CONSPECTUS:
By using transition metal catalysts, chemists have altered the ‘logic of chemical synthesis’ by enabling the functionalization of carbon-hydrogen bonds, which have traditionally been considered inert. Within this framework, our laboratory has been fascinated by the potential for aldehyde C–H bond activation. Our approach focused on generating acyl-metal-hydrides by oxidative addition of the formyl C–H bond, which is an elementary step first validated by Tsuji in 1965. In this Account, we review our efforts to overcome limitations in hydroacylation. Initial studies resulted in new variants of hydroacylation and ultimately spurred the development of related transformations (e.g., carboacylation, cycloisomerization, and transfer hydroformylation).
Sakai and coworkers demonstrated the first hydroacylation of olefins when they reported that 4-pentenals cyclized to cyclopentanones, using stoichiometric amounts of Wilkinson’s catalyst. This discovery sparked significant interest in hydroacylation, especially for the enantioselective and catalytic construction of cyclopentanones. Our research focused on expanding the asymmetric variants to access medium-sized rings (e.g., seven- and eight-membered rings). In addition, we achieved selective intermolecular couplings by incorporating directing groups onto the olefin partner. Along the way, we identified Rh and Co catalysts that transform dienyl aldehydes into a variety of unique carbocycles, such as cyclopentanones, bicyclic ketones, cyclohexenyl aldehydes, and cyclobutanones. Building on the insights gained from olefin hydroacylation, we demonstrated the first highly enantioselective hydroacylation of carbonyls. For example, we demonstrated that ketoaldehydes can cyclize to form lactones with high region- and enantioselectivity. Following these reports, we reported the first intermolecular example that occurs with high stereocontrol. Ketoamides undergo intermolecular carbonyl hydroacylation to furnish α-acyloxyamides that contain a depsipeptide linkage.
Finally, we describe how the key acyl-metal-hydride species can be diverted to achieve a C–C bond cleaving process. Transfer hydroformylation enables the preparation of olefins from aldehydes by a dehomologation mechanism. Release of ring strain in the olefin acceptor offers a driving force for the isodesmic transfer of CO and H2. Mechanistic studies suggest that the counterion serves as a proton-shuttle to enable transfer hydroformylation. Collectively, our studies showcase how transition metal catalysis can transform a common functional group, in this case aldehydes, into structurally distinct motifs. Fine-tuning the coordination sphere of an acyl-metal-hydride species can promote C–C and C–O bond forming reactions, as well as C–C bond cleaving processes.
Graphical Abstract

1. INTRODUCTION
One of the largest applications of homogenous catalysis by volume is the Rh-catalyzed hydroformylation of olefins to generate aldehydes.5 We reasoned that metal-catalyzed transformations similar in design to hydroformylation would have the same potential for broad use. Hydroformylation involves an acyl-Rh-hydride species that undergoes reductive elimination to generate the formyl C–H bond. Over the last decade, our laboratory has been interested in the reverse elementary step: activation of the aldehyde C–H bond to generate an acyl-metal-hydride species (Figure 1). By tuning the coordination sphere of this acyl-metal-hydride species, we have discovered C–C and C–O bond forming reactions, as well as C–C bond cleaving methods. In this Account, we present a personal report of our studies on Rh- and Co-catalyzed olefin hydroacylation and ketone hydroacylation, and the related carboacylation and transfer hydroformylation. We share how some discoveries were serendipitous, while others were guided by mechanistic insights. We identified the appropriate ligand by examining representative ligands from different families, searching for trends, and then fine-tuning the most promising scaffolds. In each case, however, the choice of ligand plays a key role in promoting reactivity and selectivity (see Figure 1 for an overview of the ligands featured in this Account).
Figure 1.
Overview of divergent transformations triggered by formyl C–H bond activation and ligands featured in this Account as they appear in chronological order (black: commercially available, blue: ligands our lab designed and synthesized).
2. ALDEHYDE C–H BOND FUNCTIONALIZATION
In 1965, Tsuji reported that aldehyde C–H bonds undergo oxidative addition to generate an acyl-metal-hydride species during his studies on decarbonylation.6 Future efforts focused on diverting aldehyde reactivity away from decarbonylation. In 1972, Sakai and coworkers found that a series of 4-pentenals cyclized to the corresponding cyclopentanones when using stoichiometric Wilkinson’s complex.7 This study spurred developments in catalytic hydroacylation, which is defined as the addition of a hydrogen atom and acyl group across an alkene, alkyne, or carbonyl. To date, a range of transition metals (Rh, Ru, Ni, Co) and organic molecules (NHC’s), catalyze intramolecular and intermolecular olefin hydroacylation.8 The most efficient hydroacylation catalysts are designed around cationic Rh(I) centers, which were originally shown to be effective catalysts by Bosnich and coworkers.9 Figure 2A depicts the well-accepted mechanism for Rh-catalyzed olefin hydroacylation. Oxidative addition of I to a formyl C–H bond generates acyl-Rh(III)-hydride II. Subsequent olefin coordination (IV), followed by migratory insertion, affords either linear or branched acyl-Rh(III)-alkyl species V or VI, respectively. Reductive elimination of V or VI releases the corresponding ketone product and regenerates catalyst I. The propensity for the acyl-Rh(III)-hydride species II to undergo an off-cycle reductive decarbonylation (i.e., Tsuji-Wilkinson decarbonylation) via Rh-carbonyl III presents a problem.6
Figure 2.
(A) Olefin hydroacylation mechanism and (B) strategy for suppressing decarbonylation.
Advances in Rh-catalyzed hydroacylation focus on inhibiting reductive decarbonylation. One successful strategy uses aldehyde partners that possess proximal coordinating atoms (Figure 2B). The coordination of an additional ligand to 16-electron Rh species II results in a fully saturated organometallic species. Following initial reports of intramolecular olefin hydroacylation, Suggs showed that quinoline aldehydes do not undergo decarbonylation, but instead couple to olefins by intermolecular hydroacylation.10 Next, Jun and coworkers discovered that 2-(diphenylphosphino)benzaldehyde couples to a range of olefins.11 Miura and Willis expanded the scope of the aldehyde partner to include proximal coordinating groups such as alcohols, sulfides, and amines.12 An alternative strategy uses catalytic scaffolding groups to achieve similar levels of reactivity and selectivity. Jun and coworkers demonstrated that 2-amino-3-picoline acts as a co-catalyst for intermolecular hydroacylation by forming a chelating picolyl imine.13 After hydroacylation, the resulting imine product undergoes hydrolysis to afford the corresponding ketone and regenerate the amine catalyst.
An overview of progress in hydroacylation can be found in a Chemical Review8c by Willis and an Organic Reactions chapter8f by our laboratory. For a recent review on asymmetric hydroacylation, we direct the reader to a Chemical Communications viewpoint from our group.14 The majority of studies in the field of asymmetric hydroacylation focused on the cyclization of 4-pentenals, which bear different substitution patterns to afford cyclopentanones.8,14 For intermolecular couplings, using chelating aldehydes has allowed for the enantioselective synthesis of ketones.8,14 A rare example of intermolecular olefin hydroacylation with simple aldehydes was reported by Tanaka.15 In this example, the authors demonstrated that acrylamides are suitable olefin partners and hypothesize that the proximal amide coordinates to the Rh center during catalysis to stabilize the acyl-Rh(III)-hydride intermediate. With this background in mind, our research team focused on three main questions:
(1) Can we expand asymmetric variants by incorporating directing groups on the olefin component?
(2) Can we develop an analogous transformation where ketones could be used in place of olefins to generate the corresponding esters and lactones?
(3) Can we diverge the key acyl-Rh(III)-hydride intermediate to other pathways, including those that involve C–C bond cleavage?
2.1. Olefin Hydroacylation with Chelating Substrates
Our work in the area of olefin hydroacylation with chelating substrates centers around four themes, which are summarized in Figure 3. Our contributions include (1) developing the enantio- and regioselective synthesis of medium-sized heterocyclic ketones, (2) using ring-strain to drive intermolecular hydroacylation, (3) incorporating an additional directing group on the olefin partner to control regioselectivity for intermolecular couplings, and (4) identifying a catalyst that allows for the intermolecular hydroacylation of unactivated olefins (e.g., α-olefins).
