Abstract
Methods
In this study, qRT-PCR was used to investigate the expression levels of the SOX15 gene and of miR-182, miR-183, miR-375, and miR-96 in thyroid tumors and adjacent noncancerous tissues. We also investigated the methylation status of the SOX15 promoter by methylation-specific PCR in tumors and adjacent noncancerous tissues.
Results
We observed a statistically significant downregulation of SOX15 expression in tumors compared to noncancerous tissue samples. The methylation levels of tumors and matched noncancerous tissues were similar, but miR-182, miR-183, and miR-375 expression levels were elevated in tumor tissues compared to noncancerous tissue samples.
Conclusions
Our results indicate that SOX15 gene expression is associated with the pathogenesis of papillary thyroid carcinoma (PTC), and the epigenetic control of the SOX15 gene is regulated by miRNAs rather than by promoter methylation.
1. Introduction
Thyroid cancer (TC) is a rare type of cancer and occurs as a consequence of environmental and genetic factors [1]. Cell types from which the tumors arise determine the type of thyroid cancer [2]. Among the four main types, papillary thyroid carcinoma (PTC) is the most common [3]. However, the molecular mechanism underlying the pathogenesis of thyroid carcinoma is still unclear.
Cancer can be described as a disease of altered gene expression. Activation or silencing of a gene may alter the overall activity of the cell. This can arise as a result of gene mutations or changes in the gene expression levels. Gene expression is primarily controlled by the specific binding of transcription factors to their target DNA sequences. The expression of genes is also controlled by epigenetic mechanisms such as DNA methylation and aberrant miRNA expression.
The SOX family genes code for transcription factors; however, they need additional partner proteins for the transcriptional regulation of their target genes [4]. All SOX proteins have the high mobility group (HMG) box DNA binding domain and are divided into 8 subgroups (A-G) according to the similarities in this domain [5, 6]. Members of the SOX family are expressed in a tissue-specific manner and play a critical role in the developmental processes [7]. It is well known that developmental proteins also play crucial roles in the tumor formation.
On the other hand, SOX proteins, unlike other transcription factors, bind to the minor groove of DNA and this interaction brings regulatory elements close to each other [8, 9]. Therefore, in addition to transcriptional regulation, they also act as modulators of the chromatin structure [10]. The sequences outside the HMG box also facilitate interactions between the SOX proteins and influence their specific binding properties [11].
SOX15 is the only member of the SOXG group, and its HMG domain has a unique structure [12]. The human SOX15 gene is universally expressed in different tissues and has been mapped to the 17p13 region [13, 14]. Among the SOX family members, SOX15 function in cell biology and development is the least understood. Thu et al. [15] reported for the first time that SOX15 is downregulated in pancreatic ductal adenocarcinoma (PDAC) as a result of promoter hypermethylation. Subsequently, its downregulation has been associated with the development and progression of different types of human malignancies [15–19]. As mentioned above, there is only one study in the literature investigating the SOX15 gene in association with promoter methylation and copy number alterations [15]. However, epigenetic regulation of gene expression via miRNA molecules is an equally important mechanism. MicroRNAs (miRNAs) are endogenous noncoding RNA molecules, about 18-26 nucleotides in length, which bind to target mRNAs and posttranscriptionally regulate their expression [20]. Recent reports have indicated that deregulation of miRNAs is associated with the development and progression of various cancers including thyroid carcinoma [21, 22]. A significant number of studies have shown that miRNAs have important functions in thyroid cancer. Zhu et al. [23] reported that miR-182 exerts an oncogenic effect in thyroid cancer by downregulating CHL1 expression. More recently, it has also been reported that increased miRNA expression may have clinical and prognostic significance in thyroid cancer [21]. As a result of TargetScan database analysis, we identified a miR-96 target sequence on the SOX15 mRNA. miR-182 and miR-96 are found in the same miRNA gene cluster together with miR-183 [24]. A recent pseudogene-gene (PGG) functional association analysis indicated that miR-375 may also regulate SOX15 expression in different cancer types [25].
However, the role of SOX15 and its association with miRNAs in thyroid cancer has not been investigated thoroughly. There is only a single report in the literature which investigates miR-147b in thyroid carcinoma in association with SOX15 [26].
In the present study, to understand the epigenetic regulation of SOX15, we focused to investigate SOX15 expression in association with promoter methylation and expression of four different miRNAs in thyroid carcinoma.
