Abstract
Objective
To review the role of adjuvant hyperbaric oxygen therapy (HBOT) in the treatment of malignant otitis externa (MOE).
Data sources
PubMed, Scopus, Web of Science, Science Direct, and Cochrane Library were searched for the following concepts: “hyperbaric oxygen” and “malignant or necrotizing otitis externa.”
Methods
Studies were included if they contained (1) patients with reported evidence of MOE, (2) employment of adjuvant HBOT, (3) details on patients’ medical condition, and (4) documented survival outcomes. Extracted information included patient demographics, underlying medical conditions, infectious etiology, signs and symptoms, medical and surgical treatments, duration of medical treatment, mean follow up time, HBOT setting, number of HBOT sessions, complications, survival rate, and all-cause mortality.
Results
A total of 16 studies comprising 58 patients (mean age 68.0 years) were included. Diabetes was present in 94.7% of cases and Pseudomonas spp (64.3%) was the most common infectious agent. Cranial nerve VII was involved in 55.2% of cases. Overall, the disease cure rate with adjuvant HBOT was 91.4% and all-cause mortality was 8.6%. Among those who had cranial nerve VII involvement, 72.0% had return of function and 93.8% of them survived.
Conclusion
HBOT may be an effective treatment option for refractory or advanced MOE but its efficacy remains unproven due to lack of strong scientific evidence. However, its therapeutic value should not be underestimated given good results and few adverse events reported in this study.
Keywords: Malignant otitis externa, Necrotizing otitis externa, Diabetes mellitus, Hyperbaric oxygen, Therapy, Osteomyelitis
Introduction
Malignant otitis externa (MOE) is a necrotizing infection of the soft tissue of the external auditory canal (EAC) that can rapidly invade into adjacent structures to cause skull base osteomyelitis. Affected patients are typically elderly, uncontrolled diabetics or immunocompromised individuals. They present with severe otalgia, purulent otorrhea, and granulation tissue at the osteo-cartilaginous junction in the EAC refractory to treatment.1 The most common causative agent is Pseudomonas aeruginosa; however, other aggressive bacteria and fungi have been reported in the literature.2, 3, 4
Treatment of MOE includes topical and systemic administration of appropriate antibiotics or antifungals. Extensive disease may require surgical debridement of EAC as well as adjacent structures.5 Despite advances in medications and management, MOE remains a severe infection with significant morbidity and mortality.5, 6, 7 Adjuvant hyperbaric oxygen therapy (HBOT) has been proposed in cases where antibiotics and surgical interventions fail to cure MOE.8 However, the clinical impact of HBOT in treating MOE remains controversial.9,10
The American Undersea and Hyperbaric Medical Society (UHMS) and the European Committee for Hyperbaric Medicine (ECHM) generally accept necrotizing inflammation of soft tissues and refractory osteomyelitis as indications for HBOT.10,11 However, they provide no specific recommendation for MOE due to very low levels of evidence. Given theoretic value of HBOT in treating MOE, the goal of this study was to provide a systematic review of literature pertaining to the use of adjuvant HBOT in managing MOE and to assess its clinical impact.
Methods
Data extraction
A comprehensive review of the literature was undertaken according to PRISMA guidelines12 using Pub Med/MEDLINE, Scopus, Web of Science, ScienceDirect, and the Cochrane Database of Systematic Review. Mesh terms of “hyperbaric oxygen” and “otitis externa” were used where applicable. Additional terms encompassing “necrotizing externa”, “necrotising externa” OR “malignant otitis externa” were employed as well. Each database was searched from inception to June 18th, 2019. The titles and abstracts of the retrieved articles were reviewed for full text assessment by two authors (YJB, JP). Articles not written in English, zoological studies, reviews, and editorials were excluded. A complete review of the text was performed by both authors to assure that inclusion and exclusion criteria were met. Studies were included in the final data set if they contained (1) patients with documented evidence of MOE, (2) employment of adjuvant HBOT, (3) details on patients’ medical condition, and (4) documented survival outcomes. Due to limited availability of HBOT centers, we anticipated small number of studies that would employ HBOT; therefore, case reports or case series reporting the use of HBOT were included for further scrutiny. Individual patient data collected for the review includes patient demographic information, underlying medical conditions, infectious etiology, signs and symptoms, medical and surgical treatments, duration of medical treatment, mean follow up time, HBOT setting, number of HBOT sessions, outcomes after HBOT, complications of HBOT, survival rate, duration of survival, and all-cause mortality. Cure was defined as symptom resolution without clinical signs of infection and/or regression of inflammatory process confirmed by scintigraphy (e.g. Gallium scan). When cranial nerves were involved, recovery of nerve function was not used to determine the cure. All data was recorded as individual patient data. Data that could not be extracted was considered “missing” and was excluded from the final data set.
