Abstract
Objectives
To investigate effects of romosozumab treatment on disease activity and bone mineral density (BMD) in patients with rheumatoid arthritis (RA) and severe osteoporosis in comparison with effects of denosumab treatment.
Methods
A total of 50 women were enrolled in this study. The subjects were randomized equally into 2 groups: the romosozumab group or the denosumab group. Disease activity score in 28 joints (DAS28)-erythrocyte sedimentation rate (ESR) and BMD at lumbar spine were evaluated.
Results
The percent changes (Δ) in the BMD values at 3 and 6 months for the lumbar spine were as follows: romosozumab; 4.9% and 5.2%, denosumab: 2.3% and 3.2%. The ΔBMD for the lumbar spine at 3 months was significantly higher in the romosozumab group than in the denosumab group (P = 0.044). The DAS28-ESR at baseline, 3 and 6 months in the romosozumab group were 2.88, 2.60 (P = 0.427) and 2.58 (P = 0.588), respectively. The change from baseline in DAS28-ESR did not differ significantly between these 2 groups at any time point.
Conclusions
The present study revealed that romosozumab treatment is more effective than denosumab treatment in increasing BMD of the lumbar spine at 3 months. Furthermore, the present study suggested that romosozumab treatment has no effects on the disease activity of RA in patients with RA and severe osteoporosis for 6 months.
Keywords: Bone mineral density, Disease activity, Osteoporosis, Rheumatoid arthritis, Romosozumab
1. Introduction
Rheumatoid arthritis (RA) is an inflammatory disease in which osteoporosis is a common comorbidity [1,2]. It is a risk factor for fragility fracture due to decreased bone mineral density (BMD), the use of glucocorticoids and the inflammatory disease itself [1,[3], [4], [5]].
Romosozumab is a humanized monoclonal antibody that binds with and inhibits sclerostin; it has a dual effect of stimulating bone formation and decreasing bone resorption [6,7]. Denosumab is a fully human monoclonal antibody that specifically and avidly binds to human RANKL, leading to diminished survival and activation of osteoclasts for bone resorption inhibition [8,9]. Romosozumab and denosumab are currently used in the treatment of postmenopausal osteoporosis [7,[10], [11], [12], [13]]. Romosozumab treatment significantly increased bone volumes of vertebral body, knee, and ankle in mice model of RA compared with the control mice [14]. However, efficacy of romosozumab treatment in patients with RA is unclear in BMD. In contrast, previous studies indicated that denosumab treatment in patients with RA was effective for increasing BMD at the lumbar spine and hip [15,16].
The determination of the levels of cytokine, tumor necrosis factor (TNF)-α, and interleukin-6 in the peripheral blood and synovium is generally conducted for patients with RA. Human TNF-α transgenic mice (hTNFtg mice) with constitutive overexpression of human TNF-α leads to the development of an RA-like destructive arthritis, for which anti-sclerostin antibody treatment may lead to the worsening of clinical RA outcomes under chronic TNF-α dependent inflammatory conditions [17]. Therefore, romosozumab treatment may exacerbate the disease activity in RA. Denosumab treatment in patients with RA had no effect on RA disease activity [15,16].
Hence, the effect of romosozumab treatment on the BMD and disease activity in patients with RA is unclear. The aim of the present study is to clarify the effects of romosozumab treatment on the BMD and disease activity in patients with RA and severe osteoporosis when compared with the effects of denosumab treatment during early phase.
