Abstract
Background.
It is suggested that patients with defecation disorders (DD) strain excessively or do a Valsalva maneuver (VM) during evacuation, resulting in rectoanal discoordination, which hinders rectal evacuation. However, definitive data are lacking.
Methods.
Rectoanal pressures during evacuation and a VM were measured with seated high resolution manometry (HRM) in 64 healthy and 136 constipated women with a normal (84 women, C-normal) or prolonged (52 women, C-abnormal) balloon expulsion time (BET). The number of abnormal rectoanal parameters during evacuation and the joint distribution of pressures during evacuation and a VM were used to discriminate between controls and C-abnormal BET patients.
Key Results.
The peak anal pressure (5s) during a VM accounted for 0%, 26%, and 49% of the variance in anal pressure during evacuation in healthy women, C-normal BET, and C-abnormal BET. The association between anal pressure during a VM and evacuation was stronger in C-abnormal BET than in healthy women and C-normal BET (P for interaction < 0.001). Fifty eight of 64 controls and 33 of 52 C-abnormal BET patients had no or one abnormal parameter during evacuation; hence the probability of C-abnormal BET was 33/91 (36%). In patients with no or one abnormal parameter during evacuation, a logistic model based on anal pressures during evacuation and a VM discriminated between controls and patients with C-abnormal BET with a sensitivity and a specificity of 67% and 75%.
Conclusions.
Assessment of rectoanal pressures during evacuation and a VM uncovers rectaoanal discoordination and facilitates the diagnosis of DD in selected patients.
Keywords: Valsalva maneuver, Defecatory disorder, Constipation, High resolution manometry, Probability, Contour plot
Introduction
The pelvic floor muscles function in synergy with the abdominal muscles1–3, chest wall3, and diaphragm4. Indeed, the muscles around the abdomino-pelvic cavity form a flexible cylinder, which responds rapidly to changes in intra-abdominal pressure, trunk muscle activity and posture, and to the varied continence and respiratory demands of activities of daily living. Actions such as speaking, coughing, straining, weight lifting, and a Valsalva maneuver increase abdominal pressure and are accompanied by contraction of the external anal sphincter and pelvic floor muscles, which serves to preserve continence5–10.
In contrast, normal defecation requires increased abdominal, hence rectal pressure and relaxation of the external anal sphincter5. Defecation disorders (DD) are primarily characterized by rectoanal discoordination or dyssynergia that is often attributed to excessive straining and may be accompanied by structural disturbances (e.g., rectal intussusception), which may be clinically significant11,12. In healthy people, straining to begin defecation is unusual and straining to end defecation is rare, even for hard stools13. In contrast, 40% of constipated patients strain to begin evacuating hard stools. Perhaps excessive straining or a Valsalva maneuver are less effective than normal defecation for evacuating stool. Indeed, in DD patients, the externally-directed axial forces measured with a force transducer are lower during a Valsalva maneuver than during defecation14. The inwardly oriented (i.e., orad) force during voluntary contraction (i.e., squeeze) is also weaker in patients with DD than in healthy women14,15. Taken together, these findings suggest some patients with DD have more generalized pelvic floor dysfunction. Limited by availability, axial transducers are not used to measure rectoanal forces in clinical practice.
During anorectal manometry, the anal pressure during a Valsalva maneuver is used to evaluate the integrity of the sacral lower motor neuron reflex arc, especially in patients with fecal incontinence, but not to investigate rectoanal coordination in DD16,17. However, no studies have compared rectoanal pressures during evacuation and a Valsalva maneuver in DD. If indeed, DD patients use a Valsalva maneuver to defecate, the rectoanal pressures during a Valsalva maneuver and evacuation should more closely resemble each other in C-abnormal BET than in healthy people. Our hypotheses were that rectoanal pressures during Valsalva maneuver would uncover rectoanal discoordination and increase the utility of high resolution manometry (HRM) for diagnosing DD18–20. Our aims were to compare (i) the relationship between anal pressures during evacuation and a Valsalva maneuver in healthy people, constipated patients without a DD, and constipated patients with a DD and (ii) estimate the incremental utility of anal pressures during a Valsalva maneuver for diagnosing DD.
Methods
Study Participants
From January 2011 to April 2018, 136 patients with Rome III functional constipation or IBS-C and 64 healthy women aged between 18 to 80 years, consented to participate in the studies approved by the Institutional Review Board at Mayo Clinic21. Healthy women did not have Rome III symptom criteria for any functional bowel disorder, documented grade 3 or 4 obstetric anorectal laceration, or any previous anorectal surgery. Patients had symptoms of chronic constipation for 1 year or longer and had failed treatment with over-the-counter laxatives. None of the participants had clinically significant systemic disease (eg, cardiovascular or neurological) nor were they taking medications (eg, opioids) that might interfere with the objectives of the study or pose safety concerns. Some findings from this cohort but not the detailed analyses of anal pressures during evacuation and a Valsalva maneuver have been published previously20,22.
Anorectal manometry and rectal balloon expulsion test.
After rectal cleansing with 1–2 sodium phosphate (Fleets®,C.B. Fleet, Lynchburg, VA) enemas, rectal and anal pressures were measured with a high resolution manometry (HRM) catheter (Manoscan™; 4.2 mm diameter; currently Medtronic Inc) in the seated position at rest, during squeeze, simulated evacuation, and a Valsalva maneuver, which was performed by asking participants to exhale into a balloon attached to a sphygmomanometer to generate a pressure of 20 mmHg. Rectoanal pressures were analyzed using the commercially available software (Manoview AR v3.0; Medtronic Inc)19. Participants had up to 3 minutes to expel a 4-cm-long balloon filled with 50 ml water from the rectum in privacy while seated on a commode23,24. The balloon expulsion time (BET) was noted, and the balloon was removed if participants could not expel the balloon within 3 minutes. Normal values for the BET depend on the type of balloon24,25. For balloons similar to those used in this study, a BET greater than 60 seconds is prolonged24,25.
Data and Statistical Analysis
Sensors that recorded a resting pressure of 30 mmHg or greater were considered to be in the functional anal canal20. During evacuation, the lowest anal pressure recorded by adjacent anal sensors over 10s (i.e., between 5 and 15s) was averaged and used for further analysis. During squeeze, the highest anal pressure recorded by three adjacent anal sensors over 3s (i.e., between 2 and 5s) were averaged for further analysis. During a Valsalva maneuver, the highest anal pressure over 5s (i.e., between 2 and 7s) were averaged for further analysis; depending on the anal profile, either three or four adjacent anal sensors were used for the analysis (Figure 1)20. During a Valsalva maneuver, the pressures were averaged over 5s, which is the duration over which nearly 100% of vital capacity is expired in the absence of airway obstruction26. The Kruskal-Wallis and chi-squared tests respectively compared continuous and categorical variables among healthy women, patients with C-normal BET and C-abnormal BET.
Figure 1.
