Abstract
The potential emergence of SARS-CoV-2 variants capable of escaping vaccine-generated immune responses poses a looming threat to vaccination efforts and will likely prolong the duration of the COVID-19 pandemic. Additionally, the prevalence of beta coronaviruses circulating in animals and the precedent they have set in jumping into human populations indicates that they pose a continuous threat for future pandemics. Currently, only one therapeutic is approved by the U.S. Food and Drug Administration (FDA) for use in treating COVID-19, remdesivir, although other therapies are authorized for emergency use due to this pandemic being a public health emergency. In this review, twenty-four different treatments are discussed regarding their use against COVID-19 and any potential future coronavirus-associated illnesses. Their traditional use, mechanism of action against COVID-19, and efficacy in clinical trials are assessed. Six treatments evaluated are shown to significantly decrease mortality in clinical trials, and ten treatments have shown some form of clinical efficacy.
Keywords: Anti-viral, Protease inhibitor, Anti-inflammatory, Monoclonals
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a novel betacoronavirus (β-coronavirus or β-CoV) first identified in China in late 2019 and is the cause of coronavirus disease 2019 (COVID-19) [1]. Severity of the disease ranges from asymptomatic infection to dyspnea, pneumonia, and acute respiratory distress syndrome (ARDS) [1]. To date, over 4,660,000 people worldwide have died from this COVID-19 [2]. The regular appearance of novel variants of concern, including the B.1.1.7 (World Health Organization (WHO) designated, alpha variant), which emerged in the United Kingdom in September 2020 [3], the B.1.351 beta variant found in isolates from Nelson Mandela Bay [3], South Africa in October 2020 [4], the B.1617.2 delta variant which emerged in October 2020 in India, and the P.1 lineage of the B.1.1.28 variant that seems to have emerged from Manaus in the Amazon region in December 2020 [5] have changed the epidemiological and immunological dynamics of COVID-19 progression. More recently, the WHO added additional SARS-CoV-2 lineages of interest: eta, iota, kappa and lambda that will inevitably be extended as the pandemic continues. The persistent emergence of SARS-CoV-2 variants have placed a strain on the efficacy of available vaccines. For example, there is reduced efficacy of current vaccines to prevent human infections with the B.1.617.2 delta variant when compared to the B.1.1.7 alpha variant. [6] Hence, there is an urgent need for therapies that can be used to prevent the entry or disrupt the replication of SARS-CoV-2 with the goal of minimizing patient infections, morbidity and facilitating a rapid recovery to full health.
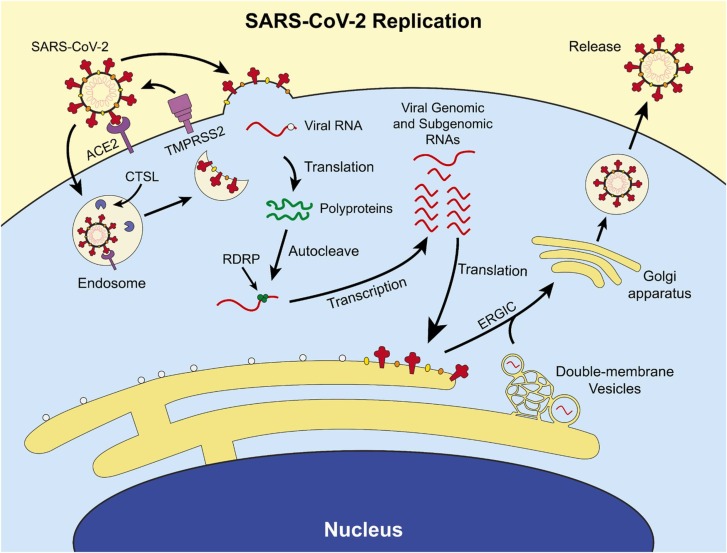
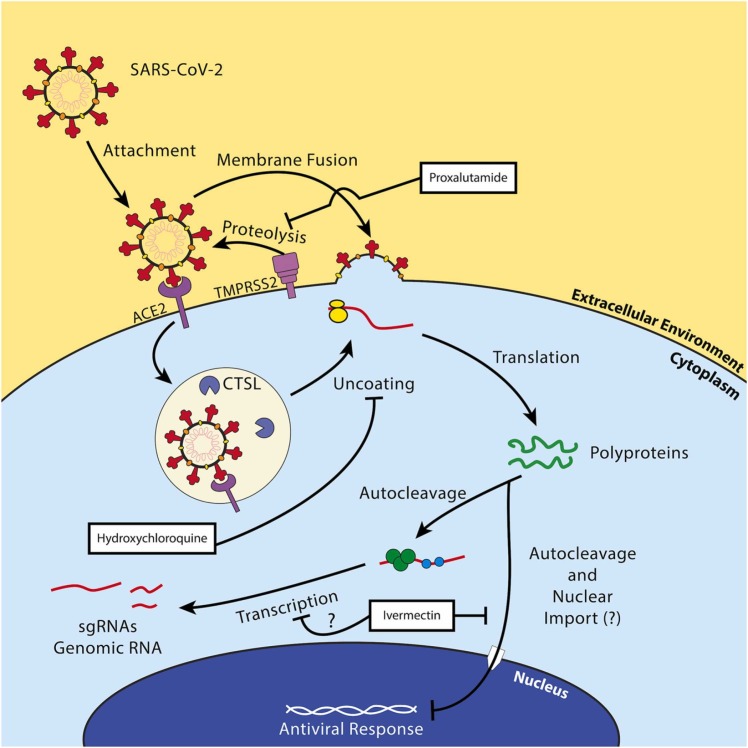
Within the life cycle of SARS-CoV-2 are potential therapeutic targets. The replication cycle of SARS-CoV-2 follows a similar route to other enveloped positive-sense RNA viruses. [7] The cell tropism of this virus is defined by the distribution of its cell receptor, angiotensin-converting enzyme 2 (ACE2), and its membrane fusion-priming proteases: transmembrane serine protease 2 (TMPRSS2), cathepsin L (CTSL), and furin [8], [9]. This indicates a primary tropism for epithelial cells in the lung and bronchus [10]. Following membrane fusion, the virus releases its genome into the cytoplasm of the host cell, the viral RNA is directly translated by host ribosomes into two viral polyproteins, and the polyproteins self-cleave into their respective non-structural proteins [11]. The virus begins producing subgenomic RNAs and genomic RNAs inside of complex structures called viral replication organelles most notably characterized by the formation of double-membrane vesicles complexed with the endoplasmic reticulum [12]. These RNAs are then translated into structural and non-structural proteins, genomic RNAs are then packaged at the endoplasmic reticulum-Golgi intermediate compartment (ERGIC). The ERGIC then buds into the Golgi apparatus, where the complete virions form, and the resulting virions are released through the traditional secretory pathway [13]. The SARS-CoV-2 viral replication cycle is summarized in Fig. 1. Multiple treatments have been used to treat patients who are infected with SARS-CoV-2. The focus of this review is to describe many of the treatments that have been used, the basic research which provides rationale for their use and the outcomes of the clinical studies.
Fig. 1.
Overview of the replication cycle of SARS-CoV-2. SARS-CoV-2 binds its cell receptor, ACE2, and enters with assistance from either cathepsin L (CTSL) or transmembrane serine protease 2 (TMPRSS2). The virus enters the cell either by the endocytic pathway or by directly fusing with the cell membrane. It replicates its RNA utilizing its encoded RNA-dependent RNA polymerase (RDRP, green circle), manufactures non-structural proteins, and produces structural proteins at the endoplasmic reticulum and in specialized double-membrane vesicles. The structural proteins traffic to the Golgi via the endoplasmic-reticulum-Golgi intermediate compartment (ERGIC) where they are assembled into a complete virion and exported out of the cell through the secretory pathway.
2. Methods
Treatments were selected by either querying the ClinicalTrials.gov website or by identifying treatments for which there were widespread NIH-sponsored clinical trials such as the Adaptive COVID-19 Treatment Trial or ACTT. These treatments were then subjected to a literature review via PubMed for their mechanism of action, in vitro and in vivo studies of their efficacy against SARS-CoV-2 and other coronaviruses, and clinical trial data.
3. Treatment strategies
3.1. Antivirals
This category of treatment strategies includes any therapeutic which directly targets SARS-CoV-2 and its lifecycle, excluding antibody-based therapies. We have included pharmaceuticals that inhibit functions of viral proteins (i.e. its replicase, proteases, etc.) or processes (i.e. translation) that are critical to the functioning of the virus in this section.
3.1.1. Remdesivir (GS-5734)
Remdesivir is a prodrug nucleoside analog that requires metabolism by the host cell to initially release the parent nucleoside (GS-441524) followed by intracellular kinase activity to produce the active nucleoside triphosphate form that inhibits the RNA-dependent RNA polymerase (RDRP) of RNA viruses [14]. RDRPs have widely conserved catalytic structural motifs that are susceptible to broad antiviral drugs that target them [13], [15]. Remdesivir specifically acts as a delayed chain terminating RDRP inhibitor because it hinders complete extension of viral RNA [16], [17]. It is currently the only FDA approved drug for use in treating COVID-19.
In vitro studies confirmed the efficacy of remdesivir or GS-441524 as coronavirus antivirals. An in vitro study of remdesivir and GS-441524, examining drug efficacy against murine hepatitis virus, a model β-2a CoV, in delayed brain tumor (DBT) cells, and against SARS-CoV-1 and MERS-CoV in primary human airway epithelial cells, revealed significant decreases in viral titers and viral RNA levels [18]. In the DBT cells, the half maximal effective concentration of GS-441524 was 1.1 µM in a viral infection assay, and at concentrations above 500 nM remdesivir, the virus was undetectable in plaque assays. This data supported observations from a previous in vitro study which showed that treatment of primary human airway epithelial cells infected with patient isolates of SARS-CoV-1 or MERS-CoV-1, led to significant reductions in viral RNA levels with EC50 of 860 nM for GS-441524 and 74 nM for remdesivir [19]. Both studies reflected a therapeutic index of more than 100, meaning the concentration necessary to reduce viral replication to 50% of normal is at least two orders of magnitude lower than the concentration that kills 50% of cultured cells. Interestingly, the overall therapeutic effect of remdesivir appears to be weakened by the proofreading exonuclease activity of non-structural protein 14 (nsp14) in vitro, a protein that also shuts down protein synthesis by the host cell [18]. Removal of the exonuclease activity of nsp14 strengthens the antiviral effects of remdesivir. However, it is important to note that remdesivir is not as strongly inhibited as nucleoside analogs that act as mutagens, such as ribavirin and 5-fluorouracil [20].
In clinical trials, remdesivir has shown clinical significance. In a randomized, double-blind, placebo-controlled trial of 1062 participants performed by the National Institute for Allergy and Infectious Disease, patients showed shortened time to recovery and a reduction in lower respiratory tract infections. In this study, remdesivir or placebo was administered intravenously at 200 mg day one/100 mg days 2–10. In comparison to placebo, remdesivir significantly decreased the mean time to recovery (p < 0.001) from a mean of 15–10 days, decreased time to one and two stages of ordinal improvement, decreased time to discharge, and decreased the occurrence of serious adverse events compared to controls [21] (NCT04280705). By day 15, Kaplan Meier estimates of mortality was 6.7% for remdesivir compared to 11.9% in placebo controls and by day 29, 11.9% with remdesivir compared to 15.2% in placebo controls (hazard ratio = 0.73, 95% CI: 0.52–1.03). In contrast, another randomized, open-label controlled trial of 594 hospitalized patients with mild COVID-19 conducted by Gilead Sciences found that patients taking remdesivir for 5 days, had a significant improvement in clinical status (p = 0.02), when compared to standard of care after 11 days [22]. However, in the same study, participants who took remdesivir for 10 days showed no significant improvement in clinical status when compared to standard of care (p = 0.18). On day 28 of the 9 patients who had died, 2 had received remdesivir for 5 days, 3 for 10 and 4 were given the standard of care. The clinical importance of these drug-induced improvements was unclear [22].
Clinical application of remdesivir should be undertaken while considering the potential for primary adverse events (nausea, hypokalemia, headache). The aforementioned clinical trial performed by Gilead Sciences revealed a significant increase in treatment-emergent adverse events among study participants who received the drug for 10 days [22]. Another randomized clinical trial of 4891 participants on remdesivir found that participants who received the prodrug for 10 days experienced statistically significant worse outcomes by day 14 of the study when baseline clinical status as a variable was not included [23]. Interestingly, this study did not report differences in treatment-emergent adverse events among participants who received the drug for 10 days versus 5 days. However, using remdesivir as a treatment for COVID-19 may be best when limiting the course of treatment to 5 days as opposed to 10 days considering the available clinical data.
3.1.2. Favipiravir (T-705)
Favipiravir (6-fluoro-3-hydroxy-2-pyrazinecarboxamide) is a pyrazinecarboxamide derivative with demonstrated activity against RNA viruses. Favipiravir is metabolized by cellular enzymes into its ribofuranosyltriphosphate active therapeutic form, RTP [24]. Clinically, favipiravir is active against a broad range of influenza viruses, which are segmented negative-strand RNA viruses, including A(H1N1) pdm09, A(H5N1) and A(H7N9) avian virus. In addition to influenza, favipiravir can target the replication of other RNA viruses, including hantaviruses, enteroviruses, flaviviruses, noroviruses, and respiratory syncytial virus, a paramyxovirus [24]. Similar to remdesivir, favipiravir can inhibit viral RNA-dependent RNA polymerase and it is thought to inhibits viral RNA elongation by two separate mechanisms. Early studies revealed that favipiravir functions as a delayed chain terminator while also acting as a mutagen [25]. The number of genomic mutations in a study of influenza viruses repeatedly passaged with favipiravir showed a significant increase compared to controls, particularly transition mutations [26]. Its mechanism of chain termination is not well elucidated, but because its ribose sugar still contains a hydroxyl group at its 3’ end, it is likely that it interacts with its paired base to sterically inhibit extension of the nascent viral RNA [27].
Favipiravir has shown a potent inhibitory effect in vitro and in animal models. The mutagenic effects of favipiravir showed a decrease in influenza virus produced over repeated passages, indicating an increase in mutations until extinction [26]. A study using a Syrian hamster model found that preemptive treatment with favipiravir decreased infectious titers, and preventive treatment with favipiravir produced an undetectable viral titer [28].
There is relatively little clinical data on the efficacy of favipiravir against COVID-19. In a currently unpublished randomized, open-label controlled trial of 200 participants, treatment with favipiravir (1600 mg (8 tablets, 2 times a day) and 600 mg (3 tablets, 2 times a day) for 14 days) compared to standard of care, revealed a statistically significant improvement in clinical status after 10 days (p = 0.0372 Chi-squared), a decrease in time to clinical improvement (8 days in the favipiravir vs. 12 days in the standard of care-controls p < 0.0001 log rank), and an increase in viral clearance by day 10 of treatment (98% treatment group vs. 79% in controls, p = 0.00016) (NCT04542694). However, there was no statistically significant decrease in mortality. No analyses of treatment-emergent adverse events were reported. Favipiravir is not currently approved by the FDA for use in treating COVID-19. Given the open label design of the study, and lack of significant changes in mortality, it is unclear that favipiravir has any effect on the clinical course of COVID-19. Moreover, considering that studies in a Syrian hamster model have shown notable toxicity at high doses via significant weight loss, further studies in vitro and in animal models to test the efficacy against coronaviruses is advised [28].
3.2. Molnupiravir (EIDD-2801, MK-4482)
Molnupiravir is an orally administered antiviral that was originally developed by Emory University for Drug Discovery (EIDD) for treating Venezuelan equine encephalitis virus (VEEV). This isopropyl-ester prodrug of a ribonucleoside analog β-D-N4-hydroxycytidine, EIDD-1931), which is phosphorylated at the N4-hydroxyl group in the intestinal tract, incorporates into RNA, inducing mutations that reduce viral viability over the duration of infection [29]. In vitro Vero cells infected with VEEV and treated with 2.5 µM EIDD-1931 had a lower number of virions released with reduced particle infectivity. EIDD-1931 has demonstrated efficacy against Ebola [30] in Vero E6 cells, against influenza in human epithelial cell cultures and in ferret models of influenza [31], MERS in Calu-3 human lung cancer, Vero cells and human primary epithelial lung cell cultures [32]. In vivo, molnupiravir reduced viral loads in C57BL/6 mouse models of MERS and SARS-CoV. Interestingly, in the same study, murine hepatitis viral mutants that were resistant to remdesivir had increased susceptibility to molnupiravir [32].
In vitro, in Calu-3 and Vero cells, EIDD-1931 has an IC50 of 80 nM and 90 nM, respectively when added at the same time as SARS-CoV-2. Using primary epithelial lung cells, a maximal titer reduction of SARS-CoV-2 by 5-logs was observed at a dose of 10 µM EIDD-1931, despite SARS-CoV-2 nsp14’s proofreading exonuclease activity.
Molnupiravir is currently being tested in phase II and phase III clinical trials in COVID-19 patients (NCT04405570 and NCT04405739). The results from these trials, which have not been completed, have not been released.
Although molnupiravir has shown promise against multiple RNA viruses in vitro, including the aforementioned β-CoVs, some potential concerns remain with antiviral mutagens as a treatment strategy at large. Using a mutagen as a form of treatment could promote resistance to itself. A study of ribavirin treatment in poliovirus infection has revealed that use of the mutagen created a selective pressure towards drug resistance [33]. Providing a higher mutation rate in an environment that selects against viruses that cannot escape the immune response generated by the COVID-19 vaccines could be a risky strategy, as it could conceivably accelerate the emergence of a new variant that escapes the vaccines developed so far. However, there is no precedence nor data that suggest the likelihood of this happening and RNA viruses are more likely to select for greater polymerase fidelity as a means of escaping mutagens as was the case for polio. This could perhaps mean that wide scale use of mutagens as a treatment strategy could lead to the emergence of less mutagenic variants. More in silico and in vitro data are necessary to properly elucidate the veracity of these concerns.
3.3. Lopinavir/ritonavir (Kaletra)
Lopinavir is an orally administered viral protease inhibitor that was developed by Abbott pharmaceuticals to treat HIV infections [34]. Ritonavir, a second protease inhibitor developed by Abbott, was more effective in vitro as a potent inhibitor of cytochrome P450 3A4 (CYP3A). When used together, ritonavir prevents CYP3A mediated metabolism of lopinavir to increase drug bioavailability [35]. Accordingly, lopinavir in combination with ritonavir was approved by the FDA to treat HIV. The combination of lopinavir/ritonavir (Kaletra) functions as a competitive inhibitor of the HIV viral Gag-Pol protease. In the absence of Gag and Pol, HIV is not infectious.
Using either wild-type or a genetically modified form of MERS, which expresses luciferase, lopinavir had antiviral activity against SARS-CoV-1 at 8–32 µg/ml and Kaltera had an EC50 of 8.5 µM against MERS in human lung epithelial Calcu-3 cells in vitro [36], [37].
At present Kaletra is being used in thirty-five clinical drug trials in the treatment of SARS-CoV-2 patients. Although clinics are still recruiting patients to examine the efficacy of Koletra in treating COVID-19, several studies were stopped early since there was no significant clinical benefit for patients treated with Koletra alone, when compared to placebo controls [38], [39] (NCT04455958). In a multi-center, open-label, randomized, clinical trial, patients treated with the combination of 400 mg of lopinavir, 100 mg of ritonavir and 400 mg ribavirin every 12 h, along with three doses of 8 million international units of interferon beta-1b on alternate days (combination group) when compared with 14 days of 400 mg lopinavir and 100 mg ritonavir alone every 12 h (control group), had a significant reduction in time to recovery (7 vs 12 days p = 0.0010) [40]. These studies illustrate the potential of using combination therapies for COVID-19 patients, an approach that has worked well with HIV.
3.3.1. PF-00835231 and protease inhibitor strategies at large
PF-00835231 is a coronavirus protease inhibitor that prevents cleavage and processing of the 1a and 1ab replicase-associated polyproteins (pp1a and pp1ab, respectively) produced by translation of the virus’ open reading frame 1b (ORF1b) [41]. SARS-CoV-2 uses two proteases to process its polyproteins: a papain-like protease (PLpro) and its 3CL main protease (Mpro). PF-00835231 specifically acts against Mpro and was initially designed against the Mpro of SARS-CoV-1. However, the two viruses’ Mpro amino acid sequences are similar enough for the drug to have a potential action against SARS-CoV-2. Its prodrug counterpart is PF-07304814 [42].
An in vitro study of PF-00835231 has shown potency against SARS-CoV-2. The study used a recombinant human airway epithelium adenocarcinoma cell line (A549) with exogenous expression of ACE2. Observing cytopathic effects and syncytia formation, the study found that PF-00835231 had a statistically smaller EC50 than remdesivir (0.221 μM vs 0.442 μM at 24 h post-infection, p = 0.002) with minimal cytotoxicity [43]. This suggests that PF-00835231 is likely to have a high therapeutic index with good potential as a drug candidate against SARS-CoV-2. Furthermore, since the Mpro of SARS-CoV-2 is either not mutated or mutations are predicted to have no functional consequences in several variants, PF-00835231 could have broad therapeutic activity against current and emerging viral variants.
Clinical trials and pharmacokinetic studies on PF-00835231 and its phosphate prodrug counterpart are either ongoing or have yet to post their results (NCT04627532 and NCT04535167). Some concerns exist regarding the bioavailability of the drug, as studies suggest it is a substrate for the P-glycoprotein based upon increases in its efficacy combined with P-glycoprotein inhibitors [43]. However, the aforementioned in vitro study of PF-00835231 investigated this possibility by analyzing RNA datasets of human airway epithelial cells, which show that the protein is not expressed by these cells [43]. The same study also highlighted the potential this drug has in a combinatorial strategy. To date, a great deal of effort has gone into investigating the efficacy of replicase inhibitors against SARS-CoV-2, but less focus has been placed on protease inhibitors as a treatment strategy.
3.3.2. Peginterferon λ-1a
Peginterferon lambda-1a (or PegIFNλ-1a) is a type III interferon therapeutic that was initially designed for treating chronic hepatitis C virus (HCV) infections [44]. It is a pegylated form of the naturally-occurring cytokine IFNλ, which has an antiviral function analogous to type 1 IFNs, and can trigger shutdown of host translation through activity of protein kinase R (PKR) [45]. While type III IFN receptors are expressed mainly by mucosal epithelial barrier cells and certain immune cells [46], type I IFN receptors are more ubiquitously expressed. A result of PegIFNλ-1a use as an antiviral for illnesses such as HCV is a decrease in adverse side effects compared to IFNλ. IFNλ signals via a heterodimeric receptor to activate the Janus kinase-signal transducer and activator of transcription 1 (JAK1/STAT1) and Tyrosine kinase 2 (Tyk2) to promote signaling via the interferon stimulated gene factor 3 (composed of signal transducer and activator of transcription 1 (STAT1), STAT2, insulin regulatory factor 9) pathway. This JAK/STAT signaling promotes transcription of IL-12, skewing toward a Th1 antiviral immune response from CD4 + Th1 cells and CD8 + T cells [45]. Given the antiviral nature of type III interferons and their concentrated activity in epithelial cells, antiviral therapies of this type are appealing.
