Summary
A series of N-alkyl-substituted polybenzimidazoles (SPBIs), synthesized by simple condensation and N-alkylation, act as functional materials with tunable microstructures and sensing performance. For their controllable morphologies, the formation of nano-/microspheres is observed at the n(RBr)/n(PBI) feed ratio of 5:1. Products with different degrees of alkylation can recognize metal ions and nitroaromatic compounds (NACs). For example, SPBI-c, obtained at the feed ratio of 1:1, can selectively detect Cu2+, Fe3+, and NACs. By contrast, SPBI-a, obtained at the feed ratio of 0.1:1, can exclusively detect Cu2+ with high sensitivity. Their sensing mechanisms have been studied by FT-IR spectroscopy, SEM, XPS, and DFT calculations. Interestingly, the SPBIs can adsorb Cu2+ in solution and show good recyclability. These results demonstrate that polymeric materials with both sensing and adsorption applications can be realized by regulating the alkylation extent of the main chain, thus providing a new approach for the facile synthesis of multifunctional materials.
Subject areas: organic chemistry, Photonics, polymers, Optical materials
Graphical abstract
Highlights
-
•
Tunable multifunctional sensor materials were synthesized by simple reaction
-
•
Morphology and sensing property can be controlled by the extent of N-alkylation
-
•
SPBIs can selectively and sensitively detect multiple analytes by dual channels
-
•
SPBIs can reversibly and efficiently adsorb Cu2+ as indicated with UV-vis signal
Organic chemistry; Photonics; Polymers; Optical Materials
Introduction
The multianalyte detection concept, which was first proposed by De Silva's group (Magri et al., 2006; Schmittel and Shu, 2012; Chen et al., 2015; Magri, 2021), has become a hotspot in the field of sensors owing to its advantages of high efficiency, rapidity, simultaneous recognition and in-situ detection (Xu et al., 2017; Park et al., 2018; Zhang et al., 2018; Chen et al., 2019a; Zhao et al., 2021; Slenders et al., 2021; Huang et al., 2021b; Nakamitsu et al., 2021). Multianalyte sensors were originally designed for multiple metal ions (Magri et al., 2006), but these systems have subsequently been developed for multiple bioactive molecules (Yin et al., 2018; Zhang et al., 2020b; Thanzeel et al., 2020), bacteria (Zheng et al., 2018), etc. In some cases, multifunctional sensors based on fluorescent gels (Zhang et al., 2018; Ozay et al., 2020) or polymers (Ozay et al., 2020; Liu et al., 2020; Huang et al., 2021a) can simultaneously remove analytes, thus reducing the cost of pollutant treatment in practical applications. However, there are still some challenges in the field of multifunctional sensor materials. For example, when recognizing multiple analytes, the sensitivity of sensor may be reduced to some extent and it is slightly lower than that of a traditional sensor (Schmittel and Shu, 2012; Lochman et al., 2019). Moreover, there is an urgent need to develop a simple, universal method for the preparation of multifunctional sensing materials.
Benzoxazole materials, which show eye-catching fluorescent properties, are not limited to the field of covalent organic frameworks (COFs) (Seo et al., 2019). For example, small benzimidazole fluorescent molecules can sensitively and rapidly detect various analytes through different interactions (Wu et al., 2016, 2018; Ge et al., 2018; Jiang et al., 2019; Chen et al., 2020b). However, the small adsorption capacities of these materials hinder adsorption applications, and thus polymeric or other macromolecular materials are required to achieve simultaneous analyte removal by benzoxazole-based sensors (Rabbani et al., 2017; Lv et al., 2021). Although polybenzimidazole (PBI) has been widely applied in a variety of areas (Liang et al., 2019; Geng et al., 2019; Wang et al., 2019; Shan et al., 2019; Cui et al., 2021; Jin et al., 2021), there are only a few reports on sensing (Park and Gong, 2017; Diao et al., 2018; Kaur et al., 2019) owing to the disadvantages of unmodified PBI, including poor solubility, weak fluorescence, and difficulties in achieving uniform dispersion in sensing systems. We speculated that the introduction of flexible chains into the backbone of PBI through simple N-alkylation would weaken π-π stacking between the polymer chains, thus reducing aggregation-caused quenching phenomena and improving the fluorescence properties of PBI. Moreover, the introduction of alkyl chains could improve the solubility of PBI, which is beneficial for multifunctional sensing (Figure 1).
Figure 1.
Construction of a multifunctional polymeric sensor based on substituted polybenzimidazole (SPBI)
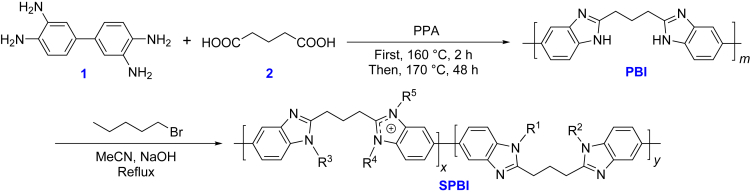
Herein, we report the design and synthesis of a series of substituted polybenzimidazole (SPBI) sensing materials via a metal-free catalytic route (Scheme 1) as well as the applications of these materials. PBI with a linear framework was constructed by dehydration condensation between 3,3′-diaminobenzidine (1) and glutaric acid (2), a dicarboxylic fatty acid. Then, a series of SPBIs with tunable microstructures and sensing performance was obtained by further modification of PBI via simple N-alkylation at different feed ratios (Table 1).
Scheme 1.
Synthetic route for substituted polybenzimidazole (SPBI)
Table 1.
Effect of feed ratio on the basic structure of SPBI
| Sample | Feed ratio (RBr/PBI) | R1 | R2 | R3 | R4 | R5 |
|---|---|---|---|---|---|---|
| SPBI-a | 0.1:1 | Pentyl- | H | H | H | – |
| SPBI-b | 0.5:1 | Pentyl- | H | H | H | – |
| SPBI-c | 1:1 | Pentyl- | Pentyl- | Pentyl- | H | – |
| SPBI-d | 2:1 | Pentyl- | Pentyl- | Pentyl- | H | – |
| SPBI-e | 3:1 | Pentyl- | Pentyl- | Pentyl- | H | – |
| SPBI-f | 4:1 | Pentyl- | Pentyl- | Pentyl- | Pentyl- | Pentyl- |
| SPBI-g | 5:1 | Pentyl- | Pentyl- | Pentyl- | Pentyl- | Pentyl- |
Results and discussion
Characterization and basic properties of SPBIs
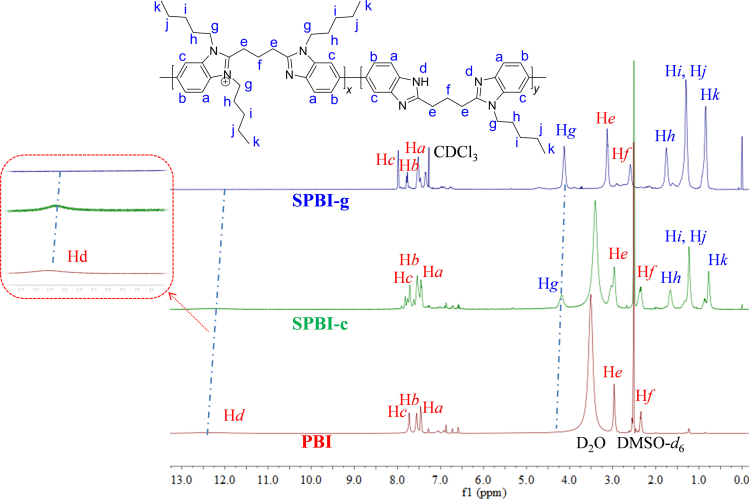
The structures of materials were systematically characterized by 1H NMR (Figures S1–S9 and Table S1), FT-IR spectroscopy (Figure S10 and Table S2), PXRD, and XPS. In 1H NMR spectrum of PBI, the chemical shifts at 7.41–7.48, 7.51–7.61, and 7.65–7.82 ppm were assigned to aromatic hydrogens (Ha, Hb, and Hc) in the repeating unit, and that at 12.35 ppm was assigned to -NH- (Hd) in the benzimidazole ring. Further, the chemical shifts at 2.31–2.36 and 2.92–2.98 ppm corresponded to the alkyl segment (Hf and He) in the repeating unit of PBI. The signals for -CH2- at the end of polymer chain (1.20–1.50 and 2.54–2.63 ppm; Figure S1) were used to estimate the number-average molecular weight (Mn), and the results are summarized in Table S3. Here, we discuss the results for SPBI-c and SPBI-g (Table 1) as representative examples [see the Supplemental Information for further characterization data and spectra of PBI and SPBIs (Figures S1–S8 and Table S1)]. New characteristic signals, including those at 0.69–0.90 ppm (Hk), 1.10–1.32 ppm (Hi and Hj), 1.57–1.75 ppm (Hh), and 4.28 ppm (Hg), were observed in SPBI prepared by the N-alkylation of PBI (Figures 2 and S9). Moreover, the N-H (Hd) signal was weakened gradually, indicating the successful alkylation of PBI.
Figure 2.
1H NMR spectra of PBI, SPBI-c, and SPBI-g
In the FT-IR spectra, SPBI samples exhibited similar characteristic peaks (Figure S10 and Table S2), with a gradual enhancement of the stretching vibrations (at 2957, 2931, and 2855 cm−1) of saturated C-H and its bending vibration (at 1414 cm−1), and the vibrations of the -CH2- groups in alkyl segments (at 724 cm−1) as the proportion of C5H11Br increased. These changes reveal an increase in the alkylation ratio of SPBI.
The XPS spectra of SPBI products were measured according to a previously reported method (Yu et al., 2019; Li et al., 2021). As shown in Figure S11A, the peaks located at 285.1, 397.6, and 528.1 eV were attributed to C1s, N1s, and O1s, respectively, in the backbone of SPBI-c. Moreover, the high-resolution C1s spectrum showed four peaks at 284.6, 285.1, 285.5, and 286.2 eV (Figure S11B), assigned to C-C/C=C, C-N/C-O, C=N, and C=O, respectively, in the terminal group of SPBI-c. The N1s spectrum could be divided into two peaks at 399.9 and 401.6 eV (Figure S11C), corresponding to C-N and C=N, respectively (Jiao et al., 2019; Li et al., 2019b). Thus, the XPS data further confirm the construction of a polymer skeleton.
Using reported methods (Geng et al., 2019; Zhang et al., 2019a, 2019b; To et al., 2020; Fan et al., 2021), the crystallinity and thermal stability of each material were investigated. As depicted in Figure S12, the PXRD pattern of PBI exhibited a broad peak at 15°–35°, indicating an amorphous state. This broad peak remained after alkylation, demonstrating that the SPBI materials are also amorphous. The thermal stabilities of the SPBI were investigated under a N2 atmosphere. As shown in Figure S13, after being modified by alkyl chains, the SPBI began to decompose in the range of 416.6–419.8°C, with the termination of thermal weight loss occurring at 470.0–477.4°C (Table S4), demonstrating the good thermal stability of the SPBIs.
