Abstract
The aim of this study was to investigate the efficacy and safety of minocycline (MIN) and favipiravir combination therapy in patients with coronavirus disease 2019 (COVID-19) admitted to our hospital in Fukui Prefecture, Japan, in March and April of 2020. In this retrospective study, a favipiravir monotherapy group (Control group, n = 9) was compared with a combined favipiravir plus MIN therapy group (MIN group, n = 12). No severe cases were present. The primary comparative endpoints evaluated were duration of fever, duration of hospitalization, duration from treatment initiation to severe acute respiratory syndrome coronavirus 2 polymerase chain reaction (PCR)-negative results, and changes in cytokine and chemokine production. Median duration from start of treatment to negative PCR test was significantly shorter in the MIN group than in the Control group. Mean rates of cytokine and chemokine reduction were significantly greater for interleukin-6 and interleukin-8 in the MIN group. No difference in adverse event rates were seen between groups, and only minor adverse events were encountered. MIN has been reported to have not only broad antibacterial activity, but also antiviral and anti-inflammatory activity. The present results support the efficacy and safety of MIN plus favipiravir therapy for the treatment of COVID-19.
Keywords: Minocycline, Favipiravir, COVID-19, SARS-CoV-2, Inflammatory cytokines
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) caused a massive outbreak of coronavirus disease 2019 (COVID-19) in Wuhan, China, in December 2019 [1]. The first case in Japan was reported on January 15, 2020 [2]. Due to the subsequent spread of the infection, the outbreak in Fukui Prefecture from March to April 2020 (122 cases) was defined as the first wave [3].
To date, remdesivir and baricitinib have been approved for the treatment of COVID-19 in Japan [4]. However, these agents had not been approved in time for the first wave, and existing antiviral drugs including favipiravir (FPV), immunomodulators/immunosuppressants, and anticoagulants were therefore used.
Inflammatory cytokine storm has been noted to occur in COVID-19 patients, suggesting the possibility of acute respiratory distress syndrome and multiple-organ failure [5]. Minocycline (MIN) has been reported to exert anti-inflammatory, immunomodulatory, and antiviral effects in addition to its broad antibacterial activity. The possibility of antiviral activity against SARS-CoV-2 has also been suggested by several researchers [6,7]. The objective of the present study was to investigate the efficacy and safety of combination therapy comprising MIN and FPV.
In our institution, 30 patients with COVID-19 were admitted in the first wave. FPV (day 1, 1800 mg BID; after day 2, 800 mg BID, per os) was administered to 21 patients, with MIN (100 mg BID, per os) added at the discretion of the attending physician due to the possibility of complications from bacterial pneumonia. This study was a retrospective comparison of patients who received FPV alone (Control group, n = 9) and patients who received FPV plus MIN (MIN group, n = 12). Inflammatory cytokine and chemokine production was measured in patients for whom blood remnant samples were available on admission and before discharge (Control group, n = 5; MIN group, n = 6). We also compared duration of fever, duration of hospitalization, and the interval between start of treatment and negative results from PCR testing in the MIN and Control groups.
In terms of patient background, median age tended to be higher and duration from onset to start of treatment tended to be shorter in the Control group. No severe cases (requiring either admission to the intensive care unit or mechanical ventilation) and no deaths were encountered. No differences in other clinical parameters or laboratory values on admission were evident between groups (Tables 1 and S1).
COVID-19 was diagnosed from reverse transcription polymerase chain reaction (RT-PCR) testing at our hospital or at Fukui Prefectural Institute of Public Health and Environmental Science. To detect cytokine and chemokine levels in plasma, including tumor necrosis factor α, interferon-γ, interleukin (IL)-6, and IL-8, the Human Premixed Multi-Analyte Kit (R&D Systems, Minneapolis, MN, USA) was used.