Figure 3.
Overview of the developed olefin hydroacylations with chelating substrates.
In 2009, we reported an enantioselective intramolecular olefin hydroacylation to afford seven- and eight-membered heterocycles (Figure 4).16 The heteroatom (X) in the carbon framework of 1 promotes desired reactivity over decarbonylation. Starting with aldehyde 1, a cationic Rh catalyst modified by (R,R)-Me-DuPHOS allows for the enantioselective preparation of seven- and eight-membered heterocyclic ketones 2. Matching the ancillary ligand with the aldehyde substrate enabled high reactivity and stereoselectivity.
Figure 4.
Enantioselective intramolecular hydroacylation for the synthesis of seven- and eight-membered heterocycles.
Preventing unwanted decarbonylation becomes more challenging with the intermolecular variant of olefin hydroacylation. To create a driving force for desired reactivity, we selected highly strained cyclopropenes 4 as the olefin partner (Figure 5).17 Josiphos ligands promoted the desymmetrization of cyclopropenes to set vicinal stereocenters in 5. High levels of diastereoselectivity (up to >20:1 dr) and enantioselectivity (up to >99% ee) were obtained. This method complemented the limited reports for synthesizing enantioenriched cyclopropanes bearing a quaternary stereocenter at the time. However, the optimal conditions were not applicable to linear olefins.
Figure 5.
Enantioselective desymmetrization of cyclopropenes by intermolecular hydroacylation.
To control for branched selectivity in olefin hydroacylations, we used distal directing groups on the olefin partner (Figure 6). Transition metal-catalyzed reactions involving directing groups tethered to olefins have seen large success.18a However, this approach was rarely used for enantio- and regioselective intermolecular olefin hydroacylation. Two examples were reported in 2009 at the time of our initial studies: Tanaka demonstrated that acrylamides are suitable chelating olefins15 and Suemene reported that 1,5-hexadienes promote branched selectivity.18b Building on this strategy, we used homoallylic sulfides 6 as the olefin partner because we had previously demonstrated that intramolecular olefin hydroacylation occurs in the presence of a sulfide tether (Figure 6A).19 Various salicylaldehydes 3 undergo the coupling reaction to afford aryl ketones 7 bearing α-stereocenters. Moreover, a multitude of aryl homoallylic sulfides 6 are compatible. Decarbonylation is mitigated by combining low reaction temperatures, an appropriate ligand, and the presence of directing groups on both reactants. To investigate the regioselective outcome, we subjected allylic sulfide 6a to the standard reaction conditions and observed linear ketone 7a. This result suggests that a five-membered rhodacycle intermediate influences the branched versus linear regioselectivity.
Figure 6.
(A) Regio- and enantioselective coupling of salicylaldehydes and homoallylic sulfides. (B) Analogous regioselective hydroacylations of allylic and homoallylic alcohols.
Towards branch-selective hydroacylation, we developed a tandem catalytic cycle that converts allylic and homoallylic alcohols 8 to (homo)aldol motifs 9 (Figure 6B).20 The design leverages the reversible alcoholysis of phosphinites. Methyl diphenylphosphinite undergoes exchange with an equivalent of alcohol 8 to afford an allylic phosphinite, which is a competent directing group for Rh-catalyzed hydroacylation. A variety of salicylaldehydes 3 and (homo)allylic alcohols 8 couple to form hydroxy ketones 9. Reactivity depends on the ability of the hydroxyl group to form a phosphinite; the analogous methyl ether of 8 shows no reactivity under these standard conditions.
We then discovered a Rh catalyst that allows for the regioselective preparation of linear ketones 11 (Figure 7).1 The combination of a phosphoramidite ligand (R-SIPHOS-PE) and a heterogenous base are critical for reactivity and regioselectivity. We proposed that (R)-SIPHOS-PE aids in inhibiting decarbonylation by lowering the barrier for reductive elimination of the acyl-Rh(III)-alkyl species. In agreement with this proposal, mechanistic findings suggest that hydrorhodation to form a branched acyl-Rh(III)-alkyl species is reversible, whereas linear hydrorhodation is irreversible. We prepared eight biologically active octaketide natural products (i.e., dothiorelones, cytosporones, and phomopsin C). In a related study, we synthesized twelve analogs of the cytosporone family and ultimately found an increase in cytotoxicity with a densely fluorinated acyl carbogenic chain.21
Figure 7.
Rh-catalyzed hydroacylation of unactivated olefins and octaketide natural product synthesis.
We aimed for hydroacylations with simple, non-functionalized aldehyde partners by using olefins bearing directing groups (Figure 8).22 Vinyl phenols 13 undergo Rh-catalyzed hydroacylation with a broad scope of aldehydes 12, including aryl, alkenyl, and alkyl aldehydes – none of which contained a coordinating functional group.22a Benzylic ketones 14 can then undergo an acid-mediated dehydrative cyclization to afford the corresponding benzofurans. This sequence leads to four natural products: eupomatenoids 12, 16–18. The kinetic profile shows saturation kinetics for the vinyl phenol partner.22b Chelation of the vinyl phenol aids in favoring hydroacylation over decarbonylation. Moreover, the small bite-angle ligand (dcpm) lowers the barrier of oxidative addition of the Rh catalyst to the formyl C–H bond, which we determined to be the turnover-limiting step.
Figure 8.
Olefin-directed hydroacylation with non-chelating aldehydes.
2.2. Other Strategies and Metals for Olefin Hydroacylation
James and Young reported the first enantioselective intramolecular olefin hydroacylation, a kinetic resolution of racemic 4-pentenals.23 While this was a groundbreaking result, the cyclization was limited to a theoretical yield of 50%. Nearly four decades later, we set out to achieve a related variant using dynamic kinetic resolution (DKR).24 However, examples of C–C bond forming DKR processes are rare.25 Our strategy relies on a co-catalyst (a bulky primary amine) to selectively racemize the aldehyde starting material 15, by means of enamine 16, and not the cyclopentanone product 17 (Figure 9).26 This selective racemization exploits the electrophilicity of these different carbonyls. If the amine co-catalyst condenses onto enantioenriched ketone 17, the formation of the less-substituted enamine 18 is preferred. Therefore, avoiding allylic strain prevents product epimerization to 19.
Figure 9.
Design and strategy for the intramolecular DKR hydroacylation of racemic 4-pentenals.
Similar to other intramolecular olefin hydroacylations, we observe a strong ligand dependence for the DKR hydroacylation of homoallylic aldehydes 15 (Figure 10).26 The cyclization depends on matching the ligand with the substituents on both the α-carbon atom and pendant olefin. We identified three combinations of substrate, ligand, and amine co-catalyst that allow access to various alkyl- and aryl-substituted cyclopentanones 17. In the case of α-aryl cyclopentanones, Houminer demonstrated that this motif undergoes oxidative decomposition.27 Therefore, to inhibit product decomposition and epimerization, we performed a reductive workup with L-Selectride to furnish cyclopentanols. Preliminary mechanistic findings suggest that reductive elimination is the turnover-limiting step. This DKR enables access to α,γ-disubstituted cyclopentanones that are difficult to access otherwise.28
Figure 10.
Optimal ligands for the enantioselective DKR hydroacylation.
Our laboratory,2,29 Yoshikai,30 and Vinogradov31 have found that the more earth abundant, Co-derived catalysts, can also promote olefin hydroacylation. By using Co catalysis, we accessed allylic ketones 21 with 1,3 dienes 20 acting as the olefin partner (Figure 11).29 The mechanism differs from the typical Rh-catalyzed olefin hydroacylation mechanism. In the initial step, oxidative cyclization of the two substrates and Co catalyst forges the new C–C bond. Subsequently, an endocyclic β-H elimination of the seven-membered cobaltacycle affords a Co–H species. Reductive elimination completes the cycle to afford ketone 21. We applied this method to aryl, alkenyl, and alkyl aldehydes, forming ketones 21 in high yield with excellent regioselectivity. This method contributes to the emerging hydroacylation strategies that exploit non-precious metal catalysis.8
Figure 11.