2. Methods
2.1. Study design
This study investigated the clinical course and background variables of patients with RA who fulfilled the American College of Rheumatology (ACR) classification criteria (1987) and the ACR/European League against Rheumatism criteria [18,19], as well as had a dual-energy X-ray absorptiometry (DXA) T-score of ≤ −2.5 at the lumbar spine, total hip, or femoral neck along with a previous fragility fracture, or T-score of ≤ −3.3 at the lumbar spine, or vertebral fractures ≥ 2. This study enrolled 50 postmenopausal women with RA from May 2019 to March 2020 who were randomized equally by the clinical trial center of our institution into 2 groups: romosozumab group (210 mg dose/month via subcutaneous injection [SC]) or denosumab group (60 mg dose/6 months via SC). All patients were instructed to take 0.5–0.75 μg of eldecalcitol, an active vitamin D3 analog, daily. Patients who had hypocalcemia, cardiovascular disease, and severe chronic kidney disease were excluded from study. This study was an open-label, randomized, pilot study. This study was approved by the ethical review board of this institution (TGE1220-064) and followed the Declaration of Helsinki. All patients agreed to participate in the study and provided written informed consent.
2.2. Study assessments
The clinical assessments recorded C-reactive protein (CRP), and the disease activity score in 28 joints (DAS28)-erythrocyte sedimentation rate (ESR) at the baseline, and at 3 and 6 months. The BMDs of the lumbar spine (L1-L4), total hip and femoral neck were measured by DXA (Prodigy; GE Healthcare, Madison, WI, USA) at the baseline, and at 3 and 6 months. The bone turnover makers of procollagen type I N-terminal propeptide (PINP) and tartrate-resistant acid phosphatase-5b (TRACP-5b) were recorded at baseline and at 3 and 6 months.
The primary endpoint included a comparison between romosozumab and denosumab groups in the change from the baseline (Δ) BMD at the lumbar spine, total hip, and femoral neck at 3 and 6 months. The secondary endpoint included comparisons between the romosozumab and denosumab groups for ΔDAS28-ESR in the romosozumab group at 3 and 6 months. The DAS28-ESR is a standard measurement of disease activity in RA [20].
2.3. Statistical analysis
The data analyses were performed using an observed case analysis. Comparisons between the romosozumab and denosumab groups in term of the patients' age, body weight, biological disease-modifying anti-rheumatic drugs use, methotrexate use, glucocorticoid use, rheumatoid factor positivity, CRP, DAS28-ESR, Health Assessment Questionnaire Disability Index, the estimated glomerular filtration rate calculated by creatinine, serum calcium level, prior osteoporosis treatment, the number of vertebral fractures, T-score, and turnover markers at baseline, and ΔBMD, and ΔDAS28-ESR level at 3 and 6 months were performed using the Mann-Whitney U test and Fisher's exact test, when appropriate. The ΔBMD at 3 and 6 months, and ΔDAS28-ESR level were analyzed by paired t test. A P-value of < 0.05 denoted statistical significance.
3. Results
3.1. Baseline patient characteristics
The baseline demographics and clinical characteristics in the romosozumab and denosumab groups are summarized in Table 1. The T-score in the femoral neck of the romosozumab group was significantly lower than that of the denosumab group.
Table 1.
Comparison of patient characteristics between the romosozumab and denosumab groups at baseline.
