Keywords: ACE2, bacterial metabolites, gastrointestinal RAS, microbiome, tissue RAS
Abstract
Gut microbiota is a potent biological modulator of many physiological and pathological states. The renin-angiotensin system (RAS), including the local gastrointestinal RAS (GI RAS), emerges as a potential mediator of microbiota-related effects. The RAS is involved in cardiovascular system homeostasis, water-electrolyte balance, intestinal absorption, glycemic control, inflammation, carcinogenesis, and aging-related processes. Ample evidence suggests a bidirectional interaction between the microbiome and RAS. On the one hand, gut bacteria and their metabolites may modulate GI and systemic RAS. On the other hand, changes in the intestinal habitat caused by alterations in RAS may shape microbiota metabolic activity and composition. Notably, the pharmacodynamic effects of the RAS-targeted therapies may be in part mediated by the intestinal RAS and changes in the microbiome. This review summarizes studies on gut microbiota and RAS physiology. Expanding the research on this topic may lay the foundation for new therapeutic paradigms in gastrointestinal diseases and multiple systemic disorders.
INTRODUCTION
A growing number of studies indicate the critical role of gut microbiota in health and disease. It seems that the interplay between the host and its microbiome is complex and bidirectional. On the one hand, various pathological states, like gastrointestinal diseases, diabetes, hypertension, or depression, may shape the composition and activity of microbiota (1). On the other, gut bacteria and their metabolites may influence the host homeostasis, triggering or contributing to the initiation of pathological processes. (2–4). The critical link in this interaction may be the renin-angiotensin system (RAS). The local gastrointestinal RAS (GI RAS) deserves special attention due to its proximity to the intestinal lumen—the habitat of gut microflora. The GI RAS is involved in glycemic and electrolyte homeostasis, inflammatory process, carcinogenesis, aging-related changes, and many other functions. By modulating the GI RAS, gut bacteria may impact these processes. At the same time, the effects of the RAS-targeted therapies might be mediated, in part, by their actions on the GI RAS and the gut microbiome. This review summarizes the biological role of RAS and current evidence on its reciprocal relationship with microbiota (see Fig. 1).
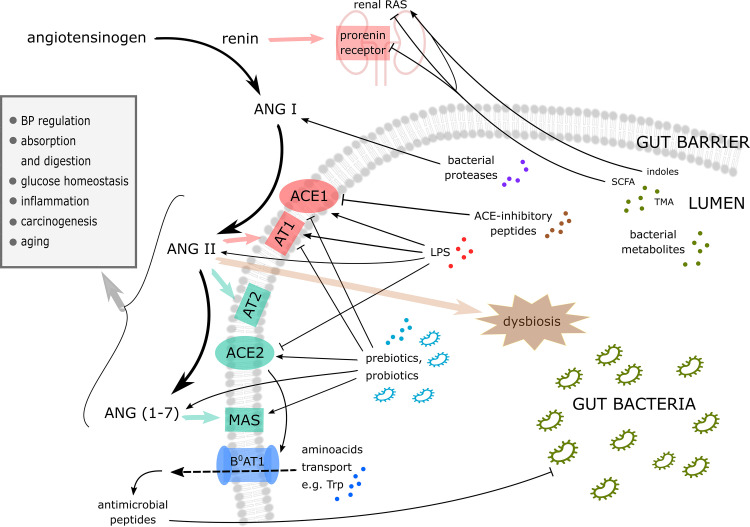
Figure 1.
Key interactions between gut microbiome and renin-angiotensin system (details are given in the text). ACE1, angiotensin-converting enzyme 1; ACE2, angiotensin-converting enzyme 2; ANG I, angiotensin I; ANG II, angiotensin II; ANG (1–7), angiotensin (1–7); AT1, type 1 angiotensin II receptor; AT2, type 2 angiotensin II receptor; BP, blood pressure; B0AT1; broad neutral amino acid transporter 1; LPS, lipopolysaccharide; MAS, Mas receptor; RAS, renin-angiotensin system; SCFA, short-chain fatty acids; TMA, trimethylamine; Trp, tryptophan.
SYSTEMIC RAS
In the mid-1970s, the classical RAS consisted of circulating renin, angiotensinogen, angiotensin I, angiotensin II, and angiotensin-converting enzyme (ACE) (5). Since then, the view of the RAS has been gradually expanded by the addition of new elements to the system. These include the (pro)renin receptor, which retains and activates renin in tissues, whereas the ACE2–MAS axis mainly counteracts angiotensin II effects (6). The systemic RAS is a major regulator of cardiovascular and renal functions and plays a crucial role in controlling blood volume and pressure (7). However, under pathophysiological conditions, the effects of the RAS can intensify, triggering inflammation and structural remodeling, promoting cardiac, and vascular damage (8).
Circulating renin, mainly produced by the kidneys, is responsible for the first and rate-limiting step in the RAS cascade. It hydrolyzes angiotensinogen, a liver-secreted peptide, to create angiotensin I. Following that, ACE1, a membrane-bound exopeptidase predominantly expressed in the pulmonary endothelium, cleaves angiotensin I to form the octapeptide angiotensin II (9). The latter is the most potent, biologically active component of the RAS, inducing all of the system's classical actions, such as blood pressure increase, vasoconstriction, tissue remodeling, and proinflammatory and profibrotic effects (10). Angiotensin II stimulates the AT1 and AT2 receptors, which usually mediate opposite functions (11). Most effects of angiotensin II are conveyed by the AT1 receptors (12), which form the pathway known as the ACE1-AT1R axis. The alternative RAS axes involve the activation of the AT2 receptor instead of AT1 and the ACE2-MAS axis; angiotensin-converting enzyme II (ACE2) can form angiotensin (1–7), which is a Mas-receptor agonist (MAS).
Blocking various RAS components proved to be an effective therapy in multiple disorders, such as hypertension, diabetes, and renal or heart failure. In fact, due to multitargeted actions, ACE-inhibitors and angiotensin receptor blockers became a cornerstone of the strategies to reduce cardiovascular risk. Their effects go beyond lowering blood pressure alone, most likely owing to the local (tissue) RAS interactions.
LOCAL GASTROINTESTINAL RAS
Components of the RAS are found locally within tissue systems, such as the brain, heart, kidney, adipose tissue, skeletal muscles, adrenal glands, and last but not least, digestive organs (13). The most significant contribution of these local systems is their activity at the cellular level via paracrine and autocrine mechanisms. They mediate cell-specific effects on growth, proliferation, and metabolism (13). It should be noted that they may interact with endocrine RAS and other peptide systems (e.g., the endothelin system) on various levels (14). Notably, the concept of tissue RAS explains the beneficial effects of ACE inhibitors and angiotensin receptor blockers that are independent of blood pressure change, e.g., the cardioprotective outcome of these drugs (15).
This review focuses on the gastrointestinal RAS (GI RAS) as the system is the most likely to relate to gut microbiota. GI RAS regulates intestinal physiological functions, such as electrolyte homeostasis, digestion, peptide transport, glucose, sodium, and water absorption, gastrointestinal motility, and secretion through the intestinal epithelium (13, 16, 17). Moreover, this system partly controls the mechanisms responsible for intestinal inflammation, apoptosis, fibrosis, and mucosal protection (16, 18). Similar to the systemic RAS, the GI RAS effects depend mainly on balance between the ACE1-AT1R and the ACE2-MAS axes (19). Generally, the activation of the ACE1-AT1R axis causes vasoconstriction and is involved in the induction of apoptosis, vascular remodeling, atherosclerosis, and inflammation (20, 21) In contrast, the ACE2-MAS axis protects the gastrointestinal mucosa and promotes its regeneration after damage (18).
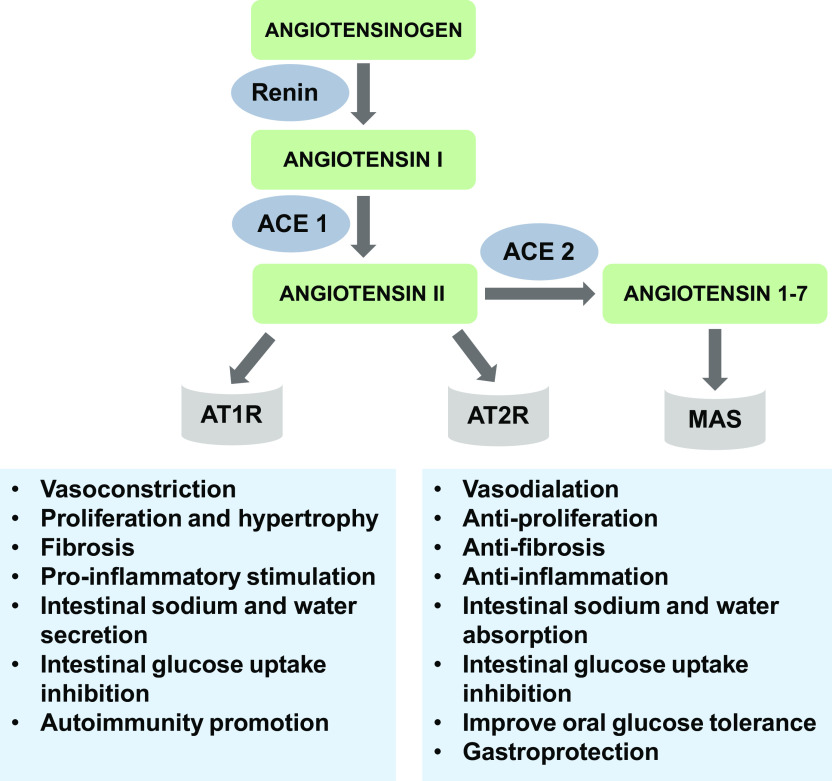
Figure 2 summarizes major pathways and their effects, and Table 1 lists the GI RAS key components expressed in the intestines.
Figure 2.
Main pathways and effects of the gastrointestinal renin-angiotensin system. ACE 1, angiotensin-converting enzyme 1; ACE 2, angiotensin-converting enzyme 2; AT1R, type 1 angiotensin II receptor; AT2R, type 2 angiotensin II receptor; MAS, Mas receptor.
Table 1.
