This editorial refers to ‘Fractional flow reserve derived from computed tomography coronary angiography in the assessment and management of stable chest pain: the FORECAST randomized trial’, by N. Curzen et al., doi:10.1093/eurheartj/ehab444.
First devised as an invasive measurement of the clinical relevance of coronary artery stenosis, fractional flow reserve (FFR) has become widely utilized for identifying coronary lesions in need of revascularization at the time of invasive coronary angiography (ICA).1 European guidelines recommend pressure wire-based FFR or instantaneous wave-free ratio measurements before revascularization, especially when prior testing for myocardial ischaemia is either inconclusive or unavailable.2 Indeed, FFR is the gold standard test to tell us whether a coronary atherosclerotic lesion is haemodynamically significant. This is achieved by measuring the ratio of the coronary artery pressure distal to the lesion divided by the coronary pressure proximal to the lesion (or at the aortic root), under maximal hyperaemic conditions during ICA. Although FFR-guided revascularization improves clinical outcomes,2 it still forms part of an invasive procedure. Only a few years ago, the clinical cardiology community could not imagine a world where FFR could be performed non-invasively, allowing only those patients eligible for revascularization to end up on the cath-lab table. The hope for non-invasive FFR is being realized with the advancement of computed tomography coronary angiography (CTCA) and the ground-breaking developments of computational fluid dynamics during the last decade.3
The growing acceptance of CTCA as a first-line diagnostic test for stable chest pain in the recent ESC guidelines4 accelerated the adoption of methods using computational fluid dynamics to interrogate the haemodynamic significance of coronary atherosclerotic lesions. Indeed, computed tomography-derived FFR (FFRCT) has emerged as the most practical non-invasive means to assess the haemodynamic significance of coronary atherosclerotic lesions. Despite its methodological limitations, FFRCT has generated the expectation among the clinical community that it will offer a safer, cheaper, and more patient-friendly way to replace invasive FFR. But is this indeed the case?
In this issue of the European Heart Journal,5 the FORECAST investigators set out to determine the cost implications of FFRCT in a population of 1400 patients with stable chest pain undergoing CTCA, compared with standard of care in the UK.6 The trial also considered secondary outcomes of upmost relevance to patient care: major adverse cardiac and cerebrovascular events (MACCE) and general well-being, including quality of life and angina status.
It is important to note that the FORECAST trial occurred following the publication of specific NICE Medical Technologies Guidance on the use of FFRCT 7 that predicted substantial cost savings for the UK’s health service with the use of the test. Given this, the major findings from FORECAST are very timely: in a cohort of patients with stable chest pain, there was no significant difference in cost over 9 months between those randomized to CTCA with FFRCT vs. standard of care. The study was only designed to investigate resource allocation between those randomized to CTCA with selective FFRCT and those randomized to usual care, not to investigate cost benefits or patient care benefits of specific alternative investigation strategies such as stress echocardiography. The findings reveal that the ICA rate and the coronary revascularization rate were not reduced enough in the experimental arm to balance the costs of the CTCA and the FFRCT.
Secondary outcomes were predominantly non-significant—an important finding. The trial found no significant difference in MACCE, angina symptoms, quality of life, or requirement for coronary revascularization. Importantly, those investigated with FFRCT were 22% less likely to receive ICA compared with those in the standard care arm. This is a key finding given the purposes of FFRCT discussed initially. Further, those investigated with FFRCT were 52% less likely to receive an ICA that showed no obstructive lesions. This is a finding worthy of note, given that CTCA tends to overestimate the severity of coronary atherosclerotic lesions. So, patients who have FFRCT seem to achieve a similar quality of life and clinical outcome to those who follow the standard care, but with fewer invasive procedures.
How does FFRCT fit into the ‘big picture’ of CTCA-driven clinical care?
The FORECAST trial does not address the critical question of when exactly FFRCT should be utilized in the clinical care pathway. FFRCT may be of most use when CTCA has been performed for stable chest pain, and angina symptoms persist despite optimum medical therapy. FFRCT could be retrospectively assessed on the original CTCA with the goal to assess suitability for revascularization. Further randomized trials are needed to elucidate the possible cost-effectiveness of FFRCT in this setting. Another factor expected to improve the accuracy of FFRCT is the technological advancement of the CT hardware. Photon-counting CT scanners are expected to eliminate the number of non-diagnostic (or low-confidence) analyses for FFRCT due the expected reduction of noise levels and beam hardening artefacts.
