Abstract
Optical endoscopy is the primary diagnostic and therapeutic tool for management of gastrointestinal (GI) malignancies. Most GI neoplasms arise from precancerous lesions; thus, technical innovations to improve detection and diagnosis of precancerous lesions and early cancers play a pivotal role in improving outcomes. Over the last few decades, the field of GI endoscopy has witnessed enormous and focused efforts to develop and translate accurate, user‐friendly, and minimally invasive optical imaging modalities. From a technical point of view, a wide range of novel optical techniques is now available to probe different aspects of light–tissue interaction at macroscopic and microscopic scales, complementing white light endoscopy. Most of these new modalities have been successfully validated and translated to routine clinical practice. Herein, we provide a technical review of the current status of existing and promising new optical endoscopic imaging technologies for GI cancer screening and surveillance. We summarize the underlying principles of light–tissue interaction, the imaging performance at different scales, and highlight what is known about clinical applicability and effectiveness. Furthermore, we discuss recent discovery and translation of novel molecular probes that have shown promise to augment endoscopists' ability to diagnose GI lesions with high specificity. We also review and discuss the role and potential clinical integration of artificial intelligence‐based algorithms to provide decision support in real time. Finally, we provide perspectives on future technology development and its potential to transform endoscopic GI cancer detection and diagnosis.
Keywords: gastrointestinal tract, machine learning, molecular probe, optical endoscopy
We provide a technical review of optical endoscopic imaging technologies for GI cancer screening and surveillance. This review summarizes the principles of light–tissue interaction, the imaging performance at different scales, and highlights what is known about clinical applicability and effectiveness. Furthermore, we discuss the translation of novel molecular probes and integration of artificial intelligence‐based algorithms to provide decision support.
![]()
Abbreviations
- ADR
adenoma detection rate
- AI
artificial intelligence
- ASGE
American Society for Gastrointestinal Endoscopy
- CADe
computer‐aided detection
- CADx
computer‐aided diagnosis
- CE
chromoendoscopy
- CLE
confocal laser endomicroscopy
- EAC
esophageal adenocarcinoma
- ESCC
esophageal squamous cell carcinoma
- ESGE
European Society of Gastrointestinal Endoscopy
- FICE
Fuji Intelligent Chromo Endoscopy
- FOV
field of view
- GI
gastrointestinal
- HRME
high‐resolution microendoscope
- NBI
narrowband imaging
- PIVI
preservation and incorporation of valuable endoscopic innovations
- RCT
randomized controlled trials
- SECM
spectrally encoded confocal endomicroscopy
- VLE
volumetric laser endomicroscopy
- WLE
white light endoscopy
1. Introduction
Cancers in the gastrointestinal (GI) tract, including the esophagus, stomach, small intestine, and colon, are among the 10 most common cancers worldwide, imposing a significant healthcare burden globally [1]. Carcinogenesis in the GI tract typically involves a cascade of molecular dysregulation and architectural alternations; thus, a significant proportion of the resulting morbidity, mortality, and healthcare cost can potentially be prevented through detection and treatment of precursor lesions and early cancers. To detect cancer at early stages, a combination of screening and surveillance programs, including both endoscopic and nonendoscopic tests, have been developed and implemented. Recommended screening and surveillance programs vary by population risk and geography.
Current strategies for early detection of esophageal neoplasia depend on the type of esophageal cancer which is most prevalent. Globally, 90% of esophageal cancers are categorized as esophageal squamous cell carcinoma (ESCC); ESCC is most prevalent in certain geographical regions such as East Asia, Iran, and Africa [1, 2]. In these regions, screening endoscopy in conjunction with Lugol's chromoendoscopy (CE) is advocated as the gold standard modality based on its high sensitivity [3, 4], although limited specificity remains a challenge. In Western countries, esophageal adenocarcinoma (EAC) is the predominant histologic subtype. Population‐based screening endoscopy is not currently recommended due to the low incidence. However, the last few decades have witnessed dramatically increasing rates of incidence of EAC [5], and guidelines in the United States and Europe recommend white light endoscopic surveillance of patient with Barrett's esophagus, the only known precursor to EAC [6, 7]. Nonetheless, conventional white light endoscopy (WLE) is found to frequently miss early cancers in BE [8]. In addition to standard endoscopy, more affordable and less invasive endoscopic and tissue sampling approaches, such as ultrathin endoscopy and Cytosponge combined with biomarkers [9], are also under evaluation as alternative methods for esophageal cancer screening.
For gastric cancer, countries with a high incidence such as Japan and South Korea have implemented national screening programs [1, 10, 11]. While less invasive nonendoscopic screening methods such as barium upper GI series can be used, endoscopy remains the primary tool in high incidence countries with higher cancer detection rates and biopsy capability [12, 13]. In regions with a low or intermediate incidence, endoscopy is only recommended for individuals at an increased risk for gastric cancer [14, 15].
Colorectal cancer is the third most common cancer globally and ranks second in mortality [1]. Adenomatous polyps are the most important precursor lesions for colorectal cancer, and their detection and removal through polypectomy are associated with reduced cancer incidence and morbidity [16, 17]. Large‐scale screening is commonly practiced in North American and European countries [18], offering a range of nonendoscopic and endoscopic screening tests, including guaiac‐based fecal occult blood test, fecal immunochemical test, sigmoidoscopy, and colonoscopy. The clinical adoption of these modalities varies in different countries [19], but colonoscopy is the only gold standard screening tool that offers direct visualization, same‐session detection, and removal of polyps across the entire colon. With recent advances in optical endoscopy, there is a significant interest in optical diagnosis of diminutive (≤ 5 mm) colorectal polyps that represent a vast majority of all polyps, yet are only linked with minimal risks of malignant progression [20].
Currently, standard endoscopes remain the primary diagnostic and therapeutic tool for GI cancer screening and surveillance (Fig. 1). Standard endoscopy, in which either upper or lower GI tract of a sedated patient is thoroughly examined with a white light endoscope by a trained endoscopist, allows for imaging, biopsies, and treatment in a single endoscopic session. However, despite its central role, many studies report that standard endoscopy frequently misses GI lesions at early stages [8, 21, 22], primarily due to the inability to visualize subtle architectural changes under conventional white light illumination. The development of novel endoscopic technologies with improved accuracy, enhanced sampling, and minimal invasiveness could play a pivotal role to advance early detection and clinical management of GI lesions. Figure 1 highlights existing and novel endoscopic technologies that offer multimodal imaging of GI lesions to improve early detection at macroscopic and microscopic scales. Overall, macroscopic modalities can interrogate a large field of view (FOV) and serve as “red‐flag” techniques to rapidly survey the entire lumen and identify suspicious lesions. In a two‐step protocol, modalities with microscopic resolution can be used to further examine suspicious lesions with cellular or subcellular detail (10 µm or higher). As a minimally invasive macroscopic modality, capsule endoscopy offers an appealing option for “red‐flag” imaging. Alternatively, high‐resolution endoscopic modalities such as confocal laser endomicroscopy (CLE) and endocytoscopy can provide histologic information in real time.
Fig. 1.
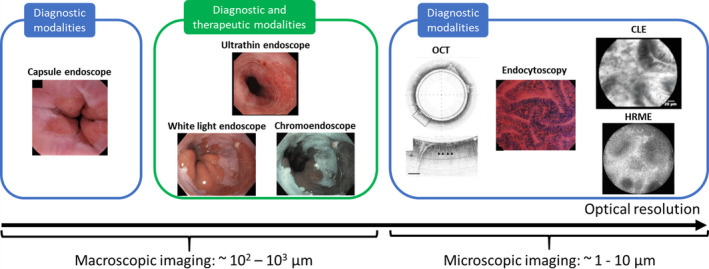
Optical endoscopic techniques for macroscopic and microscopic imaging of the GI mucosa. Existing macroscopic modalities include high‐definition endoscopy, ultrathin endoscopy, and capsule endoscopy. Microscopic resolution can be achieved using OCT, endocytoscopy, CLE, and HRME. Reproduced from [66, 81, 136] with permission from Elsevier (white light and chromoendoscope, capsule endoscope, and endocytoscopy, respectively), from [55] with permission from John Wiley and Sons (ultrathin endoscope), from [137] by permission from Springer Nature (OCT), from [69] with permission from © Georg Thieme Verlag KG (CLE).
Together, this wide range of technologies in Fig. 1 provides powerful tools for endoscopists to examine the GI tract both macroscopically and microscopically, forming the basis for the next generation of GI endoscopy. As summarized in Table 1, to help guide translation of promising new technologies, the American Society for GI Endoscopy (ASGE) created the preservation and incorporation of valuable endoscopic innovations (PIVI) performance thresholds that new technologies for assessment of Barrett's esophagus and colorectal polyps should meet prior to adoption [23, 24].
Table 1.
| Clinical condition | Imaging‐guided endoscopic management | Required performance thresholds |
|---|---|---|
| Barrett's esophagus | Perform targeted biopsies (without random biopsies) |
For diagnosis of HGD and EAC
|
| Rectosigmoid polyps | Leave suspected hyperplastic polyps 5 mm or smaller without resection |
For diagnosis of adenomatous histology
|
| Colorectal polyps | Resect and discard polyps 5 mm or smaller without histopathology evaluation |
For determining postpolypectomy surveillance intervals
|
In this technical review, we provide an overview of the current status of existing and emerging optical imaging modalities for GI endoscopy. Previous reviews of endoscopic imaging techniques in the GI tract have been provided by the ASGE technology committee, the European Society of GI Endoscopy (ESGE) research committee, and other authors [18, 24, 25, 26], with a strong focus on the performance of commercially available and commonly studied modalities such as CE and CLE, while providing detailed descriptions of available evidence of their clinical performance. In this review, we focus on the technical aspects and clinical applicability of optical endoscopy, highlighting recent advances in device and optical design, molecular probes, and machine‐learning algorithms for image interpretation. In addition to commercially available platforms, we also discuss emerging technologies at earlier stages of clinical translation, summarizing how they exploit different dimensions of light–tissue interaction to identify lesions at early stages of GI cancer progression. For each modality, we discuss the imaging capabilities while highlighting the fundamental principles of light–tissue interactions and their implications for clinical usefulness. We review imaging systems with various novel form factors (examples in Fig. 2), including capsules, balloon catheters, and probes, and discuss how they can contribute to multimodal and less invasive imaging of the GI tract with enhanced contrast and resolution. We also review the clinical translation and integration of novel molecular probes and machine‐learning algorithms.
Fig. 2.
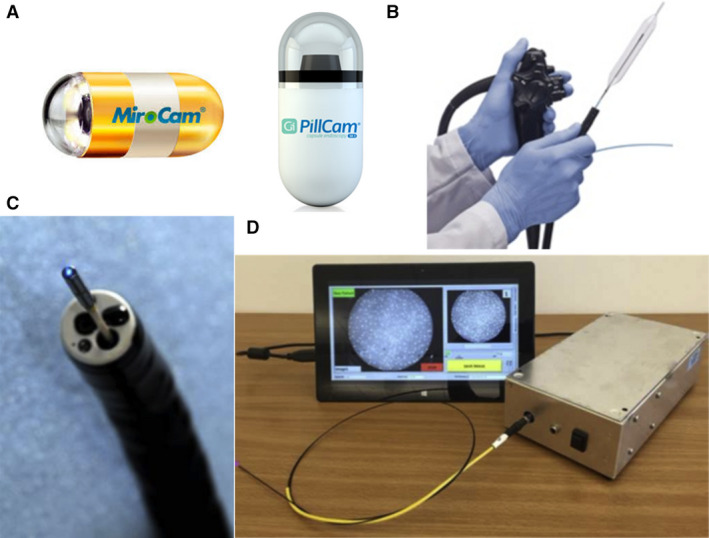
Examples of capsule‐, balloon‐, and probe‐based optical endoscopic systems. (A) Capsule endoscopes (MicroCam and PillCam). (B) VLE probe within an inflated balloon catheter. (C) Confocal laser endomicroscope through a biopsy channel. (D) A low‐cost HRME with integrated diagnostic software. Figure 2B–D reproduced from [97, 138, 139] with permission from Elsevier.
