Abstract
There are a few biological functions or phenomenon which are universally associated with majority of the cancers and hypoxia and immune systems are among them. Hypoxia often occurs in most of the cancers which helps the cells in adapting different responses with respect to the normal cells which may be the activation of signaling pathways which regulate proliferation, angiogenesis, and cell death. Similar to it, immune signaling pathways are known to play critical roles in cancers. Moreover, there are a number of genes which are known to be associated with these hypoxia and immune system and appear to direct affect the tumor growth and propagations. Cancer is among the leading cause of death and oral cancer is the tenth-leading cause due to cancer death. In this study, we were mainly interested to understand the impact of alteration in the expression of hypoxia and immune system-related genes and their contribution to head and neck squamous cell carcinoma. Moreover, we have collected the genes associated with hypoxia and immune system from the literatures. In this work, we have performed meta-analysis of the gene and microRNA expression and mutational datasets obtained from public database for different grades of tumor in case of oral cancer. Based on our results, we conclude that the critical pathways which dominantly enriched are associated with metabolism, cell cycle, immune system and based on the survival analysis of the hypoxic genes, we observe that the potential genes associated with head and neck squamous cell carcinoma and its progression are STC2, PGK1, P4HA1, HK1, SPIB, ANXA5, SERPINE1, HGF, PFKM, TGFB1, L1CAM, ELK4, EHF, and CDK2.
Subject terms: Cancer genomics, Oral cancer, Computational biology and bioinformatics, Immunology
Introduction
Hypoxia often occurs in cancer and helps the cells in adapting different responses than the normal cells such as the triggering of signaling pathways regulating critical biological processes (proliferation, angiogenesis, and cell death or apoptosis)1. So far from the previous work, a number of genes associated with these processes and functions have been explored and investigated. Similarly, different forms of cancer require different immune systems and associated signalling pathways, and the immune signalling network (ISN) may be a major component in cancer genesis and progression. Although it has been established that cancers, including head and neck cancer, are immunogenic tumours for which immunotherapy is aggressively sought by targeting immunological checkpoints, an immune-based prognostic signature remains a viable option2–4. Several prior works2–4 propose pathway-level knowledge and analytic methodologies, as well as Hansen and Iyengar’s4 computational strategy to bridge the gap between precision medicine and systems treatments. Comprehension and unravelling comprehensive and minute understanding of cell phenotypes and disease pathophysiology remains a basic problem, as do the molecular mechanisms that lead to disease initiation and oral cancer progression.
Oral cancer is the 10th most prevalence cancer globally and in general classified as head and neck squamous cell carcinoma (HNSCC). It is a malignant neoplasia which arises in oral cavity of lip, tongue, gingiva, mouth floor and glands5–7 which may originate by a number of factors such as genetic alterations, gene expression alterations, and mutations5,8–13. Furthermore, more factors may act as the potential cause of such cancer and one of the cause is HPV (Human Pappiloma Virus) infection and this virus is non-enveloped icosahedral capsid with circular double standard DNA which majorly cause cervical cancer in human12,14,15. In addition, there exit potential difference between HPV-induced oral cancers than that of HPV-negative (oral) tumors in terms of the clinical response and finally the overall survival rates16,17. In the previous study, there are lots of work which have been performed for the study of oral cancer such as mutational, gene, and miRNA expression profiling, epigenetic changes, and proteomics for HNSCC5,12,18,19.
When we’re looking for a profound understanding of something, from a computational study to a therapeutic method20–22. It provides a ray of hope for a revolutionary diagnostic technique. Thus, in HNSCC, the hypoxia and immune-based prognostic signatures maintain a diagnostic potential that can be further explored and examined. We chose a publically available gene expression dataset for this purpose and evaluated the data with the goal of understanding how signaling networks and their components are relevant to the immune system. In this study, our goal was to understand the impact of alteration in the expression of hypoxia and immune system-related genes and their contribution to head and neck cancer23,24. For this purpose, we have collected the hypoxia-associated genes based on the literature related to diverse biological processes and functions and have also collected the mutational and expression (both microRNA and gene) datasets from Gene Expression Omnibus (GEO) freely accessible public database (http://www.ncbi.nlm.nih.gov/geo/) for which we have performed comparative analysis and the clinical relevance i.e., survival analysis25–27.
Based on our work, we observe that there are certain sets of genes which are always differentially expressed irrespective of the stages and similar to it there are a number of pathways which are potentially altered in result to the differential gene expression patterns. Based on our results, we conclude that the critical pathways which are dominantly enriched are associated with metabolism, cell cycle, immune system.