Co-catalyzed hydroacylation of 1,3-dienes.
2.3. Divergent Synthesis of Carbocycles
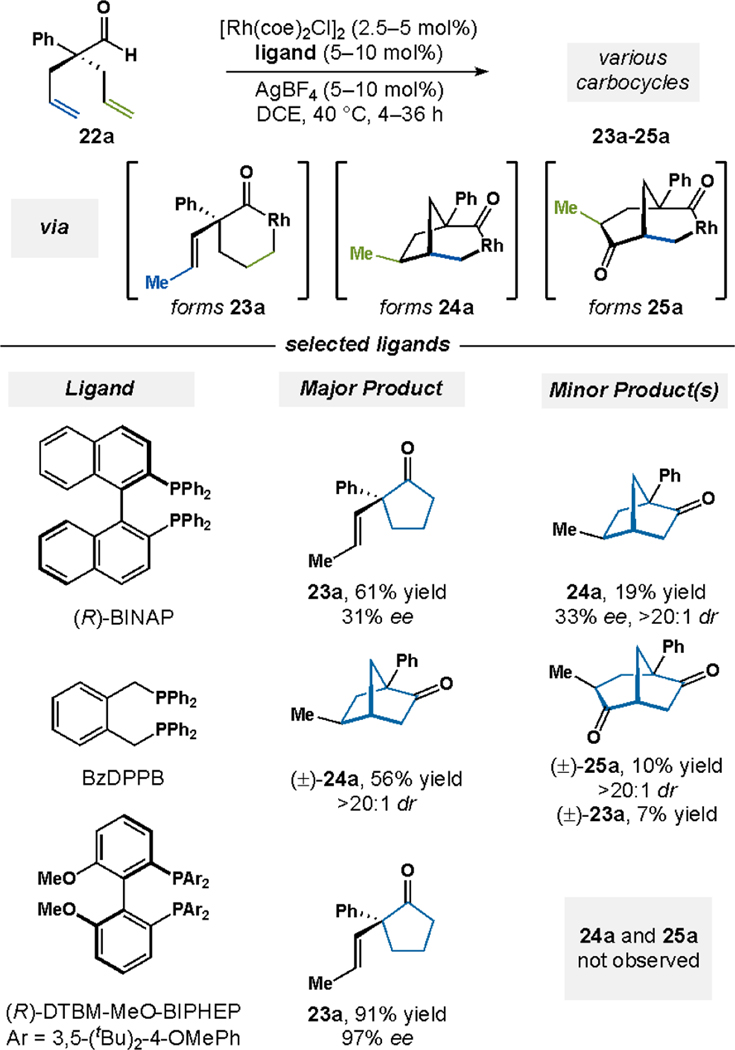
Cyclase enzymes convert geranyl pyrophosphate to a variety of structurally unique carbocycles (Figure 12A). Inspired by Nature, we hypothesized that a common dienyl aldehyde could undergo a variety of metal-catalyzed bond formations, which would all be triggered by C–H bond activation (Figure 12B). Our investigations began with a chiral Rh catalyst and symmetric dienyl aldehyde 22a (Figure 13).32 We found that changing the ancillary ligand yields different mixtures of cyclopentanone 23a, bicyclic ketone 24a, and bicyclic diketone 25a. When using (R)-BINAP, we observe cyclopentanone 23a as the major product alongside bicyclic ketone 24a; both ketones 23a and 24a are formed with low levels of enantioinduction. However, when using BzDPPB the product ratio favors bicyclic ketone 24a, which arises from a novel carboacylation pathway that is triggered by C–H bond activation.33 Lastly, the use of (R)-DTBM-MeO-BIPHEP solely affords cyclopentanone 23a with high levels of enantioselectivity. Interested by the array of products formed from 22a, we sought to develop the divergent carbocyclization chemistry and unearth the mechanistic pathways.
Figure 12.
(A) Enzyme directed cyclizations of geranyl pyrophosphate. (B) Transition metal-catalyzed hydroacylation to afford various carbocyclic frameworks.
Figure 13.
Divergent cyclizations of a dienyl aldehyde 22a based on ligand choice.
Our initial study focused on expanding the substrate scope of the enantioselective cyclopentanone synthesis (Figure 14).32 Desymmetrization of dienyl aldehydes 22 proceeds with high enantiocontrol. In the presence of lower catalyst loadings than our initial lead (see Figure 12), an array of α-aryl and α-alkyl aldehydes 22 transform to carbocycles 23. This method allows for the synthesis of cyclopentanones bearing a stereodefined quaternary center. Mechanistic findings support an irreversible and enantioselective olefin isomerization followed by hydroacylation of the remaining terminal olefin (vide infra). We proposed that the α-vinyl group, which is initially formed by olefin isomerization, not only directs hydroacylation to the remaining terminal olefin, but also coordinates to the Rh center to slow decarbonylation.
Figure 14.
Rh-catalyzed desymmetrization of quaternary centers by hydroacylation.
After observing olefin isomerization in the synthesis of cyclopentanones 23, we were interested in intercepting a related acyl-Rh-hydride intermediate (Figure 15).34 Changing the chiral ligand to a spirobiindane (SDP) backbone and the salt additive to NaBArF4 diverted the reactivity of 22 to cyclohexenyl aldehydes 26. The scope of this cycloisomerization compares favorably to the previous olefin hydroacylation to afford cyclopentanones 23. This method sets a quaternary stereocenter, as well as a distal tertiary stereocenter, with high levels of stereocontrol. Moreover, cyclohexenes 26 are complementary to the regioisomers formed from a Diels-Alder reaction between terminal dienes and α,β-unsaturated aldehydes.35 The cycloisomerization proceeds by formyl C–H bond activation, regioselective carbometallation, and then endocyclic β-H elimination (vide infra).
Figure 15.
Asymmetric cycloisomerization to access cyclohexenes.
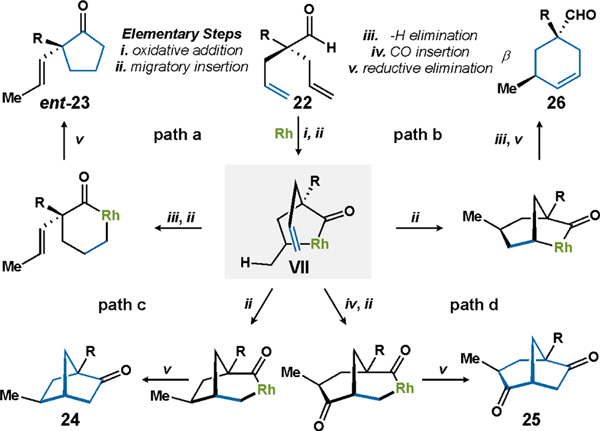
On the basis of literature precedent and our own mechanistic findings, we propose that a key intermediate can diverge into the four distinct products 23-26 (Figure 16).8,32,34 Initial oxidative addition of the Rh catalyst to the aldehyde C–H bond affords an acyl-Rh(III)-hydride intermediate. Stereoselective hydrorhodation of one olefin then affords five-membered rhodacycle VII, which is the common denominator between all four products we observe in our studies. Collectively, these findings showcase that fine-tuning the Rh catalyst can diverge the reactivity of a common aldehyde into unique carbocycles. However, at no point in our studies did we observe reductive elimination of VII to furnish a cyclobutanone product. Interested by the possibility of forming densely substituted cyclobutanones, we decided to explore Co catalysis.
Figure 16.
Mechanistic pathways for the Rh-catalyzed cyclizations of dienyl aldehydes.
