Abstract
We present new complete mitogenome sequences of Silurus glanis (S. glanis) from 4 samples such as male and female individuals from two countries (Hungary, Czech Republic). The complete mitochondria were determined from genome sequencing by using Illumina MiSeq platform resulting in long, 300 bp. paired-end reads. De novo assembly was performed resulting in one nod (scaffold) covering the total mitochondria in each sample. The mitochondrial genomes were circular, double-stranded molecules of 16,524 bp in length and consisted of 13 protein-coding genes (PCGs), 2 ribosomal RNA genes, 22 transfer RNA genes, and 1 control region. These sequences were deposited in the NCBI GeneBank under the accession numbers (MW796040, MW796041, MW796042, MW796043) and compared with the only available S. glanis mitochondrial genome (NC_014261.1) sequenced by unidentified technology and showed 99% similarity. We found in seq1 82, in seq2 82, seq3 83, seq4 82 nucleotide alterations involving 10 protein-coding genes and meaning 29 amino acid substitutions as well.
Keywords: Silurus glanis, Mitogenome, Wels catfish, Amino acid substitution
Specifications Table
| Subject | Genomics |
| Specific subject area | Mitogenomics |
| Type of data | Mitogenome sequence data in FASTA file format, tables, mitogenome map in figure format (.PNG) |
| How data were acquired | Illumina MiSeq platform |
| Data format | Raw and analyzed |
| Parameters for data collection | Genomic DNAs were extracted with Thermo Scientific™ GeneJET Genomic DNA Purification Kit from caudal fin. The DNA concentration and purity were checked by agarose gel electrophoresis and spectrophotometric quantification. Libraries were prepared using the Nextera XT DNA Sample Preparation Kit according to the manufacturer's protocol (Wagle, Berger et al. 2012). The samples were sequenced using MiSeq v2 (2 × 301 bp) chemistries (Illumina). |
| Description of data collection | Four Silurus glanis total gDNA samples were de novo sequenced and analyzed. Mitogenomes were reconstructed in silico in each sample. The circular mitochondrial genome map was drawn using Microsoft® Excel®. For sequence comparison, we used NCBI BLAST and Geneious 9.0.5. |
| Data source location | Silurus glanis samples were collected from Southern Hungary and Northern Czech Republic rivers. |
| Data accessibility | The mitogenome data is available in Genbank with the accession numbers: MW796040 (https://www.ncbi.nlm.nih.gov/nuccore/MW796040.1/), MW796041 (https://www.ncbi.nlm.nih.gov/nuccore/MW796041.1/), MW796042 (https://www.ncbi.nlm.nih.gov/nuccore/MW796042.1/), MW796043 (https://www.ncbi.nlm.nih.gov/nuccore/MW796043.1/). The raw reads are available in NCBI Sequence Read Archive with the accession numbers: SRR15503605 (https://trace.ncbi.nlm.nih.gov/Traces/sra/?run=SRR15503605), SRR15503606 (https://trace.ncbi.nlm.nih.gov/Traces/sra/?run=SRR15503606), SRR15503607 (https://trace.ncbi.nlm.nih.gov/Traces/sra/?run=SRR15503607), SRR15503608 (https://trace.ncbi.nlm.nih.gov/Traces/sra/?run=SRR15503608). |
Value of the Data
-
•
We provided 4 new whole mitochondrial genomes of the European catfish (Silurus glanis, S. glanis). Continued expansion of mitochondrial genome databases to include both a greater number of species and increased representation of populations from throughout their range will provide an improved basis for analysis.
-
•
Our data will be useful for S. glanis species monitoring, phylogenetic, population, and evolutionary studies.
-
•
Illumina long read has been chosen and applied for the NGS sequencing methodology. The sequencing using paired-end reads of 300bp were uniquely used for whole mitochondrial genome sequencing in the case of the species of the Teleostei group. Higher coverage of the nucleotide positions and proper quality values of the assemblies (see N50 values) resulted in a more accurate whole mitochondrial genome of our samples. These mitogenomes were suggested and provided as new reference sequences for further studies.
