Abstract
Objective
Our objective was to review skin prick allergy testing (SPAT) results in patients with symptomatic rhinitis in an Irish population.
Methods
A fifteen-year retrospective review of our database of symptomatic patients with rhinitis was performed. All patients who had SPAT performed during this interval were included. Data was analysed in terms of demographics and dominant allergens.
Results
1158 patients were included. 617 Females vs 541 Males. Age range five to eighty-five years old. Mean age thirty-four years. 49% of our patients tested positive to at least one aeroallergen. The most common allergens were dust mites (23%) and timothy grass (22%). Patients born during the Irish pollen season (April–July) were between 5 and 7 times more likely to be sensitive to timothy and ryegrass pollens compared to others tested. 241 patients had both SPAT and serum allergen specific IgE testing (SASIgET) performed; positive results were consistent between both groups.
Conclusion
Results demonstrated that half of our patients with symptomatic rhinitis had allergen sensitisation. Dust mites and grass were the main allergens in our area. Our nurse led clinic has allowed efficient patient education and the development of a unique Irish SPAT database. Retesting a patient with a known allergy test result it is not indicated.
Keywords: Allergic rhinitis, Skin prick allergy test, Dust mites, Grass allergy, Rhinosinusitis, Allergen specific IgE
Introduction
Allergic rhinitis (AR) can be defined, as per Allergic Rhinitis and its Impact on Asthma (ARIA) Guidelines by one or more of the following nasal symptoms: rhinorrhoea, nasal obstruction, nasal itching and sneezing.1 Also, symptoms of eye itchiness, fatigue, disturbance of sleep pattern and general reduction in quality of life are reported by affected patients.2, 3, 4, 5
AR can be classified as intermittent (e.g. pollens) or persistent (e.g. dust mites).1,6 Intermittent AR is usually caused by pollens from trees, grasses and weeds and is dependent upon the geographic area, degree of urbanization and pollination periods for certain types of plants. Persistent AR usually reflects a reaction to indoor allergens such as dust mites, mould spores or animal dander.
Symptomatically, AR can be mild (symptoms are present but not interfering with the quality of life) or moderate-severe (affecting the patients' quality of life).1,6 The severity can manifest in exacerbations of asthma symptoms, sleep disturbance, impairment of daily routine, sport or school activities. Severity fluctuates from year to year and from season to season in relation to allergen exposure. Most patients seeking medical care present with symptoms in the moderate-to-severe spectrum of AR symptoms.7, 8, 9 Symptom control is often poor due to a lack of patient knowledge, poor compliance and technique, particularly around the use of topical nasal medications.8
The diagnosis of AR is often evident from clinical history and examination, but for many patients a correlation with the triggering allergen can be challenging to recognize. In these patients, further investigation with an allergen-specific immunoglobulin E (IgE) test is needed. Allergen identification allows for patient education, avoidance measures and/or allergen immunotherapy in refractory cases.8,10,11
The symptoms exhibited in AR are present after being exposed to a sensitizing allergen and subsequent production of allergen specific IgE. It is a Type Ⅰ Gel and Coombs hypersensitivity reaction. The allergic response consists of two phases. An immediate phase peaks at 15–30 min after allergen exposure. This corresponds to mast cell degranulation and inflammatory mediators release. The second phase peaks at 6–12 h after exposure. This corresponds to infiltration of the nasal tissues by eosinophils, basophils and other inflammatory cells. Nonspecific nasal hyper-responsiveness can result due to mucosal inflammation, priming to the precipitating allergen as well as to other stimuli (allergenic and nonallergenic in nature).12,13
Several risk factors have been linked to an increased risk of developing AR. These include family history of atopy, male sex, birth during the pollen months, early use of antibiotics, parental smoking in the first year of life and early exposure to indoor allergens, such as dust mites.14,15 It is thought that the presence of each of these factors is associated with a positive likelihood ratio (from three to five) for a diagnosis of AR.16
The prevalence of AR has increased worldwide in the last 40 years.9,17, 18, 19, 20 AR is the most common form of non-infectious rhinitis, affecting up to 500 million people worldwide or approximately 20% of the population, 10–30% of all adults and up to 40% of children.18,19,21 Being a disease that is increasing globally, an understanding of dominant allergens has become crucial in its management and treatment. Frequent comorbidities such as asthma, eczema, food allergies and rhinosinusitis add to the clinical and financial burden of the disease.