Type III IFNs have some antiviral activity against SARS-CoV-2 and other β-coronaviruses in vitro. A study of in vitro inhibition of SARS-CoV-2 using two epithelial cell lines, (simian Vero E6 and human Calu-3 cells), showed a statistically significant decrease (p = 0.007 and p = 0.0223, respectively) in viral titers after pre-treatment with recombinant IFNλ at 10 ng/ml [47]. In a separate study a 24-hour pre-treatment of human primary airway epithelial cells with PegIFNλ-1a, followed by infection with SARS-CoV-2, reduced SARS-CoV-2 viral titers 48 h post infection to levels comparable to 1 µM remdesivir. In the same study, a single 2 µg IFNλ subcutaneous injection in 1 year-old BALB/c mice administered prophylactically 18 h prior to infection or therapeutically 12 h after infection, resulted in reduced viral titers in the lungs, without affecting the nasal titers [48].
It is discouraging, however that PegIFNλ-1a has not reliably produced significant results in clinical trials. A randomized, single-blind placebo-controlled trial with 120 participants injected subcutaneously with 180 µg of PegIFNλ-1a within 72 h of diagnosis, produced no significant clinical improvements in COVID-19 outpatients [49]. A second randomized, double-blind controlled trial with 60 outpatients found that PegIFNλ-1a accelerated viral clearance by individuals 7 days after a subcutaneous injection of 180 µg of PegIFNλ-1a, in patients with a high baseline viral load (>106 copies of RNA per ml) [49]. Both studies indicated more frequent increases in liver activity as measured by increases in blood aminotransferase among participants in the treatment group. Despite its lack of efficacy, PegIFNλ-1a is a treatment option with low risk for treatment-emergent adverse events because of the limited distribution of type III IFN receptors. Accordingly, healthcare professionals might consider it as an option if no other recourse is available in future pandemics. PegIFNλ-1a is not FDA-approved for use in treating COVID-19 as of writing.
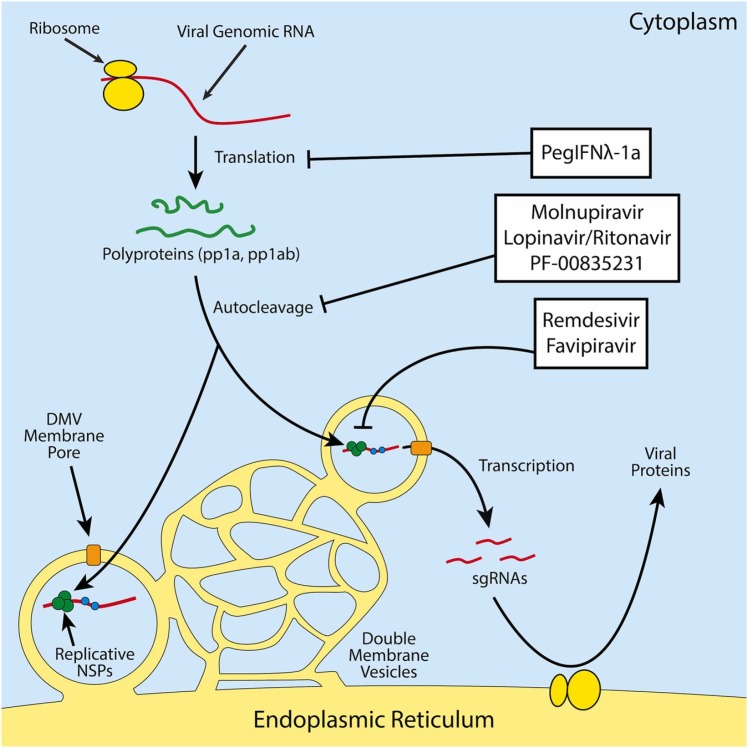
A summary of our discussion of the efficacy and mechanism of investigated antiviral treatment strategies are found below in Table 1 for treatments that have existing clinical trial data. The mechanisms of action for these therapeutics are displayed in Fig. 2.
Table 1.
Summary of antiviral treatment strategies with existing clinical data.
| Treatment | Mechanism Target | Clinical benefit? | Mortality decrease? |
|---|---|---|---|
| Remdesivir | RNA-dependent RNA polymerase | ✓ | ✓/Mixed |
| Favipiravir | RNA-dependent RNA polymerase | ✓ | X |
| Lopinavir/Ritonavir | Viral protease | ✓ | X |
| Peginterferon λ-1a | General viral replication, cell activity | Mixed | X |
✓ - The drug produced the respective column’s effect. X - The drug failed to produce the respective column’s effect. Mixed - The drug produced negative and positive outcomes or only produced positive outcomes in some trials. * - Clinical trials of this drug either failed to exceed 100 participants or lacked any blinding.
Fig. 2.
Scheme of the mechanisms of action of various antivirals against SARS-CoV-2. Protease inhibitors act against the protease activity of the two coronavirus polyproteins, which prevents its self-cleavage into various non-structural viral proteins. RDRP inhibitors prevent the production of genomic and subgenomic RNAs (sgRNAs). PegIFNλ-1a stimulates the innate antiviral state in its target cells, perhaps most notably by turning off host translation machinery through activation of protein kinase R (PKR) among other antiviral effectors.
3.4. Anti-Inflammatories and immunomodulatory drugs
In this section we describe therapeutics which limit the hyperimmune response often associated with COVID-19 progression. We discuss antibody therapies against host factors in a different section. The reviewed anti-inflammatories and immunomodulatory drugs include but are not limited to any therapeutic that modulate immunologically relevant processes including cytokine release, cytokine response, lymphocyte proliferation, clotting, or innate immune effector mechanisms.
3.4.1. Compound 21
Compound 21 (C21) is an agonist for the angiotensin II type 2 receptor (AT2R) involved in the renin-angiotensin-aldosterone system (RAS) that regulates blood pressure and vasoconstriction [50]. Angiotensin II is a ligand for two receptors which produce opposing effects. The angiotensin II type 1 receptor (AT1R) is important in promoting inflammation, vasoconstriction, an increase in blood pressure, and fibrin deposition [51]. In contrast, AT2R has an anti-inflammatory effect, decreases blood pressure, and promotes vasodilation. Angiotensin II can also be enzymatically cleaved by the SARS-CoV-2 viral receptor ACE2 into a ligand that specifically acts on AT2R [51]. As an agonist for AT2R, C21 can reduce neurological deficits and cerebral infarct sizes in a mouse model of ischemic stroke [52]. At 100 µM, C21 can also significantly reduce TNF-ɑ mediated endothelial inflammation of human vascular endothelial cells, which induced adhesion of Thp-1 leukocytes in vitro. This reduced adhesion correlates with a loss in the β2 integrin adhesion ligand, ICAM-1. Similarly, 10 µM C21 can significantly inhibit adhesion of murine leukocytes during TNF-ɑ induced inflammation in mouse aorta in vivo. Both in vitro and in vivo, treatment with the AT2R antagonist, PD 123319, reversed this effect [50]. These observations are directly related to the role of AT2R in promoting the “protective” side of the RAS over its proinflammatory counterpart, AT1R.
While there are no studies in vitro which examine the mechanism of C21 inhibition of SARS-CoV-2 during infection, theoretically, the effects of C21 on the host could be predicted by data from other coronavirus studies. Respiratory failure leads to respiratory acidosis, a condition that evolves when the lungs cannot remove enough of the carbon dioxide produced by the body. Studies of SARS-CoV-1 have demonstrated an association between exposure to the S2 spike protein in combination with acid and a decrease in ACE2 expression [53]. These studies have also showed a worsening of lung pathology among acid-treated mice, an increase in leukocyte infiltrates, and an increase in angiotensin II concentrations. Along with the role of the AT1R in fibrin deposition, these factors could contribute to thrombotic complications in severe COVID-19 infections, which includes pulmonary embolism [54]. More in vitro and in vivo studies are necessary to determine if RAS imbalances result from SARS-CoV-2 infection and if they result in an enhanced cytopathic effect.
Clinically, C21 trials have demonstrated limited success. A randomized, triple-masked, placebo-controlled trial with 106 participants given 200 mg/day (100 mg twice a day) of C21 treatment in COVID-19 infection found a significant reduction (p = 0.003) in oxygen supplementation at day 14 of the study, although there was no difference at day 7 (p = 0.0568) when utilizing a p-value cutoff of p < 0.05 (the study used a p < 0.1 cutoff). C21 did not significantly decrease C reactive protein in serum (p = 0.0881) (NCT04452435). C21 prevents the need for oxygen supplementation but does not significantly affect mortality. Interestingly, the same outcomes do not appear to be true of AT1R pathway antagonists. A retrospective study of 112 COVID-19 patients found no significant differences in Angiotensin Converting Enzyme inhibitors (ACEi)/Angiotensin Receptor Blocker (ARB) medication usage among patients with severe and mild COVID-19 [55]. ACEi is an ACE inhibitor while ARB is an AT1R antagonist, and these two medications work in tandem to reduce AT1R signaling and skew the RAS balance towards an anti-inflammatory state. Although a retrospective study is not ideal, it is possible that inhibiting the AT1R signaling pathway might be less effective than rebalancing the RAS system by activating the AT2R pathway. More clinical trials are necessary to discern the efficacy of anti-AT1R medications and possible combinatorial strategies with C21, but C21 appears to have some relevant clinical efficacy by reducing the need for oxygen.
3.4.2. Baricitinib
Baricitinib is a specific inhibitor of JAK1/2, which play important roles in regulating cytokine responses [56], [57], [58]. It has proven effective in treating autoimmune illnesses associated with excessive production of pro-inflammatory cytokines, such as rheumatoid arthritis [59]. This is because many pro-inflammatory cytokine receptors, such as IFN receptors, use the JAK/STAT pathway upon stimulation to transcribe effector genes [60]. Thus, inhibiting JAKs attenuates responses to inflammatory cytokines and acts as an anti-inflammatory.
Baricitinib’s mechanism of action against COVID-19 is slightly more complex than its role in attenuating JAK/STAT signaling. An in vitro study of proinflammatory cytokines in blood isolated from study participants with active COVID-19 infection showed that 1 µM baricitinib significantly (p < 0.0001) decreased IFN-ɣ concentrations along with other proinflammatory cytokines when blood cells were exposed to SARS-CoV-2 spike protein [61]. This response may be due to the ability of baricitinib to potentially disrupt spike protein endocytosis by binding to adaptor-associated kinase 2 (AAK2) and BMP-2 inducible kinase (BIKE) with high affinity as well as cyclin G-associated kinase (GAK) with more moderate affinity [62]. All three of these proteins are important in the clathrin-mediated endocytic pathway, and it is possible that baricitinib also acts by preventing viral entry as well. However, no in vitro studies have evaluated this mechanism, so further research is necessary.
Baricitinib has shown some clinical utility in combination with remdesivir [63]. A double-blind, randomized, placebo-controlled trial of 1033 hospitalized COVID-19 adult patients by the NIAID found that remdesivir and baricitinib combined, significantly improved time to recovery (p = 0.03) and had 30% higher odds in improvement of clinical status, compared to remdesivir monotherapy [63]. Specifically, patients who were being treated with combination therapy receiving high-flow oxygen at the time of the enrollment in the study, averaged 10 days to recovery in contrast to an average of 18 days for patients on remdesivir alone, while the 28-day mortality rate was 5.1% versus 7.8% for the controls. Further investigations of baricitinib are merited, and its effect as a monotherapy should be better characterized. Some concerns exist regarding adverse events in baricitinib therapy, including a potential increased risk of coinfection, anemia, and lymphocytopenia [64]. An additional potential benefit of baricitinib therapy, is that unlike other first generation JAK1/JAK2 inhibitors, baricitinib is cleared by the kidneys, instead of being metabolized by cytochrome P450 in the liver. This might explain discrepancies in the efficacies of different JAK1/JAK2 inhibitors discussed later, and it’s a potential explanation for a statistically significant decrease in occurrence of adverse events (p = 0.03) in the combinatorial study mentioned above [63].
3.4.3. Ruxolitinib
Ruxolitinib, a first generation JAK1/2 inhibitor structurally similar to baricitinib, promotes an anti-inflammatory response by inhibiting downstream signaling of cytokines such as interferons via STAT1 [56], [65]. It is associated with improvement in autoimmune conditions in vitro and in vivo associated with an inflammatory response, like dermatomyositis [66].
Ruxolitinib has demonstrated in vitro efficacy against the SARS-CoV-2 complement pro-inflammatory response in primary normal human lung epithelial cells or human lung carcinoma A549 cells. Complement is an innate defense mechanism that clears pathogens through its products’ effector functions, which include opsonization, inflammation and chemotaxis, and formation of the pore-forming membrane attack complex. A transcriptomic analysis in the presence of 1 µM ruxolitinib, during COVID-19 infection showed that levels of mRNA for several complement proteins including C1R, C1S, CFB, and C3 were restored to normal by treatment with ruxolitinib [65]. Notably, in vitro, production of C3a in SARS-CoV-2 infected induced alveolar epithelial cells (iAECs) was significantly reduced in cells treated with ruxolitinib; this effect was enhanced by co-treatment in the presence of 250 nM remdesivir. Activation of complement can induce tissue damage, which has been well-documented in other diseases like postinfectious glomerulonephritis [67]. It is possible that normalizing complement expression could thus prevent organ damage associated with cytokine storm and overstimulation of the immune response.
Ruxolitinib has exhibited disappointing results in clinical trials so far. A randomized, double-blind, placebo-controlled trial carried out by Novartis, examined the effects of 5 mg ruxolitinib twice a day in 432 patients with COVID-19 over a 14- or 28-day period. Novartis found no significant changes in mortality or Intensive Care Unit (ICU) usage, ventilator use, one- or two-point clinical improvements from baseline on an ordinal scale, or time to improvement in clinical status (NCT04362137). Interestingly, these results differ from the results of the clinical trial on baricitinib, with ruxolitinib failing to provide significant improvements in time to recovery. The reason for this difference is unclear, but it is possible that it relates to how the body clears the two drugs, since baricitinib is largely cleared by the kidneys and ruxolitinib is subject to a first-pass effect during liver metabolism [56]. In addition, serious adverse events have been reported for ruxolitinib, which include several cases of progressive multifocal leukoencepholapathies [68], [69], [70].
3.4.4. Dornase alfa
Dornase alfa is a recombinant form of human DNAse I, that cleaves extracellular DNA created by netosis, the expulsion of DNA by neutrophils used to clear pathogens [71]. This drug is FDA approved for use during inflammatory illnesses, such as cystic fibrosis, which are associated with neutrophil extracellular traps (NETs). The most common application is in combination with neutrophil elastases in treating the thick mucus associated with cystic fibrosis [72]. The enzyme combination promotes clearance of thick mucus in the lungs by decreasing the viscoelasticity of respiratory mucus secretions. Elevated levels of neutrophils and macrophages have been linked to extrusion of DNA extracellular traps during bacterial infection and COVID-19 [73], [74].
In a very limited study of 3 patients, dornase alfa was found to have anti-inflammatory activity in vitro analysis, as well as unanticipated antiviral activity [75]. In this in vitro study of artificially induced DNA clumps in peripheral blood mononuclear cells, the DNAse activity of dornase alfa at 100units/ml reduced the DNA NETs and the concomitant cytopathic effect in cultured mononuclear cells. Under these conditions, dornase alfa demonstrated no notable cytotoxicity within 10–100 U/ml [75]. COVID-19 serum has been shown to contain NETs, and SARS-CoV-2 has been proven to effectively induce NET formation in vitro [76]. The result of inflammation in COVID-19 infection is severe pneumonia and progression to ARDS. Dornase alfa has an immunomodulatory effect that lessens tissue damage associated with NETs as an effector of inflammation. In addition, dornase alfa decreased SARS-CoV-2 viral RNA concentrations in a Madin-Darby Bovine Kidney (MDBK) cell line compared to untreated infected cells in the aforementioned study [75]. However, there is no discernible mechanism for its potential antiviral action, so more research is necessary to validate its antiviral activity.
A lack of data from randomized controlled trials with sufficient sample sizes for dornase alfa means that its clinical utility has yet to be properly determined against COVID-19. A nonrandomized, single-masked trial of 30 COVID-19 patients with ARDS posted results that appear to suggest an increase in static lung compliance and a decrease in length of ICU admission in the dornase alpha-treated experimental group. However, no statistical analyses were performed on the study results, so it is unclear whether the study had sufficient statistical power to attain significant results (NCT04402970). Another randomized trial, with aerosol administration of 2500 U of dornase alfa, twice a day, for 7 days enrolling 100 patients is still underway and has not yet posted results [77]. Dornase alfa appears to potentially have some therapeutic benefit in treating COVID-19, but insufficient data has been generated to determine if it produces any clinical benefits.
3.4.5. Heparin
Heparin, originally described as an extract of liver in 1916 [78] by Maclean, is an acidic mucopolysaccharide which is naturally produced in the liver, lungs and mast cells and has been used clinically for close to 100 years as an anticoagulant, to inhibit blood clotting [79]. As early as 1928, the structure of heparin was described as a sulfur-containing glycosoaminoglycan [80], and it was purified in 1934 [80]. Heparin functions to inhibit formation of the C3 complement convertase, C3b,Bb [81] upstream of C5b-9, and thereby inhibits the procoagulant activity of platelet factor V and the production of the pro-thrombinase complex [82]. Inhaled heparin is mucolytic, and in part because of this effect, inhaled heparin has been used to treat cystic fibrosis to thin viscous secretions [81], [83].
Heparin has promising results in clinical trials to treat COVID-19 patients who are in the ICU and are on mechanical ventilation. Several forms of heparin have been approved from clinical use to treat and prevent blood clots and thin viscous secretions in COVID-19 patients: Tinzaparin sodium, Dalteparin, enoxaprin and Nadroparin. A retrospective clinical trial of 152 COVID-19 patients was conducted (NCT04412304) to determine the effects of thrombophylaxis in critically ill COVID-19 patients. [84] In this study 67 patients receiving low-dose thrombo-phylaxis (2500–4500IU tinzaparin or 2500–5000 IU dalteparin), 48 receiving medium-dose thrombophylaxis (>4500 IU but <175IU/kg of body weight of tinzaparin or >5000 IU but <200 IU/kg of body weight of delteparin) and 37 receiving high-dose thrombophylaxis (> 175IU/kg of body weight tinzaparin or >200IU/kg body weight dalteparin) revealed that the death rate was significantly reduced in patients who received high dose (13.5%) when compared to those receiving medium dose (25%) or low dose (38.8%) thrombophylaxis. When compared to patients who received low dose thrombophylaxis, the mortality rate was also reduced among patients who received medium dose (hazard ratio = 0.88; 95% CI: 0.43–1.83) and high dose therapies (hazard ratio = 0.33; 95% CI: 0.13–0.87). In addition, while 17.9% of the low dose patients, and 18.8% of the medium dose patients had thromboembolic events, these numbers were reduced to 2.7% in the high-dose patients. [84] A separate retrospective study of 450 COVID-19 patients receiving either a standard prophylactic enoxaparin dosage (40–60 mg daily) or an intermediate dosage (40–60 mg twice daily) showed a significant decline in mortality associated with the higher dosage (18.8% vs 5.8%, p = 0.02). [86].
While these studies are either open-label, uncontrolled, retrospective, and/or confounded by treatment of the control group, they reveal a potential use for heparin as a treatment strategy in critically ill COVID-19 patients through their possible reduction in patient mortality. In a third observational study of 2773 hospitalized patients with COVID-19, 786 (28%) were given systemic anticoagulation therapy [87] (oral, subcutaneous or intravenous administration). The median hospital stay for all treated patients was 5 days, and mortality was 22.5%, with a median survival of 21 days. This was in comparison to 22.8% survival with a median survival of 14 days in patients who did not receive anticoagulation therapy. Notably, for patients who required mechanical ventilation (n = 395), in-hospital mortality was 29.1% (median survival 21 days) for patients treated with given anti-coagulation, in contrast to 62.7% (median survival 9 days) for patients who were not given anticoagulation therapy. Notably, using a multivariate proportional hazards model, the longer anticoagulation treatments were associated with a significant reduction in mortality rates (adjusted HR = 0.86 per day; 95% CI; 0.82–0.89; p < 0.001). Unfortunately, the specific anticoagulant(s) used in this study was not described. Close to 100 clinical trials using heparin during COVID-19 are ongoing or have been recently completed and the outcomes of these studies should be closely watched. Although no data from randomized, blinded controlled trials currently exist, initial studies show that heparin may be a promising treatment strategy for critically ill COVID-19 ICU patients requiring mechanical ventilation.
3.4.6. Dexamethasone
Dexamethasone is a long-acting synthetic corticosteroid used to treat a wide variety of inflammatory illnesses [77], [85]. The anti-inflammatory properties of corticosteroids were widely characterized at a clinical level before their mechanisms of action on a cellular level were elucidated. Corticosteroids suppress expression and release of proinflammatory cytokines by either binding to negative glucocorticoid response elements in genes encoding them or by interacting with transcription factors associated with their increased expression [85]. The result is an attenuated inflammatory response and a reduction in damage associated with immune responses.
Dexamethasone has presented inconclusive results in the treatment of non-COVID-19 pneumonias since the response changes with the dosage, time of administration and duration of treatment. A randomized, controlled trial of 277 patients with ARDS found that dexamethasone (i.v. 20 mg/day on days 1–5; 10 mg/day on days 6–10) significantly (p = 0.0047) decreased mortality by day 60 (21% of patients died in the treatment group compared to 36% of patients in the control group) and time spent on a ventilator (between group difference 4–8 days, p < 0.0001) when compared to patients receiving routine intensive care [86]. A pre-COVID-19 retrospective study, examining the outcomes in 401 SARS patients, found that all non-critical patients receiving an average of 105 mg/day corticosteroids survived the disease while of the 121 of 152 critical patients who received an average daily dose of 133.5 mg corticosteroids, 25 patients died. Analysis of these 401 SARS patients failed to show a benefit of corticosteroid use in terms of death rate or days spent in the hospital [87]. However, after adjustment with multivariate analysis for possible confounders to minimize the effects of the differences in patient baselines, treatment with corticosteroids was found to shorten hospital stays, and reduce short-term and overall mortality [87]. However, a meta-analysis of ten clinical trials of dexamethasone in treating influenza-associated pneumonia found that the drug increased mortality and length of stay in the ICU, and it was associated with a higher incidence of secondary infections [88]. Given its potent anti-inflammatory nature, dexamethasone poses a risk of persistent immunosuppression at high doses of up to 1000 mg/day, resulting in reduction of CD4 and CD8 T cells and a concomitant increase in secondary infections [89]. Therefore, this immunosuppression could lead to a higher incidence of secondary infections that offset any improvement in mortality in patients with viral pneumonia. Thus, dexamethasone may be a potential therapeutic against COVID-19 when administered carefully and early in the onset of ARDS in individuals with severe disease.
