Abstract
Background
For neonates and preterm infants, in whom a transfusion dose is low, the use of red blood cells (RBC) from cord blood appears to be feasible. Standardisation of fractionation and identification and assessment of quality control parameters for such RBC are still lacking.
Materials and methods
We describe the process used to obtain RBC from cord blood for transfusion purposes, including quality controls to evaluate fractionation performance and the effects of storage. The cord RBC, to which SAG-M was added, were sampled on the day of fractionation, and 7 and 14 days (end of storage) later in order to measure the complete blood count, biochemical parameters and residual white blood cells. We also assessed microbial contamination.
Results
Data relative to 279 cord blood units were evaluated. The median gestational age at collection was 40 weeks (interquartile range [IQR] 39.1–40.7) and the median volume was 90 mL (IQR 81–103). Units were subjected to automated fractionation with Compomat, and packed RBC were suspended in SAG-M solution. The median volume of the SAG-M-suspended units was 31 mL (IQR 24.0–38.1) and the median haematocrit was 54.2% (IQR 49.4–59.5). The median volume after leukoreduction was 22 mL (IQR 17–28), with the volume decrease being similar in units leukoreduced before (n=75) or after (n=204) storage. The haematocrit of leukoreduced units was higher than that of buffy coat-depleted units. Storage at 2–6 °C for 14 days was accompanied by an increase of potassium levels and percentage of haemolysis. Microbial cultures were positive for 2.9% of the collected units.
Discussion
Fractionation of whole cord blood can provide RBC concentrates with similar baseline characteristics as units from adults. The transfusion dose and quality of the units appear safe and suitable for clinical use in neonates, with a satisfactory haematocrit and residual white blood cell content, despite a very variable collection volume.
Keywords: cord blood, red blood cells, fractionation, neonates
INTRODUCTION
Several authors have proposed the use of umbilical cord blood (UCB) for transfusion therapy in babies born prematurely and neonates. These patients often require transfusions for surgical interventions, anaemia of prematurity and iatrogenic anaemia due to laboratory tests. UCB transfusions have been used in both autologous and allogeneic settings.
In the autologous setting, cord blood has been used as an alternative to adult blood transfusions for different clinical indications, including neonatal anaemia1–8, surgery9–16 or both17. Since recipients are mostly premature, autologous cord blood transfusions are given as whole blood. Unfortunately, the volume of these units is dependent on the neonate’s body weight and gestational age and does not enable all transfusion needs to be covered.
In the allogeneic setting, both whole blood and red blood cell concentrates (RBC) have been transfused into adults and neonates for anaemic conditions18–29. For neonates and mainly for preterm infants, for whom the transfusion dose is low (generally 20 mL/kg), the use of allogeneic RBC from UCB appears to be feasible. There are only two previous reports, by Hassall et al.28 and Bianchi et al.29, of successful transfusion therapy in high-risk neonates using allogeneic RBC from term neonatal UCB, obtained by manual separation and automated fractionation and leukoreduction, respectively. No adverse events ascribable to the use of these blood components were reported in either study.
There are some previous reports on the collection and storage of whole UCB as well as fractionation and production of RBC from UCB (Table I). Bifano et al. collected autologous cord blood with a mean volume of 65 mL (30–110 mL) reporting a mean haematocrit (Hct) of 42±2% after the addition of the preservative solution to the collection bags30. Hassall et al. collected allogeneic UCB, in a fixed volume of anticoagulant, with a volume ranging from 42 to 128 mL and a variable CPDA-1 ratio (2.0–6.1)28. It is noteworthy that no standardisation was proposed for the whole blood products in these reports, but only comparisons with previously published data on whole blood storage.
Table I.
Processing and storage parameters of umbilical cord blood units
| References (Author, ref, year) | Type of donor | UCB units (N) | UCB unit volume (mL)§ | Type of blood component | Type of fractionation and length of storage | UCB-RBC volume (mL) | Additive solution (AS) (ratio blood:AS) | Microbial contamination rate (%) | Quality controls |
|---|---|---|---|---|---|---|---|---|---|
| Bifano et al.30 1994 |
Term or near-term | 31 | 65 (30–110) | Whole blood | Not applicable/28 days | - | - | 8 | CBC, iATP, 2,3 DPG s-potassium, glucose, pH, free Hb, haemolysis rate, |
| Hassal et al.28 2010 |
Term | 24 | 73 (42–128) | Whole blood | Not applicable/35 days | - | - | NA | CBC, s-potassium and s-potassium/donation volume free Hb, haemolysis rate, |
| Eichler et al.2 2000 |
Preterm | 34 | 56.4 ±32.9 | RBC | Semi-automated (Optipress II, Baxter)/14 days | 28.6±18.8 (6.5–87.0) mean | SAG-M (fixed, 8 mL) | 8.6 | CBC (only on unseparated and separated UCB, the day of fractionation) |
| Brune et al.17 2002 |
Preterm and term | 131 | 23.4 ±9.4 (<1,000)§§ 25.6 ±17.4 (1,000–1,999)§§ 51.4 ±16.4 (2,000–2,999)§§ 60.4 ± 8.5 (3,000–3,999)§§ 91.8 ±37.8 (>4,000)§§ |
RBC | Manual/35 days | see legend# | SAG-M (10:1) | none | CBC |
| Garritsen et al.32 2003 |
Preterm and term | 390 | 24.5 ±8.4 (<1,000)§§ 31.2 ±15.9 (1,000–1,999)§§ 49.7 ±18.7 (2,000–2,999)§§ 62.8 ±23.8 (3,000–3,999)§§ 88.3 ±39.2 (>4,000)§§ |
BC-depleted RBC | Manual/35 days | 27.1±7.2 median | SAG-M (5:1) | 1.8 | CBC, microbial testing, s-potassium, glucose, pH, free Hb, haemolysis rate, iATP (performed on 12 units) |
| Widing et al.33 2007 |
Term | 37 | 89.6 (3.5.–172) mean | BC-depleted and leucoreduced RBC* | Manual/35 days | NA | SAG-M and PAGGS-M (50:11) | 2.7 | CBC, microbial testing, s-potassium, free Hb, haemolysis rate |
| Brune et al.34 2007 |
Term | 12 | NA | Leucoreduced RBC** | Gravity separation with hollow-fibre filtration (Sangofer, Germany)/35 days | 62.3±13.5 | SAG-M (fixed, 10 mL) | none | CBC, microbial testing, pH, free Hb, haemolysis rate |
| Khodaboux et al.31 2011 |
Preterm | 47 | NA | BC-depleted RBC | Automated (Sepax Biosafe)/35 days | 34.4±13.4 (SAG-M) and 32.2 ±20.5 (AS-3) mean | SAG-M (34 units) and AS-3 (13 units) (AS adjusted to obtain Hct of 55–65%) | 7 | CBC, microbial testing, s-potassium, sodium, lactate, glucose, pH, free Hb, haemolysis rate, osmotic resistance |
| Bianchi et al.35 2012 |
Term | 43 | 92.3 ±18.3 | Leucoreduced RBC concentrates*** | Automated (Compomat G4, Fresenius)/14 days | 31.2±8.2 | SAG-M (2:1) | 3.9 | CBC, microbial testing, s-potassium, sodium, lactate, glucose, pH, free Hb, haemolysis rate |
Values are expressed as means, except for those reported by Bianchi et al. which are expressed as medians;
UCB volumes are expressed in relation to neonatal birth weight.
