Abstract
Background
The benefits of plasma as an adjunct to the treatment of haemorrhagic shock are well established; however, the mechanism by which plasma modulates the endotheliopathy of trauma remains unclear. Our recent data demonstrated a novel role of microRNA-19b in post-haemorrhagic shock endothelial dysfunction via targeting of syndecan-1. Additionally, fibrinogen, as a key component of plasma or an isolated haemostatic protein, protects the endothelium by stabilizing syndecan-1. We therefore hypothesized that fibrinogen would inhibit microRNA-19b to mitigate the endotheliopathy of trauma in a murine model of haemorrhagic shock.
Materials and methods
C57BL/6J mice were subjected to haemorrhagic shock (mean arterial pressure 35±5 mmHg for 90 minutes) followed by resuscitation with lactated Ringer’s, fresh frozen plasma, fibrinogen or no resuscitation. MicroRNA-19b and syndecan-1 mRNA were measured in lung tissue by qRT-PCR. Lungs were stained for histopathologic injury, and broncheoalveolar lavage was collected for protein as a permeability indicator.
Results
Pulmonary microRNA-19b was increased after haemorrhagic shock and lactated Ringers, but reduced to sham levels by plasma and fibrinogen. Conversely, pulmonary syndecan-1 mRNA was downregulated by haemorrhagic shock and lactated Ringers, but returned to sham levels by plasma and fibrinogen. Plasma and fibrinogen-based resuscitation reduced lung injury compared to haemorrhagic shock and lactated Ringers while fibrinogen also reduced broncheoalveolar lavage protein.
Discussion
We have demonstrated a novel mechanism by which fibrinogen, a key component of plasma and haemostatic agent, inhibits miR-19b, possibly by mitigating the endotheliopathy of trauma. Complete demonstration of the mechanism of fibrinogen inhibition of endotheliopathy via microRNA, however, remains to be elucidated. These findings support the early and empiric use of fibrinogen in post-haemorrhagic shock resuscitation.
Keywords: shock, haemorrhagic, fibrinogen, endothelium, microRNA
INTRODUCTION
Trauma is the leading cause of death for individuals ages 1 to 44 both in the United States and worldwide, while haemorrhagic shock (HS) remains the primary cause of early death after trauma1,2 in both the military and civilian setting3,4. Survivors of haemorrhagic shock are at risk to develop the endotheliopathy of trauma (EoT), which is a systemic response to activated endothelial cells that leads to abnormalities in coagulation, inflammation, and endothelial barrier integrity5. This is clinically manifested as a pro-inflammatory state with vascular leak and tissue edema, which contributes to multi-organ dysfunction, and ultimately, death6. Changes in strategies for resuscitation have been critically important for reducing mortality after trauma, specifically those that have included the early and empiric use of fresh frozen plasma (FFP) for patients who present with haemorrhagic shock.
A number of clinical studies have confirmed the importance and utility of balanced resuscitation for this group of patients, using a 1:1:1 ratio of red blood cells, plasma, and platelets7-10. Recent studies have also found a mortality benefit when plasma is given to patients in haemorrhagic shock in the pre-hospital environment11. The benefits of plasma as an adjunct to the treatment of haemorrhagic shock are thought to be two-fold. First, plasma contains important factors that may correct or improve trauma-induced coagulopathy12. Second, plasma appears to offer protective effects against haemorrhage-induced endothelial dysfunction, likely via restoration of the endothelial glycocalyx6,13,14. Strategies that support restoration of the endothelial glycocalyx and barrier integrity offer opportunities for further reduction of haemorrhage-related morbidity and mortality; however, the cellular and molecular mechanisms by which EoT develops and the glycolcalyx is restored are not yet fully elucidated.
The backbone of the endothelial glyocalyx is formed by syndecan-1, a heparan sulfate cell surface proteoglycan15,16. Shedding of the syndecan-1 ectodomain into the systemic circulation is associated with enhanced shock, inflammation, and endothelial damage after haemorrhage and other forms of shock6. Higher levels of circulating syndecan-1 have been independently associated with mortality after haemorrhagic shock in human studies17–19. Previously, we have found that in a rodent model of haemorrhagic shock, pulmonary syndecan-1 messenger RNA (mRNA) was reduced after HS, but increased after resuscitation with FFP, indicating that syndecan-1 mRNA expression may also be a target of haemorrhagic shock20. Alteration of syndecan-1 expression at the mRNA level indicated that microRNAs (miR) might be involved in this process. MiRNAs are short single-stranded RNA molecules that regulate gene expression by binding with the 3′-untranslated regions of target mRNAs to modulate translation. We have recently demonstrated a novel role of microRNA, miR-19b, in post-haemorrhagic shock endothelial dysfunction via targeting of syndecan-1 mRNA21,22.
Finally, fibrinogen, as a fundamental component of plasma or an isolated protein, has been shown to protect the endothelium by stabilizing syndecan-1 on the cell surface23,24. Furthermore, we demonstrated that plasma depleted of fibrinogen loses its protective effects23. We therefore hypothesised that fibrinogen would inhibit miR-19b to mitigate the endotheliopathy of trauma in a murine model of haemorrhagic shock.
MATERIALS AND METHODS
Donor plasma and fibrinogen
Plasma was obtained from healthy donors through the Bonfils/Vitalant Blood Bank Research Donor Program, Denver, Colorado. Per standard blood bank procedures, plasma was frozen and stored at −20°C within eight hours until ready for testing. RiaSTAP® (CSL Behring GmbH, Marburg, Germany), a lyophilized human fibrinogen concentrate, was obtained and reconstituted per manufacturer’s instructions at a concentration of 10 mg/mL; it was then aliquoted and stored at −20°C until ready for use.
