Abstract
CD8+ T cells have conventionally been studied in relationship to pathogen or tumor clearance. Recent reports have identified novel functions of CXCR5+CD8+ T cells that can home to lymphoid follicles, a key site of antibody production. In this review we provide an in-depth analysis of conflicting reports regarding the impact of CXCR5+CD8+ T cells on antibody production and examine the data supporting a role for antibody-enhancement (B cell “helper”) and antibody-downregulation (antibody-suppressor) by CXCR5+CD8+ T cell subsets. CXCR5+CD8+ T cell molecular phenotypes are associated with CD8-mediated effector functions including distinct subsets that regulate antibody responses. Co-inhibitory molecule PD-1, among others, distinguish CXCR5+CD8+ T cell subsets. We also provide the first in-depth review of human CXCR5+CD8+ T cells in the context of clinical outcomes and discuss the potential utility of monitoring the quantity of peripheral blood or tissue infiltrating CXCR5+CD8+ T cells as a prognostic tool in multiple disease states.
Keywords: CXCR5+CD8+T cells, Antibody
Introduction:
A review of the subset of CXCR5+CD8+ T cells must first acknowledge the abundance of work that identifies the critical role of CD8+ T cells in pathogen clearance. Cytotoxic type 1 CD8+ T cells (Tc1) that produce pro-inflammatory cytokines such as IFN-γ and TNF-α and are among the most well-defined CD8+ T cell subsets involved in clearance of intracellular pathogens and tumors. Tc1 cells kill target cells via release of cytotoxic molecules, such as perforin and granzyme, and comprise the majority of CD8+ T cell subsets in the peripheral blood of healthy human blood donors (1). These cells are critical to the defense against intracellular bacteria, viruses, and protozoa, and clear immunogenic targets in a variety of conditions including autoimmune, tumor, transplant, and other inflammatory milieus. In addition to the Tc1 cell subset, other less-abundant CD8+ T effector cell subsets include inducible IL-4- and IL-5-producing (Tc2; active in mediating allergy, asthma, as well as defense against helminths and venoms), IL-9-producing [Tc9; active in preventing CD4+ T cell-mediated inflammation in the small intestine (2), exacerbating asthma and allergic inflammation in atopic dermatitis (3), and mediating anti-tumor effects (4)], IL-17-producing (Tc17; active in the defense against extracellular bacteria and fungi), and FoxP3-expressing regulatory (active in maintaining immune homeostasis) CD8+ T cells (5, 6). Given the disparate effector functions and plasticity of CD8+ T cell subsets, a more in-depth understanding of CD8+ T cell subpopulations is critical to their phenotypic and functional classification and to determination of their potential clinical utility in monitoring, preventing, and/or treating immune mediated disease.
Recent work in various models and disease states has identified unique subsets of CD8+ T cells expressing the chemokine receptor CXCR5, which directs these cells into secondary lymphoid follicles (7). Within these secondary follicles, CXCR5+CD8+ T cells may encounter a rich milieu of antigens, antigen presenting cells, and other follicular cells, and are reported to display a range of effector functions in distinct viral, tumor, and autoimmune settings. The bulk of literature to date has focused on CXCR5+CD8+ T cell-mediated clearance of viruses that have predilection for sequestration in lymphoid follicles, including HIV/SIV (8–17) and LCMV (18–20), as well as prognostic associations of CXCR5+CD8+ T cells detected in tumors and draining lymph nodes with improved clinical outcomes for malignancies such as colorectal (21, 22) and hepatocellular carcinoma (23, 24).
This review builds upon prior analyses of CXCR5+CD8+ T cells that have examined their role in viral immunity, autoimmunity and tumor immunity (7, 25–27), by focusing on this subset’s divergent roles on humoral immunity and by categorizing molecular phenotypes reported for subsets with distinct effector functions. While the full spectrum of CXCR5+CD8+ T cell function is included, we focus on an analysis of the data supporting a role for CXCR5+CD8+ T cell subsets on enhancement of antibody production (B cell “helper” function) versus downregulation of antibody-production (antibody-suppressor). We also provide an in-depth analysis of CXCR5+CD8+ T cells in a variety of clinical conditions (including viral or bacterial infection, malignancy, autoimmunity, and transplantation) and discuss their potential clinical utility.
CXCR5+CD8+ T Cells that Enhance Antibody Production
Similar to CXCR5+PD-1hiCD4+ T follicular helper cells (TFH) that support antibody production (28, 29), CXCR5+PD-1hiCD8+ T subsets have also been reported to exhibit a B cell helper function. CXCR5+CD8+ T cells in various disease states and models, ranging from colorectal malignancy to viral hepatitis to LCMV infection, have been reported to enhance antibody production by 1) direct interaction with B cells in co-culture (23, 30–37), or 2) synergistic interactions with CD4+ T cells and B cells in co-culture (38, 39).
In the first such study to examine CXCR5+CD8+ T cell enhancement of antibody through direct interaction with B cells, Quigley et al. demonstrated that CXCR5+CD8+ T cells from human tonsillectomy specimens increase both the survival and non-specific IgG production of CD19+ B cells when co-cultured for seven days. While specific cellular mechanisms of B cell help were not explored in this study, it was noted that in vitro stimulated CXCR5+CD8+ T cells exhibited upregulated expression of molecules necessary for T cell-dependent humoral immune responses (32), including CD70 (40), OX40 (41), and ICOS (42). The capacity of human CXCR5+CD8+ T cells to upregulate antibody production nearly 2-fold or greater in culture has been reported in a number of other studies utilizing human-derived cells from patients with various diseases including chronic hepatitis B infection (33), classical Hodgkin lymphoma (34), nasal polyps (35), and hepatocellular carcinoma (23). Since then, several groups have identified CXCR5+CD8+ T cells as direct B cell helpers in murine models.
In an IL-2 KO murine model of autoimmunity (autoimmune hemolytic anemia) that develops due to the absence of CD4+ T regulatory cells (TReg), the authors observed an increase in CXCR5+PD-1hiCD4+ T cells and CXCR5+PD-1hiCD8+ T cells in lymphoid tissue. Both subsets upregulated ICOS and Bcl6, known to be important for interactions between CD4+ TFH and follicular B cells (38). Depletion of either CD4+ T cells or CD8+ T cells resulted in increased survival, improved anemia, decreased frequency of B cells, and decreased IgG autoantibody binding of erythrocytes. Interestingly, the reduction of IgG was predominantly for IgG1 (IL-4 dependent) for CD4+ T cell depletion and both IgG1 and IgG3 (IFN-γ-dependent) for CD8+ T cell depletion, suggesting some differences in CD4- and CD8-mediated enhancement of autoantibody production in this model. Furthermore, supernatant from in vitro stimulated CXCR5+PD-1hiCD8+ T cells significantly upregulated IgG production by in vitro stimulated (anti-CD40, anti-IgM) wild type B cells. Notably, these CD8+ T cells supported IgG production to levels comparable to those observed with supernatants from CD4+ TFH cells (38). In contrast to these studies, co-cultures consisting of B cells and CXCR5+PD-1+CD8+ T cells from LCMV-infected wild type mice resulted in only mild increases in the percentage of IgG1+ B cells and in vitro production of IgG1 in the supernatant compared to B cells cultured alone (and significantly less than co-cultures of B cells with CXCR5+PD-1+CD4+ TFH cells) (31).
In Bcl6 KO mice, that have functional B cells but abrogated endogenous CD4+ TFH cell responses, the adoptive transfer of Runx3-deficient transgenic (LCMV GP33/Db-reactive) P14 CD8+ T cells enriched for CXCR5+CD8+ T cells promoted antibody production in response to KLH-GP33 immunization (but to half the extent when compared to adoptive transfer of CD4+ TFH cells) (30). However, the increase in IgG antibody production occurred in the absence of germinal center (GC) formation, suggesting an extrafollicular mechanism of B cell help. This observation is consistent with studies in the IL-2 KO mouse model, where adoptive transfer of IL-2 KO CD8+ T cells (enriched for CXCR5+PD-1hiCD8+ T cells) into TCRα KO mice resulted in increased plasma cells but not GC B cells. In contrast, adoptive transfer of CD4+ TFH cells resulted in expansion of both GC B cells and plasma cells (30).
In these murine and human studies, antibody-enhancing CXCR5+CD8+ T cells have routinely been reported to express IL-21, CD40L, ICOS, PD-1, and Bcl-6, though not necessarily to the extent expressed by CD4+ TFH cells. When comparing CXCR5+CD8+ T cells isolated from peritumoral tissue versus peripheral blood, hepatocellular carcinoma-infiltrating CXCR5+CD8+ T cells were noted to express higher levels of IL-21, ICOS, and PD-1, illustrating the heterogeneity of CXCR5+CD8+ T cell subsets’ phenotypic expression and function not only between various disease states, but even in different immune locales within the same patient (23).