1. Data Description
S. glanis is the largest-bodied European freshwater fish, Inhabitant of Native in Eastern Europe and western Asia. This species is now extensively dispersed and introduced in several countries to the west and south of its endemic range. The S. glanis belongs to the family Siluridae, a group of freshwater fish indigenous to Europe, Asia, and Africa. There are 107 species from 12 genera in this family. Among the 18 Silurus species, two are native to Europe: wels catfish and Aristotle's catfish (S. aristotelis). European catfish is the largest-bodied fish of the order Siluriformes and can attain a maximum length of 500 cm, although it more commonly reaches 300 cm [1].
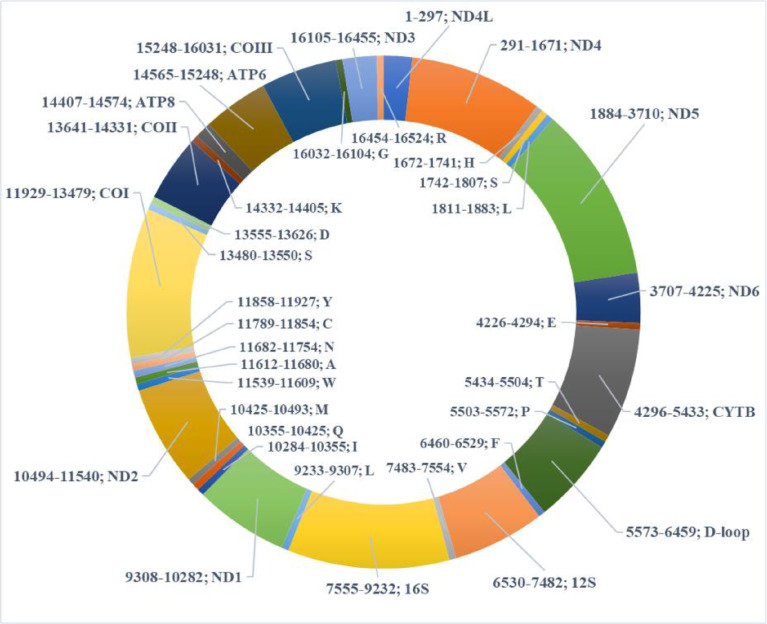
The circular mitogenomes of S. glanis (GeneBank accession numbers MW796040, MW796041, MW796042, MW796043) were 16,524 bp in length, in all 4 samples, which contained 37 genes (13 protein-coding, 22 tRNAs, 2 rRNAs) and one control region displacement loop (D-loop) (Fig. 1, Table 2, Supplementary 1.). The genes encoded by the mitogenome are characteristic to the vertebrate mitocondrial genome. The organization of the genes also tends to be conserved among vertebrates for 37 genes and the D-loop, which are arranged in the same order from hagfish to eutherian mammals [2], [3], [4], [5], [6]. Information for each individual is presented in Table 1. The representative complete mitogenome map in Fig. 1. The 4 mitochondria showed 99% similarity, twelve of 13 PCGs contained the typical ATG as a start codon, however, the gene COI started with GTG. Similar data were found by Vittas; Wu et al.; Zeng et al. [7], [8], [9]. 6 genes (nad5, nad4L, atp6, atp8, COI, nad1) of 13 PCGs ended in TAA for the stop codon. 3 genes ended in TAG (nad2, nad3, nad6). 4 genes (cytb, nad4, COII, COIII) ended in only a T residue. Such immature stop codon is completed via post-transcriptional polyadenylation [10].
Fig. 1.
S. glanis mitochondrial genome. Genes for proteins and rRNAs are shown with standard abbreviations, outside of the circle. Genes for tRNAs are designated by a single letter for the corresponding amino acid, inside of the circle. Before the genes, it shows their position in seq4.
Table 2.
