Abstract
Background/Aims:
Gut microbiota are affected by diet, country and affects outcomes in cirrhosis. Western diets are associated with dysbiosis. Comparisons with other diets is needed.
Aim:
Compare cirrhosis patients from USA to Brazil with respect to diet, microbiota, and impact on hospitalizations.
Methods:
Healthy controls and compensated/decompensated outpatients with cirrhosis from USA and Brazil underwent dietary recall and stool for 16S rRNA sequencing. Demographics, medications/cirrhosis details were compared within/between countries. Patients with cirrhosis were followed for 90-day hospitalizations. Regression for Shannon diversity was performed within cirrhosis. Regression for hospitalizations adjusting for clinical and microbial variables was performed.
Results:
MELD, diabetes, ascites, albumin was similar, but more Americans were men, had higher hepatic encephalopathy and alcohol/hepatitis C etiology with lower NAFLD than Brazilians. Brazilians had higher cereal, rice, and yogurt intake versus USA. As disease progressed cereals, rice/beans, coffee, and chocolate consumption reduced. Microbial diversity was higher in Brazilians. Within cirrhosis, high diversity was related to Brazil-origin (p<0.0001), age, and cereal intake (p=0.05) while high MELD (p=0.009) and ascites (p=0.05) did the reverse. Regardless of stage, beneficial taxa/those higher with grain/yogurt intake were higher (Ruminococcaceae, Christensenellacae, Prevotellaceae), while pathobionts (Porphyromonadaceae, Sutterellaceae, Enterobacteriaceae) were lower in Brazilians.
Hospitalizations:
More Americans were hospitalized versus Brazil (p=0.002). On regression, MELD (p=0.001) and ascites (p=0.001) associated with higher while chocolate (p=0.03) and Brazil-origin (p=0.001) associated with lower hospitalizations with/without microbiota inclusion.
Conclusions:
: Brazilian cirrhotic patients follow a diet richer in cereals and yogurt, which associates with higher microbial diversity and beneficial microbiota and could contribute towards lower hospitalizations compared to a Western-diet consuming American cohort.
Keywords: Western diet, Chocolate, Cereals, Microbial diversity, Yogurt
Graphical Abstract

BACKGROUND:
Due to the increasing prevalence of chronic liver disease worldwide, it is important to determine how population-specific characteristics can influence disease progression1. Alterations in gut microbiota composition and function play a major role in progression of cirrhosis2. In experiences from Western countries compared to Turkey and Mexico, the dietary and socio-cultural practices can impact the gut microbiota and hospitalizations3–5. Compared to the US-based diet, the Turkish diet had more fermented milk products leading to higher diversity and better outcomes3. In contrast, the Mexican diet with higher carbohydrate and low protein showed lower diversity and worse outcomes than the US-based cohort4.
Since the Brazilian population has a high genetic heterogeneity, a high burden of liver disease and cirrhosis, with unique dietary habits, the impact of gut microbiota in Brazilian patients with cirrhosis is important to evaluate6–8. This will provide insight into the interaction of diet, microbiota and cirrhosis severity and progression and further refine our understanding of dietary practices that can potentially improve outcomes. Our aim was to compare the diet and gut microbiota changes in patients with cirrhosis from Brazil and USA and study their association with hospitalizations over 90 days.
METHODS:
Subjects:
After IRB approval, we prospectively recruited outpatients from two sites in USA and Brazil. All subjects underwent 7-day dietary recall and stool collection. We included healthy controls and outpatients with cirrhosis both in compensated and decompensated stages from both countries. Controls were subjects without chronic diseases, any illicit drug use, alcohol abuse (AUDIT-10≥8), or prescription medications. Cirrhosis was diagnosed by liver biopsy, transient elastography, presence of signs of portal hypertension, varices, or thrombocytopenia in patients with chronic liver disease, or frank decompensation. Decompensation was defined as ascites, hepatic encephalopathy (HE), variceal bleeding or jaundice. Patients with an unclear history of cirrhosis, any illicit drug use, alcohol abuse (AUDIT-10≥8), and those unable to consent, provide dietary history or samples were excluded.
In addition to the structured 7-day dietary recall, we recorded clinical and demographics data, as well as medications and cirrhosis characteristics. Specifically, we collected data on alcohol use, MELD score, complications, medications such as lactulose, rifaximin, proton pump inhibitors (PPI) and beta-blockers. All patients with cirrhosis were followed for 90 days after enrollment for non-elective hospitalizations. We compared within cohort (control vs compensated vs decompensated) and between cohort (controls, compensated and decompensated in USA vs Brazil) for demographics and diet for everyone and cirrhosis-specific changes within the cirrhosis cohorts. Sample size was based on prior studies comparing the impact of diet and microbiota from Turkey and Mexico9,10.
Microbiome analysis:
All patients underwent stool collection for microbiota analysis using identical methods according to standard published techniques11. Analysis was performed using microbial 16SrRNA sequencing.