Quality review and assessment of risk of bias
Level of evidence for each included article was performed using Oxford Center for Evidence-Based Medicine (OCEBM).13 The risk of bias was assessed according to the CochraneHandbook for Systematic Reviews of Interventions. Thelatest version of this tool was updated in March 2011,version 5.1.0.14 Two authors (YJB, JP) performed a pilot assessment on three studies to check for consistency of assessment. Both then performed independent risk assessment on the remaining studies. All disagreements were resolvedby the way of discussion with a third author (SAN). Risk of bias items included thefollowing: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias.
Results
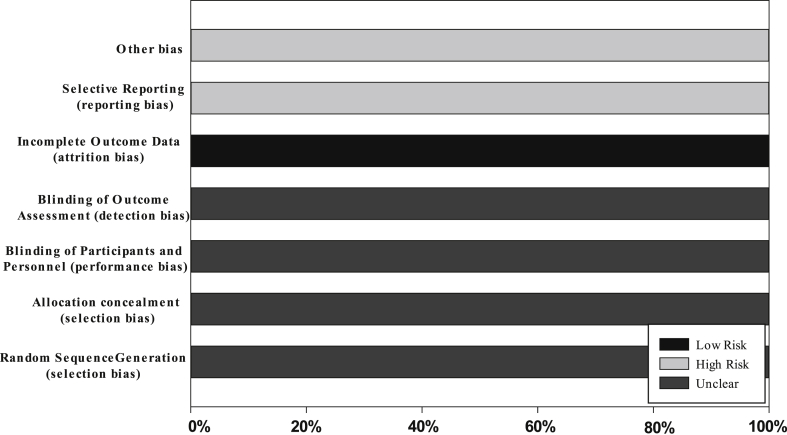
The literature search identified 286 articles and 187 remained after duplicates were removed. A total of 147 articles were removed based on title and abstract. A full text review of the remaining 40 resulted in inclusion of 16 articles.8,15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29 A PRISMA diagram outlining the process is shown in Fig. 1. All included articles were retrospective case reports/case series, representing level 4 evidence according to the Oxford Levels of Evidence.Risk of bias was assessed for each included study (Fig. 2).
Figure 1.
Prisma diagram.
Figure 2.
Risk of bias: a review of author's judgment about each item, presented as a percentage across all included studies.
Study demographics
Included studies originated from 7 different countries. There were 13 case reports or case series15, 16, 17, 18, 19, 20, 21, 22, 23, 24,26, 27, 28 and 3 retrospective observational studies,8,25,29 comprising a total of 58 subjects with an average age of 68.0 years (range 36–84). The duration of symptom before the diagnosis was made ranged from 14 to 183 days with an average of 43.2 days. The pressure setting and duration of HBOT session varied across the studies, detailed in Table 1. The average number of HBOT sessions was 30 (range 5–80). The overall disease cure rate was 91.4% and the average duration of follow-up was 25.5 months (range 1–60).
Table 1.
Demographic information of included studies.
| Author (year) | Country | OLE | Subjects (n) | Age (years) | DOS (d) | Treatment Context | HBOT setting (atm, duration) | Number of HBOT sessions | Cure rate (%) | DOF (m) |
|---|---|---|---|---|---|---|---|---|---|---|
| Mader (1982) | USA | 4 | 1 | 55 | 24 | Refractory | 2.5, 90 min | 20 | 100 | 6 |
| Joachims (1988) | Israel | 4 | 1 | 63 | 30 | Refractory | 2.8, 90 min | 40 | 0 | 12 |
| Shupak (1989) | Israel | 4 | 2 | 79a | 28 | Refractory | 2.5, 90 min | 28a | 100 | 19.5a |
| Davis (1992) | USA | 4 | 16 | 67a | – | Refractory | 2.4, 90 min | 30a | 100 | 54a |
| Gordon (1994) | USA | 4 | 2 | 75a | 183a | Refractory | 2, 90 min | 27a | 50 | 15a |
| Bath (1998) | UK | 4 | 1 | 46 | – | Refractory | 1.5, 60 min | 27 | 100 | 60 |
| Genden (1998) | UK | 4 | 1 | 80 | 35 | Refractory | – | – | 100 | – |
| Lancaster (2000) | UK | 4 | 1 | 58 | 61 | Refractory | – | 14 | 0 | – |
| Marzo (2003) | USA | 4 | 3 | 56a | 35.7a | Refractory | – | 29a | 66 | 11a |
| Okpala (2005) | UK | 4 | 1 | 72 | 14 | Concomitant | – | – | 100 | – |
| Singh (2005) | Oman | 4 | 1 | 54 | 30 | Concomitant | 2.5, 30 min | 34 | 100 | 36 |
| Narozny (2006) | Poland | 4 | 8 | 64a | – | Concomitant | 2.5, 70 min | 24a | 100 | 30.1a |
| Ling (2008) | Australia | 4 | 1 | 77 | 35 | Concomitant | 2.4, - | 30 | 100 | 24 |
| Leahy (2011) | Australia | 4 | 1 | 76 | – | Refractory | – | 54 | 100 | – |
| Manso (2016) | Portugal | 4 | 1 | 81 | 30 | Concomitant | – | – | 100 | 1 |
| Amaro (2019) | Portugal | 4 | 16 | 71a | – | Refractory | – | 34a | 100 | – |
| Total/Average | 58 | 68 (36–84) | 43 (14–183) | 30 (5–80) | 91.4 | 25.5 (1–60) |
Treatment context refers to whether HBOT was used concomitantly or after failing medical therapy or surgery (refractory).
