Successful establishment of a tumour relies on a cascade of interactions between cancer cells and stromal cells within an evolving microenvironment. Both immune and nonimmune cellular components have key roles in this process. Here, we review the major anti‐ and pro‐tumour factors in the early tumour microenvironment and discuss how understanding this process may be exploited in the clinic.

Keywords: cancer‐associated fibroblast, extracellular matrix, immune, malignant transformation, stroma, tumour microenvironment
Abstract
Successful establishment of a tumour relies on a cascade of interactions between cancer cells and stromal cells within an evolving microenvironment. Both immune and nonimmune cellular components are key factors in this process, and the individual players may change their role from tumour elimination to tumour promotion as the microenvironment develops. While the tumour–stroma crosstalk present in an established tumour is well‐studied, aspects in the early tumour or premalignant microenvironment have received less attention. This is in part due to the challenges in studying this process in the clinic or in mouse models. Here, we review the key anti‐ and pro‐tumour factors in the early microenvironment and discuss how understanding this process may be exploited in the clinic.
Abbreviations
- Arg1
arginase 1
- ATRA
all‐trans retinoic acid
- CAF
cancer‐associated fibroblast
- CC(L)
C‐C motif chemokine (ligand)
- CD
cluster of differentiation
- Chi3L1
chitinase‐3‐like protein 1
- CRC
colorectal cancer
- CXCL
C‐X‐C motif chemokine
- DAMPs
damage‐associated molecular patterns
- DCs
dendritic cells
- ECM
extracellular matrix
- EGF
epidermal growth factor
- FAP
fibroblast activation protein
- FGF(R)
fibroblast growth factor (receptor)
- FSP1
fibroblast‐specific protein 1
- Gas6
growth arrest‐specific 6
- G‐CSF
granulocyte colony‐stimulating factor
- GDF‐15
growth differentiation factor‐15
- HGF
hepatocyte growth factor
- HLA
human leucocyte antigen
- HMGB1
high‐mobility group protein 1
- HPV
human papillomavirus
- ICAM‐1
intercellular adhesion molecule‐1
- ICPI
immune checkpoint inhibitors
- IDO
indoleamine dioxygenase
- IFN
interferon
- IGF
insulin‐like growth factor
- IL
interleukin
- ILCs
innate lymphoid cells
- JAM2
junctional adhesion molecule 2
- KPC
KrasLSL.G12D/+; p53R172H/+; PdxCretg/+
- LAIR‐1
leucocyte‐associated immunoglobulin‐like receptor‐1
- LIF
leukaemia inhibitory factor
- LOX(L)
lysyl oxidase (‐like proteins)
- MAP(K)
mitogen‐activated protein (kinase)
- M‐CSF
macrophage colony‐stimulating factor
- MDSCs
myeloid‐derived suppressor cells
- MHC
major histocompatibility complex
- MICA/B
MHC class I‐related proteins A/B
- miR‐
microRNA‐
- MMP
matrix metalloproteinase
- MMTV‐PyM
mouse mammary tumour virus‐polyoma middle tumour antigen
- MYL
myosin light chain
- NET
neutrophil extracellular trap
- NF‐kB
nuclear factor kappa‐light‐chain‐enhancer of activated B cells
- NKG2D
natural killer group 2, member D
- NKs
natural killer cells
- NKTs
natural killer T cells
- NOS
nitric oxide synthase
- OAC
oesophageal adenocarcinoma
- op/op
osteoporotic
- OPN
osteopontin
- P4HA1
prolyl 4‐hydroxylase a‐subunit isoform 1
- PBMC
peripheral blood mononuclear cells
- PD‐(L)1
programmed dead‐(ligand)1
- PDAC
pancreatic ductal adenocarcinoma
- PDGF(R)
platelet‐derived growth factor (receptor)
- PDX
patient‐derived xenograft
- PGE2
prostaglandin E2
- PLOD‐1, PLOD‐3
procollagen‐lysine 2‐oxoglutarate 5‐dioxygenase 1 and 3
- PSC
pancreatic stellate cells
- ROS
reactive oxygen species
- SASP
senescence‐associated secretory phenotype
- SHH
sonic hedgehog
- SMA
smooth‐muscle actin
- SPARC
secreted protein acidic and rich in cysteine
- STAT
signal transducer and activator of transcription
- TAM
tumour‐associated macrophage
- TAN
tumour‐associated neutrophils
- TAZ
transcriptional coactivator with PDZ‐binding motif
- TCR
T‐cell receptor
- TGF‐β
transforming growth factor beta
- TH
T helper
- TKI
tyrosine kinase inhibitor
- TLR
toll‐like receptor
- TNF
tumour necrosis factor
- TRAIL
TNF‐regulated apoptosis‐inducing ligand
- TRAMP
transgenic adenocarcinoma mouse prostate
- Treg
T regulatory cell
- VEGF
vascular endothelial growth factor
- XCL‐1
X‐C motif chemokine ligand 1
- YAP
Yes‐associated protein
1. Introduction
The stepwise accumulation of genetic changes from healthy tissue through to malignancy, is accompanied by co‐option of neighbouring normal cells to support tumour development and progression. These ‘normal’ cells, which include immune and nonimmune populations, are collectively termed the stroma. Stromal cells and cancer cells collectively form the tumour microenvironment.
While much work has focused on the characterisation of oncogenic mutations within epithelial cells from the point of cancer initiation, the acquisition of mutations alone is not always sufficient to give rise to cancer, as the majority of mutagenic cells die or senesce [1, 2, 3]. Indeed, many premalignant lesions already bear a significant mutational burden; however, only a minority go on to develop cancer [4, 5, 6, 7] and considerable lag times from these initial mutations to tumour development have been reported [8]. This implies a role for the surrounding stromal environment from the point of cancer initiation, where non‐cell‐autonomous environmental cues provide a niche permissive for malignant transition.
With our increasing appreciation of the complexities of the tumour microenvironment, the concept of exploiting the stroma for therapeutic benefit is becoming more attractive. Thus, whether the characteristics of an evolving microenvironment can be exploited towards early diagnosis and patient stratification approaches in early stages of carcinogenesis remain‐ key questions to be addressed. In this review, we examine the state of our understanding of components within the microenvironment and their contribution to malignant transition. While immune components have been the subject of the most intense research, nonimmune constituents are now emerging as critical players, and here, we attempt to emphasise their role, highlighting emerging strategies to facilitate early detection.
2. Immune cell populations of the evolving tumour microenvironment: losing control
The recognition and elimination of genetically distinct transformed cells by the immune system (immunosurveillance) [9] represents a major barrier to the progression of early cancers. This is evident from numerous studies reporting a higher incidence of cancer in immunocompromised individuals [10, 11, 12, 13, 14] and in mice lacking key cytotoxic components of the immune systems such as IFN‐γ, perforin and natural killer cells (NK cells) [15, 16]. The potential of the immune system to eliminate established tumours in humans has been well‐demonstrated by the effectiveness of immune checkpoint inhibitors (ICPIs). Therefore, the immune system's role in interacting with virtually all nonimmune components of the TME as well as mediating signalling beyond the physical confines of the TME to the tumour macroenvironment has led to great interest in the role of the immune system in early tumorigenesis [17].
The presence of immune cells in tumours capable of recognising mutated cancer cells raises several important questions, which until now remain a significant challenge in oncology: How do cancer cells avoid destruction by the immune system? How is immune control lost? And when during tumorigenesis is this control lost?
2.1. Adaptive immunity in malignant transformation
T lymphocytes are key determinants in tumour fate. Elevated CD8 infiltration and high CD8:Treg ratios in established tumours are associated with favourable long‐term prognosis [18, 19, 20, 21]. In early lesions experiencing active immune surveillance, tumour neoantigens generated as a result of genetic instability are presented on MHC, alerting T cells to the presence of cancer cells leading to rapid elimination [22, 23, 24, 25, 26]. Studies using carcinogen‐driven murine models have shown that the premalignant microenvironment is significantly more immune stimulatory than established tumour microenvironments, supporting potent T‐cell activation [27, 28]. In genetically engineered mouse models, DuPage et al. [22] elegantly demonstrated antigen‐dependent accumulation of T cells was sufficient to delay malignant lung tumour progression. Premalignant lesions have increased numbers of T cells compared with fully developed invasive lesions, and those with lower infiltration exhibit higher incidence of progression to cancer, indicating a key relationship between T cells and the epithelium in this stepwise process [28, 29, 30, 31, 32, 33].
However, this is in stark contrast to established tumours which frequently present with a paucity of tumour‐infiltrating lymphocytes exhibiting an impaired cytotoxic capacity. Established tumours escape immune attack through four major mechanisms. First, mutation of constituents of the antigen presentation pathway reduces neoantigen presentation rendering cancer cells ‘invisible’ to T cells. Indeed, 40% of human non‐small‐cell lung cancers exhibit defects in human leucocyte antigens (HLAs), reducing antigen presentation and promoting immune escape [34]. Second, functional T cells eliminate immunogenic clones promoting the development of less heterogeneous, less immunogenic tumours in the process, termed immunoediting. In murine sarcoma, antigen‐specific T cells present in early lesions were critical for immunoediting to permit outgrowth of less immunogenic clones [35]. Third, tumours induce a state of dysfunction in infiltrating T cells often termed ‘immune exhaustion’. DuPage et al. [22] showed that despite an initially effective T‐cell response to delay lung tumour progression, T cells did not persist and exhibited a decreased cytotoxicity, despite continued antigen exposure. Indeed, it has been shown that persistent antigen exposure is a critical trigger for initiation of the T‐cell exhaustion signature [36, 37], which is increasingly appreciated as a graded spectrum of inactivity with its origins in the early TME. In other models examining loss of T‐cell function in premalignant lesions, increases in PD‐1/PD‐L1 expression, Treg diversity and numbers of circulating Tregs were indicative of T‐cell dysfunction [31, 38, 39]. Fourth, established tumours develop an immune‐suppressive microenvironment which protects them from T‐cell‐mediated immunity. This has been historically demonstrated by the phenomenon of ‘concomitant immunity’. Tumour formation is induced in a mouse. Following excision of the tumour, the same mouse demonstrates immune‐mediated resistance to rechallenge with cells from its own tumour. This is an elegant demonstration that the immune system may eliminate isolated tumour cells, but once the microenvironment is formed the tumour is protected from circulating immunity [40]. Within the evolving TME, transforming growth factor beta (TGF‐β), epithelial or immune derived, can further amplify loss of T‐cell‐mediated immune surveillance [41, 42]. Tregs suppress T‐cell effector functions [43, 44], downregulating genes involved in the cytotoxic function of CD8 T cells including granzyme B and IFN‐γ, ultimately tipping the immune balance towards tolerance and facilitating immune evasion. TGF‐β is key to the initiation of regulatory programmes and differentiation of CD4 T cells into Tregs [41, 45, 46, 47, 48]. Interestingly, during malignant transition TGF‐β operates in a context‐specific manner, playing opposing roles, initially functioning as a tumour suppressor capable of inducing apoptosis in premalignant cells before behaving as a potent immune suppressor, reviewed extensively elsewhere [49, 50, 51]. The wider immune functions of other microenvironment components are discussed in more detail later in this review. Thus, the perturbation of T‐cell functionality in premalignant lesions does not occur in isolation, but via crosstalk with other constituents of the immune milieu.
Collectively, these reports add to a growing body of evidence suggesting that while T cells are essential for immune control of premalignant lesions, loss of T‐cell‐mediated immune surveillance may occur prior to malignant transition and that immune involvement actively promotes progression to cancer [31, 38, 52].
2.2. Innate modulation of the transforming tumour microenvironment
Although critical fate determinants, T cells do not operate in isolation, engaging in extensive crosstalk with other immune cells within the developing microenvironment [32]. The innate immune system, comprising neutrophils, NK cells, innate lymphoid cells (ILCs), dendritic cells (DCs) and macrophages, functions primarily as a rapid response to pathogens. In the tumour context, an initially protective response switches to promote carcinogenesis. While directly contributing to tumorigenesis by selecting immunogenic clones, the innate immune compartment ultimately shapes the formation of a tumour microenvironment providing inflammatory cues capable of controlling T‐cell fate, modulating mutated cancer cells themselves and modifying underlying tissues.
2.2.1. Macrophages
Of all immune cells, macrophages are the most abundant and display arguably the highest plasticity, responding rapidly to a diverse array of environmental stimuli. Activated pro‐inflammatory (M1) and anti‐inflammatory (wound healing, M2) populations have been described extensively [53, 54]. However, in the context of a tumour, M1 and M2 nomenclatures represent a grossly oversimplified classification, with macrophages existing along a spectrum of phenotypes from pro‐ to anti‐inflammatory depending on the localised cues they receive [55, 56, 57, 58, 59]. Indeed, reflecting the breadth of diversity of tumour‐derived cues, studies have described macrophages expressing both markers typical of M1 and M2 phenotypes, and traits overlapping with myeloid‐derived suppressor cells (MDSCs). Heterogeneity within the microenvironment of a developing tumour further amplifies intratumoral macrophage diversity and diversity across cancer types.
In very early stages of tumour development, enhanced numbers of macrophages have been observed [32, 60], and those recruited exhibit a pro‐inflammatory phenotype, activated by host factors such as DAMPs and tumour cell DNA [24, 61, 62]. Such infiltrates contribute to immune surveillance and are capable of directly eliminating immunogenic tumour cell clones via release of toxic mediators such as TNF, interleukin‐2 (IL‐2), reactivate nitrogen and oxygen species [63, 64, 65, 66, 67]. This behaviour is reminiscent of their role in infection, phagocytosing and presenting tumour‐derived antigen to incoming CD8 T cells [68, 69, 70, 71, 72]. Moreover, augmenting these effects, macrophage‐derived IL‐1 activates surveilling innate and adaptive immune cells to inhibit malignant progression. As occurs for T‐cell surveillance, tumours evolve strategies to evade immune control. Recent work has identified a mechanism used by neoplastic cells to avoid macrophage‐mediated immune surveillance during the early stages of tumorigenesis. Epithelial‐derived GDF‐15 suppressed macrophage cytotoxic activity by inhibiting TNF and NO production in an NF‐κB‐dependent manner [73]. Therefore, in response to a rapidly adapting cytokine milieu within developing lesions including TGF, IL‐10, IL‐4 and M‐CSF, macrophages acquire a tumour‐promoting, immune‐suppressive phenotype, remodelling the microenvironment in the process [74, 75, 76, 77].
However, these antitumour effects of very early lesions are replaced by a tumour‐promoting phenotype at an early stage of carcinogenesis. This requirement for macrophages in malignant progression was elegantly demonstrated by Lin et al. who crossed MMTV‐PyMT mice, which develop breast tumours spontaneously, with osteoporotic (op/op) mice lacking CSF‐1, a cytokine critical for macrophage function. While they observed little impact on the development of premalignant lesions, progression to invasive carcinoma was significantly impaired [76, 78].
A key feature of once antitumour, inflammatory macrophages is the acquisition of immune‐suppressive traits to facilitate malignant progression [60, 76, 79, 80]. To support immune suppression, and thus evasion of early immune surveillance, macrophages use both direct and indirect mechanisms. Expression of immune checkpoints such as PD‐L1 inhibits T‐cell function, and release of suppressive cytokines, metabolites and proteases has far‐reaching effects on the immune compartment [81, 82]. Pro‐inflammatory cytokines such as IL‐12 are replaced with the secretion of IL‐10 [82, 83], Indoleamine dioxygenase (IDO) [20, 84] and TGF‐β [42, 49, 82, 85, 86], which together impair T‐cell effector activity [82, 87, 88] and promote induction of Treg phenotypes [47, 89]. CCL22 recruits Tregs and along with CCL5 and CCL17 can inhibit T‐cell proliferation [90, 91]. Similarly, IL‐10 and TGF‐β inhibit DC maturation, reducing antigen‐presenting capacity and downstream T‐cell responses.
Macrophages within the premalignant microenvironment may also elicit tumour‐promoting activities beyond influencing immune cells, either via direct effects on epithelial cells or by indirect effects on other nonimmune stromal populations. There are several direct epithelial mechanisms. First, factors such as ROS and NOS also induce DNA damage contributing to genomic instability and mutational burden [74, 75, 92, 93, 94]. Second, the release of cytokines and growth factors such as EGF stimulates epithelial proliferation [95, 96, 97, 98]. In both chronic inflammation and Kras‐driven models of pancreatic cancer, macrophages were shown to drive metaplasia of acinar to ductal cells. Pancreatic acinar cells with oncogenic KRASG12D upregulated the expression of ICAM‐1 to recruit macrophages, which through matrix metalloproteinase‐9 (MMP9) and TNF‐α production induced metaplasia, the earliest abnormal pancreatic lesions [60]. Also in pancreatic cancer, reshaping of the premalignant microenvironment to a PDAC phenotype has been reported to require early recruitment of macrophages through CCL9 and CCL2 [99]. Macrophage‐derived Gas6 has also recently been described as a critical regulator for the transition between premalignant breast cancer and invasive breast cancer [100]. Signalling via Axl on premalignant epithelial cells, having engaged with macrophage‐derived Gas6, induced downstream survival signals and concurrent loss of E‐cadherin.
Beyond their direct influence on epithelial cells, as for any organ, an oxygen supply needs to be established to support an expanding epithelial compartment in hyperplastic lesions [101, 102, 103]. Here, macrophages, including a subset expressing Tie2, contribute to the angiogenic switch and function as potent inducers of angiogenesis via production of diverse pro‐angiogenic factors VEGF, TNF‐α, IL‐8, MMP9 and FGF‐2 [104, 105, 106, 107, 108, 109]. These features have been demonstrated in transgenic mouse models which recapitulate the very early neoplastic lesions. In an inducible mouse model of preneoplastic changes in the mammary epithelium, macrophage recruitment was necessary for epithelial proliferation and induction of angiogenesis [110]. Similarly, in mice null for CSF1, delayed progression from premalignant to invasive lesions was due a failure to induce angiogenesis by macrophages [78, 111], a process dependent on MMP9 [112]. This was true also for intestinal tumorigenesis [113]. Moreover, macrophage‐derived MMP9 plays a pivotal role in tumour angiogenesis, regulating the bioavailability of VEGF [114, 115].
Macrophages are also involved in remodelling of the underlying tissues. The extracellular matrix (ECM), a 3D network composed of polysaccharides, proteins including collagens and glycoproteins, provides biochemical and biomechanical cues critical for tumour progression [116, 117]. Macrophages are frequently observed within collagen‐rich areas of developing tumours [118] where they are implicated in the deposition of matrix [86, 119, 120, 121, 122, 123, 124], fibril organisation [119, 125, 126, 127], cross‐linking (stiffening the ECM) and degradation to release growth factors and support cell motility [120, 128]. Here, they have been shown to upregulate expression of the matricellular glycoproteins, osteopontin, osteoactivin, fibronectin, collagen types I VI and XIV and secreted protein acidic and rich in cysteine (SPARC) needed for the assembly and organisation of collagenous matrix [119, 129, 130, 131]. Macrophages produce enzymes to modify collagen assembly such as prolyl 4‐hydroxylase a‐subunit isoform 1 (P4HA1) and procollagen‐lysine 2‐oxoglutarate 5‐dioxygenase 1 and 3 (PLOD‐1, PLOD‐3) [119], and modify secreted structure via expression of lysyl oxidase (LOX) and lysyl oxidase‐like (LOXL) proteins [126], supporting matrix stiffening needed for changes in epithelial morphology (discussed in detail later).
2.2.2. ILCs and NK cells
Within the early cancer setting, ILCs are extremely efficient at eliminating malignant cells through the expression of perforin and granzyme, as well as death ligands, such as TNF‐regulated apoptosis‐inducing ligand (TRAIL) and Fas ligand [132, 133, 134] ILCs comprise the cytotoxic NK cells, and the more recently discovered ‘helper’ ILC subsets. NK cells are key participants in immune surveillance of early lesions, ‘recognising’ malignant cells by the absence of MHC I on cancer cells evading cytotoxic T cells [135], and hence, their presence is associated with good prognosis. Evidence supporting this antitumour role came from studies showing increased cancer incidence in mice and humans lacking NK cells [136, 137], and increased cancer risk in patients with low NK activity [138].
A fluctuating balance of activating and inhibitory receptor–ligand interactions on NK cells controls their activity. The most interesting of these, from a tumour immunology perspective, has been the natural killer group 2, member D (NKG2D) receptor, which can recognise MHC class I‐related proteins A and B (MICA and MICB), ectopically expressed antigens such as Rae1 or H60, as well as DNA damage‐induced cell surface ligands [139, 140], facilitating tumour cell elimination [140, 141, 142]. Cytokines including IFN‐γ, IL‐12, IL‐18 and IL‐15, and the cell surface adhesion molecule LFA‐1 within the TME also potently activate NK cells [143, 144]. Activated NK cells produce TNF, IFN‐γ or CSF‐2, modulating leucocyte function to amplify antitumour activity, and also express chemokines, such as CCL5 and XCL1 to recruit DCs [145, 146]. As the TME establishes, however, NK cells become much less effective at killing their targets, exhibiting decreased cytotoxicity and inflammatory cytokine production. To evade destruction by NK cells, tumours employ both direct and indirect mechanisms, including coating themselves in collagen and platelets to shield themselves from NK detection [147, 148]. Cytokines also add to their newly acquired suppressive phenotype, weakening cytotoxic capacity and arresting T‐cell proliferation [145, 149, 150, 151, 152].
The role of the other ILC populations is poorly understood and varies depending on the local microenvironment, with both immune‐protective and tumour‐promoting effects demonstrated in lung, skin and colon cancer [153, 154, 155]. ILCs are largely tissue‐resident and divided into ILC1, ILC2 and ILC3 subsets functionally corresponding to the TH1, TH2 and TH17 subsets of CD4+ T cells – however this is a considerable simplification, and the full nature of these subsets' functions is still under investigation. Analogous to NK cells, ILC1s and ILC3s are also activated by IL‐15, and contribute to immune surveillance through the release of TNF, IL‐8 and IL‐2, promoting leucocyte recruitment and proliferation [156, 157]. During malignant progression, however, within an increasingly TGF‐β‐rich environment, NKs have been reported to convert into ILC1‐like cells with a reduced ability to control tumour growth [145, 149]. ILC3s have also been shown to drive epithelial proliferation in a IL‐22‐dependent manner [158].
