Abstract
Background
Meniere's disease (MD) is an idiopathic disorder of the inner ear, which manifests as cochleo-vestibular dysfunction. Hearing loss will progress to a profound levelin a subset of patients with MD, and vestibular interventions can independently cause loss of hearing. The aim of this study was to systematically review the published literature describing the safety and efficacy of CI in patients with MD.
Materials and methods
A systematic literature review was conducted in accordance PRISMA guidelines to identify articles that assessed at least one functional outcome in patients with MD who underwent CI. Demographic information, disease history, MD symptoms, outcomes measures, and complications related to CI were extracted from included studies.
Results
In total, 17 studies were included, and 182 patients with MD underwent CI. The weighted-mean age was 61.9 years (range 27–85). Study objective and methodology varied, and there was significant heterogeneity in CI outcome measures reported. In total, 179 (98.3%) of 182 patients reported objective improvements in at least one hearing metric after CI. A total of 69 patients (37.9%) reported vertigo or severe dizziness prior to CI, compared to 22 patients (15.4%) postoperatively. Two studies reported significant reductions in postoperative Tinnitus Handicap Inventory score (THI). Quality of life assessments varied between studies. Complications rates were low with only nine patients (4.9%) reporting a serious CI-related complication.
Conclusions
This systematic review evaluated 17 studies describing the safety and efficacy of CI in patients with MD and encountered many challenges due to small sample sizes, and heterogeneity in study design and outcomes measured. Despite these limitations, this study of 182 patients is to the best of our knowledge the largest systematic review evaluating the safety and efficacy of CI in MD. The results of this study support the need for a standardized approach to evaluating outcomes of CI in patients with MD in future studies.
Keywords: Cochlear implantation, Meniere's disease
Introduction
Meniere's disease (MD) is an idiopathic disorder of the inner ear. Potential etiologic sources include endolymphatichydrops, an increase in endolymphatic pressure in the membranous labyrinth, as well as a variety of anatomic or physiologic conditions such as ischemia orautoimmune injury to the inner ear. Clinically, cochleo-vestibular dysfunction manifests as a syndromic combination of sporadic vertigo, ear fullness, roaring tinnitus, and progressive sensorineural hearing loss (SNHL).1,2 Hearing loss in MDtraditionally affects low frequencies and fluctuates, whereas high-frequency hearing losses tend to be nonfluctuating.3 The severity of each symptom can vary among patients and even for the same patient overtime. In some cases, the intensity of the vestibular symptoms can be severe, with only minor impact on hearing or vice versa. Others experience the entire range of ailments in great severity, with intrusive symptoms that tremendously impact quality of life.4
MD is typically unilateral and has a reported incidence of 3.5–513 cases per 100,000 per year.5 Less is known about the bilateral variant. Treatment options for MD focus on minimizing vestibular symptoms while preserving hearing; the most effective management plan is determined on a case-by-case basis. The loss of hearing tends to be greatest at the beginning of the disease, with eventual stabilization at moderate-to-severe hearing loss.6,7 However, a large portion of this population will progress to profound hearing loss, rendering them postlingually deaf in one or both ears.3
The utility of cochlear implantation (CI) has allowed for treatment of hearing loss in patients with MD, whether due to the disease process itself or secondary to vestibular interventions.8 The role of CI in treating MD-associated hearing loss has not been thoroughly investigated to date. Although implementation of CI in MD has become more common, clinical outcomes remain unclear. The aim of this study was to systematically review the published literature describing the safety and efficacy of CI in patients with MD.
Methods
Study design
This systematic review was undertaken following the PRISMA guidelines (Fig. 1).9 Databases searched included PubMed/MEDLINE, Scopus, and Cochrane Database of Systematic Review. Search strategy consisted of two terms (cochlear implant, and Meniere disease) combined with MESH terms and variations in the spelling of MD (i.e. meniere's, menieres, meniere). This strategy was mimicked and adjusted for each database, searching from inception through July 24th, 2019. The Preferred Reporting Items for Systematic Reviews and Meta-Analysis statement was used throughout this systematic review.10 Two authors (VD, JP) screened all articles by title and abstract for relevance of CI in MD. Articles not written in English were excluded, along with zoological studies, editorials, book chapters, and reviews. After initial screening, two authors independently reviewed the full-text of remaining studies for both inclusion and exclusion criteria. References of included studies were reviewed in search of any additional studies not identified in the initial search. Studies included for qualitative synthesis included the following criteria: (1) CI in patients with MD, (2) Must assess some functional status of patients receiving CI, either through objective or subjective testing, ideally before and after implantation. Available data were extracted from each study including demographics, laterality of MD, duration of MD, CI used, MD symptoms pre and post CI, complications of CI, quality of life assessment, and any tests utilized in assessing functional status, both pre and post CI. Data that were not extractable were considered missing and were excluded from the final data set.
