Abstract
Background
During the COVID-19 pandemic, American Society for Testing and Materials level 3 and level 2 medical face masks (MFMs) have been used for most health care workers and even for the first responders owing to a shortage of N95 respirators. However, the MFMs lack effective peripheral seal, leading to concerns about their adequacy to block aerosol exposure for proper protection. The purpose of this study was to evaluate the peripheral seal of level 3 and level 2 MFMs with a 3-dimensional (3D-) printed custom frame.
Methods
Level 3 and level 2 MFMs were tested on 10 participants with and without a 3D-printed custom frame; the efficiency of mask peripheral seal was determined by means of quantitative fit testing using a PortaCount Fit Tester based on ambient aerosol condensation nuclei counter protocol.
Results
The 3D-printed custom frame significantly improved the peripheral seal of both level 3 and level 2 MFMs compared with the masks alone (P < .001). In addition, both level 3 and level 2 MFMs with the 3D-printed custom frame met the quantitative fit testing standard specified for N95 respirators.
Practical Implications
The 3D-printed custom frame over level 3 and level 2 MFMs can offer enhanced peripheral reduction of aerosols when using collapsible masks. With the shortage of N95 respirators, using the 3D-printed custom frame over a level 3 or level 2 MFM is considered a practical alternative to dental professionals.
Key Words: MFM, ASTM, level 3, level 2, peripheral seal, fit factor
Abbreviation Key: 3D, 3-dimensional; ASTM, American Society for Testing and Materials; CDC, Centers for Disease Control and Prevention; FDA, US Food and Drug Administration; L-2, Level 2; L-3, Level 3; MFM, Medical face mask; MM, Micrometer; NIOSH, National Institute of Occupational Safety and Health; OSHA, Occupational Safety and Health Administration; PPE, Personal protective equipment
According to the Centers for Disease Control and Prevention (CDC) guidance for dental setting, precautions are recommended for aerosol-generating procedures, such as wearing an N95 or higher-level respirator and ideally performing the procedure in an airborne infection–isolation room.1 With the global COVID-19 pandemic, dentists, dental hygienists, dental assistants, and other front-line dental health care personnel across the United States are experiencing a shortage of personal protective equipment (PPE).2 , 3 This unprecedented challenge has forced some health care workers to clean and reuse PPE,4 which may increase the risk of transmitting COVID-19.5 As a consequence, in February 2020, an emergency use authorization from the US Food and Drug Administration (FDA) allowed importation of N95-type masks that meet international standards to mitigate the crisis.6 , 7 However, awareness of the transmission of disease through respiratory droplets and aerosol has triggered critical examination of the safety of these masks.
The Occupational Safety and Health Administration (OSHA) regulates the selection of appropriate PPE including masks in a workplace.8 It announced that any N95 respirator has to pass certain criteria. Bacterial filtration efficiency, particulate filtration efficiency, breathability, flame spread, and fluid resistance are the most critical criteria points. Even though American Society for Testing and Materials (ASTM) level 3 and level 2 medical face masks (MFMs) have similar results compared with N95 respirators in all of the aforementioned critical points (Table 1 ),9 , 10 there are several differences among N95 and level 3 and level 2 MFMs. One of the important differences is the peripheral seal. According to the CDC, the respirator must fit the user’s face snugly (that is, create a relative seal) to minimize the number of particles that bypass the filter through gaps between the user’s skin and the respirator seal.11 A fit test is used to assess this relative seal.
Table 1.
| TEST | N95 (1860/1860S, 3M) | N95 (1870, 3M) | ASTM∗ LEVEL 3 MFM† (GCFCXS, CROSSTEX) | ASTM LEVEL 2 MFM (GCPYE, CROSSTEX) |
|---|---|---|---|---|
| Bacterial Filtration Efficiency, % | > 99 | > 99 | ≥ 98 | ≥ 98 |
| Particulate Filtration Efficiency (≈ 0.1 μm Diameter), % | > 95‡ | > 95‡ | ≥ 98§ | ≥ 98§ |
| Breathability, mm H2O/cm2 | < 6.5 | 4.9 | < 6.0 | < 6.0 |
| Flame Spread | Class 1 | Class 1 | Class 1 | Class 1 |
| Fluid Resistance, mm Hg | 120 | 160 | 160 | 120 |
ASTM: American Society for Testing and Materials.
MFM: Medical face mask.
The N95 respirator is evaluated with the standard respirator testing procedures of the National Institute for Occupational Safety and Health.
