Abstract
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) outbreak began in late 2019 in Wuhan, China, and have since spread globally. Deep sequencing analysis identified the disease within a few weeks, and on February 11, the World Health Organization (WHO) named it “COVID-19 caused by SARS-CoV-2.” SARS-CoV-2 was declared a global pandemic by the WHO in March 2020. Coronavirus disease has become a global challenge for researchers and health care workers, affecting over 174 million people and causing over 3 million deaths. Because of the widespread nature, extensive measures are being taken to reduce person-to-person contact, and special precautions are being taken to prevent the transmission of this infection to vulnerable populations such as geriatrics, pediatrics, and health care professionals. We summarized the genesis of COVID-19 spread, its pathology, clinical perspectives, and the use of natural ingredients as a possible cure for COVID-19 in this review. This article has highlighted information about current vaccines approved for emergency use as well as those in various stages of clinical trials. Vaccine availability around the world is a promising development in the fight against the SARS-CoV-2 virus. We conducted a narrative review to present the current state and research on this situation, specific diagnosis, clinical manifestation, emergency approaches, herbal-based remedies, and COVID vaccines.
Graphical abstract

Keywords: COVID-19, Severe acute respiratory syndrome, Pathology, Therapeutic strategy, Natural remedies, COVID vaccines, Clinical trials
Introduction
The novel coronavirus disease 2019 is caused by severe coronavirus (COVID-19). SARS-CoV-2 (acute respiratory syndrome coronavirus) was discovered and reported in December 2019 in Wuhan, China, and has since spread to become a global pandemic. The SARS-CoV-2 virus has a long incubation period of up to 33 days (in some studies, an incubation period of more than 14 days was used) and a fast transmission rate that is faster than that of other coronaviruses, such as SARS-CoV and middle east respiratory syndrome (MERS)-CoV. Asymptomatic carriers can spread the virus as well. The majority of SARS-CoV-2 patients experience mild-to-moderate symptoms; however, approximately 15% develop severe pneumonia, and approximately 5% develop acute respiratory distress syndrome (ARDS), septic shock, multiple organ failure, and even death. As a result of the aforementioned characteristics, COVID-19 had spread to more than 200 countries by June 1, 2021, resulting in more than 170,000,000 confirmed cases and 3,782,490 confirmed deaths. As a result of the pandemic, which has made humans more vulnerable to microbial pathogens and revealed gaps in our therapeutic arsenal, scientists are working at an unprecedented pace to understand the disease and find a cure. At the moment, two major courses are thought to be driving COVID-19 pathogenesis. SARS-CoV-2 identification, fusion, entry, and replication, also known as the replication cycle, are primarily driven by viral proteins in the early stages of infection progression. A massive inflammatory/immune response to SARS-CoV-2 that causes tissue damage drives the late stage of infection progression (Bogoch et al. 2020). As a result, both virus proteins and host factors are required for COVID-19 pathogenesis, making them promising anti-viral therapy targets.
Coronavirus (CoV) was first isolated in 1987 and is thought to be a primordial variant found in pangolins or bats. Coronaviruses cause bronchitis in birds, decimating the poultry population. In 1960, the human strand of coronaviruses (HCoV) was isolated from the noses of common cold patients. OC43 and 229E were the two main strands of the HCoV obtained. The term coronavirus refers to the crown-like projections on surfaces of coronaviruses. In Latin, the term “corona” means “halo” or “crown.” Coronaviruses are a type of virus that commonly affects the nose, upper throat, and sinuses (Rahman et al. 2021).
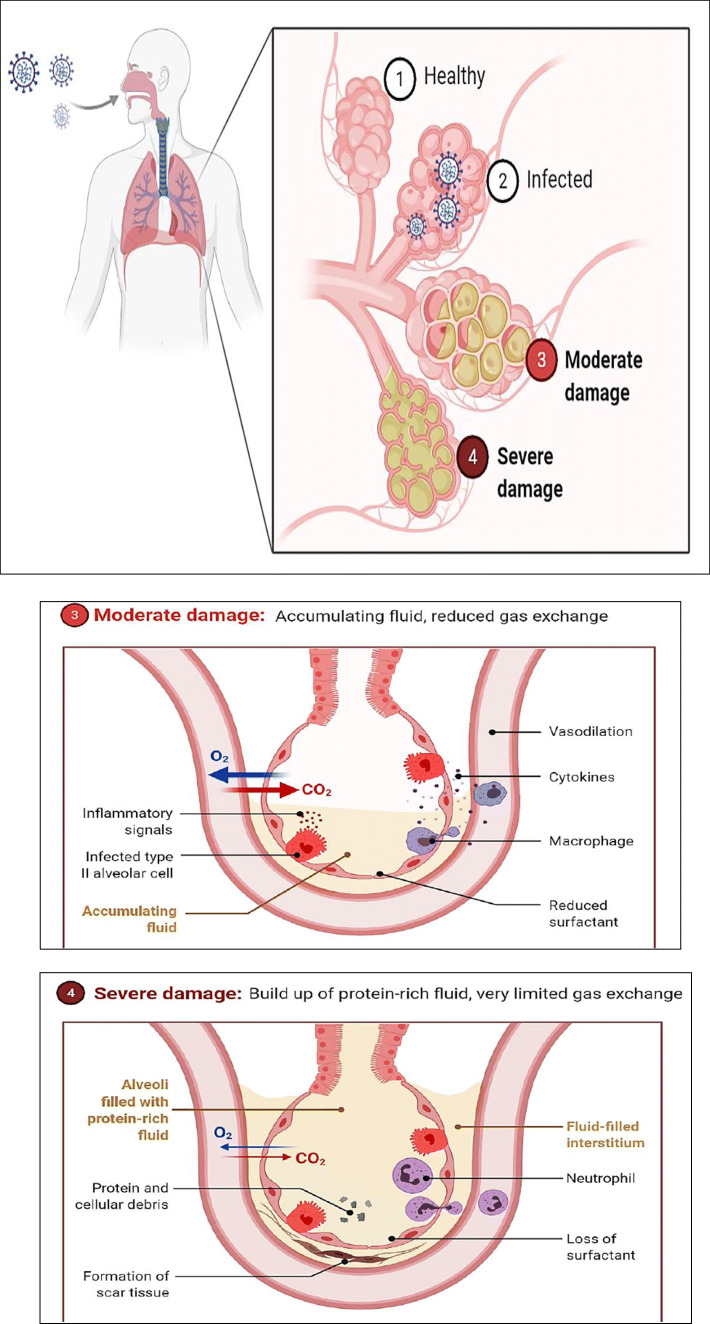
The centers for disease control and prevention (CDCP) begins to see the emergence of a new coronavirus known as SARS-CoV-2 in 2019. The first case of human transmission was discovered in Wuhan, China. Coronaviruses are linked to viruses that can cause disease in humans and animals. SARS-CoV-2 is one of seven types of coronaviruses, which include the common ones that cause severe diseases such as SARS and MERS. The other types are primarily viruses that cause colds and do not cause serious infections. SARS virus strain known as SARS-CoV is a coronavirus association. The new strain of CoV is known as SARS-CoV-2, and it is the cause of coronavirus disease (COVID-19). Other members of this virus family include SARS-CoV and MERS-CoV. COVID-19 was identified sequentially as SARS-CoV in approximately 79% of cases and MERS-CoV in approximately 50% of cases. Furthermore, homology modeling revealed that the receptor-binding domain of SARS-CoV and COVID-19 is the angiotensin-converting enzyme 2 (ACE2) receptor in humans, causing infection (Bogoch et al. 2020; Lu et al. 2020a; Zhao et al. 2020). COVID-19 is a respiratory tract infection that can affect both the upper and lower respiratory tracts. Their infection ranges from mild to fatal in humans and is spread primarily through person-to-person contact. The effect of coronavirus on the respiratory tract is given in Fig. 1.
Fig. 1.
Effect of SARS-CoV-2 on the respiratory tract
The new coronavirus strain has rapidly spread throughout the world. COVID-19 was declared a pandemic by the World Health Organization on March 11, 2020 (Du Toit 2020; Calisher et al. 2020). A pandemic occurs when an infection to which people are not immune spreads across larger geographic areas. Geriatrics, those with cardiovascular or respiratory disorders and diabetic patients, are at the greatest risk. According to various reports, nosocomial infections are more likely in the case of COVID-19 (Corman et al. 2020; Wang et al. 2020a, b, c; Nishiura et al. 2020; Rahman et al. 2020).