16S rRNA bacterial community analysis:
The V1 and V2 hyper-variable regions of the bacterial 16S ribosomal RNA (rRNA) gene were sequenced on a PGM Ion Torrent Next-generation sequencer using Multitag fusion primers targeting the V1–V2 region (27F: 5’-AGAGTTTGATCCTGGCTCAG-3’, 355R: 5’-GCTGCCTCCCGTAGGAGT-3’).
Quality Control:
A negative control (water) and a known positive control were used in each batch of microbial DNA sequencing to assess laboratory variability and contamination12.
Overall Bio-informatics analysis: We used the Microbiome Analysis Center’s Portal to organize raw data, track clinical metadata, and track analysis between the groups A sequence identity of 97% was used to generate OTUs representing bacterial species. The taxonomic identity of reference sequences were determined using the RDP11 Classifier(Ribosomal data project) 13 and QIIME2 (Quantitative Insights Into Microbial Ecology) Pipeline14. Microbiota were compared using LEfSe between and within countries at family and genus level. Alpha diversity (Shannon index) and Beta-diversity (comparison between several groups using principal coordinate analyses) were performed. In addition, we used LEfSe (Linear discriminant analysis of effect size) to compare individual taxa at the family and genus level15. We compared within cohort (control vs compensated vs decompensated) and between cohort (controls, compensated and decompensated in USA vs Brazil) for all metrics mentioned above.
Regression for Shannon diversity was performed for all subjects and then within cirrhosis. Demographics, cirrhosis details, including alcohol-related etiology and microbial diversity were co-variates. We performed stepwise linear regression for Shannon Diversity index in all subjects and then in only patients with cirrhosis using USA vs Brazil as one of the predictor variables. Demographics and diet were evaluated in all subjects while in addition, cirrhosis-related variables, including MELD score, complications, alcohol-related etiology were also assessed as contributors in the cirrhosis-only analysis.
Hospitalizations at 90 days were also analyzed using binary logistic regression using diet, demographics, cirrhosis severity and country of origin as variables. Then using MaAsLin2, we determined the additive impact of microbiota on hospitalizations using multi-variable regression16.
All authors had access to the study data and reviewed and approved the final manuscript.
RESULTS:
Subject characteristics:
We enrolled 157 US-based subjects (48 controls, 59 compensated and 50 decompensated patients) and 228 subjects from Brazil (57 controls, 114 compensated and 57 decompensated patients. As shown in table 1, while patients with cirrhosis were similar in age, Brazilian controls were younger than their US-based counterparts. There was relatively similar race-based enrollment across and within sites with most subjects being white. A relatively higher proportion of US-based subjects were men. When patients with cirrhosis were compared, while the MELD scores, PPI, diabetes, ascites presence and albumin levels were comparable between countries, US-based patients were more likely to have alcohol and hepatitis C-related rather than NAFLD etiology and higher percentage with prior HE and rifaximin use than Brazilian patients.
Table 1:
Comparison of Demographics and Diet between Cohorts in the Entire Group
| USA (n=157) | Brazil (n=228) | |||||
|---|---|---|---|---|---|---|
| Control (n=48) | Comp (n=59) | Decomp (n=50) | Control (n=57) | Comp (n=114) | Decomp (n=57) | |
| Age‡* | 62.1±13.6* | 61.0±7.9 | 61.0±6.8 | 53.1±16.9 | 61.7±10.4 | 58.0±13.7 |
| Male* | 27 | 48* | 46* | 16 | 46 | 37 |
| Race (White/Black/Other) | 36/9/3 | 39/18/2 | 34/14/2 | 43/7/7 | 90/19/5 | 41/9/7 |
| MELD score‡† | - | 8.7±2.9 | 12.6±5.3* | - | 8.4±2.3 | 14.6±5.3 |
| NAFLD etiology* | - | 10 | 4 | 69 | 14 | |
| Alcohol etiology* | - | 16 | 27 | - | 16 | 21 |
| Hepatitis C etiology* | - | 27* | 16 | - | 23 | 14 |
| Other etiologies | - | 6 | 3 | - | 6 | 8 |
| Diabetes | - | 14 | 15 | - | 63 | 18 |
| Proton pump inhibitor use*‡† | 10 | 19 | 26* | 5 | 50 | 17 |