MFM is evaluated with the ASTM standard.
During this pandemic, a novel 3-dimensional (3D-) printed custom mask frame was designed by Bellus3D and beta tested in collaboration with Loma Linda University School of Dentistry. Using a 3D facial scan, the custom frame was printed and placed over the level 3 and level 2 MFMs, which was supposed to enhance the peripheral seal. According to OSHA regulations, any workplace should select and use respirators based on a fit test, which evaluates the peripheral sealing of a respirator on a person.8 However, it was reported that few of the MFMs had passed the fit test owing to the lack of a peripheral seal.12 Therefore, it is critical to evaluate the sealing function of the level 3 and level 2 MFMs with a 3D-printed custom mask frame.
On the basis of the current pandemic situation, we hypothesize that with the addition of a 3D-printed custom frame, the peripheral sealing function of MFMs could be comparable with that of N95 respirators. Therefore, we aim to evaluate the peripheral sealing efficiency of ASTM level 3 and level 2 MFMs with a 3D-printed custom frame compared with ASTM level 3 and level 2 MFMs alone.
Methods
Participant recruitment
Our study was approved by the institutional review board of the Loma Linda University (IRB 5200314). Male and female dental faculty, staff members, and students at Loma Linda University School of Dentistry were recruited. People who met the following criteria were accepted into the study: 18 years and older, being able to read and sign the informed consent form, and having a 3D-printed custom mask frame. Exclusion criteria included participants who had COVID-19 symptoms, smoked 30 minutes before the testing, and had facial hair interfering with the mask or frame seal.
Masks and respirators
The fit or peripheral seal of commercially available level 3 MFM (GCFCXS, Crosstex) and level 2 MFM (GCPYE, Crosstex) was evaluated with and without wearing a 3D-printed custom mask frame. N95 respirator (1860/1860s/1870, 3M) was used as a positive control. One of the 3 N95 respirators was provided to the participant after an initial screening fit test. Before the fit test, the participant had to feel comfortable, and the investigator needed to accept that adequate fit was achieved with the situated N95 respirator.13 , 14 As ASTM level 3 is closer to the N95 respirators in terms of their critical properties (Table 1),9 , 10 the order of testing began with the N95 respirator, then level 3 MFM, and finally level 2 MFM with and without the customized 3D-printed frame.
3D-printed custom mask frame
A digital facial scan was generated using a 3D scan technology with a software application (Bellus3D FaceApp) on an iPad Pro (Apple) according to the manufacturer’s instructions. From the facial scan, a customized mask frame standard triangle language (STL) file was generated and was 3D printed with the SprintRay Pro 95 3D printer. The printer uses a digital light processing technology, based on a projector to cure the resin at the 405-nm wavelength. The RayWare software (Version 2.7.0.13) by SprintRay was used to slice the STL file and submit it to the printer. The printing materials used were Die and Model Gray, and Die and Model Tan—all made by SprintRay.
PortaCount respirator fit tester
The efficiency of the mask peripheral seal was determined by means of the PortaCount Fit Tester (model 8048, TSI) that provides a quantitative fit test using the ambient aerosol condensation nuclei counter protocol and is compliant with OSHA 29CFR1910.134, American National Standards Institute /ASTM Z88.10-2001, Canadian Standards Association Z94.4-2011, and Health and Safety Executive (UK) 282/28 regulations.8 , 15 TSI Particle Generator (model 8026, TSI) was also used to ensure high enough particle counts and appropriate particle size distributions in an enclosed space to meet OSHA standards. For each fit test, a disposable sampling probe kit (model 8025-N95R, TSI) was installed on the mask or respirator, which allows the probe to sample the air from inside the mask.
Fit testing
Five conditions were evaluated for each participant:
-
▪
N95 respirator (1860/1860s/1870, 3M) as the positive control;
-
▪
ASTM level 3 MFM without a frame; ASTM level 3 MFM with a frame;
-
▪
ASTM level 2 MFM without a frame;
-
▪
ASTM level 2 MFM with a frame.
