Abstract
Aims
Physical frailty is a commonly encountered geriatric syndrome among older adults without coronary heart disease (CHD). The impact of frailty on the incidence of long-term cardiovascular outcomes is not known.We aimed to evaluate the long-term association of frailty, measured by the Fried frailty phenotype, with all-cause-mortality and MACE among older adults without a history of CHD at baseline in the National Health and Aging Trends Study.
Methods and Results
We used the National Health and Aging Trends Study, a prospective cohort study linked to a Medicare sample. Participants with a prior history of CHD were excluded. Frailty was measured during the baseline visit using the Fried physical frailty phenotype. Cardiovascular outcomes were assessed during a 6-year follow-up.
Of the 4656 study participants, 3259 (70%) had no history of CHD 1 year prior to their baseline visit. Compared to those without frailty, subjects with frailty were older (mean age 82.1 vs. 75.1 years, P < 0.001), more likely to be female (68.3% vs. 54.9%, P < 0.001), and belong to an ethnic minority. The prevalence of hypertension, falls, disability, anxiety/depression, and multimorbidity was much higher in the frail and pre-frail than the non-frail participants. In a Cox time-to-event multivariable model and during 6-year follow-up, the incidences of death and of each individual cardiovascular outcomes were all significantly higher in the frail than in the non-frail patients including major adverse cardiovascular event (MACE) [hazard ratio (HR) 1.77, 95% confidence interval (CI) 1.53, 2.06], death (HR 2.70, 95% CI 2.16, 3.38), acute myocardial infarction (HR 1.95, 95% CI 1.31, 2.90), stroke (HR 1.71, 95% CI 1.34, 2.17), peripheral vascular disease (HR 1.80, 95% CI 1.44, 2.27), and coronary artery disease (HR 1.35, 95% CI 1.11, 1.65).
Conclusion
In patients without CHD, frailty is a risk factor for the development of MACEs. Efforts to identify frailty in patients without CHD and interventions to limit or reverse frailty status are needed and, if successful, may limit subsequent adverse cardiovascular events.
Keywords: Older adults, Frailty, Coronary disease
Graphical abstract
See page 3866 for the editorial comment on this article (doi:10.1093/eurheartj/ehab481)
Introduction
One challenge in the clinical management of the rapidly expanding older adult population in the USA is the increased prevalence of frailty, an important geriatric syndrome.1 This is particularly relevant in the cardiovascular sphere as the prevalence and incidence of cardiovascular disease are also markedly increased with age. The physical frailty phenotype is a clinical state in which there is increased vulnerability to stressors due to diminished reserves across multiple physiological systems, resulting in functional decline, increased mortality, and a higher likelihood of complications from disease and from therapeutic interventions.1 , 2
In prior studies that examined the influence of frailty on cardiovascular outcomes, the assessment of frailty was performed in study populations at high cardiovascular risk, including those with acute coronary syndromes, peripheral vascular disease, and valvular heart disease.3–6 For example, Farooqi et al.7 have shown that frailty can provide an incremental prognostic value in addition to traditional cardiovascular risk assessment, but this meta-analysis included a mix of studies with and without cardiovascular disease. However, among patients without known coronary heart disease (CHD), the long-term association between frailty and major adverse cardiovascular events (MACE) remains largely unknown. In this study, we aimed to evaluate the long-term association of frailty, measured by the Fried frailty phenotype, with all-cause-mortality and MACE among older adults without a history of CHD using the National Health and Aging Trends Study (NHATS).
Methods
The source and study population
We examined the 2011 NHATS baseline cohort.8 NHATS is a prospective cohort study funded by the National Institute on Aging (U01AG032947) that studies functioning in later life. The source population for this study is derived from a sample of Medicare beneficiaries aged 65 years and older, a nationally representative cohort of older patients in the community. These older adults were interviewed in 2011 during their baseline visit and annual re-interviews were performed for each participant to document changes, trends, and dynamics in later life functioning.8 Detailed information on geriatric risks, including frailty, physical and cognitive capacity, activities of daily living (ADL), and the social, physical, and technological environments were collected. African Americans and patients from older ages were oversampled from the Medicare enrolment file. For each participant, the NHATS repository is linked to Medicare data that were available prior to the 2011 baseline visit.
The study population included adults ≥65 years of age enrolled during the 2011 NHATS baseline visit who also had linked Medicare data available for analysis prior to their baseline visit. For each participant, CHD was identified 12 months prior to the 2011 NHATS baseline visit using International Classification of Diseases-9th Revision 410–414, 410.0–410.9, 410.00–410.02, 410.10–410.12, 410,20–410,22, 410.30–410.32, 410.40–410.42, 410.50–410.52, 410.60–410.62, 410.70–410.72, 410.80–410.82, 410.90–41.92, and 4292.
Frailty assessment
Frailty in each older patient in the NHATS-CMS study was assessed using the five domains of the Fried physical frailty phenotype9: exhaustion, low physical activity, weakness, slowness, and shrinking (i.e. unintentional weight loss). If three or more, out of the five criteria, were present, the individual was categorized as frail and those with one or two of the five were categorized as ‘pre-frail’. Out of the total study population (n = 3259), 16% (n = 527) were categorized as ‘frail’ and 47% (n = 1535) were categorized as ‘non-frail’. Detailed definitions of meeting each criterion were previously published.10 For missing frailty data, a multiple imputation methods was adapted, and it was similar to previously published work that used the imputed frailty dataset out of 10 replicas.10 The estimates from running separate models on the 10 replicates were pooled together to obtain the final estimates. The pooling of the estimates was performed in such a way that appropriately accounted for the uncertainty in the missing frailty data imputation (see Statistical analysis section).11
Cardiovascular outcomes
A MACE was defined as death from any cause, acute myocardial infarction, any subsequent CHD, stroke, or peripheral vascular disease, whichever came first. To address competing risks of death, MACE2 was defined as acute myocardial infarction, any subsequent CHD, stroke, or peripheral vascular disease, whichever came first, excluding all-cause mortality. Secondary cardiovascular endpoints included each of these individual components identified in the Center of Medicare and Medicaid Services database during the 6-year follow-up. Furthermore, any primary hospital admission for subsequent CHD was identified and reported separately.
Geriatric risks
For each patient, specific geriatric risks were assessed during the NHATS follow-up visits. These included measures of functioning [ADL and instrumental ADL (IADL), and functional limitations], cognitive function (any form of cognitive impairment, dementia/Alzheimer’s disease), disability, and mobility disability. For each older participant, the Katz scale was performed to assess independence in (i) self-care (ADL: bathing, dressing, eating, toileting); (ii) household activities (IADL: doing laundry, preparing meals, shopping for groceries and for personal items, medication management, handling bills and banking); and (iii) mobility (getting around inside, going outside, getting out of bed).10 Screening for cognitive dysfunction was performed to assess functions related to memory, orientation, and executive function. For patients with severe cognitive impairment, a proxy interview was conducted, and the proxy was asked about the function of the participant. Dementia status was ascertained using the following instruments: (i) a physician report indicating that the participant has dementia or Alzheimer’s disease; (ii) a scoring indicating a probable dementia administered to proxies; and (iii) results from cognitive tests that evaluate memory, orientation, and executive function.12 Disability was measured using the American Community Survey Disability Questions. Outcomes related to mobility, self-care, and household activities were performed independently for each participant during follow-up visits. Loss of independence was defined as patients reporting never or rarely going outside or the use of devices to go outside.