2. Materials and Methods
2.1. Patients
Primary thyroid tumors and adjacent nonmalignant tissue samples were collected from 52 patients, prior to any treatment at the Istanbul Education and Research Hospital between April 2016 and May 2017. The study was approved by the Ethics Committee of Istanbul University-Cerrahpasa, Cerrahpasa Medical Faculty (No: 71305). Signed informed consent was obtained from all patients before sample collection.
Table 1 lists the clinicopathological features of the patients. Pathological analysis was performed at the Pathology Department of the Istanbul Education and Research Hospital.
Table 1.
Clinicopathological characteristics of patients.
| Clinicopathological characteristics | n (%) | |
|---|---|---|
| Sex | Female | 44 (84.62) |
| Male | 8 (15.38) | |
| Age | ≤55 | 35 (67.31) |
| >55 | 16 (30.77) | |
| Unknown | 1 (1.92) | |
| TNM stage | TNM1 | 34 (65.38) |
| TNM2 | 7 (13.46) | |
| TNM3 | 6 (11.54) | |
| TNM4 | 3 (5.77) | |
| Unknown | 2 (3.85) | |
| Lymphatic invasion | Present | 17 (32.69) |
| Absent | 34 (65.38) | |
| Unknown | 1 (1.92) | |
| Vascular invasion | Present | 7 (13.46) |
| Absent | 44 (84.61) | |
| Unknown | 1 (1.92) | |
| Perineural invasion | Present | 6 (11.54) |
| Absent | 45 (86.54) | |
| Unknown | 1 (1.92) | |
| Capsule invasion | Present | 17 (32.69) |
| Absent | 33 (63.46) | |
| Unknown | 2 (3.85) | |
| Calcification | Present | 26 (50) |
| Absent | 21 (40.38) | |
| Unknown | 5 (9.62) | |
| Tumor diameter | ≤2 | 34 (65.38) |
| 2-4 | 15 (28.85) | |
| >4 | 2 (3.85) | |
| Unknown | 1 (1.92) | |
| Histologic type | Papillary | 47 (90.38) |
| Medullar | 1 (1.92) | |
| Follicular | 1 (1.92) | |
| Anaplastic | 1 (1.92) | |
| Unknown | 2 (3.85) | |
2.2. Methylation-Specific Polymerase Chain Reaction
Genomic DNA was obtained using the High Pure PCR Template Preparation Kit (Roche, Germany). After spectrophotometric quantitation, 500 ng of genomic DNA was bisulphide-treated using the EZ DNA Methylation-Gold Kit (Zymo Research, CA, USA) and finally resuspended in 10 μl TE buffer. PCR was performed in 25 μl volume containing 200 ng of modified DNA as template, 10x buffer, 100 mM dNTP, 10 pmol of each primer, and 5 U/μl AmpliTaq Gold DNA polymerase (ThermoFisher Scientific, MA, USA). Primers of methylated and unmethylated sequences were designed by the MethPrimer methylation analysis software and are listed in Table 2. The PCR products were directly loaded onto 2% agarose gels and analyzed using the Bio1D software (Vilber Lourmat, France) under UV light. The volume/area ratios were calculated to determine the methylation level of the SOX15 gene.
Table 2.
Primer sequences used in this study.
| Primer | Sequence |
|---|---|
| SOX15 qRT-PCR | F: 5′-CAGCTATGGCTCTTCCCACTG-3′ R: 5′-AGGGTTGTATGGAGTGGGAGA-3′ |
| β2M qRT-PCR | F: 5′-CTCGCGCTACTCTCTCTTTCTGG-3′ R: 5′-GCTTACATGTCTCGATCCCACTTAA-3′ |
| SOX15 methylated | F: 5′-TTATTCGCGTTTGGTAGTTGTC-3′ R: 5′-AAACCTTTACTTCCAACCTATTCG-3′ |
| SOX15 unmethylated | F: 5′-GGTTTATTTGTGTTTGGTAGTTGTT-3′ R: 5′-AAACCTTTACTTCCAACCTATTCAAC-3′ |
2.3. Reverse Transcription and Quantitative RT-PCR
Total RNA was isolated from tissue samples by using the PureLink™ RNA Mini Kit (ThermoFisher Scientific, MA, USA) according to the manufacturer's instructions. Reverse transcription was performed using 300 ng of total RNA and the Reverse Aid First-Strand cDNA synthesis kit (ThermoFisher Scientific, MA, USA). Expression levels of the SOX15 gene were analyzed by qRT-PCR using the SYBR green and LightCycler 480 system (Roche Diagnostics, Germany).