At the beginning of each test, the participant passed the quantitative fit test with 1 of the N95 respirators (1860/1860s/1870, 3M) as the US National Institute of Occupational Safety and Health (NIOSH) requires a minimum fit factor of 100 for half respirators to indicate sufficient fit (that is, no leak).15 All tests were conducted in an enclosed room. Before the tests, the TSI Particle Generator was running for at least 15 minutes to generate enough counts of particles, which are expected to possess a nominal size of 0.04 μm with a geometric standard deviation of 2.2 from a dilute (2%) solution of sodium chloride according to instrument specifications. Then the PortaCount Fit Tester was calibrated as daily checks. Before the fit testing, the information about participants, respirators, and fit test setting was entered into the FitPro Ultra Fit Test Software database.
Fit testing was performed following the manufacturer’s instructions. For each test, the sample probe was installed in the “breathing zone” of the mask. The OSHA FAST-Filtering Face protocol was selected to perform the fit testing under the N95 Companion mode, in FitPro Ultra Software. The test for each condition consisted of 2.5 minutes while wearing the mask as shown (Figure 1 ). The participant performed 4 preset movements—“Bending over,” “Talking,” “Head side to side,” and “Head up and down”—during the testing. As the participants were performing the movements while wearing a mask, the machine provided a continuous display of real-time fit factor based on the Cout/Cin counting (Cout: ambient particle concentration in space of closed room; Cin: mask particle concentration in the mask). Thus, mask or respirator leakage was measured simultaneously while the test participant moved and breathed. A set of fit factors was recorded for each movement, and an overall fit factor was presented as the final score of fit testing. The final test result was indicated as “passed” if the final score was 100 or higher, as required by the NIOSH.15 The time for each fit testing was approximately 2.5 minutes (Figure 2 ).
Figure 1.
ASTM level 3 (L-3) medical face mask (MFM) with a 3-dimensional–printed custom frame. An installed fit test probe in the L-3 MFM is connected to the fit test machine with a clear sample tube. The clear tube samples particles inside the MFM and ambient particles outside the MFM are sampled through the blue tube. ASTM: American Society for Testing and Materials. Photograph courtesy of Dr. Ahmed. Consent to use photograph was taken per Loma Linda University policy.
Figure 2.
Data collection procedures in quantitative fit testing. ASTM: American Society for Testing and Materials. MFM: Medical face mask.
Statistical analysis
The dependent variable in this study was the fit factor determined by taking the ratio of the average chamber concentration to the concentration measured inside the respirator for each test exercise. The overall fit factor is calculated using the harmonic mean value of each exercise.15 , 16 It uses individual exercise fit factors that involve converting first the exercise fit factors to penetration values, determining the average, and then converting that result back to a fit factor. This procedure is described in the following equation:
where ff is the fit factor.
A sample size of 10 was needed for an 80% chance to find a standardized effect size of 1.0 at an alpha level of 0.05, when evaluating the peripheral sealing difference between MFMs with and without a 3D-printed custom frame. We present the descriptive statistics including mean (standard deviation), median, and interquartile range for continuous and count data in Table 3 . To determine if differences in fit factor existed across MFMs with and without a frame, we conducted the Friedman test with Durbin-Conover post hoc comparisons. We performed all tests of hypotheses at an alpha level of 0.05 and conducted all analyses using R v3.6.2 (R Core Team).
Table 3.
Overall fit factor scores of 10 participants per group for N95 respirator, ASTM∗ level 3, and ASTM level 2 medical face masks.
| N95 RESPIRATOR, MEAN (SD†) | L-3‡ MFM§, MEAN (SD) | L-3 MFM +AND 3D¶ FRAME, MEAN (SD) | D1: L-3 MFM +AND 3D FRAME – L-3 MFM, MEAN (95% CI) | L-2∗∗ MFM, MEAN (SD) | L-2 MFM +AND 3D FRAME, MEAN (SD) | D2: L-2 MFM +AND 3D FRAME – L-2 MFM, MEAN (95% CI) |
|---|---|---|---|---|---|---|
| 177 (39.5) | 3 (1.5) | 145 (53.1) | 142 (106.71 to 177.29) | 7 (3.3) | 109 (31.8) | 102 (80.76 to 123.24) |
ASTM: American Society for Testing and Materials.
SD: Standard deviation.
L-3: Level 3.
MFM: Medical face mask.
3D: 3-dimensional printed.
L-2: Level 2.