Another T‐cell population with innate‐like properties are the natural killer T cells (NKTs). These are a unique population of T cells that recognise lipid antigens presented on the class I‐like molecule C1d. ‘Type I’ NKTs use a semi‐invariant TCR made of a specific alpha chain with a small number of beta chains and respond in vitro to the lipid alpha Gal‐Cer. Mice lacking type I NKTs have generally been found to have defects in tumour immune surveillance and there is interest in using alpha Gal‐Cer to stimulate antitumour immunity, though results in patients have been limited. However, in a transgenic model of intestinal polyps (‘APC min’ mouse), type I NKTs were found to promote polyp formation by recruitment of Tregs. ‘Type II’ NKTs (or ‘nonclassical’ NKTs) use diverse alpha and beta TCR chains; in contrast to type I, mouse experiments suggest they have an immune‐regulatory, tumour‐promoting function. Like the ILCs, the importance of NKTs in early tumorigenesis is not well‐understood.
2.2.3. Neutrophils
Neutrophils operate as vital first responders during infection. Given this critical early role in infection, it follows that neutrophils also contribute extensively to early tumorigenesis, with anti‐ and pro‐tumour activities reported. As for macrophages, this may in part be a consequence of their functional plasticity. Indeed, the phenotype of neutrophils in the TME depends on tumour type and stage. In early tumorigenesis, neutrophils are recruited by epithelial‐ and stroma‐derived chemokines such as G‐CSF, CXCL8, CXCL1, CXCL2, CXCL3 and CXCL5 [159, 160, 161]. Here, they elicit antitumour activity through the release of cytotoxic granules, production of TNF‐α, NO, H2O2, TRAIL and IFN‐γ and expression of costimulatory molecules [162, 163, 164]. At this stage, skewed by IFN‐β, tumour‐associated neutrophils (TANs) attract and promote activation of surveilling T cells [164, 165, 166].
However, directly contradicting this antitumour activity, TANs have also been reported to contribute to carcinogenesis from the point of tumour initiation. Local TGF‐β pushes neutrophils towards a tumour‐promoting phenotype [166, 167], inducing genetic instability [168] and MAPK‐dependent proliferation of preneoplastic cells [169, 170] as well as releasing pro‐inflammatory cytokines [97]. In particular, neutrophil‐derived IL‐6 and IL‐11 have been shown to promote cancer cell proliferation and inhibit apoptosis via STAT3 [171]. Now, TANs preferentially recruit Tregs, directly suppressing CD8 T cells via PD‐L1 and indirectly by IL‐8‐driven arginase production [172, 173, 174, 175]. Once established, TANs contribute to shaping of the microenvironment inducing angiogenesis, remodelling of ECM through deposition of neutrophil elastase and MMP release [176, 177, 178, 179]. A further feature of neutrophils is the production of neutrophil extracellular traps (NETs). These extracellular DNA structures are decorated in cytotoxic granules, MMPs and neutrophil elastase, and although a growing body of evidence describing roles in tumour circulation [180], metastatic colonisation [181] and tumour dormancy [182] exists, their role in early stages of primary tumour development remains sparse [183].
2.2.4. Dendritic cells
Dendritic cells are professional antigen‐presenting cells that bridge the innate and adaptive immune compartments, stimulating T cells in an antigen‐specific fashion. In the still inflammatory early TME, proficient DCs effectively present tumour antigen to T cells alongside costimulatory molecules CD40, CD80 and CD86, thereby playing a central role in immune surveillance [67, 184, 185, 186]. Here, their presence is associated with good prognosis [146, 184]. However, exposure to suppressive modulators such as VEGF, IL‐10, TGF‐β and prostaglandin E2 (PGE2) and microenvironmental cues such as accumulation of lipids and decreased pH inhibits DC maturation, decreasing expression of costimulatory modules and hence reducing antigen presentation capacity [82, 187, 188, 189, 190]. This renders DCs less capable of priming T cells, while more prone to promote TH2 and Treg responses [191, 192, 193]. PD‐L1 expression by DC directly suppresses infiltrating CD8 T cells [186, 194]. Cytokine‐induced changes in transcriptional and metabolic pathways further promote a tolerogenic phenotype, stimulating expression of factors such as IDO, Arg1, iNOS and STAT3 [195, 196, 197, 198, 199]. To further complicate matters, with reports of anti‐ and pro‐tumour activities depending on the site examined, our understanding of DCs may be confounded by tissue specificities in addition to stage of development [191, 200].
Thus, although our knowledge is increasing, the role of the immune infiltrate in premalignant tissues and the balance of protection vs. progression remains unclear (Fig. 1). With a large body of correlative data, detailed phenotypic and functional analyses are required. Furthermore, as discussed later in this review, the choice of model to study the microenvironment in carcinogenesis is critical. Of note, correlations between inflammation and progression of premalignant states to cancer are stronger along the gastrointestinal tract than other sites. These include Barrett's oesophagus, Crohn's disease and ulcerative colitis. This raises the questions of whether different anatomical sites exhibit different immune infiltration, whether tissue‐specific responses to inflammation exist, and how these may contribute to progression of precancerous lesions at different sites. The pro‐tumour functions of the innate immune system in established tumours have been particularly well‐studied, but this in part reflects the technical challenges of mechanistic studies of very early lesions.
Fig. 1.
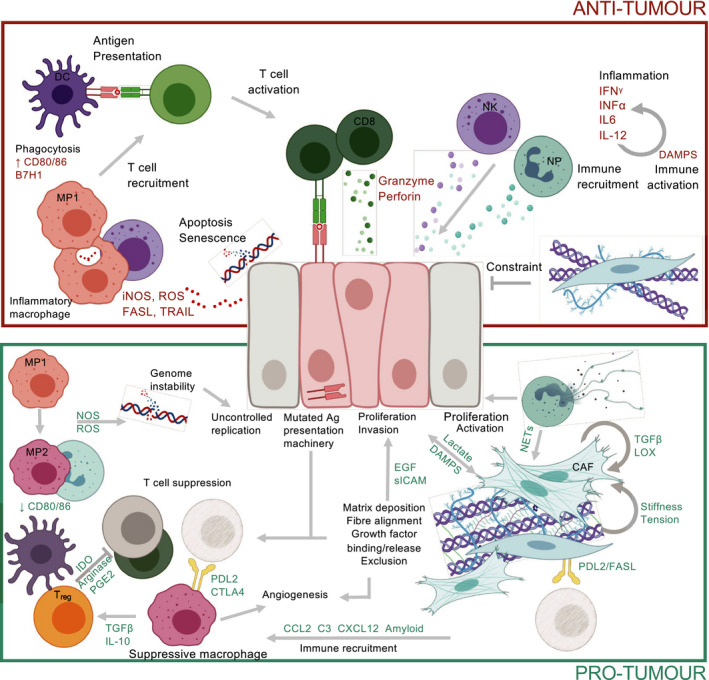
Functions of the early tumour microenvironment. Recruitment and activation of cytotoxic CD8 T cells, NK cells, neutrophils and macrophages result in the elimination of mutated epithelial cells. However, these initial antitumour events driven by immune surveillance give way to a suppressive environment with tumour‐promoting functions. Extensive crosstalk between stromal populations and cancer cells elicit and amplify pro‐tumour functions. These include fibroblast activation and ECM remodelling; induction of the angiogenic switch; release of growth factors and intermediates to support genomic instability and proliferation; immune exclusion; collaboration between immune cells to prevent antitumour responses including impaired antigen presentation, reducing cytotoxic capacity; release of suppressive cytokines; and T‐cell deletion/induction of anergy. CD8, cytotoxic T cell; Treg, regulatory T cell; MP1, inflammatory macrophage; MP2, suppressive macrophage; DC, dendritic cell; NP, neutrophil; NK, natural killer cell.
The mechanisms underlying the transition from early immune surveillance by innate immune cells to tumour‐promoting phenotypes of macrophages, NK cells, neutrophils and DCs, later during tumorigenesis, are poorly understood. This is in part due to the technical challenges of studying very early lesions in a ‘true’ epithelial setting, which requires transgenic mouse models. Much of the evidence for early innate immune surveillance comes from macroscopic observations of increased cancer rates in immune‐suppressed states, rather than direct observation of the premalignant epithelium. One could hypothesise two key reasons for this shift. According to a cancer cell centric view, premalignant lesions that acquire the ability to co‐opt pro‐tumorigenic innate immunity can grow into tumours [201, 202]. Notably, cancer presents a paradox to the standard alarm signal sensors of the immune system: although tumours are genetically different and, thus, capable of promoting antigenic stimulation of T cells, they also release endogenous danger signals known as damage‐associated molecular patterns (DAMPs) to alert the innate immune system. DAMPs tend to generate an innate response compatible with immune regulation, T‐cell suppression and healing. The latter involves the secretion of epithelial growth factors and angiogenic signals which both support tumour growth. Indeed, tumours have been famously likened to ‘wounds that do not heal’ [203]. From a wider point of view, this makes sense, as the innate system has evolved to protect damaged epithelial cells under normal circumstances. Importantly, the inflammatory insult often precedes the premalignant transformation. It is well known that chronically inflamed nonmalignant pathologies such as ulcerative colitis are at high risk of generating malignant lesions, because the inflammatory microenvironment provides an ideal context for premalignant lesions to ‘flourish’ into an invasive cancer [204].
3. Stroma in malignant transformation: landscaping the tumour microenvironment
While the interactions between malignant cells and the immune system play a key role in establishing the tumour microenvironment during malignant transition, other components of the host tissue play a critical and historically overlooked part. Indeed, the accumulation of stromal components including fibroblasts and vessels is also reported in premalignant lesions including oesophageal, pancreatic and skin cancers [205, 206, 207, 208, 209, 210]. Here, we will briefly discuss emerging roles and our current understanding of these populations to carcinogenesis (Fig. 2). Cancer‐associated fibroblasts (CAFs) are one of the most abundant stromal components in the developing TME and are recognised by numerous markers such as fibroblast‐specific protein 1 (FSP1/S100A4), vimentin, α‐smooth‐muscle actin (αSMA), fibroblast activation protein (FAP), PDGF receptor‐α (PDGFR‐α) and podoplanin. CAFs largely originate as a result of the chronic wound‐healing response present in the early TME and display a genomic, epigenomic and secretomic profile distinct from untransformed fibroblasts [211, 212, 213].
Fig. 2.

Fibroblast adaptation in the early tumour microenvironment. Within a rapidly changing environment, local cues either biochemical or biophysical support accumulation and further alterations to an already heterogeneous compartment. With few reports of antitumour activity, most CAF traits reported across known subsets are pro‐tumour. These include release of factors to support cancer cell proliferation and the angiogenic switch, cytokines to modulate immune recruitment, polarisation, suppression and ECM remodelling. CAF‐induced matrix remodelling operates on multiple levels, binding and releasing cytokine sinks, excluding immune cells and altering functionality, supporting cell migration and further activating CAFs in a feedforward loop.
Cancer‐associated fibroblasts exhibit diverse pro‐tumour functions including growth factor secretion, angiogenesis, immunoregulation [214, 215, 216, 217], ECM remodelling [218, 219] and promoting cancer stemness [220], invasion [221, 222, 223] and chemoresistance [216, 224, 225, 226, 227]. To enable such diversity, the CAF compartment is highly heterogeneous, composed of multiple subpopulations displaying functional specialisation from potentially different origins [228, 229, 230, 231]. However, observations of fibroblast accumulation and expansion around premalignant lesions prior to malignant transformation indicate that, at initial stages, fibroblasts are predominantly tissue‐resident [207, 229]. Although the majority of studies have cited pro‐tumour effects of CAFs, pancreatic cancer studies using the KPC model have yielded more contradictory results, highlighting specific roles of CAF subsets. Here, Feig et al. [216] depleted CAFs based on expression of FAP, reducing tumour burden. In contrast, studies ablating αSMA CAFs resulted in poorly differentiated tumours and shortened survival implying that αSMA‐expressing CAFs, at least in early pancreatic lesions, exhibit tumour‐suppressive activity mediated via immune activation and physical constraint [232, 233]. Together, with observations of CAF accumulation in early lesions, these data indicate that the initial fibroblast response to mutated cells can be tumour‐suppressive, before conversion to a tumour‐promoting phenotype [234, 235]. That said, fibroblasts developing with premalignant lesions can significantly influence adjacent epithelial cells via paracrine signalling [210, 220, 236, 237, 238, 239]. Fibroblast‐derived factors including HGF, IGF, EGF, TGF‐β, IL‐6, LIF, oncostatin M, FGF and metabolites elicit both transformative and proliferative effects on their targets [236, 240, 241, 242]. Early in vitro and xenograft studies in prostate cancer showed that tumour fibroblasts but not normal counterparts could stimulate proliferation and transformation of epithelial cells from benign prostate hyperplasia. Interestingly, CAFs alone were unable to induce growth of normal epithelium, providing early indications of a requirement for reciprocal communication between the two compartments for malignant transformation to occur. Indeed, epithelial cells in precancerous states have been shown to use TGF‐β, PDGF‐D and FGF‐2 to recruit, activate and transform tissue‐resident fibroblasts towards a CAF phenotype [234, 235]. And, while genetically stable, epigenetic alterations and changes in expression of noncoding RNA underpin many gene expression changes following the development of CAF traits [243, 244, 245, 246]. For example, changes in miR‐31, miR‐214 and miR‐155 facilitate conversion of normal fibroblasts into CAFs, whereas the upregulation of miR‐21 impacts TGF‐β signalling to support acquisition of a CAF phenotype.
Increasing genomic stress can promote the production of activin and TGF‐β, supporting CAF activation [247]. Similarly, the release of ‘danger’ signals such as IL‐1α or DAMPs by epithelial cells is sufficient to promote CAF activation and upregulation of pro‐inflammatory genes, with IL‐1β for example, released to promote epithelial proliferation [248, 249, 250]. Physical stresses created in a rapidly changing environment also support formation and expansion CAFs [218]. For example, responses to environmental stiffness via YAP‐TAZ signalling and heat shock factor 1 have been reported to reprogramme fibroblasts towards a more tumour‐promoting phenotype [251, 252]. In response to epithelial‐derived factors, CAFs may proliferate, become ‘activated’ or become senescent [253], a process which itself can exert significant effects to adjacent cells via the senescence‐associated secretory phenotype (SASP; reviewed extensively in Ref. [238, 254]).
Cancer‐associated fibroblasts exhibit a capacity to modulate lymphatic vessels, their presence correlating with lymphatic vessel density and metastasis [255]. Here, CAF‐secreted factors such as hyaluronan [256], FGF, HGF and VEGF‐C directly induce lymphangiogenesis [257, 258, 259], while tumour‐derived lysyl oxidase‐like protein 2 (LOXL2) and sonic hedgehog have been reported to stimulate CAFs to upregulate VEGF‐C and SDF‐1α to support lymphatic expansion [260, 261]. CAF‐derived ECM components such as hyaluronan, fibronectin, collagen, laminin and osteopontin have further been identified as lymphangiogenic drivers [257, 262], as have CAF‐derived microvesicles which are reported to exert pro‐lymphangiogenic effects via angiopoietin and Tie2 driving VEGFR3 expression on lymphatics [263]. Whether similar communications are established in the early TME to support malignant transition remains to be determined.
3.1. Crosstalk of CAFs with immune cells
Cancer‐associated fibroblasts are emerging as key immune modulators in the tumour context, with increasing evidence to suggest that crosstalk between the nonimmune stroma and leukocytes is essential for escape from early immune control and tumour progression [205, 214, 215, 264, 265, 266]. CAFs contribute to initial tumour growth via promotion of an initial local inflammatory microenvironment through upregulation of NF‐κB‐controlled pathways in response to IL‐1β produced by tissue‐resident immune cells in the skin [205]. This gene signature was observed in the preneoplastic ‘hyperplastic’ stage, suggesting that early CAF transformation is critical for both the induction and maintenance of tumour‐promoting inflammation in the skin. Intriguingly when CAFs from a similar time point in cervical cancer were examined, no such gene signature was found, hinting at the importance of the tissue specificity of both the fibroblastic origin and the resident immune microenvironment when driving early‐stage tumour progression.
Immune‐modulatory fibroblasts within the developing tumour microenvironment can impact recruitment, retention and polarisation of the innate immune compartment [183, 205, 249, 266, 267, 268, 269]. We have shown that in early stages of cancer, immune‐regulatory CAFs recruit macrophages through a variety of signals including complement C3, CSF and CXCL12 [269] and others have shown similar to CXCL12 and CXCL16, and via the CCL2–CCR2 axis [270, 271, 272, 273]. Functioning as potent modulators of macrophage behaviour [205, 265, 267, 272], fibroblast‐derived chitinase‐3‐like protein 1 (Chi3L1) also drives macrophages towards a suppressive phenotype in mammary lesions [266] as does CXCL12 in prostate cancer [270]. Similarly, pancreatic stellate cells (PSCs) isolated from human pancreatic tumours, but not healthy tissue, were able to induce an MDSC phenotype in peripheral blood mononuclear cells (PBMCs) in an IL‐6‐dependent manner [274]. CAFs also upregulate TLRs, enabling them to respond to environmental triggers such as DAMPs and release‐soluble mediators that support both tumour proliferation and immune suppression during tumorigenesis [250, 275, 276]. This impact, however, appears to be tissue‐ and TLR‐specific, with TLR7 and TLR9 instead promoting effective immune responses required for immune surveillance [277, 278, 279].
Notably, mounting evidence supports a role for CAFs in modulation of adaptive immune components, shaping T‐cell dysfunction associated with loss of immune surveillance. The production of soluble factors such as IL‐10 and TGF‐β, and metabolic mediators prostaglandin E2 (PGE2), indoleamine 2,3‐dioxygenase (IDO) and arginase can impair activation and cytotoxicity or push responses towards tumour‐promoting TH2 and TH17 responses [280, 281, 282, 283]. FAP‐expressing CAFs can modulate T cells in an NO‐dependent manner, their depletion resulting in increased CD8 T‐cell tumour infiltration [215], and podoplanin expressing CAFs cross‐presented tumour antigen in MHC I to directly induce antigen‐specific antigen‐dependent T‐cell deletion and anergy via PD‐L2 and Fas interactions [214]. Recently, a population expressing MHC II and CD74 has also been identified implying a similar capacity to modulate CD4 T cells [217]. However, indicative that these suppressive behaviours develop as the microenvironment becomes more established, and CAFs may initially elicit T‐cell stimulatory effects, reports have also suggested a role for CAF‐derived IL‐6‐activating T‐cell production of IFN‐γ and IL‐17A [281]. Moreover, CAF‐driven recruitment and differentiation of Tregs contribute to the onset of an immune‐suppressive environment [46]. In human breast and ovarian cancers, subpopulations of CAF were able to recruit Tregs via CXCL12, mediate retention by OX40L, PD‐L2 and JAM2 interactions and support survival [284, 285]. CAFs also exhibit the capacity to disrupt NK function either by abolishing production of cytotoxic granules, granzyme B and perforin by PGE2 and IDO, or by secretion of MMPs to cleave NKG2D ligands on target tumour cells [286, 287, 288]. While these studies were performed in metastatic lesions, such behaviours may also apply to the loss of antitumour activity contributing to immune evasion during malignant transition.
As alluded to previously, a complex interplay exists between CAFs and immune cells, the generation of the tumour microenvironment relies on reciprocal signals. For example, while CAFs impact macrophages and neutrophils, neutrophils also release ROS and NETs to deliver pro‐fibrotic signals [183, 289]. Likewise, CAFs drive macrophage recruitment and polarisation, yet IL‐4‐polarised suppressive macrophages induce expression of the collagen cross‐linker enzyme lysyl hydroxylase 2 in adjacent fibroblasts to promote stiffening of the ECM [281, 290].
3.2. CAFs and the extracellular matrix
Following tissue damage, fibroblasts of healthy tissues rapidly synthesise and deposit ECM as part of the wound‐healing response. In cancer, however, these mechanisms are perturbed to generate a fibrotic, permissive niche [117, 123, 291]. Here, fibroblasts serve as the dominant source of ECM and remodelling enzymes, producing amongst others, collagen family members, laminins, tenascin C, fibronectin, versican, hyaluronic acid, lysyl oxidase (LOX) family members and MMPs [292, 293, 294, 295, 296]. The deposition of ECM, even at very early stages of tumorigenesis, changes the mechanics of a tissue, contributing to transition from premalignant disease to cancer [206, 297, 298]. For example, in breast cancer, a collagen‐dense microenvironment frequently presenting as high mammographic density represents a significant risk factor. This was confirmed when fibroblasts derived from healthy women with high mammographic density (but no breast cancer) exhibited significantly higher desmoplastic and pro‐tumorigenic phenotypes compared with counterparts isolated from women with low mammographic density [117, 299, 300]. Also in breast cancer, tissue‐resident fibroblasts exposed to local secretion of granulin increase the expression of a variety of ECM components [301], promoting the desmoplastic response and malignant progression of otherwise indolent tumours [302, 303]. The increases in ECM reorganisation are catalysed by CAF‐derived MMPs and LOX proteins, which cross‐link collagens and mediate fibre elongation and fibre realignment to promote a stiffer medium conducive with malignant progression [304, 305, 306, 307]. Importantly, CAF‐driven changes to the ECM in the developing TME do not occur in isolation. Instead, reciprocal, dynamic communication exists. Upon remodelling the environment to create a dense, more rigid matrix, fibroblasts sense the modified mechanical cues, in turn stimulating production of cross‐linkers LOX to further stiffen the ECM, signal proliferation, or promote acquisition of a CAF phenotype via the SRC‐YAP‐MYL9/MYL2 axis [218, 308].
Thus, within the developing TME, CAFs evolve to alter cellular, structural and chemical aspects of tissues, but the physical changes they afford through deposition and remodelling of the ECM themselves also play a pivotal role in malignant transition.