A few studies have investigated the specific cellular mechanisms by which CXCR5+CD8+ T cell-mediated upregulation of antibody production occurs. CXCR5+CD8+ T cells isolated from human tonsillectomy specimens and co-cultured with CD19+ B cells alone significantly increased levels of IgG, IgM, and IgA in a dose-dependent manner, albeit not to the level of CD4+ T cell:B cell co-cultures (36). Interestingly, the addition of anti-IL-21 or anti-CD40L to CXCR5+CD8+ T:B cell co-cultures reduced production of IgG and IgM by 40–60%, supporting a contact-dependent (CD40L-dependent) and soluble factor-mediated (IL-21-dependent) mechanism of antibody upregulation by these cells (36). The importance of CD40/CD40L interactions in CXCR5+CD8+ T cell-mediated help to B cells has also been reported in an LCMV mouse model, where addition of anti-CD40L neutralizing antibody to CXCR5+PD-1+CD8+ T:B cell cultures, or co-culture with CD40-deficient B cells, abrogated IgG1 production similar to levels observed in cultures of B cells alone (31). In this study, contact dependent B cell help for in vitro (and in vivo) antibody production provided by CXCR5+PD-1+CD8+ T cells was further supported by the requirement for MHC-I matching between CD8+ T cells and B cells.
In contrast to the preceding data suggesting provision of direct help to B cells by CXCR5+PD-1+CD8+ T cells, two groups have demonstrated CD8+ T cell-mediated help to B cells through synergistic interactions with CD4+ TFH cells to enhance antibody production (38, 39). In one study, when CXCR5+CD8+ T cells from human patients (with hepatitis B) were incubated with either CD19+CD27− naïve B cells or CD19+CD27+ memory B cells alone (stimulated with staphylococcal enterotoxin B), they failed to upregulate in vitro antibody production (despite expressing IL-21, a cytokine known to induce B cell antibody production). However, addition of the CXCR5+CD8+ T cells to CXCR5+CD4+ T:B cell co-culture elicited significantly increased IgG antibody production (~2-fold) compared to CXCR5+CD4+ T:B cell co-culture alone (39). In TCRα KO mice immunized with keyhole limpet hemocyanin (KLH), followed by adoptive transfer of IL-2 KO CD8+ T cells (CXCR5+PD-1hiBcl-6+IL-21+CD8+ T cells) alone, was associated with increased frequency of B220intCD138+ plasma cells but not B220+GL-7+Fas+ GC B cells in recipient spleens, and did not promote IgG production. However, when IL-2 KO CD8+ T cells were transferred along with IL-2 KO CD4+ TFH cells, a synergistic effect resulting in increased IgG2b titer (to a greater extent than IgG1 and no effect on IgG2a titer) was observed (38). The results of these studies suggest that CXCR5+CD8+T cell subsets under some circumstances may act synergistically with CD4+ TFH cells (rather than through independent stimulation of B cells) to enhance antibody production.
It remains elusive whether antigen immunogenicity, genetic, and/or acquired immune conditions or immune locales play a key role in driving CXCR5+CD8+ T cell subsets’ differentiation, effector function, and capacity to influence antibody production. Phenotypic markers, cytokines, effector molecules, and transcription factors characterizing CXCR5+CD8+ T cell subsets investigated in specific models are shown in Table 1. Most CXCR5+CD8+ T cell subsets that are reported to enhance humoral immunity or regulate viral, tumor, and autoimmunity express some similar transcription factors, co-inhibitory molecules, co-stimulation molecules, cytokines, and effector molecules. In contrast, antibody-suppressor CXCR5+CD8+ T cells identified in transplantation models are distinguished from all of these other subsets by the absence of the co-inhibitory molecule PD-1, absence of the co-stimulatory molecules ICOS/ICOSL, and lack of production of IL-10 or IL-21 (43). Furthermore, antibody-suppressor CXCR5+CD8+ T cells are distinguished from CD8+ T regulatory cells (44–49) by their expression of IFN-γ, perforin/granzyme, and CD28 and the lack of expression of PD-1, IL-10, and the transcription factor FoxP3 (Table 1). Further investigation of CXCR5+CD8+ T cells with complex immunophenotyping may improve the categorization of these heterogeneous subsets, clarify mechanisms of action, and implicate lineage relationships.
Table 1.
Phenotype of CXCR5+CD8+ T cell Subsets by Function
| CD8+ T cell subsets | ||||||||
|---|---|---|---|---|---|---|---|---|
| CXCR5+CD8+ T cells Evidence for Antibody Regulation |
CXCR5+CD8+ T Cells Antibody Regulation Not Reported |
CD8+ T Regulatory cells (for reference) |
||||||
| Effector Function | Antibody-enhancer** | Antibody-Suppression | Anti-viral | Anti-tumor | CD8+ T regs | |||
| Qa-1-restricted (Anti-autoimmune) |
Follicular regulatory (Anti-Viral immunity) |
Antibody-suppressor (Transplant Alloimmunity) |
||||||
| Transcription factors | Bcl-6 | (30, 31, 34–37) | nr | nr | * | (9, 12, 17, 20, 73) | (74) | nr |
| Tbet | (30, 35) | nr | nr | * | (19) | nr | nr | |
| Tcf1 | (30) | nr | nr | * | (19) | nr | nr | |
| Eomes | (30, 38) | (59) | nr | * | (19) | (74) | (44) | |
| FoxP3 | nr | (58) | nr | (51) | nr | nr | (44, 45) | |
| Co-inhibitory molecules | PD-1 | (23, 31, 33–35) | (58) | nr | (51) | (8, 12, 18, 19, 66, 73, 75) | (24, 74, 76) | (46) |
| Tim-3 | (33) | nr | (55) | * | (9, 18, 19) | (24) | nr | |
| CTLA-4 | (33) | (58) | nr | * | (17) | (76) | (44, 45) | |
| Co-stimulatory molecules | CD27 | (32, 36) | nr | nr | * | nr | (74, 76) | nr |
| CD28 | (32, 38) | nr | nr | * | (73) | (74) | (47, 48) | |
| CD40L | (31, 36) | nr | nr | * | (12) | nr | nr | |
| ICOS | (23, 31, 32, 34, 35) | nr | nr | * | (9, 20, 73) | nr | (45) | |
| ICOSL | nr | (58) | nr | (51) | (9) | nr | nr | |
| Cytokines | IFN-γ | (30, 32, 33, 35–37, 39) | nr | nr | (51, 53, 54) | (12, 18, 66) | (22, 76, 77) | (49) |
| IL-10 | (39) | (57) | (55) | (51) | (17, 66) | (77) | (46) | |
| IL-21 | (31, 33–36, 39) | nr | nr | * | (12) | nr | nr | |
| Effector molecules | Perf | (32, 34, 36) | (58) | (55) | (52) | (18) | (76, 78) | (45) |
| Gzmb | (34, 36) | nr | nr | * | (18) | (22, 24, 76, 78) | (45) | |
| Chemokine Receptor | CXCR5 | (30, 31, 34–37) | (58) | (55) | (51, 53) | (9, 12, 17, 19, 20, 73) | (20, 23, 24, 74, 76) | nr |
Red highlight = Positive expression; Green highlight = Not expressed.
Unpublished data; nr = Not reported
CXCR5+CD8+ T cells enhance antibody in models of viral infection, autoimmunity, and tumor
CXCR5+CD8+ T Cells that Mediate Downregulation of Antibody Production
In contrast to the literature supporting an antibody enhancing function of CXCR5+CD8+ T cells, our research group has identified a novel subset of antibody-suppressor CXCR5+IFN-γ+CD8+ T cells (CD8+ TAb-supp cells) using in vivo transplant experimental murine models. We have reported that this subset suppresses in vivo alloantibody production 5- and 10-fold following allogeneic kidney (50) and hepatocyte transplant (51), respectively. This reduction in alloantibody formation is associated with significant enhancement of graft survival following CD8+ TAb-supp adoptive cellular therapy in both kidney (15 days to 52 days) (50) and hepatocyte (14 days to 35 days) (51) transplant recipients using models in which graft loss occurs by antibody-mediated rejection. Alloantibody suppression is mediated, in part, by CD8-mediated clearance of antibody-producing B cells through both FasL and perforin mechanisms (52). This cytotoxic clearance is antigen-specific, as CD8+ TAb-supp cells do not kill naïve or third-party primed IgG+ B cells in vitro or in vivo (51, 52). Recently, our group has identified CXCR5+CD8+ T cells in the peripheral blood of human kidney transplant recipients. The quantity of both CXCR5+CD8+ and CXCR5+IFN-γ+CD8+ T cells inversely associates with the incidence of de novo donor-specific antibody (53), suggesting a human correlate of murine CD8+ TAb-supp cells. In experimental murine models, we found that IFN-γ is critical to antibody-suppressor CD8+ T cell function (54), since CD8+ T cells isolated from IFN-γ KO mice do not develop antibody-suppressor function. Approximately 70% of CD44+CXCR5+CD8+ T cells in both murine (51) and human (53) transplant recipients express IFN-γ. The CD8+ TAb-supp cells that our group has investigated lack expression of PD-1, ICOS/ICOSL, and CD8+ T regulatory cell (CD8+ TReg) markers IL-10, CD103, and FoxP3 (51), and thus represent a subset of CXCR5+CD8+ T cells that are distinct from CD8+ TReg cells (44–49) as well as PD-1+CXCR5+CD8+ T cell subsets with, antiviral, anti-tumor, anti-autoimmune (Qa-1 restricted) and antibody-enhancing functions (Table 1). Furthermore, while antibody suppressor CXCR5+CD8+ T cells are antigen-specific, the antigen specificity of the CXCR5+PD-1hiCD8+ T cells that enhance antibody production has not been tested. Collectively, these findings raise the prospect of further refining and developing this CD8+ T cell subset as a cellular therapy for the prevention or treatment of antibody mediated rejection.
