87(7):486–90. [PubMed] [Google Scholar]
- 68.Deng S, Sun Z, Zhang C, Chen G, Li J. Surgical Treatment Versus Conservative Management for Acute Achilles Tendon Rupture: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J Foot Ankle Surg. Nov-Dec 2017;56(6):1236–1243. 10.1053/j.jfas.2017.05.036. [DOI] [PubMed] [Google Scholar]
- 69.Loebel C, Burdick JA. Engineering Stem and Stromal Cell Therapies for Musculoskeletal Tissue Repair. Cell Stem Cell. March12018;22(3):325–339. 10.1016/j.stem.2018.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gaspar D, Spanoudes K, Holladay C, Pandit A, Zeugolis D. Progress in cell-based therapies for tendon repair. Adv Drug Deliv Rev. April2015;84:240–56. 10.1016/j.addr.2014.11.023. [DOI] [PubMed] [Google Scholar]
- 71.Costa-Almeida R, Calejo I, Gomes ME. Mesenchymal Stem Cells Empowering Tendon Regenerative Therapies. Int J Mol Sci. June192019;20(12). 10.3390/ijms20123002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bianco P. “Mesenchymal” stem cells. Annu Rev Cell Dev Biol. 2014;30:677–704. 10.1146/annurev-cellbio-100913-013132. [DOI] [PubMed] [Google Scholar]
- 73.Caplan AI. Mesenchymal stem cells. J Orthop Res. September1991;9(5):641–50. 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 74.Friedenstein AJ. Bone marrow osteogenic stem cells. In: Cohn DV, Glorieux FH, Martin TJ, eds. Calcium Regulation and Bone Metabolism. Amsterdam: Elsevier Sci.; 1990:353–361. [Google Scholar]
- 75.Owen M, Friedenstein AJ. Stromal stem cells: marrow-derived osteogenic precursors. Ciba Found Symp. 1988;136:42–60. 10.1002/9780470513637.ch4. [DOI] [PubMed] [Google Scholar]
- 76.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. April21999;284(5411):143–7. 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 77.Ambrosi TH, Longaker MT, Chan CKF. A Revised Perspective of Skeletal Stem Cell Biology. Front Cell Dev Biol. 2019;7:189. 10.3389/fcell.2019.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kwon DR, Park GY. Adult mesenchymal stem cells for the treatment in patients with rotator cuff disease: present and future direction. Ann Transl Med. November2018;6(22):432. 10.21037/atm.2018.09.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yokoya S, Mochizuki Y, Natsu K, Omae H, Nagata Y, Ochi M. Rotator cuff regeneration using a bioabsorbable material with bone marrow-derived mesenchymal stem cells in a rabbit model. Am J Sports Med. June2012;40(6):1259–68. 10.1177/0363546512442343. [DOI] [PubMed] [Google Scholar]
- 80.Geburek F, Roggel F, van Schie HTM, et al. Effect of single intralesional treatment of surgically induced equine superficial digital flexor tendon core lesions with adipose-derived mesenchymal stromal cells: a controlled experimental trial. Stem Cell Res Ther. June52017;8(1):129. 10.1186/s13287-017-0564-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ju YJ, Muneta T, Yoshimura H, Koga H, Sekiya I. Synovial mesenchymal stem cells accelerate early remodeling of tendon-bone healing. Cell Tissue Res. June2008;332(3):469–78. 10.1007/s00441-008-0610-z. [DOI] [PubMed] [Google Scholar]
- 82.Park GY, Kwon DR, Lee SC. Regeneration of Full-Thickness Rotator Cuff Tendon Tear After Ultrasound-Guided Injection With Umbilical Cord Blood-Derived Mesenchymal Stem Cells in a Rabbit Model. Stem Cells Transl Med. November2015;4(11):1344–51. 10.5966/sctm.2015-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hernigou P, Flouzat Lachaniette CH, Delambre J, et al. Biologic augmentation of rotator cuff repair with mesenchymal stem cells during arthroscopy improves healing and prevents further tears: a case-controlled study. Int Orthop. September2014;38(9):1811–8. 10.1007/s00264-014-2391-1. [DOI] [PubMed] [Google Scholar]