Materials and methods
In the first step, we have selected the data of our interest for mutation, gene, and miRNA expression analysis. For the gene expression and miRNA expression datasets, the samples have been analyzed by using the inbuilt tool GEO2R28,29 and the mutational dataset which have been obtained from TCGA database have been analyzed from in-house MATLAB code. For pathway enrichment analysis, the similar protocols have been followed as per DAVID and panther databases30–33. For differential gene expression analysis, we have compared the tumor samples with normal samples to generate differentially expressed genes and miRNAs lists and to generate the list of mutated genes the threshold has been set i.e., 5% of the samples showing the mutation for specific genes. After preparing the lists (overexpressed genes and microRNAs) and the mutated genes, we proceed for our goal which is to understand the expression and mutational patterns10,34 and its inferred functions33,34. All the pathways have p-values less than 0.05, blue color means highest p-values and the yellow color for lowest p-value. For generating DEGs network, FunCoup2.035 has been used for all the networks throughout the work and cytoscape36 has been used for network visualization. For most of our coding and calculations MATLAB has been used. FunCoup predicts four different classes of functional coupling or associations such as protein complexes, protein–protein physical interactions, metabolic, and signaling pathways35.
Results
Gene expression profiling reveals that there are selected sets of pathways are mostly affected as a result of alteration in gene expression
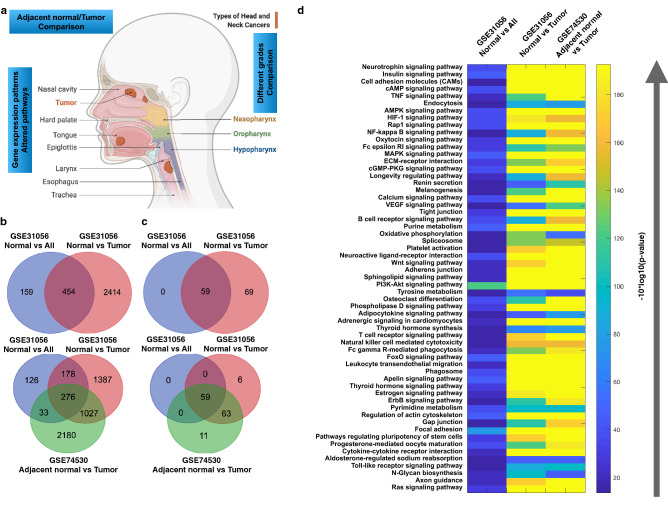
In the first step, the major goal was to understand the gene expression patterns and profiles between different tumor stages for which we have used the oral cancer expression dataset from GEO GSE848467. The dataset contains the samples which have tongue squamous cells carcinoma cells of both male and female of different ages and different stages from stage I to IV. We have performed comparative analysis of grade I with grade II, III, and IV to investigate the evolved DEGs and altered functions from grade I to IV and the total number of samples were 99. Here, we observe that the number of DEGs is comparatively low for grades I and II i.e., 24, for grades I and III is 40 and for grades I and IV is 175 and number of shared genes are quite low (Fig. 1a) and alterations in gene expression pattern increases exponentially from grade I to IV. Irrespective of the tumor location and the number of DEGs there are 59 pathways which are commonly enriched even in combination with the dataset GSE31056 (Fig. 1b,c). All these 59 enriched pathways have been displayed with their respective p-values under different conditions (Fig. 1d) and more details have been presented as Supplementary Data 1. Further, we have also compared this data with another dataset (GSE37991)37–41 in Supplementary Data 2. Here, we also observe that both the datasets share a large number of vital DEGs and the pathways.
Figure 1.
Differential gene expression profiling of different grades of oral cancer. (a) Venn diagram for differentially expressed genes, inferred, and enriched pathways; (b) Plot to show the overall number of DEGs, inferred, and enriched pathways.
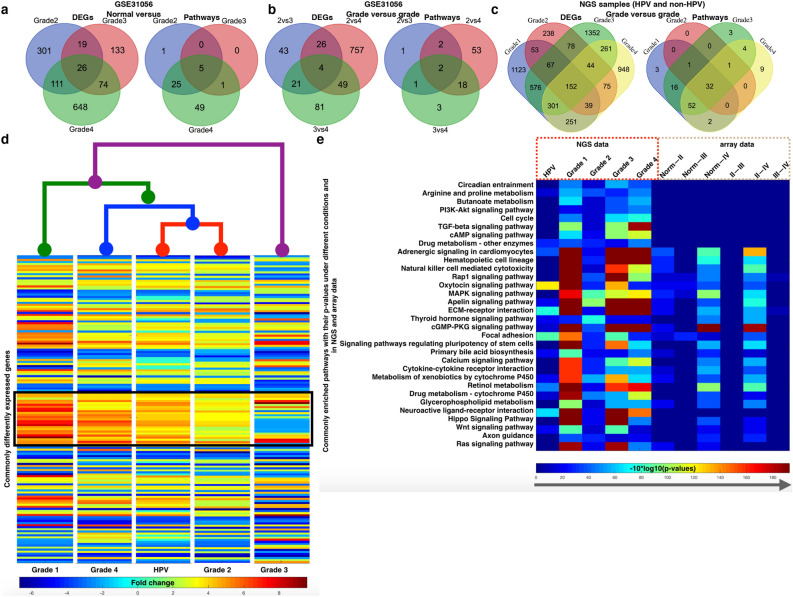
We have compared gene expression pattern of overlapped genes in HPV infected samples from grade I–IV (Fig. 2a–c). Here, we tried to present a list of genes (table/supplementary) irrespective of tumor grades, at the same time it also shows dissimilarity in level of gene expression and functional impact. Figure 2d states that Grade I and IV are having similar pattern of the expression, grade II shares their pattern with all four grades of HPV infected tumor whereas grade III showed distinct from all other grades (Fig. 2a–c). It claims that grade II is important stage where all HPV mediated oncogenic components are expressed. In Fig. 2d, we have inserted a box with black line which is just to show the reverse behavior in terms of expression for the selected genes grade 3 versus all other conditions. Overlapped, DEGs of oral tumor also showed difference in functional effect. Figure 2e shows that there are 32 pathways which are mainly altered in HPV-infected oral cancer and those p-value were compared with altered pathways of non-infected oral cancer (GEO datasets) pathways, it revealed that few of the pathways such as circadian entrainment, arginine and proline metabolism, butanoate metabolism, PI3K-AKT, cell cycle, TGF-beta, cAMP, neuro active ligand receptor interaction are highly altered in HPV-infected tumor than non-infected oral cancer which implies enhanced vulnerability of HPV infection in oral cancer. Here, we also observe that functional effect and gene expression followed the same pattern (Fig. 2d,e). Thus, we conclude that different grades of oral tumor lead to diverse impact on gene expression pattern and their functions.
