Abstract
Objectives
To determine the prevalence of osteoporosis and the proportion who needed treatment after screening women aged 65 years or older; their treatment acceptance and continuation.
Methods
This is an observational study conducted between May 2017 and April 2020.
Participants underwent clinical assessment and bone mineral density measurement of lumbar spine, total hip, and femoral neck by dual energy X-ray absorptiometry. Those with osteoporosis at any site or osteopenia with 10-year major fracture risk ≥ 20% or hip fracture risk ≥ 3% by Fracture Risk Assessment Tool® were offered drug treatment.
Results
Among 1800 participants, 15.9% were normal, 33.2% were low-risk osteopenic, 27.2% were high-risk osteopenic, and 23.7% were osteoporotic. Their mean age was 69.4 years and 6.3% had low-energy fractures after menopause. After stepwise logistic regression analysis, only prior low-energy fractures after menopause and low body mass index (BMI) remained significantly correlated with osteoporosis. Those who needed treatment were significantly older, menopaused at age 45 years or earlier, had a parent with hip fracture, had low-energy fractures after menopause, and low BMI. Drug was offered to 916 women but 67.6% refused because they worried about side effects, interaction with existing drugs, and were reluctant to take more drugs. Treatment acceptance was significantly higher among osteoporotic patients. Treatment continuation at 6th and 12th months was also significantly higher in osteoporotic patients.
Conclusions
Osteoporosis screening in elderly women identified a significant proportion who needed treatment. Encouraging them to initiate drug, especially high-risk osteopenic patients, remained a challenge.
Keywords: Osteoporosis, Female, Bone density, Risk factors, Treatment
1. Introduction
Osteoporosis is an age-related disease characterized by low bone mass and micro-architectural deterioration, leading to bone fragility and an increased risk of fractures. Osteoporotic fractures commonly occur at the wrist, spine, hip, and humerus. Hip fractures are associated with the highest health and social care costs due to functional limitation, decreased mobility, and increased need for residential care even after the fractures are fixed. Mortality after hip fractures approaches 20% in the first year [1].
There is a well-established relationship between fracture risk and bone mineral density (BMD). With each standard deviation fall in BMD, fracture risk increases by 1.5 to 3-fold [2]. BMD measurement by dual energy X-ray absorptiometry (DXA) is the gold standard for diagnosing osteoporosis. A recent report from China showed 45.9% of women 65 years and older had osteoporosis in the lumbar spine, femoral neck or total hip in 2008–2018 [3]. In the United States, 27.1% of women in the same age group had osteoporosis in 2017–2018 [4]. The prevalence of osteoporosis in Canadian women was 30.1% in those aged 65–69, 36.2% in those aged 70–74, 41.4% in those aged 75–79, 45.6% in those aged 80–84, 48.1% in those aged 85–89, and 47.6% in those aged 90 and above [5]. The prevalence of osteoporosis in women 65 years and older is largely unknown in Hong Kong. According to the latest population projection, the percentage of women 65 years and older will increase from 9.5% in 2019 to 14% in 2029 and 17.7% in 2039. The actual life expectancy of a 60 year old woman in 2019 was 90 years [6]. The total number of osteoporotic hip fractures in Hong Kong women was projected to increase by 2.4 fold between 2018 and 2040 [7]. With the increase in the elderly population and longevity, offering DXA and prompt treatment for osteoporosis is important for the reduction of osteoporotic fractures.
The objectives of this pilot screening program are to determine the prevalence of osteoporosis and the proportion for whom treatment was required after screening, and the risk factors associated with osteoporosis and need for treatment. The treatment acceptance and continuation among those who needed treatment were also evaluated. The results will help public health specialists and clinicians estimate the burden of osteoporosis and workload involved with treatment and follow-up after the implementation of screening for elderly women.
2. Methods
This screening program was funded by the Time Limited Project of The Community Chest of Hong Kong (Grant ID: TLP-FPAH-EBC). Due to funding restrictions, this pilot screening program could only recruit 1800 subjects between May 2017 and April 2020. Eligibility criteria included female, aged 65 or above, never had osteoporosis treatment, and never had DXA. The program was announced at a press conference attended by local media. Eligible women were recruited on a first come, first served basis until all quotas were filled.