- 84.Liu Y, Suen CW, Zhang JF, Li G. Current concepts on tenogenic differentiation and clinical applications. J Orthop Translat. April2017;9:28–42. 10.1016/j.jot.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lui PP, Rui YF, Ni M, Chan KM. Tenogenic differentiation of stem cells for tendon repair-what is the current evidence? J Tissue Eng Regen Med. August2011;5(8):e144–63. 10.1002/term.424. [DOI] [PubMed] [Google Scholar]
- 86.Alberton P, Popov C, Pragert M, et al. Conversion of human bone marrow-derived mesenchymal stem cells into tendon progenitor cells by ectopic expression of scleraxis. Stem Cells Dev. April102012;21(6):846–58. 10.1089/scd.2011.0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lui PP. Stem cell technology for tendon regeneration: current status, challenges, and future research directions. Stem Cells Cloning. 2015;8:163–74. 10.2147/SCCAA.S60832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wilson A, Hodgson-Garms M, Frith JE, Genever P. Multiplicity of Mesenchymal Stromal Cells: Finding the Right Route to Therapy. Front Immunol. 2019;10:1112. 10.3389/fimmu.2019.01112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Magne D, Bougault C. What understanding tendon cell differentiation can teach us about pathological tendon ossification. Histol Histopathol. August2015;30(8):901–10. 10.14670/HH-11-614. [DOI] [PubMed] [Google Scholar]
- 90.Harris MT, Butler DL, Boivin GP, Florer JB, Schantz EJ, Wenstrup RJ. Mesenchymal stem cells used for rabbit tendon repair can form ectopic bone and express alkaline phosphatase activity in constructs. J Orthop Res. September2004;22(5):998–1003. 10.1016/j.orthres.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 91.Caplan H, Olson SD, Kumar A, et al. Mesenchymal Stromal Cell Therapeutic Delivery: Translational Challenges to Clinical Application. Front Immunol. 2019;10:1645. 10.3389/fimmu.2019.01645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Garcia-Sanchez D, Fernandez D, Rodriguez-Rey JC, Perez-Campo FM. Enhancing survival, engraftment, and osteogenic potential of mesenchymal stem cells. World J Stem Cells. October262019;11(10):748–763. 10.4252/wjsc.v11.i10.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Caplan AI. Medicinal signalling cells: they work, so use them. Nature. February2019;566(7742):39. 10.1038/d41586-019-00490-6. [DOI] [PubMed] [Google Scholar]
- 94.Pagliuca FW, Millman JR, Gurtler M, et al. Generation of functional human pancreatic beta cells in vitro. Cell. October92014;159(2):428–39. 10.1016/j.cell.2014.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhao C, Wang Q, Temple S. Stem cell therapies for retinal diseases: recapitulating development to replace degenerated cells. Development. April152017;144(8):1368–1381. 10.1242/dev.133108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chien KR, Frisen J, Fritsche-Danielson R, Melton DA, Murry CE, Weissman IL. Regenerating the field of cardiovascular cell therapy. Nat Biotechnol. March2019;37(3):232–237. 10.1038/s41587-019-0042-1. [DOI] [PubMed] [Google Scholar]
- 97.NIH. NIH launches first U.S. clinical trial of patient-derived stem cell therapy to replace dying cells in retina. 2019.