Expression of key components of the gastrointestinal renin-angiotensin system in the intestine
| Tissue | ACE1 | ACE2 | AT1R | AT2R | ANG II | MAS | References |
|---|---|---|---|---|---|---|---|
| Small intestine | |||||||
| Brush border of epithelial cells | + | + | + | (22–24) | |||
| Mesenteric microvascular endothelium | + | + | (23, 24) | ||||
| Muscularis mucosa | + | + | + | (24) | |||
| Muscularis propria | + | (24) | |||||
| Circular and longitudinal muscle layers | + | + | + | (25–27) | |||
| Myenteric plexus | + | + | (25–27) | ||||
| Small vessels in the muscularis propria | + | (25, 26) | |||||
| Crypt and crypt-villus junction epithelial cells | + | (22) | |||||
| Mucosal mast cells | + | (27) | |||||
| Large intestine | (28) | ||||||
| Surface epithelial cells | + | + | + | (23, 29) | |||
| Crypts | + | + | (29) | ||||
| Lamina propria macrophages | + | (29) | |||||
| Myofibroblasts | + | (29) | |||||
| Mucosal vessel walls | + | (29) | |||||
| Mesenteric microvascular walls | + | + | (29) | ||||
| Colonic dorsal root ganglion neurons | + | (30) |
ACE1, angiotensin-converting enzyme 1; ACE2, angiotensin-converting enzyme 2; AT1R, type 1 angiotensin II receptor; AT2R, type 2 angiotensin II receptor; ANG II, angiotensin II; MAS, Mas-receptor.
RAS AND GUT MICROBIOTA IN HEALTH AND DISEASE
Cardiovascular System
Recent studies show that there is a crosstalk between the gut microbiome and the cardiovascular system. Gut microbiota-derived molecules may affect cardiovascular homeostasis acting directly on the blood vessels and the heart (31, 32). Reciprocally, changes in the cardiovascular system may affect gut microbiota by altering the structure and function of bacterial habitat, i.e., intestines (33). It has been found that gut bacteria may affect the circulatory system indirectly by modulating gut sympathetic activity (34) and RAS. In this regard, both the sympathetic nervous system and RAS are key factors contributing to numerous cardiovascular pathologies, including hypertension and heart failure (35).
The interplay between the microbiome and RAS is most evident in the regulation of blood pressure. Experimental studies suggest an important role for gut microbiota in developing angiotensin II-induced hypertension and organ damage related to hypertension. For example, Karbach et al. (36) showed that germ-free mice infused with angiotensin II had lower blood pressure and less cardiac fibrosis than mice that received a fecal transplant before the experiment or sham germ-free group. In addition, it has been found that the administration of probiotics, i.e., bacteria intended to have health benefits, reduces blood pressure (37–39).
It has been found that bacterial proteases interact in vitro with human RAS peptides (40). Furthermore, research indicates that ACE inhibitory peptides are released during the bacterial fermentation processes (41, 42) producing a blood pressure-lowering effect (43). For example, Nakamura et al. showed that two tripeptides (Val-Pro-Pro and Ile-Pro-Pro) with an inhibitory effect on ACE were produced in milk fermented by the Lactobacillus helveticus (44), which resulted in a decrease in blood pressure (45). The ability of individual Lactobacillus helveticus to produce antihypertensive peptides is most likely related to their proteolytic system's completeness and efficiency (46). A study conducted by Ramchandran and Shah (47) suggests that among the probiotic organisms, Bifidobacterium longum 5022 has the maximal ACE-inhibitory potential.
Moreover, prebiotics and probiotics may shift GI RAS balance towards the ACE2-MAS axis. It has been shown that inulin or Lactobacillus casei supplementation received by mothers prevented high-fat diet-induced hypertension in offspring, which was associated with the lower ACE1 and AT1 receptor expression and activation of ACE2 (48). Those data suggest that dietary approaches with probiotic and prebiotic supplementation may be an attractive therapeutic option in the treatment or primary prevention of hypertension. However, further investigation is warranted.
Importantly, gut microbiota produces many biologically active compounds, i.e., bacterial metabolites that may interact with the GI RAS and systemic RAS. Short-chain fatty acids (SCFA), such as butyrate, acetate or propionate, affect blood pressure by modulating local RAS in the kidneys. For example, sodium butyrate inhibits angiotensin II-induced hypertension by suppressing the (pro)renin receptor and the intrarenal RAS (49). Acetate supplementation also resulted in the downregulation of the local RAS in the kidney and heart (50). On the contrary, succinate, an intermediate in microbial propionate synthesis, has recently emerged as an activator of the renal RAS through the SUCNR1 signaling (51). In addition, gut bacteria-derived uremic toxins such as indoxyl sulfate probably cause chronic kidney injury by activating intrarenal RAS (52).
Another important group of bacterial metabolites is methylamines, i.e., trimethylamine (TMA) and its oxide (TMAO). Their pathological role, especially in the cardiovascular system, is widely discussed; however, the potential mechanisms of action remain obscure (3). Several lines of evidence suggest that RAS, at least in part, may mediate their biological effects. It has been shown that TMAO infusion alone did not increase blood pressure but combined with a low dose of angiotensin II, TMAO prolonged the hypertensive effect (53). We have recently shown that TMAO causes a favorable shift in the RAS axes in heart failure-induced remodeling. Namely, chronic treatment with TMAO in rats reduced expression of AT1 receptor with a concomitant increase in the expression of AT2 receptor in the heart. Similar changes were observed in the kidneys (32).
It must be emphasized that changes in RAS activity, presumably locally in the intestines, may reciprocally modulate gut microbes. Angiotensin II treatment significantly altered gut bacteria and their metabolites in plasma and feces (54). In line with these findings, chronic angiotensin II infusion in the rat model of hypertension is associated with gut microbiota changes. Specifically, there was a decrease in microbial richness and an increase in Firmicutes/Bacteroidetes ratio. Moreover, treatment with minocycline significantly lowered blood pressure in angiotensin II–infused rats (4). However, the possible role of microbiota in the latter findings should be interpreted with caution as minocycline is known to have bacteria-independent effects on neuro-immune signaling (55). Finally, changes in RAS activity may modify the gut-blood barrier permeability to bacterial metabolites, limiting their systemic effects. It has been shown that treatment with enalapril, an ACE inhibitor, attenuated hypertension-induced disturbances in the intestines, including leaky gut, and decreased TMA passage into the circulation (56).
Absorption and Digestion
Most GI RAS-mediated effects have been shown with respect to water and sodium transport through the intestinal epithelium. In the jejunum, angiotensin II at low doses increased sodium and fluid absorption by stimulating sympathetic activity (57). Conversely, a higher dose with simultaneous pressure response reduced absorption or induced net fluid secretion, probably by forming prostaglandins (58). A possible mechanism for this inconsistent outcome may be the activation of different axes; Jin and others showed that stimulation of AT2 receptors improves intestinal sodium and water absorption, whereas AT1 receptors inhibited absorption or stimulated fluid secretion (59). In the proximal colon, angiotensin II promotes water and sodium absorption by stimulating conjugated transport of NaCl (60). It is worth noting that disturbances in endogenous ion transport alter the intestinal microenvironment and thereby modulate the gut microbiome composition. This concept has been elegantly demonstrated using various ion transport knockout models (61). For example, sodium/proton exchanger NHE3 deficiency results in a higher luminal pH, promoting dysbiotic and proinflammatory shifts in the intestinal microbiome (62). On the other hand, gut microbes may reciprocally affect water and ion homeostasis. Traditionally, clinical medicine's focus was on the water-electrolyte disturbances caused by the local action of pathological bacteria producing diarrhea. However, accumulating evidence points to the critical role of gut bacteria composition and their metabolites in the systemic RAS-dependent control of water-electrolyte balance. A perfect example is the above-mentioned control of renin release by SCFA. It has been found that SCFA receptors (i.e., Olfr78 and Gpr41) in the renal juxtaglomerular apparatus mediate renin secretion in response to the signals from gut microbiota, which is weakened by antibiotic treatment and in Olfr78 and Gpr41 knockout mice (63).
Apart from water-electrolyte balance, the GI RAS plays a role in peptides digestion. ACE1 and ACE2 of the intestinal brush border are considered peptidases, enabling digestion and absorption of peptides (64). ACE2 increases the amino acid transporter activity (B0AT1), which has been recently linked to the microbial ecology in the gut. Hashimoto et al. (65) demonstrated that the reduction of tryptophan uptake in ACE2-knock out mice resulted in impaired expression of antimicrobial peptides and consequently altered gut microbial composition. Since angiotensin (1–7) has been shown to increase jejunal tryptophan absorption (66), it may similarly modulate the gut microbiome. In this regard, a very recent study by Oliveira et al. (67) showed that deletion of MAS, angiotensin (1–7) receptor, in mouse model produced lower neutral amino acids absorption and changes in the gut microbiome. Specifically, the malnourishment profile leads to a compensatory increase in intestinal villi length and an unfavorable shift in bacterial composition.
Glucose Homeostasis
Patients with diabetes are characterized by an unfavorable shift in bacterial composition, especially with regards to butyrate and endotoxin-producing bacteria (68). Moreover, an altered microbiome has been observed in prediabetic patients (69) and recent findings highlight the role of the gut microbiome in glucose homeostasis. Notably, bacterial metabolites like short-chain fatty acids can affect blood glucose levels and insulin release (70). A study conducted by Lu et al. (61) showed that acetate produced by intestinal microbiota might be involved in the diabetic-induced early kidney injury acting via RAS modulation. Antibiotic treatment in diabetic rats markedly lowered plasma acetate, inhibited intrarenal RAS activity, and reduced kidney damage. Also, several probiotic strains have been found to exert antidiabetic modulatory effects, i.e., by inhibiting enzymes that increase glucose absorption in the intestines (71) and by regulating postprandial blood glucose (72). In this regard, it is worth noting that alterations in GI RAS activity induced by diabetes disturb intestinal glucose transport and thereby contribute to postprandial hyperglycemia (73). Diabetes promotes glucose transport via sodium/glucose cotransporter 1 (SGLT1) and glucose transporter 2 (GLUT2) (74). Angiotensin II inhibits intestinal glucose uptake in a rapid and dose‐dependent manner through the SGLT-1 (75). Interestingly, in diabetic rats, ACE1 and AT1 receptors on the brush border were reduced. There was a disproportionate increase in GLUT2 expression compared with SGLT1, which significantly reduced RAS-dependent inhibition of glucose uptake via SGLT1 (76).
On the other hand, diabetes increases the expression of ACE2 and MAS receptors in the jejunum. Angiotensin (1–7), a critical component of the ACE2-MAS axis, was also able to inhibit glucose uptake and significantly improved oral glucose tolerance in type 1 diabetes (17). Therefore, the activity of ACE1 and ACE2 determines the overall rate of glucose transport through the intestinal epithelium. In this respect, recent studies showed that probiotics could activate the ACE2-MAS axis. Bifidobacterium longum supplementation increased the expression of ACE2 and MAS and had beneficial effects on the glycemic and lipid profiles (77). Interestingly, Verma et al. (78, 79) showed that the engineered probiotic species Lactobacillus paracasei may serve as a live vector for oral delivery of ACE2 and angiotensin (1–7) to attenuate diabetic retinopathy in animal models. Therefore, probiotics-based regulation of RAS emerges as an important therapeutic approach in managing metabolic diseases and diabetic complications.