Reference genome gene organization.
| Gene/Element | Abbreviation | Position | Size(bp) | Startcodon | Stopcodon |
|---|---|---|---|---|---|
| NADH dehydrogenase subunit 4L | ND4L | 1–297 | 297 | ATG | TAA |
| NADH dehydrogenase subunit 4 | ND4 | 291–1671 | 1381 | ATG | T* |
| tRNAHis | H | 1672–1741 | 70 | ||
| tRNASer | S | 1742–1807 | 66 | ||
| tRNALeu | L | 1811–1883 | 73 | ||
| NADH dehydrogenase subunit 5 | ND5 | 1884–3710 | 1827 | ATG | TAA |
| NADH dehydrogenase subunit 6 | ND6 | 3707–4225 | 519 | ATG | TAG |
| tRNAGlu | E | 4226–4294 | 69 | ||
| cytochrome b | CYTB | 4296–5433 | 1138 | ATG | T* |
| tRNAThr | T | 5434–5504 | 71 | ||
| tRNAPro | P | 5503–5572 | 70 | ||
| control region | D-loop | 5573–6459 | 887 | ||
| tRNAPhe | F | 6460–6529 | 70 | ||
| 12S ribosomal RNA | 12S | 6530–7482 | 953 | ||
| tRNAVal | V | 7483–7554 | 72 | ||
| 16S ribosomal RNA | 16S | 7555–9232 | 1678 | ||
| tRNALeu | L | 9233–9307 | 75 | ||
| NADH dehydrogenase subunit 1 | ND1 | 9308–10282 | 975 | ATG | TAA |
| tRNAIle | I | 10284–10355 | 72 | ||
| tRNAGln | Q | 10355–10425 | 71 | ||
| tRNAMet | M | 10425–10493 | 69 | ||
| NADH dehydrogenase subunit 2 | ND2 | 10494–11540 | 1047 | ATG | TAG |
| tRNATrp | W | 11539–11609 | 71 | ||
| tRNAAla | A | 11612–11680 | 69 | ||
| tRNAAsn | N | 11682–11754 | 73 | ||
| tRNACys | C | 11789–11854 | 66 | ||
| tRNATyr | Y | 11858–11927 | 70 | ||
| cytochrome c oxidase subunit I | COI | 11929–13479 | 1551 | GTG | TAA |
| tRNASer | S | 13480–13550 | 71 | ||
| tRNAAsp | D | 13555–13626 | 72 | ||
| cytochrome c oxidase subunit II | COII | 13641–14331 | 691 | ATG | T* |
| tRNALys | K | 14332–14405 | 74 | ||
| ATP synthase F0 subunit 8 | ATP8 | 14407–14574 | 168 | ATG | TAA |
| ATP synthase F0 subunit 6 | ATP6 | 14565–15248 | 684 | ATG | TAA |
| cytochrome c oxidase subunit III | COIII | 15248–16031 | 784 | ATG | T* |
| tRNAGly | G | 16032–16104 | 73 | ||
| NADH dehydrogenase subunit 3 | ND3 | 16105–16455 | 351 | ATG | TAG |
| tRNAArg | R | 16454–16524 | 71 |
Table 1.
Reported mitogenome samples.
In seq1 and seq3, which are female samples, control region, trnP, trnT, CYTB, ND5, trnL, trnS, trnH, ND4, ND4L, trnR, ND3, trnG, COX3, ATP6, ATP8, trnK, COX2, trnD, COX1, trnW, ND2, trnM, trnI, ND1, trnL, rrnL, trnV, rrnS, trnF were encoded by the H-strand, trnQ, trnA, trnN, trnC, trnY, trnS, ND6, trnE, were encoded by the L-strand. On the other hand, in seq2 and seq4, which are males, trnQ, trnA, trnN, trnC, trnY, trnS, ND6, trnE, were encoded by the H-strand, control region, trnP, trnT, CYTB, ND5, trnL, trnS, trnH, ND4, ND4L, trnR, ND3, trnG, COX3, ATP6, ATP8, trnK, COX2, trnD, COX1, trnW, ND2, trnM, trnI, ND1, trnL, rrnL, trnV, rrnS, trnF were encoded by the L-strand.
