Main text
To the Editor,
The coronavirus disease 2019 (COVID-19) pandemic continues to pose a serious threat to people’s lives, owing to the variants, particularly the B.1.617.2 (delta) variant. We recently published a paper in Molecular Therapy that studies the efficacy and safety of COVID-19 vaccines in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).1 Here, we report analyses of the efficacy of COVID-19 vaccines against the delta variant. We evaluated the efficacy of COVID-19 vaccines from the perspective of overall infection, severe infection, and fatal infection, following receipt of 3 types of vaccines: messenger RNA (mRNA)-based vaccine; viral vector (non-replicating) vaccine; and inactivated vaccine (no data have been reported for other types of vaccines).
We identified records by searching Google Scholar, PubMed, Medline, Excerpt Medica Database (EMBASE), and the Cochrane Central Register of Controlled Trials (CENTRAL) for “(COVID-19 vaccine), B.1.617.2, and delta variant” on 11 August 2021. They comprised 7 clinical trials, 3 categories of vaccine (comprising 6 different vaccines), and a total of 504,781 cases (69,315 versus 435,466).2, 3, 4, 5, 6, 7, 8 A meta-analysis was performed using R statistical software (details are shown in supplemental information, Figure S1, and Table S1).9
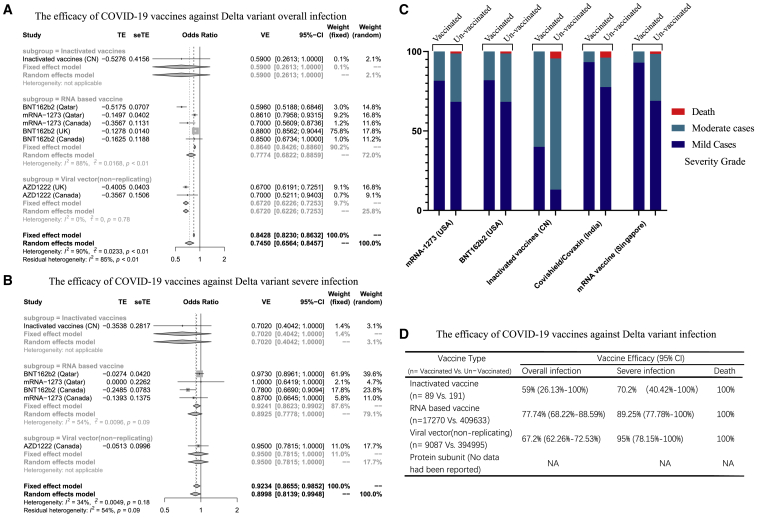
We first investigated the efficacy of COVID-19 vaccines against overall infection by the delta variant. The efficacy was 59% (95% confidence interval [CI]: 26.13; 100) for inactivated vaccines, 67.74% (95% CI: 62.26; 72.53) for viral vector vaccines, and 77.74% (95% CI: 68.22; 88.59) for mRNA-based vaccines (Figures 1A and 1D). We next evaluated the efficacy against severe infection (defined as per World Health Organization guidelines).2 The efficacy of inactivated vaccines was 70.2% (95% CI: 40.42; 100), of viral vector (non-replicating) vaccines 95% (95% CI: 78.15; 100), and of RNA-based vaccines 89.25% (95% CI: 77.78; 100; Figures 1B and 1D).
Figure 1.
The efficacy of COVID-19 vaccines against the B.1.617.2 (delta) variant
(A) Overall infection. (B) Severe infection is shown. (C) The incidence of severity grade in vaccinated and un-vaccinated cases is shown. Death (red), moderate cases (dark blue), and mild cases (light blue) are shown. (D) The details of efficacy of vaccines are shown.
To further evaluate the efficacy of COVID-19 vaccines against severe infection with delta variant, we compared vaccinated and un-vaccinated patients. Because the clinical trials of viral vector (non-replicating) vaccines lack sufficient numbers of mild and moderate cases and deaths, only mRNA-based vaccines and inactivated vaccines were included in this analysis. The results showed that the incidence of death in the entire vaccinated group was 0%, although the rate in the un-vaccinated group was 1.25%–4.55%. Meanwhile, the incidence of moderate infection in the vaccinated group (6.73%–60%) was lower than that in un-vaccinated group (19.32%–86.37%). The RNA-based vaccines showed better protection than inactivated vaccines (Figures 1C and 1D).