Figure 1.
PRISMA diagram.
Quality review and assessment of risk of bias
Level of evidence for each included article was performed using Oxford Center for Evidence-Based Medicine (OCEBM).11 The risk of bias was assessed according to the Cochrane Handbook for Systematic Reviews of Interventions version 5.1.0.12 Two authors (VD and JP) performed a pilot assessment on three studies to check for consistency of assessment. Both then performed independent risk assessment on the remaining studies. All disagreements were resolved by the way of discussion with a third author (SAN). Risk of bias items included the following: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias. The risk of bias for each aspect is graded as low, unclear, or high.
Statistical methods
Given the heterogeneity and lack of adequate data in outcome metrics, no meta-analysis or statistical tests were performed.
Results
Study selection and characteristics
Initial search returned 332 articles. After 53 duplicates were removed, the remaining 279 articles were reviewed by title and abstract. Full text review was performed for 29 articles, and 17 met criteria for inclusion (Table 1).3,13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28 The date of publication ranged from 1999 to 2018, and studies ranged in size from one to twenty-seven patients. The studies were conducted in seven different countries, with the United States of America being most prevalent (n = 7). There were three Australian studies, two from the United Kingdom and two from Germany. There was one article from each of Spain, Italy and Belgium. Study types varied, and included 13 retrospective studies (76.5%) and four prospective studies (23.5%). In terms of design, six studies (35.3%) compared CI outcomes between patients with MD and a CI control group of patients without MD. One study (5.9%) compared CI outcomes of patients with active MD symptoms and without active MD symptoms to a control population. A total of 39 patients (21.4%) underwent ipsilateral surgical labyrinthectomy in addition to CI, and one study (5.9%) directly compared CI outcomes in patients with and without labyrinthectomy.
Table 1.
Study details and patient characteristics.
| Author (year) | Country | Study Design | OLE | n | Male (n) | Mean Age (Range) | MD Ear | Mean Duration (year) |
|---|---|---|---|---|---|---|---|---|
| Manrique-Huarte21 (2018) | Spain | Prospective | 2b | 23 | 15 | 59 | U-22 BL-1 |
11 |
| Canzi13 (2017) | Italy | Prospective | 4 | 4 | 2 | 58.2 (48–69) | U-4 | 10.5 |
| Mukherjee24 (2017) | ||||||||
| A | Australia | Retrospective | 2b | 22 | 13 | 62.7 (42–76) | U-3 BL-19 | 20.2 |
| B | 6 | 2 | 65.6 (50–75) | U-5 BL-1 |
28.5 | |||
| C | 3 | 3 | 71.6 (59–84) | U-2 BL-1 |
30 | |||
| Prenzler25 (2017) | Germany | Retrospective | 2b | 27 | 15 | 57.2 | U-27 | 29.5 |
| Heywood17(2016) | Australia | Retrospective | 4 | 2 | 0 | 73.5 (63–84) | U-2 | – |
| Doobe14 (2015) | Germany | Prospective | 4 | 5 | 2 | 61 (46–76) | U-5 | – |
| Samy26 (2015) | USA | Retrospective | 3b | 8 | – | 63.5 | – | 10 |
| Shi27 (2015) | Australia | Retrospective | 4 | 1 | 1 | 53 | BL-1 | 30 |
| Fife15 (2014) | USA | Retrospective | 4 | 10 | 6 | 65 (42–84) | U-9 BL-1 |
– |
| MacKeith20 (2014) | UK | Retrospective | 4 | 2 | 2 | 45 (43–47) | U-2 | – |
| McRackan3 (2014) | USA | Retrospective | 4 | 21 | 10 | 65.3 (27–85) | U-2 BL-19 |
– |
| Mick22 (2014) | USA | Retrospective | 2b | 20 | 12 | 68.2 | U-4 BL-16 |
26.6 |
| Vermeire28 (2014) | Belgium | Retrospective | 4 | 7 | – | 67 (57–72) | – | 18 |
| Hansen16 (2013) | USA | Prospective | 4 | 10 | – | 54.3 (42–63) | U-10 | 2.5a |
| Holden18 (2012) | USA | Retrospective | 4 | 1 | 0 | 45 | BL-1 | 8 |
| Lustig19 (2003) | USA | Retrospective | 4 | 9 | 3 | 61 (30–77) | U-2 BL-7 |
27 |
| Morgan23 (1999) | UK | Retrospective | 4 | 1 | 0 | 57 | BL-1 | 30 |
Mukherjee (2017): Group A = CI without labyrinthectomy, Group B = CI with labyrinthectomy performed simultaneously, Group C = CI with prolonged delay after labyrinthectomy; OLE, Oxford Level of Evidence; MD, Meniere's Disease; U, Unilateral; BL, Bilateral.
