Abstract
Methods
The mice were randomly distributed into four groups: (a) control (CTRL) group, (b) ETEC group, (c) IQW-ETEC group, and (d) IRW-ETEC group. Villus length and crypt depth were measured after hematoxylin and eosin staining. The inflammatory reaction was analyzed via inflammatory cytokines (i.e., TNF-α, IL-1β, IL-6, and IL-10) using the enzyme-linked immunosorbent assay (ELISA). The microbiota in the colon was sequenced using 16S ribosomal RNA.
Results
The villus length decreased, the crypt depth decreased, and the expression of inflammatory cytokines (i.e., TNF-α, IL-1β, IL-6, and IL-10) increased due to ETEC. In the IRW-ETEC and IQW-ETEC groups, the Shannon index decreased (P < 0.05). IQW and IRW increased the abundance of Firmicutes, Proteobacteria, Clostridiales, Lachnospiraceae, and Alloprevotella; contrastingly, it decreased the abundance of Epsilonproteobacteria, Erysipelotrichales, Prevotellaceae, and Flavobacteriaceae compared to the ETEC group (P <0.05).
Conclusion
This study ascertained that the addition of IQW and IRW could alleviate jejunal inflammation and increase microbiota community diversity.
1. Introduction
According to records, diarrhea kills approximately 800,000 children each year [1]. One in ten children worldwide has died due to diarrhea [2]. The cause of diarrhea is complicated; however, pathogenic bacterial infections are one of the major causes. Pathogenic bacteria were detected in 80% of diarrhea patients in Southeast Asia and 72% of diarrhea patients in South America [3].
The traditional treatment of infectious diarrhea involves antibiotics (and sometimes antiviral or antiparasitic medications), which may increase pathogen resistance to drugs. Simultaneously, antibiotics ruin the intestinal microenvironment of the host. Thus, there is much concern about determining alternatives to antibiotics in diarrhea treatment. Natural products, polypeptides, polysaccharides, probiotics, and other substances can be added to the host diet to treat infectious diarrhea; these have received much attention in recent years [4–6]. For example, one study revealed that adding plant polysaccharides to one's diet can alleviate diarrhea symptoms of mice infected with enterotoxigenic Escherichia coli (ETEC) [7].
Ile-Gln-Trp (IQW) and Ile-Arg-Trp (IRW) are two active tripeptides extracted from egg whites. Some studies have proved that IQW and IRW have several functions, including lowering blood pressure and cholesterol and antioxidant and anti-inflammatory properties [8–11]. Studies have shown that IQW and IRW can reduce the TNF-induced inflammatory and oxidative stress responses in endothelial cells; these anti-inflammatory and antioxidative effects of IRW and IQW are regulated through the NF-κB signal pathway [12, 13]. It has been shown that IRW can display anti-inflammatory effects by inhibiting p65 protein activity in NF-κB [14]. Additionally, research has shown that IRW can upregulate the expression of nicotinamide phosphoribosyltransferase (NAMPT) protein in mouse muscle cells, improving metabolic levels and alleviating obesity [15]. Our team focused on adding this active peptide to the host diet, exploring the prevention and treatment of intestinal damage. We explored the impact of colitis by adding IRW and IQW to the diet of a DSS-induced colitis mouse model; research showed that IQW could adjust the amino acid levels in serum and regulate intestinal immune function to relieve inflammation; IQW and IRW could reduce oxidative stress induced by DSS by increasing antioxidant enzyme activity; IQW and IRW achieved this by increasing the diversity of the host's intestinal microorganisms and increasing the probiotic biomass [16, 17]. In view of the IQW and IRW effect in the DSS-induced mouse model, we expect IQW and IRW can perform a similar effect in the ETEC-induced mouse model. In this study, we explore the effects of IQW and IRW on jejunal inflammation in an ETEC-induced mouse model.
2. Materials and Methods
2.1. Experiment Design
The Chinese guidelines for animal welfare were observed for the experimental strategy design. Approval from the Animal Care and Use Committee of Hunan Agricultural University was obtained. In total, 24 male mice (8 weeks, average weight: 23 g) were used in the experiment. All mice were raised at Hunan Agricultural University and fed in a comfortable environment (relative humidity: 53%; average temperature: 24 degrees). In order to simulate natural light conditions, the animals were fed under 12 h of light and 12 h without light. In order to alleviate the stress response caused by the environmental change, mice were given a 3-day adaptation period before the experiment. After 3 days, the mice were randomly divided into four groups: (a) control (CTRL) group (n = 6), (b) ETEC group (n = 6), (c) IQW-ETEC group (n = 6), and (d) IRW-ETEC group (n = 6). Groups a and b were given basal diet and natural drinking water for the first 7 days. Group a was given 0.1 mL saline in the first 7 days. Group b was given 0.1 mL 5 × 109 CFU/mL ETEC for 7 days. Mice in groups c and d were put on a basal diet of IQW (93.04% purity, 0.03% mass concentration) and 0.03% IRW (87.91% purity; 0.03% mass concentration), respectively. Meanwhile, 0.1 mL ETEC was given to groups c and d 7 days after the first day of the feeding experiment; this lasted for 7 days. At the end of the 15th day of the feeding experiment, all mice fasted for 12 h and were subsequently weighed before being sacrificed. The acute blood loss method is used to collect blood in mice. The jejunal tissues and colon contents were collected after autopsy; all samples were frozen using liquid nitrogen and stored in a -80°C freezer for further experimentation.