This applies only to five units re-suspended in PAGGS-M;
post-storage filtration.
the collected cord blood was sufficient to obtain at least one 10 mL/kg packed red blood cell unit;
AS: additive solution; BC-depleted: buffy coat was removed; CBC: complete blood count; 2,3 DPG: 2,3 diphosphoglycerate; free Hb: free haemoglobin; iATP: intracellular adenosine triphosphate; s-potassium: supernatant potassium
At present, there are only a few papers on UCB processing to obtain packed RBC as well as data on quality controls2,17,31–35. Eichler et al. reported an experience with 34 autologous UCB units fractionated with a semi-automated separator (Optipress II, Baxter, Germany), which had a mean RBC volume of 28.6 mL (6.5–87.0) and a Hct of 62.7% (31–82)2. Khodabux et al. fractionated units with an automated separator (Biosafe, Eysins, Switzerland) in 15 minutes, obtaining a product with a final Hct of 60.5±4%31. Other authors reported data on RBC production obtained by centrifugation and manual separation17,32–33. Garritsen et al. produced packed RBC units with a mean RBC volume of 27.1±7.2 mL and a Hct of 56.5±1.3%32. In the other two experiences, there were no data on the volume of the RBC17,33.
Although transfusion of packed RBC from UCB appears to be feasible and safe, a full standardisation of fractionation and identification and assessment of quality control parameters, superimposable to those for the adult counterparts, are still lacking.
In this study, we describe the process to obtain a packed RBC unit for transfusion purposes from UCB, and quality controls implemented to check the fractionation performance and effects of storage. From this point on, packed RBC from UCB are named UCB-RBC.
MATERIALS AND METHODS
Study design
We developed and evaluated a technique to obtain red blood cells from cord blood through whole cord blood fractionation in a top-and-bottom set. After this phase, the red cells were stored for 14 days at 2–6 °C to perform quality controls and to assess parameters during storage. We evaluated the results of quality controls in both UCB-RBC from which the buffy coat had been removed and pre-storage leukoreduced UCB-RBC.
Since UCB-RBC may be considered a blood component “under evaluation”, the following parameters were assessed for quality control: Hct (50–70%), residual white blood cells (WBC) after filtration (<1×106) and percentage of haemolysis at the end of the storage (<0.8%)36.
Donor enrolment and cord blood collection
Cord blood units were collected from donors who were eligible for cord and blood donation according to national and international regulations. Candidate donors with a history of foetal-maternal incompatibility, maternal infections (hepatitis B virus [HBV], hepatitis C virus [HCV], human immunodeficiency virus [HIV], syphilis) and risks of congenital infections (cytomegalovirus [CMV], toxoplasmosis) were excluded. Informed consent to collect cord blood units was obtained from parents before delivery. Informed consent for cord blood collection was approved by the Ethics Committee of the “A. Gemelli” University Hospital Foundation. All data about parents’ clinical history and informed consent were stored at the UNICATT Cord Blood Bank, according to standard operating procedures.
Criteria for eligibility for UCB collection were: ≥37 weeks of gestation, absence of infection in the mother, or fever within 24 hours of delivery, and no staining of the amniotic fluid. In both vaginal and Caesarean deliveries, the placenta was still in utero when blood was harvested. The umbilical cord was double-clamped, transected, and cleaned with povidone iodine and a chlorhexidine-alcohol preparation. Cord blood was collected by gravity with a collection system including a 200 mL double-bag collection set (CB Collect™, Fresenius HemoCare, Bad Homburg, Germany) containing 20 mL of citrate-phosphate-dextrose (CPD) as an anticoagulant solution plus an additional 10 mL CPD bag. The cord blood collection set was placed on a blood mixer to avoid clot formation. After collection, all units were maintained at 2–6 °C before being transferred to the Cord Blood Bank for further processing. Maternal blood samples were also collected to perform serological studies (HBsAg, anti-HCV, anti-HIV, TPHA, IgG and IgM for CMV) and molecular investigations (nucleic acid amplification testing for HBV, HCV, HIV) for transfusion-transmissible infections. Additional tests were performed according to seasonal outbreaks (e.g. West Nile virus).
Cord blood processing
UCB units were eligible for processing when they had a volume >60 mL, no signs of clots or haemolysis and if no more than 24 hours had passed since collection. After volume determination, samples (1 mL) of the cord blood units were taken in order to assess haematological parameters and blood group type (ABO, Rh type and phenotype, Kell antigens). Blood grouping was performed with column agglutination technology (DG Gel Cards, Grifols, Spain): in a case of mixed population, the UCB unit was excluded from processing.
UCB units were initially transferred into a processing set (Compoflex®, T&B processing set, Fresenius HemoCare, Bad Homburg, Germany) by a sterile device. All units underwent centrifugation in a “sandwich” mode (Figure 1) at 2,151 g for 12 minutes without break. After centrifugation, UCB units were fractionated using either Compomat G4® or Compomat G5® (Fresenius HemoCare, Bad Homburg, Germany). Configuration parameters for Compomat G4® and Compomat G5® were: (i) upper press to mm, which indicates the exit of the upper press was 52 for G4 and 53 for G5; (ii) lower press out, which indicates the levels of exit of the detectors was DA1-DA7 for G4 and DA2-DA5 for G5; (iii) speed (%) was 4 in both cases.