On the day of consultation, participants had to fill in the Fracture Risk Assessment Tool® (FRAX®) checklist after registration. One clinician was responsible for the whole program to ensure consistency of data collection and uniformity of management. All participants were interviewed using a standardized questionnaire to collect data on clinical risk factors for osteoporosis such as age, age at menopause, duration of menopause, history of osteoporosis related low-energy fracture(s) after menopause, parental hip fracture, smoking habit, drinking habit, medical disease(s) and use of regular medication(s) that cause bone loss, number of falls in the past 2 years, daily calcium intake, duration of exercise (walking or Tai Chi or stretching exercise) performed each day, and duration of sun exposure every day. The total daily calcium intake included diet and supplementation. Dietary calcium intake was calculated using a reference chart produced by the dietetic department of Queen Mary Hospital, a tertiary referral center for osteoporosis management. Body weight and height were measured with participants wearing light clothing and without shoes to calculate their body mass index (BMI). BMD was measured at the lumbar spine, the total hip, and the neck of femur using the Hologic Discovery densitometer (QDR 4500; Hologic, Inc., Bedford, MA, USA) operated by a single operator. T-scores were determined with reference to local southern Chinese normative database. Further investigations would be arranged for those with a Z-score less than −2.0 to exclude secondary osteoporosis. Spine X-ray would be performed when a vertebral fracture was suspected.
Treatment was offered to those with osteoporosis at any one site and those with osteopenia plus FRAX® 10-year major osteoporotic fracture risk ≥ 20% or hip fracture risk ≥ 3% [8]. Three drugs were available: oral daily raloxifene, oral weekly alendronate, and 6-monthly denosumab injection. The clinician provided personalised advice on the goals of treatment, treatment duration, side effects and efficacy of appropriate drug(s) to be used, possible causes of treatment failure, importance of lifestyle modifications and fall prevention, alternatives to pharmacologic therapy, and the morbidity and mortality of osteoporotic fractures to each participant. Participants and their accompanying relatives were encouraged to discuss their concerns with the clinician and share the decision-making in choosing the management option they preferred. Reasons for refusal of treatment were documented. Since this is a service program, not a research study, participants are not required to sign any consent. The use of clinical records for analysis was approved by the Association's Ethics Panel (equivalent to Institutional Review Board) as well as the Health Services Subcommittee (a governing board on all health services programs) with approval number of OA1-2 and followed the Declaration of Helsinki.
2.1. Statistical analysis
Data analysis was performed with IBM® Statistical Package for Social Science (SPSS®) (Windows version 27; IBM Corp., Armonk, New York, US). Bone status was categorized into normal (T-score ≥ -1.0), osteopenia (T-score between −1.0 and −2.5), and osteoporosis (T-score ≤ -2.5) according to the World Health Organization recommendation [9]. Those with osteopenia were further subdivided into high and low risk by FRAX®, available at http://www.shef.ac.uk/FRAX. Those with 10-year major osteoporotic fracture risk ≥ 20% or hip fracture risk ≥ 3% were classified as high risk [8]. Primary outcomes studied were: need for treatment, acceptance of treatment, and continuation of treatment. Descriptive statistics on continuous variables (age, age at menopause, duration of menopause) were presented as mean ± standard deviation and categorical variables (parental hip fracture, history of osteoporosis related low-energy fracture(s) after menopause, low BMI (< 18.5 kg/m2), had medical disease(s) that cause bone loss, on regular medication(s) that cause bone loss, ever fell in the past 2 years) were presented as proportion. Associations between demographic characteristics, clinical characteristics and lifestyle habits (calcium intake, exercise, sun exposure, cigarette use, and alcohol use) with osteoporosis and the 3 primary outcomes were analyzed using Student t test for normally distributed continuous variables and Chi-square test for normally distributed categorical variables. Pearson correlation analysis was performed to examine the correlation between significant independent variables (demographic characteristics/clinical characteristics/lifestyle habits), and outcome variables (osteoporosis/need treatment). Similar correlation analyses between outcome variables (treatment acceptance and continuation) and independent variables (demographic and clinical characteristics, lifestyle habits and bone status) were also performed. All analyses were two-tailed, and P-values ≤ 0.05 were considered statistically significant. Multivariate stepwise logistic regression analysis was performed to delineate which independent variable best predicted osteoporosis, the need for treatment, acceptance of treatment, and continuation of treatment. The results from logistic regression were presented as odds ratios (ORs) and 95% confidence intervals (CIs).