- 98.Chen X, Song XH, Yin Z, et al. Stepwise differentiation of human embryonic stem cells promotes tendon regeneration by secreting fetal tendon matrix and differentiation factors. Stem Cells. June2009;27(6):1276–87. 10.1002/stem.61. [DOI] [PubMed] [Google Scholar]
- 99.Cohen S, Leshansky L, Zussman E, et al. Repair of full-thickness tendon injury using connective tissue progenitors efficiently derived from human embryonic stem cells and fetal tissues. Tissue Eng Part A. October2010;16(10):3119–37. 10.1089/ten.TEA.2009.0716. [DOI] [PubMed] [Google Scholar]
- 100.Chen X, Yin Z, Chen JL, et al. Force and scleraxis synergistically promote the commitment of human ES cells derived MSCs to tenocytes. Sci Rep. 2012;2:977. 10.1038/srep00977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Xu W, Wang Y, Liu E, et al. Human iPSC-derived neural crest stem cells promote tendon repair in a rat patellar tendon window defect model. Tissue Eng Part A. November2013;19(21–22):2439–51. 10.1089/ten.TEA.2012.0453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dale TP, Mazher S, Webb WR, et al. Tenogenic Differentiation of Human Embryonic Stem Cells. Tissue Eng Part A. March2018;24(5–6):361–368. 10.1089/ten.TEA.2017.0017. [DOI] [PubMed] [Google Scholar]
- 103.Komura S, Satake T, Goto A, et al. Induced pluripotent stem cell-derived tenocyte-like cells promote the regeneration of injured tendons in mice. Sci Rep. March42020;10(1):3992. 10.1038/s41598-020-61063-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Freedman BR, Mooney DJ. Biomaterials to Mimic and Heal Connective Tissues. Adv Mater. May2019;31(19):e1806695. 10.1002/adma.201806695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chen L, Liu JP, Tang KL, et al. Tendon derived stem cells promote platelet-rich plasma healing in collagenase-induced rat achilles tendinopathy. Cell Physiol Biochem. 2014;34(6):2153–68. 10.1159/000369659. [DOI] [PubMed] [Google Scholar]
- 106.Gelberman RH, Linderman SW, Jayaram R, et al. Combined Administration of ASCs and BMP-12 Promotes an M2 Macrophage Phenotype and Enhances Tendon Healing. Clin Orthop Relat Res. September2017;475(9):2318–2331. 10.1007/s11999-017-5369-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Docheva D, Muller SA, Majewski M, Evans CH. Biologics for tendon repair. Adv Drug Deliv Rev. April2015;84:222–39. 10.1016/j.addr.2014.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shoaib A, Mishra V. Surgical repair of symptomatic chronic achilles tendon rupture using synthetic graft augmentation. Foot Ankle Surg. September2017;23(3):179–182. 10.1016/j.fas.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 109.Derwin KA, Baker AR, Spragg RK, Leigh DR, Iannotti JP. Commercial extracellular matrix scaffolds for rotator cuff tendon repair. Biomechanical, biochemical, and cellular properties. J Bone Joint Surg Am. December2006;88(12):2665–72. 10.2106/JBJS.E.01307. [DOI] [PubMed] [Google Scholar]
- 110.Linderman SW, Shen H, Yoneda S, et al. Effect of connective tissue growth factor delivered via porous sutures on the proliferative stage of intrasynovial tendon repair. J Orthop Res. July2018;36(7):2052–2063. 10.1002/jor.23842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhou Y, Wang JH. PRP Treatment Efficacy for Tendinopathy: A Review of Basic Science Studies. Biomed Res Int. 2016;2016:9103792. 10.1155/2016/9103792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Patel S, Caldwell JM, Doty SB, et al. Integrating soft and hard tissues via interface tissue engineering. J Orthop Res. April2018;36(4):1069–1077. 10.1002/jor.23810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Liu CF, Aschbacher-Smith L, Barthelery NJ, Dyment N, Butler D, Wylie C. Spatial and temporal expression of molecular markers and cell signals during normal development of the mouse patellar tendon. Tissue Eng Part A. March2012;18(5–6):598–608. 10.1089/ten.TEA.2011.0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nguyen PK, Pan XS, Li J, Kuo CK. Roadmap of molecular, compositional, and functional markers during embryonic tendon development. Connect Tissue Res. September2018;59(5):495–508. 10.1080/03008207.2018.1511710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Schneider M, Angele P, Jarvinen TAH, Docheva D. Rescue plan for Achilles: Therapeutics steering the fate and functions of stem cells in tendon wound healing. Adv Drug Deliv Rev. April2018;129:352–375. 10.1016/j.addr.2017.12.016. [DOI] [PubMed] [Google Scholar]