Average years of deafness reported.
Clinical data and outcome measures for each study are detailed in Table 2. All 17 studies reported at least one hearing outcome measure, although audiometric evaluation varied in each. Four articles (23.5%) included quality of life outcome measures. One study, assessed quality of life using the Hearing Handicap Inventory (HHI) and MD-Functional level scale (MD-FLS), which were both administered postoperatively. One study evaluated quality of life by comparing pre and postoperative scores for Tinnitus Handicap Inventory (THI), Dizziness Handicap Inventory (DHI), MD Patient-Oriented Severity Index (MDPOSI) and MD-FLS. One used the Nijmegen Cochlear Implant Questionnaire (NCIQ) to assess health-related quality of life after CI, and one compared THI and 36-item Short Form (SF-26) scores before and after CI.
Table 2.
Clinical data and outcome measures reported by studies.
| Author (year) | Treatment (n) | Device (n) | Outcomes | Complications (n) |
|---|---|---|---|---|
| Manrique-Huarte21 (2018) | Unreported | Unreported | PTA, WRS, VHIT, VMP | None reported |
| Canzi13 (2017) | IT Gent (4) | Unreported | HIT, PTA, Open-set SRS, THI, MD-FLS, DHI, MDPOSI | None reported |
| Mukherjee24 (2017) | CI w/o Lab (22), CI + Lab(6), CI + Lab delayed (3) |
CN: CI512 (5) | PTA, BKB, CUNY | Bilateral vestibular failure, oscillopsia, vertigo (1) |
| Prenzler25 (2017) | IT Gent (4), VN (1), ELS (5), 1 Myringotomy, 15 BH | Unreported | FES65, HSM | None reported |
| Heywood17 (2016) | Unreported | CN: Freedom (1), ME: Concerto (1) | CNC, CUNY, BKB | None reported |
| Doobe14 (2015) | CI + Lab(5) | Unreported | DHI, SRS | None reported |
| Samy26 (2015) | Unreported | Unreported | CNC, HINT | Dizziness and disabling tinnitus (1) |
| Shi27 (2015) | IT HCTZ, BH, AH (1) | Unreported | Serial audiometry, BKB | None reported |
| Fife15 (2014) | Lab (2), ELS (2) | CN: N24 (1), CI512 (8), CI24RE (3) | HHI, MD-FLS, HINT, AzBio | Device failure (1) |
| MacKeith20 (2014) | CI + Lab(2) | CN: CI500 (2) | PTA, BKB | None reported |
| McRackan3 (2014) | Medical management (13)a, ITCS (2), IT Gent (1), ELS (3), Lab (2) | Unreported | CNC | None reported |
| Mick22 (2014) | Unreported | AB: Clarion 1.2 (2), HR90K (11), HFI(1), CN: N22 (1), N24 (2), ME: Pulsar CI100 (1), Sonata (2) | CID, CUNY, HINT. CNC, THI, SF-36 | New-onset vertigo (1), electrode extrusion (1) |
| Vermeire28 (2014) | Unreported | AB: HR90K (3), CN: CI512 (2), CI24RE (2) | NVA, LIST, NCIQ | New-onset, disabling vertigo (1) |
| Hansen16 (2013) | CI + Lab(10) | CN: CI512 (1), Freedom (2), CI422 (6), AB: HR90K (1) | CNC, AzBio | None reported |
| Holden18 (2012) | IT Dexa, IT Gent, ELS (1) | CN: N24 Contour (1), Freedom (1) | CNC, BKB, sound localization | None reported |
| Lustig19 (2003) | ELS (5), PF (1), Lab (1) | AB: Clarion 1.2 (3), HFI (2), HFCII (2), CN: N22 (1) | Monosyllabic words/phonemes, CID, HINT | Skin flap revision (1), extreme dizziness (1), device failure (1) |
| Morgan23 (1999) | CI + Lab (1) | Digisonic: DX10 (1) | PTA, BKB | None reported |
IT, Intratympanic; Gent, Gentamicin; CI, Cochlear Implant; Lab, Labyrinthectomy;VN, Vestibular Neurectomy; ELS, Endolymphatic Sac Surgery; BH, Betahistine; HCTZ, Hydrochlorothiazide; ITCS, Intratympanic corticosteroid; PF, Perilymphatic fistula; CN, Cochlear (Nucleus); ME, MED-EL; AB, Advanced Bionics; AH, Amiloride Hydrochloride; PTA, Pure tone auditory threshold average; WRS, Word Recognition Score; VHIT, video head impulse test; VMP, vestibular myogenic potentials; HIT, Head impulse test and caloric testing; THI, Tinnitus Handicap Inventory; SRS, Speech Recognition Score; MD-FLS, MD - Functional Level Scale; DHI, Dizziness Handicap Inventory; MDPOSI, MDPatient-Oriented Severity Index; BKB, Bamford-Kowal-Bench test; CUNY, City University of New York test; FES65, Freiburger Einsilber test; HSM, Hochmair-Schulz-Moser test; CNC, Consonant-Nucleus-Consonant word score; HINT, Hearing In Noise Test; HHI, Hearing Handicap Inventory; CID, Central Institute for the Deaf test; SF-36, 36-item Short Form; NVA, Nederlandse Vereniging voor Audiologie monosyllabic word test; LIST, Sentences in quiet and noise test.