2.2. Histological Analysis of the Jejunum
Samples contained different alcohol concentrations (50%, 70%, 80%, 90%, and 95%). For dehydration, a dimethyl benzene ethanol and paraffin (1 : 1) solution was used for the sample embedding processing. The jejunal tissue morphology and tissue damage were analyzed, and the height of intestinal villi and crypt depth were microscope measured using hematoxylin-eosin-stained samples.
2.3. Jejunal Tissue Inflammatory Cytokine Detection
Jejunal TNF-α, IL-1β, IL-6, and IL-10 were detected via enzyme-linked immunosorbent assay (ELISA). Antibodies (anti-TNF-α, IL-1β, IL-6, and IL-10) were added into the polystyrene HRP-plate well after dilution with carbonate-coated buffer (1 : 100) and placed at 4°C for 12 h overnight. The solution was poured out, and the plates were washed with PBS solution three times. The blocking solution was added to each plate well, incubated at 37°C for 1.5 h. After washing the samples three times using PBS solution, they were incubated at 37°C for 1.5 h. Diluted biotinylated antibodies (goat against mice) were added for 30 min at 37°C. TMB (3,3′,5,5′-Tetramethylbenzidine) substrate was added to each reaction well for the color reaction; the reaction lasted 20 min at 37°C. Sulfuric acid was added to each reaction well for the termination reaction. The absorbance of each reaction was measured at 450 nm.
2.4. Microbial Community Analysis
The DNA of colon content samples was extracted, and the purified DNA was used as a template to amplify the variable region of V3+V4 of bacterial 16S rDNA gene by PCR. The PCR products were sent to MicroBio for sequencing analysis. The obtained sequencing results were optimized for OTU-operational taxonomic unit cluster analysis. Alpha diversity analysis (species richness statistics, such as Chao and Ace, and species diversity statistics, such as Shannon and Simpson) was performed using Mothur (version 1.33.3) software. Microbial Ecology (QIIME) was an open-source tool for analyzing the original sequence. PycGootookit6 software was used to deal with the sequence errors and database redundancy in the original data [18]. The optimal overlapping sequence was found by splicing the original sequence [19]. For each sample, the sequence was analyzed using QIIME and the UPARSE application to determine the operational classification unit (OTUs), and the classification data were assigned to each OTUs using the RDP classifier (version 2.2) [20]. The selected sequence of representatives. RDP classifier and Greengenes database were used for classification. Alpha diversity analysis of the jejunal bacterial community was performed using the abundance-based coverage estimator (ACE), bias-corrected Chao richness estimator, Shannon index, and Good's coverage.
2.5. Data Analysis
The statistical software package (SPSS V16.0) was used for statistical analysis. The one-way ANOVA method was used to analyze significant differences among groups, and the data were represented as mean ± SD. GraphPad Prism 8 was used to make graphs. P values < 0.05 were regarded as significantly different.
3. Results
As shown in Figure 1, compared to the CTRL group, the length of intestinal villi in the ETEC group was significantly decreased (P < 0.05), and crypt depth was increased considerably (P < 0.05), which indicated that ETEC could cause severe jejunal damage. However, compared to the ETEC group, in the IQW-ETEC and IRW-ETEC groups, the length of intestinal villi increased while the crypt depth decreased (P < 0.05), which might indicate that IQW and IRW dramatically improve the status of intestinal injury, improving damage recovery.
Figure 1.

H&E staining results of mouse jejunum (n = 6): (a) CTRL, (b) ETEC, (c) IQW+ETEC, and (d) IRW+ETEC. The villus length (e), crypt depth (f), and the ratio of villi length to crypt depth (g); the change in letter denotes a significant difference.
As shown in Figure 2, the contents of inflammatory cytokines (TNF-α, IL-1β, IL-6, and IL-10) in the ETEC group were significantly higher than those in the CTRL group. The contents of TNF-α, IL-1β, IL-6, and IL-10 in the IQW-ETEC and IRW-ETEC groups were significantly lower than those in the ETEC group (P < 0.05).
Figure 2.

Inflammatory cytokines in the mouse jejunum in each group (n = 6): (a) TNF-α, (b) IL-1β, (c) IL-6, and (d) IL-10; the change in letter denotes a significant difference.
Sequencing analysis was from the V3-V4 region of 16S rRNA in collected colon content samples. Figure 3 shows the α-diversity analysis of each group. In the ETEC group, the Ace index, Shannon index, Chao index, Sobs index, and Good's coverage were significantly lower than in the CTRL group. Additionally, compared to the ETEC group, the α-diversity of the IQW-ETEC and IRW-ETEC groups was significantly increased (P < 0.05).
Figure 3.