Mouse model of haemorrhagic shock
All procedures performed were approved by the University of Maryland School of Medicine’s Institutional Animal Care and Use Committee. The experiments were conducted in compliance with the National Institutes of Health guidelines on the use of laboratory animals. All animals were housed at constant room temperature with a 12:12-h light-dark cycle, with access to food and water ad libitum. Male C57BL/6J mice were used at 8 to 10 weeks of age and weighing approximately 25 g. Our established coagulopathic model of trauma and haemorrhagic shock was utilised25,26. Briefly, under isoflurane anesthesia, a midline laparotomy incision was made, the intestines were inspected and then the incision was closed. This was followed by femoral artery cannulation for blood withdrawal, blood pressure monitoring, and resuscitation. Mice were subjected to haemorrhagic shock (mean arterial pressure (MAP) 35±5 mmHg for 90 minutes) followed by resuscitation with lactated ringer’s (LR), fresh frozen plasma (FFP), fibrinogen (fib) or no resuscitation. MAP was recorded for 30 minutes after resuscitation. Mice resuscitated with LR were given 3x shed blood volume, FFP 1x shed blood volume, and fibrinogen 1x shed blood volume at a concentration of 10 mg/mL25,27. The dose of fibrinogen used in this study approximates haemostatic doses used in humans for treatment of haemorrhagic shock. Experimental groups were compared to shams, which underwent femoral artery cannulation but no laparotomy or HS. Mice were euthanized three hours after resuscitation by exsanguination under isoflurane anesthesia and lungs were harvested for further analysis. Lungs were chosen as the target organ to study, as they are the most commonly injured organ after haemorrhagic shock28. The three-hour time point was chosen based on previous investigations demonstrating restoration of pulmonary syndecan-1 by FFP at this time point20.
Pulmonary microRNA and mRNA measurement
Fresh frozen lung tissue was harvested for miR-19b and syndecan-1 mRNA which was measured by qRT-PCR. The primer sequences used for detecting syndecan-1 were: forward: 5′-GAAGAAGAAGGACGAAGGCAG-3′; and reverse: 5′-CCTCCTGTTTGGTGGGC-3′. U6 small nuclear RNA and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were used as endogenous controls for miR-19b and syndecan-1, respectively. Relative RNA amount was calculated using the 2^-ΔΔCt method.
Lung histopathologic injury
The left lung was embedded in optimal cutting temperature compound (OCT) at the time of harvest and stored at −80°C. Lung tissue was sectioned and stained with hematoxylin and eosin (H&E) and scored on a three-point scale for alveolar thickness, capillary congestion, and cellularity as described by Hart et al. and as we have reported27,29,30. The overall lung injury score was calculated by averaging the three parameters.
Lung Permeability
After euthanasia, the trachea of each mouse was cannulated and bronchoalveolar lavage (BAL) fluid was collected by three injections of 0.4 mL of phosphate-buffered saline into the right lungs. The BAL fluid was then centrifuged at 10,000 g and 4°C for 10 minutes. Supernatant from the BAL fluid was stored at −80°C for subsequent testing. Total protein in the BAL fluid was measured with the BCA Protein Assay (Thermo Fisher Scientific, Waltham, MA, USA).
Statistical analysis
All data were analysed by one-way analysis of variance (ANOVA) with Bonferroni correction; p values <0.05 were considered significant. Data are expressed as mean ± standard deviation (SD), n=3–10/group. Sample size was based on our previous study using the same mouse model of haemorrhagic shock31.
RESULTS
Mean arterial pressure improved by fibrinogen
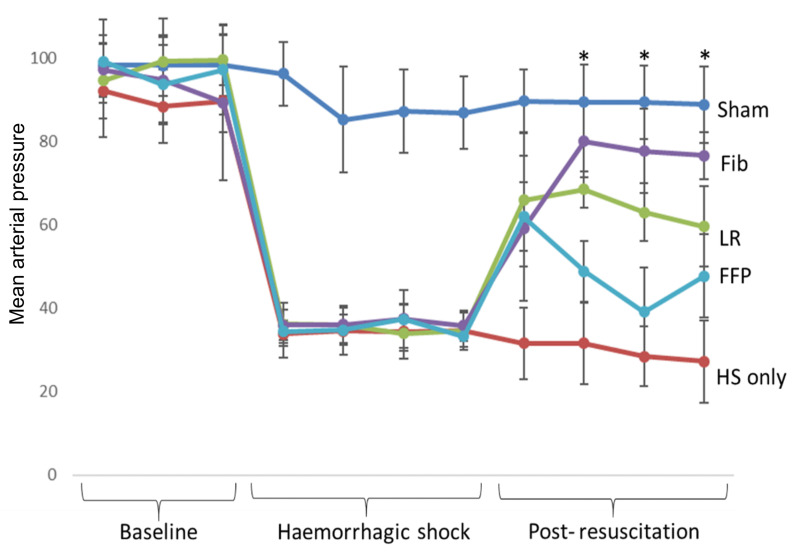
Mean arterial pressure was similar for all groups at baseline and for the HS, LR, FFP, and fibrinogen groups during haemorrhagic shock (Figure 1). Animals resuscitated with fibrinogen had a significantly higher MAP than animals resuscitated with FFP or LR at three of the four post-resuscitation time points (p<0.05, two-way ANOVA).
Figure 1.
Mean arterial pressure over time
Mice underwent 90 minutes of haemorrhagic shock followed by resuscitation with either fibrinogen, FFP, or LR, and were compared to mice undergoing haemorrhagic shock only and sham mice. Data is reported as mean ± SD with n=6–10/group; two-way ANOVA.