Amino acid sequences were compared to the only available S. glanis mitogenome (NC_014261.1). From the 13 PCGs, we found differences in 10, which are nad2, nad3, nad4, nad4L, nad5, nad6, cytb, COI, COII, COIII. Atp6, atp8, and nad1 were the same in the 4 examined samples and in the S. glanis mitochondrial genome (NC_014261.1) sequence as well. In cytb and nad6 was 1, in nad2 and COIII were 2, in COI were 3, in nad4L and nad5 were 4, in nad4 and nad3 were 5 amino acid changes comparing to the only available S. glanis sequence (NC_014261.1). COII showed differences in seq3 there were 2 amino acid changes, in seq1, seq2, and seq4 was only 1. The amino acid changes are shown in Table 3 (Supplementary 2.). Sequencing multiple mitochondrial genomes from the same species, S. glanis revealed varying levels of intraspecies genetic variation.
Table 3.
The observed amino acid changes in the 13 protein-coding genes in the examined S. glanis samples. Black and red colors indicate the changes of NC_014261.1 to examined amino acids at the position of the gene sequence. Each indicated amino acid changes were detected uniformly in the four newly identified mitogenomes. *The Val154Ile substitution in the cox2 gene was observed only in seq3.
| Protein coding genes | Amino acid changes |
|---|---|
| nad1 | - |
| cytb | Val145Ile |
| nad6 | Val42Ile |
| nad5 | Asn76Ser Val211Ile Ile538Met Thr599Ala |
| nad4 | Ser71Cys Phe73Leu Val76Leu Ala338Thr Pro339His |
| nad4L | Phe53Ser Cys56Phe Met61Ile Arg62Leu |
| nad3 | Pro36Ser Asp39Glu Ser44Pro His55Arg Ser56Phe |
| cox3 | Pro140Ser Gly142Val |
| atp6 | - |
| atp8 | - |
| cox1 | Met1Val Gln111Leu His260Tyr |
| cox2 | Ile76Val Val154Ile* |
| nad2 | Ser179Leu Pro326Leu |
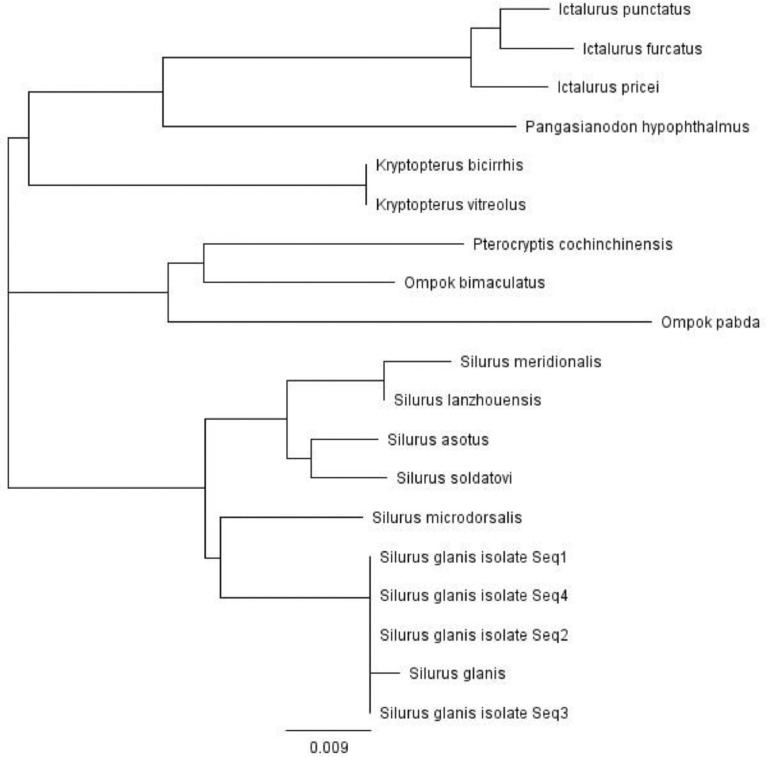
The phylogenetic relationship of S. glanis was compared with previously analyzed mitogenomes of other Siluriformes, Silurus, Kryptopterus, Ompok, and Pterocryptis genus in Siluridae family and on two other genera, Ictalurus in Ictaluridae, Pangasianodon in Pangasiidae family. The phylogenetic tree is shown in Fig. 2. The phylogenetic location of S. glanis was the closest to S. microdorsalis. Similar results were found by Park et al.; Yang et al. [11,12].