| Variables; median (Q1, Q3) | Romosozumab group (n = 25) | Denosumab group (n = 25) | P-value |
|---|---|---|---|
| Age, yr | 74 (70, 80) | 73 (68, 77) | 0.497 |
| Disease duration, yr | 10 (5, 16) | 11 (6, 17) | 0.606 |
| Height, cm | 151.5 (147, 156) | 152.0 (146.5, 154) | 0.711 |
| Body weight, kg | 47.9 (43, 54) | 51.4 (45, 54.6) | 0.593 |
| bDMARD use, n (%) | 15 (60) | 14 (56) | 1.000 |
| MTX use, n (%) | 16 (64) | 13 (52) | 0.567 |
| Glucocorticoid use, n (%) | 5 (20) | 6 (24) | 1.000 |
| RF positive, n (%) | 15 (60) | 18 (72) | 0.551 |
| CRP, mg/dL | 0.16 (0.03, 0.69) | 0.06 (0.04, 0.15) | 0.122 |
| DAS28-ESR | 2.68 (2.05, 3.52) | 2.75 (2.22, 3.5) | 0.769 |
| HAQ-DI | 0.25 (0, 1) | 0.375 (0, 0.5) | 0.902 |
| Cr-eGFR, mL/min/1.73m2 | 68.3 (57, 75.4) | 61.3 (20.275, 77.55) | 0.503 |
| Serum calcium level, mg/dL | 9.4 (9.2, 9.6) | 9.3 (9, 9.55) | 0.084 |
| Prior osteoporosis treatment, n (%) | 11 (44) | 13 (52) | 0.572 |
| Bisphosphonates, n (%) | 8 (32) | 9 (36) | |
| SERM, n (%) | 1 (4) | 1 (4) | |
| Active vitamin D3 analog, n (%) | 0 (0) | 3 (12) | |
| Oral calcium, n (%) | 2 (8) | 0 (0) | |
| Prevalent vertebral fractures, n (%) | 14 (56) | 14 (56) | 1.000 |
| T-score in lumbar spine | −2.1 (−2.9, −1.1) | −1.8 (−2.5, −1.1) | 0.560 |
| T-score in total hip | −1.9 (−2.9, −1.1) | −2.2 (−2.3, −1.8) | 0.814 |
| T-score in femoral neck | −3 (−3.4, −2.5) | −2.7 (−2.9, −2.3) | 0.047 |
| P1NP, ng/mL | 41.4 (25.7, 57) | 35.5 (21.1, 46.55) | 0.275 |
| TRACP-5b, mU/dL | 458 (302, 528) | 368 (263, 451) | 0.195 |
Q1, 25th percentile; Q3, 75th percentile; bDMARD, biological disease-modifying antirheumatic drug; MTX, methotrexate; RF, rheumatoid factor; CRP, C-reactive protein; DAS, disease activity score; ESR, erythrocyte sedimentation rate; HAQ-DI, Health Assessment Questionnaire Disability Index; Cr-eGFR, estimated glomerular filtration rate calculated by creatinine; SERM, selective estrogen receptor modulator; P1NP, N-terminal propeptide of type I procollagen; TRACP-5b, tartrate-resistant acid phosphatase-5b.
3.2. Comparison between the romosozumab and denosumab groups for ΔBMD at lumbar spine, total hip, and femoral neck
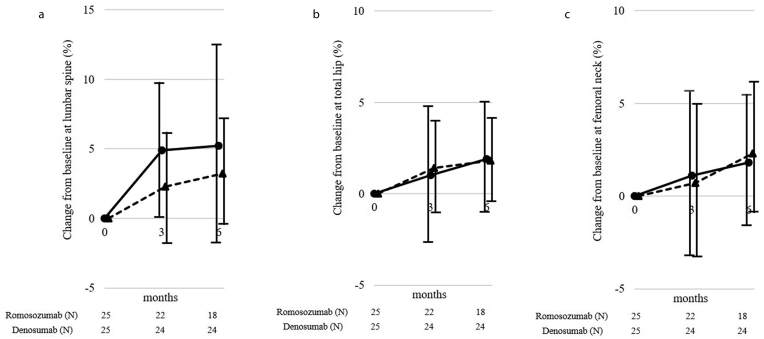
The ΔBMD values at 3 and 6 months in the romosozumab group were significantly increased by 4.9% ± 4.7% (P < 0.001) and 5.2% ± 7.3% (P = 0.013) for the lumbar spine; 1.0% ± 3.7% (P = 0.294) and 1.9% ± 3.2% (P = 0.038) for the total hip; and 1.1% ± 4.6% (P = 0.335), and 1.8% ± 3.6% (P = 0.226) for the femoral neck from the baseline, respectively. The ΔBMD values at 3 and 6 months in the denosumab group were significantly increased by 2.3% ± 4.3% (P = 0.066) and 3.2% ± 3.6% (P = 0.014) for the lumbar spine; 1.4% ± 2.6% (P = 0.072) and 1.8% ± 2.3% (P = 0.007) for the total hip; and 0.7% ± 4.3% (P = 0.728) and 2.3% ± 3.3% (P = 0.046) for the femoral neck from baseline, respectively (Fig. 1). The ΔBMD for the lumbar spine at 3 months in the romosozumab group was significantly higher than that in the denosumab group (P = 0.044). The ΔBMD for total hip and femoral neck did not differ significantly between these 2 groups at any time point.