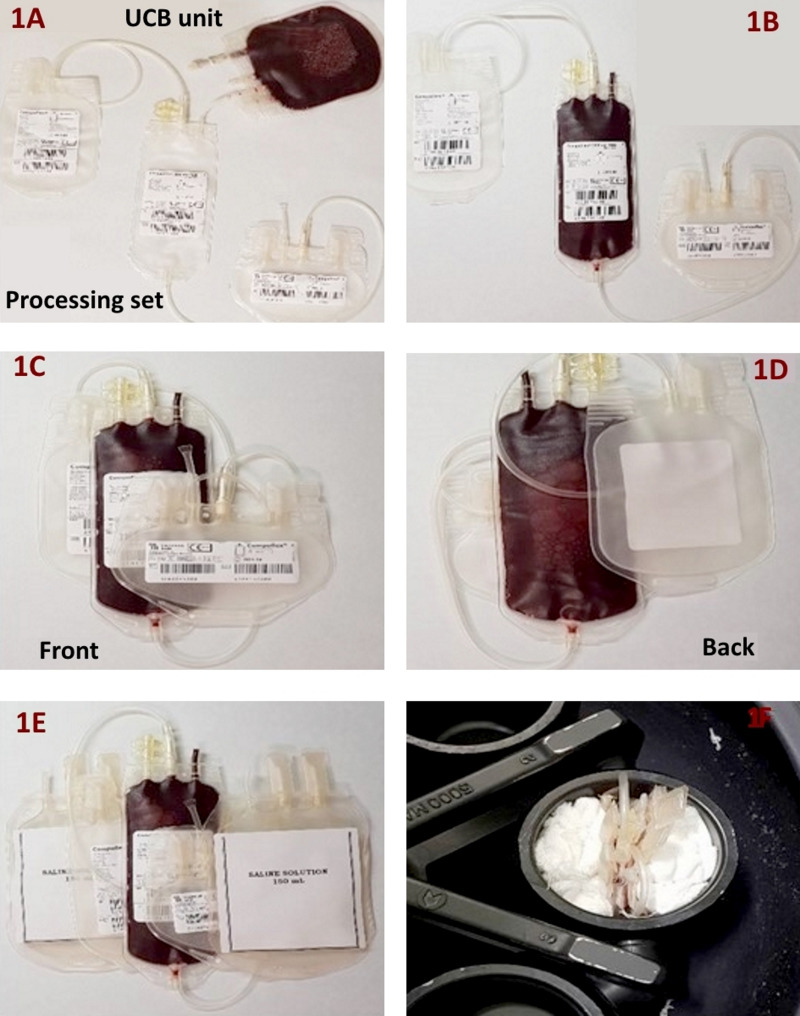
Figure 1.
Cord blood unit centrifugation
The umbilical cord blood unit in the picture was transferred into the Compoflex®, T&B processing set (1A, 1B); the processing set was the folded (1C, 1D) and positioned between two transfer bags containing saline solution (1E). The three bags were then put into the basket of the centrifuge (1F).
After fractionation, a saline, adenine, glucose-mannitol (SAG-M) solution was added to the packed RBC units, with a ratio of blood: SAG-M of 2:1. After re-suspension, UCB-RBC from which the buffy coat was removed underwent filtration with paediatric red cell filters (Pall Purecell® RN1, Pall Corporation, New York, NY, USA) to obtain a leukocyte-depleted red cell unit. The units were filtered either before storage (pre-storage filtered units), or after storage (post-storage filtered units).
All UCB-RBC units were stored for only 14 days (2–6 °C) to perform γ-irradiation (137Cs), according to national regulations and guidelines. We recorded all collection and processing data. Blood group (ABO and Rh type) was confirmed on the attached segment before UCB-RBC validation.
Quality control assays
UCB-RBC units underwent multiple tests to assess biochemical, haematological and microbial characteristics at the time of collection and after fractionation (time 0) and on days 7 and 14. We conducted quality controls both on units from which the buffy coat had been removed and on pre-storage leukoreduced UCB-RBC. Aliquots (1 mL) for laboratory testing were obtained from units by sterile attachment (Terumo Sterile tubing welder, Terumo Medical Corp., Elkton, MD, USA) of a sampling bag.
Biochemical and haematological parameters
Haematological parameters (haemoglobin [Hb], Hct, WBC count) were determined with an XE-2100 (Sysmex Europe GmbH, Germany) at time 0 and on days 7 and 14. We also calculated red cell mass (expressed in mL) at the end of every step using the following formula:
At the end of storage (day 14), the percentage haemolysis was assessed by evaluating supernatant free Hb according to the following formula:
where Hb is expressed in g/dL. Supernatant free Hb was evaluated with a HemoCue Plasma Low Hemoglobin (HemoCue AB, Kuvettgatan 1, 262 71 Ängelholm, Sweden). Hct and Hb were obtained by the XE-2100 (Sysmex Europe GmbH, Germany).
Biochemical parameters, measured on undiluted supernatant, included pH (Radiometer ABL 800 Flex, Denmark), potassium, sodium, glucose and lactate (Hitachi Cobas® 8000, Roche Diagnostics, France).
Evaluation of residual white blood cells
Residual WBC after filtration were evaluated by flow cytometry (LeucoFinder™, Cytognos, Salamanca, Spain). LeucoFinder™ is a single-platform, flow cytometric method for residual WBC enumeration in leukoreduced blood products which combines detection of the fluorescence signal from a DNA marker incorporated into the nucleus of residual WBC allowing their discrimination, with the use of Perfect-Count Microspheres™, for their absolute count. After staining with propidium iodide, samples were acquired by the flow cytometer (FacsCanto BD, BD Biosciences, Becton Dickinson, San Jose, CA, USA) within 48 hours from filtration according to manufacturer’s instructions.
The absolute number of residual WBC in the leukoreduced sample was calculated using the following formula:
where the N. of Perfect–Count is expressed in μL, at a known concentration and rWBC are residual leukocytes.
Microbial testing
Two samples of 1 mL from the UCB-RBC units were examined for the presence of aerobic and anaerobic bacterial and fungal contamination after fractionation and after 14 days of storage (BacT/ALERT, bioMérieux, Craponne, France). The procedure was the same as that used at the UNICATT Cord Blood Bank to test UCB units for transplantation. To adhere to the NetCord-FACT Standard37, this method was internally validated using UCB contaminated with known concentrations of aerobic and anaerobic organisms and fungi. A minimum contamination of 10 CFU/mL in 1 mL could be detected. The analytical sensitivity was 98%. A cord blood unit was considered eligible for allogeneic transfusion when microbial culture and the mother’s serological and molecular viral (HBV, HCV, HIV) markers, syphilis, and CMV-IgM status were negative.
Statistical analyses
Continuous variables are expressed as the median ± interquartile range (IQR) and compared by the Mann-Whitney test (GraphPad Prism v. 6.01, GraphPad Software Inc. La Jolla, CA, USA).