α-Diversity of colon microorganisms of mice in each group (n = 6), letter (b) denotes a significant difference: (a) Ace index, (b) Shannon index, (c) Chao index, (d) Sobs index, and (e) coverage index.
Microbiota analysis was conducted on colon content samples; Figure 4(a) shows the relative abundance of collected microorganisms at the phylum level. The abundance of Bacteroidetes, Firmicutes, and Proteobacteria showed advantages in the phylum level. In the CTRL group, the relative abundance of microbiota was Bacteroidetes (55.20%), Firmicutes (35.33%), and Proteobacteria (4.08%). In the IQW-ETEC group, the relative abundance of microbiota was Bacteroidetes (58.24%), Firmicutes (29.92%), and Proteobacteria (6.11%). In the IRW-ETEC group, the relative abundance of microbiota was Bacteroidetes (70.21%), Firmicutes (20.66%), and Proteobacteria (4.77%). In Figure 4(b), IQW and IRW significantly increased the abundance of Bacteroidetes compared with the ETEC group (P < 0.05). In Figure 4(c), IQW and IRW significantly reduced the abundance of Firmicutes in mice compared with the ETEC group (P < 0.05). The IRW-ETEC and IQW-ETEC groups were significantly different from the ETEC group (P < 0.05), and the proportion of Firmicutes in the colon of mice in the IRW-ETEC group also increased to a certain extent. In Figure 4(d), the abundance of Proteobacteria in ETEC is significantly higher than in the CTRL group (P < 0.05). The abundance of Proteobacteria in the IRW-ETEC and IQW-ETEC groups is significantly lower than in the ETEC group (P < 0.05).
Figure 4.
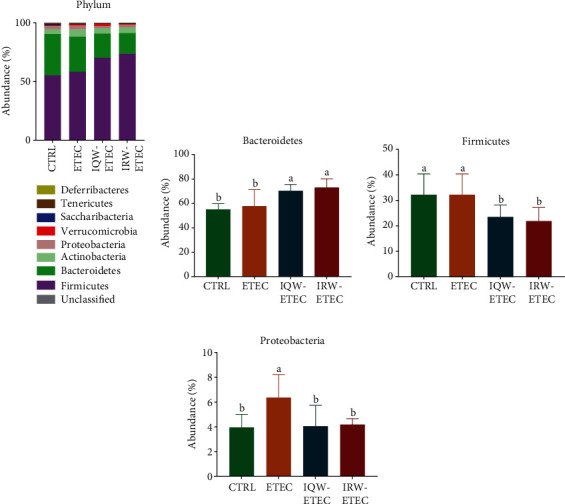
Effect of IQW and IRW treatment on the microorganisms at the phylum level: (a) microbiota of the colon in the four groups at the phylum level (n = 6), (b) Bacteroidetes, (c) Firmicutes, and (d) Proteobacteria; the change in letter denotes a significant difference.
Figure 5(a) shows the nine classes with the most abundant abundance in colon contents. In the CTRL group, Bacteroides (54.95%), Clostridia (23.7%), and Bacilli (10.7%) were the most abundant. In the ETEC group, Bacteroidetes (57.5%), Clostridium (12.96%), Bacillus (12.99%), and Erysipelotrichia (3.95%) were the most abundant groups. In the IQW-ETEC group, Bacteroidetes (70.1%), Clostridium (11.6%), and Bacillus (7.1%) were the largest groups. In the IRW-ETEC group, Bacteroides (72.9%), Clostridium (9.1%), Bacillus (5.4%), and Erysipelas (3.0%) were the most abundant groups. The results in Figure 5(b) show that both IQW and IRW treatments increased the abundance of Bacteroidia in the intestine of mice, and both groups showed significant differences compared with the ETEC group (P < 0.05). At the same time, it can be seen from Figure 5(c) that ETEC treatment significantly increased the abundance of Epsilonproteobacteria (P < 0.05). IQW and IRW treatment could significantly reduce the growth of Epsilonproteobacteria (P < 0.05). In Figure 5(d), the relative abundance of Clostridia in the ETEC group is significantly decreased compared to the CTRL group; IQW and IRW significantly increased the abundance of Clostridia compared to the ETEC group.
Figure 5.

Effect of IQW and IRW treatment on the microorganisms at the class level: (a) microbiota of the colon in the four groups at the class level (n = 6), (b) Bacteroidia, (c) Epsilonproteobacteria, and (d) Clostridia; the change in letter denotes a significant difference.