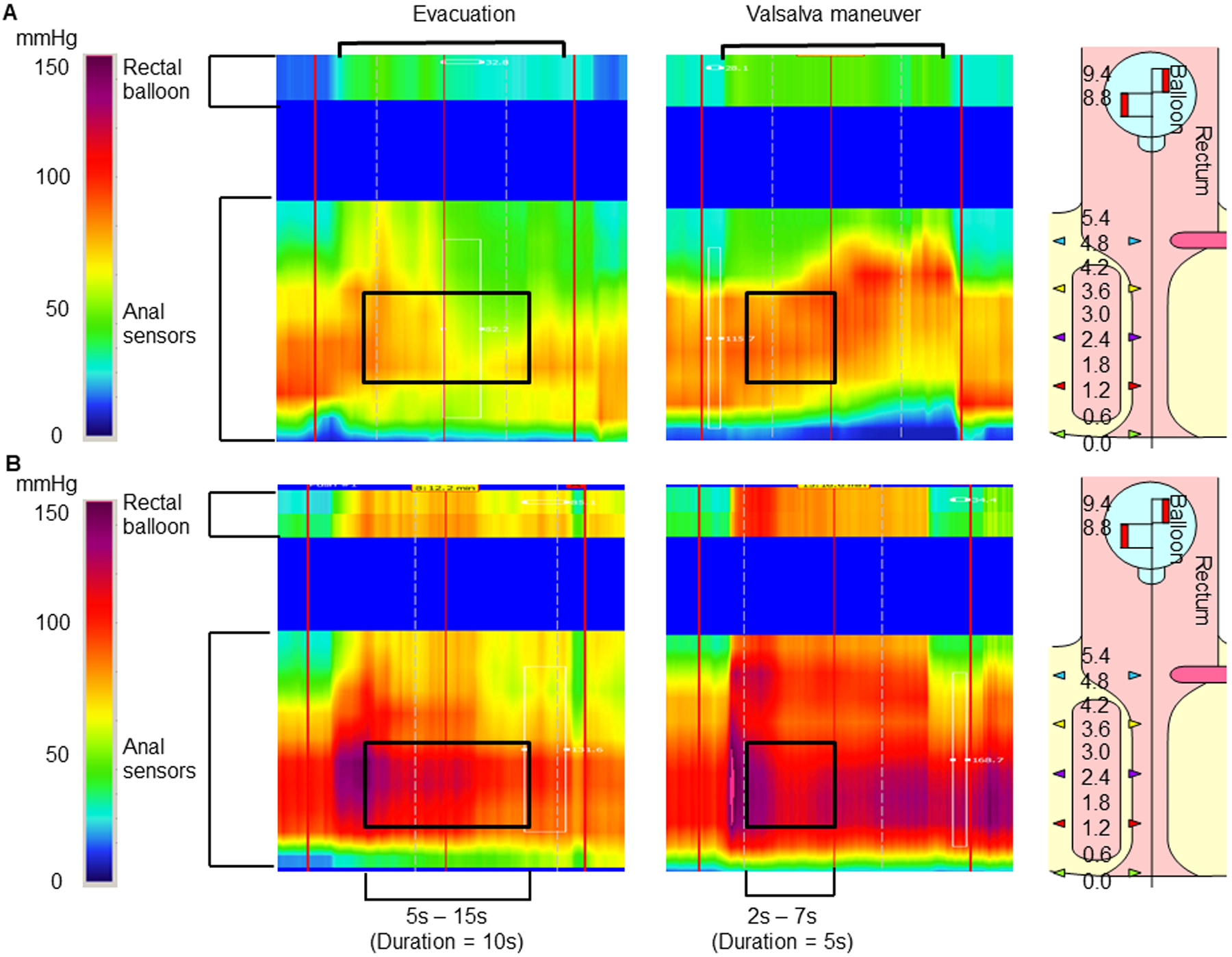
Anal pressures during evacuation (left) and a Valsalva maneuver (right) in patients with C-normal BET (A) and C-abnormal BET (B). Anal pressure was averaged over 4 sensors for a duration of 10s during evacuation and, depending on the anal profile, either 3 or 4 sensors for a duration of 5s during a Valsalva maneuver. During a Valsalva maneuver, there is anal contraction in both patients. During evacuation, there is anal relaxation and contraction respectively in the upper and lower panels.
The statistical tests are presented in chronological order below and in Table 1. Linear regression models analyzed whether anal pressures during evacuation, squeeze, and a Valsalva maneuver discriminated between controls and patients with C-abnormal BET (Approach 1, Table 1). [In these models, the anal squeeze pressure did not discriminate between controls and patients with C-abnormal BET.] Hence, subsequent linear regression models used only anal pressures during a Valsalva maneuver and evacuation to discriminate among healthy women, patients with C-normal BET, and C-abnormal BET (Approach 2, Table 1) and the variance explained by the regression models were presented.
Table 1.
Statistical Analyses
| No | Question | Analysis | Results |
|---|---|---|---|
| 1 | Do anal pressures during evacuation, squeeze, and a VM discriminate between healthy controls and C- abnormal BET? | Three linear regression models in healthy women, patients with C-normal BET, and C-abnormal BET
|
Table 3 |
| 2 | Do anal pressures during evacuation and a VM discriminate between C- abnormal BET versus healthy controls and separately versus C-normal BET | Two linear regression models
|
Table 4 |
| 3 | Assess the joint distribution of pressures during evacuation and a VM in patients with (a) no or one and (b) two or more abnormal parameters during evacuation |
|
Table 6, Figures 2–4 |
VM = Valsalva maneuver
The joint distribution of anal pressures during evacuation and a Valsalva maneuver was used to estimate the theoretical likelihood of C-abnormal BET versus health, summarized as a log density–ratio (Approach 3, Table 1). The incremental utility of this log density–ratio for discriminating between C-abnormal BET and health was considered separately in participants with “no or one” and “two or more” abnormal parameters during evacuation. That analysis utilized a multivariate logistic regression model, which combined the number of abnormal parameters during evacuation (no or one versus two or more abnormal parameters) and the log density ratio to predict C-abnormal BET (versus health) (Approach 3, Table 1). The log-odds of C-abnormal BET (versus health) from this model were exponentiated to give the odds, and expressed as the probability for diagnosis of C-abnormal BET versus health using the formula probability = odds/[odds + 1])27. These predicted probabilities were depicted as contour plots, and displayed as boxplots. Finally, the diagnostic value of the predicted probabilities was evaluated using receiver operating characteristic (ROC) curves.
The statistical analyses were performed with JMP software (version 9.4, SAS Cary, NC) and the contour plot was prepared with SAS software (SAS Institute Inc, Cary, NC).
Results
Study participants
Of the 200 participants, 64 were healthy women with a normal BET (32%), 84 were constipated women with a normal BET (C-normal BET, 42%) and 52 were constipated women with an abnormal BET (C-abnormal BET, 26%). The healthy women (age, 35 [13] years) were younger (P = 0.01) than C-normal BET (age, 42 [17] years) and C-abnormal BET (age, 41 [14] years) (Table 2). The BMI was comparable among groups.
Table 2.
