Abstract
Purpose
Racial/ethnic minoritized groups, women, and economically disadvantaged groups are disproportionately affected by the COVID-19 pandemic. We investigated racial/ethnic differences by gender in correlates of COVID-19 infection among veterans seeking health care services at the Veterans Health Administration. Little is known about gender-specific factors associated with infection among veterans. This study seeks to fill this gap.
Methods
The sample was veterans with results from a COVID-19 test (polymerase chain reaction) conducted at Veterans Health Administration facilities between March 1, 2020, and August 5, 2020, and linked to the Centers for Disease Control and Prevention Social Vulnerability Index data (39,223 women and 316,380 men). Bivariate, multivariate logistic, and predicted probability analyses were conducted. All analyses were stratified by gender.
Results
Similar percentages of women and men tested positive for COVID-19 (9.6% vs. 10.0%). In multivariate analysis, compared with non-Hispanic White women, American Indian/Alaska Native, Black, and Hispanic women all had significantly higher odds of infection. Similar racial/ethnic differences were found for men. Both older men and women (>40 years) had lower odds of infection, but the age cut points differed (40 for women, 55 for men). Men 80 years and older had a higher odds than those aged less than 40 years of age. For men, but not for women, being employed (vs. unemployed) was associated with an increased odds of infection, and having comorbidities was associated with decreased odds. There were significant differences within and across gender-by-race/ethnicity in infection, after adjusting for covariates.
Conclusions
American Indian/Alaska Native, Hispanic, and Black women and men veterans are disproportionately impacted by COVID-19 infection. Widespread testing and tracking, education, and outreach regarding COVID-19 mitigation and vaccination efforts are recommended.
The COVID-19 pandemic has exposed long-standing structural, social, and economic inequities that disproportionately affect minoritized racial/ethnic groups, women, and economically disadvantaged groups. Minoritized racial and ethnic groups and the economically disadvantaged have higher rates of COVID-19 infection, greater health consequences, and higher mortality than non-Hispanic White people and the more affluent (Chowkwanyun & Reed, 2020; Moore et al., 2020; Price-Haywood, Burton, Fort, & Seoane, 2020; Richardson et al., 2020; Wadhera et al., 2020). These groups are also more likely to be essential workers, have less of an ability to practice physical distancing because of work requirements and/or living situations, and have more limited access to testing and health care (Artiga, Rae, Pham, Hamel, & Munana, 2020; Dodds & Fakoya, 2020; McCormack, Avery, Spitzer, & Chandra, 2020). They also may be more likely to have underlying health conditions, putting them at greater risk of serious complications and death (Raifman & Raifman, 2020; Richardson et al., 2020). Although women, on average, have less severe COVID-19 health consequences than men (Bhopal & Bhopal, 2020; Griffith et al, 2020; Sharma, Volgman, & Michos, 2020; Wenham, Smith, Morgan, & Gender and COVID-19 Working Group, 2020), the social, economic, and professional consequences of the pandemic disproportionally affect women (Alon, Doepke, Olmstead-Rumsey, & Tertilt, 2020).
State-level case rates show women have similar infection rates as men (Harvard GenderSci Lab, 2020), but less is known about the extent to which racial/ethnic differences may depend on gender. This is important to consider because the intersection of gender and race/ethnicity might leave some groups at even higher risk. For example, women of color are more likely to be employed in jobs that put them at increased risk of exposure to the virus, including essential workers and caregiving positions (Artiga, et al., 2020; Kambhampati et al., 2020). For these reasons, there may be an excess burden with respect to positive cases especially among Black and Hispanic women.
Two recent COVID-19 studies investigating racial/ethnic differences found Black and Hispanic veterans have an excess burden of infection (Ferguson et al., 2021a; Rentsch et al., 2020). We further examine racial/ethnic differences in COVID-19 infection by gender using a large, national, racially/ethnically diverse sample of veterans who received a COVID-19 test from the Veterans Health Administration (VHA) between March and August 2020. The VHA, which provides health care to eligible veterans without requirement for an insurance premium, provides a national setting for evaluating gender-specific racial/ethnic differences that are independent of financial healthcare access (Washington, Villa, Brown, Damron-Rodriguez, & Harada, 2005).
Although the veteran population is projected to decrease over time, the number of women veterans is projected to increase from approximately 1.9 million to 2.2 million over the next 25 years (National Center for Veterans Analysis and Statistics, 2017). Relative to men, women veterans are younger, more racially diverse, and more educated, but have similar physical and mental illness burden as well as gender-specific health care needs (e.g., related to military sexual trauma and reproductive care) (Frayne et al., 2006; Han, Yano, Watson, & Ebrahimi, 2019; Harrington et al., 2019; Lehavot, Hoerster, Nelson, Jakupcak, & Simpson, 2012; Washington, Davis, Der-Martirosian, & Yano, 2013). Although there is a growing research agenda specifically focused on women veterans’ health and well-being (Frayne et al., 2006; Washington, Farmer, Mor, Canning, & Yano, 2015; Yano et al., 2006), little is known about factors associated with COVID-19 infection among women veterans. This study seeks to fill this gap.
We use a social determinants of health framework to guide our research questions and analysis (Braveman, Egerter, & Williams, 2011a). Informed by this framework, we propose that structural and economic factors shape the opportunities and resources available to individuals that can be used to invest in health, economic well-being, and overall quality of life, thus allowing individuals to avoid or minimize exposure to COVID-19. These opportunities and resources are unequally distributed across groups, with lower access among minoritized racial/ethnic groups, women, and the economically disadvantaged (Braveman et al., 2011a; Braveman et al., 2011b; Link & Phelan, 1995; Williams, Lawrence, & Davis, 2019). Our framework is also informed by intersectionality theory such that the impact of multiple dimensions of social placement are nonadditive in nature (Bowleg, 2012; Crenshaw, 1991). To date, few COVID-19 infection studies have included gender-stratified analyses, potentially obscuring importance differences between women and men (Griffith et al., 2020; Jin et al., 2020; Wenham et al., 2020). We therefore examined COVID-19 infection in veterans by race/ethnicity, separately for women and men. We conducted additional analyses allowing for comparisons across all gender-by-race/ethnicity groups. We hypothesized that, in general, men and women who are minoritized will be more likely to test positive for COVID-19 than White veterans and anticipate a gender-by-race/ethnicity interaction.
Methods
Data and Sample
Our sample consisted of veterans with results from a valid COVID-19 test conducted at any VHA facility between March 1, 2020, and August 5, 2020, excluding veteran VHA employees. Data came from a national database of individuals evaluated in the VHA for respiratory illness or COVID-19 exposure, linked to VHA's administrative records and the Centers for Disease Control and Prevention (CDC) 2018 Social Vulnerability Index (SVI) (most recent SVI available). We used county-level FIPS codes (county-level geographic identifiers) for the patient's residential address to link patient data to the SVI. The SVI is used to assess community-level preparedness for and response to a variety of hazardous events, such as infectious disease outbreaks, including the current COVID-19 pandemic (Karaye & Horney, 2020). This work received a Determination of Non-Research form the Institutional Review Board of the VA Greater Los Angeles Healthcare System.
Measures
Our dependent measure was COVID-19 test status: positive or negative using polymerase chain reaction tests obtained via nasopharyngeal swabs. Our main independent measure was veterans’ race/ethnicity: non-Hispanic White (White) (reference), non-Hispanic American Indian and Alaska Native (AI/AN), non-Hispanic Asian (Asian), non-Hispanic Black (Black), Hispanic, non-Hispanic Native Hawaiian and Other Pacific Islander (NH/OPI), Multiracial, and unknown/missing.