Another subset with antibody suppressive function, identified as CD3+CD8+CXCR5hiCD44hi T “follicular regulatory” (TFR) cells, has been reported to inhibit ex vivo CD4+ TFH IL-21 production and GC B cell (CPG-TLR9 stimulated CD19+CD38+) IgG production in the setting of HIV infection (55). These CD8+ TFR cells exhibit enhanced expression of IL-10, as well as Tim-3, CD122, and GITR, but reduced expression of perforin compared to conventional CD8+ T cells. Many other markers shown in Table 1 have not been reported for this subset. CD8+ TFR cell suppression of CD4+ TFH IL-21 production in culture was Tim-3 dependent, since addition of anti-Tim3 antibody abrogated the effect. Virus-primed tonsillar CD8+ TFR cells reduced IgG production in co-culture greater than 3-fold when compared to IgG production by virus infected GC B cells alone (55). However, the exact mechanism of human CXCR5+CD8+ T cell-mediated inhibition of antibody production in these studies, whether via cytokine-facilitated suppression or direct cytotoxicity to B cells, was not explored. CD8+ TFR cells are distinguished from CD8+ TAb-supp cells by expression of IL-10.
An additional subset of antibody downregulating CXCR5+CD8+ T cells has been identified in the setting of murine autoimmunity models and is characterized by the triad of CD44, CD122, and Ly49 (a MHC-I inhibitory receptor) surface marker expression (56). These cells express ICOSL, PD-1, and are restricted to interaction with Qa-1+CD4+ T cells and protect against autoimmunity (many other markers shown in Table 1 have not been reported for this subset). Qa-1, a murine homolog of HLA-E, is expressed on activated T and B lymphocytes and dendritic cells, and promotes expansion of antigen-specific CD8+ T cells when bound to their TCR (57). Adoptive transfer of Qa-1-restricted effector CD8+ T cells (CD44+CD122+Ly49+) into Rag2−/− hosts reconstituted with Qa-1+ B and Qa-1+CD4+ T cells inhibits primary autoantibody responses by 10-fold (58). In the setting of rheumatoid arthritis, cellular therapy with Qa-1-restricted effector CD8+ T cells resulted in clearance of autoreactive CD4+Qa-1+ TFH cells, suppression of downstream autoantibody, and amelioration of disease progression (as evidenced by assessment score of limb swelling and erythema) (56). Recently, CXCR5+CD44+CD122+Ly49+CD8+ Tregs were reported to mediate key protection against lethal autoimmunity that is distinct from CD4+ TReg cells (59). In this study, genetic deletion of TGF-β receptor (TGF-βR) and the transcription factor Eomesodermin (Eomes) resulted in enhanced spontaneous GCs in the spleen, increased percentage of GC B cells, increased CD4+ TFH cells, increased anti-double stranded DNA autoantibodies, and reduced overall survival. These double knockout (DKO) mice had unaltered CD4+ TFR cells but significantly decreased quantity of CXCR5+CD44+CD122+Ly49+CD8+Helios+ Tregs, and only adoptive transfer of the latter CD8+ Treg population could rescue the autoimmune phenotype. CD122hiLy49hiCD8+ T cells from DKO mice displayed increased effector molecules and markers such as granzyme A, granzyme B, and KLRG-1, consistent with their lower expression of the transcription factor Helios that is associated with TReg identity. Furthermore, these authors reported that while TGF-βR is critical for expression of the transcription factor Helios and CD8+ TReg development, the transcription factor Eomes regulates the expression of CXCR5 critical for follicular localization and GC regulation. However, both TGF-βR and Eomes contribute to expression of pro-survival Bcl-2 and in vivo CD8+ TReg cell survival and homeostasis. Interestingly, Choi et al reported that host mice with a mutation impairing Qa1 interaction with the CD8+ T cell receptor (60), resulted in robust alloantibody response after full MHC disparate heart transplant and accelerated rejection despite peri-transplant treatment with CTLA-4 Ig, owing to disruption of Qa-1 restricted CD8+ TReg cell inhibition of CD4+ TFH expansion (61). In contrast, CD8+ TAb-supp cells that also significantly suppress alloantibody production after transplant express KLRG-1 (associated with effector cell function) and mediate antigen specific antibody suppression, features that distinguish them from KLRG-1-negative Qa-1-restricted CD8+ T follicular regulatory cells (CD8+ TFR) that downregulate excessive or dysregulated Qa1+-expressing CD4+ TFH cells (59). Furthermore, while these Qa-1-restricted CD8+ TFR have been reported to eliminate target Qa-1+CD4+ TFH cells in a perforin-dependent manner, their inhibitory effect on antibody production does not occur through direct interaction with B cells. Thus, while Qa-1-restricted CD8+ TFR have capacity to inhibit antibody production, their exclusive targeting of CD4+ TFH cells is decidedly different from B cell helper CXCR5+PD-1hiCD8+ T cells and antibody-suppressor CXCR5+PD-1−CD8+ T cells that exert direct effects on B cells (Figure 1).
Figure 1. Distinct Mechanisms by which CXCR5+CD8+ T Cell Subsets Impact Humoral Immunity.
