Abstract
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) attacks pulmonary alveolar cells via angiotensin-converting enzyme 2 (ACE2) receptors and causes pulmonary infections that result in coronavirus disease (COVID-19), inducing immune responses that can result in severe pneumonia. We reviewed the clinical experiences of lung diseases during the COVID-19 pandemic to offer insights into the adaptations made by experts in the diagnosis and treatment of these comorbidities. Various lung comorbidities increase the severity of COVID-19 and associated mortality by amplifying ACE2 expression. Additionally, the COVID-19 pandemic has changed the use of routine diagnostic pulmonary imaging methods, making chest sonography scoring the most convenient, as it can be conducted bedside. Treatment protocols for SARS-CoV-2 infection and the underlying lung diseases are also affected owing to potential interactions. The optimal diagnostic methods and treatment protocols for lung diseases have been adapted worldwide to increase survival rates and attenuate acute lung injuries during the COVID-19 pandemic.
Keywords: Coronavirus disease-19, Severe acute respiratory syndrome coronavirus-2, Pathophysiology, Angiotensin-converting enzyme 2, Lung comorbidities, Pulmonary images
Introduction
The coronavirus disease (COVID-19) outbreak occurred by the end of 2019 [1], and thereafter, the COVID-19 burden has significantly increased worldwide. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection was declared a pandemic with clinical symptoms such as fever, cough, dyspnea, pneumonia, and even acute respiratory distress syndrome (ARDS) [1]. The initial analysis of disease severity revealed that 31.7% of patients with COVID-19 needed intensive care unit (ICU) care, 29% had ARDS, 5% needed extracorporeal membrane oxygenation, and 15% had died [1]. As of February 29, 2020, the overall mortality rate in Wuhan, the epicenter of infection, was less than 1.4%. The median mortality rate of patients with COVID-19 in each country was calculated to be 1.5%–2% by Johns Hopkins university [2]. Recently, COVID-19 has become the leading cause of death in the U.S., especially among elderly individuals [3].
SARS-CoV-2 infects the nasopharynx and lungs, resulting in various symptoms of varying severity. The infected patients range from asymptomatic individuals to those with upper respiratory tract infections, smell and taste abnormalities, diarrhea, pneumonia, and even ARDS. Angiotensin-converting enzyme 2 (ACE2) receptors [4], to which the coronavirus binds, are found on type 2 alveolar, ciliated, and goblet cells in the airways [5]. Hypoxia is frequently experienced by patients with COVID-19, especially those with comorbidities that affect the lungs. Furthermore, SARS-CoV-2 infection can induce cytokine storms that damage the lungs and result in ARDS and pulmonary fibrosis [6]. In contrast, SARS-CoV-2 may be able to escape the innate immune response [6]. COVID-19 may disturb the immune function; worsen underlying pulmonary diseases, such as chronic obstructive pulmonary disease (COPD) and cancer, and transplantation outcome; and cause additive mortality. Furthermore, COVID-19 may influence the diagnosis and treatment of pulmonary diseases, which need imaging techniques, invasive procedures, and chemoimmunotherapy. Information regarding the risks and benefits of clinical diagnostic methods and treatment protocols is limited. Therefore, we reviewed the clinical diagnostic methods and treatment protocols of pulmonary diseases that were affected by the COVID-19 pandemic during 2020. For this purpose, we searched PUBMED for relevant articles using the keywords COVID-19, ACE2, pathophysiology, COPD, cancer, transplantation, image, and histopathology. One hundred and seven review and research articles were obtained for pathophysiology with the keywords COVID-19, ACE2, pathophysiology, and histopathology. To obtain review articles, case reports, clinical study reports, and guidelines regarding COVID-19 and pulmonary diseases, we searched the database with COVID-19 and COPD, or lung cancer, or lung transplantation as keywords. In total 158, 299, and 175 articles were obtained for COVID-19 and COPD, lung cancer, and lung transplantation, respectively. To determine the effect of pulmonary imaging on COVID-19, the keywords images (sonography, tomography) and COVID-19 were used to search for review articles; 108 and 322 articles were retrieved, respectively. Additionally, this review offers insights into the pathogenesis of COVID-19 and the adaptations made by experts to pulmonary disease diagnosis and treatment.
Pathophysiology of COVID-19
Innate immunity/inflammation/cytokine storm
Initially, the spike protein of SARS-CoV-2 binds to the ACE2 receptor on the surface of lung epithelial cells, type 2 alveolar cells, and endothelial cells [4] (Fig. 1 ). A human type 2 transmembrane serine protease (TMPRSS2) prime S glycoprotein facilitates virus entry into these host cells. The virus enters the cell cytoplasm and releases its RNA for replication using the host cell machinery, which results in multiplication of the virus that spreads to other host cells. In the human immune system, the cells that recognize the viral antigens become antigen-presenting cells that process the viral antigens to attract natural killer (NK) cells and CD8+ cytotoxic T cells. Therefore, both innate and adaptive immune systems are activated, and pro-inflammatory cytokines and chemokines are produced.