Figure 2.
Differentially expressed genes and the enriched pathways for HPV infected oral cancer. Venn diagram for the different combinations of DEGs and the enriched pathways for (a,b) GSE31056 and (c) NGS dataset. (d) Heatmap and cluster for the 152 commonly DEGs in case of NGS data. (e) Commonly enriched 32 pathways for NGS dataset and the p-values for these pathways including the array dataset.
Higher mutations leads to the potential change in critical biological functions associated with oral cancer
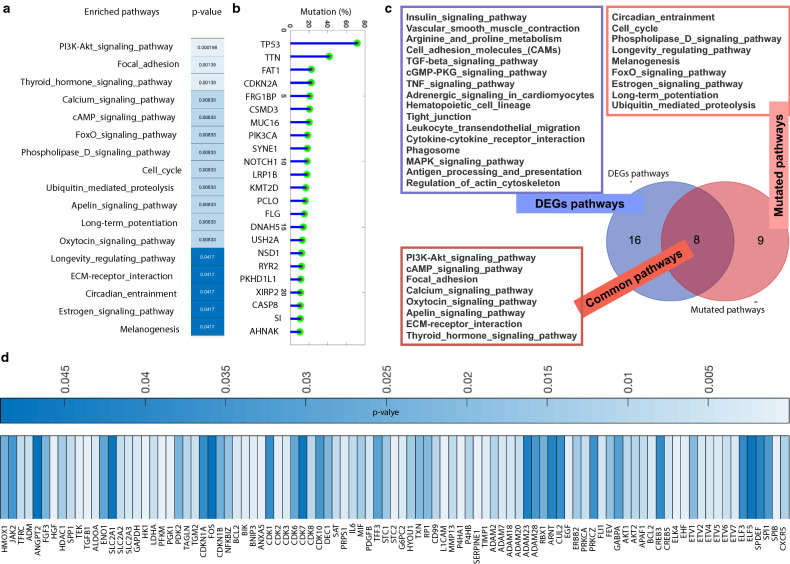
For mutational profiling, we have used the datasets from TCGA database which contains 530 samples (Head and Neck Squamous Cell Carcinoma, Firehose Legacy) and from here, we have selected the mutated genes which appear minimum in 5% of the samples and performed the pathway enrichment analysis where PI3K-Akt signaling, focal adhesion, thyroid hormone signaling, calcium signaling, cAMP signaling, FoxO signaling, phospholipase-d signaling, cell cycle, ubiquitin mediated proteolysis, apelin signaling, long-term potentiation, oxytocin signaling, Longevity regulating, ECM-receptor interaction, circadian entrainment, estrogen signaling, and melanogenesis (Fig. 3a) are among the enriched pathways for the selected genes which were mutated in minimum of the 5% of the samples. Furthermore, the top mutated (≥ 10%) genes and observe that TP53, TTN, FAT1, CDKN2A, FRG1BP, CSMD3, MUC16, PIK3CA, SYNE1, NOTCH1, LRP1B, KMT2D, PCLO, FLG, DNAH5, USH2A, NSD1, RYR2, PKHD1L1, XIRP2, CASP8, SI, and AHNAK (Fig. 3b) are among the highly mutated genes. Majority of these genes are well known to be associated with a number of cancers including the head and neck cancer and the similar case is with the enriched pathways. Furthermore, we have drawn a venn diagram to look over the commonly and specific altered pathways both because of altered expression or mutations (Fig. 3c). PI3K-Akt signaling, cAMP signaling, Focal adhesion, Calcium signaling, Oxytocin signaling, Apelin signaling, ECM-receptor interaction, and thyroid hormone signaling are those pathways which are commonly altered in terms of gene over expression and mutations which gives more significance to these pathways while circadian entrainment, cell cycle, phospholipase-d signaling, longevity regulating pathway, melanogenesis, FoxO signaling, estrogen signaling, long-term potentiation, and ubiquitin mediated proteolysis signaling pathways are specifically altered due to mutation. There are 16 signaling pathways which are exclusively altered due to over expression and some of them are insulin signaling, CAMs, TGF, TNF, tight junction, cGMP-PKG, phagosome signaling pathways and more.