OLE: oxford level of evidence; DOS: duration of symptoms; HBOT: hyperbaric oxygen therapy; DOF: duration of follow-up; d: days; n: number of subjects; atm: atmosphere; min: minutes; m: months.
Average of reported cases.
Patient factors and outcomes
A summary of patient demographics, microbiology, and outcomes are presented in Table 2. Males comprised 71.4% of reported gender with male to female ratio of 2.5:1. Diabetes was the predominant underlying condition, present in 94.7% of patients. Patients with history of cancer and organ transplantation comprised 4.9% and 3.5%, respectively. The most commonly reported infectious organism was Pseudomonas spp (64.3%), followed by Aspergillus spp (16.1%), Staphylococcus aureus (12.5%), and Candida spp (12.5%). Culture results were negative in 8.9% of reported cases.
Table 2.
Patient factors, management, and outcomes.
| Variable | Total (%) |
|---|---|
| Total cases | 100 (n = 58) |
| Mean age (years) | 68.0 |
| Gender | |
| Male | 71.4 |
| Female | 27.6 |
| Underlying Condition | |
| Diabetes mellitus | 94.7 |
| History of cancer | 4.9 |
| History of transplant | 3.5 |
| Microbiology | |
| Pseudomonas spp. | 64.3 |
| Aspergillus spp. | 16.1 |
| S. aureus | 12.5 |
| Candida spp. | 12.5 |
| Culture negative | 8.9 |
| CN Involvement | |
| CN VII | 55.2 (n = 32) |
| CN function recovery | 72.0 |
| Disease cure rate | 87.5 |
| Survival | 93.8 |
| Multiple CN involvement | 25.9 |
| Surgery or Procedure | 50.0 |
| Overall outcomes | |
| Disease cure rate | 91.4 |
| Survival | 91.4 |
| All-cause mortality | 8.6 |
CN: cranial nerve; HBOT: hyperbaric oxygen therapy.
Surgical management includes extensive debridement.
Mastoidectomy, or facial nerve decompression.
Procedure includes local external auditory canal debridement.
Cranial nerve (CN) VII involvement causing facial palsy was reported in 55.2% of cases. After employing HBOT, CN function recovered in 72% of the cases. Among those who had CN VII involvement, the disease cure rate was 87.5% and reported survival was 93.4%. Multiple CN involvement was present in 25.9% of patients but recovery of CN function, disease cure rate, and survival for this group could not be assessed due to reporting bias.
Half of the patients underwent procedural or surgical management of the disease. Procedures included local EAC debridement andsurgical management included extensive debridement beyond the EAC including mastoidectomy or facial nerve decompression. All patients received antibiotic regimen with adjuvant HBOT. Overall, the disease was cured in 91.4% of cases and an all-cause mortality rate was 8.6%. The average duration of survival was 7.3 months (range 1–13).
Outcomes based on microbiology were as follows: among those with Pseudomonasspp. infection, 97% survived; 75% survived from Aspergillus spp. infection; 67% survived from Candida spp.; and 67% survived from polymicrobial infection. All patients who had culture negative results survived. The microbiology for those who succumbed to polymicrobial infection included a combination of Staphylococcus spp., Aspergillus spp., and Candida spp.