In term of efficacy against overall infection by the delta variant, RNA-based vaccines ranked first, followed by viral vector (non-replicating) vaccines and inactivated vaccines, which is consistent with the results against the original SARS-CoV-2 strain. RNA-based vaccines may be more effective against these mutant strains, owing to their complete presentation of the full immunogenicity of SARS-CoV-2. However, the efficacy of all vaccines was decreased against the delta variant. The level of neutralizing antibodies is an important factor in determining the effectiveness of vaccines. For all types of vaccine, the titers of neutralizing antibodies against the delta variant after infection were decreased compared with that of original virus strains.10 At the same time, some studies have found that the peak of delta virus burden is the same in vaccinated people compared to those who have not been vaccinated.11 These findings may explain the reduced effectiveness of the vaccine. Chen et al.10 found that mRNA-based vaccines produced the highest level of neutralizing antibodies in both normal vaccinated humans and in patients infected with delta variant, which is consistent with the efficacy analysis. However, for viral vector (non-replicating) vaccines and inactivated vaccines, the titers of neutralizing antibodies in vaccinated patients were significantly lower than those in previously infected patients,10 suggesting that they carry insufficient immunogenicity, resulting in decreased efficacy.
Although vaccination cannot completely prevent infection by the COVID-19 delta variant, protection against moderate and severe infection remains satisfactory, and all vaccines exhibit efficacy greater than 70%. The viral vector (non-replicating) vaccines exhibit the highest efficacy, but due to the small sample size, this result is somewhat biased. Vaccination can prevent death, and the proportion of severe cases is also greatly reduced compared to the un-vaccinated. Therefore, vaccination remains an important preventive method for the delta variant. However, a noteworthy problem remains. Whereas the vaccine could prevent moderate and severe infections, the viral burden was unchanged.11 This may delay diagnosis to a certain extent and may thus increase the risk of transmission, particularly to un-vaccinated people.12 Booster shots may address this problem. Pouwels et al.11 comprehensively compared the viral load and neutralizing antibody levels of the various strains among previously infected, vaccinated, and un-vaccinated people. They observed that the risk of infection by the delta variant in previously infected people was lower than in vaccinated people and that the level of neutralizing antibodies in previously infected people was greater than in vaccinated people,11 suggesting that antibodies against the original virus strains still play an important role in the control of current variant strains, as do the COVID-19 vaccines. Nevertheless, because efficacy is weakened because of different levels of immunogenicity, booster shots may strengthen protection by the vaccines.13,14 Some countries have already approved the use of booster shots, with prioritization of the immunocompromised.13 Another option is the development of new vaccines; however, the virus that causes COVID-19 mutates almost once a week,15 and the rate of vaccine development may be insufficient against this rate of mutation, unless a vaccine with stronger immunogenicity can be developed.
Our analysis has some limitations. Due to the limited data for the delta variant and the fact that all studies were test-negative design, researchers have corrected vaccine efficiency for different reasons, which results in a large heterogeneity of the data. In addition, most of the studies are published on pre-printed platforms and have not yet undergone peer review. Thus, there may be design flaws leading to data bias. Therefore, further real-world data are needed for verification.
We conclude that the efficacy of all COVID-19 vaccines has dropped against the delta variant. Fortunately, the COVID-19 vaccines exhibit satisfactory efficacy against severe infection. Undoubtedly, vaccination is the most promising means to control COVID-19, including that caused by the delta variant. Nonetheless, the vaccine booster shots should be considered to combat COVID-19 variants.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.ymthe.2021.09.024.
Contributor Information
Hong Shen, Email: hongshen2000@csu.edu.cn.
Ying Han, Email: yinghan@csu.edu.cn.