Results
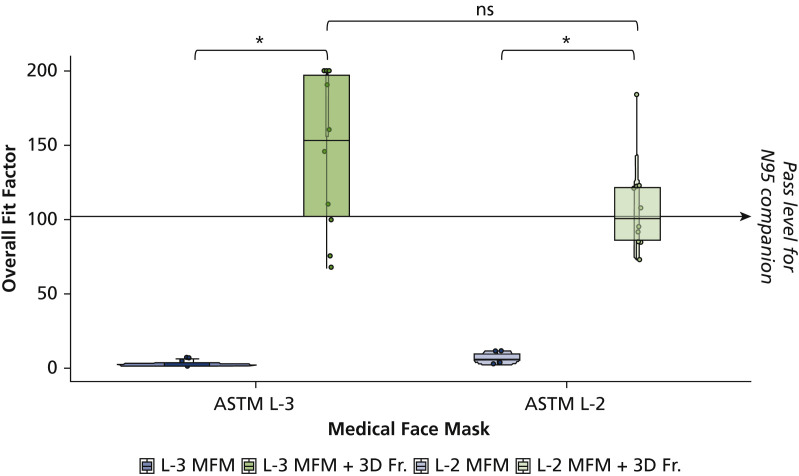
We recruited 10 participants for the study. Fit testing with the instrument began with an N95 respirator, followed by the medical masks with and without the 3D-printed frame. For all tested participants, level 3 and level 2 MFMs without frame failed the fit testing (Figure 3 ). The mean overall fit factor for these masks were 3 and 7, which were far below the standard pass level of 100.
Figure 3.
Overall fit factor scores of 10 participants per group for ASTM level 3 (L-3) and level 2 (L-2) medical face masks (MFMs). Data are shown as boxplots of median, 25th and 75th percentile, and minimum-maximum whiskers. ∗ P < .001 versus respective face masks by Durbin-Conover pairwise comparisons. ASTM: American Society for Testing and Materials. 3D Fr.: 3-dimensional–printed custom frame.
ASTM level 3 MFMs showed low fit factor scores in all 4 movements (Table 2). Similarly, ASTM level 2 MFMs showed similarly low fit factor scores in all 4 movements (Table 2). The fit factor dramatically increased when a 3D-printed custom frame was used on both ASTM level 3 and level 2 MFMs (Table 2).
Table 2.
Fit factor scores of 10 participants per group for N95 respirator, ASTM∗ level 3, and ASTM level 2 medical face masks.
| FIT TEST MOVEMENTS | N95 RESPIRATOR, MEAN (SD†) | LEVEL 3 MFM‡, MEAN (SD) | LEVEL 3 MFM + 3D§ FRAME, MEAN (SD) | LEVEL 2 MFM, MEAN (SD) | LEVEL 2 MFM + 3D FRAME, MEAN (SD) |
|---|---|---|---|---|---|
| Bending Over | 185 (42.4) | 4 (1.9) | 160 (46.9) | 7 (5.1) | 160 (60.6) |
| Talking | 177 (38.4) | 6 (3.7) | 137 (61.5) | 13 (7.7) | 70 (31.4) |
| Head Side to Side | 197 (9.8) | 3 (1.0) | 152 (55.4) | 10 (8.6) | 130 (41.0) |
| Head Up and Down | 184 (50.0) | 3 (1.8) | 151 (58.2) | 6 (2.7) | 167 (45.1) |
ASTM: American Society for Testing and Materials.
SD: Standard deviation.
MFM: Medical face mask.
3D: 3-dimensional printed.
The overall fit factor scores of ASTM level 3 MFM with a 3D-printed custom frame showed significant improvement in the sealing compared with ASTM level 3 MFM alone (P < .001) (Figure 3 and Table 3), passing with the mean value of 145. Likewise, the test results of ASTM level 2 MFM with a 3D-printed custom frame showed significant improvement in the sealing compared with ASTM level 2 MFM alone (P < .001) (Figure 3 and Table 3). The results of the overall fit factor for level 2 MFM with the 3D-printed custom frame also passed with the mean of 109. The results of the overall fit factor of ASTM level 3 MFM with the 3D-printed custom frame provided approximately 33% better seal than ASTM level 2 MFM with the frame.
As the positive control to validate fit testing procedures, the N95 respirator achieved an average of 177 for the overall fit factor, with high fit factor scores in all 4 movements (Figure 4 and Tables 2 and 3).
Figure 4.
Overall fit factor scores of 10 participants per group for N95 respirator and American Society for Testing and Materials level 2 (L-2) and level 3 (L-3) medical face masks (MFMs). 3D Fr.: 3-dimensional–printed custom frame.