3.3. Mechanical cues in the developing tumour microenvironment
A gradual shift in the rigidity of the ECM from that of normal tissue is implicated in malignant progression due to its far‐reaching impact on cell morphology, migration, alignment, angiogenesis and immune responses [116, 123, 309, 310, 311, 312]. In addition to diverse paracrine stimuli, epithelial cells respond to physical stimuli to promote proliferation and morphological adaptations conducive with acquisition of cancer traits. The impact of increasing ECM stiffness alone on epithelial behaviour was elegantly shown by Paszek et al. in 2005 [309], a concept that was built upon more recently using methacrylated hyaluronic acid hydrogels [312]. Here, Ondeck et al dynamically tuned gels from normal (< 150 Pascals) to malignant (> 3000 Pascals) stiffness to demonstrate coincident mechanical sensing and loss of mammary epithelial morphology through TGF‐β and YAP signalling. Changes in stiffness together with increasingly aligned fibres promote tumour cell migration, leveraging traction against remodelled matrix [294, 310, 313]. Indeed, the mechanical coupling of ECM with epithelial cells and CAF cytoskeleton is critical to cell motility in transforming tissues, with benign and malignant cells exhibiting very different mechanical properties [309, 314].
Extracellular matrix tethers and immobilises many soluble mediators, serving as a valuable reservoir for pro‐tumour, CAF‐activating and immune‐suppressive factors such as EGF, FGF, PDGF and critically TGF‐β [119, 296, 315, 316]. Many of these provide subtle guidance cues when tethered, but are fully bioactive once released from proteoglycans upon proteolytic degradation. Moreover, matrix remodelling and resulting increases in stiffness occurring as desmoplasia progresses in developing tumours can support the angiogenic switch. This may be a direct consequence of mechanical compression of vessels driving a hypoxic response [317, 318, 319], through mechanosensing properties of endothelial cells [320, 321, 322], or through MMP‐mediated remodelling via release of matrix‐bound factors such as VEGF, periostin and tenascin C [323]. Disruption of matrix structure, for example inhibition of modifying enzymes such as LOXL family members and reduction in cross‐linked collagenous ECM matrix, has been reported to impair both tumorigenesis and angiogenesis [324].
Remodelling of the ECM modulates immune cell trafficking and functional status [325, 326, 327]. This may occur via direct signals from components of the TME, but also as a consequence of age. Most cancers arise in individuals over the age of 60 [328], and in aged tissues, both fibroblasts and immune populations undergo significant alterations [329, 330, 331]. Altered secretomes, and metabolic and ECM profiles have the potential to combine, propagating pro‐tumour dysfunctional states. An increasingly stiff matrix can induce CAFs to produce chemoattractants such as CCL2 and CSF‐1, attracting innate immune cells, which may deposit further collagen [119]. This ECM also serves a source of DAMPs. Proteolytic cleavage of matrix components such as fibrinogen, fibronectin domains, versican and decorin creates fragments recognised by TLR2 and TLR4 on immune cells [332, 333, 334, 335, 336, 337]. Interactions can promote both differentiation and dysfunction; however, whether such interactions mediate anti‐ vs. pro‐tumour behaviour remains less clear. Across melanoma, lung, colon and liver cancers, versican has been reported to drive an immunosuppressive DC phenotype via TLR2, but has also been reported to direct DCs into an inflammatory phenotype, critical for T‐cell infiltration and antitumour immunity [338].
The physical properties of remodelled ECM exert diverse effects on epithelial, stromal and immune cells it surrounds [116, 310, 339, 340, 341, 342, 343]. The ECM can determine macrophage shape and polarisation [344], and collagen‐rich matrices bias towards pro‐tumorigenic phenotypes, whereas ECM rich in fibronectin promotes antitumorigenic phenotypes [345, 346, 347, 348]. Indeed, increased deposition of type I collagen has been shown to directly stimulate inhibitory receptors such as LAIR‐1 on immune cells [349]. Moreover, YAP activation in cells sensing tensional cues induces the expression of cytokines to recruit MDSCs and TAMs [350]. A modified ECM, rich in fibrillar collagen, further contributes to immune suppression by acting as a physical barrier. T cells are frequently more abundant within ECM‐rich areas surrounding epithelial cells, and such physical exclusion is associated with poor prognosis [216, 325, 351, 352]. Stiffer substrates have been associated with impaired effector function [353, 354]; within dense matrices, T cells are less motile since migration, which is protease‐independent, relies on a rapid ability to deform and squeeze through pores and along fibres [355, 356]. And while impeded, they may experience prolonged exposure to immune‐suppressive cytokines.
Beyond any direct effects on cell behaviour within the developing TME, an accumulation of collagens, increased cross‐linking and stiffening, and rapidly expanding blood vessels lead to a gradual increase in interstitial fluid pressure [317, 319]. This feature itself can contribute to transformation of the local environment. Increases to the movement of fluid through a tissue, called interstitial flow, are sufficient to drive changes in fibroblast and collagen fibres towards a more aligned orientation associated with increasing stiffness [357, 358]. Similarly, fluid movement stimulates the production of TGF‐β by fibroblasts [359] contributing to the alterations in CAF activation status and ongoing immunosuppressive activity. Interstitial flow supports the formation of subtle transcellular chemokine gradients guiding cells out of physical contact with the developing tumour and towards draining lymphatic vessels, which also sense fluid flux, upregulating immune‐homing chemokines and cell adhesion molecules in the process [360, 361, 362, 363]. Such cues are key for efficient immune trafficking, particularly antigen‐presenting cells, to and from the local environment during tumorigenesis.
A multiplicity of mechanisms exists for CAFs to influence, and be influenced by, the developing TME. With growing evidence and technical precision, this is being ascribed to an increasing number of unique CAF subpopulations, defined by tissue‐specific markers. This overwhelming complexity can be distilled, however, into the recurrent functional observations of these CAFs performing either immune‐modulatory, ECM deposition or mechanical remodelling roles. Whether these specialised subpopulations are present from the outset of malignancy as tissue‐resident fibroblasts or whether they are plastic and interconvert with developing cues in the TME remains an open question in the field.
4. Outreach activities: systemic effects of the tumour microenvironment
This review has focussed on the reciprocal microenvironment changes that facilitate early tumour growth. However, as practising oncologists are aware, cancer manifests as a systemic illness. The molecular details of how cancer ‘reaches out’ to influence whole‐body physiology are beginning to be understood [364, 365]. Again, much of the focus has been on immunity. Systemic immunity is impaired in patients with advanced cancer, and this is clinically relevant with both success of immunotherapy and mortality from intercurrent infections [366, 367]. Several recent mouse‐based studies have described widespread immune alterations present in cancer [17, 368]. The microenvironment of the tumour draining lymph node changes in parallel to tumour development [269, 369], which may impair onset of immunity or facilitate metastasis. Immune cytokines may also influence bone marrow function to release immature myeloid cells which traffic to the TME to become MDSCs. There has also been interest in these effects as a prognostic or predictive marker, in particular the neutrophil:lymphocyte ratio which is associated with patient outcomes and may be a crude measure of systemic immune dysfunction [370]. In addition to the immunological effects, cancer induces systemic metabolic changes that manifest as cachexia. Intriguingly, this may be mediated by reciprocal systemic stromal changes: Roberts et al. demonstrated that the depletion of CAF‐like fibroblast populations from muscle and fat is responsible for loss of mass in these organs in cancer [371]. There is also overlap between immune and metabolic reprogramming: the inflammatory mediators that induce cachexia in turn elevate immune‐suppressive factors such as cortisol, which further impairs antitumour immunity [372]. It will be fascinating to see how far these systemic changes can be traced to early tumour development.
5. Capturing a changing tumour microenvironment, from mice to humans
Historical mouse models of cancer often use injectable human cell lines in immunodeficient mice. While these models have been key to the progress in developing cancer treatments for the past half‐century, they do not model the early microenvironment or the adaptive immune component of the TME. Immune‐based treatments are now central to the practice of oncology, and so, it is essential to use immune‐competent models that reflect the whole TME. Advances over the last decade in genetic engineering have increased the feasibility of spontaneous tumour models, which have a neoantigen profile and local immune infiltrate more representative of human cases than injectable tumour models [373, 374, 375, 376]. However, 65 million years of evolution have resulted in a number of significant mechanistic differences between mice and humans, for example Ly49 vs. killer immunoglobulin receptor expression on NK cells [377], differing antibody isotypes [378, 379] and differing transcriptional programmes for B‐cell and T‐cell development [378]. There are also genomic differences between mice and humans that complicate developing spontaneous models.
Concerted efforts are now being made to ‘humanise’ murine models as far as reasonably possible. Patient‐derived xenografts (PDX) in combination with an immune system reconstituted from an autologous haematopoietic stem cell are increasingly used in tumour immunology, especially to predict patient response to therapy [380]. However, the injection of large numbers of late‐stage cells to create a viable xenograft limits their use for the study of the early TME. Organoid technology meanwhile maintains many of the advantages of PDX, including maintaining heterogeneity [381] and tumour–stroma interactions [382, 383], while also allowing for high‐throughput investigation of the earliest stages of tumour development. For example, it was shown that extended TNF‐α treatment of human ovarian surface epithelial cell‐derived organoids led to loss of normal structural organisation and development of an early cancer legion, thus suggesting a link between a pro‐inflammatory microenvironment and the initiation of tumorigenesis [384]. Similarly, a library of human CRC organoids was created from a range of tumour stages including normal epithelium up to metastatic cancer. Sequencing of each organoid highlighted a high dependency on culture ‘niche factors’ was present in early organoids, and a subsequent mutational loss of this requirement was a critical stage in development into an adenocarcinoma. The authors then went on to validate this suggestion that transition from early cancer to invasive cancer requires increased growth factor autonomy, xenografting the organoids onto mice [385]. It is becoming increasingly clear therefore that improved understanding of the early TME will require utilisation and expansion of both the in vivo and in vitro toolkit (Table 1).
Table 1.
Advantages and limitations of mouse models in the early TME.
| Model system | Advantages | Limitations |
|---|---|---|
| Subcutaneous xenograft (cell line or patient‐derived) |
Rapid turnaround of experiments, high throughput Human cancer cells Tractable: genetic manipulation of cell lines to study pathways accessible |
Does not capture organ‐specific or early TME Requires immunocompromised mice Replacement of stroma by host |
| Subcutaneous mouse cell lines |
Rapid turnaround of experiments, high throughput Tractable: genetic manipulation of cell lines to study pathways accessible Intact immune system |
Does not capture organ‐specific or early TME Species differences in immunity Highly transformed cells not representative of early stages or heterogeneity |
| Orthotopic injection models |
Models the organ‐specific tumour microenvironment If mouse cells used, intact immunity |
Injection of highly transformed cells, large numbers, does not model early TME |
| Genetically engineered mouse models |
Models full development of lesions, including premalignant stages Intact immune system Accurate modelling of organ‐specific and early microenvironment |
Expensive and time‐consuming Specialised techniques needed for tumour monitoring ‘Clean’ – defined oncogenic drivers decrease mutational spectrum Mouse genomic differences mean mutating homologs of human oncogenes does not necessarily produce the same organ cancer Mouse immune differences may be significant Timescales of months still do not match the many years of tumorigenesis in humans Not available for all cancers yet |
| Humanised Mouse models |
Allows study of human cancer‐immune interactions in an animal system May overcome species differences in immunity |
Expensive and time‐consuming Transplantation of transformed tissue, which may not reflect early lesions Stromal elements are mouse‐derived Marrow transplantation may produce off‐target graft vs. host effects |
6. Early detection and therapeutic intervention
For many cancers, early detection and intervention significantly improve survival. In oesophageal cancer, the detection of the premalignant condition Barrett's oesophagus can dramatically increase dismal 5‐year survival rates of less than 20%. However, in the case of Barrett's, monitoring by endoscopic surveillance represents a significant cost and disruption to patients' lives. Thus, identifying the minority that will go on to develop OAC is a priority. Examining the underlying microenvironment may help to define and stratify patients at greater risk. Indeed, molecular imaging has been used to exploit changes in tissue glycosylation for the identification of dysplastic tissues [386, 387, 388]. Early detection of cancers also requires the discovery and use of robust biomarkers to identify lesions likely to progress. Thus, strategies to screen at‐risk groups in a minimally invasive fashion are increasingly focusing on analysis of the premalignant lesion. Although currently focused on detecting early changes in the epithelial compartment [389, 390], our increasing understanding of the complex interactions at play within tissues undergoing malignant transformation presents the opportunity to exploit changes within the surrounding stroma for early detection and patient stratification (Table 2). Changes in immune content and localisation [18], ECM characteristics [391, 392, 393] and functional gene signatures [145, 394, 395] all offer potential. Indeed, sequencing approaches have enabled large‐scale, single‐cell resolution characterisation of the immune landscape of over 30 different tumour types [396].
Table 2.
Pro‐ and antitumour hallmarks of the early TME. SHH, sonic hedgehog; TKI, tyrosine kinase inhibitors.
| Function | Stromal mechanism | Therapeutic strategies |
|---|---|---|
| Antitumour early functions | ||
| Immune surveillance of nascently transformed epithelial cells |
T cells – cytokines: TNFs, IFN‐γ, granzyme B, receptors: FasL, cell contact: perforin, granzyme B. NKs – cytokines: TNFs, IFN‐γ, CSF‐2, granzyme B, receptors: NKG2D, KIRs, KKp44, cell contact: perforin. ILCs – cytokines: TNFs, IL‐2, IL‐8. Neutrophils – cytokines: IFNs TNF‐⍺, NO, H2O2, receptors: TRAIL, costimulatory molecules |
|
| Recruitment of tumour neoantigen‐specific T cells |
CAFs – cytokines: TNF‐⍺, IL‐1β, chemokines: CXCL9, CXCL10, CXCL11, DAMPs, matrix remodelling. T cells – cytokines: IFN‐g, TNFs. Macrophages – chemokines: CXCL9, CXCL10, CXCL11, DAMPs, matrix remodelling. DCs – chemokines: CCL2, CCL3, CCL17, CCL21, XCL1, receptors: costimulatory receptors, MHC |
|
| Promotion of ‘M1’ macrophages |
CAFs – cytokines: VEGF, receptors: ICAM‐1, chemokines – CCL2, CCL9, DAMPs. T cells – cytokines: TNF‐⍺, IFN‐γ, chemokines: NO, ROS, IL‐2, DAMPs. Macrophages – cytokines: IFN‐γ, DAMPs. Neutrophils – cytokines: MIPs, IFN‐γ, DAMPs |
|
| Pro‐tumour early functions: impact on cancer cells | ||
| Sustained proliferative signalling and transformation |
CAFs – cytokines: TGF‐β, IL‐1b, IL‐6, growth factors: HGF, IGF, EGF, LIF, oncostatin M, FGF, exosomes, MMPs. ECM – growth factors: following matrix degradation, increases in stiffness. mutagens: ROS, NOS. TAMs – growth factors: TGF‐β, EGFs, VEGF, PDGF, MMPs. TANs – cytokines: TGF‐β, MMPs ILCs – cytokines: IL‐22 |
|
| Resisting cell death |
CAFs – cytokines: TGF‐β, IL‐6, IL‐10, IL‐11, receptors: PD‐L1, FasL, matrix deposition. T cells – receptors: reduced TRAIL, FasL. TAMs – cytokines: IL‐6, IL‐11, TNF‐⍺, matrix deposition. TANs – cytokines: IL‐6, IL‐11. ILCs – receptors: reduced TRAIL, FasL |
|
| Pro‐tumour early functions: impact on stromal cells | ||
| Metabolic support of growing tumour |
CAFs – cytokines: TGF‐β metabolites: alanine, glutamine, lactate. Endothelial cells – angiogenic switch. TAMs – metabolites: IDO, Arg1, lactate, angiogenic switch |
|
| Recruitment of immune‐suppressive cells |
CAFs – cytokines: C3, CSF, IL‐6, IL‐10, TGF‐β, GDF‐15, chemokines: CXCL12 and CXCL16. Endothelial cells – receptors: CLEVER‐1. T cells – conversion to Tregs. TAMs – conversion to MDSCs, cytokines: IL‐6, IL‐10 and TGF‐β, chemokines: CCL5, CCL17, CCL22, metabolites: IDO, arginase. ILCs – conversion to ILC2 and ILC3 |
|
| Impairing T‐cell activity |
CAFs – cytokines: TGF‐β and IL‐6, metabolites: PGE2 and IDO, receptors: PD‐L1, PD‐L2 and FasL, inducing fibrosis. T cells – cytokines: TGF‐β, reduced pro‐inflammatory cytokines, receptors: PD‐L1, CTLA‐4, Fas, LAG3, TIGIT, conversion to Tregs. Macrophages – cytokines: IL‐10, chemokines: CCL22, metabolites: IDO, Arg1, lactate, receptors: PD‐L1, inducing fibrosis. DCs – cytokines: TGF‐β, IL‐10, metabolites: IDO, Arg1 receptors: PD‐L1, PD‐L2, reduced costimulation. ILCs – cytokines: IL‐12, IL‐22, receptors: NKp46 |
|
| Fibroblast function |
Fibroblasts – cytokines: TGF‐β, IL‐1a, growth factors: PDGF‐2,FGF‐2, microRNA ECM – increases in stiffness, signalling: YAP‐TAZ, HSF‐1 T cells – cytokines: IL‐1, IL‐6 and TNF. TAMs – cytokines: TGF‐β, IL‐6, growth factors: PDGFs, FGF2, mutagen: ROS |
|
Developing our comprehension of how immune and fibroblastic components contribute to the transition from premalignant to cancer affords a range of clinical approaches for early intervention. Understanding kinetics of the host responses in malignant transition, such as loss of immune surveillance, may provide a unique therapeutic window. Preclinical models have explored strategies to prevent or reverse the switch in innate cells to maintain or reinstate an antitumour phenotype. However, while attractive, many pathways disrupted during immune suppression are key to maintain immune tolerance and protection against autoimmunity, thus global approaches to inhibit the innate immune system in early cancer may be counterproductive. Instead, due to their diverse array of functions, targeting the fibroblasts may provide an alternative method to stall malignant transition. While eradication strategies have been investigated, such an approach may in practice be hampered by the lack of specific markers and complexities stemming from heterogeneous pro‐ vs. antitumour subsets [214, 216, 232, 233]. Reprogramming or ‘normalisation’ methods are proving more attractive. Off the back of studies identifying novel stromal targets, methods to interfere with CAF activation status and improve traits such as mechanical properties via inhibition of factors including FGFR, hedgehog, NOX, TGF‐β, LOX, hyaluronic acid and all‐trans retinoic acid (ATRA) pathways have progressed from preclinical models to clinical trial. Utilisation of re‐normalising the TME for enhancement of existing therapy is well‐demonstrated by a recent report in murine breast cancer. By modifying an angiotensin blocker to be pH‐dependent, the drug was active in the more acidic microenvironment of a developing tumour but not in normal neutral pH tissue. Therefore, only CAFs in the TME were reprogrammed to a normalised state, and not activated fibroblasts in adjacent tissue. This normalisation enhanced the efficacy of ICPI. Likewise, the repurposing of existing agents shown to have effects on stroma and wider TME is under trial [397, 398, 399, 400]. For example, in PDAC, the drug ATRA (which is widely used to treat acute promyelocytic leukaemia) was shown to restore pancreatic stellate cells to a quiescent state only seen in the very early stages of tumour initiation, and these treated cells displayed significantly decreased pro‐tumour functions. However, the ability of mouse models to predict therapeutic effect is not straightforward. Preclinical models suggest that hedgehog inhibitors may be useful for the treatment of pancreatic cancer by restricting stromal growth and improving drug penetration [401]. However, subsequent clinical trials have been disappointing [402]. Further preclinical studies have shown that while hedgehog inhibition reduces desmoplasia, it can also enhance epithelial growth. The unpredictable balance of these factors in humans may then determine whether the treatment has a positive or negative effect [403].
Beyond the design of novel therapeutic targets, a thorough understanding of the composition and roles of stroma of the early tumour microenvironment may inform patient prognosis or predict response to treatment [404, 405, 406]. For example, a stromal gene signature has been shown to predict poor survival and metastasis in high‐grade serous ovarian cancer. In summary, although the early stroma offers many opportunities for intervention for patient benefit, key barriers to advancing this are the early detection of these lesions, and a more thorough understanding of the relationship between animal models and different human tumours at early stages of development.
7. Perspectives and conclusions
Tissues surrounding mutated cells play a pivotal role in carcinogenesis, from initial elimination responses through to supporting growth and development of a permissive niche. Recent technological advances have dramatically increased our appreciation of the complex networks at play, the duality of our immune system, the increasing diversity and functional specialisation of fibroblasts, and the impact of changing tissue mechanics. Even so, outstanding challenges remain. We know relatively little about the plasticity, heterogeneity and interdependencies that define the evolving stromal landscape. Nevertheless, it is likely that these complex tumour–stroma interactions compliment the well‐documented tumour heterogeneity and cumulatively contribute to increased robustness of early tumours [407], and we now face the challenge of dissecting these roles, addressing impact of tissue specificities and previously overlooked traits such as the ageing microenvironment. A better understanding facilitated by the development mouse models that more faithfully recapitulate early stages of cancer, the installation of extensive patient sample repositories and inclusion of stromal parameters in trials will help to unravel underpinning mechanisms in real time, and most importantly stand to inform new approaches to better identify and treat patients early.
Conflict of interest
The authors declare no conflict of interest.
Author contributions
JDS conceived the review, and WMM, JOJ and JDS wrote the paper.