Figure 6 shows the highest abundance of colon contents at the order level (Figure 6(a)). And in the CTRL group, Bacteroidales (54.95%), Clostridiales (23.7%), Lactobacillales (5.78%), and Bacillales (4.9%) were the most abundant groups. In the ETEC group, Bacteroidetes (57.53%), Clostridium (12.96%), Lactobacillus (10.3%), and Erysipelotrichales (3.95%) were the most abundant groups. In the IQW-ETEC group, Bacteroidetes (70.1%), Clostridium (11.6%), Lactobacillus (3.1%), and Bacillus (4.0%) were the most abundant groups. In the IRW-ETEC group, Bacteroidetes (72.9%), Clostridium (9.12%), and Lactobacillus (3.99%) were the largest groups. Figure 6(b) shows that ETEC significantly increases the abundance of Bacteroidales (P < 0.05). The effects of IQW-ETEC and IRW-ETEC were not significant according to the ETEC group (P > 0.05). Figure 6(c) shows the levels of Clostridiales in the colon of mice: the group treated with ETEC had significantly decreased amounts compared to the CTRL group (P < 0.05); in contrast, the abundance of Clostridiales in the IQW-ETEC and IRW-ETEC groups was significantly increased compared to the ETEC group (P < 0.05). Thus, the effects of IQW and IRW treatment were effective. Figure 6(d) shows that ETEC can significantly increase the abundance of Erysipelotrichales (P < 0.05). The addition of IQW and IRW had an obvious trend in reducing the Erysipelotrichales content compared with the ETEC group.
Figure 6.

Effect of IQW and IRW treatment on the microorganisms at the order level: (a) microbiota of the colon in the four groups (order level; n = 6), (b) Bacteroidales, (c) Clostridiales, and (d) Erysipelotrichales; the change in letter denotes a significant difference.
At the family level, nine families had the highest abundance (Figure 7(a)). In the CTRL group, Lachnospiraceae (12.9%), Prevotellaceae (8.1%), and Bacteroidaceae (5.6%) were the most abundant microorganisms. Lactobacillaceae (5.8%) was the most abundant microorganisms in the ETEC group. Lachnospiraceae (6.4%), Prevotellaceae (9.1%), Bacteroidaceae (8.3%), and Lactobacillaceae (7.5%) were the most abundant microorganisms in the IQW-ETEC group. The most abundant microorganisms in the IRW-ETEC group included Lachnospiraceae (9.6%), Prevotellaceae (6.2%), Bacteroidaceae (9.2%), and Staphylococcaceae (3.9%). Lachnospiraceae (7.9%), Prevotellaceae (7.6%), Bacteroidaceae (7.6%), and Ruminococcaceae (4.8%) were the most abundant microorganisms in the IQW-ETEC group. It can be concluded from Figure 7(b) that the Lachnospiraceae in the ETEC group is significantly lower than in the CTRL and IQW-ETEC groups (P < 0.05). However, there was no significant difference between the ETEC and IRW-ETEC groups. There was a clear trend on increasing the abundance of Lachnospiraceae. In Figure 7(c), ETEC significantly increases the abundance of Prevotellaceae (P < 0.05). And the effect of IQW and IRW was effective (P < 0.05). In Figure 7(d), the abundance of Flavobacteriaceae is significantly increased by ETEC (P < 0.05) compared to the CTRL group. There is a significant decrease of Flavobacteriaceae in the IQW-ETEC group compared to the ETEC group. However, there is no significant difference between the ETEC group, IRW-ETEC group, and CTRL group, despite the obvious presence of a trend of Flavobacteriaceae decrease in the IRW-ETEC group.
Figure 7.
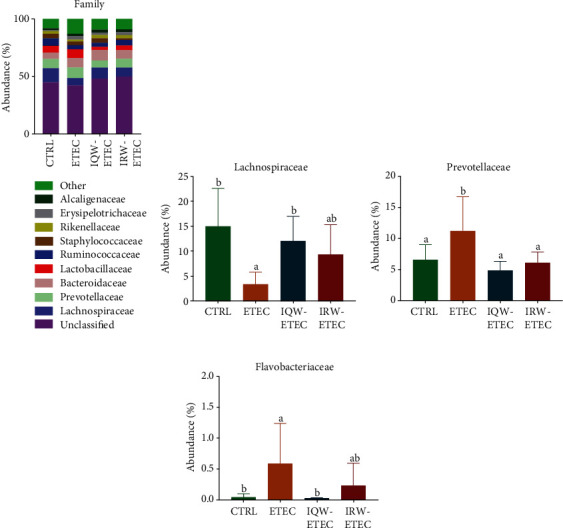
Effect of IQW and IRW treatment on the microorganisms at the family level: (a) microbiota of the colon in the four groups (family level; n = 6), (b) content of Lachnospiraceae, (c) Prevotellaceae, and (d) Flavobacteriaceae; the letter change denotes a significant difference.
At the genus level, nine genera have the highest abundance (Figure 8(a)). In the CTRL group, Bacteroides (5.3%), Alloprevotella (5.6%), Lactobacillus (5.4%), and Staphylococcus (4.7%) were the most abundant groups. In the ETEC group, Bacteroides (5.3%), Prevotella (4.8%), Lactobacillus (9.1%), and Helicobacter (2.8%) were the most abundant groups. In the IQW-ETEC group, Bacteroides (9.96%), Prevotella (4.6%), Lactobacillus (2.7%), and Staphylococcus (3.8%) were the most abundant groups. In the IRW-ETEC group, Bacteroides (8.97%), Prevotella (6.4%), and Lactobacillus (3.4%) were the largest groups. As shown in Figure 8(b), the abundance of Bacteroides in intestinal microorganisms was significantly increased by the IQW compared to the ETEC group (P < 0.05), and there was still an obvious trend of Bacteroides increase in the IRW-ETEC group compared to the ETEC group. Figure 8(c) shows that ETEC significantly increased the abundance of Helicobacter compared to the CTRL group (P < 0.05), and the IQW and IRW significantly increased the abundance of Helicobacter compared to the ETEC group (P < 0.05). In Figure 8(d), there is a trend of decreasing abundance of Alloprevotella after ETEC treatment; there was also a recovery effect after IQW and IRW treatment.