Demographics and Patient Characteristics
| Parameter† | Healthy women | Constipation, normal BET | Constipation abnormal BET | P value |
|---|---|---|---|---|
| N | 64 | 84 | 52 | |
| Age, y | 35 (13) | 42 (17) | 41 (14) | .01 |
| BMI, kg/m2 | 26 (5) | 25 (5) | 24 (5) | .2 |
| Symptoms | ||||
| < 3 bowel movements/week, n (%) | 0 | 33 (39%) | 22 (42%) | .7 |
| Incomplete evacuation, n (%) | 0 | 62 (74%) | 37 (71%) | .7 |
| Straining, n (%) | 0 | 72 (88%) | 45 (88%) | .7 |
| Hard stool, n (%) | 0 | 58 (69%) | 38 (73%) | .6 |
| Sensation of blockage, n (%) | 0 | 56 (66%) | 36 (69%) | .7 |
| Manual evacuation, n (%) | 0 | 32 (38%) | 24 (46%) | .3 |
| IBS-C, n (%) | 0 | 41 (49%)‡ | 17 (33%) | .04 |
| Functional constipation, n (%) | 0 | 40 (48%)‡ | 35 (67%) | .04 |
| Live births | 0.95 (1.4) | 1.3 (1.3) | 1.25 (1.4) | .2 |
| Vaginal deliveries | 0.65 (1.2) | 0.9 (1.1) | 1 (1.4) | .2 |
| Cesarean section | 0.3 (0.7) | 0.4 (1) | 0.2 (0.6) | .5 |
| Vaginal deliveries requiring pelvic sutures | 0.3 (0.8) | 0.5 (0.9) | 0.6 (0.9) | .04 |
| Traumatic births, n (%) | 1 (2%) | 4 (5%) | 1(2%) | .4 |
| Anal trauma, other than during childbirth, n (%) | 1 (2%) | 4 (5%) | 0(0%) | .08 |
| Pressures, mmHg | ||||
| Anal resting pressure | 85 (26) | 86 (27) | 98 (27) | .06 |
| 10th, 90th percentile | 40, 104 | |||
| Increased, reduced§ | 16, 5 | 25, 5 | 22, 0 | |
| Squeeze increment | 60 (36) | 46 (41) | 48 (37) | .052 |
| 10th, 90th percentile | 16, 104 | |||
| Increased, reduced§ | 5, 6 | 7, 19 | 5, 11 | |
| Rectal pressure – evacuation | 58 (29) | 64 (30) | 48 (25) | .007 |
| 10th, 90th percentile | 26, 98 | |||
| Increased, reduced§ | 6, 6 | 11, 3 | 2, 13 | |
| Anal pressure – evacuation | 62 (20) | 69 (21) | 88 (32) | <.0001 |
| 10th, 90th percentile | 41, 96 | |||
| Increased, reduced§ | 6, 6 | 8, 7 | 17, 2 | |
| Rectoanal gradient – evacuation | −4 (29) | −5 (31) | −40 (38) | <.0001 |
| 10th, 90th percentile | −36, 30 | |||
| Increased, reduced§ | 6, 7 | 12, 10 | 1, 25 | |
| Anal pressure – Valsalva maneuver | 92 (25) | 89 (34) | 105 (35) | .1 |
| 10th, 90th percentile | 61, 121 | |||
| Increased, reduced§ | 6, 6 | 12, 18 | 13, 6 | |
| Number of abnormal rectoanal parameters during evacuation | ||||
| No or one | 58 | 80 | 33 | <.0001 |
| Two or more | 6 | 4 | 19 | <.0001 |
Values are Mean (SD) unless stated otherwise.
Three out of 84 constipated women with normal BET had chronic constipation but did not satisfy ROME criteria as they were taking laxatives.
Values are number of patients with abnormal pressures relative to 10th-90th percentile values in healthy women.
The proportion of patients with specific bowel symptoms was not different between C-normal BET and C-abnormal BET. However, more patients with C-abnormal BET (67%) than C-normal BET (48%) had functional constipation (P = .04) (Table 2). Among obstetric variables, only the number of vaginal deliveries requiring pelvic sutures was associated (P=.04) with group status, being greater in constipated than healthy women.
Rectoanal pressures during squeeze, evacuation, and Valsalva maneuver.
The average anal resting pressure and squeeze increment were not significantly different among the groups (Table 2). However, 22 C-abnormal BET (42%) and 25 C-normal BET (30%) patients had anal hypertension, i.e., the anal resting pressure was greater than the 90th percentile value for healthy women in this study, which was 85 mmHg. The anal squeeze increment was reduced (i.e. <10th percentile value in healthy women [16 mmHg]) in 19 C-normal BET (23%) and 11 C-abnormal BET (21%) patients (Table 2).
The rectal pressure during evacuation was different (P = .007) among groups, being lower in C-abnormal BET (48 [25] mmHg) than in C-normal BET (64 [30] mmHg) and healthy women (58 [29] mmHg). Thirteen C-abnormal BET (29%) had reduced rectal pressure during evacuation. The anal pressure during evacuation was also different (P < .0001) among groups, being greater in C-abnormal BET (88 [32] mmHg) than in C-normal BET (69 [21] mmHg) and healthy women (62 [20] mmHg). Seventeen of 52 C-abnormal BET patients (33%) had increased anal pressure during evacuation. The rectoanal gradient was different (P < .0001) among groups, being more negative in C-abnormal BET (−40 [38] mmHg) than in C-normal BET (−5 [31] mmHg) and healthy women (−4 [29] mmHg). Twenty-five C-abnormal BET patients (48%) had a more negative rectoanal gradient during evacuation.
Of 64 controls, 51 had none, 7 had one, and 6 had two or more abnormal rectoanal parameters during evacuation. Among 84 patients with C-normal BET, 67 had none, 13 had one, and 4 had two or more abnormal rectoanal parameters during evacuation. Of 52 C-abnormal BET patients, 19 had none, 14 had one, and 19 had two or more abnormal parameters during evacuation. Thus, 33 of 91 participants (36%) with no or one abnormal rectoanal parameter during evacuation had C-abnormal BET. Hence, the (prior) probability of belonging to the C-abnormal BET in such patients was 36%. By contrast 19 of 25 participants (76%) with two or more abnormal rectoanal parameters had C-abnormal BET.
By contrast to rectoanal pressures during evacuation, the anal pressure during Valsalva maneuver was not significantly different among healthy women, C-normal BET, and C-abnormal BET.
Relationships among anal pressures during squeeze, Valsalva maneuver and evacuation
In healthy controls, the anal pressure during squeeze accounted for 8% of the variance in anal pressure during evacuation (P = .03); the anal pressure during a Valsalva maneuver did not predict anal pressure evacuation (Table 3, Model 1). By contrast, in C-normal BET and C-abnormal BET, the anal pressure during a Valsalva maneuver accounted for respectively 26% and 49% of the variance in anal pressures during evacuation (P < .001); the anal pressure during squeeze was not significant in these models (Table 3, Models 2 and 3).
Table 3.