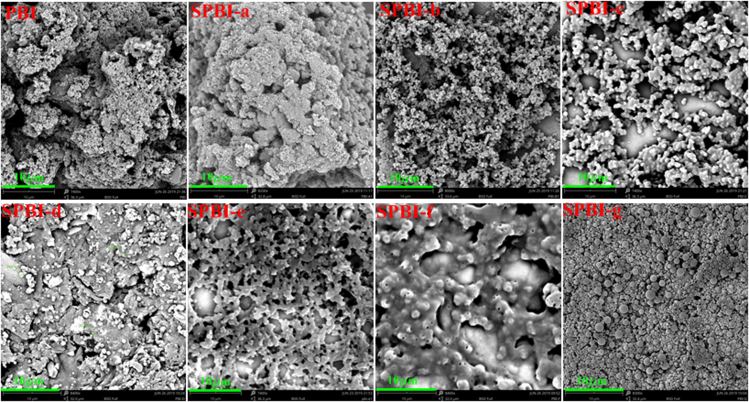
Importantly, the morphologies of materials were evaluated by SEM as reported methods (Shan et al., 2019; Zhang et al., 2019a; To et al., 2020; Fan et al., 2021) (Figure 3). The surface of PBI was uneven and loose powder with stacked micropores was located between the layers. SPBI-a, prepared using an n(C5H11Br)/n(PBI) feed ratio of 0.1:1, showed a morphology similar to that of PBI. In contrast, the morphology of SPBI-b (feed ratio of 0.5:1) began to change into small particles, indicating the effective modification with alkyl chains. Furthermore, SPBI-c (feed ratio of 1:1) formed a network structure. Some small balls were observed in SPBI-d (feed ratio of 2:1) and a regular network was observed for SPBI-e (feed ratio of 3:1). Further increasing the feed ratio to 5:1 (SPBI-g) resulted in the formation of some regular nanospheres, owing to ionization during the N-alkylation of PBI. This salt-type product was affected by electrostatic repulsion to form nanoparticles. Thus, not only the extent of PBI alkylation but also the morphology of the alkylated products can be adjusted by using the feed ratio of the reactants. In particular, nanoparticles can be formed at a high feed ratio when increasing n(C5H11Br).
Figure 3.
SEM images of SPBIs prepared using different feed ratios
Obviously, the N-alkylation of PBI is random and heterogeneous, but this randomness at different feed ratios automatically regulates the polymer structure due to the existence of the steric hindrance. Therefore, for the preparation process of SPBI, it is very simple to wholly regulate the structures and properties of the polymer. And the following researches on the performance and application of SPBI further prove this design.
The photophysical properties of serial SPBIs
The physical and spectral properties of SPBIs were investigated. The solubility was improved by the introduction of alkyl, as expected. The SPBIs could be dissolved in various common organic solvents, such as dichloromethane (DCM), EtOH, and N,N-dimethyl formamide (DMF). The extent of alkylation for the SPBIs is summarized in Table S3. As the feed ratio increased, the alkylation rate and yield increased gradually. When the feed ratio was 4:1, ionization began to occur (Table 1). The accompanying slight color change from gray to brown-red (Table S3) implied that the extent of alkylation may have an effect on the spectral properties of SPBIs.
The UV-vis and fluorescence spectra of PBI and SPBIs were explored in dimethyl sulfoxide (DMSO) or DMF (with increased alkylation, the solubility of the SPBIs in polar DMSO decreases; therefore, DMF was used as the solvent for SPBI-f and SPBI-g) (Ge et al., 2018; Chen et al., 2020b). As shown in Figure S14, the UV-vis absorption spectra of SPBIs were similar, with absorption peaks located at approximately 300 nm. The shoulder peak observed at 265 nm was mainly caused by the π-π∗ electronic transition on the conjugated skeleton. As the degree of alkylation increased, the absorbance of SPBIs decreased slightly. The fluorescence of PBI was so weak under the excitation of 328 nm and there was an obvious enhancement for the fluorescence intensity of SPBIs with the emission peaks red-shifted to approximately 447 nm at the same test conditions. The extent of alkylation had little effect on the emission peak position but changed the fluorescence intensity. The Stokes shifts of SPBI-a ∼ SPBI-g were in the range of 56–87 nm, and the relative fluorescence quantum yields were 34.1%, 31.6%, 32.7%, 43.6%, 28.9%, 50.7%, and 51.2%, respectively. The enhancement of fluorescence might be because of the decrease in π-π interaction between the benzimidazole units in the backbone of PBI (Yang et al., 2017; Xie et al., 2017).
According to the SEM analysis mentioned above, the morphologies of SPBI-c (network structure) and SPBI-g (nano-spheres) were relatively regular. Therefore, they were selected as representative compounds for evaluating the UV-vis and fluorescence spectra in THF, MeCN, EtOH, DMF, and DMSO. As shown in Figures S15 and S16, the UV-vis absorption spectra of SPBI-c and SPBI-g in various solvents showed slight differences. In particular, the absorbance of SPBI-c was largest in DMSO, and an absorption tail appeared in EtOH. For SPBI-g, an absorption tail was observed in MeCN. A comparison of the fluorescence spectra in different solvents showed that the fluorescence intensities of SPBI-c and SPBI-g were relatively strong in DMF and DMSO.
The N atoms in the SPBIs can easily coordinate metal ions, and the products with lower degrees of alkylation (e.g., SPBI-a) have strong π-π interactions and short distances between the polymer chains. Therefore, only metal ions with a suitable size can enter the framework, promoting interactions between analytes and the polymeric sensor. As the extent of alkylation increases, the π-π interactions decrease, accompanying the increase of the distance between the polymer chains. These may affect the interaction of metal ions with SPBIs and will be beneficial for interactions between the SPBIs and nitro-aromatic compounds (NACs) with the larger molecular sizes (Dou et al., 2014; Zeng et al., 2016). To explore the influence of alkylation degree, SPBI-a, SPBI-b, SPBI-c, and SPBI-g were used as representative compounds to investigate the sensing performance.
Sensing performance of SPBIs toward metal ions
Metal ions (e.g., Cu2+) play vital roles in human physiological processes. However, trace metal ions can be amplified through the food chain owing to the non-degradability of metal ions and their accumulation in organisms (Chen et al., 2021). As excess amounts of some metal ions in the body may cause various diseases (Jiang et al., 2018; Pang et al., 2019; Khairy and Duerkop, 2019), it is necessary to develop new sensor materials for the convenient detection of metal ions. The selectivity of SPBI-a (6.0% alkylation) for 16 metal ions was investigated in the DMSO/H2O system.
As shown in Figure 4, the UV-vis absorption peak of SPBI-a was located at 303 nm with a shoulder peak at 265 nm. When Cu2+ was added, the absorbance of the sensing system at 303 nm decreased slightly, and the absorbance at 275 and 350–800 nm increased significantly, causing the solution color change from colorless to indigo (Figure 4C). This result indicated that SPBI-a shows a colorimetric response to Cu2+. To rule out that this change was due to the color of Cu2+ itself, the UV-vis spectrum of an aqueous solution containing only the same amount of Cu2+ was collected. The aqueous solution containing only Cu2+ had an absorbance of less than 0.5 and appeared to be colorless. As shown in Figure 4B, the absorbance of the sensing system at 605 nm increased obviously with the addition of Cu2+, and the absorption of the system at 605 nm was unchanged with the addition of Fe3+. The other metal ions also had few effects on the systems. Therefore, SPBI-a can be used as a Cu2+ colorimetric sensor.
Figure 4.
UV-vis absorption spectra, absorbance changes at 605 nm, and color changes of SPBI-a (DMSO/H2O = 99:1, v/v) after the addition of various metal ions (50 μM)
(A) UV-vis absorption spectra changes.
(B) Absorbance changes at 605 nm.
(C) Color changes.
Interference experiments demonstrated that the UV-vis spectra of this system were not affected significantly by other ions, with the exception of Fe3+, which had only a small effect (Figure S17), indicating the good anti-interference ability of SPBI-a in response to Cu2+. Furthermore, the interaction between SPBI-a and Cu2+ was explored. As shown in Figure S18, with an increase in the Cu2+ concentration, the absorbance of SPBI-a at 303 nm decreased accompanied by a slight of redshift, and the absorbance at 250–280 and 350–800 nm increased gradually, resulting in a change in the solution color from colorless to blue. These observations indicate that there is a strong interaction between SPBI-a and Cu2+ (Aysha et al., 2019). Importantly, the system gradually reached a saturated state when the concentration of Cu2+ is approximately 80 μM. Using a reported method (Kumar and Chae, 2019; Chen et al., 2019a, 2019b; Zhao et al., 2019), the LOD was calculated as 8.76 × 10−7 M (Figure S19), which is equivalent to the sensitivity of some Cu2+ sensors reported recently (Table S6) (Han et al., 2019; Wu et al., 2020b; Wei et al., 2020).
Using reported methods (Imase et al., 2003; Kim et al., 2013), the sensing performance of SPBI materials obtained at other feed ratios were also investigated. For example, using UV-vis absorption spectroscopy, SPBI-b was found to selectively recognize two metal ions (Cu2+ and Fe3+) with LODs of 4.50 × 10−7 and 1.49 × 10−8 M, respectively (Figures S20–S26). With increased alkylation, the distance between the polymer chains might increases and the interactions of the SPBI with analytes might be affected, resulting in changes in sensitivity (Tables S6 and S7). SPBI-c (28.5% alkylation) and SPBI-g (65.0% alkylation) could also discern Cu2+ and Fe3+ but with different sensitivities (Figures S27–S39 and further discussions can be seen in the SI). The signals of SPBI-c were more sensitive to Cu2+ than those of SPBI-g, and its sensitivity was also better than those of some reported metal ion sensors (Table S6) (Kim et al., 2013; Zhang et al., 2020a; Du et al., 2020). For Fe3+, the sensitivity of SPBI-c was also higher than that of SPBI-g as well as those of some reported Fe3+ sensors (Table S7) (Li et al., 2019a, 2019b; Fan et al., 2020; Cui et al., 2020; Zheng et al., 2020; Shia et al., 2020; Jia et al., 2020; Sun et al., 2020). Therefore, the sensing performance of the SPBI is controllable, and the most sensitive material must have a suitable size for metal ions to coordinate with N atoms in SPBI. Herein, SPBI-c obtained at n(C5H11Br)/n(PBI) = 1:1 was found to exhibit the best performance.
Application of SPBIs in cyclic adsorption for Cu2+
At present, there are many types of materials used for adsorption. Especially, recycling these adsorption materials can save the cost of materials to a large extent and is conducive to environmental protection (Liu et al., 2020, 2021; Huang et al., 2021a; Wang et al., 2020b; Chen et al., 2020a), especially for multifunctional material (Chen et al., 2021). Using Cu2+ as an example, the cyclic adsorption application of SPBIs was investigated (Figure S40). Treatment with HCl (pH = 2), EDTA solution, and deionized water promoted Cu2+ desorption from the SPBIs, allowing these materials to be used for the reversible adsorption of Cu2+ under the indication by UV-vis absorbance spectra. The adsorption rates and adsorption capacities are summarized in Table S5.