Structure of COVID-19
The structure of the COVID-19 (Fehr AR 2015) mainly consists of a nucleocapsid protein, RNA, membrane protein, envelope protein, hemagglutinin-esterase dimer, and a spike protein, as represented in Fig. 2.
Fig. 2.
Structure of SARS-CoV-2
Nucleocapsid (N) protein
The nucleocapsid (N) protein possesses in vitro affinity and is highly phosphorylated, playing an important role in translation and transcription. N protein binds to the genome of the virus in a string type confirmation. This protein triggers the viral genome to replicate-transcriptase complex (RTC) and finally results in packing the encapsulated genome into viral particles.
Membrane (M) protein
Membrane (M) protein is a widely expressed structural protein and is the most abundant protein. It exhibits its action by binding to the nucleocapsid and by promoting membrane curvature. It is also called the central organizer for the coronavirus assembly.
Envelope (E) protein
Envelope (E) protein is found within the virus in a very small quantity and is mainly a transmembrane protein and activates the ion channels. It facilitates the protein assembly and release of the virus, but this protein is not involved in the viral replication process.
Hemagglutinin-esterase (HE) dimer protein
Hemagglutinin-esterase (HE) dimer protein binds to the sialic acids which are present on the surface of glycoproteins, enables its action via entering into the spike protein-mediated cells, and spreads through the mucosa.
Spike (S) protein
Spike (S) protein is located on the surface of the virus, facilitating the entry of SARS-CoV-2 into the human cells. It is highly glycosylated and employs an N-terminal signaling pathway to achieve its access to the endoplasmic reticulum and furthermost attaching to the host receptors. For the majority of coronaviruses, S-proteins are cleaved into two different polypeptide domains, N-terminal S1 subunit and C-terminal S2 subunit. The S1 contains receptor-binding domain (RBD) for binding onto host cell receptors while the S2 subunit mediates the membrane fusion.
Transmission of SARS-CoV-2
COVID-19 is thought to be of zoonotic origin based on the spread of the disease from the Wuhan animal market. Efforts are being made to locate the reservoir from which it emerged and spread throughout the human population. The initial report identified two snake species as possible origins, but no proper evidence was reported aside from the possibility of mammals and birds. COVID-19’s genomic similarities with the bat sequence accounted for 88% of the total. Evidence suggests that person-to-person contact is the most common cause of this infection’s spread (Holshue et al. 2020).
When COVID-19 patients cough or breath out, the virus is expelled in the form of tiny droplets, which can enter the nose or mouth of people who are not infected, resulting in the onset of the disease. When symptoms are at their worst, this infection is extremely contagious. The virus can settle in droplets on nearby objects or surfaces. When a person comes into contact with the virus through their eyes, nose, or mouth, they are likely to become infected (Thompson 2020). It should be noted that the COVID-19 is progressing and that the researchers are still open-ended. In the case of coronavirus spread, there are additional modes of transmission.
The initial binding of host cell receptors and subsequent fusion with the cell membrane is the primary mechanism involved in viral infection. It has been discovered that the primary target of this virus is the epithelial cells of the lungs. As a result, it has been determined that the transmission of SARS-CoV to humans, as well as its action, is linked to the binding of the virus spikes receptor-binding domain and the ACE2 cellular receptors. Because the SARS-CoV and COVID-19 receptor-binding domains are similar, the binding is most likely via ACE2 receptors (Wang et al. 2020a, b, c, Nishiura et al. 2020).
Symptoms and clinical significance
The symptoms of COVID-19 appear after an incubation period of approximately 5.2 days (Thompson 2020). The validity period ranges from 6 to 41 days, with an average of 14 days, from the onset of virus action to death. The patient’s immunity is the most important factor in determining death. The most common COVID-19 symptoms are fever, persistent cough, shortness of breath, diarrhea, lymphopenia, and hemoptysis. Symptoms should appear 2–14 days after being exposed to the virus. On day 9 of the illness, a mild symptom of progression and presentation pneumonia is observed. Clinical approach related to chest CT scan is evaluated for the effect of pneumonia, and other features like acute respiratory distress syndrome (Rothan and Byrareddy 2020; Assiri et al. 2013a, b; Lee et al. 2003), the incidence of grant-glass opacities (some cases both sides of the lungs were observed) (Assiriet al. 2013), and acute cardiac injury. The main reason for the therapeutic strategy’s failure is a decrease in immune response and inflammation, as well as opacities in the pulmonary progression.
The parallels between the earlier beta-coronavirus and COVID-19 are striking. Although symptoms such as dry cough, fever, bilateral ground-glass opacities on CT scan, and dyspnea are shared by both (Lee et al. 2003), the unique clinical characteristics of the COVID-19 such as sneezing, rhinorrhea, and sore throat distinguish them. Diarrhea is a common gastrointestinal symptom in COVID-19 patients as well as MERS-CoV or SARS-CoV patients. As a result, the patient should have urine and fecal tests performed to determine the next route of transmission (Brubaker et al. 2013; Phan et al. 2020).
Pathophysiology and clinical aspect
Coronaviruses are positive-strand RNA viruses with the largest-known RNA genome (30–32 kb) and a 5-cap and 3-poly-A-tail. While the transcription process works through randomized controlled trials (RCT) complex, which is the replication–transcription complex organized in the vesicles, the synthesis of polyproteins 1a/1ab in the host cell is taken into account. Transcription is terminated at the transcription regulatory sequence, which is located between the open reading frames (ORFs), which serve as a template for sub-genomic mRNA production. ORF1a and ORF1b guide the production of the polypeptides pp1a and pp1b which are further processed by the main protease. Aside from ORFs, structural proteins such as nucleocapsid, membrane, spike, and envelope proteins are encoded. The structural proteins, the envelope, play a role in the pathogenicity of the virus by preventing virus assembly and release. In some cases, the viral infection causes exaggerated immune responses in the host or even a “cytokine storm,” which can result in extensive tissue damage. Interleukin-6 (IL-6), which is produced by leukocytes and acts on a variety of cells and tissues, is the star of this cytokine storm. IL-6’s primary function is to promote B lymphocyte differentiation, but it also has pro-inflammatory properties. The SARS-CoV-2 virus enters the lungs via the respiratory tract and begins targeting organs that express angiotensin-converting enzyme 2, such as the heart, lungs, renal, and gastrointestinal systems (Fehr and Perlman 2015).
The majority of COVID-19 cases are associated with a patient profile that includes an elevated body temperature of 39°C for 5 days, as well as coughing and breathing difficulties (Lu et al. 2020b). A real-time polymeric chain reaction is used to analyze the sputum of a COVID-19 infected patient. The laboratory results show 2.91 × 109cells/L leukocytes, of which 70% was neutrophils, resembling leucopenia. A C-protein concentration of 16.16 mg/L was also reported (Wan et al. 2020). D-dimer and erythrocyte sedimentation rates are also elevated. Elevated levels of interleukin-1 receptor antagonist (IL-1RA), interleukin-1 (IL-1), interleukin-10 (IL-10), interleukin-9 (IL9), interleukin-8 (IL8), interleukin-7 (IL7), granulocyte-colony-stimulating factor (GCSF), granulocyte-macrophage colony-stimulating factor (GMCSF), interferon-inducible protein-10 (IP10), interferon (IFN), macrophage inflammatory proteins (MIP1), MIP1B, tumor necrosis factor (TNF), platelet-derived growth factor subunit B (PDGFB), and vascular endothelial growth factor A (VEGFA) have also been reported (Brubaker et al. 2013; Lu et al. 2020b; Wu et al. 2020). The abnormal range of pro-inflammatory cytokines can lead to disease severity in some cases. The COVID-19 clinical spectrum ranges from symptomatic to asymptomatic, with respiratory failure that may necessitate ventilation support. In COVID-19-infected patients, a chest CT scan reveals opacification with or without abnormalities.
Risk criteria
A few factors can influence the risk of virus exposure, while others may play a role in the progression of severe illness. According to the World Health Organization, the likelihood of becoming infected with COVID-19 is low in most cases (Jaimes et al. 2020). The spread is most visible in people who have frequent contact with patients and health care workers. Older adults are at a much higher risk of contracting the severe infection caused by SARS-CoV-2, as are people with certain medical conditions such as asthma, chronic kidney disease, cystic fibrosis, and diabetes.