| Ascites | - | - | 34 | - | - | 41 |
| Lactulose* | - | - | 38* | - | - | 25 |
| Rifaximin* | - | - | 31* | - | - | 5 |
| Prior hepatic encephalopathy ‡† | - | - | 40* | - | - | 27 |
| Serum albumin (g/dl) ‡† | - | 3.8±0.5 | 3.2±0.6 | - | 4.3±0.4 | 3.2±0.5 |
| Smoking within 30 days | 2 | 16 | 9 | 3 | 16 | 6 |
| Active alcohol use‡† | 10 | 3 | 2 | 12 | 9 | 2 |
| Diet | ||||||
| Coffee‡†* | 27* | 38* | 33 | 50 | 98 | 44 |
| Tea† | 18 | 18 | 16 | 30 | 42 | 26 |
| Caffeinated carbonated drinks† | 17 | 22 | 28 | 22 | 44 | 22 |
| Other carbonated drinks† | 6 | 10 | 7 | 24 | 35 | 16 |
| Milk | 30 | 30 | 30 | 35 | 72 | 35 |
| Yogurt * | 14* | 9* | 6* | 27 | 26 | 24 |
| Cheese | 35 | 40 | 33 | 48 | 61 | 32 |
| Eggs | 25 | 27 | 23 | 37 | 43 | 30 |
| Vegetables | 46 | 50 | 39 | 55 | 91 | 34 |
| Fruits | 42 | 42 | 35 | 53 | 108 | 49 |
| Multi-grain cereals † * | 24 | 21 | 17 | 48 | 64 | 34 |
| Rice † * | 12 | 10 | 7 | 55 | 109 | 54 |
| White bread | 22 | 32 | 30 | 45 | 84 | 48 |
| Wheat bread† | 20 | 11 | 4* | 31 | 35 | 17 |
| Beef | 34 | 49 | 41 | 50 | 93 | 48 |
| Pork† | 30 | 48 | 26 | 14 | 42 | 10 |
| Seafood † * | 10 | 10 | 9 | 1 | 3 | 0 |
| Poultry | 49 | 57 | 47 | 54 | 99 | 46 |
| Chocolate†‡ | 14 | 13 | 12 | 35 | 31 | 16 |
| Hospitalizations over 90 days * | - | 6 (10%) | 14 (28%) | - | 0 (0%) | 11 (19%) |
p<0.05 US vs Brazil
p<0.05 within USA
p<0.05 within Brazil
Comp: compensated, Decomp: decompensated. Rice in Brazil was interpreted as rice with beans, data are presented as raw numbers or mean±SD unless otherwise specified.
Diet:
Brazilian subjects showed higher cereal, rice with beans, and yogurt intake and lower seafood compared to Americans. As disease progressed in both countries, cereals, coffee, and chocolate reduced with decompensation. Alcohol intake was higher in controls compared to cirrhosis patients in both countries. On the other hand, alcohol intake, meat, fish, poultry, egg, milk, vegetables, and fruit intake were similar across countries (Table 1).
Gut microbiota:
Diversity:
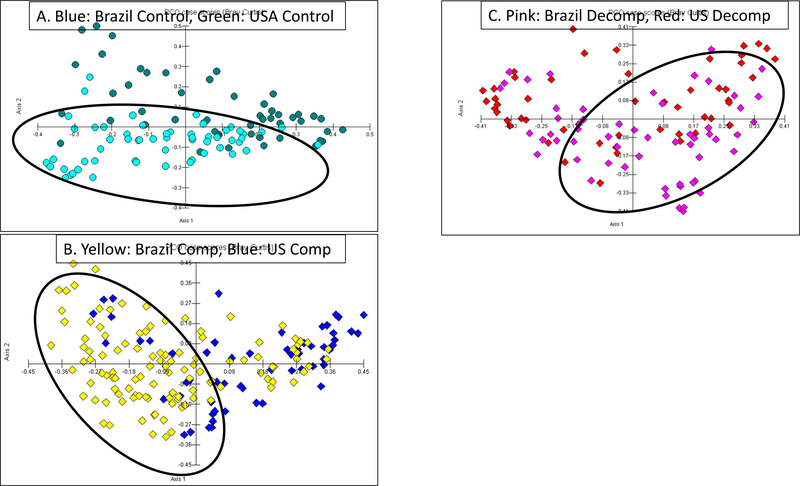
Alpha-diversity was significantly higher in all Brazilian compared to US cohorts (Figure 1A). As shown in figures 1B–C, decompensated patients clustered separately from compensated patients and controls within both countries. When patients were compared between countries, the clustering was further apart in compensated patients and controls while there was more overlap in decompensated patients’ microbiota regardless of country (Figure 2A–C).
Figure 1: Shannon Diversity and Principal Coordinate Analysis within USA and Brazil Cohorts.
A: Shannon diversity median and 95% CI shows significantly lower alpha-diversity in American versus Brazilian cohorts. USCtr: American Controls, USCom: American compensated patients, USDec: American Decompensated patients, BrCrl: Brazilian controls, BrCom: Brazilian compensated patients, BrDec: Brazilian Decompensated patients.
B: Within Brazil beta-diversity shows clustering of controls (blue) compared to compensated (yellow) and decompensated (purple) dots.
C: Within USA beta-diversity again shows clustering of controls (green) compared to compensated (blue) and decompensated (red) dots.
Figure 2: Principal Coordinate Analysis comparison between USA and Brazil Cohorts for controls, compensated and decompensated Patients with cirrhosis.
Between country comparisons of Beta-diversity
A: Control comparisons showed Brazilian controls (blue) clustered away from Americans (green)
B: Compensated patients analysis showed Brazilian patients (yellow) clustered away from Americans (blue)
C: Decompensated patients analysis showed relative lack of clustering between cohorts, Brazilian group (pink), American group (red)
On regression with all subjects included, Brazil origin (p<0.0001), control status (p=0.05), higher age (p=0.05), and higher cereal intake (p=0.05) were associated with higher Shannon diversity. Within the cirrhosis group, high Shannon diversity was related to Brazil-origin (p<0.0001), higher age and cereal intake (p=0.05) while high MELD (p=0.009) and ascites (p=0.05) did the reverse.