2. Macroscopic imaging systems
2.1. Current standard of care: white light endoscopy
Because of the unique anatomy of the GI tract, conventional endoscopy with white light illumination remains the gold standard to assess GI lesions. Despite its routine use, there is a critical need to further improve the diagnostic performance of WLE. For surveillance of Barrett's esophagus, for example, four‐quadrant random biopsy sampling (referred to as the Seattle protocol) remains an important and essential component of current guidelines [29]. Similarly, CE using Lugol's staining is generally practiced for ESCC screening in many Asian countries. In screening colonoscopy, the use of high‐definition endoscopy with advanced modalities such as virtual CE has also been recommended by the ESGE [18].
Over the last few decades, the technical performance of WLE has benefited from improvements in imaging sensors and optics that offer greater pixel density and higher magnification. Since the late 1990s, high‐definition endoscopes have become widely available, enabling more meticulous examination of mucosal patterns, and replacing standard‐definition endoscopes as the current modality of choice. Standard high‐definition endoscopy has been further complemented by less invasive ultrathin endoscopes and capsule endoscopes, and the detailed imaging features and specifications are compared in Table 2. Recent commercial systems are also augmented by advanced imaging features, including magnification endoscopes with up to 150‐fold optical magnification and various techniques to enhance mucosal features such as CE. While data comparing high‐definition with standard‐definition WLE are relatively scarce, high‐definition endoscopes have been used in numerous clinical studies, especially in tandem with virtual or dye‐based CE.
Table 2.
| Endoscope systems | High‐definition endoscope | Ultrathin endoscope | Capsule endoscope |
|---|---|---|---|
| Endoscope diameter (Approx.) | 9–13 mm | 5–6 mm | 11 mm |
| FOV | 140° to 170° | 120° to 140° | 145° to 170° |
| Camera resolution |
High definition (up to 2 million pixels) |
Standard definition (100 000–400 000 pixels) |
256 × 256 to 512 × 512 pixels |
| Scope guidance | 4‐way angulation | 2‐way or 4‐way angulation | Passive peristalsis; External magnetic steering |
| Advanced imaging capability | Yes | Yes | No |
| Sedation requirement | Yes | No | No |
| GI tract accessibility | Upper or lower GI | Upper GI | Upper or lower GI, including small bowel |
| Biopsy capability | Yes | Supported in most models except disposable versions | No |
Future development of high‐definition WLE can benefit from further improvements in the optical FOV and resolution, as well as 3D imaging capability. As shown in Table 2, current high‐definition endoscopes can support up to 2K video acquisition with an angular FOV of 140–170°. To better survey the GI anatomy, wide FOV endoscopes are being developed to provide an extra wide angular or near‐panoramic view (245–330°), and preliminary studies have shown their utility for better colon polyp detection and improved visualization of occult regions in the upper GI tract [30, 31, 32, 33]. In addition, endoscopes with UHD resolution (4k or 8k vs. current 2k) and 3D imaging capabilities, although mostly demonstrated in laparoscopic applications or animal models [34, 35, 36], also have the potential to enable more detailed mucosal examination and enhance surgical maneuverability in the GI tract.
2.2. Virtual chromoendoscopy
In contrast to WLE based on the entire visible spectrum, virtual CE exploits spectral variations in the interaction of light with tissue to highlight mucosal features, such as blood vessels or changes in light scattering. Because spectral imaging can be achieved by either optical filtering or postprocessing without modifying the imaging optics, virtual CE is seamlessly integrated in most current endoscopic systems. At the touch of a button, it provides a convenient means for endoscopists to investigate lesions with enhanced contrast. First‐generation virtual CE include narrowband imaging (NBI; Olympus, Tokyo, Japan), Fuji Intelligent Chromo Endoscopy (FICE; Fujinon, Tokyo, Japan), and iScan (Pentax, Tokyo, Japan). Newer modalities, such as blue laser imaging, have also been recently introduced. As summarized in Table 3, NBI enhances the contrast of mucosal features and microvascular networks by illuminating the GI surface with blue (415 nm) and green light (540 nm); FICE and iScan, in comparison, are based on postprocessing algorithms to improve vessel visualization and tissue type differentiation. Among these modalities, NBI is the most commonly studied virtual CE modality; thanks to its wide availability, as well as established interpretation criteria with substantial interobserver agreement [37], the modality is well accepted, especially among experts.
Table 3.
Advanced imaging modalities available in commercial endoscopic platforms.
| Imaging modality | Virtual CE | Dye‐based CE | |||||
|---|---|---|---|---|---|---|---|
| NBI | FICE | iScan | Indigo carmine | Methylene blue | Acetic acid | Lugol's iodine | |
| Source of contrast | Reflectance; hemoglobin absorption | Reflectance | Reflectance | Reflectance of exogenous dyes | Absorption by small intestine and colonic epithelium | Acetic whitening | Absorption by tissue with high glycogen |
| Targeted clinical features | Mucosal patterns and vascular network | Mucosal patterns and vascular network | Mucosal patterns and vascular network | Mucosal topology such as pits and ridges | Uptake by intestinal epithelium | Mucosal patterns | Uptake by normal squamous epithelium |
In Barrett's esophagus, CE was shown to improve diagnostic yield for detection of dysplasia/cancer by 34% in a meta‐analysis of 14 studies involving 843 patients [38]. As a widely studied modality, NBI was shown to meet the PIVI thresholds for BE surveillance (Table 1) and its conjunction use with standard WLE is recommended [25, 29]. For gastric precancerous histology, NBI is recommended by the ESGE due to its capability to significantly improve intestinal metaplasia detection compared to high‐definition endoscopy alone [15]. In screening colonoscopy, a meta‐analysis by ASGE reported a 91% NPV using NBI for detection of adenomas in academic centers, surpassing the PIVI threshold of 90% to support a “diagnosis‐and‐leave” strategy for diminutive polyps [24]. However, it should be noted that subpar results that fell short of meeting the same criteria were reported in community settings [39, 40]. Per ESGE recommendations, the use of virtual CE to provide optical diagnosis is only suggested when endoscopy is adequately documented and performed by experts [18].
In settings where the most recent platforms are available, virtual CE has shown great promise to better visualize GI lesions without incurring additional cost and applying exogenous dyes. As most high‐definition endoscope systems offer CE compatibility nowadays, it is gaining momentum and more popularity, especially among experts. To endorse their routine use, however, universal classification criteria need to be established and externally validated, especially among novice users outside of research centers. In addition, associated learning curves and interobserver reliability need to be studied. At the meantime, the importance of technical advances should be recognized. A very recent meta‐analysis of 11 randomized controlled trials (RCTs) revealed that the second‐generation and brighter NBI significantly increased the adenoma detection rate (ADR) compared to WLE, as well as to first‐generation NBI [41].
As an extension to commercially available virtual CE that exploits tissue response in specific spectral bands, multispectral or hyperspectral imaging has also shown value for GI lesion characterization with increased spectral dimensions [42, 43]. Recently, real‐time hyperspectral imaging has been enabled in endoscopic systems and initial clinical evaluation in the GI tract has been reported [44, 45, 46]. Taken together, it is expected that GI lesion detection will be further improved by virtual CE as the technology continually improves and the clinical use becomes standardized.
2.3. Dye‐based chromoendoscopy
Unlike optical or computational filtering in virtual CE, dye‐based CE takes advantage of exogenous dyes to enhance contrast of mucosal features, especially in lesions that may appear subtle, flat, or depressed under WLE. In general, two types of dyes are clinically used: absorptive dyes such as methylene blue and Lugol's iodine, and contrast stains such as indigo carmine (Table 3). Among those dyes, Lugol's iodine selectively binds to glycogen that is more abundantly stored in normal than dysplastic squamous epithelium. Methylene blue is preferentially absorbed by epithelial cells of small intestine or colon types, and indigo carmine highlights mucosal topology by filling crevices, pits, and ridges.
The source of imaging contrast, and thus the targeted mucosal features, depends on the staining or uptake mechanisms of specific dyes. Thanks to its high uptake by glycogen‐abundant cells, Lugol's iodine is routinely used as a highly sensitive (92–100% sensitivity), yet inexpensive dye for ESCC screening [2]. For surveillance of Barrett's esophagus, systematic reviews showed improved diagnostic yield and accuracy using acetic acid or methylene blue; therefore, CE is recommended as an adjunctive tool in addition to WLE [29]. For the choice of dyes, acetic acid is inexpensive and meets the ASGE threshold for BE surveillance (Table 1), with early evidence showing that it is more cost‐effective than random biopsies in a high‐risk population [29, 47]. Methylene blue also provides substantial contrast enhancement, but concerns have been raised due to its link to DNA damage [48]. In the stomach, a meta‐analysis of 10 studies using different dyes also demonstrated that dye‐based CE can better detect early gastric cancers and precursors (pooled sensitivity and specificity of 0.90 and 0.82 in 699 patients) than WLE [49].
In settings that lack access to up‐to‐date endoscopic platforms with virtual CE, dye‐based CE offers a safe and relatively inexpensive alternative to enhance mucosal contrast, even though it requires a more cumbersome procedure and relies on the quality and uniformity of the spraying. Like virtual CE, to make the best use of dye‐based CE, user expertise is critical. In addition, a consensus on validated interpretation criteria is yet to be established, which can be particularly challenging given the wide range of dyes and their different uptake or staining patterns.
2.4. Ultrathin endoscopy
Compared to standard endoscopes, ultrathin endoscopes are designed to access smaller luminal organs so that endoscopy can be performed during unsedated, outpatient procedures, even though expertise is still required for image interpretation. Thanks to a smaller diameter (6 mm or less, compared to up to 13 mm in standard endoscopes), patients have a higher acceptance and suffer from lower risks of complications and recovery time [50]. The trade‐off includes decreased pixel resolution and reduced mechanical maneuverability (Table 2); a pediatric biopsy forceps can be passed through the accessory channel to obtain biopsies, but the ability to perform more complicated surgical procedures is limited. To further facilitate clinical adoption of the technology, less costly, portable, and disposable versions of ultrathin endoscopes have also been developed, but biopsy capability is not supported [51].
The capability of ultrathin endoscopy to investigate upper GI lesions is reported in several pilot studies. As a less costly outpatient procedure, transnasal endoscopy using ultrathin endoscopes is considered a potential alternative for BE screening [7], even though its role in BE surveillance is limited by the relatively low imaging quality. In a pilot randomized cross‐over study involving 82 patients, transnasal endoscopy was found to have high diagnostic performance for BE detection (98% sensitivity and 100% specificity) comparable to standard WLE [52]; nonetheless, since an enriched surveillance population was examined in a tertiary‐care center, the generalizability of this research needs to be further studied. Ultrathin endoscopy was also evaluated for gastric neoplasia detection in 57 patients with superficial gastric neoplasia or undergoing follow‐up endoscopy after ESD, reporting significantly worse performance characteristics than high‐definition WLE [53]. This can be ascribed to the increased difficulty in scope manipulation and the relatively poor imaging quality. As the latest ultrathin models are equipped with better illumination and NBI capability [54, 55], its potential role in the upper GI tract should be further evaluated.