Discussion
As coronavirus infection continues to spread rapidly and a global shortage of masks and other protective equipment against COVID-19 surges,17 there is an emerging requirement for greater flexibility in approach, such as additive manufacturing using 3D printing.18 , 19 In fact, 3D printing has been helpful in the face of dwindling medical supplies in the fight against COVID-19, with many 3D printing companies engaged in production on a regular basis.18 , 20 Under an emergency use authorization, the FDA is working closely with the government, industry, and health care facility stakeholders on the regulatory considerations of 3D printing or purchasing 3D-printed devices in view of the broader public health emergency.19 , 21 The FDA early on had approved a 3D-printed surgical face mask (Stopgap) created by a Veterans Health Administration team to protect clinical health care providers from COVID-19.22
The evaluation and selection of a respirator are important to ensure the best protection to health care professionals. The N95 respirator is the most common type of respirator used in health care, which is used for protection against airborne bioaerosols such as tuberculosis and measles.23 In the United States, respirators are tested and certified by the NIOSH. The label “N95” indicates that the material of the mask is at least 95% effective in filtering 0.3-μm nonoily particles per the NIOSH criteria.24 According to the CDC guidelines for COVID-19 PPE for health care personnel, N95 or higher respirators are preferred, yet MFMs are an acceptable alternative.25
MFMs are cleared by the FDA for health care professionals’ protection during surgical procedures. There are 2 major differences between the MFM and N95 respirator according to NIOSH. First, the filtration efficiency of the N95 respirator and the MFM is evaluated by different agencies. The N95 respirator is evaluated with the standard respirator testing procedures of NIOSH.26, 27, 28 MFMs, in particular, are evaluated using ASTM standard F2299 by FDA.10 , 29 Even though some commercial brands of MFMs could also be at least 95% efficient for particles ranging from 0.1 through 5 μm, the CDC considers it less reliable owing to different testing methods. Moreover, the N95 respirator has a tighter fit than MFMs without a 3D-printed custom frame. This difference could largely reduce the protective function of an MFM because of the loose-fitting characteristics of MFMs, including surgical masks.30 During this unprecedented pandemic, the functionality of the MFMs could be improved with a better peripheral seal. When the 3D-printed custom frame was added to the MFM, the peripheral seal improved dramatically. In fact, with effective filtration efficiency, the use of MFM with a 3D-printed custom frame could potentially be an alternative to N95 respirators during periods of shortage. ASTM level 1 MFM was not considered in this study because the resistance to penetration by synthetic blood is much lower than levels 3 and 2 (Table 1), which provides less protection during aerosol-generating dental procedures.
According to OSHA regulations, fit testing is required for N95 respirators to ensure peripheral sealing is achieved for each person.31 The 2 most common models of N95 respirators used by health care professionals across the United States are 3M 1860 (1860 as the regular size, 1860S as the small size) and 3M 1870. According to the literature, there is no single best-fitting respirator among existing products.13 , 14 Therefore, to validate the successful fit test procedures, in our study, the most individually suitable N95 respirator from one of the 3M N95 respirators was chosen as the positive control for testing.
COVID-19 virus can be transmitted through airborne transmission (particles < 5 μm in diameter) in addition to droplet transmission (particles > 5-10 μm in diameter), which occurs when a person is in close contact (< 1 meter).32 This means it is much more effective in disease transmission as it has longer life span in the air and can be transmitted to distances greater than 1 m distance without coming in contact with the infected person.32 , 33 Quantitative fit test is one of the effective methods to measure the penetration of small particles (particles < 5 μm in diameter). Therefore, it is appropriate that the quantitative fit testing was used to evaluate the peripheral sealing of ASTM level 3 and level 2 MFMs with or without a frame. In the literature, it has been reported that quantitative fit testing is more accurate as it provides objective and numerical data, whereas qualitative fit testing is a subjective test that depends on the ability of the participant to taste sweetness or bitterness and his or her olfactory senses.34 , 35 The modified ambient aerosol condensation nuclei counter quantitative fit testing protocol for filtering facepiece respirators was used in accordance with OSHA’s Respiratory Protection Standard. The company, TSI, originally proposed this protocol to OSHA. Also, the PortaCount series machines from TSI are typically used for this test in the United States. The PortaCount model 8048 has the N95 Companion Technology built into the machine, which could be used to fit test respirators equipped with less than 99% efficient filter media. Thus, the PortaCount model 8048 was used to test the N95 respirator and ASTM level 3 and level 2 MFMs with or without the 3D-printed custom frame.