Signs, symptoms, and complications
Table 3 details presenting signs and symptoms as well as complications of HBOT. The most common presenting symptom was otorrhea (84.1%), followed by otalgia (77.3%) and headache (36.4%). Common signs found in these patients included granulation tissue in the EAC (59.1%), edema of the EAC (27.3%), and fever (2.3%). HBOT was generally well tolerated and adverse events were reported in 2 studies.8,22 A total of 4 patients (6.9%) required tympanostomy tube placement to allow middle-ear pressure equalization during HBOT chamber compression. One patient (1.7%) experienced panic attack.
Table 3.
Signs and symptoms and complications.
| Variable | Total (%) |
|---|---|
| Signs and symptoms | |
| Otorrhea | 84.1 |
| Otalgia | 77.3 |
| Granulation tissue in EAC | 59.1 |
| Headache | 36.4 |
| EAC edema | 27.3 |
| Fever | 2.3 |
| Complications of HBOT | |
| Tympanostomy tube placement | 6.9 (n = 4) |
| Panic attack | 1.7 (n = 1) |
EAC: external auditory canal; HBOT: hyperbaric oxygen therapy.
Discussion
The increasing prevalence of MOE has been noted as early as in 1988 likely attributed to the aging diabetic population.30, 31, 32 Although development of new antipseudomonal agents seemed at first to control the disease progression, their widespread use raised concern for resistant microbes.5,33 Due to high morbidity and mortality associated with MOE, it is imperative that all treatment options be explored. Hence, the purpose of this systematic review was to assess the efficacy of HBOT as an adjuvant treatment for MOE.
Diabetics have long been associated with this infection34 and its prevalence in MOE is commonly reported in 80–100% of patients.5,35,36 The pathogenesis of MOE in diabetics is largely attributed to synergistic effects of impaired leukocyte activity and microangiopathy induced hypoxia. Polymorphonuclear neutrophils and lymphocytes in diabetics characteristically have impaired cellular mechanisms that lead to poor migration, reduced chemotaxis, and defective phagocytosis.37, 38, 39, 40 In addition, diabetic microangiopathy causes tissue hypoperfusion and hypoxia, further impairing the oxygen-dependent antimicrobial activity by leukocytes.41, 42, 43 Infection further decreases oxygen levels in the tissues due to bacterial oxygen uptake and high oxygen consumption by inflammatory processes.44
Although aggressive parenteral antibiotic treatment may adequately penetrate the infected tissue in the early stages of the disease, disease progression and more extensive tissue damage may impair penetration. Thus, the exploration of HBOT as a therapeutic adjunct seems to be a theoretically sound. Hyperbaric oxygenation has been shown to increase the oxygen partial pressure in infected tissues,43 enhancing oxygen-mediated leukocyte killing of pathogens.45 Furthermore, periodic exposure to hyperbaric oxygenation enhances soft-tissue and bone healing by promoting fibroblastic division, collagen production, and capillary angiogenesis.46,47 In a clinical setting, HBOT has been reported to be a valuable adjunct in the treatment of chronic or refractory osteomyelitis.48, 49, 50
In the current study, some variations in patient characteristics and management were observed. Diabetes accounted for 95% of the underlying conditions in patients treated with HBOT. Pseudomonas spp (64%) was the most common offending microbe, followed by Aspergillus spp (16%), Staphylococcus aureus (13%), and Candida spp (13%). On average, patients were diagnosed with MOE after 43 days of symptom onset with a range of 14–183 days. Cranial nerve VII was involved in 55% of cases and multiple cranial nerve palsies were present in 26%. Furthermore, a significant variation in the therapeutic management were observed: 50% of patients underwent surgery for debridement, mastoidectomy, or facial nerve decompression; the pressure of oxygen delivery ranged from 1.5 to 2.8 atm; the duration of each HBOT session ranged from 30 min to 90 min; and the number of sessions varied between 5 and 80 sessions (Table 1).
Most patients were referred to undergo HBOT when disease progressions were seen refractory to standard antibiotic therapies. Despitethe heterogeneity in patient characteristics and management, adjuvant HBOT was associated with a high cure rate (91%). This is comparable to cure rate (92%) reported in a systematic review that conducted a thorough evaluation of the literature published between 1968 and 2011.5 However, this review noted significant heterogeneity in the included studies and could not control for HBOT usage in the assessment of the outcomes. It should be noted that the high cure rate observed in present study was achieved even thoughpatientswere at an advanced disease stage as indicated by the relatively high percentage of CN involvement. Prior investigations have found that facial nerve involvement is associated with poorer outcome with up to 3-fold increased risk of mortality as compared to patients without cranial nerve palsy.51, 52, 53, 54 One study showed that those with facial nerve palsy did not regain complete recovery of nerve function despite medical treatment.36 In the current study, 71% of patients with facial nerve palsy experienced return of CN function, 88% achieved cure from the disease, and 94% survived. Thus, these results may suggest that adjuvant HBOT may be an effective option in advanced and/or refractory cases of MOE.