There is somewhat limited data regarding the efficacy of dexamethasone in treating COVID-19 as clinical trials of the drug are still ongoing. A randomized, open-label clinical trial of 6425 patients hospitalized with COVID-19 found a significant (age adjusted ratio p < 0.001) decrease in mortality among patients receiving dexamethasone compared with the usual care group within 28 days of randomization. The survival benefits of dexamethasone were present when used in conjunction with invasive mechanical ventilation, or in patients treated with supplemental oxygen alone, but not for individuals who did not receive respiratory support [90]. While this trial has a large sample size, it suffers issues with its internal validity. Specifically, there was a lack of blinding and a significant difference in the mean age between the experimental and control groups. Despite its design flaws, it demonstrates the potential of dexamethasone use as a therapeutic for COVID-19. Overall, dexamethasone is a promising treatment strategy for individuals with severe COVID-19 due to its ability to reduce patient mortality.
3.4.7. Methylprednisolone
Methylprednisolone is mid-acting broad spectrum corticosteroid anti-inflammatory drug used to treat a variety of conditions [91]. It prevents expression and release of inflammatory cytokines and similar to dexamethasone has been used to treat inflammatory illnesses and post-surgical complications [91]. In that sense, its primary utility is in preventing excess damage caused by an immune response.
There are no in vitro studies examining the utility of methylprednisolone in COVID-19 infection, but clinical trials of the drug in participants with ARDS reveal some potential concerns. A randomized, double-blind, placebo-controlled trial of 180 patients with ARDS showed no survival benefit when compared to controls [92]. In this study patients were intravenously infused with 2 mg/kg methylprednisolone on day 1, followed by 0.5 mg/kg every 6 h for 14 days, 0.5 mg/kg every 12 h for 7 days, followed by tapering if the patient was able to breathe without assistance for 48 h. While at 60 and 180 days, the use of methylprednisolone had no effect on patient mortality (p = 1.0), use of the corticosteroid was associated with higher mortality when taken two weeks past the onset of ARDS. However, it did reduce ventilator use and length of ICU stay. At best, this study indicates that methylprednisolone could be used with caution and timed with the natural history and/or onset of a disease to maximize utility and minimize harm.
Clinical trials for methylprednisolone in COVID-19 patients show that it has some potential clinical benefit. A randomized, single-blind controlled trial of 250 mg/day (i.v.) for 3 days of methylprednisolone among 62 people in the early pulmonary phase of severe COVID-19 showed a significant decrease in death rate (5.9% in the treatment group vs. 42.9% in the control group, p < 0.001) and reduction in time to improvement among the experimental group compared to controls [93]. The timeline of this study fits the findings of the clinical trial on general ARDS study discussed above- methylprednisolone was administered only to individuals who had yet to develop ARDS but were experiencing dyspnea. However, it is important to note that this clinical trial was potentially confounded by diabetes, with diabetics consisting of a significantly higher proportion of the control group than the experimental group. Other clinical trials designed as prospective and retrospective cohort studies provide some supporting evidence of methylprednisolone efficacy, primarily by demonstrating that the corticosteroid reduces ventilator use [94], [95] (NCT04323592). These studies also support the early use of methylprednisolone to maximize its potential clinical benefits. In support of these observations, a triple-blind, randomized controlled trial of 86 hospitalized COVID-19 patients found that methylprednisolone, significantly improved clinical status on day 5 (p = 0.002) and day 10 (p = 0.0001) after admission, while reducing the need for using a ventilator (p = 0.040) and length of hospital stays (p = 0.0015) overall when compared to control patients treated with dexamethasone [96]. Overall, more controlled trials with larger sample sizes would be helpful in establishing the efficacy of this class of drugs, but existing data shows it is a potentially useful tool in combating COVID-19 and future outbreaks of coronavirus-associated pneumonia and ARDS when administered early in onset.
3.4.8. Losartan
Losartan is an antihypertensive medication that functions by antagonizing the angiotensin II type 1 receptor (AT1R) involved in RAS [97]. Its function in the pathway complements that of C21. Whereas C21 agonizes AT2R to induce its anti-inflammatory and anti-thrombotic effects, losartan inhibits binding of angiotensin II to AT1R to limit its proinflammatory and thrombotic effects. This is evidenced by studies of the effect of losartan in mouse models, which show that the drug decreases blood pressure, increases enzymatic defenses against oxidative stress, and reduces concentrations of IL-6 [98], [99].
There are no in vitro studies of losartan against SARS-CoV-2 associated inflammation, and articles on a potential antiviral activity are in preprint. However, much like C21, losartan improves lung elastance in a mouse model challenged with SARS-CoV-1 spike protein in an acidified environment [53]. The results showed that losartan limited the decrease in lung elastance, the change in the pressure of air needed to expand the lungs, which indicates reduced tissue fibrosis.
It is disappointing that losartan has failed to demonstrate a patient benefit in clinical trials against SARS-CoV-2. A phase II double-blind, randomized controlled trial of 117 outpatients with symptomatic COVID-19 found no significant differences in mortality, improvements in dyspnea, or changes in disease severity [100]. However, it is important to keep in mind that relatively few, if any of the participants were hospitalized, used supplemental oxygen, or was admitted to the ICU, and none of the patients died. Larger studies with more hospitalizations might provide some evidence for clinical utility not found in this study. Another clinical trial using losartan during SARS-CoV-2 infection with published results has been posted to the NIH clinical trials website, but no statistical analyses were included, likely because of the small sample size of 31 patients [101]. This study is in line with the observations made with ACE inhibitors and angiotensin receptor blockers mentioned with the C21 clinical trials. Based on these clinical studies, at this point, losartan and other treatments which inhibit the AT1R are likely not promising treatment strategies against COVID-19.
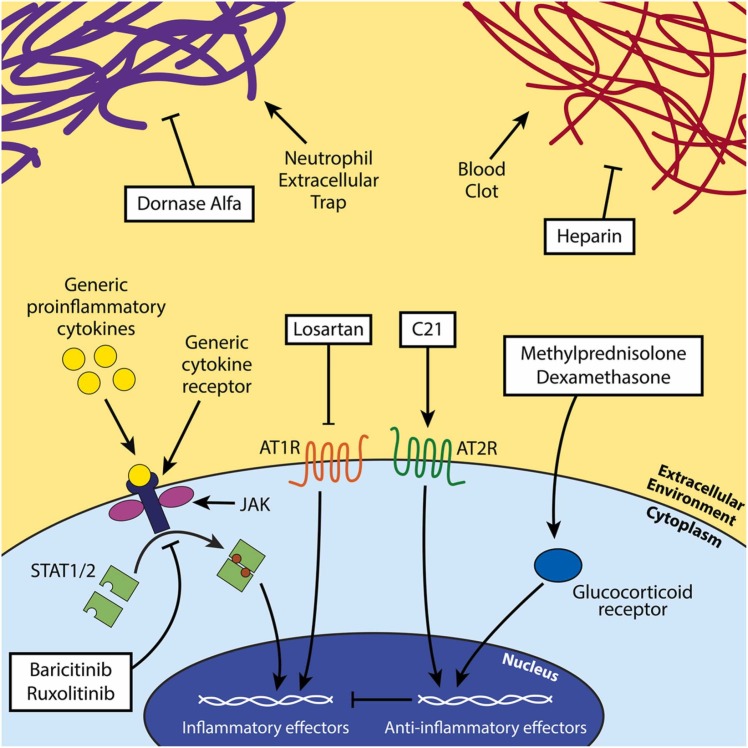
A summary of our review of the mechanism of action and efficacy of anti-inflammatory treatment strategies is described below in Table 2. Fig. 3, Fig. 4.
Table 2.
Summary of anti-inflammatory treatment strategies.
| Treatment | Mechanism Target | Clinical benefit? | Mortality decrease? |
|---|---|---|---|
| Compound 21 | AT2R agonist (renin-angiotensin system) | ✓ | X |
| Losartan | AT1R antagonist (renin-angiotensin system) | X | X |
| Baricitinib | JAK1/2 | ✓ | X |
| Ruxolitinib | JAK1/2 | X | X |
| Dornase Alfa | Neutrophil extracellular traps (mucous) | X (?) | X (?) |
| Heparin | Blood clotting | ✓* | ✓* |
| Methylprednisolone | Inflammatory cytokine production | ✓* | ✓* |
| Dexamethasone | Inflammatory cytokine production | ✓ | ✓ |
✓ - The drug produced the respective column’s effect. X - The drug failed to produce the respective column’s effect. ‐* - Clinical trials of this drug either failed to exceed 100 participants or lacked any blinding.
Fig. 3.
Scheme of the investigated anti-inflammatory therapeutics. Dornase alfa acts against NETs, baricitinib and ruxolitinib inhibit JAK/STAT signaling, losartan and C21 leverage the angiotensin-renin system to reduce inflammation, and methylprednisolone and dexamethasone act as anti-inflammatories through the action of the glucocorticoid receptor in the cytoplasm. Heparin blocks blood clotting by inhibiting clotting factors upstream of thrombin production.
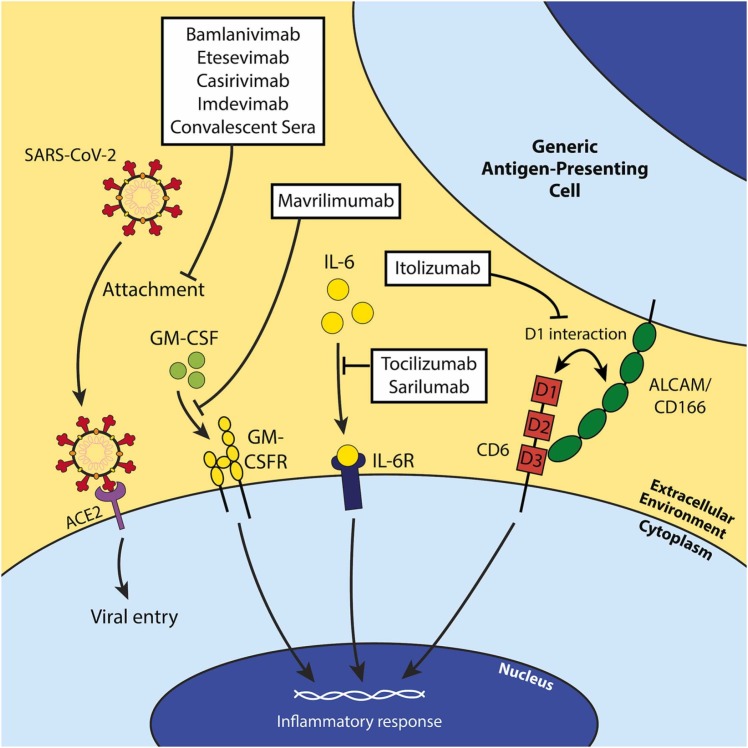
Fig. 4.
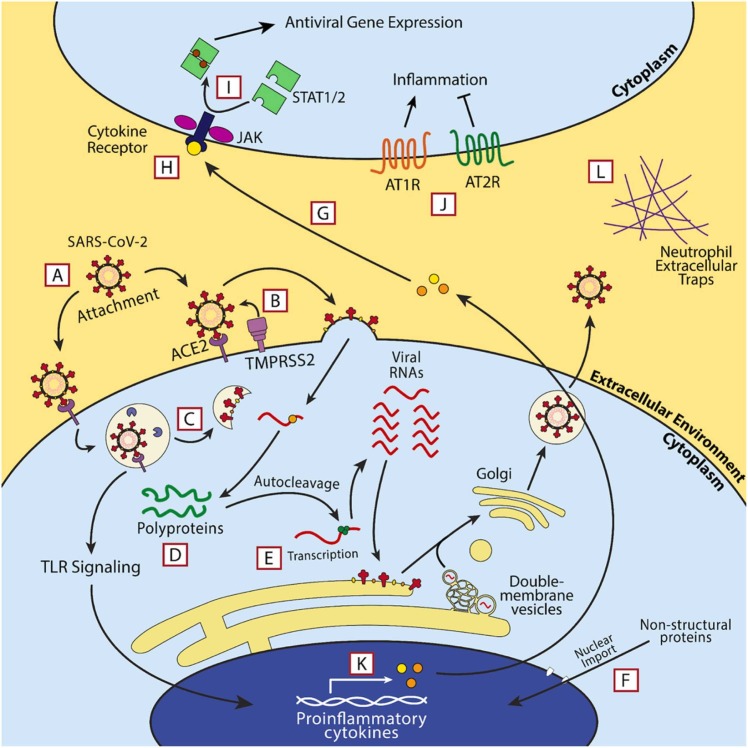
Schematic of the mechanisms of action of each investigated antibody therapy against COVID-19. Most antibody therapies directly target SARS-CoV-2 by neutralizing the virus and preventing entry of the virus into susceptible host cells (bamlanivimab, etesvimab, casirivimab, imdevimab, and convalescent sera). However, mAbs exist against host factors to attenuate inflammatory responses in COVID-19 infection. Mavrilimumab is a GM-CSFR antagonist and prevents GM-CSF’s inflammatory functions (i.e. T cell activation and proliferation, etc.). Tocilizumab and sarilumab are IL-6R antagonists and serve a similar function to mavrilimumab conceptually, and itolizumab binds domain 1 (D1) of CD6 to inhibit its non-adhesive effector functions.
3.5. Monoclonal antibodies
In this section we review antibody therapeutics that act against a variety of targets, including both SARS-CoV-2 itself as well as host factors. These treatment strategies can act either in an immunomodulatory capacity or a direct antiviral capacity.
3.5.1. Convalescent plasma
Convalescent plasma is blood plasma obtained from individuals who have recovered from an infectious disease containing antibodies against the infectious organism. It has been widely investigated in the treatment of emerging infectious diseases, including Ebola and influenza RNA viruses, and has been found to potentially reduce disease severity when administered early in illness [102], [103]. Antibodies in convalescent plasma serve the effector functions that antibodies do during any infection- they function as opsonins, activate complement, and bind to pathogens to prevent adherence or cell entry.
Convalescent plasma is capable of neutralizing SARS-CoV-2 in in vitro studies. An in vitro study of convalescent plasma using a microneutralization assay found that ~63% of 345 donated plasma specimens achieved adequate viral neutralization according to FDA guidelines [104]. However, some considerations must be considered when choosing donors. Specifically, individuals who experienced more severe symptoms and were hospitalized, presented higher titers of IgG and might be better candidates for donating plasma to ensure viral neutralization [104], [105].
Convalescent plasma, however, has not met expectations. A randomized, double-blind controlled trial with 333 hospitalized patients with severe COVID-19 found no significant differences in mortality or clinical status by ordinal scale between the group receiving convalescent plasma and the placebo group [106]. A different randomized, open label clinical trial of 103 patients with severe or life-threatening COVID-19 found no significant differences in time to discharge or mortality between the placebo and experimental groups overall. The trial, which was stopped early, may have been underpowered to be able to define clinically important differences [107]. Although its mechanism of action is well established, convalescent plasma does not appear to have the efficacy necessary to merit widespread use. However, studies with larger sample sizes and greater statistical power might find significant results as some investigated outcomes in these two low power studies were close to being significant. Regardless, issues with donor criteria and neutralizing antibody titers indicate that larger, randomized trials may provide more conclusive data on the utility of convalescent plasma in treating COVID-19.
3.5.2. Itolizumab
Itolizumab is a humanized, monoclonal antibody (mAb) that targets CD6, expressed by peripheral blood T cells, medullary thymocytes and B1 B-cells [108]. CD6 which binds activated leukocyte cell adhesion molecule (ALCAM), is crucial for leukocyte adhesion and extravasation of T cells during inflammatory responses, but it also has a separate, less understood and more controversial role in acting as a costimulator for T cells while also recognizing certain pathogen-associated molecular patterns (PAMPs) [109], [110]. Itolizumab derives its immunomodulatory effects by blocking T cell effector functions of CD6 while retaining its leukocyte-adhesive properties by binding at a separate domain from the one used for adhesion [111]. Given that these T cell effector functions of CD6 appear to promote lymphocyte proliferation and differentiation, Itolizumab would be expected to have an anti-inflammatory effect during viral or intracellular bacterial infections [109]. It has been used in treating certain autoimmune illnesses, most notably psoriasis [112].
In vitro, Itolizumab can inhibit proliferation and differentiation of CD2/CD3/CD28-stimulated T cells [113]. In addition, both in vitro and in vivo, itolizumab can prevent transcriptional changes that promote T cell replication [114]. The same study showed that a murine antibody with identical function could alleviate experimentally induced autoimmune encephalomyelitis (EAE), a murine model of multiple sclerosis, and appears to reduce expression of proinflammatory cytokines [114]. The downregulated cytokines in this study overlap with cytokines overexpressed in severe COVID-19 infections, specifically IL-6 and TNF-ɑ, so it is possible that Itolizumab may present a well-tailored immunomodulatory effect for treating COVID-19 [115]. However, the full effect of CD6 in T cell costimulation is not well understood, and studies of intracellular calcium concentrations in T cells show that CD6 stimulation can also decrease signal transduction in contrast to its postulated role as a costimulator [116].
Itolizumab does not have sufficient clinical data available as a treatment for COVID-19 to make any conclusive statements regarding how effective it is, but clinical trial data suggests that this monoclonal antibody might provide clinical benefit. A randomized, open-label clinical trial, funded by Biocon, the makers of Itolizumab, with 32 hospitalized patients with mild to severe COVID-19-associated ARDS found Itolizumab significantly decreased mortality (3 deaths in standard of care vs. 0 deaths for standard of care + Itolizumab, p = 0.0296), resulted in increased saturated oxygen levels (SpO2) without changing the fraction of inspired oxygen (FiO2). In addition, patients in the standard of care + Itolizumab group had decreased levels of two pro-inflammatory cytokines, TNF-ɑ and IL-6 [117]. The study sample size and blinding, however, are not ideal, with too few patients in the study to be able to make conclusions on the clinical usefulness of Itolizumab. In addition, the mechanism of action of this drug is not straightforward nor well-defined. Yet, this clinical trial which found that Itolizumab can reduce COVID-19 associated ARDS mortality and improve lung function may merit further study in larger, independent, controlled studies. Treatment-emergent adverse events in this trial included infusion reactions and temporary lymphopenia.
3.5.3. Tocilizumab
Tocilizumab is a monoclonal antibody (mAb) antagonist against the IL-6 receptor (IL-6R) [118]. IL-6 is a partially proinflammatory cytokine produced by detection of PAMPs, detection of damage-associated molecular patterns (DAMPs), or in conjunction with other cytokines. It derives its proinflammatory effects by binding to IL-6R, inducing IL-6R-CD130 dimerization, and causing a transduction cascade through the JAK/STAT pathway that results in the release of acute-phase proteins [119]. It biases the T-cell response towards a Th17 (extracellular pathogen) response and encourages production of IgG by B cells, and elevated levels of the cytokine are associated with autoimmune diseases like Castelman’s disease and rheumatoid arthritis [119], [120].
No in vitro studies exist that directly test the efficacy of tocilizumab in dampening the immune anti- SARS-CoV-2 response, but published studies implicate this mAb as a potential therapy for COVID-19. First, patients with severe COVID-19 cases have a statistically significant increases in proinflammatory IL-6 (p < 0.001) and TNF-ɑ (p < 0.037) levels, when compared to patients with mild disease [115]. In a murine model of influenza A that parallels COVID-19 infection, increased levels of IL-6 promote muscle dysfunction, TNF-ɑ was elevated as well [121]. A murine analog of tocilizumab decreased muscle dysfunction in this mouse model, as measured by grip strength. Though this study might not have estimated mortality or outcomes typically sought after in a clinical trial, it suggests that tocilizumab may be able to target the documented musculoskeletal sequelae of COVID-19 infection [122], [123]. However, in vitro data also suggests that blocking IL-6 activity is not an overall positive means of reducing inflammation caused by respiratory viruses. IL-6 deficient mice experienced worse outcomes from influenza virus infection, a slower recovery, and worsened viral clearance by macrophages [124].
Tocilizumab has not shown great promise in clinical trials against SARS-CoV-2. A randomized, double-blind controlled trial funded by F. Hoffmann–La Roche and the Department of Health and Human Services of 452 initial participants with severe COVID-19 found that this mAb did not significantly improve mortality nor hospital discharge by day 28 of the study [125]. The study did find a potential reduction in time to hospital discharge for the experimental group, but this finding was not statistically significant. Another randomized, double-blind controlled trial of 243 participants diagnosed with COVID-19 and a combination of two severe COVID-19 symptoms found no significant difference in mortality and intubation, decline in health status, or time to discontinuation of oxygen supplementation [126]. Tocilizumab has not yet produced results in clinical trials that merit its use in treating COVID-19.
3.5.4. Etesevimab, bamlanivimab, and anti-RBD mAbs
Etesevimab and bamlanivimab are two neutralizing mAbs which target epitopes on the receptor-binding domain (RBD) of the SARS-CoV-2 spike protein and are typically given together in a mAb cocktail [127]. Because interactions between the spike RBD and ACE2 are necessary for viral entry, anti-RBD mAbs were used therapeutically since they can either sterically hinder the interaction of SARS-CoV-2 with ACE2 or force viral antireceptors into unstable conformations [128], [129]. Monoclonal antibody therapies have been specifically developed and screened for a variety of viruses, perhaps most notably against Ebola virus [130].
The in vitro efficacies of etesevimab, bamlanivimab, and other mAbs used in monotherapies or combinatorial treatment strategies have varied widely due to escape by the proliferation of different SARS-CoV-2 variants. An in vitro study found that bamlanivimab and etesevimab both lost neutralizing activity against the B.1.351 (Beta) variant, though it retained neutralizing activity against the B.1.1.7 (Alpha) variant and wild-type SARS-CoV-2 [131]. The B.1.351 (Beta) variant has three amino acid substitutions in its receptor binding domain that explain its escape from what were previously neutralizing mAb therapies, and this study provides evidence of the potential for escaping antibodies by showing across-the-board decreases in ID50 in serum from Pfizer and Moderna vaccine recipients. A recent Nature article supports these findings [126]. Specifically, the B.1.351 (Beta) variant also escaped neutralization by etesevimab and bamlanivimab in this study, while the B.1.617.2 (Delta) variant could escape only bamlanivimab [132]. These two studies also found that casirivimab and imdevimab, two other anti-RBD mAbs were capable of neutralizing both the B.1.351(Beta) and B.1.617.2 (Delta) variants, though casirivimab’s IC50 increased markedly and indicates a reduction in its neutralization activity.