Figure 3.
Mutational profiling and functional impact in oral cancer. (a) We have performed pathway enrichment analysis for those genes which appear to have more than 5% mutation for the selected dataset from TCGA database; (b) Genes with mutations ≥ 10%; (c) Comparison of the altered functions with respect to mutations and differential expression; (d) p-value for Kaplan-Meyer plots after survival analysis of the hypoxic genes in case of head and neck cancer.
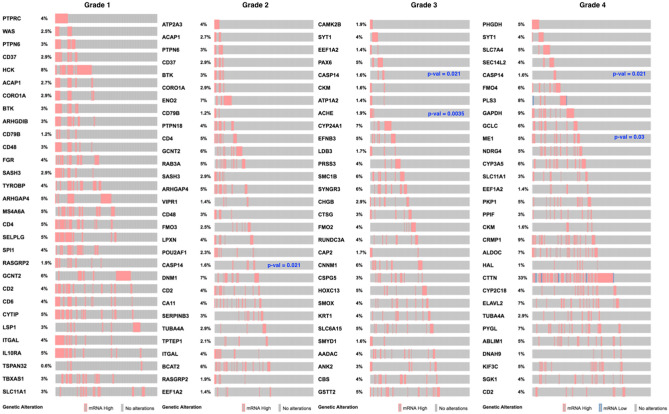
Furthermore, we have also investigated the clinical relevance of the hypoxic genes by performing survival analysis (Kaplan-Meyer plot) and plot the heatmap of the p-values of all those genes (Fig. 3d) which appear significant (p-value < 0.05). Overall, 95 genes appear significant in case of head and neck cancer and STC2, PGK1, P4HA1, HK1, SPIB, ANXA5, SERPINE1, HGF, PFKM, TGFB1, L1CAM, ELK4, EHF, and CDK2 appear to be highly significant which have p-values even lower than or equal to 0.0013. After analyzing the clinical relevance, we have fetched the inferred pathways for these top-ranked genes and we observe that these genes not only relate with the hypoxic condition but also a number of fully those pathways which directly affect the tumor initiation, propagation, and growth as shown in Table 1. Moreover, these top-ranked genes have been processed for detailed clinical relevance for which their overexpression have been checked in patients samples in TCGA database (Fig. 4) for all the four grades and the percentage of the patients with overexpressed genes have been shown.
Table 1.
Top hypoxic genes with p-values ≤ 0.0013 and the associated pathways.
| Genes | Pathways |
|---|---|
| HGF | Cytokine-cytokine_receptor_interaction |
| HGF | Focal_adhesion |
| HGF | Pathways_in_cancer |
| HGF | Renal_cell_carcinoma |
| HGF | Melanoma |
| PGK1 | Glycolysis_/_gluconeogenesis |
| PGK1 | Carbon_fixation_in_photosynthetic_organisms |
| TGFB1 | MAPK_signaling_pathway |
| TGFB1 | Cytokine-cytokine_receptor_interaction |
| TGFB1 | Cell_cycle |
| TGFB1 | TGF-beta_signaling_pathway |
| TGFB1 | Leishmaniasis |
| TGFB1 | Chagas_disease |
| TGFB1 | Pathways_in_cancer |
| TGFB1 | Colorectal_cancer |
| TGFB1 | Renal_cell_carcinoma |
| TGFB1 | Pancreatic_cancer |
| TGFB1 | Chronic_myeloid_leukemia |
| SERPINE1 | p53_signaling_pathway |
| SERPINE1 | Complement_and_coagulation_cascades |
| P4HA1 | Arginine_and_proline_metabolism |
| CDK2 | Cell_cycle |
| CDK2 | Oocyte_meiosis |
| CDK2 | p53_signaling_pathway |
| CDK2 | Progesterone-mediated_oocyte_maturation |
| CDK2 | Prostate_cancer |
| CDK2 | Small_cell_lung_cancer |
| PFKM | Glycolysis_/_gluconeogenesis |
| PFKM | Pentose_phosphate_pathway |
| PFKM | Fructose_and_mannose_metabolism |
| PFKM | Galactose_metabolism |
| PFKM | Insulin_signaling_pathway |
| HK1 | Glycolysis_/_gluconeogenesis |
| HK1 | Fructose_and_mannose_metabolism |
| HK1 | Galactose_metabolism |
| HK1 | Starch_and_sucrose_metabolism |
| HK1 | Amino_sugar_and_nucleotide_sugar_metabolism |
| HK1 | Streptomycin_biosynthesis |
| HK1 | Insulin_signaling_pathway |
| HK1 | Type_II_diabetes_mellitus |
| ELK4 | MAPK_signaling_pathway |
| L1CAM | Axon_guidance |
| L1CAM | Cell_adhesion_molecules_(CAMs) |
| TGFB1 | Hippo_Signaling_Pathway |
| SERPINE1 | Hippo_Signaling_Pathway |
| HGF | Ras_signaling_pathway |
| HGF | Rap1_signaling_pathway |
| SERPINE1 | Apelin_signaling_pathway |
| HK1 | HIF-1_signaling_pathway |
| SERPINE1 | HIF-1_signaling_pathway |
| PGK1 | HIF-1_signaling_pathway |
| CDK2 | FoxO_signaling_pathway |
| TGFB1 | FoxO_signaling_pathway |
| CDK2 | PI3K-Akt_signaling_pathway |
| HGF | PI3K-Akt_signaling_pathway |
| PFKM | AMPK_signaling_pathway |
| TGFB1 | Osteoclast_differentiation |
Figure 4.