- 116.De Micheli AJ, Swanson JB, Disser NP, et al. Single-cell Transcriptomic Analyses Identifies Extensive Heterogeneity in the Cellular Composition of Mouse Achilles Tendons. Am J Physiol Cell Physiol. September22020. 10.1152/ajpcell.00372.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Harvey T, Flamenco S, Fan CM. A Tppp3(+)Pdgfra(+) tendon stem cell population contributes to regeneration and reveals a shared role for PDGF signalling in regeneration and fibrosis. Nat Cell Biol. December2019;21(12):1490–1503. 10.1038/s41556-019-0417-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kendal AR, Layton T, Al-Mossawi H, et al. Multi-omic single cell analysis resolves novel stromal cell populations in healthy and diseased human tendon. Sci Rep. September32020;10(1):13939. 10.1038/s41598-020-70786-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Thomopoulos S, Parks WC, Rifkin DB, Derwin KA. Mechanisms of tendon injury and repair. J Orthop Res. June2015;33(6):832–9. 10.1002/jor.22806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Manning CN, Havlioglu N, Knutsen E, et al. The early inflammatory response after flexor tendon healing: a gene expression and histological analysis. J Orthop Res. May2014;32(5):645–52. 10.1002/jor.22575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Marsolais D, Cote CH, Frenette J. Neutrophils and macrophages accumulate sequentially following Achilles tendon injury. J Orthop Res. November2001;19(6):1203–9. 10.1016/S0736-0266(01)00031-6. [DOI] [PubMed] [Google Scholar]
- 122.de la Durantaye M, Piette AB, van Rooijen N, Frenette J. Macrophage depletion reduces cell proliferation and extracellular matrix accumulation but increases the ultimate tensile strength of injured Achilles tendons. J Orthop Res. February2014;32(2):279–85. 10.1002/jor.22504. [DOI] [PubMed] [Google Scholar]
- 123.Lin D, Alberton P, Caceres MD, Volkmer E, Schieker M, Docheva D. Tenomodulin is essential for prevention of adipocyte accumulation and fibrovascular scar formation during early tendon healing. Cell Death Dis. October122017;8(10):e3116. 10.1038/cddis.2017.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Collins CA, Olsen I, Zammit PS, et al. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell. July292005;122(2):289–301. 10.1016/j.cell.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 125.Barker N, van Es JH, Kuipers J, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. October252007;449(7165):1003–7. 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 126.Snippert HJ, Clevers H. Tracking adult stem cells. EMBO Rep. February2011;12(2):113–22. 10.1038/embor.2010.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Blanpain C, Fuchs E. Stem cell plasticity. Plasticity of epithelial stem cells in tissue regeneration. Science. June132014;344(6189):1242281. 10.1126/science.1242281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bi Y, Ehirchiou D, Kilts TM, et al. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat Med. October2007;13(10):1219–27. 10.1038/nm1630. [DOI] [PubMed] [Google Scholar]
- 129.Zhang J, Wang JH. Characterization of differential properties of rabbit tendon stem cells and tenocytes. BMC Musculoskelet Disord. January182010;11:10. 10.1186/1471-2474-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lee CH, Lee FY, Tarafder S, et al. Harnessing endogenous stem/progenitor cells for tendon regeneration. J Clin Invest. July12015;125(7):2690–701. 10.1172/JCI81589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wang Y, Zhang X, Huang H, et al. Osteocalcin expressing cells from tendon sheaths in mice contribute to tendon repair by activating Hedgehog signaling. Elife. December152017;6. 10.7554/eLife.30474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yin Z, Hu JJ, Yang L, et al. Single-cell analysis reveals a nestin(+) tendon stem/progenitor cell population with strong tenogenic potentiality. Sci Adv. November2016;2(11):e1600874. 10.1126/sciadv.1600874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sakabe T, Sakai K, Maeda T, et al. Transcription factor scleraxis vitally contributes to progenitor lineage direction in wound healing of adult tendon in mice. J Biol Chem. April202018;293(16):5766–5780. 