Prior to the emergence of mAb-resistant variants, etesevimab and bamlanivimab presented great clinical promise. A randomized, double-blind controlled trial of 1035 non-hospitalized participants with mild or moderate COVID-19 significantly reduced hospitalization and death, reduced viral load by day 7, and decreased time to recovery as defined by the absence of symptoms [133]. These results indicate excellent clinical utility, though the study suffers from some concerns regarding its general applicability due to a disproportionately high percentage of white participants. It is also important to note that this study took place prior to the arrival of the B.1.617.2 (Delta) variant in March, which is known to escape neutralization by bamlanivimab, indicating that these results may not hold in the presence of emerging variants. Another randomized, double-blind controlled trial with 577 non-hospitalized participants with mild or moderate COVID-19 found potential differences between bamlanivimab monotherapy at differing dosages and a combinatorial strategy with etesevimab when compared to a placebo group. Specifically, a combinatorial strategy decreased viral load by day 11 compared to baseline, improved symptoms, and decreased hospitalizations but did not improve time to resolution of symptoms compared to the placebo group [134]. However, the only significant results for bamlanivimab monotherapy groups were an improvement in symptom scores and symptom resolution by day 11 of the study for the low-dosage (700 mg) group and a decrease in viral load by day 29 of the study in the medium-dosage (2800 mg) group [134]. From these results it can be inferred that a mAb combinatorial strategy targeting separate epitopes is a more effective strategy than a mAb monotherapy, especially given the now widespread prevalence of the bamlanivimab-resistant B.1.617.2 (Delta) variant. Regardless of the existence of mAb-resistant variants, mAb treatment strategies have displayed perhaps one of the most promising clinical utilities of any biologic evaluated so far. Approval of mAbs early in future outbreaks of novel coronaviruses could be a useful strategy for reducing mortality and morbidity associated with these viruses.
A summary of our review of the efficacy and mechanism of antibody-based treatment strategies are shown below in Table 3, Table 4. Table 4 reviews mAb treatment strategies not thoroughly discussed here, but for which there is existing clinical trial data, and also describes the mechanisms of action of each reviewed antibody therapeutic.
Table 3.
Summary of antibody treatment strategies.
| Treatment | Mechanism Target | Clinical benefit? | Mortality decrease? |
|---|---|---|---|
| Convalescent Plasma | Polyclonal Abs against SARS-CoV-2 | Mixed | X |
| Itolizumab | mAb against CD-6- attenuates T-cell costimulation | ✓* | X* |
| Tocilizumab | mAb against IL-6 receptor | X | X |
| Etesevimab | mAb against the receptor-binding domain of the spike protein | ✓ | ✓ |
| Bamlanivimab | mAb against the receptor-binding domain of the spike protein | ✓ | ✓ |
✓ - The drug produced the respective column’s effect. X - The drug failed to produce the respective column’s effect. Mixed - The drug produced negative and positive outcomes. *- Clinical trials of this drug either failed to exceed 100 participants or lacked any blinding.
Table 4.
Summary of antibody treatment strategies not investigated in this study.
| Drug | Mechanism | Clinical benefit? | Mortality decrease? | References |
|---|---|---|---|---|
| Casirivimab/Imdevimab (REGN-COV2) | Anti-RBD mAb cocktail | ✓ | N/A | [135] |
| Sarilumab | IL-6R mAb antagonist | X | X | [136] |
| Mavrilimumab | mAb antagonist against human granulocyte macrophage colony-stimulating factor receptor (GM-CSFR) | X* | X* | [137] |
✓ - The drug produced the respective column’s effect. X - The drug failed to produce the respective column’s effect. Mixed - The drug produced negative and positive outcomes or only produced positive outcomes in some trials. * - Clinical trials of this drug either failed to exceed 100 participants or lacked any blinding. NA - This outcome has yet to be investigated.
✓ - The drug produced the respective column’s effect. X - The drug failed to produce the respective column’s effect. Mixed - The drug produced negative and positive outcomes or only produced positive outcomes in some trials. * - Clinical trials of this drug either failed to exceed 100 participants or lacked any blinding. N/A - This outcome has yet to be investigated.
3.6. Parasite or hormone-targeted approaches
In this category we review therapeutics whose mechanisms of action do not comfortably fit into any of the treatment categories that we have already covered. These therapeutics each have a postulated mechanism of action against SARS-CoV-2 or COVID-19 that is distinct from their traditional use, including some immunomodulatory effects. Primarily the identified targets are antiviral effects.
3.6.1. Ivermectin
Ivermectin is an anti-parasitic typically used to treat parasitic infections, including river blindness (onchocerciasis), lymphatic filariasis, scabies, and lice. One of its therapeutic mechanisms in this context is its activity against various ion channels necessary for several crucial physiological activities in disease-carrying vectors and their transmitted diseases [138]. It irreversibly opens ligand-gated ion channels to produce a constant influx of chloride ions, producing neurotoxicity by repolarizing neurons [139]. A second antiparasitic mechanism of action is its role in inhibiting the secretion of parasite proteins that allow evasion of an effective host immune response [140].
Ivermectin also has a well-documented mechanism against certain viruses, and in vitro models have shown that ivermectin is able to significantly reduce viral replication [141]. It accomplishes this by inhibiting nuclear localization of viral proteins that enter the nucleus through the nuclear pore complex with assistance from the importin-ɑ/ꞵ1 heterodimer (Imp-ɑ/ꞵ1). In vitro expression of both GFP-tagged viral (Dengue’s NS5 protein and HIV integrase) and cellular proteins with nuclear localization signals (NLS) specific to different importins have revealed that ivermectin can prevent nuclear localization of proteins specific to the Imp-ɑ/ꞵ1 heterodimer but not other importins [141]. This has been validated with other viruses that rely more on nuclear import, primarily human adenoviruses, by disrupting any protein-protein interactions with Imp-ɑ by its substrates [142], [143]. These reductions in viral titer have been evidenced in lesser detail to SARS-CoV-2, and an in vitro study of viral replication found that ivermectin significantly decreased viral reproduction [144]. The outcomes of the in vitro studies of RNA viruses are especially surprising given that much of their life cycle occurs in the cytoplasm of the host cell as opposed to the nucleus. Some proteins expressed by coronaviruses are known at times to localize to the nucleus or at the nuclear pore complex, but the Imp-ɑ/ꞵ1 heterodimer plays no apparent role in either of these processes [145], [146]. A recent in silico study posited a separate mechanism in which ivermectin binds to the virus’ RNA dependent RNA polymerase (RDRP) with high affinity [147]. More studies in vitro and in vivo are necessary to investigate its precise antiviral mechanism against SARS-CoV-2.
Despite its purported antiviral activity against some RNA viruses, ivermectin has exhibited mixed efficacy in clinical trials against COVID-19. A randomized, open-label clinical trial of ivermectin monotherapy with 60 enrolled participants experiencing severe COVID-19 only reported a decrease in persistence of viral RNA after testing 18 participants in the experimental group and 6 participants in the control group (NCT04646109). Another randomized, healthcare provider-blinded study of ivermectin monotherapy with 66 enrolled participants found no significant differences between the experimental group and controls (NCT04407507). Neither study exhibited significant decreases in mortality, and the only remaining clinical trials that report improvements in metrics like oxygen saturation are combinatorial therapy studies that, in some cases, lack randomization, blinding or have small sample sizes [148] (NCT04343092, NCT04425863). However, in a randomized, double-blind, standard of care-controlled study of 400 patients in Bangladesh, when combined with doxycycline, patients treated with ivermectin were significantly more likely to improve clinically within 7 days when compared to the control group (60.7% treatment group vs. 44.4% in the controls p < 0.03). In addition, when treated with Ivermectin significantly fewer patients deteriorated to severe illness, which was defined as severe dyspnea, respiratory distress, tachypnea (> 30 breaths/min), and hypoxia (SpO2 < 90% at room air) over the period of a month (8.7% in the treatment group vs. 17.8% in the standard of care controls; p < 0.013) and improved recovery from persistent SARS-CoV-2 as evidenced by a negative RT-PCR (7.7% in the treatment group compared to 20% in the standard-of-care controls p < 0.001). Serious adverse events were observed in 2 of the treatment patients compared to 0 in the controls [148].
Wider-scale usage of ivermectin raises two potential issues. The first is the issue of potential neurotoxicity. This drug produces severe neurological symptoms in canines (collies) and mice that are homozygous for a non-functional P-glycoprotein transporter (P-gp) present in the blood-brain barrier [149]. Indeed, a case study of a patient with nonsense mutations in both P-gp alleles revealed that ivermectin can cause acute neurotoxicity in humans [150]. This adverse outcome is likely due to accumulation of ivermectin in neurons, since ivermectin functions as a substrate for wild-type P-gp which leads to the drug being pumped out of the brain into the blood [151]. Nonsense mutations generate a truncated protein and prevent the transporter from functioning normally. A clinical trial of ivermectin for treating severe COVID-19 affirms the possibility of toxicity in individuals with mutations in the ABCB1 gene- 5 participants in the trial experienced encephalopathy as a result of genetic susceptibility to the drug (NCT04646109).
The second potential issue is promoting resistance to ivermectin which is often used to treat helminths and kill some disease vectors. Overuse of antimicrobials and antiparasitics has fueled an increase in drug resistance among many pathogens over time, and the same applies to treatment with ivermectin. Ivermectin resistance in Sarcoptes scabiei, the mite that causes scabies, has been reported among individuals with intensive ivermectin use as early as 2004 [152]. Widespread usage of the drug should, thus, be weighed against the risk of inadvertently progressing resistance to it among diseases humanity has only recently brought under greater control. Overall, given the ambiguity behind its mechanism of action and its mixed clinical efficacy, its use should be discouraged until it is further studied.
3.6.2. Hydroxychloroquine
Hydroxychloroquine (HCQ), a synthetic form of quinine, is a weak base that has long been used to treat malaria and a variety of autoimmune conditions [152]. Its mechanism of action in treating malaria involves increasing the pH of vesicles in Plasmodium species during the parasites’ asexual life cycle in red blood cells, which prevents the activity of acid proteases in degrading hemoglobin [153]. Its mechanism in treating autoimmune conditions likely involves decreasing the concentration of some pro-inflammatory cytokines in serum by interfering with the normal function of lysosomes in autophagy and toll-like receptor (TLR) activation [154], [155], [156], [157].
HCQ and chloroquine, a structurally and functionally analogous malaria treatment to HCQ, have also shown some antiviral properties through in vitro studies of coronaviruses. A study of chloroquine-treated Vero E6 cells infected with SARS-CoV-1 demonstrated an IC50 of 8.8 µM and 99% inhibition of viral reproduction at 16 µM [158]. The antiviral activity of HCQ is a similar mechanism to its antimalarial activities. An in vitro study of Vero E6 cells infected with SARS-CoV-2 and treated with HCQ revealed that the drug inhibits early endosome acidification and maturation into endolysosomes [159]. The resulting effect was a higher retention of virions in early endosomes and a failure to release viral RNA into the cytoplasm of the infected cells.
The proposed immunomodulatory effects of HCQ, in lessening the severity of COVID-19 infection, are less well understood in the context of viral infections. In an in vitro study of DENV-2, one of four dengue viruses and another positive-sense RNA virus [160], HCQ treatment of J744A.1 murine macrophages led to an increase in the retinoic acid inducible gene-I (RIG-I) and mitochondrial antiviral signaling protein (MAVS) pattern recognition receptors (PRRs) as well as interferon-ꞵ (IFN-ꞵ) and tumor necrosis factor ɑ (TNF-ɑ) [161]. In the human A549 lung carcinoma cell line, knockdown of MAVS expression reversed the therapeutic response to the HCQ. Similarly, antagonizing interferon-ɑ and -ꞵ receptors in the presence of HCQ limited the efficiency of HCQ in blocking dengue infection of A549 cells. From these studies it could be inferred that in part, the role of HCQ as an antiviral in vitro is to increase pattern recognition receptor (PRR) signaling leading to increased inflammation. In contrast, a clinical trial, which used HCQ in patients with HIV had no significant changes in serum interleukin 6 (IL-6) or D-dimer levels along with a significant reduction in CD4 + T-cells when compared to placebo controls [162]. This conflicts with the proposed therapeutic mechanism of action of HCQ in treating autoimmune diseases and its widely postulated immunomodulatory effects for COVID-19. However, virtually no in vivo or in vitro studies on HCQ treatment in COVID-19 infection have evaluated changes in proinflammatory cytokine concentrations in serum, so this aspect of HCQ’s mechanism of action has yet to be scientifically validated for COVID-19.
Despite its potential multiple mechanisms of action against COVID-19, clinical trials using HCQ monotherapy have yet to produce any significant clinical benefit. A randomized, quadruple-masked controlled trial of 479 inpatient participants (242 experimental, 237 controls) revealed no significant changes in all-cause mortality, oxygen-free days by day 28, or ventilator-free days by day 28 [163]. A second randomized, quadruple-masked controlled study comparing HCQ to HCQ combined with azithromycin, found that the 20 participants showed no significant changes in viral clearance or clinical response, although the study did not provide any statistical analyses (NCT04358081). The lack of a clinical response to HCQ could be explained by differences in the expression of the two proteins that mediate internalization of SARS-CoV-2 in tissue culture and in vivo in the human body. Specifically, HCQ inhibits endosomal protease cathepsin L-mediated infection by SARS-CoV-2 via the endocytic pathway, while TMPRSS2, which permits SARS-CoV-2 infection via cell membrane fusion rather than endolysosome fusion, reduces the efficiency of HCQ’s inhibition of viral entry [164]. SARS-CoV-2’s spike protein is cleaved by TMPRSS2 to prime membrane fusion by exposing the S2 subunit’s fusion peptide, allowing for entry of the virus directly into the cytoplasm. Entry through this pathway does not require endocytosis, which is the step at which cathepsin L activity is inhibited by HCQ. Thus, combinatorial strategies that also target TMPRSS2 might better enhance HCQ’s efficacy by targeting both entry mechanisms. Clinical trials on HCQ as a treatment for COVID-19 have not revealed any striking adverse events.
Hydroxychloroquine has also been investigated as a form of post-exposure prophylaxis against COVID-19, but to little avail. A randomized, quadruple-blind controlled trial of 1312 participants with a known exposure to someone with COVID-19 investigated a tapered 800–600 mg regimen of HCQ treatment and evaluated onset of symptoms and clinical outcomes. No significant differences were found between the experimental and placebo groups regarding development of active disease or disease severity over 14 days [165]. This further supports the notion that SARS-CoV-2 is not particularly susceptible to HCQ in vivo.
3.6.3. Proxalutamide
Proxalutamide is a nonsteroidal antiandrogen drug that acts as an antagonist to the cytoplasmic androgen receptor, and it was initially designed with the intent of treating prostate cancer [166]. Prostate cancer is associated with over signaling of androgen receptors which promotes excessive transcriptional activity [167]. Thus, androgen receptor antagonists prevent excessive transcription and progression to cancer in the prostate.
The potential antiviral activity of proxalutamide parallels its activity in treating prostate cancer. As mentioned, TMPRSS2, a transmembrane serine protease, binds the coronavirus spike protein for direct membrane fusion through its S2 subunit [8]. A luciferase reporter assay showed that the TMPRSS2 promoter contains an androgen-response element that increases expression of the transmembrane protein in the presence of the androgen, methyltrienolone [168]. The result is a possible causal relationship between a higher COVID-19 severity among men when compared to females of the same age, especially men experiencing hyper-androgenic alopecia [169], [170], [171], [172], [173], [174], [175]. Thus, proxalutamide’s antiviral activity would result from a decrease in androgen-associated expression of TMPRSS2, which would hinder SARS-CoV-2 membrane fusion. However, no in vitro studies have validated this mechanism.
Despite a lack of in vitro studies on its mechanism of action, proxalutamide has exhibited promise in SARS-CoV-2 clinical trials. A randomized, quadruple-masked controlled clinical trial by Applied Biology Inc. evaluated hospitalization among 268 male patients diagnosed with COVID-19. It found that proxalutamide decreased hospitalization rates from 26.1% of participants in the control group to 2.2% of participants in the experimental group. The groups were randomized equally, with 134 participants in each group, though the statistical significance of the observation was not tested [176]. A second randomized, double-blinded clinical trial of 236 non-hospitalized participants (108 women and 128 men) reported statistically significant decreases in time to clinical remission, increases in viral clearance by day 7, and increases in clinical remission by day 7 of the study [177]. In the future, additional studies in vitro and/or in vivo would be useful in further validating the mechanism of proxalutamide and other anti-androgen drugs in reducing the severity of COVID-19. It is notable that both clinical trials mentioned above reported increases in gastrointestinal treatment-related adverse events, primarily diarrhea [176], [177].
A summary of each antiparasitic and each anti-androgen treatment strategy is provided ( Table 5). In addition, we include known information on identified clinical benefit and effects on COVID-19 patient mortality. The mechanisms of action are also depicted schematically in Fig. 5. Fig. 6.
Table 5.
Summary of anti-parasitic and anti-androgen treatment strategies.
| Treatment | Mechanism Target | Clinical benefit? | Mortality decrease? |
|---|---|---|---|
| Ivermectin | Nuclear import | X | X |
| Proxalutamide | TMPRSS2 Expression | ✓ | N/A |
| Hydroxychloroquine | Endosomal acidification | X | X |
✓ - The drug produced the respective column’s effect. X - The drug failed to produce the respective column’s effect. Clinical trials of this drug either failed to exceed 100 participants or lacked any blinding. N/A - This outcome has yet to be investigated.
Fig. 5.
Scheme of the mechanisms of action for hydroxychloroquine, ivermectin, and proxalutamide. Hydroxychloroquine increases the pH of the endosome, which prevents its maturation and stops conformational changes in the spike protein that permit viral entry. Ivermectin has two postulated mechanisms of action, the first being inhibition of nuclear localization of coronavirus proteins (though nuclear localization is not widely thought to be important to the SARS-CoV-2’s lifecycle) and the second, more theoretical being inhibition of the virus’s replicase. Finally, proxalutamide decreases expression of TMPRSS2, which is encoded by a gene containing an androgen response element, by antagonizing the cytoplasmic androgen receptor.
Fig. 6.
Ongoing or published clinical trials of therapeutics used during different phases of COVID-19. Each letter in a red box describes the step in viral replication or the immune response that at least one investigated drug targets. Each letter’s corresponding treatments are listed below. A - Etesevimab, Bamlanivimab, Convalescent sera. B – Proxalutamide. C – Hydroxychloroquine. D - Lopinavir/Ritonavir. E - Remdesivir, Favipiravir, Molnupiravir. F – Ivermectin. G – Tocilizumab. H - Peginterferon – λ. I - Baricitinib, Ruxolitinib. J - Losartan, Compound 21 (C21). K - Dexamethasone, Methylprednisolone. L - Dornase alfa.
4. Discussion
Worldwide, a large number of therapeutic strategies have been investigated in treating COVID-19 and yet, to date, there is only one treatment approved by the FDA to treat this disease, remdesivir. This is likely because many FDA-approved drugs described in this review that are used clinically to treat SARS CoV-2 symptoms, do not directly target the virus. In addition to remdesivir, five of the treatments described in this review- methylprednisolone, dexamethasone, etesevimab, bamlanivimab, heparin and - have reported a significant decrease in mortality in clinical trials when evaluated, though the ability of remdesivir to reduce mortality beyond 15 days was not significant. Other therapeutics have not produced significant reductions in mortality while others have yet to investigate reductions in mortality, particularly mAb therapies directly against SARS-CoV-2, as well as proxalutamide.
Each of the individual therapies described, were administered during specific phases of the progression of COVID-19 to have the best patient outcome. Two outpatient treatments that have provided the most promising courses of treatment for non-hospitalized patients are proxalutamide, and monoclonal antibody therapies. Monoclonal antibody therapies, Etesevimab and bamlanivimab, are used in patients with mild-moderate COVID-19. These therapies, which target and neutralize SARS-CoV-2, reduce hospital admissions, decrease viral loads, and decrease time to recovery. As a result, ultimately Etesevimab and bamlanivimab decrease mortality rates [127], [133], [134]. Male pattern hair loss has been associated with hospitalization due to COVID-19 [171], which led clinicians to question the roles of androgens in COVID-19 progress. It is exciting that Proxalutamide, an androgen antagonist has reduced cardiac, and respiratory disorders, while significantly improving survival when used in outpatients [176]. Anti-viral therapies, such as remdesivir, provided the most benefit when administered during the first 5 days of hospitalization [22]. In addition, co-administration of baricitinib, which likely disrupts internalization of the virus when combined with remdesivir improved time to recovery in hospitalized patients who were receiving high flow oxygen [21], [63]. Anti-inflammatories such as C21, when administered to hospitalized patients requiring oxygen, significantly reduced the need for oxygen supplementation by day 14, but provided no significant benefit at day 7, which may indicate that this therapy prevents persistent lung damage (NCT04452435). During the progression of an inflammatory response such as found in COVID-19 patients, release of acute phase proteins can lead to emboli that can complicate recovery. Accordingly, patients treated with heparin therapies had significant improvements in mortality rates, likely due to the reduction of blood clots and the potential improvement in lung function due to the mucolytic activities of heparin. The benefits of heparin were limited to critically ill patients who were in the ICU or on mechanical ventilation. Methylprednisolone provided the most benefit when used during the first 5–10 days after hospital admission to reduce the need for ventilators and hospital stays, suggesting that limiting the patient’s own immune response early during COVID-19 provided significant clinical benefits. Monoclonals that also limit the extent of the immune response by limiting T cell proliferation, such as Itolizumab decreased mortality in hospitalized patients with mild to severe COVID-associated ARDS.
Given the regular emergence of novel respiratory viruses over the past 20 years starting with SARS-Co-V-1, followed by Middle Eastern Respiratory Syndrome (MERS) and now SARS-CoV-2, it is logical to conclude that the need for potent, multi-use therapeutics that can be used to treat emerging viruses will continue for years to come. Apart from monoclonal antibody therapies, which can be administered in an out-patient setting, all other clinical trials were conducted on patients that had been admitted to the hospital. Moving forward, the NIH SARS-CoV-2 Antiviral Therapeutics Summit members outlined multiple principles that should go into future development of antivirals that could be taken at home [178]. Moreover, the drug should be potent against SARS-CoV-2, have bioavailability to the sites of infection, and have limited cytotoxicity. Moreover, the successes in using drug combinations of antivirals (remdesivir) with immunosuppressives, similar to successful combination therapy treatments of HIV [179] or Hepatitis C [180], has been proposed as a promising approach in the treatment of COVID-19. Although studies with proxalutamide were conducted in males, since both males and females have male pattern hair loss during aging, it would be prudent to investigate therapies that prevent activation of the Ace2 protease TMPRSS2 in women as well.
A separate area of great concern is the persistence of “long COVID”-associated morbidity (long-haul COVID-19). A meta-analysis of 21 studies of COVID-19 sequelae found that 80% of 47,910 individuals who recovered from infection experienced one or more long-term symptoms like fatigue, headaches, or anosmia [181]. The etiology of these sequelae are not well defined and there are currently no biomarkers or even a general consensus of what constitutes long COVID but many of the symptoms resemble chronic fatigue syndrome (CFS), also known as myalgic encephalomyelitis, which is known to occur in other viral infections [182], [183]. As global COVID-19 infections exceed 200 million in total, as of writing, greater focus will need to be placed on identifying the cause of long COVID symptoms. Addressing these sequelae as long-term morbidity grows will be a priority, particularly since many sufferers are relatively young. Long COVID treatments will likely require a combination of repurposed drugs or the development of new therapeutics to alleviate the symptoms.