HBOT is not without side effects, although these were usually minor and acceptable when confronting a life threatening condition such as MOE. In a large retrospective analysis of 2334 patient who underwent HBOT, 9.2% of patients experienced middle ear barotrauma while hypoglycemia, oxygen toxicity, dizziness, anxiety reactions, dyspnea, and chest pain occurred in 0.5–1.5% of patients.55 Another study investigated otological complications of HBOT and found 14.8% of 1115 patients experienced symptoms characterized by otalgia, aural fullness, hearing loss, or tinnitus.56 In the present study, 4 patients (7%) required tympanostomy tube placement and 1 patient (2%) experienced panic attack with HBOT.
To our knowledge, there is only one retrospective observational study that compared the outcomes of antibiotic therapy alone to antibiotic therapy with HBOT.57 This study compared 23 patients who were only treated with antibiotics to 19 patients who were treated with HBOT with antibiotic therapy and found that group with adjuvant HBOT had a shorter time to symptom improvement, lower recurrence rate, and higher cure rate. Interestingly, improvement in facial nerve palsy was observed in 75% (n = 4) in those treated with adjuvant HBOT as compared to 0% (n = 2) in the antibiotic only group.57 Although the treatment protocol in this observational study was not standardized, it suggests potential efficacy of HBOT in treating MOE and highlights the need for further investigation.
Limitations
To date, there are no completed randomized controlled trials that evaluate the efficacy of adjuvant HBOT in the treatment of MOE. Most of the available data are drawn from case reports or observational studies; hence, no studies with high levels of evidence were included and the included studies are subject to reporting bias. Furthermore, the variabilities in the treatment modalities and settings limit the generalizability of the results. Although lack of rigorous trials compromise's the level of evidence and the strength of recommendation, it draws attention to the need for investigations to evaluate the efficacy of HBOT in MOE. The rarity of disease as well as accessibility to hyperbaric chambers pose barriers in conducting prospective robust trials to determine the value of HBOT in MOE.
Conclusion
HBOT may be an effective treatment option for refractory or advanced MOE but its efficacy remains unproven due to lack of strong scientific evidence. However, its therapeutic value should not be underestimated given good results and few adverse events reported in this study.
Conflicts of interest
None.
Funding
None.
Edited by Yu-Xin Fang
Footnotes
Peer review under responsibility of Chinese Medical Association.
Reference
- 1.Cohen D., Friedman P. The diagnostic criteria of malignant external otitis. J Laryngol Otol. 1987;101:216–221. doi: 10.1017/s0022215100101562. [DOI] [PubMed] [Google Scholar]
- 2.Bowles P.F., Perkins V., Schechter E. Fungal malignant otitis externa. BMJ Case Rep. 2017;2017 doi: 10.1136/bcr-2016-218420. bcr2016218420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tarazi A.E., Al-Tawfiq J.A., Abdi R.F. Fungal malignant otitis externa: pitfalls, diagnosis, and treatment. Otol Neurotol. 2012;33:769–773. doi: 10.1097/MAO.0b013e3182565b46. [DOI] [PubMed] [Google Scholar]
- 4.Bovo R., Benatti A., Ciorba A., Libanore M., Borrelli M., Martini A. Pseudomonas and Aspergillus interaction in malignant external otitis: risk of treatment failure. Acta Otorhinolaryngol Ital. 2012;32:416–419. [PMC free article] [PubMed] [Google Scholar]
- 5.Mahdyoun P., Pulcini C., Gahide I. Necrotizing otitis externa: a systematic review. Otol Neurotol. 2013;34:620–629. doi: 10.1097/MAO.0b013e3182804aee. [DOI] [PubMed] [Google Scholar]