In this study, according to the manufacturer's instruction, the overall fit factor was calculated from 4 preset movements with a minimum pass level of 100 for a half-mask respirator. The value from each section can give a better understanding of the mask sealing (Table 2). ASTM level 3 and level 2 masks without the frame failed in fit testing (Figure 3), with a mean overall fit factor score of 3 and 7, respectively. Similar results were found before and was considered reasonable as none of the level 3 and level 2 MFMs come with peripheral sealing similar to that of N95 respirators.12 In contrast, the peripheral sealing was increased when the 3D-printed frame was added. The mean overall fit factor score achieved the pass level, that is, 145 and 109, respectively (Figure 3 and Table 3).
Although ASTM level 3 MFM with a 3D-printed custom frame showed 33% higher mean scores of the overall fit factor than ASTM level 2, it was not significant (P = .428).
During the initial screening fit test, there was a 60% failure rate for 3M N95 1860 masks (data not shown); this is comparable to the reports in the literature, which range from 50% through 75%.36 , 37 However, the failure rate of ASTM level 3 MFM with a 3D-printed custom frame was 20%. It indicates the importance of fit testing and proper peripheral sealing for all respirators and MFMs for adequate protection.
As proof of a valid testing system, the 2 most common models of N95 respirator, that is, 3M 1860 (1860 as the regular size, 1860S as the small size) and 3M 1870, were tested. As expected, we found that the mean score of the overall fit factor was 177 (Figure 4 and Tables 2 and 3). Although all of the tested masks passed the fit testing with the 3D-printed custom frame, a larger sample size would be needed to evaluate the similarity between the sealing of N95 respirators and the sealing of ASTM level 3 and level 2 MFMs with a 3D-printed custom frame.
Conclusions
The results of our study show that a 3D-printed custom frame can considerably increase the fit factor scores in a quantitative fit test specified by the OSHA. This new application may improve the peripheral sealing efficiency lacking in MFM alone and may provide an alternative solution to enhance PPE for aerosol protection. Thus, improving the peripheral seal of ASTM level 3 and level 2 MFMs using a 3D-printed custom frame is expected to markedly reduce potential contamination that may reach the patient and the dental practitioner and help offset urgent respirator supply shortages during the COVID-19 pandemic.
Biographies
Dr. Ahmed is a research associate, Center for Dental Research, Loma Linda University School of Dentistry, A1019 Chan Shun Pavilion, 11175 Campus Street, Loma Linda, CA 92350.
Dr. Zhong is an assistant professor, Center for Dental Research, Loma Linda University School of Dentistry, Loma Linda, CA.
Dr. Suprono is an associate professor, Center for Dental Research, Loma Linda University School of Dentistry, Loma Linda, CA.
Dr. Savignano is an assistant professor, Center for Dental Research, Loma Linda University School of Dentistry, Loma Linda, CA.
Dr. Riter is an associate professor, Loma Linda University School of Dentistry, Loma Linda, CA.
Mr. Oyoyo is an assistant professor, Loma Linda University School of Dentistry, Loma Linda, CA.
Ms. Wilson is a manager, Infection Control & Safety, Loma Linda University School of Dentistry, Loma Linda, CA.
Ms. Reece is an occupational safety specialist, Environmental Health & Safety, Loma Linda University, Loma Linda, CA.
Dr. Kim is an assistant professor, Loma Linda University School of Dentistry, Loma Linda, CA.
Dr. Cho is an assistant professor, Loma Linda University School of Dentistry, Loma Linda, CA.
Dr. Handysides is an associate professor, Loma Linda University School of Dentistry, Loma Linda, CA.
Dr. Richardson is an associate professor, Loma Linda University School of Dentistry, Loma Linda, CA.
Dr. Caruso is a distinguished professor, Loma Linda University School of Dentistry, Loma Linda, CA.
Dr. Li is a distinguished professor, Loma Linda University School of Dentistry, Loma Linda, CA.
Footnotes
Disclosures. None of the authors reported any disclosures.
A.A. and Z.Z. equally contributed as co–first authors.
References
- 1.Centers for Disease Control and Prevention Emergency preparedness and response: guidance for dental settings during the COVID-19 response. 2020. https://emergency.cdc.gov/coca/calls/2020/callinfo_060320.asp Accessed June 26, 2020.