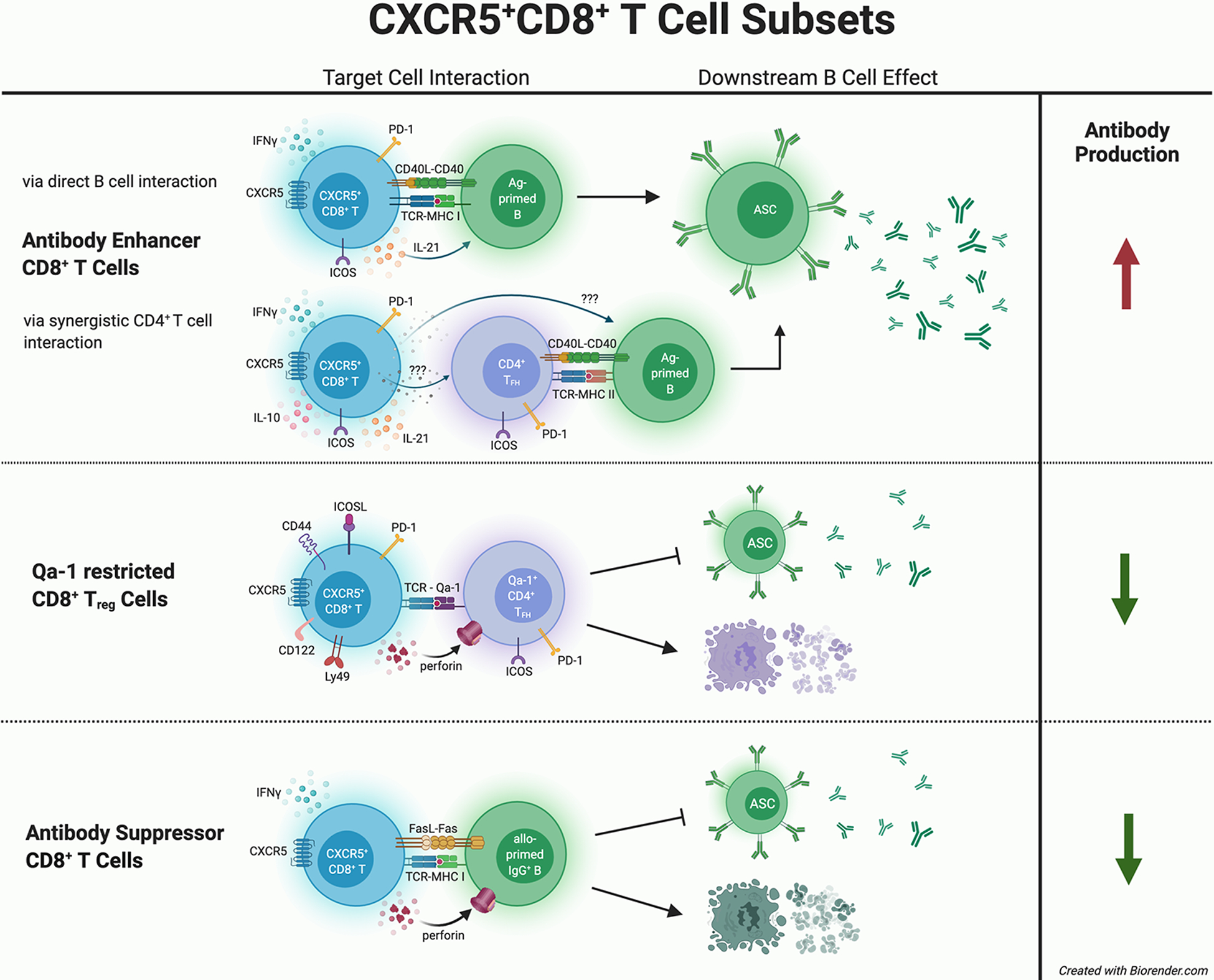
CXCR5+CD8+ T cell subsets are reported to mediate different effects upon antibody production. Antibody-enhancing CXCR5+CD8+ T cells express PD-1, ICOS, CD40L, and cytokines such as IFN-γ and IL-21. In vitro co-culture studies suggest that antibody-enhancing CXCR5+CD8+ T cells interact directly with antibody-producing B cells, or synergistically with CD4+ TFH cells, to increase antibody production by antibody secreting cells (ASC). In contrast, one subset of CXCR5+CD8+ T cells that protects against autoimmunity is Qa-1 restricted (CD44+ICOSL+PD-1+CD122+Ly49+) and exerts cytotoxic killing of autoreactive CD4+ TFH cells resulting in decreased autoantibody production. Another subset of antibody-suppressor CXCR5+CD8+ T cells (CD44+ICOSL−PD-1−IFN-γ+) is MHC-I restricted, antigen-specific, and directly mediates killing of allo-primed IgG+ B cells resulting in reduction of alloantibody production after transplant.
Clinical Correlations of Human CXCR5+CD8+ T Cells
Given the identification of CXCR5+CD8+ T cells in a wide array of both clinical diseases and murine models, their potential to serve as indicators of disease severity and/or as useful predictive tools warrants consideration. Table 2 provides a comprehensive listing of all reports of human CXCR5+CD8+ T cells and their clinical correlations in patients with viral infection, bacterial infection, malignancy, autoimmune disease or after kidney transplantation. Owing to their unique CD8+ T cell follicular homing capabilities, several groups have noted a direct correlation between the quantity of CXCR5+CD8+ T cells with the suppression of viral load for viruses known to sequester in lymphoid follicles, such as HIV and SIV (8, 14, 18, 33, 62, 63). CXCR5+CD8+ T cell quantity is increased in HIV infected patients compared to healthy controls and their frequency is inversely correlated with HIV viral load (8). In addition, CXCR5+CD8+ T cell quantity has been introduced as an indicator of disease severity since among HIV infected patients, those with lower frequencies of circulating CXCR5+CD8+ T cells also have lower CD4+ T cell counts (8). None of the aforementioned studies reported concurrent anti-viral antibody titers. However, Roider et al. identified a significant positive correlation between the frequency of circulating CXCR5+CD8+ T cells and HIV neutralization by plasma from HIV infected and antiretroviral therapy naïve children who reportedly have higher titers of broadly neutralizing antibody compared to adults (64). Perinatally infected HIV-positive children exhibit significantly lower CXCR5+CD8+T cell frequencies compared to HIV-negative unexposed children (65).
Table 2.
Clinical Correlations and Phenotype of Human CXCR5+CD8+ T cell subsets
| Clinical Condition/Number of Patients (n) | CXCR5+CD8+ T cell phenotype | CXCR5+CD8+ T cell Clinical Correlation | Reference | |
|---|---|---|---|---|
| Anti-viral | HIV (n=36) |
IL-2+IFN-γ+IL-17+ | High circulating CXCR5+CD8+ T cell quantity correlates with HIV neutralization by plasma from HIV-infected children | (64) |
| HIV (n=115) |
CCR7−/loCD62LhiIL-21hi TCF1hiBCL6hiBLIMP1− | High circulating CXCR5+CD8+ T cell quantity correlates with reduced HIV viral load | (63) | |
| HIV (n=101) |
PD-1+ | High circulating HIV-specific CXCR5+CD8+ T cell quantity correlates with reduced HIV viral load | (8) | |
| HIV (n=14) |
PD-1+CD44+KLRG1+ IFN-γ+CD107a+ | High circulating HIV-specific CXCR5+CD8+ T cell quantity correlates with reduced HIV viral load | (18) | |
| Dengue Virus 2 (n=57) |
PD-1+ | High circulating anti-viral CXCR5+CD8+ T cell quantity correlates with reduced viral load and protection from associated kidney injury | (66) | |
| Bacterial Infection | Bacterial Pneumonia (n=65) |
PD-1+CD103lo | High circulating CXCR5+CD8+ T cell quantity correlates with exacerbation of pneumonia severity | (67) |
| Anti-tumor | Colorectal cancer (n=44) |
PD1hiTim3hiLAG3hi IFN-γhiTNF-αhiIL-2hi PRF1hiGZMBhi | High CXCR5+CD8+ T cell quantity in tumors or tumor draining lymph nodes correlates with increased cancer-free survival times | (22) |
| HCC (n=40) |
PD-1hiICOShi IL-21hi | High tumor-infiltrating CXCR5+CD8+ T cell quantity negatively correlates with microvascular invasion, TNM staging and early recurrence | (23) | |
| Pancreatic cancer (n=12) |
PD-1+TIM-3+ | High tumor-infiltrating and circulating CXCR5+CD8+ T cell quantity correlates with disease-free survival after tumor resection | (68) | |
| Bladder cancer (n=11, fresh tissue for flow cytometry; n=249 bioinformatics gene signature) |
PD-1+CTLA-4+ CD62L+LAG3+ IFN-γ+GZMB+ TNF-α+CD107a+ | High tumor-infiltrating CXCR5+CD8+ T cell quantity correlates with lower tumor stage and prolonged overall survival and cancer-free survival | (69) | |
| Follicular (Germinal Center B cell) lymphoma (n=187, bioinformatics gene expression) |
PD-1+IFN-γ+TNF-α+ | High tumor expression of CXCR5+CD8+ T cell signature genes correlates with prolonged survival | (70) | |
| Autoimmunity | SLE (n=79) |
CD158e+ (Inhibitory, Killer cell Ig-like receptor, KIR+) | Reduced quantity of circulating CD158e+CD8+ T cells in SLE patients compared to healthy controls; KIR+CD8+ T cells potential human counterpart to murine Ly49+ Qa-1-restricted CD122hiCD8+ T cell related to protection from autoimmunity | (59) |
| Transplantation | Kidney Transplant (n=95) |
CD44+CD62L−IFN-γ+ | High circulating CXCR5+CD8+ T cell quantity correlates with lower incidence of de novo donor specific antibody production and improved clinical outcomes at 1 year post transplant | (53) |
Qiu et al. noted, in a study of 36 patients infected with Dengue virus 2 (DENV2), that higher quantity of peripheral blood PD-1+CXCR5+CD8+ T cells correlated with decreased viral load at time of diagnosis. In addition, their quantity was protective for virally mediated kidney injury (a common complication of DENV2 infection), as patients without kidney injury exhibited significantly higher quantities of CXCR5+CD8+ T cells compared to those with kidney injury (66). These results were not correlated with anti-viral antibody responses.
In a study of 65 patients hospitalized for bacterial pneumonia, Shen et al. found that quantity of peripheral blood CXCR5+CD8+ T cells correlated with the severity of community-acquired and hospital-acquired pneumonia, noting a nearly 3-fold or greater increase in circulating CXCR5+CD8+ T cells during community-acquired and hospital-acquired pneumonia exacerbations, respectively, compared to patients with controlled disease. Furthermore, in assessing the diagnostic correlation associated with monitoring of these cells, the AUC for the ratio of CXCR5+CD8+ T/CD8+ T cells was determined to be 0.944 (Sensitivity = 90.5%; Specificity = 93%), leading the authors to suggest that CXCR5-expressing CD8+ T cell quantity alone can predict exacerbation of community- or hospital-acquired pneumonia (67). This direct correlation with disease severity suggests a deleterious pro-inflammatory immunity or an inhibitory impact of these cells on protective immunity. However, the correlation of CXCR5+CD8+ T cell quantity with the strength of humoral immunity to specific pathogens was not assessed in these studies.