Fig. 1.
The percent changes from the baseline in the bone mineral densities of the (a) lumbar spine, (b) total hip, and (c) femoral neck regions. Circle and solid line, romosozumab group; triangle and dotted line, denosumab group.
3.3. Changes in bone turnover markers levels
The changes from baseline in the romosozumab group were 116.5% ± 229.1% at 3 months and 106.9% ± 245.8% at 6 months for P1NP; and −30.7% ± 45.2% at 3 months and −21.5% ± 56.1% at 6 months for TRACP-5b, respectively. The changes from baseline in the denosumab group were −47.4% ± 26.9% at 3 months and −43.4% ± 29.7% at 6 months for P1NP; and −63.6% ± 25.8% at 3 months and −49.4% ± 27.8% at 6 months for TRACP-5b, respectively.
3.4. Clinical fracture
The clinical fracture was vertebral fracture in 1 patient in the denosumab group. In the romosozumab group, there was no clinical fracture.
3.5. Comparison between the romosozumab and denosumab groups for disease activity
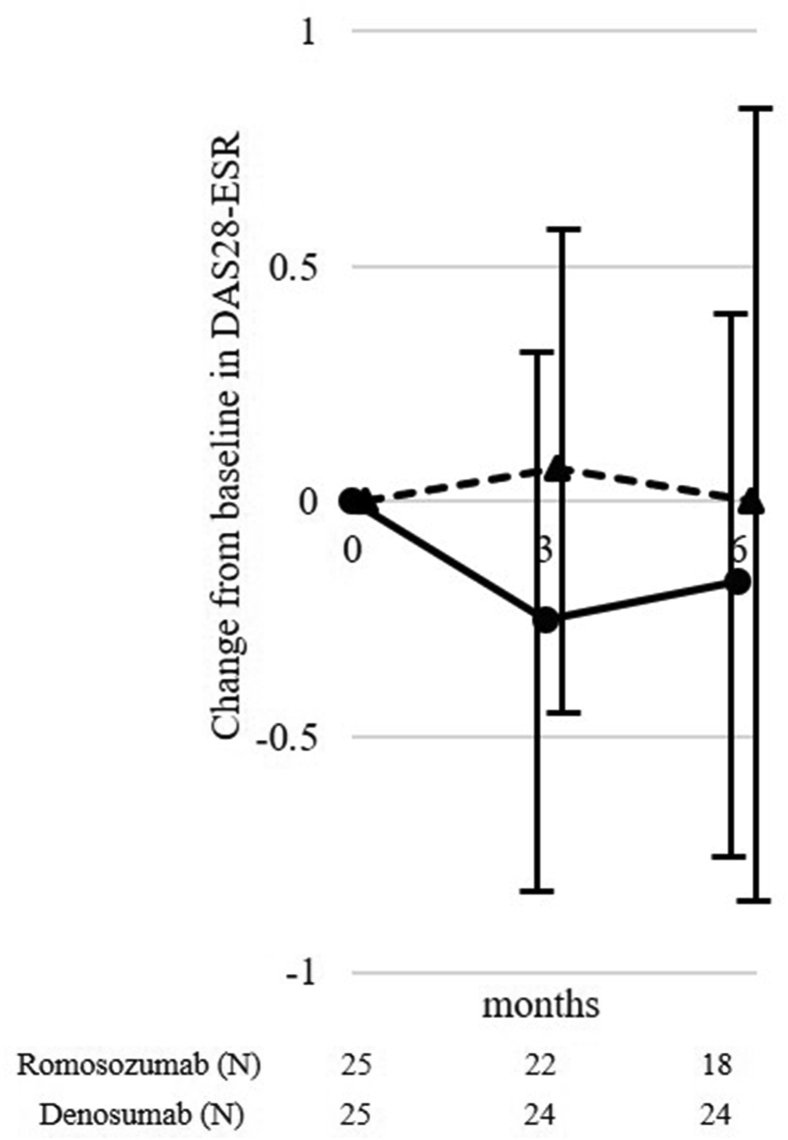
The ΔDAS28-ESR at 3 and 6 months in the romosozumab group were −0.25 ± 0.58 and −0.17 ± 0.58 and that in the denosumab group were 0.07 ± 0.53 and 0.00 ± 0.78, respectively (Fig. 2). The ΔDAS28-ESR did not differ significantly between these 2 groups at any time point.