Prevention
Recommending effective approaches to halt the spread of SARS-CoV-2 is now a global challenge (Hui et al. 2020). Anti-viral vaccination primarily inhibits viral action, but developing a vaccine or any treatment modalities for a newly developed strain of virus takes time (Brubaker et al. 2013). The formulation against these viral strands should be developed safely and effectively. The best way to prevent this virus from spreading is to avoid close contact with the infected person and to practice good personal hygiene (Cui et al. 2019; Zhu et al. 2020; Ngandu et al. 2015).
The CDC recommends washing hands with water and soap for 20 seconds each time after public exposure. In the absence of soap, hand sanitizers containing at least 60% alcohol are recommended and avoid touching your face with your hands before washing them (Wang et al. 2020a, b, c).
Public bodies and the government are developing preventive measures to break the chain of transmission of SARS-CoV-2 and to educate the public about the disease. The high mortality rate in the population is primarily due to patients’ low immunity.
In addition, to the evolution, adaptation, and spread of the disease, the major challenges that should be effectively monitored are epidemiological changes and mutation. It has also been reported that the disease has spread to pet animals such as dogs. In this pandemic situation, there are still several unanswered questions (Bassetti et al. 2020). Personal protective equipment (PPE) is a method of infection prevention and control that is commonly used by passengers on flights and health care professionals as a preventive measure against becoming infected. The PPE acts as a face shield and is worn by healthcare professionals or other individuals to prevent infection.
Is there a cure for COVID-19?
According to the CDC, there is no specific medication used to treat COVID-19 at this time; instead, patients are given supportive care. The primary reason for the isolation of patients suffering from this disorder is the transmission of this disease from person to person. This approach is primarily used to prevent the virus from spreading to the community and to reduce the severity of the disorder globally (Toots et al. 2019, Jin et al. 2020). The current treatment strategy that doctors are using to reduce viral infection is a combination of HIV protease inhibition and broad-spectrum anti-viral drugs such as nucleotide analogs. The anti-viral drug is administered in the course of oral twice-daily administration of oseltamivir (75 mg), lopinavir (500 mg), and intravenous administration of ganciclovir (0.25 g) for 3–14 days (Ngandu et al. 2015, Lu et al. 2020b). According to research published in 2019, chloroquine and broad-spectrum anti-viral drugs are effective against CoV infection. These medical strategies were found to be safe and are being considered for the treatment of COVID-19 infection. Until and unless an invention for the therapy of COVID-19 is made, RNA synthase inhibitors, peptide (EK1), neuraminidase inhibitors, and ritonavir/lopinavir were included in the patients’ medication history.
It is important to note that more researchers needed the drug discovery and drug development process to create a medication for the safe and effective treatment of COVID-19. There is a need for pharmacological evaluation of the developed drug and for that animal model to be replicated with the disease as of the human epidermis for evaluating the pre-and post-exposure prophylaxis parameters against COVID-19 (Jaimes et al. 2020; Toots et al. 2019; Rothan and Byrareddy 2020; Shah et al. 2021). This SARS-CoV-2 outbreak caused a devastating economic, social, political, and global health problem. However, several vaccines are being developed, and some are undergoing clinical trials. These may include
nucleic acid vaccine: these are mRNA-based vaccines including Moderna;
viral vector vaccines including AstraZeneca and sputnik V;
inactivated virus vaccine including Sinovac vaccine; and
antigen-based vaccine including EpiVac corona.
Use of natural products for COVID-19
Natural remedies have been used for the treatment and cure of viral infections all over the world since ancient times. They are safe and effective treatments for viral infections, and research on natural ingredients that can be used for the management and treatment of various disorders is ongoing (Lalani and Poh 2020). Such natural products have a favorable efficacy and a tolerable level of toxicity (Huang et al. 2020). Figure 3 depicts some examples of possible natural remedies for the management of COVID-19, as well as possible formulation approaches.
Fig. 3.
Possible formulation aspects and natural remedies for COVID-19
Use of flavonoids as a natural remedy
Flavonoids are a type of phenolic natural product that is widely used in both traditional and modern medicine to treat various viral infections. Flavonoids inhibit coronavirus replication in silico, and the main protease is required for virus replication. A docking study revealed that naringenin can effectively bind to the protease by forming a hydrogen bond with the amino acids of the main protease, indicating that naringenin can inhibit the SARS-CoV-2 protease (Khaerunnisa et al. 2020). Flavonoids have been studied extensively in a wide variety of RNA and DNA viruses (Zakaryan et al. 2017; Dou et al. 2011). In silico studies also demonstrated quercetin’s inhibitory action against SARS-CoV-2, owing to its high affinity for binding to the main protease. Another study found that flavonoids like kaempferol, luteolin-7-glucoside, catechin, naringenin, catechin, quercetin, and epigallocatechin were effective against SARS-CoV-2. Rutin was also thought to be effective against SARS-CoV-2. Flavonoids’ mechanisms of action are as follows:
block the binding and influx of the virus into the cells,
inhibit the process of viral replication, and
polyprotein or translation process to inhibit the attack of the virus to various cells.
Other inhibitory activity of the flavonoids includes
flavonoids that act on the specific extracellular region of the virus-like the viral protein visible on capsid;
prevention of the viral host attachment and the entry of the virus; some flavonoids can even change the structure of the virus;
inhibition of the replication at an early stage;
inhibition of transcription and translation;
last stage mutation blocking; and
modulating the immune system and reduce viral load.
The following Table 1 includes a summary of some bioactive compounds with their mechanisms (Pastor et al. 2021).
Table 1.
Summary of some bioactive compounds with their mechanisms
| Bioactive | Food | In vitro properties | Mechanism |
|---|---|---|---|
| Curcumin | Curcuma longa (turmeric) |
Anti-oxidant,anti-fibrosis, and anti-inflammation |
Good binding affinity toward the main protease; inhibit aminopeptidase; reduction of AT1 and AT2 receptors; decrease macrophage population |
| Kaempferol | Cabbage, spinach, dill, beans |
Anti-cancer, anti-oxidant, and anti-inflammatory |
The affinity of binding with ACE-2; inhibit ion channel receptor and regulation of T-cell receptor |
| Quercetin | Onion, tea, grape, Hypericum perforatum |
Anti-oxidant, anti-viral, and anti-inflammatory |
Affinity against ACE-2 and protease |
| Apigenin | Celery, oranges, parsley, Matricaria chamomilla |
Anti-hyperglycemic, anti-oxidant, anti-viral, and anti-inflammatory |
Reduction of COX-2; decreasing interleukin-6 level; target ACE-2 |
AT1, angiotensin II type 1; AT2, angiotensin II type 2; ACE-2, angiotensin-converting enzyme 2; COX-2, cyclooxygenase-2
Role of vaccines against COVID-19
Millions of people have already contracted the virus, and around 2 million deaths have been reported. The researchers are working round the clock for the development of effective vaccines (Hossain et al. 2020; Sharma et al. 2021). The vaccine passes through several stages before the manufacturer can get approval. In the USA, Food and Drug Administration (FDA) approves and the CDC works to ensure the safety of the public. Vaccines mimic the infectious agent which may be a virus, bacteria, or any other microorganism causing the disease to which our immune system rapidly responds. Earlier, the weak form of the infectious agent was used which allows the immune system to work based on “memory” so as when the actual infection happens, the immune system quickly recognizes it and prepares antibodies (What You Need to Know about COVID-19 Vaccines | UNICEF, n.d.). Now, other different types of vaccine approaches, including mRNA vaccines, inactivated vaccines, protein adjuvant vaccines, or live vaccines, were also developed; firstly, three vaccines were authorized by the FDA also (UPDATED Comparing COVID-19 Vaccines: Timelines, Types and Prices | BioSpace, n.d.). Data from several phase III vaccine efficacy trials were reported at the end of 2020, paving the way for these vaccines to be approved and rolled out. The following organizations have reported vaccine efficacy data. Pfizer-BioNTech, Moderna, AstraZeneca–University of Oxford, Johnson & Johnson, Gamaleya, Sinovac Biotech, Sinopharm, Novavax, and Bharat Biotech are among the companies involved. Except for the Novavax vaccine, each of these vaccines had been approved for rollout to adults and, in some cases, adolescents as of June 14, 2021, through a variety of approval processes based on region and regulatory agency.