References
- 1.Martincorena I, Roshan A, Gerstung M, Ellis P, Van Loo P, McLaren S, Wedge DC, Fullam A, Alexandrov LB, Tubio JMet al. (2015) High burden and pervasive positive selection of somatic mutations in normal human skin. Science 348, 880–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martincorena I, Fowler JC, Wabik A, Lawson ARJ, Abascal F, Hall MWJ, Cagan A, Murai K, Mahbubani K, Stratton MRet al. (2018) Somatic mutant clones colonize the human esophagus with age. Science 362, 911–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tomasetti C & Vogelstein B (2015) Variation in cancer risk among tissues can be explained by the number of stem cell divisions. Science 347, 78–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weaver JMJ, Ross‐Innes CS, Shannon N, Lynch AG, Forshew T, Barbera M, Murtaza M, Ong CAJ, Lao‐Sirieix P, Dunning MJet al. (2014) Ordering of mutations in preinvasive disease stages of esophageal carcinogenesis. Nat Genet 46, 837–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wajapeyee N, Serra RW, Zhu X, Mahalingam M & Green MR (2008) Oncogenic BRAF induces senescence and apoptosis through pathways mediated by the secreted protein IGFBP7. Cell 132, 363–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pollock PM, Harper UL, Hansen KS, Yudt LM, Stark M, Robbins CM, Moses TY, Hostetter G, Wagner U, Kakareka Jet al. (2003) High frequency of BRAF mutations in nevi. Nat Genet 33, 19–20. [DOI] [PubMed] [Google Scholar]
- 7.Michaloglou C, Vredeveld LCW, Soengas MS, Denoyelle C, Kuilman T, Van Der Horst CMAM, Majoor DM, Shay JW, Mooi WJ & Peeper DS (2005) BRAFE600‐associated senescence‐like cell cycle arrest of human naevi. Nature 436, 720–724. [DOI] [PubMed] [Google Scholar]
- 8.Mitchell TJ, Turajlic S, Rowan A, Nicol D, Farmery JHR, O'Brien T, Martincorena I, Tarpey P, Angelopoulos N, Yates LRet al. (2018) Timing the landmark events in the evolution of clear cell renal cell cancer: TRACERx renal. Cell 173, 611–623.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burnet FM (1970) The concept of immunological surveillance. Prog Exp Tumor Res 13, 1–27. [DOI] [PubMed] [Google Scholar]
- 10.Penn I (1996) Malignant melanoma in organ allograft recipients. Transplantation 61, 247–248. [DOI] [PubMed] [Google Scholar]
- 11.Penn I & Starzl TE (1972) Malignant tumors arising de novo in immunosuppressed organ transplant recipients. Transplantation 14, 407–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stutman O (1976) Immunodepression and malignancy. Adv Cancer Res 22, 261–422. [DOI] [PubMed] [Google Scholar]
- 13.Pham SM, Kormos RL, Landreneau RJ, Kawai A, Gonzalez‐Cancel I, Hardesty RL, Hattler BG & Griffith BP (1995) Solid tumors after heart transplantation: lethality of lung cancer. Ann Thorac Surg 60, 1623–1626. [DOI] [PubMed] [Google Scholar]
- 14.Lateef N, Basit KA, Abbasi N, Kazmi SMH, Ansari AB & Shah M (2016) Malignancies after heart transplant. Exp Clin Transplant 14, 12–16. [DOI] [PubMed] [Google Scholar]
- 15.Kaplan DH, Shankaran V, Dighe AS, Stockert E, Aguet M, Old LJ & Schreiber RD (1998) Demonstration of an interferon gamma‐dependent tumor surveillance system in immunocompetent mice. Proc Natl Acad Sci USA 95, 7556–7561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ & Schreiber RD (2001) IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature 410, 1107–1111. [DOI] [PubMed] [Google Scholar]
- 17.Allen BM, Hiam KJ, Burnett CE, Venida A, DeBarge R, Tenvooren I, Marquez DM, Cho NW, Carmi Y & Spitzer MH (2020) Systemic dysfunction and plasticity of the immune macroenvironment in cancer models. Nat Med 26, 1125–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galon J, Costes A, Sanchez‐Cabo F, Kirilovsky A, Mlecnik B, Lagorce‐Pages C, Tosolini M, Camus M, Berger A, Wind Pet al. (2006) Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 313, 1960–1964. [DOI] [PubMed] [Google Scholar]
- 19.Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, Jungbluth AA, Frosina D, Gnjatic S, Ambrosone Cet al. (2005) Intraepithelial CD8+ tumor‐infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci USA 102, 18538–18543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gruosso T, Gigoux M, Manem VSK, Bertos N, Zuo D, Perlitch I, Saleh SMI, Zhao H, Souleimanova M, Johnson RMet al. (2019) Spatially distinct tumor immune microenvironments stratify triple‐negative breast cancers. J Clin Invest 129, 1785–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Angell HK, Bruni D, Barrett JC, Herbst R & Galon J (2020) The immunoscore: colon cancer and beyond. Clin Cancer Res 26, 332–339. [DOI] [PubMed] [Google Scholar]
- 22.DuPage M, Cheung AF, Mazumdar C, Winslow MM, Bronson R, Schmidt LM, Crowley D, Chen J & Jacks T (2011) Endogenous T cell responses to antigens expressed in lung adenocarcinomas delay malignant tumor progression. Cancer Cell 19, 72–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McGranahan N, Furness AJS, Rosenthal R, Ramskov S, Lyngaa R, Saini SK, Jamal‐Hanjani M, Wilson GA, Birkbak NJ, Hiley CTet al. (2016) Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science 351, 1463–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koebel CM, Vermi W, Swann JB, Zerafa N, Rodig SJ, Old LJ, Smyth MJ & Schreiber RD (2007) Adaptive immunity maintains occult cancer in an equilibrium state. Nature 450, 903–907. [DOI] [PubMed] [Google Scholar]
- 25.Loeser S, Loser K, Bijker MS, Rangachari M, Van Der Burg SH, Wada T, Beissert S, Melief CJM & Penninger JM (2007) Spontaneous tumor rejection by cbl‐b‐deficient CD8+ T cells. J Exp Med 204, 879–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eyles J, Puaux AL, Wang X, Toh B, Prakash C, Hong M, Tan TG, Zheng L, Ong LC, Jin Yet al. (2010) Tumor cells disseminate early, but immunosurveillance limits metastatic outgrowth, in a mouse model of melanoma. J Clin Invest 120, 2030–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mohammed J, Ryscavage A, Perez‐Lorenzo R, Gunderson AJ, Blazanin N & Glick AB (2010) TGFΒ1‐induced inflammation in premalignant epidermal squamous lesions requires IL‐17. J Invest Dermatol 130, 2295–2303. [DOI] [PubMed] [Google Scholar]
- 28.Johnson SD, Levingston C & Young MRI (2016) Premalignant oral lesion cells elicit increased cytokine production and activation of T‐cells. Anticancer Res 36, 3261–3270. [PMC free article] [PubMed] [Google Scholar]
- 29.Freeman A, Bridge JA, Maruthayanar P, Overgaard NH, Jung JW, Simpson F, Prow TW, Soyer HP, Frazer IH, Freeman Met al. (2014) Comparative immune phenotypic analysis of cutaneous squamous cell carcinoma and intraepidermal carcinoma in immune‐competent individuals: proportional representation of CD8+ T‐cells but not FoxP3+ regulatory T‐cells is associated with disease stage. PLoS One 9, e110928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Öhman J, Mowjood R, Larsson L, Kovacs A, Magnusson B, Kjeller G, Jontell M & Hasseus B (2015) Presence of CD3‐positive T‐cells in oral premalignant leukoplakia indicates prevention of cancer transformation. Anticancer Res 35, 311–318. [PubMed] [Google Scholar]
- 31.Beatty PL, van der Geest R, Hashash JG, Kimura T, Gutkin D, Brand RE & Finn OJ (2016) Immunobiology and immunosurveillance in patients with intraductal papillary mucinous neoplasms (IPMNs), premalignant precursors of pancreatic adenocarcinomas. Cancer Immunol Immunother 65, 771–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Degnim AC, Hoskin TL, Arshad M, Frost MH, Winham SJ, Brahmbhatt RA, Pena A, Carter JM, Stallings‐Mann ML, Murphy LMet al. (2017) Alterations in the immune cell composition in premalignant breast tissue that precede breast cancer development. Clin Cancer Res 23, 3945–3952. [DOI] [PubMed] [Google Scholar]
- 33.Maglietta A, Maglietta R, Staiano T, Bertoni R, Ancona N, Marra G & Resta L (2016) The immune landscapes of polypoid and nonpolypoid precancerous colorectal lesions. PLoS One 11, e0159373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McGranahan N, Rosenthal R, Hiley CT, Rowan AJ, Watkins TBK, Wilson GA, Birkbak NJ, Veeriah S, Van Loo P, Herrero Jet al. (2017) Allele‐specific HLA loss and immune escape in lung cancer evolution. Cell 171, 1259–1271.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dupage M, Mazumdar C, Schmidt LM, Cheung AF & Jacks T (2012) Expression of tumour‐specific antigens underlies cancer immunoediting. Nature 482, 405–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blank CU, Haining WN, Held W, Hogan PG, Kallies A, Lugli E, Lynn RC, Philip M, Rao A, Restifo NPet al. (2019) Defining ‘T cell exhaustion’. Nat Rev Immunol 19, 665–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McLane LM, Abdel‐Hakeem MS & Wherry EJ (2019) CD8 T cell exhaustion during chronic viral infection and cancer. Annu Rev Immunol 37, 457–495. [DOI] [PubMed] [Google Scholar]
- 38.Hatam LJ, DeVoti JA, Rosenthal DW, Lam F, Abramson AL, Steinberg BM & Bonagura VR (2012) Immune suppression in premalignant respiratory papillomas: enriched functional CD4+Foxp3+ regulatory T cells and PD‐1/PD‐L1/L2 expression. Clin Cancer Res 18, 1925–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li A, Herbst RH, Canner D, Schenkel JM, Smith OC, Kim JY, Hillman M, Bhutkar A, Cuoco MS, Rappazzo CGet al. (2019) IL‐33 signaling alters regulatory T cell diversity in support of tumor development. Cell Rep 29, 2998–3008.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berendt MJ & North RJ (1980) T‐cell‐mediated suppression of anti‐tumor immunity. An explanation for progressive growth of an immunogenic tumor. J Exp Med 151, 69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moo‐Young TA, Larson JW, Belt BA, Tan MC, Hawkins WG, Eberlein TJ, Goedegebuure PS & Linehan DC (2009) Tumor‐derived TGF‐β mediates conversion of CD4+Foxp3+ regulatory T cells in a murine model of pancreas cancer. J Immunother 32, 12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thomas DA & Massagué J (2005) TGF‐β directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer Cell 8, 369–380. [DOI] [PubMed] [Google Scholar]
- 43.Chen ML, Pittet MJ, Gorelik L, Flavell RA, Weissleder R, Von Boehmer H & Khazaie K (2005) Regulatory T cells suppress tumor‐specific CD8 T cell cytotoxicity through TGF‐β signals in vivo. Proc Natl Acad Sci USA 102, 419–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mempel TR, Pittet MJ, Khazaie K, Weninger W, Weissleder R, von Boehmer H & von Andrian UH (2006) Regulatory T cells reversibly suppress cytotoxic T cell function independent of effector differentiation. Immunity 25, 129–141. [DOI] [PubMed] [Google Scholar]
- 45.Strainic MG, Shevach EM, An F, Lin F & Medof ME (2013) Absence of signaling into CD4+ cells via C3aR and C5aR enables autoinductive TGF‐β1 signaling and induction of Foxp3+ regulatory T cells. Nat Immunol 14, 162–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen WJ, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G & Wahl SM (2003) Conversion of peripheral CD4+CD25‐ naive T cells to CD4+CD25+ regulatory T cells by TGF‐β induction of transcription factor Foxp3. J Exp Med 198, 1875–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fantini MC, Becker C, Monteleone G, Pallone F, Galle PR & Neurath MF (2004) Cutting edge: TGF‐β induces a regulatory phenotype in CD4+ CD25− T cells through Foxp3 induction and down‐regulation of Smad7. J Immunol 172, 5149–5153. [DOI] [PubMed] [Google Scholar]
- 48.Ravi R, Noonan KA, Pham V, Bedi R, Zhavoronkov A, Ozerov IV, Makarev E, Artemov AV, Wysocki PT, Mehra Ret al. (2018) Bifunctional immune checkpoint‐targeted antibody‐ligand traps that simultaneously disable TGFβ enhance the efficacy of cancer immunotherapy. Nat Commun 9, 741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Batlle E & Massagué J (2019) Transforming growth factor‐β signaling in immunity and cancer. Immunity 50, 924–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.David CJ & Massagué J (2018) Contextual determinants of TGFβ action in development, immunity and cancer. Nat Rev Mol Cell Biol 19, 419–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bardeesy N, Cheng KH, Berger JH, Chu GC, Pahler J, Olson P, Hezel AF, Horner J, Lauwers GY, Hanahan Det al. (2006) Smad4 is dispensable for normal pancreas development yet critical in progression and tumor biology of pancreas cancer. Genes Dev 20, 3130–3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kavanagh ME, Conroy MJ, Clarke NE, Gilmartin NT, O'Sullivan KE, Feighery R, MacCarthy F, O'Toole D, Ravi N, Reynolds JVet al. (2016) Impact of the inflammatory microenvironment on T‐cell phenotype in the progression from reflux oesophagitis to Barrett oesophagus and oesophageal adenocarcinoma. Cancer Lett 370, 117–124. [DOI] [PubMed] [Google Scholar]
- 53.Balkwill F, Charles KA & Mantovani A (2005) Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell 7, 211–217. [DOI] [PubMed] [Google Scholar]
- 54.Pinto ML, Rios E, Durães C, Ribeiro R, Machado JC, Mantovani A, Barbosa MA, Carneiro F & Oliveira MJ (2019) The two faces of tumor‐associated macrophages and their clinical significance in colorectal cancer. Front Immunol 10, 1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aras S & Raza Zaidi M (2017) TAMeless traitors: macrophages in cancer progression and metastasis. Br J Cancer 117, 1583–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Canè S, Ugel S, Trovato R, Marigo I, De Sanctis F, Sartoris S & Bronte V (2019) The endless saga of monocyte diversity. Front Immunol 10, 1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Penny HL, Sieow JL, Adriani G, Yeap WH, See Chi Ee P, San Luis B, Lee B, Lee T, Mak SY, Ho YSet al. (2016) Warburg metabolism in tumor‐conditioned macrophages promotes metastasis in human pancreatic ductal adenocarcinoma. Oncoimmunology 5, e1191731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gonzalez H, Hagerling C & Werb Z (2018) Roles of the immune system in cancer: from tumor initiation to metastatic progression. Genes Dev 32, 1267–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Martinez FO & Gordon S (2014) The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep 6, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liou GY, Döppler H, Necela B, Edenfield B, Zhang L, Dawson DW & Storz P (2015) Mutant KRAS–induced expression of ICAM‐1 in pancreatic acinar cells causes attraction of macrophages to expedite the formation of precancerous lesions. Cancer Discov 5, 52–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Woo SR, Fuertes MB, Corrales L, Spranger S, Furdyna MJ, Leung MYK, Duggan R, Wang Y, Barber GN, Fitzgerald KAet al. (2014) STING‐dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity 41, 830–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Galluzzi L, Buqué A, Kepp O, Zitvogel L & Kroemer G (2017) Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol 17, 97–111. [DOI] [PubMed] [Google Scholar]
- 63.Cui S, Reichner JS, Mateo RB & Albina JE (1994) Activated murine macrophages induce apoptosis in tumor cells through nitric oxide‐dependent or ‐independent mechanisms. Cancer Res 54, 2462–2467. [PubMed] [Google Scholar]
- 64.Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W, Huhn RD, Song W, Li D, Sharp LLet al. (2011) CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science 331, 1612–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lamagna C, Aurrand‐Lions M & Imhof BA (2006) Dual role of macrophages in tumor growth and angiogenesis. J Leukoc Biol 80, 705–713. [DOI] [PubMed] [Google Scholar]
- 66.Ribatti D (2017) The concept of immune surveillance against tumors. The first theories. Oncotarget 8, 7175–7180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Woo SR, Corrales L & Gajewski TF (2015) Innate immune recognition of cancer. Annu Rev Immunol 33, 445–474. [DOI] [PubMed] [Google Scholar]
- 68.Ong SM, Tan YC, Beretta O, Jiang D, Yeap WH, Tai JJY, Wong WC, Yang H, Schwarz H, Lim KHet al. (2012) Macrophages in human colorectal cancer are pro‐inflammatory and prime T cells towards an anti‐tumour type‐1 inflammatory response. Eur J Immunol 42, 89–100. [DOI] [PubMed] [Google Scholar]
- 69.Ålgars A, Irjala H, Vaittinen S, Huhtinen H, Sundström J, Salmi M, Ristamäki R & Jalkanen S (2012) Type and location of tumor‐infiltrating macrophages and lymphatic vessels predict survival of colorectal cancer patients. Int J Cancer 131, 864–873. [DOI] [PubMed] [Google Scholar]
- 70.Ohri CM, Shikotra A, Green RH, Waller DA & Bradding P (2009) Macrophages within NSCLC tumour islets are predominantly of a cytotoxic M1 phenotype associated with extended survival. Eur Respir J 33, 118–126. [DOI] [PubMed] [Google Scholar]
- 71.Ma J, Liu L, Che G, Yu N, Dai F & You Z (2010) The M1 form of tumor‐associated macrophages in non‐small cell lung cancer is positively associated with survival time. BMC Cancer 10, 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mantovani A, Sozzani S, Locati M, Allavena P & Sica A (2002) Macrophage polarization: tumor‐associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol 23, 549–555. [DOI] [PubMed] [Google Scholar]
- 73.Ratnam NM, Peterson JM, Talbert EE, Ladner KJ, Rajasekera PV, Schmidt CR, Dillhoff ME, Swanson BJ, Haverick E, Kladney RDet al. (2017) NF‐κB regulates GDF‐15 to suppress macrophage surveillance during early tumor development. J Clin Invest 127, 3796–3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Modi BG, Neustadter J, Binda E, Lewis J, Filler RB, Roberts SJ, Kwong BY, Reddy S, Overton JD, Galan Aet al. (2012) Langerhans cells facilitate epithelial DNA damage and squamous cell carcinoma. Science 335, 104–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Radisky DC, Levy DD, Littlepage LE, Liu H, Nelson CM, Fata JE, Leake D, Godden EL, Albertson DG, Nieto MAet al. (2005) Rac1b and reactive oxygen species mediate MMP‐3‐induced EMT and genomic instability. Nature 436, 123–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Carron EC, Homra S, Rosenberg J, Coffelt SB, Kittrell F, Zhang Y, Creighton CJ, Fuqua SA, Medina D & Machado HL (2017) Macrophages promote the progression of premalignant mammary lesions to invasive cancer. Oncotarget 8, 50731–50746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Colotta F, Allavena P, Sica A, Garlanda C & Mantovani A (2009) Cancer‐related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis 30, 1073–1081. [DOI] [PubMed] [Google Scholar]
- 78.Lin EY, Nguyen AV, Russell RG & Pollard JW (2001) Colony‐stimulating factor 1 promotes progression of mammary tumors to malignancy. J Exp Med 193, 727–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Clark CE, Hingorani SR, Mick R, Combs C, Tuveson DA & Vonderheide RH (2007) Dynamics of the immune reaction to pancreatic cancer from inception to invasion. Cancer Res 67, 9518–9527. [DOI] [PubMed] [Google Scholar]
- 80.Liou GY, Bastea L, Fleming A, Döppler H, Edenfield BH, Dawson DW, Zhang L, Bardeesy N & Storz P (2017) The presence of interleukin‐13 at pancreatic ADM/PanIN lesions alters macrophage populations and mediates pancreatic tumorigenesis. Cell Rep 19, 1322–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kuang DM, Zhao Q, Peng C, Xu J, Zhang JP, Wu C & Zheng L (2009) Activated monocytes in peritumoral stroma of hepatocellular carcinoma foster immune privilege and disease progression through PD‐L1. J Exp Med 206, 1327–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ruffell B, Chang‐Strachan D, Chan V, Rosenbusch A, Ho CMT, Pryer N, Daniel D, Hwang ES, Rugo HS & Coussens LM (2014) Macrophage IL‐10 blocks CD8+ T cell‐dependent responses to chemotherapy by suppressing IL‐12 expression in intratumoral dendritic cells. Cancer Cell 26, 623–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zeni E, Mazzetti L, Miotto D, Lo Cascio N, Maestrelli P, Querzoli P, Pedriali M, De Rosa E, Fabbri LM, Mapp CEet al. (2007) Macrophage expression of interleukin‐10 is a prognostic factor in nonsmall cell lung cancer. Eur Respir J 30, 627–632. [DOI] [PubMed] [Google Scholar]
- 84.Munn DH & Mellor AL (2016) IDO in the tumor microenvironment: inflammation, counter‐regulation, and tolerance. Trends Immunol 37, 193–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sica A, Saccani A, Bottazzi B, Polentarutti N, Vecchi A, Van Damme J & Mantovani A (2000) Autocrine production of IL‐10 mediates defective IL‐12 production and NF‐κB activation in tumor‐associated macrophages. J Immunol 164, 762–767. [DOI] [PubMed] [Google Scholar]