The association between AR and asthma was recognised over two centuries ago. Significantly up to 40% of patients with a diagnosis of AR also have a diagnosis of asthma.7,10 Furthermore, 80% of people who have asthma will also be affected by AR. These patients reported increased usage of their asthma controlling medication if they did not manage their rhinitis appropriately.5,22 This is of particular relevance to the Irish population, having the fourth highest prevalence of asthma in the World.21 Galway is a thriving cultural city located on the west coast of Ireland. A buzzing cosmopolitan city centre leads to a popular seaside destination and beyond to a rugged rural and agricultural backdrop. Tourism and agriculture are important industries with a catchment population for this study of approximately 400,000.
Investigations for AR centre on determining the allergen specific IgE sensitivity. This can be performed by skin or haematology testing techniques, depending on the availability and setting. Skin prick/percutaneous (SPAT) or intradermal technique (IDT) are the two main testing procedures used on the patient's skin. IDT is usually only carried out when SPAT is negative but the suspicious of AR persists. Nasal challenges for specific aeroallergens are undertaken particularly when considering specific allergen immunotherapy.
Introducing an extract of the suspected allergen percutaneously induces an IgE hypersensitivity reaction in the skin. This is termed ‘wheal-and-flare reaction', which is characterised by an irregular blanched elevated wheal surrounded by an area of erythema, which appears within 15–20 min after the injection of an allergen.
It assesses the specific allergen immediate IgE-mediated sensitivity.23 Results are measured in mm, with positive results measuring more than 3 mm.
Once the diagnosis of AR is made, treatment options needs to be discussed with the patient and family. Treatment consists in allergen avoidance methods, pharmacological therapy and depends on symptoms severity and seasonal variation. For most patients, nasal saline irrigation in conjunction with an intranasal corticosteroid, and or oral H1-antihistaminic medication, is encouraged and is usually enough to control the disease. Education and technique is particularly important when prescribing topical nasal medication.8 Leukotriene-receptor antagonists and allergen immunotherapy administered subcutaneously, or sublingually are the next steps in poorly controlled symptoms but should be considered on an individual basis.24, 25, 26, 27 In recalcitrant cases a variety of surgical procedures mainly to the inferior turbinates can be undertaken (e.g. coblation, laser, debridement, cryotherapy) as an adjunct to medical treatment.
AR is one of the most underestimated diseases in terms of severity, lifestyle impact and treatment costs. AR is in fact a global health problem and is associated with significant economic burden and impaired quality of life.2, 3, 4, 5,21,28,29 AR symptoms are reflected by working and school absenteeism days, decrease in productivity and increased psychosocial burden on these patients.2, 3, 4, 5 In a recent UK study patients reported 4.1 days per year AR-related workplace absenteeism.5 The economic costs are huge on both patient and the health system. A 2014 analysis from The European Union determined that avoidable indirect costs per untreated patient with AR per year to be €2405.28
Our overall objective was to review the demographics and the main sensitizing allergens of symptomatic AR patients tested with SPAT in our region, the West of Ireland.
Methods
We undertook a retrospective review of all patients symptomatic for rhinitis, who presented at our nurse led allergy clinic between January 2003 and December 2018. All patients were initially seen in the Otorhinolaryngology (ORL) outpatients by an ORL clinician, who referred the patients for SPAT, based on the clinical suspicion of AR.
SPAT is preferred at our institution due to its simplicity, rapidity of performance and ability to counsel patients at the time of testing. A standard 20-aeroallergen kit test with positive (histamine) and negative (saline) control was used.
The 20 allergens tested were: dog, cat, horse, weed mix, mugworth (Artemisia vulgaris), tree mix 1 (early blossom), tree mix 2 (mid blossom), Aspergillus fumigates, ash, oak, maize, Timothy grass, Ryegrass, Cladosporium Herbarum, wheat, grass mix, Candida, barley, house dust mite Der p 1 & 2.