Bergman and coworkers characterized a five-membered cobaltacycle that undergoes oxidatively-induced reductive elimination to form cyclobutanones.36 In addition, Vinogradov showed that paramagnetic Co(0)-complexes catalyze intramolecular hydroacylation of 4-pentenal to afford cyclopentanone.31 This precedent, paired with our previous success in Co catalysis,29 led us to investigate hydroacylation to form cyclobutanones. We identified that a Co catalyst, modified by (S,S)-BDPP, and substoichiometric amounts of Zn reductant formed cyclobutanones 27 with high selectivity (Figure 17).2 A variety of α-aryl bisallyl aldehydes 22 undergo the transformation to favor cyclobutanones 27 over cyclopentanones iso-23 (≥10:1 rr). This study features enantioselective construction of cyclobutanones 27 by hydroacylation and complements previous methods that rely upon parallel kinetic resolutions.23b,c Mechanistic findings support a canonical hydroacylation mechanism that involves reductive elimination of a five-membered cobaltacycle to afford 27.
Figure 17.
Co-catalyzed hydroacylation affords enantioenriched cyclobutanones.
3. CARBONYL HYDROACYLATION
In comparison to olefin hydroacylation, the corresponding carbonyl hydroacylation for asymmetric ester synthesis remains much less explored.37 Bosnich reported the first example of ketone hydroacylation.38 In this study, 1,4-ketoaldehydes cyclize to racemic γ-lactones under Rh catalysis. While distinct in mechanism, hydroacylation to generate esters bears similarities to the Tishchenko reaction,39 which is a process catalyzed by base. For example, the industrial synthesis of ethyl acetate involves a Tishchenko reaction of acetaldehyde with an alkoxide catalyst (Figure 18A).40 While industrially relevant, enantioselective variants of the Tishchenko reaction were rare at the time we began our studies.41 We imagined that functionalization of aldehyde C–H bonds would represent an attractive and unified approach to making both ketones and esters. Preventing reductive decarbonylation and controlling regioselectivity (Tishchenko-like versus Benzoin-like products) are main obstacles shared between carbonyl and olefin hydroacylation (Figure 18B). However, an additional challenge arises when attempting to couple two carbonyl starting materials. The aldol and aldol condensation reactions represent well-established pathways that compete with carbonyl hydroacylation.
Figure 18.
(A) Inspiration for carbonyl hydroacylation and (B) challenges to overcome.
3.1. Intramolecular Carbonyl Hydroacylation
We started investigations with the intramolecular cyclization of ketoaldehydes 28, which contain an oxygen atom in the tether (Figure 19A).3 Despite all possible products, we identified a Rh catalyst that affords the Tishchenko-type ester 29, with only minor amounts of decarbonylation. A variety of ketoaldehydes 28 undergo the desired cyclization to afford seven-membered heterocyclic lactones 29, with high reactivity and selectivity. In a related study, we expanded the scope of the ketoaldehydes to include nitrogen atom tethers that cyclize to afford benzoxazecinones 31 (Figure 19B).42 Therefore, both oxygen and nitrogen atoms bind to Rh to inhibit decarbonylation and lower the entropic cost for medium ring formation. While limited in scope, this breakthrough afforded an enantioselective preparation of medium-sized rings and provided an opportunity to study the mechanism of ketone hydroacylation.
Figure 19.
Rh-catalyzed carbonyl hydroacylations furnishes medium-sized heterocyclic lactones.
While the mechanism was assumed to mirror olefin hydroacylation, experiments were necessary to confirm. Kinetic isotope effects and Hammett plot studies suggest that ketone insertion into the Rh–H bond is the turnover-limiting step.43 Moreover, the absence of cross-over products supports an intramolecular hydrorhodation of the ketone. This elementary step affords an acyl-Rh-alkoxide species, which then undergoes reductive elimination to afford heterocyclic lactones 29 and 31. Density functional theory (DFT) studies support our experimental observations.
While we successfully identified a chiral Rh catalyst for enantioselective carbonyl hydroacylation, only medium-sized lactones could be accessed. Therefore, to identify a catalyst for small-membered ring construction we focused on substrates without a coordinating atom in the tether (Figure 20). We hypothesized that tuning the counterion for the Rh catalyst would expand the scope of intramolecular carbonyl hydroacylation. For this study, we chose the intramolecular hydroacylation of ketoaldehyde 32 to afford phthalides 33 (Figure 21).44 Fine-tuning of a silver salt resulted in inhibiting decomposition pathways. Specifically, the coordinating ability of the anion (depicted as X) needed to be matched with the ketone substituent (depicted as R). Empirical studies revealed a nitrate anion is optimal for alkyl-substituted ketones, whereas aryl-substituted ketones require a mesylate counterion. In general, stronger coordinating counterions can help prevent decarbonylation. However, the resulting catalysts are often more sluggish. As a result, the counterion remains a valuable parameter for tuning both reactivity and selectivity. Currently, the substrate scope is limited to aryl-substituted ketoaldehydes 32. Careful selection of the bisphosphine ligand alongside the counterion resulted in high reactivity and enantioselectivity. With this method, we prepared (S)-3-n-butylphthalide in 93% yield and 97% ee. This natural product imparts the flavor of celery and its racemate reached phase-III clinical trials for treating strokes.45
Figure 20.
Design of a strategy uses coordinating counterions to expand the scope to non-chelating aldehydes.
Figure 21.
Enantioselective preparation of phthalides by Rh-catalyzed carbonyl hydroacylation.
In a subsequent study, we focused on desymmetrization of bisketoaldehydes 34 for the enantioselective preparation of bicyclic lactones 35 and 36 (Figure 22).46 Choosing the appropriate Rh source, solvent, and temperature allowed for the diastereodivergent synthesis of anti- and syn-bicyclic lactones (35 and 36, respectively). With dimeric [Rh(nbd)Cl]2, an ethereal solvent, and a lower reaction temperature we observed selective formation of the anti-lactone 35. Changing to cationic [Rh(cod)2]SbF6, an alcoholic solvent, and increasing the temperature furnished syn-fused lactones 36. Both transformations progress with high levels of reactivity and selectivity. We propose that the use of polar, coordinating solvents (i.e., DME and tAmOH) inhibits decarbonylation. This strategy enables an enantioselective formal synthesis of (–)-mesembrine.47 Starting with bisketoaldehyde 34a, carbonyl hydroacylation affords syn-fused lactone 36a. Redox manipulations and an allylic alcohol transposition affords lactone 37 and completes the formal synthesis.48
Figure 22.
Diastereodivergent construction of bicyclic lactones by enantioselective carbonyl hydroacylation.
3.2. Intermolecular Carbonyl Hydroacylation
Like Rh-catalyzed olefin hydroacylation, intermolecular carbonyl hydroacylation poses more challenges compared to the intramolecular counterpart. We hypothesized that a directing group on the carbonyl partner could address these challenges. We selected ketoamides 38 as the carbonyl partner and identified a Rh catalyst that could afford the corresponding α-acyloxyamides 39 with high regio- and enantiocontrol (Figure 23).49 Key to the success of this coupling was the design and synthesis of a new Josiphos ligand (L4) that possessed both a π-accepting diarylphosphine and a σ-donating dialkylphosphine substituent. We propose that the π-accepting phosphine substituent is positioned trans to the carbonyl partner, making it more prone to migratory insertion. Likewise, the σ-donating dialkylphosphine is positioned trans to the hydride ligand therefore increasing hydricity. This dual activation explains why the Josiphos ligand class was uniquely effective for the transformation. In a follow-up study, we prepared a novel dcpp-inspired bisphosphine ligand (L5) that contains two P-stereogenic centers.50 This bidentate ligand for Rh expands the scope to include isatins and linear α-ketoamides with aliphatic aldehydes.
Figure 23.
Enantioselective coupling of aldehydes and ketoamides.