Asterisk indicates resuscitation time points at which MAP of fibrinogen resuscitated mice was significantly higher than MAP of FFP, LR and HS only groups. fib: fibrinogen, FFP: fresh frozen plasma, LR: lactated ringer’s, HS: haemorrhagic shock.
Pulmonary miR-19b reduced by fibrinogen and FFP
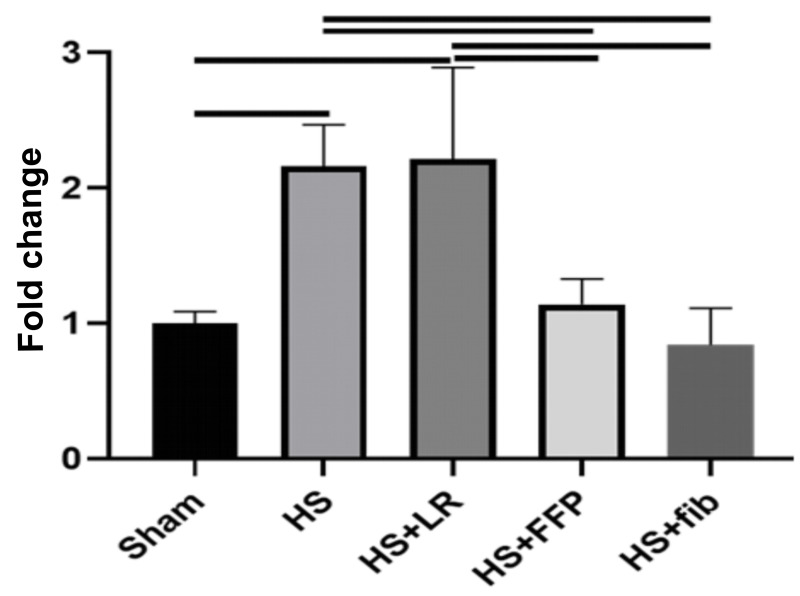
Pulmonary miR-19b was quantitated following haemorrhagic shock and resuscitation. MiR-19b was increased after HS and HS+LR when compared to sham (2.16±0.31 HS vs 1.00±0.09 sham, p=0.03 and 2.21±0.68 HS+LR vs 1.00±0.09 sham, p=0.02). However, pulmonary miR-19b was significantly reduced by HS+FFP and HS+fibrinogen and was comparable to shams (1.14±0.19 HS+FFP vs 1.00±0.09 sham, p>0.99) and (0.84±0.27 HS+fibrinogen vs 1.00±0.09 sham, p>0.99) (Figure 2).
Figure 2.
Pulmonary miR-19b
Mice underwent 90 minutes of haemorrhagic shock followed by resuscitation with either fibrinogen, FFP, or LR, and were compared to mice undergoing haemorrhagic shock only and sham mice. Pulmonary miR-19b was measured by qRT-PCR. Data is reported as mean ± SD with n=3/group; one-way ANOVA. Bars indicate relationships with p<0.05. fib: fibrinogen, FFP: fresh frozen plasma, LR: lactated ringer’s, HS: haemorrhagic shock.
Pulmonary syndecan-1 mRNA increased by fibrinogen and FFP
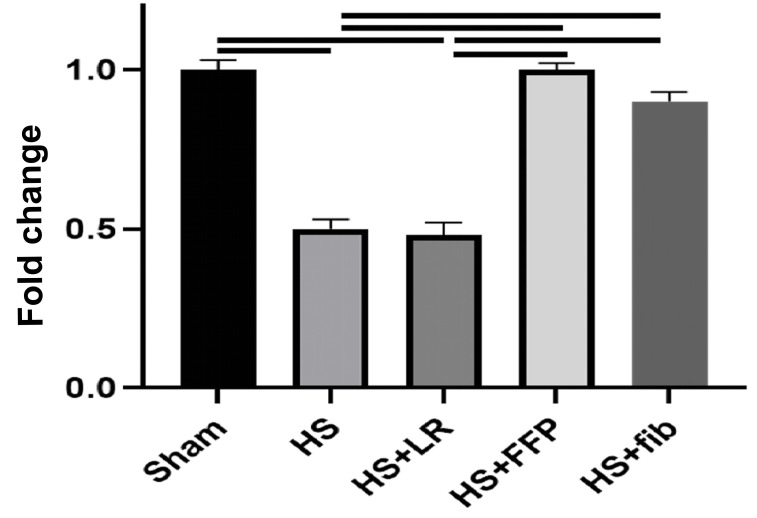
Conversely, syndecan-1 mRNA was downregulated by HS and HS+LR when compared to sham (0.5±0.03 HS vs 1.0±0.03 sham, p<0.0001 and 0.48±0.04 HS+LR vs 1.0±0.03 sham, p<0.0001). However, syndecan-1 mRNA returned to sham levels by HS+FFP (1.0±0.02 HS+FFP vs 1.0±0.03 sham, p>0.99) and HS+fibrinogen (0.9±0.03 vs 1.0±0.03 sham, p=0.06) (Figure 3).
Figure 3.
Pulmonary Syndecan-1
Mice underwent 90 minutes of haemorrhagic shock followed by resuscitation with either fibrinogen, FFP, or LR, and were compared to mice undergoing haemorrhagic shock only and sham mice. Pulmonary syndecan-1 mRNA was measured by qRT-PCR. Data is reported as mean ± SD with n=3/group; one-way ANOVA. Bars indicate relationships with p<0.05. fib: fibrinogen, FFP: fresh frozen plasma, LR: lactated ringer’s, HS: haemorrhagic shock.