Composition of medical management was not provided.
Quality assessment
Based on the Oxford Level of Evidence (Table 1), four of the 17 studies are deemed as level 2b evidence, one is deemed level 3b evidence and 12 are deemed level 4 evidence.
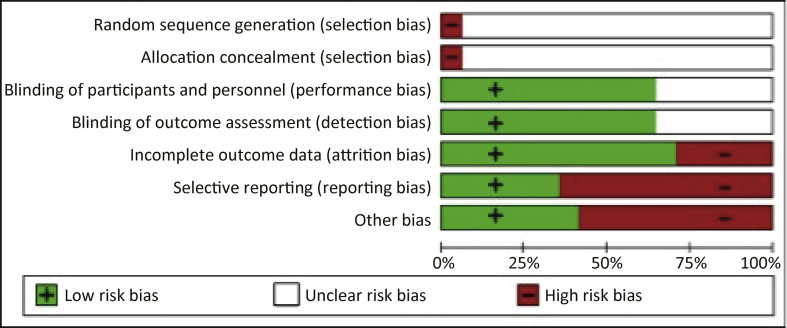
The risks of bias in the included trials are summarized in Figs. 2 and 3.
Figure 2.
Risk of bias graph: review authors' judgments about each risk of bias item for all studies collectively. Percentage of all studies with high risk of bias, low risk of bias or unclear risk of bias for each risk of bias item.
Figure 3.
Risk of bias summary: review authors' judgments about each risk of bias item for each individual study.
Cohort characteristics
A total of 182 patients with MD underwent CI and were included in this review. The weighted-mean age of patients was 61.9 years with a range of 27–85 years. Of the studies that reported gender, there were 88 males (56%) and 69 females (44%). Unilateral disease was reported in 99 patients (59%), while 68 patients (41%) had bilateral MD. The weighted-mean time from symptom onset to CI was 20.2 years with a range of 8–30 years.
Audiologic benefits of cochlear implantation in Meniere's disease
Overall, the hearing outcomes for patients with MD were positive with the vast majority demonstrating objective improvements in at least one hearing metric following implantation. In total, 179 (98.4%) of 182 patients were reported to have good hearing outcomes or improved auditory function in measures reported by their respective study following CI.
Six studies compared CI outcomes for patients with MD to a control group of patients with CI without MD. The majority of comparisons showed no statistically significant difference in hearing outcomes between the MD and control groups. One study reported a statistically significant difference in postoperative Consonant-Nucleus-Consonant (CNC) in favor of the control group rather than patients with MD.3 A subset analysis revealed that patients with inactive MD had statistically significant lower CNC scores than the controls, while there was no significant difference between the controls and those with active MD.3 In one article, patients with MD outperformed the control group in Central Institute for the Deaf (CID) sentences at six-months and one-year.19 A third study demonstrated that patients with MD had significantly better audiometric performance than the control group after first fitting.25 However, repeat assessment at one-year follow-up failed to show significant differences between the groups. The remaining studies compared post-CI hearing outcomes for patients with MD versus control but failed to demonstrate significant differences.
A decline in speech perception scores was reported in only three (1.6%) of 182 total patients. Two had undergone simultaneous ipsilateral surgical labyrinthectomy at the time of CI, and chemical labyrinthectomy was performed in the other. One patient demonstrated a 60% increase in Bench-Koval-Bamford (BKB) score in 50 dB of background noise, but also decreased from 98% to 84% in quiet conditions.20 After preoperative BKB scores were measured, the author reported that the patient's hearing had worsened leading up to CI. However, repeat BKB scores reflecting the change were not available.20 Thus, the comparison of the BKB scores might be an inaccurate result. Another patient was reported to have a 38% decrease in BKB score when lip-reading and listening with CI turned on.23 This was measured six-weeks postoperatively, before the full effects of active rehabilitation were likely to be observed.23,29 In the third patient with a reportedly poor hearing outcome, supplemental lip-reading was required even after CI.24 This patient had undergone five prior intratympanic applications of gentamicin, and there was a two-year delay between labyrinthectomy and CI.