4. TRANSFER HYDROFORMYLATION
Nature uses cytochrome P450 enzymes to oxidize C–H bonds in a highly selective manner.51 Within this family of enzymes, the demethylases excise methyl groups in the biosynthesis of sterols (Figure 24A).52 In this cascade, an aldehyde intermediate undergoes dehydroformylation to access the olefin product. Inspired by this biosynthetic sequence, we envisaged developing a dehydroformylation of aldehydes as a complementary tool for organic synthesis (Figure 24B). Notably this transformation would occur via the cleavage of one C–C bond to generate olefins. This approach stands in stark contrast to staple olefinations where aldehydes transform to olefins via C–C bond formations, such as the Wittig, Julia-Lythgoe, and Horner-Wadsworth-Emmons reactions.53
Figure 24.
(A) Nature’s approach to dehydroformylation. (B) Rh-catalyzed transfer hydroformylation.
Our proposal relied on triggering C–C bond cleavage by chemoselective activation of formyl C–H bonds using Rh catalysis. This process requires trapping the acyl-Rh(III)-hydride species in a pathway that outcompetes known hydroacylation and decarbonylation. Olefins generated by dehydroformylation have been observed in reactions that use stoichiometric catalysts or require elevated reaction temperatures (160–300 °C).54 We imagined that a Rh catalyst could work in tandem with a sacrificial strained olefin acceptor to transfer a hydrogen atom and formyl group from the aldehyde substrate. We postulated that the release of substantial ring strain in the olefin acceptor could offer a driving force for the isodesmic reaction55 and allow for selective access to the desired olefin product. Notably, this process would avoid the intermediacy of CO gas, which could potentially act as a catalyst poison. If successful, this strategy could pave the way for future transfer hydroformylations that use alcohols and alkanes as substrates with an oxidizing agent.
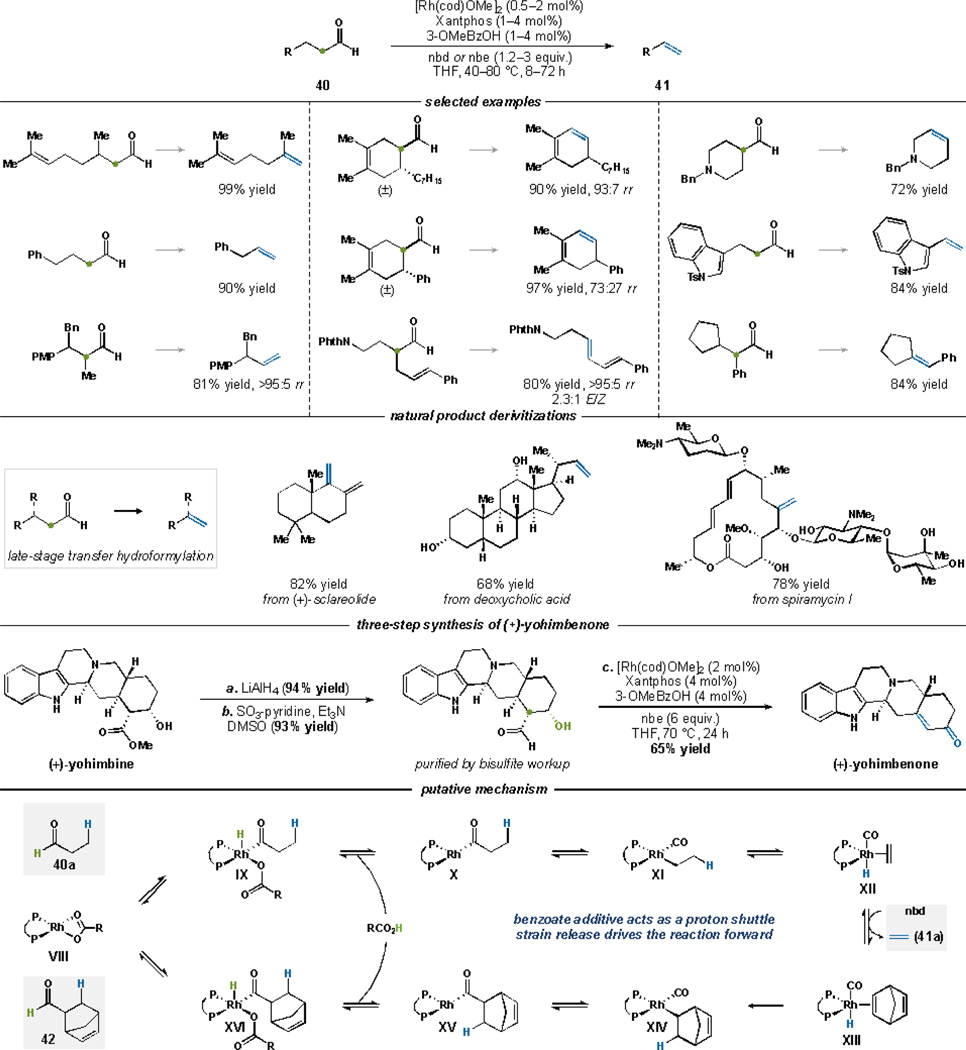
A Rh catalyst promotes selective transfer hydroformylation of a variety of aldehydes 40 (Figure 25).4 Crucial to the success of this process was the appropriate counterion (i.e., 3-methoxy-benzoate) and strained olefin acceptor (nbd = norbornadiene or nbe = norbornene). An array of aldehydes 40 transform to terminal and internal olefins, conjugated dienes, as well as cyclic and trisubstituted olefins (41). The transformation tolerates a range of functional groups. We demonstrated a late-stage transfer hydroformylation of natural product derivatives. Specifically, we prepared indole alkaloid (+)-yohimbenone in three-steps from the inexpensive, commercially available precursor (+)-yohimbine. Our synthesis starts with reduction of the exocyclic ester to afford the corresponding aldehyde. Subjecting this aldehyde to the transfer hydroformylation conditions affords (+)-yohimbenone in 65% yield. The cascade initially forms the corresponding allylic alcohol, but after prolonged reaction time a transfer hydrogenation event occurs to afford the enone functionality in (+)-yohimbenone.
Figure 25.
Rh-catalyzed C–C bond cleavage by transfer hydroformylation.
We sought to understand the mechanistic underpinnings of transfer hydroformylation. Deuterium labeling studies and characterization of organometallic intermediates support the catalytic cycle shown in Figure 25. Oxidative addition of Rh(benzoate) species VIII to aldehyde 40a affords acyl-Rh-hydride IX. Reductive elimination of IX releases an equivalent of 3-OMeBzOH and affords coordinatively unsaturated complex X. Species X then undergoes CO-migratory extrusion followed by β-hydride elimination to yield olefin complex XII. Ligand exchange with the strained olefin partner releases olefin 41a. Migratory insertion of the olefin into the Rh–H bond of XIII yields XIV. CO-insertion, oxidative addition to 3-OMeBzOH, and finally reductive elimination furnishes the acceptor byproduct 42 and regenerates the active catalyst VIII.
The proposed transfer hydroformylation mechanism highlights why the judicious choice of counterion and olefin acceptor facilitate productive chemistry. The counterion effectively acts as a proton-shuttle between the two distinct acyl-Rh-hydride species IX and XVI. Therefore, fine tuning the basicity of this chemical species is paramount. Moreover, the olefin acceptor needs to be sufficiently strained to drive the reaction in the forward direction. Following our study, Morandi and coworkers have unlocked an array of exciting transformations that bear similar design features, which they refer to as shuttle catalysis.56 Also, the Nozaki laboratory has reported an Ir-catalyzed dehydroformylation of aldehydes that directly expels CO gas instead of transferring it to a strained acceptor.57 Recently, Sorenson and coworkers have developed a Co catalyst that works in tandem with photoredox catalysis to transform aldehydes to olefins by dehydroformylation.58 Our laboratory has also developed a cascade that enables the conversion of alcohols to dehomologated olefins via the intermediacy of an aldehyde.59
5. OUTLOOK
Discovered in the late 1700s and coined in 1835, the aldehyde represents one of the most fundamental functional groups in organic synthesis.60 Aside from oxidations and reductions, aldehydes act as both nucleophiles and electrophiles. In addition, they engage in atom economical transformations with olefins (e.g., the Paternò-Büchi reaction) and umpolung chemistry (e.g., the Stetter reaction and Benzoin condensation).35 Chemists today continue to develop strategies to both synthesize and transform aldehydes. This Account summarizes our efforts in diverting the reactivity of an organometallic intermediate that arises from aldehyde C–H bond activation to prepare novel motifs. The resulting methodologies allow for the rapid construction of C–C and C–O bonds and also C–C bond cleavage. The idea of taking common functional groups and discovering new ways to couple them with other partners is an emerging area of theoretical and experimental research.61
Our efforts have aided in the development of (1) intramolecular hydroacylations to afford carbocycles other than five-membered systems and (2) regio- and enantioselective intermolecular hydroacylations. There remains significant opportunities to (1) expand the substrate scope of directed hydroacylations and (2) identify more earth-abundant catalysts. The use of earth-abundant catalysts that promote formal hydroacylation via distinct mechanisms represents an emerging area of research.62 We hope these insights will guide the use of transition metal catalysis to enable divergent reaction pathways for aldehydes and other common functional groups.