Statistical analysis was performed using GraphPad Prism version 9.1.1 software (GraphPad Software, San Diego, CA, USA). Continuous variables are presented as median and interquartile range and were evaluated using the unpaired t-test (parametric) or Mann-Whitney U test (non-parametric). Categorical variables were evaluating using Fisher's exact test. Differences between groups were evaluated using the Wilcoxon matched-pairs signed-rank test. A two-tailed P value < 0.05 was considered a significant result.
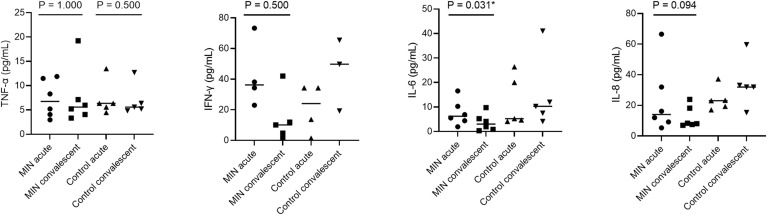
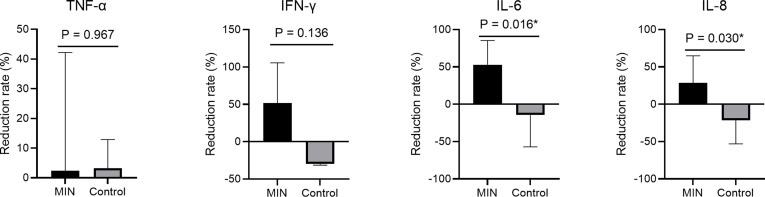
Inflammatory cytokine/chemokine assay showed a significant decrease in IL-6 concentration in the MIN group (P = 0.031, Fig. 1 ). In terms of the mean reduction rate of cytokines and chemokines, IL-6 and IL-8 were suppressed significantly more in the MIN group than in the Control group (P = 0.016 and P = 0.030, respectively; Fig. S1). As clinical outcomes, no significant differences were seen in median durations of fever or hospitalization, but the median interval from the start of treatment to negative PCR results was significantly shorter in the MIN group (P = 0.017, Table 1 ). Although there was no significant difference in the types of symptoms and their duration, dyspnea and low appetite were slightly more common in the control group, and one patient still had residual dyspnea at the time of discharge. As for the course of symptoms, nasal discharge and headache were rather slightly longer in the MIN group. Since only approximately 40% of the patients in both groups were followed up with imaging studies, the overall trend was unclear, but the percentage of patients who were completely improved was slightly higher in the MIN group, although the difference was not significant (Table 2 ). Adverse events were minor in both groups and rates did not differ significantly between groups (Table S2).
Fig. 1.
Plasma concentration of inflammatory cytokine/chemokine levels in COVID-19 patients who received favipiravir with or without minocycline. Sample size was 6 for the MIN group and 5 for the control group. Horizontal bars represent median values. Statistical significance was only tested between values on admission (acute) and before discharge (convalescent) in the Control group and MIN group. However, for IFN-γ, IL-6, and IL-8, only the MIN group was tested because they were clearly elevated in the convalescent phase in the control group. *Values of P < 0.05 were deemed significant. IFN-γ, interferon-gamma; IL, interleukin; MIN, minocycline; TNF-α, tumor necrosis factor alpha.
Table 1.
Characteristics of patients in the minocycline and control groups.