2.5. Capsule endoscopy
First approved by the FDA in 2001, capsule endoscopy provides easy and safe access to the GI tract (Fig. 2A). In the last two decades, capsule endoscopy has revolutionized the management of small bowel disease [56]. Most commercially available capsule endoscopes consist of a miniaturized imaging sensor, illumination, and imaging optics, and an internal power supply encased in a disposable enclosure. Data transmission is usually achieved wirelessly via radio telemetry or electric‐field propagation [57], and wired data retrieval has also been reported in tethered capsules [58]. Typical imaging specifications of commercially available capsule endoscopy are shown in Table 2.
Given the successful application of capsule endoscopy in the small bowel, there is an increasing interest to investigate its role in other parts of the GI tract. In the upper GI tract, capsule endoscopy is challenging due to the unique anatomy. The average capsule transit time through the esophagus can be as low as about 30 s [59], mandating a fast frame rate to capture high‐quality images; once it enters the stomach, the uncontrolled capsule movement further complicates image acquisition. Using the Pillcam UGI capsule that captures 35 frames per second, capsule endoscopy has been shown to visualize important clinical landmarks of the esophagus; in addition, feasibility to view the entire stomach was demonstrated with patients positioned at different planes and angles on an examination bed in a nurse‐led protocol [59]. Owing to its moderate accuracy (sensitivity 78% and specificity 73% for BE detection in a meta‐analysis of nine studies involving 618 patients by Bhardwaj et al.), however, it is not recommended for BE screening [7, 60]. In the lower GI tract, capsule endoscopy has also been found to be useful for detecting additional polyps in patients with incomplete colonoscopy [61].
A major drawback of capsule endoscopy is its lack of active locomotion, which compromises its imaging quality and limits its use in luminal organs. Recent technical efforts have been made to overcome this barrier with internal stabilizing or external control mechanisms [62, 63]. Coupled with an external magnetic steering system, capsule endoscopy has been used to survey more capacious parts of the GI tract [64]. A recent study reported its safe use in 3182 asymptomatic participants to visualize focal lesions in the stomach, suggesting its potential for gastric cancer screening [65]. While in an early stage of development, very recent clinical evidence using a updated magnetically controlled capsule system has shown improved imaging quality and maneuverability [66, 67], and future studies to assess its diagnostic value are warranted.
3. Microscopic imaging systems
While macroscopic imaging modalities form the fundamentals of GI endoscopy, the gold standard for cancer diagnosis remains microscopic examination. In the routine practice, this is achieved by acquisition of biopsies followed by standard histology procedures. This process can lead to unnecessary biopsies and related healthcare costs, delay of diagnosis, and loss to follow‐up. As a result, there has been a long‐standing interest in developing in vivo endoscopic techniques to facilitate lesion characterization with microscopic or near‐microscopic resolution. In this section, we will review endomicroscopic imaging techniques (Table 4), including commercially available systems such as CLE and volumetric laser endomicroscopy (VLE), and investigative research systems such as the high‐resolution microendoscope (HRME) and capsules based on optical coherence tomography (OCT).
Table 4.
| Imaging modality | CLE | Endocytoscopy | OCT | HRME |
|---|---|---|---|---|
| Endoscopic form factor | Endoscope‐ or probe‐based | Endoscope‐ or probe‐based | Probe‐, capsule‐ or balloon‐based | Probe‐based |
| Source of contrast | Fluorescence | Reflectance | Reflectance | Fluorescence |
| Contrast agent | Fluorescein | Methylene blue and crystal violet | NA | Proflavine |
| Targeted clinical features | Extracellular matrix | Cellular architectural morphology | Cellular architectural morphology | Cell nuclei |
| Resolution | 1–3.5 μm | 1.7–4.2 μm | ~ 10 μm | 4.4 μm |
| Imaging depth | Up to 70 μm | Surface | 1–2.5 mm | Surface |
| FOV | 200–300 μm | 120–700 μm | Large FOV with pullback | 790 μm |
| Phase of development | Commercially available; extensively evaluated in the entire GI tract | Commercially available; clinically evaluated in the entire GI tract | Commercially available; mostly evaluated in esophagus | Evaluated in the esophagus and colon |
| Comments on clinical applicability | Compatible with molecular probes; high cost | Compatible with exogenous dyes and advanced imaging such as NBI; high cost | Label‐free and allows large‐area scanning; incompatible with dyes or molecular probe; high cost | Potentially compatible with molecular probes; low cost |
3.1. Confocal laser endomicroscopy
By illuminating stained tissue with a scanning low‐power laser, CLE (Fig. 2C) can generate fluorescence images of the mucosal layer with micron‐level resolution, providing histologic information similar to standard pathology (Table 4). To image deep epithelial layers with high contrast, confocal scanning is implemented for optical sectioning. Two types of fluorescent contrast agents can be used, including fluorescein that is intravenously administered, and topically applied vital dyes such as acriflavine, tetracycline, or cresyl violet. Fluorescein enhances the contrast of extracellular matrix such as mucosal crypts and villi, as well as vascular structures. Topically applied dyes stain nuclei and thus visualize nuclear morphometry. In clinical practice, fluorescein is FDA‐approved and most widely used without adverse effects [68], and consensus classification criteria referred to as the Miami Classification have been established [69].
Overall, high‐performance characteristics have been achieved using CLE for detection of BE‐related dysplasia (pooled sensitivity and specificity of 89% and 83% in a meta‐analysis of 789 patients) [70], gastric neoplasia (pooled sensitivity and specificity of 81% and 98% in 657 patients) [71], and CRC neoplasms (pooled sensitivity and specificity of 83% and 90% in 376 patients) [72]. Nonetheless, concerns regarding the heterogeneity in performance characteristics and diagnostic yield were raised in recent meta‐analyses [25, 29, 70], indicating the importance of expertise and need for comprehensive training. While providing favorable diagnostic performance in many studies conducted in academic research centers, the routine use of CLE is largely hindered by the significant up‐front investment. In addition, its small FOV can be prone to sampling error for targeted biopsies, especially when operated by novice users to probe small lesions with a stable FOV. Mosaicking in the GI tract to image regions with a larger surface area has been demonstrated in preliminary feasibility studies, but the clinical utility is still limited [73, 74]. Overall, CLE, like many other emerging microscopic modalities, is mostly evaluated in tertiary centers. Without a better understanding of its learning curve and a thorough cost–utility analysis, the practicality for community use is still unknown.
3.2. Endocytoscopy
Endocytoscopy is in principle similar to magnification WLE that offers reflectance imaging with up to 150‐fold optical magnification, except that the optical magnification is further improved for imaging at the cellular level (Table 4). Commercially available systems are either endoscope‐based or probe‐based [75], and they support optical magnification of approximately 500‐fold (endoscope‐based) to > 1000‐fold (probe‐based). To enhance mucosal surface contrast under white light illumination, methylene blue or its combination with crystal violet has been found useful for visualizing nuclear and glandular patterns [76].
Commercial endocytoscopy systems have been shown to allow for cellular‐level characterization of GI lesions [77, 78, 79]. Recent studies investigated the effectiveness of endocytoscopy to tackle challenging tasks such as diagnosing adenomatous diminutive polyps and low‐grade adenoma in the colon, reporting high diagnostic performance (96.8% accuracy in 39 patients and 86.4% accuracy in 573 patients, respectively) [80, 81]. To facilitate its broader application, efforts have been made to develop classification systems for standardized clinical interpretation [82]. Different from fluorescence‐based CLE, endocytoscopy provides a reflectance modality to investigate suspicious lesions at ultrahigh resolution. In addition to exogenous dyes, it offers compatibility with virtual CE such as NBI. While clinical evidence from large‐scale RCTs is still unavailable, its further evaluation is supported by initial studies reporting performance characteristics comparable to CLE.
3.3. Optical coherence tomography
Using a low‐coherence light source to probe tissue reflectance at varied transverse locations, OCT generates depth‐resolved, near‐microscopic images with axial resolution of about 10 µm and lateral resolution of approximately 30 µm (Table 4). Since OCT images are acquired via a single‐mode fiber, it is inherently compatible with endoscopic imaging of luminal organs including the GI tract and cardiovascular systems. To access lesions in the GI tract, OCT systems of different form factors have been developed, including probe‐ and balloon‐based OCT that can pass through an endoscope working channel, and a capsule‐based design that can be operated in the primary care setting (Table 4). Using OCT in different forms, depth‐resolved and high‐resolution imaging has been reported in Barrett's esophagus, stomach, small intestine, and colon polyps.
In 2013, an OCT‐based imaging system became commercially available for esophageal imaging, known as the VLE (NvisionVLE, NinePoint Medical, Bedford, MA, USA; Fig. 2B). In the VLE, a balloon catheter is used to center the OCT probe, enabling stable circumferential scanning of a 6 cm segment of esophagus with a 3 mm imaging depth in 90 seconds. Since its introduction, studies have been conducted to establish image interpretation criteria, reporting a diagnostic accuracy of 87% for BE‐related dysplasia in a pilot study of 27 patients [83]. Moreover, artificial intelligence (AI)‐based algorithms are developed and clinical evaluation is underway in a multicenter RCT [84]. Another important extension of the VLE technology is the introduction of laser marking in 2016, enabling a targeted biopsy protocol shown to lead to a higher yield of dysplasia in BE compared with the standard Seattle protocol (33.7% in 106 patients vs. 19.6% in 95 patients) [85]. While the majority of OCT studies focused on the esophagus, successful imaging of the colon has been reported in case studies or case series [86, 87]. Unlike the tubular esophagus, good tissue contact with the balloon catheter to ensure VLE imaging quality can be more difficult in the colon.
Thanks to the scanning and pullback mechanism, OCT systems can generate depth‐resolved and near‐microscopic maps of a long esophageal segment, and cross‐sectional visualization of multiple histologic layers can provide important information for detecting disease progression beneath the superficial surface. Combined with a laser marking system, VLE is uniquely poised to reduce sampling errors, a major limitation for other endomicroscopic modalities. Nonetheless, commercial VLEs are costly and clinical interpretation of reflectance‐based volumetric images remains a challenging process, even though computer‐aided systems are under evaluation. In addition, as OCT captures tissue reflectance, it precludes the use of specific contrast agents and molecular probes.
Many recent technical innovations in endoscopic OCT have embraced a capsule enclosure, allowing for miniature optomechanical components to be included at distal end of the probe and improving imaging quality and contrast. Spatial resolution, for example, can be improved with a miniaturized actuation system to minimize rotational distortion [88], or using a shorter wavelength to enable ultrahigh‐resolution OCT [89]. OCT‐based endoscopy also benefited from an increased scanning speed, allowing for ultra‐fast OCT angiography to visualize subsurface microvasculature in the GI tract [90]; briefly, circumferential OCT images are acquired at 400 frames per second, and decorrelation between sequential frames is calculated to highlight circulating erythrocytes and thus the 3D microvasculature. In addition to circumferential imaging of luminal organs, a piezoelectric probe was also developed to enable forward‐viewing OCT imaging of colorectal polyps [91]. Enabled by different scanning mechanisms such as microelectromechanical system and piezoelectric transducers to form a 2D sampling pattern, forward‐viewing OCT probes can generate high‐resolution and label‐free images with an intuitive view similar to standard endoscopy.