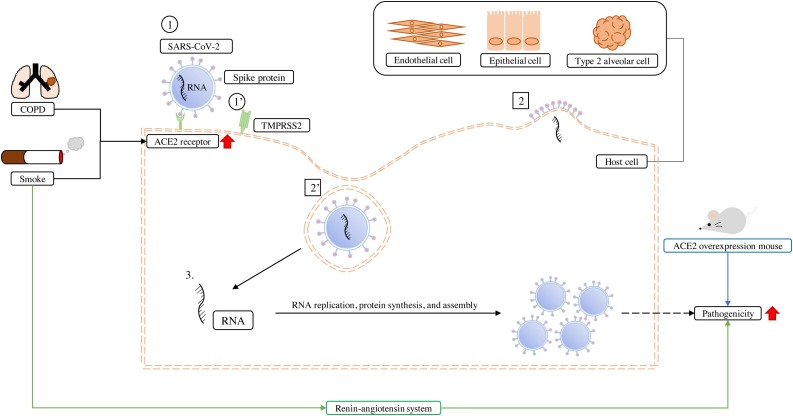
Fig. 1.
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection of lung cells and the associated complications.
The SARS-CoV-2 surface spike protein, S glycoprotein, can act through two pathways to enter the host cells, such as lung epithelial cells, endothelial cells, and type 2 alveolar cells. (1) One is via the spike protein binding to the angiotensin-converting enzyme 2 (ACE2) receptor, (2) using endocytosis to enter the cell, whereas (1’) the other is through the activation of the host cell surface type 2 transmembrane serine protease (TMPRSS2) by the spike protein, (2’) leading to SARS-CoV-2 fusion into the host cells. (3) Thereafter, SARS-CoV-2 replicates its RNA, synthesizes proteins, and assembles, resulting in the multiplication of viruses that spread to other host cells. Clinical statistical analysis showed that chronic obstructive pulmonary disease (COPD) and smoking are risk factors of SARS-CoV-2 infection as they can cause epithelial cells and type 2 alveolar cells to express more ACE2 receptors at the gene, mRNA, and protein levels. In addition, smoke could also pass through the renin-angiotensin system to enhance the pathogenicity of SARS-CoV-2. The same result was observed in human ACE2 over-expressing transgene mice, which had more severe SARS-CoV-2 pathogenicity.
The traditional innate immune system response to virus infection involves macrophages, monocytes, dendritic cells, and neutrophils (Fig. 2 ). These immune cells express a variety of pattern recognition receptors (PRRs) that can detect virus-induced pathogen-associated molecular patterns (PAMPs). The endosomal PRRs are toll-like receptors (TLRs) 3, 7, and 8, which recognize extracellular PAMPs, such as viral RNA [7]. The other PRRs are cytoplasmic RNA sensors, such as retinoic acid-inducible gene I (RIG-I) and melanoma differentiation-associated protein 5 (MDA5). The TLRs trigger the expression of pro-inflammatory cytokine-inducing transcription factors, such as nuclear factor-kappa B (NF-κB) and RIG-1/MDA5, and activate interferon response factor 3 (IRF3), or activation protein 1. IRF3 triggers the increased expression of type I interferon (T1IFN), which is a typical antiviral cytokine, whereas the NF-κB pathway triggers the expression of innate pro-inflammatory cytokines (interleukin [IL]-1, IL-6, and tumor necrosis factor-alpha [TNF-α]). Interestingly, SARS-CoV, Middle East respiratory syndrome coronavirus (MERS-CoV), and possibly SARS-CoV-2 may suppress these immune responses, including TLR3/7 and T1IFN [8], resulting in a poor prognosis.
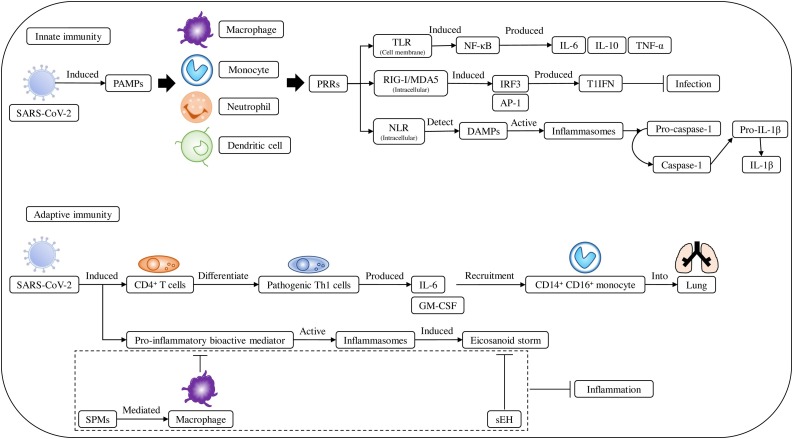
Fig. 2.
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection induces immune reaction.