KEY REFERENCES.
Von Delius, M.; Le, C. M.; Dong, V. M. Rhodium-Phosphoramidite Catalyzed Alkene Hydroacylation: Mechanism and Octaketide Natural Product Synthesis. J. Am. Chem. Soc. 2012, 134, 15022–15032.1 Design of a Rh catalyst that furnishes linear regioselectivity in the intermolecular hydroacylation of unactivated olefins with chelating aldehydes. Mechanistic studies guided catalyst development and ultimately led to the synthesis of eight octaketide natural products.
Kim, D. K.; Riedel, J.; Kim, R. S.; Dong, V. M. Cobalt Catalysis for Enantioselective Cyclobutanone Construction. J. Am. Chem. Soc. 2017, 139, 10208–10211.2 The identification of a Co catalyst for intramolecular hydroacylation allows for the enantioselective synthesis of cyclobutanones from dienyl aldehydes.
Shen, Z.; Khan, H. A.; Dong, V. M. Rh-Catalyzed Carbonyl Hydroacylation: An Enantioselective Approach to Lactones. J. Am. Chem. Soc. 2008, 130, 2916–2917.3 The first report of a highly enantioselective carbonyl hydroacylation; ketoaldehydes cyclize to the corresponding lactones under Rh catalysis.
Murphy, S. K.; Park, J.-W.; Cruz, F. A.; Dong, V. M. Rh-catalyzed C–C bond cleavage by transfer hydroformylation. Science 2015, 347, 56–60.4 The design and development of transfer hydroformylation, which is a method for transferring CO and H2 from an aldehyde substrate to a strained olefin acceptor. The isodesmic reaction is driven by the release of ring strain in the olefin acceptor.
ACKNOWLEDGMENT
We are grateful to past and present students, postdoctoral fellows, collaborators, and colleagues. We acknowledge these funding agencies: National Institutes of Health (R35GM127071) and National Science Foundation (CHE-1956457) for their support.
Biographies
Ryan T. Davison obtained his B.S. in Chemistry from Hobart and William Smith Colleges in 2017 under the guidance of Prof. Justin Miller. After his undergraduate studies, Ryan joined Prof. Vy Dong’s group at the University of California, Irvine as a PhD student.
Erin L. Kuker obtained a B.A. in Chemistry from Indiana University Bloomington in 2019 under the guidance of Prof. Kevin Brown. In 2019, she began her PhD studies with Prof. Vy Dong at the University of California, Irvine.
Vy M. Dong is a Professor of Chemistry at the University of California, Irvine. She earned her B.S. in Chemistry at University of California, Irvine, M.S. at UC Berkeley, and Ph.D. at the California Institute of Technology. She began her independent career at the University of Toronto in 2006, where she was promoted to Associate Professor. In 2012, she returned to the University of California, Irvine.
Footnotes
Notes
The authors declare no competing financial interests.
REFERENCES
- (1).von Delius M; Le CM; Dong VM Rhodium-Phosphoramidite Catalyzed Alkene Hydroacylation: Mechanism and Octaketide Natural Product Synthesis. J. Am. Chem. Soc 2012, 134, 15022–15032. [DOI] [PubMed] [Google Scholar]
- (2).Kim DK; Riedel J; Kim RS; Dong VM Cobalt Catalysis for Enantioselective Cyclobutanone Construction. J. Am. Chem. Soc 2017, 139, 10208–10211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Shen Z; Khan HA; Dong VM Rh-Catalyzed Carbonyl Hydroacylation: An Enantioselective Approach to Lactones. J. Am. Chem. Soc 2008, 130, 2916–2917. [DOI] [PubMed] [Google Scholar]
- (4).Murphy SK; Park J-W; Cruz FA; Dong VM Rh-Catalyzed C–C Bond Cleavage by Transfer Hydroformylation. Science 2015, 347, 56–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Franke R; Selent D; Börner A. Applied Hydroformylation. Chem. Rev 2012, 112, 5675–5732. [DOI] [PubMed] [Google Scholar]
- (6).Tsuji J; Ohno K. Organic Syntheses by Means of Noble Metal Compounds XXI. Decarbonylation of Aldehydes Using Rhodium Complex. Tetrahedron Lett. 1965, 6, 3969–3971. [Google Scholar]
- (7).Sakai K; Ide J; Oda O; Nakamura N. Synthetic Studies on Prostanoids 1 Synthesis of Methyl 9-Oxoprostanoate. Tetrahedron Lett. 1972, 13, 1287–1290. [Google Scholar]
- (8).(a) For reviews, see:Jun C-H; Hong J-B; Lee D-Y Chelation-Assisted Hydroacylation. Synlett 1999, 1–12. [Google Scholar]; (b) Jun C-H; Jo E-A; Park J-W Intermolecular Hydroacylation by Transition-Metal Complexes. Eur. J. Org. Chem 2007, 2007, 1869–1881. [Google Scholar]; (c) Willis MC. Transition Metal Catalyzed Alkene and Alkyne Hydroacylation. Chem. Rev 2010, 110, 725–748. [DOI] [PubMed] [Google Scholar]; (d) Willis MC In Comprehensive Organic Synthesis II, 2nd ed.; Knochel P, Molander GA, Elsevier: Amsterdam, 2014; pp 961–994. [Google Scholar]; (e) Leung JC; Krische MJ Catalytic Intermolecular Hydroacylation of C–C π-Bonds in the Absence of Chelation Assistance. Chem. Sci 2012, 3, 2202–2209. [Google Scholar]; (f) Dong VM; Kou KGM; Le DN Transition-Metal-Catalyzed Hydroacylation. In Organic Reactions; Vol. 96; Denmark SE, Ed.; Wiley: Hoboken, New Jersey, 2018; pp 229–592. [Google Scholar]
- (9).(a) Fairlie DP; Bosnich B. Homogenous Catalysis. Conversion of 4-Pentenals to Cyclopentanones by Efficient Rhodium-Catalyzed Hydroacylation. Organometallics 1988, 7, 936–945. [Google Scholar]; (b) Fairlie DP; Bosnich B. Homogenous Catalysis. Mechanism of Catalytic Hydroacylation: The Conversion of 4-Pentenals to Cyclopentanones. Organometallics 1988, 7, 946–954. [Google Scholar]
- (10).Suggs JW Isolation of a Stable Acylrhodium(III) Hydride Intermediate Formed during Aldehyde Decarbonylation. Hydroacylation. J. Am. Chem. Soc 1978, 100, 640–641. [Google Scholar]
- (11).Lee H; Jun C-H Hydroacylation of 1-Alkene with 2-(Diphenylphosphino)benzaldehyde by Rh(I). Bull. Korean Chem. Soc 1995, 16, 66–68. [Google Scholar]
- (12).(a) For an example that uses an alcohol coordinating group, see:Kokobu K; Matsumasa K; Miura M; Nomura M. Rhodium-Catalyzed Coupling Reaction of Salicyl Aldehydes with Alkynes via Cleavage of the Aldehyde C–H Bond. J. Org. Chem 1997, 62, 4564–4565. For an example that uses a sulfide coordinating group, see: [Google Scholar]; (b) Willis MC; McNally SJ; Beswick PJ Chelation-Controlled Intermolecular Hydroacylation: Direct Addition of Alkyl Aldehydes to Functionalized Alkenes. Angew. Chem., Int. Ed 2004, 43, 340–343. For an example that uses an amine coordinating group, see: [DOI] [PubMed] [Google Scholar]; (c) Castaing M; Wason SL; Estepa B; Hooper JF; Willis MC 2-Aminobenzaldehydes as Versatile Substrates for Rhodium-Catalyzed Alkyne Hydroacylation: Application to Dihydroquinolone Synthesis. Angew. Chem., Int. Ed 2013, 52, 13280–13283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Jun C-H; Lee H; Hong J-B Chelation-Assisted Intermolecular Hydroacylation: Direct Synthesis of Ketone from Aldehyde and 1-Alkene. J. Org. Chem 1997, 62, 1200–1201. [Google Scholar]