Lung histopathologic injury reduced by fibrinogen and FFP
Both FFP (1.17±0.28) and fibrinogen-based resuscitation (0.33±0.27) reduced lung injury compared to HS alone (2.78±0.45, p<0.0001 for both) and HS+LR (1.94±0.44, p=0.01 and p<0.0001, respectively). Lung histopathologic injury was similar between HS+FFP and HS+fibrinogen when compared to sham (1.17±0.28 HS+FFP vs 0.89±0.17 sham, p>0.99; and 0.33±0.27 HS+fibrinogen vs 0.89±0.17 sham, p=0.2) (Figure 4).
Figure 4.
Lung histopathologic injury
Mice underwent 90 minutes of haemorrhagic shock followed by resuscitation with either fibrinogen, FFP, or LR, and were compared to mice undergoing haemorrhagic shock only and sham mice. Shown are representative images and the corresponding lung injury scores (scale of 0–3). Data is reported as mean ± SD with n=4–6/group; one-way ANOVA. Bars indicate relationships with p<0.05.
fib: fibrinogen, FFP: fresh frozen plasma, LR: lactated ringer’s, HS: haemorrhagic shock.
Lung permeability reduced by fibrinogen and FFP
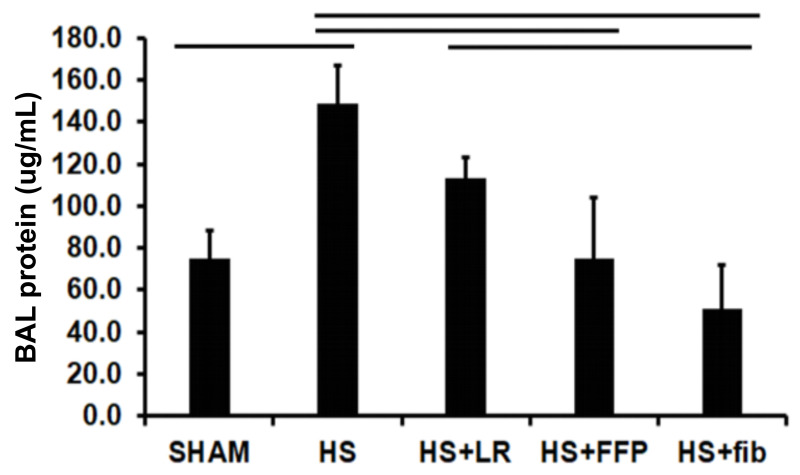
Lung permeability was assessed by BAL protein which was significantly increased by HS and HS+LR compared to shams (147±18 HS vs 75±13 shams, p>0.01) and 113±10 HS+LR vs 75±13 shams, p>0.05, respectively) (Figure 5). HS+FFP reduced BAL protein compared to HS (75±12 HS+FFP vs 147±18 HS, p<0.01) but fibrinogen based resuscitation further decreased protein (51±21 HS+fib) and was comparable to shams (75±13 shams, p>0.05).
Figure 5.
Lung permeability
Mice underwent 90 minutes of haemorrhagic shock followed by resuscitation with either fibrinogen, FFP, or LR, and were compared to mice undergoing haemorrhagic shock only and sham mice. BAL protein concentration is shown as an indicator of lung permeability. Data is reported as mean ± SD with n=6/group; one-way ANOVA. Bars indicate relationships with p<0.05.
fib: fibrinogen, FFP: fresh frozen plasma, LR: lactated ringer’s, HS: haemorrhagic shock.
DISCUSSION
We have previously identified that miR-19b targets syndecan-1 in both in vitro and in vivo models of haemorrhagic shock and have confirmed the findings of Uhlich et al. that miR19-b is upregulated in patients in haemorrhagic shock21,32. We now sought to determine the role of resuscitation in modulating this pathologic microRNA and hypothesized that fibrinogen, a key component of FFP and an important haemostatic agent, would inhibit miR-19b to mitigate the endotheliopathy of trauma in a murine model of haemorrhagic shock. Resuscitation with fibrinogen or FFP was associated with decreases in pulmonary miR-19b, lung histopathologic injury and pulmonary permeability. Conversely, resuscitation with fibrinogen or FFP was associated with a reciprocal increase in pulmonary syndecan-1 mRNA.
MicroRNAs have predominantly been investigated in the setting of infection, cancer, and autoimmune disorders. They are small non-coding RNA that bind to target messenger RNA to modulate translation or stability, and although they have been determined to be powerful regulators of inflammation, limited data exist regarding their expression and role after injury32,33. MiR-19b is part of the miR-17-92 family of miRNA clusters34. It has previously been implicated in a number of cancers and inflammatory diseases such as atherosclerosis and coronary artery disease and has been shown to be downregulated in human aging34–41. We have previously demonstrated that syndecan-1 is a novel target of miR-19b after haemorrhagic shock21. We have now shown that pulmonary miR-19b is upregulated after haemorrhagic shock but that its pathologic expression can be altered by resuscitation with FFP or fibrinogen.
A number of studies have found that patients in haemorrhagic shock have increased shedding of the syndecan-1 ectodomain into the circulation and that measurement of shed syndecan-1 can serve as a biomarker for endothelial injury and as an independent predictor of mortality16,17,31,42,43. Shed syndecan-1 is also detected with sepsis, cardiac arrest, burns and after emergency surgical procedures44–47. Fresh frozen plasma, and more recently solvent/detergent-treated plasma, has been shown to mitigate shedding and provide protection48–50. Much less is known about the regulation and expression of syndecan-1 mRNA in the pulmonary endothelium. The current study confirms our prior finding of decreased syndecan-1 mRNA expression after haemorrhagic shock20, and now extends that work to demonstrate a decrease in syndecan-1 mRNA by resuscitation with fibrinogen or FFP. In both the present study and previous work, we have shown that a decrease in pulmonary syndecan-1 mRNA is associated with endothelial dysfunction20,31.