Demographic characteristics, medical conditions, and healthcare utilization
Each older adult enrolled in the study was asked whether their physician had ever told them they had any of the following medical conditions: high blood pressure, diabetes mellitus, stroke, any cardiac disorder, arthritis, lung or bone disease, and cognitive impairment or dementia. Hospitalization within the past 12 months and baseline assessment on self-care, mobility, and household activities were collected.10
Statistical analysis
Participants with a history of CHD and stroke were excluded. During the 2011 baseline NHATS visit, participants were categorized into three distinct groups: no frailty, pre-frailty, and frailty as assessed by the Fried physical frailty phenotype. Demographics, smoking status, comorbidity, hospitalizations, emergency department visits, falls, self-care, mobility, household activities, depression, anxiety, and cognitive impairment at baseline were reported for the frail and the non-frail. Frequencies and percentages were calculated for categorical variables and mean ± standard deviation for continuous variables. Data on self-care, mobility, and household activities are presented as cumulative proportions at 6 years for the frail vs. the non-frail group (Table 1).
Table 1.
Characteristics of the study population of patients without a history of coronary heart disease enrolled in the National Health and Aging Trends Study by physical frailty phenotype
| Characteristics | Total (n = 3259) | No frailty (n = 1197) | Pre-frailty (n = 1535) | Frailtya (n = 527) | P-value |
|---|---|---|---|---|---|
| Age, years, mean | 77.6 | 75.1 | 78.0 | 82.1 | <0.001 |
| Age, years, % | |||||
| 65–69 | 18.8 | 25.7 | 16.4 | 9.0 | |
| 70–74 | 20.7 | 26.6 | 19.3 | 11.6 | |
| 75–79 | 20.0 | 21.1 | 21.0 | 14.6 | <0.001 |
| 80–84 | 19.6 | 16.3 | 22.2 | 19.8 | |
| 85–89 | 12.6 | 6.7 | 13.4 | 23.9 | |
| ≥90 | 8.2 | 3.5 | 7.8 | 20.2 | |
| Sex, % | |||||
| Female | 60.7 | 54.9 | 62.6 | 68.3 | <0.001 |
| Male | 39.3 | 45.1 | 37.4 | 31.7 | |
| Race, % | |||||
| Non-Hispanic white | 72.0 | 76.9 | 72.3 | 60.3 | |
| Non-Hispanic black | 21.2 | 17.4 | 20.9 | 31.2 | <0.001 |
| Hispanic | 4.2 | 3.3 | 4.0 | 6.5 | |
| Others | 2.6 | 2.4 | 2.8 | 2.0 | |
| BMI, kg/m2, mean | 27.1 | 26.9 | 27.5 | 26.5 | 0.001 |
|
Smoking status, % Smoke at least 1 cigarette/day |
49.2 |
50.9 |
49.7 |
44.2 |
0.044 |
| Comorbidities, % | |||||
| Arthritis | 54.6 | 41.4 | 57.7 | 75.5 | <0.001 |
| Diabetes mellitus | 21.2 | 16.5 | 21.5 | 30.8 | <0.001 |
| Hypertension | 63.8 | 56.6 | 66.8 | 71.3 | <0.001 |
| Lung disease | 13.7 | 8.1 | 15.5 | 20.9 | <0.001 |
| Osteoporosis | 21.3 | 15.9 | 21.8 | 32.0 | <0.001 |
| Dementia | 6.1 | 1.0 | 5.0 | 20.9 | <0.001 |
| No. chronic diseases, % | |||||
| 0–1 | 35.4 | 52.3 | 30.7 | 10.6 | |
| 2–3 | 49.4 | 41.8 | 54.5 | 51.7 | <0.001 |
| ≥4 | 15.2 | 5.9 | 14.8 | 37.7 | |
| Cancer, % | 26.6 | 24.4 | 28.4 | 26.6 | 0.071 |
| Hospital stay past 12 months, % | 16.7 | 8.9 | 17.2 | 32.8 | <0.001 |
| Any fall past month, % | 30.1 | 18.6 | 32.2 | 50.2 | <0.001 |
| Disability, % | |||||
| No difficulty | 74.6 | 94.0 | 73.7 | 33.2 | |
| Difficulty but no help | 11.9 | 4.5 | 15.0 | 20.1 | <0.001 |
| Help | 13.4 | 1.5 | 11.3 | 46.7 | |
| Mobility disability, % | |||||
| No difficulty | 67.6 | 90.2 | 65.9 | 21.2 | |
| Difficulty but no help | 17.5 | 8.8 | 22.2 | 23.5 | <0.001 |
| Help | 14.9 | 1.0 | 11.9 | 55.3 | |
| Household activities disability, % | |||||
| No difficulty | 62.2 | 86.4 | 58.9 | 16.7 | |
| Difficulty but no help | 12.3 | 8.0 | 16.1 | 11.1 | <0.001 |
| Help | 25.5 | 5.6 | 25.0 | 72.2 | |
| Overall disability level, % | |||||
| No difficulty | 51.0 | 77.7 | 44.4 | 9.4 | |
| Difficulty but no help | 20.3 | 15.4 | 26.4 | 13.6 | <0.001 |
| Help | 28.7 | 6.8 | 29.2 | 77.0 | |
| Depression, % (PHQ2 score ≥3) | 13.8 | 4.8 | 14.1 | 33.8 | <0.001 |
| Anxiety, % | |||||
| GAD2 score ≥3 | 11.3 | 4.5 | 11.7 | 26.1 | <0.001 |
| No. ED visits, % | |||||
| 0 | 76.0 | 84.1 | 75.3 | 59.8 | <0.001 |
| 1 | 15.9 | 12.7 | 17.0 | 19.7 | |
| ≥2 | 8.1 | 3.2 | 7.7 | 20.6 | |
| No. hospitalizations, % | <0.001 | ||||
| 0 | 88.5 | 94.4 | 88.4 | 75.4 | |
| 1 | 8.9 | 4.8 | 9.5 | 16.4 | |
| ≥2 | 2.6 | 0.8 | 2.1 | 8.2 | |
| Total LOS in hospital, days, mean | 1.00 | 0.31 | 0.93 | 2.74 | <0.001 |
| No. physician visits, mean | 7.24 | 5.99 | 7.65 | 8.89 | <0.001 |
| No. ADL impairment, % | <0.001 | ||||
| 0 | 63.4 | 88.3 | 59.4 | 18.4 | |
| 1–2 | 21.8 | 10.5 | 28.9 | 27.1 | |
| ≥3 | 14.8 | 1.3 | 11.7 | 54.5 | |
| No. IADL impairments, % | |||||
| 0 | 63.5 | 87.0 | 61.0 | 17.6 | |
| 1–2 | 20.7 | 11.6 | 25.7 | 26.4 | <0.001 |
| ≥3 | 15.8 | 1.4 | 13.3 | 56.1 | |
| Cognitive impairment, % | 8.6 | 2.6 | 8.0 | 26.8 | <0.001 |
| AD8 dementia, % | 5.8 | 0.6 | 3.6 | 23.8 | <0.001 |
| Dementia (probable), % | 13.1 | 3.5 | 11.3 | 40.0 | <0.001 |
AD8, AD8 dementia screening interview; ADL, activities of daily living; BMI, body mass index; ED, emergency department; GAD2, generalized anxiety disorder 2-item; IADL, instrumental activities of daily living; LOS, length of stay; PHQ2, patient health questionnaire-2.