Dizziness/vertigo
In all, there were a total of 69 patients (37.9%) reportedly experiencing vertigo or severe dizziness within the one-year prior to CI.3,13,14,16,19,20,22, 23, 24,28 After CI, this decreased resulting in only 22 patients (12.1%) reporting vertigo. None of the 39 patients (21.4%) who underwent CI with simultaneous labyrinthectomy or CI sequential to labyrinthectomy experienced long-term vertigo or dizziness after CI. Of the 143 patients (78.6%) in whom no labyrinthectomy was performed, 30 patients (16.5%) reported preoperative vertigo, and 22 patients (15.4%) reported vertigo postoperatively. Vertigo resolved within three-months after CI in 11 of the 22 patients, and long-term or chronic vertigo was reported in the remaining 11 patients (6%).
Tinnitus
Two articles used the Tinnitus Handicap Inventory (THI) to assess the impact of tinnitus on patient perceived quality of life.13,22 In one study of four patients, THI scores improved from a mean score of 77.3 preoperatively to 6.0 postoperatively.13 Another study reported THI scores for 15 patients, which improved from a median of 37.0 preoperatively to a median of 9.0 one-year postoperatively.22 Two studies reported a total of 13 patients that endorsed tinnitus preoperatively, which decreased to only six experiencing postoperative tinnitus.19,28 One patient in a separate study subjectively reported a significant reduction in tinnitus after CI.23 Worsening tinnitus was reported by Samy et al in one patient that developed persistent debilitating tinnitus following CI, which ultimately lead to explantation.27
Quality of life
Two studies used the Meniere's Disease Functional Level Scale (MD-FLS), which is a six-point scaling system used to evaluated patient functionality related to their vestibular symptoms.13,15 In one study containing ten patients, the mean MD-FLS score improved only slightly from 3.9 preoperatively to 3.4 postoperatively.15 The same ten patients also reported a collective eight-fold reduction in annual vertigo episodes following CI. Another study reported an improvement in MD-FLS in four patients from a mean of 4.8 prior to implantation to a mean of 1.5 postoperatively.13 The Meniere's Disease Patient-Oriented Severity Index (MDPOSI) is a 23-item instrument designed to assess the disease-specific quality of life of people with MD both during, and between attacks. One study reported an improvement in MDPOSI scores from a preoperative mean of 73.5 to a mean of 18.75 postoperatively.13 Another study reported median pre- and postoperative Short-Form Health Survey (SF-36) scores for each of the eight domains.22 For seven of the eight SF-36 domains, the median scores were improved one-year postoperatively compared to preoperative scores. Median scores for the energy/vitality domain did not change, and none of the median domain scores worsened after CI. Improvements were also reported in THI and DHI, each by two studies.13,14,22
Complications
The overall rate of reported complications was low. In total, 182 patients were reviewed, and complications were reported in nine patients (4.9%). Of these nine, five (55.6%) had bilateral disease, three (33.3%) had unilateral MD, and laterality was not reported for one (11.1%). None had undergone concurrent labyrinthectomy, while four (44.4%) had previously undergone endolymphatic sac decompressions. One of the seven (14.3%) patients reported by Vermiere et al developed incapacitating vestibular attacks after implantation, which was not present prior to implantation.28 Fife et al reported that one of the ten patients (10%) experienced a device failure one-year after implantation.15 The patient complained of episodic ear pain, headache, vertigo and electrical shocks upon device activation, which began following a hip surgery, for which monopolar electrocautery was used. The device was explanted, and a new device was re-implanted with a good result. Of the 20 patients reported by Mick et al,22 two (10%) experienced an unfavorable long-term outcome following CI. One patient had reported no vertigo episodes in the year prior to implantation but developed near-constant imbalance and spinning vertigo that required the use of a cane. Throughout the five-year follow-up period, the symptoms reportedly improved only slightly. The second patient had bilateral MD, and complained of imbalance and dizziness for one year following CI, before being diagnosed with electrode extrusion. Three of nine patients (33.3%) reported by Lustig et al19 experienced postoperative complications. One required revision of a skin flap, and another was hospitalized for several days due to extreme dizziness. The third experienced a device failure and was scheduled for an explanation and reimplantation with a new device. One of the 31 patients (3.2%) reported by Mukherjee et al24 developed symptoms of bilateral vestibular failure, oscillopsia, and active vertigo postoperatively. This patient reported a poor quality of life and could no longer work. One out of eight patients (12.5) reported by Samy et al26 encountered a complication. The patient underwent explantation and reimplantation due to dizziness and debilitating tinnitus. After reimplantation with a different type of implant, the symptoms persisted, and the patient requested the second device be explanted as well.