The innate immune system includes macrophages, monocytes, dendritic cells, and neutrophils, which act through pattern recognition receptors (PRRs), detecting virus-induced pathogen-associated molecular patterns (PAMPs). PRRs included TLRs 3, 7, and 8, which can induce the production of cytokines such as IL-6, IL-10, and TNF-α. Other PRRs, retinoic acid-inducible gene I (RIG-I) and melanoma differentiation-associated protein 5 (MDA5), could induce interferon response factor 3 (IRF3) or activation protein 1 (AP-1) producing type I IFNs (T1IFN) to inhibit infection. Nucleotide-binding domain leucine-rich repeat (NLR) proteins also act as PRRs, which can detect endogenous danger-associated molecular patterns (DAMPs), activate inflammasomes, and covert pro-caspase-1 to AP-1, which then induces the conversion of pro-IL-1β to IL-1β. In adaptive immunity, SARS-CoV-2 induced CD4+ T cell differentiation to pathogenic Th1 cells, which produced IL-6 and granulocyte macrophage-colony-stimulating factor (GM-CSF) stimulating the recruitment of CD14+ CD16+ monocytes to the lungs. In addition, pro-inflammatory bioactive mediators such as eicosanoids, prostaglandins, and leukotrienes activate an inflammasome-induced eicosanoid storm. However, specialized pro-resolution mediators (SPMs) may mediate macrophage clearance of pro-inflammatory cytokine production, and soluble epoxide hydrolase (sEH) which could degrade arachidonic acid-derived epoxyeicosatrienoic acids (EETs) to inhibit inflammation.
NF-κB can also be triggered by the renin-angiotensin-aldosterone system (RAAS). Initially, SARS-CoV-2 downregulates ACE2, which is important for the conversion of angiotensin (Ang) II to angiotensin (1–7) [9] (Fig. 3 ). Thus, Ang II accumulates and binds to the angiotensin type 1 receptor (AT1R) on the immune cell membrane; this process activates the NF-κB pathway. This, in turn, induces IL-6, TNF-α, IL-1β, and IL-10 production. Ang II activates AT1R and regulates mitogen-activated protein kinases (MAPK) (ERK1/2, JNK, p38MPK) to release the cytokines IL-1, IL-10, IL-12, and TNF-α.
Fig. 3.
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infections induces cytokine storm.
During infection, SARS-CoV-2 induces lung epithelial cells, endothelial cells, and macrophages to produce cytokines such as IL-1, IL-2, IL-6, IL-7, IL-10, TNF-α, granulocyte colony-stimulating factor (GCSF), interferon-gamma-inducible protein 10 (CXCL10), monocyte chemoattractant protein 1 (MCP-1), and macrophage inflammatory protein 1-alpha (MIP-1α). This causes a cytokine storm, leading to macrophage, monocyte, and neutrophil recruitment from circulation into the lungs inducing endothelial cell, vascular barrier, capillary, and alveolar damage, resulting in acute lung injury. SARS-CoV-2, through the renin-angiotensin-aldosterone system (RAAS), inhibits angiotensin-converting enzyme 2 (ACE2) convert angiotensin (Ang) II to Ang 1–7, resulting in higher Ang II levels in the plasma, and through AT1R binding to macrophages and monocytes, results in NF-κB-induced IL-1β, IL-6, IL-10, and TNF-α expression. Active AT1R through the MAPK-ERK1/2, JNK, and p38MPK pathways regulates IL-1, IL-10, IL-12, and TNF-α release, causing a cytokine storm. In addition, SARS-CoV-2 causes lymphocytopenia, decreasing memory helper T, CD4+ and CD8+ lymphocytes, and NK cells.
The other pathogen recognition sensors are in the cytosol, such as nucleotide-binding domain leucine-rich repeat (NLR) proteins. NLRs detect endogenous danger-associated molecular patterns (DAMPs) within cells, and then trigger inflammasomes. Inflammasomes convert pro-caspase-1 to active caspase-1. Caspase-1 converts pro-IL-1β to active IL-1β. Additionally, NK cells are considered a part of the innate immune system as they destroy virus-infected cells.
The adaptive immune cells recognize and protect against invading viruses after the activation of innate immunity; these cells induce CD4+ T lymphocyte-derived cytokine expression, CD8+ T lymphocyte-mediated cytotoxicity, and B cell antibody production (Fig. 2). SARS-CoV-2 may stimulate the CD4+ T lymphocytes, which differentiate into Th1 cells. IL-6 and granulocyte macrophage colony-stimulating factor (GM-CSF) are then released from these Th1 cells. Thereafter, the CD14+ CD16+ monocytes in the blood circulate into the lungs. These specific monocytes may become alveolar macrophages. In adaptive immunity, CD8+ T cells (cytolytic) secrete granulysin, which is mediated by perforin. This process triggers the apoptosis of cytotoxic T and antigen-presenting cells. Finally, the over-activation of antigenic activity is prevented.