Frailty was assessed by the physical frailty phenotype paradigm that is grounded in five criteria: exhaustion, low physical activity, weakness, slowness, and shrinking (www.nhats.org).
Proportional hazard models were used to assess the association between frailty and cardiovascular outcomes among older adults at 6-year follow-up. Patients were censored if they developed the cardiovascular outcomes of interest or if they were lost to follow-up. To address confounding by age, demographics, and other risk factors, we performed three additional multivariable Cox models. Model 2 adjusted for age and sex; Model 3 adjusted for age, sex, race/ethnicity, body mass index (BMI), and smoking status; and Model 4 adjusted for age, sex, race/ethnicity, BMI, smoking status, diabetes, hypertension, number of comorbid diseases, and dependency status (as a surrogate measure for composite functional status). To explore sensitivity of findings to dementia status, we performed a sensitivity analysis by excluding those with probable or definitive dementia (Supplementary material online, Tables S1). The assumption for Cox proportional hazard models was checked by plotting the Schoenfeld residuals against survival time for each primary and secondary cardiovascular outcome by frailty group. As sensitivity analysis, we fitted stratified Cox models that allowed the form of the underlying baseline hazard function to vary across age categories and between sexes (i.e. violation of the proportion hazard assumption) (see Supplementary material online, Tables S2 and S3). Kaplan–Meier curves were constructed to evaluate the association of frailty status at baseline with MACE and each individual cardiovascular outcome. Log-rank statistic was calculated for each curve. To test for interaction between frailty, as categorical variable, with each individual cardiovascular risk factors, likelihood ratio tests were performed to compare models with and without the interaction term. We have tested interactions of frailty with cardiovascular disease risk factors (i.e. BMI, smoking, diabetes, and hypertension) in Model 4 with each individual MACE outcome (Supplementary material online, Tables S4–S7). To facilitate interpretation, the hazard ratios of frailty and pre-frailty were presented separately by the level of each significant modifier in the supplementary material. For missing data on frailty, we adopted a two-step approach. First, if a test (grip or walking test) was not done because of health/safety concerns, a value of zero was assigned to indicate worst performance. Second, for remaining missing values, we employed multiple imputation (10 replicates) using chained equations (see details in Bandeen-Roche et al.10). A separate model was fitted using each imputed dataset, and the parameter estimates (i.e. regression coefficients and standard errors) obtained from each model were then combined into one set of inferential statistics via the STATA ‘mi estimate’ command that accounted for the uncertainty in the imputed values.
All tests are two-sided, and the statistically significant level is set at P < 0.05. Data analyses were conducted using SAS (v.9.4; SAS Institute Inc, Cary, NC, USA) and STATA version 15 MP (Stata Corp., College Station, TX, USA). The Johns Hopkins Medicine Institutional Review Board approved this study.
Results
Of the 4656 patients enrolled in the 2011 NHATS baseline visit, the mean age was 75 years and 60% of the study population was ≥75 years of age. Female participants constituted 61% of the cohort and the majority enrolled was non-Hispanic Whites. On average, the majority was overweight, and more than half of the cohort smoked at least one cigarette per day. The majority of this older population had multiple chronic conditions and 15% of the cohort had four or more chronic comorbidities. The most prevalent medical conditions were hypertension, arthritis, and osteoporosis. Approximately 21% of the study population was living with diabetes mellitus, and 6.1% had dementia at baseline.
Of the 3259 patients who had no history of CHD or stroke prior to their baseline NHATS visits, 1535 (47%) patients were pre-frail and 527 (16%) patients had physical frailty according to the Fried frailty phenotype. Of the total study population, 478 (15%) had missing frailty data at baseline and these estimates were imputed (see Methods section). Patients who were frail were older, more likely to be women and belong to an ethnic minority as compared to non-frail patients. Frail patients had higher prevalence of hypertension, diabetes mellitus, history of prior cardiovascular risk factors, dementia, lung disease, and arthritis than non-frail patients. The overall number of chronic comorbid conditions was also higher among frail patients with approximately one in three patients reported having four or more chronic medical conditions (Table 1). Frail patients were more likely to be admitted to the hospital and had more emergency department visits in 12 months prior to their baseline NHATS visits, than did non-frail patients. When evaluating measures of disability at baseline, including self-care, mobility disability, and household activities disability, patients with frailty were more likely to report significant impairment, than did non-frail patients. The overall disability level (i.e. having difficulties requiring help) among the frail group was as high as 76.8%, but only 10.0% reported having difficulties requiring help in the non-frail group. Frail patients also had high cognitive impairment at baseline and ∼40% had probable dementia at baseline (Table 1).