Each allergen was introduced with an individual sterile lancet into the superficial epidermis of the patients arm. Results of the SPAT were read and measured in mm after 20 min. These results were recorded in terms of size and the severity of the reaction. Severity was determined visually based on the degree of wheal and flare reaction to a specific allergen, using comparison of the reaction to the positive control as a baseline. A 3 mm or larger wheel was considered positive. A single specialist nurse performed the tests and recorded the results in our protected database saved on the hospital system. No severe adverse reactions were recorded during the fifteen years of testing. All patients were counselled about their sensitised allergen/s at the time of the result; oral and written allergen avoidance advice was provided. Data was analysed for prevalence in terms of demographics, geographic location and dominant allergens. We also compared the results with the Serum allergen specific IgE testing (SASIgET) or previous SPAT testing when available. Some of our patients also had SASIgET via Enzyme-linked immunosorbent assay (ELISA) prior to our skin testing.
Data was processed using Statistical Package for the Social Science (SPSS).
Ethical approval of our study was granted by Galway University Hospital Ethics Committee.
Results
1158 patients had SPAT performed during this fifteen-year interval. 617 females and 541 males were tested. Age ranged between five and eighty-five years with a mean of thirty-four years. Patients younger than ten years of age represented 8% of all our subjects.
On average eighty patients were tested per year over the fifteen year period.
Our results revealed that 49% of the patients showed a positive reaction to at least one tested allergen.
On analysis of the positive results, 56% males tested positive compared to 44% females. We observed that males appeared to have more severe (61% vs 40%) or very severe reaction (67% vs 33%) to house dust mites compared to their female counterparts. Over our study period, there were eighteen patients who had repeated SPAT. This was performed on average at 2.7 years after the initial test. All positive results were the same on both occasions. There was no clear reason for repeat SPAT testing recorded and this weakness has been corrected on our data base.
Twenty-one percent of patients (241 cases) also underwent SASIgET testing prior to SPAT. We observed that positive tests correlated and were consistent between both groups.
The most frequently encountered allergens in our population are dust mites and grass (Table 1).
Table 1.
Percentages of the main positive allergens in our study population.
| Allergen | Positive (%) |
|---|---|
| House dust mites | 23 |
| Timothy grass | 22 |
| Dog | 17 |
| Cat | 15 |
Seventy-five percent of patients with a positive SPAT result lived in an urban location. House dust mites was the most common allergen across all tested locations. One region (West Meath) had a high prevalence of barley sensitisation (19%). Barley is the primary crop propagated in that region, primarily used as malt for alcoholic beverages.
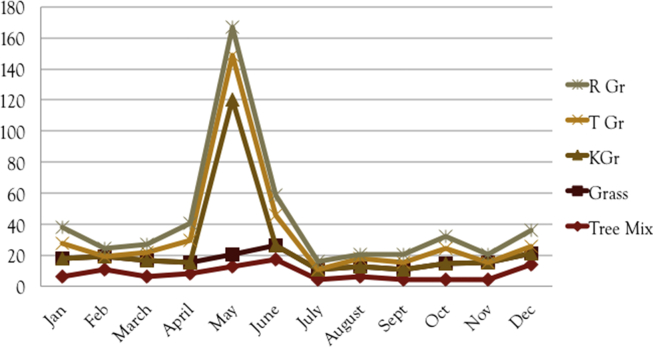
Interestingly, patients born during the Irish pollen season (April–July) were between 5 and 7 times more likely to be sensitive to timothy and ryegrass pollens compared to others tested (Fig. 1).
Figure 1.
The number of positive patients to specific grass allergens in respect to their birth month.
Discussion
The prevalence of AR is increasing worldwide.21 Implementation of clinical guidelines and focused history taking leads to improved referral efficiency. Our findings might also reflect an increase degree of urbanization and pollution implicit on our island, as Ireland moves from a more agricultural background to an industrial future.