Several components of plasma appear to be important to restoring endothelial barrier integrity after haemorrhagic shock23,51,52. The present study demonstrated a beneficial effect of resuscitation with both fibrinogen and FFP. Previously, we have shown in vitro that fibrinogen enhances endothelial barrier integrity by associating with cell surface syndecan-1, and plasma that depleted of fibrinogen lost its protective effects23. Additionally, Yu et al. recently identified fibrinogen as the key anti-apoptotic factor in plasma24. In the clinical setting, low fibrinogen levels have been shown to be an independent predictor of mortality in severely injured patients53,54. It is interesting to note that fibrinogen resulted in an increase MAP compared to FFP. The mechanism for this finding is not known but may be related to improved restoration of barrier integrity by fibrinogen. Another possible explanation relates to restoration of cellular syndecan-1 by fibrinogen. We have shown that fibrinogen restores syndecan-1 in endothelial cells23 but it also possible it may restore its expression in vascular smooth muscles cells, and thus augment vascular tone55.
Transfusion of fibrinogen concentrate has also been shown to reduce blood loss, transfusion requirements, and the length of intensive care and hospital stay in severely injured and post-surgical patients56. Additionally, compared to FFP, fibrinogen does not require cross matching and offers unique opportunities for pathogen reduction and far fewer storage constraints6,56. Fibrinogen is the first coagulation factor to fall to critically low levels during massive bleeding, which suggests that it may be beneficial to administer fibrinogen early after severe injury and haemorrhage. This, however, has not consistently been shown in clinical studies6. A systematic review of the use of fibrinogen concentrate found that it was generally associated with improved outcomes when used for perioperative bleeding or in a massive trauma setting, although more studies are needed57. A meta-analysis of six randomised controlled trials in patients undergoing elective surgery found that fibrinogen concentrate reduced transfusions without an increase in thrombotic events or mortality58. Several other randomised trials of fibrinogen in trauma have unfortunately focused on feasibility rather than benefit59,60. Lastly, two recent meta-analysis, one on postoperative blood loss and the other in trauma-related bleeding concluded there was low quality evidence and heterogeneity which limited recommendations for its use61,62. In the United States, the Food and Drug Administration has not yet approved fibrinogen concentrate for use in massively bleeding patients after injury. Instead, cryoprecipitate is used to replace fibrinogen as part of massive transfusion protocols. Cryoprecipitate is prepared from human plasma and contains fibrinogen, as well as von Willebrand factor, factor VIII, factor XIII, and fibronectin. Although there is evidence that fibrinogen levels decrease early after haemorrhage, massive transfusion protocols typically do not include products specifically meant to replete fibrinogen, such as cryoprecipitate, until much later in the course of resuscitation. The current study did not include assessment of coagulation but rather focused on endothelial protection by fibrinogen. Nonetheless, results suggest that early administration of fibrinogen could be beneficial.
There are several limitations to the current study. We have demonstrated beneficial effects of both FFP and fibrinogen after haemorrhagic shock; however, fibrinogen concentrate is not universally clinically available for use in patients under this indication. We also used human plasma and human fibrinogen concentrate in a murine model of haemorrhagic shock. The use of human blood products in a small animal model of haemorrhagic shock allows us to study the clinical product used in humans, which we consider offering a significant translational advantage, although xeno-incompatibility is a possible confounder. However, we have previously demonstrated a lack of species-specific differences in pulmonary indices after haemorrhagic shock30. Additionally, while our model of trauma and haemorrhagic shock does result in coagulopathy, we did not examine or monitor for any alterations in coagulopathy between groups63. Lastly, we only examined effects of resuscitation in pulmonary tissue, as the lungs are the most frequently injured organ after trauma64, and only at one point in time.
Conclusions
This study presents a new and important relationship between microRNA-19b and fibrinogen resuscitation during haemorrhagic shock, but complete demonstration of the mechanism of fibrinogen inhibition of endotheliopathy via microRNA remains to be elucidated.
Footnotes
FUNDING
This work was supported by the National Institutes of Health RO1GM107482.
Authorship contributions
AMC wrote the article and conducted analyses. FW accrued data and conducted analyses. AMC and FW performed the experiments. RAK developed the idea, interpreted data and assisted in writing the article. All Authors reviewed and edited the manuscript.
The Authors declare no conflicts of interest.