The prevalence and phenotypic characteristics of CXCR5+CD8+ T cell subsets also correlate with patient outcomes in non-viral disease states, such as with malignancy. In patients with hepatocellular carcinoma (HCC), a high percentage of tumor-infiltrating CXCR5+CD8+ T cells correlated with a lower risk of early recurrence (within three years) and metastasis (23). Interestingly, the authors of this study also noted a co-localization of IL-21+CXCR5+CD8+ T cells and CD19+ B cells in some patients and a positive correlation between the frequency of tumor infiltrating CXCR5+CD8+ T cells and tumor-infiltrating CD19+ B cells. Furthermore, co-culture of tumor-infiltrating CXCR5+CD8+ T cells with autologous CD19+ B cells resulted in significantly higher in vitro IgG production than co-culture of CXCR5+CD8+ T cells and B cells from healthy donors. The frequency of tumor-infiltrating CD138+ plasmablasts in these HCC patients also positively correlated with overall and disease-free survival. However, these authors did not investigate tumor-antigen specific antibody production in these patients.
In a study of twelve patients with pancreatic cancer, high quantities of tumor-infiltrating CXCR5+CD8+ T cells (at the time of tumor resection) correlated with prolonged disease free survival time (68). CXCR5+CD8+ T cells are a small population of total CD8+ T cells in the peripheral blood of healthy controls (0.4–5.0%). However, in pancreatic cancer patients, circulating CXCR5+CD8+ T cells were significantly increased and comprised 7–21% of total peripheral CD8+ T cells. Furthermore, CXCR5+CD8+ T cells comprised more than 50% of total CD8+ T cells in pancreatic tumor specimens. CXCR5+CD8+ T cells in this study were PD-1+Tim-3+ and mediated higher in vitro cytotoxicity to target cells compared to CXCR5−CD8+ T cells. Interestingly, these investigators utilized autologous human B cells as targets in these in vitro cytotoxicity assays which is analogous to the CD8:B cell cytotoxicity assays used in our murine studies to investigate CD8+Ab-supp T cell mediated B cell killing.
In patients with bladder cancer, high quantities of tumor-infiltrating CXCR5+CD8+ T cells also correlated with higher overall and disease-free survival (69). In another study involving 44 patients with Stage II colorectal cancer, patients with high quantity of CXCR5+CD8+ T cells in tumors or tumor-draining lymph node and patients with high IFN-γ, perforin, and granzyme B expression by tumor CXCR5+CD8+ T cells exhibited significantly longer disease-free survival (22). Taken together, these studies highlight an important positive correlation between quantity of CXCR5+CD8+ T cells detected in tumors and/or tumor draining lymph nodes with enhanced tumor immunity and raises the potential for including quantitative assessment of tumor-infiltration by CXCR5+CD8+ T cells as an additional prognostic tool for some malignancies. More studies are warranted to investigate the cellular and humoral immune mechanism(s) associated with these improved clinical outcomes.
One study investigated CXCR5+CD8+ T cells in human follicular lymphoma, a non-Hodgkin lymphoma that arises from malignant GC B cells (70). The authors found that CXCR5+CD8+ T cells were more abundant in follicular lymphoma compared to normal human tonsillar tissue. Co-culture of autologous flow-sorted CXCR5+CD8+ T cells (expanded in vitro with α−CD3/CD28, TGF-β and IL-23) with autologous follicular lymphoma tumor cells (activated with soluble CD40L and IL-4) revealed in vitro tumor killing. They also determined that autologous CXCR5+CD8+ T cells suppressed CD4+ TFH mediated differentiation of CD38+CD19+ plasmablasts (stimulated with staphylococcal enterotoxin B) in a cell contact independent mechanism. Thus, while this study focused on CXCR5+CD8+ T cell-mediated cytotoxic killing of tumor cells, these tumor cells were malignant GC B cells, and is consistent with the CD8+ TAb-supp cells that our group has reported has the functional capacity to kill alloprimed B cells and reduce alloantibody production after transplant in mice (51).
A recent study reported the critical roles of TFG-β receptor and transcription factor Eomes on development, homeostasis and survival of Ly49+CD122hiCXCR5+CD8+ Tregs that control GC reactions and mediate protection against lethal autoimmunity in mice. These authors also analyzed peripheral blood mononuclear cells (PBMCs) from patients with the autoimmune disease systemic lupus erythematosus (SLE) and found that SLE patients compared to healthy controls had reduced quantity of CD158e+CD8+ T cells with reduced expression of Helios, a key Treg associated transcription actor. Corresponding autoantibody titers in these SLE patients were not reported (59). The CD158 gene family encodes killer cell immunoglobulin-like receptors (KIR). In these studies, CD158e+CD8+ T cells were analyzed based on their presumed similarity to human CD122hiKIR+Eomes+CD8+ T cells described in other studies (71) and resemblance to murine anti-autoimmune Qa-1-restricted Ly49+CD122hiCD8+ T cells.
In a prospective study of 95 first-time kidney transplant recipients, our group demonstrated an inverse association between the quantity of peripheral blood CXCR5+CD8+ T cells (and CXCR5+IFN-γ+CD8+ T cells) and risk for development of de novo donor-specific antibody (DSA) after transplant. Transplant recipients who developed de novo DSA exhibited 2-fold lower quantity of CXCR5+CD8+ T cells (and CXCR5+IFN-γ+CD8+ T cells) both pre-transplant as well as post-transplant compared to recipients who did not develop DSA. Furthermore, pre-transplant quantity of CXCR5+IFN-γ+CD8+ T cells less than 3,300 per million peripheral blood mononuclear cells was highly associated with the development of de novo DSA [Area Under the Curve (AUC) = 0.81; Sensitivity = 93%; Specificity = 62%] (53). This pre-transplant immune assessment suggests that prospective kidney transplant recipients could be stratified into groups that are low or high risk for de novo DSA production. This is the only prospective study reporting a direct correlation between the quantity of human CXCR5+CD8+ T cells with in vivo antibody responses.
While the clinical utility of monitoring CXCR5+CD8+ T cells is promising, several potential barriers exist to their routine use in assessing clinical disease or for their development as a cellular therapy. First, the relatively low percentage of CXCR5+CD8+ T cells, comprising 1.9% of all peripheral blood mononuclear cells (PBMC) in healthy individuals (32) and ranging from less than 1% of all PBMC (22, 37) to 5% of CD3+ T cells even in active disease (68, 72) renders the isolation, consistent quantification, and characterization of these cells challenging. Second, although expression of CXCR5 differentiates a subset of effector CD8+ T cells in various models and diseases, its expression in peripheral blood lymphocytes may fluctuate over time as CXCR5+CD8+ T cells traffic in and out of the circulation from their functional niches in lymphoid follicles and other sites. More research is needed to understand how the quantity of peripheral blood CXCR5+CD8+ T cells correlates with their quantity and differentiated phenotype in lymphoid depots or other tissues. Third, the marked phenotypic heterogeneity of CXCR5+CD8+ T cell subsets, as highlighted in this review, further complicates the categorization and separation of disease related CXCR5+CD8+ T cell subsets by effector (including regulatory) functions. Finally, factors that contribute to expansion of the various CXCR5+CD8+ T cell subsets (in vivo or in vitro) require further investigation. Despite these and other challenges ahead, significant potential exists to enhance existing knowledge and to develop the prognostic and therapeutic potential of CXCR5+CD8+ T cells. Promising results from murine models in which CXCR5+CD8+ T cells tested as a cellular therapy reduced autoantibody (56, 58) or alloantibody (50, 51) levels and associated antibody-mediated tissue damage encourage the clinical translation of these studies. This review highlights the need for further studies to elucidate the biology of this newly emerging subset including its role in modulating antibody responses in a variety of clinical conditions, including transplantation.
Conclusions:
Emerging interest in immune properties of CXCR5+CD8+ T cells has led to investigation of their role in a variety of experimental and clinical settings in the past few years. These cells, owing to their follicular-homing capabilities unique amongst known CD8+ T cell subsets, participate in a wide variety of functions in lymphoid-associated viral, tumor, auto- and allo-immune disease processes. CXCR5+CD8+ T cell subsets in some studies have been reported to enhance antibody production, while in others have been reported to downregulate antibody production. It is likely then that phenotypic and functional differentiation of distinct CXCR5+CD8+ T cell subsets account for these differences. It remains to be determined how the diverse phenotypes and effector functions reported to date for CXCR5+CD8+ T cells correlate with their lineage, differentiation, and plasticity. Knowledge gained from these future studies is critical to refine the application of CXCR5+CD8+ T cell subsets for potential diagnostic, prognostic, and therapeutic purposes.