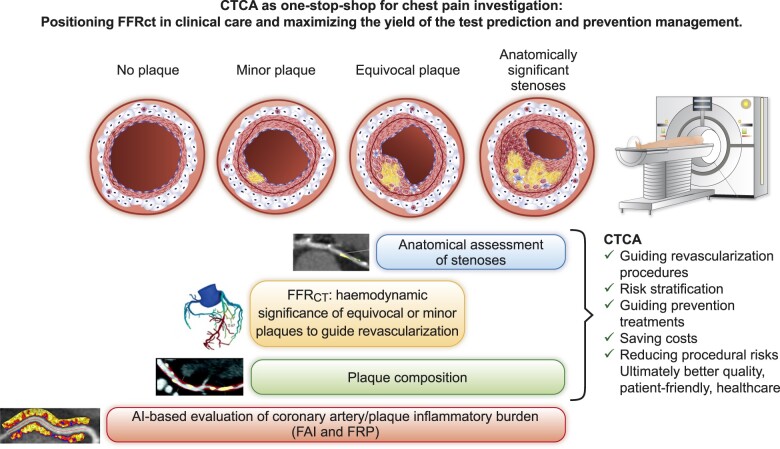
Given the upgrade of CTCA to a class I indication for the investigation of stable chest pain in the 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes,4 it is important to map out the new opportunities that modern CTCA has to offer. CTCA-guided management of chest pain inevitably generates four categories of patients that may require a different therapeutic approach (Graphical Abstract).
Graphical Abstract.
CTCA is rapidly becoming a ‘one-stop shop’ for the investigation of patients with stable chest pain. With the rise of CTCA worldwide, distinct groups of patients will be frequently encountered: those with no detectable atherosclerosis, those with atherosclerosis which is minor, those with atherosclerosis of questionable haemodynamic importance, and those with obstructive disease clearly visible on anatomical assessment. FFRCT is likely to be most useful for patient care and resource minimization in the minor and equivocal stenosis groups where FFRCT can be a gatekeeper for invasive procedures. Within the group with no atherosclerosis, only new AI-based technologies, such as FAI, will be useful for adding yield to the CTCA through enhanced risk stratification, and such technologies can also be applied across all stages of disease. AI, artificial intelligence; CTCA, computed tomography coronary angiography; FAI, fat attenuation index; FFRCT, fractional flow reserve computed tomography; FRP, fat radiomic profile.
Patients where obstructive coronary artery disease is evident, who can be referred for revascularization or start medical management. Following the results of the ISCHAEMIA trial,8 if left main stem stenosis is excluded, the patient could be treated with tablets, but an FFRCT could be requested on the existing CTCA only if symptoms persist, to guide the revascularization strategy, especially in multivessel/multilesion disease. With the advance of CTCA hardware technologies and the introduction of FFRCT, the revascularization decision is expected to be made more confidently, without the need for ICA. The CTCA images can be analysed for the total plaque burden, while identifying the vulnerable or inflamed plaques may help us detect the ‘vulnerable patient’ and guide aggressive medical management. Detecting the inflammatory burden of plaques or the overall inflammatory burden of the coronary tree evaluated by technologies studying perivascular fat attenuation [e.g. perivascular fat attenuation index (FAI)]9 or perivascular fat radiomic profile (FRP)] offers new opportunities for personalizing treatment decisions focused on improving prognosis.10
Patients with coronary atherosclerosis of questionable haemodynamic importance. These are the patients whose treatment can be guided by FFRCT. The default use of FFRCT to interrogate these plaques does not yield a health economic benefit.5 However, if the patient is treated with tablets and an FFRCT is requested on the existing CTCA only if symptoms persist, the cost-effectiveness of the test is expected to be much higher. This population may also benefit from more advanced plaque characterization by studying plaque composition (e.g. calcified/non-calcified plaque burden) and by analysing the inflammatory burden of the plaques or the overall inflammatory burden of the coronary tree with perivascular fat analysis,9 , 10
Patients with atherosclerosis which is clearly not haemodynamically significant. These patients are often dismissed as of limited interest, but they can be a source of future heart attacks when minor (but inflamed) plaques rupture. This is a new population created as a result of widespread use of CTCA who sit between primary and secondary prevention and for which the current clinical guidelines give conflicting messages. FFRCT may be important in this population as it may provide the cumulative haemodynamic impact of diffuse disease along a coronary artery, the prognostic value of which needs to be confirmed in outcome trials. The same population will benefit from plaque characterization11 as well as measurement of the coronary inflammatory burden using the FAI score12 or radiomic phenotyping,10 to allow accurate risk stratification that will then inform medical decision-making. Coronary calcium scoring may also add some value as a first investigation in untreated patients in this category, although its value in secondary prevention (statin-treated populations) is limited by the fact that it detects stable, calcified plaques.