Owing to the small distance between the polymer chains of SPBI-a with a low alkylation degree, Cu2+ could not easily enter into the polymer framework to coordinate (Imase et al., 2003), but the surface adsorption still occurred. The initial Cu2+ adsorption rate was only 75.07% with poor recyclability. For SPBI-g with a high alkylation degree, the large distance between polymer chains was also not conducive to interactions with metal ions (Imase et al., 2003; Kim et al., 2013), resulting in an adsorption performance similar to that of SPBI-a.
Interestingly, for SPBI-c, the moderate alkylation degree provided a suitable distance between polymer chains that allowed Cu2+ to enter into the framework and coordinate with N atoms, resulting in good adsorption performance with an initial Cu2+ adsorption rate of 96.81%. Moreover, this material maintained an adsorption rate of more than 80% in five cycles (Figure 5). Importantly, the recycling performance of SPBI was similar to those of some reported reusable adsorption materials (Table S9) (Liu et al., 2020; Huang et al., 2021a; Wang et al., 2020b; Chen et al., 2020a).
Figure 5.
Reversible adsorption for Cu2+ by SPBI-c
Sensing performance of SPBIs toward NACs
NACs are important raw materials for some blasting equipment and in the leather industry. Owing to the significant impact of these compounds on public safety, human life, and property, the trace detection of NACs is also a hot topic in the sensor field (Chen et al., 2020c; Wang et al., 2019; Zhuang et al., 2020b; Kasthuri et al., 2019). When the extent of alkylation increases, the π-π interactions in SPBIs decrease, accompanying by an increase in distance between polymer chains (Imase et al., 2003; Kim et al., 2013) These may be beneficial to interactions between C=N in SPBI and NACs with the larger molecular sizes. Thus, SPBI might be applicable to the recognition of electron-deficient NACs, and the selectivity of SPBI-c (28.5% alkylation) toward different NACs (the structures are shown in Figure S41) was tested.
SPBI-c was found to selectively detect PA, DNP, and NP by fluorescence quenching (Figure S42). Using a reported method (Jiao et al., 2019; Zhang et al., 2019a), the fluorescence quenching efficiencies of three typical NACs (PA, DNP, and NP) for SPBI-c were studied (Figure 6 and S43). With 160 μM PA, a maximum quenching efficiency of 96.8% was obtained, whereas higher concentrations of DNP and NP were required to achieve similar quenching efficiencies, demonstrating the high sensitivity of SPBI-c to PA. Further-more, using a reported approach (Gao et al., 2019; Nandi et al., 2020), the LODs of SPBI-c for PA, DNP, and NP were calculated to be 1.81 × 10−7, 2.29 × 10−7 and 2.62 × 10−7 M, respectively, which are equivalent to the LODs of some reported NAC sensors (Gao et al., 2019; Nandi et al., 2020; Ghorai et al., 2019; Dutta et al., 2020).
Figure 6.
Fluorescence spectra, linear plot, and Stern-Volmer plot for SPBI-c (DMSO/H2O = 99:1, V/V) upon the addition of different concentrations of PA
(A) Fluorescence spectra for SPBI-c with the addition of PA (λex = 331 nm).
(B) Linear plot for SPBI-c with the addition of PA.
(C) Stern-Volmer plot for SPBI-c with the addition of PA
The sensitivity of fluorescence-quenching sensors to analytes can also be judged from the Stern-Volmer (S-V) constant, Ksv, which can be calculated as follows: I0/I = 1 + Ksv[Q]. As shown in Figures 6C and S43C, for PA, DNP, or NP, the S-V plot curved upward at higher concentrations, indicating that this process involves a combination of dynamic and static quenching (Goswami et al., 2019; Rajak et al., 2019). In the low concentration range, the S-V plot is linear, and the Ksv values for SPBI-c toward PA, DNP, and NP were 6.5631 × 104, 4.5122 × 104, and 3.8651 × 104 M−1, respectively. These Ksv values are higher than those of the most reported fluorescent polymer sensors for NACs detection (Ghorai et al., 2019; Dutta et al., 2020).
Moreover, SPBI-g was also found to sensitively detect PA, DNP, and NP (Figure S44), with LODs of 1.68 × 10−7, 1.93 × 10−7, and 2.15 × 10−7 M, respectively (Figures S45 and S46), and Ksv values of 3.75 × 104, 3.85 × 104, and 2.68 × 104 M−1, respectively (Figure S47). The sensitivities of SPBI-g and SPBI-c to PA, DNP, and NP were on the same order of magnitude, but SPBI-g showed higher sensitivity for the detection of PA (Chen et al., 2020c; Wu et al., 2020a) (for a detailed comparison, see Table S8).
Sensing mechanism of SPBIs for multianalyte
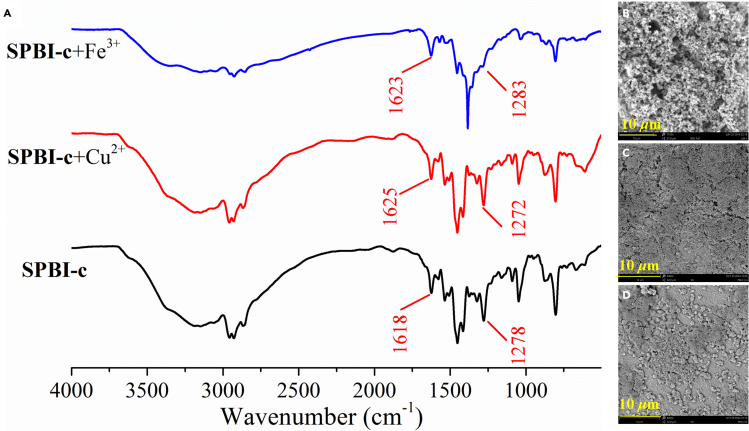
According to the reported method (Li et al., 2019b; Zhang et al., 2019b; Chen et al., 2021; Chen et al., 2020c; Wang, et al., 2020), the effects of analytes on the FT-IR spectra and morphology of SPBIs were determined. Using SPBI-c as a representative compound, the changes in FT-IR spectra and SEM images before and after the addition of Cu2+ or Fe3+ were observed. As depicted in Figure 7, the stretching vibration of C-N and C=N in SPBI-c are located at 1278 and 1618 cm−1, respectively. After the addition of Cu2+, these stretching vibrations moved to 1272 and 1625 cm−1, respectively, indicating an interaction between the N atoms in SPBI-c and Cu2+ (Wu et al., 2016; Li et al., 2019b). Similarly, these stretching vibrations also moved after the addition of Fe3+ to SPBI-c. In addition, the morphology of SPBI-c changed from a network structure to a dense honeycomb structure, confirming an interaction between SPBI-c and metal ions (Zeng et al., 2016). The interactions of SPBI-a (Figure S48), SPBI-b (Figure S49), SPBI-c (Figure S50), and SPBI-g (Figures S51 and S52) with different analytes (metal ions and NACs) were also examined by FT-IR spectroscopy and SEM.
Figure 7.
FT-IR spectra, SEM images of SPBI-c before and after the addition of Cu2+ or Fe3+
(A) FT-IR spectra of SPBI-c before and after the addition of Cu2+ or Fe3+.
(B) SEM images of SPBI-c.
(C) SEM images of SPBI-c after the addition of Cu2+.
(D) SEM images of SPBI-c after the addition of Fe3+.
In particular, for the detection of NACs, the quenching process at low concentrations can be determined from the fluorescence lifetime changes (Figure S53) (Kasthuri et al., 2019; Zhuang et al., 2020b). The fluorescence lifetime decay curves of SPBI-c and SPBI-g were less affected by NACs. Therefore, the quenching of NACs in the low concentration range can be considered a static quenching process (Jiang et al., 2019; Chen et al., 2020c).
In addition, the interactions between polymeric sensors and analytes can be measured by XPS analysis (Li et al., 2019b; Zhao et al., 2019; Wang et al., 2020c). Using SPBI-c as an example, the C1s and N1s binding energies in the system after the addition of Cu2+, Fe3+, and PA were determined. As shown in Figures 8A and 8D, upon the addition of Cu2+, the C1s binding energy of C=N in SPBI-c moved from 286.1 to 286.6 eV, and the N1s binding energy moved from 401.6 to 402.3 eV. These changes demonstrate the presence of a coordination effect between Cu2+ and C=N in SPBI-c (Li et al., 2019b). Similar interactions were confirmed between SPBI-c and Fe3+ (Figures 8B and 8E) or PA (Figures 8C and 8F).
Figure 8.
C1s and N1s XPS spectra of SPBI-c after the addition of Cu2+, Fe3+, and PA
(A) C1s XPS spectra of SPBI-c after the addition of Cu2+.
(B) C1s XPS spectra of SPBI-c after the addition of Fe3+.
(C) C1s XPS spectra of SPBI-c after the addition of PA.
(D) N1s XPS spectra of SPBI-c after the addition of Cu2+.
(E) N1s XPS spectra of SPBI-c after the addition of Fe3+.
(F) N1s XPS spectra of SPBI-c after the addition of PA.
The structures of the representative compound SPBI-c, its Cu2+ complex and PA were optimized by DFT-B3LYP/6-31G in Gaussian 09 software. As shown in Figure S54, the electron cloud of the frontier orbital in polymer is mainly distributed on the benzimidazole unit and the energy of the highest occupied molecular orbital (HOMO) is −0.39 eV. Although the energy of the HOMO orbital for PA is −4.01 eV, which is lower than the HOMO energy level of the polymer. This facilitates the photoinduced electron transfer (PET) effect produced between the polymers and PA, leading to the fluorescence quenching (Zhuang et al., 2020a, 2020b). Meanwhile, the structure of the metal complex also has been optimized by taking the Cu2+ complex as a representative. It can be found that, when coordinated with Cu2+, the energy gap of HOMO-LUMO orbital for the polymer (SPBI-c) is reduced (Figure S54), forming a more stable metal complex (Park and Gong, 2017; Zeng et al., 2016; Pang et al., 2019). This further proves the coordination between the metal ion and the polymer.
Based on the above analysis, interaction models for the SPBIs with metal ions (Cu2+ or Fe3+) or PA were proposed (Figure 9). The metal ions can coordinate with the C=N bonds in the polymer chains to form a stable metal complex, leading to a change in UV-vis absorption signal of the system and realizing the colorimetric detection of Cu2+ or Fe3+ by SPBIs. For PA, the -OH group can form hydrogen bonds with multiple C=N groups in the polymer backbone (Jiang et al., 2019; Chen et al., 2020c; Kasthuri et al., 2019), inducing fluorescence quenching of the sensing system by photoinduced electron transfer (PET). Thus, the SPBIs can also be used for the recognition of PA.