3.4. High‐resolution microendoscope
While offering superior resolution compared to conventional endoscopes, the high cost of the above‐mentioned endomicroscopic modalities limits their clinical use in tertiary medical centers. As an alternative shown in Fig. 2D, the HRME is a low‐cost ($1500), fiber‐optic fluorescence microscope that can image nuclear morphology at subcellular resolution [92]. In pilot clinical trials, the clinical utility of HRME was demonstrated for accurate colon polyp characterization using proflavine as an inexpensive and topically applied dye for vital staining [93, 94]; in a prospective single‐center trial, HRME was shown to have a high accuracy and specificity of 94% and 95% for detecting adenomatous and neoplastic polyps during routine screening or surveillance colonoscopy of 94 patients [95]. In the upper GI tract, HRME has also shown value for detection of BE‐related dysplasia and ESCC [96, 97]. In a prospective trial of 147 high‐risk patients, adjunctive HRME following Lugol's CE was shown to increase the specificity from 48% to 88% for ESCC detection while achieving a sensitivity of 91% [98].
To further facilitate dissemination of this high‐resolution yet inexpensive technology, quantitative and automated diagnostic algorithms have been developed and prospectively validated [97, 99, 100]. Recent development focused on further improvement of its axial resolution with optical sectioning techniques [101, 102]. Compared with commercial CLE, HRME offers a low‐cost alternative to visualize important histologic features in community and resource‐constrained settings.
3.5. Other in vivo microscopy technologies
Various emerging technologies at earlier stages of development have also been reported in animal models or case studies. Multiphoton endomicroscopy, for example, is a novel technology that has gained attention as a high‐resolution and label‐free imaging modality. Taking advantage of near‐infrared laser excitation and endogenous autofluorescence, recent studies in rodent animal models reported deeper penetration (120 µm) than CLE without administration of contrast agent [103, 104].
Spectrally encoded confocal endomicroscopy (SECM) is another fiber‐optic imaging modality that can image tissue surface reflectance using a single‐mode fiber coupled with a diffraction grating that encodes tissue locations with distinct wavelengths. Integrated with a rotary junction and a pullback mechanism, high‐speed line scanning of large areas can be achieved [105] Like OCT‐based capsules, a tethered SECM capsule was shown feasible for imaging of the esophagus [106].
In addition to imaging modalities that offer direct visualization, spectroscopic probes have also been developed to study the subcellular architecture and molecular constituents of GI lesions. Briefly, endoscopic spectroscopy measures the spectral composition of backscattered light from tissue under varied illumination wavelengths and geometries, exploiting three major types of light–tissue interactions—elastic scattering, absorption, and inelastic scattering. Elastic scattering, as the dominant type of scattering, occurs without wavelength change and is sensitive to alterations in cell and tissue architecture. In addition, tissue absorption, mostly from hemoglobin, lipids, and water, also contributes to elastic scattering spectra in the visible and near‐infrared spectral regions. In comparison, inelastic (or Raman) scattering with wavelength shifts is relatively weaker, but can be employed to characterize chemical fingerprints of specific molecular components. A broad range of spectroscopic techniques, including diffuse reflectance spectroscopy and Raman spectroscopy, have been clinically evaluated with varied results in the GI tract [107, 108, 109, 110]. Compared to imaging modalities, spectroscopic techniques provide label‐free, quantitative, and objective measurements of GI mucosa. Nonetheless, its clinical interpretability is limited by the complexity of high‐dimensional data from a point‐to‐point FOV. With recent studies reporting technical advances such as novel fiber design and nanoparticles [111, 112], further evaluation in larger clinical trials is warranted for this broad range of technologies.
4. Molecular imaging
Most existing modalities detect early cancer lesions based on the presence of macroscopic or microscopic morphological changes. By studying the altered biomolecular cascades that may precede architectural changes, molecular imaging is an emerging field in modern oncology that has the potential to detect GI lesions at earlier stages with higher specificity than morphologic alterations. Driven by genetic and molecular profiling of tumors, specific biomarkers can be targeted using a wide range of molecular probes, including antibodies, peptides, nanoparticles, aptamers, and affibodies [113]. Since molecular probes are typically fluorescently labeled, they are mostly used in conjunction with fluorescence imaging modalities such as CLE or widefield fluorescence endoscopes. Ex vivo near‐infrared imaging has also been employed to suppress confounding tissue autofluorescence.
While most studies are focused on proof of concept in rodent animal models, recent studies have demonstrated potential for ex vivo and in vivo applications in human trials. In Barrett's esophagus, lectin‐based probes were found useful for differentiating dysplastic from benign lesions in ex vivo human specimens [114, 115]. Sturm et al. [116] developed a peptide that binds specifically to BE‐related dysplasia and demonstrated its safe in vivo application using the CLE. A similar study was conducted for CLE‐based targeted imaging of EGFR in the colon [117]. Thanks to its specific binding, molecular probes can also been used with widefield fluorescence endoscopes for detection of flat colon lesions [118, 119].
Molecular probes in combination with fluorescence endoscopic imaging provide novel routes to targeted imaging with high specificity, addressing a key limitation of many widefield endoscopic modalities. Another advantage of this approach, as demonstrated in animal models, is the safe use for longitudinal studies without tissue removal [120]. Recent successful translation of this approach into human trials shows promising results for functional characterization of GI lesions, thereby holding potential for personalized treatment recommendations. Future studies in this intrinsically multidisciplinary field will benefit from collective efforts to develop novel probes and imaging devices, while strictly monitoring safety during clinical use.
5. Machine learning for computer‐aided detection and diagnosis
With the advent of various novel endoscopic techniques, objective and confident interpretation of multiscale and multidimensional clinical data is a crucial, yet cumbersome and challenging task. In the meantime, the abundance of large‐volume imaging data presents numerous opportunities to employ machine learning to assist novice endoscopists and experts alike. From an algorithm development perspective, two approaches have been used to facilitate clinical decision making. First, in classical machine‐learning methods, as illustrated in Fig. 3A, a broad range of features can be explored and extrapolated, such as shape, texture, color, and clinically inspired features [121]. After feature extraction, a classifier can be developed for computer‐aided diagnosis (CADx). Second, recent implementation of deep learning algorithms has been shown to contribute to more sophisticated examination of the feature space, while enabling accelerated processing of large datasets in real time (Fig. 3B). Compared with classical machine‐learning approaches, deep learning scales more effectively with large datasets and thus is well suited for processing a series of images with high dimensions (e.g. multimodal images); in addition, it also alleviates the burden of complicated feature engineering. Nonetheless, a large and standardized dataset is usually required for effective training of deep learning algorithms, and their clinical interpretability is relatively poor. In this section, we discuss recent advances of machine‐learning approaches to perform specific clinical tasks.
Fig. 3.
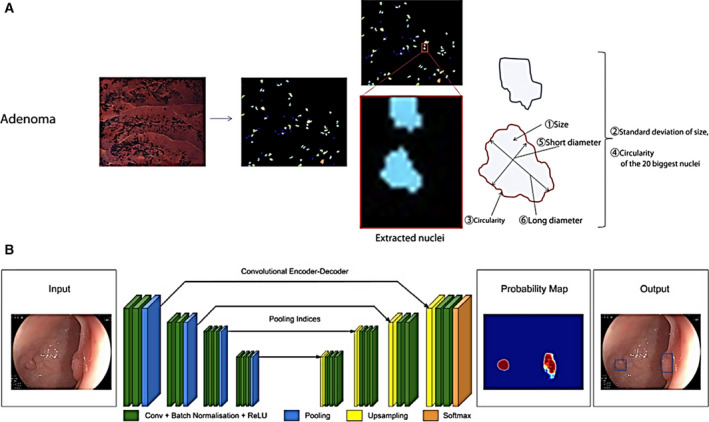
Examples of computer‐aided algorithms for detection and diagnosis of colorectal polyps. (A) In microscopic images of polyps collected with endocytoscopy, clinically inspired nuclear morphology features were extracted and quantified for diagnosing advanced histology. (B) A data‐driven algorithm is trained using a convolutional neural network to highlight adenomatous CRC polyps in WLE images. Figure 3A reproduced from [134] with permission from Elsevier. Figure 3Breproduced from [125] with permission from BMJ Publishing Group Ltd.
In terms of clinical output, algorithms are generally trained to perform either computer‐aided detection (CADe) such as lesion detection using macroscopic modalities, or CADx based on microscopic or high‐definition widefield imaging. To date, CADe algorithms have been most extensively applied to improve automated colorectal polyp detection [122]. Initial studies in the field focused on polyp detection rate as the primary performance measure, and recent advancement in computing hardware and algorithms has significantly accelerated image processing for real‐time and video‐rate applications [123, 124]. Very recently, in a prospective RCT involving 1057 patients, Wang et al. reported significantly increased ADR in WLE images using an AI‐based system than standard colonoscopy (29.1% vs. 20.3%) [125]. Of note, the ADR improvement is attributed to detection of more diminutive polyps. Diagnostic algorithms (CADx) to differentiate adenomatous from benign colorectal polyps were also developed in retrospective studies [126], with a recent study reporting a high accuracy of 94% for diagnosing adenomatous diminutive polyps in unaltered NBI videos [127]. Similarly, excellent accuracies were reported to diagnose neoplastic lesions in the esophagus and stomach in widefield modalities, including WLE [128], NBI [129], or their combination [130]. When integrated with standard WLE or advanced widefield modalities such as NBI, these diagnostic algorithms can facilitate more quantitative and objective interpretation of clinical data; for novice users, such algorithms can provide critical decision support that can reduce the learning curve and improve the diagnostic accuracy. Microscope imaging modalities, including CLE, HRME, endocytoscopy, and VLE, have also benefited from automated algorithms [99, 100, 131, 132, 133, 134, 135].
It is evident that machine learning is playing an increasingly significant role in the present field of GI endoscopy. While most algorithms are developed and evaluated retrospectively, prospective studies with real‐time and low‐latency algorithms are emerging. With the integration of AI systems in recently launched commercial systems, including EndoBrain in endocytoscopy systems (Olympus) and Intelligent real‐time image segmentation in the NvisionVLE imaging system (NinePoint Medical), it is hoped that machine learning will contribute to palpable changes in routine practice in the coming years. As machine learning in GI endoscopy is experiencing rapid progress, there remain several barriers for clinical implementation. First, it is of crucial importance to validate the generalizability of computer‐assisted algorithms, especially when images are acquired by users in varied settings. Second, for users to make best use of computer‐aided decision support, interpretability and training are also important factors when designing the algorithm architecture. Finally, the intricate nature of computational algorithms can raise new ethical and regulatory concerns, and their role during practical use will ultimately depend on acceptance by the healthcare community.
6. Conclusion and outlook
The past few decades have witnessed substantial evolution of optical endoscopy techniques and their continuous clinical translation to enable in vivo characterization of the GI mucosa with unpreceded imaging contrast, resolution, depth, and speed. The multidimensional, volumetric, and real‐time imaging data open new opportunities for improved detection of GI pathology, potentially leading to important shifts in diagnostic and therapeutic algorithms. Moreover, novel molecular probe and machine‐learning methods are under clinical evaluation, showing great promises to improve detection accuracy and reliability. Taken together, the constantly evolving landscape of modern GI endoscopy presents numerous opportunities and demands a multidisciplinary and collaborative effort in the academic and healthcare community. In the meantime, with academic‐industrial partnerships playing an increasingly important role in prototype development and commercialization, close integration of academia and industry is also called for to accelerate technology translation, standardization, and dissemination.
The rapid advent of novel techniques also presents new challenges. While offering direct visualization of GI lesions, acquisition and interpretation of clinical data using optical imaging techniques require adequate training and expertise. Since a large proportion of these clinical studies are conducted in research or academic centers, it is of paramount importance to standardize their clinical use and validate diagnostic performance in varied clinical settings. Once validated in large‐scale studies, there are challenges to balance increased cost, learning curves, and user resistance to new technology. Therefore, in addition to technical performance metrics, clinical barriers for technology adoption should be assessed when placing novel devices into the hands of practitioners. Nonetheless, clinical adoption of novel technologies has taken place rapidly; for example, the use of capsule endoscopy for small bowel disease and NBI for colorectal polyp characterization shows that wide scale adoption is possible in short time frames. With an ever‐increasing amount of technical innovations and clinical data, we expect that novel optical imaging technologies will lead to significant changes in the clinical workflow in the coming decade, thus improving outcomes for a large at‐risk population through more accurate and less invasive endoscopic imaging.