3. Results
A total of 1800 treatment-naïve elderly women joined this pilot screening program. Overall, 15.9% had normal bone mineral density, 33.2% had osteopenia and were at low risk of fracture, 27.2% had osteopenia and were at high risk of fracture, and 23.7% were osteoporotic. Out of the 489 osteopenic women, 44 had major osteoporotic fracture risk of ≥ 20% in 10 years, 400 had hip fracture risk of ≥ 3% in 10 years, and 45 were at high risk for both major osteoporotic and hip fractures. The demographic characteristics, clinical characteristics and lifestyle habits in each of the above bone status subgroup is compared in Table 1. Pearson correlation showed age ≥ 70 years old (r = 0.055, P < 0.05), longer duration of menopause (r = 0.113, P < 0.01), history of osteoporosis related low-energy fracture(s) after menopause (r = 0.066, P < 0.01), and low BMI (r = 0.188, P < 0.01) were significant risk factors for osteoporosis. After stepwise logistic regression analysis, only history of osteoporosis related low-energy fracture(s) after menopause (OR, 1.764; 95% CI, 1.178–2.643; P = 0.005), and low BMI (OR, 6.194; 95% CI, 3.746–10.252; P < 0.001) remained significantly associated with osteoporosis.
Table 1.
Demographic characteristics, clinical characteristics and lifestyle habits by bone status (% within group).
| Risk factor | Whole group (n = 1800) | Normal1 (n = 287) | Osteopenia, low risk2 (n = 597) | Osteopenia, high risk3 (n = 489) | Osteoporosis4 (n = 427) | P-value (Groups 1&2 vs 3&4) |
|---|---|---|---|---|---|---|
| Mean age, yr (SD) | 69.4 (4.2) | 69.1 (3.8) | 67.5 (2.3) | 71.1 (4.4) | 70.1 (5.0) | < 0.001 |
| Mean menopause age, yr (SD) | 50.8 (4.5) | 51.6 (4.3) | 50.8 (4.0) | 50.7 (4.7) | 50.3 (4.8) | 0.027 |
| Mean duration of menopause, yr (SD) | 18.5 (6.2) | 17.5 (5.9) | 16.7 (4.6) | 20.3 (6.6) | 19.8 (7.1) | < 0.001 |
| Parental hip fracture | 195 (10.8%) | 38 (13.2%) | 31 (5.2%) | 79 (16.2%) | 47 (11%) | < 0.001 |
| Had osteoporosis related fracture after menopause | 113 (6.3%) | 6 (2.1%) | 18 (3.0%) | 56 (11.5%) | 39 (9.1%) | < 0.001 |
| Body mass index < 18.5 kg/m2 | 69 (3.8%) | 2 (0.7%) | 14 (2.3%) | 9 (1.8%) | 44 (10.3%) | < 0.001 |
| Have medical disease(s) associated with osteoporosis | 271 (15.1%) | 54 (18.8%) | 83 (13.9%) | 84 (17.2%) | 50 (11.7%) | 0.606 |
| Taken drug that causes BMD loss | 264 (14.7%) | 41 (14.3%) | 73 (12.2%) | 84 (17.2%) | 66 (15.5%) | 0.037 |
| Had one or more falls in past 2 years | 521 (28.9%) | 78 (27.2%) | 169 (28.3%) | 143 (29.2%) | 131 (30.7%) | 0.377 |
| Daily calcium intake | ||||||
| < 600 mg | 874 (48.6%) | 135 (47.0%) | 302 (50.6%) | 234 (47.9%) | 203 (47.6%) | 0.761 |
| 600- < 800 mg | 312 (17.3%) | 52 (18.1%) | 102 (17.1%) | 84 (17.2%) | 74 (17.3%) | |
| 800- < 1200 mg | 505 (28.1%) | 80 (27.9%) | 164 (27.5%) | 141 (28.8%) | 120 (28.1%) | |
| 1200 mg or more | 109 (6.1%) | 20 (7.0%) | 29 (4.9%) | 30 (6.1%) | 30 (7.0%) | |
| Less than 20 min of walking or Tai Chi or stretching exercise daily | 113 (6.3%) | 20 (7.0%) | 39 (6.5%) | 30 (6.1%) | 24 (5.6%) | 0.496 |
| Less than 20 min of sun exposure daily | 609 (33.8%) | 104 (36.2%) | 204 (34.2%) | 157 (32.1%) | 144 (33.7%) | 0.374 |
| Smoke 20 or more cigarettes daily | 7 (0.4%) | 1 (0.3%) | 2 (0.3%) | 2 (0.4%) | 2 (0.5%) | 0.740 |
| Drink 3 units of alcohol daily | 1 (0.06%) | 0 | 0 | 1 | 0 | – |
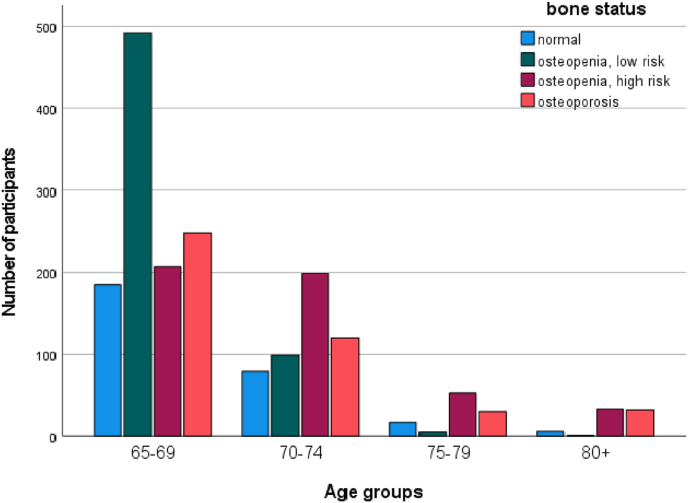
The age-related prevalence of the 4 bone status groups is illustrated in Fig. 1. The proportion of elderly women with osteoporosis increased gradually from 21.9% for those aged 65–69, to 24.0% for those aged 70–74, to 28.2% for those aged 75–79. Among those aged 80 years and older, the proportion sharply increased to 44.3%.