β2M was used as the reference to normalize the mRNA levels of each sample for quantification. CT values of the target and reference genes in tumor and normal thyroid tissues were analyzed by the LightCycler Software. Expression changes were determined by the relative mRNA levels using the 2-∆∆Ct method [27].
2.4. miRNA Quantification
TaqMan microRNA RT kit (ThermoFisher Scientific, MA, USA) was used for cDNA synthesis according to the manufacturer's instructions. The expression levels of miRNAs were analyzed with TaqMan MicroRNA Assay (hsa-mir-182-5p ID: 002334, hsa-mir-183-5p ID: 002269, hsa-mir-375-3p ID: 000564, and hsa-mir96-3p ID: 002140) (ThermoFisher Scientific, MA, USA). qRT-PCR amplification was performed using the protocol for TaqMan™ Small RNA Assays user guide (Publication Number 4364031, Revision Date 10 December 2019 Rev. H). qRT-PCR was performed using the LightCycler 480 system (Roche Diagnostics, Mannheim, Germany). Ct values of target miRNAs were normalized to U6 small nuclear RNA (ID: 001973), and the fold changes in expression levels of each miRNA were calculated using the 2-∆∆Ct method. The target and reference miRNAs were coamplified in the same reaction.
2.5. Statistical Analyses
Statistical analysis was performed using the SPSS 21.0 (IBM® SPSS® Statistics, IBM Corporation Somers, NY, USA) program. The paired sample t-test was used, and p < 0.05 was considered statistically significant for data showing normal distribution. The nonparametric counterpart of the paired sample t-test, Wilcoxon Signed Rank Test, was used for unequally distributed expression levels.
3. Results
The expression level of the SOX15 gene was analyzed in 49 pairs of thyroid tumors and adjacent noncancerous tissues. We detected the SOX15 transcript in both tumor and normal tissue samples. However, SOX15 gene expression was significantly downregulated in 64.6% (34/49) of TC tissues compared to their normal counterparts (Figure 1). Next, we explored the relationship between SOX15 expression levels and the clinicopathological features of TC patients. The level of SOX15 expression was not associated with any particular clinicopathological parameter.
Figure 1.
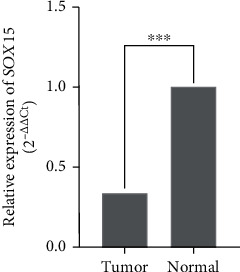
SOX15 gene expression in thyroid cancer tumor tissues compared to their normal counterparts. ∗∗∗p ≤ 0.001.
One of the mechanisms which leads to downregulation of gene expression is promoter methylation. Further, we investigated the methylation status of the SOX15 promoter (-158 to -382 region) by Methylation-Specific PCR (MSP) in 44 paired tumors and adjacent noncancerous tissues. In TC samples, 15 cases (15/44) revealed both moderately unmethylated and highly methylated forms, and 3 cases revealed both methylated and unmethylated forms. 25 of the remaining 26 samples were completely methylated, and one sample was completely unmethylated. The noncancerous adjacent counterpart of the completely unmethylated tumor sample was also completely unmethylated. 21 of the remaining noncancerous samples were completely methylated, and 22 revealed both methylated and unmethylated forms.
We did not observe statistically significant differences between the methylation levels of tumors and matched noncancerous tissues (p = 0.115). Our results indicate that the downregulation of the SOX15 gene is not associated with promoter methylation (Table 3).
Table 3.
Correlation between SOX15 mRNA expression and promoter methylation.
| SOX15 expression n (%) |
|||||
|---|---|---|---|---|---|
| Increase | Decrease | No change | ∗ p | ||
| Methylation | Increase | 4 (9.1) | 9 (20.5) | 3 (6.8) | 0.162 |
| Decrease | 1 (2.3) | 8 (18.2) | 0 (0) | ||
| No change | 5 (11.4) | 13 (29.5) | 1 (2.3) | ||
∗Statistical analysis was performed by using Spearman correlation.
Thus, we concluded that a different mechanism such as miRNAs might modulate SOX15 expression. For this purpose, we investigated the expression rates of miRNA-182, miRNA-183, miRNA-96, and miRNA-375 in 45 matched tumor and noncancerous tissue pairs. Significant differences in miRNA-182, miRNA-183, and miRNA-375 levels were observed between TC tumors and matched normal tissues. However, miRNA-96 levels were similar both in tumor and in normal tissues. For the tumor and normal tissues, the levels of miRNAs are presented in Table 4.
Table 4.