Acknowledgements:
BioRender was used to generate Figure 1.
Support:
This work was supported by The Ohio State University Department of Surgery, Division of Transplant Surgery, Comprehensive Transplant Center, The Ohio State University College of Medicine, and the National Institutes of Health grant AI139913. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Nonstandard Abbreviations:
- Ab
Antibody
- DSA
Donor specific antibody
- GC
Germinal center
- Tc1
Cytotoxic type 1 CD8+ T cell
- TFH
Follicular helper CD4+ T cell
- TFR
Follicular regulatory CD8+ T cell
Footnotes
Disclosure: The authors have no financial conflicts of interest.
References:
- 1.Amancha PK, Ackerley CG, Duphare C, Lee M, Hu YJ, Amara RR, and Kelley CF. 2019. Distribution of Functional CD4 and CD8 T cell Subsets in Blood and Rectal Mucosal Tissues. Sci. Rep 9: 6951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang SY, Song JH, Guleng B, Cotoner CA, Arihiro S, Zhao Y, Chiang HS, O’Keeffe M, Liao G, Karp CL, Kweon MN, Sharpe AH, Bhan A, Terhorst C, and Reinecker HC. 2013. Circulatory antigen processing by mucosal dendritic cells controls CD8(+) T cell activation. Immunity. 38: 153–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Visekruna A, Ritter J, Scholz T, Campos L, Guralnik A, Poncette L, Raifer H, Hagner S, Garn H, Staudt V, Bopp T, Reuter S, Taube C, Loser K, and Huber M. 2013. Tc9 cells, a new subset of CD8(+) T cells, support Th2-mediated airway inflammation. Eur. J. Immunol 43: 606–618. [DOI] [PubMed] [Google Scholar]
- 4.Lu Y, Hong B, Li H, Zheng Y, Zhang M, Wang S, Qian J, and Yi Q. 2014. Tumor-specific IL-9-producing CD8+ Tc9 cells are superior effector than type-I cytotoxic Tc1 cells for adoptive immunotherapy of cancers. Proc. Natl. Acad. Sci. USA 111: 2265–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mittrucker HW, Visekruna A, and Huber M. 2014. Heterogeneity in the differentiation and function of CD8(+) T cells. Arch. Immunol. Ther. Exp. (Warsz) 62: 449–458. [DOI] [PubMed] [Google Scholar]
- 6.Annunziato F, Romagnani C, and Romagnani S. 2015. The 3 major types of innate and adaptive cell-mediated effector immunity. J. Allergy Clin. Immunol 135: 626–635. [DOI] [PubMed] [Google Scholar]
- 7.Fousteri G, and Kuka M. 2020. The elusive identity of CXCR5(+) CD8 T cells in viral infection and autoimmunity: Cytotoxic, regulatory, or helper cells? Mol. Immunol 119: 101–105. [DOI] [PubMed] [Google Scholar]
- 8.Jiao YM, Yang HG, Huang HH, Tu B, Xing SJ, Mao L, Xia W, He R, Zhang JY, Xu RN, Jin L, Shi M, Xu Z, Qin EQ, Wang XC, Wu H, Ye L, and Wang FS. 2017. Dichotomous Roles of Programmed Cell Death 1 on HIV-Specific CXCR5(+) and CXCR5(–) CD8(+) T Cells during Chronic HIV Infection. Front. Immunol 8: 1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leong YA, Chen Y, Ong HS, Wu D, Man K, Deleage C, Minnich M, Meckiff BJ, Wei Y, Hou Z, Zotos D, Fenix KA, Atnerkar A, Preston S, Chipman JG, Beilman GJ, Allison CC, Sun L, Wang P, Xu J, Toe JG, Lu HK, Tao Y, Palendira U, Dent AL, Landay AL, Pellegrini M, Comerford I, McColl SR, Schacker TW, Long HM, Estes JD, Busslinger M, Belz GT, Lewin SR, Kallies A, and Yu D. 2016. CXCR5(+) follicular cytotoxic T cells control viral infection in B cell follicles. Nat. Immunol 17: 1187–1196. [DOI] [PubMed] [Google Scholar]
- 10.Yang HG, Jiao YM, Huang HH, Zhang C, Zhang JY, Xu RN, Song JW, Fan X, Jin L, Shi M, and Wang FS. 2020. Transforming growth factor-beta promotes the function of HIV-specific CXCR5(+) CD8 T cells. Microbiol. Immunol 64: 458–468. [DOI] [PubMed] [Google Scholar]
- 11.Velu V, Mylvaganam G, Ibegbu C, and Amara RR. 2018. Tfh1 Cells in Germinal Centers During Chronic HIV/SIV Infection. Front. Immunol 9: 1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perdomo-Celis F, Feria MG, Taborda NA, and Rugeles MT. 2019. Induction of Follicular-Like CXCR5(+) CD8(+) T Cells by TGF-beta1/IL-23 Is Limited During HIV Infection. Viral Immunol. 32: 278–288. [DOI] [PubMed] [Google Scholar]
- 13.Reuter MA, Del Rio Estrada PM, Buggert M, Petrovas C, Ferrando-Martinez S, Nguyen S, Sada Japp A, Ablanedo-Terrazas Y, Rivero-Arrieta A, Kuri-Cervantes L, Gunzelman HM, Gostick E, Price DA, Koup RA, Naji A, Canaday DH, Reyes-Teran G, and Betts MR. 2017. HIV-Specific CD8(+) T Cells Exhibit Reduced and Differentially Regulated Cytolytic Activity in Lymphoid Tissue. Cell Rep. 21: 3458–3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Starke CE, Vinton CL, Ladell K, McLaren JE, Ortiz AM, Mudd JC, Flynn JK, Lai SH, Wu F, Hirsch VM, Darko S, Douek DC, Price DA, and Brenchley JM. 2020. SIV-specific CD8+ T cells are clonotypically distinct across lymphoid and mucosal tissues. J. Clin. Invest 130: 789–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiao M, Chen X, He R, and Ye L. 2018. Differentiation and Function of Follicular CD8 T Cells During Human Immunodeficiency Virus Infection. Front. Immunol 9: 1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mylvaganam GH, Chea LS, Tharp GK, Hicks S, Velu V, Iyer SS, Deleage C, Estes JD, Bosinger SE, Freeman GJ, Ahmed R, and Amara RR. 2018. Combination anti-PD-1 and antiretroviral therapy provides therapeutic benefit against SIV. JCI Insight 3: e122940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mylvaganam GH, Rios D, Abdelaal HM, Iyer S, Tharp G, Mavigner M, Hicks S, Chahroudi A, Ahmed R, Bosinger SE, Williams IR, Skinner PJ, Velu V, and Amara RR. 2017. Dynamics of SIV-specific CXCR5+ CD8 T cells during chronic SIV infection. Proc. Natl. Acad. Sci. USA 114: 1976–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He R, Hou S, Liu C, Zhang A, Bai Q, Han M, Yang Y, Wei G, Shen T, Yang X, Xu L, Chen X, Hao Y, Wang P, Zhu C, Ou J, Liang H, Ni T, Zhang X, Zhou X, Deng K, Chen Y, Luo Y, Xu J, Qi H, Wu Y, and Ye L. 2016. Follicular CXCR5- expressing CD8(+) T cells curtail chronic viral infection. Nature. 537: 412–428. [DOI] [PubMed] [Google Scholar]
- 19.Im SJ, Konieczny BT, Hudson WH, Masopust D, and Ahmed R. 2020. PD-1+ stemlike CD8 T cells are resident in lymphoid tissues during persistent LCMV infection. Proc. Natl. Acad. Sci. USA 117: 4292–4299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang Z, Zak J, Pratumchai I, Shaabani N, Vartabedian VF, Nguyen N, Wu T, Xiao C, and Teijaro JR. 2019. IL-27 promotes the expansion of self-renewing CD8(+) T cells in persistent viral infection. J. Ex. Med 216: 1791–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lofroos AB, Kadivar M, Resic Lindehammer S, and Marsal J. 2017. Colorectal cancer-infiltrating T lymphocytes display a distinct chemokine receptor expression profile. Eur. J. Med. Res 22: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.E J, Yan F, Kang Z, Zhu L, Xing J, and Yu E. 2018. CD8(+)CXCR5(+) T cells in tumor-draining lymph nodes are highly activated and predict better prognosis in colorectal cancer. Human Immunol. 79: 446–452. [DOI] [PubMed] [Google Scholar]
- 23.Ye L, Li Y, Tang H, Liu W, Chen Y, Dai T, Liang R, Shi M, Yi S, Chen G, and Yang Y. 2019. CD8+CXCR5+T cells infiltrating hepatocellular carcinomas are activated and predictive of a better prognosis. Aging. (Albany NY) 11: 8879–8891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jin Y, Lang C, Tang J, Geng J, Song HK, Sun Z, and Wang J. 2017. CXCR5(+)CD8(+) T cells could induce the death of tumor cells in HBV-related hepatocellular carcinoma. Int. Immunopharmacol 53: 42–48. [DOI] [PubMed] [Google Scholar]