RESULTS
Processing of umbilical cord blood units
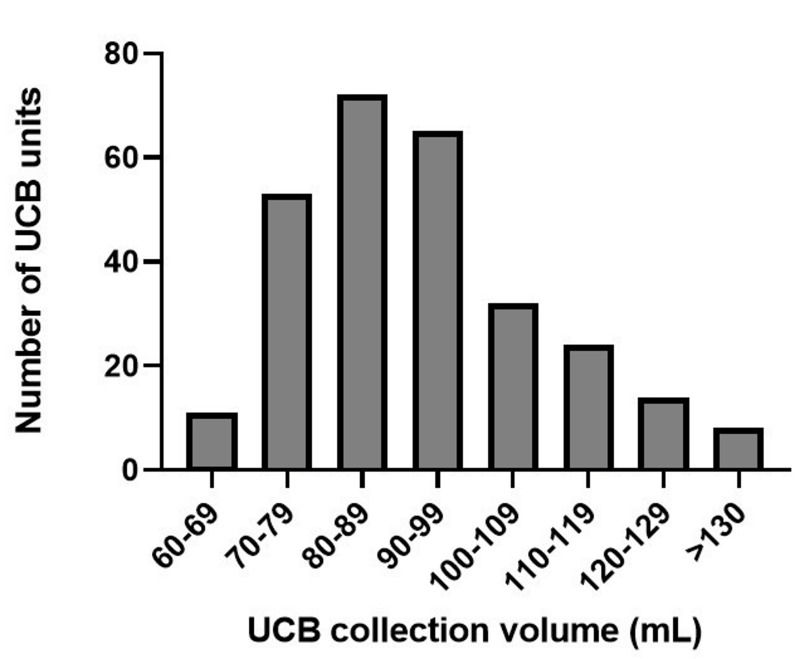
We evaluated data obtained from 279 UCB units (Table II) collected at a median gestational age of 40 weeks (IQR 39.1–40.7). The distribution of the volumes of the processed units (including anticoagulant solution) is shown in Figure 2. All units were fractionated in a median time of 218 seconds. After fractionation, there was a median RBC loss of 38% (31–47); this loss was greater in UCB units with a volume <80 mL than in units with a volume >80 mL (43.5%, IQR 36–54 vs 37%, IQR 30–45, p<0.05). Data on RBC mass (in mL) are reported in Table II (for unfractionated UCB units) and in Table III (for units from which the buffy coat had been removed and leukoreduced units). Compomat G4® and Compomat G5® (n=10) elicited similar RBC losses.
Table II.
Blood component characteristics before (UCB) and after cord blood unit fractionation (UCB-RBC)
| Whole UCB units | All (n=279) | <80 mL (n=64) | >80 mL (n=215) |
|---|---|---|---|
| Volume (mL) | 90 (81–103) | 74 (70.3–76.8) | 93 (87–107) |
| WBC (×10 9 /L) | 10.1 (8.7–12.1) | 10.3 (8.9–12.5) | 10.3 (8.7–12.0) |
| Haemoglobin (g/dL) | 11.8 (11.2–12.9) | 11.5 (10.7–12.2) | 12 (11.2–13.1) |
| Haematocrit (%) | 36.7 (34.9–39.5) | 36.5 (33.8–37.6) | 37.5 (35–40.1) |
| RBC mass (mL) | 33.4 (28.8–39.2) | 26.5 (24.2–28.4) | 35.9 (31.5–40.8) |
| SAG-M UCB-RBC units | |||
| Buffy coat-depleted units, volume (mL) | 31 (24.0–38.1) | 22 (18–27) | 33 (28.5–40) |
| Leukoreduced units, volume (mL) | 22* (17–28) | 16** (13–20.5) | 25*** (18–29) |
Umbilical cord blood data are also classified according to the initial volume of the cord blood. Values are expressed as median ± interquartile range.
Data from 75 units;
Data from 12 units;
Data from 63 units.
UCB: umbilical cord blood before fractionation; WBC: white blood cells, RBC: red blood cells; UCB-RBC: umbilical cord blood packed red blood cells; SAG-M: saline, adenine, glucose, mannitol.
Figure 2.
Volume of umbilical cord blood units, including anticoagulant solution, at collection
UCB: umbilical cord blood.
Table III.
Haematological parameters of fractionated umbilical cord red blood cell units from which the buffy coat was removed (a) or which underwent leukoreduction (b)
| Day 0 | Day +7 | Day +14 | |
|---|---|---|---|
| a. Buffy coat-depleted RBC | |||
| WBC (x10 3 /μL) | 4.36 (2.4–7.5) | 2.97 (1.8–4.9) | 0.02 (0.01–0.05) |
| Haemoglobin (g/dL) | 18.1 (16.5–19.8) | 19.8 (18.7–21.1) | 19.9 (18.4–20.8) |
| Haematocrit (%) | 54.3* (49.4–59.5) | 58.2 (54.8–60) | 59.1 (54.9–62.3) |
| Haemolysis (%) | - | - | 0.21 (0.16–0.35) |
| RBC mass (mL) | 17.1 (11.5–20.9) | - | - |
| b. Leukoreduced RBC | |||
| WBC (×10 3 /μL) | 0.01 (0.0–0.02) | 0.03 (0.02–0.04) | 0.02 (0.01–0.04) |
| Haemoglobin (g/dL) | 20.1 (18.5–21.5) | 21 (18.7–22.1) | 21.2 (19.3–21.8) |
| Haematocrit (%) | 60.3* (55.5–64.5) | 60.2 (56.7–64.7) | 62.2 (56.8–65.9) |
| RBC mass (mL) | 18.6 (15.8–23.1) | - | - |
| Haemolysis (%) | - | - | 1.3 (0.7–1.8) |
| Residual WBC (×10 6 ) | 0.25 (0.14–0.40) | - | - |
Values are expressed as median ± interquartile range.
p<0.05, comparing values at day 0.
RBC: red blood cells, WBC: white blood cells.
At the end, all units were suspended in SAG-M: 204 units had the buffy-coat removed and were filtered post-storage while 75 UCB-RBC underwent pre-storage leukoreduction. After leukoreduction, the volume decreased from 31 mL (IQR 24–38.1) to 22 mL (IQR 17–28). Volume reduction was the same in both pre-storage and post-storage leukoreduced units.
After addition of SAG-M, leukoreduced UCB-RBC units showed a higher Hct than UCB-RBC from which the buffy coat was removed (60.3%, IQR 55.5–64.5, vs 54.3%, IQR 49.4–59.5, p<0.05).
Quality controls of umbilical cord red blood cell units
The units of UCB-RBC to which SAG-M had been added were sampled at time 0 (day of fractionation), at day +7 (7 days after fractionation) and at day +14 (at the end of storage) to perform a complete blood count, measure biochemical parameters and determine, by flow cytometry, the number of residual WBC (only for leukoreduced units). Data are presented in Table III for haematological parameters and Table IV for biochemical parameters. These data are shown separately for units undergoing only buffy coat removal before storage and for units undergoing pre-storage leukoreduction.
Table IV.
Biochemical parameters of fractionated umbilical cord red blood cell units from which the buffy coat was removed (a) or which underwent leukoreduction (b)
| Day 0 | Day +7 | Day +14 | LR vs BC p values | |
|---|---|---|---|---|
| a. Buffy coat-depleted RBC | ||||
| pH | 7.8 (7.5–7.9) | 7.5 (7.2–7.7) | 7.1* (6.8–7.3)* | - |
| Lactate (mmol/L) | 1.9 (1.4–3.3) | 9.1 (7.8–10.8) | 13.6 (12.4–14.9)** | - |
| Glucose (mg/dL) | 671 (644–710) | 603 (570–652) | 556 (537–599)* | - |
| Na+ (mmol/L) | 135 (130–138) | 114 (108–120) | 104 (99–110)* | - |
| K+ (mmol/L) | 1.7 (1.5–2.0) | 2.5 (2.1–2.7) | 2.8 (2.5–3.1)* | - |
| b. Leukoreduced RBC | ||||
| pH | 6.9 (6.8–6.9) | 6.8 (6.7–6.8) | 6.6* (6.6–6.7) | 0.0001 |
| Lactate (mmol/L) | 4 (3.6–5.2) | 12.5 (11–14.4) | 16.4* (15.2–18.6) | 0.001 |
| Glucose (mg/dL) | 725 (683–775) | 631 (578–701) | 535** (504–628) | Ns |
| Na+ (mmol/L) | 144.4 (141–146) | 134 (132–137) | 130* (129–132) | 0.0001 |
| K+ (mmol/L) | 2.4 (2.3–3.8) | 15.5 (13.6–18.1) | 25.9* (23.3–28.5) | 0.0001 |
Values are expressed as median ± interquartile range.
p<0.001,
p<0.05, comparing values at day 0 and day +14.