Eicosanoids are pro-inflammatory bioactive lipid mediators, such as prostaglandins and leukotrienes. SARS-CoV-2 may activate inflammasomes and induce eicosanoid storms (Fig. 2). During the eicosanoid storm inflammation, the levels of specialized pro-resolving mediators (SPMs) are reduced. SPMs induce the macrophage-mediated clearance of debris and counter pro-inflammatory cytokine production, which reduces inflammation [10]. They alleviate inflammation in influenza. Arachidonic acid-derived epoxyeicosatrienoic acids (EETs) also reduce and alleviate inflammation. EETs may induce the production of mediators in the anti-inflammatory processes. Treatment with SPMs or EETs may stimulate macrophage phagocytosis, decrease pro-inflammatory cytokine levels, diminish leukocytosis, and attenuate inflammatory cell infiltration in organs. These anti-inflammatory processes may result in an adaptive immune response and anti-SARS-CoV-2 antibody production. Interestingly, soluble epoxide hydrolase (sEH) produces EETs as metabolites. In some studies, SPMs and sEH inhibitors downregulate the expression of transcription regulator NF-κB and diminish the eicosanoid storms [10].
There is an increase in the monocyte and macrophage levels in patients with COVID-19 [1]. Furthermore, epithelial cells, macrophages, and endothelial cells secrete cytokines in the lungs. The levels of IL-1, IL-2, IL-6, IL-7, IL-10, TNF-α, granulocyte colony-stimulating factor (GCSF), interferon-gamma-inducible protein 10, monocyte chemoattractant protein 1, and macrophage inflammatory protein 1-alpha are elevated in patients with COVID-19 [1,11] (Fig. 3). Some of these cytokines are associated with lung injury in severe COVID-19 pneumonia. For example, an increased level of IL-6 is a poor prognostic factor in patients with COVID-19 and IL-10 is an anti-inflammatory cytokine that downregulates neutrophil and monocyte functions. Although IL-10 is beneficial for sepsis, the persistent downregulation of human leukocyte antigen-DR isotype (HLA-DR) by monocytes may lead to higher mortality. The cytokine storm results in the influx of various immune cells (macrophages, neutrophils, and T cells) from circulation to the lungs, thereby damaging endothelial cells, vascular barriers, capillaries, and alveoli, resulting in acute lung injury [1]. In patients with severe COVID-19, T lymphocytes produce less memory helper T cells and more naïve helper T cells [11]. With the occurrence of lymphocytopenia, the levels of subtypes of CD4+ and CD8+ T lymphocytes and NK cells decreases in patients with severe COVID-19. Despite the low level of lymphocytes in patients with severe COVID-19 and lymphocytopenia, the lymphocytes express high levels of HLA-DR and CD38.
Clinical lung injuries in patients with COVID-19
COVID-19 in patients with COPD and smokers
According to the clinical characteristics of patients with COVID-19 pneumonia, comorbidities, such as COPD, are among the most important risk factors [12]. Although the initial meta-analysis of Lippi et al. (2020) showed that smoking is not a proven risk factor of severe COVID-19, the authors only enrolled five study populations [13]. Alqahtani et al. studied both COPD and smoking status as risk factors for COVID-19 [14]. They analyzed 2473 patients with COVID-19 and found that those with COPD have a higher risk of severe disease than those without COPD (relative risk (RR) = 1.88, 95% confidence interval: 1.4–2.4), whereas current smokers have more severe complications than former smokers and non-smokers (RR = 1.45, 95% CI: 1.03–2.04). Leung et al. [15] validated ACE2 expression in the Cornell (n = 211) and the British Columbia Cancer Agency (n = 238) cohorts and showed that ACE2 expression in current smokers is significantly higher than that in the controls. Jacobs et al. retrieved 134 subjects from lung tissue biobanks at Ghent University Hospital in Belgium [5] and reported that ACE2 mRNA expression is higher in current smokers than in non-smokers. The authors also concluded that smoking status and COPD are associated with upregulated ACE2 mRNA expression independently, after adjusting for other factors. The same results were obtained in immunohistochemical (IHC) staining of ACE2 and analysis of protein levels in type II alveolar cells, bronchial epithelium, and alveolar tissue. One possible explanation is that smoking may upregulate the expression of the ACE2 receptor or that nicotine may influence the renin-angiotensin system and downregulate the expression of the ACE2 receptor (Fig. 1). Additionally, ACE2 expression in patients with COPD is higher than that in patients with asthma, and it causes more severe COVID-19, as 961 patients were hospitalized [16].
Interestingly, SARS-CoV cannot infect ACE2 knockout mice [17]. Moreover, the overexpression of human ACE2 in transgenic mouse models enhances the pathogenicity of SARS-CoV-2. This difference could be caused by the spike protein (S protein); although SARS-CoV and SARS-CoV-2 have similar structures, but SARS-Cov-2 S protein have higher occupancy (>90%) hydrogen bonds at S protein receptor-binding domain of ACE2 interface area. The mechanism indicates that SARS-Cov-2 can easily bind to ACE2 compared with SARS-CoV [18].
Upon SARS-CoV-2 infection, the virus downregulates the expression of ACE2. ACE2 is a negative regulator of the renin-angiotensin system that lowers the expression of Ang II. Decreased levels of ACE2, which is important for vasodilation, induce pneumonia and ARDS.