Discussion
This systematic review evaluated 17 studies describing the safety and efficacy of CI in patients with MD and encountered many challenges due to small sample sizes, and heterogeneity in study design and outcomes measured. Even when all studies were combined, the sample size remained small. Due to high variability in study objective and methodology, CI outcome measures varied greatly and made direct comparisons difficult in an already limited sample. Patient characteristics were not uniformly reported and allowed for limited evaluation of factors associated with poor outcomes and adverse events. Despite these limitations, our study of 182 patients is to the best of our knowledge the largest systematic review demonstrating the safety and efficacy of CI in MD.
The literature published to date demonstrated that CI is a safe and effective rehabilitation therapy for hearing loss in patients with MD. Outcomes were similar to those experienced by the general CI population and were obtained in patients with and without ipsilateral labyrinthectomy. This supports early histological findings that reported MD ears with end-stage disease, and after labyrinthectomy, had sufficient spiral ganglion cells to benefit from electrical stimulation.30,31 A decline in speech perception scores was reported in only three (1.6%) of 182 total patients. For patients with SNHL directly related to the disease process of MD, CI serves as an effective primary treatment. For those with uncontrolled vertigo, this might allow for effective treatment of vestibular symptoms with secondary rehabilitation of labyrinthectomy-induced unilateral deafness.
The direct effect of CI on vestibular symptoms is less clear and should be managed on a case-to-case basis.13,14,17,20,23,24 Despite an overall reduction in frequency, outcomes for dizziness and vertiginous symptoms varied greatly between individual studies. One factor largely affecting the prevalence or improvement of vertigo postoperatively was the specific surgical technique applied at the time of CI. Of the patients reporting vertigo postoperatively, none had undergone simultaneous or delayed labyrinthectomy with CI. Vertigo resolved within three-months after CI in half of the patients, and long-term or chronic vertigo was reported in the remainder. Surgical labyrinthectomy was efficacious in eliminating vertigo and also associated with similar hearing outcomes to CI alone.13,14,17,20,23,24 The incidence of bilateral vestibular hypofunction was low, and occurred almost exclusively in patients with bilateral MD. This highlights the concept that not all patients with MD make good candidates for CI, and labyrinthectomy should only be performed in selected patients.13,17,20,24 The collective prevalence of tinnitus was decreased after CI, despite limited use of well-structured or validated reporting methods.
Quality of life improvements after CI have been well demonstrated in postlingually deaf patients.32 As a whole, quality of life improved in this cohort of patients with MD after CI. The assessment of quality of life varied greatly between studies, with few reporting outcomes using the validated measures. One study reported only minor reduction in MD-FLS score after CI. Interestingly, the same ten patients also reported a collective eight-fold reduction in annual vertigo episodes following CI. In another study, median scores for the energy/vitality domain of SF-36 did not change. The heterogeneity in quality of life assessment measures reported by this collection of studies does not allow for adequate comparisons and can only be reported on a study-by-study basis. This points to the need for uniformity in future investigations evaluating quality life after CI in patients with MD.
The overall rate of complications was low in the existing literature. Less than 5% of the patients experienced a serious or long-term complication related to CI. A previous study of 403 CI patients reported the rate of major complications at 5%.33 This suggests that patients with MD face a similar complication rate, compared to the general CI population. Complications were more likely in those with bilateral MDand patients with endolymphatic sac decompressions prior to CI. Two of the four complications reported in patients with bilateral MD were likely the result of factors not attributable to CI or MD.19
Conclusion
This systematic review evaluated 17 studies describing the safety and efficacy of CI in patients with MD and encountered many challenges due to small sample sizes, and heterogeneity in study design and outcomes measured. Even when all studies are combined, the sample size remains small with limited individual patient data available. Despite these limitations, this study of 182 patients is to the best of our knowledge the largest systematic review evaluating the safety and efficacy of CI in MD. Based on existing data, patients with MD demonstrated significant hearing improvements following CI and a complication rate similar to non-MD CI recipients. The results of this study support the need for a standardized approach to evaluating outcomes of CI in patients with MD in future studies.
Author contribution
Vincent M. Desiato: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Writing – Original Draft, Writing – Review & Editing, Project administration.