Individual taxa:
As shown in tables 2 and 3, there were major differences at the family level on LEfSe between and within cohorts. Potentially beneficial taxa and those associated with higher multi-grain intake were higher in Brazilians (Ruminococcaceae, Christensenellacae, Prevotellaceae), while potential pathobionts (Porphyromonadaceae, Sutterellaceae, Enterobacteriaceae) were higher in USA. Specific genera belonging to these families further separated between and within groups in USA and Brazil (Tables S1–S9 and figures S1–3).
Table 2:
LEfSe Analysis of Families Different Between Cohorts
| Higher in USA | Higher in Brazil | |
|---|---|---|
| Controls |
Porphyromonadaceae
Streptococcaceae Pastuerellaceae Bacteroidaceae Peptostreptococcaceae |
Ruminococcaceae
Prevotellaceae Clostridiales Incertae Sedis IV Clostridaceae Enterococcaceae Veillonellaceae Heliobacteriaceae Carnobacteriaceae Synergistiaceae Erysipelothricaceae Christensenellacae |
| Compensated |
Porphyromonadaceae
Streptococcaceae Sutterellaceae Bacteroidaceae Peptostreptococcaceae Enterobacteriaceae Clostridiales Cluster XI, XIII Acidaminococcaceae |
Heliobacteriaceae
Carnobacteriaceae Ruminococcaceae Prevotellaceae Clostridiales Incertae Sedis IV Synergistiaceae Erysipelothricaceae Christensenellacae |
| Decompensated |
Acidaminococcaceae
Sutterellaceae |
Streptococcaceae
Carnobacteriaceae Ruminococcaceae Prevotellaceae Christensenellacae Catabacteriaceae |
Table 3:
LEfSe Analysis of Families Different Within Countries
| USA | Higher in Controls | Higher in Compensated |
| Controls vs compensated USA |
Clostriales Incertae Sedis IV
Rikenellaceae Defluvitalaceae Ruminococcaceae Peptococcaceae |
Lactobacillaceae
Streptococcaceae Veillonellaceae Fusobacteriaceae Actinomycetaceae |
| Higher in Controls | Higher in Decompensated | |
| Controls vs Decompensated USA |
Rikenellaceae
Peptococcaceae Peptostreptococcacae Bacteroidaceae Clostriales Incertae Sedis IV Clostriales Incertae Sedis XII Clostriales Incertae Sedis XIII Bradyrhizobiaceae Ruminococcaceae Porphyromonadaceae Defluvitalaceae Thermodesulfobiaceae |
Campylobacteriaceae
Lactobacillaceae Bifidobacteriaceae Veillonellaceae Fusobacteriaceae |
| Higher in Compensated | Higher in Decompensated | |
| Compensated vs Decompensated USA |
Lachnospiraceae
Clostriales Incertae Sedis XI Clostriales Incertae Sedis XII Clostriales Incertae Sedis XIII Rikenellaceae Coriobacteriaceae |
Peptostreptococcacae
Bifidobacteriaceae Veillonellaceae |
| Brazil | Higher in Controls | Higher in Compensated |
| Controls vs compensated Brazil |
Catabacteriaceae
Porphyromonadaceae Blastocatella Synergistaceae Acidaminococcaceae Sutterellaceae Desulfovibrionaceae Clostridiales_IncertaeSedisIV Rikenellaceae Burkholderiales_incertaesedis Acidimicrobiaceae Bacteroidaceae Christensenellaceae Thermodesulfobiaceae Peptococcaceae Clostridiaceae Marinilabiliaceae Heliobacteriaceae Ruminococcaceae CandidatusCloacamonas Clostridiales_IncertaeSedisXIII |
Lactobacillaceae
Streptococcaceae Micrococcaceae Clostridiales_IncertaeSedisXI Fusobacteriaceae Enterobacteriaceae Pasteurellaceae Leuconostocaceae Erysipelotrichaceae |
| Higher in Controls | Higher in Decompensated | |
| Controls vs Decompensated Brazil |
Catabacteriaceae
Porphyromonadaceae Blastocatella Clostridiales_IncertaeSedisXI Synergistaceae Acidaminococcaceae Sutterellaceae Desulfovibrionaceae Clostridiales_IncertaeSedisIV Rikenellaceae Clostridiales_IncertaeSedisXII Burkholderiales_incertaesedis Acidimicrobiaceae Chloroplast Christensenellaceae Hyphomonadaceae Flammeovirgaceae Cyclobacteriaceae Peptococcaceae Clostridiaceae Chitinophagaceae Marinilabiliaceae Heliobacteriaceae Ruminococcaceae Clostridiales_IncertaeSedisXIII Bacteroidales_incertaesedis |
Lactobacillaceae
Bifidobacteriaceae Fusobacteriaceae Enterobacteriaceae Pasteurellaceae Leuconostocaceae Micrococcaceae Streptococcaceae Actinomycetaceae |
| Higher in Compensated | Higher in Decompensated | |
| Compensated vs Decompensated Brazil |
Catabacteriaceae
Prevotellaceae Synergestaceae Acidaminococcaceae Desulfovibrionaceae Rikenellaceae Chloroplast Cyclobacteriaceae Rikenellaceae Clostridiales_IncertaeSedisIV Clostridiales_IncertaeSedisXII Ruminococcaceae Heliobacteriaceae Clostridiaceae |
Bacteroidaceae
Micrococcaceae Streptococcaceae |
Hospitalizations:
Within 90 days, 21 (19%) patients with cirrhosis from USA were admitted compared to 11 (6%) from Brazil (p<0.001) were hospitalized. The leading reason for hospitalizations in US were HE (n=7) followed by ascites-related (n=4), renal/electrolyte (n=4) and infections (n=3) and others (n=3). In Brazil the leading cause was HE (n=5) followed by ascites-related (n=3), renal/electrolyte (n=2), and others (n=1).