Finally, ∼25% of the patients having CTCA in the modern era have no detectable atherosclerosis. In these patients the risk for future heart attacks is very low but not zero. Analysis of coronary inflammation using artificial intelligence models such as perivascular fat phenotyping (FAI score and FRP)10 , 12 , 13 could provide meaningful information, allowing accurate refinement of their future cardiovascular risk, building imaging on top of current risk stratification models such as the ESC SCORE2.14
CTCA is becoming the first-line test for investigation of the patient with stable chest pain. Through the multiple approaches outlined above, CTCA can guide revascularization procedures (using FFRCT), allowing simultaneous extraction of information not achievable by any other non-invasive test. This includes information about plaque composition and plaque/arterial inflammation which can facilitate re-allocation of resources and treatments to the vulnerable plaque and the vulnerable patient. CTCA has great potential to render redundant other widely used tests for ischaemia and significantly reduce the number of unnecessary invasive diagnostic procedures. The complete health, economic, and socioeconomic consequences of CTCA can only be evaluated when CTCA is used as a ‘one-stop shop’, to provide information about ischaemia, the vulnerable plaque, but—most importantly—the vulnerable patient.
Funding
C.A. is supported by the British Heart Foundation (FS/16/15/32047, TG/16/3/32687, TG/19/2/34831, and RG/F/21/110040), the National Institute for Health Research Oxford Biomedical Research Centre (Oxford, UK), the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre, and Innovate UK.
Conflicts of interest: C.A. is an inventor on various patents in the cardiac CT field: Patent PCT /GB2015/052359 and patent applications PCT/GB2017/053262, GB2018/1818049.7, GR20180100490, and GR20180100510, licensed through exclusive licence to Caristo Diagnostics. C.A. is a founder, shareholder, and Director of Caristo Diagnostics, a CT image analysis company. H.W. has no relationships relevant to the contents of this paper to disclose.
Contributor Information
Charalambos Antoniades, Division of Cardiovascular Medicine, Radcliffe Department of Medicine, University of Oxford, Oxford, UK; Acute Vascular Imaging Centre, University of Oxford, Oxford, UK.
Henry W West, Division of Cardiovascular Medicine, Radcliffe Department of Medicine, University of Oxford, Oxford, UK; Acute Vascular Imaging Centre, University of Oxford, Oxford, UK.
The opinions expressed in this article are not necessarily those of the Editors of the European Heart Journal or of the European Society of Cardiology.
References
- 1. Achenbach S, Rudolph T, Rieber J, Eggebrecht H, Richardt G, Schmitz T, Werner N, Boenner F, Mollmann H. Performing and interpreting fractional flow reserve measurements in clinical practice: an expert consensus document. Interv Cardiol 2017;12:97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet JP, Falk V, Head SJ, Jüni P, Kastrati A, Koller A, Kristensen SD, Niebauer J, Richter DJ, Seferovic PM, Sibbing D, Stefanini GG, Windecker S, Yadav R, Zembala MO. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J 2019;40:87–165. [DOI] [PubMed] [Google Scholar]
- 3. Koo BK, Erglis A, Doh JH, Daniels DV, Jegere S, Kim HS, Dunning A, DeFrance T, Lansky A, Leipsic J, Min JK. Diagnosis of ischemia-causing coronary stenoses by noninvasive fractional flow reserve computed from coronary computed tomographic angiograms. Results from the prospective multicenter DISCOVER-FLOW (Diagnosis of Ischemia-Causing Stenoses Obtained Via Noninvasive Fractional Flow Reserve) study. J Am Coll Cardiol 2011;58:1989–1997. [DOI] [PubMed] [Google Scholar]
- 4. Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, Prescott E, Storey RF, Deaton C, Cuisset T, Agewall S, Dickstein K, Edvardsen T, Escaned J, Gersh BJ, Svitil P, Gilard M, Hasdai D, Hatala R, Mahfoud F, Masip J, Muneretto C, Valgimigli M, Achenbach S, Bax JJ, Group ESD. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes: The Task Force for the diagnosis and management of chronic coronary syndromes of the European Society of Cardiology (ESC). Eur Heart J 2019;41:407–477. [DOI] [PubMed] [Google Scholar]