Other individual-level variables included age (<40 years, 40–54 years, 55–64 years, 65–80 years, and ≥80 years); individual-level socioeconomic status (SES) (low, high, and military service-connected disability); employment (employed, not employed, and retired); prior diagnosis of any (vs. no) comorbidities initially listed by CDC as risk factors for severe COVID-19 (chronic kidney disease stage 5 or end-stage renal disease, chronic pulmonary disease, diabetes, heart disease, immunocompromised state, liver disease, hypertension, asthma, and severe obesity) (Stokes et al., 2020); and a time period indicator of when the COVID-19 test was conducted. We determined individual-level SES based on the VA's enrollment priority group: low individual-level SES included veterans with an income below the VA's threshold for required copayment for care and high individual-level SES included those above the threshold. Individual-level SES is unknown for veterans with service-connected disability, because these veterans do not have to provide income information to determine their VA copayment eligibility; this group constituted the third income category. We obtained information about prior diagnosis of CDC's COVID-19–related comorbidities from International Classification of Diseases, 10th edition, codes between March 3, 2018, and February 1, 2020. We classified individuals who did not have a primary care visit during this time period (and thus have less opportunity for assessment of comorbidities) as missing comorbidity information. Because testing and spread of the pandemic has changed over time (Boehmer et al., 2020; Stokes et al., 2020), we also included an indicator of when the COVID-19 test occurred in 2020 corresponding with general changes in case rates and social distancing policies: earlier (March through May) and later (June through August) (Kaiser Family Foundation, 2020).
Because COVID-19 infection varies at the county and community levels, we also adjusted for three county-level SVI domains: county-level SES, household composition/disability, and minoritized status/non-English speakers (Chen & Krieger, 2021; Kim & Bostwick, 2020; Nayak et al., 2020). The county-level SES domain included the following U.S. Census measures of county-level characteristics: the percent of the population with an income below poverty, the percent of the population that is unemployed, the median household income, and the percent of the population with no high school diploma. The household composition/disability domain included the following county characteristics: the percent of the population age 65 years and older, the percent of the population age 17 years and younger, the percent of the population older than age 5 with a disability, and the percent of households with a single parent. The minoritized status/non-English speakers domain comprised of two county-level characteristics: the percent of the population who were of racial/ethnic groups that are minoritized, and the percent of the population who spoke English “less than well” (combined response categories of speaks English “not well” and “not at all”).
Statistical Analysis
We calculated descriptive statistics of means and proportions for the sample and the percentages positive for COVID-19 separately for women and men. To investigate the extent to which some racial/ethnic groups were disproportionately represented in the COVID-19 positive sample, we first qualitatively compared racial/ethnic composition of the study sample (i.e., those who received a COVID-19 test) with those testing positive for COVID-19, for women and men. We fit separate logistic regression models for women and men to examine the relationship between race/ethnicity and COVID-19 test positivity. Because few studies have conducted multivariate gender-stratified models and because of its conceptual importance, we report our findings separately for women and men. Informed by our conceptual framework, we sequentially fit the following models: 1) unadjusted association between race/ethnicity and COVID-19 test positivity, 2) adjusted for individual-level characteristics (age, individual-level SES, employment, CDC comorbidity status, and time period of testing), and 3) further adjusted for county-level SVI. We clustered standard errors at the VA facility level to account for facility-level and regional variation in COVID-19 infection and testing. To further explore gender and race/ethnicity intersectionalities, we used postestimation commands to calculate predicted probabilities from model 3 and compared probabilities of COVID positivity across all gender-by-race/ethnicity groups. To address multiple comparisons, we used a higher confidence threshold than is typical and considered differences across models to be statically significant if the 99% confidence intervals (CIs) of the predicted probabilities did not overlap. All analyses were conducted using Stata 15.1 (StataCorp LLC, College Station, TX).
Results
Table 1 shows the respondent characteristics of the sample, overall and by gender. In total, 355,603 veterans were tested between March and August and had valid test results. Overall, 89% of the sample were men and 11% were women, which is comparable with the veteran population (National Center for Veterans Analysis and Statistics, 2017). There were significant gender differences for all demographic, health, and SVI county-level variables. Relative to men, a lower percentage of women were White (49.5% vs. 61.3%) and a higher percentage were Black (31.8% vs. 22.6%). Approximately 9% of men and women were Hispanic. A higher percentage of women (26.0%) were less than age 40 compared with men (11.1%), and only 13.7% of women were ages 65–79 years compared with 43.3% of men. About 1 in 10 women and men had high individual-level SES. Fourteen percent of women and 20.2% of men had low individual-level SES. Slightly more than one-third of women (37.3%) and 29.4% of men were employed. A higher percentage of men were retired (23.0%) compared with women (8.3%). Seventy-eight percent of men reported one comorbidity or more; 59.3% of women reported one or more. The majority of women and men were tested in the June to August time period. Among county-level SVI domains, there were statistically significant, although not necessarily clinically relevant, differences in mean SVI between women and men. Last, overall, 9.9% of veterans tested positive for COVID-19: 9.6% of women and 10.0% of men.
Table 1.
Characteristics of Veterans who Obtained COVID-19 Tests, March 1, 2020–August 5, 2020
| Sex |
Total % or Mean (N = 355,603) |
|||
|---|---|---|---|---|
| Women % or Mean (n = 39,223) |
Men % or Mean (n = 316,380) |
Difference p Value | ||
| Race/ethnicity, % | ||||
| White | 49.5 | 61.3 | <.001 | 60.0 |
| AI/AN | 1.0 | 0.7 | 0.7 | |
| Asian | 1.5 | 1.1 | 1.1 | |
| Black | 31.8 | 22.6 | 23.6 | |
| Hispanic | 9.2 | 8.5 | 8.6 | |
| NH/OPI | 0.9 | 0.7 | 0.7 | |
| Multiracial | 1.4 | 0.8 | 0.9 | |
| Unknown/missing | 4.7 | 4.2 | 4.3 | |
| Age, % | ||||
| <40 years | 26.0 | 11.1 | <.001 | 12.7 |
| 40–54 years | 32.3 | 15.1 | 17.0 | |
| 55–64 years | 26.3 | 20.9 | 21.5 | |
| 65–79 years | 13.7 | 43.3 | 40.1 | |
| ≥80 years | 1.8 | 9.6 | 8.7 | |
| Individual SES/service-connected disability, % | ||||
| High individual SES | 10.6 | 11.4 | <.001 | 11.3 |
| Low individual SES | 14.0 | 20.2 | 19.5 | |
| Service-connected disability | 75.4 | 68.4 | 69.2 | |
| Employment status, % | ||||
| Not employed | 45.2 | 41.5 | <.001 | 41.9 |
| Employed | 37.3 | 29.4 | 30.3 | |
| Retired | 8.3 | 23.0 | 21.3 | |
| Unknown | 9.2 | 6.2 | 6.5 | |
| Comorbidity status, % | ||||
| None | 34.6 | 16.5 | <.001 | 18.5 |
| ≥1 comorbidity | 59.3 | 78.3 | 76.2 | |
| Missing | 6.1 | 5.1 | 5.2 | |
| Time period, % | ||||
| March–May | 28.5 | 29.3 | .001 | 29.2 |
| June–August | 71.5 | 70.7 | 70.8 | |
| Social vulnerability index domains,∗ mean (SD) | ||||
| Residential SES | 0.43 (0.23) | 0.44 (0.24) | <.001 | 0.39 (7.62) |
| Household composition/disability | 0.36 (0.25) | 0.36 (0.26) | .02 | 0.36 (0.26) |
| Minoritized status/non-English speakers | 0.78 (0.21) | 0.75 (0.24) | <.001 | 0.75 (0.24) |
| COVID-19 test status, % | ||||
| Negative | 90.4 | 90.0 | .02 | 90.1 |
| Positive | 9.6 | 10.0 | 9.9 | |
Abbreviations: AI/AN, American Indian and Alaska Native; NH/OPI, Native Hawaiian and Other Pacific Islander; SD, standard deviation; SES, socioeconomic status; SVI, Social Vulnerability Index.