On binary logistic regression, MELD score (OR 3.3, p=0.001) and ascites (OR: 2.9, p=0.001) were associated with hospitalizations, while chocolate intake (OR 0.3, p=0.03) and Brazil origin (OR 0.2, p=0.001) were protective.
When microbiota were added using MaAsLin2 at family and genus levels, both models included cirrhosis severity (MELD score, prior HE), Brazil origin, dietary constituents (rice, coffee) and independent contributions from potentially pathogenic and autochthonous taxa were contributors towards hospitalizations (Table 4).
Table 4:
MaAsLin2 Analysis of Hospitalizations in Patients with Cirrhosis
| Genus-level | Family-level | ||||||
|---|---|---|---|---|---|---|---|
| Feature | Coefficient | P-value | Q-value | Feature | Coefficient | P-value | Q-value |
| Brachyspira | 7.710336 | 5.44E-09 | 1.87E-06 | Brachyspiraceae | 7.729541 | 5.01E-09 | 6.66E-07 |
| MELD score | 0.389873 | 3.32E-07 | 5.70E-05 | Initial MELD | 0.389873 | 3.32E-07 | 2.21E-05 |
| Kosakonia | 6.368388 | 1.20E-06 | 0.000137 | Enterococcaceae | 3.228292 | 1.02E-06 | 4.50E-05 |
| Anaerofilum | 2.340699 | 1.61E-06 | 0.000138 | Prior HE | 1.079602 | 1.97E-05 | 0.000655 |
| Prior HE | 1.079602 | 1.97E-05 | 0.001352 | Rice | −0.76889 | 0.000281 | 0.007471 |
| Ruminococcus | −2.46194 | 5.68E-05 | 0.002783 | USA1-BRA2 | −0.22077 | 0.00076 | 0.016853 |
| Salmonella | 3.196077 | 5.08E-05 | 0.002783 | Coffee | −0.42314 | 0.001514 | 0.028762 |
| Enterococcus | 3.742249 | 7.30E-05 | 0.003132 | ||||
| Rice | −0.76889 | 0.000281 | 0.010705 | ||||
| Atopobium | 2.989837 | 0.000526 | 0.016407 | ||||
| Dorea | −1.07421 | 0.000526 | 0.016407 | ||||
| USA1-BRA2 | −0.22077 | 0.00076 | 0.021732 | ||||
| Coffee | −0.42314 | 0.001514 | 0.039941 | ||||
Positive coefficient indicates associated with hospitalization while negative coefficient indicates protection from hospitalization. Brazil was coded as 2 and USA as 1 so negative indicates lower hospitalizations in Brazil-origin patients with cirrhosis.
DISCUSSION:
We found that despite differences in cirrhosis severity, patients with cirrhosis from Brazil follow a diet richer in cereals, rice and beans, and yogurt that is associated with higher gut microbial diversity. Brazilian patients, regardless of disease or compensated stage and likely related to their diet showed higher beneficial bacteria, and lower hospitalizations than US-based patients with cirrhosis. Cereals and chocolate intake were linked with higher diversity and lower hospitalizations regardless of cohort. The Brazilian diet is associated with higher microbial diversity and lower hospitalizations in cirrhosis compared to the Western diet followed in USA.
These findings underscore the major role that diet, and socio-cultural practices play in determining the microbiota composition and their specific association even with end-stage organ damage such as cirrhosis. The racial composition was similar across countries. The diet in subjects from Brazil consistently showed a greater consumption of cereals, rice, multi-grains, and yogurt with lower seafoods compared to the USA regardless of controls or cirrhosis. This reflects the traditional Brazilian meals that have a foundation of rice and beans that is then topped with varying amounts of multi-grains, vegetables, manioc flour and overlying meat or other proteins, as necessary. These contrast with the predominantly meat and carbohydrate-focused Western diet with relatively lower yogurt consumption. In addition, there is an emphasis on greater unprocessed and natural foods in Brazilian universal dietary guidelines that could be critical for consistency regarding dietary advice in their national healthcare system17. Rice is often consumed in conjunction with black beans, a good source of plant-based protein in Brazil which has lower ammoniagenicity than animal proteins and could be potentially related to lower hospitalizations in Brazilian patients with cirrhosis.18–21 The higher consumption of whole grains, rice and beans and yogurt could be associated with engendering microbial diversity, which was seen in all cohorts within the Brazilian compared to US-based subjects. This higher consumption of grains likely led to a greater variety of bacteria that metabolize complex carbohydrates to short-chain fatty acids (SCFA)22. These SCFAs are important for the integrity of the intestinal barrier, preventing bacterial translocation and stimulating the immunity and enteric nervous system22, 23. The bacteria over-represented in the Brazilian co horts are also associated with secondary bile acid production that are bacteriostatic and indicate greater gut health24. Alcohol intake but not misuse, was higher in hea lthy controls in both countries compared to cirrhosis patients. Prior studies have shown that dysbiosis is higher with alcohol use25 but since our controls (regardless of country) had higher diversity that patients with cirrhosis, it is unlikely that this amount of alcohol was a factor in microbiota analyses.