- 86.Rivera LB, Bradshaw AD & Brekken RA (2011) The regulatory function of SPARC in vascular biology. Cell Mol Life Sci 68, 3165–3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bayne LJ, Beatty GL, Jhala N, Clark CE, Rhim AD, Stanger BZ & Vonderheide RH (2012) Tumor‐derived granulocyte‐macrophage colony‐stimulating factor regulates myeloid inflammation and T cell immunity in pancreatic cancer. Cancer Cell 21, 822–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Qiu SQ, Waaijer SJH, Zwager MC, de Vries EGE, van der Vegt B & Schröder CP (2018) Tumor‐associated macrophages in breast cancer: innocent bystander or important player? Cancer Treat Rev 70, 178–189. [DOI] [PubMed] [Google Scholar]
- 89.Schmidt A, Zhang XM, Joshi RN, Iqbal S, Wahlund C, Gabrielsson S, Harris RA & Tegnér J (2016) Human macrophages induce CD4+ Foxp3+ regulatory T cells via binding and re‐release of TGF‐β. Immunol Cell Biol 94, 747–762. [DOI] [PubMed] [Google Scholar]
- 90.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon‐Hogan M, Conejo‐Garcia JR, Zhang L, Burow Met al. (2004) Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med 10, 942–949. [DOI] [PubMed] [Google Scholar]
- 91.Movahedi K, Laoui D, Gysemans C, Baeten M, Stangé G, Van Den Bossche J, Mack M, Pipeleers D, In't Veld P, De Baetselier Pet al. (2010) Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Res 70, 5728–5739. [DOI] [PubMed] [Google Scholar]
- 92.Hussain SP, Hofseth LJ & Harris CC (2003) Radical causes of cancer. Nat Rev Cancer 3, 276–285. [DOI] [PubMed] [Google Scholar]
- 93.Jaiswal M, LaRusso NF, Burgart LJ & Gores GJ (2000) Inflammatory cytokines induce DNA damage and inhibit DNA repair in cholangiocarcinoma cells by a nitric oxide‐dependent mechanism. Cancer Res 60, 184–190. [PubMed] [Google Scholar]
- 94.Jaiswal M, Larusso NF & Gores GJ (2001) Nitric oxide in gastrointestinal epithelial cell carcinogenesis: linking inflammation to oncogenesis. Am J Physiol Gastrointest Liver Physiol 281, G626–G634. [DOI] [PubMed] [Google Scholar]
- 95.O'Sullivan C, Lewis CE, McGee JOD & Harris AL (1993) Secretion of epidermal growth factor by macrophages associated with breast carcinoma. Lancet 342, 148–149. [DOI] [PubMed] [Google Scholar]
- 96.Lu H, Clauser KR, Tam WL, Fröse J, Ye X, Eaton EN, Reinhardt F, Donnenberg VS, Bhargava R, Carr SAet al. (2014) A breast cancer stem cell niche supported by juxtacrine signalling from monocytes and macrophages. Nat Cell Biol 16, 1105–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Greten FR & Grivennikov SI (2019) Inflammation and cancer: triggers, mechanisms, and consequences. Immunity 51, 27–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Goswami S, Sahai E, Wyckoff JB, Cammer M, Cox D, Pixley FJ, Stanley ER, Segall JE & Condeelis JS (2005) Macrophages promote the invasion of breast carcinoma cells via a colony‐stimulating factor‐1/epidermal growth factor paracrine loop. Cancer Res 65, 5278–5283. [DOI] [PubMed] [Google Scholar]
- 99.Sodir NM, Kortlever RM, Barthet VJA, Campos T, Pellegrinet L, Kupczak S, Anastasiou P, Swigart LB, Soucek L, Arends MJet al. (2020) MYC instructs and maintains pancreatic adenocarcinoma phenotype. Cancer Discov 10, 588–607. [DOI] [PubMed] [Google Scholar]
- 100.Gomes AM, Carron EC, Mills KL, Dow AM, Gray Z, Fecca CR, Lakey MA, Carmeliet P, Kittrell F, Medina Det al. (2019) Stromal Gas6 promotes the progression of premalignant mammary cells. Oncogene 38, 2437–2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bossi P, Coggi G, Viale G, Viale G, Alfano RM, Lee AKC, Lee AKC & Bosari S (1995) Angiogenesis in colorectal tumors: microvessel quantitation in adenomas and carcinomas with clinicopathological correlations. Cancer Res 55, 5049–5053. [PubMed] [Google Scholar]
- 102.Bluff JE, Menakuru SR, Cross SS, Higham SE, Balasubramanian SP, Brown NJ, Reed MW & Staton CA (2009) Angiogenesis is associated with the onset of hyperplasia in human ductal breast disease. Br J Cancer 101, 666–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.De Palma M, Biziato D & Petrova TV (2017) Microenvironmental regulation of tumour angiogenesis. Nat Rev Cancer 17, 457–474. [DOI] [PubMed] [Google Scholar]
- 104.De Palma M, Venneri MA, Galli R, Sergi LS, Politi LS, Sampaolesi M & Naldini L (2005) Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell 8, 211–226. [DOI] [PubMed] [Google Scholar]
- 105.Coffelt SB, Tal AO, Scholz A, De Palma M, Patel S, Urbich C, Biswas SK, Murdoch C, Plate KH, Reiss Yet al. (2010) Angiopoietin‐2 regulates gene expression in TIE2‐expressing monocytes and augments their inherent proangiogenic functions. Cancer Res 70, 5270–5280. [DOI] [PubMed] [Google Scholar]
- 106.Linde N, Casanova‐Acebes M, Sosa MS, Mortha A, Rahman A, Farias E, Harper K, Tardio E, Reyes Torres I, Jones Jet al. (2018) Macrophages orchestrate breast cancer early dissemination and metastasis. Nat Commun 9, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lewis JS, Landers RJ, Underwood JCE, Harris AL & Lewis CE (2000) Expression of vascular endothelial growth factor by macrophages is up‐regulated in poorly vascularized areas of breast carcinomas. J Pathol 192, 150–158. [DOI] [PubMed] [Google Scholar]
- 108.Shojaei F, Wu X, Zhong C, Yu L, Liang XH, Yao J, Blanchard D, Bais C, Peale FV, Van Bruggen Net al. (2007) Bv8 regulates myeloid‐cell‐dependent tumour angiogenesis. Nature 450, 825–831. [DOI] [PubMed] [Google Scholar]
- 109.Stockmann C, Doedens A, Weidemann A, Zhang N, Takeda N, Greenberg JI, Cheresh DA & Johnson RS (2008) Deletion of vascular endothelial growth factor in myeloid cells accelerates tumorigenesis. Nature 456, 814–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Schwertfeger KL, Xian W, Kaplan AM, Burnett SH, Cohen DA & Rosen JM (2006) A critical role for the inflammatory response in a mouse model of preneoplastic progression. Cancer Res 66, 5676–5685. [DOI] [PubMed] [Google Scholar]
- 111.Lin EY, Li JF, Gnatovskiy L, Deng Y, Zhu L, Grzesik DA, Qian H, Xue XN & Pollard JW (2006) Macrophages regulate the angiogenic switch in a mouse model of breast cancer. Cancer Res 66, 11238–11246. [DOI] [PubMed] [Google Scholar]
- 112.Huang S, Van Arsdall M, Tedjarati S, McCarty M, Wu W, Langley R & Fidler IJ (2002) Contributions of stromal metalloproteinase‐9 to angiogenesis and growth of human ovarian carcinoma in mice. J Natl Cancer Inst 94, 1134–1142. [DOI] [PubMed] [Google Scholar]
- 113.Oguma K, Oshima H, Aoki M, Uchio R, Naka K, Nakamura S, Hirao A, Saya H, Taketo MM & Oshima M (2008) Activated macrophages promote Wnt signalling through tumour necrosis factor‐α in gastric tumour cells. EMBO J 27, 1671–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Deryugina EI & Quigley JP (2010) Pleiotropic roles of matrix metalloproteinases in tumor angiogenesis: contrasting, overlapping and compensatory functions. Biochim Biophys Acta 1803, 103–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, Tanzawa K, Thorpe P, Itohara S, Werb Zet al. (2000) Matrix metalloproteinase‐9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol 2, 737–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SFT, Csiszar K, Giaccia A, Weninger Wet al. (2009) Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 139, 891–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Provenzano PP, Inman DR, Eliceiri KW, Knittel JG, Yan L, Rueden CT, White JG & Keely PJ (2008) Collagen density promotes mammary tumor initiation and progression. BMC Med 6, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wyckoff JB, Wang Y, Lin EY, Li JF, Goswami S, Stanley ER, Segall JE, Pollard JW & Condeelis J (2007) Direct visualization of macrophage‐assisted tumor cell intravasation in mammary tumors. Cancer Res 67, 2649–2656. [DOI] [PubMed] [Google Scholar]
- 119.Afik R, Zigmond E, Vugman M, Klepfish M, Shimshoni E, Pasmanik‐Chor M, Shenoy A, Bassat E, Halpern Z, Geiger Tet al. (2016) Tumor macrophages are pivotal constructors of tumor collagenous matrix. J Exp Med 213, 2315–2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Liguori M, Solinas G, Germano G, Mantovani A & Allavena P (2011) Tumor‐associated macrophages as incessant builders and destroyers of the cancer stroma. Cancers (Basel) 3, 3740–3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Shao R, Bao S, Bai X, Blanchette C, Anderson RM, Dang T, Gishizky ML, Marks JR & Wang X‐F (2004) Acquired expression of periostin by human breast cancers promotes tumor angiogenesis through up‐regulation of vascular endothelial growth factor receptor 2 expression. Mol Cell Biol 24, 3992–4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Saupe F, Schwenzer A, Jia Y, Gasser I, Spenlé C, Langlois B, Kammerer M, Lefebvre O, Hlushchuk R, Rupp Tet al. (2013) Tenascin‐C downregulates Wnt inhibitor Dickkopf‐1, promoting tumorigenesis in a neuroendocrine tumor model. Cell Rep 5, 482–492. [DOI] [PubMed] [Google Scholar]
- 123.Bonnans C, Chou J & Werb Z (2014) Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol 15, 786–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mammoto A, Connor KM, Mammoto T, Yung CW, Huh D, Aderman CM, Mostoslavsky G, Smith LEH & Ingber DE (2009) A mechanosensitive transcriptional mechanism that controls angiogenesis. Nature 457, 1103–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Burke RM, Madden KS, Perry SW, Zettel ML & Brown EB (2013) Tumor‐associated macrophages and stromal TNF‐α regulate collagen structure in a breast tumor model as visualized by second harmonic generation. J Biomed Opt 18, 086003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhu Y, Herndon JM, Sojka DK, Kim KW, Knolhoff BL, Zuo C, Cullinan DR, Luo J, Bearden AR, Lavine KJet al. (2017) Tissue‐resident macrophages in pancreatic ductal adenocarcinoma originate from embryonic hematopoiesis and promote tumor progression. Immunity 47, 323–338.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ingman WV, Wyckoff J, Gouon‐Evans V, Condeelis J & Pollard JW (2006) Macrophages promote collagen fibrillogenesis around terminal end buds of the developing mammary gland. Dev Dyn 235, 3222–3229. [DOI] [PubMed] [Google Scholar]
- 128.Jiang D & Lim SY (2016) Influence of immune myeloid cells on the extracellular matrix during cancer metastasis. Cancer Microenviron 9, 45–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Atai NA, Bansal M, Lo C, Bosman J, Tigchelaar W, Bosch KS, Jonker A, De Witt Hamer PC, Troost D, McCulloch CAet al. (2011) Osteopontin is up‐regulated and associated with neutrophil and macrophage infiltration in glioblastoma. Immunology 132, 39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sangaletti S, Stoppacciaro A, Guiducci C, Torrisi MR & Colombo MP (2003) Leukocyte, rather than tumor‐produced SPARC, determines stroma and collagen type IV deposition in mammary carcinoma. J Exp Med 198, 1475–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Varol C & Sagi I (2018) Phagocyte—extracellular matrix crosstalk empowers tumor development and dissemination. FEBS J 285, 734–751. [DOI] [PubMed] [Google Scholar]
- 132.Glasner A, Levi A, Enk J, Isaacson B, Viukov S, Orlanski S, Scope A, Neuman T, Enk CD, Hanna JHet al. (2018) Mandelboim, NKp46 receptor‐mediated interferon‐γ production by natural killer cells increases fibronectin 1 to alter tumor architecture and control metastasis. Immunity 48, 107–119.e4. [DOI] [PubMed] [Google Scholar]
- 133.Smyth MJ, Thia KYT, Street SEA, Cretney E, Trapani JA, Taniguchi M, Kawano T, Pelikan SB, Crowe NY & Godfrey DI (2000) Differential tumor surveillance by natural killer (NK) and NKT cells. J Exp Med 191, 661–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Finnberg N, Klein‐Szanto AJP & El‐Deiry WS (2008) TRAIL‐R deficiency in mice promotes susceptibility to chronic inflammation and tumorigenesis. J Clin Invest 118, 111–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kärre K, Ljunggren HG, Piontek G & Kiessling R (1986) Selective rejection of H‐2‐deficient lymphoma variants suggests alternative immune defence strategy. Nature 319, 675–678. [DOI] [PubMed] [Google Scholar]
- 136.Hsu J, Hodgins JJ, Marathe M, Nicolai CJ, Bourgeois‐Daigneault MC, Trevino TN, Azimi CS, Scheer AK, Randolph HE, Thompson TWet al. (2018) Contribution of NK cells to immunotherapy mediated by PD‐1/PD‐L1 blockade. J Clin Invest 128, 4654–4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Miller GM, Andres ML & Gridley DS (2003) NK cell depletion results in accelerated tumor growth and attenuates the antitumor effect of total body irradiation. Int J Oncol 23, 1585–1592. [PubMed] [Google Scholar]
- 138.Imai K, Matsuyama S, Miyake S, Suga K & Nakachi K (2000) Natural cytotoxic activity of peripheral‐blood lymphocytes and cancer incidence: an 11‐year follow‐up study of a general population. Lancet 356, 1795–1799. [DOI] [PubMed] [Google Scholar]
- 139.Soriani A, Zingoni A, Cerboni C, Lannitto ML, Ricciardi MR, Di Gialleonardo V, Cippitelli M, Fionda C, Petrucci MT, Guarini Aet al. (2009) ATM‐ATR‐dependent up‐regulation of DNAM‐1 and NKG2D ligands on multiple myeloma cells by therapeutic agents results in enhanced NK‐cell susceptibility and is associated with a senescent phenotype. Blood 113, 3503–3511. [DOI] [PubMed] [Google Scholar]
- 140.Gasser S, Orsulic S, Brown EJ & Raulet DH (2005) The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature 436, 1186–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bauer S, Groh V, Wu J, Steinle A, Phillips JH, Lanier LL & Spies T (1999) Activation of NK cells and T cells by NKG2D, a receptor for stress‐ inducible MICA. Science 285, 727–729. [DOI] [PubMed] [Google Scholar]
- 142.Guerra N, Tan YX, Joncker NT, Choy A, Gallardo F, Xiong N, Knoblaugh S, Cado D, Greenberg NR & Raulet DH (2008) NKG2D‐deficient mice are defective in tumor surveillance in models of spontaneous malignancy. Immunity 28, 571–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Müller L, Aigner P & Stoiber D (2017) Type I interferons and natural killer cell regulation in cancer. Front Immunol 8, 304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lakshmikanth T, Burke S, Ali TH, Kimpfler S, Ursini F, Ruggeri L, Capanni M, Umansky V, Paschen A, Sucker Aet al. (2009) NCRs and DNAM‐1 mediate NK cell recognition and lysis of human and mouse melanoma cell lines in vitro and in vivo. J Clin Invest 119, 1251–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Dadi S, Chhangawala S, Whitlock BM, Franklin RA, Luo CT, Oh SA, Toure A, Pritykin Y, Huse M, Leslie CSet al. (2016) Cancer immunosurveillance by tissue‐resident innate lymphoid cells and innate‐like T cells. Cell 164, 365–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Böttcher JP, Bonavita E, Chakravarty P, Blees H, Cabeza‐Cabrerizo M, Sammicheli S, Rogers NC, Sahai E, Zelenay S & Reis e Sousa C(2018) NK cells stimulate recruitment of cDC1 into the tumor microenvironment promoting cancer immune control. Cell 172, 1022–1037.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Maurer S, Kropp KN, Klein G, Steinle A, Haen SP, Walz JS, Hinterleitner C, Märklin M, Kopp HG & Salih HR (2018) Platelet‐mediated shedding of NKG2D ligands impairs NK cell immune‐surveillance of tumor cells. Oncoimmunology 7, e1364827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Nieswandt B, Hafner M, Echtenacher B & Männel DN (1999) Lysis of tumor cells by natural killer cells in mice is impeded by platelets. Cancer Res 59, 1295–1300. [PubMed] [Google Scholar]
- 149.Gao Y, Souza‐Fonseca‐Guimaraes F, Bald T, Ng SS, Young A, Ngiow SF, Rautela J, Straube J, Waddell N, Blake SJet al. (2017) Tumor immunoevasion by the conversion of effector NK cells into type 1 innate lymphoid cells. Nat Immunol 18, 1004–1015. [DOI] [PubMed] [Google Scholar]
- 150.Bengsch B, Ohtani T, Herati RS, Bovenschen N, Chang KM & Wherry EJ (2018) Deep immune profiling by mass cytometry links human T and NK cell differentiation and cytotoxic molecule expression patterns. J Immunol Methods 453, 3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lee J‐C, Lee K‐M, Kim D‐W & Heo DS (2004) Elevated TGF‐β1 secretion and down‐modulation of NKG2D underlies impaired NK cytotoxicity in cancer patients. J Immunol 172, 7335–7340. [DOI] [PubMed] [Google Scholar]
- 152.Schwinn N, Vokhminova D, Sucker A, Textor S, Striegel S, Moll I, Nausch N, Tuettenberg J, Steinle A, Cerwenka Aet al. (2009) Interferon‐γ down‐regulates NKG2D ligand expression and impairs the NKG2D‐mediated cytolysis of MHC class I‐deficient melanoma by natural killer cells. Int J Cancer 124, 1594–1604. [DOI] [PubMed] [Google Scholar]
- 153.Chiossone L, Dumas PY, Vienne M & Vivier E (2018) Natural killer cells and other innate lymphoid cells in cancer. Nat Rev Immunol 18, 671–688. [DOI] [PubMed] [Google Scholar]
- 154.Simoni Y, Fehlings M, Kløverpris HN, McGovern N, Koo SL, Loh CY, Lim S, Kurioka A, Fergusson JR, Tang CLet al. (2017) Human innate lymphoid cell subsets possess tissue‐type based heterogeneity in phenotype and frequency. Immunity 46, 148–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wagner M & Koyasu S (2019) Cancer immunoediting by innate lymphoid cells. Trends Immunol 40, 415–430. [DOI] [PubMed] [Google Scholar]
- 156.Eisenring M, Vom Berg J, Kristiansen G, Saller E & Becher B (2010) IL‐12 initiates tumor rejection via lymphoid tissue‐inducer cells bearing the natural cytotoxicity receptor NKp46. Nat Immunol 11, 1030–1038. [DOI] [PubMed] [Google Scholar]
- 157.Carrega P, Loiacono F, Di Carlo E, Scaramuccia A, Mora M, Conte R, Benelli R, Spaggiari GM, Cantoni C, Campana Set al. (2015) NCR + ILC3 concentrate in human lung cancer and associate with intratumoral lymphoid structures. Nat Commun 6, 8280. [DOI] [PubMed] [Google Scholar]
- 158.Kirchberger S, Royston DJ, Boulard O, Thornton E, Franchini F, Szabady RL, Harrison O & Powrie F (2013) Innate lymphoid cells sustain colon cancer through production of interleukin‐22 in a mouse model. J Exp Med 210, 917–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Jamieson T, Clarke M, Steele CW, Samuel MS, Neumann J, Jung A, Huels D, Olson MF, Das S, Nibbs RJBet al. (2012) Inhibition of CXCR2 profoundly suppresses inflammation‐driven and spontaneous tumorigenesis. J Clin Invest 122, 3127–3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Katoh H, Wang D, Daikoku T, Sun H, Dey SK & DuBois RN (2013) CXCR2‐expressing myeloid‐derived suppressor cells are essential to promote colitis‐associated tumorigenesis. Cancer Cell 24, 631–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Masucci MT, Minopoli M & Carriero MV (2019) Tumor associated neutrophils. Their role in tumorigenesis, metastasis, prognosis and therapy. Front Oncol 9, 1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Granot Z, Henke E, Comen EA, King TA, Norton L & Benezra R (2011) Tumor entrained neutrophils inhibit seeding in the premetastatic lung. Cancer Cell 20, 300–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Granot Z (2019) Neutrophils as a therapeutic target in cancer. Front Immunol 10, 1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Eruslanov EB, Bhojnagarwala PS, Quatromoni JG, Stephen TL, Ranganathan A, Deshpande C, Akimova T, Vachani A, Litzky L, Hancock WWet al. (2014) Tumor‐associated neutrophils stimulate T cell responses in early‐stage human lung cancer. J Clin Invest 124, 5466–5480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Andzinski L, Kasnitz N, Stahnke S, Wu CF, Gereke M, Von Köckritz‐Blickwede M, Schilling B, Brandau S, Weiss S & Jablonska J (2016) Type I IFNs induce anti‐tumor polarization of tumor associated neutrophils in mice and human. Int J Cancer 138, 1982–1993. [DOI] [PubMed] [Google Scholar]
- 166.Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, Worthen GS & Albelda SM (2009) Polarization of tumor‐associated neutrophil phenotype by TGF‐β: “N1” versus “N2” TAN. Cancer Cell 16, 183–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Shaul ME, Levy L, Sun J, Mishalian I, Singhal S, Kapoor V, Horng W, Fridlender G, Albelda SM & Fridlender ZG (2016) Tumor‐associated neutrophils display a distinct N1 profile following TGFβ modulation: a transcriptomics analysis of pro‐ vs. antitumor TANs. Oncoimmunology 5, e1232221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Canli Ö, Nicolas AM, Gupta J, Finkelmeier F, Goncharova O, Pesic M, Neumann T, Horst D, Löwer M, Sahin Uet al. (2017) Myeloid cell‐derived reactive oxygen species induce epithelial mutagenesis. Cancer Cell 32, 869–883.e5. [DOI] [PubMed] [Google Scholar]