| Variable | Minocycline group (n = 12) | Control group (n = 9) | P value |
|---|---|---|---|
| Demographic parameters | |||
| Age, years (median, IQR) | 54.0 (51.0–58.3) | 67.0 (64.0–72.0) | 0.012* |
| ≥65 (n, %) | 2 (16.7%) | 6 (66.7%) | |
| <65 (n, %) | 10 (83.3%) | 3 (33.3%) | 0.032* |
| Sex (male, n, %) | 7 (58.3%) | 5 (55.6%) | 1.000 |
| Body mass index, kg/m2 (median, IQR) | 22.8 (21.6–24.3) | 25.0 (23.2–27.4) | 0.079 |
| Clinical parameters | |||
| Highest temperature, °C (median, IQR) | 38.3 (37.5–38.6) | 38.3 (38.0–38.8) | 0.950 |
| Heart rate, beats/min (median, IQR) | 81 (73–93) | 75 (72–96) | 0.910 |
| Respiratory rate, breaths/min (median, IQR) | 15 (14–18) | 16 (13–19) | 0.654 |
| Systolic blood pressure, mmHg (median, IQR) | 120 (109–129) | 124 (120–130) | 0.403 |
| Oxygen support (n, %) | 1 (8.3%) | 2 (22.2%) | 0.553 |
| Duration from first symptoms to diagnosis, days (median, IQR) | 5.0 (3.5–5.0) | 3.0 (1.0–4.3) | 0.161 |
| Duration from first symptoms to admission, days (median, IQR) | 6.0 (3.5–7.0) | 4.0 (1.8–6.0) | 0.182 |
| Underlying diseases (n, %) | 6 (50.0%) | 6 (66.7%) | 0.661 |
| Cardiovascular disease (n, %) | 4 (33.3%) | 5 (55.6%) | 0.396 |
| Diabetes mellitus (n, %) | 1 (8.3%) | 4 (44.4%) | 0.119 |
| Endocrine disease (n, %) | 3 (25.0%) | 0 | 0.229 |
| Chronic kidney disease (n, %) | 0 | 0 | |
| Chronic obstructive pulmonary disease (n, %) | 0 | 0 | |
| Cancer (n, %) | 0 | 0 | |
| Chest imaging findings, infiltrate and/or ground-glass opacity (n, %) | 11 (91.7%) | 9 (100.0%) | 1.000 |
| Treatment | |||
| Duration from first symptoms to therapy†, days (median, IQR) | 8.5 (6.8–9.3) | 4.5 (1.8–7.0) | 0.010* |
| Duration of favipiravir administration, days (median, IQR) | 13.0 (10.0–14.0) | 11.0 (10.0–14.0) | 0.566 |
| Duration from admission to minocycline therapy, days (median, IQR) | 0 (0–0) | – | |
| Duration of minocycline administration, days (median, IQR) | 14.0 (12.8–15.3) | – | |
| Clinical outcomes | |||
| Duration of fever, days (median, IQR) | 2.0 (0–5.0) | 3.0 (2.0–5.0) | 0.903 |
| Duration from start of therapy† to viral RT-PCR negative result, days (median, IQR) | 14.0 (12.0–14.0) | 17.0 (15.0–20.0) | 0.017* |
| Hospital stay, days (median, IQR) | 19.0 (17.0–22.8) | 22.0 (19.0–32.0) | 0.120 |
| Mortality (n, %) | 0 | 0 | |
†Favipiravir with or without minocycline therapy.
*Values of P < 0.05 were deemed significant.
IQR, interquartile range.
Table 2.
Course of symptoms and chest imaging findings of patients in the minocycline and control groups.