COVID-19 in lung cancer
In China, cancer comorbidity is a risk factor for COVID-19, providing a poorer prognosis. In the Thoracic Cancers International COVID-19 Collaboration registry multicenter observational study, lung cancer was associated with high mortality and low ICU admission rate with COVID-19 infection [19]. Lung cancer patients with COVID-19 have high hospitalization, ARDS, and mortality rates. COVID-19 is more severe in patients with lung cancer, smoking status, and COPD, indicating that they are risk factors.
During immunotherapy, the immune system changes, which increases the risk of COVID-19 and its severity. Conversely, the adverse effects of anti-cancer therapies on the lungs may mimic COVID-19 pneumonia. Nivolumab (a programmed cell death protein-1 [PD-1] checkpoint inhibitor) immunotherapy may cause a paradoxical immunologic response to the influenza virus and increase T cell-activated systemic inflammation. Moreover, antineoplastic therapies may increase COVID-19 vulnerability in patients with lung cancer. For example, bevacizumab may increase the risk of thrombosis. Gemcitabine, cisplatin, and taxanes may increase the risk of myelosuppression and immunosuppression. Gemcitabine and epidermal growth factor receptor-targeted agents and anti-PD-1 inhibitors increase the risk of interstitial pneumonitis. Radiotherapy increases interstitial fibrosis in the lungs. For patients with non-small-cell lung cancer with an MET exon 14 skipping mutation, the c-Met kinase inhibitor crizotinib is administered. However, crizotinib-induced interstitial lung disease (ILD) can develop at the same time as SARS-CoV-2 infection. In real-world situations, before SARS-CoV-2 polymerase chain reaction (PCR) confirmation, lung adenocarcinoma, drug-induced ILD, and COVID-19 could be mimicked in images (for examples computerized tomography (CT) of the chest) that show ground-glass opacity and consolidation [20,21].
Elective surgery is indicated for early-stage adenocarcinoma of the lung, such as stages I and II. An incidental finding of SARS-CoV-2 pneumonia after resection of lung cancer was reported by Tian et al. [22].
Operating rooms are associated with a higher risk of nosocomial infection to other inpatients and healthcare providers. Tian et al. reported that one patient survived and the other died owing to the deteriorated oxygenation capacity due to COVID-19 pneumonia after surgery. The pathological results of the resected lung tissue were alveolar edema, focal type II pneumocytes hyperplasia, protein-rich exudate, and inflammatory cell patchy infiltration without hyaline membrane formation. The early phase of COVID-19 pneumonia could be identified in pathological reports and CT scan of the chest, which reveal ground-glass opacity near the peripheral lung.
Patients with lung cancer have experienced changes in their treatment plans during the COVID-19 pandemic. Therefore, decisions on adjuvant or palliative therapy during the COVID-19 pandemic should be exercised with caution. The European Society for Medical Oncology (ESMO) provides recommendations for the management and treatment of patients with lung cancer during the COVID-19 pandemic [23]. The ESMO, Cancer Care Ontario, Huntsman Cancer Institute, and Magnitude of Clinical Benefit Scale defined patients with lung cancer based on three different priorities—high, medium, and low. High priority: clinically unstable and/or the magnitude of benefit qualifies the intervention as high priority because the patients’ condition is immediately life-threatening; medium priority: delay beyond 6–8 weeks could potentially affect the overall outcome and/or the magnitude of benefit qualifies for intermediate priority although the patients’ condition is non-critical; low priority: allowing services to be delayed for the duration of the COVID-19 pandemic and/or the intervention is non-priority based on the magnitude of benefit because the patients’ condition is stable enough. The priorities of diagnosis, staging, and treatment for lung cancer decrease the possible negative effect of management during the COVID-19 pandemic. The termination of treatment plans has been suggested for lung cancer patients with COVID-19 infection, and impaired pulmonary function and cardiopulmonary comorbidities will increase COVID-19 mortality in patients with lung cancer.
During the treatment of patients with lung cancer and COVID-19, chemo-immunotherapy (carboplatin, pemetrexed, and pembrolizumab) is frequently used.
Tocilizumab is a recombinant humanized monoclonal antibody directed against the IL-6 receptor. Bonomi et al. reported a 65-year-old man with a history of smoking who was diagnosed with stage IV lung adenocarcinoma (bilateral lung metastases). He received tocilizumab (anti-IL-6 receptor) twice in ASST Cremona, Lombardia, Italy in 2020. After treatment, the oxygen support was decreased and CT scan showed ground glass opacity reduction. Finally, the patient was discharged, and he resumed chemoimmunotherapy without complications [24]. This strategy is compatible with the ESMO guideline that COVID19 treatment should be prioritized and chemoimmunotherapy can be resumed after stabilization of COVID-19. Chemoimmunotherapy should not combined with tocilizumab; chemoimmunotherapy is also not suggested during COVID-19 in patients with lung cancer.