- 169.Gong L, Cumpian AM, Caetano MS, Ochoa CE, De la Garza MM, Lapid DJ, Mirabolfathinejad SG, Dickey BF, Zhou Q & Moghaddam SJ (2013) Promoting effect of neutrophils on lung tumorigenesis is mediated by CXCR2 and neutrophil elastase. Mol Cancer 12, 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Lerman I, De La Luz Garcia‐Hernandez M, Rangel‐Moreno J, Chiriboga L, Pan C, Nastiuk KL, Krolewski JJ, Sen A & Hammes SR (2017) Infiltrating myeloid cells exert protumorigenic actions via neutrophil elastase. Mol Cancer Res 15, 1138–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Johnson DE, O'Keefe RA & Grandis JR (2018) Targeting the IL‐6/JAK/STAT3 signalling axis in cancer. Nat Rev Clin Oncol 15, 234–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Wang TT, Zhao YL, Peng LS, Chen N, Chen W, Lv YP, Mao FY, Zhang JY, Cheng P, Teng YSet al. (2017) Tumour‐activated neutrophils in gastric cancer foster immune suppression and disease progression through GM‐CSF‐PD‐L1 pathway. Gut 66, 1900–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Mishalian I, Bayuh R, Levy L, Zolotarov L, Michaeli J & Fridlender ZG (2013) Tumor‐associated neutrophils (TAN) develop pro‐tumorigenic properties during tumor progression. Cancer Immunol Immunother 62, 1745–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Mishalian I, Bayuh R, Eruslanov E, Michaeli J, Levy L, Zolotarov L, Singhal S, Albelda SM, Granot Z & Fridlender ZG (2014) Neutrophils recruit regulatory T‐cells into tumors via secretion of CCL17 – a new mechanism of impaired antitumor immunity. Int J Cancer 135, 1178–1186. [DOI] [PubMed] [Google Scholar]
- 175.Rotondo R, Barisione G, Mastracci L, Grossi F, Orengo AM, Costa R, Truini M, Fabbi M, Ferrini S & Barbieri O (2009) IL‐8 induces exocytosis of arginase 1 by neutrophil polymorphonuclears in nonsmall cell lung cancer. Int J Cancer 125, 887–893. [DOI] [PubMed] [Google Scholar]
- 176.Queen MM, Ryan RE, Holzer RG, Keller‐Peck CR & Jorcyk CL (2005) Breast cancer cells stimulate neutrophils to produce oncostatin M: Potential implications for tumor progression. Cancer Res 65, 8896–8904. [DOI] [PubMed] [Google Scholar]
- 177.Ancelin M, Chollet‐Martin S, Hervé MA, Legrand C, El Benna J & Perrot‐Applanat M (2004) Vascular endothelial growth factor VEGF189 induces human neutrophil chemotaxis in extravascular tissue via an autocrine amplification mechanism. Lab Investig 84, 502–512. [DOI] [PubMed] [Google Scholar]
- 178.Nozawa H, Chiu C & Hanahan D (2006) Infiltrating neutrophils mediate the initial angiogenic switch in a mouse model of multistage carcinogenesis. Proc Natl Acad Sci USA 103, 12493–12498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Coussens LM & Werb Z (1996) Matrix metalloproteinases and the development of cancer. Chem Biol 3, 895–904. [DOI] [PubMed] [Google Scholar]
- 180.Razak NA, Elaskalani O & Metharom P (2017) Pancreatic cancer‐induced neutrophil extracellular traps: a potential contributor to cancer‐associated thrombosis. Int J Mol Sci 18, 487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Park J, Wysocki RW, Amoozgar Z, Maiorino L, Fein MR, Jorns J, Schott AF, Kinugasa‐Katayama Y, Lee Y, Won NHet al. (2016) Cancer cells induce metastasis‐supporting neutrophil extracellular DNA traps. Sci Transl Med 8, 361ra138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Albrengues J, Shields MA, Ng D, Park CG, Ambrico A, Poindexter ME, Upadhyay P, Uyeminami DL, Pommier A, Küttner Vet al. (2018) Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science 361, eaao4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Munir H, Jones J, Janowitz T, Martins C, Welsh S & Shields J (2020) Stromal amyloid β drives neutrophil extracellular trap formation to augment tumour growth. BioRxiv Cancer Biol. 10.1101/2020.01.10.901686 [DOI] [Google Scholar]
- 184.Broz ML, Binnewies M, Boldajipour B, Nelson AE, Pollack JL, Erle DJ, Barczak A, Rosenblum MD, Daud A, Barber DLet al. (2014) Dissecting the tumor myeloid compartment reveals rare activating antigen‐presenting cells critical for T cell immunity. Cancer Cell 26, 638–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Roberts EW, Broz ML, Binnewies M, Headley MB, Nelson AE, Wolf DM, Kaisho T, Bogunovic D, Bhardwaj N & Krummel MF (2016) Critical role for CD103+/CD141+ dendritic cells bearing CCR7 for tumor antigen trafficking and priming of T cell immunity in melanoma. Cancer Cell 30, 324–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Salmon H, Idoyaga J, Rahman A, Leboeuf M, Remark R, Jordan S, Casanova‐Acebes M, Khudoynazarova M, Agudo J, Tung Net al. (2016) Expansion and activation of CD103+ dendritic cell progenitors at the tumor site enhances tumor responses to therapeutic PD‐L1 and BRAF inhibition. Immunity 44, 924–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Colegio OR, Chu NQ, Szabo AL, Chu T, Rhebergen AM, Jairam V, Cyrus N, Brokowski CE, Eisenbarth SC, Phillips GMet al. (2014) Functional polarization of tumour‐associated macrophages by tumour‐derived lactic acid. Nature 513, 559–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Cubillos‐Ruiz JR, Silberman PC, Rutkowski MR, Chopra S, Perales‐Puchalt A, Song M, Zhang S, Bettigole SE, Gupta D, Holcomb Ket al. (2015) ER stress sensor XBP1 controls anti‐tumor immunity by disrupting dendritic cell homeostasis. Cell 161, 1527–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Herber DL, Cao W, Nefedova Y, Novitskiy SV, Nagaraj S, Tyurin VA, Corzo A, Cho HI, Celis E, Lennox Bet al. (2010) Lipid accumulation and dendritic cell dysfunction in cancer. Nat Med 16, 880–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Veglia F, Tyurin VA, Mohammadyani D, Blasi M, Duperret EK, Donthireddy L, Hashimoto A, Kapralov A, Amoscato A, Angelini Ret al. (2017) Lipid bodies containing oxidatively truncated lipids block antigen cross‐presentation by dendritic cells in cancer. Nat Commun 8, 2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Aspord C, Pedroza‐Gonzalez A, Gallegos M, Tindle S, Burton EC, Su D, Marches F, Banchereau J & Palucka AK (2007) Breast cancer instructs dendritic cells to prime interleukin 13‐secreting CD4+ T cells that facilitate tumor development. J Exp Med 204, 1037–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Sisirak V, Faget J, Gobert M, Goutagny N, Vey N, Treilleux I, Renaudineau S, Poyet G, Labidi‐Galy SI, Goddard‐Leon Set al. (2012) Impaired IFN‐α production by plasmacytoid dendritic cells favors regulatory T‐cell expansion that may contribute to breast cancer progression. Cancer Res 72, 5188–5197. [DOI] [PubMed] [Google Scholar]
- 193.Shalapour S & Karin M (2019) Pas de Deux: control of anti‐tumor immunity by cancer‐associated inflammation. Immunity 51, 15–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Lu C, Redd PS, Lee JR, Savage N & Liu K (2016) The expression profiles and regulation of PD‐L1 in tumor‐induced myeloid‐derived suppressor cells. Oncoimmunology 5, e1247135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Pallotta MT, Orabona C, Volpi C, Vacca C, Belladonna ML, Bianchi R, Servillo G, Brunacci C, Calvitti M, Bicciato Set al. (2011) Indoleamine 2,3‐dioxygenase is a signaling protein in long‐term tolerance by dendritic cells. Nat Immunol 12, 870–878. [DOI] [PubMed] [Google Scholar]
- 196.Sumpter TL, Dangi A, Matta BM, Huang C, Stolz DB, Vodovotz Y, Thomson AW & Gandhi CR (2012) Hepatic stellate cells undermine the allostimulatory function of liver myeloid dendritic cells via STAT3‐dependent induction of IDO. J Immunol 189, 3848–3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Geiger R, Rieckmann JC, Wolf T, Basso C, Feng Y, Fuhrer T, Kogadeeva M, Picotti P, Meissner F, Mann Met al. (2016) L‐arginine modulates T cell metabolism and enhances survival and anti‐tumor activity. Cell 167, 829–842.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Rodriguez PC, Quiceno DG & Ochoa AC (2007) L‐arginine availability regulates T‐lymphocyte cell‐cycle progression. Blood 109, 1568–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Molon B, Ugel S, Del Pozzo F, Soldani C, Zilio S, Avella D, De Palma A, Mauri PL, Monegal A, Rescigno Met al. (2011) Chemokine nitration prevents intratumoral infiltration of antigen‐specific T cells. J Exp Med 208, 1949–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Chaput N, Conforti R, Viaud S, Spatz A & Zitvogel L (2008) The Janus face of dendritic cells in cancer. Oncogene 27, 5920–5931. [DOI] [PubMed] [Google Scholar]
- 201.Kusmartsev S & Gabrilovich DI (2006) Effect of tumor‐derived cytokines and growth factors on differentiation and immune suppressive features of myeloid cells in cancer. Cancer Metastasis Rev 25, 323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Fukuyama T, Ichiki Y, Yamada S, Shigematsu Y, Baba T, Nagata Y, Mizukami M, Sugaya M, Takenoyama M, Hanagiri Tet al. (2007) Cytokine production of lung cancer cell lines: correlation between their production and the inflammatory/immunological responses both in vivo and in vitro. Cancer Sci 98, 1048–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Flier JS, Underhill LH & Dvorak HF (1986) Tumors: wounds that do not heal. N Engl J Med 315, 1650–1659. [DOI] [PubMed] [Google Scholar]
- 204.De Visser KE & Coussens LM (2005) The interplay between innate and adaptive immunity regulates cancer development. Cancer Immunol Immunother 54, 1143–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Erez N, Truitt M, Olson P & Hanahan D (2010) Cancer‐associated fibroblasts are activated in incipient neoplasia to orchestrate tumor‐promoting inflammation in an NF‐κB‐dependent manner. Cancer Cell 17, 135–147. [DOI] [PubMed] [Google Scholar]
- 206.Frede J, Greulich P, Nagy T, Simons BD & Jones PH (2016) A single dividing cell population with imbalanced fate drives oesophageal tumour growth. Nat Cell Biol 18, 967–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Lockwood DSR, Yeadon TM, Clouston AD, Crawford DG, Fawcett J, Callaghan SA & Gotley DC (2003) Tumor progression in hepatocellular carcinoma: relationship with tumor stroma and parenchymal disease. J Gastroenterol Hepatol 18, 666–672. [DOI] [PubMed] [Google Scholar]
- 208.Neesse A, Algül H, Tuveson DA & Gress TM (2015) Stromal biology and therapy in pancreatic cancer: a changing paradigm. Gut 64, 1476–1484. [DOI] [PubMed] [Google Scholar]
- 209.Bhowmick NA, Neilson EG & Moses HL (2004) Stromal fibroblasts in cancer initiation and progression. Nature 432, 332–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Olumi AF, Grossfeld GD, Hayward SW, Carroll PR, Tlsty TD & Cunha GR (1999) Carcinoma‐associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res 59, 5002–5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Olumi A, Grossfeld G, Hayward S, Carroll P, Cunha G, Hein P & Tlsty T (2000) Carcinoma‐associated fibroblasts stimulate tumor progression of initiated human epithelium. Breast Cancer Res 2, S19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Pidsley R, Lawrence MG, Zotenko E, Niranjan B, Statham A, Song J, Chabanon RM, Qu W, Wang H, Richards Met al. (2018) Enduring epigenetic landmarks define the cancer microenvironment. Genome Res 28, 625–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Bauer M, Su G, Casper C, He R, Rehrauer W & Friedl A (2010) Heterogeneity of gene expression in stromal fibroblasts of human breast carcinomas and normal breast. Oncogene 29, 1732–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Lakins MA, Ghorani E, Munir H, Martins CP & Shields JD (2018) Cancer‐associated fibroblasts induce antigen‐specific deletion of CD8+ T Cells to protect tumour cells. Nat Commun 9, 948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Kraman M, Bambrough PJ, Arnold JN, Roberts EW, Magiera L, Jones JO, Gopinathan A, Tuveson DA & Fearon DT (2010) Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein‐α. Science 330, 827–830. [DOI] [PubMed] [Google Scholar]
- 216.Feig C, Jones JO, Kraman M, Wells RJB, Deonarine A, Chan DS, Connell CM, Roberts EW, Zhao Q, Caballero OLet al. (2013) Targeting CXCL12 from FAP‐expressing carcinoma‐associated fibroblasts synergizes with anti‐PD‐L1 immunotherapy in pancreatic cancer. Proc Natl Acad Sci USA 110, 20212–20217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Elyada E, Bolisetty M, Laise P, Flynn WF, Courtois ET, Burkhart RA, Teinor JA, Belleau P, Biffi G, Lucito MSet al. (2019) Cross‐species single‐cell analysis of pancreatic ductal adenocarcinoma reveals antigen‐presenting cancer‐associated fibroblasts. Cancer Discov 9, 1102–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Calvo F, Ege N, Grande‐Garcia A, Hooper S, Jenkins RP, Chaudhry SI, Harrington K, Williamson P, Moeendarbary E, Charras Get al. (2013) Mechanotransduction and YAP‐dependent matrix remodelling is required for the generation and maintenance of cancer‐associated fibroblasts. Nat Cell Biol 15, 637–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Goetz JG, Minguet S, Navarro‐Lérida I, Lazcano JJ, Samaniego R, Calvo E, Tello M, Osteso‐Ibáñez T, Pellinen T, Echarri Aet al. (2011) Biomechanical remodeling of the microenvironment by stromal caveolin‐1 favors tumor invasion and metastasis. Cell 146, 148–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Chen WJ, Ho CC, Chang YL, Chen HY, Lin CA, Ling TY, Yu SL, Yuan SS, Louisa Chen YJ, Lin CYet al. (2014) Cancer‐associated fibroblasts regulate the plasticity of lung cancer stemness via paracrine signalling. Nat Commun 5, 3472. [DOI] [PubMed] [Google Scholar]
- 221.Yu Y, Xiao CH, Tan LD, Wang QS, Li XQ & Feng YM (2014) Cancer‐associated fibroblasts induce epithelial‐mesenchymal transition of breast cancer cells through paracrine TGF‐β signalling. Br J Cancer 110, 724–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Zhuang J, Lu Q, Shen B, Huang X, Shen L, Zheng X, Huang R, Yan J & Guo H (2015) TGFβ1 secreted by cancer‐associated fibroblasts induces epithelial‐mesenchymal transition of bladder cancer cells through lncRNA‐ZEB2NAT. Sci Rep 5, 11924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Gaggioli C, Hooper S, Hidalgo‐Carcedo C, Grosse R, Marshall JF, Harrington K & Sahai E (2007) Fibroblast‐led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nat Cell Biol 9, 1392–1400. [DOI] [PubMed] [Google Scholar]
- 224.Tao L, Huang G, Wang R, Pan Y, He Z, Chu X, Song H & Chen L (2016) Cancer‐associated fibroblasts treated with cisplatin facilitates chemoresistance of lung adenocarcinoma through IL‐11/IL‐11R/STAT3 signaling pathway. Sci Rep 6, 38408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Su S, Chen J, Yao H, Liu J, Yu S, Lao L, Wang M, Luo M, Xing Y, Chen Fet al. (2018) CD10+GPR77+ cancer‐associated fibroblasts promote cancer formation and chemoresistance by sustaining cancer stemness. Cell 172, 841–856.e16. [DOI] [PubMed] [Google Scholar]
- 226.Vennin C, Mélénec P, Rouet R, Nobis M, Cazet AS, Murphy KJ, Herrmann D, Reed DA, Lucas MC, Warren SCet al. (2019) CAF hierarchy driven by pancreatic cancer cell p53‐status creates a pro‐metastatic and chemoresistant environment via perlecan. Nat Commun 10, 3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Marusyk A, Tabassum DP, Janiszewska M, Place AE, Trinh A, Rozhok AI, Pyne S, Guerriero JL, Shu S, Ekram Met al. (2016) Spatial proximity to fibroblasts impacts molecular features and therapeutic sensitivity of breast cancer cells influencing clinical outcomes. Cancer Res. 76, 6495–6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Raz Y, Cohen N, Shani O, Bell RE, Novitskiy SV, Abramovitz L, Levy C, Milyavsky M, Leider‐Trejo L, Moses HLet al. (2018) Bone marrow‐derived fibroblasts are a functionally distinct stromal cell population in breast cancer. J Exp Med 215, 3075–3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Arina A, Idel C, Hyjek EM, Alegre ML, Wang Y, Bindokas VP, Weichselbaum RR & Schreiber H (2016) Tumor‐associated fibroblasts predominantly come from local and not circulating precursors. Proc Natl Acad Sci USA 113, 7551–7556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Bochet L, Lehuédé C, Dauvillier S, Wang YY, Dirat B, Laurent V, Dray C, Guiet R, Maridonneau‐Parini I, Le Gonidec Set al. (2013) Adipocyte‐derived fibroblasts promote tumor progression and contribute to the desmoplastic reaction in breast cancer. Cancer Res 73, 5657–5668. [DOI] [PubMed] [Google Scholar]
- 231.Bartoschek M, Oskolkov N, Bocci M, Lövrot J, Larsson C, Sommarin M, Madsen CD, Lindgren D, Pekar G, Karlsson Get al. (2018) Spatially and functionally distinct subclasses of breast cancer‐associated fibroblasts revealed by single cell RNA sequencing. Nat Commun 9, 5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Rhim AD, Oberstein PE, Thomas DH, Mirek ET, Palermo CF, Sastra SA, Dekleva EN, Saunders T, Becerra CP, Tattersall IWet al. (2014) Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell 25, 735–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Özdemir BC, Pentcheva‐Hoang T, Carstens JL, Zheng X, Wu CC, Simpson TR, Laklai H, Sugimoto H, Kahlert C, Novitskiy SVet al. (2014) Depletion of carcinoma‐associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell 25, 719–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Cadamuro M, Nardo G, Indraccolo S, Dall'Olmo L, Sambado L, Moserle L, Franceschet I, Colledan M, Massani M, Stecca Tet al. (2013) Platelet‐derived growth factor‐D and Rho GTPases regulate recruitment of cancer‐associated fibroblasts in cholangiocarcinoma. Hepatology 58, 1042–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Procopio MG, Laszlo C, Al Labban D, Kim DE, Bordignon P, Jo SH, Goruppi S, Menietti E, Ostano P, Ala Uet al. (2015) Combined CSL and p53 downregulation promotes cancer‐associated fibroblast activation. Nat Cell Biol 17, 1193–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Bhowmick NA, Chytil A, Plieth D, Gorska AE, Dumont N, Shappell S, Washington MK, Neilson EG & Moses HL (2004) TGF‐β signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science 303, 848–851. [DOI] [PubMed] [Google Scholar]
- 237.Demir IE, Kujundzic K, Pfitzinger PL, Saricaoglu ÖC, Teller S, Kehl T, Reyes CM, Ertl LS, Miao Z, Schall TJet al. (2017) Early pancreatic cancer lesions suppress pain through CXCL12‐mediated chemoattraction of Schwann cells. Proc Natl Acad Sci USA 114, E85–E94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Krtolica A, Parrinello S, Lockett S, Desprez PY & Campisi J (2001) Senescent fibroblasts promote epithelial cell growth and tumorigenesis: a link between cancer and aging. Proc Natl Acad Sci USA 98, 12072–12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Wu D, Zhuo L & Wang X (2017) Metabolic reprogramming of carcinoma‐associated fibroblasts and its impact on metabolic heterogeneity of tumors. Semin Cell Dev Biol 64, 125–131. [DOI] [PubMed] [Google Scholar]
- 240.Shekhar MPV, Werdell J, Santner SJ, Pauley RJ & Tait L (2001) Breast stroma plays a dominant regulatory role in breast epithelial growth and differentiation: implications for tumor development and progression. Cancer Res 61, 1320–1326. [PubMed] [Google Scholar]
- 241.Roswall P, Bocci M, Bartoschek M, Li H, Kristiansen G, Jansson S, Lehn S, Sjölund J, Reid S, Larsson Cet al. (2018) Microenvironmental control of breast cancer subtype elicited through paracrine platelet‐derived growth factor‐CC signaling. Nat Med 24, 463–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Osuala KO, Sameni M, Shah S, Aggarwal N, Simonait ML, Franco OE, Hong Y, Hayward SW, Behbod F, Mattingly RRet al. (2015) Il‐6 signaling between ductal carcinoma in situ cells and carcinoma‐associated fibroblasts mediates tumor cell growth and migration. BMC Cancer 15, 584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Tanaka K, Miyata H, Sugimura K, Fukuda S, Kanemura T, Yamashita K, Miyazaki Y, Takahashi T, Kurokawa Y, Yamasaki Met al. (2015) miR‐27 is associated with chemoresistance in esophageal cancer through transformation of normal fibroblasts to cancer‐associated fibroblasts. Carcinogenesis 36, 894–903. [DOI] [PubMed] [Google Scholar]