Fig. 1.
Bone status by age groups with Fracture Risk Assessment Tool®.
A total of 113 (6.3%) women reported 1 or more osteoporosis related low-energy fractures after menopause: 108 women fractured once, four fractured twice, and one fractured thrice. The predominant fracture site was the distal forearm (50 women, 44.2% of those who had fractures), followed by tibia/fibula (19 women, 16.8%), and vertebrae (18 women, 15.9%). Among these 113 women, 6 had normal BMD and 12 were osteopenic with low fracture risk by FRAX®. In the group with normal BMD, 3 had distal forearm fractures, 1 had humerus fracture, 1 had tibia/fibula fracture, and 1 had a rib fracture. Among the low-risk osteopenic women, 6 had distal forearm fracture, 4 had tibia/fibula fracture, and 2 had rib fractures. All participants with prior hip or vertebral fractures were included in either the high-risk osteopenia or osteoporosis groups.
According to the local guideline, individuals at high risk of fracture should be treated, namely those with prior low-energy hip or vertebral fractures, osteoporosis, and osteopenia with 10 years major osteoporotic fracture risk ≥ 20%, and hip fracture risk ≥ 3% [10]. There was a substantial increase in the proportion of women who needed treatment from 40.1% in the 65–69 years old to 64.1% in the 70–74 years old groups. The proportion progressively increased to 78.7% and 90% for those aged 75–79 years and 80+ years, respectively. Pearson correlation showed significant correlations between the need for treatment and age ≥ 70 years old (r = 0.280, P < 0.01), menopausal age at 45 years old or younger (r = 0.055, P < 0.05), longer duration of menopause (r = 0.252, P < 0.01), parental hip fracture (r = 0.096, P < 0.01), history of osteoporosis related low-energy fracture(s) after menopause (r = 0.172, P < 0.01), low BMI (r = 0.104, P < 0.01), and on drugs that cause bone loss (r = 0.049, P < 0.05). After stepwise logistic regression analysis, aged 70 years and older (OR, 3.328; 95% CI, 2.718–4.075; P < 0.001), menopause at 45 years old or below (OR, 1.602; 95% CI, 1.039–2.471; P = 0.020), parental hip fracture (OR, 1.884; 95% CI, 1.383–2.567; P < 0.001), history of osteoporosis related low energy fracture(s) after menopause (OR, 5.567; 95% CI, 3.334–9.297; P < 0.001), and low BMI (OR, 3.332; 95% CI, 1.890–5.873; P < 0.001) remained significantly associated with the need for treatment.
Among the 916 women in whom osteoporosis treatment was indicated, 619 (67.6%) refused treatment because they were concerned about the side effects of drugs, worried about interaction with existing medications, and reluctant to take more medication. For the 297 women who agreed to start treatment, 34 requested referrals to out-patient medical clinics in public hospitals because of financial concerns and the remaining 263 patients started treatment in the Association on a self-finance basis. Most of them (227) chose generic oral alendronate, 31 chose branded raloxifene, and 5 chose denosumab. Treatment acceptance was significantly higher among those with osteoporosis (50%) than those with osteopenia with high fracture risk (17.2%) (P < 0.001). Pearson correlation analysis found significant correlations between treatment acceptance and history of osteoporosis related low-energy fracture(s) after menopause (r = 0.071, P = 0.032), low BMI (r = 0.069, P = 0.038), and having medical disease(s) that cause bone loss (r = 0.075, P = 0.023), but not with age, age at menopause, duration of menopause, parental hip fracture, ever fell in the past 2 years, and use of medication(s) that cause bone loss. After stepwise logistic regression analysis, only history of osteoporosis related low-energy fracture(s) after menopause (OR, 1.602; 95% CI, 1.039–2.471; P = 0.016), and diagnosis of osteoporosis (OR, 2.904; 95% CI, 2.338–3.606; P < 0.001) remained significant in predicting treatment acceptance.