Clinical relevance. Clinical Relevance for the top-ranked genes (based on connectivity of the genes within the network generated through network database) and respective inferred pathways. p-value represents the clinical significance in terms of survival analysis and the TCGA database and cBioPortal have been used.
Differential microRNAs expression also potentially impact the cancer associated functions
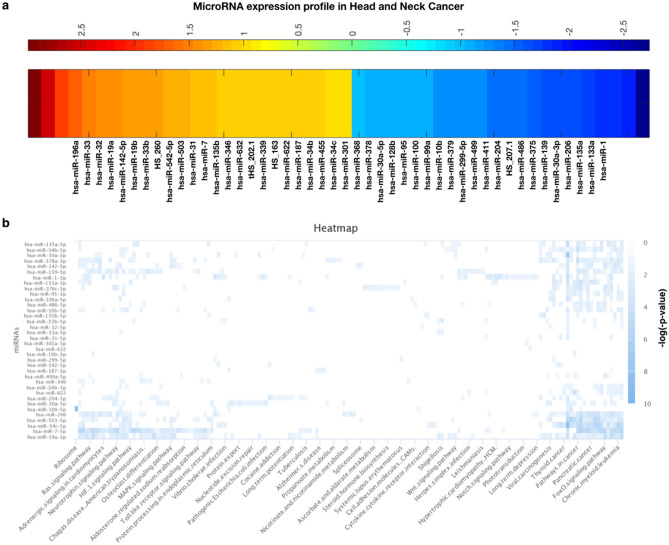
After analyzing the gene expression and mutational profiling, we have performed miRNA expression profiling and for this purpose, the dataset was collected from GEO database i.e., GSE31227 and the platform was GPL977019. This dataset contains 15 patient surgical margin as controls and 15 patient Oral Squamous Cell Carcinoma (OSCC) and we found that there are 46 miRNAs out of 739 miRNAs which are overexpressed in case of head and neck cancer (Fig. 5a) and in terms of functions the most affected biological pathways are thyroid cancer, pathways in cancer, pancreatic cancer, Foxo signaling, chronic myeloid leukemia, HIF1 signaling and more and most of these pathways appears to strongly associated with head and neck cancer and oral cancer (Fig. 5b). Here, it can be clearly seen that the pathways enriched in DEGs and the miRNA pathways list have a number of common pathways such as HIF-1 signaling, Ras, MAPK, immune system associated pathways, and directly cancer-associated pathways.
Figure 5.
miRNA expression profiling and their functional impact in head and neck cancer. (a) Differentially expressed miRNAs and (b) the enriched pathways with the respective miRNAs.
Discussion
Hypoxia and ISN often help the cells in adapting different responses than the normal cells such as the triggering of signaling pathways regulating critical biological processes (proliferation, angiogenesis, and cell death or apoptosis)15,20,42–49. These two processes have been explored in a number of human diseases and different from the previous works, here these two systems have been explored simultaneously in HNSCC and also the role of microRNAs have been analyzed with their effect on biological processes and functions and furthermore the survival analysis12,50–52 has been analyzed for the hypoxic genes. The reason to focus on hypoxia and mainly the ISN was that the associated signaling pathways with these two systems are considered as a master regulator for cancer initiation and progression and has also been proven that HNSCC is an immunogenic tumor and immunotherapy is strongly pursued through targeting on the immune checkpoints the immune based prognostic signature remains a potential that can be applied2–4. In the previous works2–4, pathway-level understanding and analysis approaches has also been presented which present computational approach to bridge between precision medicine and systems therapeutics.