We found similarly higher diversity in the Turkish population, where the traditional diet including another fermented milk product called Ayran compared to American patients5. On the other hand, high population rates of lactose intolerance in Mexico showed a major reduction in milk products compared to USA4. The multigrain cereal increase in Brazil was linked with higher diversity independent of cirrhosis severity and demographic parameters. Moreover, cereal intake decreased even further in decompensated compared to compensated patients with cirrhosis, which further likely contributed towards the lower diversity with disease progression. In addition to cereals, coffee, and chocolate intake reduced in decompensated compared to compensated patients. The reasons for this reduced intake are unclear but potential anorexia and ascites, which predominate in decompensated patients could contribute26. This is important because both coffee and chocolate are associated with beneficial impacts on portal pressure, liver disease progression and microbiota.27–30 In addition to the country of origin, personal food preferences and a diagnosis of cirrhosis can also encourage patients to alter their ultimate dietary intake.
This could also contribute to the relative narrowing of the gap in beta-diversity of microbiome between USA and Brazil in the decompensated group compared to the compensated and control subjects. The increasing overlap between USA versus Brazilian cohorts as the disease progresses may indicate that with advancing disease, the impact of diet, socio-cultural practices and country may reduce. Therefore, this encourages the clinicians to increase the intake of specific potential beneficial foods such as fermented milk products, multigrain cereals, chocolate, and coffee in compensated patients to potentially prevent progression to decompensation. In addition to diversity that was higher in all subjects in Brazil compared to USA, there was a higher relative abundance of beneficial, autochthonous families such as Ruminococcaceae, Christensenellacae and Prevotellaceae and lower potential pathobionts such as Porphyromonadaceae, Sutterellaceae and Enterobacteriaceae31. These bacteria have been shown in prior studies across the world to be associated with clinically relevant outcomes and could potentiate the changes in the hospitalizations in our cohort between countries10, 32–34. When only diet, demographics and country were considered, Brazil origin and chocolate were protective while cirrhosis severity was associated with hospitalizations.
Even though several aspects of the population differed at baseline between USA and Brazil groups such as cirrhosis etiology, HE, rifaximin use and demographics, none of these were significant on the multi-variable analysis. This was confirmed with MAAsLin2 analysis where Brazil-origin was associated with lower hospitalizations, as was coffee and rice intake, and beneficial genera such as Dorea and Ruminococcus. On the other hand, higher MELD score, US origin, prior HE, and potential pathobionts such as Salmonella, Enterococcus, Kosakonia, Brachyspira, Atopobium and Anaerofilum were associated with hospitalizations. Several of these (Salmonella, Enterococcus and Kosakonia, previously part of Enterobacter), contain known pathogens31, 35. These genera are often related with cirrhosis pro gression and despite the country and diet, remain associated with hospitalizations. Other genera such as Atopobium is higher in patients with cirrhosis in the small bowel36, while Anaerofilum, associated with seafood consumption, which was higher in the US-based cohort and is also higher in depression, which is prevalent in cirrhosis37–39. Brachyspira is an interesting genus associated with impaired gut barrier in irritable bowel syndrome40. Alterations in gut barrier function are important in cirrhosis and could be a reason why higher Brachyspira may be associated with hospitalizations. While chocolate, yogurt, cereal, rice, and coffee were relatively higher in Brazilian patients with cirrhosis, only rice and coffee were associated with lower hospitalization despite including microbiota in the regression. This likely implies that the impact of cereals, chocolate, and yogurt were related to microbial change, while rice and coffee could have additional non-microbial associations with hospitalizations.
The study has limitations since it evaluates only associations, has differences between the two cohorts, does not account for socio-economic status, uses a food questionnaire that collected only qualitative data and has a high proportion of men. In addition, there is a higher number of NAFLD patients and lower hepatitis C and alcohol-related patients, lower age of the controls and lower percentage with HE and rifaximin therapy in the Brazilian compared to the US-based cohort. However, these demographic and other variables were adjusted in the multi-variable analyses and microbial and dietary changes were still associated with better outcomes. In addition, the determinants of higher alpha diversity also included these variables and did not demonstrate independent contributions from these differing variables between countries.