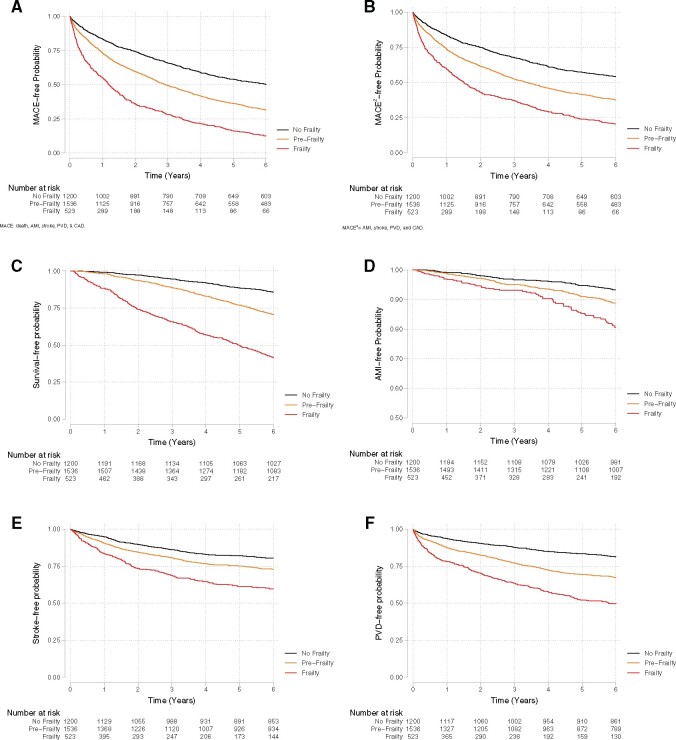
The age-adjusted incidence of cardiovascular outcomes at the 6-year follow-up is presented in Table 2. Frail patients developed more cardiovascular outcomes than did the pre-frail and non-frail groups over the 6-year follow-up, including a MACE, death, acute myocardial infarction, stroke, peripheral vascular disease, or any coronary artery disease (Figure 1). In an unadjusted Cox proportional hazards model, frailty and pre-frailty were associated with MACE and with each individual component of cardiovascular outcomes: all-cause death, acute myocardial infarction, peripheral vascular disease, and any subsequent coronary artery disease, as compared to non-frail patients. After adjusting for age, sex, race/ethnicity, census division, residence and income, BMI, traditional cardiovascular risk factors, dependency, and the number of concomitant chronic medical conditions, frailty remains highly associated with MACE, death, and peripheral vascular disease at the 6-year follow-up in the NHATS study (Table 3). In a sensitivity analysis excluding those patients with definitive or probable dementia (n = 2832), both frailty and pre-frailty were associated with MACE and with each individual cardiovascular outcome during follow-up when compared to the non-frail group (Supplementary material online, Table S1). In a stratified Cox model that allowed the form of the underlying baseline hazard function to vary across age categories and between sexes, the results largely remained the same (Supplementary material online, Tables S2 and S3). Modified association of frail by smoking status on all-cause mortality, acute myocardial infarction, and coronary artery disease as outcomes are presented in Supplementary material online, Tables S4 –S6 and that of frailty on peripheral vascular disease by hypertension is presented in Supplementary material online, Table S7.
Table 2.
The age-adjusted incidence of major adverse cardiovascular events by physical frailty phenotype among older adults without history of coronary heart disease outcomes in the National Health and Aging Trends Study during the 6-year follow-up
| Outcome | Total (n = 3259) | No frailty (n = 1197) | Pre-frailty (n = 1535) | Frailty a (n = 527) |
|---|---|---|---|---|
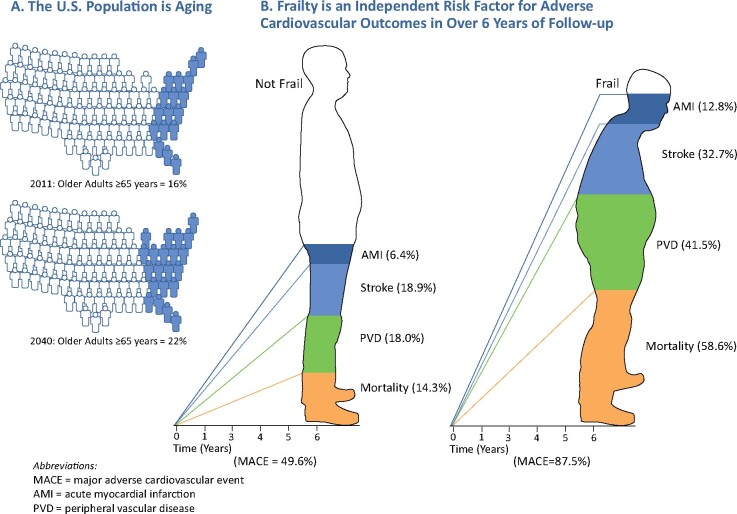
| MACE1, % | 64.7 | 49.6 | 68.6 | 87.5 |
| MACE2, % | 56.3 | 45.0 | 60.1 | 70.8 |
| Death, % | 28.6 | 14.3 | 29.5 | 58.6 |
| AMI, % | 9.0 | 6.4 | 9.7 | 12.8 |
| Stroke, % | 24.2 | 18.9 | 25.3 | 32.7 |
| PVD, % | 27.7 | 18.0 | 30.6 | 41.5 |
| CAD, % | 37.0 | 30.4 | 39.3 | 45.2 |
MACE1: a composite of acute myocardial infarction, stroke, peripheral vascular disease, coronary artery disease, and all-cause mortality; MACE2: a composite of acute myocardial infarction, stroke, peripheral vascular disease, coronary artery disease.
AMI, acute myocardial infarction; CAD, coronary artery disease; MACE, major adverse cardiovascular event; PVD, peripheral vascular disease.
Frailty and pre-frailty were assessed by the physical frailty phenotype paradigm that is grounded in five criteria: exhaustion, low physical activity, weakness, slowness, and shrinking (www.nhats.org).
Figure 1.
(A) Kaplan–Meier survival curve illustrating major adverse cardiovascular event (MACE)-free over 6-year follow-up by frailty status at baseline in the NHATS-CMS study among patients without a history of coronary heart disease (log-rank P < 0.001). MACE was defined as a composite of all-cause mortality, acute myocardial infarction, stroke, peripheral vascular disease, and subsequent coronary disease. (B) Kaplan–Meier survival curve illustrating MACE2-free over 6-year follow-up by frailty status at baseline in the NHATS-CMS study among patients without a history of coronary heart disease (log-rank P < 0.001). MACE2 was defined as a composite of acute myocardial infarction, stroke, peripheral vascular disease, and subsequent coronary disease. (C) Kaplan–Meier survival curve illustrating the survival over 6-year follow-up by frailty status at baseline in the NHATS-CMS study among patients without a history of coronary heart disease (log-rank P < 0.001). (D) Kaplan–Meier survival curve illustrating acute myocardial infarction-free survival over 6-year follow-up by frailty status at baseline in the NHATS-CMS study among patients without a history of coronary heart disease (log-rank P = 0.003). (E) Kaplan–Meier survival curve illustrating stroke-free survival over 6-year follow-up by frailty status at baseline in the NHATS-CMS study among patients without a history of coronary heart disease (log-rank P < 0.001). (F) Kaplan–Meier survival curve illustrating peripheral vascular disease-free survival over 6-year follow-up by frailty status at baseline in the NHATS-CMS study among patients without a history of coronary heart disease (log-rank P < 0.001).
Table 3.