Fig. 2.
Changes in disease activity score in 28 joints (DAS28)-erythrocyte sedimentation rate (ESR). Circle and solid line, romosozumab group; triangle and dotted line, denosumab group.
3.6. Safety
The retention rates in the romosozumab and denosumab groups were 72.0% and 96.0% at 6 months. In the romosozumab group, adverse events were joint pain, rash, nausea, headache, hypertension and itching in 2, 2, 1, 1, 1, and 1 patient, respectively. In the denosumab group, adverse events were injection site reaction and nausea in 1 and 1 patient, respectively.
4. Discussion
The present study revealed that romosozumab treatment is more effective than denosumab treatment in patients with RA for increasing BMD of the lumbar spine during the early phase. Romosozumab and denosumab are currently used in the treatment of postmenopausal osteoporosis. The romosozumab treatment of postmenopausal osteoporosis increased 9.7%–13.1% at the lumbar spine, 2.3%–6.8% at the total hip, and 2.1%–2.3% at the femoral neck at 6 months [[10], [11], [12]]. In the present study, the ΔBMD was 5.2% for the lumbar spine, 1.9% for the total hip; and 1.8% for the femoral neck at 6 months in the romosozumab group. We thus speculated that the use of a steroid could be one of the reasons for the difference in these results. In patients with early RA, treatment with methyl-prednisolone resulted in a decrease in the sclerostin level [21]. Similarly, glucocorticoid suppressed the sclerostin level in human mesenchymal cells lines. Moreover, patients with RA who were administrated with glucocorticoid showed lower serum levels of sclerostin when compared to the healthy controls [22]. In comparison between romosozumab and denosumab groups, the romosozumab group showed significantly increased BMD in the lumbar spine at 3 months. To date, no studies have comparatively examined the effects on BMD by romosozumab and denosumab treatments. In a previous comparative study on romosozumab and alendronate in postmenopausal women, increased BMD was recorded in the lumbar spine at 12 months of romosozumab and alendronate treatments (13.7%–14.0% and 5.0%–5.8%, respectively) [13,23]. In a previous comparative study between denosumab and alendronate treatment effects in postmenopausal women, increased BMD in the lumbar spine with denosumab and alendronate treatments were 5.3% and 4.2% at 12 months, and 9.1% and 7.5% at 24 months, respectively [24,25]. The changes of bone turnover markers levels in this study were similar to the previous reports [11,12,23]. The P1NP increased rapidly and the TRACP-5b decreased continuously in the romosozumab group. This mechanism is involved in early increasing BMD. Based on these results, treatment with romosozumab showed more effectiveness in increasing the BMD in the lumbar spine than treatment with denosumab. Hence, we believe that our present results revealed this speculation in patients with RA and severe osteoporosis.