Inflammation
Inflammatory bowel disease (IBD), including Crohn’s disease and ulcerative colitis, is a group of serious gastrointestinal disorders, where inflammation plays a central role. Although the IBD etiology is not fully understood, it is generally believed that improper activation of the intestinal mucosal immune system is the main contributor to this disorder (80). Recent years have provided more evidence that the diverse microbiota-host interactions influence the immune response and inflammation within the intestine (81). It is postulated that altered composition of gut microbiota contributes to inflammatory diseases like IBD. In fact, clinical trials show promising results of the use of fecal microbiota transplantation in ulcerative colitis, but the evidence for its efficacy is still limited (82). In addition, there is currently little knowledge of the exact immune mechanisms stimulated by microbiota modulation. However, the interplay with RAS is highly expected since extensive literature suggests a pivotal role of RAS in the inflammatory process.
In general, the renin-angiotensin II cascade promotes colitis. Overexpression of renin significantly increases the large intestine’s susceptibility to inflammation (83). Also, many experimental studies have shown that ACE inhibitors or AT1 receptors blockers alleviate chemically induced colitis (84–86). There are several potential mechanisms by which RAS promotes inflammation. Among them, stimulating TH17 activation seems to be of particular interest in terms of a possible microbiota-RAS-inflammation linkage. Angiotensin II both directly (via JAK2/STAT1/3) and indirectly (via cytokine production) promotes lymphocyte polarization in TH17 but not in TH1 (87). Also, data show that activated CD4+ T cells have high expression of AT1 receptors on their surface, and the RAS promotes autoimmunity through TH1/TH17 (88). Recent studies suggest that TH1/TH17 balance is modulated by gut microbiota. Namely, fecal transplants from IBD donors induce a greater proportion of Th17 cells in the gut than healthy donors (89). In line with this, the proportion of Th17 cells was reduced following microbiota transplant from healthy individuals (90). Also, colonization with a certain Escherichia coli induced systemic TH17 immunity and aggravated colitis development (91).
RAS may also contribute to the translocation of leukocytes into extravascular compartments during inflammation by increasing leukocyte adhesion to the endothelium. Activating AT1 receptor signaling, angiotensin II promotes the colonization of leukocytes, followed by an increase in cell adhesion molecules (86). Other studies also confirmed that pharmacological inhibition of ACE and angiotensin receptors decreases the severity of colitis by reducing the infiltration of inflammatory cells (84). Recently, mucosal vascular addressin cell adhesion molecule 1 (MAdCAM-1), which belongs to endothelial cell adhesion molecules, has become a potential target for IBD treatment (92). MAdCAM-1 increases dramatically during inflammation (93) and may contribute to the development of colitis. It seems that both GI RAS and gut microbiota play a role in MAdCAM-1 regulation. Western diet-induced dysbiosis has increased its expression (94), whereas probiotics exerted the opposite effect (95). AT1 receptor also affects the expression of MAd-CAM-1 by controlling the translocation of NF-κB into the nucleus (86, 96). Therefore, appropriate modulation of bacterial composition and GI RAS activity may be a new target in IBD therapy through the modification of MAdCAM-1.
It is worth noting that the ACE2-MAS axis protects the mucous membrane and aids in the repair process after damage. In particular, angiotensin (1–7) protects the gastric mucosa by reducing inflammatory cytokines (97). It can be speculated that inflammatory response to bacterial endotoxemia is related to ACE2 actions. Ye et al. (98) showed that cells challenged with lipopolysaccharide (LPS) had upregulated expression of renin, angiotensin II, ACE1, and AT1 receptors. At the same time, the mice model of LPS-induced lung injury exhibited lower expression of ACE2. Injection of ACE2 attenuated inflammation and significantly improved the pathological injury.
Taking together, the GI RAS seems to play an essential role in the inflammatory process in the intestine and, therefore, may be a mediator of microbiota-induced effects. Proper selection of microbiome-based therapeutics, i.e., probiotics or prebiotics, in terms of activity against RAS, may introduce new strategies in inflammatory or autoimmune diseases management. However, it is necessary to gain a complete understanding of the microbiome-RAS-related effects before the widespread implementation of such therapeutic interventions.
On the other hand, it needs to be stressed that such interactions are bidirectional. Changes in the intestinal environment caused by inflammation promote dysbiotic shifts in bacterial composition. For example, inflammation produces an expansion of Enterobacteriaceae populations (99), which exacerbates inflammation and epithelial damage (100). At least, in theory, it may be the altered activation of GI RAS during inflammation that makes the intestinal habitat more propathogenic. The already mentioned study by Oliveira et al. (67) demonstrated that MAS receptor deletion produced dysbiosis, which was associated with increased proliferation and cell inflammation, most likely due to overproduction of LPS.
Carcinogenesis
Much attention has been paid to the pro- and anticarcinogenic role of the gut microbiome. A broad description of the topic is beyond the scope of this review and has been elegantly summarized elsewhere (101). We would like to highlight the existing evidence for an association between cancer and microbiota composition changes (102–104). Some studies revealed distinct commensal bacteria that promote tumor development in genetically predisposed animals (105). Others demonstrate that targeted changes in gut bacteria composition reduces carcinogenesis in mouse models of colorectal cancer (106). A number of possible mechanisms behind bacterial actions have been proposed, including inflammation, metabolic activity, and genotoxicity (107).
Likewise, by stimulating the ACE1-AT1R axis, the RAS plays a role in angiogenesis, cell proliferation, and fibrosis, which constitute crucial carcinogenesis processes (108, 109).
In fact, the GI RAS and microbiome may share the same signaling pathways. A prominent example is the Toll-like receptors (TLRs) which recognize pathogen-derived molecules like LPS and have an established role in tumor development. For example, prebiotic treatment counteract neoplastic lesions via downregulation of TLR4 (110). Furthermore, carcinogenic actions of pathogens like Fusobacterium nucleatum or Escherichia coli have been linked to TLR4 signaling (111, 112). Likewise, angiotensin II upregulates TLR4 expression, thereby contributing to the development of liver fibrosis (113). Importantly, several lines of evidence suggest a synergy between RAS and microbiome effects on TLR. Simultaneous treatment with angiotensin II and LPS exerted a much higher level of TGF-β in hepatic fibrogenesis than either agent alone (113). Analogously, cotreatment with probiotic and AT1 receptor blocker exerted a more potent and synergistic inhibitory effect on liver fibrosis than either agent administered independently (114).
Another important mechanism by which RAS exerts a carcinogenic effect is the stimulation of tumor angiogenesis - angiotensin II activates the expression of several angiogenesis and growth factors, including vascular endothelial growth factor (VEGF) (109) and angiopoietin 2 (115). Moreover, cancer-related angiogenesis was decreased after administrating captopril (ACE inhibitor) and irbesartan (AT1 antagonist) in a mouse model of colorectal cancer (116). In light of this, bacterial ligands also promoted angiogenic response by the upregulation of VEGF receptor 2 (117), whereas probiotics exerted the opposite effect downregulating VEGF and angiopoietin 2 (118).
As described in Local Gastrointestinal RAS, the ACE2-MAS axis with angiotensin (1–7) has an inhibitory effect on many angiotensin II-induced processes. However, its role with respect to cancer development is complex and not fully elucidated. Generally, angiotensin (1–7) has antimetastatic, antiproliferative and antiangiogenic effects (119). Surprisingly though, it also promoted migration and invasion of renal cell carcinoma (120). Nevertheless, the ACE2-MAS axis constitutes a promising therapeutic target and represents a potential factor that underlies microbiome-related effects. It has been shown that modulation of bacterial composition may impact the amount of ACE2 in the intestine (121–123). In line with this, it has been shown that ACE2 gene expression in tissues from colorectal cancers was positively correlated with the abundance of specific bacterial taxa, most prominently Chlamydia (124). Therefore, pro- or anticarcinogenic features of a microbial community may be, at least in part, due to changes in the RAS activity.
Aging
Aging is associated with various physiological and pathophysiological changes in local RAS activity and other gastrointestinal functions, including dysfunction of the intestinal epithelium, gastrointestinal motility, and absorption (125, 126), as well as increased susceptibility to enteritis (125, 127). RAS components may cause or aggravate the degenerative changes associated with aging in the intestine (19). On the other hand, the physiological aging process affects the local expression of RAS. Garrido-Gil et al. (128) reported that older rats showed higher colonic expression of the AT1 receptor but lower expression of the AT2 receptor than younger rats. Also, the ACE1/ACE2 ratio was elevated in adult rats compared with juvenile animals in the jejunum and colon. This finding suggests that the ACE2 activity decreases with age and the GI RAS balance moves towards the ACE1-AT1R axis (19).
Such pathological changes induced by aging are likely to alter intestinal habitat and presumably affect the microbial community (129). On that account, some studies have reported that composition, diversity, and metabolic function of gut microbiota change with age (130, 131). Alternatively, studies using animal models suggest that pathogenic gut bacteria and their metabolites contribute to frailty, aging-associated diseases, and reduced longevity (130, 132, 133). Therefore, therapeutic interventions aimed at healthier aging and lifespan extension should target microbiota and their habitat, i.e., GI RAS activity and other intestinal functions. Importantly, microbiome-based therapy may act by shifting the GI RAS balance towards the ACE2-MAS axis. It has been demonstrated that recombinant probiotics can increase circulating levels of angiotensin (1–7) and decrease levels of angiotensin II in a well-characterized rodent model of aging (134). All in all, the knowledge of the microbiome-RAS relationship in aging-related pathologies is still limited but may prove useful in the effective healthcare of the elderly.
COVID-19
ACE2 has recently gained special attention due to a novel coronavirus outbreak. Published data confirmed that ACE2 is the receptor for SARS-CoV-2 (135). Since the expression of ACE2 is very high in enterocytes, the intestines and gut microbiota emerge as important players in SARS-CoV-2 infection (136). Zang et al. (137) demonstrated explicitly that SARS-CoV-2 could infect ACE2+ mature enterocytes in the human small intestine, a mechanism mediated by TMPRSS2 and TMPRSS4 proteases. Importantly, the infection reduces ACE2 expression in the lung and other tissues due to spike protein-mediated downregulation of ACE2 (138), which may explain multiorgan failure in COVID-19 (139, 140). Therefore, the administration of ACE2 soluble forms may provide a double beneficial effect during SARS-CoV-2 infection by slowing virus entry into host cells while ensuring the putative preservation of ACE2 contraregulatory functions on RAS, thus assisting in lung injury defense (141).
Ample evidence indicates that SARS-CoV-2 infection causes a defect of the gut-blood barrier, increasing the penetration of microbes, bacterial lipopolysaccharide, or peptidoglycan into the circulation, possibly disrupting the immunological reaction to COVID-19 infection, and resulting in multisystem dysfunction or septic shock (139, 142, 143). It was suggested that in the pathogenesis of SARS-CoV-2, any disturbances in host-microbiota crosstalk could act as an initiating or reinforcing factor (144).