Mean (±SD) expression values of miRNA-182, miRNA-183, miRNA-96, and miRNA-375 in tumor and noncancerous tissues.
| Target Ct Mean ± SD |
Reference Ct Mean ± SD |
∆Ct Mean ± SD |
∆∆Ct | 2-∆∆Ct | p | ||
|---|---|---|---|---|---|---|---|
| miRNA-182 | Tumor | 34.46 ± 1.85 | 24.16 ± 1.34 | 10.32 ± 2.03 | -1.64 | 3.11 | ∗ <0.001 |
| Noncancerous | 35.74 ± 1.55 | 23.9 ± 1.09 | 11.85 ± 1.53 | 0 | 1 | ||
| miRNA-183 | Tumor | 33.67 ± 2.06 | 24.53 ± 1.35 | 9.14 ± 1.98 | -1.27 | 2.41 | ∗0.003 |
| Noncancerous | 34.59 ± 1.59 | 24.22 ± 1.16 | 10.38 ± 1.57 | 0 | 1 | ||
| miRNA-375 | Tumor | 32.19 ± 3.19 | 24.56 ± 1.37 | 7.63 ± 3.55 | -2.04 | 4.1 | ∗∗0.001 |
| Noncancerous | 33.93 ± 2.14 | 24.26 ± 1.19 | 9.67 ± 2.25 | 0 | 1 | ||
| miRNA-96 | Tumor | 36.47 ± 1.5 | 23.27 ± 1.6 | 12.93 ± 2.33 | 0.24 | 0.85 | ∗∗0.628 |
| Noncancerous | 36.31 ± 1.42 | 23.05 ± 1.69 | 12.7 ± 1.79 | 0 | 1 |
∗Statistical analyses were performed by paired sample t-test. ∗∗Statistical analyses were performed by the Wilcoxon Signed Rank Test. SD: standard deviation.
4. Discussion
Thyroid cancer is one of the most common endocrine malignancies. Although some genetic alterations such as BRAF, RAS, CTNNB1, TP53, and EGFR mutations have been associated with thyroid cancer, additional molecular mechanisms are thought to be involved in the formation and progression of TC [28–32]. Aberrant activation of signaling pathways is a common mechanism in human cancers. One of the important pathways is the Wnt/β-catenin signaling pathway which regulates cellular events such as proliferation, differentiation, and cell motility. Several secreted protein families activate or inhibit Wnt/β-catenin signaling. Upon activation, the Wnt/β-catenin pathway triggers the formation and progression of different types of human cancers [33]. Previous reports have shown that some of the SOX gene family members are negative regulators (or antagonists) of the Wnt/β-catenin signaling pathway [34, 35]. Thu et al. have identified SOX15 as a negative regulator of the Wnt/β-catenin pathway in PDAC [16]. In contrast to other members of the SOX family, function of the SOX15 gene is not well defined in cancer. It is well documented that SOX15 has critical functions in myogenic differentiation [36]. Following this initial report, other studies have associated aberrant SOX15 expression with other kinds of tumors such as gastric, endometrial, and colon cancer [15–19].
However, SOX15 has not been investigated in detail in TC. To our knowledge, only a single study is available in the literature, which investigates SOX15 in TC [26]. According to this report, SOX15 is underexpressed in TC tumor cells and cell lines under the influence of miR-147b and silencing of SOX15 via miR-147b activates the Wnt/β-catenin pathway.