Figure 8.

Effect of IQW and IRW treatment on the microorganisms at the genus level: (a) microbiota of the colon in the four groups at the genus level (n = 6), (b) Bacteroides, (c) Helicobacter, and (d) Alloprevotella; a letter change denotes a significant difference.
4. Discussion
The intestinal tract is one of the most important digestive organs in mammals. Equally important, the intestinal tract also plays an important role in immune function [21]. The intestinal tract is a dynamic and complex system; the intestinal microenvironment is the coexistent result of host and microbiota [22, 23]. The intestinal cells have a strong regenerative capability, with damaged cells recovering within 3 days [24]. The intestinal cells are composed of two types of cell lineages: an absorptive (enterocyte) cell lineage and a secretory (exocrine) cell lineage, both of which originate from intestinal stem cells (ISCs) [25]. Active ISCs are the major actuator for damaged intestinal cells; crypts are the storage region of ISCs [26]. Intestinal villus length and crypt depths are the two common evaluation indexes of intestinal inflammation. Studies have shown that intestinal damage can decrease villus height while increasing crypt depth [27]. Our research highlights the ability of ETEC to cause jejunal damage. Notably, IRW and IQW polypeptides can promote jejunal cell recovery.
After infection, ETEC can adhere to intestinal cells and initiate damage via toxins in a short period [28]. Additionally, ETEC, through the MAPK and NF-κB pathways, causes further inflammatory damage [29]. A previous study pointed out that ETEC infection increased the expression of IL-1β, IL-6, TNF-α, IL-17, and IL-18 [30]. Our research also determined that ETEC can significantly increase the expression of TNF-α, IL-1β, IL-6, and IL-10. Moreover, IQW and IRW can significantly alleviate the overexpression of inflammatory cytokines (P < 0.05). Accordingly, there is no significant difference between the IQW and IRW groups (P > 0.05).
The intestinal microbiota plays an important role in host immunity, digestion, and metabolism and is unique to a specific host body [31]. The mammalian intestinal tract is homeostatically an orderly symbiotic environment; adverse conditions destroy the balance between intestinal microbiota and host [32, 33]. Intestinal inflammation can lead to a disturbance of host intestinal organisms. A study showed that pathogenic bacterial infections, such as Salmonella enterica infection, can cause host intestinal inflammation, reducing intestinal microbiota diversity [34]. Our research points out that in the α-diversity test of the colon intestinal tissue of experimental mice, the microbial community richness in the ETEC group was significantly decreased compared with that in the CTRL group (P < 0.05). Figure 4 shows that the microbial abundance in the ETEC group was significantly lower than the CTRL, IQW-ETEC, and IRW-ETEC groups (P < 0.05). To a certain extent, IQW and IRW can alleviate the decrease in intestinal microbial microorganisms caused by ETEC.
IQW and IRW are two kinds of polypeptides that possess numerous excellent biological activities. A study revealed that IRW functions by regulating and improving the diversity of the intestinal microbiome of the host [35, 36]; our research confirmed this. IRW and IQW can significantly promote intestinal microbiome recovery. In the IQW-ETEC and IRW-ETEC groups, the Shannon index, Sobs index, Chao index, Ace index, and Good's coverage were significantly higher than in the ETEC group.
ETEC reduced the abundance of Bacteroidetes. Bacteroidetes are the most common intestinal microbes in the human gut, accounting for roughly 50% of the intestinal microbes in a Western person [37]. Some bacteria in the Bacteroides genus, such as Bacteroides fragilis, have been shown to prevent and treat intestinal diseases. One study showed that B. fragilis could alleviate inflammation in the DSS-induced IBD colitis model in mice and alleviate weight loss caused by IBD and inflammation [38]. Some studies have shown that Bacteroides have several probiotic effects, such as promoting the digestion of dietary-fiber polysaccharides and the host immunity [39, 40]. Our experimental results showed that IQW and IRW could facilitate the restoration of the host intestinal microbiome environment, improving the abundance of intestinal probiotics in the host intestinal tract and alleviating the jejunal inflammatory response caused by ETEC. A previous study by our research group revealed that IQW could increase the Bacteroides biomass, and IRW can increase the abundance of Clostridium [41]. And our research showed similar results. Another study pointed out that IRW and IQW intake could increase the abundance of Firmicutes and Actinobacteria and reduce the proportion of Bacteroidetes and Proteobacteria [42]. ETEC increased the abundance of Flavobacteriaceae, e.g., F. aquatile and F. meningosepticum, which can cause many diseases, including aquatic diseases (shark fin rot and equine back disease) and neonatal meningitis, respectively [43]. Flavobacteria infection is also the infectious agent for chronic skin disease and bacterial gill disease in fish [42]. The experimental results demonstrate that the proportion of Flavobacteriaceae in the intestines of IQW-treated mice significantly decreased (P < 0.05); after IRW treatment, the proportion of intestinal Flavobacteriaceae decreased. Studies have pointed out that IQW and IRW can improve the abundance of Firmicutes, Bacteroidetes, and Proteobacteria, increase the Lactobacillus and Bifidobacterium biomass, and decrease the abundance of Helicobacter pylori and Verrucomicrobia[43, 44]. Our research shows similar results, with IRW and IQW improving the abundance of probiotics such as Firmicutes, Proteobacteria, Clostridiales, Lachnospiraceae, and Alloprevotella and decreasing the abundance of pathogenic bacteria such as Epsilonproteobacteria, Erysipelotrichales, Prevotellaceae, and Flavobacteriaceae.