Pfizer-BioNTech
It was the first vaccine to be approved for emergency use by the FDA, as it was mentioned to be 95% effective for the prevention of symptomatic disease for people older than 12 years old. For this, two shots were recommended which were 21 days apart. It is a type of mRNA vaccine which delivers genetic code from SARS-CoV-2 to the host cells giving instructions for making spike protein copies. The spike protein does the work of infecting and penetrating the host cells which also helps in stimulating the immune response and also produces antibodies. The reported side effects include headache, pain, chills, headache, and swelling at the site of injection.
Moderna
This vaccine was the second one to get emergency approval from the Food and Drug Administration in December 2020. It is also an mRNA-based vaccine with high efficacy in preventing symptomatic disease. It is recommended for adults 18 years and older. This vaccine also requires two shots 28 days apart. One of the advantages offered by this vaccine is that it can be kept in storage for much longer up to 30 days with normal refrigeration, but the limitation of this vaccine was that it showed less effectiveness up to 86% in people who are 65 years and older. The side effects include pain, headache, chills, and swelling at the injection site.
Johnson & Johnson
It is a type of viral vector vaccine and got its approval in February 2021 by the Food and Drug Administration for emergency approval. It can also be administered to 18 years or older adults. As it is a type of carrier vaccine, it uses an adenovirus that carries genetic code to cells by producing spike proteins that will train the immune system of the body. In comparison to Moderna and Pfizer, it has the advantage in that it is easier to store and requires a single shot. But in July, FDA has made a warning that the Johnson & Johnson vaccine in rare cases can lead to neurological disorders.
Oxford-AstraZeneca
This vaccine is based on the principle of carrier vaccine but offers the advantage of lower cost and can be stored in normal refrigeration conditions for a period of up to 6 months. Some countries suspended its use as it showed the development of blood clots within 2 weeks after the vaccination is done. It was recommended for 18 years or older adults with two doses, 4 to 12 weeks apart. On March 25, 2021, AstraZeneca proved the efficacy against the symptomatic COVID-19 to be 76% and 100% in severe cases while 85% efficacy in people who are aged 65 years or older.
Novavax
This is the type of protein adjuvant vaccine that has been found to be effective against COVID-19 and also against the mutations. It is a type of vaccine that has spike protein itself but is being formulated as a nanoformulation. It is also recommended for adults more than 18 years of age with two doses, 3 weeks apart. The common side effects include tenderness, headache, and pain at the site of injection.
Gamaleya Sputnik V
It is a viral vector, given in two doses; the second dose is given 21 days apart from the first dose. It shows with 92% of efficacy and is recommended to the age group above 18. From the trial’s start date to the present, no variants have been identified originating from the trial locations (June 2021)
Bharath Biotech – Covaxin
It is a viral vector, given to subjects above the age of 18. Given in two doses, the second dose is given 28 days apart from the first dose. The phase III trial began on November 16, 2020, and is still ongoing in India; variants discovered include B.1.617.2 and B.1.617.1.
Sinovac Biotech – CoronaVac
It is an inactive virus, given in two doses; the second dose is given 14–28 days after the first dose. Multiple studies are conducted in various countries such as Brazil (50.7%), Indonesia (65%), Chile (56.5%), Brazil (78%), and Turkey (91%). Given to the subjects above the age of 18 is 51% efficacy against symptomatic SARS-CoV-2 infection, 100% efficacy against severe disease, and 100% efficacy against hospitalization beginning 14 days after the second dose.
Sinopharm – BBIBP-CorV
It is an inactive form of the virus. Given in two doses, the second dose is given 21 days after the first dose. It is given to the age group above 18. During this time, no variants originating from trial sites have been identified (June 2021).
Novavax
It is a protein subunit; the second dose is given 21 days after the first dose. It is given to the age group above 18, and 100% efficacies have been shown.
VECTOR – EpiVacCorona
It is a protein subunit, given in 2 doses; the second dose is given 21–28 days after the first dose. During this time, no variants originating in the trial locations have been identified (June 2021).
Clinical trial of vaccines against SARS-COV-2
There are several vaccines that are in different phases of clinical trials globally. Table 2 provides a summary of these clinical trials.
Table 2.
Summary of clinical trials being conducted on different COVID-19 vaccines in individuals with different age groups
| Title | Status | Interventions | Age | Phases | Locations | NCT number |
|---|---|---|---|---|---|---|
| Use of BCG vaccine as a preventive measure for COVID-19 in health care workers | R | Live | 18 years and older | Phase 2 | Brazil | NCT04659941 |
| BCG vaccine in reducing morbidity and mortality in elderly individuals in COVID-19 hotspots | R | Live | 60 to 80 years | Phase 3 | India | NCT04475302 |
| Safety and immunogenicity of two different strengths of the inactivated COVID-19 vaccine ERUCOV-VAC | R | Inactivated | 18 to 55 years | Phase 1 | Turkey | NCT04691947 |
| An effectiveness study of the Sinovac’s adsorbed COVID-19(inactivated) vaccine | A, NR | Inactivated vaccine | 18 years and older | Phase 4 | Brazil | NCT04747821 |
| Efficacy of hydroxychloroquine (HCQ) as post-exposure prophylaxis (PEP) for prevention of COVID-19 | C | Drug: hydroxychloroquine | 18 years and older | Phase 3 | India | NCT04408456 |
| Study on sequential immunization of recombinant COVID-19 vaccine (Ad5 vector) and RBD-based protein subunit vaccine | A, NR | Recombinant vaccine | 18 years and older | Phase 4 | China | NCT04833101 |
| Efficacy, immunogenicity, and safety of the inactivated COVID-19 vaccine (TURKOVAC) versus the CoronaVac vaccine | R | Inactivated vaccine | 18 to 55 years | Phase 3 | Turkey | NCT04942405 |
|
Clinical trial to assess safety and immunogenicity of Gam-COVID-Vac combined vector vaccine for severe acute respiratory syndrome Coronavirus 2 (SARS-Cov-2) infection |
A, NR | Viral vector | 18 years and older | Phase 2/phase 3 | India | NCT04640233 |
| Serological response to mRNA and inactivated COVID-19 vaccine in health care workers in Hong Kong | R | Inactivated vaccine | 18 years and older | Hong Kong | NCT04898946 | |
| Mix and match of the second COVID-19 vaccine dose for safety and immunogenicity | R | mRNA vaccine | 18 to 99 years | Phase 2 | Canada | NCT04894435 |
| A study to evaluate MVC-COV1901 vaccine against COVID-19 in adult | A, NR | S protein with adjuvant | 20 and older | Phase 2 | Vietnam | NCT04695652 |
| Clinical trial for SARS-CoV-2 vaccine (COVID-19) | R | Inactivated | 18 to 59 years | Phase 3 | Turkey | NCT04582344 |
| Safety and immunogenicity of the inactivated Koçak-19 Inaktif Adjuvan lı COVID-19 vaccine compared to placebo | R | Adjuvant | 18 to 55 years | Phase 1 | Turkey | NCT04838080 |
| COVID-19 vaccination of immunodeficient persons (COVAXID) | R | mRNA vaccine | 18 and older | Phase 4 | Sweden | NCT04780659 |
| Clinical trial to evaluate the efficacy, immunogenicity, and safety of the inactivated SARS-CoV-2 vaccine (COVID-19) | A, NR | Inactivated vaccine | 18 to 85 years | Phase 3 | Argentina | NCT04560881 |
| A study to assess the safety and immunogenicity of the corona vaccine against COVID-19 | A, NR | Inactivated vaccine | 18 years and older  (adult, older adult) | Phase 4 | Brazil | NCT04756830 |
| Safety and immunity of COVID-19 APC vaccine | R | Pathogen-specific APC | 6 months to 80 years | Phase 1 | China | NCT04299724 |
| A clinical trial to evaluate the recombinant SARS-CoV-2 vaccine (CHO Cell) for COVID-19 | R | Recombinant vaccine | 3 years and older  (child, adult, older adult) | Phase 1/phase 2 | China | NCT04869592 |
| Clinical trial of efficacy and safety of Sinovac’s adsorbed COVID-19(inactivated) vaccine in healthcare professionals | A, NR | Inactivated vaccine | 18 years and older | Phase 3 | Brazil | NCT04456595 |