Hospitalized patients with COVID-19 had a disparity in intestinal microflora diversity, characterized by lower levels of probiotic bacteria (e.g., Lactobacillus and Bifidobacterium) (145) and a significantly higher abundance of opportunistic pathogens (e.g., Streptococcus, Veillonella, and Actinomyces) (146). It has been proposed that gut microbes modulate colonic ACE2 and thereby influence COVID-19 infectivity. Feng et al. (121) investigated the effect of enteritis caused by Salmonella enterica and treatment with segmented filamentous bacteria, a probiotic, on ACE2 expression. They found that the coronavirus receptor expression was elevated in both cases. On the contrary, Yang et al. (122) demonstrated that reconstitution of the gut microbiota in germ-free rats markedly decreased the colonic ACE2 expression. The latest study on mice supports these results as ACE2 levels in intestines were significantly higher in germ-free animals than in conventional mice. The germ-free phenotype was partially recapitulated by antibiotic treatment (123).
PERSPECTIVES
Despite numerous reports about the influence of gut bacteria on the host and vice versa, there is still much to be elucidated regarding mechanisms determining this interaction. Current evidence suggests that gut microbiota and their metabolites may modulate the RAS. The pleiotropic actions of RAS in homeostatic processes support its role in mediating microbiota-related effects. On the other hand, changes in the intestinal habitat caused by the GI RAS disturbances related to hypertension and other diseases may shape microbiota metabolic activity and composition. Also, it can be speculated that the effects of RAS-targeted therapies can be mediated, in part, by their actions on the GI RAS and microbiome. Both the RAS and gut microbiome play a role in glycemic and electrolyte homeostasis, inflammation, carcinogenesis, and aging-related changes. Still, many of these connections remain largely underinvestigated. Nevertheless, appropriate manipulation of the RAS, either directly or by altering gut bacteria, could be beneficial in treating many pathologies. Further studies on the reciprocal relation between RAS and gut bacteria are needed to lay a foundation for new therapeutic paradigms.
GRANTS
K. Jaworska was supported by the Foundation for Polish Science (FNP).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.J. and M.U. conceived and designed research; M.K. prepared figures; K.J. and M.K. drafted manuscript; K.J., M.K., and M.U. edited and revised manuscript; K.J., M.K., and M.U. approved final version of manuscript.
REFERENCES
- 1.Tang WH, Kitai T, Hazen SL. Gut microbiota in cardiovascular health and disease. Circ Res 120: 1183–1196, 2017. doi: 10.1161/CIRCRESAHA.117.309715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Onyszkiewicz M, Gawrys-Kopczynska M, Konopelski P, Aleksandrowicz M, Sawicka A, Koźniewska E, Samborowska E, Ufnal M. Butyric acid, a gut bacteria metabolite, lowers arterial blood pressure via colon-vagus nerve signaling and GPR41/43 receptors. Pflugers Arch 471: 1441–1453, 2019. doi: 10.1007/s00424-019-02322-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Onyszkiewicz M, Jaworska K, Ufnal M. Short chain fatty acids and methylamines produced by gut microbiota as mediators and markers in the circulatory system. Exp Biol Med (Maywood) 245: 166–175, 2020. doi: 10.1177/1535370219900898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang T, Santisteban MM, Rodriguez V, Li E, Ahmari N, Carvajal JM, Zadeh M, Gong M, Qi Y, Zubcevic J, Sahay B, Pepine CJ, Raizada MK, Mohamadzadeh M. Gut dysbiosis is linked to hypertension. Hypertension 65: 1331–1340, 2015. doi: 10.1161/HYPERTENSIONAHA.115.05315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peach MJ. Renin-angiotensin system: biochemistry and mechanisms of action. Physiol Rev 57: 313–370, 1977. doi: 10.1152/physrev.1977.57.2.313. [DOI] [PubMed] [Google Scholar]
- 6.Bader M. Tissue renin-angiotensin-aldosterone systems: targets for pharmacological therapy. Annu Rev Pharmacol Toxicol 50: 439–465, 2010. doi: 10.1146/annurev.pharmtox.010909.105610. [DOI] [PubMed] [Google Scholar]
- 7.De Mello WC. Local renin angiotensin aldosterone systems and cardiovascular diseases. Med Clin North Am 101: 117–127, 2017. doi: 10.1016/j.mcna.2016.08.017. [DOI] [PubMed] [Google Scholar]
- 8.Forrester SJ, Booz GW, Sigmund CD, Coffman TM, Kawai T, Rizzo V, Scalia R, Eguchi S. Angiotensin II signal transduction: an update on mechanisms of physiology and pathophysiology. Physiol Rev 98: 1627–1738, 2018. doi: 10.1152/physrev.00038.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lavoie JL, Sigmund CD. Minireview: overview of the renin-angiotensin system—an endocrine and paracrine system. Endocrinology 144: 2179–2183, 2003. doi: 10.1210/en.2003-0150. [DOI] [PubMed] [Google Scholar]
- 10.Brasier AR, Recinos A 3rd, Eledrisi MS. Vascular inflammation and the renin-angiotensin system. Arterioscler Thromb Vasc Biol 22: 1257–1266, 2002. doi: 10.1161/01.atv.0000021412.56621.a2. [DOI] [PubMed] [Google Scholar]
- 11.Fyhrquist F, Saijonmaa O. Renin-angiotensin system revisited. J Intern Med 264: 224–236, 2008. doi: 10.1111/j.1365-2796.2008.01981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol 292: C82–C97, 2007. doi: 10.1152/ajpcell.00287.2006. [DOI] [PubMed] [Google Scholar]
- 13.Paul M, Poyan Mehr A, Kreutz R. Physiology of local renin-angiotensin systems. Physiol Rev 86: 747–803, 2006. doi: 10.1152/physrev.00036.2005. [DOI] [PubMed] [Google Scholar]
- 14.Rossi GP, Sacchetto A, Cesari M, Pessina AC. Interactions between endothelin-1 and the renin-angiotensin-aldosterone system. Cardiovasc Res 43: 300–307, 1999. doi: 10.1016/s0008-6363(99)00110-8. [DOI] [PubMed] [Google Scholar]
- 15.Borghi C, Omboni S. Angiotensin-converting enzyme inhibition: beyond blood pressure control—the role of Zofenopril. Adv Ther 37: 4068–4018, 2020. doi: 10.1007/s12325-020-01455-2. [DOI] [PubMed] [Google Scholar]
- 16.Garg M, Angus PW, Burrell LM, Herath C, Gibson PR, Lubel JS. Review article: the pathophysiological roles of the renin-angiotensin system in the gastrointestinal tract. Aliment Pharmacol Ther 35: 414–428, 2012. doi: 10.1111/j.1365-2036.2011.04971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong TP, Ho KY, Ng EK, Debnam ES, Leung PS. Upregulation of ACE2-ANG-(1-7)-Mas axis in jejunal enterocytes of type 1 diabetic rats: implications for glucose transport. Am J Physiol Endocrinol Metab 303: E669–681, 2012. doi: 10.1152/ajpendo.00562.2011. [DOI] [PubMed] [Google Scholar]
- 18.Brzozowski T. Role of renin-angiotensin system and metabolites of angiotensin in the mechanism of gastric mucosal protection. Curr Opin Pharmacol 19: 90–98, 2014. doi: 10.1016/j.coph.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 19.Pasanen L, Launonen H, Siltari A, Korpela R, Vapaatalo H, Salmenkari H, Forsgard RA. Age-related changes in the local intestinal renin-angiotensin system in normotensive and spontaneously hypertensive rats. J Physiol Pharmacol 70: 199–208, 2019. doi: 10.26402/jpp.2019.2.03. [DOI] [PubMed] [Google Scholar]
- 20.Inokuchi Y, Morohashi T, Kawana I, Nagashima Y, Kihara M, Umemura S. Amelioration of 2,4,6-trinitrobenzene sulphonic acid induced colitis in angiotensinogen gene knockout mice. Gut 54: 349–356, 2005. doi: 10.1136/gut.2003.036343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rossig L, Dimmeler S, Zeiher AM. Apoptosis in the vascular wall and atherosclerosis. Basic Res Cardiol 96: 11–22, 2001. doi: 10.1007/s003950170073. [DOI] [PubMed] [Google Scholar]
- 22.Shorning BY, Jardé T, McCarthy A, Ashworth A, de Leng WW, Offerhaus GJ, Resta N, Dale T, Clarke AR. Intestinal renin-angiotensin system is stimulated after deletion of Lkb1. Gut 61: 202–213, 2012. doi: 10.1136/gutjnl-2011-300046. [DOI] [PubMed] [Google Scholar]
- 23.Duggan KA, Mendelsohn FA, Levens NR. Angiotensin receptors and angiotensin I-converting enzyme in rat intestine. Am J Physiol Gastrointest Liver Physiol 257: G504–G510, 1989. doi: 10.1152/ajpgi.1989.257.4.G504. [DOI] [PubMed] [Google Scholar]
- 24.Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol 203: 631–637, 2004. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ewert S, Spak E, Olbers T, Johnsson E, Edebo A, Fändriks L. Angiotensin II induced contraction of rat and human small intestinal wall musculature in vitro. Acta Physiol (Oxf) 188: 33–40, 2006. doi: 10.1111/j.1748-1716.2006.01600.x. [DOI] [PubMed] [Google Scholar]
- 26.Spak E, Casselbrant A, Olbers T, Lönroth H, Fändriks L. Angiotensin II-induced contractions in human jejunal wall musculature in vitro. Acta Physiol (Oxf) 193: 181–190, 2008. doi: 10.1111/j.1748-1716.2007.01826.x. [DOI] [PubMed] [Google Scholar]
- 27.Avula LR, Buckinx R, Favoreel H, Cox E, Adriaensen D, Van Nassauw L, Timmermans JP. Expression and distribution patterns of Mas-related gene receptor subtypes A-H in the mouse intestine: inflammation-induced changes. Histochem Cell Biol 139: 639–658, 2013. doi: 10.1007/s00418-013-1086-9. [DOI] [PubMed] [Google Scholar]