Conflict of interest
The authors declare no conflict of interest.
Author contributions
YT, SA, and RR‐K wrote the paper.
Acknowledgements
This work was supported by funding from the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health under Award Number R21EB023431. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA & Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68, 394–424. [DOI] [PubMed] [Google Scholar]
- 2.Codipilly DC, Qin Y, Dawsey SM, Kisiel J, Topazian M, Ahlquist D & Iyer PG (2018) Screening for esophageal squamous cell carcinoma: recent advances. Gastrointest Endosc 88, 413–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mwachiro MM, Burgert SL, Lando J, Chepkwony R, Bett C, Bosire C, Abnet CC, Githanga J, Waweru W, Giffen CAet al. (2016) Esophageal squamous dysplasia is common in asymptomatic kenyans: a prospective, community‐based, cross‐sectional study. Am J Gastroenterol 111, 500–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dawsey SM, Fleischer DE, Wang G‐Q, Zhou B, Kidwell JA, Lu N, Lewin KJ, Roth MJ, Tio TL & Taylor PR (1998) Mucosal iodine staining improves endoscopic visualization of squamous dysplasia and squamous cell carcinoma of the esophagus in linxian, china. Cancer 83, 220–231. [PubMed] [Google Scholar]
- 5.Hur C, Miller M, Kong CY, Dowling EC, Nattinger KJ, Dunn M & Feuer EJ (2013) Trends in esophageal adenocarcinoma incidence and mortality. Cancer 119, 1149–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weusten BLAM, Bisschops R, Coron E, Dinis‐Ribeiro M, Dumonceau JM, Esteban JM, Hassan C, Pech O, Repici A, Bergman Jet al. (2017) Endoscopic management of Barrett's esophagus: European Society of Gastrointestinal Endoscopy (ESGE) position statement. Endoscopy 49, 191–198. [DOI] [PubMed] [Google Scholar]
- 7.Shaheen NJ, Falk GW, Iyer PG, Gerson LB; American College of Gastroenterology (2016). ACG Clinical Guideline: diagnosis and management of Barrett's esophagus. Am J Gastroenterol 111, 30–50; quiz 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vieth M, Ell C, Gossner L, May A & Stolte M (2004) Histological analysis of endoscopic resection specimens from 326 patients with Barrett's esophagus and early neoplasia. Endoscopy 36, 776–781. [DOI] [PubMed] [Google Scholar]
- 9.Ross‐Innes CS, Debiram‐Beecham I, O'Donovan M, Walker E, Varghese S, Lao‐Sirieix P, Lovat L, Griffin M, Ragunath K, Haidry Ret al. (2015) Evaluation of a minimally invasive cell sampling device coupled with assessment of trefoil factor 3 expression for diagnosing Barrett's esophagus: a multi‐center case‐control study. PLoS Medicine 12, 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamashima C (2018) Update version of the Japanese Guidelines for Gastric Cancer Screening. Jpn J Clin Oncol 48, 673–683. [DOI] [PubMed] [Google Scholar]
- 11.Guideline Committee of the Korean Gastric Cancer Association (KGCA), Development Working Group & Review Panel (2019) Korean practice guideline for gastric cancer 2018: an evidence‐based, multi‐disciplinary approach. J Gastric Cancer 19, 1–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim GH, Liang PS, Bang SJ & Hwang JH (2016) Screening and surveillance for gastric cancer in the United States: is it needed? Gastrointest Endosc 84, 18–28. [DOI] [PubMed] [Google Scholar]
- 13.Zakko L, Lutzke L & Wang KK (2017) Screening and preventive strategies in esophagogastric cancer. Surg Oncol Clin N Am 26, 163–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evans JA, Chandrasekhara V, Chathadi KV, Decker GA, Early DS, Fisher DA, Foley K, Hwang JH, Jue TL, Lightdale JRet al. (2015) The role of endoscopy in the management of premalignant and malignant conditions of the stomach. Gastrointest Endosc 82, 1–8. [DOI] [PubMed] [Google Scholar]
- 15.Pimentel‐Nunes P, Libânio D, Marcos‐Pinto R, Areia M, Leja M, Esposito G, Garrido M, Kikuste I, Megraud F, Matysiak‐Budnik Tet al. (2019) Management of epithelial precancerous conditions and lesions in the stomach (MAPS II): European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter and Microbiota Study Group (EHMSG), European Society of Pathology (ESP), and Sociedade Port. Endoscopy 51, 365–388. [DOI] [PubMed] [Google Scholar]
- 16.Winawer SJ, Zauber AG, Ho MN, O'Brien MJ, Gottlieb LS, Sternberg SS, Waye JD, Schapiro M, Bond JH, Panish JFet al. (1993) Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med 329, 1977–1981. [DOI] [PubMed] [Google Scholar]
- 17.Zauber AG, Winawer SJ, O'Brien MJ, Lansdorp‐Vogelaar I, van Ballegooijen M, Hankey BF, Shi W, Bond JH, Schapiro M, Panish JFet al. (2012) Colonoscopic polypectomy and long‐term prevention of colorectal‐cancer deaths. N Engl J Med 366, 687–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bisschops R, East JE, Hassan C, Hazewinkel Y, Kamiński MF, Neumann H, Pellise M, Antonelli G, Bustamante‐Balen M, Coron Eet al. (2019) Correction: advanced imaging for detection and differentiation of colorectal neoplasia: European Society of Gastrointestinal Endoscopy (ESGE) Guideline ‐ update 2019 (Endoscopy DOI: 10.1055/a‐1031‐7657). Endoscopy 51, C6. [DOI] [PubMed] [Google Scholar]
- 19.Issa IA & Noureddine M (2017) Colorectal cancer screening: an updated review of the available options. World J Gastroenterol 23, 5086–5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vleugels JLA, Hassan C, Senore C, Cassoni P, Baron JA, Rex DK, Ponugoti PL, Pellise M, Parejo S, Bessa Xet al. (2019) Diminutive polyps with advanced histologic features do not increase risk for metachronous advanced colon neoplasia. Gastroenterology 156, 623–634.e3. [DOI] [PubMed] [Google Scholar]
- 21.Yalamarthi S, Witherspoon P, McCole D & Auld C (2004) Missed diagnoses in patients with upper gastrointestinal cancers. Endoscopy 36, 874–879. [DOI] [PubMed] [Google Scholar]
- 22.van Rijn JC, Reitsma JB, Stoker J, Bossuyt PM, van Deventer SJ & Dekker E (2006) Polyp miss rate determined by tandem colonoscopy: a systematic review. Am J Gastroenterol 101, 343–350. [DOI] [PubMed] [Google Scholar]
- 23.Sharma P, Savides TJ, Canto MI, Corley DA, Falk GW, Goldblum JR, Wang KK, Wallace MB & Wolfsen HC (2012) The American Society for Gastrointestinal Endoscopy PIVI (Preservation and Incorporation of Valuable Endoscopic Innovations) on imaging in Barrett's Esophagus. Gastrointest Endosc 76, 252–254. [DOI] [PubMed] [Google Scholar]
- 24.Abu Dayyeh BK, Thosani N, Konda V, Wallace MB, Rex DK, Chauhan SS, Hwang JH, Komanduri S, Manfredi M, Maple JTet al. (2015) ASGE technology committee systematic review and meta‐analysis assessing the ASGE PIVI thresholds for adopting real‐time endoscopic assessment of the histology of diminutive colorectal polyps. Gastrointest Endosc 81, 502.e1–502.e16. [DOI] [PubMed] [Google Scholar]
- 25.Thosani N, Abu Dayyeh BK, Sharma P, Aslanian HR, Enestvedt BK, Komanduri S, Manfredi M, Navaneethan U, Maple JT, Pannala Ret al. (2016) ASGE Technology Committee systematic review and meta‐analysis assessing the ASGE preservation and incorporation of valuable endoscopic innovations thresholds for adopting real‐time imaging‐assisted endoscopic targeted biopsy during endoscopic surveillance. Gastrointest Endosc 83, 684–698.e7. [DOI] [PubMed] [Google Scholar]
- 26.East JE, Vleugels JL, Roelandt P, Bhandari P, Bisschops R, Dekker E, Hassan C, Horgan G, Kiesslich R, Longcroft‐Wheaton Get al. (2016) Advanced endoscopic imaging: European Society of Gastrointestinal Endoscopy (ESGE) technology review. Endoscopy 48, 1029–1045. [DOI] [PubMed] [Google Scholar]
- 27.Subramanian V & Ragunath K (2014) Advanced endoscopic imaging: a review of commercially available technologies. Clin Gastroenterol Hepatol 12, 368–376.e1. [DOI] [PubMed] [Google Scholar]
- 28.Ho S‐H, Uedo N, Aso A, Shimizu S, Saito Y, Yao K & Goh K‐L (2018) Development of image‐enhanced endoscopy of the gastrointestinal tract. J Clin Gastroenterol 52, 295–306. [DOI] [PubMed] [Google Scholar]
- 29.Qumseya B, Sultan S, Bain P, Jamil L, Jacobson B, Anandasabapathy S, Agrawal D, Buxbaum JL, Fishman DS, Gurudu SRet al. (2019) ASGE guideline on screening and surveillance of Barrett's esophagus. Gastrointest Endosc 90, 335–359.e2. [DOI] [PubMed] [Google Scholar]
- 30.Gralnek IM (2015) Emerging technological advancements in colonoscopy: Third Eye® Retroscope® and Third Eye® Panoramic TM, Fuse® Full Spectrum Endoscopy® colonoscopy platform, Extra‐Wide‐Angle‐View colonoscope, and NaviAid TM G‐EYE TM balloon colonoscope. Dig Endosc 27, 223–231. [DOI] [PubMed] [Google Scholar]
- 31.Kakushima N, Takizawa K, Tanaka M, Kawata N, Ito S, Imai K, Hotta K, Igarashi K, Kishida Y, Yoshida Met al. (2016) A novel wide viewing endoscope for upper gastrointestinal screening: a pilot study. Endosc Int Open 04, E190–E192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bronzwaer M, Dekker E, Weingart V, Groth S, Pioche M, Rivory J, Beyna T, Neuhaus H, Ponchon T, Allescher Het al. (2018) Feasibility, safety, and diagnostic yield of the Extra Wide Angle View (EWAVE) colonoscope for the detection of colorectal lesions. Endoscopy 50, 63–68. [DOI] [PubMed] [Google Scholar]
- 33.Yamada H, Shibata T, Terada T, Osaki H, Maeda K, Tahara T, Nagasaka M, Nakagawa Y & Ohmiya N (2020) Usefulness of full‐spectrum endoscopy for the upper gastrointestinal tract. J Clin Gastroenterol 54, 344–349. [DOI] [PubMed] [Google Scholar]
- 34.Ohigashi S, Taketa T, Shimada G, Kubota K, Sunagawa H & Kishida A (2019) Fruitful first experience with an 8K ultra‐high‐definition endoscope for laparoscopic colorectal surgery. Asian J Endosc Surg 12, 362–365. [DOI] [PubMed] [Google Scholar]