The p values are from χ2 test comparison between men and women.
The SVI ranged from 0 to 1, where higher numbers indicate greater vulnerability. Each SVI domain was created by summing percentiles of individual county-level characteristics from 2014 to 2018 U S. Census estimates. Summed percentiles were then rank-ordered by county to determine domain-specific percentile.
Table 2 presents COVID-19 positivity rates for all covariates, and significant differences across levels of each variable, stratified by gender. There were significant racial/ethnic differences in infection for both genders. Among women, Hispanic and Black women had the highest COVID-19 positivity rate (13.5% and 13.4% respectively), followed by AI/AN (10.8%), Asian (8.7%), NH/OPI (8.6%), Multiracial (6.8%), and White (6.6%) women. Patterns of COVID-19 positivity rates for men were similar, with Hispanic (15.9%) and Black (14.5%) men having the highest rates, followed by AI/AN (10.6%), NH/OPI (9.8%), Multiracial (8.9%), Asian (8.4%), and White (7.5%) men.
Table 2.
Percentage of Veteran Sample who Tested Positive for COVID-19, March 1, 2020–August 5, 2020 (N = 35,338)
| Women |
Men |
|||
|---|---|---|---|---|
| Percentage | p Value | Percentage | p Value | |
| Race/ethnicity | ||||
| White | 6.6 | <.001 | 7.5 | <.001 |
| AI/AN | 10.8 | 10.6 | ||
| Asian | 8.7 | 8.4 | ||
| Black | 13.4 | 14.5 | ||
| Hispanic | 13.5 | 15.9 | ||
| NH/OPI | 8.6 | 9.8 | ||
| Multiracial | 6.8 | 8.9 | ||
| Age | ||||
| <40 years | 12.1 | <.001 | 12.6 | <.001 |
| 40–54 years | 9.8 | 12.2 | ||
| 55–64 years | 8.1 | 9.3 | ||
| 65–79 years | 7.1 | 8.4 | ||
| ≥80 years | 10.8 | 11.8 | ||
| Individual SES/service-connected disability | ||||
| High individual SES | 11.0 | .001 | 11.1 | <.001 |
| Low individual SES | 8.9 | 8.4 | ||
| Service-connected disability | 9.6 | 10.3 | ||
| Employment status | ||||
| Not employed | 9.7 | <.001 | 9.7 | <.001 |
| Employed | 10.1 | 11.1 | ||
| Retired | 6.8 | 8.8 | ||
| Unknown | 9.6 | 10.5 | ||
| Comorbidity status | ||||
| None | 10.4 | <.001 | 11.5 | <.001 |
| ≥1 comorbidity | 9.1 | 9.5 | ||
| Missing | 10.0 | 12.6 | ||
| Time period | ||||
| March–May | 9.8 | .417 | 12.3 | <.001 |
| June–August | 9.5 | 9.0 | ||
Abbreviations: AI/AN, American Indian and Alaska Native; NH/OPI, Native Hawaiian and Other Pacific Islander; SD, standard deviation; SES, socioeconomic status.
Percents are row percentages of COVID-positive men and women; p values are from χ2 test comparison of COVID-positive versus negative for men and women.
Men and women under 40 years of age had the highest COVID-19 positivity rate, followed by men 40–54 years old, and both men and women age 80 and over. High individual-level SES women and men had higher COVID-19 positivity rates than their low individual-level SES peers. Employed women and men had the highest positivity rates. Those with no comorbid conditions had higher COVID-19 positivity rates than women and men with one or more condition. COVID-19 positivity rates for women were similar across the two time periods; conversely, men tested in the earlier period (March to May) had significantly higher positivity rates than men tested in the later period.
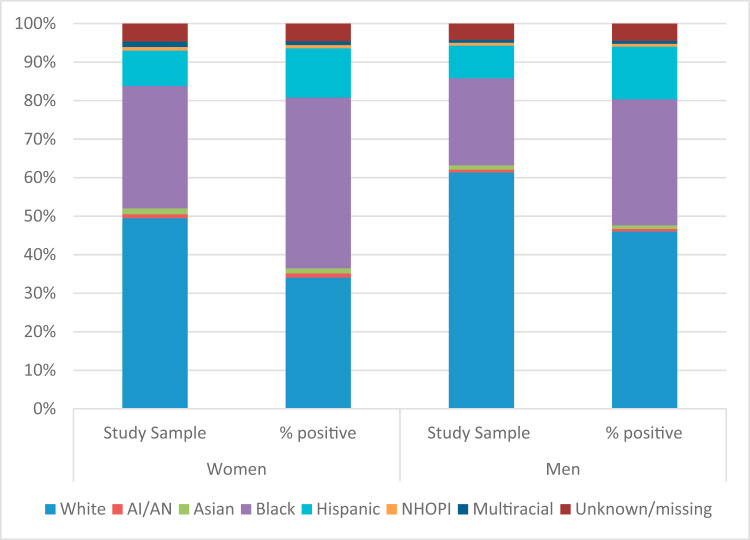
Among women, several racial/ethnic groups were over-represented with respect to COVID-19 infection (Figure 1 , Appendix A). For example, Black women comprised 31.8% of all women tested, but comprised 44.3% of those testing positive. Hispanic women comprised 9.2% of women tested but 12.8% of those who tested positive. Similarly, Black and Hispanic men were over-represented with respect to infection, with Black men representing 22.6% of those tested and 32.8% of those testing positive, and Hispanic men representing 8.5% of those tested but 13.7% of those testing positive. In contrast, White women and men were under-represented (34% White women and 46% of White men tested positive, but comprise 50% and 61% of the sample, respectively).
Figure 1.
Comparison of study sample and COVID-19–positive sample by racial/ethnic composition.
Table 3 displays the regression results for the odds of COVID-19 infection among women. In bivariate analysis (Model 1), Hispanic (odds ratio [OR], 2.20; 95% CI, 1.82–2.66), Black (OR, 2.19; 95% CI, 1.91–2.52) and AI/AN (OR, 1.71; 95% CI, 1.24–2.36) women all had significantly higher odds of infection than White women. These race/ethnicity effects remained stable after individual-level covariates adjustment (Model 2), although the ORs were slightly smaller for some groups. Relative to the youngest women, those ages 40 to 79 had lower odds of infection; women in the oldest category were not statistically different than the youngest women. Compared with high individual-level SES veterans, those who had low individual-level SES or who had service connected disability had lower odds of infection. Women who were retired had lower odds than those who were not employed, whereas there was no difference between employed and not employed women. Comorbidity status and testing period were not associated with infection. With further adjustment for SVI (Model 3), although slightly attenuated, the ORs for race/ethnicity remain largely stable and significant. Living in counties with a higher SVI for minoritized status/non-English speakers was significantly associated with odds of infection; residential SES and household composition were not significant. The effects of the other covariates did not change in this model.