| Variable | Minocycline group (n = 12) | Control group (n = 9) | P value |
|---|---|---|---|
| Symptoms in clinical course† (n, %) | 12 (100.0%) | 9 (100.0%) | 1.000 |
| Fever (n, %) | 8 (66.7%) | 8 (88.9%) | 0.338 |
| Dyspnea (n, %) | 0 | 2 (22.2%) | 0.171 |
| Cough (n, %) | 10 (83.3%) | 6 (66.7%) | 0.611 |
| Sputum (n, %) | 5 (41.7%) | 5 (55.6%) | 0.670 |
| General malaise (n, %) | 8 (66.7%) | 6 (66.7%) | 1.000 |
| Nasal discharge (n, %) | 4 (33.3%) | 1 (11.1%) | 0.338 |
| Sore throat (n, %) | 3 (25.0%) | 3 (33.3%) | 1.000 |
| Headache (n, %) | 5 (41.7%) | 2 (22.2%) | 0.642 |
| Diarrhea (n, %) | 5 (41.7%) | 6 (66.7%) | 0.387 |
| Low appetite (n, %) | 5 (41.7%) | 7 (77.8%) | 0.184 |
| Taste and smell disorder (n, %) | 6 (50.0%) | 4 (44.4%) | 1.000 |
| Myalgia (n, %) | 3 (25.0%) | 1 (11.1%) | 0.603 |
| Duration of symptoms during hospitalization | |||
| Dyspnea, days (median, IQR) | 0 (0–0) | 0 (0–0) | 0.236 |
| Cough, days (median, IQR) | 5.0 (2.0–8.0) | 7.0 (0–11.0) | 0.545 |
| Sputum, days (median, IQR) | 0 (0–4.5) | 1.0 (0–2.0) | 0.971 |
| General malaise, days (median, IQR) | 1.0 (0–8.5) | 4.0 (0–7.0) | 0.836 |
| Nasal discharge, days (median, IQR) | 0 (0–1.0) | 0 (0–0) | 0.067 |
| Sore throat, days (median, IQR) | 0 (0–0.5) | 0 (0–1.0) | 0.640 |
| Headache, days (median, IQR) | 0 (0–4.8) | 0 (0–0) | 0.079 |
| Diarrhea, days (median, IQR) | 0 (0–0.3) | 1.0 (0–1.0) | 0.255 |
| Low appetite, days (median, IQR) | 0 (0–7.0) | 4.0 (0–7.0) | 0.715 |
| Taste and smell disorder, days (median, IQR) | 1.5 (0–7.3) | 0 (0–4.0) | 0.166 |
| Myalgia, days (median, IQR) | 0 (0–0.3) | 0 (0–0) | 0.332 |
| Chest imaging findings in acute phase, infiltrate and/or ground-glass opacity (n, %) | 11 (91.7%) | 9 (100.0%) | 1.000 |
| Chest imaging findings in convalescent phase | |||
| Imaging tests available (n, %) | 5 (41.7%) | 4 (44.4%) | 1.000 |
| Complete improvement (n, %) | 3/5 (60.0%) | 1/4 (25.0%) | 0.524 |
| Partial improvement (n, %) | 2/5 (40.0%) | 3/4 (75.0%) | 0.524 |
†Symptoms at onset and during hospitalization.
*Values of P < 0.05 were deemed significant.
IQR, interquartile range.
In normal times, the use of antimicrobial agents for viral infections is not recommended clinically in view of antimicrobial overuse. However, during the COVID-19 epidemic, several antimicrobial agents have been focused on as repurposing drugs for their antiviral and anti-inflammatory effects. There are multiple therapeutic targets for SARS-CoV-2, among which RNA-dependent RNA polymerase (RdRp) and main protease (Mpro) are involved in transcription and replication of the viral genome [8]. FVP inhibited RdRp of SARS-CoV-2 and resulted in higher viral shedding at 14 days post-hospitalization compared to the control group, although the difference was not significant [9]. MIN shows potential antiviral activity by inhibiting the SARS-CoV-2 Mpro and may be effective against COVID-19 [10]. In this study, the duration of PCR negative conversion was shorter in the MIN group than in the Control group with FPV alone. It is possible that the combination of FVP and MIN, which have different virus-inhibitory mechanisms, increased viral clearance. However, more patients in the MIN group were younger than 65 years. People older than 65 years are known to be at risk for severe disease, and the older the person, the higher the mortality rate. We have found some conflicting literature, one reporting that older age is a factor in slower viral clearance [11] and others that age has no effect on viral clearance [12,13]. At this point, we judge the results to be inconclusive, however, aging is thought to cause a decline in immunity [12], which may affect viral shedding.