- 25.Perdomo-Celis F, Taborda NA, and Rugeles MT. 2017. Follicular CD8(+) T Cells: Origin, Function and Importance during HIV Infection. Front. Immunol 8: 1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu D, and Ye L. 2018. A Portrait of CXCR5(+) Follicular Cytotoxic CD8(+) T cells. Trends Immunol. 39: 965–979. [DOI] [PubMed] [Google Scholar]
- 27.Valentine KM, and Hoyer KK. 2019. CXCR5+ CD8 T Cells: Protective or Pathogenic? Front. Immunol 10: 1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Breitfeld D, Ohl L, Kremmer E, Ellwart J, Sallusto F, Lipp M, and Forster R. 2000. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J. Exp. Med 192: 1545–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schaerli P, Willimann K, Lang AB, Lipp M, Loetscher P, and Moser B. 2000. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J. Exp. Med 192: 1553–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shan Q, Zeng Z, Xing S, Li F, Hartwig SM, Gullicksrud JA, Kurup SP, Van Braeckel-Budimir N, Su Y, Martin MD, Varga SM, Taniuchi I, Harty JT, Peng W, Badovinac VP, and Xue HH. 2017. The transcription factor Runx3 guards cytotoxic CD8(+) effector T cells against deviation towards follicular helper T cell lineage. Nat. Immunol 18: 931–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen Y, Yu M, Zheng Y, Fu G, Xin G, Zhu W, Luo L, Burns R, Li QZ, Dent AL, Zhu N, Cui W, Malherbe L, Wen R, and Wang D. 2019. CXCR5(+)PD-1(+) follicular helper CD8 T cells control B cell tolerance. Nat. Commun 10: 4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quigley MF, Gonzalez VD, Granath A, Andersson J, and Sandberg JK. 2007. CXCR5+ CCR7- CD8 T cells are early effector memory cells that infiltrate tonsil B cell follicles. Eur. J. Immunol 37: 3352–3362. [DOI] [PubMed] [Google Scholar]
- 33.Li Y, Tang L, Guo L, Chen C, Gu S, Zhou Y, Ye G, Li X, Wang W, Liao X, Wang Y, Peng X, Liu G, Zhang X, Sun J, Peng J, and Hou J. 2020. CXCL13-mediated recruitment of intrahepatic CXCR5(+)CD8(+) T cells favors viral control in chronic HBV infection. J. Hepatol 72: 420–430. [DOI] [PubMed] [Google Scholar]
- 34.Le KS, Ame-Thomas P, Tarte K, Gondois-Rey F, Granjeaud S, Orlanducci F, Foucher ED, Broussais F, Bouabdallah R, Fest T, Leroux D, Yadavilli S, Mayes PA, Xerri L, and Olive D. 2018. CXCR5 and ICOS expression identifies a CD8 T-cell subset with TFH features in Hodgkin lymphomas. Blood. Adv 2: 1889–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xiao L, Jia L, Bai L, He L, Yang B, Wu C, and Li H. 2016. Phenotypic and functional characteristics of IL-21-expressing CD8(+) T cells in human nasal polyps. Sci. Rep 6: 30362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shen J, Luo X, Wu Q, Huang J, Xiao G, Wang L, Yang B, Li H, and Wu C. 2018. A Subset of CXCR5(+)CD8(+) T Cells in the Germinal Centers From Human Tonsils and Lymph Nodes Help B Cells Produce Immunoglobulins. Front. Immunol 9: 2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xing J, Zhang C, Yang X, Wang S, Wang Z, Li X, and Yu E. 2017. CXCR5(+)CD8(+) T cells infiltrate the colorectal tumors and nearby lymph nodes, and are associated with enhanced IgG response in B cells. Exp. Cell Res 356: 57–63. [DOI] [PubMed] [Google Scholar]
- 38.Valentine KM, Davini D, Lawrence TJ, Mullins GN, Manansala M, Al-Kuhlani M, Pinney JM, Davis JK, Beaudin AE, Sindi SS, Gravano DM, and Hoyer KK. 2018. CD8 Follicular T Cells Promote B Cell Antibody Class Switch in Autoimmune Disease. J. Immunol 201: 31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiang H, Li L, Han J, Sun Z, Rong Y, and Jin Y. 2017. CXCR5(+) CD8(+) T Cells Indirectly Offer B Cell Help and Are Inversely Correlated with Viral Load in Chronic Hepatitis B Infection. DNA Cell biol. 36: 321–327. [DOI] [PubMed] [Google Scholar]
- 40.Agematsu K, Kobata T, Yang FC, Nakazawa T, Fukushima K, Kitahara M, Mori T, Sugita K, Morimoto C, and Komiyama A. 1995. CD27/CD70 interaction directly drives B cell IgG and IgM synthesis. Eur. J. Immunol 25: 2825–2829. [DOI] [PubMed] [Google Scholar]
- 41.Stuber E, and Strober W. 1996. The T cell-B cell interaction via OX40-OX40L is necessary for the T cell-dependent humoral immune response. J. Exp. Med 183: 979–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wikenheiser DJ, and Stumhofer JS. 2016. ICOS Co-Stimulation: Friend or Foe? Front. Immunol 7: 304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu Y, Ma X, Gong R, Zhu J, Wei L, and Yao J. 2018. Recent advances in CD8(+) regulatory T cell research. Oncol. Letters 15: 8187–8194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Agle K, Vincent BG, Piper C, Belle L, Zhou V, Shlomchik W, Serody JS, and Drobyski WR. 2018. Bim regulates the survival and suppressive capability of CD8(+) FOXP3(+) regulatory T cells during murine GVHD. Blood. 132: 435–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Churlaud G, Pitoiset F, Jebbawi F, Lorenzon R, Bellier B, Rosenzwajg M, and Klatzmann D. 2015. Human and Mouse CD8(+)CD25(+)FOXP3(+) Regulatory T Cells at Steady State and during Interleukin-2 Therapy. Front. Immunol 6: 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Elizondo DM, Andargie TE, Haddock NL, da Silva RLL, de Moura TR, and Lipscomb MW. 2019. IL-10 producing CD8(+) CD122(+) PD-1(+) regulatory T cells are expanded by dendritic cells silenced for Allograft Inflammatory Factor-1. J. Leukoc. Biol 105: 123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barbon CM, Davies JK, Voskertchian A, Kelner RH, Brennan LL, Nadler LM, and Guinan EC. 2014. Alloanergization of human T cells results in expansion of alloantigen-specific CD8(+) CD28(–) suppressor cells. Am. J. Transplant 14: 305–318. [DOI] [PubMed] [Google Scholar]
- 48.Vuddamalay Y, Attia M, Vicente R, Pomie C, Enault G, Leobon B, Joffre O, Romagnoli P, and van Meerwijk JP. 2016. Mouse and human CD8(+) CD28(low) regulatory T lymphocytes differentiate in the thymus. Immunology. 148: 187–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Uss E, Rowshani AT, Hooibrink B, Lardy NM, van Lier RA, and ten Berge IJ. 2006. CD103 is a marker for alloantigen-induced regulatory CD8+ T cells. J. Immunol 177: 2775–2783. [DOI] [PubMed] [Google Scholar]
- 50.Han JL, Zimmerer JM, Zeng Q, Ringwald BA, Cassol C, Warren RT, Abdel-Rasoul M, Breuer CK, and Bumgardner GL. 2020. Antibody-suppressor CD8+ T cells ameliorate antibody-mediated rejection following kidney transplant in mice. J. Immunol 204: 87.16.31776205 [Google Scholar]