CRediT authorship contribution statement
Rory J. Malek: Data curation, Writing – original draft. Colin A. Bill: Conceptualization, Writing – review & editing. Charlotte M. Vines: Conceptualization, Writing – review & editing.
Conflict of interest statement
Charlotte M. Vines and Colin A. Bill are funded to conduct a research study with Gilead Sciences. Gilead Sciences was not involved in the preparation or writing of this review. The remaining author, Rory J. Malek declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
RJM and CMV are funded by NSF grant HRD-182675 provided through the The University of Texas Louis Stokes Alliance for Minority Particiaption program, and CMV, CAB are funded by NIH grant SC1-GM111172.
References
- 1.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G.F., Tan W., Team CNCIaR A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. PubMed PMID: 31978945; PMCID: PMC7092803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johns Hopkins Coronavirus Resource Center [Internet]2020 [cited December 24, 2020]. https://coronavirus.jhu.edu/.
- 3.Leung K., Shum M.H., Leung G.M., Lam T.T., Wu J.T. Early transmissibility assessment of the N501Y mutant strains of SARS-CoV-2 in the United Kingdom, October to November 2020. Eur. Surveill. 2021;26(1) doi: 10.2807/1560-7917.ES.2020.26.1.2002106. PubMed PMID: 33413740; PMCID: PMC7791602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tegally H., Wilkinson E., Giovanetti M., Iranzadeh A., Fonseca V., Giandhari J., Doolabh D., Pillay S., San E.J., Msomi N., Mlisana K., von Gottberg A., Walaza S., Allam M., Ismail A., Mohale T., Glass A.J., Engelbrecht S., Van Zyl G., Preiser W., Petruccione F., Sigal A., Hardie D., Marais G., Hsiao N.Y., Korsman S., Davies M.A., Tyers L., Mudau I., York D., Maslo C., Goedhals D., Abrahams S., Laguda-Akingba O., Alisoltani-Dehkordi A., Godzik A., Wibmer C.K., Sewell B.T., Lourenço J., Alcantara L.C.J., Kosakovsky Pond S.L., Weaver S., Martin D., Lessells R.J., Bhiman J.N., Williamson C., de Oliveira T. Detection of a SARS-CoV-2 variant of concern in South Africa. Nature. 2021;592(7854):438–443. doi: 10.1038/s41586-021-03402-9. [DOI] [PubMed] [Google Scholar]
- 5.National Center for Immunization and Respiratory Diseases (NCIRD) DvoVD. CDC COVID-19 Science Briefs, 2020.
- 6.Lopez Bernal J., Andrews N., Gower C., Gallagher E., Simmons R., Thelwall S., Stowe J., Tessier E., Groves N., Dabrera G., Myers R., Campbell C.N.J., Amirthalingam G., Edmunds M., Zambon M., Brown K.E., Hopkins S., Chand M., Ramsay M. Effectiveness of Covid-19 Vaccines against the B.1.617.2 (Delta) Variant. New Engl. J. Med. 2021;385:585–594. doi: 10.1056/NEJMoa2108891. PubMed PMID: 34289274; PMCID: PMC8314739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fehr A.R., Perlman S. Coronaviruses: an overview of their replication and pathogenesis. Methods Mol. Biol. 2015;1282:1–23. doi: 10.1007/978-1-4939-2438-7_1. PubMed PMID: 25720466; PMCID: PMC4369385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Müller M.A., Drosten C., Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280. doi: 10.1016/j.cell.2020.02.052. PubMed PMID: 32142651; PMCID: PMC7102627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao M.M., Yang W.L., Yang F.Y., Zhang L., Huang W.J., Hou W., Fan C.F., Jin R.H., Feng Y.M., Wang Y.C., Yang J.K. Cathepsin L plays a key role in SARS-CoV-2 infection in humans and humanized mice and is a promising target for new drug development. Signal Transduct. Target Ther. 2021;6(1):134. doi: 10.1038/s41392-021-00558-8. PubMed PMID: 33774649; PMCID: PMC7997800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lukassen S., Chua R.L., Trefzer T., Kahn N.C., Schneider M.A., Muley T., Winter H., Meister M., Veith C., Boots A.W., Hennig B.P., Kreuter M., Conrad C., Eils R. SARS-CoV-2 receptor ACE2 and TMPRSS2 are primarily expressed in bronchial transient secretory cells. EMBO J. 2020;39(10) doi: 10.15252/embj.20105114. PubMed PMID: 32246845; PMCID: PMC7232010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.V’kovski P., Kratzel A., Steiner S., Stalder H., Thiel V. Coronavirus biology and replication: implications for SARS-CoV-2. Nat. Rev. Microbiol. 2021;19(3):155–170. doi: 10.1038/s41579-020-00468-6. PubMed PMID: 33116300; PMCID: PMC7592455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolff G., Limpens R.W.A.L., Zevenhoven-Dobbe J.C., Laugks U., Zheng S., de Jong A.W.M., Koning R.I., Agard D.A., Grünewald K., Koster A.J., Snijder E.J., Bárcena M. A molecular pore spans the double membrane of the coronavirus replication organelle. Science. 2020;369(6509):1395–1398. doi: 10.1126/science.abd3629. PubMed PMID: 32763915; PMCID: PMC7665310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sicari D., Chatziioannou A., Koutsandreas T., Sitia R., Chevet E. Role of the early secretory pathway in SARS-CoV-2 infection. J. Cell Biol. 2020;219(9) doi: 10.1083/jcb.202006005. PubMed PMID: 32725137; PMCID: PMC7480111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cho A., Saunders O.L., Butler T., Zhang L., Xu J., Vela J.E., Feng J.Y., Ray A.S., Kim C.U. Synthesis and antiviral activity of a series of 1’-substituted 4-aza-7,9-dideazaadenosine C-nucleosides. Bioorg. Med Chem. Lett. 2012;22(8):2705–2707. doi: 10.1016/j.bmcl.2012.02.105. PubMed PMID: 22446091; PMCID: PMC7126871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Venkataraman S., Prasad B.V.L.S., Selvarajan R. RNA dependent RNA polymerases: insights from structure, function and evolution. Viruses. 2018;10(2) doi: 10.3390/v10020076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yin W., Mao C., Luan X., Shen D.D., Shen Q., Su H., Wang X., Zhou F., Zhao W., Gao M., Chang S., Xie Y.C., Tian G., Jiang H.W., Tao S.C., Shen J., Jiang Y., Jiang H., Xu Y., Zhang S., Zhang Y., Xu H.E. Structural basis for inhibition of the RNA-dependent RNA polymerase from SARS-CoV-2 by remdesivir. Science. 2020;368(6498):1499–1504. doi: 10.1126/science.abc1560. PubMed PMID: 32358203; PMCID: PMC7199908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang L., Zhou R. Structural basis of the potential binding mechanism of remdesivir to SARS-CoV-2 RNA-dependent RNA polymerase. J. Phys. Chem. B. 2020;124(32):6955–6962. doi: 10.1021/acs.jpcb.0c04198. PubMed PMID: 32521159; PMCID: PMC7309898. [DOI] [PubMed] [Google Scholar]
- 18.Agostini M.L., Andres E.L., Sims A.C., Graham R.L., Sheahan T.P., Lu X., Smith E.C., Case J.B., Feng J.Y., Jordan R., Ray A.S., Cihlar T., Siegel D., Mackman R.L., Clarke M.O., Baric R.S., Denison M.R. Coronavirus susceptibility to the antiviral remdesivir (GS-5734) is mediated by the viral polymerase and the proofreading exoribonuclease. mBio. 2018;9(2) doi: 10.1128/mBio.00221-18. PubMed PMID: 29511076; PMCID: PMC5844999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sheahan T.P., Sims A.C., Graham R.L., Menachery V.D., Gralinski L.E., Case J.B., Leist S.R., Pyrc K., Feng J.Y., Trantcheva I., Bannister R., Park Y., Babusis D., Clarke M.O., Mackman R.L., Spahn J.E., Palmiotti C.A., Siegel D., Ray A.S., Cihlar T., Jordan R., Denison M.R., Baric R.S. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci. Transl. Med. 2017;9(396) doi: 10.1126/scitranslmed.aal3653. PubMed PMID: 28659436; PMCID: PMC5567817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith E.C., Blanc H., Surdel M.C., Vignuzzi M., Denison M.R. Coronaviruses lacking exoribonuclease activity are susceptible to lethal mutagenesis: evidence for proofreading and potential therapeutics. PLoS Pathog. 2013;9(8) doi: 10.1371/journal.ppat.1003565. PubMed PMID: 23966862; PMCID: PMC3744431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C., Hohmann E., Chu H.Y., Luetkemeyer A., Kline S., Lopez de Castilla D., Finberg R.W., Dierberg K., Tapson V., Hsieh L., Patterson T.F., Paredes R., Sweeney D.A., Short W.R., Touloumi G., Lye D.C., Ohmagari N., Oh M.D., Ruiz-Palacios G.M., Benfield T., Fätkenheuer G., Kortepeter M.G., Atmar R.L., Creech C.B., Lundgren J., Babiker A.G., Pett S., Neaton J.D., Burgess T.H., Bonnett T., Green M., Makowski M., Osinusi A., Nayak S., Lane H.C. Members A-SG. Remdesivir for the treatment of COVID-19 - final report. New. Engl. J. Med. 2020;383(19):1813–1826. doi: 10.1056/NEJMoa2007764. PubMed PMID: 32445440; PMCID: PMC7262788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spinner C.D., Gottlieb R.L., Criner G.J., Arribas López J.R., Cattelan A.M., Soriano Viladomiu A., Ogbuagu O., Malhotra P., Mullane K.M., Castagna A., Chai L.Y.A., Roestenberg M., Tsang O.T.Y., Bernasconi E., Le Turnier P., Chang S.C., SenGupta D., Hyland R.H., Osinusi A.O., Cao H., Blair C., Wang H., Gaggar A., Brainard D.M., McPhail M.J., Bhagani S., Ahn M.Y., Sanyal A.J., Huhn G., Marty F.M., Investigators G-U-- Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19: A randomized clinical trial. JAMA. 2020;324(11):1048–1057. doi: 10.1001/jama.2020.16349. PubMed PMID: 32821939; PMCID: PMC7442954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldman J.D., Lye D.C.B., Hui D.S., Marks K.M., Bruno R., Montejano R., Spinner C.D., Galli M., Ahn M.Y., Nahass R.G., Chen Y.S., SenGupta D., Hyland R.H., Osinusi A.O., Cao H., Blair C., Wei X., Gaggar A., Brainard D.M., Towner W.J., Muñoz J., Mullane K.M., Marty F.M., Tashima K.T., Diaz G., Subramanian A., Investigators G-U-- Remdesivir for 5 or 10 days in patients with severe COVID-19. New Engl. J. Med. 2020;383(19):1827–1837. doi: 10.1056/NEJMoa2015301. PubMed PMID: 32459919; PMCID: PMC7377062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Furuta Y., Gowen B.B., Takahashi K., Shiraki K., Smee D.F., Barnard D.L. Favipiravir (T-705), a novel viral RNA polymerase inhibitor. Antivir. Res. 2013;100(2):446–454. doi: 10.1016/j.antiviral.2013.09.015. PubMed PMID: 24084488; PMCID: PMC3880838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Furuta Y., Takahashi K., Kuno-Maekawa M., Sangawa H., Uehara S., Kozaki K., Nomura N., Egawa H., Shiraki K. Mechanism of action of T-705 against influenza virus. Antimicrob. Agents Chemother. 2005;49(3):981–986. doi: 10.1128/AAC.49.3.981-986.2005. PubMed PMID: 15728892; PMCID: PMC549233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baranovich T., Wong S.S., Armstrong J., Marjuki H., Webby R.J., Webster R.G., Govorkova E.A. T-705 (favipiravir) induces lethal mutagenesis in influenza A H1N1 viruses in vitro. J. Virol. 2013;87(7):3741–3751. doi: 10.1128/JVI.02346-12. PubMed PMID: 23325689; PMCID: PMC3624194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sangawa H., Komeno T., Nishikawa H., Yoshida A., Takahashi K., Nomura N., Furuta Y. Mechanism of action of T-705 ribosyl triphosphate against influenza virus RNA polymerase. Antimicrob. Agents Chemother. 2013;57(11):5202–5208. doi: 10.1128/AAC.00649-13. PubMed PMID: 23917318; PMCID: PMC3811313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Driouich J.S., Cochin M., Lingas G., Moureau G., Touret F., Petit P.R., Piorkowski G., Barthélémy K., Laprie C., Coutard B., Guedj J., de Lamballerie X., Solas C., Nougairède A. Favipiravir antiviral efficacy against SARS-CoV-2 in a hamster model. Nat. Commun. 2021;12(1):1735. doi: 10.1038/s41467-021-21992-w. PubMed PMID: 33741945; PMCID: PMC7979801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Urakova N., Kuznetsova V., Crossman D.K., Sokratian A., Guthrie D.B., Kolykhalov A.A., Lockwood M.A., Natchus M.G., Crowley M.R., Painter G.R., Frolova E.I., Frolov I. β- d -N4-hydroxycytidine is a potent anti-alphavirus compound that induces a high level of mutations in the viral genome. J. Virol. 2018;92(3) doi: 10.1128/JVI.01965-17. PubMed PMID: 29167335; PMCID: PMC5774879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reynard O., Nguyen X.N., Alazard-Dany N., Barateau V., Cimarelli A., Volchkov V.E. Identification of a new ribonucleoside inhibitor of ebola virus replication. Viruses. 2015;7(12):6233–6240. doi: 10.3390/v7122934. PubMed PMID: 26633464; PMCID: PMC4690858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Toots M., Yoon J.J., Cox R.M., Hart M., Sticher Z.M., Makhsous N., Plesker R., Barrena A.H., Reddy P.G., Mitchell D.G., Shean R.C., Bluemling G.R., Kolykhalov A.A., Greninger A.L., Natchus M.G., Painter G.R., Plemper R.K. Characterization of orally efficacious influenza drug with high resistance barrier in ferrets and human airway epithelia. Sci. Transl. Med. 2019;11(515) doi: 10.1126/scitranslmed.aax5866. PubMed PMID: 31645453; PMCID: PMC6848974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sheahan T.P., Sims A.C., Zhou S., Graham R.L., Pruijssers A.J., Agostini M.L., Leist S.R., Schäfer A., Dinnon K.H., Stevens L.J., Chappell J.D., Lu X., Hughes T.M., George A.S., Hill C.S., Montgomery S.A., Brown A.J., Bluemling G.R., Natchus M.G., Saindane M., Kolykhalov A.A., Painter G., Harcourt J., Tamin A., Thornburg N.J., Swanstrom R., Denison M.R., Baric R.S. An orally bioavailable broad-spectrum antiviral inhibits SARS-CoV-2 in human airway epithelial cell cultures and multiple coronaviruses in mice. Sci. Transl. Med. 2020;12(541) doi: 10.1126/scitranslmed.abb5883. PubMed PMID: 32253226; PMCID: PMC7164393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pfeiffer J.K., Kirkegaard K. A single mutation in poliovirus RNA-dependent RNA polymerase confers resistance to mutagenic nucleotide analogs via increased fidelity. Proc. Natl. Acad. Sci. U.S.A. 2003;100(12):7289–7294. doi: 10.1073/pnas.1232294100. PubMed PMID: 12754380; PMCID: PMC165868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kempf D.J., Marsh K.C., Denissen J.F., McDonald E., Vasavanonda S., Flentge C.A., Green B.E., Fino L., Park C.H., Kong X.P. ABT-538 is a potent inhibitor of human immunodeficiency virus protease and has high oral bioavailability in humans. Proc. Natl. Acad. Sci. U.S.A. 1995;92(7):2484–2488. doi: 10.1073/pnas.92.7.2484. PubMed PMID: 7708670; PMCID: PMC42242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Heeswijk R.P., Veldkamp A., Mulder J.W., Meenhorst P.L., Lange J.M., Beijnen J.H., Hoetelmans R.M. Combination of protease inhibitors for the treatment of HIV-1-infected patients: a review of pharmacokinetics and clinical experience. Antivir. Ther. 2001;6(4):201–229. [PubMed] [Google Scholar]
- 36.Chen F., Chan K.H., Jiang Y., Kao R.Y., Lu H.T., Fan K.W., Cheng V.C., Tsui W.H., Hung I.F., Lee T.S., Guan Y., Peiris J.S., Yuen K.Y. In vitro susceptibility of 10 clinical isolates of SARS coronavirus to selected antiviral compounds. J. Clin. Virol. 2004;31(1):69–75. doi: 10.1016/j.jcv.2004.03.003. PubMed PMID: 15288617; PMCID: PMC7128415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sheahan T.P., Sims A.C., Leist S.R., Schäfer A., Won J., Brown A.J., Montgomery S.A., Hogg A., Babusis D., Clarke M.O., Spahn J.E., Bauer L., Sellers S., Porter D., Feng J.Y., Cihlar T., Jordan R., Denison M.R., Baric R.S. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat. Commun. 2020;11(1):222. doi: 10.1038/s41467-019-13940-6. PubMed PMID: 31924756; PMCID: PMC6954302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lipsitch M., Perlman S., Waldor M.K. Testing COVID-19 therapies to prevent progression of mild disease. Lancet Infect. Dis. 2020;20(12):1367. doi: 10.1016/S1473-3099(20)30372-8. PubMed PMID: 32618282; PMCID: PMC7202831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li Y., Xie Z., Lin W., Cai W., Wen C., Guan Y., Mo X., Wang J., Wang Y., Peng P., Chen X., Hong W., Xiao G., Liu J., Zhang L., Hu F., Li F., Zhang F., Deng X., Li L. Efficacy and safety of lopinavir/ritonavir or arbidol in adult patients with mild/moderate COVID-19: an exploratory randomized controlled trial. Med. 2020;1(1):105–113. doi: 10.1016/j.medj.2020.04.001. PubMed PMID: 32838353; PMCID: PMC7235585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hung I.F., Lung K.C., Tso E.Y., Liu R., Chung T.W., Chu M.Y., Ng Y.Y., Lo J., Chan J., Tam A.R., Shum H.P., Chan V., Wu A.K., Sin K.M., Leung W.S., Law W.L., Lung D.C., Sin S., Yeung P., Yip C.C., Zhang R.R., Fung A.Y., Yan E.Y., Leung K.H., Ip J.D., Chu A.W., Chan W.M., Ng A.C., Lee R., Fung K., Yeung A., Wu T.C., Chan J.W., Yan W.W., Chan J.F., Lie A.K., Tsang O.T., Cheng V.C., Que T.L., Lau C.S., Chan K.H., To K.K., Yuen K.Y. Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet. 2020;395(10238):1695–1704. doi: 10.1016/S0140-6736(20)31042-4. PubMed PMID: 32401715; PMCID: PMC7211500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoffman R.L., Kania R.S., Brothers M.A., Davies J.F., Ferre R.A., Gajiwala K.S., He M., Hogan R.J., Kozminski K., Li L.Y., Lockner J.W., Lou J., Marra M.T., Mitchell L.J., Murray B.W., Nieman J.A., Noell S., Planken S.P., Rowe T., Ryan K., Smith G.J., Solowiej J.E., Steppan C.M., Taggart B. Discovery of ketone-based covalent inhibitors of coronavirus 3CL proteases for the potential therapeutic treatment of COVID-19. J. Med. Chem. 2020;63(21):12725–12747. doi: 10.1021/acs.jmedchem.0c01063. PubMed PMID: 33054210; PMCID: PMC7571312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boras B., Jones R.M., Anson B.J., Arenson D., Aschenbrenner L., Bakowski M.A., Beutler N., Binder J., Chen E., Eng H., Hammond H., Hammond J., Haupt R.E., Hoffman R., Kadar E.P., Kania R., Kimoto E., Kirkpatrick M.G., Lanyon L., Lendy E.K., Lillis J.R., Logue J., Luthra S.A., Ma C., Mason S.W., McGrath M.E., Noell S., Obach R.S., O’Brien M.N., O’Connor R., Ogilvie K., Owen D., Pettersson M., Reese M.R., Rogers T.F., Rossulek M.I., Sathish J.G., Shirai N., Steppan C., Ticehurst M., Updyke L.W., Weston S., Zhu Y., Wang J., Chatterjee A.K., Mesecar A.D., Frieman M.B., Anderson A.S., Allerton C. Discovery of a novel inhibitor of coronavirus 3CL protease for the potential treatment of COVID-19. bioRxiv. 2021 doi: 10.1101/2020.09.12.293498. PubMed PMID: 32935104; PMCID: PMC7491518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.de Vries M., Mohamed A.S., Prescott R.A., Valero-Jimenez A.M., Desvignes L., O’Connor R., Steppan C., Devlin J.C., Ivanova E., Herrera A., Schinlever A., Loose P., Ruggles K., Koralov S.B., Anderson A.S., Binder J., Dittmann M. A comparative analysis of SARS-CoV-2 antivirals characterizes 3CLpro inhibitor PF-00835231 as a potential new treatment for COVID-19. J. Virol. 2021 doi: 10.1128/JVI.01819-20. PubMed PMID: 33622961; PMCID: PMC8139662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Andersen H., Meyer J., Freeman J., Doyle S.E., Klucher K., Miller D.M., Hausman D., Hillson J.L. Peginterferon lambda-1a, a new therapeutic for hepatitis C infection, from bench to clinic. J. Clin. Transl. Hepatol. 2013;1(2):116–124. doi: 10.14218/JCTH.2013.00014. PubMed PMID: 26357610; PMCID: PMC4521278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Donnelly R.P., Kotenko S.V. Interferon-lambda: a new addition to an old family. J. Interferon Cytokine Res. 2010;30(8):555–564. doi: 10.1089/jir.2010.0078. PubMed PMID: 20712453; PMCID: PMC2925029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wack A., Terczyńska-Dyla E., Hartmann R. Guarding the frontiers: the biology of type III interferons. Nat. Immunol. 2015;16(8):802–809. doi: 10.1038/ni.3212. PubMed PMID: 26194286; PMCID: PMC7096991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Felgenhauer U., Schoen A., Gad H.H., Hartmann R., Schaubmar A.R., Failing K., Drosten C., Weber F. Inhibition of SARS-CoV-2 by type I and type III interferons. J. Biol. Chem. 2020;295(41):13958–13964. doi: 10.1074/jbc.AC120.013788. PubMed PMID: 32587093; PMCID: PMC7549028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dinnon K.H., Leist S.R., Schäfer A., Edwards C.E., Martinez D.R., Montgomery S.A., West A., Yount B.L., Hou Y.J., Adams L.E., Gully K.L., Brown A.J., Huang E., Bryant M.D., Choong I.C., Glenn J.S., Gralinski L.E., Sheahan T.P., Baric R.S. A mouse-adapted model of SARS-CoV-2 to test COVID-19 countermeasures. Nature. 