| A study to evaluate the efficacy, safety, and immunogenicity of inactivated SARS-CoV-2 vaccines (vero cell) in a healthy population aged 18 years old and above | R | Inactivated vaccine | 18 years and older | Phase 3 | United Arab Emirates | NCT04510207 |
| The phase I clinical trial of booster vaccination of adenovirus type-5 vectored COVID-19 vaccine | A, NR | Viral vector | Child, adult, older adult | Phase 1 | China | NCT04568811 |
| Study on sequential immunization of inactivated SARS-CoV-2 vaccine and recombinant SARS-CoV-2 vaccine (Ad5 vector) | A, NR | Recombinant vaccine | 18 to 59 years | Phase 4 | China | NCT04892459 |
| Phase I trial of a recombinant COVID-19 vaccine (CHO cell) | R | Recombinant vaccine | 18 years and older | Phase 1 | China | NCT04636333 |
| Efficacy, safety, and immunogenicity of inactivated SARS-CoV-2 vaccines (vero cell) to prevent COVID-19 in a healthy adult population in Peru healthy adult population in Peru | A, NR | Inactivated | 18 years and older | Phase 3 | Peru | NCT04612972 |
| A study of SARS-CoV-2 infection and potential transmission in individuals immunized with Moderna COVID-19 vaccine | R | mRNA-based vaccine | 18 to 29 years | Phase 3 | USA | NCT04811664 |
| An immuno-bridging and immunization schedules study of COVID-19 vaccine (vero cell), inactivated | R | Inactivated | 3 years and older | Phase 4 | China | NCT04863638 |
| Hydroxychloroquine (HCQ) as post-exposure prophylaxis (PEP) for prevention of COVID-19 | R | Drug: hydroxychloroquine (HCQ) | 18 years and older | Phase 3 | India | NCT04858633 |
| Phase â…£ clinical trial of inactivated SARS-CoV-2 vaccine for prevention of COVID-19 in healthy adults | R | Inactivated vaccine | 18 to 59 years | Phase 4 | Beijing, China | NCT04962308 |
| Profiling antibody status and vaccine effectiveness in post-vaccination with SARS CoV2 in Ain Shams University | R | Viral vector | 18 years and older | Phase 2/phase 3 | Egypt | NCT04885764 |
| Clinical trial of efficacy, safety, and immunogenicity of Gam-COVID-Vac vaccine against COVID-19 in Belarus | A, NR | Viral vector | 18 to 60 years | Phase 3 | Belarus | NCT04564716 |
| COVAXIN in a pediatric cohort | R | Inactivated | 2 to 18 years | Phase 2/phase 3 | India NCT04918797 | |
| Safety and immunogenicity study of inactivated vaccine for prevention of SARS-CoV-2 infection (COVID-19) | A, NR | Inactivated vaccine | 60 and older | Phase 1/phase 2 | China | NCT04383574 |
| Impact of the immune system on response to anti-coronavirus disease 19 (COVID-19) vaccine in allogeneic stem cell recipients (COVID VaccinAllo) | R | mRNA-based vaccine | 18 to 100 years | Phase 3 | Belgium | NCT04951323 |
| Efficacy of hydroxychloroquine (HCQ) as post-exposure prophylaxis (PEP) for prevention of COVID-19 | C | Drug: hydroxychloroquine | 18 years and older | Phase 3 | India | NCT04408456 |
| A study on the safety, tolerability, and immune response of SARS-CoV-2 Sclamp (COVID-19) vaccine in healthy Adults | R | Adjuvant | 18 years and older | Phase 1 | Australia | NCT04495933 |
| Efficacy, immunogenicity, and safety of inactivated ERUCOV-VAC compared with placebo in COVID-19 | R | Inactivated vaccine | 18 to 64 years | Phase 2 | Turkey | NCT04824391 |
| Clinical trial of efficacy, safety, and immunogenicity of Gam-COVID-Vac vaccine against COVID-19 | A, NR | Viral vector | 18 to 111 years | Phase 3 | Moscow, Russia | NCT04530396 |
| Safety and efficacy of a non-replicating ChAdOx1 vector vaccine AZD1222 (COVISHIELD) for prevention of COVID-19 in patients with liver cirrhosis | R | Recombinant | 18 years and older | Not applicable | India | NCT04794946 |
| Lot-to-lot consistency of an inactivated SARS-CoV-2 vaccine for prevention of COVID-19 in healthy adults | A, NR | Inactivated | 26 to 45 years | Phase 4 | China | NCT04894227 |
| Study to evaluate the immunogenicity and safety of heterologous SARS-CoV-2 vaccine schemes | R | Drug: Gam-COVID-Vac/Gam-COVID-Vac | 21 to 65 years | Phase 2 | Argentina | NCT04962906 |
| Safety and immunogenicity study of 2019-nCoV vaccine (mRNA-1273) for prophylaxis of SARS-CoV-2 infection (COVID-19) | A, NR | mRNA vaccine | 18 to 99 years | Phase 1 | Washington, USA | NCT04283461 |
| Study of monovalent and bivalent recombinant protein vaccines against COVID-19 in adults 18 years of age and older | R | Adjuvanted recombinant protein vaccine | 18 years and older | Phase 3 | Japan | NCT04904549 |
| Safety and immunogenicity of an intranasal SARS-CoV-2 vaccine (BBV154) for COVID-19 | A, NR | Viral vector | 18 to 60 years | Phase 1 | India | NCT04751682 |
| Phase I clinical trial of a COVID-19 vaccine in 18–60 healthy adults | A, NR | Recombinant vaccine | 18 to 60 years | Phase 1 | China | NCT04313127 |
| Third dose of Moderna COVID-19 vaccine in transplant recipients | A, NR | mRNA vaccine | 18 years and older | Phase 4 | Canada | NCT04885907 |
| Safety, tolerability, and immunogenicity of the COVID-19 vaccine candidate (VBI-2902a) | A, NR | Virus-like particle | 18 years and older | Phase 1/phase 2 | Canada | NCT04773665 |
| Polio vaccine (IPV) for SARS-CoV-2 and prevention of coronavirus disease (COVID-19) | R | Polio vaccine | 18 to 80 years | Phase 4 | USA | NCT04639375 |
Post-COVID complications
Since the outbreak of this pandemic, research has focused on the rapid diagnosis and development of new therapies for the effective treatment of COVID-19, as it is not only a respiratory disease, but it has also been discovered that high levels of some endogenous substances are being produced in response to the virus’ inflammation (Andrade et al. 2021). A Guillain-Barre syndrome characterized by polyneuropathies is one of the immune system complications. It was reported in clinical trials of pediatric, adolescent, and adult patients. Other reports show that cases of COVID-19-associated arthritis condition and 456 rheumatic patients over the age of 60 were reported (Pablos et al. 2020). According to reports, an auto-inflammatory disease known as Kawasaki disease was observed, which primarily affects children under the age of five years and causes acute inflammation in caliber vessels, macrophage activation, and increased cardiac involvement. The complications associated with the hematological system result in a direct effect of SARS-CoV-2 linked hyper inflammation, which produces some endogenous substances promoting vascular hemostasis and blood coagulation, which is directly affected by the release of pro-inflammatory and procoagulant cytokines. The SARS-CoV-2 entry mechanism is mediated by the Hace2-R receptor, which is found in the lungs, intestinal smooth muscles, heart, kidneys, and liver. Binding to the h-ACE2-R causes a decrease in receptor density on vascular tissue, which is associated with the negative regulation of h-ACE2-R activity, followed by angiotensin II accumulation, which causes vasoconstriction, pro-inflammatory, and profibrotic effects. Respiratory failure, pulmonary embolism, thromboembolism, pneumonia, and post-viral fibrosis are some of the other pulmonary complications (Moniruzzaman et al. 2020).
The pathophysiological findings in COVID-19 patients indicate a high risk of myocarditis, myocardial injury, and heart failure. Myocardial injury is detected in 25% of hospitalized patients with the COVID-19 condition, which is associated with a risk of death. High levels of cytokines and mediators such as TNF-α, IL-6, and nitric oxide may cause myocardial depression. Digestive system complications and clinical manifestations such as diarrhea, abdominal pain, anorexia, gastrointestinal hemorrhage, constipation, and acid reflux were also reported. According to various reports and surveys, 20% of COVID patients developed mental health issues such as anxiety, depression, or dementia within 3 months of diagnosis, according to a doctor in the USA (Van Hees et al. 2020). Depression is a condition or disorder that primarily interferes with an individual’s normal life. In this era, especially as the coronavirus pandemic is prolonging and harming individuals, the causes of depression and its related suicidal death are becoming more prevalent. Not only in this era but also one out of every ten people suffers from depression at some point in their lives as a result of low self-esteem(Woods and Scott 2016). There is a need for a safe and effective health care regimen that can aid positive feedback on people’s mental health by increasing the country’s economy through increased participation in the work field.
