As of June 17, 2021, 21.1% of the world population has received at least one dose of any COVID-19 vaccine [1]. Dermatologists have rapidly witnessed an increasing number of cutaneous adverse events, especially “COVID-arm” [2], [3]. Cases of herpes zoster (HZ) have started to be published at an increasing rate very recently [4], [5], [6], [7], [8], [9]. McMahon et al. reported 10 cases of herpes zoster (HZ) and four cases of herpes simplex virus (HSV) flares after Moderna and Pfizer vaccination [4]. Herein, we report four additional cases, including two patients under biologics.
A 39-year-old woman was referred to the emergency unit for an acute facial rash present for five days. It began on the evening after the first injection of BNT162b2 mRNA COVID-19 vaccine (Pfizer) on the left upper arm. Her past medical history was notable for psoriasis, for which she had recently started guselkumab, a monoclonal antibody directed against interleukin 23 (100 mg, two injections one month apart, with the second injection being given five weeks prior to vaccination). She had never experienced labial herpes. The eruption started on the forehead and extended to the bridge of the nose and the side of the neck. A few sparse lesions were also noted on the lower part of the face and chin (Fig. 1 ). She reported puffiness and dusky red periorbital oedema (Fig. 2 ). Upon examination, her clinical presentation was in favour of a herpes virus infection. The patient was in good condition although tired. She denied fever, chills or loss of appetite. She had no headache and no kerato-conjunctivitis, and cranial nerve examination was normal. She had palpable cervical lymph nodes and sensitive preauricular lymph node enlargement. Although the rash was not restricted to one or several dermatomes, we chose to start valaciclovir 1 g t.i.d. for one week. Cutaneous polymerase chain reaction was positive for HSV-1 but negative for varicella zoster virus (VZV). The eruption subsided 3–4 days after the consultation. At the one-week follow-up call, the patient had only a few crusts remaining. She was authorized to receive her next guselkumab injection the following week. The second dose of BNT162b2 mRNA vaccine was planned for July 2021. To date, the patient has not contacted us as instructed to do if the eruption did not subside.
Fig. 1.

Herpetiform rash on the forehead and the left side of the neck. Scattered lesions around the mouth and chin and on the neck folds.
Fig. 2.
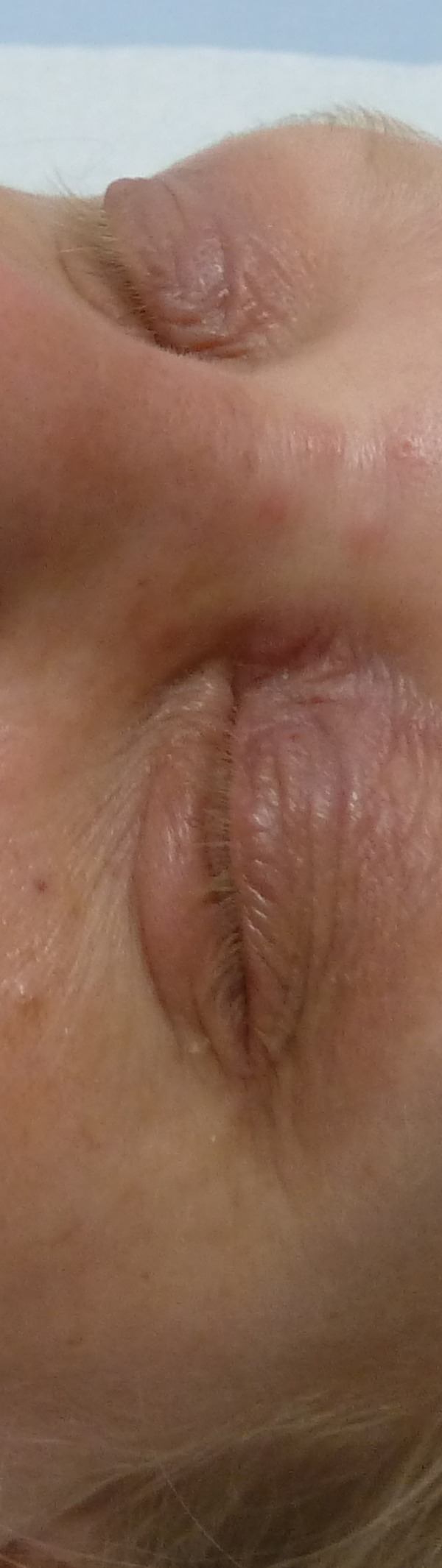
Close-up view of the eyes showing dusky red eyelids.