The present study revealed no effect of romosozumab treatment on the disease activity of patients with RA. Moreover, the change of DAS28-ESR did not differ significantly between the romosozumab and denosumab groups at 3 and 6 months. Wehmeyer et al [17] reported that in hTNFtg mice, antibody-mediated inhibition of sclerostin leads to an acceleration of RA disease. In addition, Singh et al [26] reported that the serum sclerostin level correlated with the disease activity, CRP, and ESR in patients with RA. Based on our results, the romosozumab treatment in patients with RA did not adversely affect the disease activity. In the present study, several patients had low disease activity. We thus believe that romosozumab treatment in patients with RA has no effect on the disease activity, although this result may be derived because of the low serum sclerostin level or TNF-α level.
This study has some limitations. First, the sample size was small and the study duration was short due to a pilot study. Second, we did not evaluate the 10-year probability of fracture of hip and major osteoporotic fractures using FRAX. Third, in this study, the patients showed relatively suppressed disease activity with treatment. Therefore, it remains unclear if similar results as in this study can be obtained in patients with high disease activity. Finally, we could not evaluate the levels of serum sclerostin or TNF-α. Therefore, a prospective study would be necessary to determine the effect of romosozumab in larger samples sizes and with longer treatment period of treatment. We believe that the present study provides important insights for future researches.
5. Conclusions
The present study is the first study to evaluate the comparison between romosozumab and denosumab treatments in patients with RA and severe osteoporosis. The present study revealed that romosozumab treatment is more effective than denosumab treatment in increasing BMD of the lumbar spine, and romosozumab treatment has no effects on the disease activity of RA in patients with RA who have suppressed disease activity and severe osteoporosis in the early phase. Romosozumab treatment should be considered to increase the BMD in the lumbar spine. Consequently, a better understanding of romosozumab treatment for patients with RA and severe osteoporosis is important in the clinical practice.
CRediT author statement
Takeshi Mochizuki: Conceptualization, Investigation, Data Curation, Writing-Original draft.
Koichiro Yano: Formal analysis.
Katsunori Ikari: Conceptualization, Writing-Review & editing.
Ken Okazaki: Writing-Review & editing.
Conflicts of interest
Takeshi Mochizuki received honoraria for lectures from Astellas, Bristol-Myers, Chugai, Daiichi Sankyo, Eisai, Eli Lilly, Janssen, Mochida, Pfizer, and Tanabe-Mitsubishi. Koichiro Yano received honoraria for lectures from AbbVie, Astellas, Ayumi, Bristol-Meyers, Eisai, Hisamitsu, Mochida, and Takeda. Katsunori Ikari received honoraria for lectures from AbbVie, Astellas, Bristol-Myers, Chugai, Eisai, Eli Lilly, Janssen, Takeda, Tanabe-Mitsubishi, and UCB. Ken Okazaki has no conflicts of interest. The sponsors were not involved in the study design; collection, analysis, and interpretation of data; writing of the article; and/or decision to submit the results for publication.
Acknowledgments
ORCID Takeshi Mochizuki: 0000-0002-8316-8671. Koichiro Yano: 0000-0002-9514-2719. Katsunori Ikari: 0000-0001-9066-2005. Ken Okazaki: 0000-0003-1274-8406.
Footnotes
Peer review under responsibility of The Korean Society of Osteoporosis.