- 28.Campbell DJ, Habener JF. Angiotensinogen gene is expressed and differentially regulated in multiple tissues of the rat. J Clin Invest 78: 31–39, 1986. doi: 10.1172/JCI112566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hirasawa K, Sato Y, Hosoda Y, Yamamoto T, Hanai H. Immunohistochemical localization of angiotensin II receptor and local renin-angiotensin system in human colonic mucosa. J Histochem Cytochem 50: 275–282, 2002. doi: 10.1177/002215540205000215. [DOI] [PubMed] [Google Scholar]
- 30.Van Remoortel S, Ceuleers H, Arora R, Van Nassauw L, De Man JG, Buckinx R, De Winter BY, Timmermans JP. Mas-related G protein-coupled receptor C11 (Mrgprc11) induces visceral hypersensitivity in the mouse colon: a novel target in gut nociception? Neurogastroenterol Motil 31: e13623, 2019. doi: 10.1111/nmo.13623. [DOI] [PubMed] [Google Scholar]
- 31.Restini CBA, Fink GD, Watts SW. Vascular reactivity stimulated by TMA and TMAO: are perivascular adipose tissue and endothelium involved? Pharmacol Res 163: 105273, 2021. doi: 10.1016/j.phrs.2020.105273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gawrys-Kopczynska M, Konop M, Maksymiuk K, Kraszewska K, Derzsi L, Sozanski K, Holyst R, Pilz M, Samborowska E, Dobrowolski L, Jaworska K, Mogilnicka I, Ufnal M. TMAO, a seafood-derived molecule, produces diuresis and reduces mortality in heart failure rats. Elife 9: e57028, 2020. doi: 10.7554/eLife.57028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Konopelski P, Konop M, Perlejewski K, Bukowska-Osko I, Radkowski M, Onyszkiewicz M, Jaworska K, Mogilnicka I, Samborowska E, Ufnal M. Genetically determined hypertensive phenotype affects gut microbiota composition, but not vice versa. J Hypertens 39: 1790–1799, 2021. doi: 10.1097/HJH.0000000000002864. [DOI] [PubMed] [Google Scholar]
- 34.Muller PA, Schneeberger M, Matheis F, Wang P, Kerner Z, Ilanges A, Pellegrino K, del Mármol J, Castro TBR, Furuichi M, Perkins M, Han W, Rao A, Pickard AJ, Cross JR, Honda K, de Araujo I, Mucida D. Microbiota modulate sympathetic neurons via a gut–brain circuit. Nature 583: 441–446, 2020. doi: 10.1038/s41586-020-2474-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saxena PR. Interaction between the renin-angiotensin-aldosterone and sympathetic nervous systems. J Cardiovasc Pharmacol 19: S80–S88, 1992. doi: 10.1097/00005344-199219006-00013. [DOI] [PubMed] [Google Scholar]
- 36.Karbach SH, Schönfelder T, Brandão I, Wilms E, Hörmann N, Jäckel S, Schüler R, Finger S, Knorr M, Lagrange J, Brandt M, Waisman A, Kossmann S, Schäfer K, Münzel T, Reinhardt C, Wenzel P. Gut microbiota promote angiotensin II–induced arterial hypertension and vascular dysfunction. J Am Heart Assoc 5: e003698, 2016. doi: 10.1161/JAHA.116.003698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kawase M, Hashimoto H, Hosoda M, Morita H, Hosono A. Effect of administration of fermented milk containing whey protein concentrate to rats and healthy men on serum lipids and blood pressure. J Dairy Sci 83: 255–263, 2000. doi: 10.3168/jds.S0022-0302(00)74872-7. [DOI] [PubMed] [Google Scholar]
- 38.Seppo L, Jauhiainen T, Poussa T, Korpela R. A fermented milk high in bioactive peptides has a blood pressure-lowering effect in hypertensive subjects. Am J Clin Nutr 77: 326–330, 2003. doi: 10.1093/ajcn/77.2.326. [DOI] [PubMed] [Google Scholar]
- 39.Aihara K, Kajimoto O, Hirata H, Takahashi R, Nakamura Y. Effect of powdered fermented milk with Lactobacillus helveticus on subjects with high-normal blood pressure or mild hypertension. J Am Coll Nutr 24: 257–265, 2005. doi: 10.1080/07315724.2005.10719473. [DOI] [PubMed] [Google Scholar]
- 40.Rivière G, Michaud A, Corradi HR, Sturrock ED, Acharya KR, Cogez V, Bohin J-P, Vieau D, Corvol P. Characterization of the first angiotensin-converting like enzyme in bacteria: ancestor ACE is already active. Gene 399: 81–90, 2007. doi: 10.1016/j.gene.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dave LA, Hayes M, Montoya CA, Rutherfurd SM, Moughan PJ. Human gut endogenous proteins as a potential source of angiotensin-I-converting enzyme (ACE-I)-, renin inhibitory and antioxidant peptides. Peptides 76: 30–44, 2016. doi: 10.1016/j.peptides.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 42.Donkor ON, Henriksson A, Vasiljevic T, Shah NP. Proteolytic activity of dairy lactic acid bacteria and probiotics as determinant of growth and in vitro angiotensin-converting enzyme inhibitory activity in fermented milk. Dairy Sci Technol 87: 21–38, 2007. doi: 10.1051/lait:2006023. [DOI] [Google Scholar]
- 43.Gonzalez-Gonzalez C, Gibson T, Jauregi P. Novel probiotic-fermented milk with angiotensin I-converting enzyme inhibitory peptides produced by Bifidobacterium bifidum MF 20/5. Int J Food Microbiol 167: 131–137, 2013. doi: 10.1016/j.ijfoodmicro.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 44.Nakamura Y, Yamamoto N, Sakai K, Okubo A, Yamazaki S, Takano T. Purification and characterization of angiotensin I-converting enzyme inhibitors from sour milk. J Dairy Sci 78: 777–783, 1995. doi: 10.3168/jds.S0022-0302(95)76689-9. [DOI] [PubMed] [Google Scholar]
- 45.Nakamura Y, Yamamoto N, Sakai K, Takano T. Antihypertensive effect of sour milk and peptides isolated from it that are inhibitors to angiotensin I-converting enzyme. J Dairy Sci 78: 1253–1257, 1995. doi: 10.3168/jds.S0022-0302(95)76745-5. [DOI] [PubMed] [Google Scholar]
- 46.Griffiths MW, Tellez AM. Lactobacillus helveticus: the proteolytic system. Front Microbiol 4: 30, 2013. doi: 10.3389/fmicb.2013.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ramchandran L, Shah NP. Proteolytic profiles and angiotensin-I converting enzyme and alpha-glucosidase inhibitory activities of selected lactic acid bacteria. J Food Sci 73: M75–M81, 2008. doi: 10.1111/j.1750-3841.2007.00643.x. [DOI] [PubMed] [Google Scholar]
- 48.Hsu C-N, Hou C-Y, Chan JY, Lee C-T, Tain Y-L. Hypertension programmed by perinatal high-fat diet: effect of maternal gut microbiota-targeted therapy. Nutrients 11: 2908, 2019. doi: 10.3390/nu11122908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang L, Zhu Q, Lu A, Liu X, Zhang L, Xu C, Liu X, Li H, Yang T. Sodium butyrate suppresses angiotensin II-induced hypertension by inhibition of renal (pro) renin receptor and intrarenal renin–angiotensin system. J Hypertens 35: 1899–1908, 2017. doi: 10.1097/HJH.0000000000001378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marques FZ, Nelson E, Chu P-Y, Horlock D, Fiedler A, Ziemann M, Tan JK, Kuruppu S, Rajapakse NW, El-Osta A, Mackay CR, Kaye DM. High-fiber diet and acetate supplementation change the gut microbiota and prevent the development of hypertension and heart failure in hypertensive mice. Circulation 135: 964–977, 2017. doi: 10.1161/CIRCULATIONAHA.116.024545. [DOI] [PubMed] [Google Scholar]
- 51.Toma I, Kang JJ, Sipos A, Vargas S, Bansal E, Hanner F, Meer E, Peti-Peterdi J. Succinate receptor GPR91 provides a direct link between high glucose levels and renin release in murine and rabbit kidney. J Clin Invest 118: 2526–2534, 2008. doi: 10.1172/JCI33293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sun C-Y, Chang S-C, Wu M-S. Uremic toxins induce kidney fibrosis by activating intrarenal renin–angiotensin–aldosterone system associated epithelial-to-mesenchymal transition. PLoS One 7: e34026, 2012. doi: 10.1371/journal.pone.0034026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ufnal M, Jazwiec R, Dadlez M, Drapala A, Sikora M, Skrzypecki J. Trimethylamine-N-oxide: a carnitine-derived metabolite that prolongs the hypertensive effect of angiotensin II in rats. Can J Cardiol 30: 1700–1705, 2014. doi: 10.1016/j.cjca.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 54.Cheema MU, Pluznick JL. Gut microbiota plays a central role to modulate the plasma and fecal metabolomes in response to angiotensin II. Hypertension 74: 184–193, 2019. doi: 10.1161/HYPERTENSIONAHA.119.13155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang C, Zhang Y-P, Li Y-Y, Liu B-P, Wang H-Y, Li K-W, Zhao S, Song C. Minocycline ameliorates depressive behaviors and neuro-immune dysfunction induced by chronic unpredictable mild stress in the rat. Behav Brain Res 356: 348–357, 2019. doi: 10.1016/j.bbr.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 56.Jaworska K, Huc T, Samborowska E, Dobrowolski L, Bielinska K, Gawlak M, Ufnal M. Hypertension in rats is associated with an increased permeability of the colon to TMA, a gut bacteria metabolite. PLoS One 12: e0189310, 2017. doi: 10.1371/journal.pone.0189310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Levens NR, Peach MJ, Carey RM. Interactions between angiotensin peptides and the sympathetic nervous system mediating intestinal sodium and water absorption in the rat. J Clin Invest 67: 1197–1207, 1981. doi: 10.1172/jci110135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Levens NR. Control of intestinal absorption by the renin-angiotensin system. Am J Physiol Gastrointest Liver Physiol 249: G3–G15, 1985. doi: 10.1152/ajpgi.1985.249.1.G3. [DOI] [PubMed] [Google Scholar]
- 59.Jin XH, Wang ZQ, Siragy HM, Guerrant RL, Carey RM. Regulation of jejunal sodium and water absorption by angiotensin subtype receptors. Am J Physiol Regulat Comp Integrat Physiol 275: R515–R523, 1998. doi: 10.1152/ajpregu.1998.275.2.R515. [DOI] [PubMed] [Google Scholar]