Proportional hazards regression model evaluating the influence of physical frailty status on 6-year cardiovascular outcomes among older adults without a history of coronary heart disease in the National Health and Aging Trends Study
| MACE 1 | MACE 2 | Death | AMI | Stroke | PVD | CAD | |
|---|---|---|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | |
| Model 1a | |||||||
| Pre-frailty |
1.48 (1.33, 1.64) |
1.42 (1.28, 1.59) |
1.79 (1.49, 2.15) |
1.51 (1.14, 2.01) |
1.32 (1.12, 1.57) |
1.66 (1.41, 1.97) |
1.33 (1.16, 1.53) |
| Frailty |
2.34 (2.06, 2.67) |
2.09 (1.81, 2.40) |
3.70 (3.03, 4.50) |
2.35 (1.65, 3.33) |
2.00 (1.62, 2.46) |
2.53 (2.07, 3.09) |
1.80 (1.51, 2.15) |
| Model 2b | |||||||
| Pre-frailty |
1.47 (1.32, 1.63) |
1.42 (1.27, 1.58) |
1.75 (1.46, 2.11) |
1.46 (1.10, 1.95) |
1.36 (1.15, 1.61) |
1.62 (1.37, 1.91) |
1.32 (1.16, 1.52) |
| Frailty |
2.28 (1.99, 2.61) |
2.05 (1.77, 2.37) |
3.62 (2.95, 4.44) |
2.35 (1.63, 3.38) |
2.11 (1.69, 2.62) |
2.32 (1.88, 2.85) |
1.75 (1.46, 2.10) |
| Model 3c | |||||||
| Pre-frailty |
1.43 (1.28, 1.58) |
1.36 (1.22, 1.52) |
1.78 (1.48, 2.14) |
1.42 (1.06, 1.90) |
1.32 (1.11, 1.56) |
1.56 (1.31, 1.85) |
1.27 (1.11, 1.46) |
| Frailty |
2.15 (1.88, 2.47) |
1.91 (1.65, 2.21) |
3.61 (2.93, 4.43) |
2.20 (1.52, 3.18) |
1.98 (1.58, 2.47) |
2.12 (1.72, 2.62) |
1.65 (1.37, 1.98) |
| Model 4d | |||||||
| Pre-frailty |
1.34 (1.21, 1.49) |
1.29 (1.15, 1.44) |
1.64 (1.36, 1.98) |
1.36 (1.01, 1.82) |
1.25 (1.05, 1.49) |
1.49 (1.25, 1.77) |
1.17 (1.02, 1.35) |
| Frailty |
1.77 (1.53, 2.06) |
1.59 (1.35, 1.87) |
2.70 (2.16, 3.38) |
1.95 (1.31, 2.90) |
1.71 (1.34, 2.17) |
1.80 (1.44, 2.27) |
1.35 (1.11, 1.65) |
MACE1: a composite of acute myocardial infarction, stroke, peripheral vascular disease, coronary artery disease, and all-cause mortality; MACE2: a composite of acute myocardial infarction, stroke, peripheral vascular disease, coronary artery disease.
AMI, acute myocardial infarction; CAD, coronary artery disease; MACE, major adverse cardiovascular event; PVD, peripheral vascular disease.
Model 1 was adjusted for age.
Model 2 was adjusted for age, sex, race/ethnicity, census division, residence, and income.
Model 3 was adjusted for age, sex, race/ethnicity, census division, residence, income, body mass index, smoking status, diabetes, and hypertension.
Model 4 was adjusted for age, sex, race/ethnicity, census division, residence, income, body mass index, smoking status, diabetes, hypertension, dependency, and number of chronic diseases.
Discussion
We examined the association of physical frailty phenotype with cardiovascular outcomes among older adults in the NHATS without prior CHD during the 6-year follow-up. The major findings of this study are as follows: (i) participants without CHD at baseline who exhibit pre-frailty or physical frailty, as measured by the Fried frailty phenotype, had a high prevalence of multiple chronic conditions, baseline disability, mobility disability, and cognitive dysfunction as compared to non-frail CHD participants; (ii) participants with baseline pre-frailty and physical frailty also had higher rates of healthcare utilization with more emergency department visits, admissions to the inpatient service, and longer hospital lengths of stay; and (iii) as compared to non-frail subjects, pre-frail and frail older patients had a higher risk of developing MACE, including mortality during the 6-year follow-up, even after adjusting for demographic characteristics, traditional cardiovascular risk factors, and multimorbidity at baseline (Graphical abstract).
The ageing of the US population and the influence of frailty on the incidence of cardiovascular outcomes in over 6 years of follow-up. (A) Estimates on the projected number (%) of all older adults in 2040 were obtained from www.census.gov. (B) The cumulative incidence of each cardiovascular outcome during 6 years of follow-up was derived from the National Health and Aging and Trends Study.
In this cohort of older adults free of CHD at baseline, we estimated that the prevalence of frailty is ∼16%, which is significantly lower than the prevalence of frailty among patients with preexisting cardiovascular disease.13 , 14 Consistent with our estimates, pooled analysis from 46 studies that enrolled participants with frailty showed that 1 in 6 community-dwelling older adults lives with frailty.15 While the older patients in our study were free of known cardiac disease at baseline, many older adults with frailty frequently have coexisting cardiovascular risk factors. A bidirectional association between frailty and multimorbidity exists, in which the coexistence of these two geriatric syndromes will lead to the progressive worsening of both.16 In a systematic review and meta-analysis of 48 observational studies, 70% of frail older adults examined also had multimorbidity with two or more coexisting conditions.16 In our study, 60% of older patients reported multimorbidity, defined as two or more coexisting chronic medical conditions. Hypertension was the most commonly encountered cardiovascular risk factor and 1 in 5 patients had diabetes mellitus. The burden of these cardiovascular risk factors is clearly higher in the frail, than the non-frailty cohort. Vetrano et al.16 reported the important observation that the vast majority of older adults with frailty are also multimorbid, but very few older adults with multimorbidity are also frail. The authors hypothesize that multimorbidity plays an important deterministic role in the development of frailty syndrome. Frailty and multimorbidity, including hypertension, diabetes mellitus, and other traditional cardiovascular risk factors, can potentially share common pathophysiology mechanisms that put older adults at risk for the development of cardiovascular disease including, inflammation, coagulopathy, and metabolic dysregulation.17
In a retrospective cohort study, middle aged participants from the Civil Service departments in London were examined based on their cardiovascular risk at baseline.18 Those with four different cardiovascular disease risk scores (Framingham cardiovascular disease, Framingham CHD, Framingham stroke, and Systematic Coronary Risk Evaluation) were associated with an elevated risk of frailty, measured using the physical frailty phenotype.18 Data from the British Regional Heart Study19 also showed that older adults frailty in older age was associated with a number of cardiovascular risk factors. Taken together with the results of our study, this highlights the bidirectional association between frailty and cardiovascular disease mentioned previously. In a cross-sectional study, Fernandes et al. 20 investigated the association between frailty, measured by the physical frailty phenotype, and cardiovascular risk measured by the Framingham risk score. The investigators found that frailty and pre-frailty were associated with increased cardiovascular risk. Frailty and cardiovascular disease risk were measured at the same time. Veronese et al.21 evaluated the prognostic value of a multidimensional prognostic index, an instrument grounded in comprehensive geriatric assessment, and self-reported cardiovascular outcomes over 8 years of follow-up. The multidimensional index predicted the onset of cardiovascular disease in community dwellers affected by, or at risk for, osteoarthritis. Our study complements these findings by measuring frailty and pre-frailty, using the Fried physical frailty phenotype, and incidences of the outcomes were ascertained in the CMS database during 6 years of follow-up. The cumulative knowledge continues to highlight the importance of frailty as a risk factor for cardiovascular disease and trigger