- 6.Jacobsen L.M., Antonelli P.J. Errors in the diagnosis and management of necrotizing otitis externa. Laryngoscope. 2010;120(Suppl 4):S207. doi: 10.1002/lary.21673. [DOI] [PubMed] [Google Scholar]
- 7.Marina S., Goutham M.K., Rajeshwary A., Vadisha B., Devika T. A retrospective review of 14 cases of malignant otitis externa. J Otol. 2019;14:63–66. doi: 10.1016/j.joto.2019.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis J.C., Gates G.A., Lerner C., Davis M.G., Mader J.T., Dinesman A. Adjuvant hyperbaric oxygen in malignant external otitis. Arch Otolaryngol Head Neck Surg. 1992;118:89–93. doi: 10.1001/archotol.1992.01880010093022. [DOI] [PubMed] [Google Scholar]
- 9.Phillips J.S., Jones S.E. Hyperbaric oxygen as an adjuvant treatment for malignant otitis externa. Cochrane Database Syst Rev. 2005:CD004617. doi: 10.1002/14651858.CD004617.pub2. [DOI] [PubMed] [Google Scholar]
- 10.Weaver L. 13rd ed. Undersea and Hyperbaric Medical Society; 2014. Hyperbaric Oxygen Therapy Indications. [PubMed] [Google Scholar]
- 11.Mathieu D., Marroni A., Kot J. Correction to Mathieu D, Marroni A, Kot J: tenth European Consensus Conference on Hyperbaric Medicine: recommendations for accepted and non-accepted clinical indications and practice of hyperbaric oxygen treatment. Diving Hyperb Med. 2017 Mar;47(1):24–32. doi: 10.28920/dhm47.1.24-32. Diving Hyperb Med. 2017;47:131-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8:336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 13.Howick J.C.I., Glasziou P. Oxford Centre for Evidence-Based Medicine; 2014. Explanation of the 2001 Oxford Centre for Evidence-Based Medicine (OCEBM) Levels of Evidence (Background Document) [Google Scholar]
- 14.Higgins J.P.T., Sterne J.A.C. The Cochrane Collaboration; 2011. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. [Google Scholar]
- 15.Mader J.T., Love J.T. Malignant external otitis. Cure with adjunctive hyperbaric oxygen therapy. Arch Otolaryngol. 1982;108:38–40. doi: 10.1001/archotol.1982.00790490040011. [DOI] [PubMed] [Google Scholar]
- 16.Joachims H.Z., Danino J., Raz R. Malignant external otitis: treatment with fluoroquinolones. Am J Otolaryngol. 1988;9:102–105. doi: 10.1016/s0196-0709(88)80014-0. [DOI] [PubMed] [Google Scholar]
- 17.Shupak A., Greenberg E., Hardoff R., Gordon C., Melamed Y., Meyer W.S. Hyperbaric oxygenation for necrotizing (malignant) otitis externa. Arch Otolaryngol Head Neck Surg. 1989;115:1470–1475. doi: 10.1001/archotol.1989.01860360072021. [DOI] [PubMed] [Google Scholar]
- 18.Gordon G., Giddings N.A. Invasive otitis externa due to Aspergillus species: case report and review. Clin Infect Dis. 1994;19:866–870. doi: 10.1093/clinids/19.5.866. [DOI] [PubMed] [Google Scholar]
- 19.Bath A.P., Rowe J.R., Innes A.J. Malignant otitis externa with optic neuritis. J Laryngol Otol. 1998;112:274–277. doi: 10.1017/s0022215100158335. [DOI] [PubMed] [Google Scholar]
- 20.Genden E.M., Goebel J.A. Escherichia coli osteomyelitis of the skull base. Otolaryngol Head Neck Surg. 1998;118:853–855. doi: 10.1016/S0194-5998(98)70281-5. [DOI] [PubMed] [Google Scholar]
- 21.Lancaster J., Alderson D.J., McCormick M. Non-pseudomonal malignant otitis externa and jugular foramen syndrome secondary to cyclosporin-induced hypertrichosis in a diabetic renal transplant patient. J Laryngol Otol. 2000;114:366–369. doi: 10.1258/0022215001905580. [DOI] [PubMed] [Google Scholar]
- 22.Marzo S.J., Leonetti J.P. Invasive fungal and bacterial infections of the temporal bone. Laryngoscope. 2003;113:1503–1507. doi: 10.1097/00005537-200309000-00016. [DOI] [PubMed] [Google Scholar]
- 23.Okpala N.C., Siraj Q.H., Nilssen E., Pringle M. Radiological and radionuclide investigation of malignant otitis externa. J Laryngol Otol. 2005;119:71–75. doi: 10.1258/0022215053222978. [DOI] [PubMed] [Google Scholar]