10.1074/jbc.RA118.001987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Best KT, Loiselle AE. Scleraxis lineage cells contribute to organized bridging tissue during tendon healing and identify a subpopulation of resident tendon cells. FASEB J. July2019;33(7):8578–8587. 10.1096/fj.201900130RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ackerman JE, Nichols AE, Studentsova V, Best KT, Knapp E, Loiselle AE. Cell non-autonomous functions of S100a4 drive fibrotic tendon healing. Elife. May242019;8. 10.7554/eLife.45342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Moser HL, Doe AP, Meier K, et al. Genetic lineage tracing of targeted cell populations during enthesis healing. J Orthop Res. December2018;36(12):3275–3284. 10.1002/jor.24122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Howell K, Chien C, Bell R, et al. Novel Model of Tendon Regeneration Reveals Distinct Cell Mechanisms Underlying Regenerative and Fibrotic Tendon Healing. Sci Rep. March232017;7:45238. 10.1038/srep45238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Dyment NA, Hagiwara Y, Matthews BG, Li Y, Kalajzic I, Rowe DW. Lineage tracing of resident tendon progenitor cells during growth and natural healing. PLoS One. 2014;9(4):e96113. 10.1371/journal.pone.0096113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Pearse RV 2nd, Esshaki D, Tabin CJ, Murray MM. Genome-wide expression analysis of intra- and extraarticular connective tissue. J Orthop Res. April2009;27(4):427–34. 10.1002/jor.20774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Disser NP, Ghahramani GC, Swanson JB, et al. Widespread diversity in the transcriptomes of functionally divergent limb tendons. J Physiol. April2020;598(8):1537–1550. 10.1113/JP279646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Grinstein M, Dingwall HL, Shah RR, Capellini TD, Galloway JL. A robust method for RNA extraction and purification from a single adult mouse tendon. PeerJ. 2018;6:e4664. 10.7717/peerj.4664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Baron CS, van Oudenaarden A. Unravelling cellular relationships during development and regeneration using genetic lineage tracing. Nat Rev Mol Cell Biol. December2019;20(12):753–765. 10.1038/s41580-019-0186-3. [DOI] [PubMed] [Google Scholar]
- 143.Bressler HB, Markus M, Bressler RP, Friedman SN, Friedman L. Temporal tendinosis: A cause of chronic orofacial pain. Curr Pain Headache Rep. March212020;24(5):18. 10.1007/s11916-020-00851-1. [DOI] [PubMed] [Google Scholar]
- 144.Xu Y, Murrell GA. The basic science of tendinopathy. Clin Orthop Relat Res. July2008;466(7):1528–38. 10.1007/s11999-008-0286-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Cook JL, Purdam CR. Is tendon pathology a continuum? A pathology model to explain the clinical presentation of load-induced tendinopathy. Br J Sports Med. June2009;43(6):409–16. 10.1136/bjsm.2008.051193. [DOI] [PubMed] [Google Scholar]
- 146.McBeath R, Edwards RW, O’Hara BJ, et al. Tendinosis develops from age- and oxygen tension-dependent modulation of Rac1 activity. Aging Cell. June2019;18(3):e12934. 10.1111/acel.12934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yamaguchi K, Ditsios K, Middleton WD, Hildebolt CF, Galatz LM, Teefey SA. The demographic and morphological features of rotator cuff disease. A comparison of asymptomatic and symptomatic shoulders. J Bone Joint Surg Am. August2006;88(8):1699–704. 10.2106/JBJS.E.00835. [DOI] [PubMed] [Google Scholar]
- 148.Lui PP, Maffulli N, Rolf C, Smith RK. What are the validated animal models for tendinopathy? Scand J Med Sci Sports. February2011;21(1):3–17. 10.1111/j.1600-0838.2010.01164.x. [DOI] [PubMed] [Google Scholar]
- 149.Andersson G, Backman LJ, Scott A, Lorentzon R, Forsgren S, Danielson P. Substance P accelerates hypercellularity and angiogenesis in tendon tissue and enhances paratendinitis in response to Achilles tendon overuse in a tendinopathy model. Br J Sports Med. October2011;45(13):1017–22. 10.1136/bjsm.2010.082750. [DOI] [PubMed] [Google Scholar]
- 150.Bell R, Li J, Gorski DJ, et al. Controlled treadmill exercise eliminates chondroid deposits and restores tensile properties in a new murine tendinopathy model. J Biomech. February12013;46(3):498–505. 10.1016/j.jbiomech.2012.10.020. [DOI] [PubMed] [Google Scholar]