In addition, a previous study reported MIN suppresses inflammatory cytokine and chemokine production by inhibiting inhibitor of nuclear factor kappa B-α/β in the Toll-like receptor (TLR)-4 pathway [14]. Upregulation of the TLR-4 pathway is reportedly observed in COVID-19 patients [15]. MIN administration may thus inhibit the production of inflammatory cytokines in COVID-19 patients, helping to prevent severe disease.
Regarding the course of symptoms, the MIN group tended to have less dyspnea and anorexia, although this was not significantly different, and there was no difference in cough or sputum. Only about 40% of the patients had follow-up imaging studies for pneumonia, and although there was a slight tendency toward complete improvement in the MIN group, there was a trend toward improvement in all of the imaging studies followed, with no significant differences. More samples and follow-up rates will be needed to understand the overall trend.
There are several limitations to this study. First, this was a non-randomized, single-center, retrospective study of a small cohort and the number of samples available to measure the production of inflammatory cytokines and chemokines was limited. Second, the attending physician decided on the treatment plan in a collegial manner with a team of two or more people, but that selection bias could not be ruled out.
In summary, MIN plus FPV therapy may be an effective and safe treatment for COVID-19, but the results should be validated in further studies with a larger cohort and a randomized design.
Authors’ contributions
Conceptualization, KI and HI; methodology, IS and HI; formal analysis, KI and HI; investigation, KI, TH, IS and HI; data curation, KI, TH and HI; writing—original draft preparation, KI and HI; supervision, TH, IS and HI.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethics approval
This study was performed based on the Declaration of Helsinki and its amendments and the Ethical Guidelines for Clinical Research by the Ministry of Health, Labor and Welfare, Japan. This case series study was approved by the Ethics Committee of the University of Fukui Hospital (approval no. 20200025). Written consent for favipiravir administration was obtained from all patients. The need to obtain informed consent for participation in this study was waived by the review board based on the retrospective design of the study.
Data availability
The datasets obtained and/or analyzed during the current study available from the corresponding author on reasonable request.
Declaration of competing interest
The authors have read the journal's policy on the disclosure of potential conflicts of interest and the journal's authorship agreement. The authors declare that they have neither conflicts of interest nor competing interests.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jiac.2021.09.016.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
figs1.
Mean reduction rates in inflammatory cytokine/chemokine levels between testing on admission and before discharge. Bars represent mean values. *Values of P < 0.05 were deemed significant. IFN-γ, interferon-gamma; IL, interleukin; MIN, minocycline; TNF-α, tumor necrosis factor alpha.
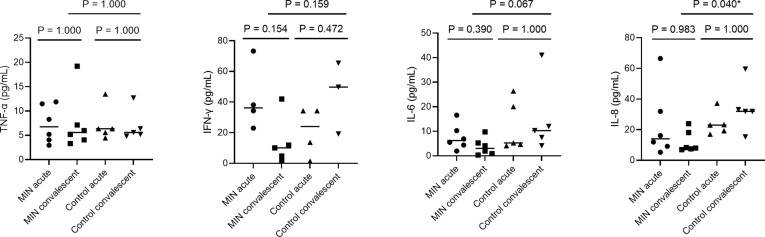
figs2.
Multiple comparison (Dunn’s) tests for plasma concentration of inflammatory cytokine/chemokine levels in COVID-19 patients who received favipiravir with or without minocycline (Fig. 1). Significance difference tests were performed only for the acute and convalescent phase in the MIN and control groups and for the convalescent phase between the MIN and control groups. *Values of P < 0.05 were deemed significant. IFN-γ, interferon-gamma; IL, interleukin; MIN, minocycline; TNF-α, tumor necrosis factor alpha.