In this study, the main focus of the study was to understand the expression pattern of both the genes and the microRNAs and the mutational profiling followed by the survival analysis for HNSCC for which the datasets have been utilized from the GEO and the TCGA database. After the expression and mutational profiling, we performed comparative analysis for the functions in both the cases. PI3K-Akt signaling, cAMP signaling, focal adhesion, calcium signaling, oxytocin signaling, apelin signaling, ECM-receptor interaction, and thyroid hormone signaling are those pathways which are commonly enriched for both the cases differential expression and mutation in HNSCC which gives higher significance to these pathways for the selected disease while there are specific pathways for DEGs and mutated genes lists which means there are pathways which may be altered only because of overexpression of the genes or higher mutations rate. Mutation-specific altered pathways are circadian entrainment, cell cycle, phospholipase-d signaling, longevity regulating pathway, melanogenesis, FoxO signaling, estrogen signaling, long-term potentiation, and ubiquitin mediated proteolysis signaling pathways while insulin signaling, CAMs, TGF, TNF, tight junction, cGMP-PKG, phagosome signaling pathways are altered gene expression specific. From OSCC microRNAs analysis, 46 miRNAs appear overexpressed and the most affected functions are thyroid cancer, pathways in cancer, pancreatic cancer, Foxo signaling, chronic myeloid leukemia, HIF1 signaling and more and most of these pathways appears to strongly associated with head and neck cancer and oral cancer. Based on the clinical relevance of the hypoxic genes, there are a large number of genes which are highly significant and STC2, PGK1, P4HA1, HK1, SPIB, ANXA5, SERPINE1, HGF, PFKM, TGFB1, L1CAM, ELK4, EHF, and CDK2 are highly significant which have p-values even lower than or equal to 0.0013. Similar to the expression and mutational profiling, the inferred pathways of the top-ranked genes are direct components of those pathways which directly affect the tumor initiation, propagation, and growth. Moreover, the RNA and miRNA expression analysis shows that there are common functions in the RNA and the miRNA pathways list such as HIF-1 signaling, Ras, MAPK, immune system associated pathways, and directly cancer-associated pathways.
Conclusions
As mentioned that the major goal of this study was to understand the role of expression profiling of genes and the microRNAs and the mutational profiling of the genes and also the clinical relevance in case of HNSCC and based on results and the analysis, it leads to the conclusion that the critical pathways which could be dominantly enriched or altered in case of HNSCC are associated with metabolism, cell cycle, immune system, and hypoxia and the three different datasets of gene expression and microRNA expression, and the mutational data also leads to the conclusion that the pathways and the pathways components potentially associated with HNSCC and its progression.
Supplementary Information
Acknowledgements
We are thankful to DSR, KAU and for providing us the resources and the facility to carry out the work to Special Infectious Agents Unit, King Fahd Medical Research Centre, King Abdulaziz University, Jeddah, Saudi Arabia, Medical Laboratory Sciences Department, Faculty of Applied Medical Sciences, King Abdulaziz University, Jeddah, Saudi Arabia, Biochemistry Department, Faculty of Sciences, King Abdulaziz University, Jeddah, Saudi Arabia, King Fahd Medical Research Center, King Abdulaziz University, P. O. Box 80216, Jeddah 21589, Saudi Arabia, and Enzymoics, 7 Peterlee Place, Hebersham, NSW 2770; Novel Global Community Educational Foundation, Australia.
Author contributions
Conceptualization, L.H.B., S.S.S., M.A.K. and E.I.A.; methodology, L.H.B., S.S.S., M.M., M.A.K., M.A.R. and E.I.A.; software, E.I.A.; validation, L.H.B. and E.I.A.; formal analysis, L.H.B., S.S.S., M.M., M.A.K., M.A.R. and E.I.A.; investigation, L.H.B., S.S.S., M.A.K. and E.I.A.; resources, S.S.S., M.A.K. and E.I.A.; data curation, S.S.S., M.M., M.A.R. and E.I.A.; writing—original draft preparation, L.H.B., S.S.S., M.M., M.A.K. and E.I.A.; writing—review and editing, L.H.B., S.S.S., M.M., M.A.K., M.A.R. and E.I.A.; visualization, L.H.B., S.S.S. and E.I.A.; supervision, M.A.K. and E.I.A.; project administration, E.I.A.; funding acquisition, M.A.K. and E.I.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah, Saudi Arabia, Grant Number FP-5-42 and The APC was funded by FP-5-42.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mohammad Mobashir, Email: m.mobashir@cdslifesciences.com.
Esam Ibraheem Azhar, Email: eazhar@kau.edu.sa.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-98031-7.