- 151.Hast MW, Zuskov A, Soslowsky LJ. The role of animal models in tendon research. Bone Joint Res. June2014;3(6):193–202. 10.1302/2046-3758.36.2000281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Millar NL, Hueber AJ, Reilly JH, et al. Inflammation is present in early human tendinopathy. Am J Sports Med. October2010;38(10):2085–91. 10.1177/0363546510372613. [DOI] [PubMed] [Google Scholar]
- 153.Jarvinen M, Jozsa L, Kannus P, Jarvinen TL, Kvist M, Leadbetter W. Histopathological findings in chronic tendon disorders. Scand J Med Sci Sports. April1997;7(2):86–95. 10.1111/j.1600-0838.1997.tb00124.x. [DOI] [PubMed] [Google Scholar]
- 154.Hashimoto T, Nobuhara K, Hamada T. Pathologic evidence of degeneration as a primary cause of rotator cuff tear. Clin Orthop Relat Res. October2003;(415):111–20. 10.1097/01.blo.0000092974.12414.22. [DOI] [PubMed] [Google Scholar]
- 155.Maffulli N, Wong J, Almekinders LC. Types and epidemiology of tendinopathy. Clin Sports Med. October2003;22(4):675–92. 10.1016/s0278-5919(03)00004-8. [DOI] [PubMed] [Google Scholar]
- 156.Jelinsky SA, Rodeo SA, Li J, Gulotta LV, Archambault JM, Seeherman HJ. Regulation of gene expression in human tendinopathy. BMC Musculoskelet Disord. May32011;12:86. 10.1186/1471-2474-12-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Cook JL, Khan KM, Purdam C. Achilles tendinopathy. Man Ther. August2002;7(3):121–30. 10.1054/math.2002.0458. [DOI] [PubMed] [Google Scholar]
- 158.Schubert TE, Weidler C, Lerch K, Hofstadter F, Straub RH. Achilles tendinosis is associated with sprouting of substance P positive nerve fibres. Ann Rheum Dis. July2005;64(7):1083–6. 10.1136/ard.2004.029876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Matthews TJ, Hand GC, Rees JL, Athanasou NA, Carr AJ. Pathology of the torn rotator cuff tendon. Reduction in potential for repair as tear size increases. J Bone Joint Surg Br. April2006;88(4):489–95. 10.1302/0301-620X.88B4.16845. [DOI] [PubMed] [Google Scholar]
- 160.Scott A, Khan KM, Cook JL, Duronio V. Human tendon overuse pathology: histopathologic and biochemical findings. Understanding and Prevention of Tendinopathy in the Athlete, Encyclopedia of Sports Medicine. Vol 12. Blackwell Publishing; 2007:69–84. [Google Scholar]
- 161.Kragsnaes MS, Fredberg U, Stribolt K, Kjaer SG, Bendix K, Ellingsen T. Stereological quantification of immune-competent cells in baseline biopsy specimens from achilles tendons: results from patients with chronic tendinopathy followed for more than 4 years. Am J Sports Med. October2014;42(10):2435–45. 10.1177/0363546514542329. [DOI] [PubMed] [Google Scholar]
- 162.Dakin SG, Martinez FO, Yapp C, et al. Inflammation activation and resolution in human tendon disease. Sci Transl Med. October282015;7(311):311ra173. 10.1126/scitranslmed.aac4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Millar NL, Akbar M, Campbell AL, et al. IL-17A mediates inflammatory and tissue remodelling events in early human tendinopathy. Sci Rep. June62016;6:27149. 10.1038/srep27149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Millar NL, Gilchrist DS, Akbar M, et al. MicroRNA29a regulates IL-33-mediated tissue remodelling in tendon disease. Nat Commun. April102015;6:6774. 10.1038/ncomms7774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Millar NL, Dean BJ, Dakin SG. Inflammation and the continuum model: time to acknowledge the molecular era of tendinopathy. Br J Sports Med. December2016;50(23):1486. 10.1136/bjsports-2016-096419. [DOI] [PubMed] [Google Scholar]
- 166.Dean BJ, Gettings P, Dakin SG, Carr AJ. Are inflammatory cells increased in painful human tendinopathy? A systematic review. Br J Sports Med. February2016;50(4):216–20. 10.1136/bjsports-2015-094754. [DOI] [PubMed] [Google Scholar]
- 167.Dakin SG, Newton J, Martinez FO, et al. Chronic inflammation is a feature of Achilles tendinopathy and rupture. Br J Sports Med. March2018;52(6):359–367. 10.1136/bjsports-2017-098161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Jomaa G, Kwan CK, Fu SC, et al. A systematic review of inflammatory cells and markers in human tendinopathy. BMC Musculoskelet Disord. February62020;21(1):78. 10.1186/s12891-020-3094-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Dakin SG, Werling D, Hibbert A, et al. Macrophage sub-populations and the lipoxin A4 receptor implicate active inflammation during equine tendon repair. PLoS One. 2012;7(2):e32333. 