- 35.Akizue N, Matsumura T, Maruoka D, Ishikawa K, Hang D, Okimoto K, Saito K, Nakagawa T, Arai M & Kato N (2018) Novel three‐dimensional imaging system may facilitate gastric endoscopic submucosal dissection procedure: an ex vivo animal study. Endosc Int Open 06, E1431–E1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Higuchi K, Kaise M, Noda H, Ikeda G, Akimoto T, Yamawaki H, Goto O, Ueki N, Futagami S & Iwakiri K (2019) Usefulness of 3‐dimensional flexible endoscopy in esophageal endoscopic submucosal dissection in an ex vivo animal model. Gastroenterol Res Pract 2019, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sharma P, Bergman JJGHM, Goda K, Kato M, Messmann H, Alsop BR, Gupta N, Vennalaganti P, Hall M, Konda Vet al. (2016) Development and validation of a classification system to identify high‐grade dysplasia and esophageal adenocarcinoma in Barrett's esophagus using narrow‐band imaging. Gastroenterology 150, 591–598. [DOI] [PubMed] [Google Scholar]
- 38.Qumseya BJ, Wang H, Badie N, Uzomba RN, Parasa S, White DL, Wolfsen H, Sharma P & Wallace MB (2013) Advanced imaging technologies increase detection of dysplasia and neoplasia in patients with Barrett's esophagus: a meta‐analysis and systematic review. Clin Gastroenterol Hepatol 11, 1562–1570.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuiper T, Marsman WA, Jansen JM, van Soest EJ, Haan YCL, Bakker GJ, Fockens P & Dekker E (2012) Accuracy for optical diagnosis of small colorectal polyps in nonacademic settings. Clin Gastroenterol Hepatol 10, 1016–1020. [DOI] [PubMed] [Google Scholar]
- 40.Ladabaum U, Fioritto A, Mitani A, Desai M, Kim JP, Rex DK, Imperiale T & Gunaratnam N (2013) Real‐time optical biopsy of colon polyps with narrow band imaging in community practice does not yet meet key thresholds for clinical decisions. Gastroenterology 144, 81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Atkinson NSS, Ket S, Bassett P, Aponte D, De Aguiar S, Gupta N, Horimatsu T, Ikematsu H, Inoue T, Kaltenbach Tet al. (2019) Narrow‐band imaging for detection of neoplasia at colonoscopy: a meta‐analysis of data from individual patients in randomized controlled trials. Gastroenterology 157, 462–471. [DOI] [PubMed] [Google Scholar]
- 42.Leavesley SJ, Walters M, Lopez C, Baker T, Favreau PF, Rich TC, Rider PF & Boudreaux CW (2016) Hyperspectral imaging fluorescence excitation scanning for colon cancer detection. J Biomed Optics 21, 104003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goto A, Nishikawa J, Kiyotoki S, Nakamura M, Nishimura J, Okamoto T, Ogihara H, Fujita Y, Hamamoto Y & Sakaida I (2015) Use of hyperspectral imaging technology to develop a diagnostic support system for gastric cancer. J Biomed Optics 20, 016017. [DOI] [PubMed] [Google Scholar]
- 44.Hohmann M, Kanawade R, Klämpfl F, Douplik A, Mudter J, Neurath MF & Albrecht H (2017) In‐vivo multispectral video endoscopy towards in‐vivo hyperspectral video endoscopy. J Biophotonics 10, 553–564. [DOI] [PubMed] [Google Scholar]
- 45.Yoon J, Joseph J, Waterhouse DJ, Luthman AS, Gordon GSD, di Pietro M, Januszewicz W, Fitzgerald RC & Bohndiek SE (2019) A clinically translatable hyperspectral endoscopy (HySE) system for imaging the gastrointestinal tract. Nat Commun 10, 1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grigoroiu A, Yoon J & Bohndiek SE (2020) Deep learning applied to hyperspectral endoscopy for online spectral classification. Sci Rep 10, 3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bhandari P, Kandaswamy P, Cowlishaw D & Longcroft‐Wheaton G (2012) Acetic acid‐enhanced chromoendoscopy is more cost‐effective than protocol‐guided biopsies in a high‐risk Barrett's population. Dis Esophagus 25, 386–392. [DOI] [PubMed] [Google Scholar]
- 48.Olliver JR, Wild CP, Sahay P, Dexter S & Hardie LJ (2003) Chromoendoscopy with methylene blue and associated DNA damage in Barrett's oesophagus. Lancet 362, 373–374. [DOI] [PubMed] [Google Scholar]
- 49.Zhao Z, Yin Z, Wang S, Wang J, Bai B, Qiu Z & Zhao Q (2016) Meta‐analysis: the diagnostic efficacy of chromoendoscopy for early gastric cancer and premalignant gastric lesions. J Gastroenterol Hepatol 31, 1539–1545. [DOI] [PubMed] [Google Scholar]
- 50.Peery AF, Hoppo T, Garman KS, Dellon ES, Daugherty N, Bream S, Sanz AF, Davison J, Spacek M, Connors Det al. (2012) Feasibility, safety, acceptability, and yield of office‐based, screening transnasal esophagoscopy (with video). Gastrointest Endosc 75, 945–953.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sami SS, Iyer PG, Pophali P, Halland M, di Pietro M, Ortiz‐Fernandez‐Sordo J, White JR, Johnson M, Guha IN, Fitzgerald RCet al. (2019) Acceptability, accuracy, and safety of disposable transnasal capsule endoscopy for Barrett's esophagus screening. Clin Gastroenterol Hepatol 17, 638–646.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shariff MK, Bird‐Lieberman EL, O'Donovan M, Abdullahi Z, Liu X, Blazeby J & Fitzgerald R (2012) Randomized crossover study comparing efficacy of transnasal endoscopy with that of standard endoscopy to detect Barrett's esophagus. Gastrointest Endosc 75, 954–961. [DOI] [PubMed] [Google Scholar]
- 53.Toyoizumi H, Kaise M, Arakawa H, Yonezawa J, Yoshida Y, Kato M, Yoshimura N, Goda K‐I & Tajiri H (2009) Ultrathin endoscopy versus high‐resolution endoscopy for diagnosing superficial gastric neoplasia. Gastrointest Endosc 70, 240–245. [DOI] [PubMed] [Google Scholar]
- 54.Kawai T, Yanagizawa K, Naito S, Sugimoto H, Fukuzawa M, Gotoda T, Matsubayashi J, Nagao T, Hoshino S, Tsuchida Aet al. (2014) Evaluation of gastric cancer diagnosis using new ultrathin transnasal endoscopy with narrow‐band imaging: preliminary study. J Gastroenterol Hepatol (Australia) 29 (S4), 33–36. [DOI] [PubMed] [Google Scholar]
- 55.Tanuma T, Morita Y & Doyama H (2016) Current status of transnasal endoscopy worldwide using ultrathin videoscope for upper gastrointestinal tract. Dig Endosc 28, 25–31. [DOI] [PubMed] [Google Scholar]
- 56.Rondonotti E, Spada C, Adler S, May A, Despott EJ, Koulaouzidis A, Panter S, Domagk D, Fernandez‐Urien I, Rahmi Get al. (2018) Small‐bowel capsule endoscopy and device‐assisted enteroscopy for diagnosis and treatment of small‐bowel disorders: European Society of Gastrointestinal Endoscopy (ESGE) Technical Review. Endoscopy 50, 423–446. [DOI] [PubMed] [Google Scholar]
- 57.Bang S, Park JY, Jeong S, Kim YH, Shim HB, Kim TS, Lee DH & Song SY (2009) First clinical trial of the “MiRo” capsule endoscope by using a novel transmission technology: electric‐field propagation. Gastrointest Endosc 69, 253–259. [DOI] [PubMed] [Google Scholar]
- 58.Ughi GJ, Gora MJ, Swager A‐F, Soomro A, Grant C, Tiernan A, Rosenberg M, Sauk JS, Nishioka NS & Tearney GJ (2016) Automated segmentation and characterization of esophageal wall in vivo by tethered capsule optical coherence tomography endomicroscopy. Biomed Opt Express 7, 409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ching HL, Healy A, Thurston V, Hale MF, Sidhu R & McAlindon ME (2018) Upper gastrointestinal tract capsule endoscopy using a nurse‐led protocol: first reported experience. World J Gastroenterol 24, 2893–2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bhardwaj A, Hollenbeak CS, Pooran NR & Mathew A (2009) A Meta‐analysis of the diagnostic accuracy of esophageal capsule endoscopy for barrett's esophagus in patients with gastroesophageal reflux disease. Gastrointest Endosc 69, AB363–AB364. [DOI] [PubMed] [Google Scholar]
- 61.Baltes P, Bota M, Albert J, Philipper M, Hörster H‐G, Hagenmüller F, Steinbrück I, Jakobs R, Bechtler M, Hartmann Det al. (2018) PillCamColon2 after incomplete colonoscopy ‐ a prospective multicenter study. World J Gastroenterol 24, 3556–3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Koulaouzidis A, Iakovidis DK, Karargyris A & Rondonotti E (2015) Wireless endoscopy in 2020: will it still be a capsule? World J Gastroenterol 21, 5119–5130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ching HL, Hale MF & McAlindon ME (2016) Current and future role of magnetically assisted gastric capsule endoscopy in the upper gastrointestinal tract. Therap Adv Gastroenterol 9, 313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Swain P, Toor A, Volke F, Keller J, Gerber J, Rabinovitz E & Rothstein RI (2010) Remote magnetic manipulation of a wireless capsule endoscope in the esophagus and stomach of humans (with ). Gastrointest Endosc 71, 1290–1293. [DOI] [PubMed] [Google Scholar]
- 65.Zhao AJ, Qian YY, Sun H, Hou X, Pan J, Liu X, Zhou W, Chen Y‐Z, Jiang X, Li Z‐Set al. (2018) Screening for gastric cancer with magnetically controlled capsule gastroscopy in asymptomatic individuals. Gastrointest Endosc 88, 466–474.e1. [DOI] [PubMed] [Google Scholar]
- 66.Jiang B, Qian Y‐Y, Pan J, Jiang X, Wang Y‐C, Zhu J‐H, Zou WB, Zhou W, Li ZS & Liao Z (2020) Second‐generation magnetically controlled capsule gastroscopy with improved image resolution and frame rate: a randomized controlled clinical trial (with video). Gastrointest Endosc 91: 1379–1387. [DOI] [PubMed] [Google Scholar]
- 67.Beg S, Card T, Warburton S, Rahman I, Wilkes E, White J & Ragunath K (2020) Diagnosis of Barrett's esophagus and esophageal varices using a magnetically assisted capsule endoscopy system. Gastrointest Endosc 91, 773–781.e1. [DOI] [PubMed] [Google Scholar]
- 68.Wallace MB, Meining A, Canto MI, Fockens P, Miehlke S, Roesch T, Lightdale CJ, Pohl H, Carr‐locke D, Löhr Met al. (2010) The safety of intravenous fluorescein for confocal laser endomicroscopy in the gastrointestinal tract. Aliment Pharmacol Ther 31, 548–552. [DOI] [PubMed] [Google Scholar]
- 69.Wallace M, Lauwers G, Chen Y, Dekker E, Fockens P, Sharma P & Meining A (2011) Miami classification for probe‐based confocal laser endomicroscopy. Endoscopy 43, 882–891. [DOI] [PubMed] [Google Scholar]
- 70.Xiong Y‐Q, Ma S‐J, Zhou J‐H, Zhong X‐S & Chen Q (2016) A meta‐analysis of confocal laser endomicroscopy for the detection of neoplasia in patients with Barrett's esophagus. J Gastroenterol Hepatol 31, 1102–1110. [DOI] [PubMed] [Google Scholar]
- 71.Qian W, Bai T, Wang H, Zhang L, Song J & Hou XH (2016) Meta‐analysis of confocal laser endomicroscopy for the diagnosis of gastric neoplasia and adenocarcinoma. J Dig Dis 17, 366–376. [DOI] [PubMed] [Google Scholar]