- 2.Livingston E., Desai A., Berkwits M. Sourcing personal protective equipment during the COVID-19 pandemic. JAMA. 2020;323(19):1912–1914. doi: 10.1001/jama.2020.5317. [DOI] [PubMed] [Google Scholar]
- 3.Ren Y., Feng C., Rasubala L., Malmstrom H., Eliav E. Risk for dental healthcare professionals during the COVID-19 global pandemic: an evidence-based assessment. J Dent. 2020;101:103434. doi: 10.1016/j.jdent.2020.103434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mahmood S.U., Crimbly F., Khan S., Choudry E., Mehwish S. Strategies for rational use of personal protective equipment (PPE) among healthcare providers during the COVID-19 crisis. Cureus. 2020;12(5) doi: 10.7759/cureus.8248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization Rational use of personal protective equipment (PPE) for coronavirus disease (COVID-19): interim guidance. 19 March 2020. http://apps.who.int/iris/handle/10665/331498 Accessed December 11, 2020.
- 6.Plana D., Tian E., Cramer A.K. Assessing the quality of nontraditional N95 filtering face-piece respirators available during the COVID-19 pandemic. medRxiv. 2020;2020 doi: 10.1186/s12879-021-06008-8. 07.25.20161968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.US Food and Drug Administration Emergency use authorizations for medical devices. https://www.fda.gov/medical-devices/emergency-situations-medical-devices/emergency-use-authorizations Accessed September 25, 2020.
- 8.Occupational Safety and Health Administration Respiratory protection. https://www.osha.gov/laws-regs/regulations/standardnumber/1910/1910.134 Accessed December 10, 2020.
- 9.Rengasamy S., Shaffer R., Williams B., Smit S. A comparison of facemask and respirator filtration test methods. J Occup Environ Hyg. 2017;14(2):92–103. doi: 10.1080/15459624.2016.1225157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.ASTM International . ASTM International; 2011. ASTM F2100-20: Standard Specification for Performance of Materials Used in Medical Face Masks; pp. 447–449. [Google Scholar]
- 11.D’Alessandro M.M., Cichowicz J.K. Proper N95 respirator use for respiratory protection preparedness. NIOSH Science Blog, Centers for Disease Control and Prevention. https://blogs.cdc.gov/niosh-science-blog/2020/03/16/n95-preparedness/ March 16, 2020. Accessed September 23, 2020.
- 12.Oberg T., Brosseau L.M. Surgical mask filter and fit performance. Am J Infect Control. 2008;36(4):276–282. doi: 10.1016/j.ajic.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shaffer R.E., Janssen L.L. Selecting models for a respiratory protection program: what can we learn from the scientific literature? Am J Infect Control. 2015;43(2):127–132. doi: 10.1016/j.ajic.2014.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suen L.K.P., Guo Y.P., Ho S.S.K., Au-Yeung C.H., Lam S.C. Comparing mask fit and usability of traditional and nanofibre N95 filtering facepiece respirators before and after nursing procedures. J Hosp Infect. 2020;104(3):336–343. doi: 10.1016/j.jhin.2019.09.014. [DOI] [PubMed] [Google Scholar]
- 15.Occupational Safety and Health Administration Fit testing procedures (mandatory) https://www.osha.gov/laws-regs/regulations/standardnumber/1910/1910.134AppA Accessed December 10, 2020.
- 16.Sietsema M., Brosseau L.M. Comparison of two quantitative fit-test methods using N95 filtering facepiece respirators. J Occup Environ Hyg. 2016;13(8):621–627. doi: 10.1080/15459624.2016.1159690. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization Disease outbreak news. 2020. https://www.who.int/csr/don/en/ Accessed December 8, 2020.
- 18.Tarfaoui M., Nachtane M., Goda I., Qureshi Y., Benyahia H. 3D printing to support the shortage in personal protective equipment caused by COVID-19 pandemic. Materials. 2020;13(15):3339. doi: 10.3390/ma13153339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tarfaoui M., Nachtane M., Goda I., Qureshi Y., Benyahia H. Additive manufacturing in fighting against novel coronavirus COVID-19. Int J Adv Manuf Tech. 2020;110(11):2913–2927. doi: 10.1007/s00170-020-06077-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Statista, Examplatory use of 3D printing to provide medical supplies during coronavirus (COVID-19) pandemic in 2020. https://www.statista.com/statistics/1107198/covid-19-3d-printing-medical-supplies Accessed December 8, 2020.