- 60.de los Rios AD, Labajos M, Manteca A, Morell M, Souviron A. Stimulatory action of angiotensin II on water and electrolyte transport by the proximal colon of the rat. J Endocrinol 86: 35–43, 1980. doi: 10.1677/joe.0.0860035. [DOI] [PubMed] [Google Scholar]
- 61.Lu C-C, Hu Z-B, Wang R, Hong Z-Hh, Lu J, Chen P-P, Zhang J-X, Li X-Q, Yuan B-Y, and Huang S-J. Gut microbiota dysbiosis-induced activation of the intrarenal renin-angiotensin system is involved in kidney injuries in rat diabetic nephropathy. Acta Pharmacologica Sinica 1–8, 2020. doi: 10.1038/s41401-019-0326-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Larmonier CB, Laubitz D, Hill FM, Shehab KW, Lipinski L, Midura-Kiela MT, McFadden R-MT, Ramalingam R, Hassan KA, Golebiewski M, Besselsen DG, Ghishan FK, Kiela PR. Reduced colonic microbial diversity is associated with colitis in NHE3-deficient mice. Am J Physiol Gastrointest Liver Physiol 305: G667–G677, 2013. doi: 10.1152/ajpgi.00189.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pluznick JL, Protzko RJ, Gevorgyan H, Peterlin Z, Sipos A, Han J, Brunet I, Wan L-X, Rey F, Wang T, Firestein SJ, Yanagisawa M, Gordon JI, Eichmann A, Peti-Peterdi J, Caplan MJ. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc Natl Acad Sci USA 110: 4410–4415, 2013. doi: 10.1073/pnas.1215927110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bai JP. Distribution of brush-border membrane peptidases along the rat intestine. Pharm Res 11: 897–900, 1994. doi: 10.1023/a:1018946228432. [DOI] [PubMed] [Google Scholar]
- 65.Hashimoto T, Perlot T, Rehman A, Trichereau J, Ishiguro H, Paolino M, Sigl V, Hanada T, Hanada R, Lipinski S, Wild B, Camargo SMR, Singer D, Richter A, Kuba K, Fukamizu A, Schreiber S, Clevers H, Verrey F, Rosenstiel P, Penninger JM. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature 487: 477–481, 2012. doi: 10.1038/nature11228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Borges EL, Lima PB, Peluso AA, Sampaio WO, de Oliveira JS, de Oliveira ML, Etelvino GM, Ruoccolo RT, Ferreira AJ, Santos RA. Angiotensin-(1-7) influences tryptophan absorption in the rat and mouse intestine. J Adv Med Med Res 19: 1–9, 2017. doi: 10.9734/BJMMR/2017/30329. [DOI] [Google Scholar]
- 67.Oliveira LP, Guimarães VHD, Oliveira JR, Guimarães ALS, de Paula AMB, Bader M, Dos Santos RAS, Santos SHS. Genetic deletion of the angiotensin-(1–7) receptor mas leads to alterations in gut villi length modulating TLR4/PI3K/AKT and produces microbiome dysbiosis. Neuropeptides 82: 102056, 2020. doi: 10.1016/j.npep.2020.102056. [DOI] [PubMed] [Google Scholar]
- 68.Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 490: 55–60, 2012. doi: 10.1038/nature11450. [DOI] [PubMed] [Google Scholar]
- 69.Allin KH, Tremaroli V, Caesar R, Jensen BAH, Damgaard MTF, Bahl MI, Licht TR, Hansen TH, Nielsen T, Dantoft TM, Linneberg A, Jørgensen T, Vestergaard H, Kristiansen K, Franks PW, Consortium I-D, Hansen T, Bäckhed F, Pedersen O; IMI-DIRECT consortium. Aberrant intestinal microbiota in individuals with prediabetes. Diabetologia 61: 810–820, 2018. doi: 10.1007/s00125-018-4550-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tolhurst G, Heffron H, Lam YS, Parker HE, Habib AM, Diakogiannaki E, Cameron J, Grosse J, Reimann F, Gribble FM. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes 61: 364–371, 2012. doi: 10.2337/db11-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gulnaz A, Nadeem J, Han J-H, Lew L-C, Son J-D, Park Y-H, Rather IA, Hor Y-Y. Lactobacillus Sps in reducing the risk of diabetes in high-fat diet-induced diabetic mice by modulating the gut microbiome and inhibiting key digestive enzymes associated with diabetes. Biology 10: 348, 2021. doi: 10.3390/biology10040348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dang F, Jiang Y, Pan R, Zhou Y, Wu S, Wang R, Zhuang K, Zhang W, Li T, Man C. Administration of Lactobacillus paracasei ameliorates type 2 diabetes in mice. Food Funct 9: 3630–3639, 2018. doi: 10.1039/c8fo00081f. [DOI] [PubMed] [Google Scholar]
- 73.Fandriks L. The renin-angiotensin system and the gastrointestinal mucosa. Acta Physiol (Oxf) 201: 157–167, 2011. doi: 10.1111/j.1748-1716.2010.02165.x. [DOI] [PubMed] [Google Scholar]
- 74.Debnam ES, Karasov WH, Thompson CS. Nutrient uptake by rat enterocytes during diabetes mellitus; evidence for an increased sodium electrochemical gradient. J Physiol 397: 503–512, 1988. doi: 10.1113/jphysiol.1988.sp017015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wong TP, Debnam ES, Leung PS. Involvement of an enterocyte renin-angiotensin system in the local control of SGLT1-dependent glucose uptake across the rat small intestinal brush border membrane. J Physiol 584: 613–623, 2007. doi: 10.1113/jphysiol.2007.138578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wong TP, Debnam ES, Leung PS. Diabetes mellitus and expression of the enterocyte renin-angiotensin system: implications for control of glucose transport across the brush border membrane. Am J Physiol Cell Physiol 297: C601–C610, 2009. doi: 10.1152/ajpcell.00135.2009. [DOI] [PubMed] [Google Scholar]
- 77.Machado AS, Oliveira JR, Lelis DF, de Paula AMB, Guimarães ALS, Andrade JMO, Brandi IV, Santos SHS. Oral probiotic bifidobacterium longum supplementation improves metabolic parameters and alters the expression of the renin-angiotensin system in obese mice liver. Biol Res Nurs 23: 100–108, 2021. doi: 10.1177/1099800420942942. [DOI] [PubMed] [Google Scholar]
- 78.Verma A, Xu K, Du T, Zhu P, Liang Z, Liao S, Zhang J, Raizada MK, Grant MB, Li Q. Expression of human ACE2 in Lactobacillus and beneficial effects in diabetic retinopathy in mice. Mol Ther Methods Clin Dev 14: 161–170, 2019. [Erratum in Mol Ther Methods Clin Dev 17: 400, 2020]. doi: 10.1016/j.omtm.2019.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Verma A, Zhu P, Xu K, Du T, Liao S, Liang Z, Raizada MK, Li Q. Angiotensin-(1–7) expressed from Lactobacillus bacteria protect diabetic retina in mice. Transl Vis Sci Technol 9: 20, 2020. doi: 10.1167/tvst.9.13.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Podolsky DK. Inflammatory bowel disease (1). N Engl J Med 325: 928–937, 1991. doi: 10.1056/NEJM199109263251306. [DOI] [PubMed] [Google Scholar]
- 81.Blander JM, Longman RS, Iliev ID, Sonnenberg GF, Artis D. Regulation of inflammation by microbiota interactions with the host. Nat Immunol 18: 851–860, 2017. doi: 10.1038/ni.3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Paramsothy S, Kamm MA, Kaakoush NO, Walsh AJ, van den Bogaerde J, Samuel D, Leong RWL, Connor S, Ng W, Paramsothy R, Xuan W, Lin E, Mitchell HM, Borody TJ. Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: a randomised placebo-controlled trial. The Lancet 389: 1218–1228, 2017. doi: 10.1016/S0140-6736(17)30182-4. [DOI] [PubMed] [Google Scholar]
- 83.Shi Y, Liu T, He L, Dougherty U, Chen L, Adhikari S, Alpert L, Zhou G, Liu W, Wang J, Deb DK, Hart J, Liu SQ, Kwon J, Pekow J, Rubin DT, Zhao Q, Bissonnette M, Li YC. Activation of the renin-angiotensin system promotes colitis development. Sci Rep 6: 27552, 2016. doi: 10.1038/srep27552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Salmenkari H, Pasanen L, Linden J, Korpela R, Vapaatalo H. Beneficial anti-inflammatory effect of angiotensin-converting enzyme inhibitor and angiotensin receptor blocker in the treatment of dextran sulfate sodium-induced colitis in mice. J Physiol Pharmacol 69: 1–12, 2018. doi: 10.26402/jpp.2018.4.07. [DOI] [PubMed] [Google Scholar]
- 85.Spencer AU, Yang H, Haxhija EQ, Wildhaber BE, Greenson JK, Teitelbaum DH. Reduced severity of a mouse colitis model with angiotensin converting enzyme inhibition. Dig Dis Sci 52: 1060–1070, 2007. doi: 10.1007/s10620-006-9124-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mizushima T, Sasaki M, Ando T, Wada T, Tanaka M, Okamoto Y, Ebi M, Hirata Y, Murakami K, Mizoshita T, Shimura T, Kubota E, Ogasawara N, Tanida S, Kataoka H, Kamiya T, Alexander JS, Joh T. Blockage of angiotensin II type 1 receptor regulates TNF-alpha-induced MAdCAM-1 expression via inhibition of NF-kappaB translocation to the nucleus and ameliorates colitis. Am J Physiol Gastrointest Liver Physiol 298: G255–G266, 2010. doi: 10.1152/ajpgi.00264.2009. [DOI] [PubMed] [Google Scholar]