The World Health Organization (WHO) estimated in 2016 that outdoor and indoor ambient air pollution kills 7 million people annualy and is affecting all regions of the world. Approximately 90% of people breathe air that is not compliant with WHO Air Quality Guidelines.30 20% of non-infectious deaths worldwide are from respiratory illnesses and cancers related to exposure to fine particulate matter (PM2.5), the most health-harmful air pollutant.31
Allergens concentrations are frequently influenced by temperature, humidity, sunlight and air pollutants.32 House dust mite is a particular pest, preferring humid environments; it produces powerful allergens secondary to skin casts, secretions and faecal material.31, 32, 33 Most modern homes probably have detectable levels of house dust mites and their allergy producing fragments.34
To our knowledge, this is the first study to investigate SPAT/AR in an adult and paediatric population in Ireland. Our focus was to evaluate the population in the Galway and surrounding region. Our study is different in many ways to previous national studies.35,36 Duggan et al found that the severity of AR in schoolchildren from the Cork region (southern Ireland) was increasing overtime and as children grew older.35 They have also found that AR symptoms were equally distributed among sexes. Grace et al have investigated the prevalence of AR among adult male athletes from South-West Ireland.36 In their group 27% were found to have allergic rhino-conjunctivitis, with the most common positive tested allergens being house dust mites and grass, which are similar to our findings from West.
In terms of variation of results across different subpopulation, we tested across several areas: age, gender, month of birth and geographical location.
Our results revealed that the age group 10–20 is by far the most tested one for symptomatic AR. If this is due to adolescents and young adults being conscious of their symptoms and reporting them more often or if hormones play a role at this age it is yet to be investigated.
Only 8% of our population was represented by children younger than ten years of age. A percentage of young children with allergies are dealt with directly by our local paediatric department, who perform their own allergy assessments and only refer to ORL if surgical input is anticipated. This probably accounts for the lower numbers of younger children in our cohort.
House dust mites and grass are the two main allergens encountered in our area and these are in keeping with other reports in the literature.36, 37, 38 House dust mites was consistently found over the locations and years studied. One must also consider the lack of variation between what is now termed rural and urban Ireland as there is a common exposure to most allergens across both areas.35 Interestingly, a single region (West Meath) had a high prevalence of barley sensitisation, corresponding to the fact that 30% of all Irish barley production is from this region.
We now encourage local and regional testing which would help clinicians to become more familiar with the local common allergens. House dust is comprised of multiple allergens, including dust mites, cockroach, pet dander, outdoors pollens, moulds, bacteria, textile fibres and decomposing insects. Specific, purified components of house dust are tested in the various kits available. We must take note of the significance of the main allergens identified as relevant in our area: house dust mites, timothy grass, cat and dog dander. House dust mites represents almost half (42%) of the allergens in our area.
The association of higher pollen count season (April to July) with birth months although controversial has been described previously in the literature.39,40 Our symptomatic patients also showed this type of distribution. In their study Duggan et al demonstrated a shift in high AR prevalence from the winter/spring of year 2002 to late spring/early summer of 2007.35 This change represents a shift from an infection to an allergic cause, either due to climate change or pollution.
Developing an unique geographic map of allergens and their seasonal variation helps developing local policies for testing and management of AR. Testing should be carried out if initial treatment fails to control the symptoms, and there is still a high suspicious of AR after ruling out other causes. It may be possible to run a smaller range of skin testing on some patients based on focused history taking. In intractable cases, testing is useful also for allergen avoidance measures and consideration of immunotherapy. SPAT should be considered as first line investigation, due to its cost-effectiveness and rapid results. A wider range of substances can be tested by this method. We do reinforce, through our findings that neither SPAT nor SASIgET tests need to be repeated overtime, unless there is clinical suspicion of a new allergen.
Local and economic factors may ultimately decide which form of allergy testing is undertaken at a particular unit; however, SPAT is a particularly good fit for our unit in Galway. We have developed a local allergen map to assist general practitioners and clinicians in managing patients with symptomatic AR.
Being a retrospective cohort study, we acknowledge that there are recognised limitations to this type of observational research. Retrospective studies provide inferior levels of evidence as compared to prospective studies. Ultimately, we were looking back at archived data from a 15-year period. Record keeping was not designed for the study; there were gaps and inconsistencies in data collection. It was impossible to control bias; this was not a population-based study, making most of the results observations rather than having proven statistical significance. Nevertheless, we propose that this was a unique opportunity to analyse, discuss and learn from 15 years of SPAT testing in the west of Ireland. No similar study exists in the literature and we have learnt, evolved and changed where necessary, with our ultimate aim to improve patient care and outcomes.