5. Conclusions
ETEC can cause jejunal damage, exacerbating the inflammatory reaction. However, IQW and IRW can decrease the expression of inflammatory cytokines, thereby improving the abundance of probiotics such as Firmicutes, Proteobacteria, Clostridiales, Lachnospiraceae, and Alloprevotella and decreasing the abundance of pathogenic bacteria such as Epsilonproteobacteria, Erysipelotrichales, Prevotellaceae, and Flavobacteriaceae.
Acknowledgments
This research was supported by the Hunan Provincial Science and Technology Department (2020NK2004, 2019TP2004, 2018WK4025, 2020ZL2004, 2016NK2101, and 2016TP2005), National Natural Science Foundation of China (Nos. 31772642, 31672457, and 41807135), Local Science and Technology Development Project Guided by The Central Government (YDZX20184300002303 and 2018CT5002), China Postdoctoral Science Foundation (2018M632963 and 2019T120705), Scientific Research Fund of Hunan Provincial Education Department (2020JGYB112 and 18B107), Double First-Class Construction Project of Hunan Agricultural University (SYL201802003, YB2018007, and CX20190497), and Natural Science Foundation of Hunan Province, China (No. 2019JJ50220).
Contributor Information
Jun Fang, Email: fangjun1973@hunau.edu.cn.
Gang Liu, Email: gangle.liu@gmail.com.
Data Availability
The data of this study was available at the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1.Kotloff K. L., Nataro J. P., Blackwelder W. C., et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet . 2013;382(9888):209–222. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- 2.Jiang Z. D., DuPont H. L. Etiology of travellers’ diarrhea. Journal of Travel Medicine . 2017;24(suppl_1):S13–s16. doi: 10.1093/jtm/tax003. [DOI] [PubMed] [Google Scholar]
- 3.Zhang C., Iqbal J., Gómez-Duarte O. G. Murine immunization with CS21 pili or LngA major subunit of enterotoxigenic Escherichia coli (ETEC) elicits systemic and mucosal immune responses and inhibits ETEC gut colonization. Veterinary Microbiology . 2017;202:90–100. doi: 10.1016/j.vetmic.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ren W., Yin J., Duan J., et al. Mouse intestinal innate immune responses altered by enterotoxigenic Escherichia coli (ETEC) infection. Microbes and Infection . 2014;16(11):954–961. doi: 10.1016/j.micinf.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Xu Y., Lahaye L., He Z., Zhang J., Yang C., Piao X. Micro-encapsulated essential oils and organic acids combination improves intestinal barrier function, inflammatory responses and microbiota of weaned piglets challenged with enterotoxigenic Escherichia coli F4 (K88+) Animal Nutrition (Zhongguo xu mu shou yi xue hui) . 2020;6(3):269–277. doi: 10.1016/j.aninu.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang C., Chen X., Nakamura Y., Yu C., Qi H. Fucoxanthin activities motivate its nano/micro-encapsulation for food or nutraceutical application: a review. Food & Function . 2020;11(11):9338–9358. doi: 10.1039/D0FO02176H. [DOI] [PubMed] [Google Scholar]
- 7.Dong X. Y., Azzam M. M. M., Zou X. T. Effects of dietary threonine supplementation on intestinal barrier function and gut microbiota of laying hens. Poultry Science . 2017;96(10):3654–3663. doi: 10.3382/ps/pex185. [DOI] [PubMed] [Google Scholar]
- 8.Liu Y., Liu W., Wang Y., et al. Inhibitory effect of depolymerized sulfated galactans from marine red algae on the growth and adhesion of diarrheagenic Escherichia coli. Marine Drugs . 2019;17(12):p. 694. doi: 10.3390/md17120694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liao W., Chakrabarti S., Davidge S. T., Wu J. Modulatory effects of egg white ovotransferrin-derived tripeptide IRW (Ile-Arg-Trp) on vascular smooth muscle cells against angiotensin II stimulation. Journal of Agricultural and Food Chemistry . 2016;64:7342–7347. doi: 10.1021/acs.jafc.6b03513. [DOI] [PubMed] [Google Scholar]
- 10.Liao W., Fan H., Davidge S. T., Wu J. Egg white-derived antihypertensive peptide IRW (Ile-Arg-Trp) reduces blood pressure in spontaneously hypertensive rats via the ACE2/Ang (1-7)/mas receptor axis. Molecular Nutrition & Food Research . 2019;63, article e1900063 doi: 10.1002/mnfr.201900063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Majumder K., Liang G., Chen Y., Guan L., Davidge S. T., Wu J. Egg ovotransferrin-derived ACE inhibitory peptide IRW increases ACE2 but decreases proinflammatory genes expression in mesenteric artery of spontaneously hypertensive rats. Molecular Nutrition & Food Research . 2015;59:1735–1744. doi: 10.1002/mnfr.201500050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu Q., Fan H., Yu W., Hong H., Wu J. Transport study of egg-derived antihypertensive peptides (LKP and IQW) using Caco-2 and HT29 coculture monolayers. Journal of Agricultural and Food Chemistry . 2017;65(34):7406–7414. doi: 10.1021/acs.jafc.7b02176. [DOI] [PubMed] [Google Scholar]