- 87.He L, Du J, Chen Y, Liu C, Zhou M, Adhikari S, Rubin DT, Pekow J, Li YC. Renin-angiotensin system promotes colonic inflammation by inducing TH17 activation via JAK2/STAT pathway. Am J Physiol Gastrointest Liver Physiol 316: G774–G784, 2019. doi: 10.1152/ajpgi.00053.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Platten M, Youssef S, Hur EM, Ho PP, Han MH, Lanz TV, Phillips LK, Goldstein MJ, Bhat R, Raine CS, Sobel RA, Steinman L. Blocking angiotensin-converting enzyme induces potent regulatory T cells and modulates TH1- and TH17-mediated autoimmunity. Proc Natl Acad Sci USA 106: 14948–14953, 2009. doi: 10.1073/pnas.0903958106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Britton GJ, Contijoch EJ, Mogno I, Vennaro OH, Llewellyn SR, Ng R, Li Z, Mortha A, Merad M, Das A, Gevers D, McGovern DPB, Singh N, Braun J, Jacobs JP, Clemente JC, Grinspan A, Sands BE, Colombel J-F, Dubinsky MC, Faith JJ. Microbiotas from humans with inflammatory bowel disease alter the balance of gut Th17 and RORγt+ regulatory T cells and exacerbate colitis in mice. Immunity 50: 212–224.e4, 2019. doi: 10.1016/j.immuni.2018.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Britton GJ, Contijoch EJ, Spindler MP, Aggarwala V, Dogan B, Bongers G, San Mateo L, Baltus A, Das A, Gevers D, Borody TJ, Kaakoush NO, Kamm MA, Mitchell H, Paramsothy S, Clemente JC, Colombel J-F, Simpson KW, Dubinsky MC, Grinspan A, Faith JJ. Defined microbiota transplant restores Th17/RORγt+ regulatory T cell balance in mice colonized with inflammatory bowel disease microbiotas. Proc Natl Acad Sci USA 117: 21536–21545, 2020. doi: 10.1073/pnas.1922189117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Viladomiu M, Kivolowitz C, Abdulhamid A, Dogan B, Victorio D, Castellanos JG, Woo V, Teng F, Tran NL, Sczesnak A. IgA-coated E. coli enriched in Crohn's disease spondyloarthritis promote TH17-dependent inflammation. Sci Transl Med 9, 2017. doi: 10.1126/scitranslmed.aaf9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sandborn WJ, Colombel JF, Enns R, Feagan BG, Hanauer SB, Lawrance IC, Panaccione R, Sanders M, Schreiber S, Targan S, van Deventer S, Goldblum R, Despain D, Hogge GS, Rutgeerts P; International Efficacy of Natalizumab as Active Crohn's Therapy Trial Group; Evaluation of Natalizumab as Continuous Therapy Trial Group. Natalizumab induction and maintenance therapy for Crohn's disease. N Engl J Med 353: 1912–1925, 2005. doi: 10.1056/NEJMoa043335. [DOI] [PubMed] [Google Scholar]
- 93.Sasaki M, Bharwani S, Jordan P, Joh T, Manas K, Warren A, Harada H, Carter P, Elrod JW, Wolcott M, Grisham MB, Alexander JS. The 3-hydroxy-3-methylglutaryl-CoA reductase inhibitor pravastatin reduces disease activity and inflammation in dextran-sulfate induced colitis. J Pharmacol Exp Ther 305: 78–85, 2003. doi: 10.1124/jpet.102.044099. [DOI] [PubMed] [Google Scholar]
- 94.Rai RP, Liu Y, Iyer SS, Liu S, Gupta B, Desai C, Kumar P, Smith T, Singhi AD, Nusrat A, Parkos CA, Monga SP, Czaja MJ, Anania FA, Raeman R. Blocking integrin α4β7-mediated CD4 T cell recruitment to the intestine and liver protects mice from western diet-induced non-alcoholic steatohepatitis. J Hepatol 73: 1013–1022, 2020. doi: 10.1016/j.jhep.2020.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cheng Y, Tang S, Huang Y, Liang F, Fang Y, Pan S, Wu T, Xu X. Lactobacillus casei-fermented blueberry pomace augments sIgA production in high-fat diet mice by improving intestinal microbiota. Food Funct 11: 6552–6564, 2020. doi: 10.1039/d0fo01119c. [DOI] [PubMed] [Google Scholar]
- 96.Shigematsu T, Specian RD, Wolf RE, Grisham MB, Granger DN. MAdCAM mediates lymphocyte-endothelial cell adhesion in a murine model of chronic colitis. Am J Physiol Gastrointest Liver Physiol 281: G1309–G1315, 2001. doi: 10.1152/ajpgi.2001.281.5.G1309. [DOI] [PubMed] [Google Scholar]
- 97.Brzozowski T, Ptak-Belowska A, Kwiecien S, Krzysiek-Maczka G, Strzalka M, Drozdowicz D, Pajdo R, Olszanecki R, Korbut R, Konturek SJ, Pawlik WW. Novel concept in the mechanism of injury and protection of gastric mucosa: role of renin-angiotensin system and active metabolites of angiotensin. Curr Med Chem 19: 55–62, 2012. doi: 10.2174/092986712803413953. [DOI] [PubMed] [Google Scholar]
- 98.Ye R, Liu Z. ACE2 exhibits protective effects against LPS-induced acute lung injury in mice by inhibiting the LPS-TLR4 pathway. Exp Mol Pathol 113: 104350, 2020. doi: 10.1016/j.yexmp.2019.104350. [DOI] [PubMed] [Google Scholar]
- 99.Lupp C, Robertson ML, Wickham ME, Sekirov I, Champion OL, Gaynor EC, Finlay BB. Host-mediated inflammation disrupts the intestinal microbiota and promotes the overgrowth of Enterobacteriaceae. Cell Host Microbe 2: 119–129, 2007. doi: 10.1016/j.chom.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 100.Carvalho FA, Koren O, Goodrich JK, Johansson MEV, Nalbantoglu I, Aitken JD, Su Y, Chassaing B, Walters WA, González A, Clemente JC, Cullender TC, Barnich N, Darfeuille-Michaud A, Vijay-Kumar M, Knight R, Ley RE, Gewirtz AT. Transient inability to manage proteobacteria promotes chronic gut inflammation in TLR5-deficient mice. Cell Host Microbe 12: 139–152, 2012. doi: 10.1016/j.chom.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tsilimigras MC, Fodor A, Jobin C. Carcinogenesis and therapeutics: the microbiota perspective. Nat Microbiol 2: 1–10, 2017. doi: 10.1038/nmicrobiol.2017.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Feng Q, Liang S, Jia H, Stadlmayr A, Tang L, Lan Z, Zhang D, Xia H, Xu X, Jie Z, Su L, Li X, Li X, Li J, Xiao L, Huber-Schönauer U, Niederseer D, Xu X, Al-Aama JY, Yang H, Wang J, Kristiansen K, Arumugam M, Tilg H, Datz C, Wang J. Gut microbiome development along the colorectal adenoma–carcinoma sequence. Nat Commun 6: 6528, 2015. doi: 10.1038/ncomms7528. [DOI] [PubMed] [Google Scholar]
- 103.Hussen BM, Al-Marzoqi AH, Ghasemian A. Assessment of oncogenic role of intestinal microbiota in colorectal cancer patients. J Gastrointest Cancer 52:1016–1021, 2020. doi: 10.1007/s12029-020-00531-8. [DOI] [PubMed] [Google Scholar]
- 104.Sun C-H, Li B-B, Wang B, Zhao J, Zhang X-Y, Li T-T, Li W-B, Tang D, Qiu M-J, Wang X-C, Zhu C-M, Qian Z-R. The role of Fusobacterium nucleatum in colorectal cancer: from carcinogenesis to clinical management. Chronic Dis Transl Med 5: 178–187, 2019. doi: 10.1016/j.cdtm.2019.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Viljoen KS, Dakshinamurthy A, Goldberg P, Blackburn JM. Quantitative profiling of colorectal cancer-associated bacteria reveals associations between fusobacterium spp., enterotoxigenic Bacteroides fragilis (ETBF) and clinicopathological features of colorectal cancer. PLoS One 10: e0119462, 2015. doi: 10.1371/journal.pone.0119462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhu W, Miyata N, Winter MG, Arenales A, Hughes ER, Spiga L, Kim J, Sifuentes-Dominguez L, Starokadomskyy P, Gopal P, Byndloss MX, Santos RL, Burstein E, Winter SE. Editing of the gut microbiota reduces carcinogenesis in mouse models of colitis-associated colorectal cancer. J Exp Med 216: 2378–2393, 2019. doi: 10.1084/jem.20181939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Louis P, Hold GL, Flint HJ. The gut microbiota, bacterial metabolites and colorectal cancer. Nat Rev Microbiol 12: 661–672, 2014. doi: 10.1038/nrmicro3344. [DOI] [PubMed] [Google Scholar]
- 108.Deshayes F, Nahmias C. Angiotensin receptors: a new role in cancer? Trends Endocrinol Metab 16: 293–299, 2005. doi: 10.1016/j.tem.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 109.Pupilli C, Lasagni L, Romagnani P, Bellini F, Mannelli M, Misciglia N, Mavilia C, Vellei U, Villari D, Serio M. Angiotensin II stimulates the synthesis and secretion of vascular permeability factor/vascular endothelial growth factor in human mesangial cells. J Am Soc Nephrol 10: 245–255, 1999. doi: 10.1681/ASN.V102245. [DOI] [PubMed] [Google Scholar]
- 110.Masanobu F, Yutaka K, Mitsuyama K, Andoh A, Aoyama T, Matsumoto Y, Kanauchi O. Prebiotic treatment reduced preneoplastic lesions through the downregulation of toll like receptor 4 in a chemo-induced carcinogenic model. J Clin Biochem Nutr 49: 57–61, 2011. doi: 10.3164/jcbn.10-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wu Y, Wu J, Chen T, Li Q, Peng W, Li H, Tang X, Fu X. Fusobacterium nucleatum potentiates intestinal tumorigenesis in mice via a Toll-like receptor 4/p21-activated kinase 1 cascade. Dig Dis Sci 63: 1210–1218, 2018. doi: 10.1007/s10620-018-4999-2. [DOI] [PubMed] [Google Scholar]
- 112.Zaidi AH, Kelly LA, Kreft RE, Barlek M, Omstead AN, Matsui D, Boyd NH, Gazarik KE, Heit MI, Nistico L, Kasi PM, Spirk TL, Byers B, Lloyd EJ, Landreneau RJ, Jobe BA. Associations of microbiota and toll-like receptor signaling pathway in esophageal adenocarcinoma. BMC Cancer 16: 52, 2016. doi: 10.1186/s12885-016-2093-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shirai Y, Yoshiji H, Noguchi R, Kaji K, Aihara Y, Douhara A, Moriya K, Namisaki T, Kawaratani H, Fukui H. Cross talk between toll‐like receptor‐4 signaling and angiotensin‐II in liver fibrosis development in the rat model of non‐alcoholic steatohepatitis. J Gastroenterol Hepatol 28: 723–730, 2013. doi: 10.1111/jgh.12112. [DOI] [PubMed] [Google Scholar]
- 114.Sawada Y, Kawaratani H, Kubo T, Fujinaga Y, Furukawa M, Saikawa S, Sato S, Seki K, Takaya H, Okura Y, Kaji K, Shimozato N, Mashitani T, Kitade M, Moriya K, Namisaki T, Akahane T, Mitoro A, Yamao J, Yoshiji H. Combining probiotics and an angiotensin‐II type 1 receptor blocker has beneficial effects on hepatic fibrogenesis in a rat model of non‐alcoholic steatohepatitis. Hepatol Res 49: 284–295, 2019. doi: 10.1111/hepr.13281. [DOI] [PubMed] [Google Scholar]
- 115.Yasumatsu R, Nakashima T, Masuda M, Ito A, Kuratomi Y, Nakagawa T, Komune S. Effects of the angiotensin-I converting enzyme inhibitor perindopril on tumor growth and angiogenesis in head and neck squamous cell carcinoma cells. J Cancer Res Clin Oncol 130: 567–573, 2004. doi: 10.1007/s00432-004-0582-7. [DOI] [PubMed] [Google Scholar]
- 116.Neo JH, Malcontenti-Wilson C, Muralidharan V, Christophi C. Effect of ACE inhibitors and angiotensin II receptor antagonists in a mouse model of colorectal cancer liver metastases. J Gastroenterol Hepatol 22: 577–584, 2007. doi: 10.1111/j.1440-1746.2006.04797.x. [DOI] [PubMed] [Google Scholar]