- 244.Li Q, Zhang D, Wang Y, Sun P, Hou X, Larner J, Xiong W & Mi J (2013) MiR‐21/Smad 7 signaling determines TGF‐β1‐induced CAF formation. Sci Rep 3, 2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Albrengues J, Bertero T, Grasset E, Bonan S, Maiel M, Bourget I, Philippe C, Herraiz Serrano C, Benamar S, Croce Oet al. (2015) Epigenetic switch drives the conversion of fibroblasts into proinvasive cancer‐associated fibroblasts. Nat Commun 6, 10204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Perugorria MJ, Wilson CL, Zeybel M, Walsh M, Amin S, Robinson S, White SA, Burt AD, Oakley F, Tsukamoto Het al. (2012) Histone methyltransferase ASH1 orchestrates fibrogenic gene transcription during myofibroblast transdifferentiation. Hepatology 56, 1129–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Fordyce C, Fessenden T, Pickering C, Jung J, Singla V, Berman H & Tlsty T (2010) DNA damage drives an activin A‐dependent induction of cyclooxygenase‐2 in premalignant cells and lesions. Cancer Prev Res 3, 190–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Ershaid N, Sharon Y, Doron H, Raz Y, Shani O, Cohen N, Monteran L, Leider‐Trejo L, Ben‐Shmuel A, Yassin Met al. (2019) NLRP3 inflammasome in fibroblasts links tissue damage with inflammation in breast cancer progression and metastasis. Nat Commun 10, 4375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Biffi G, Oni TE, Spielman B, Hao Y, Elyada E, Park Y, Preall J & Tuveson DA (2019) Il1‐induced Jak/STAT signaling is antagonized by TGFβ to shape CAF heterogeneity in pancreatic ductal adenocarcinoma. Cancer Discov 9, 282–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Zambirinis CP, Levie E, Nguy S, Avanzi A, Barilla R, Xu Y, Seifert L, Daley D, Greco SH, Deutsch Met al. (2015) TLR9 ligation in pancreatic stellate cells promotes tumorigenesis. J Exp Med 212, 2077–2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Scherz‐Shouval R, Santagata S, Mendillo ML, Sholl LM, Ben‐Aharon I, Beck AH, Dias‐Santagata D, Koeva M, Stemmer SM, Whitesell Let al. (2014) The reprogramming of tumor stroma by HSF1 is a potent enabler of malignancy. Cell 158, 564–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Ferrari N, Ranftl R, Chicherova I, Slaven ND, Moeendarbary E, Farrugia AJ, Lam M, Semiannikova M, Westergaard MCW, Tchou Jet al. (2019) Dickkopf‐3 links HSF1 and YAP/TAZ signalling to control aggressive behaviours in cancer‐associated fibroblasts. Nat Commun 10, 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Demaria M, O'Leary MN, Chang J, Shao L, Liu S, Alimirah F, Koenig K, Le C, Mitin N, Deal AMet al. (2017) Cellular senescence promotes adverse effects of chemotherapy and cancer relapse. Cancer Discov 7, 165–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Fane M & Weeraratna AT (2020) How the ageing microenvironment influences tumour progression. Nat Rev Cancer 20, 89–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Zhang Y, Tang H, Cai J, Zhang T, Guo J, Feng D & Wang Z (2011) Ovarian cancer‐associated fibroblasts contribute to epithelial ovarian carcinoma metastasis by promoting angiogenesis, lymphangiogenesis and tumor cell invasion. Cancer Lett 303, 47–55. [DOI] [PubMed] [Google Scholar]
- 256.Koyama H, Kobayashi N, Harada M, Takeoka M, Kawai Y, Sano K, Fujimori M, Amano J, Ohhashi T, Kannagi Ret al. (2008) Significance of tumor‐associated stroma in promotion of intratumoral lymphangiogenesis: pivotal role of a hyaluronan‐rich tumor microenvironment. Am J Pathol 172, 179–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Shiga K, Hara M, Nagasaki T, Sato T, Takahashi H & Takeyama H (2015) Cancer‐associated fibroblasts: Their characteristics and their roles in tumor growth. Cancers (Basel) 7, 2443–2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Monteran L & Erez N (2019) The dark side of fibroblasts: cancer‐associated fibroblasts as mediators of immunosuppression in the tumor microenvironment. Front Immunol 10, 1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Peng Q, Zhao L, Hou Y, Sun Y, Wang L, Luo H, Peng H & Liu M (2013) Biological characteristics and genetic heterogeneity between carcinoma‐associated fibroblasts and their paired normal fibroblasts in human breast cancer. PLoS One 8, e60321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Wang C, Xu S, Tian Y, Ju A, Hou Q, Liu J, Fu Y & Luo Y (2019) Lysyl oxidase‐like protein 2 promotes tumor lymphangiogenesis and lymph node metastasis in breast cancer. Neoplasia 21, 413–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Wei R, Lv M, Li F, Cheng T, Zhang Z, Jiang G, Zhou Y, Gao R, Wei X, Lou Jet al. (2017) Human CAFs promote lymphangiogenesis in ovarian cancer via the Hh‐VEGF‐C signaling axis. Oncotarget 8, 67315–67328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Santi A, Kugeratski FG & Zanivan S (2018) Cancer associated fibroblasts: the architects of stroma remodeling. Proteomics 18, 1700167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Zhao T, Yan Z, Liu J, Sun H, Chen Y, Tao Y, Xu W, Qian H & Yan Y (2018) Mesenchymal stem cell derived exosomes enhance lymphangiogenesis via exosomal transfer of Ang‐2/Tie2. BioRxiv 466987. 10.1101/466987 [DOI] [Google Scholar]
- 264.Shields JD, Kourtis IC, Tomei AA, Roberts JM & Swartz MA (2010) Induction of lymphoid like stroma and immune escape by tumors that express the chemokine CCL21. Science 328, 749–752. [DOI] [PubMed] [Google Scholar]
- 265.Takahashi H, Sakakura K, Kudo T, Toyoda M, Kaira K, Oyama T & Chikamatsu K (2017) Cancer‐associated fibroblasts promote an immunosuppressive microenvironment through the induction and accumulation of protumoral macrophages. Oncotarget 8, 8633–8647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Cohen N, Shani O, Raz Y, Sharon Y, Hoffman D, Abramovitz L & Erez N (2017) Fibroblasts drive an immunosuppressive and growth‐promoting microenvironment in breast cancer via secretion of chitinase 3‐like 1. Oncogene 36, 4457–4468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Augsten M, Sjöberg E, Frings O, Vorrink SU, Frijhoff J, Olsson E, Borg Å & Östman A (2014) Cancer‐associated fibroblasts expressing CXCL14 rely upon NOS1‐derived nitric oxide signaling for their tumor‐ Supporting properties. Cancer Res 74, 2999–3010. [DOI] [PubMed] [Google Scholar]
- 268.Bott A, Erdem N, Lerrer S, Hotz‐Wagenblatt A, Breunig C, Abnaof K, Wörner A, Wilhelm H, Münstermann E, Ben‐Baruch Aet al. (2017) miRNA‐1246 induces pro‐inflammatory responses in mesenchymal stem/stromal cells by regulating PKA and PP2A. Oncotarget 8, 43897–43914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Davidson S, Efremova M, Riedel A, Mahata B, Pramanik J, Huuhtanen J, Kar G, Vento‐Tormo R, Hagai T, Chen Xet al. (2020) Single‐cell RNA sequencing reveals a dynamic stromal niche that supports tumor growth. Cell Rep 31, 107628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Comito G, Giannoni E, Segura CP, Barcellos‐De‐Souza P, Raspollini MR, Baroni G, Lanciotti M, Serni S & Chiarugi P (2014) Cancer‐associated fibroblasts and M2‐polarized macrophages synergize during prostate carcinoma progression. Oncogene 33, 2423–2431. [DOI] [PubMed] [Google Scholar]
- 271.Allaoui R, Bergenfelz C, Mohlin S, Hagerling C, Salari K, Werb Z, Anderson RL, Ethier SP, Jirström K, Påhlman Set al. (2016) Cancer‐associated fibroblast‐secreted CXCL16 attracts monocytes to promote stroma activation in triple‐negative breast cancers. Nat Commun 7, 13050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Ren G, Zhao X, Wang Y, Zhang X, Chen X, Xu C, Yuan ZR, Roberts AI, Zhang L, Zheng Bet al. (2012) CCR2‐dependent recruitment of macrophages by tumor‐educated mesenchymal stromal cells promotes tumor development and is mimicked by TNFα. Cell Stem Cell 11, 812–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Yu PF, Huang Y, Han YY, Lin LY, Sun WH, Rabson AB, Wang Y & Shi YF (2017) TNFα‐activated mesenchymal stromal cells promote breast cancer metastasis by recruiting CXCR2+ neutrophils. Oncogene 36, 482–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Mace TA, Ameen Z, Collins A, Wojcik S, Mair M, Young GS, Fuchs JR, Eubank TD, Frankel WL, Bekaii‐Saab Tet al. (2013) Pancreatic cancer‐associated stellate cells promote differentiation of myeloid‐derived suppressor cells in a StAT3‐dependent manner. Cancer Res 73, 3007–3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Eiró N, González L, González LO, Fernandez‐Garcia B, Andicoechea A, Barbón E, García‐Muñiz JL & Vizoso FJ (2013) Toll‐like receptor‐4 expression by stromal fibroblasts is associated with poor prognosis in colorectal cancer. J Immunother 36, 342–349. [DOI] [PubMed] [Google Scholar]
- 276.Koliaraki V, Chalkidi N, Henriques A, Tzaferis C, Polykratis A, Waisman A, Muller W, Hackam DJ, Pasparakis M & Kollias G (2019) Innate sensing through mesenchymal TLR4/MyD88 signals promotes spontaneous intestinal tumorigenesis. Cell Rep 26, 536–545.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.González‐Reyes S, Marín L, González L, González LO, Del Casar JM, Lamelas ML, González‐Quintana JM & Vizoso FJ (2010) Study of TLR3, TLR4 and TLR9 in breast carcinomas and their association with metastasis. BMC Cancer 10, 665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Sheyhidin I, Nabi G, Hasim A, Zhang RP, Ainiwaer J, Ma H & Wang H (2011) Overexpression of TLR3, TLR4, TLR7 and TLR9 in esophageal squamous cell carcinoma. World J Gastroenterol 17, 3745–3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Ni YH, Ding L, Zhang DY, Hou YY, Huang X & Hu Q (2015) Distinct expression patterns of Toll‐like receptor 7 in tumour cells and fibroblast‐like cells in oral squamous cell carcinoma. Histopathology 67, 730–739. [DOI] [PubMed] [Google Scholar]
- 280.Ino Y, Yamazaki‐Itoh R, Oguro S, Shimada K, Kosuge T, Zavada J, Kanai Y & Hiraoka N (2013) Arginase II expressed in cancer‐associated fibroblasts indicates tissue hypoxia and predicts poor outcome in patients with pancreatic cancer. PLoS One 8, e55146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Barnas JL, Simpson‐Abelson MR, Brooks SP, Kelleher RJ & Bankert RB (2010) Reciprocal functional modulation of the activation of T lymphocytes and fibroblasts derived from human solid tumors. J Immunol 185, 2681–2692. [DOI] [PubMed] [Google Scholar]
- 282.De Monte L, Reni M, Tassi E, Clavenna D, Papa I, Recalde H, Braga M, Di Carlo V, Doglioni C & Protti MP (2011) Intratumor T helper type 2 cell infiltrate correlates with cancer‐associated fibroblast thymic stromal lymphopoietin production and reduced survival in pancreatic cancer. J Exp Med 208, 469–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Su X, Ye J, Hsueh EC, Zhang Y, Hoft DF & Peng G (2010) Tumor microenvironments direct the recruitment and expansion of human Th17 cells. J Immunol 184, 1630–1641. [DOI] [PubMed] [Google Scholar]
- 284.Costa A, Kieffer Y, Scholer‐Dahirel A, Pelon F, Bourachot B, Cardon M, Sirven P, Magagna I, Fuhrmann L, Bernard Cet al. (2018) Fibroblast heterogeneity and immunosuppressive environment in human breast cancer. Cancer Cell 33, 463–479.e10. [DOI] [PubMed] [Google Scholar]
- 285.Givel AM, Kieffer Y, Scholer‐Dahirel A, Sirven P, Cardon M, Pelon F, Magagna I, Gentric G, Costa A, Bonneau Cet al. (2018) MiR200‐regulated CXCL12β promotes fibroblast heterogeneity and immunosuppression in ovarian cancers. Nat Commun 9, 1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Balsamo M, Scordamaglia F, Pietra G, Manzini C, Cantoni C, Boitano M, Queirolo P, Vermi W, Facchetti F, Moretta Aet al. (2009) Melanoma‐associated fibroblasts modulate NK cell phenotype and antitumor cytotoxicity. Proc Natl Acad Sci USA 106, 20847–20852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Ziani L, Ben Safta‐Saadoun T, Gourbeix J, Cavalcanti A, Robert C, Favre G, Chouaib S & Thiery J (2017) Melanoma‐associated fibroblasts decrease tumor cell susceptibility to NK cell‐mediated killing through matrix‐metalloproteinases secretion. Oncotarget 8, 19780–19794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Li T, Yang Y, Hua X, Wang G, Liu W, Jia C, Tai Y, Zhang Q & Chen G (2012) Hepatocellular carcinoma‐associated fibroblasts trigger NK cell dysfunction via PGE2 and IDO. Cancer Lett 318, 154–161. [DOI] [PubMed] [Google Scholar]
- 289.Barnes JL & Gorin Y (2011) Myofibroblast differentiation during fibrosis: role of NAD(P)H oxidases. Kidney Int 79, 944–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Knipper JA, Willenborg S, Brinckmann J, Bloch W, Maaß T, Wagener R, Krieg T, Sutherland T, Munitz A, Rothenberg MEet al. (2015) Interleukin‐4 receptor α signaling in myeloid cells controls collagen fibril assembly in skin repair. Immunity 43, 803–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Páez D, Labonte MJ, Bohanes P, Zhang W, Benhanim L, Ning Y, Wakatsuki T, Loupakis F & Lenz HJ (2012) Cancer dormancy: a model of early dissemination and late cancer recurrence. Clin Cancer Res 18, 645–653. [DOI] [PubMed] [Google Scholar]
- 292.Faouzi S, Le Bail B, Neaud V, Boussarie L, Saric J, Bioulac‐Sage P, Balabaud C & Rosenbaum J (1999) Myofibroblasts are responsible for collagen synthesis in the stroma of human hepatocellular carcinoma: an in vivo and in vitro study. J Hepatol 30, 275–284. [DOI] [PubMed] [Google Scholar]
- 293.Yoshimura H, Michishita M, Ohkusu‐Tsukada K, Matsuda Y, Ishiwata T, Naito Z & Takahashi K (2015) Cellular sources of tenascin‐C in canine mammary carcinomas. Vet Pathol 52, 92–96. [DOI] [PubMed] [Google Scholar]
- 294.Erdogan B, Ao M, White LM, Means AL, Brewer BM, Yang L, Washington MK, Shi C, Franco OE, Weaver AMet al. (2017) Cancer‐associated fibroblasts promote directional cancer cell migration by aligning fibronectin. J Cell Biol 216, 3799–3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Attieh Y, Clark AG, Grass C, Richon S, Pocard M, Mariani P, Elkhatib N, Betz T, Gurchenkov B & Vignjevic DM (2017) Cancer‐associated fibroblasts lead tumor invasion through integrin‐β3‐dependent fibronectin assembly. J Cell Biol 216, 3509–3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Pickup MW, Laklai H, Acerbi I, Owens P, Gorska AE, Chytil A, Aakre M, Weaver VM & Moses HL (2013) Stromally derived lysyl oxidase promotes metastasis of transforming growth factor‐β‐deficient mouse mammary carcinomas. Cancer Res 73, 5336–5346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Eble JA & Niland S (2019) The extracellular matrix in tumor progression and metastasis. Clin Exp Metastasis 36, 171–198. [DOI] [PubMed] [Google Scholar]
- 298.Venning FA, Wullkopf L & Erler JT (2015) Targeting ECM disrupts cancer progression. Front Oncol 5, 224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.DeFilippis RA, Fordyce C, Patten K, Chang H, Zhao J, Fontenay GV, Kerlikowske K, Parvin B & Tlsty TD (2014) Stress signaling from human mammary epithelial cells contributes to phenotypes of mammographic density. Cancer Res 74, 5032–5044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.DeFilippis RA, Chang H, Dumont N, Rabban JT, Chen YY, Fontenay GV, Berman HK, Gauthier ML, Zhao J, Hu Det al. (2012) CD36 repression activates a multicellular stromal program shared by high mammographic density and tumor tissues. Cancer Discov 2, 826–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Elkabets M, Gifford AM, Scheel C, Nilsson B, Reinhardt F, Bray MA, Carpenter AE, Jirström K, Magnusson K, Ebert BLet al. (2011) Human tumors instigate granulin‐expressing hematopoietic cells that promote malignancy by activating stromal fibroblasts in mice. J Clin Invest 121, 784–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Fullár A, Dudás J, Oláh L, Hollósi P, Papp Z, Sobel G, Karászi K, Paku S, Baghy K & Kovalszky I (2015) Remodeling of extracellular matrix by normal and tumor‐associated fibroblasts promotes cervical cancer progression. BMC Cancer 15, 256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Yeung TL, Leung CS, Wong KK, Samimi G, Thompson MS, Liu J, Zaid TM, Ghosh S, Birrer MJ & Mok SC (2013) TGF‐β modulates ovarian cancer invasion by upregulating CAF‐Derived versican in the tumor microenvironment. Cancer Res 73, 5016–5028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Hanley CJ, Noble F, Ward M, Bullock M, Drifka C, Mellone M, Manousopoulou A, Johnston HE, Hayden A, Thirdborough Set al. (2016) A subset of myofibroblastic cancer‐associated fibroblasts regulate collagen fiber elongation, which is prognostic in multiple cancers. Oncotarget 7, 6159–6174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Navab R, Strumpf D, To C, Pasko E, Kim KS, Park CJ, Hai J, Liu J, Jonkman J, Barczyk Met al. (2016) Integrin α11β1 regulates cancer stromal stiffness and promotes tumorigenicity and metastasis in non‐small cell lung cancer. Oncogene 35, 1899–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Zeltz C, Pasko E, Cox TR, Navab R & Tsao MS (2019) LOXL1 is regulated by integrin α11 and promotes non‐small cell lung cancer tumorigenicity. Cancers (Basel) 11, 705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Pankova D, Chen Y, Terajima M, Schliekelman MJ, Baird BN, Fahrenholtz M, Sun L, Gill BJ, Vadakkan TJ, Kim MPet al. (2016) Cancer‐associated fibroblasts induce a collagen cross‐link switch in tumor stroma. Mol Cancer Res 14, 287–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Ge J, Burnier L, Adamopoulou M, Kwa MQ, Schaks M, Rottner K & Brakebusch C (2018) RhoA, Rac1, and Cdc42 differentially regulate SMA and collagen i expression in mesenchymal stem cells. J Biol Chem 293, 9358–9369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart‐King CA, Margulies SS, Dembo M, Boettiger Det al. (2005) Tensional homeostasis and the malignant phenotype. Cancer Cell 8, 241–254. [DOI] [PubMed] [Google Scholar]
- 310.Wei SC, Fattet L, Tsai JH, Guo Y, Pai VH, Majeski HE, Chen AC, Sah RL, Taylor SS, Engler AJet al. (2015) Matrix stiffness drives epithelial‐mesenchymal transition and tumour metastasis through a TWIST1‐G3BP2 mechanotransduction pathway. Nat Cell Biol 17, 678–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Yeung T, Georges PC, Flanagan LA, Marg B, Ortiz M, Funaki M, Zahir N, Ming W, Weaver V & Janmey PA (2005) Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil Cytoskeleton 60, 24–34. [DOI] [PubMed] [Google Scholar]
- 312.Ondeck MG, Kumar A, Placone JK, Plunkett CM, Matte BF, Wong KC, Fattet L, Yang J & Engler AJ (2019) Dynamically stiffened matrix promotes malignant transformation of mammary epithelial cells via collective mechanical signaling. Proc Natl Acad Sci USA 116, 3502–3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Aung A, Seo YN, Lu S, Wang Y, Jamora C, del Álamo JC & Varghese S (2014) 3D traction stresses activate protease‐dependent invasion of cancer cells. Biophys J 107, 2528–2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Rigato A, Miyagi A, Scheuring S & Rico F (2017) High‐frequency microrheology reveals cytoskeleton dynamics in living cells. Nat Phys 13, 771–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Öhlund D, Handly‐Santana A, Biffi G, Elyada E, Almeida AS, Ponz‐Sarvise M, Corbo V, Oni TE, Hearn SA, Lee EJet al. (2017) Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J Exp Med 214, 579–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Mariathasan S, Turley SJ, Nickles D, Castiglioni A, Yuen K, Wang Y, Kadel EE, Koeppen H, Astarita JL, Cubas Ret al. (2018) TGFβ attenuates tumour response to PD‐L1 blockade by contributing to exclusion of T cells. Nature 554, 544–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Jain RK, Martin JD & Stylianopoulos T (2014) The role of mechanical forces in tumor growth and therapy. Annu Rev Biomed Eng 16, 321–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Dufort CC, Delgiorno KE & Hingorani SR (2016) Mounting pressure in the microenvironment: fluids, solids, and cells in pancreatic ductal adenocarcinoma. Gastroenterology 150, 1545–1557.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Dufort CC, DelGiorno KE, Carlson MA, Osgood RJ, Zhao C, Huang Z, Thompson CB, Connor RJ, Thanos CD, Scott Brockenbrough Jet al. (2016) Interstitial pressure in pancreatic ductal adenocarcinoma is dominated by a gel‐fluid phase. Biophys J 110, 2106–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Liu J & Agarwal S (2010) Mechanical signals activate vascular endothelial growth factor receptor‐2 to upregulate endothelial cell proliferation during inflammation. J Immunol 185, 1215–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Myers KA, Applegate KT, Danuser G, Fischer RS & Waterman CM (2011) Distinct ECM mechanosensing pathways regulate microtubule dynamics to control endothelial cell branching morphogenesis. J Cell Biol 192, 321–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Edgar LT, Maas SA, Guilkey JE & Weiss JA (2015) A coupled model of neovessel growth and matrix mechanics describes and predicts angiogenesis in vitro. Biomech Model Mechanobiol 14, 767–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Deryugina EI, Zajac E, Juncker‐Jensen A, Kupriyanova TA, Welter L & Quigley JP (2014) Tissue‐infiltrating neutrophils constitute the major in vivo source of angiogenesis‐inducing MMP‐9 in the tumor microenvironment. Neoplasia 16, 771–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Chang J, Lucas MC, Leonte LE, Garcia‐Montolio M, Singh LB, Findlay AD, Deodhar M, Foot JS, Jarolimek W, Timpson Pet al. (2017) Pre‐clinical evaluation of small molecule LOXL2 inhibitors in breast cancer. Oncotarget 8, 26066–26078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Salmon H, Franciszkiewicz K, Damotte D, Dieu‐Nosjean MC, Validire P, Trautmann A, Mami‐Chouaib F & Donnadieu E (2012) Matrix architecture defines the preferential localization and migration of T cells into the stroma of human lung tumors. J Clin Invest 122, 899–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Binder MJ, McCoombe S, Williams ED, McCulloch DR & Ward AC (2017) The extracellular matrix in cancer progression: role of hyalectan proteoglycans and ADAMTS enzymes. Cancer Lett 385, 55–64. [DOI] [PubMed] [Google Scholar]