LR: leukoreduced, BC: buffy coat; RBC: red blood cells; Na+: sodium; K+: potassium.
Microbial contamination
All UCB-RBC were tested for microbial contamination. After 5 days of incubation, eight out of 279 were positive (2.9%). We identified the following microbial species: Staphylococcus aureus, Bacteroides distasonis, Bacteroides vulgatus and Enterococcus faecium with a maximum time to positivity of 4 days plus 4 hours and 18 minutes.
DISCUSSION
UCB units unsuitable for haematopoietic stem cell transplantation might be a valuable resource for allogeneic transfusion therapy, since most of the discarded units have an adequate volume for subsequent fractionation into blood components such as RBC. This may also guarantee blood resources in developing countries in which the recruitment of donors is insufficient or in clinical emergencies such as outbreaks. In Italy, in 2019, only just under 6% of cord blood units collected were suitable for transplantation, leaving a substantial number of units to be destined to other clinical applications38.
At our institution, we were able to obtain UCB-RBC with an automated blood separator guaranteeing a closed system, a rapid method of fractionation (in less than 5 minutes) with standardised protocols. In addition, RBC-UCB were filtered, in the first phase at the time of clinical use (post-storage filtration) and more recently at the time of production (pre-storage filtration) to obtain leukoreduced RBC. UCB-RBC units had a median volume of 31 mL (IQR 28.8–39.2) after buffy coat removal. Such a variable volume must take into account the initial collection volume of the UCB units but reported data showed that the collection of variable volumes of UCB in a fixed anticoagulant volume is feasible and units may be stored for up to 35 days from collection28. This might be considered for example for autologous use, if whole cord blood fractionation is not performed.
Fractionation of UCB caused a loss of RBC mass which was greater for UCB with a collection volume <80 mL. RBC mass loss might be increased using new leukoreduction filters for such small RBC volumes or whole blood filters. Brune et al. published the findings of an interesting study34 in which RBC units were obtained by gravity separation after whole-blood filtration. Following the leukoreduction, blood was separated into plasma and RBC after passing through a hollow-fibre filter system. Twenty minutes later, the process was completed, and RBC were suspended in 10 mL of SAG-M. Leukoreduced UCB-RBC had a volume of 62.3±13.5 mL and a Hct of 56.0±5.6%. These units contained significantly lower concentrations of WBC (0.5±0.4/μL) and platelets (958±101/μL) compared to the concentrations in unfiltered RBC from cord blood (WBC 6.3±2.6/μL and platelets 62,685±24,100/μL) and RBC from adult donors (WBC 2.1±0.9/μL and platelets 3,568±1,193/μL). The authors considered this technique less expensive than semi-automated or automated blood separators and highly feasible for developing countries in which there are high birth rates and transfusion needs, e.g. for malaria and sickle cell crises34. We reported a significantly higher Hct in leukoreduced UCB-RBC units (60.3%) than in buffy-coat-depleted UCB-RBC units (54.3%). We assumed that leukoreduced units might be more concentrated than buffy coat-depleted ones, since the initial Hct and collection volume were not statistically different between these two groups.
At our institution, we also collected data on residual WBC after leukoreduction (Table II). Filtration allowed us to obtain a satisfactory UCB-RBC unit (WBC <1×106) in 12 of 13 (92.5%) filtered units with the median number of residual WBC being 0.25×106 (IQR 0.14–0.40). These values fulfil the international standard that a minimum of 90% of the units undergoing quality control should meet these criteria36. Since UCB contains a high number of nucleated RBC, there may be some interference in the residual WBC count. In the UCB-RBC units undergoing this quality control, the number of nucleated RBC was almost 5% of total WBC. There was no correlation between initial nucleated RBC count and residual WBC in leukoreduced UCB-RBC units. Residual leukocytes, which unavoidably contaminate RBC products despite leukoreduction, as well as soluble mediators derived from leukocytes, are among the main factors responsible for some detrimental effects on nitric oxide availability, immune regulation, inflammatory response and coagulation39.. As compared with their adult counterparts, however, neutrophils, monocytes and immune lymphoid cells in foetal blood exhibit highly tolerant phenotypes, and are much less capable of evoking a proficient inflammatory response. After separation, buffy coat removal leads to a 93% (78–98%) reduction of total WBC. Following filtration, it is possible to obtain a further decrease of residual WBC with a final reduction of 99.8% (99.7–99.9). Currently, leukoreduction filters used for adult RBC are high-efficiency filters that allow reductions of 3–4 log. In the case of UCB-RBC the efficiency of filtration (4–5 log) must take into account some factors such as the higher concentration of WBC in UCB units and the shorten filter length (around 15 cm). It is well known that filtration efficiency is influenced by several factors such as the volume of the filter, pre-filtration number of WBC, temperature, speed, pressure, priming and rinsing technique, presence of haemoglobin S and number of platelets40. Widing et al. also filtered UCB-RBC units but did not provide data on residual WBC. They showed that leukoreduced units were less subject to haemolysis compared to non-leukoreduced units (p=0.001), and assumed that dead WBC may have an effect on RBC destruction during storage33. Moreover, they showed that non-leukoreduced autologous units had lower levels of tumour necrosis factor-α and higher transforming growth factor-β1 levels compared to leukoreduced units or units stored for a shorter time, hypothesising that these cytokines might have a role in modulating immune response to infections.
In terms of additive solutions, SAG-M has been the main choice, albeit at highly variable ratios. Some chose a fixed volume of additive solution2,34 while others decided a defined ratio and adjusted the volume of the additive solution to the volume of the packed RBC17,32–33,35 or the desired final Hct (55–65%)31. In only two experiences packed RBC were suspended in phosphate, adenine, glucose, guanosine, saline-mannitol33 (PAGGS-M) and additive solution-3 containing phosphate, adenine, glucose, saline-mannitol (AS-3)31. PAGGS-M is an isotonic solution, whereas SAG-M is hypertonic. During RBC storage, PAGGS-M determines a lower rate of haemolysis after 42 days, whereas the decrease in RBC deformability and aggregability, and increase in blood viscosity are the same in units containing PAGGS-M or SAG-M41. SAG-M is the standard additive solution in Europe while AS-3 is the third additive solution licensed in the USA and Canada. To date, there are not sufficient data to state that AS-3 has a better performance than SAG-M42. When UCB-RBC in SAG-M were transfused into neonates no adverse events associated with the additive solution were reported2,17,29.