- 117.Schirbel A, Kessler S, Rieder F, West G, Rebert N, Asosingh K, McDonald C, Fiocchi C. Pro-angiogenic activity of TLRs and NLRs: a novel link between gut microbiota and intestinal angiogenesis. Gastroenterology 144: 613–623.e9, 2013. doi: 10.1053/j.gastro.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Li J, Sung CYJ, Lee N, Ni Y, Pihlajamäki J, Panagiotou G, El-Nezami H. Probiotics modulated gut microbiota suppresses hepatocellular carcinoma growth in mice. Proc Natl Acad Sci USA 113: E1306–E1315, 2016. doi: 10.1073/pnas.1518189113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Xu J, Fan J, Wu F, Huang Q, Guo M, Lv Z, Han J, Duan L, Hu G, Chen L, Liao T, Ma W, Tao X, Jin Y. The ACE2/angiotensin-(1–7)/Mas receptor axis: pleiotropic roles in cancer. Front Physiol 8: 276, 2017. doi: 10.3389/fphys.2017.00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zheng S, Yang Y, Song R, Yang X, Liu H, Ma Q, Yang L, Meng R, Tao T, Wang S, He J. Ang-(1-7) promotes the migration and invasion of human renal cell carcinoma cells via Mas-mediated AKT signaling pathway. Biochem Biophys Res Commun 460: 333–340, 2015. doi: 10.1016/j.bbrc.2015.03.035. [DOI] [PubMed] [Google Scholar]
- 121.Feng Z, Wang Y, Qi W. The small intestine, an underestimated site of SARS-CoV-2 infection: from red queen effect to probiotics. Preprints 2020030161, 2020. doi: 10.20944/preprints202003.0161.v1. [DOI] [Google Scholar]
- 122.Yang T, Chakraborty S, Saha P, Mell B, Cheng X, Yeo J-Y, Mei X, Zhou G, Mandal J, Golonka R, Yeoh BS, Putluri V, Piyarathna DWB, Putluri N, McCarthy CG, Wenceslau CF, Sreekumar A, Gewirtz AT, Vijay-Kumar M, Joe B. Gnotobiotic rats reveal that gut microbiota regulates colonic mRNA of Ace2, the receptor for SARS-CoV-2 infectivity. Hypertension 76: e1–e3, 2020. doi: 10.1161/HYPERTENSIONAHA.120.15360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Koester ST, Li N, Lachance DM, Morella NM, Dey N. Variability in digestive and respiratory tract Ace2 expression is associated with the microbiome. PLoS One 16: e0248730, 2021. doi: 10.1371/journal.pone.0248730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bao R, Hernandez K, Huang L, Luke JJ. ACE2 and TMPRSS2 expression by clinical, HLA, immune, and microbial correlates across 34 human cancers and matched normal tissues: implications for SARS-CoV-2 COVID-19. J Immunother Cancer 8, 2020. doi: 10.1136/jitc-2020-001020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ren WY, Wu KF, Li X, Luo M, Liu HC, Zhang SC, Hu Y. Age-related changes in small intestinal mucosa epithelium architecture and epithelial tight junction in rat models. Aging Clin Exp Res 26: 183–191, 2014. doi: 10.1007/s40520-013-0148-0. [DOI] [PubMed] [Google Scholar]
- 126.Elderman M, Sovran B, Hugenholtz F, Graversen K, Huijskes M, Houtsma E, Belzer C, Boekschoten M, de Vos P, Dekker J, Wells J, Faas M. The effect of age on the intestinal mucus thickness, microbiota composition and immunity in relation to sex in mice. PLoS One 12: e0184274, 2017. doi: 10.1371/journal.pone.0184274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Thevaranjan N, Puchta A, Schulz C, Naidoo A, Szamosi JC, Verschoor CP, Loukov D, Schenck LP, Jury J, Foley KP, Schertzer JD, Larché MJ, Davidson DJ, Verdú EF, Surette MG, Bowdish DME. Age-associated microbial dysbiosis promotes intestinal permeability, systemic inflammation, and macrophage dysfunction. Cell Host Microbe 21: 455–466.e4, 2017. doi: 10.1016/j.chom.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Garrido-Gil P, Dominguez-Meijide A, Moratalla R, Guerra MJ, Labandeira-Garcia JL. Aging-related dysregulation in enteric dopamine and angiotensin system interactions: implications for gastrointestinal dysfunction in the elderly. Oncotarget 9: 10834–10846, 2018.doi: 10.18632/oncotarget.24330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Li H, Qi Y, Jasper H. Preventing age-related decline of gut compartmentalization limits microbiota dysbiosis and extends lifespan. Cell Host Microbe 19: 240–253, 2016. doi: 10.1016/j.chom.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Biagi E, Franceschi C, Rampelli S, Severgnini M, Ostan R, Turroni S, Consolandi C, Quercia S, Scurti M, Monti D, Capri M, Brigidi P, Candela M. Gut microbiota and extreme longevity. Curr Biol 26: 1480–1485, 2016.doi: 10.1016/j.cub.2016.04.016. [DOI] [PubMed] [Google Scholar]
- 131.Claesson MJ, Cusack S, O'Sullivan O, Greene-Diniz R, de Weerd H, Flannery E, Marchesi JR, Falush D, Dinan T, Fitzgerald G, Stanton C, van Sinderen D, O'Connor M, Harnedy N, O'Connor K, Henry C, O'Mahony D, Fitzgerald AP, Shanahan F, Twomey C, Hill C, Ross RP, O'Toole PW. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc Natl Acad Sci USA 108, Suppl 1: 4586–4591, 2011. doi: 10.1073/pnas.1000097107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bárcena C, Valdés-Mas R, Mayoral P, Garabaya C, Durand S, Rodríguez F, Fernández-García MT, Salazar N, Nogacka AM, Garatachea N, Bossut N, Aprahamian F, Lucia A, Kroemer G, Freije JMP, Quirós PM, López-Otín C. Healthspan and lifespan extension by fecal microbiota transplantation into progeroid mice. Nat Med 25: 1234–1242, 2019. doi: 10.1038/s41591-019-0504-5. [DOI] [PubMed] [Google Scholar]
- 133.Smith P, Willemsen D, Popkes M, Metge F, Gandiwa E, Reichard M, Valenzano DR. Regulation of life span by the gut microbiota in the short-lived African turquoise killifish. Elife 6: e27014, 2017. doi: 10.7554/eLife.27014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Carter CS, Morgan D, Verma A, Lobaton G, Aquino V, Sumners E, Raizada M, Li Q, Buford TW. Therapeutic delivery of Ang (1–7) via genetically modified probiotic: a dosing study. J Gerontol A Biol Sci Med Sci 75: 1299–1303, 2020. doi: 10.1093/gerona/glz222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Letko M, Marzi A, Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat Microbiol 5: 562–569, 2020. doi: 10.1038/s41564-020-0688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lugito NPH, Kurniawan A, Damay V, Chyntya H, Sugianto N. The role of gut microbiota in SARS-CoV-2 infection: focus on angiotensin-converting enzyme 2. Curr Med Issues 18: 261, 2020. doi: 10.4103/cmi.cmi_80_20. [DOI] [Google Scholar]
- 137.Zang R, Gomez Castro MF, McCune BT, Zeng Q, Rothlauf PW, Sonnek NM, Liu Z, Brulois KF, Wang X, Greenberg HB, Diamond MS, Ciorba MA, Whelan SPJ, Ding S. TMPRSS2 and TMPRSS4 promote SARS-CoV-2 infection of human small intestinal enterocytes. Sci Immunol 5, 2020. doi: 10.1126/sciimmunol.abc3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lei Y, Zhang J, Schiavon CR, He M, Chen L, Shen H, Zhang Y, Yin Q, Cho Y, Andrade L, Shadel GS, Hepokoski M, Lei T, Wang H, Zhang J, Yuan JX-J, Malhotra A, Manor U, Wang S, Yuan Z-Y, Shyy JY-J. SARS-CoV-2 spike protein impairs endothelial function via downregulation of ACE 2. Circ Res 128: 1323–1326, 2021. doi: 10.1161/CIRCRESAHA.121.318902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Penninger JM, Grant MB, Sung JJY. The role of angiotensin converting enzyme 2 in modulating gut microbiota, intestinal inflammation, and coronavirus infection. Gastroenterology 160: 39–46, 2021. doi: 10.1053/j.gastro.2020.07.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gurwitz D. Angiotensin receptor blockers as tentative SARS-CoV-2 therapeutics. Drug Dev Res 81: 537–540, 2020. doi: 10.1002/ddr.21656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Viana SD, Nunes S, Reis F. ACE2 imbalance as a key player for the poor outcomes in COVID-19 patients with age-related comorbidities - Role of gut microbiota dysbiosis. Ageing Res Rev 62: 101123, 2020. doi: 10.1016/j.arr.2020.101123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gheblawi M, Wang K, Viveiros A, Nguyen Q, Zhong JC, Turner AJ, Raizada MK, Grant MB, Oudit GY. Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: celebrating the 20th anniversary of the discovery of ACE2. Circ Res 126: 1456–1474, 2020. doi: 10.1161/CIRCRESAHA.120.317015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ; China Medical Treatment Expert Group for Covid-19. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 382: 1708–1720, 2020. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Matos A, da Silva AP, Ferreira J, Santos AC, Bicho MC, Bicho M. Renin angiotensin system, gut-lung cross talk and microbiota. Lessons from SARS-CoV infections. In: Some RNA Viruses, edited by Shah Y., Abuelzein E.. London: IntechOpen, 2021, p. 37–52. doi: 10.5772/intechopen.94325. [DOI] [Google Scholar]
- 145.Xu K, Cai H, Shen Y, Ni Q, Chen Y, Hu S, Li J, Wang H, Yu L, Huang H, Qiu Y, Wei G, Fang Q, Zhou J, Sheng J, Liang T, Li L. [Management of corona virus disease-19 (COVID-19): the Zhejiang experience]. Zhejiang Da Xue Xue Bao Yi Xue Ban 49: 147–157, 2020. doi: 10.3785/j.issn.1008-9292.2020.02.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Gu S, Chen Y, Wu Z, Chen Y, Gao H, Lv L, Guo F, Zhang X, Luo R, Huang C, Lu H, Zheng B, Zhang J, Yan R, Zhang H, Jiang H, Xu Q, Guo J, Gong Y, Tang L, Li L. Alterations of the gut microbiota in patients with coronavirus disease 2019 or H1N1 influenza. Clin Infect Dis 71: 2669–2678, 2020. doi: 10.1093/cid/ciaa709. [DOI] [PMC free article] [PubMed] [Google Scholar]