We conclude that despite cohorts from US and Brazil differing on some aspects of cirrhosis severity, the Brazilian diet which is richer in multi-grain cereals, rice, and yogurt associates with higher gut microbial diversity and greater relative abundance of beneficial microbiota on multi-variable analysis. Changes in diet focused on multi-grain cereals, yogurt, coffee, and chocolate intake which was higher in Brazilian patients and those with compensated disease regardless of country could modulate microbiota composition and potentially associate with lower hospitalizations. Unique country-level changes in the diet and microbiome composition can impact outcomes in cirrhosis and these changes should acknowledged when planning cirrhosis-related microbial interventions across countries and cultures. Encouraging the consumption of dietary yogurt, multi-grain cereals and coffee could potentially improve outcomes in patients with cirrhosis currently following a Western diet.
Supplementary Material
What You Need to Know.
Background
Gut microbiota are affected by diet and cultural practices, and in turn can impact disease progression in patients with cirrhosis. However, interaction of diet, microbiota and cirrhosis-related outcomes in cohorts from US and Brazil need to be explored.
Findings
In 157 American and 228 Brazilian subjects, we found that despite baseline differences in cirrhosis severity, Brazilian patients with cirrhosis, who follow a diet richer in cereals, rice and beans, and yogurt had higher gut microbial diversity. Brazilian patients, regardless of disease or compensated stage and likely related to their diet showed higher beneficial bacteria, and lower hospitalizations than US-based patients with cirrhosis.
Implications for patient care
The intake of multi-grains, plant-based proteins, chocolate, and yogurt should be encouraged in patients with cirrhosis currently on a Western diet. Country-level differences in microbiota and diet should be kept in mind before extrapolating results between populations.
Acknowledgments
Grant Support: Supported in part by VA Merit Review 2I0CX001076, NCATS R21TR003095 and AHRQ RO1025412 to JSB
Abbreviations:
- HE
hepatic encephalopathy
- PPI
proton pump inhibitors
- rRNA
ribosomal RNA
- LEfSe
Linear discriminant function analysis effect size
- RDP
Ribosomal Data Project
- QIIME2
Quantitative Insights Into Microbial Ecology
- SCFA
short-chain fatty acids
Footnotes
Preprint server: not applicable
Disclosures: none for any author
Writing Assistance: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Asrani SK, Devarbhavi H, Eaton J, et al. Burden of liver diseases in the world. J Hepatol 2019;70:151–171. [DOI] [PubMed] [Google Scholar]
- 2.Acharya C, Bajaj JS. Chronic Liver Diseases and the Microbiome-Translating Our Knowledge of Gut Microbiota to Management of Chronic Liver Disease. Gastroenterology 2021;160:556–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bajaj JS, Idilman R, Mabudian L, et al. Diet affects gut microbiota and modulates hospitalization risk differentially in an international cirrhosis cohort. Hepatology 2018;68:234–247. [DOI] [PubMed] [Google Scholar]
- 4.Bajaj JS, Torre A, Rojas ML, et al. Cognition and hospitalizations are linked with salivary and faecal microbiota in cirrhosis cohorts from the USA and Mexico. Liver Int 2020;40:1395–1407. [DOI] [PubMed] [Google Scholar]
- 5.Cox IJ, Idilman R, Fagan A, et al. Metabolomics and microbial composition increase insight into the impact of dietary differences in cirrhosis. Liver Int 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nader LA, de Mattos AA, Bastos GA. Burden of liver disease in Brazil. Liver Int 2014;34:844–9. [DOI] [PubMed] [Google Scholar]
- 7.Lins TC, Vieira RG, Abreu BS, et al. Genetic composition of Brazilian population samples based on a set of twenty-eight ancestry informative SNPs. Am J Hum Biol 2010;22:187–92. [DOI] [PubMed] [Google Scholar]
- 8.Parra FC, Amado RC, Lambertucci JR, et al. Color and genomic ancestry in Brazilians. Proc Natl Acad Sci U S A 2003;100:177–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bajaj JS, Idilman R, Mabudian L, et al. Diet affects gut microbiota and modulates hospitalization risk differentially in an international cirrhosis cohort. Hepatology 2018. [DOI] [PubMed] [Google Scholar]
- 10.Bajaj JS, Torre A, Rojas ML, et al. Cognition and hospitalizations are linked with salivary and faecal microbiota in cirrhosis cohorts from the USA and Mexico. Liver Int 2020. [DOI] [PubMed] [Google Scholar]
- 11.Bajaj JS, Betrapally NS, Hylemon PB, et al. Salivary microbiota reflects changes in gut microbiota in cirrhosis with hepatic encephalopathy. Hepatology 2015;62:1260–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sikaroodi M, Gillevet PM. Quality control in multi-tag pyrosequencing of microbial communities. Biotechniques 2012;53:381–3. [DOI] [PubMed] [Google Scholar]
- 13.Wang Q, Garrity GM, Tiedje JM, et al. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol 2007;73:5261–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.http://qiime.org/tutorials/tutorial.html.
- 15.Segata N, Izard J, Waldron L, et al. Metagenomic biomarker discovery and explanation. Genome Biol 2011;12:R60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.https://huttenhower.sph.harvard.edu/maaslin/.