Table 3.
Logistic Regression Results of Relationship Between COVID-19 Infection and Veterans’ Characteristics, Women, March 1, 2020, to August 5, 2020 (n = 39,223)
| Model 1 OR (95% CI) | Model 2 OR (95% CI) | Model 3 OR (95% CI) | |
|---|---|---|---|
| Race/ethnicity | |||
| White | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| AI/AN | 1.71 (1.24–2.36) | 1.68 (1.22–2.32) | 1.59 (1.14–2.20) |
| Asian | 1.35 (0.90–2.03) | 1.25 (0.83–1.87) | 1.20 (0.83–1.73) |
| Black | 2.19 (1.91–2.52) | 2.23 (1.94–2.57) | 2.01 (1.76–2.30) |
| Hispanic | 2.20 (1.82–2.66) | 2.03 (1.69–2.45) | 1.82 (1.58–2.09) |
| NH/OPI | 1.34 (0.91–1.98) | 1.33 (0.90–1.96) | 1.17 (0.78–1.76) |
| Multiracial | 1.04 (0.70–1.53) | 0.99 (0.67–1.46) | 0.93 (0.65–1.34) |
| Age | |||
| <40 years | 1.00 (ref) | 1.00 (ref) | |
| 40–54 years | 0.77 (0.71–0.85) | 0.78 (0.71–0.86) | |
| 55–64 years | 0.63 (0.57–0.71) | 0.64 (0.57–0.72) | |
| 65–79 years | 0.62 (0.53–0.73) | 0.63 (0.54–0.73) | |
| ≥80 years | 1.25 (0.89–1.76) | 1.20 (0.86–1.68) | |
| Individual SES/service-connected disability | |||
| High individual-level SES | 1.00 (ref) | 1.00 (ref) | |
| Low individual-level SES | 0.79 (0.69–0.91) | 0.78 (0.67–0.89) | |
| Service-connected disability | 0.74 (0.66–0.84) | 0.73 (0.64–0.83) | |
| Employment status | |||
| Not employed | 1.00 (ref) | 1.00 (ref) | |
| Employed | 1.02 (0.95–1.09) | 1.04 (0.97–1.12) | |
| Retired | 0.75 (0.64–0.89) | 0.77 (0.66–0.90) | |
| Unknown | 0.94 (0.82–1.08) | 0.92 (0.79–1.07) | |
| CDC comorbidity status | |||
| None | 1.00 (ref) | 1.00 (ref) | |
| ≥1 comorbidities | 0.96 (0.89–1.05) | 0.96 (0.88–1.04) | |
| Missing | 0.90 (0.75–1.08) | 0.84 (0.68–1.03) | |
| Time period | |||
| March–May | 1.00 (ref) | 1.00 (ref) | |
| June–August | 0.95 (0.77–1.17) | 0.93 (0.75–1.15) | |
| SVI | |||
| Residential SES | 1.56 (0.95–2.52) | ||
| Household composition/disability | 1.19 (0.74–1.93) | ||
| Minoritized status/non-English speakers | 1.95 (1.31–2.89) | ||
Abbreviations: AI/AN, American Indian and Alaska Native; CDC, Centers for Disease Control and Prevention; CI, confidence interval; NH/OPI, Native Hawaiian and Other Pacific Islander; OR, odds ratio; SES, socioeconomic status; SVI, Social Vulnerability Index.
Model 1: bivariate associations with race/ethnicity; Model 2: further adjusted for age, individual-level SES, employment status, comorbidities; Model 3: further adjusted for SVI for the SES, household composition, and minoritized status domains. All models clustered standard errors at the VA facility level. Bold denotes statistical significance at p < .05.
Table 4 shows the results for men. In Model 1, relative to White men, all racial/ethnic groups of men (except for Asians) had significantly higher odds of infection. Hispanic men had the highest odds of COVID-19 positivity (OR, 2.34; 95% CI–1.68, 3.27), followed by Black (OR, 2.08; 95% CI, 1.88–2.31), NH/OPI (OR, 1.34; 95% CI, 1.10–1.65), and Multiracial (OR, 1.21; 95% CI, 1.03–1.42) men. The racial/ethnic differences remain similar with adjustment for demographic characteristics (Model 2). Men aged 55–79 years had lower odds of infection compared with the youngest men; there were no other significant age differences. Men with low individual-level SES or service-connected disability had a lower odds of infection than high individual-level SES men. Unlike women, men who were employed had higher odds of infection than men who were not employed, and men with comorbidities had lower odds of infection than those with none. Men who were tested during the summer of 2020 had a lower odds of infection than men tested earlier in the year. When county SVI domains were added (Model 3), Asian, NH/OPI, and Multiracial groups were no longer significantly different and AI/AN, Black, and Hispanic differences were reduced but remained significant. Men 80 and over had significantly higher odds of infection (OR, 1.20; 95% CI, 1.03–1.38). Individual-level SES, employment, and comorbidity status differences remained similar. Living in a county with higher SVI minoritized status/non-English speakers indices was associated with a higher odds of infection; the effects of residential SES and household composition were not significant.
Table 4.
Logistic Regression Results of Relationship Between COVID-19 Infection and Veterans’ Characteristics, Men, March 1, 2020, to August 5, 2020 (n = 316,380)
| Model 1 OR (95% CI) | Model 2 OR (95% CI) | Model 3 OR (95% CI) | |
|---|---|---|---|
| Race/ethnicity | |||
| White | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| AI/AN | 1.46 (1.21–1.77) | 1.46 (1.21–1.76) | 1.30 (1.09–1.55) |
| Asian | 1.13 (0.87–1.48) | 1.03 (0.79–1.33) | 0.88 (0.70–1.11) |
| Black | 2.08 (1.88–2.31) | 2.13 (1.93–2.36) | 1.82 (1.64–2.01) |
| Hispanic | 2.34 (1.68–3.27) | 2.24 (1.60–3.15) | 2.03 (1.73–2.38) |
| NH/OPI | 1.34 (1.10–1.65) | 1.31 (1.07–1.61) | 1.17 (0.97–1.42) |
| Multiracial | 1.21 (1.03–1.42) | 1.18 (1.01–1.39) | 1.06 (0.90–1.26) |
| Age | |||
| <40 years | 1.00 (ref) | 1.00 (ref) | |
| 40–54 years | 0.96 (0.90–1.02) | 0.98 (0.92–1.04) | |
| 55–64 years | 0.73 (0.68–0.79) | 0.75 (0.70–0.80) | |
| 65–79 years | 0.77 (0.70–0.85) | 0.78 (0.71–0.86) | |
| ≥80 years | 1.17 (0.99–1.39) | 1.20 (1.03–1.38) | |
| Individual SES/service-connected disability | |||
| High individual-level SES | 1.00 (ref) | 1.00 (ref) | |
| Low individual-level SES | 0.75 (0.71–0.79) | 0.74 (0.71–0.78) | |
| Service-connected disability | 0.92 (0.87–0.97) | 0.92 (0.88–0.97) | |
| Employment status | |||
| Not employed | 1.00 (ref) | 1.00 (ref) | |
| Employed | 1.15 (1.09–1.21) | 1.17 (1.12–1.23) | |
| Retired | 0.95 (0.87–1.05) | 1.02 (0.95–1.09) | |
| Unknown | 1.07 (0.96–1.19) | 1.09 (0.997–1.20) | |
| CDC comorbidity status | |||
| None | 1.00 (ref) | 1.00 (ref) | |
| ≥1 comorbidities | 0.90 (0.86–0.95) | 0.91 (0.86–0.95) | |
| Missing | 1.09 (0.999–1.19) | 1.08 (0.98–1.19) | |
| Time period | |||
| March–May | 1.00 (ref) | 1.00 (ref) | |
| June–August | 0.71 (0.56–0.90) | 0.70 (0.55–0.87) | |
| SVI | |||
| Residential SES | 1.29 (0.79–2.10) | ||
| Household composition/disability | 1.33 (0.85–2.07) | ||
| Minoritized status/non-English speakers | 2.86 (2.08–3.92) | ||
Abbreviations: AI/AN, American Indian and Alaska Native; CDC, Centers for Disease Control and Prevention; CI, confidence interval; NH/OPI, Native Hawaiian and Other Pacific Islander; OR, odds ratio; SES, socioeconomic status; SVI, Social Vulnerability Index.