REFERENCES
- 1.Kochanek KD, Xu J, Murphy SL, et al. Deaths: final data for 2009. Centers for Disease Control and Prevention (CDC) - National Center for Health Statistics - National Vital Statistics System; [Accessed on: 02/09/2020]. Available at: https://www.cdc.gov/nchs/data/nvsr/nvsr60/nvsr60_03.pdf. [PubMed] [Google Scholar]
- 2.Kauvar DS, Wade CE. The epidemiology and modern management of traumatic hemorrhage: US and international perspectives. Crit Care. 2005;9(Suppl 5):S1–9. doi: 10.1186/cc3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eastridge BJ, Mabry RL, Seguin P, et al. Death on the battlefield ( 2001–2011): implications for the future of combat casualty care. J Trauma. 2012;73:S431–7. doi: 10.1097/TA.0b013e3182755dcc. [DOI] [PubMed] [Google Scholar]
- 4.Kauvar DS, Lefering R, Wade CE. Impact of hemorrhage on trauma outcome: an overview of epidemiology, clinical presentations, and therapeutic considerations. J Trauma. 2006;60:S3–11. doi: 10.1097/01.ta.0000199961.02677.19. [DOI] [PubMed] [Google Scholar]
- 5.Jenkins DH, Rappold JF, Badloe JF, et al. Trauma hemostasis and oxygenation research position paper on remote damage control resuscitation. Shock. 2014;41:3–12. doi: 10.1097/SHK.0000000000000140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu F, Chipman A, Pati S, Miyasawa B, Corash L, Kozar RA. Resuscitative strategies to modulate the endotheliopathy of trauma: from cell to patient. Shock. 2020;53:575–84. doi: 10.1097/SHK.0000000000001378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holcomb JB, Tilley BC, Baraniuk S, et al. the PROPPR Study Group. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA. 2015;313:471–82. doi: 10.1001/jama.2015.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holcomb JB, Wade CE, Michalek JE, et al. Increased plasma and platelet to red blood cell ratios improves outcome in 466 massively transfused civilian trauma patients. Ann Surg. 2008;248:447–58. doi: 10.1097/SLA.0b013e318185a9ad. [DOI] [PubMed] [Google Scholar]
- 9.Holcomb JB, del Junco DJ, Fox EE, et al. The Prospective, Observational, Multicenter, Major Trauma Transfusion (PROMMTT) study. JAMA Surg. 2013;148:127. doi: 10.1001/2013.jamasurg.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borgman MA, Spinella PC, Perkins JG, et al. The ratio of blood products transfused affects mortality in patients receiving massive transfusions at a combat support hospital. J Trauma Inj Infect Crit Care. 2007;63:805–13. doi: 10.1097/TA.0b013e3181271ba3. [DOI] [PubMed] [Google Scholar]
- 11.Sperry JL, Guyette FX, Brown JB, et al. Prehospital plasma during air medical transport in trauma patients at risk for hemorrhagic shock. N Engl J Med. 2018;379:315–26. doi: 10.1056/NEJMoa1802345. [DOI] [PubMed] [Google Scholar]
- 12.Chang R, Cardenas JC, Wade CE, Holcomb JB. Advances in the understanding of trauma-induced coagulopathy. Blood. 2016;128:1043–9. doi: 10.1182/blood-2016-01-636423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Torres LN, Sondeen JL, Ji L, et al. Evaluation of resuscitation fluids on endothelial glycocalyx, venular blood flow, and coagulation function after hemorrhagic shock in rats. J Trauma. 2013;75:759–66. doi: 10.1097/TA.0b013e3182a92514. [DOI] [PubMed] [Google Scholar]
- 14.Barelli S, Alberio L. The role of plasma transfusion in massive bleeding: protecting the endothelial glycocalyx? Front Med. 2018;18(5):91. doi: 10.3389/fmed.2018.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reitsma S, Slaaf DW, Vink H, et al. The endothelial glycocalyx: composition, functions, and visualization. Pflügers Arch Eur J Physiol. 2007;454:345–59. doi: 10.1007/s00424-007-0212-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kozar RA, Pati S. Syndecan-1 restitution by plasma after hemorrhagic shock. J Trauma. 2015;78:S83–6. doi: 10.1097/TA.0000000000000631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johansson PI, Stensballe J, Rasmussen LS, Ostrowski SR. A high admission syndecan-1 level, a marker of endothelial glycocalyx degradation, is associated with inflammation, protein C depletion, fibrinolysis, and increased mortality in trauma patients. Ann Surg. 2011;254:194–200. doi: 10.1097/SLA.0b013e318226113d. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez Rodriguez E, Ostrowski SR, Cardenas JC, et al. Syndecan-1: a quantitative marker for the endotheliopathy of trauma. J Am Coll Surg. 2017;225:419–27. doi: 10.1016/j.jamcollsurg.2017.05.012. [DOI] [PubMed] [Google Scholar]
- 19.Johansson PI, Stensballe J, Rasmussen LS, Ostrowski SR. High circulating adrenaline levels at admission predict increased mortality after trauma. J Trauma. 2012;72:428–36. doi: 10.1097/ta.0b013e31821e0f93. [DOI] [PubMed] [Google Scholar]
- 20.Kozar RA, Peng Z, Zhang R, et al. Plasma restoration of endothelial glycocalyx in a rodent model of hemorrhagic shock. Anesth Analg. 2011;112:1289–95. doi: 10.1213/ANE.0b013e318210385c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu F, Wang JY, Chao W, et al. MiR-19b targets pulmonary endothelial syndecan-1 following hemorrhagic shock. Sci Rep. 2020;10:15811. doi: 10.1038/s41598-020-73021-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Felekkis K, Touvana E, Stefanou C, Deltas C. MicroRNAs: a newly described class of encoded molecules that play a role in health and disease. Hippokratia. 2010;14:236–40. [PMC free article] [PubMed] [Google Scholar]