When using UCB for transfusion purposes, various issues need to be considered, which are not only connected with the volume and the haematological characteristics after processing but also red cell lesions during storage. In whole blood UCB stored for 28 days, there was no significant decline in Hct and variation of haemolysis30. Intracellular ATP levels decreased slightly (from 4.32±0.58 to 3.46±0.56 μmol/g Hb) and there was a marked reduction of 2,3-diphosphoglycerate (2,3-DPG) levels (from 13.30±1.00 to 1.31±0.28 μmol/g Hb)30. There was also a fall in plasma glucose (from 29.1±3.7 to 18.4±4.7 mmol/L) associated with a decline of extracellular pH (from 6.85±0.14 to 6.51±0.12) resulting from metabolic glycolysis by red cells during storage and an increase of potassium levels (from 8.2±3.4 to 32±5.9 mmol/L). Hassall et al. also reported that, at day 14 of storage, there was an increase of supernatant potassium (median 18.05 mmol/L) even if standardised by donation volume (median 15.10 mmol/L)43.
Our quality control data showed a similar pattern. Storage at 2–6 °C for 14 days led to an increase of potassium levels which were significantly higher in leukoreduced (median 25.9 mmol/L, 23.3–28.5) than in buffy coat-depleted units (median 2.8 mmol/L, 2.5–3.1) as well as a different percentage of haemolysis (1.3 vs 0.21, in leukoreduced and buffy coat-depleted UCB-RBC, respectively). These data allow speculation on the role of leukoreduction in determining such different characteristics: it is well known that filtration may have an effect on the content of the RBC mass. However, several randomised clinical trials showed no differences in terms of morbidity and mortality when comparing fresh RBC or RBC that had been stored for longer times, either in adult44–46 or paediatric47–48 settings. Accordingly, in our clinical experience there were no adverse events related to this issue since the units were transfused before the increase of potassium, generally within 7 days of collection29. It might be advisable in high-risk neonates, i.e. in cases of renal failure, to modify the potassium supplementation of a nutritional plan, if applicable. In consideration that neonatal RBC are more fragile than adult ones, an earlier expiry date (at +14 days) may be advisable, even in the autologous setting in which leukoreduction and irradiation are not required.
Potassium levels increase independently of the additive solution, as shown by comparisons of RBC stored in SAG-M vs PAGGS-M33 as well as SAG-M vs AS-331. The increment is due to haemolytic phenomena during storage, as demonstrated by the increase of the percentage of haemolysis. At our facility, leukoreduced UCB-RBC showed a median of 1.3% (0.7–1.8) haemolysis at day 14 of storage. Garritsen et al. showed a statistically significant increased level of free Hb (from 19.4±19.2 to 416.9±254.5 mg/dL, p<0.05), an increased percentage of haemolysis (from 0.0±0.0 to 1.1±0.8%, p<0.05) and a reduction of intracellular ATP (from 3.7±0.9 to 1.2±0.5 mg/dL, p<0.05) after 35 days32. Widing et al. reported that haemolysis was low during the first 3 weeks of storage but increased in the subsequent 2 weeks. Haemolysis was greater in nine of 14 (64.2%) SAG-M units than in one of 12 (8.3%) PAGGS-M units (p=0.0053, Fisher’s exact text)33. Khodabux et al. compared fractionated UCB-RBC in SAG-M and AS-3, unfractionated whole cord blood and pedi-pack units from adult leukoreduced RBC, sampling every 7 days from collection until 35 days. Only 21% of these UCB-RBC had less than 0.8% haemolysis showing that UCB-RBC have a shorter shelf-life when compared to pedi-pack units: 2.2±0.67 (1.1–3.5) and 0.7±0.14 (0.32–0.84), respectively31. Moreover, we found a more pronounced lactate increase and pH and glucose decreases, as also reported by others31,32,35.
With regards to microbial contamination, UCB units have a higher rate of contamination than blood collected from adult donors. The rate of microbial contamination in cord blood is highly variable (0–8.6%)2,17,30–35 and the problem is more frequent in vaginal deliveries and when the collecting staff are not well-trained. Surbek et al. suggested that some clinical conditions at the time of the delivery, such as chorioamnionitis should be regarded as a contraindication to autologous cord blood collection because they are associated with a high incidence of cord blood contamination49. In this paper, Bacteroides spp., Staphylococcus spp., and Propionibacterium spp., accounting for 13.7% of all positive results, were the most common microbial contaminants. Indeed, in accordance with international regulations and standards37, all UCB units for transplantation, transfusion (whole blood and RBC) and topical purposes (e.g. cord blood platelet gel) must undergo bacterial and fungal cultures before release for clinical use. This causes a delay in the release of a cord RBC unit, estimated at 5–6 days after collection, and is generally associated with a longer mean storage time at the time of transfusion of cord RBC (7 days, range 3.2–10), compared to that of adult RBC units (3.5 days, range 1–6)35.
CONCLUSIONS
Previous experiences of the collection, fractionation, and transfusion of cord RBC have shown that these practices are feasible and safe50–51.
Fractionation of whole UCB can produce RBC concentrates with similar baseline characteristics as units from adult donors. The data support the feasibility of obtaining leukoreduced UCB-RBC units. The clinical use of such units in the neonatal setting, especially for extremely low birth weight neonates, appears to be safe and adequate in terms of transfusion dose (at least 20 mL/kg) and quality of the units, with a good Hct (58.9%) and a reduced content of residual WBC after filtration (<1×106), despite great variety in the volumes collected. On the other hand, fractionated units seem to have a shorter shelf life than that of unfractionated ones. Based on the quality controls performed, a different expiry date should be defined for UCB-RBC, earlier than that for adult RBC. This should also allow blood component irradiation, if required. At the same time, reallocated UCB units, unsuitable for haematopoietic stem cell transplantation, might be a valuable resource for transfusion therapy as well as helping the economic sustainability of cord blood banks.
ACKNOWLEDGEMENTS
We thank Genitin ONLUS and Gruppo Donatori di Sangue F.Olgiati ONLUS for their support.
Footnotes
AUTHORSHIP CONTRIBUTIONS
MB and LT designed the study. CGV, SS and BC enrolled the donors and selected the units. NO and OB carried out the cord blood unit fractionation and quality control assays. MB wrote the manuscript. LT supervised the project and reviewed the manuscript. All the authors approved the final version of the paper.
The Authors declare no conflicts of interest.