10.1371/journal.pone.0032333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kietrys DM, Barr-Gillespie AE, Amin M, Wade CK, Popoff SN, Barbe MF. Aging contributes to inflammation in upper extremity tendons and declines in forelimb agility in a rat model of upper extremity overuse. PLoS One. 2012;7(10):e46954. 10.1371/journal.pone.0046954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Pingel J, Wienecke J, Kongsgaard M, et al. Increased mast cell numbers in a calcaneal tendon overuse model. Scand J Med Sci Sports. December2013;23(6):e353–60. 10.1111/sms.12089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sunwoo JY, Eliasberg CD, Carballo CB, Rodeo SA. The role of the macrophage in tendinopathy and tendon healing. J Orthop Res. August2020;38(8):1666–1675. 10.1002/jor.24667. [DOI] [PubMed] [Google Scholar]
- 173.Maeda T, Sakabe T, Sunaga A, et al. Conversion of mechanical force into TGF-beta-mediated biochemical signals. Curr Biol. June72011;21(11):933–41. 10.1016/j.cub.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Thorpe CT, Chaudhry S, Lei II, et al. Tendon overload results in alterations in cell shape and increased markers of inflammation and matrix degradation. Scand J Med Sci Sports. August2015;25(4):e381–91. 10.1111/sms.12333. [DOI] [PubMed] [Google Scholar]
- 175.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol. January2007;81(1):1–5. 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- 176.Rider P, Voronov E, Dinarello CA, Apte RN, Cohen I. Alarmins: Feel the Stress. J Immunol. February152017;198(4):1395–1402. 10.4049/jimmunol.1601342. [DOI] [PubMed] [Google Scholar]
- 177.Millar NL, Murrell GA, McInnes IB. Alarmins in tendinopathy: unravelling new mechanisms in a common disease. Rheumatology (Oxford). May2013;52(5):769–79. 10.1093/rheumatology/kes409. [DOI] [PubMed] [Google Scholar]
- 178.Akbar M, Gilchrist DS, Kitson SM, et al. Targeting danger molecules in tendinopathy: the HMGB1/TLR4 axis. RMD Open. 2017;3(2):e000456. 10.1136/rmdopen-2017-000456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Mosca MJ, Carr AJ, Snelling SJB, Wheway K, Watkins B, Dakin SG. Differential expression of alarmins-S100A9, IL-33, HMGB1 and HIF-1alpha in supraspinatus tendinopathy before and after treatment. BMJ Open Sport Exerc Med. 2017;3(1):e000225. 10.1136/bmjsem-2017-000225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Zhao G, Zhang J, Nie D, et al. HMGB1 mediates the development of tendinopathy due to mechanical overloading. PLoS One. 2019;14(9):e0222369. 10.1371/journal.pone.0222369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Favata M, Beredjiklian PK, Zgonis MH, et al. Regenerative properties of fetal sheep tendon are not adversely affected by transplantation into an adult environment. J Orthop Res. November2006;24(11):2124–32. 10.1002/jor.20271. [DOI] [PubMed] [Google Scholar]
- 182.Tang QM, Chen JL, Shen WL, et al. Fetal and adult fibroblasts display intrinsic differences in tendon tissue engineering and regeneration. Sci Rep. July32014;4:5515. 10.1038/srep05515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Notari M, Ventura-Rubio A, Bedford-Guaus SJ, et al. The local microenvironment limits the regenerative potential of the mouse neonatal heart. Sci Adv. May2018;4(5):eaao5553. 10.1126/sciadv.aao5553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Segel M, Neumann B, Hill MFE, et al. Niche stiffness underlies the ageing of central nervous system progenitor cells. Nature. September2019;573(7772):130–134. 10.1038/s41586-019-1484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Niu X, Subramanian A, Hwang TH, Schilling TF, Galloway JL. Tendon Cell Regeneration Is Mediated by Attachment Site-Resident Progenitors and BMP Signaling. Curr Biol. June292020. 10.1016/j.cub.2020.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kaji DA, Howell KL, Balic Z, Hubmacher D, Huang AH. Tgfbeta signaling is required for tenocyte recruitment and functional neonatal tendon regeneration. Elife. June52020;9. 10.7554/eLife.51779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Kragl M, Knapp D, Nacu E, et al. Cells keep a memory of their tissue origin during axolotl limb regeneration. Nature. July22009;460(7251):60–5. 10.1038/nature08152. [DOI] [PubMed] [Google Scholar]