- (14).Murphy SK; Dong VM Enantioselective Hydroacylation of Olefins with Rhodium Catalysts. Chem. Commun 2014, 50, 13645–13649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Shibata Y; Tanaka K. Rhodium-Catalyzed Highly Enantioselective Direct Intermolecular Hydroacylation of 1,1-Disubstituted Alkenes with Unfunctionalized Aldehydes. J. Am. Chem.Soc 2009, 131, 12552–12553. [DOI] [PubMed] [Google Scholar]
- (16).Coulter MM; Dornan PK; Dong VM Rh-Catalyzed Intramolecular Olefin Hydroacylation: Enantioselective Synthesis of Seven- and Eight-Membered Heterocycles. J. Am. Chem. Soc 2009, 131, 6932–6933. [DOI] [PubMed] [Google Scholar]
- (17).Phan DHT; Kou KGM; Dong VM Enantioselective Desymmetrization of Cyclopropenes by Hydroacylation. J. Am. Chem. Soc 2010, 132, 16354–16355. [DOI] [PubMed] [Google Scholar]
- (18).For a review on using directing groups in chemical reactions, see: [Google Scholar]; (a) Hoveyda AH; Evans DA; Fu GC Substrate-Directable Chemical Reactions. Chem. Rev 1993, 93, 1307–1370. For an early report of hydroacylation that uses a directing group on the olefin partner, see: [Google Scholar]; (b) Inui Y; Tanaka M; Imai M; Tanaka K; Suemune H. Asymmetric Rh-Catalyzed Intermolecular Hydroacylation of 1,5-Hexadiene with Salicylaldehyde. Chem. Pharm. Bull 2009, 57, 1158–1160. [DOI] [PubMed] [Google Scholar]
- (19).Coulter MM; Kou KGM; Galligan B; Dong VM Regio- and Enantioselective Intermolecular Hydroacylation: Substrate-Directed Addition of Salicylaldehydes to Homoallylic Sulfides. J. Am. Chem. Soc 2010, 132, 16330–16333. [DOI] [PubMed] [Google Scholar]
- (20).(a) Murphy SK; Petrone DA; Coulter MM; Dong VM Catalytic Hydroacylation as an Approach to Homoaldol Products. Org. Lett 2011, 13, 6216–6219. [DOI] [PubMed] [Google Scholar]; (b) Murphy SK; Coulter MM; Dong VM β-Hydroxy Ketones Prepared by Regioselective Hydroacylation. Chem. Sci 2012, 3, 355–358. [Google Scholar]
- (21).von Delius M; Le CM; Ellinger B; Kuzikov M; Gul S; Dong VM Synthesis and Biological Activity of Octaketides from the Cytosporone Family. Isr. J. Chem 2017, 57, 975–981. [Google Scholar]
- (22).(a) Murphy SK; Bruch A; Dong VM Substrate-Directed Hydroacylation: Rhodium-Catalyzed Coupling of Vinylphenols and Nonchelating Aldehydes. Angew. Chem., Int. Ed 2014, 53, 2455–2459. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Murphy SK; Bruch A; Dong VM Mechanistic Insights into Hydroacylation with Non-Chelating Aldehydes. Chem. Sci 2015, 6, 174–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).(a) James BR; Young CG The Asymmetric Cyclisation of Substituted Pent-4-enals by a Chiral Rhodium Phosphine Catalyst. J. Chem. Soc., Chem. Commun 1983, 1215–1216. For related parallel kinetic resolutions, see: [Google Scholar]; (b) Tanaka K; Fu GC Parallel Kinetic Resolution of 4-Alkynals Catalyzed by Rh(I)/Tol-BINAP: Synthesis of Enantioenriched Cyclobutanones and Cyclopentenones. J. Am. Chem. Soc 2003, 125, 8078–8079. [DOI] [PubMed] [Google Scholar]; (c) Yip SYY; Aïssa C. Isomerization of Olefins Triggered by Rhodium-Catalyzed C–H Bond Activation: Control of Endocyclic β-Hydrogen Elimination. Angew. Chem., Int. Ed 2015, 54, 6870–6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).For a more detailed discussion on dynamic kinetic resolutions, see:Bhat V; Welin ER; Guo X; Stoltz BM Advances in Stereoconvergent Catalysis from 2005 to 2015: Transition-Metal-Mediated Stereoablative Reactions, Dynamic Kinetic Resolutions, and Dynamic Kinetic Asymmetric Transformations. Chem. Rev 2017, 117, 4528–4561.
- (25).For a review on C–C bond forming processes that use DKR, see: [Google Scholar]; (a) Barlett SL; Johnson JS Synthesis of Complex Glycolates by Enantioconvergent Addition Reactions. Acc. Chem. Res 2017, 50, 2284–2296. For an example, see: [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Doyle AG; Jacobsen EN Enantioselective Alkylation of Acyclic α,α-Disubstituted Tributyltin Enolates Catalyzed by a [Cr(salen)] Complex. Angew. Chem., Int. Ed 2007, 46, 3701–3705. [DOI] [PubMed] [Google Scholar]
- (26).Chen Z; Aota Y; Nguyen HMH; Dong VM Dynamic Kinetic Resolution of Aldehydes by Hydroacylation. Angew. Chem., Int. Ed 2019, 58, 4705–4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Houminer Y. Preparation and Thermolysis of cis- and trans-1-Hydroxy-2-(2-pyridyl)cyclopentanes and cis- and trans-1-Hydroxy-2-(2-pyrazyl)cyclopentanes. J. Org. Chem 1985, 50, 786–789. [Google Scholar]
- (28).For a complementary method that prepares α,γ-disubstituted cyclopentanones from racemic cyclopentenones, see: [Google Scholar]; Jurkauskas V; Buchwald SL Dynamic Kinetic Resolution via Asymmetric Conjugate Reduction: Enantio- and Diastereoselective Synthesis of 2,4-Dialkyl Cyclopentanones. J. Am. Chem. Soc 2002, 124, 2892–2893. [DOI] [PubMed] [Google Scholar]
- (29).Chen Q-A; Kim DK; Dong VM Regioselective Hydroacylation of 1,3-Dienes by Cobalt Catalysis. J. Am. Chem. Soc 2014, 136, 3772–3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Yang J; Yoshikai N; Cobalt-Catalyzed Enantioselective Intramolecular Hydroacylation of Ketones and Olefins. J. Am. Chem. Soc 2014, 136, 16748–16751. [DOI] [PubMed] [Google Scholar]
- (31).Vinogradov MG; Tuzikov AB; Nikishin GI Intramolecular Hydroacylation Catalyzed by Cobalt Complexes. Russ. Chem. Bull 1985, 34, 325–329. [Google Scholar]