- 13.Grootaert C., Matthijs B., Voorspoels S., Possemiers S., Smagghe G., Van Camp J. Egg-derived bioactive peptides with ACE-inhibitory properties: a literature update. Food & Function . 2017;8(11):3847–3855. doi: 10.1039/C7FO00839B. [DOI] [PubMed] [Google Scholar]
- 14.Son M., Chan C. B., Wu J. Egg white ovotransferrin-derived ACE inhibitory peptide ameliorates angiotensin II-stimulated insulin resistance in skeletal muscle cells. Molecular Nutrition & Food Research . 2018;62(4) doi: 10.1002/mnfr.201700602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Majumder K., Chakrabarti S., Davidge S. T., Wu J. Structure and activity study of egg protein ovotransferrin derived peptides (IRW and IQW) on endothelial inflammatory response and oxidative stress. Journal of Agricultural and Food Chemistry . 2013;61(9):2120–2129. doi: 10.1021/jf3046076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhullar K. S., Son M., Kerek E., et al. Tripeptide IRW upregulates NAMPT protein levels in cells and obese C57BL/6J mice. Journal of Agricultural and Food Chemistry . 2021;69(5):1555–1566. doi: 10.1021/acs.jafc.0c07831. [DOI] [PubMed] [Google Scholar]
- 17.Ma Y., Jiang H., Fang J., Liu G. IRW and IQW reduce colitis-associated cancer risk by alleviating DSS-induced colonic inflammation. BioMed Research International . 2019;2019:9. doi: 10.1155/2019/6429845.6429845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu G., Yan W., Ding S., et al. Effects of IRW and IQW on oxidative stress and gut microbiota in dextran sodium sulfate-induced colitis. Cellular Physiology and Biochemistry: International Journal of Experimental Cellular Physiology, Biochemistry, and Pharmacology . 2018;51(1):441–451. doi: 10.1159/000495240. [DOI] [PubMed] [Google Scholar]
- 19.Caporaso J. G., Kuczynski J., Stombaugh J., et al. QIIME allows analysis of high-throughput community sequencing data. Nature Methods . 2010;7(5):335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Magoč T., Salzberg S. L. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics (Oxford, England) . 2011;27(21):2957–2963. doi: 10.1093/bioinformatics/btr507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huse S. M., Mark Welch D. B., Voorhis A., et al. VAMPS: a website for visualization and analysis of microbial population structures. BMC Bioinformatics . 2014;15(1) doi: 10.1186/1471-2105-15-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O'Hara A. M., Shanahan F. The gut flora as a forgotten organ. EMBO Reports . 2006;7(7):688–693. doi: 10.1038/sj.embor.7400731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maynard C. L., Elson C. O., Hatton R. D., Weaver C. T. Reciprocal interactions of the intestinal microbiota and immune system. Nature . 2012;489(7415):231–241. doi: 10.1038/nature11551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jeurissen S. H., Lewis F., van der Klis J. D., Mroz Z., Rebel J. M., ter Huurne A. A. Parameters and techniques to determine intestinal health of poultry as constituted by immunity, integrity, and functionality. Current Issues in Intestinal Microbiology . 2002;3(1):1–14. [PubMed] [Google Scholar]
- 25.Weichselbaum L., Klein O. D. The intestinal epithelial response to damage. Science China. Life Sciences . 2018;61(10):1205–1211. doi: 10.1007/s11427-018-9331-y. [DOI] [PubMed] [Google Scholar]
- 26.Lu L., Li W., Chen L., et al. Radiation-induced intestinal damage: latest molecular and clinical developments. Future Oncology . 2019;15(35):4105–4118. doi: 10.2217/fon-2019-0416. [DOI] [PubMed] [Google Scholar]
- 27.Ding S., Ma Y., Liu G., Yan W., Jiang H., Fang J. Lactobacillus brevis alleviates DSS-induced colitis by reprograming intestinal microbiota and influencing serum metabolome in murine model. Frontiers in Physiology . 2019;10:p. 1152. doi: 10.3389/fphys.2019.01152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ondrackova P., Alexa P., Matiasovic J., Volf J., Faldyna M. Interaction of porcine neutrophils with different strains of enterotoxigenic Escherichia coli. Veterinary Microbiology . 2012;160(1-2):108–116. doi: 10.1016/j.vetmic.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 29.Zanello G., Berri M., Dupont J., et al. Saccharomyces cerevisiae modulates immune gene expressions and inhibits ETEC-mediated ERK1/2 and p38 signaling pathways in intestinal epithelial cells. PLoS One . 