Treatment continuation among the 263 women who agreed to start treatment is listed in Table 2. Treatment continuation was significantly higher in the osteoporosis group than the high-risk osteopenic group at both 6th month and 12th month (P = 0.037 at 6th month and P = 0.007 at 12th month). Drug continuation at 12th month was not associated with age, menopause age, duration of menopause, history of osteoporosis related low-energy fracture(s) after menopause, parental hip fracture, history of fall in the past 2 years, having medical disease(s) that cause bone loss, and use of drug(s) that cause bone loss and low BMI. Among the 166 women who attended the 6th month follow-up, 11 stopped drug treatment. Among these 166 women, 67 (40.4%) reported daily intakes of 800 to less than 1200 mg calcium and 40 (24.1%) achieved the daily target of 1200 mg calcium. At 12th month, 157 women returned for follow-up but 19 stopped drug treatment. The proportion who achieved daily calcium intake of 800 mg to < 1200 mg and 1200 mg increased to 73 (46.5%) and 44 (28.0%), respectively.
Table 2.
Treatment continuation patient numbers among those who agreed to start treatment.
| Drug chosen | Bone status | Start | 6th month | 12th month |
|---|---|---|---|---|
| Raloxifene | Osteoporosis | 31 | 16 (51.6%) | 14 (45.2%) |
| Alendronate | Osteoporosis | 155 | 99 (63.9%) | 91 (58.7%) |
| Alendronate | Osteopenia, high risk | 72 | 35 (48.6%) | 28 (38.9%) |
| Denosumab | Osteoporosis | 5 | 5 (100%) | 5 (100%) |
4. Discussion
According to a local epidemiological study published in 1999, 45.8% of women aged 60–69 years old had osteoporosis of the spine and 22.9% had osteoporosis of the neck of femur. The prevalence of osteoporosis increased to 55.6% at the spine and 71.1% at the neck of femur in those aged 80 and above [11]. In another local study published in 2015, the prevalence of osteoporosis and osteopenia among 6099 women aged 50–89 years old was 24.9% and 51.7%, respectively [12]. In this study, the prevalence of osteoporosis and osteopenia was 23.7% and 60.4%, respectively. It is difficult to conclude whether women in this cohort had better bones than those in the previous decades because the studies were conducted in different centers using different DXA machines and reference curves.
Ageing is a strong independent risk factor for osteoporosis [13,14]. In this cohort, the prevalence of osteoporosis in those aged 80 years and above doubled that in those aged 65–69 years old. We also found low BMI and history of fracture being significant predictors for osteoporosis, which was in accordance with previous studies [15,16]. Other published studies demonstrated the protective effect of exercise on osteoporosis [16,17]. Over 90% of our participants exercised daily but we could not detect any significant association with osteoporosis. The plausible explanation was that majority of our cohort only strolled and stretched, which was too gentle to cause any effect on the bones.
Osteoporosis only partially accounts for fractures [18]. The majority of fractures occur in patients with osteopenia rather than osteoporosis, because there are more individuals with osteopenia in the population [8]. FRAX® is a computer-based algorithm that calculates the 10-year probability of a major fracture risk and hip fracture risk in those with osteopenia. Its use of clinical risk factors in conjunction with femoral neck BMD and age improves the sensitivity of fracture prediction. Hong Kong specific FRAX® is available and the Osteoporosis Society of Hong Kong [10] suggested local clinicians to adopt the National Osteoporosis Foundation (NOF) recommended treatment threshold [8] of ≥ 20% major osteoporotic fracture risk or ≥ 3% hip fracture risk. Overtreatment may exist in this cohort as the proportion of women with hip fracture risk of ≥ 3% was disproportionately high. The NOF recommended treatment threshold was based on the cost-effectiveness analysis in the United States. Many countries had their own country-specific treatment threshold. One paper from Taiwan proposed the threshold for major osteoporotic fractures to be 15% for women and 12.5% for men; and that for hip fracture to be 7% for women and 6% for men [19]. The cut-off proposed by academics from Japan was 10% for major osteoporotic fractures and 5% for hip fractures [20] and their national guideline adopted 15% for major osteoporotic fracture as the cut-off [21]. Many countries also adopted a general fixed intervention threshold [22], but each country or region should develop their own threshold.