Model 1: bivariate associations with race/ethnicity; Model 2: further adjusted for age, individual-level SES, employment status, comorbidities; Model 3: further adjusted for Social Vulnerability Indices for the SES, household composition, and minoritized status domains. All models clustered standard errors at the VA facility level. Bold denotes statistical significance at p < .05.
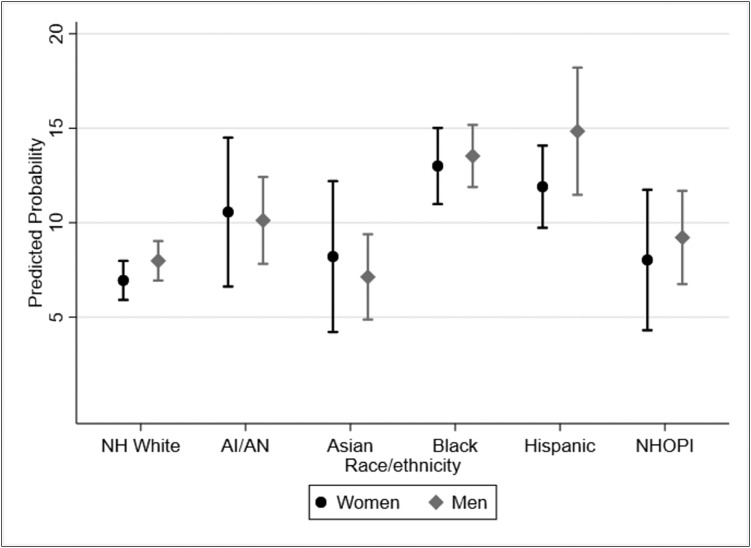
Figure 2 graphs the fully adjusted (Model 3) predicted probabilities of infection for each racial/ethnic group separately by gender and allows us to compare the probability of COVID-19 infection by race/ethnicity and across genders. Black and Hispanic women and men had higher predicted probabilities than any other racial/ethnic/gender group and the 99% CIs did not overlap with those of White women and men and Asian men. We also tested for gender-by-race/ethnicity interactions, and these findings were not significant (data not shown).
Figure 2.
Predicted probability and 99% confidence intervals of COVID-19 test positivity by gender and race/ethnicity, adjusted for demographic characteristics, comorbidities, time, and Social Vulnerability Index. Gender-stratified models are controlled for age, individual-level socioeconomic status (SES), employment status, and Social Vulnerability Indices for the SES, household composition, and minoritized status domains. All models clustered standard errors at the VA facility level.
Discussion
In this large, national study of veterans tested for COVID-19 infection at VHA facilities, we found similar racial/ethnic patterns by gender. Namely, for both women and men, AI/AN, Black, and Hispanic veterans were more likely to test positive than White veterans after adjusting for demographics, comorbidity status, and county SVI. This highlights persistent disadvantage among minoritized racial/ethnic women and men (Chowkwanyun & Reed, 2020; Moore et al., 2020; Price-Haywood et al., 2020). In particular, in comparisons across race/ethnicity and gender, Black and Hispanic women had higher predicted probabilities of infection than White men, White women, and Asian men. Thus, although there may not be an excess burden for Black and Hispanic women relative to Black and Hispanic men, there is an excess burden for these women relative to White men and women and Asian men.
Our study builds on and is consistent with earlier studies of veteran VHA users that found Black and Hispanic veterans were more likely than White veterans to be COVID-19 positive (Ferguson et al., 2021a; Rentsch et al., 2020), but did not consider gender differences. Although we hypothesized that there would be gender differences in racial/ethnic inequities in COVID-19 infection, we actually found few differences between men and women veteran VHA users. That is, for each racial/ethnic group, infection positivity was similar for men and women. Moreover, minoritized racial/ethnic men and women veterans are overrepresented among those who test positive for COVID-19 relative to our sample of veterans tested in this study as well the overall VHA user population (Hoerster et al., 2012; Lehavot et al, 2012; National Center for Veterans Analysis and Statistics, 2017; Washington et al., 2015).
An important contribution of this study is our ability to examine pair-wise comparisons for COVID-19 infections across both gender and race/ethnicity, without using White as the only reference group. In so doing, we found that not only did Hispanic women have higher predicted probability of infection than White women, they also had higher probability than White men and Asian men. Further, Black women had higher predicted probabilities than White women and men and Asian men. This finding may be because Hispanic and Black women have less ability to socially distance and/or engage in other mediating practices because of employment, caregiving responsibilities, and housing situations, among other reasons (Artiga, et al., 2020; Kambhampati et al., 2020). It is important to continue to monitor variation in COVID-19 infections for men and women as the pandemic continues to evolve. A recent study found that patterns in racial/ethnic COVID-19 infection disparities change over time; in fact, while numerous groups experienced higher COVID-19 infections in summer 2020 relative to non-Hispanic White veteran VHA users, by fall 2020, there were few racial/ethnic differences in COVID-19 infection (Wong et al., 2021a). Although Wong and colleagues’ study did not analyze men and women separately, we might similarly anticipate finding attenuated racial/ethnic differences for both men and women as the pandemic progressed through the fall and winter 2020 surge.
Our findings among Black and Hispanic women have implications for pregnant women, who might be at higher risk for severe COVID-19 outcomes (Flannery et al., 2020; Wastnedge et al., 2020). In the VHA population, more than one-quarter of women veterans are still of reproductive age and many of these women are Hispanic or Black (Washington et al., 2015). Recent reports suggest that Hispanic and Black pregnant women have higher COVID-19 positivity than White or Asian pregnant women (Flannery et al., 2020). These findings point to the need for targeted education, culturally responsive health care, and focused outreach not only based on gender and race/ethnicity, but also by age group (Harrington et al., 2019).