the need for integration of frailty assessment in the cardiovascular profile of older adults.22 , 23 Similar to our findings, Marinus et al.24 reported a higher prevalence of frailty in older female than male participants. This propensity to frailty in older female patients may have differential power of prediction when compared to older patients at risk for cardiovascular disease. Newman and colleagues examined participants enrolled in the Cardiovascular Health Study and reported that the physical frailty phenotype at baseline was strongly associated with imaging markers of subclinical atherosclerosis including carotid stenosis, impaired ankle-brachial index, and other electro- and echocardiographic variables.25 Progression of these subclinical atherosclerotic cardiovascular conditions to overt clinical events is likely driven by the pathophysiologic mechanisms present in frail older adults including higher oxidative stress26; elevated circulating inflammatory biomarkers including C-reactive protein, and neutrophils, white cell counts, and interleukin-6, and measures of coagulopathy, including D-dimer and fibrinogen.27–32 Prior research has shown that even among patients who meet only one or two of the Fried criteria, also referred to as ‘pre-frail’, there is a higher risk of developing cardiovascular disease after adjustment for traditional risk factors, inflammatory markers, and glycated haemoglobin during a follow-up period of 4.4 years.33 In the NHATS study, we adjusted for baseline demographic variables, BMI, multimorbidity, and other traditional risk factors for cardiovascular disease. Over 6 years of follow-up in the NHATS study, we found that older patients with pre-frailty and frailty exhibit higher incidences of all-cause mortality and MACE, as a composite mainly driven by death, stroke, and peripheral vascular disease. In a meta-analysis that included cross-sectional and prospective cohort studies, Veronese and colleagues23 reported that frailty was associated with an approximate three-fold increased risk of cardiovascular disease when compared to robust patients. To complement these findings, our study population enables the evaluation of frailty in older adults as predictor of MACE in patients with or without previous cardiovascular disease. Efforts to study geriatric syndromes during acute cardiovascular illnesses are well recognized by the cardiovascular community at large,2 , 34 but the ability to integrate the assessment of frailty in the care for older patients at risk for cardiovascular disease is limited because of the lack of efficacious therapies to prevent or reverse the development of physical frailty. However, several initiatives are underway to test the influence of physical activity programs, nutritional interventions, cognitive training, and a combination of these to prevent or reverse frailty in older adults.35 Because cardiovascular disease remains the most common cause of mortality in older adults, efforts to establish the efficacy and safety of such interventions in cardiovascular practice, similar to other therapies targeting traditional cardiovascular risk factors, are needed. The 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation acknowledged the importance of integrating frailty in the management and balancing the risks of each individual treatment (i.e. medical therapy plus invasive strategy) against the risk of harm from not offering therapy given perceived risk of complications.36 Our work underscores the urgent need to establish robust clinical trial data to inform management for older patients with frailty at risk for cardiovascular disease.
This study has potential limitations. First, MACEs were diagnosed using data obtained from the Medicare claims database from hospital and outpatient encounters after the 2011 baseline NHATS visit. While this method of studying cardiovascular outcomes is widely used in health services research, the severity and degree of acute myocardial infarction, stroke, vascular disease, and cardiac-specific mortality could not be ascertained. Despite this limitation, this large study is novel because it is the first to evaluate the temporal relationship between frailty and incident cardiovascular disease during 6 years of follow-up among patients without a history of CHD in the Medicare database. Second, it is plausible that patients with frailty have undiagnosed cardiovascular disease, which in turn led to a higher incidence of cardiovascular events during follow-up. Third, physical frailty was measured at the baseline visit as a binary variable with impairment of three or more domains in the Fried criteria. However, frailty can be a reversible and dynamic physiologic process and may change over time.37 When evaluating the association between physical frailty and cardiovascular outcomes, significant confounding exists, and caution is needed when interpreting the unadjusted estimates presented in Table 3. To provide a comprehensive assessment of the influence of frailty on cardiovascular outcomes, a multivariable Cox regression model, stratified by Cox modelling techniques conditioning on age, and other sensitivity analyses were provided to mitigate the influence of confounding by indication.
Conclusion
In the NHATS study, we found that pre-frailty and physical frailty phenotype are associated with a significant risk for mortality and the development of MACE during 6 years of follow-up, even after controlling for traditional cardiovascular risk factors. Efforts to integrate frailty assessment as part of primary cardiovascular prevention programs in older adults at risk for cardiovascular disease are essential in daily clinical cardiovascular practice. Testing the efficacy and safety of physical activity programs, nutritional interventions, and cognitive training to prevent or reverse physical frailty in patients at risk for the development of cardiovascular disease is needed as the US older adult population expands rapidly in the coming decades.
Supplementary material
Supplementary material is available at European Heart Journal online.
Supplementary Material
Acknowledgements
The authors would like to acknowledge the Jane and Stanley F. Rodbell family for their generous research support aimed at improving outcomes for older Americans living with cardiovascular disease.
Funding
A.A.D. receives research funding from the Pepper Scholars Program of the Johns Hopkins University Claude D. Pepper Older Americans Independence Center funded by the National Institute on Aging P30-AG021334 and mentored patient-oriented research career development award from the National Heart, Lung, and Blood Institute K23-HL153771-01. This study was also funded in part by a research grant from the Jane and Stanley F. Rodbell family in support of Geriatric Cardiology research at Sinai Hospital of Baltimore. G.G. receives funding from the Johns Hopkins University Claude D.l Pepper Older American Independence Center funded by the National Institute on Aging P30-AG021334.
Conflict of interest: The authors declare no conflict of interest.
Data availability
Data cannot be made available for sharing because of a data use agreement between Centers for Medicare & Medicaid Services and Johns Hopkins Center on Aging and Health.
Contributor Information
Abdulla A Damluji, The Inova Center of Outcomes Research, Inova Heart and Vascular Institute, 3300 Gallows Road, I-465, Falls Church, VA 22042, USA; Division of Cardiology, Department of Medicine, Johns Hopkins School of Medicine, Baltimore, MD 21205, USA.
Shang-En Chung, Division of Geriatric Medicine and Gerontology, Department of Medicine, Johns Hopkins School of Medicine, Baltimore, MD 21224, USA.