Supplemental information
References
- 1.Cai C., Peng Y., Shen E., Huang Q., Chen Y., Liu P., Guo C., Feng Z., Gao L., Zhang X., Gao Y. A comprehensive analysis of the efficacy and safety of COVID-19 vaccines. Mol. Ther. 2021;29:2794–2805. doi: 10.1016/j.ymthe.2021.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tang P., Hasan M.R., Chemaitelly H., Yassine H.M., Benslimane F.M., Al Khatib H.A., AlMukdad S., Coyle P., Ayoub H.H., Al Kanaani Z., Al Kuwari E. BNT162b2 and mRNA-1273 COVID-19 vaccine effectiveness against the Delta (B.1.617.2) variant in Qatar. medRxiv. 2021 doi: 10.1101/2021.08.11.21261885. [DOI] [PubMed] [Google Scholar]
- 3.Puranik A., Lenehan P.J., Silvert E., Niesen M.J.M., Corchado-Garcia J., O’Horo J.C., Virk A., Swift M.D., Halamka J., Badley A.D., Venkatakrishnan A.J. Comparison of two highly-effective mRNA vaccines for COVID-19 during periods of Alpha and Delta variant prevalence. medRxiv. 2021 doi: 10.1101/2021.08.06.21261707. [DOI] [Google Scholar]
- 4.Lopez Bernal J., Andrews N., Gower C., Gallagher E., Simmons R., Thelwall S., Stowe J., Tessier E., Groves N., Dabrera G. Effectiveness of Covid-19 vaccines against the B.1.617.2 (Delta) variant. N. Engl. J. Med. 2021;385:585–594. doi: 10.1056/NEJMoa2108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nasreen S., Chung H., He S., Brown K.A., Gubbay J.B., Buchan S.A., Fell D.B., Austin P.C., Schwartz K.L., Sundaram M.E., Calzavara A. Effectiveness of COVID-19 vaccines against variants of concern in Ontario, Canada. medRxiv. 2021 doi: 10.1101/2021.06.28.21259420. [DOI] [Google Scholar]
- 6.Li X.N., Huang Y., Wang W., Jing Q.L., Zhang C.H., Qin P.Z., Guan W.J., Gan L., Li Y.L., Liu W.H. Effectiveness of inactivated SARS-CoV-2 vaccines against the Delta variant infection in Guangzhou: a test-negative case-control real-world study. Emerg. Microbes Infect. 2021;10:1751–1759. doi: 10.1080/22221751.2021.1969291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thangaraj J.W.V., Yadav P., Kumar C.G., Shete A., Nyayanit D.A., Rani D.S., Kumar A., Kumar M.S., Sabarinathan R., Kumar V.S. Predominance of delta variant among the COVID-19 vaccinated and unvaccinated individuals, India, May 2021. J. Infect. 2021 doi: 10.1016/j.jinf.2021.08.006. Published online August 5, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chia P.Y., Xiang Ong S.W., Chiew C.J., Ang L.W., Chavatte J.-M., Mak T.-M., Cui L., Kalimuddin S., Chia W.N., Tan C.W., Chai L.Y.A. Virological and serological kinetics of SARS-CoV-2 Delta variant vaccine-breakthrough infections: a multi-center cohort study. medRxiv. 2021 doi: 10.1101/2021.07.28.21261295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belongia E.A., Simpson M.D., King J.P., Sundaram M.E., Kelley N.S., Osterholm M.T., McLean H.Q. Variable influenza vaccine effectiveness by subtype: a systematic review and meta-analysis of test-negative design studies. Lancet Infect. Dis. 2016;16:942–951. doi: 10.1016/S1473-3099(16)00129-8. [DOI] [PubMed] [Google Scholar]
- 10.Chen X., Chen Z., Azman A.S., Sun R., Lu W., Zheng N., Zhou J., Wu Q., Deng X., Zhao Z. Neutralizing antibodies against SARS-CoV-2 variants induced by natural infection or vaccination: a systematic review and pooled meta-analysis. Clin. Infect. Dis. 2021:ciab646. doi: 10.1093/cid/ciab646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pouwels K.B., Pritchard E., Matthews P.C., Stoesser N., Eyre D.W., Vihta K.-D., House T., Hay J., Bell J.I., Newton J.N., Farrar J. Impact of Delta on viral burden and vaccine effectiveness against new SARS-CoV-2 infections in the UK. medRxiv. 2021 doi: 10.1101/2021.08.18.21262237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Subbaraman N. How do vaccinated people spread Delta? What the science says. Nature. 2021;596:327–328. doi: 10.1038/d41586-021-02187-1. [DOI] [PubMed] [Google Scholar]
- 13.Vogel G. Unethical? Unnecessary? The booster debate intensifies. Science. 2021;373:949–950. doi: 10.1126/science.373.6558.949. [DOI] [PubMed] [Google Scholar]
- 14.Tregoning J.S., Flight K.E., Higham S.L., Wang Z., Pierce B.F. Progress of the COVID-19 vaccine effort: viruses, vaccines and variants versus efficacy, effectiveness and escape. Nat. Rev. Immunol. 2021 doi: 10.1038/s41577-021-00592-1. Published online August 9, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morales A.C., Rice A.M., Ho A.T., Mordstein C., Mühlhausen S., Watson S., Cano L., Young B., Kudla G., Hurst L.D. Causes and consequences of purifying selection on SARS-CoV-2. Genome Biol. Evol. 2021 doi: 10.1093/gbe/evab196. Published online August 24, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.