- 23.Wu F, Kozar RA. Fibrinogen protects against barrier dysfunction through maintaining cell surface syndecan-1 in vitro. Shock. 2019;51:740–4. doi: 10.1097/SHK.0000000000001207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu Q, Yang B, Davis JM, et al. Identification of fibrinogen as a key anti-apoptotic factor in human fresh frozen plasma for protecting endothelial cells in vitro. Shock. 2020;53:646–52. doi: 10.1097/SHK.0000000000001399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Potter DR, Baimukanova G, Keating SM, et al. Fresh frozen plasma and spray-dried plasma mitigate pulmonary vascular permeability and inflammation in hemorrhagic shock. J Trauma. 2015;78:S7–17. doi: 10.1097/TA.0000000000000630. [DOI] [PubMed] [Google Scholar]
- 26.Peng Z, Pati S, Potter D, et al. Fresh frozen plasma lessens pulmonary endothelial inflammation and hyperpermeability after hemorrhagic shock and is associated with loss of syndecan 1. Shock. 2013;40:195–202. doi: 10.1097/SHK.0b013e31829f91fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pati S, Peng Z, Wataha K, et al. Lyophilized plasma attenuates vascular permeability, inflammation and lung injury in hemorrhagic shock. PLoS One. 2018;13:e0192363. doi: 10.1371/journal.pone.0192363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.MacCallum NS, Evans TW. Epidemiology of acute lung injury. Curr Opin Crit Care. 2005;11:43–9. doi: 10.1097/00075198-200502000-00007. [DOI] [PubMed] [Google Scholar]
- 29.Hart ML, Ceonzo KA, Shaffer LA, et al. Gastrointestinal ischemia-reperfusion injury is lectin complement pathway dependent without involving C1q. J Immunol. 2005;174:6373–80. doi: 10.4049/jimmunol.174.10.6373. [DOI] [PubMed] [Google Scholar]
- 30.Peng Z, Pati S, Fontaine MJ, et al. Lack of species-specific difference in pulmonary function when using mouse versus human plasma in a mouse model of hemorrhagic shock. J Trauma. 2016;81:S171–6. doi: 10.1097/TA.0000000000001221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu F, Peng Z, Park PW, Kozar RA. Loss of syndecan-1 abrogates the pulmonary protective phenotype induced by plasma after hemorrhagic shock. Shock. 2017;48:340–5. doi: 10.1097/SHK.0000000000000832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uhlich RM, Konie JA, Davis JW, et al. Novel microRNA correlations in the severely injured. Surgery. 2014;156:834–41. doi: 10.1016/j.surg.2014.06.017. [DOI] [PubMed] [Google Scholar]
- 33.O’Brien J, Hayder H, Zayed Y, Peng C. Overview of microRNA biogenesis, mechanisms of actions, and circulation. Front Endocrinol (Lausanne) 2018;9:402. doi: 10.3389/fendo.2018.00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mogilyansky E, Rigoutsos I. The miR-17/92 cluster: a comprehensive update on its genomics, genetics, functions and increasingly important and numerous roles in health and disease. Cell Death Differ. 2013;20:1603–14. doi: 10.1038/cdd.2013.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Niu S, Ma X, Zhang Y, et al. MicroRNA-19a and microRNA-19b promote the malignancy of clear cell renal cell carcinoma through targeting the tumor suppressor RhoB. PLoS One. 2018;13:e0192790. doi: 10.1371/journal.pone.0192790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li C, Zhang J, Ma Z, et al. MiR-19b serves as a prognostic biomarker of breast cancer and promotes tumor progression through PI3K/AKT signaling pathway. Onco Targets Ther. 2018;11:4087–95. doi: 10.2147/OTT.S171043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lv YC, Tang YY, Peng J, et al. MicroRNA-19b promotes macrophage cholesterol accumulation and aortic atherosclerosis by targeting ATP-binding cassette transporter A1. Atherosclerosis. 2013;236:215–26. doi: 10.1016/j.atherosclerosis.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 38.Xue Y, Wei Z, Ding H, et al. MicroRNA-19b/221/222 induces endothelial cell dysfunction via suppression of PGC-1α in the progression of atherosclerosis. Atherosclerosis. 2015;241:671–81. doi: 10.1016/j.atherosclerosis.2015.06.031. [DOI] [PubMed] [Google Scholar]
- 39.Li C, Li S, Zhang F, et al. Endothelial microparticles-mediated transfer of microRNA-19b promotes atherosclerosis via activating perivascular adipose tissue inflammation in apoE −/− mice. Biochem Biophys Res Commun. 2018;495:1922–9. doi: 10.1016/j.bbrc.2017.11.195. [DOI] [PubMed] [Google Scholar]
- 40.Li S, Ren J, Xu N, et al. MicroRNA-19b functions as potential anti-thrombotic protector in patients with unstable angina by targeting tissue factor. J Mol Cell Cardiol. 2014;75:49–57. doi: 10.1016/j.yjmcc.2014.06.017. [DOI] [PubMed] [Google Scholar]
- 41.Hackl M, Brunner S, Fortschegger K, et al. MiR-17, miR-19b, miR-20a, and miR-106a are down-regulated in human aging. Aging Cell. 2010;9:291–6. doi: 10.1111/j.1474-9726.2010.00549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haywood-Watson RJ, Holcomb JB, Gonzalez EA, et al. Modulation of syndecan-1 shedding after hemorrhagic shock and resuscitation. PLoS One. 2011;6:e23530. doi: 10.1371/journal.pone.0023530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ostrowski SR, Haase N, Müller RB, et al. Association between biomarkers of endothelial injury and hypocoagulability in patients with severe sepsis: a prospective study. Crit Care. 2015;19:191. doi: 10.1186/s13054-015-0918-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Puskarich MA, Cornelius DC, Tharp J, et al. Plasma syndecan-1 levels identify a cohort of patients with severe sepsis at high risk for intubation after large-volume intravenous fluid resuscitation. J Crit Care. 2016;36:125–9. doi: 10.1016/j.jcrc.2016.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bro-Jeppesen J, Johansson PI, Kjaergaard J, et al. Level of systemic inflammation and endothelial injury is associated with cardiovascular dysfunction and vasopressor support in post-cardiac arrest patients. Resuscitation. 2017;121:179–86. doi: 10.1016/j.resuscitation.2017.09.019. [DOI] [PubMed] [Google Scholar]