Therefore, the awareness of lung cancer patients with COVID-19 during this pandemic is important for managing their treatment strategy. Another problem experienced has been a delayed diagnosis of lung cancer during the COVID-19 pandemic. In the UK Lung Cancer Coalition report, the proportion of patients with late-stage lung cancer has increased owing to possible overlapping symptoms with COVID-19 and the increasing stress on the respiratory healthcare services.
COVID-19 and lung transplantations
Lang et al. (2020) reported successful lung transplantation in a 44-year-old female with COVID-19 and had developed ARDS in Austria [25].
Post-lung transplantation, asymptomatic or mild symptoms may be exhibited during SARS-CoV-2 infection in patients treated with immunosuppression agents. However, lung transplant recipients with COVID-19 pneumonia may have different disease outcomes, which warrant further confirmational studies. In lung transplant recipients with COVID-19, immunosuppressant and anti-virus agents may be combined [26]. Tacrolimus (T cell proliferation inhibitor) is a popular treatment after lung transplantation, and lopinavir/ritonavir has been used for lung transplantation patients with COVID-19. Saez-Gimenez et al. (2020) found a strong interaction between tacrolimus and lopinavir/ritonavir; lopinavir/ritonavir inhibits tacrolimus metabolism and enhances the effect of tacrolimus. Overall, 37 (84%) patients needed oxygen therapy or ventilator support and 17 (39%) died.
Furthermore, according to lung tissue studies from patients after transplantation and post-mortem of patients who died with COVID-19, extensive end-stage pulmonary fibrosis may be observed. In this situation, lung transplantation is the only way for a patient with COVID-19-induced end-stage pulmonary fibrosis to survive. Cypel et al. (2020) noted 10 considerations regarding lung transplantation for COVID-19-associated ARDS [27]. These considerations included lung disease, patients aged <65 years with only one organ dysfunction and an estimated good recovery, such as a 5-year survival rate of 60%, patients should be cognitively aware and can consent for lung transplantation discussion, physical rehabilitation after transplantation, adequate body mass index, and patients should test negative for SARS-CoV-2 PCR or absence of viable virus shown by an infectivity assay. Additionally, the transplantation center should have a high-risk experience and low waitlist mortality.
Image study (sonography/CT/X-ray)
Chest X-ray, especially in portable radiography, may be convenient and time saving for suspected pneumonia. However, it also increases the risk of contamination during portable chest radiographs. Conversely, chest radiographs correlated poorly with the clinical condition of COVID-19 compared with CT and ultrasound imaging [28].
Lung ultrasound may help evaluate the severity of COVID-19 pneumonia and even ARDS. The characteristics of peripheral lung involvement in COVID-19 pneumonia increase the use of lung ultrasound for this evaluation. Vetrugno et al. (2020) first used lung ultrasound to evaluate COVID-19 in the lungs [29]. The authors calculated the pneumonia severity score from 0 to 36. The A lines are normal, B1 lines are interstitial change and decreased lung aeration, B2 lines are white lung such as ground-glass opacities on CT, and C is hepatization of lung parenchyma presented as lung atelectasis or pneumonia consolidation. Lung ultrasound may reduce the use of chest radiography and CT. The lung ultrasound images included A lines, B lines, and consolidations with the severity of pneumonia, such as moderate, severe, and critical.
CT data collected from patients with COVID-19 pneumonia showed bilateral patchy consolidation or ground-glass opacity, which may have a 10% crazy-paving appearance [30]. The consolidation may have an air-bronchogram and the pleural effusion is usually absent. CT helps to identify COVID-19 pneumonia in the early phases. Ground-glass opacities were observed in the peripheral lung initially. Later, large consolidations were located over the basal or dependent part of the lung. The authors of the study also concluded that the maximum lung involvement peak was 10 days following the onset of initial symptoms and defined the disease stage using lung CT as stage 1: ground-glass opacities, stage 2: increased crazy-paving pattern, stage 3: consolidation, and stage 4: gradual resolution of consolidation without a crazy-paving pattern. The Dutch Association for Radiology created a standardized assessment of chest CT in COVID-19 pneumonia, the COVID-19 Reporting and Data System, with scores from 1 (highly unlikely) to 5 (highly likely) [20]. However, the specificity of CT remains low for differentiating COVID-19 in lung cancer patients [21].
Lung histopathology
There are pathological reports based on radiologic presentation [31] or autopsy [32,33]. The gross appearance of an autopsy showed patchy peripheral hemorrhage of lung parenchyma [33]; the alveoli lose their elasticity. On the cut surfaces, fibrous cords are found with sticky secretion exudate from the pulmonary alveoli, as well as the bronchus and trachea. By hematoxylin-eosin staining and IHC, diffuse alveolar damage, both alveolar exudative inflammation and interstitial inflammation, was found to be an ARDS [33]. Alveolar septa is where edematous, widened, congested, dilated blood vessels are present, and monocytes, lymphocytes, and macrophage infiltrate (interstitial mononuclear inflammatory infiltrates) the alveoli. Type II alveolar epithelial proliferation (hyperplasia) and focal desquamation of alveolar epithelia (denuded alveolar lining cells) were observed. Focal hemorrhage and occasional organization of exudates in alveolar cavities (intra-alveolar fibrinous exudates) and loose pulmonary interstitial fibrosis have been found. Hyaline membrane formation and partial bronchial epithelial exfoliation have also been found. Organizing pneumonia was diagnosed by intra-alveolar loose fibrous plugs and organizing fibrin.