- 72.Fugazza A, Gaiani F, Carra MC, Brunetti F, Lévy M, Sobhani I, Azoulay D, Catena F, de'Angelis GL & de'Angelis N(2016) Confocal laser endomicroscopy in gastrointestinal and pancreatobiliary diseases: a systematic review and meta‐analysis. Biomed Res Int 2016, 1–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Becker V, Vercauteren T, von Weyhern CH, Prinz C, Schmid RM & Meining A (2007) High‐resolution miniprobe‐based confocal microscopy in combination with video mosaicing (with video). Gastrointest Endosc 66, 1001–1007. [DOI] [PubMed] [Google Scholar]
- 74.De Palma GD, Staibano S, Siciliano S, Persico M, Masone S, Maione F, Siano M, Mascolo M, Esposito D, Salvatori Fet al. (2010) In vivo characterisation of superficial colorectal neoplastic lesions with high‐resolution probe‐based confocal laser endomicroscopy in combination with video‐mosaicing: a feasibility study to enhance routine endoscopy. Dig Liver Dis 42, 791–797. [DOI] [PubMed] [Google Scholar]
- 75.Kwon RS, Song WK, Adler DG, Conway JD, Diehl DL, Farraye FA, Kantsevoy SV, Kaul V, Kethu SR, Mamula Pet al. (2009) Endocytoscopy. Gastrointest Endosc 70, 610–613. [DOI] [PubMed] [Google Scholar]
- 76.Ichimasa K, Kudo SE, Mori Y, Wakamura K, Ikehara N, Kutsukawa M, Takeda K, Misawa M, Kudo T, Miyachi Het al. (2014) Double staining with crystal violet and methylene blue is appropriate for colonic endocytoscopy: an in vivo prospective pilot study. Dig Endosc 26, 403–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tomizawa Y, Abdulla HM, Prasad GA, Wong Kee Song LM, Lutzke LS, Borkenhagen LS & Wang KK (2009) Endocytoscopy in esophageal cancer. Gastrointest Endosc Clin N Am 19, 273–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tomizawa Y, Iyer PG, Wongkeesong LM, Buttar NS, Lutzke LS, Wu TT & Wang KK (2013) Assessment of the diagnostic performance and interobserver variability of endocytoscopy in Barrett's esophagus: a pilot ex‐vivo study. World J Gastroenterol 19, 8652–8658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Abad MRA, Inoue H, Ikeda H, Manolakis A, Rodriguez de Santiago E, Sharma A, Fujiyoshi Y, Fukuda H, Sumi K, Onimaru Met al. (2019) Utilizing fourth‐generation endocytoscopy and the 'enlarged nuclear sign' for in vivo diagnosis of early gastric cancer. Endosc Int Open 07, E1002–E1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Utsumi T, Sano Y, Iwatate M, Sunakawa H, Teramoto A, Hirata D, Hattori S, Sano W, Hasuike N, Ichikawa Ket al. (2018) Prospective real‐time evaluation of diagnostic performance using endocytoscopy in differentiating neoplasia from non‐neoplasia for colorectal diminutive polyps (≤ 5 mm). World J Gastrointest Oncol 10, i–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kudo T, Suzuki K, Mori Y, Misawa M, Ichimasa K, Takeda K, Nakamura H, Maeda Y, Ogawa Y, Hayashi Tet al. (2020) Endocytoscopy for the differential diagnosis of colorectal low‐grade adenoma: a novel possibility for the “resect and discard” strategy. Gastrointest Endosc 91, 676–683. [DOI] [PubMed] [Google Scholar]
- 82.Kudo T, Kudo S‐E, Mori Y, Wakamura K, Misawa M, Hayashi T, Miyachi H, Katagiri A, Ishida F & Inoue H(2017) Classification of nuclear morphology in endocytoscopy of colorectal neoplasms. Gastrointest Endosc 85, 628–638. [DOI] [PubMed] [Google Scholar]
- 83.Leggett CL, Gorospe EC, Chan DK, Muppa P, Owens V, Smyrk TC, Anderson M, Lutzke LS, Tearney G & Wang KK (2016) Comparative diagnostic performance of volumetric laser endomicroscopy and confocal laser endomicroscopy in the detection of dysplasia associated with Barrett's esophagus. Gastrointest Endosc 83, 880–888.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Trindade AJ, McKinley MJ, Fan C, Leggett CL, Kahn A & Pleskow DK (2019) Endoscopic surveillance of Barrett's esophagus using volumetric laser endomicroscopy with artificial intelligence image enhancement. Gastroenterology 157, 303–305. [DOI] [PubMed] [Google Scholar]
- 85.Alshelleh M, Inamdar S, McKinley M, Stewart M, Novak JS, Greenberg RE, Sultan K, Devito B, Cheung M, Cerulli MAet al. (2018) Incremental yield of dysplasia detection in Barrett's esophagus using volumetric laser endomicroscopy with and without laser marking compared with a standardized random biopsy protocol. Gastrointest Endosc 88, 35–42. [DOI] [PubMed] [Google Scholar]
- 86.Adler DC, Zhou C, Tsai T‐H, Schmitt J, Huang Q, Mashimo H & Fujimoto JG (2009) Three‐dimensional endomicroscopy of the human colon using optical coherence tomography. Opt Express 17, 784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Trindade AJ, Sultan K, Vamadevan AS, Sejpal DV & Fan C (2016) Successful use of volumetric laser endomicroscopy in imaging a rectal polyp. Therap Adv Gastroenterol 9, 128–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liang K, Ahsen OO, Lee H‐C, Wang Z, Potsaid BM, Figueiredo M, Jayaraman V, Cable AE, Huang Q, Mashimo Het al. (2016) Volumetric mapping of Barrett's esophagus and dysplasia with en face optical coherence tomography tethered capsule. Am J Gastroenterol 111, 1664–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yuan W, Mavadia‐Shukla J, Xi J, Liang W, Yu X, Yu S & Li X (2016) Optimal operational conditions for supercontinuum‐based ultrahigh‐resolution endoscopic OCT imaging. Opt Lett 41, 250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tsai TH, Ahsen OO, Lee HC, Liang K, Figueiredo M, Tao YK, Giacomelli MG, Potsaid BM, Jayaraman V, Huang Qet al. (2014) Endoscopic optical coherence angiography enables 3‐dimensional visualization of subsurface microvasculature. Gastroenterology 147, 1219–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liang K, Ahsen OO, Wang Z, Lee H‐C, Liang W, Potsaid BM, Tsai T‐H, Giacomelli MG, Jayaraman V, Mashimo Het al. (2017) Endoscopic forward‐viewing optical coherence tomography and angiography with MHz swept source. Opt Lett 42, 3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pierce M, Yu D & Richards‐Kortum R (2011) High‐resolution fiber‐optic microendoscopy for in situ cellular imaging. J Vis Exp 47:e2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chang SS, Shukla R, Polydorides AD, Vila PM, Lee M, Han H, Kedia P, Lewis J, Gonzalez S, Kim Met al. (2013) High resolution microendoscopy for classification of colorectal polyps. Endoscopy 45, 553–559. [DOI] [PubMed] [Google Scholar]
- 94.Parikh ND, Perl D, Lee MH, Chang SS, Polydorides AD, Moshier E, Godbold J, Zhou E, Mitcham J, Richards‐Kortum Ret al. (2015) In vivo classification of colorectal neoplasia using high‐resolution microendoscopy: improvement with experience. J Gastroenterol Hepatol (Australia) 30, 1155–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Parikh ND, Perl D, Lee MH, Shah B, Young Y, Chang SS, Shukla R, Polydorides AD, Moshier E, Godbold Jet al. (2014) In vivo diagnostic accuracy of high‐resolution microendoscopy in differentiating neoplastic from non‐neoplastic colorectal polyps: a prospective study. Am J Gastroenterol 109, 68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Muldoon TJ, Thekkek N, Roblyer D, Maru D, Harpaz N, Potack J, Anandasabapathy S &Richards‐Kortum R (2010) Evaluation of quantitative image analysis criteria for the high‐resolution microendoscopic detection of neoplasia in Barrett's esophagus. J Biomed Optics 15, 26027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Quang T, Schwarz RA, Dawsey SM, Tan MC, Patel K, Yu X, Wang G, Zhang F, Xu H, Anandasabapathy Set al. (2016) A tablet‐interfaced high‐resolution microendoscope with automated image interpretation for real‐time evaluation of esophageal squamous cell neoplasia. Gastrointest Endosc 84, 834–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Protano M‐A, Xu H, Wang G, Polydorides AD, Dawsey SM, Cui J, Xue L, Zhang F, Quang T, Pierce MCet al. (2015) Low‐cost high‐resolution microendoscopy for the detection of esophageal squamous cell neoplasia: an international trial. Gastroenterology 149, 321–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shin D, Protano M‐A, Polydorides AD, Dawsey SM, Pierce MC, Kim MK, Schwarz RA, Quang T, Parikh N, Bhutani MSet al. (2015) Quantitative analysis of high‐resolution microendoscopic images for diagnosis of esophageal squamous cell carcinoma. Clin Gastroenterol Hepatol 13, 272–279.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shin D, Lee MH, Polydorides AD, Pierce MC, Vila PM, Parikh ND, Rosen DG, Anandasabapathy S & Richards‐Kortum RR (2016) Quantitative analysis of high‐resolution microendoscopic images for diagnosis of neoplasia in patients with Barrett's esophagus. Gastrointest Endosc 83, 107–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Keahey P, Ramalingam P, Schmeler K & Richards‐Kortum RR (2016) Differential structured illumination microendoscopy for in vivo imaging of molecular contrast agents. Proc Natl Acad Sci USA 113, 10769–10773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tang Y, Kortum A, Parra SG, Vohra I, Milbourne A, Ramalingam P, Toscano PA, Schmeler KM & Richards‐Kortum RR (2020) In vivo imaging of cervical precancer using a low‐cost and easy‐to‐use confocal microendoscope. Biomed Opt Express 11, 269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liang W, Hall G, Messerschmidt B, Li MJ & Li X (2017) Nonlinear optical endomicroscopy for label‐free functional histology in vivo . Light Sci Appl 6, e17082–e17087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dilipkumar A, Al‐Shemmary A, Kreiß L, Cvecek K, Carlé B, Knieling F, Gonzales Menezes J, Thoma O‐M, Schmidt M, Neurath MFet al. (2019) Label‐free multiphoton endomicroscopy for minimally invasive in vivo imaging. Adv Sci 6, 1801735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Schlachter SC, Kang D, Gora MJ, Vacas‐Jacques P, Wu T, Carruth RW, Tearney GJ, Wilsterman EJ, Bouma BE, Woods K & et al. (2013) Spectrally encoded confocal microscopy of esophageal tissues at 100 kHz line rate. Biomed Opt Express 4, 1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kang D, Do D, Ryu J, Grant CN, Giddings SL, Rosenberg M, Hesterberg PE, Yuan Q, Garber JJ, Katz AJet al. (2019) A miniaturized, tethered, spectrally‐encoded confocal endomicroscopy capsule. Lasers Surg Med 51, 452–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wan Q‐S, Wang T & Zhang K‐H (2017) Biomedical optical spectroscopy for the early diagnosis of gastrointestinal neoplasms. Tumor Biol 39, 101042831771798. [DOI] [PubMed] [Google Scholar]