Fig. 2.
Phylogenetic tree of Silurus glanis with other catfishes. Based on the mitochondrial 12S rRNA.
2. Experimental Design, Materials and Methods
S. glanis samples were collected from Hungary and Czech Republic rivers. The genomic DNAs were extracted with Thermo Scientific™ GeneJET Genomic DNA Purification Kit from caudal fin and stored at −70 °C. After the extraction, we checked the DNA concentration and purity by agarose gel electrophoresis and spectrophotometric quantification. Four S. glanis total gDNA samples were de novo sequenced and analyzed. Mitogenomes were reconstructed in silico in each sample. Libraries were prepared using the Nextera XT DNA Sample Preparation Kit according to the manufacturer's protocol [13], unless otherwise stated. Sequencing reactions were carried out using the MiSeq v2 (2 × 301 bp) chemistries (Illumina). Similar sequencing technique was used by Austin et al.; Tabassum et al.; Alam et al. [14], [15], [16]. The raw reads were cleaned by the trimming of adaptor sequences, empty reads, and ambiguous nucleotides (‘N’ at the end of the reads). The reads obtained were then assembled using the SPAdes (St Petersburg genome assembler) assembly toolkit containing various assembly pipelines based on the Bruijn Graph [17,18]. Total genome sizes of four individuals were approximately between 800–810 Mb, with 4383–4388 scaffolds and N50 varied between 3.1–3.4 Mb. The predicted genome size was corresponding to the most closely related Silurus asotus (831–1411 Mb) [19] and other Siluriformes, whose genome sizes vary from 599 Mb in Bagarius yarrelli [20] to 1200 Mb in Clarias batrachus [21]. The longest individual scaffolds were 9–9.5 Mb. Mitochondrial genomes were separated into individual scaffolds: NODE_3 (seq1), NODE_4 (seq3), NODE_6 (seq4), NODE_8 (seq2) with the same length 16,524 and k-mer coverage for the last (largest) k values used were 46.985597, 56.102578, 65.455701 and 50.835330. The sequencing coverage varied between 140–150x of the four mitochondrial genomes. The base composition was GC 44, 86% and AT 55, 14% in the samples from Czech Republic and GC 44, 87% and AT 55, 13% in the sample from Hungary. The mitogenome contigs were identified by BLAST+ [22] alignments to the previously published S. glanis mitochondrial genome (NC_014261.1). For sequence comparison, we used NCBI BLAST [23] and Geneious 9.0.5 [24]. The phylogenetic analysis was performed using Geneious 9.0.5 with the Geneious Tree Builder, the Alignment type was Global alignment with free end gaps, the Genetic Distance Model was Tamura-Nei, and the Tree built Method was Neighbor-Joining. The analysis is based on the mitochondrial 12S rRNA, because this gene sequence is frequently used in molecular taxonomy and phylogeny [25], [26], [27], [28]. For phylogenetic analysis, nucleotide sequences were downloaded from the NCBI database.
Ethics Statement
This study is based on non-living animal experiments, only tissue samples. Do not require an ethics statement.
CRediT authorship contribution statement
Kinga Székvári: Visualization, Investigation. Zoltán Szabolcsi: Conceptualization, Methodology. Barbara Kutasy: Data curation, Writing – original draft. Géza Hegedűs: Software, Resources. Eszter Virág: Software, Resources, Supervision, Writing – review & editing.