Conclusions
SPAT is a valuable tool. Forty-nine percent of our symptomatic allergic rhinitic population had documented evidence of being sensitised to at least one aeroallergen. House dust mites and timothy grass are the primary allergens in the West of Ireland. Being born during the pollen season shows a significant predisposition to intermittent AR, particularly to grass pollens. We discourage retesting patients with a known allergy test result. Patient education and allergen avoidance are fundamental tenets to the successful management of AR.
Credit author statement
A. Nae: Data curation; Software; Validation; Visualization; Resources; Writing – original draft; Writing – review & editing, K. Hinchion: Formal analysis; Methodology; Software; Resources, I.J. Keogh: Conceptualization; Project administration; Supervision; Validation; Visualization; Writing – review & editing.
Conflict of interest
Authors have no funding, financial or other conflicts of interest, including competing interests to disclose.
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Edited by Yu-Xin Fang
Footnotes
Peer review under responsibility of Chinese Medical Association.
References
- 1.Bousquet J., Van Cauwenberge P., Khaltaev N. Allergic rhinitis and its impact on asthma. J Allergy Clin Immunol. 2001;108:S147–S334. doi: 10.1067/mai.2001.118891. [DOI] [PubMed] [Google Scholar]
- 2.Bousquet J., Khaltaev N., Cruz A.A. Allergic rhinitis and its impact on asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen) Allergy. 2008;63(suppl 86):8–160. doi: 10.1111/j.1398-9995.2007.01620.x. [DOI] [PubMed] [Google Scholar]
- 3.Canonica G.W., Bousquet J., Mullol J., Scadding G.K., Virchow J.C. A survey of the burden of allergic rhinitis in Europe. Allergy. 2007;62(suppl 85):17–25. doi: 10.1111/j.1398-9995.2007.01549.x. [DOI] [PubMed] [Google Scholar]
- 4.Valovirta E., Myrseth S.E., Palkonen S. The voice of the patients: allergic rhinitis is not a trivial disease. Curr Opin Allergy Clin Immunol. 2008;8:1–9. doi: 10.1097/ACI.0b013e3282f3f42f. [DOI] [PubMed] [Google Scholar]
- 5.Price D., Scadding G., Ryan D. The hidden burden of adult allergic rhinitis: UK healthcare resource utilisation survey. Clin Transl Allergy. 2015;5:39. doi: 10.1186/s13601-015-0083-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bousquet J., Schünemann H.J., Samolinski B. Allergic rhinitis and its impact on asthma (ARIA): achievements in 10 years and future needs. J Allergy Clin Immunol. 2012;130:1049–1062. doi: 10.1016/j.jaci.2012.07.053. [DOI] [PubMed] [Google Scholar]
- 7.Bousquet J., Bodez T., Gehano P. Implementation of guidelines for allergic rhinitis in specialist practices. A randomized pragmatic controlled trial. Int Arch Allergy Immunol. 2009;150:75–82. doi: 10.1159/000210383. [DOI] [PubMed] [Google Scholar]
- 8.Corbett M., Garry S., Mc Gloughlin E., Hinchion K., Keogh I. Treating rhinitis with topical nasal sprays: patient knowledge, use and satisfaction. Ir Med J. 2020;113:154–157. [Google Scholar]
- 9.Jáuregui I., Dávila I., Sastre J. Validation of ARIA (allergic rhinitis and its impact on asthma) classification in a pediatric population: the PEDRIAL study. Pediatr Allergy Immunol. 2011;22:388–392. doi: 10.1111/j.1399-3038.2010.01108.x. [DOI] [PubMed] [Google Scholar]