2020;586(7830):560–566. doi: 10.1038/s41586-020-2708-8. PubMed PMID: 32854108; PMCID: PMC8034761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jagannathan P., Andrews J.R., Bonilla H., Hedlin H., Jacobson K.B., Balasubramanian V., Purington N., Kamble S., de Vries C.R., Quintero O., Feng K., Ley C., Winslow D., Newberry J., Edwards K., Hislop C., Choong I., Maldonado Y., Glenn J., Bhatt A., Blish C., Wang T., Khosla C., Pinsky B.A., Desai M., Parsonnet J., Singh U. Peginterferon Lambda-1a for treatment of outpatients with uncomplicated COVID-19: a randomized placebo-controlled trial. Nat. Commun. 2021;12(1):1967. doi: 10.1038/s41467-021-22177-1. PubMed PMID: 33785743; PMCID: PMC8009873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sampson A.K., Irvine J.C., Shihata W.A., Dragoljevic D., Lumsden N., Huet O., Barnes T., Unger T., Steckelings U.M., Jennings G.L., Widdop R.E., Chin-Dusting J.P. Compound 21, a selective agonist of angiotensin AT2 receptors, prevents endothelial inflammation and leukocyte adhesion in vitro and in vivo. Br. J. Pharmacol. 2016;173(4):729–740. doi: 10.1111/bph.13063. PubMed PMID: 25560767; PMCID: PMC4742292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Forrester S.J., Booz G.W., Sigmund C.D., Coffman T.M., Kawai T., Rizzo V., Scalia R., Eguchi S. Angiotensin II signal transduction: an update on mechanisms of physiology and pathophysiology. Physiol. Rev. 2018;98(3):1627–1738. doi: 10.1152/physrev.00038.2017. PubMed PMID: 29873596; PMCID: PMC6335102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Joseph J.P., Mecca A.P., Regenhardt R.W., Bennion D.M., Rodríguez V., Desland F., Patel N.A., Pioquinto D.J., Unger T., Katovich M.J., Steckelings U.M., Sumners C. The angiotensin type 2 receptor agonist Compound 21 elicits cerebroprotection in endothelin-1 induced ischemic stroke. Neuropharmacology. 2014;81:134–141. doi: 10.1016/j.neuropharm.2014.01.044. PubMed PMID: 24508710; PMCID: PMC7472595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B., Huan Y., Yang P., Zhang Y., Deng W., Bao L., Zhang B., Liu G., Wang Z., Chappell M., Liu Y., Zheng D., Leibbrandt A., Wada T., Slutsky A.S., Liu D., Qin C., Jiang C., Penninger J.M. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat. Med. 2005;11(8):875–879. doi: 10.1038/nm1267. PubMed PMID: 16007097; PMCID: PMC7095783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., Arbous M.S., Gommers D.A.M.P., Kant K.M., Kaptein F.H.J., van Paassen J., Stals M.A.M., Huisman M.V., Endeman H. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. PubMed PMID: 32291094; PMCID: PMC7146714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Peng Y.D., Meng K., Guan H.Q., Leng L., Zhu R.R., Wang B.Y., He M.A., Cheng L.X., Huang K., Zeng Q.T. Clinical characteristics and outcomes of 112 cardiovascular disease patients infected by 2019-nCoV. Zhonghua Xin Xue Guan Bing. Za Zhi. 2020;48(6):450–455. doi: 10.3760/cma.j.cn112148-20200220-00105. [DOI] [PubMed] [Google Scholar]
- 56.Schwartz D.M., Kanno Y., Villarino A., Ward M., Gadina M., O’Shea J.J. JAK inhibition as a therapeutic strategy for immune and inflammatory diseases. Nat. Rev. Drug Discov. 2017;17(1):78. doi: 10.1038/nrd.2017.267. PubMed PMID: 29282366; PMCID: PMC6168198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shi J.G., Chen X., Lee F., Emm T., Scherle P.A., Lo Y., Punwani N., Williams W.V., Yeleswaram S. The pharmacokinetics, pharmacodynamics, and safety of baricitinib, an oral JAK 1/2 inhibitor, in healthy volunteers. J. Clin. Pharm. 2014;54(12):1354–1361. doi: 10.1002/jcph.354. [DOI] [PubMed] [Google Scholar]
- 58.Fridman J.S., Scherle P.A., Collins R., Burn T.C., Li Y., Li J., Covington M.B., Thomas B., Collier P., Favata M.F., Wen X., Shi J., McGee R., Haley P.J., Shepard S., Rodgers J.D., Yeleswaram S., Hollis G., Newton R.C., Metcalf B., Friedman S.M., Vaddi K. Selective inhibition of JAK1 and JAK2 is efficacious in rodent models of arthritis: preclinical characterization of INCB028050. J. Immunol. 2010;184(9):5298–5307. doi: 10.4049/jimmunol.0902819. PubMed PMID: 20363976. [DOI] [PubMed] [Google Scholar]
- 59.Genovese M.C., Kremer J., Zamani O., Ludivico C., Krogulec M., Xie L., Beattie S.D., Koch A.E., Cardillo T.E., Rooney T.P., Macias W.L., de Bono S., Schlichting D.E., Smolen J.S. Baricitinib in patients with refractory rheumatoid arthritis. New Engl. J. Med. 2016;374(13):1243–1252. doi: 10.1056/NEJMoa1507247. [DOI] [PubMed] [Google Scholar]
- 60.Seif F., Khoshmirsafa M., Aazami H., Mohsenzadegan M., Sedighi G., Bahar M. The role of JAK-STAT signaling pathway and its regulators in the fate of T helper cells. Cell Commun. Signal. 2017;15(1):23. doi: 10.1186/s12964-017-0177-y. PubMed PMID: 28637459; PMCID: PMC5480189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Petrone L., Petruccioli E., Alonzi T., Vanini V., Cuzzi G., Najafi Fard S., Castilletti C., Palmieri F., Gualano G., Vittozzi P., Nicastri E., Lepore L., Grifoni A., Antinori A., Vergori A., Ippolito G., Cantini F., Goletti D. In-vitro evaluation of the immunomodulatory effects of Baricitinib: implication for COVID-19 therapy. J. Infect. 2021;82(4):58–66. doi: 10.1016/j.jinf.2021.02.023. PubMed PMID: 33639176; PMCID: PMC7904476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sorrell F.J., Szklarz M., Abdul Azeez K.R., Elkins J.M., Knapp S. Family-wide structural analysis of human numb-associated protein kinases. Structure. 2016;24(3):401–411. doi: 10.1016/j.str.2015.12.015. PubMed PMID: 26853940; PMCID: PMC4780864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kalil A.C., Patterson T.F., Mehta A.K., Tomashek K.M., Wolfe C.R., Ghazaryan V., Marconi V.C., Ruiz-Palacios G.M., Hsieh L., Kline S., Tapson V., Iovine N.M., Jain M.K., Sweeney D.A., El Sahly H.M., Branche A.R., Regalado Pineda J., Lye D.C., Sandkovsky U., Luetkemeyer A.F., Cohen S.H., Finberg R.W., Jackson P.E.H., Taiwo B., Paules C.I., Arguinchona H., Erdmann N., Ahuja N., Frank M., Oh M.D., Kim E.S., Tan S.Y., Mularski R.A., Nielsen H., Ponce P.O., Taylor B.S., Larson L., Rouphael N.G., Saklawi Y., Cantos V.D., Ko E.R., Engemann J.J., Amin A.N., Watanabe M., Billings J., Elie M.C., Davey R.T., Burgess T.H., Ferreira J., Green M., Makowski M., Cardoso A., de Bono S., Bonnett T., Proschan M., Deye G.A., Dempsey W., Nayak S.U., Dodd L.E., Beigel J.H. Members A-SG. Baricitinib plus remdesivir for hospitalized adults with Covid-19. New Engl. J. Med. 2021;384(9):795–807. doi: 10.1056/NEJMoa2031994. PubMed PMID: 33306283; PMCID: PMC7745180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Praveen D., Puvvada R.C., Janus M.V.A. Janus kinase inhibitor baricitinib is not an ideal option for management of COVID-19. Int J. Antimicrob. Agents. 2020;55(5) doi: 10.1016/j.ijantimicag.2020.105967. PubMed PMID: 32259575; PMCID: PMC7128600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yan B., Freiwald T., Chauss D., Wang L., West E., Mirabelli C., Zhang C.J., Nichols E.M., Malik N., Gregory R., Bantscheff M., Ghidelli-Disse S., Kolev M., Frum T., Spence J.R., Sexton J.Z., Alysandratos K.D., Kotton D.N., Pittaluga S., Bibby J., Niyonzima N., Olson M.R., Kordasti S., Portilla D., Wobus C.E., Laurence A., Lionakis M.S., Kemper C., Afzali B., Kazemian M. SARS-CoV-2 drives JAK1/2-dependent local complement hyperactivation. Sci. Immunol. 2021;6(58) doi: 10.1126/sciimmunol.abg0833. PubMed PMID: 33827897; PMCID: PMC8139422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ladislau L., Suárez-Calvet X., Toquet S., Landon-Cardinal O., Amelin D., Depp M., Rodero M.P., Hathazi D., Duffy D., Bondet V., Preusse C., Bienvenu B., Rozenberg F., Roos A., Benjamim C.F., Gallardo E., Illa I., Mouly V., Stenzel W., Butler-Browne G., Benveniste O., Allenbach Y. JAK inhibitor improves type I interferon induced damage: proof of concept in dermatomyositis. Brain. 2018;141(6):1609–1621. doi: 10.1093/brain/awy105. [DOI] [PubMed] [Google Scholar]
- 67.Wyatt R.J., Forristal J., West C.D., Sugimoto S., Curd J.G. Complement profiles in acute post-streptococcal glomerulonephritis. Pedia Nephrol. 1988;2(2):219–223. doi: 10.1007/BF00862594. [DOI] [PubMed] [Google Scholar]
- 68.Reoma L.B., Trindade C.J., Monaco M.C., Solis J., Montojo M.G., Vu P., Johnson K., Beck E., Nair G., Khan O.I., Quezado M., Hewitt S.M., Reich D.S., Childs R., Nath A. Fatal encephalopathy with wild-type JC virus and ruxolitinib therapy. Ann. Neurol. 2019;86(6):878–884. doi: 10.1002/ana.25608. PubMed PMID: 31600832; PMCID: PMC8189164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wathes R., Moule S., Milojkovic D. Progressive multifocal leukoencephalopathy associated with ruxolitinib. New Engl. J. Med. 2013;369(2):197–198. doi: 10.1056/NEJMc1302135. [DOI] [PubMed] [Google Scholar]
- 70.Nakayama K., Nakamura M., Konishi A., Kaneko S., Nakamichi K., Saijo M., Yakushiji Y., Kusaka H. JC virus granule cell neuronopathy associated with Ruxolitinib: a case report and review of the literature. eNeurologicalSci. 2020;21 doi: 10.1016/j.ensci.2020.100269. PubMed PMID: 32954021; PMCID: PMC7486434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Guimarães-Costa A.B., Nascimento M.T., Froment G.S., Soares R.P., Morgado F.N., Conceição-Silva F., Saraiva E.M. Leishmania amazonensis promastigotes induce and are killed by neutrophil extracellular traps. Proc. Natl. Acad. Sci. U.S.A. 2009;106(16):6748–6753. doi: 10.1073/pnas.0900226106. PubMed PMID: 19346483; PMCID: PMC2672475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Papayannopoulos V., Staab D., Zychlinsky A. Neutrophil elastase enhances sputum solubilization in cystic fibrosis patients receiving DNase therapy. PLoS One. 2011;6(12):28526. doi: 10.1371/journal.pone.0028526. PubMed PMID: 22174830; PMCID: PMC3235130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Doster R.S., Rogers L.M., Gaddy J.A., Aronoff D.M. Macrophage extracellular traps: a scoping review. J. Innate Immun. 2018;10(1):3–13. doi: 10.1159/000480373. PubMed PMID: 28988241; PMCID: PMC6757166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Boeltz S., Amini P., Anders H.J., Andrade F., Bilyy R., Chatfield S., Cichon I., Clancy D.M., Desai J., Dumych T., Dwivedi N., Gordon R.A., Hahn J., Hidalgo A., Hoffmann M.H., Kaplan M.J., Knight J.S., Kolaczkowska E., Kubes P., Leppkes M., Manfredi A.A., Martin S.J., Maueröder C., Maugeri N., Mitroulis I., Munoz L.E., Nakazawa D., Neeli I., Nizet V., Pieterse E., Radic M.Z., Reinwald C., Ritis K., Rovere-Querini P., Santocki M., Schauer C., Schett G., Shlomchik M.J., Simon H.U., Skendros P., Stojkov D., Vandenabeele P., Berghe T.V., van der Vlag J., Vitkov L., von Köckritz-Blickwede M., Yousefi S., Zarbock A., Herrmann M. To NET or not to NET:current opinions and state of the science regarding the formation of neutrophil extracellular traps. Cell Death Differ. 2019;26(3):395–408. doi: 10.1038/s41418-018-0261-x. PubMed PMID: 30622307; PMCID: PMC6370810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Okur H.K., Yalcin K., Tastan C., Demir S., Yurtsever B., Karakus G.S., Kancagi D.D., Abanuz S., Seyis U., Zengin R., Hemsinlioglu C., Kara M., Yildiz M.E., Deliceo E., Birgen N., Pelit N.B., Cuhadaroglu C., Kocagoz A.S., Ovali E. Preliminary report of in vitro and in vivo effectiveness of dornase alfa on SARS-CoV-2 infection. New Microbes New Infect. 2020;37 doi: 10.1016/j.nmni.2020.100756. PubMed PMID: 32922804; PMCID: PMC7476504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Arcanjo A., Logullo J., Menezes C.C.B., de Souza Carvalho Giangiarulo T.C., Dos Reis M.C., de Castro G.M.M., da Silva Fontes Y., Todeschini A.R., Freire-de-Lima L., Decoté-Ricardo D., Ferreira-Pereira A., Freire-de-Lima C.G., Barroso S.P.C., Takiya C., Conceição-Silva F., Savino W., Morrot A. The emerging role of neutrophil extracellular traps in severe acute respiratory syndrome coronavirus 2 (COVID-19) Sci. Rep. 2020;10(1):19630. doi: 10.1038/s41598-020-76781-0. PubMed PMID: 33184506; PMCID: PMC7665044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Desilles J.P., Gregoire C., Le Cossec C., Lambert J., Mophawe O., Losser M.R., Lambiotte F., Le Tacon S., Cantier M., Engrand N., Trouiller P., Pottecher J. Efficacy and safety of aerosolized intra-tracheal dornase alfa administration in patients with SARS-CoV-2-induced acute respiratory distress syndrome (ARDS): a structured summary of a study protocol for a randomised controlled trial. Trials. 2020;21(1):548. doi: 10.1186/s13063-020-04488-8. PubMed PMID: 32560746; PMCID: PMC7303591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.J M The thromboplastic action of cephalin. Am. J. Physiol. 1916;41:250–257. [Google Scholar]
- 79.Shionoya T. Studies in experimental extracorporeal thrombosis: iii. effects of certain anticoagulants (Heparin and Hirudin) on extracorporeal thrombosis and on the mechanism of thrombus formation. J. Exp. Med. 1927;46(1):19–26. doi: 10.1084/jem.46.1.19. PubMed PMID: 19869325; PMCID: PMC2131257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Baird R.J. “Give us the tools.”. The story of heparin--as told by sketches from the lives of William Howell, Jay McLean, Charles Best, and Gordon Murray. J. Vasc. Surg. 1990;11(1):4–18. [PubMed] [Google Scholar]
- 81.Weiler J.M., Yurt R.W., Fearon D.T., Austen K.F. Modulation of the formation of the amplification convertase of complement, C3b, Bb, by native and commercial heparin. J. Exp. Med. 1978;147(2):409–421. doi: 10.1084/jem.147.2.409. PubMed PMID: 624904; PMCID: PMC2184494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wiedmer T., Esmon C.T., Sims P.J. Complement proteins C5b-9 stimulate procoagulant activity through platelet prothrombinase. Blood. 1986;68(4):875–880. [PubMed] [Google Scholar]
- 83.Serisier D.J., Shute J.K., Hockey P.M., Higgins B., Conway J., Carroll M.P. Inhaled heparin in cystic fibrosis. Eur. Respir. J. 2006;27(2):354–358. doi: 10.1183/09031936.06.00069005. [DOI] [PubMed] [Google Scholar]
- 84.Jonmarker S., Hollenberg J., Dahlberg M., Stackelberg O., Litorell J., Everhov Å., Järnbert-Pettersson H., Söderberg M., Grip J., Schandl A., Günther M., Cronhjort M. Dosing of thromboprophylaxis and mortality in critically ill COVID-19 patients. Crit. Care. 2020;24(1):653. doi: 10.1186/s13054-020-03375-7. PubMed PMID: 33225952; PMCID: PMC7680989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schwiebert L.M., Beck L.A., Stellato C., Bickel C.A., Bochner B.S., Schleimer R.P., Schwiebert L.A. Glucocorticosteroid inhibition of cytokine production: relevance to antiallergic actions. J. Allergy Clin. Immunol. 1996;97(1 Pt 2):143–152. doi: 10.1016/s0091-6749(96)80214-4. [DOI] [PubMed] [Google Scholar]
- 86.Villar J., Ferrando C., Martínez D., Ambrós A., Muñoz T., Soler J.A., Aguilar G., Alba F., González-Higueras E., Conesa L.A., Martín-Rodríguez C., Díaz-Domínguez F.J., Serna-Grande P., Rivas R., Ferreres J., Belda J., Capilla L., Tallet A., Añón J.M., Fernández R.L., González-Martín J.M. network diA. Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir. Med. 2020;8(3):267–276. doi: 10.1016/S2213-2600(19)30417-5. PubMed PMID: 32043986. [DOI] [PubMed] [Google Scholar]
- 87.Chen R.C., Tang X.P., Tan S.Y., Liang B.L., Wan Z.Y., Fang J.Q., Zhong N. Treatment of severe acute respiratory syndrome with glucosteroids: the Guangzhou experience. Chest. 2006;129(6):1441–1452. doi: 10.1378/chest.129.6.1441. PubMed PMID: 16778260; PMCID: PMC7094735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ni Y.N., Chen G., Sun J., Liang B.M., Liang Z.A. The effect of corticosteroids on mortality of patients with influenza pneumonia: a systematic review and meta-analysis. Crit. Care. 2019;23(1):99. doi: 10.1186/s13054-019-2395-8. PubMed PMID: 30917856; PMCID: PMC6437920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li X.W., Jiang R.M., Guo J.Z. Glucocorticoid in the treatment of severe acute respiratory syndrome patients: a preliminary report. Zhonghua Nei Ke Za Zhi. 2003;42(6):378–381. [PubMed] [Google Scholar]
- 90.Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L., Linsell L., Staplin N., Brightling C., Ustianowski A., Elmahi E., Prudon B., Green C., Felton T., Chadwick D., Rege K., Fegan C., Chappell L.C., Faust S.N., Jaki T., Jeffery K., Montgomery A., Rowan K., Juszczak E., Baillie J.K., Haynes R., Landray M.J., Group R.C. Dexamethasone in hospitalized patients with COVID-19. New Engl. J. Med. 2021;384(8):693–704. doi: 10.1056/NEJMoa2021436. PubMed PMID: 32678530; PMCID: PMC7383595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sackstein R. Effects of methylprednisolone administration on lymphocyte LECAM-1, CD44, and LFA-1 expression. Implications for steroid-induced lymphopenia. Ann. N.Y. Acad. Sci. 1993;696:417–419. doi: 10.1111/j.1749-6632.1993.tb17183.x. [DOI] [PubMed] [Google Scholar]
- 92.Steinberg K.P., Hudson L.D., Goodman R.B., Hough C.L., Lanken P.N., Hyzy R., Thompson B.T., Ancukiewicz M. National heart Ln, and blood institute acute respiratory distress syndrome (ARDS) clinical trials network. efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N. Engl. J. Med. 2006;354(16):1671–1684. doi: 10.1056/NEJMoa051693. [DOI] [PubMed] [Google Scholar]
- 93.Edalatifard M., Akhtari M., Salehi M., Naderi Z., Jamshidi A., Mostafaei S., Najafizadeh S.R., Farhadi E., Jalili N., Esfahani M., Rahimi B., Kazemzadeh H., Mahmoodi Aliabadi M., Ghazanfari T., Sattarian M., Ebrahimi Louyeh H., Raeeskarami S.R., Jamalimoghadamsiahkali S., Khajavirad N., Mahmoudi M., Rostamian A. Intravenous methylprednisolone pulse as a treatment for hospitalised severe COVID-19 patients: results from a randomised controlled clinical trial. Eur. Respir. J. 2020;56(6) doi: 10.1183/13993003.02808-2020. PubMed PMID: 32943404; PMCID: PMC7758541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Papamanoli A., Yoo J., Grewal P., Predun W., Hotelling J., Jacob R., Mojahedi A., Skopicki H.A., Mansour M., Marcos L.A., Kalogeropoulos A.P. High-dose methylprednisolone in nonintubated patients with severe COVID-19 pneumonia. Eur. J. Clin. Invest. 2021;51(2):13458. doi: 10.1111/eci.13458. PubMed PMID: 33219551; PMCID: PMC7744876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Salton F., Confalonieri P., Meduri G.U., Santus P., Harari S., Scala R., Lanini S., Vertui V., Oggionni T., Caminati A., Patruno V., Tamburrini M., Scartabellati A., Parati M., Villani M., Radovanovic D., Tomassetti S., Ravaglia C., Poletti V., Vianello A., Gaccione A.T., Guidelli L., Raccanelli R., Lucernoni P., Lacedonia D., Foschino Barbaro M.P., Centanni S., Mondoni M., Davì M., Fantin A., Cao X., Torelli L., Zucchetto A., Montico M., Casarin A., Romagnoli M., Gasparini S., Bonifazi M., D’Agaro P., Marcello A., Licastro D., Ruaro B., Volpe M.C., Umberger R., Confalonieri M. Prolonged low-dose methylprednisolone in patients with severe COVID-19 pneumonia. Open Forum Infect. Dis. 2020;7(10):421. doi: 10.1093/ofid/ofaa421. PubMed PMID: 33072814; PMCID: PMC7543560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ranjbar K., Moghadami M., Mirahmadizadeh A., Fallahi M.J., Khaloo V., Shahriarirad R., Erfani A., Khodamoradi Z., Gholampoor Saadi M.H. Methylprednisolone or dexamethasone, which one is superior corticosteroid in the treatment of hospitalized COVID-19 patients: a triple-blinded randomized controlled trial. BMC Infect. Dis. 2021;21(1):337. doi: 10.1186/s12879-021-06045-3. PubMed PMID: 33838657; PMCID: PMC8035859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pimentel J.L., Martinez-Maldonado M., Wilcox J.N., Wang S., Luo C. Regulation of renin-angiotensin system in unilateral ureteral obstruction. Kidney Int. 1993;44(2):390–400. doi: 10.1038/ki.1993.257. [DOI] [PubMed] [Google Scholar]