Electron microscopy has revealed the presence of coronavirus particles in the bronchial mucosal epithelia and type II alveolar epithelia. SARS-CoV-2 antigen immunohistochemistry staining could be positive in alveolar epithelial cells and macrophages, and desquamated cells within the alveolar space. The real-time PCR of SARS-CoV-2 could also be positive.
SARS-CoV-2 may infect endothelial cells via the ACE2 receptor and induce thrombotic tendency. Increased levels of inflammatory mediators and immunoglobulins may induce high blood viscosity. Anti-cardiolipin antibodies have also been found in small groups. These findings suggest immune complex-mediated vasculitis via monocytes and lymphocytes within and around blood vessels, wall thickening, and focal hemorrhage [1]. Therefore, patients with COVID-19 may have a risk of deep vein thrombosis and pulmonary thromboembolism.
Albarello et al. (2020) reported that ARDS CT scan findings included pleural effusions, pulmonary vessel tubular enlargement, and sudden reduction in the caliber of the dichotomic tracts [31].
Conclusions
The lung is an important organ in humans; SARS-CoV-2 attacks the lungs via the ACE2 receptors and this infection is associated with the development of pneumonia, ARDS, and multiple organ failure. Moreover, COVID-19 pulmonary infection induces immune responses, such as cytokine storms which deteriorate the lung conditions, especially in patients with lung comorbidities such as COPD, cancer, and transplantation. The COVID-19 pandemic has also changed the routine diagnostic methods, including imaging (sonography/CT/X-ray) and histopathology. The optimal diagnosis and suitable treatment protocols for lung diseases have also been adapted to protect patients and healthcare workers worldwide, resulting in increased survival rates and reduced acute lung injuries.
Funding
This work was supported by the Taipei Tzu Chi Hospital [grant number TCRD-TPE-109-16] and the Buddhist Tzu Chi Medical Foundation [grant number TCMF-A 109–05 (110)].
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author contributions
YCC conceived the study, designed the review structure, and supervised critical revisions. WLS prepared and drafted the manuscript. KCL conducted the research and investigation. CYC designed and created the figures. All authors contributed substantially to the manuscript revision and have read and approved the final manuscript.
Conflict of interest
The authors have no conflicts of interest to declare.
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing and publication support.
References
- 1.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johns Hopkins University Coronavirus Resource Center . 2021. Mortality analyses.https://coronavirus.jhu.edu/data/mortality [accessed 26 January 2021] [Google Scholar]
- 3.Woolf S.H., Chapman D.A., Lee J.H. COVID-19 as the leading cause of death in the United States. Jama. 2021;325:123–124. doi: 10.1001/jama.2020.24865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jacobs M., Van Eeckhoutte H.P., Wijnant S.R.A., Janssens W., Joos G.F., Brusselle G.G., et al. Increased expression of ACE2, the SARS-CoV-2 entry receptor, in alveolar and bronchial epithelium of smokers and COPD subjects. Eur Respir J. 2020;56 doi: 10.1183/13993003.02378-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Catanzaro M., Fagiani F., Racchi M., Corsini E., Govoni S., Lanni C. Immune response in COVID-19: addressing a pharmacological challenge by targeting pathways triggered by SARS-CoV-2. Signal Transduct Target Ther. 2020;5:84. doi: 10.1038/s41392-020-0191-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barrat F.J., Elkon K.B., Fitzgerald K.A. Importance of nucleic acid recognition in inflammation and autoimmunity. Annu Rev Med. 2016;67:323–336. doi: 10.1146/annurev-med-052814-023338. [DOI] [PubMed] [Google Scholar]
- 8.de Wit E., van Doremalen N., Falzarano D., Munster V.J. SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol. 2016;14:523–534. doi: 10.1038/nrmicro.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuba K., Imai Y., Ohto-Nakanishi T., Penninger J.M. Trilogy of ACE2: a peptidase in the renin–angiotensin system, a SARS receptor, and a partner for amino acid transporters. Pharmacol Ther. 2010;128:119–128. doi: 10.1016/j.pharmthera.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Serhan C.N. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510:92–101. doi: 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y., et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020;71:762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lippi G., Henry B.M. Active smoking is not associated with severity of coronavirus disease 2019 (COVID-19) Eur J Intern Med. 2020;75:107–108. doi: 10.1016/j.ejim.2020.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alqahtani J.S., Oyelade T., Aldhahir A.M., Alghamdi S.M., Almehmadi M., Alqahtani A.S., et al. Prevalence, severity and mortality associated with COPD and smoking in patients with COVID-19: a rapid systematic review and meta-analysis. PLoS One. 2020;15 doi: 10.1371/journal.pone.0233147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leung J.M., Yang C.X., Tam A., Shaipanich T., Hackett T.L., Singhera G.K., et al. ACE-2 expression in the small airway epithelia of smokers and COPD patients: implications for COVID-19. Eur Respir J. 2020;55 doi: 10.1183/13993003.00688-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song J., Zeng M., Wang H., Qin C., Hou H.Y., Sun Z.Y., et al. Distinct effects of asthma and COPD comorbidity on disease expression and outcome in patients with COVID-19. Allergy. 2021;76:483–496. doi: 10.1111/all.14517. [DOI] [PubMed] [Google Scholar]