References
- 1.Ye IC, et al. Molecular portrait of hypoxia in breast cancer: A prognostic signature and novel HIF-regulated genes. Mol. Cancer Res. 2018;16:1889–1901. doi: 10.1158/1541-7786.MCR-18-0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang K, Li M, Hakonarson H. Analysing biological pathways in genome-wide association studies. Nat. Rev. Genet. 2010;11:843–854. doi: 10.1038/nrg2884. [DOI] [PubMed] [Google Scholar]
- 3.Li Y, Agarwal P, Rajagopalan D. A global pathway crosstalk network. Bioinformatics. 2008;24:1442–1447. doi: 10.1093/bioinformatics/btn200. [DOI] [PubMed] [Google Scholar]
- 4.Hansen J, Iyengar R. Computation as the mechanistic bridge between precision medicine and systems therapeutics. Clin. Pharmacol. Ther. 2012;93:117–128. doi: 10.1038/clpt.2012.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kang H, Kiess A, Chung CH. Emerging biomarkers in head and neck cancer in the era of genomics. Nat. Rev. Clin. Oncol. 2014 doi: 10.1038/nrclinonc.2014.192. [DOI] [PubMed] [Google Scholar]
- 6.Wood O, et al. Gene expression analysis of TIL rich HPV-driven head and neck tumors reveals a distinct B-cell signature when compared to HPV independent tumors. Oncotarget. 2016;7:56781–56797. doi: 10.18632/oncotarget.10788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mes SW, et al. Prognostic modeling of oral cancer by gene profiles and clinicopathological co-variables. Oncotarget. 2017;8:59312–59323. doi: 10.18632/oncotarget.19576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 9.West J, Bianconi G, Severini S, Teschendorff AE. Differential network entropy reveals cancer system hallmarks. Sci. Rep. 2012 doi: 10.1038/srep00802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lapointe J, et al. Gene expression profiling identifies clinically relevant subtypes of prostate cancer. Proc. Natl. Acad. Sci. U.S.A. 2004;101:811–816. doi: 10.1073/pnas.0304146101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rivera C. Essentials of oral cancer. Int. J. Clin. Exp. Pathol. 2015;8:11884–11894. [PMC free article] [PubMed] [Google Scholar]
- 12.Reis PP, et al. A gene signature in histologically normal surgical margins is predictive of oral carcinoma recurrence. BMC Cancer. 2011;11:437–511. doi: 10.1186/1471-2407-11-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nat. Med. 2004;10:789–799. doi: 10.1038/nm1087. [DOI] [PubMed] [Google Scholar]
- 14.Feng Z, et al. A pathogenic picornavirus acquires an envelope by hijacking cellular membranes. Nature. 2013;496:367–371. doi: 10.1038/nature12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bristow RG, Hill RP. Hypoxia and metabolism: Hypoxia, DNA repair and genetic instability. Nat. Rev. Cancer. 2008;8:180–192. doi: 10.1038/nrc2344. [DOI] [PubMed] [Google Scholar]
- 16.Nana-Sinkam SP, Croce CM. MicroRNA regulation of tumorigenesis, cancer progression and interpatient heterogeneity: Towards clinical use. Genome Biol. 2014;15:1–9. doi: 10.1186/s13059-014-0445-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duan F, et al. Genomic and bioinformatic profiling of mutational neoepitopes reveals new rules to predict anticancer immunogenicity. J. Exp. Med. 2014;211:2231–2248. doi: 10.1084/jem.20141308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Watson IR, Takahashi K, Futreal PA, Chin L. Emerging patterns of somatic mutations in cancer. Nat. Rev. Genet. 2013;14:703–718. doi: 10.1038/nrg3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Severino P, et al. MicroRNA expression profile in head and neck cancer: HOX-cluster embedded microRNA-196a and microRNA-10b dysregulation implicated in cell proliferation. BMC Cancer. 2013;13:1–15. doi: 10.1186/1471-2407-13-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Restifo NP, Smyth MJ, Snyder A. Acquired resistance to immunotherapy and future challenges. Nat. Rev. Cancer. 2016;16:121–126. doi: 10.1038/nrc.2016.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zigler M, Shir A, Levitzki A. Targeted cancer immunotherapy. Curr. Opin. Pharmacol. 2013;13:504–510. doi: 10.1016/j.coph.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 22.Luo J, Solimini NL, Elledge SJ. Principles of cancer therapy: Oncogene and non-oncogene addiction. Cell. 2009;136:823–837. doi: 10.1016/j.cell.2009.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar PP, et al. In-silico study reveals immunological signaling pathways, their genes, and potential herbal drug targets in ovarian cancer. Inform. Med. Unlock. 2020;20:100422. doi: 10.1016/j.imu.2020.100422. [DOI] [Google Scholar]
- 24.Kamal MA, et al. Gene expression profiling and clinical relevance unravel the role hypoxia and immune signaling genes and pathways in breast cancer: Role of hypoxia and immune signaling genes in breast cancer. JIMSA. 2020 doi: 10.36013/jimsa.v1i1.3. [DOI] [Google Scholar]
- 25.Werner HMJ, Mills GB, Ram PT. Cancer systems biology: A peek into the future of patient care? Nat. Rev. Clin. Oncol. 2014;11:167–176. doi: 10.1038/nrclinonc.2014.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu J, et al. An integrated TCGA pan-cancer clinical data resource to drive high-quality survival outcome analytics. Cell. 2018;173:400–416.e11. doi: 10.1016/j.cell.2018.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anaya J. OncoLnc: Linking TCGA survival data to mRNAs, miRNAs, and lncRNAs. PeerJ Comput. Sci. 2016;2:e67. doi: 10.7717/peerj-cs.67. [DOI] [Google Scholar]