- 98.Lin C.H., Yang H., Xue Q.L., Chuang Y.F., Roy C.N., Abadir P., Walston J.D. Losartan improves measures of activity, inflammation, and oxidative stress in older mice. Exp. Gerontol. 2014;58:174–178. doi: 10.1016/j.exger.2014.07.017. PubMed PMID: 25077714; PMCID: PMC4252828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Habashi J.P., Judge D.P., Holm T.M., Cohn R.D., Loeys B.L., Cooper T.K., Myers L., Klein E.C., Liu G., Calvi C., Podowski M., Neptune E.R., Halushka M.K., Bedja D., Gabrielson K., Rifkin D.B., Carta L., Ramirez F., Huso D.L., Dietz H.C.Losartan. an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science. 2006;312(5770):117–121. doi: 10.1126/science.1124287. PubMed PMID: 16601194; PMCID: PMC1482474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Puskarich M.A., Cummins N.W., Ingraham N.E., Wacker D.A., Reilkoff R.A., Driver B.E., Biros M.H., Bellolio F., Chipman J.G., Nelson A.C., Beckman K., Langlois R., Bold T., Aliota M.T., Schacker T.W., Voelker H.T., Murray T.A., Koopmeiners J.S., Tignanelli C.J. A multi-center phase II randomized clinical trial of losartan on symptomatic outpatients with COVID-19. EClinicalMedicine. 2021;37 doi: 10.1016/j.eclinm.2021.100957. PubMed PMID: 34195577; PMCID: PMC8225661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Geriak M., Haddad F., Kullar R., Greenwood K.L., Habib M., Habib C., Willms D., Sakoulas G. Randomized prospective open label study shows no impact on clinical outcome of adding losartan to hospitalized COVID-19 patients with mild hypoxemia. Infect. Dis. Ther. 2021;10(3):1323–1330. doi: 10.1007/s40121-021-00453-3. PubMed PMID: 33977506; PMCID: PMC8112834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.van Griensven J., Edwards T., de Lamballerie X., Semple M.G., Gallian P., Baize S., Horby P.W., Raoul H., Magassouba N., Antierens A., Lomas C., Faye O., Sall A.A., Fransen K., Buyze J., Ravinetto R., Tiberghien P., Claeys Y., De Crop M., Lynen L., Bah E.I., Smith P.G., Delamou A., De Weggheleire A., Haba N., Consortium E.-T. Evaluation of convalescent plasma for ebola virus disease in guinea. New Engl. J. Med. 2016;374(1):33–42. doi: 10.1056/NEJMoa1511812. PubMed PMID: 26735992; PMCID: PMC5856332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hung I.F., To K.K., Lee C.K., Lee K.L., Chan K., Yan W.W., Liu R., Watt C.L., Chan W.M., Lai K.Y., Koo C.K., Buckley T., Chow F.L., Wong K.K., Chan H.S., Ching C.K., Tang B.S., Lau C.C., Li I.W., Liu S.H., Chan K.H., Lin C.K., Yuen K.Y. Convalescent plasma treatment reduced mortality in patients with severe pandemic influenza A (H1N1) 2009 virus infection. Clin. Infect. Dis. 2011;52(4):447–456. doi: 10.1093/cid/ciq106. PubMed PMID: 21248066; PMCID: PMC7531589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Salazar E., Kuchipudi S.V., Christensen P.A., Eagar T., Yi X., Zhao P., Jin Z., Long S.W., Olsen R.J., Chen J., Castillo B., Leveque C., Towers D., Lavinder J., Gollihar J., Cardona J., Ippolito G., Nissly R., Bird I., Greenawalt D., Rossi R.M., Gontu A., Srinivasan S., Poojary I., Cattadori I.M., Hudson P.J., Josleyn N.M., Prugar L., Huie K., Herbert A., Bernard D.W., Dye J.M., Kapur V., Musser J.M. Convalescent plasma anti-SARS-CoV-2 spike protein ectodomain and receptor-binding domain IgG correlate with virus neutralization. J. Clin. Invest. 2020;130(12):6728–6738. doi: 10.1172/JCI141206. PubMed PMID: 32910806; PMCID: PMC7685744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Santiago L., Uranga-Murillo I., Arias M., González-Ramírez A.M., Macías-León J., Moreo E., Redrado S., García-García A., Taleb V., Lira-Navarrete E., Hurtado-Guerrero R., Aguilo N., Del Mar Encabo-Berzosa M., Hidalgo S., Galvez E.M., Ramirez-Labrada A., de Miguel D., Benito R., Miranda P., Fernández A., Domingo J.M., Serrano L., Yuste C., Villanueva-Saz S., Paño-Pardo J.R., Pardo J. Determination of the concentration of IgG against the spike receptor-binding domain that predicts the viral neutralizing activity of convalescent plasma and serum against SARS-CoV-2. Biology. 2021;10(3) doi: 10.3390/biology10030208. PubMed PMID: 33801808; PMCID: PMC8001978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Simonovich V.A., Burgos Pratx L.D., Scibona P., Beruto M.V., Vallone M.G., Vázquez C., Savoy N., Giunta D.H., Pérez L.G., Sánchez M.D.L., Gamarnik A.V., Ojeda D.S., Santoro D.M., Camino P.J., Antelo S., Rainero K., Vidiella G.P., Miyazaki E.A., Cornistein W., Trabadelo O.A., Ross F.M., Spotti M., Funtowicz G., Scordo W.E., Losso M.H., Ferniot I., Pardo P.E., Rodriguez E., Rucci P., Pasquali J., Fuentes N.A., Esperatti M., Speroni G.A., Nannini E.C., Matteaccio A., Michelangelo H.G., Follmann D., Lane H.C., Belloso W.H., Group P.S. A Randomized Trial of Convalescent Plasma in Covid-19 Severe Pneumonia. N. Engl. J. Med. 2021;384(7):619–629. doi: 10.1056/NEJMoa2031304. PubMed PMID: 33232588; PMCID: PMC7722692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li L., Zhang W., Hu Y., Tong X., Zheng S., Yang J., Kong Y., Ren L., Wei Q., Mei H., Hu C., Tao C., Yang R., Wang J., Yu Y., Guo Y., Wu X., Xu Z., Zeng L., Xiong N., Chen L., Man N., Liu Y., Xu H., Deng E., Zhang X., Li C., Wang C., Su S., Zhang L., Wu Y., Liu Z. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial. JAMA. 2020;324(5):460–470. doi: 10.1001/jama.2020.10044. PubMed PMID: 32492084; PMCID: PMC7270883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Krupashankar D.S., Dogra S., Kura M., Saraswat A., Budamakuntla L., Sumathy T.K., Shah R., Gopal M.G., Narayana Rao T., Srinivas C.R., Bhat R., Shetty N., Manmohan G., Sai Krishna K., Padmaja D., Pratap D.V., Garg V., Gupta S., Pandey N., Khopkar U., Montero E., Ramakrishnan M.S., Nair P., Ganapathi P.C. Efficacy and safety of itolizumab, a novel anti-CD6 monoclonal antibody, in patients with moderate to severe chronic plaque psoriasis: results of a double-blind, randomized, placebo-controlled, phase-III study. J. Am. Acad. Dermatol. 2014;71(3):484–492. doi: 10.1016/j.jaad.2014.01.897. PubMed PMID: 24703722. [DOI] [PubMed] [Google Scholar]
- 109.Nair P., Melarkode R., Rajkumar D., Montero E. CD6 synergistic co-stimulation promoting proinflammatory response is modulated without interfering with the activated leucocyte cell adhesion molecule interaction. Clin. Exp. Immunol. 2010;162(1):116–130. doi: 10.1111/j.1365-2249.2010.04235.x. PubMed PMID: 20726988; PMCID: PMC2990937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sarrias M.R., Farnós M., Mota R., Sánchez-Barbero F., Ibáñez A., Gimferrer I., Vera J., Fenutría R., Casals C., Yélamos J., Lozano F. CD6 binds to pathogen-associated molecular patterns and protects from LPS-induced septic shock. Proc. Natl. Acad. Sci. U.S.A. 2007;104(28):11724–11729. doi: 10.1073/pnas.0702815104. PubMed PMID: 17601777; PMCID: PMC1913855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Menon R., David B.G. Itolizumab - a humanized anti-CD6 monoclonal antibody with a better side effects profile for the treatment of psoriasis. Clin. Cosmet. Invest. Dermatol. 2015;8:215–222. doi: 10.2147/CCID.S47784. PubMed PMID: 25945063; PMCID: PMC4407739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pai G., Pai A.H. Itolizumab - a new biologic for management of psoriasis and psoriatic arthritis. Case Rep. Dermatol. 2017;9(2):141–145. doi: 10.1159/000475519. PubMed PMID: 29033818; PMCID: PMC5624235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Aira L.E., López-Requena A., Fuentes D., Sánchez L., Pérez T., Urquiza A., Bautista H., Falcón L., Hernández P., Mazorra Z. Immunological and histological evaluation of clinical samples from psoriasis patients treated with anti-CD6 itolizumab. MAbs. 2014;6(3):783–793. doi: 10.4161/mabs.28376. PubMed PMID: 24594862; PMCID: PMC4011922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bughani U., Saha A., Kuriakose A., Nair R., Sadashivarao R.B., Venkataraman R., Patel S., Deshchougule A.T., S S.K., Montero E., Pai H.V., Palanivelu D.V., Melarkode R., Nair P. T cell activation and differentiation is modulated by a CD6 domain 1 antibody Itolizumab. PLoS One. 2017;12(7) doi: 10.1371/journal.pone.0180088. PubMed PMID: 28672038; PMCID: PMC5495335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y., Xie C., Ma K., Shang K., Wang W., Tian D.S. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin. Infect. Dis. 2020;71(15):762–768. doi: 10.1093/cid/ciaa248. PubMed PMID: 32161940; PMCID: PMC7108125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Oliveira M.I., Gonçalves C.M., Pinto M., Fabre S., Santos A.M., Lee S.F., Castro M.A., Nunes R.J., Barbosa R.R., Parnes J.R., Yu C., Davis S.J., Moreira A., Bismuth G., Carmo A.M. CD6 attenuates early and late signaling events, setting thresholds for T-cell activation. Eur. J. Immunol. 2012;42(1):195–205. doi: 10.1002/eji.201040528. PubMed PMID: 21956609; PMCID: PMC3298641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kumar S., De Souza R., Nadkar M., Guleria R., Trikha A., Joshi S.R., Loganathan S., Vaidyanathan S., Marwah A., Athalye S.N. A two-arm, randomized, controlled, multi-centric, open-label phase-2 study to evaluate the efficacy and safety of Itolizumab in moderate to severe ARDS patients due to COVID-19. Expert Opin. Biol. Ther. 2021;21(5):675–686. doi: 10.1080/14712598.2021.1905794. PubMed PMID: 33835886; PMCID: PMC8040494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ohsugi Y., Kishimoto T. The recombinant humanized anti-IL-6 receptor antibody tocilizumab, an innovative drug for the treatment of rheumatoid arthritis. Expert Opin. Biol. Ther. 2008;8(5):669–681. doi: 10.1517/14712598.8.5.669. [DOI] [PubMed] [Google Scholar]
- 119.Pandolfi F., Franza L., Carusi V., Altamura S., Andriollo G., Nucera E. Interleukin-6 in rheumatoid arthritis. Int J. Mol. Sci. 2020;21(15) doi: 10.3390/ijms21155238. PubMed PMID: 32718086; PMCID: PMC7432115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wang H., Wang Z., Wang L., Sun L., Liu W., Li Q., Wang J. IL-6 promotes collagen-induced arthritis by activating the NLRP3 inflammasome through the cathepsin B/S100A9-mediated pathway. Int. Immunopharmacol. 2020;88 doi: 10.1016/j.intimp.2020.106985. PubMed PMID: 33182050. [DOI] [PubMed] [Google Scholar]
- 121.Radigan K.A., Nicholson T.T., Welch L.C., Chi M., Amarelle L., Angulo M., Shigemura M., Shigemura A., Runyan C.E., Morales-Nebreda L., Perlman H., Ceco E., Lecuona E., Dada L.A., Misharin A.V., Mutlu G.M., Sznajder J.I., Budinger G.R.S. Influenza A virus infection induces muscle wasting via IL-6 regulation of the E3 ubiquitin ligase atrogin-1. J. Immunol. 2019;202(2):484–493. doi: 10.4049/jimmunol.1701433. PubMed PMID: 30530483; PMCID: PMC6324970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Disser N.P., De Micheli A.J., Schonk M.M., Konnaris M.A., Piacentini A.N., Edon D.L., Toresdahl B.G., Rodeo S.A., Casey E.K., Mendias C.L. Musculoskeletal consequences of COVID-19. J. Bone Jt. Surg. Am. 2020;102(14):1197–1204. doi: 10.2106/JBJS.20.00847. PubMed PMID: 32675661; PMCID: PMC7508274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Nalbandian A., Sehgal K., Gupta A., Madhavan M.V., McGroder C., Stevens J.S., Cook J.R., Nordvig A.S., Shalev D., Sehrawat T.S., Ahluwalia N., Bikdeli B., Dietz D., Der-Nigoghossian C., Liyanage-Don N., Rosner G.F., Bernstein E.J., Mohan S., Beckley A.A., Seres D.S., Choueiri T.K., Uriel N., Ausiello J.C., Accili D., Freedberg D.E., Baldwin M., Schwartz A., Brodie D., Garcia C.K., Elkind M.S.V., Connors J.M., Bilezikian J.P., Landry D.W., Wan E.Y. Post-acute COVID-19 syndrome. Nat. Med. 2021;27(4):601–615. doi: 10.1038/s41591-021-01283-z. PubMed PMID: 33753937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yang M.L., Wang C.T., Yang S.J., Leu C.H., Chen S.H., Wu C.L., Shiau A.L. IL-6 ameliorates acute lung injury in influenza virus infection. Sci. Rep. 2017;7:43829. doi: 10.1038/srep43829. PubMed PMID: 28262742; PMCID: PMC5338329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rosas I.O., Bräu N., Waters M., Go R.C., Hunter B.D., Bhagani S., Skiest D., Aziz M.S., Cooper N., Douglas I.S., Savic S., Youngstein T., Del Sorbo L., Cubillo Gracian A., De La Zerda D.J., Ustianowski A., Bao M., Dimonaco S., Graham E., Matharu B., Spotswood H., Tsai L., Malhotra A. Tocilizumab in Hospitalized patients with severe COVID-19 pneumonia. New Engl. J. Med. 2021;384(16):1503–1516. doi: 10.1056/NEJMoa2028700. PubMed PMID: 33631066; PMCID: PMC7953459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Stone J.H., Frigault M.J., Serling-Boyd N.J., Fernandes A.D., Harvey L., Foulkes A.S., Horick N.K., Healy B.C., Shah R., Bensaci A.M., Woolley A.E., Nikiforow S., Lin N., Sagar M., Schrager H., Huckins D.S., Axelrod M., Pincus M.D., Fleisher J., Sacks C.A., Dougan M., North C.M., Halvorsen Y.D., Thurber T.K., Dagher Z., Scherer A., Wallwork R.S., Kim A.Y., Schoenfeld S., Sen P., Neilan T.G., Perugino C.A., Unizony S.H., Collier D.S., Matza M.A., Yinh J.M., Bowman K.A., Meyerowitz E., Zafar A., Drobni Z.D., Bolster M.B., Kohler M., D’Silva K.M., Dau J., Lockwood M.M., Cubbison C., Weber B.N., Mansour M.K., Investigators B.B.T.T. Efficacy of tocilizumab in patients hospitalized with COVID-19. N. Engl. J. Med. 2020;383(24):2333–2344. doi: 10.1056/NEJMoa2028836. PubMed PMID: 33085857; PMCID: PMC7646626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hurt A.C., Wheatley A.K. Neutralizing antibody therapeutics for COVID-19. Viruses. 2021;13(4) doi: 10.3390/v13040628. PubMed PMID: 33916927; PMCID: PMC8067572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Walls A.C., Xiong X., Park Y.J., Tortorici M.A., Snijder J., Quispe J., Cameroni E., Gopal R., Dai M., Lanzavecchia A., Zambon M., Rey F.A., Corti D., Veesler D. Unexpected receptor functional mimicry elucidates activation of coronavirus fusion. Cell. 2020;183(6):1732. doi: 10.1016/j.cell.2020.11.031. PubMed PMID: 33306956; PMCID: PMC7834669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wrapp D., De Vlieger D., Corbett K.S., Torres G.M., Wang N., Van Breedam W., Roose K., van Schie L., Hoffmann M., Pöhlmann S., Graham B.S., Callewaert N., Schepens B., Saelens X., McLellan J.S., Team V-CC-R Structural basis for potent neutralization of betacoronaviruses by single-domain camelid antibodies. Cell. 2020;181(6):1436–1441. doi: 10.1016/j.cell.2020.05.047. PubMed PMID: 32531248; PMCID: PMC7289117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Saphire E.O., Schendel S.L., Fusco M.L., Gangavarapu K., Gunn B.M., Wec A.Z., Halfmann P.J., Brannan J.M., Herbert A.S., Qiu X., Wagh K., He S., Giorgi E.E., Theiler J., Pommert K.B.J., Krause T.B., Turner H.L., Murin C.D., Pallesen J., Davidson E., Ahmed R., Aman M.J., Bukreyev A., Burton D.R., Crowe J.E., Davis C.W., Georgiou G., Krammer F., Kyratsous C.A., Lai J.R., Nykiforuk C., Pauly M.H., Rijal P., Takada A., Townsend A.R., Volchkov V., Walker L.M., Wang C.I., Zeitlin L., Doranz B.J., Ward A.B., Korber B., Kobinger G.P., Andersen K.G., Kawaoka Y., Alter G., Chandran K., Dye J.M., Consortium V.H.F.I. Systematic analysis of monoclonal antibodies against ebola virus GP defines features that contribute to protection. Cell. 2018;174(4):938–952. doi: 10.1016/j.cell.2018.07.033. PubMed PMID: 30096313; PMCID: PMC6102396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wang P., Nair M.S., Liu L., Iketani S., Luo Y., Guo Y., Wang M., Yu J., Zhang B., Kwong P.D., Graham B.S., Mascola J.R., Chang J.Y., Yin M.T., Sobieszczyk M., Kyratsous C.A., Shapiro L., Sheng Z., Huang Y., Ho D.D. Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7. Nature. 2021;593(7857):130–135. doi: 10.1038/s41586-021-03398-2. PubMed PMID: 33684923. [DOI] [PubMed] [Google Scholar]
- 132.Planas D., Veyer D., Baidaliuk A., Staropoli I., Guivel-Benhassine F., Rajah M.M., Planchais C., Porrot F., Robillard N., Puech J., Prot M., Gallais F., Gantner P., Velay A., Le Guen J., Kassis-Chikhani N., Edriss D., Belec L., Seve A., Courtellemont L., Péré H., Hocqueloux L., Fafi-Kremer S., Prazuck T., Mouquet H., Bruel T., Simon-Lorière E., Rey F.A., Schwartz O. Reduced sensitivity of SARS-CoV-2 vAriant Delta to antibody neutralization. Nature. 2021;596:276–280. doi: 10.1038/s41586-021-03777-9. PubMed PMID: 34237773. [DOI] [PubMed] [Google Scholar]
- 133.Dougan M., Nirula A., Azizad M., Mocherla B., Gottlieb R.L., Chen P., Hebert C., Perry R., Boscia J., Heller B., Morris J., Crystal C., Igbinadolor A., Huhn G., Cardona J., Shawa I., Kumar P., Adams A.C., Van Naarden J., Custer K.L., Durante M., Oakley G., Schade A.E., Holzer T.R., Ebert P.J., Higgs R.E., Kallewaard N.L., Sabo J., Patel D.R., Dabora M.C., Klekotka P., Shen L., Skovronsky D.M., Investigators B.- Bamlanivimab plus etesevimab in mild or moderate COVID-19. N. Engl. J. Med. 2021 doi: 10.1056/NEJMoa2102685. PubMed PMID: 34260849; PMCID: PMC8314785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gottlieb R.L., Nirula A., Chen P., Boscia J., Heller B., Morris J., Huhn G., Cardona J., Mocherla B., Stosor V., Shawa I., Kumar P., Adams A.C., Van Naarden J., Custer K.L., Durante M., Oakley G., Schade A.E., Holzer T.R., Ebert P.J., Higgs R.E., Kallewaard N.L., Sabo J., Patel D.R., Klekotka P., Shen L., Skovronsky D.M. Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial. JAMA. 2021;325(7):632–644. doi: 10.1001/jama.2021.0202. PubMed PMID: 33475701; PMCID: PMC7821080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Weinreich D.M., Sivapalasingam S., Norton T., Ali S., Gao H., Bhore R., Musser B.J., Soo Y., Rofail D., Im J., Perry C., Pan C., Hosain R., Mahmood A., Davis J.D., Turner K.C., Hooper A.T., Hamilton J.D., Baum A., Kyratsous C.A., Kim Y., Cook A., Kampman W., Kohli A., Sachdeva Y., Graber X., Kowal B., DiCioccio T., Stahl N., Lipsich L., Braunstein N., Herman G., Yancopoulos G.D., Investigators T. REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19. N. Engl. J. Med. 2021;384(3):238–251. doi: 10.1056/NEJMoa2035002. PubMed PMID: 33332778; PMCID: PMC7781102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lescure F.X., Honda H., Fowler R.A., Lazar J.S., Shi G., Wung P., Patel N., Hagino O. SC-GS. Sarilumab in patients admitted to hospital with severe or critical COVID-19: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. Respir. Med. 2021;9(5):522–532. doi: 10.1016/S2213-2600(21)00099-0. PubMed PMID: 33676590; PMCID: PMC8078879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cremer P.C., Abbate A., Hudock K., McWilliams C., Mehta J., Chang S.Y., Sheng C.C., Van Tassell B., Bonaventura A., Vecchié A., Carey B., Wang Q., Wolski K.E., Rajendram P., Duggal A., Wang T.S., Paolini J.F., Trapnell B.C., group M-Cs Mavrilimumab in patients with severe COVID-19 pneumonia and systemic hyperinflammation (MASH-COVID): an investigator initiated, multicentre, double-blind, randomised, placebo-controlled trial. Lancet Rheumatol. 2021;3(6):410. doi: 10.1016/S2665-9913(21)00070-9. PubMed PMID: 33754144; PMCID: PMC7969143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Meyers J.I., Gray M., Kuklinski W., Johnson L.B., Snow C.D., Black W.C., Partin K.M., Foy B.D. Characterization of the target of ivermectin, the glutamate-gated chloride channel, from Anopheles gambiae. J. Exp. Biol. 2015;218(Pt 10):1478–1486. doi: 10.1242/jeb.118570. PubMed PMID: 25994631; PMCID: PMC4448665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.McCavera S., Rogers A.T., Yates D.M., Woods D.J., Wolstenholme A.J. An ivermectin-sensitive glutamate-gated chloride channel from the parasitic nematode Haemonchus contortus. Mol. Pharmacol. 2009;75(6):1347–1355. doi: 10.1124/mol.108.053363. PubMed PMID: 19336526; PMCID: PMC2684884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Moreno Y., Nabhan J.F., Solomon J., Mackenzie C.D., Geary T.G. Ivermectin disrupts the function of the excretory-secretory apparatus in microfilariae of Brugia malayi. Proc. Natl. Acad. Sci. U.S.A. 2010;107(46):20120–20125. doi: 10.1073/pnas.1011983107. PubMed PMID: 21041637; PMCID: PMC2993382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wagstaff K.M., Sivakumaran H., Heaton S.M., Harrich D., Jans D.A. Ivermectin is a specific inhibitor of importin α/β-mediated nuclear import able to inhibit replication of HIV-1 and dengue virus. Biochem J. 2012;443(3):851–856. doi: 10.1042/BJ20120150. PubMed PMID: 22417684; PMCID: PMC3327999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.King C.R., Tessier T.M., Dodge M.J., Weinberg J.B., Mymryk J.S. Inhibition of human adenovirus replication by the importin α/β1 nuclear import inhibitor ivermectin. J. Virol. 2020;94(18) doi: 10.1128/JVI.00710-20. PubMed PMID: 32641484; PMCID: PMC7459547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yang S.N.Y., Atkinson S.C., Wang C., Lee A., Bogoyevitch M.A., Borg N.A., Jans D.A. The broad spectrum antiviral ivermectin targets the host nuclear transport importin α/β1 heterodimer. Antivir. Res. 2020;177 doi: 10.1016/j.antiviral.2020.104760. PubMed PMID: 32135219. [DOI] [PubMed] [Google Scholar]