Qian-Li Xue, Division of Geriatric Medicine and Gerontology, Department of Medicine, Johns Hopkins School of Medicine, Baltimore, MD 21224, USA; Department of Biostatistics, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD 21205, USA.
Rani K Hasan, Division of Cardiology, Department of Medicine, Johns Hopkins School of Medicine, Baltimore, MD 21205, USA.
Mauro Moscucci, Division of Cardiovascular Medicine, Department of Medicine, University of Michigan, Ann Arbor, MI 48109, USA.
Daniel E Forman, Geriatric Cardiology Section, University of Pittsburgh, Pittsburgh, PA, USA; Geriatric Research, Education, and Clinical Center, VA Pittsburgh Healthcare System, Pittsburgh, PA, USA.
Karen Bandeen-Roche, Department of Biostatistics, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD 21205, USA.
Wayne Batchelor, The Inova Center of Outcomes Research, Inova Heart and Vascular Institute, 3300 Gallows Road, I-465, Falls Church, VA 22042, USA.
Jeremy D Walston, Division of Geriatric Medicine and Gerontology, Department of Medicine, Johns Hopkins School of Medicine, Baltimore, MD 21224, USA.
Jon R Resar, Division of Cardiology, Department of Medicine, Johns Hopkins School of Medicine, Baltimore, MD 21205, USA.
Gary Gerstenblith, Division of Cardiology, Department of Medicine, Johns Hopkins School of Medicine, Baltimore, MD 21205, USA.
References
- 1. Walston J, Robinson TN, Zieman S, McFarland F, Carpenter CR, Althoff KN, Andrew MK, Blaum CS, Brown PJ, Buta B, Ely EW, Ferrucci L, High KP, Kritchevsky SB, Rockwood K, Schmader KE, Sierra F, Sink KM, Varadhan R, Hurria A. Integrating frailty research into the medical specialties—report from a U13 conference. J Am Geriatr Soc 2017;65:2134–2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Damluji AA, Forman DE, van Diepen S, Alexander KP, Page RL 2nd, Hummel SL, Menon V, Katz JN, Albert NM, Afilalo J, Cohen MG; American Heart Association Council on Clinical Cardiology and Council on Cardiovascular and Stroke Nursing. Older adults in the cardiac intensive care unit: factoring geriatric syndromes in the management, prognosis, and process of care: a scientific statement from the American Heart Association. Circulation 2020;141:e6–e32. [DOI] [PubMed] [Google Scholar]
- 3. Schaller MS, Ramirez JL, Gasper WJ, Zahner GJ, Hills NK, Grenon SM. Frailty is associated with an increased risk of major adverse cardiac events in patients with stable claudication. Ann Vasc Surg 2018;50:38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kunadian V, Veerasamy M, Sinclair H, Qiu W, Das R, Ahmed J, Purcell I, Edwards R, Zaman A, Bagnall A. 5 major adverse cardiovascular events at 30-days were not significantly different between frail and non-frail older (≥75 years) patients with non ST elevation acute coronary syndrome managed by invasive strategy: an analysis from the ICON1 study. Heart 2015;101:A3–A4. [Google Scholar]
- 5. Campo G, Maietti E, Tonet E, Biscaglia S, Ariza-Solè A, Pavasini R, Tebaldi M, Cimaglia P, Bugani G, Serenelli M, Ruggiero R, Vitali F, Formiga F, Sanchis J, Galvani M, Minarelli M, Lucchi GR, Ferrari R, Guralnik J, Volpato S. The assessment of scales of frailty and physical performance improves prediction of major adverse cardiac events in older adults with acute coronary syndrome. J Gerontol A Biol Sci Med Sci 2020;75:1113–1119. [DOI] [PubMed] [Google Scholar]
- 6. Ewe SH, Ajmone Marsan N, Pepi M, Delgado V, Tamborini G, Muratori M, Ng AC, van der Kley F, de Weger A, Schalij MJ, Fusari M, Biglioli P, Bax JJ. Impact of left ventricular systolic function on clinical and echocardiographic outcomes following transcatheter aortic valve implantation for severe aortic stenosis. Am Heart J 2010;160:1113–1120. [DOI] [PubMed] [Google Scholar]
- 7. Farooqi MAM, Gerstein H, Yusuf S, Leong DP. Accumulation of deficits as a key risk factor for cardiovascular morbidity and mortality: a pooled analysis of 154 000 individuals. J Am Heart Assoc 2020;9:e014686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kasper JD, Freedman VA. National Health and Aging Trends Study User Guide: Rounds 1-8 Final Release. Baltimore: Johns Hopkins University School of Public Health. www.NHATS.org.
- 9. Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56:M146–56. [DOI] [PubMed] [Google Scholar]
- 10. Bandeen-Roche K, Seplaki CL, Huang J, Buta B, Kalyani RR, Varadhan R, Xue QL, Walston JD, Kasper JD. Frailty in older adults: a nationally representative profile in the United States. J Gerontol A Biol Sci Med Sci 2015;70:1427–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rubin D, Multiple imputation for nonresponse in surveys. In: Rubin DB, ed. Multiple Imputation for Nonresponse in Surveys: New York: Wiley; 1987. p1–244. [Google Scholar]
- 12. Galvin JE, Roe CM, Powlishta KK, Coats MA, Muich SJ, Grant E, Miller JP, Storandt M, Morris JC. The AD8: a brief informant interview to detect dementia. Neurology 2005;65:559–564. [DOI] [PubMed] [Google Scholar]
- 13. Damluji AA, Huang J, Bandeen-Roche K, Forman DE, Gerstenblith G, Moscucci M, Resar JR, Varadhan R, Walston JD, Segal JB. Frailty among older adults with acute myocardial infarction and outcomes from percutaneous coronary interventions. J Am Heart Assoc 2019;8:e013686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Damluji AA, Chung SE, Xue QL, Hasan RK, Walston JD, Forman DE, Bandeen-Roche K, Moscucci M, Batchelor W, Resar JR, Gerstenblith G. Physical frailty phenotype and the development of geriatric syndromes in older adults with coronary heart disease. Am J Med 2021;134:662–671.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ofori-Asenso R, Chin KL, Mazidi M, Zomer E, Ilomaki J, Zullo AR, Gasevic D, Ademi Z, Korhonen MJ, LoGiudice D, Bell JS, Liew D. Global incidence of frailty and prefrailty among community-dwelling older adults: a systematic review and meta-analysis. JAMA Netw Open 2019;2:e198398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vetrano DL, Palmer K, Marengoni A, Marzetti E, Lattanzio F, Roller-Wirnsberger R, Lopez Samaniego L, Rodríguez-Mañas L, Bernabei R, Onder G; Joint Action ADVANTAGE WP4 Group. Frailty and multimorbidity: a systematic review and meta-analysis. J Gerontol A Biol Sci Med Sci 2019;74:659–666. [DOI] [PubMed] [Google Scholar]