Multiple variable linear regression models to predict anal pressure during evacuation using anal pressures during squeeze and a Valsalva maneuver
| Group | Predictor variables | Model P value | Variance (%) explained by model | |||
|---|---|---|---|---|---|---|
| Anal pressure during squeeze | Anal pressure during Valsalva maneuver | |||||
| Estimate (95% CI) | P value | Estimate (95% CI) | P value | |||
| Healthy women (Model 1) | 0.14 (0.02, 0.3) | 0.02 | 0.07 (−0.1, 0.3) | 0.5 | 0.03 | 8% |
| Constipated women - normal BET (Model 2) | 0.05 (−0.04, 0.1) | 0.29 | 0.27 (0.1, 0.4) | <0.001 | <0.001 | 26% |
| Constipated women - abnormal BET (Model 3) | 0.08 (−0.09, 0.2) | 0.35 | 0.58 (0.3, 0.8) | <0.001 | <0.001 | 49% |
BET – Balloon expulsion time; CI – confidence interval
Both models in Table 4 include all 3 groups; the reference groups are healthy people (Table 4, Model 1) and C-normal BET (Table 4, Model 2). In both models, the anal pressure during a Valsalva maneuver, the group status and the interaction terms explained 41% of the variance in anal pressure during evacuation. The interaction term (Valsalva maneuver*constipation with abnormal BET) was significant in both models, which indicates that the relationship between anal pressure during evacuation and Valsalva maneuver was stronger in C-abnormal BET than in healthy women (Model 1, estimate (95% confidence interval) = 0.52 (0.3, 0.8), P < .001) and in C-normal BET (Model 2, estimate (95% confidence interval) = 0.33 (0.1, 0.5), P = .002). This suggests that for every 1 mmHg increase in pressure during a Valsalva maneuver, the difference in the mean anal pressure during evacuation was 0.52 mmHg and 0.33 mmHg for (C-abnormal BET-healthy women) and (C-abnormal BET-C-normal BET).
Table 4.
Multiple variable linear regression models to predict anal pressure during evacuation using anal pressures during a Valsalva maneuver
| Comparisons | Predictor variables | Estimate (95% CI) | P value | Variance (%) explained by model; P value |
|---|---|---|---|---|
| Anal pressure during evacuation in constipation-abnormal BET and constipation-normal BET versus healthy women (Model 1) | Healthy women (Reference group) | NA | NA | |
| Constipation with normal BET | −10.9 (−34, 12) | 0.34 | 41% <0.001 |
|
| Constipation with abnormal BET | −30.8 (−57, −5) | 0.02 | ||
| Anal pressure (VM) | 0.12 (−0.07, 0.3) | 0.24 | ||
| Anal pressure (VM)*constipation with normal BET† | 0.19 (−0.04, 0.4) | 0.10 | ||
| Anal pressure (VM)*constipation with abnormal BET† | 0.52 (0.3, 0.8) | <0.001 | ||
| Anal pressure during evacuation in constipation-abnormal BET and healthy women vs constipation-normal BET (Model 2) | Constipated women (Reference group) | NA | NA | |
| Healthy women | 8.67 (−14, 31) | 0.25 | 41% <0.001 |
|
| Constipation with abnormal BET | −20.5 (−42, 0.8) | 0.06 | ||
| Anal pressure (VM) | 0.31 (0.2, 0.4) | <0.001 | ||
| Anal pressure (VM)*healthy women† | 0.18 (−0.4, 0.06) | 0.14 | ||
| Anal pressure (VM)*constipation with abnormal BET† | 0.33 (0.1, 0.5) | 0.001 |
BET – Balloon expulsion time; VM – Valsalva maneuver; CI – Confidence interval
Interaction terms
Utility of anal pressures during evacuation and a Valsalva maneuver for predicting C-abnormal BET
The interaction terms in Table 4 are significant, which indicates that the relationship between anal pressures during a Valsalva maneuver and evacuation is influenced by group status. Expressed differently, the slope for anal pressure during a Valsalva maneuver and evacuation is different in C-abnormal BET versus healthy women. However, when the specific regression lines for each group were used, they intersected inside the plot (data not shown). Consequently, it was challenging to predict group status (eg, C-abnormal or C-normal BET) for selected combinations of anal pressures, for example, pressures less than 25 mmHg during a Valsalva maneuver and 25 mmHg during evacuation.
In all but one participant, the anal pressure during a Valsalva maneuver was greater than 25 mmHg. Hence, this plot and the regression models were revised by re-centering the data at 25 mm Hg, i.e., by subtracting 25 mmHg from all pressures during a Valsalva maneuver. In the revised model (Table 5, Model 1), the group terms (i.e., “C-normal BET” and “C-abnormal BET”) were not different versus zero. This suggests that the data are consistent with a model in which the lines converge at Valsalva maneuver pressure of 25 mmHg.
Table 5.
Modified multiple variable models to predict anal pressure during evacuation from anal pressures during a Valsalva Maneuver 1
| Comparisons | Predictor variable | Estimate (95% CI) | P value | Model P value | Adjusted R2 value |
|---|---|---|---|---|---|
| Anal pressure during evacuation in constipation-abnormal BET and constipation-normal BET versus healthy women (Model 1) | Intercept | 54 (39, 68) | <.0001 | <.0001 | 41% |
| VM–25 | 0.12 (−0.08, 0.3) | .2 | |||
| VM–25*C-normal BET | 0.2 (−0.04, 0.4) | .1 | |||
| VM–25*C-abnormal BET | 0.52 (0.3, 0.7) | <.0001 | |||
| C-normal BET | −6 (−23, 11) | .5 | |||
| C-abnormal BET | −18 (−38, 2) | .08 | |||
| Anal pressure during evacuation in constipation-abnormal BET and constipation-normal BET versus healthy women (Model 2) | Intercept | 46 (39, 53) | <.0001 | <.0001 | 40% |
| VM–25 | 0.22 (0.1, 0.3) | .0002 | |||
| VM–25*C-normal BET | 0.12 (0.02, 0.2) | .01 | |||
| VM–25*C-abnormal BET | 0.32 (0.2, 0.4) | <.0001 |
Therefore, these group terms were removed from the next model (Table 5, Model 2). Similar to Models 1 and 2 in Table 4 and Model 1 in Table 5, the 2 interaction terms, which represent the group-specific slopes, were significantly different versus controls. Assuming normally distributed residuals, the results of these multiple variable linear regression models (Table 5, Model 2), were used to calculate group-specific log densities for the joint distribution of anal pressures during evacuation and Valsalva maneuver. As stated above, this model is limited to situations where the anal pressure during a Valsalva maneuver is 25 mm Hg or greater. The mean square residual was 412. The formula is as follows:
Where y = Anal pressure during evacuation and
Log density (health) = − (1 / 824)* (Anal pressure during evacuation − [46.3 + 0.22* (Anal pressure during Valsalva maneuver −25)])2
Log density (C-abnormal BET) = − (1 / 824)* (Anal pressure during evacuation − [46.3 + 0.54* (Anal pressure during Valsalva maneuver – 25)])2
For example, in a patient with anal pressures of 144 mmHg and 113 mmHg during evacuation and a Valsalva maneuver, the log densities of being healthy or having C-abnormal BET were −7.4 and −3 respectively. The difference between these log density values [log density(C-abnormal BET) − log density(health)] provides the log density ratio, which is the relative likelihood that a patient has C-abnormal BET versus health. In this patient, the log density ratio is 4.4.
Both variables (i.e., number of abnormal parameters during evacuation and the “log density–ratio”, which is derived from the joint distribution of anal pressures during evacuation and a Valsalva maneuver), independently discriminated between C-abnormal BET and health (Table 5). The results of this logistic model were used to estimate the probability of C-abnormal BET in patients with no or one and separately, two or more abnormal rectoanal parameters. Figure 2 provides the estimated probability of C-abnormal BET for any given combination of anal pressures during evacuation and a Valsalva maneuver. For example, values that are between the 60% and 70% probability lines represent a 60–70% likelihood of having C-abnormal BET.
Figure 2.