- 46.Osuka A, Kusuki H, Yoneda K, et al. Glycocalyx shedding is enhanced by age and correlates with increased fluid requirement in patients with major burns. Shock. 2018;50:60–5. doi: 10.1097/SHK.0000000000001028. [DOI] [PubMed] [Google Scholar]
- 47.Stensballe J, Ulrich AG, Nilsson JC, et al. Resuscitation of endotheliopathy and bleeding in thoracic aortic dissections: the VIPER-OCTA randomized clinical pilot trial. Anesth Analg. 2018;127:920–7. doi: 10.1213/ANE.0000000000003545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cardenas JC, Cap AP, Swartz MD, et al. Plasma resuscitation promotes coagulation homeostasis following shock-induced hypercoagulability. Shock. 2016;45:166–73. doi: 10.1097/SHK.0000000000000504. [DOI] [PubMed] [Google Scholar]
- 49.Sperry JL, Guyette FX, Brown JB, et al. Prehospital plasma during air medical transport in trauma patients at risk for hemorrhagic shock. N Engl J Med. 2018;379:315–26. doi: 10.1056/NEJMoa1802345. [DOI] [PubMed] [Google Scholar]
- 50.Wei S, Gonzalez Rodriguez E, Chang R, et al. Elevated syndecan-1 after trauma and risk of sepsis: a secondary analysis of patients from the Pragmatic, Randomized Optimal Platelet and Plasma Ratios (PROPPR) trial. J Am Coll Surg. 2018;227:587–95. doi: 10.1016/j.jamcollsurg.2018.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lopez E, Peng Z, Kozar RA, et al. Antithrombin III Contributes to the protective effects of fresh frozen plasma following hemorrhagic shock by preventing syndecan-1 shedding and endothelial barrier disruption. Shock. 2020;53:156–63. doi: 10.1097/SHK.0000000000001432. [DOI] [PubMed] [Google Scholar]
- 52.Deng X, Cao Y, Huby MP, et al. Adiponectin in fresh frozen plasma contributes to restoration of vascular barrier function after hemorrhagic shock. Shock. 2016;45:50–4. doi: 10.1097/SHK.0000000000000458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McQuilten ZK, Wood EM, Bailey M, et al. Fibrinogen is an independent predictor of mortality in major trauma patients: a five-year statewide cohort study. Injury. 2017;48:1074–81. doi: 10.1016/j.injury.2016.11.021. [DOI] [PubMed] [Google Scholar]
- 54.Rourke C, Curry N, Khan S, et al. Fibrinogen levels during trauma hemorrhage, response to replacement therapy, and association with patient outcomes. J Thromb Haemost. 2012;10:1342–51. doi: 10.1111/j.1538-7836.2012.04752.x. [DOI] [PubMed] [Google Scholar]
- 55.Chaterji S, Lam CH, Ho DS, et al. Syndecan-1 regulates vascular smooth muscle cell phenotype. PLoS One. 2014;9:e89824. doi: 10.1371/journal.pone.0089824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kozek-Langenecker S, Sørensen B, Hess JR, Spahn DR. Clinical effectiveness of fresh frozen plasma compared with fibrinogen concentrate: a systematic review. Crit Care. 2011;15:R239. doi: 10.1186/cc10488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mengoli C, Franchini M, Marano G, et al. The use of fibrinogen concentrate for the management of trauma-related bleeding: a systematic review and meta-analysis. Blood Transfus. 2017;15:318–24. doi: 10.2450/2017.0094-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fominskiy E, Nepomniashchikh VA, Lomivorotov VV, et al. Efficacy and safety of fibrinogen concentrate in surgical patients: a meta-analysis of randomized controlled trials. J Cardiothorac Vasc Anesth. 2016;30:1196–204. doi: 10.1053/j.jvca.2016.04.015. [DOI] [PubMed] [Google Scholar]
- 59.Curry N, Foley C, Wong H, et al. Early fibrinogen concentrate therapy for major haemorrhage in trauma (E-FIT 1): results from a UK multi-centre, randomised, double blind, placebo-controlled pilot trial. Crit Care. 2018;22:164. doi: 10.1186/s13054-018-2086-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nascimento B, Callum J, Tien H, et al. Br J Anaesth. 2016;117:775–82. doi: 10.1093/bja/aew343. [DOI] [PubMed] [Google Scholar]
- 61.Ng KT, Yap JLL, Kwok PE. The effect of fibrinogen concentrate on postoperative blood loss: a systematic review and meta-analysis of randomized controlled trials. J Clin Anesth. 2020;63:109782. doi: 10.1016/j.jclinane.2020.109782. [DOI] [PubMed] [Google Scholar]
- 62.Stabler SN, Li SS, Karpov A, Vu EN. Use of fibrinogen concentrate for trauma-related bleeding: a systematic-review and meta-analysis. J Trauma Acute Care Surg. 2020;89:1212–24. doi: 10.1097/TA.0000000000002920. [DOI] [PubMed] [Google Scholar]
- 63.Chesebro BB, Rahn P, Carles M, et al. Increase in activated protein C mediates acute traumatic coagulopathy in mice. Shock. 2009;32:659–65. doi: 10.1097/SHK.0b013e3181a5a632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sauaia A, Moore EE, Johnson JL, et al. Temporal trends of postinjury multiple-organ failure: Still resource intensive, morbid, and lethal. J Trauma. 2014;76:582–93. doi: 10.1097/TA.0000000000000147. [DOI] [PMC free article] [PubMed] [Google Scholar]