A 70-year-old man with a past history of myeloma under a VCD protocol (bortezomib, cyclophosphamide, dexamethasone, every 21 days) presented for follow-up of his eczematous dermatitis in May 2021. He had first been seen two months earlier and was instructed to apply highly potent corticosteroid ointment. Although the treatment was efficient, in April 2021, 10 days after receiving BNT162b2 mRNA COVID-19 vaccine, he developed a typical HZ eruption on his right arm along the C8 dermatome, which was confirmed by polymerase chain reaction. He complained of post-zoster neuralgia. He continued to present some active lesions a month after symptom onset, for which he was given valaciclovir 3 g per day for a week and which proved effective.
A 63-year-old woman with a past history of childhood chickenpox and rheumatoid arthritis treated by etanercept for the past 10 years (50 mg every 9 days) developed an HZ infection on the right side of the abdomen 24 hours after a first dose of ChAdOx1 nCoV-19 vaccine (Oxford-Astra-Zeneca) in her left arm. She presented at consultation with a typical HZ eruption that had been present for 3 days. She was in good condition and HZ was not a source of discomfort, so no treatment was initiated. A favourable outcome was achieved within seven days without treatment. The outcome after her second vaccine injection is unknown.
A 51-year-old woman with a history of confirmed relapsing palatal herpes developed HZ of the scalp. This HZ was followed by pain in the face and possibly in the oral cavity. These events occurred 2–3 weeks after her first dose of BNT162b2 mRNA vaccine. The case was not documented further.
The rapid increase in reporting since April 2021 suggests a possible immunomodulatory effect of COVID-19 vaccines on reactivation of herpes viruses Varicella Zoster Virus or HSV). According to reports by the French Medicines Agency (ANSM – Agence nationale de sécurité du médicament et des produits de santé), HZ is among the “potential signs or events under surveillance” associated with SARS-CoV-2 vaccines [10]. To date, 184 cases of HZ have been reported following administration of BNT162b2 mRNA vaccine (Pfizer), 66 cases after mRNA-1273 vaccine (Moderna), and 216 cases following administration of ChAdOx1 nCoV-19 vaccine (Oxford-Astra-Zeneca) [10]. In our series, two patients were treated with biologics for psoriasis and rheumatoid arthritis, and one patient was treated for myeloma. In the first case, we hypothesize that dissemination of HSV-1 infection may have been related to the immunosuppressive effect of guselkumab, which had not yet reached its peak efficacy. In an Israeli series of patients with autoinflammatory rheumatic diseases, 1.2% of patients (6 of 491) developed HZ two days to two weeks after injection of BNT162b2 mRNA vaccine, compared to no subjects in the control group (n = 99). None had ever had HZ previously. HZ was considered mild in most cases, and a second dose of vaccine was given without side effects [6], [9]. HZ can develop after the first or second dose of vaccine [6], [7]. Although vaccination of patients under biologics is mandatory, our first case illustrates that herpes virus reactivation may be more extensive in the event of recent initiation of biologics. Caution is thus warranted wherever initiation of a biotherapy and vaccination are planned concurrently within a short period. We recommend first injecting the initial dose of SARS-CoV-2 vaccine and starting the biotherapy afterwards, wherever the situation allows. Finally, our first patient presented a dusky red periorbital oedema already described in a previous case report and attributed to COVID-19 [11]. While our observation may have been fortuitous, the palpebral redness was striking and the patient did not present this symptom before vaccination. Interestingly, Mazzatenta et al. reported a case of purpura of the eyelids following BTN162b2 vaccination [12]. Further, cases of chilblains after administration of BNT162b2 vaccine have also been recently described [13], [14]. Immune response to vaccine may trigger symptoms similar to those observed during COVID-19. We felt that our findings were noteworthy and worth reporting in case other colleagues have encountered similar situations.