References
- 1.Zerbini C.A.F., Clark P., Mendez-Sanchez L., Pereira R.M.R., Messina O.D., Uña C.R. Biologic therapies and bone loss in rheumatoid arthritis. Osteoporos Int. 2017;28:429–446. doi: 10.1007/s00198-016-3769-2. [DOI] [PubMed] [Google Scholar]
- 2.Tanaka S., Tanaka Y., Ishiguro N., Yamanaka H., Takeuchi T. RANKL: a therapeutic target for bone destruction in rheumatoid arthritis. Mod Rheumatol. 2018;28:9–16. doi: 10.1080/14397595.2017.1369491. [DOI] [PubMed] [Google Scholar]
- 3.van Staa T.P., Geusens P., Bijlsma J.W., Leufkens H.G., Cooper C. Clinical assessment of the long-term risk of fracture in patients with rheumatoid arthritis. Arthritis Rheum. 2006;54:3104–3112. doi: 10.1002/art.22117. [DOI] [PubMed] [Google Scholar]
- 4.Arai K., Hanyu T., Sugitani H., Murai T., Fujisawa J., Nakazono K. Risk factors for vertebral fracture in menopausal or postmenopausal Japanese women with rheumatoid arthritis: a crosssectional and longitudinal study. J Bone Miner Metabol. 2006;24:118–124. doi: 10.1007/s00774-005-0657-9. [DOI] [PubMed] [Google Scholar]
- 5.Blavnsfeldt A.G., de Thurah A., Thomsen M.D., Tarp S., Langdahl B., Hauge E.M. The effect of glucocorticoids on bone mineral density in patients with rheumatoid arthritis: a systematic review and meta-analysis of randomized, controlled trials. Bone. 2018;114:172–180. doi: 10.1016/j.bone.2018.06.008. [DOI] [PubMed] [Google Scholar]
- 6.Padhi D., Jang G., Stouch B., Fang L., Posvar E. Single-dose, placebo controlled, randomized study of AMG 785, a sclerostin monoclonal antibody. J Bone Miner Res. 2011;26:19–26. doi: 10.1002/jbmr.173. [DOI] [PubMed] [Google Scholar]
- 7.McClung M.R., Grauer A., Boonen S., Bolognese M.A., Brown J.P., Diez-Perez A. Romosozumab in postmenopausal women with low bone mineral density. N Engl J Med. 2014;370:412–420. doi: 10.1056/NEJMoa1305224. [DOI] [PubMed] [Google Scholar]
- 8.Boyle W.J., Simonet W.S., Lacey D.L. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 9.Eghbali-Fatourechi G., Khosla S., Sanyal A., Boyle W.J., Lacey D.L., Riggs B.L. Role of RANK ligand in mediating increased bone resorption in early postmenopausal women. J Clin Invest. 2003;111:1221–1230. doi: 10.1172/JCI17215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cosman F., Crittenden D.B., Adachi J.D., Binkley N., Czerwinski E., Ferrari S. Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med. 2016;375:1532–1543. doi: 10.1056/NEJMoa1607948. [DOI] [PubMed] [Google Scholar]
- 11.Ishibashi H., Crittenden D.B., Miyauchi A., Libanati C., Maddox J., Fan M. Romosozumab increases bone mineral density in postmenopausal Japanese women with osteoporosis: a phase 2 study. Bone. 2017;103:209–215. doi: 10.1016/j.bone.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 12.Langdahl B.L., Libanati C., Crittenden D.B., Bolognese M.A., Brown J.P., Daizadeh N.S. Romosozumab (sclerostin monoclonal antibody) versus teriparatide in postmenopausal women with osteoporosis transitioning from oral bisphosphonate therapy: a randomised, open-label, phase 3 trial. Lancet. 2017;390:1585–1594. doi: 10.1016/S0140-6736(17)31613-6. [DOI] [PubMed] [Google Scholar]