- 17.http://189.28.128.100/dab/docs/portaldab/publicacoes/guia_alimentar_populacao_ingles.pdf.
- 18.Dias DM, Kolba N, Hart JJ, et al. Soluble extracts from carioca beans (Phaseolus vulgaris L.) affect the gut microbiota and iron related brush border membrane protein expression in vivo (Gallus gallus). Food Res Int 2019;123:172–180. [DOI] [PubMed] [Google Scholar]
- 19.Sanchez-Tapia M, Hernandez-Velazquez I, Pichardo-Ontiveros E, et al. Consumption of Cooked Black Beans Stimulates a Cluster of Some Clostridia Class Bacteria Decreasing Inflammatory Response and Improving Insulin Sensitivity. Nutrients 2020;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu H, Huang L, Pei X. Effects of sorghum rice and black rice on genes associated with cholesterol metabolism in hypercholesterolemic mice liver and intestine. Food Sci Nutr 2021;9:217–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bianchi GP, Marchesini G, Fabbri A, et al. Vegetable versus animal protein diet in cirrhotic patients with chronic encephalopathy. A randomized cross-over comparison. J Intern Med 1993;233:385–92. [DOI] [PubMed] [Google Scholar]
- 22.Pascale A, Marchesi N, Marelli C, et al. Microbiota and metabolic diseases. Endocrine 2018;61:357–371. [DOI] [PubMed] [Google Scholar]
- 23.Smith PM, Howitt MR, Panikov N, et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013;341:569–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ridlon JM, Kang DJ, Hylemon PB. Bile salt biotransformations by human intestinal bacteria. J Lipid Res 2006;47:241–59. [DOI] [PubMed] [Google Scholar]
- 25.Dubinkina VB, Tyakht AV, Odintsova VY, et al. Links of gut microbiota composition with alcohol dependence syndrome and alcoholic liver disease. Microbiome 2017;5:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. EASL Clinical Practice Guidelines on nutrition in chronic liver disease. J Hepatol 2019;70:172–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jaquet M, Rochat I, Moulin J, et al. Impactof coffee consumption on the gut microbiota: a human volunteer study. Int J Food Microbiol 2009;130:117–21. [DOI] [PubMed] [Google Scholar]
- 28.Gonzalez S, Salazar N, Ruiz-Saavedra S, et al. Long-Term Coffee Consumption is Associated with Fecal Microbial Composition in Humans. Nutrients 2020;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DeGottardi A, Berzigotti A, Seijo S, et al. Postprandial effects of dark chocolate on portal hypertension in patients with cirrhosis: results of a phase 2, double-blind, randomized controlled trial. Am J Clin Nutr 2012;96:584–90. [DOI] [PubMed] [Google Scholar]
- 30.Whitfield JB, Masson S, Liangpunsakul S, et al. Obesity, Diabetes,Coffee,Tea,an dCannabis Use Alter Risk for Alcohol-Related Cirrhosis in 2 Large Cohorts of High-Risk Drinkers. Am J Gastroenterol 2021;116:106–115. [DOI] [PubMed] [Google Scholar]
- 31.Trebicka J, Bork P, Krag A, et al. Utilizing the gut microbiome in decompensated cirrhosis and acute-on-chronic liver failure. Nat Rev Gastroenterol Hepatol 2020. [DOI] [PubMed] [Google Scholar]
- 32.Acharya C, Bajaj JS. Chronic Liver Diseases and the Microbiome: Translating Our Knowledge of Gut Microbiota to Management of Chronic Liver Disease. Gastroenterology 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sung CM, Lin YF, Chen KF, et al. Predicting Clinical Outcomes of Cirrhosis Patients With Hepatic Encephalopathy From the Fecal Microbiome. Cell Mol Gastroenterol Hepatol 2019;8:301–318 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qin N, Yang F, Li A, et al. Alterations of the human gut microbiome in liver cirrhosis. Nature 2014;513:59–64. [DOI] [PubMed] [Google Scholar]
- 35.Bajaj JS, Heuman DM, Hylemon PB, et al. Altered profile of human gut microbiome is associated with cirrhosis and its complications. J Hepatol 2014;60:940–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen Y, Ji F, Guo J, et al. Dysbiosis of small intestinal microbiota in liver cirrhosis and its association with etiology. Sci Rep 2016;6:34055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sandhu KV, Sherwin E, Schellekens H, et al. Feeding the microbiota-gut-brain axis: diet, microbiome, and neuropsychiatry. Transl Res 2017;179:223–244. [DOI] [PubMed] [Google Scholar]
- 38.Lapidot Y, Amir A, Nosenko R, et al. Alterations in the Gut Microbiome in the Progression of Cirrhosis to Hepatocellular Carcinoma. mSystems 2020;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hernaez R, Kramer JR, Khan A, et al. Depression and Anxiety Are Common Among Patients With Cirrhosis. Clin Gastroenterol Hepatol 2020. [DOI] [PMC free article] [PubMed]
- 40.Jabbar KS, Dolan B, Eklund L, et al. Association between Brachyspira and irritable bowel syndrome with diarrhoea. Gut 2020. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.