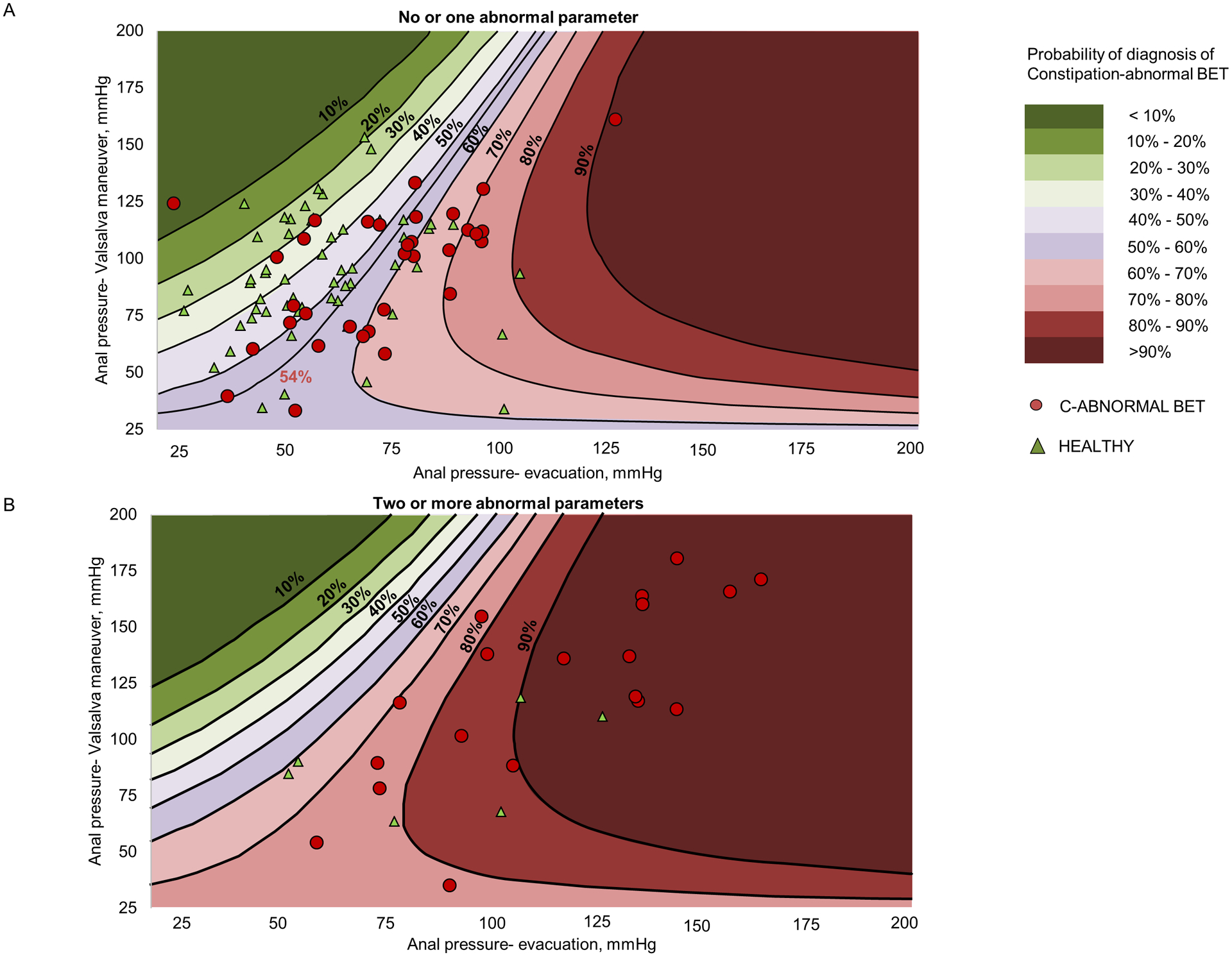
Probability of C-abnormal BET versus health in patients with no or one abnormal rectoanal parameter (upper panel) and two or more abnormal rectoanal parameters during evacuation (lower panel). The probability is determined by the joint distribution of anal pressures during evacuation and a Valsalva maneuver. In the upper panel, a probability of 54% optimally discriminated between controls and patients with C-abnormal BET, with a sensitivity of 67% and a specificity of 75%.
Figure 3 provides the distribution of these probabilities in controls and in patients with C-abnormal BET. The mean [SD] probability of having C-abnormal BET is greater (P = .0002) in C-abnormal BET (56 [16] %) than in controls (44 [15] %) with considerable overlap between groups. For example, the model suggests that 8/57 controls (14%) had an approximately 60% probability of having C-abnormal BET. The ROC curve for this model indicates that a probability threshold of 54% had an area under the curve of 0.73 (P = .0003) versus a null hypothesis value of 0.5. This model discriminated between controls and patients with C-abnormal BET with a sensitivity of 67% and a specificity of 75% (Figure 4). By contrast, the ROC curve was not statistically significant (area under curve, AUC = 0.71, P = .09) in participants with two or more abnormal parameters. Hence, an optimum cut off for discriminating between controls and C-abnormal BET could not be determined in patients with two or more abnormal parameters.
Figure 3.
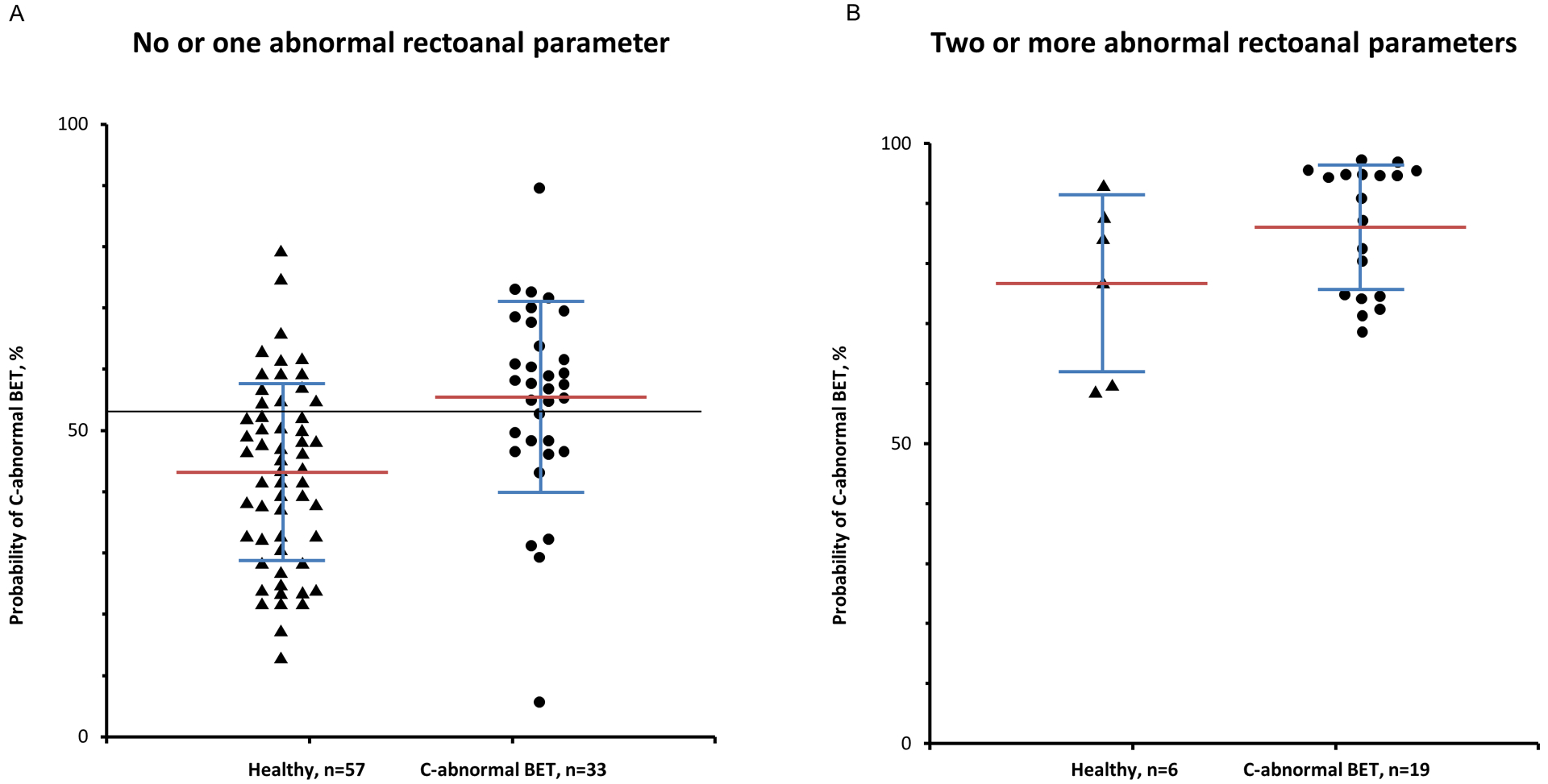
Distribution of probabilities of C-abnormal BET versus healthy controls in participants with no or one (Panel A) and two or more abnormal rectoanal parameters during evacuation (Panel B). The horizontal black line shows a probability threshold of 54%, which optimally discriminated between controls and C-abnormal BET patients (Panel A). By contrast, an optimum probability threshold could not be determined in Panel B.