References
- 1.Guan W.-J., Ni Z.-Y., Hu Y., Liang W.-H., Ou C.-Q., He J.-X., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Japanese Ministry of Health, Labor and Welfare Occurrence of pneumonia associated with the new coronavirus (1st case) Jpn Minist Health Labor Welfare. 2020 https://www.mhlw.go.jp/stf/newpage_08906.html Available at: Accessed May 25, 2021. [Google Scholar]
- 3.Fukui Prefectural Government Novel coronavirus infection, review of the first wave in Fukui prefecture and future response. Fukui Prefectural Government. 2020 https://www.pref.fukui.lg.jp/doc/kenkou/corona/jyoukyou_d/fil/0730.pdf Available at: Accessed May 25, 2021. [Google Scholar]
- 4.Japanese Ministry of Health, Labor and Welfare Medical treatment of novel coronavirus infection. Jpn Minist Health Labor Welfare. 2021 https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/kenkou_iryou/dengue_fever_qa_00001.html#Q5-10 Available at: Accessed May 26, 2021. [Google Scholar]
- 5.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Kuraishy H.M., Al-Gareeb A.I., Alqarni M., Cruz-Martins N., El-Saber Batiha G. Pleiotropic effects of tetracyclines in the management of COVID-19: emerging perspectives. Front Pharmacol. 2021;12:642822. doi: 10.3389/fphar.2021.642822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh H., Kakkar A.K., Chauhan P. Repurposing minocycline for COVID-19 management: mechanisms, opportunities, and challenges. Expert Rev Anti Infect Ther. 2020;18:997–1003. doi: 10.1080/14787210.2020.1782190. [DOI] [PubMed] [Google Scholar]
- 8.Zhou L.S., Wang M.S., Yang J.F., Xu H.C., Huang W., Shang L.Q., et al. Insights into SARS-CoV-2: medicinal chemistry approaches to combat its structural and functional biology. Top Curr Chem. 2021;379:23. doi: 10.1007/s41061-021-00335-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hassanipour S., Arab-Zozani M., Amani B., Heidarzad F., Fathalipour M., Martinez-de-Hoyo R. The efficacy and safety of Favipiravir in treatment of COVID-19: a systematic review and meta-analysis of clinical trials. Sci Rep. 2021;11:11022. doi: 10.1038/s41598-021-90551-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bharadwaj S., Lee K.E., Dwivedi V.D., Kang S.G. Computational insights into tetracyclines as inhibitors against SARS-CoV-2 Mpro via combinatorial molecular simulation calculations. Life Sci. 2020;257:118080. doi: 10.1016/j.lfs.2020.118080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu X., Xing Y., Jia J., Ni W., Liang J., Zhao D., et al. Factors associated with negative conversion of viral RNA in patients hospitalized with COVID-19. Sci Total Environ. 2020;728:138812. doi: 10.1016/j.scitotenv.2020.138812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abrahim S.A., Tessema M., Defar A., Hussen A., Ejeta E., Demoz G., et al. Time to recovery and its predictors among adults hospitalized with COVID-19: a prospective cohort study in Ethiopia. PLoS One. 2020;15 doi: 10.1371/journal.pone.0244269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bennasrallah C., Zemni I., Dhouib W., Sriha H., Mezhoud N., Bouslama S., et al. Factors associated with a prolonged negative conversion of viral RNA in patients with COVID-19. Int J Infect Dis. 2021;105:463–469. doi: 10.1016/j.ijid.2021.02.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tai K., Iwasaki H., Ikegaya S., Ueda T. Minocycline modulates cytokine and chemokine production in lipopolysaccharide-stimulated THP-1 monocytic cells by inhibiting IκB kinase α/β phosphorylation. Transl Res. 2013;161:99–109. doi: 10.1016/j.trsl.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 15.Sohn K.M., Lee S.G., Kim H.J., Cheon S., Jeong H., Lee J., et al. COVID-19 patients upregulate toll-like receptor 4-mediated inflammatory signaling that mimics bacterial sepsis. J Kor Med Sci. 2020;35:e343. doi: 10.3346/jkms.2020.35.e343. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets obtained and/or analyzed during the current study available from the corresponding author on reasonable request.