Menthol or peppermint oil has already been shown to be effective in the treatment of depression, even in severe health conditions such as myocardial infarction (Törnblom and Drossman 2018). We are moving forward with a hypothesis that includes a combination of peppermint oil, an essential oil, and a proven anti-depressant herbal extract of Hypericum perforatum (Setorki 2020; Du Toit 2020) in omega 3-fatty acid-based nano-droplets to be evaporated in a vaporized form inside the room or offices to keep people happy and improve their social lives.
The above-mentioned strategic plan is innovative in the following ways.
The combination of the strategy of anti-depressants such as menthol, Hypericum perforatum, and omega-3 fatty acid provides a synergistic effect, which is a novel concept working behind.
The conversion of these combinations into nano-droplets and converting into vapors using the active machine similar to the mosquito coil machine.
Using the nano-droplets as a room freshener and anti-depressant safely and effectively.
The lipid pro-drug approach is used which can increase the bioavailability of the drug into the brain by targeting the olfactory receptors and helps in crossing blood–brain barriers.
The individual drugs or compounds such as menthol, Hypericum perforatum, and omega-3 fatty acid are proven anti-depressant drugs, but this combination strategy is unique and novel.
The combination of menthol, Hypericum perforatum, and omega-3 fatty acid is novel and can be used safely and effectively. This combination can be used as a mind relaxant and also as an anti-depressant, as a medicament.
The in vitro and in vivo results should be supportive such as nasal toxicity, histopathological studies, and bioavailability. The target of the drug is to reach the brain and the ability to cross blood–brain barrier. The main approach needed is the assurance of quality, safety, and efficacy before producing to the market.
The combination approach used with the aid of natural ingredients is easily accessed, safe, and economical with lesser side effects. This concept can produce a health care approach and also as a placebo effect. The social life of the people can be improved and also the mental health.
This economical, safe, user-friendly combination strategy can be used by all individuals as a health care regime and also as a placebo effect. This combination can help in improving the social life of the people and improved focus on the workplace, which helps in improving the country’s economy.
The parent component used in this formulation is easily accessible and economical. Since the input is economical, the output is also economical and it can be easily accessed too.
The herbal or natural products are environment-friendly and accessible. The waste material and by-products are biodegradable and environment-friendly. The main concept behind is the mother nature that has provided the remedy for all the disease and ailments what the living things face (Karthika and Sureshkumar 2021). The other natural regimen used for the treatment of depression is given in Table 3.
Table 3.
Natural remedies used for the treatment of depression
| SL. no | Natural remedies | Short presentation | References |
|---|---|---|---|
| 1 | Wu Wei: principle of non-resistance | Let the things happen in their way, accept. If there is a storm, let it roar; one day, it will calm down by itself. This is a Taoist saying, before 2500 years ago by a Chinese Philosopher. | Chen et al. 2016 |
| 2 | Lavender | Blue–violet inflorescence acts as a relaxant, useful as an anti-depressant. Infusions can be used for internal and external use. Tea with lavender helps to calm down, act as anger soothe, aid relaxation and is useful with depression and insomnia. | Kianpour et al. 2016 |
| 3 | Aerobic exercise against depression | Reports suggest that walking and exercise can reduce depression. But in this pandemic, walking outside is not considered to be safe; hence, doing exercise and practicing Yoga indoor safely is advisable. | Herbert et al. 2020 |
| 4 | Chromotherapy, the power to heal with colors | Chromotherapy is mainly based on the idea that colors can have a positive impact on our health and our mood. There is the hypothetic report that colors can produce an electromagnetic wave which indeed can help with the synthesis of hormones such as melatonin and serotonin. A room with white lights than dim lights can help inward off depression. | Chappaz et al. 2017 |
| 5 | Osmanthus essential oil | Osmanthus fragrance can produce an anxiolytic effect with its sedative and calming action and can be used as diffusion in rooms with an oil burner. | Pai et al. 2020 |
| 6 | Vitamin D | Vitamin D can improve mood, will support the immune system, and can improve brain function. The potent candidate as an anti-depressant and in COVID treatment. | Menon et al. 2020, Mitchell 2020 |
| 7 | Hyssop | Hyssop plant can be used in the cases of bronchitis or cough condition, as an expectorant; in addition, it has an anti-inflammatory property and as anti-depressants. It can be used for the management and mitigation of depression and against the coronavirus. | Irshad et al. 2020 |
| 8 | Clove essential oil | An anti-viral and anti-depressant combination is effective during this COVID-19 situation. | Hussain et al. 2017 |
| 9 | Angelica essential oil | With its antispasmodic, anti-inflammatory, and anti-depressant effect, it revitalizes itself during the COVID crisis. | Zhou et al. 2020 |
| 10 | YlangYlang essential oil | Its relaxing and calming action is helpful during insomnia, irritability, anxiety, and depression conditions. | Rezaie-Keikhaie et al. 2019 |
Current aspects and future perspective
Several sensitive measures should be proposed to control COVID-19 transmission in the population. The first and most important strategy is to limit personal contact and maintain personal hygiene. The governments of various countries had taken a variety of preventive measures to halt the virus’ global spread. Special precautions should be taken to prevent disease spread and transmission to vulnerable populations such as pediatrics, geriatrics, and health care professionals. The government is introducing various schemes and funding to entice researchers to work in the COVID-19 field (Zhu et al. 2020, Ngandu et al. 2015, Wang et al. 2020a, b, c, Toots et al. 2019). When physical contact is made with contaminated and wet objects, such as urine and fecal matters, which are thought to effectively deal with the virus, an alternative route of transmission of this disorder is established. As a result, physicians and other healthcare workers should wash their hands regularly. According to various reports, health care workers and their families are also becoming infected with this virus. According to reports, coronavirus cases are seen twice or three times in the same person. Almost every country has implemented travel screening as a control and preventive measure to prevent the virus from spreading further on a global scale; in the meantime, they have imposed restrictions on airway transport (Cui et al. 2019; Ngandu et al. 2015).
The high mortality rate in the population is primarily due to patients weakened immune systems. On the other hand, the patient’s recovery rates must also be taken into account. Approaches or remedies are used to boost an individual’s immune system. One such method is to drink lemon water. The lemon is regarded as a natural immune booster and can serve as a preventive measure against this viral attack to some extent. Another measure is to gargle with hot water, which can wash out the virus in the throat and prevent its growth and multiplication. However, it is unclear how far these remedies will go. In addition, to the evolution, adaptation, and spread of the disease, the major challenges that should be effectively monitored are epidemiological changes and mutation. It has also been reported that the disease has spread to pet animals such as dogs (Cui et al. 2019; Bassetti et al. 2020). Other questions about this pandemic situation remain unanswered. Despite this, there is a belief that mother nature has a cure for all diseases and pain in humans and animals. Will she also have a cure for this pandemic situation? The mother is healing herself, which is good news during this situation.
Conclusion
COVID-19 is a condition that must be investigated and carefully considered. There is a strong push for the development of novel formulations or drug development to combat the infection’s attack. Vaccines and new drugs require time to be developed in order to pass quality, safety, and efficacy tests. As a result, it is advised to rely on herbal remedies that are considered safe and can be used as an effective tool for the management of this infection. Nature has endowed us with a tremendous inherent ability to combat pandemic issues such as COVID-19. A combination strategy could be an effective method to use. The combination of the two drugs can be two herbals such as flavonoids or a chemical moiety and herbal remedy, but the compatibility studies should come first. One such example is the use of quercetin in conjunction with a prophylactic agent to produce a synergistic effect. Quercetin has the ability to inhibit hemagglutinin-esterase, which is found in the virus structure. Curcumin is also an effective agent with anti-viral and anti-inflammatory properties. The use of volatile anti-viral agents can also aid in the treatment of corona viral infection to some extent. Because the lungs and upper respiratory tract are the primary organs affected by this infection, the volatile nature of the drugs can be useful in the management and prevention of this infection. To some extent, selecting the appropriate delivery or formulation could also help. The use of nanoparticles in the treatment of pulmonary infections is well documented. The surfactants used in the formulation of the nanoparticles aid in reducing the interfacial tension between cells and even the alveoli, making drug delivery much more beneficial when using nanotechnology. Several vaccines have been developed, and others are in different phases of clinical trials, but still, any treatment options were not developed which can completely prevent the COVID-19 infection. Hence, further research will continue to make successful treatment options to eradicate this virus.