Figure 4.
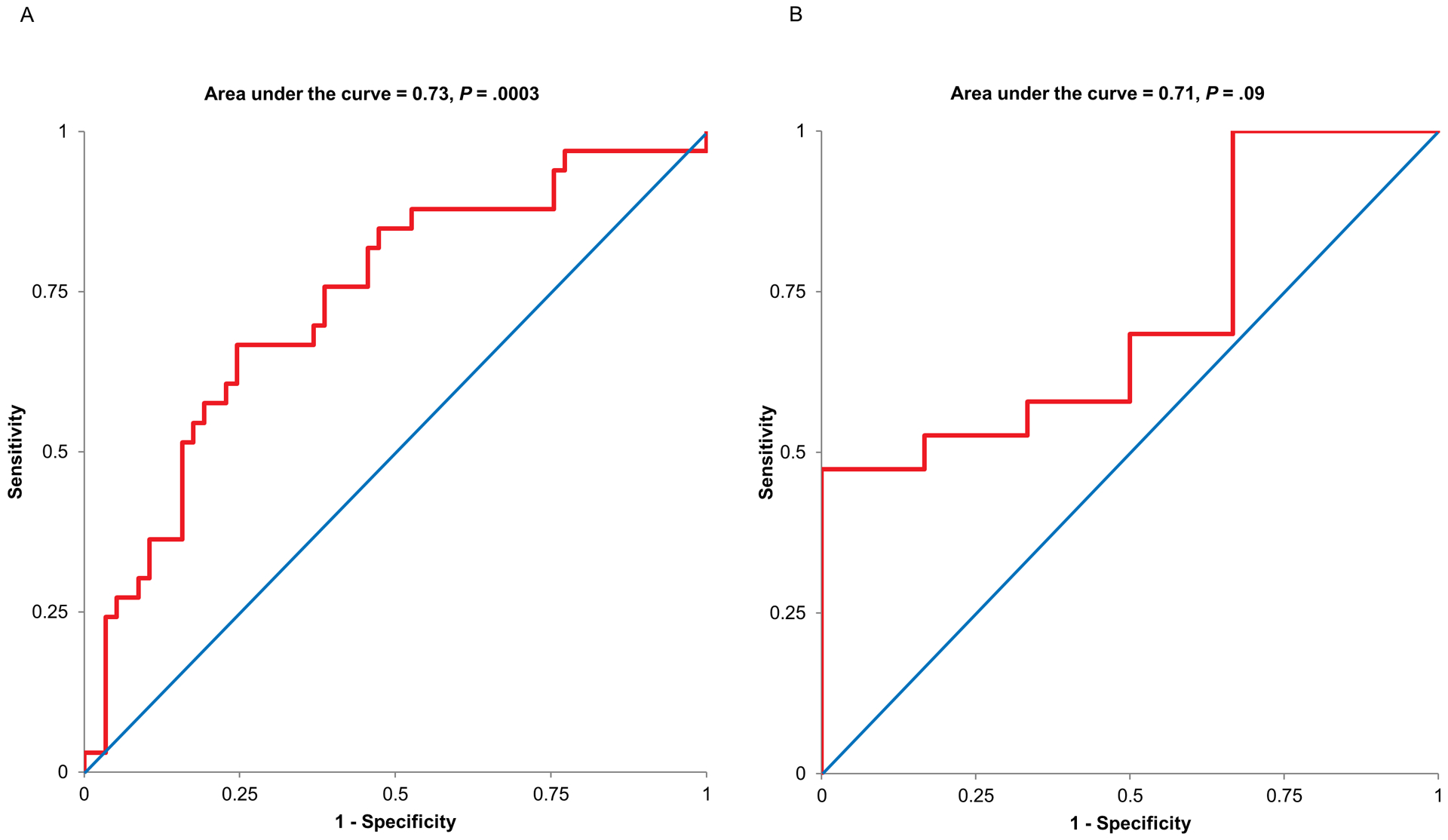
ROC curves showing the utility of anal pressures during evacuation and a Valsalva maneuver to discriminating between controls and C-abnormal BET patients in participants with no or one (Panel A) and two or more abnormal rectoanal parameters during evacuation (Panel B). In Panel A, a probability threshold of 54% distinguished between controls and C-abnormal BET patients (area under curve AUC = 0.73, sensitivity = 0.67, specificity = 0.75, P = .0003). By contrast, the ROC curve was not significant in participants with two or more abnormal recto anal parameters.
Discussion
The external anal sphincter relaxes during defecation and contracts to maintain continence28. DD are broadly attributed to inadequate rectal propulsive forces and/or dyssynergia. While dyssynergia is implicated to abnormal contraction of the external anal sphincter during defecation, this postulate is unproven29,30. Indeed, the underlying mechanism(s) of dyssynergia are not understood. Towards the objectives of enhancing our understanding of the pathogenesis and diagnosis of DD, we measured rectoanal pressures during evacuation and a Valsalva maneuver in healthy controls, C-normal BET, and C-abnormal BET.
The anal pressure during a Valsalva maneuver explained 0%, 26%, and 49% of the variance in anal pressures during evacuation in healthy women, C-normal BET and C-abnormal BET. Hence, anal pressures during evacuation more closely resemble pressures during a Valsalva maneuver in patients with C-abnormal BET than in C-normal BET and in healthy women. Confirming that finding, the interaction term (anal pressure during Valsalva maneuver*constipation with abnormal BET) was significant versus healthy controls and C-normal BET (Table 3). Taken together, these findings suggest that during defecation, some patients with C-abnormal BET resort to a Valsalva maneuver, which may at least partly explain incomplete evacuation.