- (32).Park J-W; Kou KGM; Kim DK; Dong VM Rh-Catalyzed Desymmetrization of α-Quaternary Centers by Isomerization-Hydroacylation. Chem. Sci 2015, 6, 4479–4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).(a) For an example of carboacylation triggered by C–C bond activation, see:Dreis AM; Douglas CJ Catalytic Carbon–Carbon σ Bond Activation: An Intramolecular Carbo-Acylation Reaction with Acylquinolines. J. Am. Chem. Soc 2009, 131, 412–413. For an example of carboacylation triggered by C–N bond activation, see: [DOI] [PubMed] [Google Scholar]; (b) Walker JA; Vickerman KL; Humke JN; Stanley LM Ni-Catalyzed Alkene Carboacylation via Amide C–N Bond Activation. J. Am. Chem. Soc 2017, 139, 10228–10231. [DOI] [PubMed] [Google Scholar]
- (34).Park J-W; Chen Z; Dong VM Rhodium-Catalyzed Enantioselective Cycloisomerization to Cyclohexenes Bearing Quaternary Carbon Centers. J. Am. Chem. Soc 2016, 138, 3310–3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Carey FA; Sundberg RJ Advanced Organic Chemistry, Part A: Structure and Mechanisms, 5th ed.; Springer: NY, 2007; pp 843–848. [Google Scholar]
- (36).Theopold KH; Becker PN; Bergman RG Asymmetric Induction in Reactions Employing Enolates Generated from Cyclic Organo Transition-Metal Acyl Complexes. J. Am. Chem. Soc 1982, 104, 5250–5252. [Google Scholar]
- (37).Horino H; Ito T; Yamamoto A. A New Tishchenko-Type Ester Formation Catalyzed by Ruthenium Complexes. Chem. Lett 1978, 17–20. [Google Scholar]
- (38).Bergens SH; Fairlie DP; Bosnich B. Homogenous Catalysis: Catalytic Intramolecular Conversion of 1,4-Dialdehydes to γ-Lactones. Organometallics 1990, 9, 566–571. [Google Scholar]
- (39).For a review on the Tishchenko reaction, see: [Google Scholar]; Mahrwald R. The Aldol-Tishchenko Reaction: A Tool in Stereoselective Synthesis. Curr. Org. Chem 2003, 7, 1713–1723. [Google Scholar]
- (40).Eckert M; Fleischmann G; Jira R; Bolt HM; Golka K. Acetaldehyde. In Ullmann’s Encyclopedia of Industrial Chemistry. Vol 1. Wiley-VCH: Verlag, 2006; pp 191–207. [Google Scholar]
- (41).For an asymmetric Tishchenko reaction, see:Hsu J-L Fang J-M . Stereoselective Synthesis of δ-Lactones from 5-Oxoalkanals via One-Pot Sequential Acetalization, Tishchenko Reaction, and Lactonization by Cooperative Catalysis of Samarium Ion and Mercaptan. J. Org. Chem 2001, 66, 8573–8584. [DOI] [PubMed] [Google Scholar]
- (42).Khan HA; Kou KGM; Dong VM Nitrogen-Directed Ketone Hydroacylation: Enantioselective Synthesis of Benzoxazecinones. Chem. Sci 2011, 2, 407–410. [Google Scholar]
- (43).Shen Z; Dornan PK; Khan HA; Woo TK; Dong VM Mechanistic Insights into the Rhodium-Catalyzed Intramolecular Ketone Hydroacylation. J. Am. Chem. Soc 2009, 131, 1077–1091. [DOI] [PubMed] [Google Scholar]
- (44).Phan DHT; Kim B; Dong VM Phthalides by Rhodium-Catalyzed Ketone Hydroacylation. J. Am. Chem. Soc 2009, 131, 15608–15609. [DOI] [PubMed] [Google Scholar]
- 45.Zhao C; He Z; Cui S; Zhang R. Determination of 3-n-Butylphthalide in Rabbit Plasma by HPLC with Fluorescence Detection and its Application in Pharmacokinetic Study. Biomed. Chromatogr 2003, 17, 391–395. [DOI] [PubMed] [Google Scholar]
- (46).Wu X; Chen Z; Bai Y-B; Dong VM Diastereodivergent Construction of Bicyclic γ-Lactones via Enantioselective Ketone Hydroacylation. J. Am. Chem. Soc 2016, 138, 12013–12016. [DOI] [PubMed] [Google Scholar]
- (47).Popelak A; Haack E; Lettenbauer G; Spingler H. Zur Konstitution des Mesembrins. Naturwissenschaften 1960, 47, 156. [Google Scholar]
- (48).Kulkarni MG; Rasne RM; Davawala SI; Doke AK Allyl Vinyl Ethers via Wittig Olefination: A Short and Efficient Synthesis of (±)-Mesembrine. Tetrahedron Lett. 2002, 43, 2297–2298. [Google Scholar]
- (49).Kou KGM; Le DN Dong VM Rh(I)-Catalyzed Intermolecular Hydroacylation: Enantioselective Cross-Coupling of Aldehydes and Ketoamides. J. Am. Chem. Soc 2014, 136, 9471–9476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Kou KGM; Longobardi LE; Dong VM Rhodium(I)-Catalyzed Intermolecular Hydroacylation of α-Keto Amides and Isatins with Non-Chelating Aldehydes. Adv. Synth. Catal 2015, 357, 2233–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Groves JT The Bioinorganic Chemistry of Iron in Oxygenases and Supramolecular Assemblies. Proc. Natl. Acad. Sci 2003, 100, 3569–3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Sigel A; Sigel H; Sigel RKO The Ubiquitous Roles of Cytochrome P450 Proteins: Metal Ions in Life Sciences, Vol. 3; Wiley: New York, 2007, pp 1–619. [Google Scholar]
- (53).For a review on olefination reactions in organic synthesis, see:Kelly SE Alkene Synthesis. In Comprehensive Organic Synthesis, Vol 1.; Trost BM; Fleming I; Ed.; Pergamon: Oxford, 1991, pp 729–817. [Google Scholar]
- (54).(a) For examples, see:Prince RH; Raspin KA Olefin Formation from Saturated Aldehydes and Acids by Reaction with Ruthenium and Rhodium Complexes. Chem. Commun 1966, 156–157. [Google Scholar]; (b) McCombs CA; Foster CH Dehydroformylation of Steroidal Aldehydes. Eastman Kodak Company, U.S. Patent 4272444A, 14 August 1980.
- (55).Bhawal BN; Morandi B. Isodesmic Reactions in Catalysis – Only the Beginning? Isr. J. Chem 2018, 58, 94–103. [Google Scholar]
- (56).Bhawal BN; Morandi B. Catalytic Isofunctional Reactions—Expanding the Repertoire of Shuttle and Metathesis Reactions. Angew. Chem., Int. Ed 2019, 58, 10074–10103. [DOI] [PubMed] [Google Scholar]
- (57).Kusumoto S; Tatsuki T; Nozaki K. The Retro-Hydroformylation Reaction. Angew. Chem., Int. Ed 2015, 54, 8458–8461. [DOI] [PubMed] [Google Scholar]
- (58).Abrams DJ; West JG; Sorenson EJ Toward a Mild Dehydroformylation Using Base-Metal Catalysis. Chem. Sci 2017, 8, 1954–1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Wu X; Cruz FA; Lu A; Dong VM Tandem Catalysis: Transforming Alcohols to Alkenes by Oxidative Dehydroxymethylation. J. Am. Chem. Soc 2018, 140, 10126–10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).(a) Scheele CW Om Brunsten eller Magnesia, och dess Egenskaper. Kungl. Svenska Vetenskapsakad. Handl 1774, 35, 89–116; 177–194. [Google Scholar]; (b) Liebig J. Ueber die Producte der Oxydation des Alkohols. Annalen 1835, 14, 133–167. [Google Scholar]
- (61).Mahjour B; Shen Y; Liu W; Cernak T. A Map of the Amine-Carboxylic Acid Coupling System. Nature 2020, 580, 71–75. [DOI] [PubMed] [Google Scholar]
- (62).Bandar JS; Ascic E; Buchwald SL Enantioselective CuH–Catalyzed Reductive Coupling of Aryl Alkenes and Activated Carboxylic Acids. J. Am. Chem. Soc 2016, 138, 5821–5824. [DOI] [PMC free article] [PubMed] [Google Scholar]