- 108.Almond LM, Hutchings J, Lloyd G, Barr H, Shepherd N, Day J, Stevens O, Sanders S, Wadley M, Stone N (2014) Endoscopic Raman spectroscopy enables objective diagnosis of dysplasia in Barrett's esophagus. Gastrointest Endosc 79, 37–45. [DOI] [PubMed] [Google Scholar]
- 109.Sharma N, Takeshita N & Ho KY (2016) Raman spectroscopy for the endoscopic diagnosis of esophageal, gastric, and colonic diseases. Clin Endosc 49, 404–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Douplik A, Zanati S, Saiko G, Streutker C, Loshchenov M, Adler D, Cho S, Chen D, Cirocco M, Marcon Net al. (2014) Diffuse reflectance spectroscopy in Barrett's esophagus: developing a large field‐of‐view screening method discriminating dysplasia from metaplasia. J Biophotonics 7, 304–311. [DOI] [PubMed] [Google Scholar]
- 111.Lombardini A, Mytskaniuk V, Sivankutty S, Andresen ER, Chen X, Wenger J, Fabert M, Joly N, Louradour F, Kudlinski Aet al. (2018) High‐resolution multimodal flexible coherent Raman endoscope. Light Sci Appl 7, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Harmsen S, Rogalla S, Huang R, Spaliviero M, Neuschmelting V, Hayakawa Y, Lee Y, Tailor Y, Toledo‐Crow R, Kang JWet al. (2019) Detesction of premalignant gastrointestinal lesions using surface‐enhanced resonance raman scattering‐nanoparticle endoscopy. ACS Nano 13, 1354–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Klenske E, Neurath MF, Atreya R & Rath T (2018) Molecular imaging in gastroenterology: a route for personalized endoscopy. Dig Liver Dis 50, 878–885. [DOI] [PubMed] [Google Scholar]
- 114.Bird‐Lieberman EL, Neves AA, Lao‐Sirieix P, O'Donovan M, Novelli M, Lovat LB, Eng WS, Mahal LK, Brindle KM & Fitzgerald RC (2012) Molecular imaging using fluorescent lectins permits rapid endoscopic identification of dysplasia in Barrett's esophagus. Nat Med 18, 315–321. [DOI] [PubMed] [Google Scholar]
- 115.Neves AA, Di Pietro M, O'Donovan M, Waterhouse DJ, Bohndiek SE, Brindle KM & Fitzgerald RC (2018) Detection of early neoplasia in Barrett's esophagus using lectin‐based near‐infrared imaging: an ex vivo study on human tissue. Endoscopy 50, 618–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sturm MB, Joshi BP, Lu S, Piraka C, Khondee S, Elmunzer BJ, Kwon RS, Beer DG, Appelman HD, Turgeon DKet al. (2013) Targeted imaging of esophageal neoplasia with a fluorescently labeled peptide: first‐in‐human results. Sci Transl Med 5, 184ra61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Liu J, Zuo X, Li C, Yu T, Gu X, Zhou C, Li Z, Goetz M, Kiesslich R &Li Y (2013) In vivo molecular imaging of epidermal growth factor receptor in patients with colorectal neoplasia using confocal laser endomicroscopy. Cancer Lett 330, 200–207. [DOI] [PubMed] [Google Scholar]
- 118.Burggraaf J, Kamerling IMC, Gordon PB, Schrier L, De Kam ML, Kales AJ, Bendiksen R, Indrevoll B, Bjerke RM, Moestue SAet al. (2015) Detection of colorectal polyps in humans using an intravenously administered fluorescent peptide targeted against c‐Met. Nat Med 21, 955–961. [DOI] [PubMed] [Google Scholar]
- 119.Joshi BP, Dai Z, Gao Z, Lee JH, Ghimire N, Chen J, Prabhu A, Wamsteker EJ, Kwon RS, Elta GHet al. (2017) Detection of sessile serrated adenomas in the proximal colon using wide‐field fluorescence endoscopy. Gastroenterology 152, 1002–1013.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ahmed S, Strand S, Weinmann‐Menke J, Urbansky L, Galle PR & Neumann H (2018) Molecular endoscopic imaging in cancer. Dig Endosc 30, 719–729. [DOI] [PubMed] [Google Scholar]
- 121.Alagappan M, Brown JRG, Mori Y & Berzin TM (2018) Artificial intelligence in gastrointestinal endoscopy: the future is almost here. World J Gastrointest Endosc 10, 239–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gulati S, Patel M, Emmanuel A, Haji A, Hayee B & Neumann H (2019) The future of endoscopy: advances in endoscopic image innovations. Dig Endosc 32, 512–522. [DOI] [PubMed] [Google Scholar]
- 123.Tajbakhsh N, Gurudu SR & Liang J (2016) Automated polyp detection in colonoscopy videos using shape and context information. IEEE Trans Med Imaging 35, 630–644. [DOI] [PubMed] [Google Scholar]
- 124.Misawa M, Kudo S, Mori Y, Cho T, Kataoka S, Yamauchi A, Ogawa Y, Maeda Y, Takeda K, Ichimasa Ket al. (2018) Artificial intelligence‐assisted polyp detection for colonoscopy: initial experience. Gastroenterology 154, 2027–2029.e3. [DOI] [PubMed] [Google Scholar]
- 125.Wang P, Berzin TM, Glissen Brown JR, Bharadwaj S, Becq A, Xiao X, Liu P, Li L, Song Y, Zhang Det al. (2019) Real‐time automatic detection system increases colonoscopic polyp and adenoma detection rates: a prospective randomised controlled study. Gut 68, 1813–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kominami Y, Yoshida S, Tanaka S, Sanomura Y, Hirakawa T, Raytchev B, Tamaki T, Koide T, Kaneda K & Chayama K (2016) Computer‐aided diagnosis of colorectal polyp histology by using a real‐time image recognition system and narrow‐band imaging magnifying colonoscopy. Gastrointest Endosc 83, 643–649. [DOI] [PubMed] [Google Scholar]
- 127.Byrne MF, Chapados N, Soudan F, Oertel C, Pérez ML, Kelly R, Iqbal N, Chandelier F & Rex DK (2019) Real‐time differentiation of adenomatous and hyperplastic diminutive colorectal polyps during analysis of unaltered videos of standard colonoscopy using a deep learning model. Gut 68, 94–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hirasawa T, Aoyama K, Tanimoto T, Ishihara S, Shichijo S, Ozawa T, Ohnishi T, Fujishiro M, Matsuo K, Fujisaki Jet al. (2018) Application of artificial intelligence using a convolutional neural network for detecting gastric cancer in endoscopic images. Gastric Cancer 21, 653–660. [DOI] [PubMed] [Google Scholar]
- 129.Kanesaka T, Lee TC, Uedo N, Lin KP, Chen HZ, Lee JY, Wang H‐P & Chang HT (2018) Computer‐aided diagnosis for identifying and delineating early gastric cancers in magnifying narrow‐band imaging. Gastrointest Endosc 87, 1339–1344. [DOI] [PubMed] [Google Scholar]
- 130.Horie Y, Yoshio T, Aoyama K, Yoshimizu S, Horiuchi Y, Ishiyama A, Hirasawa T, Tsuchida T, Ozawa T, Ishihara Set al. (2019) Diagnostic outcomes of esophageal cancer by artificial intelligence using convolutional neural networks. Gastrointest Endosc 89, 25–32. [DOI] [PubMed] [Google Scholar]
- 131.Swager AF, van der Sommen F, Klomp SR, Zinger S, Meijer SL, Schoon EJ, Bergman JJGHM, de With PH& Curvers WL (2017) Computer‐aided detection of early Barrett's neoplasia using volumetric laser endomicroscopy. Gastrointest Endosc 86, 839–846. [DOI] [PubMed] [Google Scholar]
- 132.Tang Y, Polydorides AD, Anandasabapathy S & Richards‐Kortum RR (2018) Quantitative analysis of in vivo high‐resolution microendoscopic images for the detection of neoplastic colorectal polyps. J Biomed Optics 23, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ştefănescu D, Streba C, Cârţână ET, Săftoiu A, Gruionu G & Gruionu LG (2016) Computer aided diagnosis for confocal laser endomicroscopy in advanced colorectal adenocarcinoma. PLoS One 11, e0154863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mori Y, Kudo SE, Wakamura K, Misawa M, Ogawa Y, Kutsukawa M, Kudo T, Hayashi T, Miyachi H, Ishida Fet al. (2015) Novel computer‐aided diagnostic system for colorectal lesions by using endocytoscopy (with videos). Gastrointest Endosc 81, 621–629. [DOI] [PubMed] [Google Scholar]
- 135.Kudo S, Misawa M, Mori Y, Hotta K, Ohtsuka K, Ikematsu H, Saito Y, Takeda K, Nakamura H, Ichimasa Ket al. (2020) Artificial intelligence‐assisted system improves endoscopic identification of colorectal neoplasms. Clin Gastroenterol Hepatol 18, 1874–1881.e2. [DOI] [PubMed] [Google Scholar]
- 136.Manfredi MA, Abu Dayyeh BK, Bhat YM, Chauhan SS, Gottlieb KT, Hwang JH, Komanduri S, Konda V, Lo SK, Maple JTet al. (2015) Electronic chromoendoscopy. Gastrointest Endosc 81, 249–261. [DOI] [PubMed] [Google Scholar]
- 137.Gora MJ, Sauk JS, Carruth RW, Gallagher KA, Suter MJ, Nishioka NS, Kava LE, Rosenberg M, Bouma BE & Tearney GJ (2013) Tethered capsule endomicroscopy enables less invasive imaging of gastrointestinal tract microstructure. Nat Med 19, 238–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Trindade AJ, Navaneethan U, Aslanian HR, Bhutani MS, Krishnan K, Lichtenstein DR, Melson J, Pannala R, Parsi MA, Schulman ARet al. (2019) Advances in the diagnosis and surveillance of Barrett's esophagus (with videos). Gastrointest Endosc 90, 325–334. [DOI] [PubMed] [Google Scholar]
- 139.Chauhan SS, Abu Dayyeh BK, Bhat YM, Gottlieb KT, Hwang JH, Komanduri S, Konda V, Lo SK, Manfredi MA, Maple JTet al. (2014) Confocal laser endomicroscopy. Gastrointest Endosc 80, 928–938. [DOI] [PubMed] [Google Scholar]
- 140.Bhat YM, Abu Dayyeh BK, Chauhan SS, Gottlieb KT, Hwang JH, Komanduri S, Konda V, Lo SK, Manfredi MA, Maple JTet al. (2014) High‐definition and high‐magnification endoscopes. Gastrointest Endosc 80, 919–927. [DOI] [PubMed] [Google Scholar]
- 141.Rodriguez SA, Banerjee S, Desilets D, Diehl DL, Farraye FA, Kaul V, Kwon RS, Mamula P, Pedrosa MC, Varadarajulu Set al. (2010) Ultrathin endoscopes. Gastrointest Endosc 71, 893–898. [DOI] [PubMed] [Google Scholar]
- 142.Wang A, Banerjee S, Barth BA, Bhat YM, Chauhan S, Gottlieb KT, Konda V, Maple JT, Murad F, Pfau PRet al. (2013) Wireless capsule endoscopy. Gastrointest Endosc 78, 805–815. [DOI] [PubMed] [Google Scholar]
- 143.Neumann H, Fuchs FS, Vieth M, Atreya R, Siebler J, Kiesslich R & Neurath MF (2011) Review article: in vivo imaging by endocytoscopy. Aliment Pharmacol Ther 33, 1183–1193. [DOI] [PubMed] [Google Scholar]
- 144.Gora MJ, Suter MJ, Tearney GJ & Li X (2017) Endoscopic optical coherence tomography: technologies and clinical applications [Invited]. Biomed Opt Express 8, 2405. [DOI] [PMC free article] [PubMed] [Google Scholar]