- 5. Curzen N, Nicholas Z, Stuart B, Wilding S, Hill K, Shambrook J, Eminton Z, Ball D, Barrett C, Johnson L, Nuttall J, Fox K, Connolly D, O’Kane P, Hobson A, Chauhan A, Uren N, Mccann GP, Berry C, Carter J, Roobottom C, Mamas M, Rajani R, Ford I, Douglas P, Hlatky MA; FORECAST Investigators. Fractional flow reserve derived from computed tomography coronary angiography in the assessment and management of stable chest pain: the FORECAST randomized trial. Eur Heart J 2021;42:3844–3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Institute for Health and Care Excellence. Resource Impact Report: Chest Pain of Recent Onset: Assessment and Diagnosis (CG95). NICE; 2016. [PubMed] [Google Scholar]
- 7.National Institute for Health and Care Excellence. Medical Technologies Guidance: HeartFlow FFRCT for estimating fractional flow reserve from coronary CT angiography (MTG32). NICE; 2017. [Google Scholar]
- 8. Maron DJ, Hochman JS, Reynolds HR, Bangalore S, O’Brien SM, Boden WE, Chaitman BR, Senior R, López-Sendón J, Alexander KP, Lopes RD, Shaw LJ, Berger JS, Newman JD, Sidhu MS, Goodman SG, Ruzyllo W, Gosselin G, Maggioni AP, White HD, Bhargava B, Min JK, Mancini GBJ, Berman DS, Picard MH, Kwong RY, Ali ZA, Mark DB, Spertus JA, Krishnan MN, Elghamaz A, Moorthy N, Hueb WA, Demkow M, Mavromatis K, Bockeria O, Peteiro J, Miller TD, Szwed H, Doerr R, Keltai M, Selvanayagam JB, Steg PG, Held C, Kohsaka S, Mavromichalis S, Kirby R, Jeffries NO, Harrell FE, Rockhold FW, Broderick S, Ferguson TB, Williams DO, Harrington RA, Stone GW, Rosenberg Y. Initial invasive or conservative strategy for stable coronary disease. N Engl J Med 2020;382:1395–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Antonopoulos AS, Sanna F, Sabharwal N, Thomas S, Oikonomou EK, Herdman L, Margaritis M, Shirodaria C, Kampoli AM, Akoumianakis I, Petrou M, Sayeed R, Krasopoulos G, Psarros C, Ciccone P, Brophy CM, Digby J, Kelion A, Uberoi R, Anthony S, Alexopoulos N, Tousoulis D, Achenbach S, Neubauer S, Channon KM, Antoniades C. Detecting human coronary inflammation by imaging perivascular fat. Sci Transl Med 2017;9;doi: 10.1126/scitranslmed.aal2658. [DOI] [PubMed] [Google Scholar]
- 10. Oikonomou EK, Williams MC, Kotanidis CP, Desai MY, Marwan M, Antonopoulos AS, Thomas KE, Thomas S, Akoumianakis I, Fan LM, Kesavan S, Herdman L, Alashi A, Centeno EH, Lyasheva M, Griffin BP, Flamm SD, Shirodaria C, Sabharwal N, Kelion A, Dweck MR, Van Beek EJR, Deanfield J, Hopewell JC, Neubauer S, Channon KM, Achenbach S, Newby DE, Antoniades C. A novel machine learning-derived radiotranscriptomic signature of perivascular fat improves cardiac risk prediction using coronary CT angiography. Eur Heart J 2019;40:3529–3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.SCOTHEART Investigators. CT coronary angiography in patients with suspected angina due to coronary heart disease (SCOT-HEART): an open-label, parallel-group, multicentre trial. Lancet 2015;385:2383–2391. [DOI] [PubMed] [Google Scholar]
- 12. Oikonomou EK, Marwan M, Desai MY, Mancio J, Alashi A, Hutt Centeno E, Thomas S, Herdman L, Kotanidis CP, Thomas KE, Griffin BP, Flamm SD, Antonopoulos AS, Shirodaria C, Sabharwal N, Deanfield J, Neubauer S, Hopewell JC, Channon KM, Achenbach S, Antoniades C. Non-invasive detection of coronary inflammation using computed tomography and prediction of residual cardiovascular risk (the CRISP CT study): a post-hoc analysis of prospective outcome data. Lancet 2018;392:929–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Antoniades C, Asselbergs FW, Vardas P. The year in cardiovascular medicine 2020: digital health and innovation. Eur Heart J 2021;42:732–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.SCORE2 working group of the ESC Cardiovascular Risk Collaboration. SCORE2 risk prediction algorithms: new models to estimate 10-year risk of cardiovascular disease in Europe. Eur Heart J 2021;42:2439–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]