- 144.Caly L., Druce J.D., Catton M.G., Jans D.A., Wagstaff K.M. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antivir. Res. 2020;178 doi: 10.1016/j.antiviral.2020.104787. Epub 20200403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sharma K., Åkerström S., Sharma A.K., Chow V.T., Teow S., Abrenica B., Booth S.A., Booth T.F., Mirazimi A., Lal S.K. SARS-CoV 9b protein diffuses into nucleus, undergoes active Crm1 mediated nucleocytoplasmic export and triggers apoptosis when retained in the nucleus. PLoS One. 2011;6(5):19436. doi: 10.1371/journal.pone.0019436. PubMed PMID: 21637748; PMCID: PMC3103500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Timani K.A., Liao Q., Ye L., Zeng Y., Liu J., Zheng Y., Yang X., Lingbao K., Gao J., Zhu Y. Nuclear/nucleolar localization properties of C-terminal nucleocapsid protein of SARS coronavirus. Virus Res. 2005;114(1–2):23–34. doi: 10.1016/j.virusres.2005.05.007. PubMed PMID: 15992957; PMCID: PMC7114095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sen Gupta P.S., Biswal S., Panda S.K., Ray A.K., Rana M.K. Binding mechanism and structural insights into the identified protein target of COVID-19 and importin-α with. J. Biomol. Struct. Dyn. 2020:1–10. doi: 10.1080/07391102.2020.1839564. PubMed PMID: 33111618; PMCID: PMC7605516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mahmud R., Rahman M.M., Alam I., Ahmed K.G.U., Kabir A.K.M.H., Sayeed S.K.J.B., Rassel M.A., Monayem F.B., Islam M.S., Islam M.M., Barshan A.D., Hoque M.M., Mallik M.U., Yusuf M.A., Hossain M.Z. Ivermectin in combination with doxycycline for treating COVID-19 symptoms: a randomized trial. J. Int Med Res. 2021;49(5) doi: 10.1177/03000605211013550. PubMed PMID: 33983065; PMCID: PMC8127799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Dowling P. Pharmacogenetics: it’s not just about ivermectin in collies. Can. Vet. J. 2006;47(12):1165–1168. PubMed PMID: 17217086; PMCID: PMC1636591. [PMC free article] [PubMed] [Google Scholar]
- 150.Baudou E., Lespine A., Durrieu G., André F., Gandia P., Durand C., Cunat S. Serious ivermectin toxicity and human ABCB1 nonsense mutations. New Engl. J. Med. 2020;383(8):787–789. doi: 10.1056/NEJMc1917344. [DOI] [PubMed] [Google Scholar]
- 151.Mealey K.L., Bentjen S.A., Gay J.M., Cantor G.H. Ivermectin sensitivity in collies is associated with a deletion mutation of the mdr1 gene. Pharmacogenetics. 2001;11(8):727–733. doi: 10.1097/00008571-200111000-00012. [DOI] [PubMed] [Google Scholar]
- 152.Ben-Zvi I., Kivity S., Langevitz P., Shoenfeld Y. Hydroxychloroquine: from malaria to autoimmunity. Clin. Rev. Allergy Immunol. 2012;42(2):145–153. doi: 10.1007/s12016-010-8243-x. PubMed PMID: 21221847; PMCID: PMC7091063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Krogstad D.J., Schlesinger P.H., Gluzman I.Y. Antimalarials increase vesicle pH in Plasmodium falciparum. J. Cell Biol. 1985;101(6):2302–2309. doi: 10.1083/jcb.101.6.2302. PubMed PMID: 3905824; PMCID: PMC2113995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Willis R., Seif A.M., McGwin G., Martinez-Martinez L.A., González E.B., Dang N., Papalardo E., Liu J., Vilá L.M., Reveille J.D., Alarcón G.S., Pierangeli S.S. Effect of hydroxychloroquine treatment on pro-inflammatory cytokines and disease activity in SLE patients: data from LUMINA (LXXV), a multiethnic US cohort. Lupus. 2012;21(8):830–835. doi: 10.1177/0961203312437270. PubMed PMID: 22343096; PMCID: PMC3808832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kuznik A., Bencina M., Svajger U., Jeras M., Rozman B., Jerala R. Mechanism of endosomal TLR inhibition by antimalarial drugs and imidazoquinolines. J. Immunol. 2011;186(8):4794–4804. doi: 10.4049/jimmunol.1000702. PubMed PMID: 21398612. [DOI] [PubMed] [Google Scholar]
- 156.An N., Chen Y., Wang C., Yang C., Wu Z.H., Xue J., Ye L., Wang S., Liu H.F., Pan Q. Chloroquine autophagic inhibition rebalances Th17/Treg-mediated immunity and ameliorates systemic lupus erythematosus. Cell Physiol. Biochem. 2017;44(1):412–422. doi: 10.1159/000484955. PubMed PMID: 29141242. [DOI] [PubMed] [Google Scholar]
- 157.Liang N., Zhong Y., Zhou J., Liu B., Lu R., Guan Y., Wang Q., Liang C., He Y., Zhou Y., Song J. Immunosuppressive effects of hydroxychloroquine and artemisinin combination therapy via the nuclear factor-κB signaling pathway in lupus nephritis mice. Exp. Ther. Med. 2018;15(3):2436–2442. doi: 10.3892/etm.2018.5708. PubMed PMID: 29456648; PMCID: PMC5795753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Keyaerts E., Vijgen L., Maes P., Neyts J., Van, Ranst M. In vitro inhibition of severe acute respiratory syndrome coronavirus by chloroquine. Biochem Biophys. Res. Commun. 2004;323(1):264–268. doi: 10.1016/j.bbrc.2004.08.085. PubMed PMID: 15351731; PMCID: PMC7092815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Liu J., Cao R., Xu M., Wang X., Zhang H., Hu H., Li Y., Hu Z., Zhong W., Wang M. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020;6(1):16. doi: 10.1038/s41421-020-0156-0. PubMed PMID: 33731711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Bäck A.T., Lundkvist A. Dengue viruses - an overview. Infect. Ecol. Epidemiol. 2013;3 doi: 10.3402/iee.v3i0.19839. PubMed PMID: 24003364; PMCID: PMC3759171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wang L.F., Lin Y.S., Huang N.C., Yu C.Y., Tsai W.L., Chen J.J., Kubota T., Matsuoka M., Chen S.R., Yang C.S., Lu R.W., Lin Y.L., Chang T.H. Hydroxychloroquine-inhibited dengue virus is associated with host defense machinery. J. Interferon Cytokine Res. 2015;35(3):143–156. doi: 10.1089/jir.2014.0038. PubMed PMID: 25321315; PMCID: PMC4350140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Paton N.I., Goodall R.L., Dunn D.T., Franzen S., Collaco-Moraes Y., Gazzard B.G., Williams I.G., Fisher M.J., Winston A., Fox J., Orkin C., Herieka E.A., Ainsworth J.G., Post F.A., Wansbrough-Jones M., Kelleher P., Team H.T. Effects of hydroxychloroquine on immune activation and disease progression among HIV-infected patients not receiving antiretroviral therapy: a randomized controlled trial. JAMA. 2012;308(4):353–361. doi: 10.1001/jama.2012.6936. PubMed PMID: 22820788; PMCID: PMC3821003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Self W.H., Semler M.W., Leither L.M., Casey J.D., Angus D.C., Brower R.G., Chang S.Y., Collins S.P., Eppensteiner J.C., Filbin M.R., Files D.C., Gibbs K.W., Ginde A.A., Gong M.N., Harrell F.E., Hayden D.L., Hough C.L., Johnson N.J., Khan A., Lindsell C.J., Matthay M.A., Moss M., Park P.K., Rice T.W., Robinson B.R.H., Schoenfeld D.A., Shapiro N.I., Steingrub J.S., Ulysse C.A., Weissman A., Yealy D.M., Thompson B.T., Brown S.M., Steingrub J., Smithline H., Tiru B., Tidswell M., Kozikowski L., Thornton-Thompson S., De Souza L., Hou P., Baron R., Massaro A., Aisiku I., Fredenburgh L., Seethala R., Johnsky L., Riker R., Seder D., May T., Baumann M., Eldridge A., Lord C., Shapiro N., Talmor D., O’Mara T., Kirk C., Harrison K., Kurt L., Schermerhorn M., Banner-Goodspeed V., Boyle K., Dubosh N., Filbin M., Hibbert K., Parry B., Lavin-Parsons K., Pulido N., Lilley B., Lodenstein C., Margolin J., Brait K., Jones A., Galbraith J., Peacock R., Nandi U., Wachs T., Matthay M., Liu K., Kangelaris K., Wang R., Calfee C., Yee K., Hendey G., Chang S., Lim G., Qadir N., Tam A., Beutler R., Levitt J., Wilson J., Rogers A., Vojnik R., Roque J., Albertson T., Chenoweth J., Adams J., Pearson S., Juarez M., Almasri E., Fayed M., Hughes A., Hillard S., Huebinger R., Wang H., Vidales E., Patel B., Ginde A., Baduashvili A., McKeehan J., Finck L., Higgins C., Howell M., Douglas I., Haukoos J., Hiller T., Lyle C., Cupelo A., Caruso E., Camacho C., Gravitz S., Finigan J., Griesmer C., Park P., Hyzy R., Nelson K., McDonough K., Olbrich N., Williams M., Kapoor R., Nash J., Willig M., Ford H., Gardner-Gray J., Ramesh M., Moses M., Ng Gong M., Aboodi M., Asghar A., Amosu O., Torres M., Kaur S., Chen J.T., Hope A., Lopez B., Rosales K., Young You J., Mosier J., Hypes C., Natt B., Borg B., Salvagio Campbell E., Hite R.D., Hudock K., Cresie A., Alhasan F., Gomez-Arroyo J., Duggal A., Mehkri O., Hastings A., Sahoo D., Abi Fadel F., Gole S., Shaner V., Wimer A., Meli Y., King A., Terndrup T., Exline M., Pannu S., Robart E., Karow S., Hough C., Robinson B., Johnson N., Henning D., Campo M., Gundel S., Seghal S., Katsandres S., Dean S., Krol O., Jouzestani M., Huynh P., Yealy D., Scholl D., Adams P., McVerry B., Huang D., Angus D., Schooler J., Moore S., Files C., Miller C., Gibbs K., LaRose M., Flores L., Koehler L., Morse C., Sanders J., Langford C., Nanney K., MdalaGausi M., Yeboah P., Morris P., Sturgill J., Seif S., Cassity E., Dhar S., de Wit M., Mason J., Goodwin A., Hall G., Grady A., Chamberlain A., Brown S., Bledsoe J., Leither L., Peltan I., Starr N., Fergus M., Aston V., Montgomery Q., Smith R., Merrill M., Brown K., Armbruster B., Harris E., Middleton E., Paine R., Johnson S., Barrios M., Eppensteiner J., Limkakeng A., McGowan L., Porter T., Bouffler A., Leahy J.C., deBoisblanc B., Lammi M., Happel K., Lauto P., Self W., Casey J., Semler M., Collins S., Harrell F., Lindsell C., Rice T., Stubblefield W., Gray C., Johnson J., Roth M., Hays M., Torr D., Zakaria A., Schoenfeld D., Thompson T., Hayden D., Ringwood N., Oldmixon C., Ulysse C., Morse R., Muzikansky A., Fitzgerald L., Whitaker S., Lagakos A., Brower R., Reineck L., Aggarwal N., Bienstock K., Freemer M., Maclawiw M., Weinmann G., Morrison L., Gillespie M., Kryscio R., Brodie D., Zareba W., Rompalo A., Boeckh M., Parsons P., Christie J., Hall J., Horton N., Zoloth L., Dickert N., Diercks D. National heart Ln, and blood institute PETAL clinical trials network. effect of hydroxychloroquine on clinical status at 14 days in hospitalized patients with COVID-19: a randomized clinical trial. JAMA. 2020;324(21):2165–2176. doi: 10.1001/jama.2020.22240. PubMed PMID: 33165621; PMCID: PMC7653542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ou T., Mou H., Zhang L., Ojha A., Choe H., Farzan M. Hydroxychloroquine-mediated inhibition of SARS-CoV-2 entry is attenuated by TMPRSS2. PLoS Pathog. 2021;17(1) doi: 10.1371/journal.ppat.1009212. PubMed PMID: 33465165; PMCID: PMC7845965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Boulware D.R., Pullen M.F., Bangdiwala A.S., Pastick K.A., Lofgren S.M., Okafor E.C., Skipper C.P., Nascene A.A., Nicol M.R., Abassi M., Engen N.W., Cheng M.P., LaBar D., Lother S.A., MacKenzie L.J., Drobot G., Marten N., Zarychanski R., Kelly L.E., Schwartz I.S., McDonald E.G., Rajasingham R., Lee T.C., Hullsiek K.H. A randomized trial of hydroxychloroquine as postexposure prophylaxis for COVID-19. New Engl. J. Med. 2020;383(6):517–525. doi: 10.1056/NEJMoa2016638. PubMed PMID: 32492293; PMCID: PMC7289276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Qu F., Gu Y., Wang Q., He M., Zhou F., Sun J., Wang G., Peng Y. Metabolomic profiling to evaluate the efficacy of proxalutamide, a novel androgen receptor antagonist, in prostate cancer cells. Invest New Drugs. 2020;38(5):1292–1302. doi: 10.1007/s10637-020-00901-w. [DOI] [PubMed] [Google Scholar]
- 167.Heinlein C.A., Chang C. Androgen receptor in prostate cancer. Endocr. Rev. 2004;25(2):276–308. doi: 10.1210/er.2002-0032. [DOI] [PubMed] [Google Scholar]
- 168.Clinckemalie L., Spans L., Dubois V., Laurent M., Helsen C., Joniau S., Claessens F. Androgen regulation of the TMPRSS2 gene and the effect of a SNP in an androgen response element. Mol. Endocrinol. 2013;27(12):2028–2040. doi: 10.1210/me.2013-1098. PubMed PMID: 24109594; PMCID: PMC5426606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Okwan-Duodu D., Lim E.C., You S., Engman D.M. TMPRSS2 activity may mediate sex differences in COVID-19 severity. Signal Transduct. Target Ther. 2021;6(1):100. doi: 10.1038/s41392-021-00513-7. PubMed PMID: 33649313; PMCID: PMC7919249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Goren A., McCoy J., Wambier C.G., Vano-Galvan S., Shapiro J., Dhurat R., Washenik K., Lotti T. What does androgenetic alopecia have to do with COVID-19? An insight into a potential new therapy. Dermatol. Ther. 2020;33(4):13365. doi: 10.1111/dth.13365. PubMed PMID: 32237190; PMCID: PMC7228378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Goren A., Vaño-Galván S., Wambier C.G., McCoy J., Gomez-Zubiaur A., Moreno-Arrones O.M., Shapiro J., Sinclair R.D., Gold M.H., Kovacevic M., Mesinkovska N.A., Goldust M., Washenik K. A preliminary observation: Male pattern hair loss among hospitalized COVID-19 patients in Spain - a potential clue to the role of androgens in COVID-19 severity. J. Cosmet. Dermatol. 2020;19(7):1545–1547. doi: 10.1111/jocd.13443. PubMed PMID: 32301221. [DOI] [PubMed] [Google Scholar]
- 172.McCoy J., Wambier C.G., Vano-Galvan S., Shapiro J., Sinclair R., Ramos P.M., Washenik K., Andrade M., Herrera S., Goren A. Racial variations in COVID-19 deaths may be due to androgen receptor genetic variants associated with prostate cancer and androgenetic alopecia. Are anti-androgens a potential treatment for COVID-19? J. Cosmet. Dermatol. 2020;19(7):1542–1543. doi: 10.1111/jocd.13455. PubMed PMID: 32333494; PMCID: PMC7267367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wambier C.G., Goren A., Vaño-Galván S., Ramos P.M., Ossimetha A., Nau G., Herrera S., McCoy J. Androgen sensitivity gateway to COVID-19 disease severity. Drug Dev. Res. 2020;81(7):771–776. doi: 10.1002/ddr.21688. PubMed PMID: 32412125; PMCID: PMC7273095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wambier C.G., Vaño-Galván S., McCoy J., Gomez-Zubiaur A., Herrera S., Hermosa-Gelbard Á., Moreno-Arrones O.M., Jiménez-Gómez N., González-Cantero A., Fonda-Pascual P., Segurado-Miravalles G., Shapiro J., Pérez-García B., Goren A. Androgenetic alopecia present in the majority of patients hospitalized with COVID-19: the “Gabrin sign”. J. Am. Acad. Dermatol. 2020;83(2):680–682. doi: 10.1016/j.jaad.2020.05.079. PubMed PMID: 32446821; PMCID: PMC7242206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Montopoli M., Zumerle S., Vettor R., Rugge M., Zorzi M., Catapano C.V., Carbone G.M., Cavalli A., Pagano F., Ragazzi E., Prayer-Galetti T., Alimonti A. Androgen-deprivation therapies for prostate cancer and risk of infection by SARS-CoV-2: a population-based study (N = 4532) Ann. Oncol. 2020;31(8):1040–1045. doi: 10.1016/j.annonc.2020.04.479. PubMed PMID: 32387456; PMCID: PMC7202813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.McCoy J., Goren A., Cadegiani F.A., Vaño-Galván S., Kovacevic M., Situm M., Shapiro J., Sinclair R., Tosti A., Stanimirovic A., Fonseca D., Dorner E., Onety D.C., Zimerman R.A., Wambier C.G. Proxalutamide reduces the rate of hospitalization for COVID-19 male outpatients: a randomized double-blinded placebo-controlled trial. Front. Med. 2021;8 doi: 10.3389/fmed.2021.668698. PubMed PMID: 34350193; PMCID: PMC8326462. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 177.Cadegiani F.A., McCoy J., Gustavo Wambier C., Vaño-Galván S., Shapiro J., Tosti A., Zimerman R.A., Goren A. Proxalutamide significantly accelerates viral clearance and reduces time to clinical remission in patients with mild to moderate COVID-19: results from a randomized, double-blinded, placebo-controlled trial. Cureus. 2021;13(2):13492. doi: 10.7759/cureus.13492. PubMed PMID: 33633920; PMCID: PMC7899267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Hall M.D., Anderson J.M., Anderson A., Baker D., Bradner J., Brimacombe K.R., Campbell E.A., Corbett K.S., Carter K., Cherry S., Chiang L., Cihlar T., de Wit E., Denison M., Disney M., Fletcher C.V., Ford-Scheimer S.L., Götte M., Grossman A.C., Hayden F.G., Hazuda D.J., Lanteri C.A., Marston H., Mesecar A.D., Moore S., Nwankwo J.O., O’Rear J., Painter G., Singh Saikatendu K., Schiffer C.A., Sheahan T.P., Shi P.Y., Smyth H.D., Sofia M.J., Weetall M., Weller S.K., Whitley R., Fauci A.S., Austin C.P., Collins F.S., Conley A.J., Davis M.I. Report of the national institutes of health SARS-CoV-2 antiviral therapeutics summit. J. Infect. Dis. 2021;224(Supplement_1):S1–S21. doi: 10.1093/infdis/jiab305. PubMed PMID: 34111271; PMCID: PMC8280938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Misson J., Clark W., Kendall M.J. Therapeutic advances: protease inhibitors for the treatment of HIV-1 infection. J. Clin. Pharm. Ther. 1997;22(2):109–117. doi: 10.1111/j.1365-2710.1997.tb00004.x. [DOI] [PubMed] [Google Scholar]
- 180.Kowdley K.V., Gordon S.C., Reddy K.R., Rossaro L., Bernstein D.E., Lawitz E., Shiffman M.L., Schiff E., Ghalib R., Ryan M., Rustgi V., Chojkier M., Herring R., Di Bisceglie A.M., Pockros P.J., Subramanian G.M., An D., Svarovskaia E., Hyland R.H., Pang P.S., Symonds W.T., McHutchison J.G., Muir A.J., Pound D., Fried M.W., Investigators I.- Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. New Engl. J. Med. 2014;370(20):1879–1888. doi: 10.1056/NEJMoa1402355. PubMed PMID: 24720702. [DOI] [PubMed] [Google Scholar]
- 181.Jacobson K.B., Rao M., Bonilla H., Subramanian A., Hack I., Madrigal M., Singh U., Jagannathan P., Grant P. Patients with uncomplicated coronavirus disease 2019 (COVID-19) have long-term persistent symptoms and functional impairment similar to patients with severe COVID-19: a cautionary tale during a global pandemic. Clin. Infect. Dis. 2021;73(3):e826–e829. doi: 10.1093/cid/ciab103. PubMed PMID: 33624010; PMCID: PMC7929039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Chia J., Chia A., Voeller M., Lee T., Chang R. Acute enterovirus infection followed by myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and viral persistence. J. Clin. Pathol. 2010;63(2):165–168. doi: 10.1136/jcp.2009.070466. PubMed PMID: 19828908. [DOI] [PubMed] [Google Scholar]
- 183.Shikova E., Reshkova V., Kumanova А, Raleva S., Alexandrova D., Capo N., Murovska M., (EUROMENE) ENoMC Cytomegalovirus, Epstein-Barr virus, and human herpesvirus-6 infections in patients with myalgic еncephalomyelitis/chronic fatigue syndrome. J. Med. Virol. 2020 doi: 10.1002/jmv.25744. PubMed PMID: 32129496; PMCID: PMC7687071. [DOI] [PMC free article] [PubMed] [Google Scholar]