Declaration of Competing Interest
The authors report no conflict of interest. The authors alone are responsible for the content and writing of the paper. The first and second authors participated in equal proportions of the work.
Acknowledgment
We express our thanks to the EduCOmat Ltd, Hungary to perform bioinformatics analysis and support the publication.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.dib.2021.107418.
Appendix. Supplementary materials
References
- 1.Copp G.H. Voracious invader or benign feline? A review of the environmental biology of European catfish Silurus glanis in its native and introduced ranges. Fish Fish. 2009;10(3):252–282. doi: 10.1111/j.1467-2979.2008.00321.x. [DOI] [Google Scholar]
- 2.Anderson S., Bankier A., Barrell B. Sequence and organization of the human mitochondrial genome. Nature. 1981;290:457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- 3.Roe B.A., Ma D.P., Wilson R.K., Wong J.F. The complete nucleotide sequence of the Xenopus laevis mitochondrial genome. J. Biol. Chem. 1985;260:9759–9774. [PubMed] [Google Scholar]
- 4.Tzeng C.S., Hui C.F., Shen S.C., Huang P.C. The complete nucleotide sequence of the Crossostoma lacustre mitochondrial genome: conservation and variations among vertebrates. Nucleic Acids Res. 1992;20:4853–4858. doi: 10.1093/nar/20.18.4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang Ys., Huang Fl., Lo Tb. The complete nucleotide sequence and gene organization of carp (Cyprinus carpio) mitochondrial genome. J. Mol. Evol. 1994;38:138–155. doi: 10.1007/BF00166161. [DOI] [PubMed] [Google Scholar]
- 6.Clayton D.A. Vertebrate mitochondrial DNA—a circle of surprises. Exp. Cell Res. 2000;255:4–9. doi: 10.1006/excr.1999.4763. [DOI] [PubMed] [Google Scholar]
- 7.Vittas S. The mitochondrial genome of the European catfish Silurus glanis (Siluriformes, Siluridae) J. Biol. Res. 2011;15:25. http://www.jbr.gr/papers20111/03-Vittas-et-al.pdf [Google Scholar]
- 8.Wu J., Lei C., Zhao J., Jin F., Gao H., Fu S., Zhou R., Luo Y., Leng Y., Xue S., Zhang W., Li G. The complete mitochondrial genome of Silurus grahami regan, 1907 (Siluriformes: Siluridae), a native catfish in Fuxian lake. Mitochondrial DNA Part B. 2021;6(3):835–836. doi: 10.1080/23802359.2021.1884024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zeng Q., Wang Z., Peng Z. Mitochondrial genome of Silurus asotus (Teleostei:Siluriformes) Mitochondrial DNA. 2011;22:5–6. doi: 10.3109/19401736.2011.636435. (2011) [DOI] [PubMed] [Google Scholar]
- 10.Ojala D., Montoya J., Attardi G. tRNA punctuation model of RNA processing in human mitochondria. Nature. 1981;290:470–474. doi: 10.1038/290470a0. [DOI] [PubMed] [Google Scholar]
- 11.Park C.E., Park Y.J., Kim M.C., Park M.K., Jung Y.G., Choi S.D., Jo Y.J., Kang G.U., Kim M.J., Li Q.X., Yoza B.A., Kim K.H., Park H.C., Shin J.H. The first complete mitochondrial genome sequence of the korean endemic catfish Silurus microdorsalis (Actinopteri, Siluriformes, Siluridae) Mitochondrial DNA Part B. 2020;5(1):131–132. doi: 10.1080/23802359.2019.1698336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang Na, Li Y., Liu Z., Chen Q., Shen Y. The complete mitochondrial genome of Silurus asotus (Siluriformes: Siluridae: Silurus) and its phylogenetic analysis. Mitochondrial DNA Part B. 2019;4(2):2377–2378. doi: 10.1080/23802359.2019.1630335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wagle N. High-throughput detection of actionable genomic alterations in clinical tumor samples by targeted, massively parallel sequencing. Cancer Discov. 