The Rome IV consensus criteria suggest that two abnormal tests should be used to diagnose DD31. HRM exclusively relies on measurement of rectoanal pressures during evacuation. However, many patients with C-abnormal BET have normal rectoanal pressures during evacuation in the left lateral and seated positions20,32. If it were necessary to diagnose a DD by Rome IV criteria in such patients, another test (e.g. defecography) would be required. Hence, we sought to increase the utility of HRM for diagnosing DD by considering pressures during a Valsalva maneuver and evacuation. Similar to other conditions (eg, coronary heart disease), these findings suggest that a stepwise approach may increase the utility of HRM for diagnosing DD33. In the first step, patients are categorized into 2 groups with no or one, or, two or more abnormal rectoanal parameters during evacuation. Thereafter, the joint distribution of anal pressures during evacuation and a Valsalva maneuver is useful for estimating the likelihood of C-abnormal BET in patients with no or one abnormal rectoanal parameter during evacuation. [Of note, anal pressures during a Valsalva maneuver alone are not significantly different among the groups.] In this study, 33 patients with C-abnormal BET and 58 healthy people had no or only one abnormal parameter during evacuation. Hence, the prior probability of C-abnormal BET in patients with no or one abnormal parameter during evacuation was 33/91 or 36%. The joint distribution of anal pressures during evacuation and a Valsalva maneuver may facilitate the diagnosis of a DD in selected patients with C-abnormal BET and no or one abnormal parameter during evacuation. For example, further testing may be unnecessary in patients with typical clinical features of a DD and a probability threshold greater than 70%. Conversely, additional tests (eg, defecography)11 should be considered in patients with typical clinical features and a probability threshold lower than 50%. By contrast, the ROC curves were not useful for discriminating between C-abnormal BET and healthy controls in patients with two or more abnormal parameters during evacuation. Indeed, the estimated likelihood of C-abnormal BET was 80% or greater in four healthy controls (Figure 3, right panel). These healthy controls either have asymptomatic pelvic floor dysfunction or have normal anorectal functions but find it challenging to simulate evacuation during a manometry. While all healthy controls in this cohort had a normal BET, we previously observed that approximately 15% of healthy controls have a prolonged seated BET. These patients have a lower (ie more negative) rectoanal gradient during left lateral HRM than healthy controls with a normal BET19.
Among patients with C-normal BET, the anal pressure during a Valsalva maneuver explained 26% of the variance in anal pressure during evacuation compared to 0% in healthy people. Perhaps the overlapping pressure profiles between patients with C-normal BET and healthy controls suggests that some constipated women with a normal BET have a DD; the latter may be uncovered by defecography11,15,34. However, in the combined models (Table 4), the interaction term (anal pressure (VM)*C-abnormal BET) but not (anal pressure (VM)*C-normal BET) was significant, which indicates that the anal pressure change during a Valsalva maneuver in C-normal BET was not different versus healthy controls or C-abnormal BET.
While these observations are based on upright anorectal manometry in a large cohort of healthy women, constipated women with a normal BET, and with an abnormal BET, there are some limitations. The prevalence of structural disturbances (e.g., rectal intussusception), which may be clinically significant, is unknown11. The joint distribution of anal pressures during evacuation and a Valsalva maneuver facilitates the diagnosis of DD in selected patients with high (>70%) or low (<40%) probability of C-abnormal BET. Limited by the overlapping values between patients and controls, this metric is less useful when the probability is between 40 and 70%. While an abnormal BET is a reasonably accurate marker of a DD, defecography may disclose a DD in some patients with a normal BET11. Further studies are necessary to determine how the joint assessment of anal pressures during evacuation and a Valsalva maneuver complements other newer approaches to analyze rectoanal pressure profiles during evacuation20.
In summary, this study suggests that some patients with C-abnormal BET resort to a Valsalva maneuver during evacuation. In selected patients, the joint distribution of anal pressures during evacuation and a Valsalva maneuver facilitates the diagnosis of DD.
Table 6.
Multiple variable logistic regression model to predict C-abnormal BET
| Variable | Odds Ratio (95% CI) | P value |
|---|---|---|
| Two or more abnormal recto anal parameters during evacuation 1 | 4.6 (1.5, 14) | .008 |
| Log density–ratio (C-abnormal BET vs healthy controls) 2 | 2 (0.5, 2.8) | 0.0003 |
CI – Confidence interval
Study highlights.
What is known?
Defecation disorders (DD) are attributed to excessive straining and rectoanal dyssynergia.
Many patients with DD have normal rectoanal pressures during evacuation. Hence, HRM is of limited utility for diagnosing DD.
What is new here?
Anal pressures during evacuation more closely resemble pressures during a Valsalva maneuver in patients with C-abnormal BET than in C-normal BET and in healthy women, which supports the hypothesis that some patients with C-abnormal BET resort to a Valsalva maneuver during evacuation.
In selected patients, the joint distribution of anal pressures during evacuation and a Valsalva maneuver facilitates the diagnosis of DD.
Financial support:
This study was supported by USPHS NIH Grant R01 DK78924.
Abbreviations
- BET
balloon expulsion time
- BMI
body mass index
- CI
confidence interval
- C-NORMAL BET
constipated women with a normal balloon expulsion time
- C-ABNORMAL BET
constipated women with a prolonged balloon expulsion time
- DD
defecatory disorder
- HRM
high-resolution manometry
- IBS-C
constipation-predominant irritable bowel syndrome
Footnotes
Potential competing interests: Dr. Bharucha holds a patent jointly with Medtronic Inc for the anorectal manometry catheter fixation device used in this study and a patent for another anorectal manometry catheter. Other authors do not have any conflicts of interest.
Data availability: The data are available from the authors upon reasonable request.
Contributor Information
Sushmitha Grama Srinivasan, Division of Gastroenterology and Hepatology, Mayo Clinic, Rochester, Minnesota..
Mayank Sharma, Division of Gastroenterology and Hepatology, Mayo Clinic, Rochester, Minnesota..
Kelly Feuerhak, Division of Gastroenterology and Hepatology, Mayo Clinic, Rochester, Minnesota..
Kent R. Bailey, Division of Biomedical Statistics and Informatics, Mayo Clinic, Rochester, Minnesota..
Adil E. Bharucha, Division of Gastroenterology and Hepatology, Mayo Clinic, Rochester, Minnesota..