2011;6(4, article e18573) doi: 10.1371/journal.pone.0018573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Messori S., Trevisi P., Simongiovanni A., Priori D., Bosi P. Effect of susceptibility to enterotoxigenic Escherichia coli F4 and of dietary tryptophan on gut microbiota diversity observed in healthy young pigs. Veterinary Microbiology . 2013;162(1):173–179. doi: 10.1016/j.vetmic.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 31.Brown R. L., Sequeira R. P., Clarke T. B. The microbiota protects against respiratory infection via GM-CSF signaling. Nature Communications . 2017;8(1):p. 1512. doi: 10.1038/s41467-017-01803-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Videnska P., Sisak F., Havlickova H., Faldynova M., Rychlik I. Influence of Salmonella enterica serovar Enteritidis infection on the composition of chicken cecal microbiota. BMC Veterinary Research . 2013;9(1):p. 140. doi: 10.1186/1746-6148-9-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiao H., Zhang Q., Lin Y., Gao Y., Zhang P. The ovotransferrin-derived peptide IRW attenuates lipopolysaccharide-induced inflammatory responses. BioMed Research International . 2019;2019:7. doi: 10.1155/2019/8676410.8676410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu W., Ren L., Zhang L., Qiao Q., Farooq M. Z., Xu Q. The potential of food protein-derived bioactive peptides against chronic intestinal inflammation. Mediators of Inflammation . 2020;2020:15. doi: 10.1155/2020/6817156.6817156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jacobson A. N., Choudhury B. P., Fischbach M. A., Tang Y., Dorrestein P. The biosynthesis of lipooligosaccharide fromBacteroides thetaiotaomicron. mBio . 2018;9(2) doi: 10.1128/mBio.02289-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Delday M., Mulder I., Logan E. T., Grant G. Bacteroides thetaiotaomicron ameliorates colon inflammation in preclinical models of Crohn’s disease. Inflammatory Bowel Diseases . 2019;25(1):85–96. doi: 10.1093/ibd/izy281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Porter N. T., Luis A. S., Martens E. C. _Bacteroides thetaiotaomicron_. Trends in Microbiology . 2018;26(11):966–967. doi: 10.1016/j.tim.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 38.Ndeh D., Baslé A., Strahl H., et al. Author Correction: Metabolism of multiple glycosaminoglycans by Bacteroides thetaiotaomicron is orchestrated by a versatile core genetic locus. Nature Communications . 2020;11(1):p. 4396. doi: 10.1038/s41467-020-18097-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ma Y., Ding S., Liu G., et al. Egg protein transferrin-derived peptides IRW and IQW regulate Citrobacter rodentium-induced. Inflammation-Related Microbial and Metabolomic Profiles. Frontiers in microbiology . 2019;10:p. 643. doi: 10.3389/fmicb.2019.00643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gómez E., Méndez J., Cascales D., Guijarro J. A. Flavobacterium psychrophilum vaccine development: a difficult task. Microbial Biotechnology . 2014;7(5):414–423. doi: 10.1111/1751-7915.12099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sebastião F. A., Nomura D., Sakabe R., Pilarski F. Hematology and productive performance of nile tilapia (Oreochromis niloticus) naturally infected with Flavobacterium columnare. Brazilian Journal of Microbiology: [publication of the Brazilian Society for Microbiology] . 2011;42(1):282–289. doi: 10.1590/S1517-83822011000100036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jean S. S., Lee W. S., Chen F. L., Ou T. Y., Hsueh P. R. Elizabethkingia meningoseptica : an important emerging pathogen causing healthcare-associated infections. The Journal of Hospital Infection . 2014;86(4):244–249. doi: 10.1016/j.jhin.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 43.Liu G., Ma Y., Yang Q., Deng S. Modulation of inflammatory response and gut microbiota in ankylosing spondylitis mouse model by bioactive peptide IQW. Journal of Applied Microbiology . 2020;128(6):1669–1677. doi: 10.1111/jam.14588. [DOI] [PubMed] [Google Scholar]
- 44.Ma Y., Ding S., Liu G., et al. Egg protein transferrin-derived peptides IRW and IQW regulate Citrobacter rodentium-induced, inflammation-related microbial and metabolomic profiles. Frontiers in Microbiology . 2019;10 doi: 10.3389/fmicb.2019.00643. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data of this study was available at the corresponding author upon reasonable request.