- 327.Sorokin L (2010) The impact of the extracellular matrix on inflammation. Nat Rev Immunol 10, 712–723. [DOI] [PubMed] [Google Scholar]
- 328.Siegel RL, Miller KD & Jemal A (2018) Cancer statistics, 2018. CA Cancer J Clin 68, 7–30. [DOI] [PubMed] [Google Scholar]
- 329.Chandra T, Ewels PA, Schoenfelder S, Furlan‐Magaril M, Wingett SW, Kirschner K, Thuret JY, Andrews S, Fraser P & Reik W (2015) Global reorganization of the nuclear landscape in senescent cells. Cell Rep 10, 471–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Mosteiro L, Pantoja C, Alcazar N, Marión RM, Chondronasiou D, Rovira M, Fernandez‐Marcos PJ, Muñoz‐Martin M, Blanco‐Aparicio C, Pastor Jet al. (2016) Tissue damage and senescence provide critical signals for cellular reprogramming in vivo. Science 354, aaf4445. [DOI] [PubMed] [Google Scholar]
- 331.Salzer MC, Lafzi A, Berenguer‐Llergo A, Youssif C, Castellanos A, Solanas G, Peixoto FO, Stephan‐Otto Attolini C, Prats N, Aguilera Met al. (2018) Identity noise and adipogenic traits characterize dermal fibroblast aging. Cell 175, 1575–1590.e22. [DOI] [PubMed] [Google Scholar]
- 332.Frevert CW, Felgenhauer J, Wygrecka M, Nastase MV & Schaefer L (2018) Danger‐associated molecular patterns derived from the extracellular matrix provide temporal control of innate immunity. J Histochem Cytochem 66, 213–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Schaefer L, Babelova A, Kiss E, Hausser HJ, Baliova M, Krzyzankova M, Marsche G, Young MF, Mihalik D, Götte Met al. (2005) The matrix component biglycan is proinflammatory and signals through Toll‐like receptors 4 and 2 in macrophages. J Clin Invest 115, 2223–2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Merline R, Moreth K, Beckmann J, Nastase MV, Zeng‐Brouwers J, Tralhão JG, Lemarchand P, Pfeilschifter J, Schaefer RM, Iozzo RVet al. (2011) Signaling by the matrix proteoglycan decorin controls inflammation and cancer through PDCD4 and microRNA‐21. Sci Signal 4, ra75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Kelsh R, You R, Horzempa C, Zheng M & McKeown‐Longo PJ (2014) Regulation of the innate immune response by fibronectin: synergism between the III‐1 and EDA domains. PLoS One 9, e102974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Smith ML, Gourdon D, Little WC, Kubow KE, Eguiluz RA, Luna‐Morris S & Vogel V (2007) Force‐induced unfolding of fibronectin in the extracellular matrix of living cells. PLoS Biol 5, e268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Sofat N, Robertson SD & Wait R (2011) Fibronectin III 13–14 domains induce joint damage via Toll‐like receptor 4 activation and synergize with interleukin‐1 and tumour necrosis factor. J Innate Immun 4, 69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Hope C, Emmerich PB, Papadas A, Pagenkopf A, Matkowskyj KA, Van De Hey DR, Payne SN, Clipson L, Callander NS, Hematti Pet al. (2017) Versican‐derived matrikines regulate Batf3–dendritic cell differentiation and promote T cell infiltration in colorectal cancer. J Immunol 199, 1993–1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Williams CM, Engler AJ, Slone RD, Galante LL & Schwarzbauer JE (2008) Fibronectin expression modulates mammary epithelial cell proliferation during acinar differentiation. Cancer Res 68, 3185–3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Kao RT, Hall J, Engel L & Stern R (1984) The matrix of human breast tumor cells is mitogenic for fibroblasts. Am J Pathol 115, 109–116. [PMC free article] [PubMed] [Google Scholar]
- 341.Ishihara S, Inman DR, Li WJ, Ponik SM & Keely PJ (2017) Mechano‐signal transduction in mesenchymal stem cells induces prosaposin secretion to drive the proliferation of breast cancer cells. Cancer Res 77, 6179–6189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Wang K, Andresen Eguiluz RC, Wu F, Seo BR, Fischbach C & Gourdon D (2015) Stiffening and unfolding of early deposited‐fibronectin increase proangiogenic factor secretion by breast cancer‐associated stromal cells. Biomaterials 54, 63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Huse M (2017) Mechanical forces in the immune system. Nat Rev Immunol 17, 679–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.McWhorter FY, Wang T, Nguyen P, Chung T & Liu WF (2013) Modulation of macrophage phenotype by cell shape. Proc Natl Acad Sci USA 110, 17253–17258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Mazur A, Holthoff E, Vadali S, Kelly T & Post SR (2016) Cleavage of type I collagen by fibroblast activation protein‐α enhances class a scavenger receptor mediated macrophage adhesion. PLoS One 11, e0150287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Perri RT, Kay NE, McCarthy J, Vessella RL, Jacob HS & Furcht LT (1982) Fibronectin enhances in vitro monocyte‐macrophage‐mediated tumoricidal activity. Blood 60, 430–435. [PubMed] [Google Scholar]
- 347.Stahl M, Schupp J, Jäger B, Schmid M, Zissel G, Müller‐Quernheim J & Prasse A(2013) Lung collagens perpetuate pulmonary fibrosis via CD204 and M2 macrophage activation. PLoS One 8, e81382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Jiang H, Hegde S & DeNardo DG (2017) Tumor‐associated fibrosis as a regulator of tumor immunity and response to immunotherapy. Cancer Immunol Immunother 66, 1037–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Lebbink RJ, De Ruiter T, Adelmeijer J, Brenkman AB, Van Helvoort JM, Koch M, Farndale RW, Lisman T, Sonnenberg A, Lenting PJet al. (2006) Collagens are functional, high affinity ligands for the inhibitory immune receptor LAIR‐1. J Exp Med 203, 1419–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Murakami S, Shahbazian D, Surana R, Zhang W, Chen H, Graham GT, White SM, Weiner LM & Yi C (2017) Yes‐associated protein mediates immune reprogramming in pancreatic ductal adenocarcinoma. Oncogene 36, 1232–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Ford K, Hanley CJ, Mellone M, Szyndralewiez C, Heitz F, Wiesel P, Wood O, Machado M, Lopez M‐A, Ganesan A‐Pet al. (2020) NOX4 inhibition potentiates immunotherapy by overcoming cancer‐associated fibroblast‐mediated CD8 T‐cell exclusion from tumors. Cancer Res 80, 1846–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Hartmann N, Giese NA, Giese T, Poschke I, Offringa R, Werner J & Ryschich E (2014) Prevailing role of contact guidance in intrastromal T‐cell trapping in human pancreatic cancer. Clin Cancer Res 20, 3422–3433. [DOI] [PubMed] [Google Scholar]
- 353.O'Connor RS, Hao X, Shen K, Bashour K, Akimova T, Hancock WW, Kam LC & Milone MC (2012) Substrate rigidity regulates human T cell activation and proliferation. J Immunol 189, 1330–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Pearce OMT, Delaine‐Smith RM, Maniati E, Nichols S, Wang J, Böhm S, Rajeeve V, Ullah D, Chakravarty P, Jones RRet al. (2018) Deconstruction of a metastatic tumor microenvironment reveals a common matrix response in human cancers. Cancer Discov 8, 304–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Bougherara H, Mansuet‐Lupo A, Alifano M, Ngô C, Damotte D, Le Frère‐Belda MA, Donnadieu E & Peranzoni E (2015) Real‐time imaging of resident T cells in human lung and ovarian carcinomas reveals how different tumor microenvironments control T lymphocyte migration. Front Immunol 6, 500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Wolf K, te Lindert M, Krause M, Alexander S, te Riet J, Willis AL, Hoffman RM, Figdor CG, Weiss SJ & Friedl P (2013) Physical limits of cell migration: control by ECM space and nuclear deformation and tuning by proteolysis and traction force. J Cell Biol 201, 1069–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Ng CP & Swartz MA (2003) Fibroblast alignment under interstitial fluid flow using a novel 3‐D tissue culture model. Am J Physiol Heart Circ Physiol 284, H1771–H1777. [DOI] [PubMed] [Google Scholar]
- 358.Ng CP, Hinz B & Swartz MA (2005) Interstitial fluid flow induces myofibroblast differentiation and collagen alignment in vitro. J Cell Sci 118, 4731–4739. [DOI] [PubMed] [Google Scholar]
- 359.Shieh AC, Rozansky HA, Hinz B & Swartz MA (2011) Tumor cell invasion is promoted by interstitial flow‐induced matrix priming by stromal fibroblasts. Cancer Res 71, 790–800. [DOI] [PubMed] [Google Scholar]
- 360.Helm CLE, Fleury ME, Zisch AH, Boschetti F & Swartz MA (2005) Synergy between interstitial flow and VEGF directs capillary morphogenesis in vitro through a gradient amplification mechanism. Proc Natl Acad Sci USA 102, 15779–15784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Shields JD, Fleury ME, Yong C, Tomei AA, Randolph GJ & Swartz MA (2007) Autologous chemotaxis as a mechanism of tumor cell homing to lymphatics via interstitial flow and autocrine CCR7 signaling. Cancer Cell 11, 526–538. [DOI] [PubMed] [Google Scholar]
- 362.Polacheck WJ, Charest JL & Kamm RD (2011) Interstitial flow influences direction of tumor cell migration through competing mechanisms. Proc Natl Acad Sci USA 108, 11115–11120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Miteva DO, Rutkowski JM, Dixon JB, Kilarski W, Shields JD & Swartz MA (2010) Transmural flow modulates cell and fluid transport functions of lymphatic endothelium. Circ Res 106, 920–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.McAllister SS & Weinberg RA (2014) The tumour‐induced systemic environment as a critical regulator of cancer progression and metastasis. Nat Cell Biol 16, 717–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Laplane L, Duluc D, Larmonier N, Pradeu T & Bikfalvi A (2018) The multiple layers of the tumor environment. Trends Cancer 4, 802–809. [DOI] [PubMed] [Google Scholar]
- 366.Klastersky J & Aoun M (2004) Opportunistic infections in patients with cancer. Ann Oncol 15, iv329–iv335. [DOI] [PubMed] [Google Scholar]
- 367.Baluch A & Pasikhova Y (2013) Influenza vaccination in oncology patients. Curr Infect Dis Rep 15, 486–490. [DOI] [PubMed] [Google Scholar]
- 368.Spitzer MH, Carmi Y, Reticker‐Flynn NE, Kwek SS, Madhireddy D, Martins MM, Gherardini PF, Prestwood TR, Chabon J, Bendall SCet al. (2017) Systemic immunity is required for effective cancer immunotherapy. Cell 168, 487–502.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Riedel A, Shorthouse D, Haas L, Hall BA & Shields J (2016) Tumor‐induced stromal reprogramming drives lymph node transformation. Nat Immunol 17, 1118–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Templeton AJ, McNamara MG, Šeruga B, Vera‐Badillo FE, Aneja P, Ocaña A, Leibowitz‐Amit R, Sonpavde G, Knox JJ, Tran Bet al. (2014) Prognostic role of neutrophil‐to‐lymphocyte ratio in solid tumors: a systematic review and meta‐analysis. J Natl Cancer Inst 106, dju124. [DOI] [PubMed] [Google Scholar]
- 371.Roberts EW, Deonarine A, Jones JO, Denton AE, Feig C, Lyons SK, Espeli M, Kraman M, McKenna B, Wells RJBet al. (2013) Depletion of stromal cells expressing fibroblast activation protein‐α from skeletal muscle and bone marrow results in cachexia and anemia. J Exp Med 210, 1137–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Flint TR, Janowitz T, Connell CM, Roberts EW, Denton AE, Coll AP, Jodrell DI & Fearon DT (2016) Tumor‐induced IL‐6 reprograms host metabolism to suppress anti‐tumor immunity. Cell Metab 24, 672–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Yarchoan M, Johnson BA, Lutz ER, Laheru DA & Jaffee EM (2017) Targeting neoantigens to augment antitumour immunity. Nat Rev Cancer 17, 209–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Olson B, Li Y, Lin Y, Liu ET & Patnaik A (2018) Mouse models for cancer immunotherapy research. Cancer Discov 8, 1358–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Ben‐David U, Ha G, Tseng YY, Greenwald NF, Oh C, Shih J, McFarland JM, Wong B, Boehm JS, Beroukhim Ret al. (2017) Patient‐derived xenografts undergo mouse‐specific tumor evolution. Nat Genet 49, 1567–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Kim J, Rhee H, Kim J & Lee S (2020) Validity of patient‐derived xenograft mouse models for lung cancer based on exome sequencing data. Genomics Inform 18, e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Kelley J, Walter L & Trowsdale J (2005) Comparative genomics of natural killer cell receptor gene clusters. PLoS Genet 1, 0129–0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Mestas J & Hughes CCW (2004) Of mice and not men: differences between mouse and human immunology. J Immunol 172, 2731–2738. [DOI] [PubMed] [Google Scholar]
- 379.Stavnezer J & Schrader CE (2014) IgH chain class switch recombination: mechanism and regulation. J Immunol 193, 5370–5378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Tentler JJ, Tan AC, Weekes CD, Jimeno A, Leong S, Pitts TM, Arcaroli JJ, Messersmith WA & Eckhardt SG (2012) Patient‐derived tumour xenografts as models for oncology drug development. Nat Rev Clin Oncol 9, 338–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Li S, Shen D, Shao J, Crowder R, Liu W, Prat A, He X, Liu S, Hoog J, Lu Cet al. (2013) Endocrine‐therapy‐resistant ESR1 variants revealed by genomic characterization of breast‐cancer‐derived xenografts. Cell Rep 4, 1116–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Wang S, Gao D & Chen Y (2017) The potential of organoids in urological cancer research. Nat Rev Urol 14, 401–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Fan H, Demirci U & Chen P (2019) Emerging organoid models: leaping forward in cancer research. J Hematol Oncol 12, 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Kwong J, Franky LC, Wong KK, Birrer MJ, Archibald KM, Balkwill FR, Berkowitz RS & Mok SC (2009) Inflammatory cytokine tumor necrosis factor α confers precancerous phenotype in an organoid model of normal human ovarian surface epithelial cells. Neoplasia 11, 529–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Fujii M, Shimokawa M, Date S, Takano A, Matano M, Nanki K, Ohta Y, Toshimitsu K, Nakazato Y, Kawasaki Ket al. (2016) A Colorectal tumor organoid library demonstrates progressive loss of niche factor requirements during tumorigenesis. Cell Stem Cell 18, 827–838. [DOI] [PubMed] [Google Scholar]
- 386.Bird‐Lieberman EL, Neves AA, Lao‐Sirieix P, O'Donovan M, Novelli M, Lovat LB, Eng WS, Mahal LK, Brindle KM & Fitzgerald RC (2012) Molecular imaging using fluorescent lectins permits rapid endoscopic identification of dysplasia in Barrett's esophagus. Nat Med 18, 315–321. [DOI] [PubMed] [Google Scholar]
- 387.Neves AA, Di Pietro M, O'Donovan M, Waterhouse DJ, Bohndiek SE, Brindle KM & Fitzgerald RC (2018) Detection of early neoplasia in Barrett's esophagus using lectin‐based near‐infrared imaging: an ex vivo study on human tissue. Endoscopy 50, 618–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Yoon J, Joseph J, Waterhouse DJ, Luthman AS, Gordon GSD, di Pietro M, Januszewicz W, Fitzgerald RC & Bohndiek SE (2019) A clinically translatable hyperspectral endoscopy (HySE) system for imaging the gastrointestinal tract. Nat Commun 10, 1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Frankell AM, Jammula SG, Li X, Contino G, Killcoyne S, Abbas S, Perner J, Bower L, Devonshire G, Ococks Eet al. (2019) The landscape of selection in 551 esophageal adenocarcinomas defines genomic biomarkers for the clinic. Nat Genet 51, 506–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Ross‐Innes CS, Debiram‐Beecham I, O'Donovan M, Walker E, Varghese S, Lao‐Sirieix P, Lovat L, Griffin M, Ragunath K, Haidry Ret al. (2015) Evaluation of a minimally invasive cell sampling device coupled with assessment of trefoil factor 3 expression for diagnosing Barrett's esophagus: a multi‐center case‐control study. PLoS Medicine 12, 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Sand JM, Larsen L, Hogaboam C, Martinez F, Han ML, Larsen MR, Nawrocki A, Zheng Q, Karsdal MA & Leeming DJ (2013) MMP mediated degradation of type IV collagen alpha 1 and alpha 3 chains reflects basement membrane remodeling in experimental and clinical fibrosis – validation of two novel biomarker assays. PLoS One 8, e84934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Resovi A, Bani MR, Porcu L, Anastasia A, Minoli L, Allavena P, Cappello P, Novelli F, Scarpa A, Morandi Eet al. (2018) Soluble stroma‐related biomarkers of pancreatic cancer. EMBO Mol Med 10, e8741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Tarney CM, Wang G, Bateman NW, Conrads KA, Zhou M, Hood BL, Loffredo J, Tian C, Darcy KM, Hamilton CAet al. (2019) Biomarker panel for early detection of endometrial cancer in the prostate, lung, colorectal, and ovarian cancer screening trial. Am J Obstet Gynecol 472.e1–472.e10. [DOI] [PubMed] [Google Scholar]
- 394.Gentles AJ, Bratman SV, Lee LJ, Harris JP, Feng W, Nair RV, Shultz DB, Nair VS, Hoang CD, West RBet al. (2015) Integrating tumor and stromal gene expression signatures with clinical indices for survival stratification of early‐stage non‐small cell lung cancer. J Natl Cancer Inst 107, djv211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Calon A, Lonardo E, Berenguer‐Llergo A, Espinet E, Hernando‐Momblona X, Iglesias M, Sevillano M, Palomo‐Ponce S, Tauriello DVF, Byrom Det al. (2015) Stromal gene expression defines poor‐prognosis subtypes in colorectal cancer. Nat Genet 47, 320–329. [DOI] [PubMed] [Google Scholar]
- 396.Thorsson V, Gibbs DL, Brown SD, Wolf D, Bortone DS, Ou Yang TH, Porta‐Pardo E, Gao GF, Plaisier CL, Eddy JAet al. (2018) The Immune landscape of cancer. Immunity 48, 812–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Chronopoulos A, Robinson B, Sarper M, Cortes E, Auernheimer V, Lachowski D, Attwood S, Garciá R, Ghassemi S, Fabry Bet al. (2016) ATRA mechanically reprograms pancreatic stellate cells to suppress matrix remodelling and inhibit cancer cell invasion. Nat Commun 7, 12630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Kim MG, Shon Y, Kim J & Oh YK (2017) Selective activation of anticancer chemotherapy by cancer‐associated fibroblasts in the tumor microenvironment. J Natl Cancer Inst 109, djw186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Chauhan VP, Chen IX, Tong R, Ng MR, Martin JD, Naxerova K, Wu MW, Huang P, Boucher Y, Kohane DSet al. (2019) Reprogramming the microenvironment with tumorselective angiotensin blockers enhances cancer immunotherapy. Proc Natl Acad Sci USA 166, 10674–10680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Hingorani SR, Bullock AJ, Seery TE, Zheng L, Sigal D, Ritch PS, Braiteh FS, Zalupski M, Bahary N, Harris WPet al. (2017) Randomized phase II study of PEGPH20 plus nab‐paclitaxel/gemcitabine (PAG) vs AG in patients (Pts) with untreated, metastatic pancreatic ductal adenocarcinoma (mPDA). J Clin Oncol 35, 4008. [DOI] [PubMed] [Google Scholar]
- 401.Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, Madhu B, Goldgraben MA, Caldwell ME, Allard Det al. (2009) Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science 324, 1457–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Catenacci DVT, Junttila MR, Karrison T, Bahary N, Horiba MN, Nattam SR, Marsh R, Wallace J, Kozloff M, Rajdev Let al. (2015) Randomized phase Ib/II study of gemcitabine plus placebo or vismodegib, a hedgehog pathway inhibitor, in patients with metastatic pancreatic cancer. J Clin Oncol 33, 4284–4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Lee MJ, Hatton BA, Villavicencio EH, Khanna PC, Friedman SD, Ditzler S, Pullar B, Robison K, White KF, Tunkey Cet al. (2012) Hedgehog pathway inhibitor saridegib (IPI‐926) increases lifespan in a mouse medulloblastoma model. Proc Natl Acad Sci USA 109, 7859–7864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.García‐Pravia C, Galván JA, Gutiérrez‐Corral N, Solar‐García L, García‐Pérez E, García‐Ocaña M, Del Amo‐Iribarren J, Menéndez‐Rodríguez P, García‐García J, de los Toyos JRet al. (2013) Overexpression of COL11A1 by cancer‐associated fibroblasts: clinical relevance of a stromal marker in pancreatic cancer. PLoS One 8, e78327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Cheon DJ, Tong Y, Sim MS, Dering J, Berel D, Cui X, Lester J, Beach JA, Tighiouart M, Walts AEet al. (2014) A collagen‐remodeling gene signature regulated by TGF‐β signaling is associated with metastasis and poor survival in serous ovarian cancer. Clin Cancer Res 20, 711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Maniati E, Berlato C, Gopinathan G, Heath O, Kotantaki P, Lakhani A, McDermott J, Pegrum C, Delaine‐Smith RM, Pearce OMTet al. (2020) Mouse ovarian cancer models recapitulate the human tumor microenvironment and patient response to treatment. Cell Rep 30, 525–540.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Kitano H (2004) Cancer as a robust system: implications for anticancer therapy. Nat Rev Cancer 4, 227–235. [DOI] [PubMed] [Google Scholar]