Ensuring treatment acceptance and adherence has been the major challenge in osteoporosis management. In the United States, the use of bisphosphonates in postmenopausal women had decreased by 50% from 2008 to 2012, the so-called “crisis in osteoporosis” [23]. Similar decline in the prescription of osteoporosis medications was observed in the United Kingdom [24]. Poor acceptance and adherence had been attributed to the bad reputation of bisphosphonates and denosumab in causing atypical femoral fractures and osteonecrosis of the jaw, drug costs, patients’ underestimation of their fracture risk and their concerns about drug safety [[25], [26], [27]]. A previous publication from our center on a younger cohort of postmenopausal women with an average age of 58.2 years old showed that 57.1% of osteoporotic patients accepted treatment, with the major reason for treatment refusal being fear of side effects [28]. In this cohort of older women, concern with serious side effects was still a major reason for refusal and the overall treatment refusal rate was higher than the younger cohort in the same centre. Another significant predictor for refusal found in this study was presence of medical disease(s) that cause bone loss such as diabetes mellitus, thyroid disease, rheumatoid arthritis, and inflammatory bowel diseases. These women also needed regular medications for their pre-existing disease(s) and they were concerned about drug interactions with existing medications and they were reluctant to take more medications. Besides, some women believed that fracture was avoidable by being more careful while others believed falls and fractures were inevitable in old age. Although drug cost had dropped substantially with the introduction of generic alendronate and the small absolute risk of atypical femoral fracture and osteonecrosis of the jaw had been emphasised during counselling, it was still difficult to persuade older women to accept treatment. It was apparent that they were overwhelmed by the rare side effects of osteoporosis drugs but ignored the threat of fragility fractures.
Achieving adequate calcium intake is also a major challenge in Asia. NOF recommends 1200 mg calcium for women aged 50 and beyond [8]. Most Asian countries were far below this recommendation, with daily calcium intakes between 300 and 500 mg [29]. The poor dietary calcium intake may be due to lactose intolerance that had been reported in 50–100% of Asia populations [30]. The International Osteoporosis Foundation calcium calculator, available at https://www.osteoporosis.foundation/educational-hub/topic/calcium-calculator, is a convenient tool but less adaptable to Asian diet. Furthermore, elderly women who do not know how to use computers or read English would not be able to use it. Therefore, we provided a calcium food chart featuring local products to each participant to help them select calcium-rich food. Those who were unable to consume 1200 mg calcium daily from food because of dietary restrictions or lactose intolerance were encouraged to use calcium supplements. In this cohort, almost half of the participants took less than 600 mg calcium daily on their first visit. After education, almost two-thirds of those who returned for follow-up at 6th month could consume more than 800 mg calcium daily and the proportion further increased to almost three-quarters by 1 year.
The strength of this study was the reasonable sample size and random recruitment that allowed results to be generalized. The main limitation of this study was the selection bias because it was not based on a community cohort. Those who had not read the news coverage on the program announcement, as well as those who had read the news coverage but were not interested to participate, were left out. Second, there could be recall bias, as we depended on our participants to report history of fracture, age of menopause and parental history of hip fracture, and thus their responses could not be verified. Third, this was a cross-sectional study, and we lacked long-term fracture data in those who opted for treatment versus those who refused treatment. Lastly, the monitoring period was not long enough to provide fracture data to compare the different outcomes in women who adhered to treatment and those who did not.
5. Conclusions
Almost one in four elderly women aged 65 and above had osteoporosis. The proportion of elderly women aged 80 years and above with osteoporosis doubled that in those aged 65–69 years. The workload after osteoporosis screening for elderly women is substantial, with around half of them requiring treatment. However, only one-third of these women agreed to start treatment. It is difficult to convince patients to accept treatment for a silent disease, especially when they are preoccupied with negative perceptions on the drugs and they are on a number of drugs for pre-existing diseases. Women who had prior osteoporosis related fractures after menopause were more likely to accept treatment. Those who had osteoporosis rather than high-risk osteopenia were more likely to accept and continue with treatment.
CRediT author statement
The sole author is responsible for the conceptualization, study design, implementation, formal analysis, data validation, and preparation of the manuscript.