In accordance with the previous results, we observed a significant downregulation of the SOX15 gene in the PTC tumor samples compared to normal tissue. Our data analysis revealed that mutation is not a frequent event in SOX15 inactivation. As a result of the multiomics approach, it has been reported that SOX15 is inactivated by concurrent hypermethylation and DNA copy number loss in PDAC [15]. However, it should be noted that regulation of expression is also controlled by other genetic and epigenetic mechanisms. Therefore, we investigated promoter methylation of the SOX15 gene in PTC tumor cells in association with its expression levels. In contrast to PDAC, our results indicate that downregulation of SOX15 is not caused by promoter hypermethylation. On the other hand, increasing evidence indicates that miRNAs play important roles in gene inactivation. Accumulating data show that various miRNAs are dysregulated in thyroid carcinoma and most of these miRNAs are involved in the regulation of malignancy and metastasis in TC [21–23, 26, 37, 38]. Hitu et al. [22] have reported that 106 of 139 miRNAs which have been investigated were upregulated in PTC while 33 were downregulated. In a previous report, Zhu et al. [23] revealed that overexpression of miR-182 regulates PTC proliferation and invasion through downregulating CHL1 expression. More recently, another report also associated miR-182 overexpression with extrathyroidal invasion, cervical lymph node metastasis, and TNM staging in PTC [38]. In accordance with these reports, we observed 3.11 times higher miR-182 levels in PTC tumor samples compared to normal tissues. However, overexpression of miR-182 was not associated with any clinicopathological characteristics of the patients. miRNAs frequently reside in clusters, and members of clusters are generally transcribed in the same direction [24]. miR-182/183/96 also are usually found as a miRNA cluster. Therefore, we investigated expression levels of miR183 and miR-96 together with miR-182 in our study cohort. Although the expression level of miR-183 increased similar to miR-182, miR-96 expression was at the same level in the tumors and normal noncancerous tissues. Our TargetScan analysis revealed that a possible target sequence for miR-96 was present in the SOX15 gene; however, we did not observe altered miRNA-96 expression or any association between the SOX15 and miRNA-96 expression levels. This result is in contrast to a report which suggested that high miRNA-96 expression in PTC tissue and cell lines promotes cell proliferation, migration, and invasion via downregulating the Deup1 protein expression [37]. This difference may be due to processing of tissues under different conditions. We used tissue samples as soon as they were surgically removed. It should also be noted that not all target sequences predicted by TargetScan are actually valid. Indeed, the estimates for false-positive rates for target prediction are at the level of 50% and the results of the target prediction programs are inconsistent [39–41].
miR-375 is one of the highly conserved miRNAs in humans through the evolution [42]. According to PGG network analysis, Johnson et al. have reported that differential expression of SOX15 was associated with miR-375 expression in prostate cancer [25]. Although in our study downregulation in SOX15 expression was not correlated with miR-375 overexpression, we detected a statistically significant increase in miR-375 expression in tumor samples compared to noncancerous ones. Our expression analysis also showed that miRNA-375 expression levels are as high as miRNA-182 and miRNA-183.
As indicated above, SOX15 has been shown to modulate the Wnt/β-catenin pathway [16–18]. Likewise, miR-183 has been shown to act as an important target in the regulation of the Wnt/β-catenin pathway in different cancer types [43–45]. On the other hand, miR-182 has been associated with tumor progression and chemoresistance in various tumors [46–48] and with suppression of apoptosis in papillary thyroid cancer [49]. miR-375 and miR-96 were shown to affect various pathways in different tumors and have been reported to regulate the PI3K/Akt pathway in thyroid cancer [50, 51].
In view of lack of studies on the function of SOX proteins in thyroid cancer, we believe that our data suggest a role for SOX15 and increased miR-182, miR-183, and miR-375 levels in the tumor samples. The results of the present study indicate that the correlation between the miRNAs and SOX15 warrants further research to reveal their role and detailed mechanism in thyroid carcinogenesis. Although investigating expression of the SOX15 protein to confirm the mRNA expression levels would corroborate our results, unfortunately, most of the samples made available for this study were not sufficient to analyze SOX15 protein expression by western blotting in matched pairs of tissue specimens. Concordance of the SOX15 mRNA expression levels with cellular protein levels in the tissue remains to be shown/confirmed. However, for SOX15, a high degree of agreement between the mRNA and protein expression has been shown in pancreatic [16] and colorectal [18] cancers as well as in gliomas [35]. Deciphering the role and mutual interaction of SOX15 with specific miRNAs will certainly help to provide further insight implicating the cellular signals and pathways involved in thyroid carcinogenesis.
5. Conclusion
In conclusion, our results indicate that the SOX15 gene is associated with PTC pathogenesis and the epigenetic control of this gene is regulated by miRNAs rather than promoter methylation. The results of the study need to be verified by the analysis of a larger number of TC samples and further correlation studies of miRNAs.
Acknowledgments
This work was supported by the Scientific Research Projects Coordination Unit of Istanbul University, project numbers: TSA-2019-33994 and TYL-2016-21433.
Data Availability
Data are available on request.
Conflicts of Interest
No conflict of interest exists in this study.