REFERENCES
- 1.Ballin A, Arbel E, Kenet G, et al. Autologous umbilical cord blood transfusion. Arch Dis Child. 1995;73:F181–93. doi: 10.1136/fn.73.3.f181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eichler H, Schaibe T, Richter E, et al. Cord blood as a source of autologous RBCs for transfusion to preterm infants. Transfusion. 2000;40:1111–7. doi: 10.1046/j.1537-2995.2000.40091111.x. [DOI] [PubMed] [Google Scholar]
- 3.Surbek DV, Glanzmann R, Senn HP, et al. Can cord blood be used for autologous transfusion in preterm neonates? Eur J Pediatr. 2000;159:790–1. doi: 10.1007/s004310000524. [DOI] [PubMed] [Google Scholar]
- 4.Brune T, Garritsen H, Hentschel R, et al. Efficacy, recovery, and safety of RBCs from autologous placental blood: clinical experience in 52 newborns. Transfusion. 2003;43:1210–5. doi: 10.1046/j.1537-2995.2003.00503.x. [DOI] [PubMed] [Google Scholar]
- 5.Jansen M, Brand A, von Lindern JS, et al. Potential use of autologous umbilical cord blood red cells for early transfusion needs in premature infants. Transfusion. 2006;46:1049–56. doi: 10.1111/j.1537-2995.2006.00841.x. [DOI] [PubMed] [Google Scholar]
- 6.Khodabux CM, von Lindern JS, van Hilten JA, et al. A clinical study on the feasibility of autologous cord blood transfusion for anemia of prematurity. Transfusion. 2008;48:1634–43. doi: 10.1111/j.1537-2995.2008.01747.x. [DOI] [PubMed] [Google Scholar]
- 7.Yavuz BA, Okulu E, Arsan S, et al. Is autologous cord blood transfusion effective and safe in preterm infants? Turk J Pediatr. 2017;59:352–4. doi: 10.24953/turkjped.2017.03.022. [DOI] [PubMed] [Google Scholar]
- 8.Kotowski M, Litwinska Z, Klos P, et al. Autologous cord blood transfusion in preterm infants - could its humoral effect be the key to control prematurity-related complications? A preliminary study. J Physiol Pharmacol. 2017;68:921–7. [PubMed] [Google Scholar]
- 9.Imura K, Kawahara H, Kitayama Y, et al. Usefulness of cord-blood harvesting for autologous transfusion in surgical newborns with antenatal diagnosis of congenital anomalies. J Ped Surg. 2001;36:851–4. doi: 10.1053/jpsu.2001.23952. [DOI] [PubMed] [Google Scholar]
- 10.Domanović D, Zavrsnik T, Vesel S. Autologous placental blood transfusion after a planned neonatal pacemaker implantation. Transfus Med. 2001;11:459–61. doi: 10.1046/j.1365-3148.2001.00333.x. [DOI] [PubMed] [Google Scholar]
- 11.Taguchi T, Suita S, Nakamura M, et al. The efficacy of autologous cord-blood transfusions in neonatal surgical patients. J Ped Surg. 2003;38:604–7. doi: 10.1053/jpsu.2003.50131. [DOI] [PubMed] [Google Scholar]
- 12.Hosono S, Mugishima H, Nakano Y, et al. Autologous cord blood transfusion in an infant with a huge sacrococcygeal teratoma. J Perinat Med. 2004;32:187–9. doi: 10.1515/JPM.2004.035. [DOI] [PubMed] [Google Scholar]
- 13.Chasovskyi K, Fedevych O, Vorobiova G, et al. Arterial switch operation in the first hours of life using autologous umbilical cord blood. Ann Thor Surg. 2012;93:1571–6. doi: 10.1016/j.athoracsur.2012.01.104. [DOI] [PubMed] [Google Scholar]
- 14.Titkov KV. Autotransfusion of cord blood erythrocytes in newborns with malformations requiring early surgical intervention. Anesteziol Reanimatol. 2014;59:38–43. [PubMed] [Google Scholar]
- 15.Chasovskyi K, Fedevych O, McMullan DM, et al. Tissue perfusion in neonates undergoing open-heart surgery using autologous umbilical cord blood or donor blood components. Perfusion. 2015;30:499–506. doi: 10.1177/0267659114550234. [DOI] [PubMed] [Google Scholar]
- 16.Choi ES, Cho S, Jang WS, et al. Cardiopulmonary bypass priming using autologous cord blood in neonatal congenital cardiac surgery. Korean Circ J. 2016;46:714–8. doi: 10.4070/kcj.2016.46.5.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brune T, Garritsen H, Witteler R, et al. Autologous placental blood transfusion for the therapy of anaemic neonates. Biol Neonate. 2002;81:236–43. doi: 10.1159/000056754. [DOI] [PubMed] [Google Scholar]
- 18.Bhattacharya N, Mukherijee K, Chettri MK, et al. A study report of 174 units of placental umbilical cord whole blood transfusion in 62 patients as a rich source of fetal hemoglobin supply in different indications of blood transfusion. Clin Exp Obstet Gynecol. 2001;28:47–52. [PubMed] [Google Scholar]
- 19.Hassall O, Bedu-Addo G, Adarkwa M, et al. Umbilical-cord blood for transfusion in children with severe anaemia in under-resourced countries. Lancet. 2003;361:678–9. doi: 10.1016/S0140-6736(03)12565-2. [DOI] [PubMed] [Google Scholar]
- 20.Bhattacharya N. Placental umbilical cord whole blood transfusion: a safe and genuine blood substitute for patients of the under-resourced world at emergency. J Am Coll Surg. 2005 Apr;200:557–63. doi: 10.1016/j.jamcollsurg.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 21.Bhattacharya N. Placental umbilical cord blood transfusion in transfusion-dependent beta thalassemic patients: a preliminary communication. Clin Exp Obstet Gynecol. 2005;32:102–6. [PubMed] [Google Scholar]
- 22.Bhattacharya N. A preliminary study of placental umbilical cord whole blood transfusion in under resourced patients with malaria in the background of anaemia. Malar J. 2006;5:20. doi: 10.1186/1475-2875-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhattacharya N. Placental umbilical cord whole blood transfusion to combat anemia in the background of advanced rheumatoid arthritis and emaciation and its potential role as immunoadjuvant therapy. Clin Exp Obstet Gynecol. 2006;33:28–33. [PubMed] [Google Scholar]
- 24.Bhattacharya N. Placental umbilical cord whole blood transfusion to combat anemia in the background of tuberculosis and emaciation and its potential role as an immuno-adjuvant therapy for the under-resourced people of the world. Clin Exp Obstet Gynecol. 2006;33:99–104. [PubMed] [Google Scholar]
- 25.Bhattacharya N. A preliminary report of 123 units of placental umbilical cord whole blood transfusion in HIV-positive patients with anemia and emaciation. Clin Exp Obstet Gynecol. 2006;33:117–21. [PubMed] [Google Scholar]
- 26.Bhattacharya N. Transient spontaneous engraftment of CD34 hematopoietic cord blood stem cells as seen in peripheral blood: treatment of leprosy patients with anemia by placental umbilical cord whole blood transfusion. Clin Exp Obstet Gynecol. 2006;33:159–63. [PubMed] [Google Scholar]