- 28.Davis S, Meltzer PS. GEOquery: A bridge between the gene expression omnibus (GEO) and BioConductor. Bioinformatics. 2007;23:1846–1847. doi: 10.1093/bioinformatics/btm254. [DOI] [PubMed] [Google Scholar]
- 29.Calon A, et al. Stromal gene expression defines poor-prognosis subtypes in colorectal cancer. Nat. Genet. 2015;47:320–329. doi: 10.1038/ng.3225. [DOI] [PubMed] [Google Scholar]
- 30.Huang DW, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: Paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37:1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li J, et al. An integrated catalog of reference genes in the human gut microbiome. Nat. Biotechnol. 2014;32:834–841. doi: 10.1038/nbt.2942. [DOI] [PubMed] [Google Scholar]
- 32.Kanehisa M, Goto S, Sato Y, Furumichi M, Tanabe M. KEGG for integration and interpretation of large-scale molecular data sets. Nucleic Acids Res. 2011;40:D109–D114. doi: 10.1093/nar/gkr988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mi H, Poudel S, Muruganujan A, Casagrande JT, Thomas PD. PANTHER version 10: Expanded protein families and functions, and analysis tools. Nucleic Acids Res. 2016;44:D336–D342. doi: 10.1093/nar/gkv1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Subramanian A, et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. U.S.A. 2005;102:15545. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alexeyenko A, Sonnhammer ELL. Global networks of functional coupling in eukaryotes from comprehensive data integration. Genome Res. 2009;19:1107–1116. doi: 10.1101/gr.087528.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Okawa S, Angarica VE, Lemischka I, Moore K, del Sol A. A differential network analysis approach for lineagespeci. Nat. Publ. Group. 2015 doi: 10.1038/npjsba.2015.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sheu JJ-C, et al. LRIG1 modulates aggressiveness of head and neck cancers byregulating EGFR-MAPK-SPHK1 signaling and extracellularmatrix remodeling. Oncogene. 2019 doi: 10.1038/onc.2013.98. [DOI] [PubMed] [Google Scholar]
- 38.Lee C-H, et al. IL-1β promotes malignant transformation and tumor aggressiveness in oral cancer. J. Cell. Physiol. 2015;230:875–884. doi: 10.1002/jcp.24816. [DOI] [PubMed] [Google Scholar]
- 39.Lee C-H, et al. Epigenetic regulation of the X-linked tumour suppressors BEX1 and LDOC1 in oral squamous cell carcinoma. J. Pathol. 2013;230:298–309. doi: 10.1002/path.4173. [DOI] [PubMed] [Google Scholar]
- 40.Chou S-T, et al. MicroRNA-486-3p functions as a tumorsuppressor in oral cancer by targeting DDR1. J. Exp. Clin. Cancer Res. 2019;38:1–14. doi: 10.1186/s13046-019-1283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peng H-Y, et al. MiR-944/CISH mediated inflammation via STAT3 is involved in oral cancer malignance by cigarette smoking. Neoplasia. 2020;22:554–565. doi: 10.1016/j.neo.2020.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harris AL. Hypoxia—A key regulatory factor in tumour growth. Nat. Rev. Cancer. 2002;2:38–47. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- 43.Pouysségur J, Dayan F, Mazure NM. Hypoxia signalling in cancer and approaches to enforce tumour regression. Nat. Cell Biol. 2006;441:437–443. doi: 10.1038/nature04871. [DOI] [PubMed] [Google Scholar]
- 44.Zhong H, et al. Overexpression of hypoxia-inducible factor 1alpha in common human cancers and their metastases. Can. Res. 1999;59:5830–5835. [PubMed] [Google Scholar]
- 45.Bhandari V, et al. Molecular landmarks of tumor hypoxia across cancer types. Nat. Genet. 2018 doi: 10.1038/s41588-018-0318-2. [DOI] [PubMed] [Google Scholar]
- 46.Qutub AA, Popel AS. Reactive oxygen species regulate hypoxia-inducible factor 1 differentially in cancer and ischemia. Mol. Cell. Biol. 2008;28:5106–5119. doi: 10.1128/MCB.00060-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gutierrez-Arcelus M, Rich SS, Raychaudhuri S. Autoimmune diseases—Connecting risk alleles with molecular traits of the immune system. Nat. Rev. Genet. 2016;17:160–174. doi: 10.1038/nrg.2015.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ma Y, Galluzzi L, Zitvogel L, Kroemer G. Autophagy and cellular immune responses. Immunity. 2013;39:211–227. doi: 10.1016/j.immuni.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 49.Hendriks G, van de Water B, Schoonen W, Vrieling H. Cellular-signaling pathways unveil the carcinogenic potential of chemicals. J. Appl. Toxicol. 2013;33:399–409. doi: 10.1002/jat.2845. [DOI] [PubMed] [Google Scholar]
- 50.Clarke C, et al. Correlating transcriptional networks to breast cancer survival: A large-scale coexpression analysis. Carcinogenesis. 2013;34:2300–2308. doi: 10.1093/carcin/bgt208. [DOI] [PubMed] [Google Scholar]
- 51.Kandoth C, et al. Mutational landscape and significance across 12 major cancer types. Nature. 2013;502:333–339. doi: 10.1038/nature12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rahman N. Realizing the promise of cancer predisposition genes. Nature. 2014;505:302–308. doi: 10.1038/nature12981. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.