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Acknowledgments
The Community Chest of Hong Kong Time Limited Project sponsored the DXA test fee for all participants. The funder had no role in study design, data collection/analysis/interpretation or manuscript preparation. ORCID Sue Seen-Tsing Lo: 0000-0003-1259-1680.
Footnotes
Peer review under responsibility of The Korean Society of Osteoporosis.
References
- 1.Bliuc D., Nguyen N.D., Alarkawi D., Nguyen T.V., Eisman J.A., Center J.R. Accelerated bone loss and increased post-fracture mortality in elderly women and men. Osteoporos Int. 2015;26:1331–1339. doi: 10.1007/s00198-014-3014-9. [DOI] [PubMed] [Google Scholar]
- 2.Ammann P., Rizzoli R. Bone strength and its determinants. Osteoporos Int. 2003;14(Suppl 3):S13–S18. doi: 10.1007/s00198-002-1345-4. [DOI] [PubMed] [Google Scholar]
- 3.Zeng Q., Li N., Wang Q., Feng J., Sun D., Zhang Q. The prevalence of osteoporosis in China, a nationwide, multicenter DXA survey. J Bone Miner Res. 2019;34:1789–1797. doi: 10.1002/jbmr.3757. [DOI] [PubMed] [Google Scholar]
- 4.Sarafrazi N., Wambogo E.A., Shepherd J.A. Osteoporosis or low bone mass in older adults: United States, 2017–2018. CDC national center for health statistics data brief No. 405. March 2021. https://www.cdc.gov/nchs/data/databriefs/db405-H.pdf [PubMed]
- 5.Government of Canada Osteoporosis and related fractures in Canada: report from the Canadian chronic disease surveillance system. 2020. https://www.canada.ca/en/public-health/services/publications/diseases-conditions/osteoporosis-related-fractures-2020.html#a2.1.1
- 6.Census and Statistics Department HKSAR government. Hong Kong population projections 2019-2069. https://www.censtatd.gov.hk/en/data/stat_report/product/B1120015/att/B1120015082020XXXXB0100.pdf
- 7.Cheung C.L., Ang S.B., Chadha M., Chow E.S.L., Chung Y.S., Hew F.L. An updated hip fracture projection in Asia: the Asian Federation of Osteoporosis Societies study. Osteoporos Sarcopenia. 2018;4:16–21. doi: 10.1016/j.afos.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cosman F., de Beur S.J., LeBoff M.S., Lewiecki E.M., Tanner B., Randall S. Clinician's guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25:2359–2381. doi: 10.1007/s00198-014-2794-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization (WHO) Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. WHO Tech Rep Ser. 1994;843 [PubMed] [Google Scholar]
- 10.Ip TP and the Osteoporosis Society of Hong Kong (OSHK) Task Group OSHK guideline for clinical management of postmenopausal osteoporosis in Hong Kong. Hong Kong Med J. 2013;19(Suppl 2):1–40. [PubMed] [Google Scholar]
- 11.Ho S.C., Lau E.M.C., Woo J., Sham A., Chan K.M., Lee S. The prevalence of osteoporosis in the Hong Kong Chinese female population. Maturitas. 1999;32:171–178. doi: 10.1016/s0378-5122(99)00026-2. [DOI] [PubMed] [Google Scholar]
- 12.Lau E.M., Chung H.L., Ha P.C., Tang H., Lam D. Bone mineral density, anthropometric indices, and the prevalence of osteoporosis in northern (Beijing) Chinese and southern (Hong Kong) Chinese women–the largest comparative study to date. J Clin Densitom. 2015;18:519–524. doi: 10.1016/j.jocd.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 13.Koh L.K., Sedrine W.B., Torralba T.P., Kung A., Fujiwara S. Chan SP et al and the Osteoporosis Self-Assessment Tool for Asians (OSTA) Research Group. A simple tool to identify Asian women at increased risk of osteoporosis. Osteoporos Int. 2001;12:699–705. doi: 10.1007/s001980170070. [DOI] [PubMed] [Google Scholar]
- 14.Huo Y.R., Suriyaarachchi P., Gomez F., Curcio C.L., Boersma D., Muir S.W. Phenotype of osteosarcopenia in older individuals with a history of falling. J Am Med Dir Assoc. 2015;16:290–295. doi: 10.1016/j.jamda.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 15.Orwoll E.S., Bauer D.C., Vogt T.M., Fox K.M. Axial bone mass in older women. Study of osteoporotic fractures research group. Ann Intern Med. 1996;124:187–196. doi: 10.7326/0003-4819-124-2-199601150-00001. [DOI] [PubMed] [Google Scholar]