Other gender differences in the odds of infection also emerged, demonstrating the usefulness of using a gender-stratified approach when exploring the association between social determinants of health and COVID-19 infection. For men and women, the youngest age group had the highest odds of infection, but cut points differed (>40 for women, >55 for men). Further, men aged 80 or older had higher odds than men aged less than 40; this difference was not found among women. In the current pandemic, younger adults are the drivers of community transmission (Boehmer et al., 2020), so it is not unexpected that they had higher rates of COVID-19 infection in VHA as well. It is important to note the oldest veteran men had higher risk, perhaps owing to gendered behavioral differences in this age group. Barber and Kim (2021) found that although older adults perceived their risk for COVID-19 to be high, older men were less likely to practice protective behaviors. Although there were no differences among women, men who were employed (vs. not employed) had an increased odds of infection, and men with comorbidities had lower odds of infection than men without. One possible explanation is that women are more likely to practice recommended mitigation behaviors including social distancing and wearing masks (Barber & Kim, 2021; Ritter & Brenan, 2020). Additionally, we did not have information on the type of occupation and there may have been gender differences in the occupation-related risk of exposure. For example, male veterans may be more likely to be essential workers in jobs that are less able to social distance (e.g., construction, police, fire fighters), be more likely to encounter individuals not wearing masks, or be less likely to wear masks themselves compared with employed women.
We found that, for both women and men veterans, those of lower individual-level SES or with service-connected disability had a lower odds of infection than those of a higher individual-level SES, contrary to what our social determinants of health model would expect. Most COVID-19–related reports and studies do not collect data on individual-level SES, although there is some evidence that those with Medicaid and those living in lower income households are at greater risk of COVID-19 infection, COVID-19 complications, and severe illness (James, Kishore, & Lee, 2020; Richardson et al., 2020; Raifman & Raifman, 2020). The VHA serves as a safety net for many veterans, providing care to a population of veterans that are lower income and more likely to be disabled than the overall veteran population and the general population (Agha, Lofgren, VanRuiswyk, & Layde, 2000; Meffert et al., 2019; Wilson & Kizer, 1997). It may be that our sample includes a larger proportion of low SES veterans who were tested as compared with veterans with higher SES, thus the pool of low SES veterans was perhaps more heterogeneous with respect to risk. Ferguson, Abdel Magid, Purnell, Kiang, and Osborne (2021b) found that, early in the pandemic, women, Black, Hispanic, and low-income veterans were more likely to be tested for COVID-19, and Wong, Yuan, Haderlein, Jones, and Washington (2021b) found that testing increased over time, particularly for Black and Hispanic veterans. Thus, their findings lend support to this possibility. Additionally, their findings underscore our own in that, although Black and Hispanic veterans are more likely to be tested, they are over-represented among those who test positive. Last, both women and men living in counties with a greater concentration of minoritized households had higher odds of infection, controlling for individual-level factors. Our findings confirm recent regional studies that found similar increased risk for those living in zip codes and counties with greater numbers of minoritized and low-income households (Chen & Krieger, 2021; Kim & Bostwich, 2020; Figueroa, Wadhera, Lee, Yeh, & Sommers, 2020; Wadhera et al., 2020).
There are limitations to our study. VHA users differ from the general U.S. population in ways that may both enable and hinder test-seeking behavior, including being older and sicker, on average, than veterans in the community (Hoerster et al., 2012; Lehavot et al., 2012; RAND Health, 2015) and more likely to have experienced military sexual trauma or homelessness (Han et al., 2019; Washington et al., 2010; Washington et al., 2013). Our sample is limited to those who sought COVID-19 testing; thus, if some groups are more likely to seek testing than others, our results might not reflect true differences in infection (see Ferguson et al., 2021a,b; Wong et al., 2021b). Nevertheless, an advantage of the current study is that we are able to disaggregate and investigate differences in racial/ethnic groups that are often combined in national and state surveillance efforts (Muñoz-Price et al., 2020) by including Asian, AI/AN, Multiracial, and Native Hawaiian and Other Pacific Islander veterans. Access to health care has been posited as a factor contributing to racial/ethnic disparities in COVID-19 testing and treatment. Using data from the VHA, the largest health care system in the United States serving more than 9 million veterans, allowed us to examine social determinants of COVID-19 infection rates while minimizing the effects of differential access to health insurance and healthcare.
Conclusions
Although women veterans comprise only about 10% of the VHA population, they represent a rapidly growing group that is demographically and medically distinct from male veterans and warrants its own investigation. The men and women veteran populations exhibited similar racial/ethnic inequities in positive COVID-19 rates, and these disparities were similar to those in the general population. This study highlights the specific risks for women of color, particularly Hispanic and Black women, that may have been overlooked in studies focused on overall gender comparisons.
Implications for Policy and/or Practice
These findings underscore the importance of widespread VHA screening, tracking, and vaccinating for COVID-19 and education and outreach programs specific for veteran women by race/ethnicity, especially Hispanic and Black women. Given the substantial demographic differences between women and men veterans, gender-tailored, culturally relevant education and outreach is warranted, for example, tailoring messaging strategies specific to women of reproductive age and older men.
Biographies
Dawn M. Upchurch, PhD, LAc, is Professor and Vice Chair in the Department of Community Health Sciences at UCLA Fielding School of Public Health. She studies the social determinants of women's health over the life course.
Michelle S. Wong, PhD, is a Health Science Specialist at the VA Greater Los Angeles Healthcare System's Center for the Study of Healthcare Innovation, Implementation & Policy. Her research focuses on improving health equity and addressing social determinants of health.
Anita H. Yuan, PhD, MPH, Quantitative Methods Lead at VA HSR&D Center for the Study of Healthcare Innovation, Implementation, and Policy, conducts research using electronic health records to examine issues in women's health, health disparity, and complementary and integrative health.
Taona P. Haderlein, PhD, is an investigator at the VA HSR&D Center for the Study of Healthcare Innovation, Implementation, & Policy. Her research examines telehealth access disparities in underserved populations.
Juliette McClendon, PhD, is the Director of Medical Affairs at Big Health. She is a Clinical Psychologist with expertise in mental health equity and culturally-responsive evidence-based practice.
Alicia Christy, MD, MHSCR. As Deputy Director of Reproductive Health, Dr. Alicia Christy supports the portfolio addressing Veteran's reproductive health priorities. Her research focuses on health disparities and the reproductive health of women Veterans. She holds the rank of professor at the Uniformed Services University.
Donna L. Washington, MD, MPH, Women's Health Focused Research Area Lead at the VA Greater Los Angeles HSR&D Center of Innovation, is Professor of Medicine at UCLA. Her research examines healthcare access, quality, and equity for women, minoritized, and other vulnerable populations.
Footnotes
Supported by the VA Office of Health Equity (OHE) and VA Quality Enhancement Research Initiative (QUERI) through grant #PEC-15-239 to the OHE/QUERI National Partnered Evaluation Center, and by VA HSR&D (#IIR-17-289 and #SDR-20-402).
The views expressed within represent those of the authors and do not necessarily represent those of the Department of Veterans Affairs or the United States Government.
All coauthors have no financial conflict of interest to declare.
Supplementary Data
Supplementary data related to this article can be found at https://doi.org/10.1016/j.whi.2021.09.006.