- 24.Singh A., Al K.M., Hyder M.J. Skull base osteomyelitis: diagnostic and therapeutic challenges in atypical presentation. Otolaryngol Head Neck Surg. 2005;133:121–125. doi: 10.1016/j.otohns.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 25.Narozny W., Kuczkowski J., Stankiewicz C., Kot J., Mikaszewski B., Przewozny T. Value of hyperbaric oxygen in bacterial and fungal malignant external otitis treatment. Eur Arch Otorhinolaryngol. 2006;263:680–684. doi: 10.1007/s00405-006-0033-y. [DOI] [PubMed] [Google Scholar]
- 26.Ling S.S., Sader C. Fungal malignant otitis externa treated with hyperbaric oxygen. Int J Infect Dis. 2008;12:550–552. doi: 10.1016/j.ijid.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 27.Leahy T.W., Sader C. A rare case of bilateral malignant otitis externa and osteomyelitis with lower cranial nerve sequelae. BMJ Case Rep. 2011;2011 doi: 10.1136/bcr.03.2011.3957. bcr0320113957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manso M.C., Rodeia S.C., Rodrigues S., Cavilhas P., Domingos R. Malignant otitis externa and stroke. Eur J Case Rep Intern Med. 2016;3 doi: 10.12890/2016_000387. 000387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amaro C.E., Espiney R., Radu L., Guerreiro F. Malignant (necrotizing) externa otitis: the experience of a single hyperbaric centre. Eur Arch Otorhinolaryngol. 2019;276:1881–1887. doi: 10.1007/s00405-019-05396-7. [DOI] [PubMed] [Google Scholar]
- 30.World Health Organization Global report on diabetes. https://www.who.int/publications-detail/global-report-on-diabetes
- 31.Bhasker D., Hartley A., Agada F. Is malignant otitis externa on the increase? A retrospective review of cases. Ear Nose Throat J. 2017;96:E1–E5. doi: 10.1177/014556131709600211. [DOI] [PubMed] [Google Scholar]
- 32.Rubin J., Yu V.L. Malignant external otitis: insights into pathogenesis, clinical manifestations, diagnosis, and therapy. Am J Med. 1988;85:391–398. doi: 10.1016/0002-9343(88)90592-x. [DOI] [PubMed] [Google Scholar]
- 33.Berenholz L., Katzenell U., Harell M. Evolving resistant pseudomonas to ciprofloxacin in malignant otitis externa. Laryngoscope. 2002;112:1619–1622. doi: 10.1097/00005537-200209000-00017. [DOI] [PubMed] [Google Scholar]
- 34.Chandler J.R. Malignant external otitis. Laryngoscope. 1968;78:1257–1294. doi: 10.1288/00005537-196808000-00002. [DOI] [PubMed] [Google Scholar]
- 35.Levenson M.J., Parisier S.C., Dolitsky J., Bindra G. Ciprofloxacin: drug of choice in the treatment of malignant external otitis (MEO) Laryngoscope. 1991;101:821–824. doi: 10.1288/00005537-199108000-00004. [DOI] [PubMed] [Google Scholar]
- 36.Mani N., Sudhoff H., Rajagopal S., Moffat D., Axon P.R. Cranial nerve involvement in malignant external otitis: implications for clinical outcome. Laryngoscope. 2007;117:907–910. doi: 10.1097/MLG.0b013e318039b30f. [DOI] [PubMed] [Google Scholar]
- 37.Eliashiv A., Olumide F., Norton L., Eiseman B. Depression of cell-mediated immunity in diabetes. Arch Surg. 1978;113:1180–1183. doi: 10.1001/archsurg.1978.01370220066011. [DOI] [PubMed] [Google Scholar]
- 38.Mowat A., Baum J. Chemotaxis of polymorphonuclear leukocytes from patients with diabetes mellitus. N Engl J Med. 1971;284:621–627. doi: 10.1056/NEJM197103252841201. [DOI] [PubMed] [Google Scholar]
- 39.Naghibi M., Smith R.P., Baltch A.L. The effect of diabetes mellitus on chemotactic and bactericidal activity of human polymorphonuclear leukocytes. Diabetes Res Clin Pract. 1987;4:27–35. doi: 10.1016/s0168-8227(87)80030-x. [DOI] [PubMed] [Google Scholar]
- 40.Geerlings S.E., Hoepelman A.I. Immune dysfunction in patients with diabetes mellitus (DM) FEMS Immunol Med Microbiol. 1999;26:259–265. doi: 10.1111/j.1574-695X.1999.tb01397.x. [DOI] [PubMed] [Google Scholar]