- 16.Shin C.S., Choi H.J., Kim M.J., Kim J.T., Yu S.H., Koo B.K. Prevalence and risk factors of osteoporosis in Korea: a community-based cohort study with lumbar spine and hip bone mineral density. Bone. 2010;47:378–387. doi: 10.1016/j.bone.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 17.Lunt M., Masaryk P., Scheidt-Nave C., Nijs J., Poor G., Pols H. The effects of lifestyle, dietary dairy intake and diabetes on bone density and vertebral deformity prevalence: the EVOS study. Osteoporos Int. 2001;12:688–698. doi: 10.1007/s001980170069. [DOI] [PubMed] [Google Scholar]
- 18.Ito K., Leslie W.D. Cost-effectiveness of fracture prevention in rural women with limited access to dual-energy X-ray absorptiometry. Osteoporos Int. 2015;26:2111–2119. doi: 10.1007/s00198-015-3107-0. [DOI] [PubMed] [Google Scholar]
- 19.Chan D.C., McCloskey E.V., Chang C.B., Lin K.P., Lim L.C., Tsai K.S. Establishing and evaluating FRAX ® probability thresholds in Taiwan. J Formos Med Assoc. 2017;116:161–168. doi: 10.1016/j.jfma.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 20.Nakatoah S., Takemaru Y. Application of the fracture risk assessment tool (FRAX®) and determination of suitable cut-off values during primary screening I specific health check-ups in Japan. J Bone Miner Metabol. 2013;31:674–680. doi: 10.1007/s00774-013-0457-6. [DOI] [PubMed] [Google Scholar]
- 21.Orimo H., Nakamura T., Hosoi T., Iki M., Uenishi K., Endo N. Japanese 2011 guidelines for prevention and treatment of osteoporosis--executive summary. Arch Osteoporos. 2012;7:3–20. doi: 10.1007/s11657-012-0109-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kanis J.A., Harvey N.C., Cooper C., Johansson H., Odén A., McCloskey E.V. Advisory board of the national osteoporosis guideline group. A systematic review of intervention thresholds based on FRAX: a report prepared for the national osteoporosis guideline group and the international osteoporosis foundation. Arch Osteoporos. 2016;11:25. doi: 10.1007/s11657-016-0278-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khosla S., Shane E. A crisis in the treatment of osteoporosis. J Bone Miner Res. 2016;31:1485–1487. doi: 10.1002/jbmr.2888. [DOI] [PubMed] [Google Scholar]
- 24.van der Velde R.Y., Wyers C.E., Teesselink E., Geusens P.P.M.M., van den Bergh JPW, de Vries F. Trends in oral anti-osteoporosis drug prescription in the United Kingdom between 1990 and 2012: variation by age, sex, geographic location and ethnicity. Bone. 2017;94:50–55. doi: 10.1016/j.bone.2016.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jha S., Wang Z., Laucis N., Bhattacharyya T. Trends in media reports, oral bisphosphonate prescriptions, and hip fractures 1996-2012: an ecological analysis. J Bone Miner Res. 2015;30:2179–2187. doi: 10.1002/jbmr.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fraser L.A., Ionnidis G., Adachi J.D., Pickard L., Kaiser S.M., Prior J., the CaMos Research Group Fragility fractures and the osteoporosis care gap in women: the Canadian Multicentre Osteoporosis Study. Osteoporos Int. 2011;22:789–796. doi: 10.1007/s00198-010-1359-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lindsay B.R., Olufade T., Bauer J., Babrowicz J., Hahn R. Patient-reported barriers to osteoporosis therapy. Arch Osteoporos. 2016;11:1–8. doi: 10.1007/s11657-016-0272-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lo S.S. Bone health status of postmenopausal Chinese women. Hong Kong Med J. 2015;21:536–541. doi: 10.12809/hkmj154527. [DOI] [PubMed] [Google Scholar]
- 29.International Osteoporosis Foundation The Asian Audit. Epidemiology, costs and burden of osteoporosis in Asia 2009. September 2009. http://www.iofbonehealth.org/sites/default/files/PDFs/Audit%20Asia/Asian_regional_audit_2009.pdf
- 30.Vesa T.H., Marteau P., Korpela R. Lactose intolerance. J Am Coll Nutr. 2000;19(2 suppl) doi: 10.1080/07315724.2000.10718086. 165S-75S. [DOI] [PubMed] [Google Scholar]