2012;2(1):82–93. doi: 10.1158/2159-8290.CD-11-0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Austin C.M., Hua Tan M., Harrisson KA., Lee Y.P., Croft L.J., Sunnucks P., Pavlova A., Gan H.M. De novo genome assembly and annotation of Australia's largest freshwater fish, the Murray cod (Maccullochella peelii), from illumina and nanopore sequencing read. GigaScience. 2017;6(8) doi: 10.1093/gigascience/gix063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tabassum N., Alam Md.J., Kim J.H., Lee S.R., Lee J.H., Park H., Kim H.W. Characterization of complete mitochondrial genome of Pogonophryne albipinna (Perciformes: Artedidraconidae) Mitochondrial DNA Part B. 2020;5(1):156–157. doi: 10.1080/23802359.2019.1698361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alam Md.J., Andriyono S., Sektiana S.P., Rahman Md.M., Kim H.W. The molecular characterization of complete mitochondrial genome of spotted snakehead fish, Channa punctata (Bloch 1793) Mitochondrial DNA Part B. 2019;4(1):547–548. doi: 10.1080/23802359.2018.1553520. [DOI] [Google Scholar]
- 17.Bankevich A. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012;19(5):455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nurk S. Proceedings of the Annual International Conference on Research in Computational Molecular Biology. 2013. Assembling genomes and mini-metagenomes from highly chimeric reads. Springer. [DOI] [Google Scholar]
- 19.Jianxun C., Xiuhai R., Qixing Y. Nuclear DNA content variation in fishes. Cytologia. 1991;56:425–429. doi: 10.1508/cytologia.56.425. (Tokyo) [DOI] [Google Scholar]
- 20.Jiang W., Lv Y., Cheng L., Yang K., Bian C. Whole-genome sequencing of the giant devil catfish, Bagarius yarrelli. Genome Biol. Evol. 2019;11:2071–2077. doi: 10.1093/gbe/evz143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li N., Bao L., Zhou T., Yuan Z., Liu S. Genome sequence of walking catfish (Clarias batrachus) provides insights into terrestrial adaptation. BMC Genom. 2018;19:952. doi: 10.1186/s12864-018-5355-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Camacho C., Coulouris G., Avagyan V. BLAST+: architecture and applications. BMC Bioinform. 2009;10:421. doi: 10.1186/1471-2105-10-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Altschul S.F., Gish W., Miller W., Myers E.W., Lipman D.J. Basic local alignment search tool. J. Mol. Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 24.Kearse M. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28(12) doi: 10.1093/bioinformatics/bts199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patwardhan A., Ray S., Roy A. Molecular markers in phylogenetic studies – a review. J. Phylogen Evol. Biol. 2014;2:131. doi: 10.4172/2329-9002.1000131. [DOI] [Google Scholar]
- 26.Widayanti R., Kusumaastuti K.A., Novi J.M., Adani F.K., Gultom C.R.P., Prastiti A.D., Nugroho H.A., Pakpahan S. Genetic variation and phylogenetic analysis of Indonesian indigenous catfish (baung fish) based on mitochondrial 12S rRNA gene. Vet. World. 2021;14(3):751–757. doi: 10.14202/vetworld.2021.751-757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leonidovna F.L., Husainov A.M., Sverdrup A.E. The correlation between saprobity and mitochondrial genes of indicator fish species based on molecular phylogeny. Int. J. Green Pharm. 2017;11:S856–S862. doi: 10.22377/IJGP.V11I04.1372. [DOI] [Google Scholar]
- 28.Wang H.Y., Tsai M.P., Dean J., Lee S.C. Molecular phylogeny of Gobioid fishes (Perciformes: Gobioidei) based on mitochondrial 12S rRNA sequences. Mol. Phylogenet. Evol. 2001;20(3):390–408. doi: 10.1006/mpev.2001.0957. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.