Figure 9.
Interaction models for SPBI with metal ions (Cu2+, Fe3+) or PA
Conclusions
In summary, PBI, a linear polymer with poor solubility, was prepared by a simple condensation reaction. Subsequently, its solubility was improved via N-alkylation, and a series of SPBI were developed for the first time as colorimetric and ratiometric sensing materials. SPBI with different extents of alkylation were obtained by controlling the feed ratio of C5H11Br and PBI. Furthermore, the morphology of SPBI could be adjusted, and a tendency toward nano-/microsphere formation was observed with increased alkylation. It was found that the interactions between polymer molecules decreased and the distance between polymer chains increased at a high degree of alkylation, causing variations in the sensing performance of SPBI toward metal ions and NACs. Moreover, the SPBI were capable of adsorbing Cu2+ in solution, and good cyclability was achieved with the aid of an acid and a strong coordinating agent. SPBI-c, obtained at the feed ratio of 1:1, exhibited the best adsorption performance. This work provides a new idea for the facile synthesis of multifunctional materials.
Limitations of the study
Reversible experiments need to judge the adsorption effect and desorption degree of the material, mainly through the color changes of the material before and after adsorption, and controlling the time of adsorption and desorption for comparison. Among them, the color discrimination has certain subjective factors, and it may affect the adsorption effect and the degree of desorption in the research process. Therefore, the time was controlled to make the adsorption and desorption effect have certain comparability. Of course, since the adsorption capacity of conjugate organic polymers is influenced by the factors like temperature, pH, adsorption time, and so on, the adsorption experiment may be further optimized.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, peptides, and recombinant proteins | ||
| 3,3′-diaminobenzidine | Energy chemical technology | CAS: 91-95-2 |
| Glutaric acid | Energy chemical technology | CAS: 110-94-1 |
| Bromopentane (n-C5H11Br) | Energy chemical technology | CAS: 110-53-2 |
| Polyphosphoric acid (PPA) | Macklin biochemical technology | CAS: 8017-16-1 |
| Potassium nitrate (KNO3) | Guangzhou Chemical reagent factory | CAS: 7757-79-1 |
| Sodium nitrate (NaNO3) | Guangzhou Chemical reagent factory | CAS: 7631-99-4 |
| Silver nitrate (AgNO3) | Guangzhou Chemical reagent factory | CAS: 7761-88-8 |
| Barium nitrate [Ba(NO3)2] | Guangzhou Chemical reagent factory | CAS: 10,022-31-8 |
| Calcium nitrate [Ca(NO3)2] | Guangzhou Chemical reagent factory | CAS: 10,124-37-5 |
| Manganese nitrate tetrahydrate [Mn(NO3)2·4H2O] | Guangzhou Chemical reagent factory | CAS: 20,694-39-7 |
| Copper Sulfate (CuSO4) | Guangzhou Chemical reagent factory | CAS: 7758-98-7 |
| Ferrous chloride [FeCl2] | Guangzhou Chemical reagent factory | CAS: 7758-94-3 |
| Lead nitrate [Pb(NO3)2] | Guangzhou Chemical reagent factory | CAS: 10,099-74-8 |
| Mercury nitrate [Hg(NO3)2] | Guangzhou Chemical reagent factory | CAS: 10,045-94-0 |
| Zinc nitrate [Zn(NO3)2] | Guangzhou Chemical reagent factory | CAS: 7779-88-6 |
| Aluminum trichloride (AlCl3) | Guangzhou Chemical reagent factory | CAS: 7446-70-0 |
| Ferric chloride (FeCl3) | Guangzhou Chemical reagent factory | CAS: 7705-08-0 |
| Chromium chloride hexahydrate (CrCl3·6H2O) | Guangzhou Chemical reagent factory | CAS: 10,060-12-5 |
| Deposited data | ||
| Raw and analyzed data | This paper | NA |
| Software and algorithms | ||
| Gaussian 09 | Gaussian Inc. | Yimo Information Technology Co., Ltd |
Resource availability
Lead contact
Further requests for resources regarding this study will be fulfilled by the corresponding author, Zhao-Yang Wang (wangzy@scnu.edu.cn).
Materials availability
This work did not produce any new unique reagents.
Experimental model and subject details
This work did not need any unique experimental model.
Method details
Materials
3,3′-Diaminobenzidine, glutaric acid, and bromopentane (n-C5H11Br) were purchased from Energy chemical technology (Shanghai) Co. Ltd. Polyphosphoric acid (PPA) was purchased from Macklin biochemical technology Co. Ltd. All soluble metal ion salts and sodium hydroxide (NaOH) are purchased from Guangzhou Chemical reagent factory. All the nitroaromatic compounds purchased from Guangzhou Chemical reagent factory. Tetrahydrofuran (THF), acetonitrile (MeCN), anhydrous ethanol (EtOH), dimethyl formamide (DMF), dichloro-methane (DCM), and dimethyl sulfoxide (DMSO) were purchased from Tianjin Damao chemical reagent factory. DMSO-d6 and CDCl3 were purchased from Energy chemical technology (Shanghai) Co. Ltd. All these reagents were used without further purification.
Apparatus
The scanning electron microscopy (SEM) image was obtained on Phenom Pro X Desktop Scanning Electron Microscope and Energy Spectrum Integrated Machine. The thermogravimetric analysis was tested in TG-209 F3 thermogravimetric analyzer. X-ray photoelectron spectroscopic (XPS) analysis was performed by an Axis Ultra-DLD X-ray photoelectron spectrometer. The fluorescent spectra were obtained with a Hitachi F-4600 spectrophotometer at room temperature using the xenon lamp as light source; the slit width was 5 nm for both excitation and emission and voltage was 400 V. The UV-vis absorption spectra were carried out with an SHIMADZU UV-2700 UV spectrometer. The pH values were recorded by a PHS-25C meter. Fluorescent lifetime was measured by FLS 920 Fluorescence Spectrometer. The metal ion adsorption detection was carried out by AAS-990 atomic absorption spectrophotometer.
Synthesis of intermediate PBI
As the reported method (Wu et al., 2016, 2018; Ge et al., 2018; Jiang et al., 2019; Chen et al., 2020b), 3.0 mmol 3,3′-diaminobenzidine, 3.0 mmol glutaric acid and 20 mL polyphosphoric acid (PPA) were added into a 50 mL round-bottom flask. The mixture was stirred at 120°C for 2 h, and then heated to 170°C for 48 h. Once the reaction was stopped, the pH of the mixture was adjusted to alkaline with NaOH solution till cooling to room temperature. A crude solid product was obtained by vacuum filtration. Subsequently, the crude product was washed several times with water, ethyl acetate, ethanol and other solvents to remove the unreacted raw materials and some by-products with the lower molecular weight. Finally, the product was dried in a vacuum drying oven at 50°C for 24 h.
Synthesis of serial SPBIs
According to reported methods (Wu et al., 2016, 2018; Ge et al., 2018; Jiang et al., 2019; Chen et al., 2020b), 1 mmol PBI, different molar n-C5H11Br, 10 mL MeCN and moderate NaOH were added into the reaction flask. After refluxing for 24 h, the solvent was removed by vacuum distillation. The product was washed with water several times to remove the NaOH. Then, the alkylated product was rinsed alternately with dichloromethane and ethanol, collecting the organic phase and removing the solvent to obtain the purified product. Finally, the anticipated product was dried in a vacuum drying oven at 40°C for 24 h.
General procedure for optical spectral measurements
The SPBI samples were dissolved in DMSO or DMF to acquire a stock solution. Then, the test solution (DMSO/H2O or DMF/H2O as V/V = 99/1) of SPBI was prepared for the experiments of both UV-vis absorption spectra and fluorescence spectra at room temperature. Among them, 1 mg sample was dissolved into 15 mL mixed solvent.
Limit of detection
As the reported (Seo et al., 2014; Giri et al., 2018), the limit of detection (LOD) was measured by the equation: LOD = 3δ/K. Therein, δ is the standard deviation of the blank measurements (n = 12), and the K is the slope of the calibration curve.
SEM analysis
According to the reported method (Zhang et al., 2019a; To et al., 2020), the morphology for SPBI and the morphology changes of SPBI combined with metal ions or nitroaromatic compounds (NACs) were determined by field emission scanning electron microscope.
XPS analysis
As reported method (Wang et al., 2019; Li et al., 2019b), the combining energy of N1s, O1s and C1s in the product and complex were measured by Axis Ultra-DLD X-Ray photoelectron spectrometer
Cyclic adsorption and desorption
According to the literature (Wang et al., 2019; Ozay et al., 2020), to realize the recycle adsorption of SPBI, the coordinated solid was treated with HCl (pH = 2) and EDTA solution, and the mixture was stirred for 30 min. Once the solid color was changed from blue to colourless, the regenerating SPBI solid was obtained by filtering. After neutralizing the acid remained on the surface of SPBI with NaOH solution (pH = 10), and rinsing with the deionized water to a neutral environment, the coordinated N atoms in SPBI were restored to their original state. The concentrations of samples were tested by AAS.
Quantification and statistical analysis
The limit of detection was obtained by the formula “LOD = 3σ/k” and σ was the relative standard deviation. Differences were considered significant at p < 0.05. The statistical analyses were performed with Origin software.
Additional resources
There are no additional resources needed to be declared in this manuscript, additional requests for this can be made by contacting the lead contact.
Acknowledgments
Financial support from the National Natural Science Foundation of China (20772035), Guangdong Basic and Applied Basic Research Foundation (No. 2021A1515012342), the Open Fund of the Key Laboratory of Functional Molecular Engineering of Guangdong Province in SCUT (No. 2017kf01) and Guangdong Provincial Science and Technology Project (No. 2017A010103016) is greatly appreciated.
Author contributions
Conceptualization, C.P. and Z.W.; Investigation, C.P., X.C. and Y.X.; Writing-Original Draft, C.P.; Writing-Review & Editing, C.P., X.C., Y.X., S.L, Q.C., Y.Z. and Z.W.; Funding Acquisition, Z.W. and S.L.; Supervision, S.L. and Z.W.; Project Administration, Z.W.
Declaration of interests
The authors declare no competing interests.
Published: October 22, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2021.103126.
Contributor Information
Shi-He Luo, Email: pinky_r@163.com.
Zhao-Yang Wang, Email: wangzy@scnu.edu.cn.
Supporting Citations
The following references appear in the Supplemental information: Abbasi et al., 2019; Abuhatab et al., 2020; Ansari et al., 2020; Bora et al., 2019; Chiou et al., 2020; Coldur et al., 2020; Darmayanti et al., 2019; Fang et al., 2020; Feng et al., 2020; Ge et al., 2020; Ghosh et al., 2019; He et al., 2021; Hosseinjani-Pirdehi et al., 2020; Jiang et al., 2020; Jigyasa et al., 2020; Joseph et al., 2020; Kadian and Manik, 2020; Ke et al., 2020; Krishnan and Suneesh, 2019; Landge et al., 2020; Li et al., 2020a; Li et al., 2020b; Ma et al., 2020a; Ma et al., 2020b; Mal et al., 2020; Mathivanan et al., 2020; Miao, 2019; Nan et al., 2020; Podasca et al., 2019; Qian et al., 2019; Qu et al., 2020; Rajalakshmi and Palanisami, 2020; Ran et al., 2020; Roja et al., 2020; Sharma et al., 2019; Tian et al., 2019; Xiao et al., 2020; Xiong et al., 2019; Xu et al., 2019; Zhu et al., 2020.