- 51.Zimmerer JM, Ringwald BA, Elzein SM, Avila CL, Warren RT, Abdel-Rasoul M, and Bumgardner GL. 2019. Antibody-suppressor CD8+ T Cells Require CXCR5. Transplantation. 103: 1809–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zimmerer JM, Pham TA, Wright CL, Tobin KJ, Sanghavi PB, Elzein SM, Sanders VM, and Bumgardner GL. 2014. Alloprimed CD8(+) T Cells Regulate Alloantibody and Eliminate Alloprimed B Cells Through Perforin- and FasL-Dependent Mechanisms. Am. J. Transplant 14: 295–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zimmerer JM, Basinger MW, Ringwald BA, Abdel-Rasoul M, Pelletier RP, Rajab A, El-Hinnawi A, Parekh H, Washburn K, and Bumgardner GL. 2020. Inverse Association Between the Quantity of Human Peripheral Blood CXCR5+IFN-γ+CD8+T cells with De Novo DSA Production in the First Year After Kidney Transplant. Transplantation. 104: 2424–2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zimmerer JM, Pham TA, Sanders VM, and Bumgardner GL. 2010. CD8+ T cells negatively regulate IL-4-dependent, IgG1-dominant posttransplant alloantibody production. J. Immunol 185: 7285–7292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miles B, Miller SM, Folkvord JM, Levy DN, Rakasz EG, Skinner PJ, and Connick E. 2016. Follicular Regulatory CD8 T Cells Impair the Germinal Center Response in SIV and Ex Vivo HIV Infection. PLoS Pathog. 12: e1005924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Leavenworth JW, Tang X, Kim HJ, Wang X, and Cantor H. 2013. Amelioration of arthritis through mobilization of peptide-specific CD8+ regulatory T cells. J. Clin. Invest 123: 1382–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim HJ, and Cantor H. 2011. Regulation of self-tolerance by Qa-1-restricted CD8(+) regulatory T cells. Sem. Immunol 23: 446–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim HJ, Verbinnen B, Tang X, Lu L, and Cantor H. 2010. Inhibition of follicular T-helper cells by CD8(+) regulatory T cells is essential for self tolerance. Nature. 467: 328–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mishra S, Liao W, Liu Y, Yang M, Ma C, Wu H, Zhao M, Zhang X, Qiu Y, Lu Q, and Zhang N. 2021. TGF-beta and Eomes control the homeostasis of CD8+ regulatory T cells. J. Exp. Med 218: e20200030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Choi JY, Eskandari SK, Cai S, Sulkaj I, Assaker JP, Allos H, AlHaddad J, Muhsin SA, Alhussain E, Mansouri A, Yeung MY, Seelen MAJ, Kim HJ, Cantor H, and Azzi JR. 2020. Regulatory CD8 T cells that recognize Qa-1 expressed by CD4 T-helper cells inhibit rejection of heart allografts. Proc. Natl. Acad. Sci. USA 117: 6042–6046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lu L, Kim HJ, Werneck MB, and Cantor H. 2008. Regulation of CD8+ regulatory T cells: Interruption of the NKG2A-Qa-1 interaction allows robust suppressive activity and resolution of autoimmune disease. Proc. Natl. Acad. Sci. USA 105: 19420–19425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Connick E, Folkvord JM, Lind KT, Rakasz EG, Miles B, Wilson NA, Santiago ML, Schmitt K, Stephens EB, Kim HO, Wagstaff R, Li S, Abdelaal HM, Kemp N, Watkins DI, MaWhinney S, and Skinner PJ. 2014. Compartmentalization of simian immunodeficiency virus replication within secondary lymphoid tissues of rhesus macaques is linked to disease stage and inversely related to localization of virus-specific CTL. J. Immunol 193: 5613–5625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Perdomo-Celis F, Taborda NA, and Rugeles MT. 2018. Circulating CXCR5-Expressing CD8+ T-Cells Are Major Producers of IL-21 and Associate With Limited HIV Replication. J. Acquir. Immune Defic. Syndr 78: 473–482. [DOI] [PubMed] [Google Scholar]
- 64.Roider J, Maehara T, Ngoepe A, Ramsuran D, Muenchhoff M, Adland E, Aicher T, Kazer SW, Jooste P, Karim F, Kuhn W, Shalek AK, Ndung’u T, Morris L, Moore PL, Pillai S, Kloverpris H, Goulder P, and Leslie A. 2018. High-Frequency, Functional HIV-Specific T-Follicular Helper and Regulatory Cells Are Present Within Germinal Centers in Children but Not Adults. Front. Immunol 9: 1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McCarty B, Mwamzuka M, Marshed F, Generoso M, Alvarez P, Ilmet T, Kravietz A, Ahmed A, Borkowsky W, Unutmaz D, and Khaitan A. 2018. Low Peripheral T Follicular Helper Cells in Perinatally HIV-Infected Children Correlate With Advancing HIV Disease. Front. Immunol 9: 1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Qiu L, Wang H, Yu Q, Liu J, Chen S, and Zhao Z. 2019. Protective role of follicular CXCR5(+)CD8(+) T cells against dengue virus 2 infection. Int. J. Infect. Dis 83: 12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shen Y, Qu QX, Jin MN, and Chen C. 2019. Investigating the role of circulating CXCR5-expressing CD8+ T-cells as a biomarker for bacterial infection in subjects with pneumonia. Respiratory Res. 20: 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bai M, Zheng Y, Liu H, Su B, Zhan Y, and He H. 2017. CXCR5(+) CD8(+) T cells potently infiltrate pancreatic tumors and present high functionality. Exp. Cell Res 361: 39–45. [DOI] [PubMed] [Google Scholar]
- 69.Huang Q, Zhou Q, Zhang H, Liu Z, Zeng H, Chen Y, Qu Y, Xiong Y, Wang J, Chang Y, Xia Y, Wang Y, Liu L, Zhu Y, Xu L, Dai B, Guo J, Wang Z, Bai Q, and Zhang W. 2020. Identification and validation of an excellent prognosis subtype of muscle-invasive bladder cancer patients with intratumoral CXCR5(+) CD8(+) T cell abundance. Oncoimmunology. 9: 1810489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chu F, Li HS, Liu X, Cao J, Ma W, Ma Y, Weng J, Zhu Z, Cheng X, Wang Z, Liu J, Jiang ZY, Luong AU, Peng W, Wang J, Balakrishnan K, Yee C, Dong C, Davis RE, Watowich SS, and Neelapu SS. 2019. CXCR5(+)CD8(+) T cells are a distinct functional subset with an antitumor activity. Leukemia. 33: 2640–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jacomet F, Cayssials E, Basbous S, Levescot A, Piccirilli N, Desmier D, Robin A, Barra A, Giraud C, Guilhot F, Roy L, Herbelin A, and Gombert JM. 2015. Evidence for eomesodermin-expressing innate-like CD8(+) KIR/NKG2A(+) T cells in human adults and cord blood samples. Eur. J. Immunol 45: 1926–1933. [DOI] [PubMed] [Google Scholar]
- 72.Xing J, Li X, E J, Wang C, and Wang H. 2020. Inverse relationship between CD40L expression and cytolytic molecule expression by CD8(+)CXCR5(+) T follicular cytotoxic cells in colorectal cancer. Exp. Cell Res 389: 111892. [DOI] [PubMed] [Google Scholar]
- 73.Im SJ, Hashimoto M, Gerner MY, Lee J, Kissick HT, Burger MC, Shan Q, Hale JS, Lee J, Nasti TH, Sharpe AH, Freeman GJ, Germain RN, Nakaya HI, Xue HH, and Ahmed R. 2016. Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature. 537: 417–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brummelman J, Mazza EMC, Alvisi G, Colombo FS, Grilli A, Mikulak J, Mavilio D, Alloisio M, Ferrari F, Lopci E, Novellis P, Veronesi G, and Lugli E. 2018. High-dimensional single cell analysis identifies stem-like cytotoxic CD8(+) T cells infiltrating human tumors. J. Exp. Med 215: 2520–2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chatterjee B, Deng Y, Holler A, Nunez N, Azzi T, Vanoaica LD, Muller A, Zdimerova H, Antsiferova O, Zbinden A, Capaul R, Dreyer JH, Nadal D, Becher B, Robinson MD, Stauss H, and Munz C. 2019. CD8+ T cells retain protective functions despite sustained inhibitory receptor expression during Epstein-Barr virus infection in vivo. PLoS Pathog. 15: e1007748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhou Y, Guo L, Sun H, Xu J, and Ba T. 2018. CXCR5(+) CD8 T cells displayed higher activation potential despite high PD-1 expression, in tumor-involved lymph nodes from patients with thyroid cancer. Int. Immunopharmacol 62: 114–119. [DOI] [PubMed] [Google Scholar]
- 77.Ma QY, Chen J, and Zhao J. 2020. Follicular cytotoxic CD8 T cells present high cytokine expression, and are more susceptible to Breg-mediated suppression in non-small cell lung cancer. Immunologic Res. 68: 54–62. [DOI] [PubMed] [Google Scholar]
- 78.Tang J, Zha J, Guo X, Shi P, and Xu B. 2017. CXCR5(+)CD8(+) T cells present elevated capacity in mediating cytotoxicity toward autologous tumor cells through interleukin 10 in diffuse large B-cell lymphoma. Int. Immunopharmacol 50: 146–151. [DOI] [PubMed] [Google Scholar]