- 188.Gerber T, Murawala P, Knapp D, et al. Single-cell analysis uncovers convergence of cell identities during axolotl limb regeneration. Science. September272018. 10.1126/science.aaq0681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Currie JD, Kawaguchi A, Traspas RM, Schuez M, Chara O, Tanaka EM. Live Imaging of Axolotl Digit Regeneration Reveals Spatiotemporal Choreography of Diverse Connective Tissue Progenitor Pools. Dev Cell. November212016;39(4):411–423. 10.1016/j.devcel.2016.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Leigh ND, Dunlap GS, Johnson K, et al. Transcriptomic landscape of the blastema niche in regenerating adult axolotl limbs at single-cell resolution. Nat Commun. December42018;9(1):5153. 10.1038/s41467-018-07604-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Gemberling M, Bailey TJ, Hyde DR, Poss KD. The zebrafish as a model for complex tissue regeneration. Trends Genet. November2013;29(11):611–20. 10.1016/j.tig.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Yun MH, Davaapil H, Brockes JP. Recurrent turnover of senescent cells during regeneration of a complex structure. Elife. May52015;4. 10.7554/eLife.05505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Kikuchi K. New function of zebrafish regulatory T cells in organ regeneration. Curr Opin Immunol. April2020;63:7–13. 10.1016/j.coi.2019.10.001. [DOI] [PubMed] [Google Scholar]
- 194.Abnave P, Ghigo E. Role of the immune system in regeneration and its dynamic interplay with adult stem cells. Semin Cell Dev Biol. March2019;87:160–168. 10.1016/j.semcdb.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 195.Tsai SL. The molecular interplay between progenitors and immune cells in tissue regeneration and homeostasis. Journal of Immunology and Regenerative Medicine. 2020;7:100024. 10.1016/j.regen.2019.100024. [DOI] [Google Scholar]
- 196.Godwin J, Kuraitis D, Rosenthal N. Extracellular matrix considerations for scar-free repair and regeneration: insights from regenerative diversity among vertebrates. Int J Biochem Cell Biol. November2014;56:47–55. 10.1016/j.biocel.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 197.Lai SL, Marin-Juez R, Moura PL, et al. Reciprocal analyses in zebrafish and medaka reveal that harnessing the immune response promotes cardiac regeneration. Elife. June202017;6. 10.7554/eLife.25605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Mescher AL, Neff AW, King MW. Inflammation and immunity in organ regeneration. Dev Comp Immunol. January2017;66:98–110. 10.1016/j.dci.2016.02.015. [DOI] [PubMed] [Google Scholar]
- 199.Kadler KE, Holmes DF, Trotter JA, Chapman JA. Collagen fibril formation. Biochem J. May151996;316 (Pt 1):1–11. 10.1042/bj3160001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Taylor SH, Al-Youha S, Van Agtmael T, et al. Tendon is covered by a basement membrane epithelium that is required for cell retention and the prevention of adhesion formation. PLoS One. January262011;6(1):e16337. 10.1371/journal.pone.0016337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Degen RM, Conti MS, Camp CL, Altchek DW, Dines JS, Werner BC. Epidemiology and Disease Burden of Lateral Epicondylitis in the USA: Analysis of 85,318 Patients. HSS J. February2018;14(1):9–14. 10.1007/s11420-017-9559-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.de Jong JP, Nguyen JT, Sonnema AJ, Nguyen EC, Amadio PC, Moran SL. The incidence of acute traumatic tendon injuries in the hand and wrist: a 10-year population-based study. Clin Orthop Surg. June2014;6(2):196–202. 10.4055/cios.2014.6.2.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Reito A, Paloneva J, Mattila VM, Launonen AP. The increasing incidence of surgically treated quadriceps tendon ruptures. Knee Surg Sports Traumatol Arthrosc. November2019;27(11):3644–3649. 10.1007/s00167-019-05453-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Lemme NJ, Li NY, DeFroda SF, Kleiner J, Owens BD. Epidemiology of Achilles Tendon Ruptures in the United States: Athletic and Nonathletic Injuries From 2012 to 2016. Orthop J Sports Med. November2018;6(11):2325967118808238. 10.1177/2325967118808238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.White JJ, Titchener AG, Fakis A, Tambe AA, Hubbard RB, Clark DI. An epidemiological study of rotator cuff pathology using The Health Improvement Network database. Bone Joint J. March2014;96-B(3):350–3. 10.1302/0301-620X.96B3.32336. [DOI] [PubMed] [Google Scholar]
- 206.Hsu H, Siwiec RM. Patellar Tendon Rupture. StatPearls. Treasure Island (FL): 2020. [PubMed] [Google Scholar]