- 13.Lau E.M.C., Dinavahi R., Woo Y.C., Wu C.H., Guan J., Maddox J. Romosozumab or alendronate for fracture prevention in East Asian patients: a subanalysis of the phase III, randomized ARCH study. Osteoporos Int. 2020;31:677–685. doi: 10.1007/s00198-020-05324-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marenzana M., Vugler A., Moore A., Robinson M. Effect of sclerostin-neutralising antibody on periarticular and systemic bone in a murine model of rheumatoid arthritis: a microCT study. Arthritis Res Ther. 2013;15:R125. doi: 10.1186/ar4305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takeuchi T., Tanaka Y., Ishiguro N., Yamanaka H., Yoneda T., Ohira T. Effect of denosumab on Japanese patients with rheumatoid arthritis: a dose-response study of AMG 162 (Denosumab) in patients with RheumatoId arthritis on methotrexate to Validate inhibitory effect on bone Erosion (DRIVE)-a 12-month, multicentre, randomised, double-blind, placebo-controlled, phase II clinical trial. Ann Rheum Dis. 2016;75:983–990. doi: 10.1136/annrheumdis-2015-208052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mochizuki T., Yano K., Ikari K., Hiroshima R., Kawakami K., Koenuma N. Effects of denosumab treatment on bone mineral density and joint destruction in patients with rheumatoid arthritis. J Bone Miner Metabol. 2018;36:431–438. doi: 10.1007/s00774-017-0848-1. [DOI] [PubMed] [Google Scholar]
- 17.Wehmeyer C., Frank S., Beckmann D., Bottcher M., Cromme C., Konig U. Sclerostin inhibition promotes TNF-dependent inflammatory joint destruction. Sci Transl Med. 2016;8:330ra35. doi: 10.1126/scitranslmed.aac4351. [DOI] [PubMed] [Google Scholar]
- 18.Arnett F.C., Edworthy S.M., Bloch D.A., McShane D.J., Fries J.F., Cooper N.S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;16:65–67. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 19.Aletaha D., Neogi T., Silman A.J., Funovits J., Felson D.T., Bingham C.O., 3rd Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62:2569–2581. doi: 10.1002/art.27584. 2010. [DOI] [PubMed] [Google Scholar]
- 20.Prevoo M.L., van ‘t Hof M.A., Kuper H.H., van Leeuwen M.A., van de Putte L.B., van Riel P.L. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38:44–48. doi: 10.1002/art.1780380107. [DOI] [PubMed] [Google Scholar]
- 21.Fassio A., Adami G., Giollo A., Viapiana O., Malavolta N., Saviola G. Acute Effects of glucocorticoid treatment, TNF alpha or IL-6R blockade on bone turnover markers and Wnt inhibitors in early rheumatoid arthritis: a pilot study. Calcif Tissue Int. 2020;106:371–377. doi: 10.1007/s00223-019-00649-3. [DOI] [PubMed] [Google Scholar]
- 22.Thiele S., Hannemann A., Winzer M., Baschant U., Weidner H., Nauck M. Regulation of sclerostin in glucocorticoid-induced osteoporosis (GIO) in mice and humans. Endocr Connect. 2019;8:923–934. doi: 10.1530/EC-19-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saag K.G., Petersen J., Brandi M.L., Karaplis A.C., Lorentzon M., Thomas T. Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med. 2017;377:1417–1427. doi: 10.1056/NEJMoa1708322. [DOI] [PubMed] [Google Scholar]
- 24.Brown J.P., Prince R.L., Deal C., Recker R.R., Kiel D.P., de Gregorio L.H. Comparison of the effect of denosumab and alendronate on BMD and biochemical markers of bone turnover in postmenopausal women with low bone mass: a randomized, blinded, phase 3 trial. J Bone Miner Res. 2009;24:153–161. doi: 10.1359/jbmr.0809010. [DOI] [PubMed] [Google Scholar]
- 25.Nakamura T., Matsumoto T., Sugimoto T., Hosoi T., Miki T., Gorai I. Clinical Trials Express: fracture risk reduction with denosumab in Japanese postmenopausal women and men with osteoporosis: denosumab fracture intervention randomized placebo controlled trial (DIRECT) J Clin Endocrinol Metab. 2014;99:2599–2607. doi: 10.1210/jc.2013-4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh A., Gupta M.K., Mishra S.P. Study of correlation of level of expression of Wnt signaling pathway inhibitors sclerostin and dickkopf-1 with disease activity and severity in rheumatoid arthritis patients. Drug Discov Ther. 2019;13:22–27. doi: 10.5582/ddt.2019.01011. [DOI] [PubMed] [Google Scholar]