- 10.Hamilton R.G., Adkinson N.F., Jr. 23. Clinical laboratory assessment of IgE-dependent hypersensitivity. J Allergy Clin Immunol. 2003;111:S687–S701. doi: 10.1067/mai.2003.123. [DOI] [PubMed] [Google Scholar]
- 11.Oppenheimer J., Nelson H.S. Skin testing. Ann Allergy Asthma Immunol. 2006;96(2):S6–S12. doi: 10.1016/s1081-1206(10)60895-2. [DOI] [PubMed] [Google Scholar]
- 12.Plaut M., Valentine M.D. Clinical practice. Allergic rhinitis. N Engl J Med. 2005;353:1934–1944. doi: 10.1056/NEJMcp044141. [DOI] [PubMed] [Google Scholar]
- 13.Barnes P.J. Pathophysiology of allergic inflammation. Immunol Rev. 2011;242:31–50. doi: 10.1111/j.1600-065X.2011.01020.x. [DOI] [PubMed] [Google Scholar]
- 14.Saulyte J., Regueira C., Montes-Martínez A., Khudyakov P., Takkouche B. Active or passive exposure to tobacco smoking and allergic rhinitis, allergic dermatitis, and food allergy in adults and children: a systematic review and meta-analysis. PLoS Med. 2014;11 doi: 10.1371/journal.pmed.1001611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matheson M.C., Dharmage S.C., Abramson M.J. Early-life risk factors and incidence of rhinitis: results from the European Community Respiratory Health Study – an international population-based cohort study. J Allergy Clin Immunol. 2011;128 doi: 10.1016/j.jaci.2011.05.039. 816–823.e5. [DOI] [PubMed] [Google Scholar]
- 16.Gendo K., Larson E.B. Evidence-based diagnostic strategies for evaluating suspected allergic rhinitis. Ann Intern Med. 2004;140:278–289. doi: 10.7326/0003-4819-140-4-200402170-00010. [DOI] [PubMed] [Google Scholar]
- 17.Aberg N., Hesselmar B., Aberg B., Eriksson B. Increase of asthma, allergic rhinitis and eczema in Swedish schoolchildren between 1979 and 1991. Clin Exp Allergy. 1995;25:815–819. doi: 10.1111/j.1365-2222.1995.tb00023.x. [DOI] [PubMed] [Google Scholar]
- 18.Mallol J., Crane J., von Mutius E., Odhiambo J., Keil U., Stewart A. ISAAC phase three study group, the international study of asthma and allergies in childhood (ISAAC) phase three: a global synthesis. Allergol Immunopathol. 2013;41:73. doi: 10.1016/j.aller.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 19.Linneberg A., Nielsen N.H., Madsen F., Frølund L., Dirksen A., Jørgensen T. Secular trends of allergic asthma in Danish adults. The Copenhagen Allergy Study. Respir Med. 2001;95:258–264. doi: 10.1053/rmed.2001.1031. [DOI] [PubMed] [Google Scholar]
- 20.Pawankar R, Holgate ST, Canonica GW, Lockey RF, Blaiss MS. WAO White Book on Allergy 2013 Update. https://www.worldallergy.org/wao-white-book-on-allergy.
- 21.Asthma in Ireland. Dublin, Ireland: The Asthma Society of Ireland. http://asthmasociety.ie/asthma-information/asthma-in-ireland.
- 22.Clatworthy J., Price D., Ryan D., Haughney J., Horne R. The value of self-report assessment of adherence, rhinitis and smoking in relation to asthma control. Prim Care Respir J. 2009;18:300–305. doi: 10.4104/pcrj.2009.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.ASCIA Allergen Immunotherapy and Skin Testing Working Party. Skin Prick Testing for the Diagnosis of Allergic Disease. First published 2006, revised September 2020. https://www.allergy.org.au/images/ASCIA_HP_SPT_Guide_2020.pdf.