References
- 1.Sapsford RR, Hodges PW, Richardson CA, Cooper DH, Markwell SJ, Jull GA. Co-activation of the abdominal and pelvic floor muscles during voluntary exercises. Neurourology & Urodynamics. 2001;20(1):31–42. [DOI] [PubMed] [Google Scholar]
- 2.Neumann P, Gill V. Pelvic floor and abdominal muscle interaction: EMG activity and intra-abdominal pressure. International Urogynecology Journal. 2002;13(2):125–132. [DOI] [PubMed] [Google Scholar]
- 3.Thompson JA, O’Sullivan PB, Briffa K, Neumann P, Court S. Assessment of pelvic floor movement using transabdominal and transperineal ultrasound. International Urogynecology Journal. 2005;16(4):285–292. [DOI] [PubMed] [Google Scholar]
- 4.Hemborg B, Moritz U, Hamberg J, Holmstrom E, Lowing H, Akesson I. Intra-abdominal pressure and trunk muscle activity during lifting. III. Effect of abdominal muscle training in chronic low-back patients. Scand J Rehabil Med. 1985;17(1):15–24. [PubMed] [Google Scholar]
- 5.Floyd WF, Walls EW. Electromyography of the sphincter ani externus in man. The Journal of physiology. 1953;122(3):599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bo K, Stien R. Needle EMG registration of striated urethral wall and pelvic floor muscle activity patterns during cough, Valsalva, abdominal, hip adductor, and gluteal muscle contractions in nulliparous healthy females. Neurourol Urodyn. 1994;13(1):35–41. [DOI] [PubMed] [Google Scholar]
- 7.Iscoe S. Control of abdominal muscles. Prog Neurobiol. 1998;56(4):433–506. [DOI] [PubMed] [Google Scholar]
- 8.Peschers UM, Vodusek DB, Fanger G, Schaer GN, DeLancey JO, Schuessler B. Pelvic muscle activity in nulliparous volunteers. Neurourol Urodyn. 2001;20(3):269–275. [DOI] [PubMed] [Google Scholar]
- 9.Thompson JA, O’Sullivan PB, Briffa NK, Neumann P. Differences in muscle activation patterns during pelvic floor muscle contraction and Valsalva maneuver. Neurourol Urodyn. 2006;25(2):148–155. [DOI] [PubMed] [Google Scholar]
- 10.Orno AK, Dietz HP. Levator co-activation is a significant confounder of pelvic organ descent on Valsalva maneuver. Ultrasound Obstet Gynecol. 2007;30(3):346–350. [DOI] [PubMed] [Google Scholar]
- 11.Bharucha AE, Lacy BE. Chronic Constipation: Mechanisms, Evaluation and Management. Gastroenterology. 2020;158(5):1232–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heinrich H, Sauter M, Fox M, et al. Assessment of Obstructive Defecation by High-Resolution Anorectal Manometry Compared With Magnetic Resonance Defecography. Clinical Gastroenterology and Hepatology. 2015;13(7):1310–1317.e1311. [DOI] [PubMed] [Google Scholar]
- 13.Bharucha AE, Seide BM, Zinsmeister AR, Melton LJ 3rd. Insights into normal and disordered bowel habits from bowel diaries. Am J Gastroenterol. 2008;103(3):692–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bharucha AE, Croak AJ, Gebhart JB, et al. Comparison of rectoanal axial forces in health and functional defecatory disorders. American Journal of Physiology - Gastrointestinal & Liver Physiology. 2006;290(6):G1164–1169. [DOI] [PubMed] [Google Scholar]
- 15.Bharucha AE, Fletcher JG, Seide B, Riederer SJ, Zinsmeister AR. Phenotypic Variation in Functional Disorders of Defecation. Gastroenterology. 2005;128:1199–1210. [DOI] [PubMed] [Google Scholar]
- 16.Azpiroz F, Enck P, Whitehead WE. Anorectal functional testing: review of collective experience. Am J Gastroenterol. 2002;97(2):232–240. [DOI] [PubMed] [Google Scholar]
- 17.Lee TH, Bharucha AE. How to Perform and Interpret a High-resolution Anorectal Manometry Test. J Neurogastroenterol Motil. 2016;22(1):46–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Basilisco G, Bharucha AE. High-resolution anorectal manometry: An expensive hobby or worth every penny? Neurogastroenterol Motil. 2017;29(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oblizajek NR, Gandhi S, Sharma M, et al. Anorectal pressures measured with high-resolution manometry in healthy people—Normal values and asymptomatic pelvic floor dysfunction. Neurogastroenterology and Motility : the official journal of the European Gastrointestinal Motility Society. 2019:e13597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sharma M, Muthyala A, Feuerhak K, Narayanan SP, Bailey KR, Bharucha AE. Improving the Utility of High Resolution Manometry for the Diagnosis of Defecatory Disorders in Women with Chronic Constipation. Neurogastroenterol and Motility. 2020. July 01:e13910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bharucha AE, Wald A, Enck P, Rao S. Functional anorectal disorders. Gastroenterology. 2006;130(5):1510–1518. [DOI] [PubMed] [Google Scholar]
- 22.Sharma M, Feuerhak KJ, Corner SM, Manduca A, Bharucha AE. A New Method for Assessing Anal Distensibility with a Barostat and MRI in Healthy and Constipated Women. Neurogastroenterol and Motility. 2020;under revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prichard DO, Lee T, Parthasarathy G, Fletcher JG, Zinsmeister AR, Bharucha AE. High-resolution Anorectal Manometry for Identifying Defecatory Disorders and Rectal Structural Abnormalities in Women. Clinical Gastroenterology & Hepatology. 2017;15(3):412–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ratuapli S, Bharucha AE, Harvey D, Zinsmeister AR. Comparison of rectal balloon expulsion test in seated and left lateral positions. Neurogastroenterol Motil. 2013;25(12):e813–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mazor Y, Prott G, Jones M, Kellow J, Ejova A, Malcolm A. Anorectal physiology in health: A randomized trial to determine the optimum catheter for the balloon expulsion test. Neurogastroenterol Motil. 2019;31(4):e13552. [DOI] [PubMed] [Google Scholar]
- 26.Lal S, Ferguson AD, Campbell EJ. Forced Expiratory Time: A Simple Test for Airways Obstruction. Br Med J. 1964;1(5386):814–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jaeschke R, Guyatt GH, Sackett DL. Users’ guides to the medical literature. III. How to use an article about a diagnostic test. B. What are the results and will they help me in caring for my patients? The Evidence-Based Medicine Working Group. JAMA. 1994;271(9):703–707. [DOI] [PubMed] [Google Scholar]
- 28.Palit S, Lunniss PJ, Scott SM. The physiology of human defecation. Dig Dis Sci. 2012;57(6):1445–1464. [DOI] [PubMed] [Google Scholar]
- 29.Preston DM, Lennard-Jones JE. Anismus in chronic constipation. Digestive Diseases & Sciences. 1985;30(5):413–418. [DOI] [PubMed] [Google Scholar]
- 30.Duthie GS, Bartolo DC. Anismus: the cause of constipation? Results of investigation and treatment. World Journal of Surgery. 1992;16(5):831–835. [DOI] [PubMed] [Google Scholar]
- 31.Rao SS, Bharucha AE, Chiarioni G, et al. Functional Anorectal Disorders. Gastroenterology. 2016;25:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ratuapli S, Bharucha AE, Noelting J, Harvey D, Zinsmeister AR. Phenotypic Identification and Classification of Functional Defecatory Disorders Using High Resolution Anorectal Manometry Gastroenterology. 2013;144:314–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Diamond GA, Forrester JS. Analysis of probability as an aid in the clinical diagnosis of coronary-artery disease. N Engl J Med. 1979;300(24):1350–1358. [DOI] [PubMed] [Google Scholar]
- 34.Grossi U, Di Tanna GL, Heinrich H, Taylor SA, Knowles CH, Scott SM. Systematic review with meta-analysis: defecography should be a first-line diagnostic modality in patients with refractory constipation. Aliment Pharmacol Ther. 2018;48(11–12):1186–1201. [DOI] [PubMed] [Google Scholar]