Jaimin J. Patel: Methodology, Validation, Formal analysis, Investigation, Writing – Original Draft, Writing – Review & Editing.
Shaun A. Nguyen: Conceptualization, Methodology, Validation, Formal analysis, Writing – Review & Editing, Project administration.
Ted A. Meyer:Conceptualization, Methodology, Validation, Writing – Review & Editing.
Paul R. Lambert: Conceptualization, Methodology, Validation, Writing – Review & Editing.
Financial disclosures
There are no financial conflicts of interest to disclose.
Declaration of Competing Interest
No conflicts of interest to report.
Edited by Yu-Xin Fang
Footnotes
Peer review under responsibility of Chinese Medical Association.
References
- 1.Committee on Hearing and Equilibrium guidelines for the diagnosis and evaluation of therapy in Menière's disease. Am Acad Otolaryngol Head Neck Found Inc Otolaryngol Head Neck Surg. 1995;113:181–185. doi: 10.1016/S0194-5998(95)70102-8. [DOI] [PubMed] [Google Scholar]
- 2.Berlinger N.T. Meniere's disease: new concepts, new treatments. Minn Med. 2011;94:33–36. [PubMed] [Google Scholar]
- 3.McRackan T.R., Gifford R.H., Kahue C.N. Cochlear implantation in Ménière's disease patients. Otol Neurotol. 2014;35:421–425. doi: 10.1097/MAO.0000000000000247. [DOI] [PubMed] [Google Scholar]
- 4.Ghavami Y., Haidar Y.M., Moshtaghi O., Lin H.W., Djalilian H.R. Evaluating quality of life in patients with Meniere's disease treated as migraine. Ann Otol Rhinol Laryngol. 2018;127:877–887. doi: 10.1177/0003489418799107. [DOI] [PubMed] [Google Scholar]
- 5.Alexander T.H., Harris J.P. Current epidemiology of Meniere's syndrome. Otolaryngol Clin North Am. 2010;43:965–970. doi: 10.1016/j.otc.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Stahle J. Advanced Meniere's disease. A study of 356 severely disabled patients. Acta Otolaryngol. 1976;81:113–119. doi: 10.3109/00016487609107484. [DOI] [PubMed] [Google Scholar]
- 7.Wright T. Menière's disease. BMJ Clin Evid. 2015;2015 [PMC free article] [PubMed] [Google Scholar]
- 8.Wareing M.J., O'Connor A.F. The role of labyrinthectomy and cochlear implantation in Menière's disease. Ear Nose Throat J. 1997;76:664–666. 668, 671-672. [PubMed] [Google Scholar]
- 9.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8:336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 10.Moher D., Liberati A., Tetzlaff J., Altman D.G., PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Plos Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Medicine OCfE-B . The Oxford 2011 levels of evidence; 2011. OCEBM Levels of Evidence Working Group. [Google Scholar]
- 12.Higgins J.P., Green S. John Wiley & Sons; 2011. Cochrane Handbook for Systematic Reviews of Interventions. [Google Scholar]
- 13.Canzi P., Manfrin M., Perotti M. Translabyrinthine vestibular neurectomy and simultaneous cochlear implant for Ménière's disease. Acta Neurochir (Wien) 2017;159:123–130. doi: 10.1007/s00701-016-2996-9. [DOI] [PubMed] [Google Scholar]
- 14.Doobe G., Ernst A., Ramalingam R., Mittmann P., Todt I. Simultaneous labyrinthectomy and cochlear implantation for patients with single-sided Ménière's disease and profound sensorineural hearing loss. Biomed Res Int. 2015;2015:457318. doi: 10.1155/2015/457318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fife T.A., Lewis M.P., May J.S., Oliver E.R. Cochlear implantation in Meniere's disease. JAMA Otolaryngol Head Neck Surg. 2014;140:535–539. doi: 10.1001/jamaoto.2014.550. [DOI] [PubMed] [Google Scholar]
- 16.Hansen M.R., Gantz B.J., Dunn C. Outcomes after cochlear implantation for patients with single-sided deafness, including those with recalcitrant Ménière's disease. Otol Neurotol. 2013;34:1681–1687. doi: 10.1097/MAO.0000000000000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heywood R.L., Atlas M.D. Simultaneous cochlear implantation and labyrinthectomy for advanced Ménière's disease. J Laryngol Otol. 2016;130:204–206. doi: 10.1017/S0022215115003345. [DOI] [PubMed] [Google Scholar]