Informed consent
The patient gave her consent for use of the pictures.
Disclosure of interest
The authors declare that they have no competing interest.
References
- 1.Our World in data https://ourworldindata.org/covid-vaccinations.[accessed June 18, 2021].
- 2.Blumenthal K.G., Freeman E.E., Saff R.R., Robinson L.B., Wolfson A.R., Foreman R.K., et al. Delayed large local reactions to mRNA-1273 vaccine against SARS-CoV-2. N Engl J Med. 2021;384:1273–1277. doi: 10.1056/NEJMc2102131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fernandez-Nieto D., Hammerle J., Fernandez-Escribano M., Moreno-Del Real C.M., Garcia-Abellas P., Carretero-Barrio I., et al. Skin manifestations of the BNT162b2 mRNA COVID-19 vaccine in healthcare workers. “COVID-arm”: a clinical and histological characterization. J Eur Acad Dermatol Venereol. 2021 doi: 10.1111/jdv.17250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McMahon D.E., Amerson E., Rosenbach M., Lipoff J.B., Moustafa D., Tyagi A., et al. Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: a registry-based study of 414 cases. J Am Acad Dermatol. 2021 doi: 10.1016/j.jaad.2021.03.092. [S0190-9622(21)00658-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bostan E., Yalici-Armagan B. Herpes zoster following inactivated COVID-19 vaccine: a coexistence or coincidence? J Cosmet Dermatol. 2021 doi: 10.1111/jocd.14035. [DOI] [PubMed] [Google Scholar]
- 6.Furer V., Zisman D., Kibari A., Rimar D., Paran Y., Elkayam O. Herpes zoster following BNT162b2 mRNA COVID-19 vaccination in patients with autoimmune inflammatory rheumatic diseases: a case series. Rheumatology (Oxford) 2021:keab345. doi: 10.1093/rheumatology/keab345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodríguez-Jiménez P., Chicharro P., Martos-Cabrera L., Seguí M., Caballero ÁM, Llamas-Velasco M., et al. Varicella-zoster virus reactivation after SARS-Cov2 BNT162b2 mRNA vaccination: report of five cases. JAAD Case Rep. 2021;15:62–63. doi: 10.1016/j.jdcr.2021.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eid E., Abdullah L., Kurban M., Abbas O. Herpes zoster emergence following mRNA COVID-19 vaccine. J Med Virol. 2021 doi: 10.1002/jmv.27036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tessas I., Kluger N. Ipsilateral herpes zoster after the first dose of BNT162b2 mRNA COVID-19 vaccine. J Eur Acad Dermatol Venereol. 2021 doi: 10.1111/jdv.17422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.ANSM. Enquête de pharmacovigilance et comité de suivi. https://ansm.sante.fr/dossiers-thematiques/covid-19-vaccins/covid-19-suivi-hebdomadaire-des-cas-deffets-indesirables-des-vaccins.[accessed June 18, 2021].
- 11.Kalner S., Vergilis I.J. Periorbital erythema as a presenting sign of COVID-19. JAAD Case Rep. 2020;6:996–998. doi: 10.1016/j.jdcr.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mazzatenta C., Piccolo V., Pace G., Romano I., Argenziano G., Bassi A. Purpuric lesions on the eyelids developed after BNT162b2 mRNA COVID-19 vaccine: another piece of SARS-CoV-2 skin puzzle? J Eur Acad Dermatol Venereol. 2021 doi: 10.1111/jdv.17340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lopez S., Vakharia P., Vandergriff T., Freeman E.E., Vasquez R. Pernio after COVID-19 vaccination. Br J Dermatol. 2021 doi: 10.1111/bjd.20404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lesort C., Kanitakis J., Donzier L., Jullien D. Chilblain-like lesions after BNT162b2 mRNA COVID-19 vaccine: a case report suggesting that “COVID toes” are due to the immune reaction to SARS-CoV-2. J Eur Acad Dermatol Venereol. 2021 doi: 10.1111/jdv.17451. [DOI] [PMC free article] [PubMed] [Google Scholar]