Appendix
References
- Agha Z., Lofgren R.P., VanRuiswyk J.V., Layde P.M. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Archives of Internal Medicine. 2000;160(21):3252–3257. doi: 10.1001/archinte.160.21.3252. [DOI] [PubMed] [Google Scholar]
- Alon T., Doepke M., Olmstead-Rumsey J., Tertilt M. National Bureau of Economic Research; Cambridge, MA: 2020. Impact of COVID-19 on gender equality (Research Working Paper 26947) [Google Scholar]
- Artiga S., Rae M., Pham O., Hamel L., Munana C. Kaiser Family Foundation; San Francisco: 2020. COVID-19 risks and impacts among health care workers by race/ethnicity. [Google Scholar]
- Barber S.J., Kim H. COVID-19 worries and behavior changes in older and younger men and women. Journal of Gerontology Series B Psychological Sciences and Social Sciences. 2021;76(2):e17–e23. doi: 10.1093/geronb/gbaa068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhopal S.S., Bhopal R. Sex differential in COVID-19 mortality varies markedly by age. Lancet. 2020;396(10250):532–533. doi: 10.1016/S0140-6736(20)31748-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehmer T.K., DeVies J., Caruso E., van Santen K.L., Tang S., Black C.L.…Gundlapalli A.V. Changing age distribution of the COVID-19 pandemic - United States, May-August 2020. MMWR. Morbidity and Mortality Weekly Report. 2020;69(39):1404–1409. doi: 10.15585/mmwr.mm6939e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowleg L. The problem with the phrase women and minorities: intersectionality-an important theoretical framework for public health. American Journal of Public Health. 2012;102(7):1267–1273. doi: 10.2105/AJPH.2012.300750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braveman P., Egerter S., Williams D.R. The social determinants of health: Coming of age. Annual Review of Public Health. 2011;32:381–398. doi: 10.1146/annurev-publhealth-031210-101218. [DOI] [PubMed] [Google Scholar]
- Braveman P.A., Kumanyika S., Fielding J., Laveist T., Borrell L.N., Manderscheid R., Troutman A. Health disparities and health equity: The issue is justice. American Journal of Public Health. 2011;101(Suppl. 1):S149–155. doi: 10.2105/AJPH.2010.300062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J.T., Krieger N. Revealing the unequal burden of COVID-19 by income, race/ethnicity, and household crowding: US county versus zip code analyses. Journal of Public Health Management and Practice. 2021;27(Supple. 1):S43–S56. doi: 10.1097/PHH.0000000000001263. [DOI] [PubMed] [Google Scholar]
- Chowkwanyun M., Reed A.L., Jr. Racial health disparities and Covid-19 – Caution and context. New England Journal of Medicine. 2020;383(3):201–203. doi: 10.1056/NEJMp2012910. [DOI] [PubMed] [Google Scholar]
- Crenshaw K.W. Mapping the margins: Intersectionality, identity politics, and violence against women of color. Stanford Law Review. 1991;43(6):1241–1299. [Google Scholar]
- Dodds C., Fakoya I. Covid-19: Ensuring equality of access to testing for ethnic minorities. BMJ. 2020;369:m2122. doi: 10.1136/bmj.m2122. [DOI] [PubMed] [Google Scholar]
- Ferguson J.M., Justice A.C., Osborne T.F., Abdel Magid H.S., Purnell A.L., Rentsch C.T. Racial and ethnic disparities for SARS-CoV2 positivity in the United States: a generalizing pandemic. medRxiv. 2021 doi: 10.1101/2021.04.27.21256215. [DOI] [Google Scholar]
- Ferguson J.M., Abdel Magid H.S., Purnell A.L., Kiang M.V., Osborne T.F. Differences in COVID-19 testing and test positivity among veterans, United States, 2020. Public Health Reports. 2021;134(4):483–492. doi: 10.1177/00333549211009498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa J.F., Wadhera R.K., Lee D., Yeh R.W., Sommers B.D. Community-level factors associated with racial and ethnic disparities in COVID-19 rates in Massachusetts. Health Affairs (Millwood) 2020;39:1984–1992. doi: 10.1377/hlthaff.2020.01040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flannery D.D., Gouma S., Dhudasia M.B., Mukhopadhyay S., Pfeifer M.R., Woodford E.C.…Hensley S.E. SARS-CoV-2 seroprevalence among parturient women in Philadelphia. Science Immunology. 2020;5(49):eabd5709. doi: 10.1126/sciimmunol.abd5709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frayne S.M., Parker V.A., Christiansen C.L., Loveland S., Seaver M.R., Kazis L.E., Skinner K.M. Health status among 28,000 women veterans. The VA Women’s Health Program Evaluation Project. Journal of General Internal Medicine. 2006;21(Suppl. 3):S40–S46. doi: 10.1111/j.1525-1497.2006.00373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith D.M., Sharman G., Holliday C.S., Enyia O.K., Valliere M., Semlow A.R.…Blumenthal R.S. Men and COVID-19: A biopsychosocial approach to understanding sex differences in mortality and recommendations for practice and policy intervention. Prevention of Chronic Disease. 2020;17:E63. doi: 10.5888/pcd17.200247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J.K., Yano E.M., Watson K.E., Ebrahimi R. Cardiovascular care in women Veterans: A call to action. Circulation. 2019;139:1102–1109. doi: 10.1161/CIRCULATIONAHA.118.037748. [DOI] [PubMed] [Google Scholar]
- Harrington K.M., Nguyen X.T., Song R.J., Hannagan K., Quaden R., Gagnon D.R.…Whitbourne S.B. VA Million Veteran Program. Gender differences in demographic and health characteristics of the Million Veteran Program Cohort. Women’s Health Issues. 2019;29(Suppl 1):S56–S66. doi: 10.1016/j.whi.2019.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvard GenderSci Lab US gender/sex COVID-19 data tracker. 2020. www.genderscilab.org/gender-and-sex-in-covid19 Available: Accessed: December 10, 2020.