- 27.Bhattacharya N. Placental umbilical cord blood transfusion: a new method of treatment of patients with diabetes and microalbuminuria in the background of anemia. Clin Exp Obstet Gynecol. 2006;33:164–8. [PubMed] [Google Scholar]
- 28.Hassall OW, Thitiri J, Fegan G, et al. Safety and efficacy of allogeneic umbilical cord blood red cell transfusion for children with severe anaemia in a Kenyan hospital: an open-label single-arm trial. Lancet Haematol. 2015;2:e101–7. doi: 10.1016/S2352-3026(15)00005-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bianchi M, Giannantonio C, Spartano S, et al. Allogeneic umbilical cord blood red cell concentrates: an innovative blood product for transfusion therapy of preterm infants. Neonatology. 2015;107:81–6. doi: 10.1159/000368296. [DOI] [PubMed] [Google Scholar]
- 30.Bifano EM, Dracker RA, Lorah K, et al. Collection and 28-day storage of human placental blood. Pediatr Res. 1994;36:90–4. doi: 10.1203/00006450-199407001-00016. [DOI] [PubMed] [Google Scholar]
- 31.Khodabux CM, van Beckhoven JM, Scharenberg JG, et al. Processing cord blood from premature infants into autologous red-blood-cell products for transfusion. Vox Sang. 2011;100:367–73. doi: 10.1111/j.1423-0410.2010.01440.x. [DOI] [PubMed] [Google Scholar]
- 32.Garritsen HS, Brune T, Louwen F, et al. Autologous red cells derived from cord blood: collection, preparation, storage and quality controls with optimal additive storage medium (Sag-mannitol) Transfus Med. 2003;13:303–10. doi: 10.1046/j.1365-3148.2003.00457.x. [DOI] [PubMed] [Google Scholar]
- 33.Widing L, Bechensteen AG, Mirlashari MR, et al. Evaluation of nonleukoreduced red blood cell transfusion units collected at delivery from the placenta. Transfusion. 2007;47:1481–7. doi: 10.1111/j.1537-2995.2007.01287.x. [DOI] [PubMed] [Google Scholar]
- 34.Brune T, Fill S, Heim G, et al. Quality and stability of red cells derived from gravity-separated placental blood with a hollow-fiber system. Transfusion. 2007;47:2271–5. doi: 10.1111/j.1537-2995.2007.01456.x. [DOI] [PubMed] [Google Scholar]
- 35.Bianchi M, Teofili L, Giannantonio C, et al. Transfuse neonates with cord blood-derived red blood cells: a feasibility study to assess allogeneic cord blood unit fractionation and validation. Blood. 2012;120:275. [Google Scholar]
- 36.European Directorate for the Quality of Medicines & HealthCare. European Committee on Blood Transfusion. Guide to the preparation, use and quality assurance of blood components. 19th edition. [Accessed on 09/05/2020]. Available at https://www.edqm.eu/sites/default/files/list_of_contents_19th_ed-blood-quality.pdf.
- 37.NetCord-FACT International Standards for Cord Blood Collection, Banking, and Release for Administration. 7th edition. Oct 15, 2019. [Accessed on 09/05/2020.]. Available at http://www.factwebsite.org/Standards/
- 38.Centro Nazionale Sangue [internet] [Accessed on 09/05/2020]. Available at https://www.centronazionalesangue.it/sites/default/files/Report%202019.pdf. [In Italian.]
- 39.Youssef LA, Spitalnik SL. Transfusion-related immunomodulation: a reappraisal. Curr Opin Hematol. 2017;24:551–7. doi: 10.1097/MOH.0000000000000376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dzik WH. Leukoreduced blood components: laboratory and clinical aspects. In: Simon TL, Dzik WH, Snyder EL, Stowell CP, Strauss RG, editors. Rossi’s Principles of Transfusion Medicine. Lippincott, Williams and Wilkins; 2002. pp. 270–87. [Google Scholar]
- 41.Zehnder L, Schulzki T, Goede JS, et al. Erythrocyte storage in hypertonic (SAGM) or isotonic (PAGGSM) conservation medium: influence on cell properties. Vox Sang. 2008;95:280–7. doi: 10.1111/j.1423-0410.2008.01097.x. [DOI] [PubMed] [Google Scholar]
- 42.D’Amici GM, Mirasole C, D’Alessandro A, et al. Red blood cell storage in SAGM and AS3: a comparison through the membrane two-dimensional electrophoresis proteome. Blood Transfus. 2012;10(Suppl 2):S46–54. doi: 10.2450/2012.008S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hassall O, Maitland K, Fegan G, et al. The quality of stored umbilical cord and adult-donated whole blood in Mombasa, Kenya. Transfusion. 2010;50:611–6. doi: 10.1111/j.1537-2995.2009.02489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Steiner ME, Ness PM, Assmann SF, et al. Effects of red-cell storage duration on patients undergoing cardiac surgery. N Engl J Med. 2015;372:1419–29. doi: 10.1056/NEJMoa1414219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lacroix J, Hébert PC, Fergusson DA, et al. Age of transfused blood in critically ill adults. N Engl J Med. 2015;372:1410–8. doi: 10.1056/NEJMoa1500704. [DOI] [PubMed] [Google Scholar]
- 46.Heddle NM, Cook RJ, Arnold DM, et al. Effect of short-term vs. long-term blood storage on mortality after transfusion. N Engl J Med. 2016;372:1937–45. doi: 10.1056/NEJMoa1609014. [DOI] [PubMed] [Google Scholar]
- 47.Dhabangi A, Ainomugisha B, Cserti-Gazdewich C, et al. Effect of transfusion of red blood cells with longer vs shorter storage duration on elevated blood lactate levels in children with severe anemia: the TOTAL randomized clinical trial. JAMA. 2015;314:2514–23. doi: 10.1001/jama.2015.13977. [DOI] [PubMed] [Google Scholar]
- 48.Fergusson DA, Hébert P, Hogan DL, et al. Effect of fresh blood cell transfusions on clinical outcomes in premature, very low-birth-weight-infants: the ARIPI randomized trial. JAMA. 2012;30:1443–51. doi: 10.1001/2012.jama.11953. [DOI] [PubMed] [Google Scholar]
- 49.Surbek DV, Glanzmann R, Senn HP, et al. Can cord blood be used for autologous transfusion in preterm neonates? Eur J Pediatr. 2000;159:790–1. doi: 10.1007/s004310000524. [DOI] [PubMed] [Google Scholar]
- 50.Khodabux CM, Brand A. The use of cord blood for transfusion purposes: current status. Vox Sang. 2009;97:281–93. doi: 10.1111/j.1423-0410.2009.001212.x. [DOI] [PubMed] [Google Scholar]
- 51.Bianchi M, Papacci P, Valentini CG, et al. Umbilical cord blood as a source for red-blood-cell transfusion in neonatology: a systematic review. Vox Sang. 2018;113:713–25. doi: 10.1111/vox.12720. [DOI] [PubMed] [Google Scholar]