Supplemental information
Data and code availability
All data are published in this manuscript and supplement; additional requests for data can be made by contacting the lead contact, Zhao-Yang Wang (wangzy@scnu.edu.cn).
References
- Abbasi F., Akbarinejad A., Alizadeh N. CdS QDs/N-methylpolypyrrole hybrids as fluorescent probe for ultrasensitive and selective detection of picric acid. Spectrochim. Acta A. 2019;216:230–235. doi: 10.1016/j.saa.2019.03.032. [DOI] [PubMed] [Google Scholar]
- Abuhatab S., El-Qanni A., Al-Qalaq H., Hmoudah M., Al-Zerei W. Effective adsorptive removal of Zn2+, Cu2+, and Cr3+ heavy metals from aqueous solutions using silica-based embedded with NiO and MgO nanoparticles. J. Environ. Manage. 2020;268:110713. doi: 10.1016/j.jenvman.2020.110713. [DOI] [PubMed] [Google Scholar]
- Ansari M., Hassan A., Alam A., Jana A., Das N. Triptycene based fluorescent polymers with azo motif pendants: eflect of alkyl chain on fluorescence, morphology and picric acid sensing. React. Funct. Polym. 2020;146:104408. [Google Scholar]
- Aysha T.S., El-Sedik M.S., Mohamed M.B.I., Gaballah S.T., Kamel M.M. Dual functional colorimetric and turn-off fluorescence probe based on pyrrolinone ester hydrazone dye derivative for Cu2+ monitoring and pH change. Dyes Pigm. 2019;170:107549. [Google Scholar]
- Bora A., Mohan K., Dolui S.K. Carbon dots as cosensitizers in dye-sensitized solar cells and fluorescence chemosensors for 2,4,6-trinitrophenol detection. Ind. Eng. Chem. Res. 2019;58:22771–22778. [Google Scholar]
- Chen H.F., Zhou Y., Wang J.Y., Lu J., Zhou Y.B. Polydopamine modified cyclodextrin polymer as efficient adsorbent for removing cationic dyes and Cu2+ J. Hazard. Mater. 2020;388:121897. doi: 10.1016/j.jhazmat.2019.121897. [DOI] [PubMed] [Google Scholar]
- Chen K., Shu Q.H., Schmittel M. Design strategies for lab-on-a-molecule probes and orthogonal sensing. Chem. Soc. Rev. 2015;44:136–160. doi: 10.1039/c4cs00263f. [DOI] [PubMed] [Google Scholar]
- Chen S.-H., Jiang K., Xiao Y., Cao X.-Y., Arulkumar M., Wang Z.-Y. Recent endeavors on design, synthesis, fluorescence mechanisms and applications of benzazole- based molecular probes toward miscellaneous species. Dyes Pigm. 2020;175:108157. [Google Scholar]
- Chen S.-H., Pang C.-M., Chen X.-Y., Yan Z.-H., Huang S.,-M., Li X.-D., Zhong Y.-T., Wang Z.-Y. Research progress in design, synthesis and application of multifunctional fluorescent probes. Chin. J. Org. Chem. 2019;39:1846–1857. [Google Scholar]
- Chen X.F., Sun C.M., Liu Y., Yu L., Zhang K., Asiri A.M., Marwani H.M., Tan H., Ai Y.J., Wang X.K., Wang S.H. All-inorganic perovskite quantum dots CsPbX3 (Br/I) for highly sensitive and selective detection of explosive picric acid. Chem. Eng. J. 2020;379:122360. [Google Scholar]
- Chen X., Huang Z., Luo S.-Y., Zong M.-H., Lou W.-Y. Multi-functional magnetic hydrogels based on Millettia speciosa Champ residue cellulose and Chitosan: highly efficient and reusable adsorbent for Congo red and Cu2+ removal. Chem. Eng. J. 2021;423:130198. [Google Scholar]
- Chen X.-B., Qi C.-X., Li H., Ding J.-Y., Yan S., Lei H., Xu L., Liu B. Highly sensitive and selective Fe3+ detection by a water-stable Tb3+-doped nickel coordination polymer-based turn-off fluorescence sensor. J. Solid State Chem. 2019;281:121030. [Google Scholar]
- Chiou Y.-R., Yan H.B., Wan C.-F., Huang C.Y., Wu A.-T. A Schiff-based fluorescence sensor for the detection of Cu2+ and its application in living cells. J. Photochem. Photobiol. A. 2020;390:112326. [Google Scholar]
- Coldur M., Oguzlar S., Ongun M.Z., Oter O., Yıldırım S. Usage of thiocyanate-based ionic liquid as new optical sensor reagent: absorption and emission based selective determination of Fe (III) ions. Spectrochim. Acta A. 2020;224:117385. doi: 10.1016/j.saa.2019.117385. [DOI] [PubMed] [Google Scholar]
- Cui X., Si Z.J., Li Y.H., Duan Q. Synthesis of telechelic PNIPAM ended with 9,10-dihydroacridine group as a recyclable and specific Fe3+ detection fluorescent sensor. Dyes Pigm. 2020;173:107873. [Google Scholar]
- Cui Y.H., Wang S., Wang D., Liu G., Liu F.X., Liang D., Wang X.D., Yong Z.P., Wang Z. HT-PEMs based on carbazole grafted polybenzimidazole with high proton conductivity and excellent tolerance of phosphoric acid. J. Membr. Sci. 2021;637:119610. [Google Scholar]
- Darmayanti L., Kadja G.T.M., Notodarmojo S., Damanhuri E., Mukti R.R. Structural alteration within fly ash-based geopolymers governing the adsorption of Cu2+ from aqueous environment: Effect of alkali activation. J. Hazard. Mater. 2019;377:305–314. doi: 10.1016/j.jhazmat.2019.05.086. [DOI] [PubMed] [Google Scholar]
- Diao H.P., Guo L.X., Liu W., Feng L.H. A novel polymer probe for Zn(II) detection with ratiometric fluorescence signal. Spectrochim. Acta A. 2018;196:274–280. doi: 10.1016/j.saa.2018.02.036. [DOI] [PubMed] [Google Scholar]
- Dou Z.S., Yu J.C., Cui Y.J., Yang Y., Wang Z.Y., Yang D.R., Qian G.D. Luminescent metal-organic framework films as highly sensitive and fast-response oxygen sensors. J. Am. Chem. Soc. 2014;136:5527–5530. doi: 10.1021/ja411224j. [DOI] [PubMed] [Google Scholar]
- Du T., Wang J., Zhang T.S., Zhang L., Yang C.Y., Yue T.L., Sun J., Li T., Zhou M.G., Wang J.L. An integrating platform of ratiometric fluorescent adsorbent for unconventional real-time removing and monitoring of copper ions. ACS Appl. Mater. Inter. 2020;12:13189–13199. doi: 10.1021/acsami.9b23098. [DOI] [PubMed] [Google Scholar]
- Dutta B., Hazra A., Dey A., Sinha C., Ray P.P., Banerjee P., Mir M.H. Construction of a succinate-bridged Cd(II)-based two-dimensional coordination polymer for efficient optoelectronic device fabrication and explosive sensing application. Cryst. Growth Des. 2020;20:765–776. [Google Scholar]
- Fan C.Y., Wu H., Guan J.Y., You X.D., Yang C., Wang X.Y., Cao L., Shi B.B., Peng Q., Kong Y. Scalable fabrication of crystalline COF membranes from amorphous polymeric membranes. Angew. Chem. Int. Ed. 2021;60:2–10. doi: 10.1002/anie.202102965. [DOI] [PubMed] [Google Scholar]
- Fan J., Zhang S.F., Xu Y.S., Wei N., Wan B., Qian L.W., Liu Y. A polyethylenimine/salicylaldehyde modified cellulose Schiff base for selective and sensitive Fe3+ detection. Carbohydr. Polym. 2020;228:115379. doi: 10.1016/j.carbpol.2019.115379. [DOI] [PubMed] [Google Scholar]
- Fang X.J., Zhu S.D., Ma J.Z., Wang F.Y., Xu H.H., Xia M.Z. The facile synthesis of zoledronate functionalized hydroxyapatite amorphous hybrid nanobiomaterial and its excellent removal performance on Pb2+ and Cu2+ J. Hazard. Mater. 2020;392:122291. doi: 10.1016/j.jhazmat.2020.122291. [DOI] [PubMed] [Google Scholar]
- Feng B.X., Xu Z., Qi C.G., Guo X.M., Gai L.G. Fluorescence quenching of photo- luminescent organic polymer nanoflms by ferric ions. Microchem. J. 2020;154:104639. [Google Scholar]
- Gao J.M., Chen X.X., Chen S.Q., Meng H., Wang Y., Li C.S., Feng L. The BODIPY-based chemo-sensor for fluorometric/colorimetric dual channel detection of RDX and PA. Anal. Chem. 2019;91:13675–13680. doi: 10.1021/acs.analchem.9b02888. [DOI] [PubMed] [Google Scholar]
- Ge F.-Y., Sun G.-H., Meng L., Ren S.-S., Zheng H.-G. Four new luminescent metal- organic frame-works as multi- functional sensors for detecting Fe3+, Cr2O72- and nitromethane. Cryst. Growth Des. 2020;20:1898–1904. [Google Scholar]
- Ge J.Y., Fan L., Zhang K., Ou T., Li Y.H., Zhang C.H., Dong C., Shuang S.M., Wong M.S. A two-photon ratiometric fluorescent probe for effective monitoring of lysosomal pH in live cells and cancer tissues. Sens. Actuators B. 2018;262:913–921. [Google Scholar]
- Geng K., Li Y., Xing Y., Wang L.H., Li N.W. A novel polybenzimidazole membrane containing bulky naphthalene group for vanadium flow battery. J. Membr. Sci. 2019;586:231–239. [Google Scholar]
- Ghorai P., Dey A., Hazra A., Dutta B., Brandao P., Ray P.P., Banerjee P., Saha A. Cd(II) based coordination polymer series: fascinating structures, efficient semi-conductors, and promising nitro aromatic sensing. Cryst. Growth Des. 2019;19:6431–6447. [Google Scholar]
- Ghosh S., Manna R., Dey S. Epoxy-based polymer incorporating 1-naphthylamine and sebacic acid moieties: a selective fluorescent sensor for ferric ions. J. Mol. Struct. 2019;1180:406–410. [Google Scholar]
- Giri D., Bankura A., Patra S.K. Poly(benzodithieno-imidazole- alt-carbazole) based π-conjugated copolymers: highly selective and sensitive turn-off fluorescent probes for Hg2+ Polymer. 2018;158:338–353. [Google Scholar]