- 21.US Food and Drug Administration Coronavirus (COVID-19) and medical devices. https://www.fda.gov/medical-devices/emergency-situations-medical-devices/coronavirus-covid-19-and-medical-devices Accessed December 8, 2020.
- 22.Ryan F. FDA approves first 3D-printed mask for COVID-19 support. Government CIO Media & Research. https://www.governmentciomedia.com/fda-approves-first-3d-printed-mask-COVID-19-support April 6, 2020. Accessed December 8, 2020.
- 23.Danyluk Q., Hon C.Y. Workers’ Compensation Board of British Columbia; 2011. Strengthening N95 filtering facepiece respirator protection programs by evaluating the contribution of each of the program elements. [Google Scholar]
- 24.Brosseau L., Ann R.B. N95 respirators and surgical masks. NIOSH Science Blog, Centers for Disease Control and Prevention. https://blogs.cdc.gov/niosh-science-blog/2009/10/14/n95/ October 14, 2009. Accessed September 23, 2020.
- 25.Centers for Disease Control and Prevention Use personal protective equipment (PPE) when caring for patients with confirmed or suspected COVID-19. https://www.cdc.gov/coronavirus/2019-ncov/downloads/A_FS_HCP_COVID19_PPE.pdf Accessed September 23, 2020.
- 26.Rengasamy S., Eimer B.C., Shaffer R.E. Comparison of nanoparticle filtration performance of NIOSH-approved and CE-marked particulate filtering facepiece respirators. Ann Occup Hyg. 2009;53(2):117–128. doi: 10.1093/annhyg/men086. [DOI] [PubMed] [Google Scholar]
- 27.NIOSH: Procedure No TEB-APR-STP-0059, Revision 2.0. Determination of particulate filter efficiency level for N95 series filters against solid particulates for non-powered, air-purifying respirators standard testing procedure (STP) https://www.cdc.gov/niosh/npptl/stps/pdfs/TEB-APR-STP-0059-508.pdf Accessed September 23, 2020.
- 28.Respiratory protective devices Final rules and notice. Fed Regist. 1995;60:30336–30398. 42 CFR 84. [Google Scholar]
- 29.ASTM International . ASTM International; 2017. ASTM F2299 / F2299M-03(2017): Standard Test Method for Determining the Initial Efficiency of Materials Used in Medical Face Masks to Penetration by Particulates Using Latex Spheres. [Google Scholar]
- 30.Isaacs D., Britton P., Howard-Jones A. Do facemasks protect against COVID-19? J Paediatr Child Health. 2020;56(6):976–977. doi: 10.1111/jpc.14936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Occupational Safety and Health Administration Respiratory fit testing. https://www.osha.gov/laws-regs/regulations/standardnumber/1910/1910.134 Accessed May 13, 2021.
- 32.World Health Organization Modes of transmission of virus causing COVID-19: implications for IPC precaution recommendations. https://reliefweb.int/sites/reliefweb.int/files/resources/WHO-2019-nCoV-Sci_Brief-Transmission_modes-2020.1-eng.pdf March 27, 2020. Accessed December 9, 2020.
- 33.Ong S.W.X., Tan Y.K., Chia P.Y. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. JAMA. 2020;323(16):1610–1612. doi: 10.1001/jama.2020.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Myong J.P., Byun J., Cho Y. The education and practice program for medical students with quantitative and qualitative fit test for respiratory protective equipment. Ind Health. 2016;54(2):177–182. doi: 10.2486/indhealth.2015-0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coffey C.C., Lawrence R.B., Zhuang Z., Campbell D.L., Jensen P.A., Myers W.R. Comparison of five methods for fit-testing N95 filtering-facepiece respirators. Appl Occup Environ Hyg. 2002;17(10):723–730. doi: 10.1080/10473220290107002. [DOI] [PubMed] [Google Scholar]
- 36.Coffey C.C., Lawrence R.B., Campbell D.L., Zhuang Z., Calvert C.A., Jensen P.A. Fitting characteristics of eighteen N95 filtering-facepiece respirators. J Occup Environ Hyg. 2004;1(4):262–271. doi: 10.1080/15459620490433799. [DOI] [PubMed] [Google Scholar]
- 37.Lee K., Slavcev A., Nicas M. Respiratory protection against Mycobacterium tuberculosis: quantitative fit test outcomes for five type N95 filtering-facepiece respirators. J Occup Environ Hyg. 2004;1(1):22–28. doi: 10.1080/15459620490250026. [DOI] [PubMed] [Google Scholar]