Acknowledgements
All authors thanks to the Department of Pharmaceutics, JSS College of Pharmacy, Ooty, India, and the Department of Pharmacy, Southeast University, Banani, 1213, Dhaka, Bangladesh, for support for this study.
Author contribution
Chenmala Karthika – concept behind the article and review; Swathy Krishna R – literature survey; Md. Habibur Rahman – editing; Rokeya Akter and Deepak Kaushik – literature survey and editing. All the authors had read the manuscript and approved for submission.
Data availability
Not applicable
Declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
Not applicable
Author information
Not applicable
Scientific and ethical concern
Not applicable
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Chenmala Karthika and Rokeya Akter contributed equally to this work.
References
- Andrade BS, Siqueira S, Soares WR d A, Rangel F d S, Santos NO, Freitas A d S, Silveira PR d, Tiwari S, Alzahrani KJ, Góes-Neto A, Azevedo V, Ghosh P, Barh D. Long-COVID and post-COVID health complications: an up-to-date review on clinical conditions and their possible molecular mechanisms. Viruses. 2021;13(4):700. doi: 10.3390/V13040700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assiri A, Al-Tawfiq JA, Al-Rabeeah AA, Al-Rabiah FA, Al-Hajjar S, Al-Barrak A, Flemban H, Al-Nassir WN, Balkhy HH, Al-Hakeem RF, Makhdoom HQ. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis. 2013;13(9):752–761. doi: 10.1016/S1473-3099(13)70204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assiri A, McGeer A, Perl TM, Price CS, Al Rabeeah AA, Cummings DA, Alabdullatif ZN, Assad M, Almulhim A, Makhdoom H, Madani H. Hospital outbreak of Middle East respiratory syndrome coronavirus. New England J of Med. 2013;369(5):407–416. doi: 10.1056/NEJMoa1306742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassetti M, Vena A, Giacobbe DR. The novel Chinese coronavirus (2019-nCoV) infections: challenges for fighting the storm Eur. J Clin Invest. 2020;50(3):e13209. doi: 10.1111/eci.13209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogoch II, Watts A, Thomas-Bachli A, Huber C, Kraemer MU, Khan K. Pneumonia of unknown etiology in Wuhan, China: potential for international spread via commercial air travel. J Travel Med. 2020;27(2):taaa008. doi: 10.1093/jtm/taaa008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brubaker MD, Amatea EA, Torres-Rivera E, Miller MD, Nabors L. Barriers and supports to substance abuse service use among homeless adults. J Addict Offender Couns. 2013;34(2):81–98. doi: 10.1002/j.2161-1874.2013.00017.x. [DOI] [Google Scholar]
- Calisher C, Carroll D, Colwell R, Corley RB, Daszak P, Drosten C, Enjuanes L, Farrar J, Field H, Golding J, Gorbalenya A. Statement in support of the scientists, public health professionals, and medical professionals of China combatting COVID-19. Lancet. 2020;395(10226):e42–e43. doi: 10.1016/S0140-6736(20)30418-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappaz C, Gall A, Nuzzo C, Billonnet L (2017) A 4-In-1 Chromotherapy, Aromatherapy, light therapy, music therapy product for well-being of people. Telemed. J. E Health 5:GKR-e20.
- Chen JZ, Ji Y, Fo L, Fusing Western and Daoist Poetics (2016) In Canadian-Daoist poetics, ethics, and aesthetics Springer. Berlin, Heidelberg:49–72
- Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DK, Bleicker T, Brünink S, Schneider J, Schmidt ML, Mulders DG (2020) Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill 25(3) [DOI] [PMC free article] [PubMed]
- Cui J, Li F, Shi ZL. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol. 2019;17(3):181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou J, Chen L, Xu G, Zhang L, Zhou H, Wang H, Su Z, Ke M, Guo Q, Zhou C. Effects of baicalein on Sendai virus in vivo are linked to serum baicalin and its inhibition of hemagglutininneuraminidase. Arch Virol. 2011;156(5):793–801. doi: 10.1007/s00705-011-0917-z. [DOI] [PubMed] [Google Scholar]
- Du Toit A. Outbreak of a novel coronavirus. Nat Rev Microbiol. 2020;18(3):123. doi: 10.1038/s41579-020-0332-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehr AR, Perlman S. Coronaviruses: an overview of their replication and pathogenesis. Mol Biol 2015. 2015;1282:1–23. doi: 10.1007/978-1-4939-2438-7_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert C, Meixner F, Wiebking C, Gilg V. Regular physical activity, short-term exercise, mental health, and well-being among university students: the results of an online and a laboratory study. Front Psychol. 2020;11:509. doi: 10.3389/fpsyg.2020.00509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holshue MLD, Lindquist SL, Wiesman JB, Spitters CE, Wilkerson ST. First case of 2019 novel corona virus in the United States. NEngl J Med. 2020;2020:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Tao G, Liu J, Cai J, Huang Z, Chen J. Current prevention of COVID-19: natural products and herbal medicine. Front Pharmacol. 2020;11:1635. doi: 10.3389/FPHAR.2020.588508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui DS, Azhar IE, Madani TA, Ntoumi F, Kock R, Dar O, Ippolito G, Mchugh TD, Memish ZA, Drosten C, Zumla A. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health—the latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis. 2020;91:264–266. doi: 10.1016/j.ijid.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain MF, Hasana S, Al Mamun A, Uddin MS, Wahed MI, Sarker S, Behl T, Ullah I, Begum Y, Bulbul IJ, Amran MS (2020)COVID-19 outbreak: pathogenesis, current therapies, and potentials for future management. Front Pharmacol 11 [DOI] [PMC free article] [PubMed]
- Hussain S, Rahman R, Mushtaq A, Belaskri AE. Clove: a review of a precious species with multiple uses. Int J Boil Chem. 2017;11:129–133. [Google Scholar]
- Irshad M, Subhani MA, Ali S, Hussain A (2020) Biological importance of essential oils. Essential Oils-Oils of Nature
- Jaimes JA, Millet JK, Stout AE, André NM, Whittaker GR. A tale of two viruses: the distinct spike glycoproteins of feline coronaviruses. Viruses. 2020;12(1):83. doi: 10.3390/v12010083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin YH, Cai L, Cheng ZS, Cheng H, Deng T, Fan YP, Fang C, Huang D, Huang LQ, Huang Q, Han Y. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version) Mil Med Res. 2020;7(1):4. doi: 10.1186/s40779-020-0233-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaerunnisa S, Kurniawan H, Awaluddin R, Suhartati S, Soetjipto S (2020) Potential inhibitor of COVID-19 main protease (Mpro) from several medicinal plant compounds by molecular docking study. 10.20944/PREPRINTS202003.0226.V1
- Karthika C, Sureshkumar R (2021) Can nature heal and be a possible remedy for the management of Covid-19? Infect Disord Drug Targets 21. 10.2174/1871526521999210111202954 [DOI] [PubMed]
- Kianpour M, Mansouri A, Mehrabi T, Asghari G. Effect of lavender scent inhalation on prevention of stress, anxiety and depression in the postpartum period. Iran J Nurs Midwifery Res. 2016;21(2):197–201. doi: 10.4103/1735-9066.178248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalani S, Poh CL. Flavonoids as antiviral agents for Enterovirus A71 (EV-A71) Viruses. 2020;12(2):184. doi: 10.3390/v12020184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee N, Hui D, Wu A, Chan P, Cameron P, Joynt GM, Ahuja A, Yung MY, Leung CB, To KF. Lui SF. A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348(20):1986–1994. doi: 10.1056/NEJMoa030685. [DOI] [PubMed] [Google Scholar]
- Lu H, Stratton CW, Tang YW. Outbreak of pneumonia of unknown etiology in Wuhan China: the mystery and the miracle. J Med Virol. 2020;92(4):401–402. doi: 10.1002/jmv.25678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, Wang W, Song H, Huang B, Zhu N, Bi Y. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, Kar SK, Suthar N, Nebhinani N. Vitamin D and depression: a critical appraisal of the evidence and future directions. Indian J Psychol Med. 2020;42(1):11–21. doi: 10.4103/IJPSYM.IJPSYM_160_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell F. Vitamin-D and COVID-19: do deficient risk a poorer outcome? (2020) The Lancet Diabetes & Endocrinology 8(7):570. [DOI] [PMC free article] [PubMed]