- 18.Holden L.K., Neely J.G., Gotter B.D., Mispagel K.M., Firszt J.B. Sequential bilateral cochlear implantation in a patient with bilateral Ménière's disease. J Am Acad Audiol. 2012;23:256–268. doi: 10.3766/jaaa.23.4.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lustig L.R., Yeagle J., Niparko J.K., Minor L.B. Cochlear implantation in patients with bilateral Ménière's syndrome. Otol Neurotol. 2003;24:397–403. doi: 10.1097/00129492-200305000-00009. [DOI] [PubMed] [Google Scholar]
- 20.MacKeith S.A., Bottrill L.D., Ramsden J.D. Simultaneous labyrinthectomy with cochlear implantation in patients with bilateral Ménière's disease. Ann Otol Rhinol Laryngol. 2014;123:485–489. doi: 10.1177/0003489414527226. [DOI] [PubMed] [Google Scholar]
- 21.Manrique-Huarte R., Calavia D., Alvarez-Gomez L., Huarte A., Perez-Fernández N., Manrique M. Vestibulo-cochlear function after cochlear implantation in patients with Meniere's disease. J Int Adv Otol. 2018;14:18–21. doi: 10.5152/iao.2018.4536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mick P., Amoodi H., Arnoldner C. Cochlear implantation in patients with advanced Ménière's disease. Otol Neurotol. 2014;35:1172–1178. doi: 10.1097/MAO.0000000000000202. [DOI] [PubMed] [Google Scholar]
- 23.Morgan M., Flood L., Hawthorne M., Raje S. Chemical labyrinthectomy and cochlear implantation for Menière's disease--an effective treatment or a last resort. J Laryngol Otol. 1999;113:666–669. doi: 10.1017/s0022215100144792. [DOI] [PubMed] [Google Scholar]
- 24.Mukherjee P., Eykamp K., Brown D. Cochlear implantation in Ménière's disease with and without labyrinthectomy. Otol Neurotol. 2017;38:192–198. doi: 10.1097/MAO.0000000000001278. http://www.ncbi.nlm.nih.gov/pubmed/27861194 [DOI] [PubMed] [Google Scholar]
- 25.Prenzler N.K., Bültmann E., Giourgas A. Cochlear implantation in patients with definite Meniere's disease. Eur Arch Otorhinolaryngol. 2017;274:751–756. doi: 10.1007/s00405-016-4356-z. [DOI] [PubMed] [Google Scholar]
- 26.Samy R.N., Houston L., Scott M., Choo D.I., Meinzen-Derr J. Cochlear implantation in patients with Meniere's disease. Cochlear Implants Int. 2015;16:208–212. doi: 10.1179/1754762814Y.0000000104. [DOI] [PubMed] [Google Scholar]
- 27.Shi J., Kertesz T. Contralateral cochlear implantation prior to vestibular nerve section for 'drop attacks' in the only hearing ear. J Laryngol Otol. 2015;129(Suppl 3):S58–S60. doi: 10.1017/S0022215115000699. [DOI] [PubMed] [Google Scholar]
- 28.Vermeire K., Van Yper L., De Vel E., Dhooge I. Is cochlear implantation an effective treatment for Menière's disease. B-ENT. 2014;10:93–98. [PubMed] [Google Scholar]
- 29.Philip R.D., Siti S.M.H., Zulkiflee S. Surgical and functional outcomes of cochlear implantation in post-lingual and cross-over patients: first 5-year review of the National Ministry of Health Malaysia cochlear implant programme. Med J Malaysia. 2018;73:393–396. [PubMed] [Google Scholar]
- 30.Chen D.A., Linthicum F.H., Rizer F.M. Cochlear histopathology in the labyrinthectomized ear: implications for cochlear implantation. Laryngoscope. 1988;98:1170–1172. doi: 10.1288/00005537-198811000-00004. [DOI] [PubMed] [Google Scholar]
- 31.Otte J., Schuknecht H.F., Kerr A.G. Ganglion cell populations in normal and pathological human cochleae. Implications for cochlear implantation. 1978. Laryngoscope. 2015;125:1038. doi: 10.1002/lary.25219. [DOI] [PubMed] [Google Scholar]
- 32.Sousa A.F., Miv C., Martinho-Carvalho A.C. Quality of life and cochlear implant: results in adults with postlingual hearing loss. Braz J Otorhinolaryngol. 2018;84:494–499. doi: 10.1016/j.bjorl.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Farinetti A., Ben G.D., Mancini J., Roman S., Nicollas R., Triglia J.M. Cochlear implant complications in 403 patients: comparative study of adults and children and review of the literature. Eur Ann Otorhinolaryngol Head Neck Dis. 2014;131:177–182. doi: 10.1016/j.anorl.2013.05.005. [DOI] [PubMed] [Google Scholar]