References
- 1.Jemal A., Bray F., Center M. M., Ferlay J., Ward E., Forman D. Global cancer statistics [published correction appears in CA Cancer J Clin. 2011 Mar-Apr; 61(2): 134] CA: a Cancer Journal for Clinicians . 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.DeLellis R. A., Lloyd R. V., Heitz P. U., Eng C. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of Endocrine Organs . IARC Press; 2004. [Google Scholar]
- 3.Howlader N., Noone A. M., Krapcho M., et al. SEER cancer statistics review. 1975-2011 . Bethasta, MD: National Cancer Institute; 2014. [Google Scholar]
- 4.Prior H. M., Walter M. A. SOX genes: architects of development. Molecular Medicine . 1996;2(4):405–412. doi: 10.1007/BF03401900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laudet V., Stehelin D., Clevers H. Ancestry and diversity of the HMG box superfamily. Nucleic Acids Research . 1993;21(10):2493–2501. doi: 10.1093/nar/21.10.2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bowles J., Schepers G., Koopman P. Phylogeny of the SOX family of developmental transcription factors based on sequence and structural indicators. Developmental Biology . 2000;227(2):239–255. doi: 10.1006/dbio.2000.9883. [DOI] [PubMed] [Google Scholar]
- 7.Tani M., Shindo-Okada N., Hashimoto Y., et al. Isolation of a Novel _Sry_ -Related Gene That Is Expressed in High-Metastatic K-1735 Murine Melanoma Cells. Genomics . 1997;39(1):30–37. doi: 10.1006/geno.1996.4483. [DOI] [PubMed] [Google Scholar]
- 8.Ferrari S., Harley V. R., Pontiggia A., Goodfellow P. N., Lovell-Badge R., Bianchi M. E. SRY, like HMG1, recognizes sharp angles in DNA. The EMBO Journal . 1992;11(12):4497–4506. doi: 10.1002/j.1460-2075.1992.tb05551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mertin S., McDowall S. G., Harley V. R. The DNA-binding specificity of SOX9 and other SOX proteins. Nucleic Acids Research . 1999;27(5):1359–1364. doi: 10.1093/nar/27.5.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wegner M. From head to toes: the multiple facets of Sox proteins. Nucleic Acids Research . 1999;27(6):1409–1420. doi: 10.1093/nar/27.6.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson M., Koopman P. Matching SOX: partner proteins and co-factors of the SOX family of transcriptional regulators. Current Opinion in Genetics & Development . 2002;12(4):441–446. doi: 10.1016/s0959-437x(02)00323-4. [DOI] [PubMed] [Google Scholar]
- 12.Schepers G. E., Teasdale R. D., Koopman P. Twenty pairs of sox: extent, homology, and nomenclature of the mouse and human sox transcription factor gene families. Developmental Cell . 2002;3(2):167–170. doi: 10.1016/s1534-5807(02)00223-x. [DOI] [PubMed] [Google Scholar]
- 13.Meyer J., Wirth J., Held M., Schempp W., Scherer G. SOX20, a new member of the SOX gene family, is located on chromosome 17p13. Cytogenetics and Cell Genetics . 1996;72(2-3):246–249. doi: 10.1159/000134200. [DOI] [PubMed] [Google Scholar]
- 14.Vujić M., Rajić T., Goodfellow P. N., Stevanović M. cDNA characterization and high resolution mapping of the human SOX20 gene. Mammalian Genome . 1998;9(12):1059–1061. doi: 10.1007/s003359900925. [DOI] [PubMed] [Google Scholar]
- 15.Thu K. L., Becker-Santos D. D., Radulovich N., Pikor L. A., Lam W. L., Tsao M. S. SOX15 and other SOX family members are important mediators of tumorigenesis in multiple cancer types. Oncoscience . 2014;1(5):326–335. doi: 10.18632/oncoscience.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thu K. L., Radulovich N., Becker-Santos D. D., et al. _SOX15_ is a candidate tumor suppressor in pancreatic cancer with a potential role in Wnt/ β-catenin signaling. Oncogene . 2014;33(3):279–288. doi: 10.1038/onc.2012.595. [DOI] [PubMed] [Google Scholar]
- 17.Rui X., Xu Y., Jiang X., Guo C., Jiang J. SOX15 regulates proliferation and migration of endometrial cancer cells. Bioscience Reports . 2017;37(5, article BSR20171045) doi: 10.1042/BSR20171045. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Wang S., Yang H., Chen X., Jiang Z. Effects of SOX15 on the colorectal cancer cells via downregulation of the Wnt/β-catenin signaling pathway. Future Oncology . 2018;14(19):1921–1932. doi: 10.2217/fon-2017-0688. [DOI] [PubMed] [Google Scholar]
- 19.Zhang M., Wang J., Gao T., et al. Inhibition of SOX15 sensitizes esophageal squamous carcinoma cells to paclitaxel. Current Molecular Medicine . 2019;19(5):349–356. doi: 10.2174/1566524019666190405121139. [DOI] [PubMed] [Google Scholar]
- 20.Stefani G., Slack F. J. Small non-coding RNAs in animal development. Nature Reviews. Molecular Cell Biology . 2008;9(3):219–230. doi: 10.1038/nrm2347. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y., Xu D., Pan J., et al. Dynamic monitoring of circulating microRNAs as a predictive biomarker for the diagnosis and recurrence of papillary thyroid carcinoma. Oncology Letters . 2017;13(6):4252–4266. doi: 10.3892/ol.2017.6028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hitu L., Gabora K., Bonci E. A., et al. MicroRNA in papillary thyroid carcinoma: a systematic review from 2018 to June 2020. Cancers . 2020;12(11):p. 3118. doi: 10.3390/cancers12113118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu H., Fang J., Zhang J., et al. miR-182 targets CHL1 and controls tumor growth and invasion in papillary thyroid carcinoma. Biochemical and Biophysical Research Communications . 2014;450(1):857–862. doi: 10.1016/j.bbrc.2014.06.073. [DOI] [PubMed] [Google Scholar]
- 24.Xu S., Witmer P. D., Lumayag S., Kovacs B., Valle D. MicroRNA (miRNA) transcriptome of mouse retina and identification of a sensory organ-specific miRNA cluster. The Journal of Biological Chemistry . 2007;282(34):25053–25066. doi: 10.1074/jbc.M700501200. [DOI] [PubMed] [Google Scholar]
- 25.Johnson T. S., Li S., Franz E., et al. PseudoFuN: deriving functional potentials of pseudogenes from integrative relationships with genes and microRNAs across 32 cancers. Gigascience . 2019;8(5, article giz046) doi: 10.1093/gigascience/giz046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu C., Liu J., Yao X., et al. Downregulation of microR-147b represses the proliferation and invasion of thyroid carcinoma cells by inhibiting Wnt/β-catenin signaling via targeting SOX15. Molecular and Cellular Endocrinology . 2020;501:p. 110662. doi: 10.1016/j.mce.2019.110662. [DOI] [PubMed] [Google Scholar]
- 27.Schmittgen T. D., Livak K. J. Analyzing real-time PCR data by the comparative C(T) method. Nature Protocols . 2008;3(6):1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 28.Xing M. BRAF mutation in thyroid cancer. Endocrine-Related Cancer . 2005;12(2):245–262. doi: 10.1677/erc.1.0978. [DOI] [PubMed] [Google Scholar]
- 29.Gire V., Wynford-Thomas D. RAS oncogene activation induces proliferation in normal human thyroid epithelial cells without loss of differentiation. Oncogene . 2000;19(6):737–744. doi: 10.1038/sj.onc.1203399. [DOI] [PubMed] [Google Scholar]
- 30.Garcia-Rostan G., Tallini G., Herrero A., D'Aquila T. G., Carcangiu M. L., Rimm D. L. Frequent mutation and nuclear localization of beta-catenin in anaplastic thyroid carcinoma. Cancer Research . 1999;59(8):1811–1815. [PubMed] [Google Scholar]
- 31.Fagin J. A., Matsuo K., Karmakar A., Chen D. L., Tang S. H., Koeffler H. P. High prevalence of mutations of the p53 gene in poorly differentiated human thyroid carcinomas. The Journal of Clinical Investigation . 1993;91(1):179–184. doi: 10.1172/JCI116168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murugan A. K., Dong J., Xie J., Xing M. Uncommon GNAQ, MMP8, AKT3, EGFR, and PIK3R1 mutations in thyroid cancers. Endocrine Pathology . 2011;22(2):97–102. doi: 10.1007/s12022-011-9155-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clevers H., Nusse R. Wnt/β-catenin signaling and disease. Cell . 2012;149(6):1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 34.Bernard P., Harley V. R. Acquisition of SOX transcription factor specificity through protein-protein interaction, modulation of Wnt signalling and post-translational modification. The International Journal of Biochemistry & Cell Biology . 2010;42(3):400–410. doi: 10.1016/j.biocel.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 35.Zhang D., Guo S., Wang H., Hu Y. SOX15 exerts antitumor function in glioma by inhibiting cell proliferation and invasion via downregulation of Wnt/β-catenin signaling. Life Sciences . 2020;255:p. 117792. doi: 10.1016/j.lfs.2020.117792. [DOI] [PubMed] [Google Scholar]
- 36.Lee H. J., Göring W., Ochs M., et al. Sox15 is required for skeletal muscle regeneration. Molecular and Cellular Biology . 2004;24(19):8428–8436. doi: 10.1128/MCB.24.19.8428-8436.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu Z. M., Wu Z. Y., Li W. H., Wang L. Q., Wan J. N., Zhong Y. MiR-96-5p promotes the proliferation, invasion and metastasis of papillary thyroid carcinoma through down-regulating CCDC67. European Review for Medical and Pharmacological Sciences . 2019;23(8):3421–3430. doi: 10.26355/eurrev_201904_17706. [DOI] [PubMed] [Google Scholar]
- 38.Yao X. G., Tan Q., Liu P. P., Feng L. J. Tissue microRNA-182 expression level and its potential prognostic value for papillary thyroid carcinoma. International Journal of Clinical and Experimental Pathology . 2019;12(8):3128–3133. [PMC free article] [PubMed] [Google Scholar]
- 39.Witkos T. M., Koscianska E., Krzyzosiak W. J. Practical aspects of microRNA target prediction. Current Molecular Medicine . 2011;11(2):93–109. doi: 10.2174/156652411794859250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pinzón N., Li B., Martinez L., et al. MicroRNA target prediction programs predict many false positives. Genome Research . 2017;27(2):234–245. doi: 10.1101/gr.205146.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fridrich A., Hazan Y., Moran Y. Too many false targets for microRNAs: challenges and pitfalls in prediction of miRNA targets and their gene ontology in model and non-model organisms bioessays. BioEssays . 2019;41(4, article e1800169) doi: 10.1002/bies.201800169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Poy M. N., Eliasson L., Krutzfeldt J., et al. A pancreatic islet-specific microRNA regulates insulin secretion. Nature . 2004;432(7014):226–230. doi: 10.1038/nature03076. [DOI] [PubMed] [Google Scholar]
- 43.Leung W. K., He M., Chan A. W., Law P. T., Wong N. Wnt/β-catenin activates MiR-183/96/182 expression in hepatocellular carcinoma that promotes cell invasion. Cancer Letters . 2015;362(1):97–105. doi: 10.1016/j.canlet.2015.03.023. [DOI] [PubMed] [Google Scholar]
- 44.Tang X., Zheng D., Hu P., et al. Glycogen synthase kinase 3 beta inhibits microRNA-183-96-182 cluster via the β-catenin/TCF/LEF-1 pathway in gastric cancer cells. Nucleic Acids Research . 2014;42(5):2988–2998. doi: 10.1093/nar/gkt1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cao D., Di M., Liang J., Shi S., Tan Q., Wang Z. MicroRNA-183 in cancer progression. Journal of Cancer . 2020;11(6):1315–1324. doi: 10.7150/jca.39044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu R., Li J., Teng Z., Zhang Z., Xu Y. Overexpressed microRNA-182 promotes proliferation and invasion in prostate cancer PC-3 cells by down-regulating N-myc downstream regulated gene 1 (NDRG1) PLoS One . 2013;8(7, article e68982) doi: 10.1371/journal.pone.0068982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rasheed S. A. K., Teo C. R., Beillard E. J., Voorhoeve P. M., Casey P. MicroRNA-182 and microRNA-200a control G-protein subunit α-13 (GNA13) expression and cell invasion synergistically in prostate cancer cells. The Journal of Biological Chemistry . 2013;288(11):7986–7995. doi: 10.1074/jbc.M112.437749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martinez-Ruiz H., Illa-Bochaca I., Omene C., et al. A TGF -miR-182-BRCA1 axis controls the mammary differentiation hierarchy. Science Signaling . 2016;9(457, article ra118) doi: 10.1126/scisignal.aaf5402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fu S., Zhao N., Jing G., Yang X., Liu J., Zhen D. Matrine induces papillary thyroid cancer cell apoptosis in vitro and suppresses tumor growth in vivo by downregulating miR-182-5p. Biomedicine & Pharmacotherapy . 2020;128:p. 110327. doi: 10.1016/j.biopha.2020.110327. [DOI] [PubMed] [Google Scholar]
- 50.Shi L., Zhao S.-M., Luo Y., et al. MiR-375: a prospective regulator in medullary thyroid cancer based on microarray data and bioinformatics analyses. Pathology, Research and Practice . 2017;213(11):1344–1354. doi: 10.1016/j.prp.2017.09.024. [DOI] [PubMed] [Google Scholar]
- 51.Zhao X., Li Y., Zhou Y. MicroRNA-96-3p promotes metastasis of papillary thyroid cancer through targeting SDHB. Cancer Cell International . 2019;19:p. 287. doi: 10.1186/s12935-019-1003-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available on request.