- Moniruzzaman M, Hossain MU, Islam MN, Rahman MH, Ahmed I, Rahman TA, Bhattacharjee A, Amin MR, Rashed A, Keya CA, Das KC. Coding-complete genome sequence of SARS-CoV-2 isolate from Bangladesh by sanger sequencing. Microbiology Resource Announcements. 2020;9(28):e00626–e00620. doi: 10.1128/MRA.00626-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngandu T, Lehtisalo J, Solomon A, Levälahti E, Ahtiluoto S, Antikainen R, Bäckman L, Hänninen T, Jula A, Laatikainen T, Lindström J. A 2year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385(9984):2255–2263. doi: 10.1016/S0140-6736(15)60461-5. [DOI] [PubMed] [Google Scholar]
- Nishiura H, Jung SM, Linton NM, Kinoshita R, Yang Y, Hayashi K, Kobayashi T, Yuan B, Akhmetzhanov AR. The extent of transmission of novel coronavirus in Wuhan, China. J Clin Med. 2020;9(2):330. doi: 10.3390/jcm9020330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pablos J, Abasolo Lydia, Alvaro-Gracia J, Blanco F J, Blanco R, castrejón isabel, Fernandez-Fernandez david, Fernandez-gutierrez B, galindo-izquierdo, maría, gonzalez-gay, miguel, manrique-arija, sara, mena Vázquez, natalia, mera Varela, antonio, Retuerto, miriam, & seijas-lopez, alvaro (2020) Prevalence of hospital PCR-confirmed COVID-19 cases in patients with chronic inflammatory and autoimmune rheumatic diseases. Ann Rheum Dis 0, 1–4. 10.1136/annrheumdis-2020-217763, 79 [DOI] [PMC free article] [PubMed]
- Pai SR, Sonkamble VV, Wagh NS (2020) Essential oils as effective agents against neurological disorders. InPlant-derived Bioactives 2020 Springer, Singapore. 409-433.10.1007/978-981-15-1761-7_17
- Pastor N, Collado MC, & Manzoni P (2021) Phytonutrient and nutraceutical action against COVID-19: current review of characteristics and benefits. 10.3390/nu13020464 [DOI] [PMC free article] [PubMed]
- Phan LT, Nguyen TV, Luong QC, Nguyen TV, Nguyen HT, Le HQ, Nguyen TT, Cao TM, Pham QD. Importation and human-to-human transmission of a novel coronavirus in Vietnam. N Engl J Med. 2020;382(9):872–874. doi: 10.1056/NEJMc2001272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman MH, Akter R, Behl T, Chowdhury MA, Mohammed M, Bulbul IJ, Elshenawy SE, Kamal MA. COVID-19 outbreak and emerging management through pharmaceutical therapeutic strategy. Curr Pharm Des. 2020;26(41):5224–5240. doi: 10.2174/1381612826666200713174140. [DOI] [PubMed] [Google Scholar]
- Rahman MH, Zafri NM, Ashik FR, Waliullah M, Khan A. Identification of risk factors contributing to COVID-19 incidence rates in Bangladesh: a GIS-based spatial modeling approach. Heliyon. 2021;7(2):e06260. doi: 10.1016/j.heliyon.2021.e06260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezaie-Keikhaie K, Hastings-Tolsma M, Bouya S, Shad FS, Sari M, Shoorvazi M, Barani ZY, Balouchi A. Effect of aromatherapy on post-partum complications: a systematic review. Complement Ther Clin Pract. 2019;35:290–295. doi: 10.1016/j.ctcp.2019.03.010. [DOI] [PubMed] [Google Scholar]
- Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun. 2020;109:102433. doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setorki M. Medicinal herbs with anti-depressant effects. J Herbmed Pharmacol. 2020;9(4):309–317. doi: 10.34172/jhp.2020.39. [DOI] [Google Scholar]
- Shah SA, Bungau S, Si Y, Xu H, Rahman M, Behl T, Gitea D, Pavel FM, Corb Aron RA, Pasca B, Nemeth S. Chemically diverse and biologically active secondary metabolites from marine Phylum chlorophyta. Marine Drugs. 2021;18(10):493. doi: 10.3390/md18100493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Batra S, Gupta S, Sharma VK, Rahman MH, Kamal MA (2021) Persons with co-existing neurological disorders: risk analysis, considerations and management in COVID-19 pandemic. CNS Neurol Disord Drug Targets 20 [DOI] [PubMed]
- Thompson R. Pandemic potential of 2019-nCoV. Lancet Infect Dis. 2020;20(3):280. doi: 10.1016/S1473-3099(20)30068-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toots M, Yoon JJ, Cox RM, Hart M, Sticher ZM, Makhsous N, Plesker R, Barrena AH, Reddy PG, Mitchell DG, Shean RC. Characterization of orally efficacious influenza drug with high resistance barrier in ferrets and human airway epithelia. Sci Transl Med. 2019;11(515):eaax5866. doi: 10.1126/scitranslmed.aax5866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Törnblom H, Drossman DA (2018) Psychotropics, antidepressants, and visceral analgesics in functional gastrointestinal disorders. Curr Gastroenterol Rep 20(12):1-0. [DOI] [PMC free article] [PubMed]
- UPDATED Comparing COVID-19 vaccines: timelines, types and prices | BioSpace. (n.d.). Retrieved July 21, 2021, from https://www.biospace.com/article/comparing-covid-19-vaccines-pfizer-biontech-moderna-AstraZeneca-oxford-j-and-j-russia-s-sputnik-v/
- Van Hees S, Fodjo JN, Wijtvliet V, Van den Bergh R, de MouraVillela EF, da Silva CF, Weckhuysen S, Colebunders R. Access to healthcare and prevalence of anxiety and depression in persons with epilepsy during the COVID-19 pandemic: a multicountry online survey. Epilepsy Behav. 2020;112:107350. doi: 10.1016/j.yebeh.2020.107350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Y, Shang J, Graham R, Baric RS, Li F (2020) Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J Virol 94(7) [DOI] [PMC free article] [PubMed]
- Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, Shi Z, Hu Z, Zhong W, Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Tang J, Wei F. Updated understanding of the outbreak of 2019 novel coronavirus (2019-nCoV) in Wuhan, China. J Med Virol. 2020;92(4):441–447. doi: 10.1002/jmv.25689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Chen X, Lu Y, Chen F, Zhang W. Clinical characteristics and therapeutic procedure for four cases with 2019 novel coronavirus pneumonia receiving combined Chinese and Western medicine treatment. Biosci Trends. 2020;14:64–68. doi: 10.5582/bst.2020.01030. [DOI] [PubMed] [Google Scholar]
- What you need to know about COVID-19 vaccines | UNICEF. (n.d.). Retrieved July 21, 2021, from https://www.unicef.org/coronavirus/what-you-need-to-know-covid-vaccine
- Woods HC, Scott H. Sleepyteens: Social media use in adolescence is associated with poor sleep quality, anxiety, depression and low self-esteem. J Adolesc. 2016;51:41–49. doi: 10.1016/j.adolescence.2016.05.008. [DOI] [PubMed] [Google Scholar]
- Wu P, Hao X, Lau EH, Wong JY, Leung KS, Wu JT, Cowling BJ, Leung GM (2020)Real-time tentative assessment of the epidemiological characteristics of novel coronavirus infections in Wuhan, China, as at 22 January 2020. Euro Surveill 25(3) [DOI] [PMC free article] [PubMed]
- Zakaryan H, Arabyan E, Oo A, Zandi K. Flavonoids: promising natural compounds against viral infections. Arch Virol. 2017;162(9):2539–2551. doi: 10.1007/s00705-017-3417-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Lin Q, Ran J, Musa SS, Yang G, Wang W, Lou Y, Gao D, Yang L, He D, Wang MH. Preliminary estimation of the basic reproduction number of novel coronavirus (2019-nCoV) in China, from 2019 to 2020: a data-driven analysis in the early phase of the outbreak. Int J Infect Dis. 2020;92:214–217. doi: 10.1016/j.ijid.2020.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Miao X, Lan X, Luo J, Luo T, Zhong Z, Gao X, Mafang Z, Ji J, Wang H, Tang Y (2020) angelica essential oil loaded electrospun gelatin nanofibers for active food packaging application. Polymers 12(2):299. [DOI] [PMC free article] [PubMed]
- Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P. China novel coronavirus investigating and research team. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable