Abstract
Objective
Salivary gland tumors account for 6%–8% of head and neck neoplasms with the parotid gland as the most common primary site. Pleomorphic adenomas (PA) are considered the most common benign parotid gland neoplasms, followed by Warthin tumors (WT). The goal of this study was to investigate the distribution of parotid gland neoplasms among a United States veteran population.
Design
Retrospective chart review.
Setting
Washington DC Veterans Affairs Medical Center.
Participants
Veterans who underwent fine needle aspiration (FNA) for a parotid gland mass from 2000 to 2018 were included. Medical records were reviewed for gender, age, tobacco use, surgery date, and pathology results.
Main outcome measures
Changes in the distribution of parotid neoplasms and tobacco use over an 18-year period.
Results
Of 141 patients with parotid gland masses, 86.5% (n = 122) were benign, 9.9% (n = 14) were malignant, and 3.5% (n = 5) were indeterminate. Of benign tumors, WT accounted for the majority at 51.6%, followed by PA at 40.2%. When stratified by decade (2000–2009 and 2010–2018), the proportion of WT compared to all other benign and malignant neoplasms increased from 31.6% to 53.6%, whereas the proportion of PA decreased from 36.8% to 33.3%. The rate of tobacco use was unchanged at approximately 32.0% among our cohort from 2000 to 2018.
Conclusion
Among our cohort of veteran patients, WT was the most common benign parotid tumor and has increased in incidence over the last two decades despite an unchanged smoking rate.
Keywords: Parotid neoplasm, Warthin tumor, Pleomorphic adenoma, Tobacco, Head and neck cancer, Veteran
Introduction
Salivary gland tumors account for 6%–8% of head and neck neoplasms, with the parotid gland as the most common primary site.1 An analysis of the Surveillance, Epidemiology, and End Results (SEER) Database from 1973 to 1999 showed a change in incidence of salivary gland tumors as a proportion of all head and neck neoplasms, with a significant increase from 6.3% in 1974–1976 to 8.1% in 1998–1999.1 Of parotid gland neoplasms, typically 80% are benign and the remaining 20% are malignant.2 Classically, pleomorphic adenomas (PA) are considered the most common benign parotid gland neoplasm, followed by Warthin tumor (WT) as described in Table 1.2, 3, 4, 5, 6, 7
Table 1.
Retrospective studies of pleomorphic adenoma (PA) and Warthin tumor (WT) epidemiology in the parotid gland.
| Study | Country | Time period | Benign parotid neoplasms(N) | PA frequency(%) | WT frequency(%) |
|---|---|---|---|---|---|
| Ethunandan et al, 2002.2 | England | 1974–1999 | 460 | 59.6 | 32.6 |
| Lukšić et al.3 | Croatia | 1985–2009 | 383 | 66.8 | 22.8 |
| Christensen et al.4 | Denmark | 1986–1995 | 433 | 54.0 | 28.0 |
| Gierek et al.5 | Poland | 1986–2006 | 416 | 75.0 | 24.0 |
| Luers et al.6 | Germany | 1990–2014 | 1818 | 61.1 | 38.9 |
| Coombe et al.7 | Australia | 2006–2013 | 77 | 59.7 | 24.6 |
There is a paucity of literature from the United States (US) on the epidemiology of benign salivary gland neoplasms over the last several decades. This is somewhat surprising considering significant changes in the pattern of tobacco use – a known risk factor the development of many parotid neoplasms2 – over the past several decades. With this in mind, the goal of the present study was to investigate the distribution of parotid gland neoplasms among the US veteran population over the last two decades.
Methods
Ethical considerations
This study was approved by the Washington DC Veterans Affairs Medical Center (VAMC) Institutional Review Board (IRB).
Data collection and participants
The electronic medical record (EMR) at the Washington DC Veterans Affairs Medical Center (VAMC) was queried for patients who had undergone a fine needle aspiration (FNA) for a parotid gland mass between 2000 and 2018. Veterans were included if they were older than 18 years of age with no prior history of FNA. Of those patients included, medical records were reviewed for gender, age at diagnosis, body mass index (BMI), history of tobacco use, cytology results from the FNA, and if present, surgical pathology. Both surgical and non-surgical patients were included, and when available, surgical pathology superseded FNA cytology for final diagnosis.
For review of cytology, direct smears were air dried and stained with Diff-Quik and needles were rinsed in CytoLyt® processed using the ThinPrep method and stained by Papanicolaou stain. Cell blocks were also made from the needle rinse with the plasma-thrombin method and stained with hematoxylin and eosin. All cases were reviewed by two board-certified pathologists and the vast majority of diagnoses were definite. When not definite, the final diagnosis was categorized as “indeterminate”.
Patients with parotid metastases from anatomically distinct primaries, such as cutaneous malignancies, were excluded from the analysis. Tobacco use was categorized as never, former, and current and only those patients who smoked cigarettes were considered tobacco users. Former smokers were those who had a previous history of tobacco use, however had not used tobacco products for at least one year prior to FNA. Multicentric tumors of the same pathology were only counted once. For instance, a patient with two Warthin tumors was only documented as having one tumor. Only primary diagnoses were included in the analysis, therefore patients with tumor recurrence were only included for the primary tumor pathology.
Statistical analyses
Data aggregation was completed using Microsoft Excel 360 (Microsoft Corporation, Richmond, WA). All data were summarized using descriptive statistics. Means with standard deviations were used for continuous variables, and frequencies and percentages were used for categorical variables. Continuous variables were compared by two sample t-test and ANOVA tests as appropriate, while categorical variables were compared by Chi-square and Fisher exact tests. Multivariate logistic regression analysis was used to examine factors which were associated with tumor type controlling for the variables which were found significant in the bivariate analysis. Statistical significance was defined as P < 0.05 and analyses were performed with SAS 9.4 (SAS Institute Inc., Cary, NC).
Results
One hundred and ninety-seven patients who underwent parotid gland FNA and/or parotidectomy for a solitary parotid lesion were included in the analysis. Of these, 56 (28.4%) were excluded due to non-neoplastic pathology, including benign cysts (n = 26), sialadenitis (n = 6), non-diagnostic/normal parotid tissue (n = 21), or due to metastatic nature (n = 3). Of the remaining 141 patients with parotid neoplasms, 122 (86.5%) were benign, 14 (9.9%) were malignant, and 5 (3.5%) were indeterminate (Table 2). Of benign neoplasms, WT accounted for the majority at 51.6% (63/122), followed by PA at 40.2% (49/122), lipoma at 5.7% (7/122), basal cell adenoma at 1.6% (2/122), and myoepithelioma at 0.8% (1/122).
Table 2.
Distribution of parotid neoplasms (2000–2018).
| Histologic type | N | Percentage (%) |
|---|---|---|
| Benigna | 122 | 100.0 |
| Warthin tumor | 63 | 51.6 |
| Pleomorphic adenoma | 49 | 40.2 |
| Basal cell adenoma | 2 | 1.6 |
| Lipoma | 7 | 5.7 |
| Myoepithelioma | 1 | 0.8 |
| Malignantb | 14 | 100.0 |
| Acinic cell carcinoma | 2 | 14.3 |
| Adenocarcinoma | 1 | 7.1 |
| Basal cell adenocarcinoma | 1 | 7.1 |
| Lymphoma | 4 | 28.6 |
| Mucoepidermoid carcinoma | 3 | 21.4 |
| Salivary duct carcinoma | 1 | 7.1 |
| Squamous cell carcinoma | 2 | 14.3 |
| Indeterminate neoplastic lesions (FNA only) | 5 | 100.0 |
Non-diagnostic and normal FNAs excluded.
Metastatic lesions excluded.
Of the 141 patients who met the inclusion criteria, 98 (69.5%) underwent surgery and 43 (30.5%) had FNA only. The discordance rate between FNA and surgical pathology for all tumors was 24.6%. A rate of 18.7% and 10.7% was observed for WT and PA respectively.
The clinical-demographic data among patients with WT and PA is depicted in Table 3. In our bivariate model, gender, age, and tobacco use were found to be statistically significant. A greater proportion of WT patients were male, compared to PA patients (93.7% vs. 77.6%, P = 0.0228). In addition, WT patients were significantly older than those with PA (66.8 ± 9.4 vs. 60.0 ± 12.9, P = 0.0018). Of patients with WT, 82.5% were former or current smokers, compared to 65.3% among patients with PA (P = 0.0106). Of note, after controlling for co-variates, only current tobacco use and age were found to be statistically significant. The odds of WT for current smokers was 3.7 times higher compared to never smokers after controlling for age and gender (3.744, 95%CI = 1.27–11.02, P = 0.0162). The odds of WT increased by 6.2% for a unit increase in age after controlling for gender and smoking status (1.062, 95%CI = 1.01–1.11, P = 0.0078).
Table 3.
Characteristics of benign parotid neoplasms (2000–2018).
| Bivariate mode | Pleomorphic adenoma (N = 49) | Warthin tumor (N = 63) | P value |
|---|---|---|---|
| Gender N(%) | 0.0228 | ||
| Female | 11 (22.4) | 4 (6.3) | |
| Male | 38 (77.6) | 59 (93.7) | |
| Age(year, ±s) | 60.0 ± 12.9 | 66.8 ± 9.4 | 0.0018 |
| BMI(±s) | 28.3 ± 5.3 | 28.4 ± 5.9 | 0.9189 |
| Race N(%) | 0.3437 | ||
| Asian | 0 | 2 (3.2) | |
| Black | 28 (57.1) | 29 (46.0) | |
| Native American | 0 | 1 (1.6) | |
| Other | 3 (6.1) | 9 (14.3) | |
| White | 18 (36.7) | 22 (34.9) | |
| Tobacco N(%) | 0.0106 | ||
| Current | 13 (26.5) | 34 (54.0) | |
| Former | 19 (38.8) | 18 (28.6) | |
| Never | 17 (34.7) | 11 (17.5) | |
| Surgery N(%) | 0.4373 | ||
| Yes | 36 (73.5) | 42 (66.7) | |
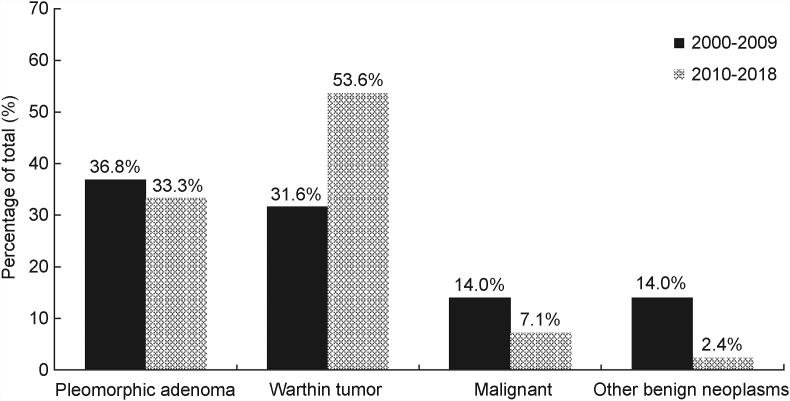
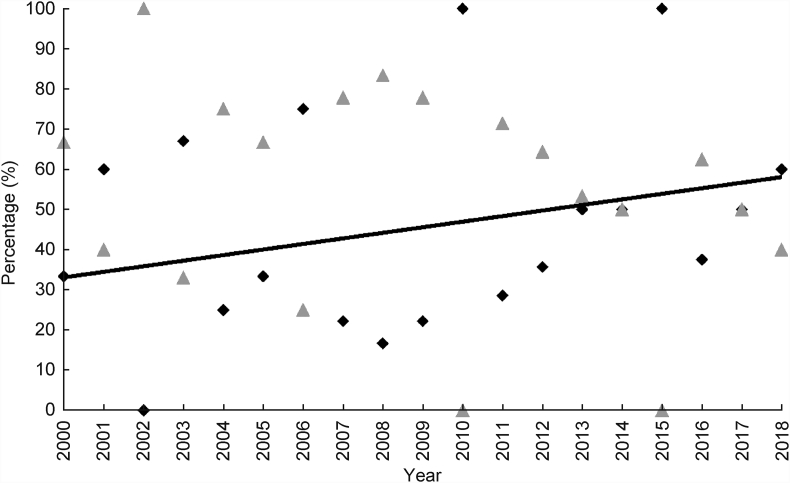
When stratified by decade (2000–2009 and 2010–2018), differences in gender, age at diagnosis, race and BMI among all comers undergoing FNA were not significant. Between the two decades, the proportion of WT increased from 31.6% to 53.6%, whereas the proportion of PA slightly decreased from 36.8% to 33.3%, respectively (Fig. 1). The incidence of WT increased significantly over the last two decades (P = 0.0422), whereas the incidence of other neoplasms remained stable (Fig. 2). Malignant neoplasms decreased in incidence over the same time period from 14.0% to 7.1% (Fig. 1).
Fig. 1.
Proportions of neoplasms over time. Absolute numbers of all tumors observed at our institution from 2000 to 2018, as diagnosed on FNA, and when available, surgical pathology. 2010 to 2018, as diagnosed on FNA, and when available, surgical pathology.
Fig. 2.
Incidence of Warthin tumor compared to all parotid neoplasms. Relative incidence of WT (diamond) compared to all other neoplasms (triangle) by year. Trend line indicates an increase in incidence of WT (P = 0.0422).
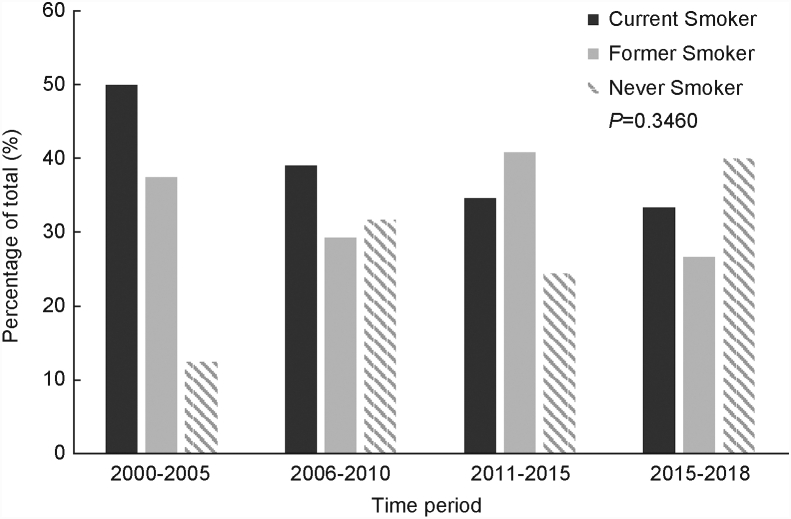
The proportion of former, current, and never smokers was also analyzed amongst all patients across four time periods (Fig. 3). Of note, the former and current smoking rates did not significantly change over time (P = 0.3460). The overall rate of current smokers across all decades was 32.0%.
Fig. 3.
Rates of tobacco use from 2000 to 2018. Incidence in tobacco use across four distinct time periods.
Discussion
A number of European studies have suggested a shifting paradigm in the once well-established epidemiology of benign parotid neoplasms, with an increasing incidence of WT compared to PA.2,6,8,9 Franzen et al8 showed a higher frequency of WT compared to PA, seen in the final decade of a 42-year long analysis in a rural German population from 1975 to 2017. Various other European studies have reported similar trends. Luers et al6 reported an increase in WT from 24% to 48% from 1990 to 2014, while Kadletz et al9 reported an increased rate of 10%–60% from 1960 to 2015. Of note, the latter study described a six-fold increase in WT incidence discordant with relatively stable smoking rates - a known risk factor for the development of WT.9,10 To our knowledge, the present investigation is the first North American study to describe the incidence of benign salivary neoplasms in several decades, as well as the first to report an overall higher incidence of WT compared to PA, which is classically considered the most common benign parotid neoplasm.
Overall, several factors may explain this apparent increase in incidence of WT. Examining risk factors for the development of WT, such as tobacco exposure, may shed some light. Once a widespread habit in the United States in the 20th century, tobacco use reached its height in 1953, with a national smoking rate of 47%,11 and has since declined. Given that the median age of patients with WT in our cohort was 66.9, increased incidence may be an artifact of widespread smoking habits during the last several decades.
Although the national smoking rate has recently declined, the U.S. veteran population has historically maintained a higher than average smoking rate.12 This observation is further corroborated by our results, which may partly account for a high incidence of WT in our cohort. However, the steady former and current smoking rate in our cohort does not explain the rising incidence of WT that we observed.
Obesity is another factor that has been implicated in the rising incidence of WT identified by European investigators.9 Among our cohort, however, there was no association between BMI and developing a WT. Furthermore, there was no difference in BMI among the two decades. It is possible that the effect of obesity on our cohort was not apparent due to the small sample size.
The rise in benign neoplasms which was observed in our cohort may be partly explained by the routine use of imaging modalities that identify small and asymptomatic parotid neoplasms. Recent studies have demonstrated a 60% overall increase in computed tomography (CT) scans utilized in the emergency room setting over the last decade, which may be responsible for incidentally noted parotid lesions, leading to further evaluation and subsequent FNA.13 However, this alone does not explain the rise in benign neoplasms and decline in malignant neoplasms in our veteran cohort.
Aside from its retrospective nature and limited sample size, a number of limitations must be considered. Of note, the percentage of patients undergoing FNA for parotid lesions is largely unknown. It is entirely possible, that the number of FNAs ordered or the compliance rate of patients completing FNAs has changed over the last two decades. An increase in the number of FNAs could explain a variance in frequencies of certain parotid pathologies. An investigation on the ordering practices of clinicians could be an avenue for future studies.
In addition, another limitation of our study is that limited data was available to compare the health of patient populations across decades. Although there was no difference among age, BMI, and smoking rates, overall cardiovascular and pulmonary status, as well as presence of diabetes may have influenced a patient's ability to undergo parotidectomy, possibly alerting true incidence of certain parotid pathologies.
Finally, as with many other studies which examine epidemiologic data on incidence and distribution of benign parotid neoplasms, the majority of information is derived from single institutions, with population-based studies less common.8 As a result, our findings may not be relatable to the general US population. Nonetheless, our results suggest a shift in the distribution of parotid gland neoplasms in the veteran population of North America.
Conclusion
In our veteran cohort who underwent FNA for parotid gland masses, Warthin tumor was the most common benign neoplasm encountered, surpassing pleomorphic adenomas in incidence. Furthermore, our results suggest that the incidence of Warthin tumor is increasing, despite relatively stable smoking rates. It remains unclear whether this is due to a higher prevalence of tobacco use or male gender among the veteran population. However, a thorough understanding of the distribution of parotid gland neoplasms in this specific population is critical to patient counseling and surgical planning. This study contributes to the demographic epidemiology of salivary gland tumors and demonstrates the shifting distribution of parotid gland neoplasms.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. Please contact the corresponding author, Adnan S. Hussaini, at adnan.s.hussaini@gunet.georgetown.edu to file a request.
Authorship contributions
Adnan S. Hussaini: Conceptualization, Methodology, Validation, Formal Analysis, Writing – Original Draft, Writing – Review & Editing, Visualization. Navin R. Prasad: Conceptualization, Methodology, Formal analysis, Investigation, Writing – Original Draft. Edina Paal: Methodology, Validation, Resources, Writing – Review & Editing. Eshetu A. Tefera: Validation, Formal Analysis, Writing – Review & Editing. Sonya Malekzadeh, MD. Conceptualization, Methodology, Writing – Review & Editing, Supervision. Jessica H. Maxwell: Conceptualization, Methodology, Writing – Review & Editing, Supervision.
Declaration of Competing Interest
None.
Edited by Qiong Wu
Footnotes
Peer review under responsibility of Chinese Medical Association.
References
- 1.Carvalho A.L., Nishimoto I.N., Califano J.A., Kowalski L.P. Trends in incidence and prognosis for head and neck cancer in the United States: a site-specific analysis of the SEER database. Int J Cancer. 2005;114:806–816. doi: 10.1002/ijc.20740. [DOI] [PubMed] [Google Scholar]
- 2.Ethunandan M., Pratt C.A., Macpherson D.W. Changing frequency of parotid gland neoplasms--analysis of 560 tumours treated in a district general hospital. Ann R Coll Surg Engl. 2002;84:1–6. [PMC free article] [PubMed] [Google Scholar]
- 3.Lukšić I., Virag M., Manojlović S., Macan D. Salivary gland tumours: 25 years of experience from a single institution in Croatia. J Craniomaxillofac Surg. 2012;40:e75–e81. doi: 10.1016/j.jcms.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 4.Christensen N.R., Charabi S., Sørensen W.T., Dethloff T., Balle V.H., Tos M. [Benign neoplasms in the parotid gland in the county of Copenhagen 1986-1995] Ugeskr Laeger. 1998;160:6066–6069. [PubMed] [Google Scholar]
- 5.Gierek T., Majzel K., Jura-Szołtys E., Slaska-Kaspera A., Witkowska M., Klimczak-Gołab L. [A 20-year retrospective histoclinical analysis of parotid gland tumors in the ENT Department AM in Katowice] Otolaryngol Pol. 2007;61:399–403. doi: 10.1016/S0030-6657(07)70451-1. [DOI] [PubMed] [Google Scholar]
- 6.Luers J.C., Guntinas-Lichius O., Klussmann J.P., Küsgen C., Beutner D., Grosheva M. The incidence of Warthin tumours and pleomorphic adenomas in the parotid gland over a 25-year period. Clin Otolaryngol. 2016;41:793–797. doi: 10.1111/coa.12694. [DOI] [PubMed] [Google Scholar]
- 7.Coombe R.F., Lam A.K., O'Neill J. Histopathological evaluation of parotid gland neoplasms in Queensland, Australia. J Laryngol Otol. 2016;130(Suppl 1):S26–S31. doi: 10.1017/S0022215115002789. [DOI] [PubMed] [Google Scholar]
- 8.Franzen A.M., Kaup F.C., Guenzel T., Lieder A. Increased incidence of Warthin tumours of the parotid gland: a 42-year evaluation. Eur Arch Otorhinolaryngol. 2018;275:2593–2598. doi: 10.1007/s00405-018-5092-3. [DOI] [PubMed] [Google Scholar]
- 9.Kadletz L., Grasl S., Perisanidis C., Grasl M.C., Erovic B.M. Rising incidences of Warthin's tumors may be linked to obesity: a single-institutional experience. Eur Arch Otorhinolaryngol. 2019;276:1191–1196. doi: 10.1007/s00405-019-05319-6. [DOI] [PubMed] [Google Scholar]
- 10.Pinkston J.A., Cole P. Cigarette smoking and Warthin's tumor. Am J Epidemiol. 1996;144:183–187. doi: 10.1093/oxfordjournals.aje.a008906. [DOI] [PubMed] [Google Scholar]
- 11.Cummings K.M., Proctor R.N. The changing public image of smoking in the United States: 1964-2014. Cancer Epidemiol Biomarkers Prev. 2014;23:32–36. doi: 10.1158/1055-9965.EPI-13-0798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Odani S., Agaku I.T., Graffunder C.M., Tynan M.A., Armour B.S. Tobacco product use among military veterans - United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2018;67:7–12. doi: 10.15585/mmwr.mm6701a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bellolio M.F., Heien H.C., Sangaralingham L.R. Increased computed tomography utilization in the emergency department and its association with hospital admission. West J Emerg Med. 2017;18:835–845. doi: 10.5811/westjem.2017.5.34152. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. Please contact the corresponding author, Adnan S. Hussaini, at adnan.s.hussaini@gunet.georgetown.edu to file a request.