- 41.Babior B.M. Oxygen-dependent microbial killing by phagocytes (second of two parts) N Engl J Med. 1978;298:721–725. doi: 10.1056/NEJM197803302981305. [DOI] [PubMed] [Google Scholar]
- 42.Knighton D.R., Halliday B., Hunt T.K. Oxygen as an antibiotic. The effect of inspired oxygen on infection. Arch Surg. 1984;119:199–204. doi: 10.1001/archsurg.1984.01390140057010. [DOI] [PubMed] [Google Scholar]
- 43.Mader J.T., Brown G.L., Guckian J.C., Wells C.H., Reinarz J.A. A mechanism for the amelioration by hyperbaric oxygen of experimental staphylococcal osteomyelitis in rabbits. J Infect Dis. 1980;142:915–922. doi: 10.1093/infdis/142.6.915. [DOI] [PubMed] [Google Scholar]
- 44.Niinikoski J., Grislis G., Hunt T.K. Respiratory gas tensions and collagen in infected wounds. Ann Surg. 1972;175:588–593. doi: 10.1097/00000658-197204000-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hohn D.C., MacKay R.D., Halliday B., Hunt T.K. Effect of O2 tension on microbicidal function of leukocytes in wounds and in vitro. Surg Forum. 1976;27:18–20. [PubMed] [Google Scholar]
- 46.Hunt T.K., Pai M.P. The effect of varying ambient oxygen tensions on wound metabolism and collagen synthesis. Surg Gynecol Obstet. 1972;135:561–567. [PubMed] [Google Scholar]
- 47.Bingham E.L., Hart G.B. Hyperbaric oxygen treatment of refractory osteomyelitis. Postgrad Med. 1977;61:70–76. doi: 10.1080/00325481.1977.11712216. [DOI] [PubMed] [Google Scholar]
- 48.Savvidou O.D., Kaspiris A., Bolia I.K. Effectiveness of hyperbaric oxygen therapy for the management of chronic osteomyelitis: a systematic review of the literature. Orthopedics. 2018;41:193–199. doi: 10.3928/01477447-20180628-02. [DOI] [PubMed] [Google Scholar]
- 49.Shields R.C., Nichols F.C., Buchta W.G., Claus P.L. Hyperbaric oxygen therapy for chronic refractory osteomyelitis of the sternum. Ann Thorac Surg. 2010;89:1661–1663. doi: 10.1016/j.athoracsur.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 50.Chen C.Y., Lin K.P., Lu S.H., Chen Y.J., Lin C.F. Adjuvant hyperbaric oxygen therapy in the treatment of hemodialysis patients with chronic osteomyelitis. Ren Fail. 2008;30:233–237. doi: 10.1080/08860220701813384. [DOI] [PubMed] [Google Scholar]
- 51.Stevens S.M., Lambert P.R., Baker A.B., Meyer T.A. Malignant otitis externa: a novel stratification protocol for predicting treatment outcomes. Otol Neurotol. 2015;36:1492–1498. doi: 10.1097/MAO.0000000000000839. [DOI] [PubMed] [Google Scholar]
- 52.Eveleigh M.O., Hall C.E., Baldwin D.L. Prognostic scoring in necrotising otitis externa. J Laryngol Otol. 2009;123:1097–1102. doi: 10.1017/S0022215109990491. [DOI] [PubMed] [Google Scholar]
- 53.Stern S.S., Soudry E., Hamzany Y., Nageris B. Malignant external otitis: factors predicting patient outcomes. Am J Otolaryngol. 2016;37:425–430. doi: 10.1016/j.amjoto.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 54.Franco-Vidal V., Blanchet H., Bebear C., Dutronc H., Darrouzet V. Necrotizing external otitis: a report of 46 cases. Otol Neurotol. 2007;28:771–773. doi: 10.1097/MAO.0b013e31805153bd. [DOI] [PubMed] [Google Scholar]
- 55.hadanny A., Meir O., Bechor Y., Fishlev G., Bergan J., Efrati S. The safety of hyperbaric oxygen treatment--retrospective analysis in 2,334 patients. Undersea Hyperb Med. 2016;43:113–122. [PubMed] [Google Scholar]
- 56.Yamamoto Y., Noguchi Y., Enomoto M., Yagishita K., Kitamura K. Otological complications associated with hyperbaric oxygen therapy. Eur Arch Otorhinolaryngol. 2016;273:2487–2493. doi: 10.1007/s00405-015-3845-9. [DOI] [PubMed] [Google Scholar]
- 57.Mardassi A., Turki S., Lahiani R., Mbarek H., Benzarti S., Gharsallah H. Is there a real benefit of hyperbaric oxygenotherapy in the treatment of necrotizing otitis externa. Tunis Med. 2016;94:863. [PubMed] [Google Scholar]