- 24.Bousquet J., Schünemann H.J., Togias A. Next-generation allergic rhinitis and its impact on asthma (ARIA) guidelines for allergic rhinitis based on grading of recommendations assessment, development and evaluation (GRADE) and real-world evidence. J Allergy Clin Immunol. 2020;145 doi: 10.1016/j.jaci.2019.06.049. 70–80.e3. [DOI] [PubMed] [Google Scholar]
- 25.Wheatly L.M., Togias A. Allergic rhinitis. N Engl J Med. 2015;372:456–463. doi: 10.1056/NEJMcp1412282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bousquet J., Schünemann H.J., Hellings P.W. MACVIA clinical decision algorithm in adolescents and adults with allergic rhinitis. J Allergy Clin Immunol. 2016;138 doi: 10.1016/j.jaci.2016.03.025. 367–374.e2. [DOI] [PubMed] [Google Scholar]
- 27.Bousquet J., Pfaar O., Togias A. 2019 ARIA care pathways for allergen immunotherapy. Allergy. 2019;74:2087–2102. doi: 10.1111/all.13805. [DOI] [PubMed] [Google Scholar]
- 28.Zuberbier T., Lötvall J., Simoens S., Subramanian S.V., Church M.K. Economic burden of inadequate management of allergic diseases in the European Union: a GA(2) LEN review. Allergy. 2014;69:1275–1279. doi: 10.1111/all.12470. [DOI] [PubMed] [Google Scholar]
- 29.Bousquet P.J., Demoly P., Devillier P., Mesbah K., Bousquet J. Impact of allergic rhinitis symptoms on quality of life in primary care. Int Arch Allergy Immunol. 2013;160:393–400. doi: 10.1159/000342991. [DOI] [PubMed] [Google Scholar]
- 30.World Health Organization. World Health Statistics 2018: Monitoring Health for the SDGs. https://www.who.int/gho/publications/world_health_statistics/2018/en/.
- 31.World Health Organization. Global Health Observatory (GHO) data. https://www.who.int/gho/phe/outdoor_air_pollution/en/.
- 32.D'Amato G., Holgate S.T., Pawankar R. Meteorological conditions, climate change, new emerging factors, and asthma and related allergic disorders. a statement of the World Allergy Organization. World Allergy Organ J. 2015;8:25. doi: 10.1186/s40413-015-0073-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Teng B., Zhang X., Yi C. The association between ambient air pollution and allergic rhinitis: further epidemiological evidence from changchun, northeastern China. Int J Environ Res Public Health. 2017;14:226. doi: 10.3390/ijerph14030226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clarke D. March 2015. An Assessment of Allergenic Mite Species and Allergens in Vehicles and Homes, with Particular Reference to Dust Mite Transfer in Clothing.http://hdl.handle.net/10379/4961 NUI Galway Thesis. [Google Scholar]
- 35.Duggan E.M., Sturley J., Fitzgerald A.P., Perry I.J., Hourihane J.O. The 2002-2007 trends of prevalence of asthma, allergic rhinitis and eczema in Irish schoolchildren. Pediatr Allergy Immunol. 2012;23:464–471. doi: 10.1111/j.1399-3038.2012.01291.x. [DOI] [PubMed] [Google Scholar]
- 36.Grace M., Hunt D., O'B Hourihane J. The prevalence of grass pollen-related allergic rhinoconjunctivitis in elite amateur Irish athletes. Ir Med J. 2016;109:448. [PubMed] [Google Scholar]
- 37.Kihlström A., Lilja G., Pershagen G., Hedlin G. Exposure to birch pollen in infancy and development of atopic disease in childhood. J Allergy Clin Immunol. 2002;110:78–84. doi: 10.1067/mai.2002.125829. [DOI] [PubMed] [Google Scholar]
- 38.Kulig M., Klettke U., Wahn V., Forster J., Bauer C.P., Wahn U. Development of seasonal allergic rhinitis during the first 7 years of life. J Allergy Clin Immunol. 2000;106:832–839. doi: 10.1067/mai.2000.110098. [DOI] [PubMed] [Google Scholar]
- 39.Salo P.M., Calatroni A., Gergen P.J. Allergy-related outcomes in relation to serum IgE: results from the national health and Nutrition examination survey 2005-2006. J Allergy Clin Immunol. 2011;127 doi: 10.1016/j.jaci.2010.12.1106. 1226–1235.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nathan R.A., Meltzer E.O., Derebery J. The prevalence of nasal symptoms attributed to allergies in the United States: findings from the burden of rhinitis in an America survey. Allergy Asthma Proc. 2008;29(6):600–608. doi: 10.2500/aap.2008.29.3179. [DOI] [PubMed] [Google Scholar]