- Goswami R., Seal N., Dash S.R., Tyagi A., Neog S. Devising chemically robust and cationic Ni(II)-MOF with nitrogen-rich micropores for moisture-tolerant CO2 capture: highly regenerative and ultrafast colorimetric sensor for TNP and multiple oxo-anions in water with theoretical revelation. ACS Appl. Mater. Inter. 2019;11:40134–40150. doi: 10.1021/acsami.9b15179. [DOI] [PubMed] [Google Scholar]
- Han T., Yuan Y., Kang H., Zhang Y., Dong L.J. Ultrafast, sensitive and visual sensing of copper ions by a dual-fluorescent film based on quantum dots. J. Mater. Chem. C. 2019;7:14904–14912. [Google Scholar]
- He Y., Gou S.H., Zhou L.H., Tang L., Liu T., Liu L., Duan M. Amidoxime- functionalized polyacrylamide-modified chitosan containing imidazoline groups for effective removal of Cu2+ and Ni2+ Carbohydr. Polym. 2021;252:117160. doi: 10.1016/j.carbpol.2020.117160. [DOI] [PubMed] [Google Scholar]
- Hosseinjani-Pirdehi H., Mahmoodi N.O.A., Taheri A., Asalemi K.A.A., Esmaeili R. Selective immediate detection of Cu2+ by a pH-sensitive rhodaminebased fluorescence probe in breast cancer cell-line. Spectrochim. Acta A. 2020;229:117989. doi: 10.1016/j.saa.2019.117989. [DOI] [PubMed] [Google Scholar]
- Huang L.L., Yu K.H., Zhou W.T., Teng Q.Y., Wang Z.Y., Dai Z.H. Quantitative principal component analysis of multiple metal ions with lanthanide coordination polymer networks. Sens. Actuators B. 2021;346:130469. [Google Scholar]
- Huang Y., Liu L.X., Yang X., Zhang X.Y., Yan B., Wu L., Feng P.J., Lou X.D., Xia F., Song Y.L., Li F.Y. A diverse micromorphology of photonic crystal chips for multianalyte sensing. Small. 2021;17:202006723. doi: 10.1002/smll.202006723. [DOI] [PubMed] [Google Scholar]
- Imase T., Ohira A., Okoshi K., Sano N., Kawauchi S., Watanabe J., Kunitake M. AFM study of two-dimensional epitaxial arrays of poly(γ-L-glutamates) with long n-alkyl side chains on graphite. Macromolecules. 2003;36:1865–1869. [Google Scholar]
- Jia T., Fu M., Zhang M.Y., Qiu J.W., Zhu H., Gao Y. A novel cholesterol conjugated fluorescence probe for Cu2+ detection and bioimaging in living cells. Spectrochim. Acta A. 2020;227:117530. doi: 10.1016/j.saa.2019.117530. [DOI] [PubMed] [Google Scholar]
- Jiang K., Chen S.-H., Luo S.-H., Pang C.-M., Wu X.-Y., Wang Z.-Y. Concise design and synthesis of water-soluble fluorescence sensor for sequential detection of Zn(II) and picric acid via cascade mechanism. Dyes Pigm. 2019;167:164–173. [Google Scholar]
- Jiang K., Wu Y.-C., Wu H.-Q., Li S.-L., Luo S.-H., Wang Z.-Y. A highly selective, pH-tolerable and fast-response fluorescent probe for Fe3+ based on star-shape benzothiazole derivative. J. Photochem. Photobiol. A. 2018;350:52–58. [Google Scholar]
- Jiang S.J., Qiu J.B., Chen S.B., Guo H.Y., Yang F.F. Double-detecting fluorescent sensor for ATP based on Cu2+ and Zn2+ response of hydrazono-bis-tetraphenylethylene. Spectrochim. Acta A. 2020;227:117568. doi: 10.1016/j.saa.2019.117568. [DOI] [PubMed] [Google Scholar]
- Jiao Y., Gao Y.F., Meng Y.T., Lu W.J., Liu Y., Han H., Shuang S.M., Li L., Dong C. One-step synthesis of label-free ratiometric fluorescence carbon dots for the detection of silver ions and glutathione and cellular imaging applications. ACS Appl. Mater. Inter. 2019;11:16822–16829. doi: 10.1021/acsami.9b01319. [DOI] [PubMed] [Google Scholar]
- Jigyasa, Kumar D., Arora P., Singh H., Rajput J.K. Polyhydroquinoline nanoaggregates: a dual fluorescent probe for detection of 2,4,6-trinitrophenol and chromium(VI) Spectrochim. Acta A. 2020;230:118087. doi: 10.1016/j.saa.2020.118087. [DOI] [PubMed] [Google Scholar]
- Jin Y., Gao B., Bian C., Meng X.X., Meng B., Wong S.I., Yang N.T., Sunarso J., Tan X.Y., Liu S.M. Elevated-temperature H2 separation using a dense electron and proton mixed conducting polybenzimidazole-based membrane with 2D sulfonated grapheme. Green. Chem. 2021;23:3374–3385. [Google Scholar]
- Joseph R., Asok A., Joseph K. Quinoline appended pillar[5]arene (QPA) as Fe3+ sensor and complex of Fe3+ (FeQPA) as a selective sensor for F-, arginine and lysine in the aqueous medium. Spectrochim. Acta A. 2020;224:117390. doi: 10.1016/j.saa.2019.117390. [DOI] [PubMed] [Google Scholar]
- Kadian S., Manik G. Green synthesis of fluorescent carbon dots using chloroplast dispersions as precursors and application for Fe3+ ion sensing. Luminescence. 2020;35:1–10. doi: 10.1002/bio.3794. [DOI] [PubMed] [Google Scholar]
- Kasthuri S., Kumar S., Raviteja S., Ramakrishna B., Maji S., Veeraiahd N., Venkatramaiah N. Influence of alkyl chains on fluoranthene ensembles towards fluorescence based detection of 2,4,6-trinitrophenol. Appl. Surf. Sci. 2019;481:1018–1027. [Google Scholar]
- Kaur H., Singh N., Kaur N., Jang D.O. Nano-aggregate-Fe3+ complex based on benzimidazole-modified calix[4] arene for amplified fluorescence detection of ADP in aqueous media. Sens. Actuators B. 2019;284:193–201. [Google Scholar]
- Ke H.S., Wei W., Yang Y.S., Wu H.P., Zhang Y.-Q., Xie G., Chen S.P. A trinuclear zinc coordination cluster exhibiting fluorescence, colorimetric sensitivity, and recycling of silver ion and detection of cupric ion. Inorg. Chem. 2020;59:2833–2842. doi: 10.1021/acs.inorgchem.9b03169. [DOI] [PubMed] [Google Scholar]
- Khairy G.M., Duerkop A. Dipsticks and sensor microtiterplate for determination of copper (II) in drinking water using reflectometric RGB readout of digital images, fluorescence or eye-vision. Sens. Actuators B. 2019;281:878–884. [Google Scholar]
- Kim K.H., Yu H., Kang H., Kang D.J., Cho C.H., Cho H.H., Oh J.H., Kim B.J. Influence of intermolecular interactions of electron donating small molecules on their molecular packing and performance in organic electronic devices. J. Mater. Chem. A. 2013;1:14538–14547. [Google Scholar]
- Krishnan S., Suneesh C.V. Fluorene-triazine conjugated porous organic polymer framework for superamplifed sensing of nitroaromatic explosives. J. Photochem. Photobiol. A. 2019;371:414–422. [Google Scholar]
- Kumar A., Chae P.S. Fluorescence tunable thiophene- bis(benzimidazole)-based probes for a cascade trace detection of Hg2+ and lysine: a molecular switch mimic. Sens. Actuators, B. 2019;281:933–944. [Google Scholar]
- Landge S.M., Lazare D.Y., Freeman C., Bunn J., Cruz J.I., Winder D., Padgett C., Aiken K.S., Ghosh D. Rationally designed phenanthrene derivatized triazole as a dual chemo- sensor for fluoride and copper recognition. Spectrochim. Acta A. 2020;228:117758. doi: 10.1016/j.saa.2019.117758. [DOI] [PubMed] [Google Scholar]
- Li A.-L., Wang Z.-L., Wang W.-Y., Liu Q.-S., Sun Y., Wang S.-F., Gu W. A novel dehydroabietic acid-based fluorescent probe for detection of Fe3+ and Hg2+ ions and its application in live-cell imaging. Microchem. J. 2021;160:105682. [Google Scholar]
- Li B., Zhou J., Bai F.Y., Xing Y.H. Lanthanide-organic framework based on a 4,4-(9,9-dimethyl-9H-fluorene-2,7-diyl) dibenzoic acid: synthesis, structure and fluorescent sensing for a variety of cations and anions simultaneously. Dyes Pigm. 2020;172:107862. [Google Scholar]
- Li X.C., Han Y.J., Sun S.S., Shan D.D., Ma X.M., He G.J., Mergu N., Park J.-S., Kim C.-H., Son Y.-A. A diaminomaleonitrile-appended BODIPY chemosensor for the selective detection of Cu2+ via oxidative cyclization and imaging in SiHa cells and zebrafish. Spectrochim. Acta A. 2020;233:118179. doi: 10.1016/j.saa.2020.118179. [DOI] [PubMed] [Google Scholar]
- Li X.S., An J.D., Zhang H.M., Liu J.J., Li Y., Du G.X., Wu X.X., Fei L., Lacoste J.D., Cai Z. Cluster-based CaII, MgII and CdII coordination polymers based on aminofunctionalized triphenyl tetra-carboxylate: bifunctional photo -luminescent sensing for Fe3+ and antibiotics. Dyes Pigm. 2019;170:107631. [Google Scholar]
- Li Y.K., He Y.L., Guo F.Y., Zhang S.P., Liu Y.Y., Lustig W.P., Bi S.M., Williams L.J., Hu J., Li J. NanoPOP: solution-processable fluorescent porous organic polymer for highly sensitive, selective, and fast naked eye detection of mercury. ACS Appl. Mater. Inter. 2019;11:27394–27401. doi: 10.1021/acsami.9b06488. [DOI] [PubMed] [Google Scholar]
- Liang N.Q., Fang J.H., Guo X.X. A facile approach for preparation of porous polybenzimidazole membranes as a promising separator for lithium ion batteries. J. Mater. Chem. A. 2019;5:15087–15095. [Google Scholar]
- Liu H.Q., Wang Y., Mo W.Q., Tang H.L., Cheng Z.Y., Chen Y., Zhang S.T., Ma H.W., Li B., Li X.B. Dendrimer-based, high-luminescence conjugated microporous polymer films for highly sensitive and selective volatile organic compound sensor arrays. Adv. Funct. Mater. 2020;30:1910275. [Google Scholar]
- Liu W., An Z., Qin L., Wang M., Liu X., Yang Y. Construction of a novel ion imprinted film to remove low concentration Cu2+ from aqueous solution. Chem. Eng. J. 2021;411:128477. [Google Scholar]