- 17. Walston J, McBurnie MA, Newman A, Tracy RP, Kop WJ, Hirsch CH, Gottdiener J, Fried LP, Cardiovascular Health S. Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: results from the Cardiovascular Health Study. Arch Intern Med 2002;162:2333–2341. [DOI] [PubMed] [Google Scholar]
- 18. Bouillon K, Batty GD, Hamer M, Sabia S, Shipley MJ, Britton A, Singh-Manoux A, Kivimäki M. Cardiovascular disease risk scores in identifying future frailty: the Whitehall II prospective cohort study. Heart 2013;99:737–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ramsay SE, Arianayagam DS, Whincup PH, Lennon LT, Cryer J, Papacosta AO, Iliffe S, Wannamethee SG. Cardiovascular risk profile and frailty in a population-based study of older British men. Heart 2015;101:616–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fernandes J, Gomes C. D S, Guerra RO, Pirkle CM, Vafaei A, Curcio C-L, Dornelas de Andrade A. Frailty syndrome and risk of cardiovascular disease: analysis from the International Mobility in Aging Study. Arch Gerontol Geriatr 2021;92:104279. [DOI] [PubMed] [Google Scholar]
- 21. Veronese N, Koyanagi A, Smith L, Musacchio C, Cammalleri L, Barbagallo M, Pilotto A. Multidimensional frailty increases cardiovascular risk in older people: an 8-year longitudinal cohort study in the Osteoarthritis Initiative. Exp Gerontol 2021;147:111265. [DOI] [PubMed] [Google Scholar]
- 22. Uchikado Y, Ikeda Y, Ohishi M. Current understanding of the role of frailty in cardiovascular disease. Circ J 2020;84:1903–1908. [DOI] [PubMed] [Google Scholar]
- 23. Veronese N, Cereda E, Stubbs B, Solmi M, Luchini C, Manzato E, Sergi G, Manu P, Harris T, Fontana L, Strandberg T, Amieva H, Dumurgier J, Elbaz A, Tzourio C, Eicholzer M, Rohrmann S, Moretti C, D'Ascenzo F, Quadri G, Polidoro A, Lourenço RA, Moreira VG, Sanchis J, Scotti V, Maggi S, Correll CU. Risk of cardiovascular disease morbidity and mortality in frail and pre-frail older adults: results from a meta-analysis and exploratory meta-regression analysis. Ageing Res Rev 2017;35:63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Marinus N, Vigorito C, Giallauria F, Haenen L, Jansegers T, Dendale P, Feys P, Meesen R, Timmermans A, Spildooren J, Hansen D. Frailty is highly prevalent in specific cardiovascular diseases and females, but significantly worsens prognosis in all affected patients: a systematic review. Ageing Res Rev 2021;66:101233. [DOI] [PubMed] [Google Scholar]
- 25. Newman AB, Gottdiener JS, McBurnie MA, Hirsch CH, Kop WJ, Tracy R, Walston JD, Fried LP; Cardiovascular Health Study Research Group. Associations of subclinical cardiovascular disease with frailty. J Gerontol A Biol Sci Med Sci 2001;56:M158–M166. [DOI] [PubMed] [Google Scholar]
- 26. Inglés M, Gambini J, Carnicero JA, García-García FJ, Rodríguez-Mañas L, Olaso-González G, Dromant M, Borrás C, Viña J. Oxidative stress is related to frailty, not to age or sex, in a geriatric population: lipid and protein oxidation as biomarkers of frailty. J Am Geriatr Soc 2014;62:1324–1328. [DOI] [PubMed] [Google Scholar]
- 27. Walker KA, Walston J, Gottesman RF, Kucharska-Newton A, Palta P, Windham BG. Midlife systemic inflammation is associated with frailty in later life: the ARIC study. J Gerontol A Biol Sci Med Sci 2019;74:343–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Baylis D, Bartlett DB, Syddall HE, Ntani G, Gale CR, Cooper C, Lord JM, Sayer AA. Immune-endocrine biomarkers as predictors of frailty and mortality: a 10-year longitudinal study in community-dwelling older people. Age (Dordr) 2013;35:963–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gale CR, Baylis D, Cooper C, Sayer AA. Inflammatory markers and incident frailty in men and women: the English Longitudinal Study of Ageing. Age (Dordr) 2013;35:2493–2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Barzilay JI, Blaum C, Moore T, Xue QL, Hirsch CH, Walston JD, Fried LP. Insulin resistance and inflammation as precursors of frailty: the Cardiovascular Health Study. Arch Intern Med 2007;167:635–641. [DOI] [PubMed] [Google Scholar]
- 31. Puts MT, Visser M, Twisk JW, Deeg DJ, Lips P. Endocrine and inflammatory markers as predictors of frailty. Clin Endocrinol (Oxf) 2005;63:403–411. [DOI] [PubMed] [Google Scholar]
- 32. Maggio M, Guralnik JM, Longo DL, Ferrucci L. Interleukin-6 in aging and chronic disease: a magnificent pathway. J Gerontol A Biol Sci Med Sci 2006;61:575–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sergi G, Veronese N, Fontana L, Rui MD, Bolzetta F, Zambon S, Corti MC, Baggio G, Toffanello ED, Crepaldi G, Perissinotto E, Manzato E. Pre-frailty and risk of cardiovascular disease in elderly men and women: the Pro.V.A. study. J Am Coll Cardiol 2015;65:976–983. [DOI] [PubMed] [Google Scholar]
- 34. Rich MW, Chyun DA, Skolnick AH, Alexander KP, Forman DE, Kitzman DW, Maurer MS, McClurken JB, Resnick BM, Shen WK, Tirschwell DL. Knowledge gaps in cardiovascular care of older adults: a scientific statement from the American Heart Association, American College of Cardiology, and American Geriatrics Society: executive summary. J Am Geriatr Soc 2016;64:2185–2192. [DOI] [PubMed] [Google Scholar]
- 35. Marcucci M, Damanti S, Germini F, Apostolo J, Bobrowicz-Campos E, Gwyther H, Holland C, Kurpas D, Bujnowska-Fedak M, Szwamel K, Santana S, Nobili A, D'Avanzo B, Cano A. Interventions to prevent, delay or reverse frailty in older people: a journey towards clinical guidelines. BMC Med 2019;17:193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Collet J-P, Thiele H, Barbato E. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: the Task Force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2020;42:1289–1367. [DOI] [PubMed] [Google Scholar]
- 37. Kitzman DW, Whellan DJ, Duncan P, Pastva AM, Mentz RJ, Reeves GR, Nelson MB, Chen H, Upadhya B, Reed SD, Espeland MA, Hewston L, O’Connor CM. Physical rehabilitation for older patients hospitalized for heart failure. N Engl J Med 2021. doi:10.1056/NEJMoa202141. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data cannot be made available for sharing because of a data use agreement between Centers for Medicare & Medicaid Services and Johns Hopkins Center on Aging and Health.