- Hoerster K.D., Lehavot K., Simpson T., McFall M., Reiber G., Nelson K.M. Health and health behavior differences: U.S. Military, veteran, and civilian men. American Journal of Preventive Medicine. 2012;43(5):483–489. doi: 10.1016/j.amepre.2012.07.029. [DOI] [PubMed] [Google Scholar]
- James M.K., Kishore M., Lee S.W. Demographic and socioeconomic characteristics of COVID-19 patients treated in the Emergency Department of a New York City hospital. Journal of Community Health. 2020;45:711–718. doi: 10.1007/s10900-020-00937-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J.M., Bai P., He W., Wu F., Liu X.F., Han D.M.…Yang J.K. Gender differences in patients with COVID-19: Focus on severity and mortality. Frontiers in Public Health. 2020;29(8):152. doi: 10.3389/fpubh.2020.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser Family Foundation State data and policy actions to address coronavirus. 2020. www.kff.org/coronavirus-covid-19/issue-brief/state-covid-19-data-and-policy-actions/ Available:
- Kambhampati A.K., O’Halloran A.C., Whitaker M., Magill S.S., Chea N., Chai S.J.…Kim L. COVID-19–associated hospitalizations among health care personnel — COVID-NET, 13 states, March 1–May 31, 2020. Morbidity and Mortality Weekly Report. 2020;69(43):1576–1583. doi: 10.15585/mmwr.mm6943e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaye I.M., Horney J.A. The impact of social vulnerability on COVID-19 in the US: An analysis of spatially varying relationship. American Journal of Preventive Medicine. 2020;59(3):317–325. doi: 10.1016/j.amepre.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.J., Bostwick W. Social vulnerability and racial inequality in COVID-19 deaths in Chicago. Health Education and Behavior. 2020;47(4):509–513. doi: 10.1177/1090198120929677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehavot K., Hoerster K.D., Nelson K.M., Jakupcak M., Simpson T.L. Health indicators for military, veteran, and civilian women. American Journal of Preventive Medicine. 2012;42(5):473–480. doi: 10.1016/j.amepre.2012.01.006. [DOI] [PubMed] [Google Scholar]
- Link B.G., Phelan J. Social conditions as fundamental causes of disease. Journal of Health and Social Behavior, Spec No. 1995:80–94. [PubMed] [Google Scholar]
- McCormack G., Avery C., Spitzer A.K., Chandra A. Economic vulnerability of households with essential workers. JAMA. 2020;324(4):388–390. doi: 10.1001/jama.2020.11366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meffert B.N., Morabito D.M., Sawicki D.A., Hausman C., Southwick S.M., Pietrzak R.H., Heinz A.J. US Veterans who do and do not utilize Veterans Affairs Health Care services: Demographic, military, medical, and psychosocial characteristics. Primary Care Companion for CNS Disorders. 2019;21(1):18m02350. doi: 10.4088/PCC.18m02350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J.T., Ricaldi J.N., Rose C.E., Fuld J., Parise M., Kang G.J.…Honein M.A., COVID-19 State, Tribal, Local, and Territorial Response Team Disparities in incidence of COVID-19 among underrepresented racial/ethnic groups in counties identified as hotspots during June 5-18, 2020 - 22 States, February-June 2020. MMWR Morbidity and Mortality Weekly Report. 2020;69(33):1122–1126. doi: 10.15585/mmwr.mm6933e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz-Price L.S., Nattinger A.B., Rivera F., Hanson R., Gmehlin C.G., Perez A.…Pezzin L.E. Racial disparities in incidence and outcomes among patients with COVID-19. JAMA Network Open. 2020;3(9):e2021892. doi: 10.1001/jamanetworkopen.2020.21892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Center for Veterans Analysis and Statistics Veteran population. Population tables. age/gender. 2017. www.va.gov/vetdata/veteran_population.asp Available:
- Nayak A., Islam S.J., Mehta A., Ko Y.A., Patel S.A., Goyal A.…Quyyumi A.A. Impact of social vulnerability on COVID-19 incidence and outcomes in the United States. MedRxiv. 2020 doi: 10.1101/2020.04.10.20060962. The Preprint Server for Health Sciences. [DOI] [Google Scholar]
- Price-Haywood E.G., Burton J., Fort D., Seoane L. Hospitalization and mortality among black patients and white patients with Covid-19. New England Journal Medicine. 2020;382(26):2534–2543. doi: 10.1056/NEJMsa2011686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raifman M.A., Raifman J.R. Disparities in the population at risk of severe illness from COVID-19 by race/ethnicity and income. American Journal of Preventive Medicine. 2020;59(1):137–139. doi: 10.1016/j.amepre.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAND Health . RAND Corporation; Santa Monica, CA: 2015. Current and projected characteristics and unique health care needs of the patient population served by the Department of Veterans Affairs. [PMC free article] [PubMed] [Google Scholar]
- Rentsch C.T., Kidwai-Khan F., Tate J.P., Park L.S., King J.T., Jr., Skanderson M.,., Justice A.C. Patterns of COVID-19 testing and mortality by race and ethnicity among United States veterans: A nationwide cohort study. PLoS Medicine. 2020;17(9):e1003379. doi: 10.1371/journal.pmed.1003379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W., Zanos T.P. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter Z., Brenan M. New April guidelines boost perceived efficacy of face masks, The Gallup/Knight Foundation Survey. 2020. https://news.gallup.com/poll/310400/new-april-guidelines-boost-perceived-efficacy-face-masks.aspx Available:
- Sharma G., Volgman A.S., Michos E.D. Sex differences in mortality from COVID-19 pandemic: Are men vulnerable and women protected? JACC Case Reports. 2020:1407–1410. doi: 10.1016/j.jaccas.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes E.K., Zambrano L.D., Anderson K.N., Marder E.P., Raz K.M., El Burai Felix S., Tie Y., Fullerton K.E. Coronavirus disease 2019 case surveillance – United States, January 22-May 30, 2020. MMWR Morbidity and Mortality Weekly Report. 2020;69:759–765. doi: 10.15585/mmwr.mm6924e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadhera R.K., Wadhera P., Gaba P., Figueroa J.F., Joynt Maddox K.E., Yeh R.W., Shen C. Variation in COVID-19 hospitalizations and deaths across New York City boroughs. JAMA. 2020;323(21):2192–2195. doi: 10.1001/jama.2020.7197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washington D.L., Davis T.D., Der-Martirosian C., Yano E.M. PTSD risk and mental health care engagement in a multi-war era community sample of women Veterans. Journal of General Internal Medicine. 2013;28(7):894–900. doi: 10.1007/s11606-012-2303-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washington D.L., Farmer M.M., Mor S.S., Canning M., Yano E.M. Assessment of the health care needs and barriers to VA use experienced by women Veterans: Findings from the National Survey of Women Veterans. Medical Care. 2015;53(4 Suppl. 1):S23–S31. doi: 10.1097/MLR.0000000000000312. [DOI] [PubMed] [Google Scholar]
- Washington D.L., Villa V., Brown A., Damron-Rodriguez J., Harada N. Racial/ethnic variations in veterans' ambulatory care use. American Journal of Public Health. 2005;95(12):2231–2237. doi: 10.2105/AJPH.2004.043570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washington D.L., Yano E.M., McGuire J., Hines V., Lee M., Gelberg L. Risk factors for homelessness among women veterans. Journal of Health Care for the Poor and Underserved. 2010;21(1):82–91. doi: 10.1353/hpu.0.0237. [DOI] [PubMed] [Google Scholar]
- Wastnedge E., Reynolds R.M., van Boeckel S.R., Stock S.J., Denison F.C., Maybin J.A., Critchley H. Pregnancy and COVID-19. Physiological Reviews. 2021;10 1(1):303–318. doi: 10.1152/physrev.00024.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenham C., Smith J., Morgan R., Gender and COVID-19 Working Group COVID-19: The gendered impacts of the outbreak. Lancet. 2020;395(10227):846–848. doi: 10.1016/S0140-6736(20)30526-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D.R., Lawrence J.A., Davis B.A. Racism and health: Evidence and needed research. Annual Review of Public Health. 2019;40:105–125. doi: 10.1146/annurev-publhealth-040218-043750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson N.J., Kizer K.W. The VA health care system: an unrecognized national safety net. Health Affairs (Millwood) 1997;16(4):200–204. doi: 10.1377/hlthaff.16.4.200. [DOI] [PubMed] [Google Scholar]
- Wong M.S., Haderlein T.P., Yuan A.H., Moy E., Jones K.T., Washington D.L. Time trends in racial/ethnic differences in COVID-19 infection and mortality. International Journal of Environmental Research and Public Health. 2021;18(9):4848. doi: 10.3390/ijerph18094848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong M.S., Yuan A.H., Haderlein T.P., Jones K.T., Washington D.L. Variations in race/ethnicity and time in COVID-19 testing among Veterans Health Administration users with COVID-19 symptoms or exposure. Preventive Medicine Reports. 2021;24:101503. doi: 10.1016/j.pmedr.2021.101503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano E.M., Bastian L.A., Frayne S.M., Howell A.L., Lipson L.R.…Fihn S.D. Toward a VA women’s health research agenda: setting evidence-based priorities to improve the health and health care of women veterans. Journal of General Internal Medicine. 2006;21(Suppl/3):S93–S101. doi: 10.1111/j.1525-1497.2006.00381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.