- Lochman L., Machacek M., Miletin M., Uhlířová Š., Lang K., Kirakci K., Zimcik P., Novakova V. Red-emitting fluorescence sensors for metal cations: the role of counter-anions and sensing of SCN- in biological materials. ACS Sensors. 2019;4:1552–1559. doi: 10.1021/acssensors.9b00081. [DOI] [PubMed] [Google Scholar]
- Lv B., Yin H., Shao Z.G., Luan Z.J., Huang Z.Y., Sun S.S., Teng Y., Miu C.H., Gao Q. Novel polybenzimidazole/graphitic carbon nitride nanosheets composite membrane for the application of acid-alkaline amphoteric water electrolysis. J. Energy Chem. 2021;64:607–614. [Google Scholar]
- Ma J.Z., Xia M.Z., Zhu S.D., Wang F.Y. A new alendronate doped HAP nanomaterial for Pb2+, Cu2+ and Cd2+ effect absorption. J. Hazard. Mater. 2020;400:123143. doi: 10.1016/j.jhazmat.2020.123143. [DOI] [PubMed] [Google Scholar]
- Ma L., Han X., Xia L., Qu F.L., Kong R.-M. A label-free G-quadruplex-based fluorescence assay for sensitive detection of alkaline phosphatase with the assistance of Cu2+ Spectrochim. Acta A. 2020;227:117607. doi: 10.1016/j.saa.2019.117607. [DOI] [PubMed] [Google Scholar]
- Magri D.C. Logical sensing with fluorescent molecular logic gates based on photoinduced electron transfer. Coord. Chem. Rev. 2021;426:213598. [Google Scholar]
- Magri D.C., Brown G.J., McClean G.D., de Silva A. Communicating chemical congregation: a molecular AND logic gate with three chemical inputs as a “lab-on-a-molecule” prototype. J. Am. Chem. Soc. 2006;128:4950–4951. doi: 10.1021/ja058295+. [DOI] [PubMed] [Google Scholar]
- Mal K., Naskar B., Chaudhuri T., Prodhan C., Goswami S., Chaudhuri K., Mukhopadhyay C. Synthesis of quinoline functionalized fluorescent chemosensor for Cu(II), DFT studies and its application in imaging in living HEK 293 cells. J. Photochem. Photobiol. A. 2020;389:112211. [Google Scholar]
- Mathivanan M., Tharmalingam B., Mani K.S., Thiagarajan V., Murugesapandia B. Simple C3-symmetric triaminoguanidine- triphenylamine conjugate as an efficient colorimetric sensor for Cu(II) and fluorescent sensor for Fe(III) ions. Spectrochim. Acta A. 2020;234:118235. doi: 10.1016/j.saa.2020.118235. [DOI] [PubMed] [Google Scholar]
- Miao C.L. Three water-stable luminescent Zn(II) coordination polymers for highly sensitive and selective sensing of acetylacetone and Fe3+ ions. J. Mol. Struct. 2019;1193:286–293. [Google Scholar]
- Nakamitsu M., Oyama K., Imai H., Fujii S., Oaki Y. Ultrahigh-sensitive compression-stress sensor using integrated stimuli-responsive materials. Adv. Mater. 2021;33:202008755. doi: 10.1002/adma.202008755. [DOI] [PubMed] [Google Scholar]
- Nan Z.Z., Hao C.C., Zhang X.G., Liu H.Y., Sun R.G. Carbon quantum dots (CQDs) modified ZnO/CdS nanoparticles based fluorescence sensor for highly selective and sensitive detection of Fe(III) Spectrochim. Acta A. 2020;228:117717. doi: 10.1016/j.saa.2019.117717. [DOI] [PubMed] [Google Scholar]
- Nandi S.K., Chowdhury S.R., Podder D., Ghorai P.K., Haldar D. A robust tripeptide for in-field selective naked eye ultratrace detection of 2,4,6-trinitrophenol. Cryst. Growth Des. 2020;20:1884–1890. [Google Scholar]
- Ozay H., Gungor Z., Yilmaz B., Ilgin P., Ozay O. Dual use of colorimetric sensor and selective copper removal from aqueous media with novel p(HEMA-co-TACYC) hydrogels: Cyclen derivative as both monomer and crosslinker. J. Hazard. Mater. 2020;388:121848. doi: 10.1016/j.jhazmat.2019.121848. [DOI] [PubMed] [Google Scholar]
- Pang C.-M., Luo S.-H., Jiang K., Wang B.-W., Chen S.-H., Wang N., Wang Z.-Y. A dual-channel sensor containing multiple nitrogen heterocycles for the selective detection of Cu2+, Hg2+ and Zn2+ in same solvent system by different mechanism. Dyes Pigm. 2019;170:107651. [Google Scholar]
- Park H., Kim J.W., Hong S.Y., Lee G., Kim D.S., Oh J.H., Yun J.Y., Ha J.S. Microporous polypyrrole-coated graphene foam for high-performance multifunctional sensors and flexible supercapacitors. Adv. Funct. Mater. 2018;28:1707013. [Google Scholar]
- Park K.-J., Gong M.-S. A water durable resistive humidity sensor based on rigid sulfonated polybenzimidazole and their properties. Sens. Actuators B. 2017;246:53–60. [Google Scholar]
- Podasca V.E., Chibac A.L., Buruiana E.C. Fluorescence quenching study of a block copolymer with uracil end units by means of nitroaromatic derivatives and metal cations. J. Mol. Struct. 2019;292:111385. [Google Scholar]
- Qian J.N., Wu D., Cai P., Xia J.B. Nitrogen atom free polythiophene derivative as an efficient chemosensor for highly selective and sensitive Cu2+ and Ag+ detection. Spectrochim. Acta A. 2019;218:75–84. doi: 10.1016/j.saa.2019.03.093. [DOI] [PubMed] [Google Scholar]
- Qu P., Li Y.C., Huang H.Y., Chen J.J., Yu Z.B., Huang J., Wang H.L., Gao B. Urea formaldehyde modified alginate beads with improved stability and enhanced removal of Pb2+, Cd2+ and Cu2+ J. Hazard. Mater. 2020;396:122664. doi: 10.1016/j.jhazmat.2020.122664. [DOI] [PubMed] [Google Scholar]
- Rabbani M.G., Islamoglu T., El-Kaderi H.M. Benzothiazole-and benzoxazole-linked porous polymers for carbon dioxide storage and separation. J. Mater. Chem. A. 2017;5:258–265. [Google Scholar]
- Rajak R., Saraf M., Verma S.K., Kumar R., Mobin S.M. Dy(III)-based metal- organic framework as a fluorescent probe for highly selective detection of picric acid in aqueous medium. Inorg. Chem. 2019;58:16065–16074. doi: 10.1021/acs.inorgchem.9b02611. [DOI] [PubMed] [Google Scholar]
- Rajalakshmi A.V., Palanisami N. Y-shaped ferrocene/non-ferrocene conjugated quino- xalines for colorimetric and fluorimetric detection of picric acid. Spectrochim. Acta A. 2020;228:117812. doi: 10.1016/j.saa.2019.117812. [DOI] [PubMed] [Google Scholar]
- Ran Y., Wang S.Y., Yin Q.Y., Wen A.L., Peng X.X., Long Y.F., Chen S. Green synthesis of fluorescent carbon dots using chloroplast dispersions as precursors and application for Fe3+ ion sensing. Luminescence. 2020;35:1–7. doi: 10.1002/bio.3794. [DOI] [PubMed] [Google Scholar]
- Roja S.S., Shylaja A., Kumar R.R. Phenothiazine-tethered 2-aminopyridine-3-carbo- nitrile: fluorescent turn-off chemosensor for Fe3+ ions and picric acid. ChemistrySelect. 2020;5:2279–2283. [Google Scholar]
- Schmittel M., Shu Q.H. A lab-on-a-molecule for anions in aqueous solution: using Kolbe electrolysis and radical methylation at iridium for sensing. Chem. Commun. 2012;48:2707–2709. doi: 10.1039/c2cc17731e. [DOI] [PubMed] [Google Scholar]
- Seo S., Kim J., Jang G., Kim D., Lee T.S. Aggregation-deaggregation-triggered, tunable fluorescence of an assay ensemble composed of anionic conjugated polymer and polypeptides by enzymatic catalysis of trypsin. ACS Appl. Mater. Interfaces. 2014;6:918–924. doi: 10.1021/am405120y. [DOI] [PubMed] [Google Scholar]
- Seo J.-M., Noh H.-J., Jeong H.Y., Baek J.-B. Converting unstable imine-linked network into stable aromatic benzoxazole-linked one via post-oxidative cyclization. J. Am. Chem. Soc. 2019;141:11786–11790. doi: 10.1021/jacs.9b05244. [DOI] [PubMed] [Google Scholar]
- Shan M.X., Liu X.L., Wang X.R., Liu Z.L., Iziyi H., Ganapathy S., Gascon J., Kapteijn F. Novel high performance poly(p-phenylene benzobisimidazole) (PBDI) membranes fabricated by interfacial polymerization for H2 separation. J. Mater. Chem. A. 2019;7:8929–8937. [Google Scholar]
- Sharma A.K., Priya, Kaith B.S., Isha, Singh A., Chandel K., Vipula Riboflavin functionalized dextrin-sodium alginate based fluorescent sensor: detoxification of Cu2+ and Ni2+ ions. ACS Appl. Polym. Mater. 2019;1:3084–3094. [Google Scholar]
- Shia W.G., Lu X.Y., Zhang S.S., Li H.W., Liu M., Dong B. C=N based PAMAM polymer dots: fluorescent property and Cu2+ sensing application. Colloids Surf. A. 2020;585:124112. [Google Scholar]
- Slenders E., Castello M., Buttafava M., Villa F., Tosi A., Lanzanò L., Koho S.V., Vicidomini G. Confocal-based fluorescence fluctuation spectroscopy with a SPAD array detector. Light Sci. Appl. 2021;10:31. doi: 10.1038/s41377-021-00475-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun R.X., Feng S.Y., Zhou B.Y., Chen Z.X., Wang D.X., Liu H.Z. Flexible cyclosiloxane-linked fluorescent porous polymers for multifunctional chemical sensors. ACS Macro Lett. 2020;9:43–48. doi: 10.1021/acsmacrolett.9b00901. [DOI] [PubMed] [Google Scholar]
- Thanzeel F.Y., Balaraman K., Wolf C. Quantitative chirality and concentration sensing of alcohols, diols, hydroxy acids, amines and amino alcohols using chlorophosphite sensors in a relay assay. Angew. Chem. Int. Ed. 2020;59:21382–21386. doi: 10.1002/anie.202005324. [DOI] [PubMed] [Google Scholar]
- Tian X.-M., Yao S.-L., Wu J., Xie H.M., Zheng T.-F., Jiang X.-J., Wu Y.Q., Mao J.G., Liu S.-J. Two benzothiadiazole-based fluorescent sensors for selective detection of Cu2+ and OH- ions. Polyhedron. 2019;171:523–529. [Google Scholar]