- 17.Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B., et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xie Y., Karki C.B., Du D., Li H., Wang J., Sobitan A., et al. Spike proteins of SARS-CoV and SARS-CoV-2 utilize different mechanisms to bind with human ACE2. Front Mol Biosci. 2020;7 doi: 10.3389/fmolb.2020.591873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garassino M.C., Whisenant J.G., Huang L.C., Trama A., Torri V., Agustoni F., et al. COVID-19 in patients with thoracic malignancies (TERAVOLT): first results of an international, registry-based, cohort study. Lancet Oncol. 2020;21:914–922. doi: 10.1016/s1470-2045(20)30314-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prokop M., van Everdingen W., van Rees Vellinga T., Quarles van Ufford H., Stoger L., Beenen L., et al. CO-RADS: a categorical CT assessment scheme for patients suspected of having COVID-19-definition and evaluation. Radiology. 2020;296:E97–104. doi: 10.1148/radiol.2020201473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fujiwara Y., Sato Y., Wang X., Oikado K., Sato Y., Fukuda N., et al. Screening for COVID-19 in symptomatic cancer patients in a cancer hospital. Cancer Cell. 2020;38:609–610. doi: 10.1016/j.ccell.2020.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tian S., Hu W., Niu L., Liu H., Xu H., Xiao S.Y. Pulmonary pathology of early-phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. J Thorac Oncol. 2020;15:700–704. doi: 10.1016/j.jtho.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Passaro A., Addeo A., Von Garnier C., Blackhall F., Planchard D., Felip E., et al. ESMO management and treatment adapted recommendations in the COVID-19 era: lung cancer. ESMO Open. 2020;5 doi: 10.1136/esmoopen-2020-000820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bonomi M., Maltese M., Brighenti M., Muri M., Passalacqua R. Tocilizumab for COVID-19 pneumonia in a patient with non-small-cell lung cancer treated with chemoimmunotherapy. Clin Lung Cancer. 2021;22:e67–e69. doi: 10.1016/j.cllc.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lang C., Jaksch P., Hoda M.A., Lang G., Staudinger T., Tschernko E., et al. Lung transplantation for COVID-19-associated acute respiratory distress syndrome in a PCR-positive patient. Lancet Respir Med. 2020;8:1057–1060. doi: 10.1016/S2213-2600(20)30361-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saez-Gimenez B., Berastegui C., Barrecheguren M., Revilla-Lopez E., Los Arcos I., Alonso R., et al. COVID-19 in lung transplant recipients: a multicenter study. Am J Transplant. 2021;21:1816–1824. doi: 10.1111/ajt.16364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cypel M., Keshavjee S. When to consider lung transplantation for COVID-19. Lancet Respir Med. 2020;8:944–946. doi: 10.1016/S2213-2600(20)30393-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoon S.H., Lee K.H., Kim J.Y., Lee Y.K., Ko H., Kim K.H., et al. Chest radiographic and CT findings of the 2019 novel coronavirus disease (COVID-19): analysis of nine patients treated in Korea. Korean J Radiol. 2020;21:494–500. doi: 10.3348/kjr.2020.0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vetrugno L., Bove T., Orso D., Barbariol F., Bassi F., Boero E., et al. Our Italian experience using lung ultrasound for identification, grading and serial follow-up of severity of lung involvement for management of patients with COVID-19. Echocardiography. 2020;37:625–627. doi: 10.1111/echo.14664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bernheim A., Mei X., Huang M., Yang Y., Fayad Z.A., Zhang N., et al. Chest CT findings in coronavirus disease-19 (COVID-19): relationship to duration of infection. Radiology. 2020;295 doi: 10.1148/radiol.2020200463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Albarello F., Pianura E., Di Stefano F., Cristofaro M., Petrone A., Marchioni L., et al. 2019-novel Coronavirus severe adult respiratory distress syndrome in two cases in Italy: an uncommon radiological presentation. Int J Infect Dis. 2020;93:192–197. doi: 10.1016/j.ijid.2020.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang H., Zhou P., Wei Y., Yue H., Wang Y., Hu M., et al. Histopathologic changes and SARS-CoV-2 immunostaining in the lung of a patient with COVID-19. Ann Intern Med. 2020;172:629–632. doi: 10.7326/M20-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou B., Zhao W., Feng R., Zhang X., Li X., Zhou Y., et al. The pathological autopsy of coronavirus disease 2019 (COVID-2019) in China: a review. Pathog Dis. 2020;78 doi: 10.1093/femspd/ftaa026. [DOI] [PMC free article] [PubMed] [Google Scholar]