- To K.C., Ben-Jaber S., Parkin I.P. Recent developments in the field of explosive trace detection. ACS Nano. 2020;14:10804–10833. doi: 10.1021/acsnano.0c01579. [DOI] [PubMed] [Google Scholar]
- Wang B.-W., Jiang K., Li J.-X., Luo S.-H., Wang Z.-Y., Jiang H.F. 1,1-Diphenylvinylsulfide as a functional AIEgen derived from the aggregation-caused- quenching molecule 1,1-diphenylethene through simple thioetherification. Angew. Chem. Int. Ed. 2020;59:2338–2343. doi: 10.1002/anie.201914333. [DOI] [PubMed] [Google Scholar]
- Wang Q.R., Zheng C.L., Cui W., He F., Zhang J.Y., Zhang T.C., He C. Adsorption of Pb2+ and Cu2+ ions on the CS2-modified alkaline lignin. Chem. Eng. J. 2020;391:123581. [Google Scholar]
- Wang X.R., Shan M.X., Liu X.L., Wang M., Doherty C.M., Osadchii D., Kapteijn F. High-performance polybenzimidazole membranes for helium extraction from natural gas. ACS Appl. Mater. Inter. 2019;11:20098–20103. doi: 10.1021/acsami.9b05548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Zhang L., Yang L., Chang G.J. An indole-based smart aerogel for simultaneous visual detection and removal of trinitrotoluene in water via synergistic effect of dipole-π and donor-acceptor interactions. Chem. Eng. J. 2020;384:123358. [Google Scholar]
- Wei C., Ding P., Nie X.R., Stuart M.C.A., Wang J.Y. Europium based coordination polyelectrolytes enable core-shell-corona micelles as luminescent probes. Soft Matter. 2020;16:5727–5733. doi: 10.1039/d0sm00598c. [DOI] [PubMed] [Google Scholar]
- Wu K., Hu J.S., Cheng X.F., Li J.X., Zhou C.H. A superior luminescent metal-organic framework sensor for sensing trace Al3+ and picric acid via disparate charge transfer behaviors. J. Lumin. 2020;219:116908. [Google Scholar]
- Wu Z.-F., Velasco E., Shan C., Tan K., Zhang Z.-Z., Hu Q.-Q., Xing K., Huang X.-Y., Li J. Robust fluorescent calcium coordination polymers as Cu2+ sensors with high sensitivity and fast response. J. Mater. Chem. C. 2020;8:6820–6825. [Google Scholar]
- Wu Y.-C., Huo J.-P., Cao L., Ding S., Wang L.-Y., Cao D.R., Wang Z.-Y. Design and application of tribenzimidazolyl star-shape molecules as fluorescent chemosensors for the fast-response detection of fluoride ion. Sens. Actuators, B. 2016;237:865–875. [Google Scholar]
- Wu Y.-C., You J.-Y., Jiang K., Wu H.-Q., Xiong J.-F., Wang Z.-Y. Novel benzimidazole-based ratiometric fluorescent probes for acidic pH. Dyes Pigm. 2018;149:1–7. [Google Scholar]
- Xiao L.Q., Shi J., Nan B.F., Chen W.L., Zhang Q., Zhang E.D., Lu M.G. Highly sensitive detection of Fe3+ ions using waterborne polyurethane-carbon dots self-healable fluorescence film. Macromol. Mater. Eng. 2020;305:1900810. [Google Scholar]
- Xie Y.J., Ge Y.W., Peng Q., Li C.G., Li Q.Q., Li Z. How the molecular packing affects the room temperature phosphorescence in pure organic compounds: the ingeniously molecular design, detailed crystal analysis, and rational theoretical calculations. Adv. Mater. 2017;29:1606829. doi: 10.1002/adma.201606829. [DOI] [PubMed] [Google Scholar]
- Xiong S.Y., Marin L., Duan L., Cheng X.J. Fluorescent chitosan hydrogel for highly and selectively sensing of pnitrophenol and 2,4,6-trinitrophenol. Carbohydr. Polym. 2019;225:115253. doi: 10.1016/j.carbpol.2019.115253. [DOI] [PubMed] [Google Scholar]
- Xu T.-Y., Wang H., Li J.-M., Zhao Y.-L., Han Y.-H., Wang X.-L., He K.-H., Wang A.-R., Shi Z.-F. A water-stable luminescent Zn(II) coordination polymer based on 5-sulfosalicylic acid and 1,4-bis(1H-imidazol-1-yl)benzene for highly sensitive and selective sensing of Fe3+ ion. Inorg. Chim. Acta. 2019;493:72–80. [Google Scholar]
- Xu X.-Y., Lian X., Hao J.-N., Zhang C., Yan B. A double-stimuli-responsive fluorescent center for monitoring of food spoilage based on dye covalently modified EuMOFs: from sensory hydrogels to logic devices. Adv. Mater. 2017;29:1702298. doi: 10.1002/adma.201702298. [DOI] [PubMed] [Google Scholar]
- Yang J., Ren Z.C., Xie Z.L., Wang C., Xie Y.J., Peng Q., Chi Z.G., Li Q.Q., Li Z. The first AIEgen with fluorescence-phosphorescence dual Mechanoluminescent at room temperature. Angew. Chem. Int. Ed. 2017;56:880–884. doi: 10.1002/anie.201610453. [DOI] [PubMed] [Google Scholar]
- Yin G.-X., Niu T.-T., Gan Y.-B., Yu T., Yin P., Chen H.-M., Zhang Y.-Y., Li H.-T., Yao S.-Z. A multi-signal fluorescent probe with multiple binding sites for simultaneous sensing of cysteine, homocysteine, and glutathione. Angew. Chem. Int. Ed. 2018;57:4991–4994. doi: 10.1002/anie.201800485. [DOI] [PubMed] [Google Scholar]
- Yu S.J., Li W., Fujii Y.K., Omuraa T., Minami H. Fluorescent spherical sponge cellulose sensors for highly selective and semiquantitative visual analysis: detection of Hg2+ and Cu2+ ions. ACS Sustain. Chem. Eng. 2019;7:19157–19166. [Google Scholar]
- Zeng G., Xing S.H., Wang X.R., Yang Y.L., Ma D.X., Liang H.W., Gao L., Hua J., Li G.H., Shi Z. 3d-4f Metal-organic framework with dual luminescent centers that efficiently discriminates the isomer and homologues of small organic molecules. Inorg. Chem. 2016;55:1089–1095. doi: 10.1021/acs.inorgchem.5b02193. [DOI] [PubMed] [Google Scholar]
- Zhang H., Dong X.Z., Wang J.H., Guan R.F., Cao D.X., Chen Q.F. Fluorescence emission of polyethylenimine-derived polymer dots and its application to detect copper and hypochlorite ions. ACS Appl. Mater. Inter. 2019;11:32489–32499. doi: 10.1021/acsami.9b09545. [DOI] [PubMed] [Google Scholar]
- Zhang M.L., Zheng Y.J., Liu M., Ren Y.X., Wang Z.X., Cao J., Wang J.J. Two Cd(II)/Mn(II) coordination polymers showing dual responsive fluorescence sensing for Fe3+ and o-NAL. J. Solid State Chem. 2019;277:693–700. [Google Scholar]
- Zhang L., Yu S., Liang X.Y., Meng X.M., Zhang Q., Li P.W., Zhou Y. Cysteamine triggered “turn-on” fluorescence sensor for total detection of fumonisin B1, B2 and B3. Food Chem. 2020;327:137058. doi: 10.1016/j.foodchem.2020.127058. [DOI] [PubMed] [Google Scholar]
- Zhang R.Q., Hu L.P., Xu Z.X., Song Y.X., Li H.Q., Zhang X., Gao X.C., Wang M.X., Xian C.Y. A highly selective probe for fluorescence turn-on detection of Fe3+ ion based on a novel spiropyran derivative. J. Mol. Struct. 2020;1204:127481. [Google Scholar]
- Zhang Y.-M., Zhu W., Huang X.-J., Qu W.-J., He J.-X., Fang H., Yao H., Wei T.-B., Lin Q. Supramolecular AIE gels based on pillar[5]arene for ultrasensitive detection and separation of multi-analytes. ACS Sustain. Chem. Eng. 2018;6:16597–16606. [Google Scholar]
- Zhao Y.J., Peng D.F., Bai G.X., Huang Y.Q., Xu S.Q., Hao J.H. Multiresponsive emissions in luminescent ions doped quaternary piezophotonic materials for mechanical -to-optical energy conversion and sensing applications. Adv. Funct. Mater. 2021;31:2010265. [Google Scholar]
- Zhao Y.T., Ouyang H., Feng S., Luo Y.N., Shi Q.R., Zhu C.Z., Chang Y.-C., Li L., Du D., Yang H.P. Rapid and selective detection of Fe(III) by using a smartphone-based device as a portable detector and hydroxyl functionalized metal organic frameworks as the fluorescence probe. Anal. Chim. Acta. 2019;1077:160–166. doi: 10.1016/j.aca.2019.05.062. [DOI] [PubMed] [Google Scholar]
- Zheng L.B., Qi P., Zhang D. Identification of bacteria by a fluorescence sensor array based on three kinds of receptors functionalized carbon dots. Sens. Actuators B. 2018;262:913–921. [Google Scholar]
- Zheng Y.T., Wang H.L., Jiang J.Z. A porous tetraphenylethylene-based polymer for fast-response fuorescence sensing of Fe(III) ion and nitrobenzene. Dyes Pigm. 2020;173:107929. [Google Scholar]
- Zhu H., Fu L.S., Liu D., Li Y.-H., Dong G.-Y. Three water-stable luminescent Zn(II) coordination polymers for highly sensitive and selective sensing of acetylacetone and Fe3+ ions. J. Solid State Chem. 2020;286:121265. [Google Scholar]
- Zhuang X.R., Zhang N.X., Zhang X., Wang Y., Zhao L.Y., Yang Q.F. A stable Cu- MOF as a dual function sensor with high selectivity and sensitivity detection of picric acid and CrO42- in aqueous solution. Microchem. J. 2020;153:104198. [Google Scholar]
- Zhuang Y.P., Yao J.Y., Zhuang Z.Y., Ni C.J., Yao H.M., Su D.L., Zhou J., Zhao Z.J. Influence of alkyl chains on fluoranthene ensembles towards fluorescence based detection of 2,4,6-trinitrophenol. AEE-active conjugated polymers based on di(naphthalen-2- yl)-1,2-diphenylethene for sensitive fluorescence detection of picric acid. Dyes Pigm. 2020;174:108041. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are published in this manuscript and supplement; additional requests for data can be made by contacting the lead contact, Zhao-Yang Wang (wangzy@scnu.edu.cn).